Abstract
Purpose
To assess health-related quality of life (HRQoL) and its associated factors in patients who survived COVID-19 and to assess a prospective evaluation of the prevalence and severity of their depression and anxiety symptoms.
Methods
We followed up a sample of hospitalized patients who survived COVID-19 at 3 and 12 months after discharge. We assessed HRQoL (Euroqol-5D-5L) through telephone interviews. Any problem in any dimension of Euroqol-5D-5L was considered as low HRQoL. The depression and anxiety symptoms were measured using the Patient Health Questionnaire-9 and Generalized Anxiety Disorder-7 tools, respectively. We estimated the adjusted prevalence ratios (aPR) to low HRQoL using Poisson regression and the changes on their depression and anxiety symptoms during the follow-up.
Results
We included 119 patients with a mean follow-up time of 363.6 days. 74% of the participants had low HRQoL at one year after hospital discharge and were associated with being ≥ 41 years old (aPR: 1.95), having a previous history of psychiatric diagnoses before COVID-19 infection (aPR: 1.47), having any COVID-19 symptom during the follow-up at one year (aPR: 1.84), and having a family member who had died from COVID-19 during the first wave (aPR: 1.24). In addition, the clinically relevant depression symptoms were frequent, and they increased from 3 (14.3%) to 12 months (18.5%).
Conclusion
One year after COVID-19 hospitalization discharge, patients had low HRQoL, and their depression symptoms increased. These findings acknowledge the need to provide services that adequately address mental health sequels and HRQoL to reduce the burden of the COVID-19.
Keywords: COVID-19, Depression, Anxiety, Quality of life, Peru
Introduction
The pandemic of the coronavirus disease 2019 (COVID-19) severely affected the general population’s physical, psychological, social, and spiritual well-being [1]. The patients who recovered and survived the disease are one of the most affected groups. Until January 2022, they are more than 250 million people [2]. However, observational studies reported that around 80% of these patients had some clinical manifestations that persisted several months after the discharge or recovery from the infection [3]. In addition, COVID-19 survivors have a low health-related quality of life (HRQoL) months after hospital discharge, especially in physical activities and pain/discomfort [4], mainly related to clinical manifestations. Furthermore, the long-term effects of COVID-19 might have a negative impact on the overall HRQoL of the COVID-19 survivors [5].
Mental health afflictions caused by the pandemic are more frequent in vulnerable groups such as children, elders, frontline workers, people with pre-existing mental pathologies, and, more specifically, individuals who recovered from COVID-19 [6, 7]. In this last group, depression, anxiety, posttraumatic stress, and poor sleep quality persist for several months after hospital discharge [8–10]. In addition, some factors associated with a higher frequency of mental disorders have also been identified, such as being female, having a greater severity of COVID-19, the persistence of physical sequelae, death of a family member due to COVID-19, and previous psychiatric diagnosis and treatment [11, 12]. Moreover, longitudinal studies reported the deterioration in their HRQoL up to six months after hospital discharge [13–15]. However, few studies described a correlation between the quality of life decline and the severity of the disease during hospital stay [13, 16, 17].
Some studies reported the effects on mental health and HRQoL one-year post-COVID-19 recovery [18], but not many in low- and middle-income countries. Latin America is one of the most affected regions by the pandemic [19, 20]. Specifically, Peru is one of the countries with the highest number of infections and deaths associated with COVID-19 per inhabitant in the world [21]. In addition, Peru’s political, health, and socioeconomic crisis has generated persisting challenges in health management during the pandemic, such as limited health infrastructure, patient care equipment, and availability of hospital beds and specialist physicians [22]. This context in the country during the pandemic impacted several populations, such as students and elders [23, 24]. However, there is still missing data about the COVID-19 survivors. Therefore, the objectives of this study were to include patients who survived COVID-19 and 1) to evaluate the HRQoL and their associated factors after the second COVID-19 wave in Peru (T2), after one year of hospital discharge, and 2) to carry out a prospective analysis of the frequency and severity of depression and anxiety symptoms after the end of the first (T1) and second COVID-19 waves in Peru (T2). We hypothesized that COVID-19 survivors 1) had an overall low HRQoL associated with age, history of psychiatric diagnoses, and persistent COVID-19 symptoms, and 2) they had persistent depression and anxiety symptoms after three months and one year of follow-up after hospital discharge.
Materials and methods
Study design and context
This observational study assessed the follow-up of individuals who survived COVID-19 and were discharged from the hospital, as evaluated in a previous study [12]. The follow-up includes two assessment moments. We conducted the first evaluation after hospital discharge at the end of the first COVID-19 infections wave in Peru, during October and November 2020 (T1), approximately three months after hospital discharge. The second evaluation was at the end of the second COVID-19 infections wave, during July and August 2021 (T2), about one year after hospital discharge. Therefore, we developed two different analyses: (a) A cross-sectional analysis of HRQoL measured at T2 and (b) a longitudinal analysis of depression and anxiety symptoms measured at T1 and T2.
The first wave of the COVID-19 pandemic in Peru was from March to November 2020 and caused more than 173 thousand deaths probably to COVID-19, one of the highest per-capita rates of excess mortality in the world [25]. During that time, the Peruvian government established a strict national lockdown from March 16th to June 30th. During October and November 2020 (T1), the Peruvian government announced the end of focalized lockdowns due to the reduction of positive and hospitalized cases. However, the night curfew and restriction of using private vehicles on Sundays remained. Also, there was a progressive opening of land and air transport in and outside the country. The second wave of the COVID-19 pandemic in Peru was from January to August 2021, reaching more than 200 thousand confirmed deaths due to COVID-19 [26]. During July and August 2021 (T2), the Peruvian government was distributing the COVID-19 vaccine to adults, and there weren’t specific restrictions on the population, besides using masks out and indoors, social events restriction, and partial obligation of COVID-19 vaccination to enter inside shopping malls, restaurants, and public transport.
