Abstract
AIM
To investigate the risk of exudative retinal detachment (ERD) morbidity in patients with pregnancy-induced hypertension (PIH) by using the logistic regression combined with the receiver operating characteristic (ROC) curve.
METHODS
A total of 46 patients with ERD and 142 patients with non-ERD were diagnosed as PIH from January 2017 to February 2020. A retrospective comparison of the clinical manifestations and laboratory tests were conducted. The risk of ERD morbidity with PIH was predicted by using logistic regression combined with an ROC curve model.
RESULTS
There was no significant difference in age and body mass index between the two groups before pregnancy (P>0.05). However, significant differences were found in gestational weeks, duration of hypertension, maximum and minimum systolic and diastolic blood pressure (BP), and plasma total protein (PTP) concentration between the two groups (P<0.05). Binary logistic regression analysis showed that the maximum systolic BP (OR=1.050, 95%CI: 1.016-1.085) and PTP concentration (OR=0.764, 95%CI: 0.702-0.832) were independent prediction risks of ERD in PIH. The sensitivities of maximum systolic BP, PTP concentration and combined diagnosis were 0.717, 0.870, and 0.870, respectively; the specificities were 0.617, 0.837, and 0.908, respectively; the area under the curve (AUC) was 0.707 (95%CI: 0.622-0.792), 0.917 (95%CI: 0.868-0.967), and 0.933 (95%CI: 0.890-0.975), respectively; the AUC of combined diagnosis was higher than that of single diagnosis (P<0.01).
CONCLUSION
Logistic regression and ROC curve model combined with maximum systolic BP and PTP can improve the early identification of high-risk PIH patients in the hospital.
Keywords: pregnancy-induced hypertension, exudative retinal detachment, plasma total protein, blood pressure
INTRODUCTION
Pregnancy-induced hypertension (PIH) is one of the most common obstetric diseases, an unresolved and unpreventable problem in obstetrics, which remains the leading risk on the health life of mother and infant[1]–[2]. PIH causes a variety of pathological vascular changes, subsequently damages the function of most of all organs, such as liver, heart, renal, brain, and eye.
It is the only one vascularity of retina which could be directly seen in the whole body. The vascular pathological changes of retina caused by PIH are considered as the ominous signs to value the progress and prognosis of PIH.
Pregnancy can induce retinal diseases or aggravate the original retinal diseases[3], a composite of severe maternal outcomes, including maternal near-miss defined by World Health Organization, cortical blindness, retinal detachment, temporary facial paralysis and maternal death, were adopted[4].
The common fundus changes include retinal artery spasm, high retinal artery reflection, arteriovenous cross indentation, retinal hemorrhage, optic disc edema, chorioretinitis[5] and exudative retinal detachment (ERD). Optical coherence tomography (OCT) manifestations retinal neuroepithelial detachment, retinal pigment epithelium (RPE) detachment and ellipsoid band change[6]–[7].
ERD is the main cause of blurred vision in PIH patients, with the incidence of 0.1%-2% worldwide[8]–[11]. Fluorescence angiographic findings support the hypothesis that retinal detachment in pre-eclampsia is secondary to choroidal ischemia from intense arteriolar vasospasm[12]–[13]. The happening of the ERD could mean a higher risk of core outcomes: stillbirth, small gestational age at delivery, lower birthweight, cardiovascular disease, neonatal mortality, seizures, admission to neonatal unit required and respiratory support[14]–[15]. Although most patients with ERD have good visual prognosis, but some patients have sustained damage in RPE layer and ellipsoid zone, which can lead to permanent loss of visual function in severe cases[16]. Therefore, identifying and intervening the risk factors related to ERD in pregnancy with hypertension can effectively reduce the abnormal pregnancy rate.
However, there is little knowledge on the risk factors of ERD in PIH patients. Here we conducted a retrospective study on the risk factors in PIH patients and found a significant correlation between the accidence of ERD and blood pressure (BP) and total protein concentration. Then we formed an algorithm with BP and total protein concentration to predict the accidence of ERD, which may extent more time before ERD and other hidden troubles occurring in PIH patients.
