Abstract
Lung transplantation is a life-saving procedure in patients with end-stage lung disease. However, it inherently depends on the availability of donor organs. The selection of suitable lungs for transplantation, management of donors to minimize further injury and improve organ function, and safe procurement remain critical for successful transplantation. In this review, we provide an update on the current understanding of donor selection, management, and lung procurement.
Keywords: Lung transplantation, Donor selection, Donor management, Lung procurement
Introduction
During the last decade, lung transplantation (LTx) has been recognized as an effective treatment option for end-stage lung disease by medical professionals and patients, and the number of LTx procedures has rapidly increased in Korea. According to the 2019 annual report of the Korean Network for Organ Sharing (KONOS) [1], the number of LTx cases increased from 18 in 2010 to 157 in 2019. However, the number of LTx candidates on the waiting list has also increased, exceeding the number of available donors. Given the shortage of suitable donors, donated organs are a limited and valuable resource. Therefore, the possibility of lung donation should be carefully evaluated, donors should be managed appropriately in the intensive care unit (ICU), and donor lungs should be procured safely in the operating room. This review aims to present an update on the current understanding of the donor selection, management, and lung procurement processes. Other strategies for expanding the donor pool, such as donation after cardiac death and ex vivo lung perfusion, are discussed elsewhere in this series of reviews.
Donor selection
Many factors should be considered when deciding to use donor lungs, such as the quality of the donor’s lungs, size matching, and the geographic distance between the donor and recipient hospital. The criteria for ideal donor lungs are as follows [2]: (1) age <55 years; (2) ABO compatibility; (3) clear chest radiograph; (4) partial pressure of oxygen >300 mm Hg on a fraction of inspired oxygen of 1.0 and a positive end-expiratory pressure of 5 cmH2O; (5) tobacco history <20 pack-years; (6) absence of chest trauma; (7) no evidence of aspiration/sepsis; (8) no prior cardiopulmonary surgery; (9) sputum Gram stain showing an absence of organisms; and (10) absence of purulent secretions on bronchoscopy.
However, stringent criteria for donor lungs can extend the waiting time and increase waiting list mortality. With clinical experience and advances in post-transplant care, the criteria for donor lungs have been extended over time. Some previous studies reported that more than half of the transplanted donors did not meet all these criteria (extended donors), and extended donor lungs showed acceptable outcomes [3-5].
Size matching is an essential component in donor selection. Although various size-matching criteria have been proposed, predicted total lung capacity (pTLC), which is calculated using sex and height, is the most commonly used criterion [6,7].
For males: pTLC=7.99×height (m)–7.08
For females: pTLC=6.60×height (m)–5.79
A donor pTLC between 75% and 125% of the recipient pTLC is considered acceptable and does not cause adverse clinical or functional effects [8]. The wide range of acceptable sizes of the donor lungs suggests that the chest wall is able to adjust to the newly transplanted lungs [9]. In cases of unusually small chests or pediatric patients, graft volume reduction, such as multiple peripheral wedge resections of the donor lungs or lobar transplantation, can be considered [10,11].
Due to ischemic time, geographic distance and transportation should also be considered in donor selection. The lung allograft ischemic time is defined as the time between the aortic cross-clamp performed during organ procurement surgery and lung allograft reperfusion during LTx surgery [12]. This usually depends on the travel time from the procurement hospital to the transplant hospital. Less than 6 hours of ischemic time is generally considered safe for LTx [8]. The 2017 report by the International Society for Heart and Lung Transplantation showed that the median allograft ischemic times have steadily increased. The ischemic time exceeded 6 hours in one-third of all transplants, and those LTx procedures showed acceptable long-term outcomes [12].
Donor management
Severe brain injury can lead to neurogenic pulmonary edema, which is a form of acute respiratory distress syndrome [13]. Increased pulmonary hydrostatic pressure due to a sudden increase in circulating catecholamines and increased expression of inflammatory mediators leads to increased lung capillary permeability and interstitial edema [13]. Donors with brain death also have a high risk of aspiration, injection, and injury from mechanical ventilation [13]. Owing to the vulnerability to injury, only a small number of lungs from these donors can be transplanted. According to the KONOS data, lungs are procured and transplanted from only 12% of the overall brain-dead donor population [14].