We carry out this study at Hospital Nacional Guillermo Almenara Irigoyen (HNGAI) in Lima, Peru, which is the second-largest hospital in the “Peruvian Social Health Insurance” (EsSalud, in Spanish), with a total of 815 hospital beds. Furthermore, it is a third-level hospital and has all medical specialties. EsSalud is one of the leading Peruvian medical insurances and treats patients who are formal employees and their relatives. During the COVID-19 pandemic, HNGAI is a national referral center for the care of COVID-19 patients.
Participants and sampling
We assessed a simple random sample of 318 patients who had been hospitalized due to COVID-19 during March and September 2020 and survived. The original randomized sampling and recruitment techniques were explained in a previous report [12]. In brief, we identified this sample after reviewing the list of 1910 adult patients diagnosed with COVID-19 and discharged from the HNGAI during that period. Then, we excluded deceased patients, those referred to another center, those with voluntary discharge, and those who had two or more hospitalizations since March 2020. The original sample size for T1 considered a margin error of 5%, a design effect of 1, and a 95% confidence interval. Then, for T2, we excluded patients who didn’t answer the follow-up call, refused to participate, or died due to an illness unrelated to COVID-19 since November 2020.
Variables
At T1, we only assessed depression and anxiety symptoms as the main outcomes. Then, at T2, we evaluated depression, anxiety symptoms, and HRQoL as the main outcomes.
Health-related quality of life
The Euroqol-5D-5L (EQ-5D) scale applies to patients and the general population for describing and assessing HRQoL [27]. It can be applied through a personalized interview or telephone [28]. This scale had two parts where individuals self-assess their health status: the EQ-5D descriptive system and the Visual Analogue Scale (VAS). The EQ-5D descriptive system comprises five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension is encoded in 1 (No problems) to 5 (extreme problems), where the combination 1–1-1–1-1 indicates the best health state possible in the five dimensions and 5–5-5–5-5, the worst health state. Therefore, we considered the result of 1–1-1–1-1 as adequate HRQoL while any other combination as low HRQoL. On the other hand, in the VAS system, subjects could self-assess their health condition through a scoring system ranging from 0 to 100, representing the worst and best possible health conditions, respectively [27]. Widely used globally, the EQ-5D-5L version is available in more than 130 languages, including Spanish, and it has a Cronbach's alpha reliability score of 0.90 [27].
Depression symptoms
For the assessment of depression symptoms, we used the Patient Health Questionnaire-9 (PHQ-9). As is known, PHQ-9 is also a self-administered scale consisting of 9 items, rated on a Likert scale ranging from 0 (“not at all”) to 3 (“almost every day”). The PHQ-9 score reflects five categories of severity of depression: Normal (0 to 4), Mild (5 to 9), Moderate (10 to 14), Moderately severe (15 to 19), and Severe (20 to 27). We used the PHQ-9 version adapted to Peruvians [29]. In studies carried out in Latin America, PHQ-9 had a Cronbach's alpha reliability score higher than 0.80 and a ROC curve of 0.86 to identify depression assessed by DSM-IV criteria. We considered moderate, moderately severe, or severe depression symptoms as clinically relevant depression symptoms [30, 31].
Anxiety symptoms
Generalized Anxiety Disorder-7 (GAD-7) is a universally valid and efficient self-administered scale to assess the severity of anxiety disorders in clinical practice. This scale has a Cronbach's alpha reliability score of 0.92 and a test–retest reliability correlation of 0.83 [32], and it has been translated and validated into Spanish [33]. It consists of seven items to measure anxiety symptoms during the two weeks before self-application. We rated each item on a Likert scale ranging from 0 (“not at all”) to 3 (“almost every day”). GAD-7 ratings reflect four levels of severity of anxiety symptoms: Normal (0 to 4), Mild (5 to 9), Moderate (10 to 14), and Severe (15 to 21). A score of 10 or more has a sensitivity of 86.8% and a specificity of 93.4% to diagnosed general anxiety disorder assessed by DSM-IV criteria. We considered moderate or severe anxiety symptoms as clinically relevant anxiety symptoms [33].
Covariates
We also collected information regarding the following covariates: (a) sociodemographic data, including sex, age, job status, with whom they lived during the hospitalization, history of relative deceased due to COVID-19 during the first wave; (b) clinical and hospitalization data, including diagnosis and/or treatment history for psychiatric diagnosis before hospitalization (made by a health professional: physician, psychologist, or psychiatric), and self-perception of COVID-19 severity during hospitalization; and the presence of COVID-19-related symptoms at follow-up at T1 and T2. The interviewer asked for the self-report of fever, dyspnea, myalgia, rhinorrhea, cough, or headache at follow-up. Then, these symptoms were categorized into no symptoms, only general symptoms (at least reported fever, myalgia, or headache, and no respiratory symptoms), only respiratory symptoms (at least reported dyspnea, rhinorrhea, or cough, and no general symptoms), and both symptoms (at least reported one general symptom and one respiratory symptom). All covariates were self-responded after direct singles questions.
Data collection
We measured all the covariates and the depression and anxiety symptoms outcomes during the first follow-up at T1. In addition, we extracted the hospitalization duration from the patients’ electronic clinical history. Then at T2, we only measured the COVID-19-related symptoms, and the outcomes of HRQoL, depression, and anxiety symptoms. Finally, we also collected the days from discharge to the interview at T2 (follow-up time) and presence of COVID-19 symptoms during the interview (at T1 and T2, independently).
Both T1 and T2 evaluations were collected in a virtual file through telephone calls to the patients, using the cell phone number registered in each patient's electronic medical record. Researchers, who were psychiatrists with clinical and research experience, called the participants to collect their data. These calls lasted approximately 20 min. If the participant had some acute anxiety or depression event during the call, the interviewers stopped the questionnaire and offered them psychiatric help. This help included a psychiatric consultation and the management of the acute event. In addition, the psychiatrist interviewer offered the participant to continue the psychiatric consultations during the following weeks and consider them as patients, following the usual health care by hospital protocols. We not collected this information about the psychiatric help, and the confidentiality was not affected.
