Abstract
SecA, the translocation ATPase of the preprotein translocase, accounts for 0.25% of the total protein in a degU32(Hy) Bacillus subtilis strain in logarithmic phase. The SecA level remained constant irrespective of the demand for exoprotein production but dropped about 12-fold during the late stationary phase. Modulation of the level of functional SecA during the exponential phase of growth affected differently the secretion of levansucrase and α-amylase overexpressed under the control of the sacB leader region. The level of SecA was reduced in the presence of sodium azide and in the div341 thermosensitive mutant at nonpermissive temperatures. Overproduction of SecA was obtained with a multicopy plasmid bearing secA. The gradual decrease of the SecA level reduced the yield of secreted levansucrase with a concomitant accumulation of unprocessed precursor in the cells, while an increase in the SecA level resulted in an elevation of the production of exocellular levansucrase. In contrast, α-amylase secretion was almost unaffected by high concentrations of sodium azide or by very low levels of SecA. Secretion defects were apparent only under conditions of strong SecA deprivation of the cell. These data demonstrate that the α-amylase and levansucrase precursors markedly differ in their dependency on SecA for secretion. It is suggested that these precursors differ in their binding affinities for SecA.
The protein SecA is an essential component of the general translocation pathway in bacteria. It is the peripheral subunit of the preprotein translocase, a multisubunit integral protein complex (see recent reviews in references 7 and 10). SecA is present in many gram-negative and gram-positive bacteria (2, 15, 18, 35), in primitive algae (47), and in cyanobacteria and the chloroplasts of higher plants (1, 14, 50–52). Studies with Escherichia coli have shown that the ATPase activity of SecA is essential for protein translocation (23, 33). SecA is the only ATPase involved in protein translocation, and its activity is stimulated by high-affinity interactions with preproteins (6, 8, 16, 24, 28).
The Bacillus subtilis secA homologue gene, div+, has been identified, cloned, and sequenced (39, 40). The deduced amino acid sequence is very similar to that of the E. coli SecA protein (31, 41), with 50% sequence identity. Like its E. coli SecA counterpart, Div is a homodimer possessing translocation ATPase activity (19, 45, 48). Nevertheless, Div complements secA mutants of E. coli only when it is expressed at a very low level (19, 49). The amino-terminal ATP binding domain of Div can functionally replace the corresponding region of SecA, and this region is thought to regulate the integration of SecA into the cytoplasmic membrane (25, 36).
The div-341 mutation was initially identified during screening for septum initiation mutants (38). This temperature-sensitive mutation affects not only cell division but also the initiation of sporulation and competence at nonpermissive temperatures, and it reduces protease production (39). This parallels the first secA mutation isolated in E. coli, which is also a temperature-sensitive mutation located within or near a cluster of genes responsible for cell division and septation (30). The protein translocation defect in the div-341 mutant is believed to be caused by the rapid degradation of the SecA variant (46).
The dependence of native B. subtilis proteins expressed from their chromosomal genes on SecA has never been investigated in detail. We have, therefore, compared the effects of the modulation of the SecA level on the secretion of levansucrase and α-amylase during the exponential phase of growth when these two proteins are expressed in the same genetic context and under the same regulated control. The results indicate a vast difference in SecA dependency for the secretion of levansucrase and α-amylase. While the secretion of levansucrase gradually varies with the SecA level in the cell, the secretion of α-amylase is affected only under conditions of strong SecA depletion. These results are discussed in terms of differences in affinities of the precursors for SecA.
MATERIALS AND METHODS
Strains and media.
The strains and plasmids used are listed in Table 1. B. subtilis GM96104, containing a sacR-amyE fusion, was constructed as described for strain GM96101 (21). Plasmid pGMS57 was inserted by double crossing over into the chromosome of strain NIG1156 (39). The resulting strain, GM96200, was transformed with pGMK58 (21) by a Campbell-like mechanism. Transformants were selected on Luria broth plates containing the appropriate antibiotic. One of the transformants (strain GM96104) containing the fusion sacR-amyE and having sucrose-inducible α-amylase production was used. Strain GM9801 overproducing SecA was obtained by transforming strain QB112 with pWMKL1 (17). Plasmid pWMKL1, derived from pWH1520 (37), contained the cloned secA wild-type gene of B. subtilis 168 under the inducible control of xylose.
TABLE 1.
Strains of B. subtilis and plasmids
| Strain or plasmid | Relevant genotype or characteristics | Source or reference |
|---|---|---|
| Strains | ||
| QB112 | degU32(Hy) sacA321 | 22 |
| GM96101 | degU32(Hy) sacA321 Δ(sacR-sacB) sacR-amyE | 21 |
| NIG1156 | degU32(Hy) secA(Ts) (div-341) | 39 |
| GM96200 | NIG1156 Δ(sacR-sacB) | This study |
| GM96104 | degU32(Hy) secA(Ts) Δ(sacR-sacB) sacR-amyE | This study |
| GM9801 | degU32(Hy) sacA321; pWMKL1 | This study |
| Plasmids | ||
| pGMS57 | pGM56 Spr | 21 |
| pGMK58 | pGMK50 with the BamHI-EcoRV fragment deleted and the sacR-amyE fusion ligated at these sites | 21 |
| pWH1520 | Apr Tcr | 37 |
| pWMKL1 | pWH1520 with secA under the control of the xylR promoter | 19 |
B. subtilis QB112, GM96101, and GM96801 were each grown at 37°C in minimal medium (4) supplemented with 1% (wt/vol) glucose or 1% glucitol, 0.25% Casamino Acids, and 15 μg of tetracycline ml−1, respectively. CaCl2 was added at 0.5 mM to the culture medium of strains GM96101 and GM96104. Strains NIG1156 and GM96104 were grown in the same medium at 30°C (permissive temperature) or at nonpermissive temperatures as indicated. Levansucrase and α-amylase expression was induced by sucrose at the concentrations indicated in the figure legends. SecA synthesis by the strain containing pWKLM1 was induced by 0.5% xylose.
