Abstract
During summer 2020, the Maricopa County Department of Public Health (MCDPH) responded to a surge in COVID-19 cases. We used internet-based platforms to automate case notifications, prioritized investigation of cases more likely to have onward transmission or severe COVID-19 based on available preinvestigation information, and partnered with Arizona State University (ASU) to scale investigation capacity. We assessed the speed of automated case notifications and accuracy of our investigation prioritization criteria. Timeliness of case notification—the median time between receipt of a case report at MCDPH and first case contact—improved from 11 days to <1 day after implementation of automated case notification. We calculated the sensitivity and positive predictive value (PPV) of the investigation prioritization system by applying our high-risk prioritization criteria separately to data available pre- and postinvestigation to determine whether a case met these criteria preinvestigation, postinvestigation, or both. We calculated the sensitivity as the percentage of cases classified postinvestigation as high risk that had also been classified as high risk preinvestigation. We calculated PPV as the percentage of all cases deemed high risk preinvestigation that remained so postinvestigation. During June 30 to July 31, 2020, a total of 55 056 COVID-19 cases with an associated telephone number (94% of 58 570 total cases) were reported. Preinvestigation, 8799 (16%) cases met high-risk criteria. Postinvestigation, 17 037 (31%) cases met high-risk criteria. Sensitivity was 52% and PPV was 98%. Automating case notifications, prioritizing investigations, and collaborating with ASU improved the timeliness of case contact, focused public health resources toward high-priority cases, and increased investigation capacity. Establishing partnerships between health departments and academia might be a helpful strategy for future surge capacity planning.
Keywords: COVID-19, communicable disease, contact tracing, public health practice, disease outbreaks, internet-based intervention
In the United States, the COVID-19 pandemic strained historically underfunded public health resources because of high levels and a rapidly fluctuating incidence of infection.1,2 Located in Phoenix, Arizona, the Maricopa County Department of Public Health (MCDPH) is the country’s third largest local health jurisdiction, serving approximately 4.4 million people across >9000 square miles. 3 In a typical year, an estimated 80 000 cases of reportable conditions are reported in Maricopa County. In 2020, MCDPH received more than 541 000 case reports, including more than 460 000 COVID-19 case reports.
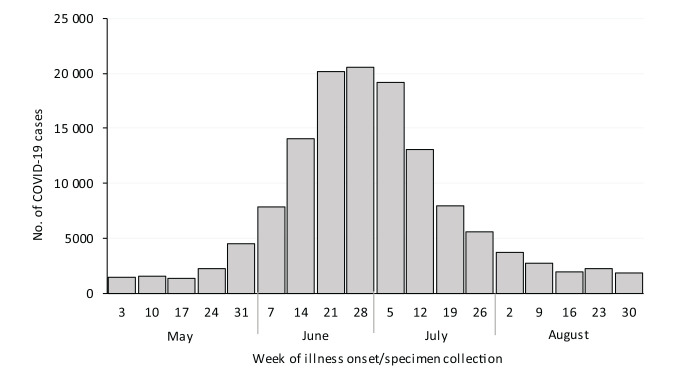
By May 2020, the number of COVID-19 cases had declined or plateaued in much of the United States; however, in Maricopa County, the number of cases began to rise rapidly after expiration of the governor’s 6-week stay-at-home order and the Memorial Day holiday weekend (Figure 1).4-6 After rapidly increasing in June, the 7-day average peaked at 3199 cases per day (71 cases per 100 000 people)—10 times the 7-day average of 328 cases per day (7 cases per 100 000 people) 1 month prior. 7 The case influx overwhelmed the existing public health infrastructure, lengthened case notification, and decreased investigation timeliness, consistent with the experience of other health departments.8,9
Figure 1.
Confirmed and probable COVID-19 cases among residents of Maricopa County, Arizona, by week of illness onset or specimen collection, May–August 2020. Confirmed and probable cases were based on Council of State and Territorial Epidemiologists case definitions. 9
MCDPH staff responded by adapting case investigation processes to use internet-based platforms to automate notifications, prioritize investigation of cases more likely to have onward transmission or severe COVID-19 outcomes based on certain high-risk criteria, and partner with Arizona State University (ASU) to maximize efficiency and meet demand.
Purpose
After the summer surge in COVID-19 cases among Maricopa County residents, we assessed the speed of our automated case notifications and the accuracy of our investigation prioritization processes during the June 30–July 31, 2020, case-reporting period.