Statistical analysis
Before analysis, we categorized the age variable according to its quartiles and the “hospitalization time” variable into 1–7, 8–14, and > 14 days. We used these last cut-off values considering that seven or fewer days of hospitalization probably is due to a mild disease with lesser antibiotic treatment and procedures [34] and that the average hospitalization time in COVID-19 patients is about 14 days [35]. The depression and anxiety symptoms outcomes were dichotomized as not clinically relevant (normal–mild) and clinically relevant (moderate–severe). In addition, the patients were categorized as asymptomatic, with general symptoms only (fever, fatigue, myalgia, or headache), with respiratory symptoms only (dyspnea, rhinorrhea, or cough), and with both types of symptoms according to their self-report regarding COVID-19 symptoms present during follow-up interviews. We measured the relative and absolute frequencies of the qualitative variables, and the median and interquartile range of the quantitative variables.
We performed Poisson regression analysis with log link function to calculate the prevalence ratios and their confidence intervals for “low HRQoL.” The variables to be adjusted in each regression model were selected considering the design of a directed acyclic graph [36]. All regression models were adjusted by follow-up time. The regression model for exposure variables “employment status,” “self-perceived severity of COVID-19,” “persistent COVID-19 symptoms,” and “depression and anxiety symptoms” were adjusted by sex and age. The regression model for the exposure variable “living with someone” was adjusted by “marital status” and “death of a relative from COVID-19.” The regression model for the “history of psychiatric disorder” variable was adjusted by sex and the variable “living with someone.” The history regression model for “psychiatric treatment” was adjusted by “history of psychiatric disorder.” The regression model for the variable “persistent symptoms due to COVID-19 at T2” was additionally adjusted by “self-perceived severity of COVID-19.” Finally, the regression models for clinically relevant depression and anxiety symptoms were adjusted by “history of psychiatric disorder,” “self-perceived severity of COVID-19,” and “death of a family member from COVID-19.”
On the other hand, Sankey diagrams were performed with the absolute frequency of the depression and anxiety symptoms categories after three months and one year of follow-up, using the free online software SankeyMATIC (https://sankeymatic.com/). A Sankey diagram includes nodes and arcs to highlight the movement from one state/time to another [37]. As transitions occur, each arc flows from its source node to the target node(s), and the node’s size and arc’s width represents the number of objects/members, thus indicating the magnitude of movement [38]. Student's t-tests were used for paired samples to calculate the mean difference of the depression and anxiety symptoms scores by comparing the measurement at three months (T1) versus one year (T2). A p-value of < 0.05 was considered statistically significant. We used Stata MP v.17.0 (StataCorp LLC, TX, United States) statistical software for all analyses.
Results
A total of 318 patients were assessed at T1. Then, at T2, 185 patients did not answer the follow-up call, eight had a wrong number, four refused to participate, and two died due to illnesses unrelated to COVID-19 since November 2020. Finally, 119 participants (37.4%) were assessed and included at T2 assessment.
General characteristics of the sample
The mean follow-up time after hospitalization discharge at T2 was 363.6 ± 48.6 days (range: 260 to 476 days). The mean days between T2 and T1 was 260 ± 33.4 (range: 203 to 307 days). The characteristics of the participants at T1 and T2 are described in Table 1. No significant differences were observed between both assessed samples.
Table 1.
Baseline characteristics of hospitalized patients for COVID-19 and discharged after the first wave (T1) (n = 318) and the second wave (T2) (n = 119)
| Variable | T1 participants (n = 318) | T2 participants (n = 119) |
|---|---|---|
| Male | 196 (61.3) | 64 (53.8) |
| Age (years)* | 53.1 (51.8–54.4) | 55.0 (41.0–67.0) |
| Job status | ||
| Unemployment | 95 (30.5) | 34 (28.6) |
| Informal employment | 31 (9.2) | 11 (9.2) |
| Formal employment | 143 (45.5) | 51 (42.9) |
| Retired | 49 (14.8) | 23 (19.3) |
| Live | ||
| Alone | 17 (5.1) | 4 (3.4) |
| With partner and/or sons/daughters | 256 (79.8) | 99 (83.2) |
| With parents and/or another family member | 45 (15.1) | 16 (13.5) |
| Death of family member for COVID-19 during first wave | 90 (30.4) | 40 (33.6) |
| History of psychiatric diagnosis | 32 (10.4) | 13 (10.9) |
| History of psychiatric treatment | 27 (8.7) | 9 (7.6) |
| Self-perception of severity of COVID-19 | ||
| Mild | 89 (29.1) | 32 (26.9) |
| Moderate | 107 (32.6) | 38 (31.9) |
| Severe | 99 (31.2) | 40 (33.6) |
| Critically ill | 23 (7) | 9 (7.6) |
| Hospitalization time | ||
| 1 to 7 days | 154 (48.4) | 58 (48.7) |
| 8 to 14 days | 84 (26.4) | 33 (27.7) |
| More than 14 days | 80 (25.2) | 28 (23.5) |
| Persistent symptoms due to COVID-19 at follow-up | ||
| No symptoms | 141 (44.3) | 44 (37.0) |
| General symptoms | 78 (24.5) | 30 (25.2) |
| Respiratory symptoms | 44 (13.8) | 11 (9.2) |
| Both symptoms | 55 (17.3) | 34 (28.6) |
| Depression symptoms | ||
| None | 222 (69) | 75 (63.0) |
| Mild | 62 (20.2) | 22 (18.5) |
| Moderate | 18 (5.6) | 13 (10.9) |
| Moderate–Severe | 11 (3.7) | 5 (4.2) |
| Severe | 5 (1.4) | 4 (3.4) |
| Anxiety symptoms | ||
| None | 223 (68.9) | 82 (68.9) |
| Mild | 71 (23.5) | 22 (18.5) |
| Moderate | 17 (5.4) | 9 (7.6) |
| Severe | 7 (2.2) | 6 (5.0) |
| Low quality of life | – | 88 (74.0) |
*Median and interquartile range
Health-related quality of life
According to the EQ-5D results, the respondents demonstrated some degree of problems when performing activities related to usual activities (14.3%), mobility (28.6%), self-care (32.8%), anxiety/depression (42%), or pain/discomfort (59.7%). Regarding HRQoL, the median score was 80 (IQR: 70–90) according to VAS. People aged 41 years or above were more likely to have a low HRQoL (adjusted prevalence ratios [aPR]: 1.95, 95% confidence intervals [95% CI]: 1.29–2.93) when compared with the younger participants. Similar results were observed for those patients with a history of psychiatric diagnosis (aPR: 1.47, 95%CI: 1.25–1.73) and those who reported only general, only respiratory, or both kinds of COVID-19 symptoms during the follow-up at one year (aPR: 1.42, 95% CI: 1.00–2.03; aPR: 1.83, 95% CI: 1.33–2.52; aPR: 1.84, 95%CI: 1.36–2.48, respectively) than asymptomatic. Finally, those with a family member who died due to COVID-19 were more likely to have a lower HRQoL (aPR: 1.24, 95% CI: 1.02–1.52) (Table 2).