Several studies have shown that the appropriate and aggressive management of potential lung donors with brain death can increase the number of eligible and harvested lungs [15-17]. A protective ventilation strategy (low tidal, high positive end-expiratory pressure [PEEP], the recruitment maneuver) is key to respiratory management in potential lung donors to reduce ventilator-induced lung injury further [18]. Using a protective ventilation strategy with low tidal volume and high PEEP increased the number of harvested lungs from 27% to 54% compared to the conventional strategy in a randomized trial [15]. Conservative fluid management is recommended [16]. To prevent aspiration, the head of the bed needs to be elevated to 30°, and the endotracheal tube balloon needs to be inflated to a pressure of 25 cmH2O [16]. Bronchoscopy should be performed early to clear stagnant secretions and blood clots and to obtain sputum samples and bronchoalveolar lavage [18]. Corticosteroid replacement is known to reduce inflammation and improve oxygenation in brain-dead donors [18,19]. The University Hospital Marqués de Valdecilla (HUMV), an organ recovery center in Spain, proposed a strict donor management protocol, which increased lung utilization from 20% to 50% [17]. The protocol is described below:
Lung donor-management protocol at HUMV
(1) Apnea test performed with a ventilator (continuous positive pressure mode)
(2) Mechanical ventilation with positive end-expiratory pressure of 8–10 cmH2O and tidal volume of 6–8 mL/kg
(3) Recruitment maneuvers once per hour and after any disconnection from the ventilator
(4) Bronchoscopy with bilateral bronchoalveolar lavage
(5) Hemodynamics are closely monitored with the PICCO System (PULSION Medical Systems SE, Munich, German), with the goal of extravascular lung water <10 mL/kg (administering diuretics if necessary) and a central venous pressure objective <8 mm Hg.
(6) Methylprednisolone (15 mg/kg) after brain death declaration
(7) Alveolar recruitment involved controlled ventilation (peak pressure limit of 35 mm Hg) with positive end- expiratory pressure of 18–20 cmH2O for 1 minute, and a 2 cmH2O decrease each minute, followed by increasing the tidal volume by 50% for 10 breaths.
Lung procurement
Upon arrival at the procurement hospital, the procurement team must assess the donor, including an updated donor history, clinical events, management imaging, and laboratory results [18]. Communication with an anesthesiologist is vital for maintaining a lung-protective ventilation strategy and conservative fluid management during procurement. The procurement surgeon should also communicate the order, duration, and procurement procedures with other procurement teams [20].
Median sternotomy is the preferred approach for thoracic organ procurement. Extension of the incision cephalad to the thyroid cartilage helps expose the head vessel and trachea [18]. After opening the pericardium, preliminary dissection for cardiac venting and cannulation is to be performed as follows: (1) circumferential dissection of the superior vena cava (SVC) at the proximal azygos vein and encircling a silk suture for ligation; (2) circumferential dissection of the inferior vena cava (IVC); and (3) separation of the aorta and pulmonary artery (PA) to allow for cross-clamp application. If the heart is procured, the heart and lung procurement teams must discuss and agree on venting strategies and PA cannulation sites.
After preliminary dissection of the heart, the bilateral pleura is widely opened below the sternum for an assessment of the lung. The lungs are inspected for edema, pleural adhesion, lung atelectasis, or contusion, and palpated for masses [20]. When atelectasis is encountered, a gentle sustained Valsalva maneuver and massage can be performed for recruitment. At this time, the procurement surgeon and anesthesiologist need to work together to maintain blood pressure and heart rate. If atelectasis does not respond to recruitment, bronchoscopy should be considered to search for a mucus plug or infection [18]. After the lung examination, abdominal organ dissection is performed.
After all procurement teams are ready for cannulation, 400 U/kg of intravenous heparin is administered for anticoagulation. PA cannulation is placed on the main PA 1.5 cm proximal to its bifurcation with the agreement of the heart procurement surgeon [18]. Care should be taken to prevent the cannula tip from entering either the right or left PA [20]. After all teams complete cannulation, the lung procurement surgeon checks the atelectasis in both lungs and performs recruitment, and 500 µg of prostaglandin E1 is injected through the PA. Next, venting and perfusion are initiated as follows.