Methods
Automation of Case Notification
To improve the timeliness of COVID-19 case notifications and investigations, MCDPH staff used Health Insurance Portability and Accountability Act (HIPAA)–compliant internet-based platforms to automate telephone calls (Everbridge) and text messages (Qualtrics). We sent automated notifications to Maricopa County residents within 24 hours of being reported as having a confirmed or probable 10 COVID-19 case. The messages in English and Spanish, which did not include protected health information or personalized laboratory results, stated, “[MCDPH] recently learned that a person with this phone number tested positive for COVID-19. If you have not been tested for COVID-19, please ignore this message.” The notification then instructed the person to isolate at home and notify household members to quarantine. It directed the person to the MCDPH website for additional infection control recommendations and resources, including how to request MCDPH-funded hotel space if they were unable to isolate at home and information to share with their close contacts. People without a telephone number were assigned to a separate team to determine contact information through standard operating procedures; those who resided in a known congregate setting (eg, long-term care, correctional facility), were aged <18 years, or died were excluded from automated case notification.
In addition, the automated text message included a secure link to a case questionnaire (also via Qualtrics). Information collected in the questionnaire included date of illness onset, symptoms experienced, hospitalization status, and type of residence and worksite (eg, congregate setting, health care facility, correctional facility). Preinvestigation data present in the statewide communicable disease database, Medical Electronic Disease Surveillance Intelligence System (MEDSIS), or collected from the case questionnaire (if available) were used to prioritize case investigations. If a case did not meet high-risk criteria (defined hereinafter) after completing the questionnaire, data collected were used in place of a telephone interview, as the questionnaire contained most standard interview questions. Any close contacts provided in the questionnaire were automatically forwarded to the MCDPH contact tracing team for notification and voluntary enrollment in symptom monitoring (not described in this article).
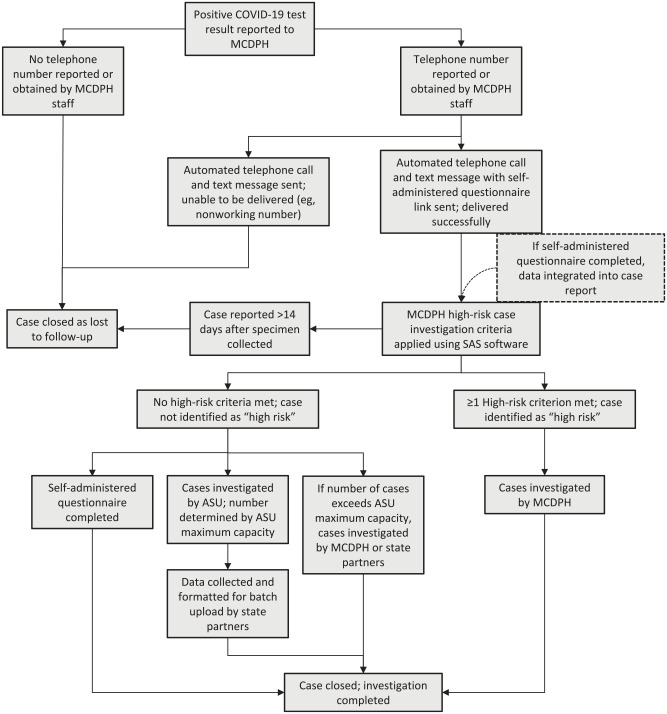
All data collected in the questionnaire corresponded to case investigation variables in MEDSIS and were uploaded daily to the person’s case report by deterministic matching using a unique identification variable created from the person’s name and date of birth (Figure 2). Duplicates were manually reviewed during daily data processing.
Figure 2.
Process flow chart for Maricopa County Department of Public Health (MCDPH) COVID-19 automated case notification, prioritization, and assignment to Arizona State University (ASU) partner investigators. Cases meeting high-risk criteria included being aged <18 years or ≥65 years, residing in a known congregate setting (eg, long-term care, correctional facility), working in a high-risk setting (eg, health care, correctional facility), being hospitalized for COVID-19, dying within 14 days of the case being reported to MCDPH, or being associated with an ongoing outbreak.