Table 2.
Association between characteristics and low quality of life (n = 119)
| Characteristics | Quality of life | ||
|---|---|---|---|
| Good | Low | aPR (95%CI) | |
| Sex | |||
| Men | 16 (25.0) | 48 (75.0) | Ref |
| Women | 15 (27.3) | 40 (72.7) | 0.97 (0.78–1.21) |
| Age (years) | |||
| 20 to 41 years | 17 (54.8) | 14 (45.2) | Ref |
| 42 to 53 years | 4 (12.1) | 29 (87.9) | 1.95 (1.29–2.93) |
| 54 to 65 years | 6 (20.7) | 23 (79.3) | 1.75 (1.13–2.70) |
| 66 to 94 years | 4 (15.4) | 22 (84.6) | 1.87 (1.22–2.85) |
| Marital status | |||
| Single | 7 (38.9) | 11 (61.1) | Ref |
| Married | 21 (25.0) | 63 (75.0) | 1.22 (0.83–1.80) |
| Divorced or widower | 3 (17.7) | 14 (82.4) | 1.34 (0.87–2.06) |
| Job status* | |||
| Unemployment | 8 (23.5) | 26 (76.5) | Ref |
| Informal employment | 4 (36.4) | 7 (63.6) | 0.80 (0.49–1.30) |
| Formal employment | 16 (31.4) | 35 (68.6) | 1.02 (0.79–1.32) |
| Retired | 3 (13.0) | 20 (87.0) | 1.20 (0.84–1.70) |
| Profess a religion | |||
| No | 4 (36.4) | 7 (63.6) | Ref |
| Yes | 27 (25.0) | 81 (75.0) | 1.20 (0.75–1.90) |
| Live with** | |||
| Alone | 0 (0.0) | 4 (100.0) | Ref |
| Couple and/or children | 26 (26.3) | 73 (73.7) | 0.79 (0.57–1.10) |
| Fathers and/or other family members | 5 (31.3) | 11 (68.8) | 0.56 (0.57–1.22) |
| Death of family member for COVID-19 during first wave | |||
| No | 25 (31.7) | 54 (38.4) | Ref |
| Yes | 6 (15.0) | 34 (85.0) | 1.24 (1.02–1.52) |
| History of psychiatric diagnosis*** | |||
| No | 31 (29.3) | 75 (70.8) | Ref |
| Yes | 0 (0.0) | 13 (100.0) | 1.47 (1.25–1.73) |
| History of psychiatric treatment† | |||
| No | 31 (28.2) | 79 (71.8) | Ref |
| Yes | 0 (0.0) | 9 (100.0) | 0.98 (0.85–1.13) |
| Self-perception of the severity of COVID-19* | |||
| Mild | 11 (34.4) | 21 (65.6) | Ref |
| Moderate | 6 (15.8) | 32 (84.2) | 1.02 (0.78–1.33) |
| Severe | 12 (30.0) | 28 (70.0) | 0.92 (0.69–1.24) |
| Critically ill | 2 (22.2) | 7 (77.8) | 0.96 (0.61–1.49) |
| Symptoms due to COVID-19 at one-year follow-up*‡ | |||
| No symptoms | 22 (50.0) | 22 (50.0) | Ref |
| General symptoms | 8 (26.7) | 22 (73.3) | 1.42 (1.00–2.03) |
| Respiratory symptoms | 0 (0.0) | 11 (100.0) | 1.83 (1.33–2.52) |
| Both symptoms | 1 (2.9) | 33 (97.1) | 1.84 (1.36–2.48) |
| Depression symptoms at one-year follow-up*†‡£ | |||
| Normal–Mild | 29 (29.9) | 68 (70.1) | Ref |
| Moderate–Severe | 2 (9.1) | 20 (90.9) | 1.09 (0.91–1.31) |
| Anxiety symptoms at one-year follow-up*†‡£ | |||
| Normal–Mild | 31 (29.8) | 73 (70.2) | Ref |
| Moderate–Severe | 0 (0.0) | 15 (100.0) | 1.17 (0.99–1.37) |
aPR Adjusted prevalence ratio by time of follow-up
Bold values denote statistical significance at the p-value < 0.05
CI 95%: 95% Confidence Intervals
*Adjusted prevalence ratio for sex and age
**Adjusted prevalence ratio for civil status and death of a family member due COVID-19
***Adjusted prevalence ratio for sex and living with
†Adjusted prevalence ratio for history of psychiatric diagnosis; ‡Adjusted prevalence ratio for self-perception of severity of COVID-19
£Adjusted prevalence ratio for death of a family member due COVID-19
Follow-up of psychiatric symptoms
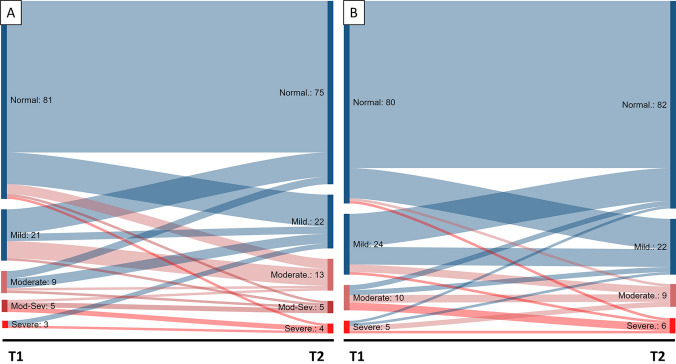
Of the patients who completed both follow-ups, 14.3% reported clinically relevant depression symptoms at T1 and 18.5% at T2, while 12.6% showed clinically relevant anxiety symptoms at both T1 and T2. Regarding the changes in the severity of depression (Fig. 1A) and anxiety (Fig. 1B) symptoms between T1 and T2, we observed the persistence of moderate and severe symptoms in both outcomes. However, concerning depression symptoms, we observed that an important group of patients had moderate or severe symptoms at T2, although they had mild or no depression symptoms at T1. So, we assessed this change by calculating the mean difference in the PHQ-9 depression symptoms score between T2 and T1, which was 0.94 units higher (p = 0.04). In contrast, the mean difference in the GAD-7 anxiety symptoms score between T2 and T1 was 0.13 units higher (p = 0.72).