(1) SVC ligation
(2) Incision at the left atrial appendage or the Waterston’s interatrial groove
(3) IVC partial transection
(4) Aortic cross-clamp
(5) Perfusion
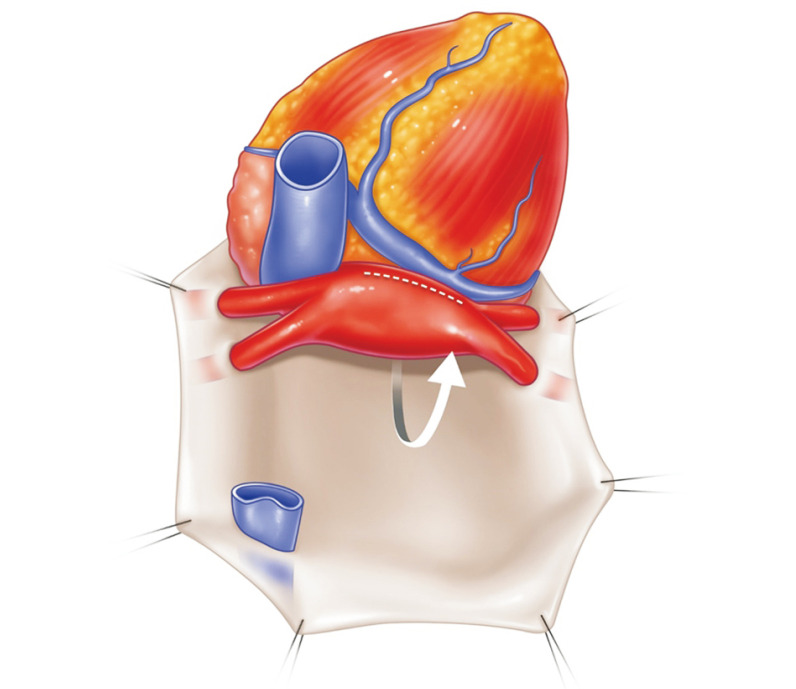
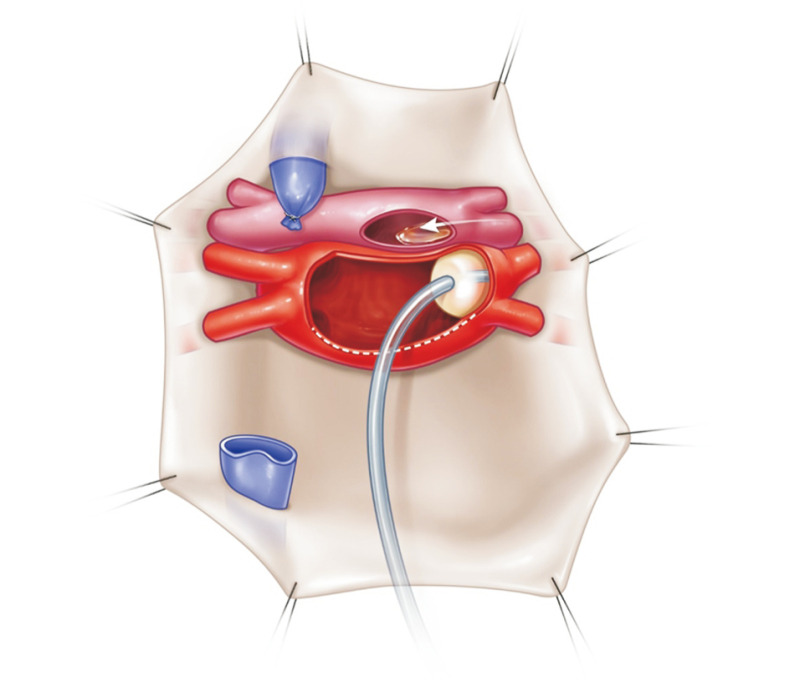
After confirming sufficient venting of the left atrium (LA), lung preservation solution (PERFADEX; XVIVO Perfusion, Gothenburg, Sweden) is perfused through the PA (Fig. 1). A total of 4 L of PERFADEX is administered in an anterograde manner until the effluent from the LA becomes clear [21]. An ice slush is placed in the thoracic cavity to lower the temperature. After perfusion, the SVC, IVC, ascending aorta, and main PA are cut. By retracting the apex of the heart, the LA anterior wall can be easily seen and opened (Fig. 2); then, through the incision of the LA, the surgeon can see each orifice of the pulmonary veins and excise the LA wall with sufficient LA muscular tissue for LA anastomosis. If the heart is also procured, it is recommended that the heart and lung procurement team should discuss and cooperate to preserve a sufficient muscular cuff around the opening of the pulmonary veins. Subsequently, retrograde perfusion through the pulmonary veins is performed with a Foley catheter (250–500 mL per vein) until the effluent from the PA becomes clear (Fig. 3) [21].