Enzyme assays.
Levansucrase activity was assayed in an acetone-water mixture (vol/vol), pH 6, containing 50 mM sucrose (5). Under these conditions, one enzyme unit corresponds to 6 mg of pure protein. α-Amylase activity was assayed at 37°C, with p-nitrophenyl-maltotrioside as the substrate (bioMerieux) at pH 6.3 in 0.1 M potassium phosphate or potassium acetate. One enzyme unit corresponds to 25 mg of pure protein. Differential rates of synthesis were evaluated by measuring enzyme production as a function of growth (4).
Quantification of proteins in cell extracts.
Samples of 2-ml culture were centrifuged, and the pellets were resuspended in electrophoresis sample buffer and sonicated (three 30-s pulses). Aliquots of the extracts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and quantitative immunoblotting by using a standard calibration curve of the pure protein. Radioactive bands were quantified with a PhosphorImager (Molecular Dynamics). The precursors of levansucrase and α-amylase and the SecA protein were analyzed by immunoblotting (34). Proteins were measured by the Bradford assay (3).
Pulse-labelling and chase experiments.
Culture samples (0.5 ml) were pulse-labelled with [35S]methionine for 3 min, and reactions were stopped in ice-cold stopping buffer (0.1 M sodium phosphate, pH 7, containing 2.4 M KCl, 200 μg of chloramphenicol ml−1, and 0.2 mM phenylmethylsulfonyl fluoride). Cell suspensions were centrifuged, and the supernatants were dialyzed against 1 mM sodium phosphate at pH 6 for 150 min at 4°C and then lyophilized. The dry samples were resuspended in electrophoresis sample buffer, boiled for 3 min, and analyzed by SDS-PAGE.
Cells were pulse-labelled at an optical density at 600 nm (OD600) of 2 by adding 0.25 mCi (9 mBq) of [35S]methionine (800 mCi mmol−1) to a 1-ml culture suspension maintained at 37°C for 45 s. Nonradioactive methionine (4 mM final concentration) was then added. Samples (0.2 ml) were withdrawn at intervals, and all reactions were immediately stopped by diluting the samples threefold with ice-cold stopping buffer. Cell suspensions and bacterial pellets were treated as described previously (21). The samples were finally analyzed by SDS-PAGE, and the bands were quantified with a PhosphorImager.
RESULTS
SecA level in a degU32(Hy) strain of B. subtilis.
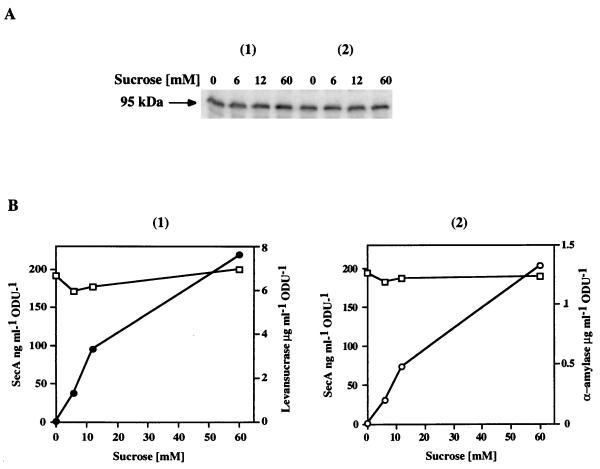
B. subtilis produced exoproteins during the exponential and stationary phases of growth. During the logarithmic phase, SecA accounts for approximately 0.25% of the total cellular protein (225 ng per optical density unit), but the level decreases after the cells enter the stationary phase, reaching a basal level about 12-fold lower. To determine the influence of exocellular protein production on the level of SecA in the cell during exponential growth, the production of levansucrase or α-amylase was examined as a function of the SecA level in strain QB112 or GM96101, respectively (Fig. 1A and B). In these strains, the synthesis of levansucrase and α-amylase is under the control of the sucrose-inducible sacB promoter, and production of SecA increases almost linearly with the sucrose concentration (4, 21). The level of intracellular SecA remained unchanged (about 1.8 μM) regardless of the yield of levansucrase or α-amylase produced. The induction of α-amylase expression at 60 mM sucrose in GM96101 cells and of levansucrase at 8 mM sucrose in QB112 cells resulted in similar yields of the two gene products. This permits modulation of the level of SecA under conditions in which both exoproteins are secreted at the same rate.
FIG. 1.