Fig. 1.
Changes in the intensity of depression (A) and anxiety (B) symptoms from 3-month follow-up (T1) to 1 year (T2). In Blue: Changes to normal or mild depression and anxiety symptoms at T2. In Red: Changes to moderate, moderate-severe, or severe depression and anxiety symptoms at T2
Discussion
Main results
The objectives of the study were (1) to cross-sectionally evaluate HRQoL and its associated factors one year after hospital discharge (T2) and (2) to perform a prospective assessment of depression and anxiety symptoms at three months (T1) and one year (T2) after hospitalization discharge, in a sample of patients who survived COVID-19. We found that most included patients had a low health-related quality of life one year after hospital discharge, especially in those with greater age, with self-reported previous history of psychiatric diagnoses, and with persistent COVID-19 symptoms. In addition, the depression and anxiety symptoms remained high one year after hospital discharge.
Three-quarters of the included patients reported some problems in their usual activities, mobility, self-care, anxiety/depression, or pain/discomfort. Their prevalence of clinically relevant depression and anxiety symptoms was 18.5% and 12.6%, respectively. COVID-19 can negatively impact the respiratory, motor, and nervous systems [39]. Thus, it causes lower physical resistance, incapacities, and permanent symptoms in those with COVID-19 infection [40]. In addition, due to the saturation of the health system and other restrictions during the pandemic, the COVID-19 survivors didn’t have access to healthcare, rehabilitation services, and follow-up after discharge, persisting their health problems [41]. Therefore, these problems might affect their daily life activities, HRQoL, and mental health.
Associated variables to low HRQoL
We found that older patients reported worse HRQoL at one-year follow-up, similarly reported by a study conducted in a sample of 361 COVID-19 patients, where age was negatively associated with the dimensions of physical strength and physical role of HRQoL at the one-month follow-up [42]. This could be due to older people’s higher prevalence of chronic diseases [43]. Other factors to consider are their loss of immune function, reduced protection against infectious agents [44], and poor family support network [45]. So, elderly COVID-19 survivors will need better access to healthcare for earlier identification and rehabilitation of their impairments.
In addition, we found that a history of psychiatric disorders was independently associated with a greater probability of having a low HRQoL. Any patient with a psychiatric diagnosis had an impairment in their HRQoL [46]. So, in addition to their mental illness, these patients had to afford the effects of COVID-19 health sequels and traumatic events during hospitalization. Similarly, the patients with persistent COVID-19 symptoms were more likely to experience a low HRQoL, as reported in patients from Italy, Germany, and the United States, where low HRQoL is associated with the persistence of COVID-19 symptoms up to one year after hospital discharge [47–49]. The post-COVID-19 syndrome significantly impacts people’s health, and symptoms such as dyspnea, myalgia, or headache have become factors that obstruct or limit daily activities [50]. This presents a further challenge for the attempts of health systems to prepare for the diagnosis, management, and follow-up of such patients [51].
The patients with a relative who died due to COVID-19 during the first wave in Peru were more likely to have a low HRQoL. The direct relatives of a COVID-19 patient suffer a deterioration of their own HRQoL and generate concern, frustration, sadness, and sleep disorders [52]. Thus, we can infer an even more significant impact on HRQoL because of the loss of a family member. This stressful event may affect individuals since it affects the depression- and anxiety-related domains of HRQoL. Thus, the psychological impact of personal and family stresses during the pandemic would be crucial in the HRQoL of the patients who survived COVID-19.
Depression and anxiety symptoms
The depression symptoms significantly increased from T1 (three months) to T2 (one year after discharge). A study conducted on COVID-19 survivors in China reported a higher frequency of depression or anxiety symptoms 12 months following the hospital discharge (26%) compared with after six months (23%) [53]. In addition, another study conducted on Italian patients who survived COVID-19 found an increase in depression and anxiety symptoms in men [18]. Consistent results demonstrate the plausible underlying mechanisms of psychiatric sequelae from COVID-19 infection, such as neurotropism, interrupted neuronal circuits, neuroinflammation, and neuronal death [11]. Thus, several interventions were proposed during the pandemic to prevent and manage the mental health symptoms in COVID-19 survivors. These mainly include technology or distance methodologies, such as telehealth, chat support groups, and hotlines [54].
However, to our knowledge, our study is one of the first reports of one-year follow-ups of patients who survived COVID-19 in Latin America and low- and middle-income countries [55]. Moreover, these countries had limited access to mental healthcare, which worsened during the pandemic [56], and they cannot fully extrapolate most technology-based interventions [57]. For this reason, the potential long-term impact of COVID-19 on mental health in patients from these countries could be even more critical and may need low-budget interventions capable of accessing all patients.
