Abstract
Ex vivo lung perfusion (EVLP) is a technique that enables active metabolism of the lung by creating an environment similar to that inside the body, even though the explanted lungs are outside the body. The EVLP system enables the use of lung grafts that do not satisfy the acceptance criteria for lung transplantation (LTx) by making it possible to evaluate the function of the lung grafts and repair lungs in poor condition, thereby reducing the waiting time of patients requiring LTx and consequently mortality.
Keywords: Lung transplantation, Donor pool, Ex vivo lung perfusion
Introduction
The increasing number of lung transplantations (LTx) has been accompanied by an increasing number of patients waiting for LTx, resulting in a chronic shortage of lungs for transplantation [1]. To overcome this insufficiency, marginal lungs that fail to meet the standard donor selection criteria or donation after circulatory death (DCD) donors are used [2]. Ex vivo lung perfusion (EVLP), introduced in the late 2000s, has significantly contributed to increasing the donor pool by enabling the evaluation and preservation of lungs explanted from the donors [3].
Cold static preservation versus ex vivo lung perfusion
Cold static preservation is currently a common method of lung preservation. In cold static preservation, a cold lung preservation solution (Perfadex) is administered through the pulmonary vessels, and the lung is stored in Perfadex at 4°C after retrieval. Theoretically, lung grafts could be preserved for up to 12 hours at 4°C [4], as cellular metabolism is reduced to 5% of that occurring at a normal temperature [5,6]. However, because active metabolism does not occur in this process, the recovery of lung function after injury, which may occur during the lung procurement process, is impossible. Additionally, irreversible and permanent lung injury may occur after a certain period of time.
Contrarily, EVLP supplies various nutrients required for graft survival at a normal body temperature through the pulmonary vessels, as well as oxygen through the airway, creating an environment for the lungs similar to that inside the body, even though they were explanted from the body, thus resulting in active metabolism. Therefore, even lungs that are slightly unsuitable for LTx can recover with EVLP. Moreover, EVLP can protect the graft from a systemic inflammatory reaction or coagulation during the repair process in the in vivo state.
Composition and techniques of the ex vivo lung perfusion system
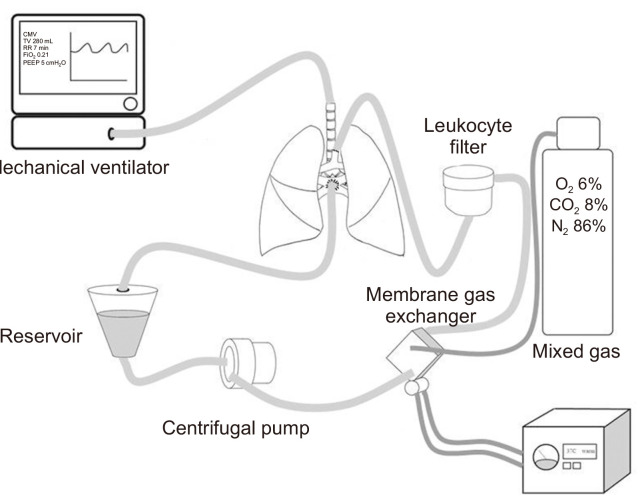
The EVLP system is mainly divided into ventilation and perfusion systems. The ventilation system utilizes a commonly used mechanical ventilator connected to the endotracheal tube that is directly inserted into the trachea. The perfusate (perfusion solution) circulates inside the circuit connected to the pulmonary artery (PA) and left atrium (LA) by a centrifugal pump. The perfusate passes through the membrane gas exchanger connected to the circuit. The gas exchanger induces deoxygenation with mixed gas (O2, 6%; CO2, 8%; N2, 86%). A leukocyte filter is also connected to the circuit to remove leukocytes before the perfusate enters the lungs through the PA. A heater–cooler unit is connected to the gas exchanger to control and maintain the temperature of the perfusate. The perfusate, which enters the lungs through the PA, is collected in the reservoir via the pulmonary vein and LA, and circulates again inside the perfusion system by a centrifugal pump (Fig. 1).
Fig. 1.
Schematic diagram of ex vivo lung perfusion (EVLP). The EVLP system consists of ventilation and perfusion system. Normothermic perfusate circulates inside the circuit connected to the membrane gas exchanger and leukocyte filter.