Academic Health Department Partnership
MCDPH expanded its preexisting partnership with ASU to include COVID-19 case investigations and rapidly scale investigation capacity. An intergovernmental agreement between MCDPH and ASU was executed and included financial payment to ASU per case investigated. ASU houses the Student Outbreak Response Team, which has been in continuous operation since 2018 and uses a graduate-level course to train global health students to conduct case investigations and contact tracing for various infectious disease outbreaks. ASU rapidly converted this group from a small in-person team to a large, virtual, dedicated COVID-19 case investigation team.
During summer 2020, 71 ASU investigators completed more than 550 call hours interviewing people in English and Spanish using an internet-based, HIPAA-compliant case investigation form designed by MCDPH investigators for use by ASU investigators. The investigation form included most interview questions in MEDSIS. All interview data were securely shared through the MCDPH Qualtrics account; MCDPH staff formatted investigation data to upload to corresponding variables in the MEDSIS case report. All data collected outside of MEDSIS were uploaded to the system, which allowed for analysis of exported MEDSIS data.
Case Investigation Prioritization
Certain cases were classified as having a higher-than-typical risk for onward transmission or severe COVID-19 outcomes; these cases were automatically assigned to MCDPH staff for case investigation (Figure 2). The risk assessment was based on data available from the MEDSIS preinvestigation, which could include information from the testing health care provider or laboratory case reports, facility reporters (eg, long-term care facility, correctional facility; if applicable), databases linked by the Arizona Department of Health Services (ADHS; eg, Hospital Information Exchange), self-administered questionnaire (if available), and death certificate (if applicable). During our analysis period, “high risk” was defined as a case among people aged <18 years or ≥65 years, who resided in a known congregate setting (eg, long-term care facility, correctional facility), who worked in a high-risk setting (eg, health care facility, correctional facility), who were hospitalized for COVID-19, who died within 14 days of the case being reported to MCDPH, or who were associated with an ongoing outbreak. A subset of cases appearing not to have any of these high-risk criteria could be assigned to ASU investigators (up to 100 cases per day). All remaining cases were assigned to either MCDPH or ADHS investigators.
Case Notification Automation and Investigation Prioritization Assessment
Timeliness of case notification was defined as the number of days from the time the case report was first received at MCDPH to the time of first public health outreach to the case patients. We examined the difference in median case notification timeliness before and after implementation of the automated system.
We calculated the sensitivity and positive predictive value (PPV) of the case investigation prioritization system to determine the accuracy of risk determination based on preinvestigation data in MEDSIS and, if available, self-administered questionnaire information. Our assumption was that we could use the complete set of data available to us postinvestigation to correctly classify cases as high risk. We applied the high-risk criteria separately to the data available to us pre- and postinvestigation to determine whether a case met high-risk criteria at preinvestigation, postinvestigation, or both. We calculated sensitivity as the percentage of cases classified postinvestigation as high risk (denominator = true positives + false negatives) that had also been classified as high risk preinvestigation (numerator = true positives). We calculated PPV as the percentage of all cases deemed high risk preinvestigation (denominator = true positives + false positives) that remained in that category postinvestigation (numerator = true positives).
These activities involved the collection and analysis of health data by a public health authority as required under Arizona Administrative Code 11 and were performed to control the spread of SARS-CoV-2 in our jurisdiction and improve an existing public health program. Therefore, this work qualified as public health practice, which did not require review by an institutional review board.
Outcomes
During June 30–July 31, 2020, a total of 58 570 COVID-19 cases (median [interquartile range (IQR)], 1415 [1088-2650] cases per day) were reported to MCDPH. During the 2 weeks before implementation of automatic case notification, median (IQR) case notification timeliness was 11 (7-14) days. After implementation of automated daily case notification, timeliness was <1 day for all cases.
Ninety-four percent of cases (55 056 of 58 570) were reported with an associated telephone number and were sent automated notifications by telephone and text message. Of those, 51 522 (88%) text message notifications were received (median [IQR], 1534 [1265-179] per day). Among people receiving these text message notifications, 17 385 (34%) opened the questionnaire link and 8670 (17%) completed the questionnaire.