The cellular SecA level is not affected by the high-level production of levansucrase and α-amylase. Strains QB112 and GM96101 were induced for synthesis of levansucrase or α-amylase with various concentrations of sucrose inducer at an OD600 of 0.25. Samples of 2-ml cell suspensions were withdrawn after three generations (final OD600 of 2) and centrifuged. Levansucrase or α-amylase activities in the supernatants were assayed. The cell pellets were treated as described in Materials and Methods and analyzed by immunoblotting with SecA antibodies. Known quantities of pure SecA were used as standards for quantitative immunoblotting and quantified with a PhosphorImager. (A) SecA immunoblotting from strains QB112 (panel 1) and GM96101 (panel 2) induced with the sucrose concentrations indicated; (B) levansucrase (●) and α-amylase (○) activities assayed in QB112 and GM96101 supernatants, respectively, and SecA in cells (□) as a function of sucrose concentration. ODU, optical density unit.
Different dose-dependent effects of sodium azide on the processing and production of levansucrase and α-amylase.
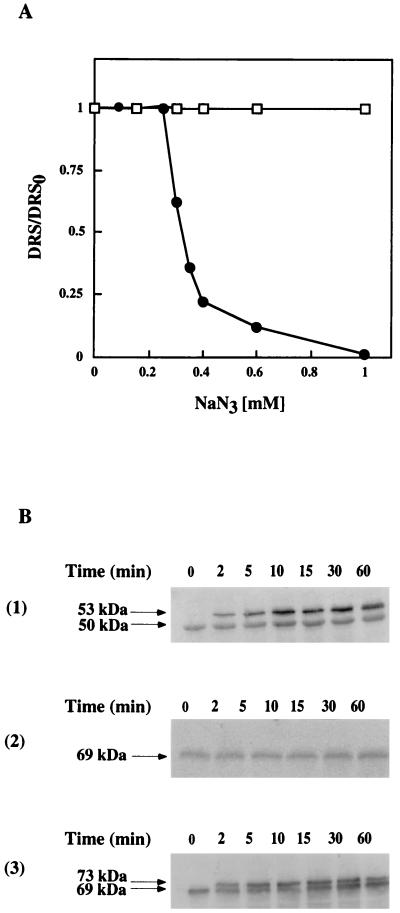
The effect of low levels of functional SecA on levansucrase and α-amylase secretion was studied by using sodium azide to specifically inhibit SecA ATPase activity (11, 32). The growth of QB112 and GM96101 was similarly affected in the presence of sodium azide. It remained unmodified up to 0.4 mM NaN3, was slightly reduced by higher concentrations, and was completely blocked by 3 mM; cells started to lyse at 10 mM (data not shown). The production of exocellular α-amylase was not affected when cells were grown in the presence of 0 to 1 mM sodium azide, while the levansucrase secretion decreased rapidly at NaN3 concentrations above 0.25 mM (Fig. 2A). To determine if α-amylase secretion is totally insensitive to sodium azide, the effect of 3 mM NaN3 was tested. Under these drastic conditions, cells no longer divided and α-amylase was not secreted (data not shown). Next, the effect of sodium azide on the fate of the precursors of each protein after the addition of 0.3 mM sodium azide to culture was monitored. This concentration inhibited levansucrase production by about 50% but had no effect on α-amylase (Fig. 2A). Unprocessed levansucrase precursor rapidly accumulated in the cells (Fig. 2B, panel 1), but there was no detectable unprocessed precursor of α-amylase (Fig. 2B, panel 2). The unprocessed precursor of α-amylase accumulated only when azide was added at a 10-fold-higher azide concentration (3 mM) (Fig. 2B, panel 3). This suggests that different levels of functional SecA are required for the efficient processing of levansucrase and α-amylase precursors. However, this suggestion is based on a specific effect of sodium azide on the SecA ATPase activity (11, 32), leaving open the possibility that this metabolic poison has other effects on cell physiology, which could complicate the interpretation.
FIG. 2.
(A) Effect of sodium azide on the production of exocellular levansucrase and α-amylase by strains QB112 and GM96101, respectively. Cells at an OD600 of 0.2 were induced with 8 mM sucrose for levansucrase synthesis and 60 mM sucrose for α-amylase synthesis. After two generation times (90 min), the cell suspensions were divided and supplemented with various concentrations of sodium azide. Levansucrase (●) and α-amylase (□) were assayed in the supernatants of samples taken from cultures grown in the presence of sodium azide concentrations up to 1 mM. DRS and DRS0, differential rates of the enzyme released in supernatant after the addition of sodium azide and in the absence of azide, respectively. (B) Processing of the precursors of levansucrase and α-amylase in strains QB112 and GM96101 grown in the presence of sodium azide was analyzed by immunoblotting of cell extracts. Strains QB112 (panel 1) and GM96101 (panels 2 and 3) were induced with 8 and 60 mM sucrose, respectively, at an OD600 of 0.5. After two generation times, sodium azide was added (panels 1 and 2, 0.3 mM final concentration; panel 3, 3 mM), and at the times indicated, samples of 5 ml were withdrawn, centrifuged, and analyzed by immunoblotting as described in Materials and Methods.
The yield of exocellular levansucrase production is proportional to the amount of SecA.