SUBJECTS AND METHODS
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and all the patients signed the informed consent.
From January 2017 to February 2020, 188 patients with PIH hospitaled in the Obstetrics Department of the Third Affiliated Hospital of Guangzhou Medical University, were enrolled and divided into two groups according to the results of fundus examination, namely, 46 ERD cases (ERD group) and 142 non-ERD ones (non-ERD group).
Best corrected visual acuity (BCVA) of 188 patients ranged from 0.1 to 1.0, with an average of 0.59±0.18. The diagnostic criteria for ERD were retinal neurodermis detachment, which was detected by fundus photography, retinal prescopy, and OCT. At the same time, the retinal detachment caused by tear and pull factor was excluded. Patients with ERD in one eye or more were enrolled to the ERD group. ERD fundus photography and OCT were shown in Figures 1 and 2.
Figure 1. Fundus photography showed that the retina was edema and bulging. OCT suggested detachment and edema of the neuroepithelium.
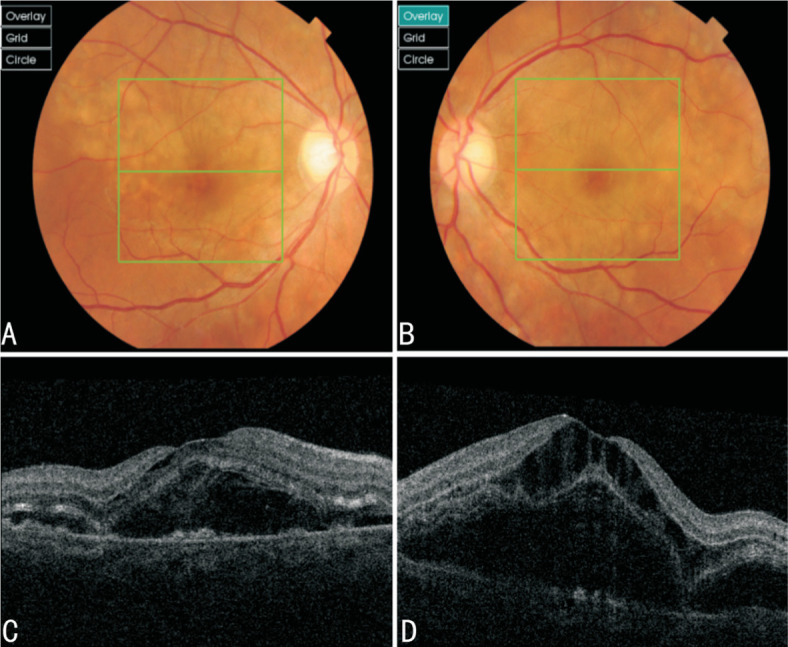
A, B: Fundus imaging results of right and left eyes of case 1; C, D: OCT results of right and left eyes of the patient.
Figure 2. Fundus imaging suggested retinal edema, retinal eminence and hemorrhage. OCT suggested detachment and edema of the neuroepithelium.