Limitations of the study
The study’s main limitation is that only 37.4% of the patients completed the one-year follow-up, which implies that the results do not adequately represent the original population of patients from the hospital who survived COVID-19 during the first wave. However, we found no significant differences between the currently analyzed sample and all the patients evaluated at T1, so we can consider these missing data were at random. Future studies need to assess larger and more representative samples from different hospital centers to estimate the long-term consequences of COVID-19. In addition, we have not evaluated the mental health and HRQoL status of patients who survived COVID-19 before acute infection or at hospital discharge. This could cause the interpretation of the follow-up outcomes to be biased due to the patients’ baseline levels before the disease.
Furthermore, the time between T2 and T1 was different for each patient (203 to 307 days), causing a mismatch of time points. However, more than 200 days are sufficient to observe changes in mental health outcomes, especially during the COVID-19 pandemic [58]. Finally, despite considering the self-perceived COVID-19 severity and COVID-19 symptoms during T1 and T2 interviews, which partially are proxies of comorbidities, there may be remaining residual confusion bias in addition to other non-collected variables such as education level, lifestyle, marital status, and economic status associated to mental health and HRQoL outcomes [59, 60]. However, this study is one of the first to assess these outcomes in survivors of COVID-19 after one year following their hospital discharge and find associations with the relevant variables, especially in low- and middle-income countries.
Conclusions
One year after COVID-19 hospital discharge, patients show a high frequency of low HRQoL. This is associated with increased age, a history of psychiatric diagnosis, the death of a relative from COVID-19, and persistent COVID-19 symptoms. In addition, the clinically relevant depression symptoms were prevalent, showing an increase from 3 to 12 months. These findings reveal the need to provide services that adequately address mental health sequelae and HRQoL to reduce the burden of the disease due to COVID-19.
Acknowledgements
None.
Author contributions
JH-V: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—review and editing. CAA-R: Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing—review and editing. WB-F: Data curation, Investigation, Writing—original draft, Writing—review and editing. CC-B: Data curation, Investigation, Writing—original draft, Writing—review and editing. MC-E: Data curation, Investigation, Writing—original draft, Writing—review and editing. GA-M: Data curation, Investigation, Writing—original draft, Writing—review and editing. FC-Q: Data curation, Investigation, Writing—original draft, Writing—review and editing. JS-B: Data curation, Investigation, Writing—original draft, Writing—review and editing. BV: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The Hospital Nacional Guillermo Almenara Irigoyen’s Institutional Review Board (548-OfyD-GRPA-ESSALUD-2021) approved the original study and the current analysis.
Consent to participate
Before including them in the study, we obtained verbal informed consent from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shek DTL. COVID-19 and quality of life: twelve reflections. Appl Res Quality Life. 2021;16(1):1–11. doi: 10.1007/s11482-020-09898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldometer (2022). COVID live—coronavirus statistics, February 12, 2022, available at https://www.worldometers.info/coronavirus/
- 3.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Science and Reports. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nandasena HMRKG, Pathirathna ML, Atapattu AMMP, Prasanga PTS. Quality of life of COVID 19 patients after discharge: systematic review. PLoS ONE. 2022;17(2):e0263941. doi: 10.1371/journal.pone.0263941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryson WJ. Long-term health-related quality of life concerns related to the COVID-19 pandemic: a call to action. Quality of Life Research. 2021;30(3):643–645. doi: 10.1007/s11136-020-02677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar UV. Post-COVID-19 sequelae. Indian J Respir Care. 2021;10(4):60–63. doi: 10.4103/ijrc.ijrc_30_21. [DOI] [Google Scholar]
- 7.Villarreal-Zegarra D, Copez-Lonzoy A, Vilela-Estrada AL, Huarcaya-Victoria J. Depression, post-traumatic stress, anxiety, and fear of COVID-19 in the general population and health-care workers: prevalence, relationship, and explicative model in Peru. BMC Psychiatry. 2021;21(1):455. doi: 10.1186/s12888-021-03456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betschart M, Rezek S, Unger I, Ott N, Beyer S, Böni A, Gisi D, Shannon H, Sprui MA, Sieber C. One year follow-up of physical performance and quality of life in patients surviving COVID-19: a prospective cohort study. Swiss Medical Weekly. 2021;151:43–44. doi: 10.4414/smw.2021.w30072. [DOI] [PubMed] [Google Scholar]
- 9.Naik S, Haldar SN, Soneja M, Mundadan NG, Garg P, Mittal A, Desai D, Trilangi PK, Chakraborty S, Begam NN, Bhattacharya B, Maher G, Mahishi N, Rajanna C, Kumar SS, Arunan B, Kirtana J, Gupta A, Patidar D, Kodan P, Sethi P, Ray A, Jorwal P, Kumar A, Nischal N, Sinha S, Biswas A, Wig N. Post COVID-19 sequelae: a prospective observational study from Northern India. Drug Discov Ther. 2021;15(5):254–260. doi: 10.5582/ddt.2021.01093. [DOI] [PubMed] [Google Scholar]