Using preinvestigation data, we classified 8948 cases as high risk; postinvestigation, we reclassified these cases as 8799 true positives and 149 false positives for the high-risk category. Postinvestigation, we classified 17 037 cases (ie, the 8799 true positives plus 8238 false negatives) as high risk. Examples of cases inaccurately classified as high risk were laboratory reports with incorrect dates of birth and people erroneously marked as associated with a congregate living setting. Examples of cases initially missed as high risk were people who did not complete the case questionnaire, were aged <65 years, and resided in a congregate setting that were later reported to MCDPH by the facility or were hospitalized and later identified as such by linking of Health Information Exchange data or worked in a health care setting and were later reported by their employer. If a case did not initially meet high-risk criteria and was triaged to ASU for investigation, the ASU investigator completed the interview, and the case was routed back to MCDPH investigators for case and/or facility follow-up, if needed. Overall, the sensitivity of the preinvestigation prioritization of high-risk cases was 52% (ie, 8799 of 17 037) and the PPV was 98% (ie, 8799 of 8948).
Lessons Learned
Innovations to information technology platforms routinely used by health department staff can improve the timeliness of case notification, identify cases to prioritize for investigation, and distribute investigation workload. Our multiplatform approach using outreach by both telephone and text message included a spectrum of technological literacy to maximize the number of people receiving information about what to do after receiving a positive test result. Those who received a text message could complete a self-administered questionnaire, providing MCDPH with additional preinvestigation information, which benefited both Maricopa County residents and MCDPH investigators by minimizing the number of telephone calls. Using preinvestigation information to categorize risk and prioritize investigations was computationally simple and achieved without any additional resource-intensive case interviews. Our criteria were highly predictive of a case being high risk for ongoing transmission or severe COVID-19; however, a system limitation was the relatively low sensitivity (52%) in detecting high-priority cases preinvestigation. Sensitivity could be improved if additional data, such as whether a person resides in a congregate setting, were included in electronic laboratory reports. Enhanced data in electronic laboratory reports could have future utility for prioritizing investigations of reportable conditions other than COVID-19.
This analysis was limited by the fact that pre- and postinvestigation data were available for people with confirmed or probable cases who completed the preinvestigation questionnaire; were interviewed by MCDPH, ADHS, or ASU investigators; were reported by a partner organization (eg, long-term care facility, jail, homeless shelter); or had additional data linked to their case in MEDSIS by external data-merging processes by ADHS. A case was classified as not being high risk if preinvestigation high-risk criteria were not met and no additional high-risk criteria were met postinvestigation, including if the case patient was not interviewed. This classification assumption may have increased our sensitivity and PPV estimates.
Systems to manage rapidly evolving outbreaks and epidemics should be adaptable at the local level while also producing data that can easily integrate into state and federal data-reporting systems. During a constantly evolving pandemic, MCDPH staff used information technology resources that could be locally customized with updates possible in <24 hours to case notification messaging, infection control recommendations, and case interview scripts. These adaptations were critical to ensure MCDPH resources were responsive to guideline updates (eg, Centers for Disease Control and Prevention [CDC] isolation and quarantine recommendations 12 ), reprioritization (eg, when in-person school resumed), and changing data collection needs. As a large local health department serving 4.4 million people, 3 MCDPH had to be able to pivot its messaging, guidance, and investigations quickly without relying on external partners to change the functionality of its systems.
Partnering with a local university was mutually beneficial. The partnership between MCDPH and ASU helped rapidly scale investigation capacity while providing a valuable learning experience for students interested in pursuing a public health career. ASU’s organizational structure with a faculty leader, supported by a graduate student leadership team, facilitated clear and rapid communication with MCDPH. This structure was needed to navigate university requirements, such as ensuring investigators were able to earn university credit, if applicable. The most substantial challenge we encountered was communicating rapidly changing federal guidance to our ASU counterparts and adapting our processes accordingly. 12 Establishing consistent teams at the health department and ASU with whom to communicate all guidance and process changes made this process more efficient. Preexisting collaboration with ASU made it easier to scale up the workforce during COVID-19 case surges, and the operational flexibility of the student and volunteer workforce allowed investigations to occur outside traditional business hours. ASU was initially assigned 100 cases per day and increased to a maximum of 500 cases per day during 2 subsequent case surges in 2020-2021. Developing such partnerships might be a helpful strategy in surge capacity planning. Intergovernmental agreements between health departments and academic partners might allow affiliates to be credentialed to routinely investigate a subset of reportable conditions and then smoothly transition to a larger role should an outbreak of greater need arise. During contract renewal processes, it was challenging to predict the scope of future case investigation support that would be needed. Incorporating some degree of flexibility into a contract with an academic partner may be beneficial. In addition, consistent increased public health funding is likely necessary to sustain this type of partnership.