Out of several EVLP protocols, the 3 most commonly used are the Lund, Toronto, and Organ Care System (OCS) protocols. Although the Lund protocol was developed first, the Toronto protocol is most commonly used. The OCS is a portable device that allows EVLP to be implemented while transporting the lungs, which dramatically reduces the cold ischemic time. The Lund and Toronto protocols use a commercialized perfusate called the STEEN solution. This perfusate contains human albumin, which reduces the occurrence of pulmonary edema even in long-lasting EVLP by maintaining a normal oncotic pressure. Similar to Perfadex, this perfusate is a low-potassium chloride solution that reduces free radical generation and prevents vascular spasms. The dextran included in it also protects the endothelium from leukocyte interactions. It also contains glucose and several electrolytes required for cellular metabolism [7]. In the Lund protocol, blood is mixed in the perfusate to obtain a hematocrit of approximately 15%; however, in the Toronto protocol, only a pure perfusate without cell components is used because red blood cells can cause mechanical injury. In the OCS protocol, blood is mixed with the OCS solution to obtain a hematocrit between 15% and 25%. The 3 protocols seem similar, but slight differences in perfusion flow, PA pressure maintenance, perfusate temperature, and respiratory rate can be observed (Table 1) [8]. Commercialized devices such as the XVIVO perfusion system (Fig. 2), Lung Assist, Vivoline LS1, and OCS, suitable for each protocol, are available for purchase.
Table 1.
Ex vivo lung perfusion protocols in clinical lung transplantation [8]
| Variable | Lund | Toronto | OCS |
|---|---|---|---|
| Perfusion | |||
| Target flow | 100% of cardiac output (70 mL/kg/min) | 40% of cardiac output | 2–2.5 L/min |
| Pulmonary arterial pressure (mm Hg) | ≤20 | ≤15 | ≤20 |
| Left atrial pressure (mm Hg) | 0 (Open LA) | 3–5 | 0 (Open LA) |
| Pump | Roller | Centrifugal | Piston (pulsatile) |
| Perfusate | 2 L of Steen solution with red-cell concentrates (hematocrit 10%–15%) | 2 L of Steen solution | 1.5 L of OCS lung solution with red-cell concentrates (hematocrit 15%–25%) |
| Ventilation | |||
| Mode | Volume controlled | Volume controlled | Volume controlled |
| Tidal volume (mL/kg) | 6–8 | 7 | 6 |
| Frequency (bpm) | 10–15 | 7 | 10 |
| Peak end-expiratory pressure (cmH2O) | 5 | 5 | 5 |
| Fraction of inspired oxygen (%) | 50 | 21 | 21 |
| Temperature (°C) | |||
| Start of ventilation | 32 | 32 | 32 |
| Start of perfusion | 15 | 25 | 32 |
| Start of evaluation | 37 | 37 | 37 |
OCS, Organ Care System; LA, left atrium.
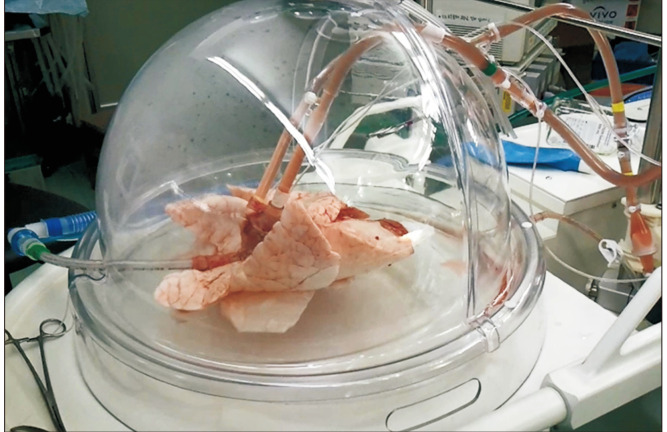
Fig. 2.
Application of ex vivo lung perfusion to a porcine lung using the XVIVO perfusion system.
Evaluation of graft function using ex vivo lung perfusion
Since DCD donors do not have blood circulation, the functional evaluation of the lung graft, including arterial blood gas analysis, is inevitably limited. The use of EVLP allows a more objective evaluation of the function of DCD lung grafts [9,10]. Assessment of the partial pressure of oxygen in the perfusate, pulmonary vascular resistance, airway pressure, and lung compliance are useful indicators for evaluating lung injury during EVLP. Lung radiography or bronchoscopy may also be performed during EVLP [11].