Fig. 1.
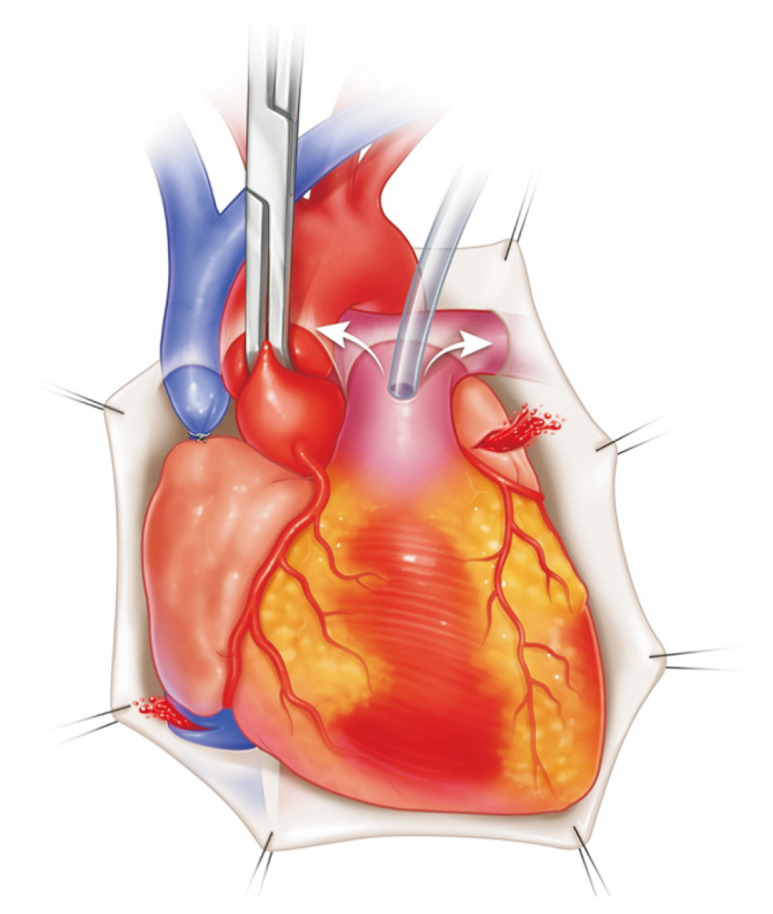
Pulmonary artery (PA) cannulation is placed on the main PA 1.5 cm proximal to its bifurcation with the agreement of the heart procurement surgeon. Care should be taken to prevent the cannula tip from entering either the right or left PA. Before perfusion, the superior vena cava is ligated, and the left atrial appendage and inferior vena cava are opened for venting. Once aortic cross clamp is placed, perfusion starts.
Fig. 2.
By retracting the apex of the heart, the anterior wall of the left atrium can be easily seen and opened.
Fig. 3.
Retrograde perfusion through the pulmonary veins is performed with a Foley catheter (250–500 mL per vein) until the effluent from the pulmonary artery becomes clear.