In Maricopa County, a rapid increase in COVID-19 cases required innovative solutions to ensure timely case notification, prioritize investigations, and rapidly scale capacity. The early implementation, cumulative efficiency, and adaptability of these systems, together with an expanded university partnership, helped MCDPH respond to subsequent COVID-19 case surges resulting from variable vaccination rates and highly transmissible viral variants. We will continue to use these systems and adapt our high-priority and investigation assignment criteria as we follow CDC’s guidance to shift from universal case investigation to focus on high-risk settings. 13
Acknowledgments
The authors appreciate the hard work of all members of the MCDPH COVID-19 Intelligence Section and ASU Student Outbreak Response Team, especially that of Tim Dennehy, Gloria Kaririwe, Daniela Ledesma, Hanna Maroofi, Laura Meyers, and Jasmine Truong. We thank our Arizona Department of Health Services colleagues for their partnership. We are grateful for the support and mentorship of our Centers for Disease Control and Prevention colleagues Kris Bisgard, Ariella Dale, and Rebecca Sunenshine.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: This work was supported by the US Treasury as part of the Coronavirus Aid, Relief, and Economic Security Act.
ORCID iD: Sarah E. Scott, MD  https://orcid.org/0000-0002-3325-8169
https://orcid.org/0000-0002-3325-8169
References
- 1. Maani N, Galea S. COVID-19 and underinvestment in the health of the US population. Milbank Q. 2020;98(2):239-249. doi: 10.1111/1468-0009.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janes C, Wan W. The nation’s public health agencies are ailing when they’re needed most. The Washington Post. Published August 31, 2020. Accessed July 3, 2021. https://www.washingtonpost.com/health/coronavirus-public-health-system/2020/08/31/4a6edec0-d662-11ea-aff6-220dd3a14741_story.html
- 3. US Census Bureau. QuickFacts: Maricopa County, Arizona, population, census, April 1, 2020. Accessed March 7, 2022. https://www.census.gov/quickfacts/fact/table/maricopacountyarizona
- 4. Centers for Disease Control and Prevention. COVID-19 data tracker: trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Accessed October 1, 2021. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases|New_case|select
- 5. Ducey DA. Executive order 2020-18: Stay Home, Stay Healthy, Stay Connected. April 30, 2020. Accessed July 3, 2021. https://azgovernor.gov/sites/default/files/eo_2020-18_stay_home_stay_healthy_stay_connected_1.0.pdf
- 6. Ducey DA. Executive order 2020-36: Stay Healthy, Return Smarter, Return Stronger. May 12, 2020. Accessed July 3, 2021. https://azgovernor.gov/sites/default/files/executive_order_2020-36_return_stronger.pdf
- 7. Maricopa County Department of Public Health. COVID-19 epidemic curve. Accessed March 7, 2022. https://phdata.maricopa.gov/Dashboard/e10a16d8-921f-4aac-b921-26d95e638a45?e=false&vo=viewonly
- 8. Spencer KD, Chung CL, Stargel A, et al. COVID-19 case investigation and contact tracing efforts from health departments—United States, June 25–July 24, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(3):83-87. doi: 10.15585/mmwr.mm7003a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Prioritizing case investigations and contact tracing for COVID-19 in high burden jurisdictions. Updated February 19, 2021. Accessed January 4, 2021. https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/prioritization.html
- 10. Council of State and Territorial Epidemiologists. Update to the standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). August 2020. Accessed August 1, 2021. https://cdn.ymaws.com/www.cste.org/resource/resmgr/ps/positionstatement2020/Interim-20-ID-02_COVID-19.pdf
- 11. Ariz Adm Code, Title 9, Ch 6, §206. Local health agency responsibilities regarding communicable disease reports (July 1, 2021). Accessed March 2, 2022. https://apps.azsos.gov/public_services/Title_09/9-06.pdf
- 12. Centers for Disease Control and Prevention. Quarantine and isolation. Updated January 4, 2022. Accessed January 27, 2022. https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html
- 13. Centers for Disease Control and Prevention. Prioritizing case investigation and contact tracing for COVID-19. Updated February 28, 2022. Accessed March 25, 2022. https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/prioritization.html