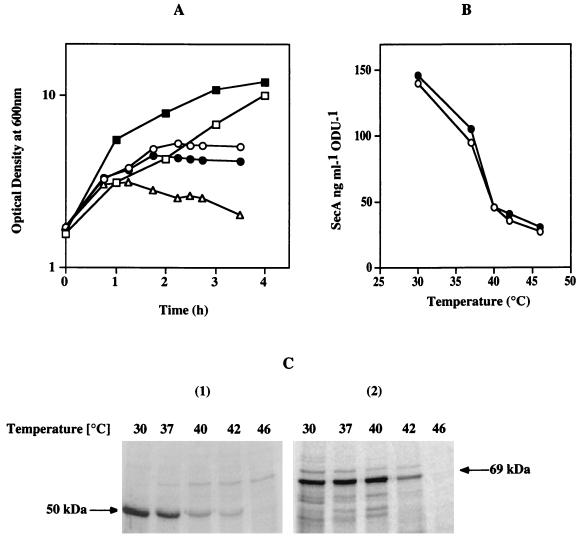
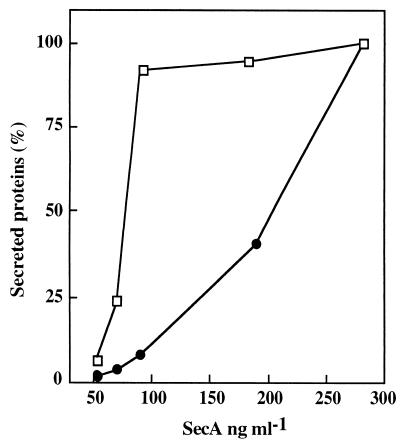
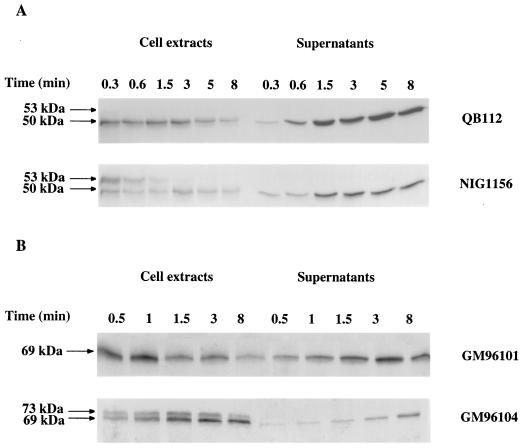
To correlate the production of exoproteins with the amount of SecA in the cells, further experiments were performed with the secA thermosensitive mutant (the div-341 mutant). The residual SecA was estimated by quantitative immunoblotting, and the yield of exocellular α-amylase and levansucrase was determined by pulse experiments at various nonpermissive temperatures. For this purpose, strain GM96104 was derived from strain NIG1156 (39) by first deleting the chromosomal region sacB, yielding strain GM96200, and then introducing the sacR-amyE fusion by Campbell-like integration of the plasmid pGMK58 (Table 1) (21). Strain GM96200 was used as a control. Growth of double-mutant strains which were secA(Ts) degU32(Hy) and had been transferred from 30°C (permissive temperature) to nonpermissive temperatures was affected after one generation at 40 and 43°C and more rapidly at 46°C (Fig. 3A). The levels of SecA in cell extracts of both strains were identical, and they decreased sharply at temperatures above 30°C (Fig. 3B), in agreement with results reported by Nakane et al. (29). The production of exocellular levansucrase and α-amylase after incubation for 8 min at each temperature was measured by means of a 3-min pulse experiment with radioactive methionine. α-Amylase production remained constant up to 40°C, while levansucrase production decreased at temperatures above 30°C (Fig. 3C). The yield of secreted levansucrase and α-amylase plotted against the residual level of SecA (Fig. 4) again reveals the differential requirements of SecA for levansucrase and α-amylase secretion.
FIG. 3.
Effect of SecA depletion on the secretion of levansucrase or α-amylase by the secA(Ts) strains NIG1156 and GM96104. (A) Growth of secA(Ts) strains at permissive and nonpermissive temperatures. Cells of strains NIG1156 and GM96104 were grown at 30°C (□) in minimal medium supplemented with 1% glucose, and levansucrase or α-amylase synthesis was induced with 8 or 60 mM sucrose, respectively. At an OD600 of 2, the initial cultures were divided into parts, and each part was transferred at the following temperatures: 37°C (■), 40°C (○), 43°C (●), and 46°C (▵). (B) SecA levels in cell extracts of secA(Ts) strains NIG1156 (●) and GM96104 (○) at various temperatures. Samples were processed as described above, analyzed for SecA by immunoblotting, and quantified with a PhosphorImager. (C) Production of exocellular levansucrase and α-amylase in secA(Ts) strains NIG1156 (panel 1) and GM96104 (panel 2) at various temperatures. Cells were grown to an OD600 of 2 as described above, shifted to the temperature indicated, and incubated for 8 min. Then 2 ml of each was pulse-labelled for 3 min with 0.15 mCi of [35S]methionine as described in Materials and Methods. The supernatants were analyzed by SDS-PAGE.
FIG. 4.
Production of levansucrase (●) and α-amylase (□) as a function of SecA. Data were taken from Fig. 3C, panels 1 and 2.