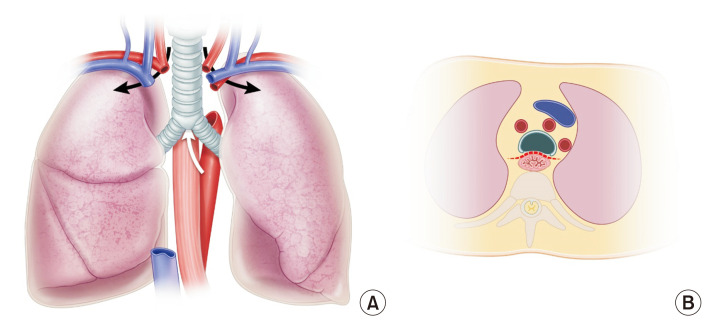
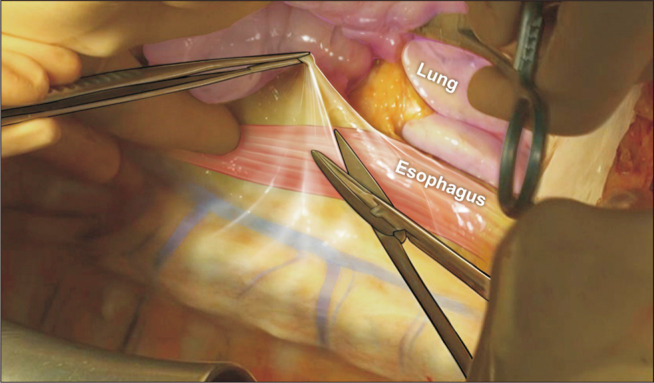
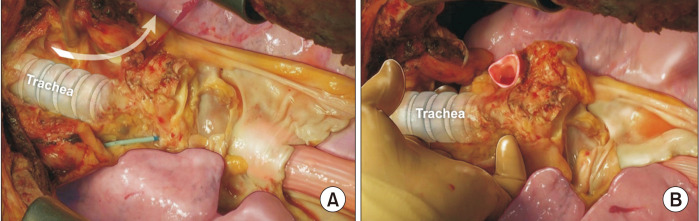
The esophagus is the most important anatomical landmark for en bloc resection of the lungs and trachea. From the diaphragm and thoracic inlet, the esophagus is the dissection plane, which is located just below the pericardium and trachea (Fig. 4). After completing retrograde perfusion, the pericardium is resected transversely at the diaphragm level. The esophagus could be easily seen, and the left lung retracted to the right side. The dissection is continued along the anterior wall of the esophagus upward up to the thoracic inlet. During the dissection, the descending aorta is divided. Then, the right lung is retracted to the left side. Dissection is performed at the same plane of the anterior wall of the esophagus (Fig. 5). The azygos vein is divided. After dissection, the lung is placed in its natural position. The trachea is then exposed (Fig. 6). A hole can be made using a finger below the head vessel (innominate and jugular vein and carotid and subclavian artery) from both sides of the trachea to the apex of both thoracic cavities, and all the mediastinal tissue and the head vessel can be cut easily. Next, the trachea is freely dissected from the esophagus, the lungs are inflated moderately, and the trachea is divided using a stapler. The procured lungs are stored in a cold lung preservation solution, enveloped in an ice box filled with ice, and then transported to the transplant hospital.
Fig. 4.
The esophagus is the most important anatomical landmark for en bloc resection of the lungs and trachea. From the diaphragm and thoracic inlet, the esophagus is the dissection plane, which is located just below the pericardium and trachea (A, B). The head vessels and superior mediastinal tissue on the right and the left side of the trachea need to be cut for en bloc resection of the lung and trachea (A).
Fig. 5.
After retracting the lungs to the opposite side, the anterior wall of the esophagus is the dissection plane from the diaphragm to the thoracic inlet. During the dissection, the descending aorta on the left and the azygos vein on the right are cut.
Fig. 6.
After exposure of the trachea, a hole can be made using a finger below the head vessel (innominate and jugular veins and carotid and subclavian arteries) from both sides of the trachea to the apex of both thoracic cavities (A), and all the mediastinal tissue and the head vessel can be cut easily. Then, the trachea is freely dissected from the esophagus (B).
Conclusion
Donor selection is the first step in successful LTx and should be balanced with the risk of death on the waiting list. In addition, active donor management can prevent further injury and improve donor lung quality. Finally, the procurement surgeon should understand the relevant anatomy and surgical procedure to safely procure the lungs and avoid unintended lung injury.
Author contributions
Conceptualization: WSY. Data curation: WSY. Formal analysis: WSY. Methodology: WSY. Project administration: WSY. Visualization: WSY, JAS. Writing–original draft: WSY, JAS. Writing–review & editing: WSY.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors are grateful to Woohyun Cho from Ajou University School of Medicine, Medical Information & Media Center, for his valuable contribution to creating the figures in this article.