- 10.Vlake JH, Van Bommel J, Hellemons ME, Wils E-J, Bienvenu OJ, Schut AFC, Klijn E, Van Bavel MP, Gommers D, Van Genderen ME. Psychologic distress and quality of life after ICU treatment for coronavirus disease 2019: a multicenter observational cohort study. Crit Care Explor. 2021 doi: 10.1097/CCE.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putri C, Arisa J, Hananto JE, Hariyanto TI, Kurniawan A. Psychiatric sequelae in COVID-19 survivors: a narrative review. World J Psychiatry. 2021;11(10):821–829. doi: 10.5498/wjp.v11.i10.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huarcaya-Victoria J, Barreto J, Aire L, Podestá A, Caqui M, Guija-Igreda R, Castillo C, Alarcon-Ruiz CA. Mental health in COVID-2019 survivors from a general hospital in Peru: sociodemographic, clinical, and inflammatory variable associations. Int J Mental Health Addict. 2021 doi: 10.1007/s11469-021-00659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arab-Zozani M, Hashemi F, Safari H, Yousefi M, Ameri H. Health-related quality of life and its associated factors in COVID-19 Patients. Osong Public Health Res Perspect. 2020;11(5):296–302. doi: 10.24171/j.phrp.2020.11.5.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taboada M, Moreno E, Cariñena A, Rey T, Pita-Romero R, Leal S, Leal S, Sanduende Y, Rodríguez A, Nieto C, Vilas E, Ochoa M, Cid M, Seoane-Pillado T. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesthesia. 2021;126(3):e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santus P, Tursi F, Croce G, Di Simone C, Frassanito F, Gaboardi P, Airoldi A, Pecis M, Negretto G, Radovanovic D. Changes in quality of life and dyspnoea after hospitalization in COVID-19 patients discharged at home. Multidiscip Respir Med. 2020;15(1):713. doi: 10.4081/mrm.2020.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talman S, Boonman-de Winter LJM, De Mol M, Hoefman E, Van Etten RW, De Backer IC. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respiratory Medicine. 2021;176:106272. doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazza MG, Palladini M, De Lorenzo R, Bravi B, Poletti S, Furlan R, Ciceri F, Vai B, Bollettini I, Melloni EMT, Mazza EB. One-year mental health outcomes in a cohort of COVID-19 survivors. Journal of Psychiatric Research. 2022;145:118–124. doi: 10.1016/j.jpsychires.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancet T. COVID-19 in Latin America-emergency and opportunity. Lancet. 2021;398(10295):93. doi: 10.1016/S0140-6736(21)01551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. (2022). WHO coronavirus (COVID-19) dashboard, February 12, 2022, available at https://covid19.who.int
- 21.Ministry of Health. (2022). Coronavirus (COVID-19) dashboard in Peru, February 12, 2022, available at https://covid19.minsa.gob.pe/sala_situacional.asp
- 22.Córdova-Aguilar A, Rossani G. COVID-19: literature review and its impact on the Peruvian health reality. Revista de la Facultad de Medicina Humana. 2020;20(3):471–477. doi: 10.25176/rfmhv20i3.2984. [DOI] [Google Scholar]
- 23.Tenorio-Mucha J, Romero-Albino Z, Vidal V, Cuba-Fuentes S. Quality of life of older adults in Peruvian Social Security during the COVID-19 pandemic. Revista del Cuerpo Médico del HNAAA. 2021;14(Sup1):41–48. doi: 10.35434/rcmhnaaa.2021.14sup1.1165. [DOI] [Google Scholar]
- 24.Figueroa-Quiñones J, Cjuno J, Machay-Pak D, Ipanaqué-Zapata M. Quality of life and depressive symptoms among peruvian university students during the COVID-19 pandemic. Frontiers in Psychology. 2022 doi: 10.3389/fpsyg.2022.781561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sempé L, Lloyd-Sherlock P, Martínez R, Ebrahim S, McKee M, Acosta E. Estimation of all-cause excess mortality by age-specific mortality patterns for countries with incomplete vital statistics: a population-based study of the case of Peru during the first wave of the COVID-19 pandemic. Lancet Regional Health Am. 2021;2:100039. doi: 10.1016/j.lana.2021.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns Hopkins Coronavirus Resource Center (2022). Mortality analyses, April 24, 2022, available at https://coronavirus.jhu.edu/data/mortality
- 27.Feng Y-S, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Quality of Life Research. 2021;30(3):647–673. doi: 10.1007/s11136-020-02688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EuroQol Research Foundation. (2022). EQ-5D-5L | Telephone interview version, available at https://euroqol.org/eq-5d-instruments/eq-5d-5l-available-modes-of-administration/telephone-interview/
- 29.Calderón M, Gálvez-Buccollini JA, Cueva G, Ordoñez C, Bromley C, Fiestas F. Validation of the peruvian version of the PHQ-9 for diagnosing depression. Revista Peruana de Medicina Experimental y Salud Publica. 2012;29(4):578–578. doi: 10.1590/s1726-46342012000400027. [DOI] [PubMed] [Google Scholar]
- 30.Cassiani-Miranda CA, Vargas-Hernández MC, Pérez-Anibal E, Herazo-Bustos MI, Hernández-Carrillo M. Reliability and dimensionality of PHQ-9 in screening depression symptoms among health science students in Cartagena, 2014. Biomédica. 2017;37(Suppl 1):112–120. doi: 10.7705/biomedica.v37i0.3221. [DOI] [PubMed] [Google Scholar]
- 31.Saldivia S, Aslan J, Cova F, Vicente B, Inostroza C, Rincón P. Psychometric characteristics of the Patient Health Questionnaire (PHQ-9) in primary health care from Chile. Revista médica de Chile. 2019;147(1):53–60. doi: 10.4067/S0034-98872019000100053. [DOI] [PubMed] [Google Scholar]
- 32.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Int Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 33.García-Campayo J, Zamorano E, Ruiz MA, Pardo A, Pérez-Páramo M, López-Gómez V, Freire O, Rejas J. Cultural adaptation into Spanish of the generalized anxiety disorder-7 (GAD-7) scale as a screening tool. Health and Quality of Life Outcomes. 2010;8:8. doi: 10.1186/1477-7525-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baek H, Cho M, Kim S, Hwang H, Song M, Yoo S. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS ONE. 2018;13(4):e0195901. doi: 10.1371/journal.pone.0195901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rees EM, Nightingale ES, Jafari Y, Waterlow NR, Clifford S, Pearson CAB, Jombart T, Procter SR, Knight GM. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Medicine. 2020;18(1):270. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans D, Chaix B, Lobbedez T, Verger C, Flahault A. Combining directed acyclic graphs and the change-in-estimate procedure as a novel approach to adjustment-variable selection in epidemiology. BMC Medical Research Methodology. 2012;12(1):156. doi: 10.1186/1471-2288-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otto E, Culakova E, Meng S, Zhang Z, Xu H, Mohile S, Flannery MA. Overview of Sankey flow diagrams: focusing on symptom trajectories in older adults with advanced cancer. J Geriatr Oncol. 2022 doi: 10.1016/j.jgo.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamer, A., Laurent, G., Pelayo, S., El Amrani, M., Chazard, E., & Marcilly, R. (2020). Exploring patient path through sankey diagram: a proof of concept, In Digital Personalized Health and Medicine (Vol. 270, 218–222). Presented at the 30th Medical Informatics Europe Conference, MIE 2020, Geneva: IOS Press, doi: 10.3233/SHTI200154 [DOI] [PubMed]