To determine at which step secretion was affected after depletion of the SecA function, pulse-chase experiments were performed at nonpermissive temperatures. At 37°C, processing remained too fast to detect α-amylase precursor (results not shown), whereas at the same temperature, the rate of levansucrase precursor processing decreased dramatically from a half-life of 5 s for the wild type (34) to 60 s for the thermosensitive mutant (Fig. 5A). Pulse-chase experiments at 42°C with the thermosensitive mutant producing α-amylase indicate that a large decrease in SecA also results in the blockage of the processing of α-amylase precursor (Fig. 5B). These results further support our notion that α-amylase secretion and levansucrase secretion have different SecA dependencies in vivo.
FIG. 5.
Kinetics of levansucrase (A) or α-amylase (B) processing and release by strains NIG1156 and QB112 or GM96104 and GM96101, respectively. Four generations after induction of the strains with sucrose (8 mM for NIG1156 and QB112 or 60 mM for GM96104 and GM96101), cultures were shifted to 37°C (A) or 42°C (B) for 8 min. Samples (2 ml) were labelled with 0.15 mCi of [35S]methionine for 45 s and chased with an excess of nonradioactive methionine. Cell extracts were immunoprecipitated, and supernatants were dialyzed, lyophilized, and finally analyzed by SDS-PAGE.
Effect of SecA overproduction on levansucrase production.
The marked sensitivity of levansucrase production to small changes in functional SecA suggests that the endogenous level of SecA does not saturate one or more steps in the secretion of levansucrase. Hence, overproduction of SecA might increase the levels of levansucrase secretion. The effect of increasing the SecA concentration on the secretion of levansucrase was examined by using the multicopy plasmid pWKML1, which bears the secA gene under the regulated control of xylR (17). Strain QB112 was transformed with pWKML1 (strain GM9801), and SecA was overproduced after induction with 0.5% xylose. The presence of xylose did not modify the growth or rate of levansucrase synthesis of cells grown in minimal medium, since xylose is not metabolized by B. subtilis (25). SecA overproduction in this strain was blocked by glucose, since xylR is controlled by catabolite repression (20). Glucitol was thus used as a carbon source because it does not modify the levansucrase synthesis or secretion in the control strain QB112. Induction of SecA gene expression by pWKML1 led to a sevenfold overproduction of SecA. The rates of levansucrase production by strain GM9801 induced by 60 mM sucrose were determined in the presence and absence of xylose. Measurements were made one generation after sucrose induction to reduce catabolite repression by the glucose generated by levansucrase sucrose hydrolysis. Under these conditions, levansucrase production was about 40% greater in the presence of xylose than in its absence.
DISCUSSION
In prokaryotic cells, precursor proteins with a typical signal sequence are secreted by a common system, the preprotein translocase. They enter the translocase via the peripheral subunit SecA, which utilizes the energy of ATP binding and hydrolysis to drive the stepwise translocation of the precursor across the membrane (7). In this work, we compared the dependence of levansucrase and α-amylase secretion on SecA. For this purpose, the respective structural genes of these two native B. subtilis exoproteins were expressed under the control of the sucrose-inducible sacR promoter. We obtained similar yields of gene expression products by using different concentrations of the inducer, 8 mM for levansucrase and 60 mM for α-amylase. Under such conditions, the effects of SecA expression on secretion are directly comparable. Our study demonstrates that various precursors may exhibit major differences in their dependency on the amount of functional SecA in the cell. The amount of functional SecA was varied in three independent ways: (i) by means of the inhibitory effect of sodium azide, which selectively blocks the preprotein-stimulated ATPase activity of SecA (27, 29, 32); (ii) by the use of the secA(Ts) div-341 mutant at nonpermissive temperatures (39); and (iii) by overproduction of SecA using pWKML1 (17). The data show that the yield of levansucrase secreted by B. subtilis cells is proportional to the amount of SecA present, whereas α-amylase secretion is rather insensitive to a large decrease in the SecA level. This main difference in the secretion potential in responses to the modulation of the amount of functional SecA in the cell is most likely due to a difference in the affinity of the precursors for SecA.
In E. coli, SecA is involved in the initial steps of the protein secretion, and it mediates the entry of the preprotein into the export pathway (9, 10). SecA directly recognizes the signal sequence and unknown elements of the mature domain of the precursor (6, 16, 24). The levansucrase and α-amylase sequence signals are very different (42). The hydrophobic domain of the α-amylase signal sequence is 1.5 times longer than that of levansucrase, and its overall hydrophobicity is much greater. Also, the net charge of the first two amino acids immediately downstream of the signal sequence is negative for α-amylase and positive for levansucrase. These aspects of the signal sequence can be crucial for binding to SecA (16, 28), and to a large extent, they explain the difference in SecA dependency.
Cells of B. subtilis are straight rods (1.5 μm long and 0.65 μm wide). The SecA concentration is approximately 1.8 μM during the exponential phase and about 0.15 μM during the stationary phase. The levansucrase precursor has an affinity constant of about 1.106 M−1 (Kd = 1 μM), since the secretion efficiency is half maximal at this SecA concentration. The α-amylase precursor affinity is at least 1 order of magnitude greater since α-amylase is normally secreted during the stationary phase (46) and, as shown in this work, is efficiently secreted even when the level of SecA during the exponential phase is very low. It is worthy to note that ProOmpA binds to SecA in vitro with a Kd of 0.06 μM (13). Therefore, it appears that levansucrase precursor is a poor affinity substrate for the translocase.