References
- 1.The National Institute of Organ, Tissue and Blood Management, author. Annual report for 2019 [Internet] The National Institute of Organ, Tissue and Blood Management; Seoul: 2020. [cited 2022 Jun 20]. Available from: https://www.konos.go.kr/board/boardListPage.do?page=sub4_2_1&boardId=30 . [Google Scholar]
- 2.Sundaresan S, Trachiotis GD, Aoe M, Patterson GA, Cooper JD. Donor lung procurement: assessment and operative technique. Ann Thorac Surg. 1993;56:1409–13. doi: 10.1016/0003-4975(93)90699-I. [DOI] [PubMed] [Google Scholar]
- 3.Bhorade SM, Vigneswaran W, McCabe MA, Garrity ER. Liberalization of donor criteria may expand the donor pool without adverse consequence in lung transplantation. J Heart Lung Transplant. 2000;19:1199–204. doi: 10.1016/S1053-2498(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 4.Pierre AF, Sekine Y, Hutcheon MA, Waddell TK, Keshavjee SH. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg. 2002;123:421–8. doi: 10.1067/mtc.2002.120345. [DOI] [PubMed] [Google Scholar]
- 5.Reyes KG, Mason DP, Thuita L, et al. Guidelines for donor lung selection: time for revision? Ann Thorac Surg. 2010;89:1756–65. doi: 10.1016/j.athoracsur.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 6.Barnard JB, Davies O, Curry P, et al. Size matching in lung transplantation: an evidence-based review. J Heart Lung Transplant. 2013;32:849–60. doi: 10.1016/j.healun.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Ouwens JP, van der Mark TW, van der Bij W, Geertsma A, de Boer WJ, Koeter GH. Size matching in lung transplantation using predicted total lung capacity. Eur Respir J. 2002;20:1419–22. doi: 10.1183/09031936.02.00294402. [DOI] [PubMed] [Google Scholar]
- 8.Orens JB, Boehler A, de Perrot M, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183–200. doi: 10.1016/S1053-2498(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 9.Yu WS, Park CH, Paik HC, et al. Changes in thoracic cavity volume after bilateral lung transplantation. Front Med (Lausanne) 2022;9:881119. doi: 10.3389/fmed.2022.881119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aigner C, Mazhar S, Jaksch P, et al. Lobar transplantation, split lung transplantation and peripheral segmental resection: reliable procedures for downsizing donor lungs. Eur J Cardiothorac Surg. 2004;25:179–83. doi: 10.1016/j.ejcts.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Shigemura N, Bermudez C, Hattler BG, et al. Impact of graft volume reduction for oversized grafts after lung transplantation on outcome in recipients with end-stage restrictive pulmonary diseases. J Heart Lung Transplant. 2009;28:130–4. doi: 10.1016/j.healun.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Chambers DC, Yusen RD, Cherikh WS, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36:1047–59. doi: 10.1016/j.healun.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Busl KM, Bleck TP. Neurogenic pulmonary edema. Crit Care Med. 2015;43:1710–5. doi: 10.1097/CCM.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 14.Yeo HJ, Yoon SH, Lee SE, et al. Current status and future of lung donation in Korea. J Korean Med Sci. 2017;32:1953–8. doi: 10.3346/jkms.2017.32.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304:2620–7. doi: 10.1001/jama.2010.1796. [DOI] [PubMed] [Google Scholar]
- 16.Angel LF, Levine DJ, Restrepo MI, et al. Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 2006;174:710–6. doi: 10.1164/rccm.200603-432OC. [DOI] [PubMed] [Google Scholar]
- 17.Minambres E, Coll E, Duerto J, et al. Effect of an intensive lung donor-management protocol on lung transplantation outcomes. J Heart Lung Transplant. 2014;33:178–84. doi: 10.1016/j.healun.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Copeland H, Hayanga JW, Neyrinck A, et al. Donor heart and lung procurement: a consensus statement. J Heart Lung Transplant. 2020;39:501–17. doi: 10.1016/j.healun.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Follette DM, Rudich SM, Babcock WD. Improved oxygenation and increased lung donor recovery with high-dose steroid administration after brain death. J Heart Lung Transplant. 1998;17:423–9. [PubMed] [Google Scholar]
- 20.Hwang HP, Kim JM, Shin S, et al. Organ procurement in a deceased donor. Korean J Transplant. 2020;34:134–50. doi: 10.4285/kjt.2020.34.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen DC, Loor G, Carrott P, Shafii A. Review of donor and recipient surgical procedures in lung transplantation. J Thorac Dis. 2019;11(Suppl 14):S1810–6. doi: 10.21037/jtd.2019.06.31. [DOI] [PMC free article] [PubMed] [Google Scholar]