In addition to these functional parameters, lung function can be evaluated by measuring lung tissue, bronchoalveolar lavage fluid, or biologic markers in the perfusate during EVLP. Measurements of interleukin (IL)-1β, IL-6, IL-8 (proinflammatory cytokines), and IL-10 (anti-inflammatory cytokine) reflect the degree of lung injury [12-14]. In addition, allograft function can be predicted by measuring endothelial nitric oxide synthase or cyclic guanosine monophosphate [15]. Based on these results, a decision is made to use or discard the graft for LTx after performing EVLP for approximately 4 hours.
Lung repair using ex vivo lung perfusion
An important purpose of EVLP is to evaluate lung function objectively, repair injured lungs, and protect them from inflammatory and immune reactions, so that they can eventually be used for LTx.
Lungs with infections can be treated by administering high-dose antibiotics during EVLP. As has been experimentally proven, in lungs with aspiration of gastric contents, it is also possible to actively remove the contents using a bronchoscope or reduce inflammation by injecting an exogenous surfactant into the bronchus [16]. Donor lungs with confirmed thromboembolism are challenging to use immediately for LTx; however, thrombolysis can be performed during EVLP to make these lungs suitable for transplantation [17].
In addition to these treatment modalities already used in clinical practice, various methods to improve lung function during ELVP have been experimentally studied. The 3 main methods are as follows: first, a trans-tracheal approach, such as ventilation with a medical gas, such as carbon monoxide, hydrogen or administration of biodegradable nanoparticle-containing drugs [18-20]; second, a method of using perfusion itself, such as mixing a drug that has an anti-inflammatory effect with a perfusate or changing the osmotic or oncotic pressure; and third, gene therapy, such as adenoviral-mediated IL-10 gene transfection [21]. Various other methods have also been attempted, such as changing the graft to be in a prone position or using stem cells [22,23].
Ex vivo lung perfusion impact on lung transplantation
The introduction of EVLP has increased the volume of LTx and the lung usage rate. EVLP has been used to induce recovery in lungs discarded because they failed to meet the acceptance criteria before performing EVLP, and these lungs have been used. Several institutions worldwide have reported that the utilization of donor organs increased up to 70% after performing EVLP [24]. The increased utilization of donor lungs reduces the waiting list time, which is expected to decrease waiting list mortality.
In addition to increasing the donor pool, EVLP provides time flexibility during the strict LTx process. Before the introduction of EVLP, lung function in DCD donors could not be evaluated within sufficient time to reduce the warm ischemic time. However, EVLP can enable evaluations of lung function using lung radiography or bronchoscopy. The recipient can be prepared for transplantation and the surgeons preparing for transplantation can perform scheduled surgery with sufficient time. Moreover, the time margin for organ transportation has increased with the development of portable devices such as OCS.
Conclusion
Although the development and implementation of EVLP protocols are still in progress, many institutions have published the results of studies on the safety and usefulness of EVLP. EVLP provides an opportunity to evaluate graft function accurately and expands the donor pool by enabling the repair of lungs in poor condition, leading to shorter waiting list times and reduced mortality. Several studies are now being conducted to improve graft function during EVLP, and EVLP is expected to significantly impact the active use of LTx in the future.