In E. coli, a subset of precursor proteins is stabilized in an unfolded state by the chaperone SecB. SecB targets these precursors to the translocase by direct binding to SecA (13). Through these events, SecB facilitates the proper recognition of the precursor protein by the translocase. So far, no SecB homologue has been identified in B. subtilis, despite the availability of the complete genome sequence. One could argue that a chaperone function for stabilizing the partially folded precursors might not be required in this bacterium. This hypothesis is supported by evidence that α-amylase and levansucrase from B. subtilis are spontaneously stabilized in an intermediate folding state under cytosolic conditions of pH and calcium concentration (12, 43). This emphasizes the importance of information borne by the signal sequence and mature domains in the secretion of B. subtilis proteins.
ACKNOWLEDGMENTS
We thank Yoshito Sadaie (National Institute of Genetics, Mishima-shi, Japan) for kindly providing secA(Ts) mutants.
This work was supported in part by the European Commission (Biotech program BIO4-CT96-0097) and was carried out within the framework of the European Bacillus Secretion Group.
REFERENCES
- 1.Berghöfer J, Karnauchov I, Hermann R G, Klösgen R B. Isolation and characterization of a cDNA encoding the SecA protein from spinach chloroplasts. J Biol Chem. 1995;270:18341–18346. doi: 10.1074/jbc.270.31.18341. [DOI] [PubMed] [Google Scholar]
- 2.Blanco J, Coque J J, Martin J F. Characterization of the secA gene of Streptomyces lividans encoding a protein translocase which complements an Escherichia coli mutant defective in the ATPase activity of SecA. Gene. 1996;176:61–65. doi: 10.1016/0378-1119(96)00220-x. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Chambert R, Petit-Glatron M F. Hyperproduction of exocellular levansucrase by Bacillus subtilis: examination of the phenotype of a sacUh strain. J Gen Microbiol. 1984;130:3143–3152. doi: 10.1099/00221287-130-12-3143. [DOI] [PubMed] [Google Scholar]
- 5.Chambert R, Petit-Glatron M F. Study of the effect of organic solvents on the synthesis of levan and the hydrolysis of sucrose by Bacillus subtilis levansucrase. Carbohydr Res. 1989;191:117–123. [Google Scholar]
- 6.Cunningham K, Wickner W. Specific recognition of the leader region of precursor proteins is required for the activation of translocation ATPase of Escherichia coli. Proc Natl Acad Sci USA. 1989;86:8630–8634. doi: 10.1073/pnas.86.22.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Blauwen T, Driessen A J M. Sec-dependent preprotein translocation in bacteria. Arch Microbiol. 1996;165:1–8. doi: 10.1007/s002030050289. [DOI] [PubMed] [Google Scholar]
- 8.den Blauwen T, Fekkes P, de Wit J G, Kuiper W, Driessen A J M. Domain interactions of the peripheral preprotein translocase subunit SecA. Biochemistry. 1996;35:11194–12004. doi: 10.1021/bi9605088. [DOI] [PubMed] [Google Scholar]
- 9.Driessen A J M, Fekkes P, van der Wolk J P W. The Sec system. Curr Opin Microbiol. 1998;1:216–222. doi: 10.1016/s1369-5274(98)80014-3. [DOI] [PubMed] [Google Scholar]
- 10.Economou A. Bacterial preprotein translocase: mechanism and conformational dynamics of a processive enzyme. Mol Microbiol. 1998;27:511–518. doi: 10.1046/j.1365-2958.1998.00713.x. [DOI] [PubMed] [Google Scholar]
- 11.Fortin Y, Phoenix P, Drapeau G R. Mutations conferring resistance to azide in Escherichia coli occur primarily in the secA gene. J Bacteriol. 1990;172:6607–6610. doi: 10.1128/jb.172.11.6607-6610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddaoui E, Leloup L, Petit-Glatron M F, Chambert R. Characterization of a stable intermediate trapped during reversible refolding of Bacillus subtilis α-amylase. Eur J Biochem. 1997;249:505–509. doi: 10.1111/j.1432-1033.1997.00505.x. [DOI] [PubMed] [Google Scholar]
- 13.Hartl F U, Lecker S, Schiebel E, Hendrick J P, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 14.Haward S R, Napier J A, Gray J C. Chloroplast SecA functions as a membrane-associated component of the Sec-like protein translocase of pea chloroplasts. Eur J Biochem. 1997;248:724–730. doi: 10.1111/j.1432-1033.1997.00724.x. [DOI] [PubMed] [Google Scholar]
- 15.Helde R, Wieseler B, Wachter E, Neubüser A, Hoffschulte H K, Hengelage T, Schimz K-L, Stuart R A, Müller M. Comparative characterization of SecA from the α-subclass purple bacterium Rhodobacter capsulatus and Escherichia coli reveals differences in membrane and precursor specificity. J Bacteriol. 1997;179:4003–4012. doi: 10.1128/jb.179.12.4003-4012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura E, Akita M, Matsuyama S, Mizushima S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J Biol Chem. 1991;266:6600–6606. [PubMed] [Google Scholar]