- 39.Jain U. Effect of COVID-19 on the organs. Cureus. 2020;12(8):e9540. doi: 10.7759/cureus.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barker-Davies RM, O'Sullivan O, Senaratne KPP, Baker P, Cranley M, Dharm-Datta S, Ellis H, Goodall D, Gough M, Lewis S, Norman J. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. British Journal of Sports Medicine. 2020;54(16):949–959. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negm AM, Salopek A, Zaide M, Meng VJ, Prada C, Chang Y, Heyn PC. Rehabilitation care at the time of coronavirus disease-19 (COVID-19) pandemic: a scoping review of health system recommendations. Front Aging Neurosci. 2022;13:781271. doi: 10.3389/fnagi.2021.781271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen K-Y, Li T, Gong F-H, Zhang J-S, Li X-K. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front Psychiatry. 2020;11:668. doi: 10.3389/fpsyt.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmoud M, Carmisciano L, Tagliafico L, Muzyka M, Rosa G, Signori A, Bassetti M, Nencioni A, Monacelli F, GECOVID Study Group Patterns of comorbidity and in-hospital mortality in older patients with COVID-19 infection. Frontiers in Medicine. 2021;8:726837. doi: 10.3389/fmed.2021.726837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jergović M, Coplen CP, Uhrlaub JL, Nikolich-Žugich J. Immune response to COVID-19 in older adults. Journal of Heart and Lung Transplantation. 2021;40(10):1082–1089. doi: 10.1016/j.healun.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cugmas M, Ferligoj A, Kogovšek T, Batagelj Z. The social support networks of elderly people in Slovenia during the Covid-19 pandemic. PLoS ONE. 2021;16(3):e0247993. doi: 10.1371/journal.pone.0247993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson HJ, Swan A, Nathan PR. Psychiatric diagnosis and quality of life: the additional burden of psychiatric comorbidity. Comprehensive Psychiatry. 2011;52(3):265–272. doi: 10.1016/j.comppsych.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Erber J, Wießner JR, Zimmermann GS, Barthel P, Burian E, Lohöfer F, Martens E, Mijočević H, Rasch S, Schmid RM, Spinner CD. Longitudinal assessment of health and quality of life of COVID-19 patients requiring intensive care-an observational study. Journal of Clinical Medicine. 2021;10(23):5469. doi: 10.3390/jcm10235469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gamberini L, Mazzoli CA, Prediletto I, Sintonen H, Scaramuzzo G, Allegri D, Colombo D, Tonetti T, Zani G, Capozzi C, Dalpiaz G. Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respiratory Medicine. 2021;189:106665. doi: 10.1016/j.rmed.2021.106665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabacof L, Tosto-Mancuso J, Wood J, Cortes M, Kontorovich A, McCarthy D, Rizk D, Rozanski G, Breyman E, Nasr L, Kellner C. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am J Phys Med Rehab. 2022;101(1):48–52. doi: 10.1097/PHM.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, Patel U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—A systematic review and meta-analysis. Journal of Medical Virology. 2022;94(1):253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boix V, Merino E. Post-COVID syndrome The never ending challenge. Medicina Clinica. 2021;S0025–7753(21):00607–612. doi: 10.1016/j.medcli.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah R, Ali FM, Nixon SJ, Ingram JR, Salek SM, Finlay AY. Measuring the impact of COVID-19 on the quality of life of the survivors, partners and family members: a cross-sectional international online survey. British Medical Journal Open. 2021 doi: 10.1136/bmjopen-2020-047680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, Zhang X. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Damiano RF, Di Santi T, Beach S, Pan PM, Lucchetti AL, Smith FA, Forlenza OV, Fricchione GL, Miguel EC, Lucchetti G. Mental health interventions following COVID-19 and other coronavirus infections: a systematic review of current recommendations and meta-analysis of randomized controlled trials. Brazilian J Psychiatry. 2021;43:665–678. doi: 10.1590/1516-4446-2020-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Oliveira Almeida K, Nogueira Alves IG, de Queiroz RS, de Castro MR, Gomes VA, Santos Fontoura FC, Brites C, Neto MG. A systematic review on physical function, activities of daily living and health-related quality of life in COVID-19 survivors. Chronic Illness. 2022 doi: 10.1177/17423953221089309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porter C, Favara M, Hittmeyer A, Scott D, Jiménez AS, Ellanki R, Woldehanna T, Craske MG, Stein A. Impact of the COVID-19 pandemic on anxiety and depression symptoms of young people in the global south: evidence from a four-country cohort study. British Medical Journal Open. 2021 doi: 10.1136/bmjopen-2021-049653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villarreal-Zegarra D, Alarcon-Ruiz CA, Melendez-Torres GJ, Torres-Puente R, Navarro-Flores A, Cavero V, Ambrosio-Melgarejo J, Rojas-Vargas J, Almeida G, Albitres-Flores L, Romero-Cabrera AB. Development of a framework for the implementation of synchronous digital mental health: realist synthesis of systematic reviews. JMIR Mental Health. 2022 doi: 10.2196/34760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poudel AN, Zhu S, Cooper N, Roderick P, Alwan N, Tarrant C, Ziauddeen N, Yao GL. Impact of Covid-19 on health-related quality of life of patients: A structured review. PLoS ONE. 2021 doi: 10.1371/journal.pone.0259164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen HC, Nguyen MH, Do BN, Tran CQ, Nguyen TT, Pham KM, Pham LV, Tran KV, Duong TT, Tran TV, Duong TH. People with suspected COVID-19 symptoms were more likely depressed and had lower health-related quality of life: the potential benefit of health literacy. Journal of Clinical Medicine. 2020;9(4):965. doi: 10.3390/jcm9040965. [DOI] [PMC free article] [PubMed] [Google Scholar]