Author contributions
All work was done by Seokjin Haam.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 annual data report: lung. Am J Transplant. 2020;20 Suppl s1:427–508. doi: 10.1111/ajt.15677. [DOI] [PubMed] [Google Scholar]
- 2.Van Raemdonck D, Neyrinck A, Verleden GM, et al. Lung donor selection and management. Proc Am Thorac Soc. 2009;6:28–38. doi: 10.1513/pats.200808-098GO. [DOI] [PubMed] [Google Scholar]
- 3.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjoberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76:244–52. doi: 10.1016/S0003-4975(03)00191-7. [DOI] [PubMed] [Google Scholar]
- 4.Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9:2262–9. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 5.de Perrot M, Bonser RS, Dark J, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part III: donor-related risk factors and markers. J Heart Lung Transplant. 2005;24:1460–7. doi: 10.1016/j.healun.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Weeder PD, van Rijn R, Porte RJ. Machine perfusion in liver transplantation as a tool to prevent non-anastomotic biliary strictures: rationale, current evidence and future directions. J Hepatol. 2015;63:265–75. doi: 10.1016/j.jhep.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Wierup P, Haraldsson A, Nilsson F, et al. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006;81:460–6. doi: 10.1016/j.athoracsur.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Cypel M, Sato M, Yildirim E, et al. Initial experience with lung donation after cardiocirculatory death in Canada. J Heart Lung Transplant. 2009;28:753–8. doi: 10.1016/j.healun.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Snell GI, Levvey BJ, Oto T, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transplant. 2008;8:1282–9. doi: 10.1111/j.1600-6143.2008.02231.x. [DOI] [PubMed] [Google Scholar]
- 10.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–40. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 11.Rega FR, Vanaudenaerde BM, Wuyts WA, et al. IL-1beta in bronchial lavage fluid is a non-invasive marker that predicts the viability of the pulmonary graft from the non-heart-beating donor. J Heart Lung Transplant. 2005;24:20–8. doi: 10.1016/j.healun.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Kaneda H, Waddell TK, de Perrot M, et al. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant. 2006;6:544–51. doi: 10.1111/j.1600-6143.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 13.De Perrot M, Sekine Y, Fischer S, et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–5. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 14.George TJ, Arnaoutakis GJ, Beaty CA, et al. A physiologic and biochemical profile of clinically rejected lungs on a normothermic ex vivo lung perfusion platform. J Surg Res. 2013;183:75–83. doi: 10.1016/j.jss.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima D, Liu M, Ohsumi A, et al. Lung lavage and surfactant replacement during ex vivo lung perfusion for treatment of gastric acid aspiration-induced donor lung injury. J Heart Lung Transplant. 2017;36:577–85. doi: 10.1016/j.healun.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Inci I, Yamada Y, Hillinger S, Jungraithmayr W, Trinkwitz M, Weder W. Successful lung transplantation after donor lung reconditioning with urokinase in ex vivo lung perfusion system. Ann Thorac Surg. 2014;98:1837–8. doi: 10.1016/j.athoracsur.2014.01.076. [DOI] [PubMed] [Google Scholar]
- 17.Dong B, Stewart PW, Egan TM. Postmortem and ex vivo carbon monoxide ventilation reduces injury in rat lungs transplanted from non-heart-beating donors. J Thorac Cardiovasc Surg. 2013;146:429–36. doi: 10.1016/j.jtcvs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Haam S, Lee JG, Paik HC, Park MS, Lim BJ. Hydrogen gas inhalation during ex vivo lung perfusion of donor lungs recovered after cardiac death. J Heart Lung Transplant. 2018;37:1271–8. doi: 10.1016/j.healun.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Grimm JC, Zhang F, Magruder JT, et al. Accumulation and cellular localization of nanoparticles in an ex vivo model of acute lung injury. J Surg Res. 2017;210:78–85. doi: 10.1016/j.jss.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Machuca TN, Cypel M, Bonato R, et al. Safety and efficacy of ex vivo donor lung adenoviral IL-10 gene therapy in a large animal lung transplant survival model. Hum Gene Ther. 2017;28:757–65. doi: 10.1089/hum.2016.070. [DOI] [PubMed] [Google Scholar]
- 21.Niikawa H, Okamoto T, Ayyat KS, et al. Prone ex vivo lung perfusion protects human lungs from reperfusion injury. Artif Organs. 2022 Jun 3; doi: 10.1111/aor.14328. [Epub]. https://doi.org/10.1111/aor.14328 . [DOI] [PubMed] [Google Scholar]
- 22.Nakajima D, Watanabe Y, Ohsumi A, et al. Mesenchymal stromal cell therapy during ex vivo lung perfusion ameliorates ischemia-reperfusion injury in lung transplantation. J Heart Lung Transplant. 2019;38:1214–23. doi: 10.1016/j.healun.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Cypel M, Yeung JC, Donahoe L, et al. Normothermic ex vivo lung perfusion: does the indication impact organ utilization and patient outcomes after transplantation? J Thorac Cardiovasc Surg. 2020;159:P346–55. doi: 10.1016/j.jtcvs.2019.06.123. [DOI] [PubMed] [Google Scholar]
- 24.Andreasson AS, Dark JH, Fisher AJ. Ex vivo lung perfusion in clinical lung transplantation: state of the art. Eur J Cardiothorac Surg. 2014;46:779–88. doi: 10.1093/ejcts/ezu228. [DOI] [PubMed] [Google Scholar]