- 17.Klein M, Hofmann B, Klose M, Freudl R. Isolation and characterization of a Bacillus subtilis secA mutant allele conferring resistance to sodium azide. FEMS Microbiol Lett. 1994;124:393–397. doi: 10.1111/j.1574-6968.1994.tb07314.x. [DOI] [PubMed] [Google Scholar]
- 18.Klein M, Meens J P, Freudl R. Functional characterization of the Staphylococcus carnosus SecA protein in Escherichia coli and Bacillus subtilis secA mutant strains. FEMS Microbiol Lett. 1995;131:271–277. doi: 10.1016/0378-1097(95)00267-9. [DOI] [PubMed] [Google Scholar]
- 19.Klose M, Schimz K L, van der Wolk J P W, Driessen A J M, Freudl R. Lysine 106 of the putative catalytic ATP-binding site of the Bacillus subtilis SecA protein is required for functional complementation of Escherichia coli secA mutants in vivo. J Biol Chem. 1993;268:4504–4510. [PubMed] [Google Scholar]
- 20.Kraus A, Hueck C, Gärtner D, Hillen W. Catabolite repression of the Bacillus subtilis xyl operon involves a cis element functional in the context of an unrelated sequence, and glucose exerts additional xylR-dependent repression. J Bacteriol. 1994;176:1738–1745. doi: 10.1128/jb.176.6.1738-1745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leloup L, Haddaoui E, Chambert R, Petit-Glatron M F. Characterization of the rate-limiting step of the secretion of Bacillus subtilis α-amylase overproduced during the exponential phase of growth. Microbiology. 1997;143:3295–3303. doi: 10.1099/00221287-143-10-3295. [DOI] [PubMed] [Google Scholar]
- 22.Lepesant J-A, Kunst F, Pascal M, Kejzlarová-Lepesant J, Steinmetz M, Dedonder R. Specific and pleiotropic regulatory mechanisms in the sucrose system of Bacillus subtilis 168. In: Schlessinger D, editor. Microbiology—1976. Washington, D.C: American Society for Microbiology; 1976. pp. 58–69. [Google Scholar]
- 23.Lill R, Cunningham K, Brundage L A, Ito K, Oliver D B, Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989;8:961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 25.Lindner C, Stülke J, Hecker M. Regulation of xylanolytic enzymes in Bacillus subtilis. Microbiology. 1994;140:753–757. doi: 10.1099/00221287-140-4-753. [DOI] [PubMed] [Google Scholar]
- 26.McNicholas P, Rajapandi T, Oliver D. SecA proteins of Bacillus subtilis and Escherichia coli possess homologous amino-terminal ATP-binding domains regulating integration into the plasma membrane. J Bacteriol. 1995;177:7231–7237. doi: 10.1128/jb.177.24.7231-7237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meens J P, Frings E, Klose T, Freudl R. An outer membrane protein of Escherichia coli can be translocated across the cytoplasmic membrane of Bacillus subtilis. Mol Microbiol. 1993;9:847–855. doi: 10.1111/j.1365-2958.1993.tb01743.x. [DOI] [PubMed] [Google Scholar]
- 28.Mori H, Araki M, Hikita C, Tagaya M, Mizushima S. The hydrophobic region of signal peptides is involved in the interaction with membrane-bound SecA. Biochim Biophys Acta. 1997;1326:23–36. doi: 10.1016/s0005-2736(97)00004-7. [DOI] [PubMed] [Google Scholar]
- 29.Nakane A, Takamatsu H, Oguro A, Sadaie Y, Nakamura K, Yamane K. Acquisition of azide-resistance by elevated SecA ATPase activity confers azide-resistance upon cell growth and protein translocation in Bacillus subtilis. Microbiology. 1995;141:113–121. doi: 10.1099/00221287-141-1-113. [DOI] [PubMed] [Google Scholar]
- 30.Oliver D B, Beckwith J. Escherichia coli pleiotropically defective in the export of secreted proteins. Cell. 1981;25:765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- 31.Oliver D B, Beckwith J. Identification of a new gene (secA) and gene product involved in the secretion of envelope proteins in Escherichia coli. J Bacteriol. 1982;150:686–691. doi: 10.1128/jb.150.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliver D B, Cabelli R J, Dolan K M, Jarosik G P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein translocation pathway. Proc Natl Acad Sci USA. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver D B. SecA protein: autoregulated ATPase catalysing preprotein insertion and translocation across the Escherichia coli inner membrane. Mol Microbiol. 1993;7:159–165. doi: 10.1111/j.1365-2958.1993.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 34.Petit-Glatron M F, Benyahia F, Chambert R. Bacillus subtilis levansucrase: a possible two step mechanism. Eur J Biochem. 1987;163:379–387. doi: 10.1111/j.1432-1033.1987.tb10810.x. [DOI] [PubMed] [Google Scholar]
- 35.Pöhling S, Piepersberg W, Wehmeier U F. Protein secretion in Streptomyces griseus N2-3-11: characterization of the secA gene and its growth phase-dependent expression. FEMS Microbiol Lett. 1997;156:21–29. doi: 10.1111/j.1574-6968.1997.tb12700.x. [DOI] [PubMed] [Google Scholar]
- 36.Rajapandi T, Oliver D B. Integration of SecA protein into the Escherichia coli inner membrane is regulated by its amino-terminal ATP-binding domain. Mol Microbiol. 1996;20:43–51. doi: 10.1111/j.1365-2958.1996.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 37.Rygus T, Hillen W. Inducible high-level expression of heterologous genes in Bacillus megaterium using the regulatory elements of the xylose-utilisation operon. Appl Microbiol Biotechnol. 1991;35:594–599. doi: 10.1007/BF00169622. [DOI] [PubMed] [Google Scholar]
- 38.Sadaie Y, Kada T. Effect of septum-division mutations on sporulation and competent cell formation in Bacillus subtilis. Mol Gen Genet. 1983;190:176–178. [Google Scholar]
- 39.Sadaie Y, Kada T. Bacillus subtilis gene involved in cell division, sporulation, and exoenzyme secretion. J Bacteriol. 1985;163:648–653. doi: 10.1128/jb.163.2.648-653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadaie Y, Takamatsu H, Nakamura K, Yamane K. Sequencing reveals similarity of the wild-type div+ gene of Bacillus subtilis to the Escherichia coli gene. Gene. 1991;98:101–105. doi: 10.1016/0378-1119(91)90110-w. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt M G, Rollo E E, Grodberg J, Oliver D B. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J Bacteriol. 1988;170:3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scotti P, Praestegaard M, Chambert R, Petit-Glatron M F. The targeting of Bacillus subtilis levansucrase in yeast is correlated to both the hydrophobicity of the signal peptide and the net charge of the N-terminus mature part. Yeast. 1996;12:953–963. doi: 10.1002/(SICI)1097-0061(199608)12:10%3C953::AID-YEA998%3E3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Scotti P, Chambert R, Petit-Glatron M F. Kinetics of the unfolding-folding transition of Bacillus subtilis levansucrase precursor. FEBS Lett. 1995;360:307–309. doi: 10.1016/0014-5793(95)00099-u. [DOI] [PubMed] [Google Scholar]
- 44.Steinmetz M, Kunst F, Dedonder D. Mapping of mutations affecting synthesis of exocellular enzymes in Bacillus subtilis. Identity of the sacUh, amyB and pap mutations. Mol Gen Genet. 1976;148:281–285. doi: 10.1007/BF00332902. [DOI] [PubMed] [Google Scholar]
- 45.Takamatsu H, Fuma S-I, Nakamura K, Sadaie Y, Shinkai A, Matsuyama S-I, Mizushima S, Yamane K. In vivo and in vitro characterization of the secA gene product of Bacillus subtilis. J Bacteriol. 1992;174:4308–4316. doi: 10.1128/jb.174.13.4308-4316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takamatsu H, Nakane A, Sadaie Y, Nakamura K, Yamane K. The rapid degradation of mutant SecA protein in the Bacillus subtilis secA341 (ts) mutant causes a protein translocation defect in the cell. Biosci Biotechnol Biochem. 1994;58:1845–1850. doi: 10.1271/bbb.58.1845. [DOI] [PubMed] [Google Scholar]
- 47.Valentin K. Phylogeny and expression of the secA gene from a chromophytic alga. Implications for the evolution of plastids and sec-dependent protein translocation. Curr Genet. 1997;32:300–307. doi: 10.1007/s002940050281. [DOI] [PubMed] [Google Scholar]
- 48.van der Wolk J P W, Klose M, Breukink E, Demel R A, de Krujiff B, Freudl R, Driessen A J M. Characterization of a Bacillus subtilis SecA mutant protein deficient in translocation ATPase and release from the membrane. Mol Microbiol. 1993;8:31–42. doi: 10.1111/j.1365-2958.1993.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 49.van der Wolk J P W, Klose M, de Wit J G, den Blaauwen T, Freudl R, Driessen A J M. Identification of the magnesium-binding domain of the high-affinity ATP-binding site of the Bacillus subtilis and Escherichia coli SecA protein. J Biol Chem. 1995;270:18975–18982. doi: 10.1074/jbc.270.32.18975. [DOI] [PubMed] [Google Scholar]
- 50.Varley J P, Moehrle J J, Manasse R S, Bendall D S, Howe C J. Characterization of plastocyanin from cyanobacterium Phormidium laminosum: copper-inducible expression and SecA-dependent targeting in Escherichia coli. Plant Mol Biol. 1995;27:179–190. doi: 10.1007/BF00019189. [DOI] [PubMed] [Google Scholar]
- 51.Voelker R, Mendel-Hartvig J, Barkan A. Transposon-disruption of a maize nuclear gene, tha1, encoding a chloroplast SecA homologue: in vivo role of cp-SecA in thylakoid protein targeting. Genetics. 1997;145:467–478. doi: 10.1093/genetics/145.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan J, Henry R, McCaffery M, Cline K. SecA homolog in protein transport within chloroplasts; evidence for endosymbiont-derived sorting. Science. 1994;266:796–801. doi: 10.1126/science.7973633. [DOI] [PubMed] [Google Scholar]