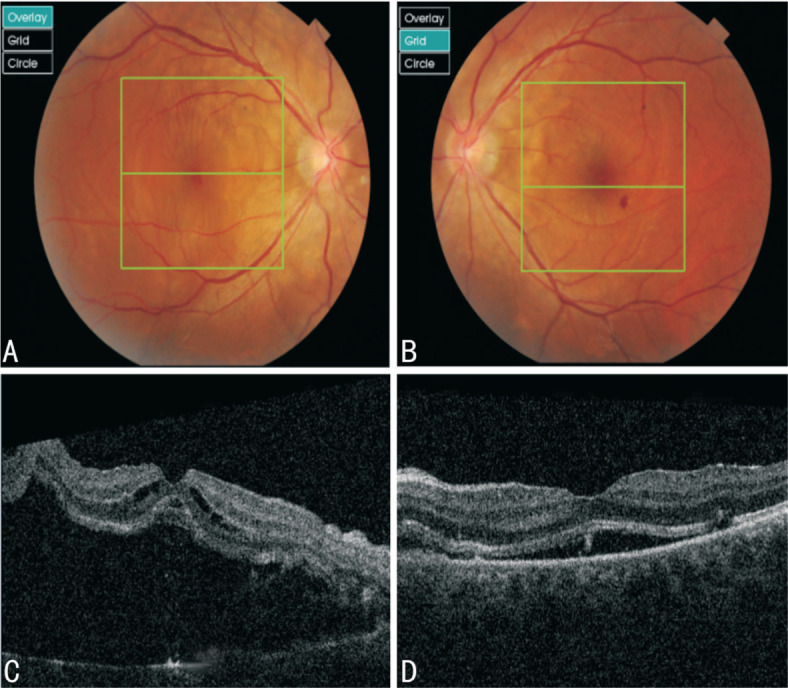
A, B: Fundus imaging results of right and left eyes of case 2; C, D: OCT results of right and left eyes of the patient.
The exclusion criteria were as follows: 1) the fundus photography and OCT examination were not completed; 2) with previous retinal and choroidal diseases; 3) the patients suffered from other conditions which could damage the retina, such as high myopia, diabetes, uremia, thrombocytopenic purpura, syphilis, dengue fever, and infection[17].
All patients were asked if there were blurred vision and dark shadow in front of their eyes. The common data of them, including age, gestational weeks, duration of hypertension, past medical history, pre-pregnancy body mass index (BMI) were recorded. And then, every case underwent 24-hour ambulatory BP monitor and the maximum systolic and diastolic BP were determined by measurement results. Visual acuity, fundus examination, fundus photography, and OCT examination were conducted by the senior ophthalmologist. Plasma total protein (PTP) concentration were also collected. The plasma colloid osmotic pressure[18] was calculated as following: plasma colloid osmotic pressure (PCOP, mm Hg)=2.1×PTP concentration (g/dL)+0.16×PTP concentration2(g/dL)+0.009×PTP concentration3(g/dL).
Statistical Analysis
Statistical analysis was performed using SPSS 16.0. The data were described by mean±standard deviation and median (interquartile interval) [M (P25, P75)], and compared between groups by independent sample t-test and Mann-Whitney U nonparametric test. Binary Logistic regression Rorward: LR method were used to value and sieve the potential risk factors of ERD in PIH patients. Receiver operating characteristic (ROC) curve was used to test the prediction and diagnostic efficiency of the algorithm.
RESULTS
There was no significant difference in age and pre-pregnancy BMI between ERD group and non-ERD group (P>0.05). Nevertheless, there were statistically significant differences in gestational weeks, duration of hypertension, maximum and minimum systolic and diastolic BP, and PTP concentration between two groups (P<0.05; Table 1).
Table 1. Comparison of general data between ERD group and non-ERD Group.
| Parameters | ERD group | Non-ERD group | Test value (t/Z) | P |
| Age (y) | 32.41±5.13 | 32.08±5.19 | -0.382 | 0.703 |
| Gestational weeks | 30.95 (28.08, 35.08) | 35.35 (31.25, 37.30) | -3.269 | 0.001 |
| Pre-pregnancy BMI (kg/m2) | 22.55 (19.45, 24.14) | 22.83 (20.55, 24.63) | -1.315 | 0.188 |
| Duration of hypertension (d) | 3.00 (1.00, 7.50) | 7.00 (1.50, 16.00) | -2.593 | 0.010 |
| Max systolic BP (mm Hg) | 170.00 (154.50, 180.50) | 155.00 (148.00, 168.25) | -4.260 | 0.000 |
| Min systolic BP (mm Hg) | 145.00 (136.75, 155.00) | 139.00 (127.00, 145.00) | -3.421 | 0.001 |
| Max diastolic BP (mm Hg) | 110.00 (101.50, 120.00) | 100.00 (95.00, 107.00) | -4.467 | 0.000 |
| Min diastolic BP (mm Hg) | 93.50 (85.75, 101.25) | 89.00 (80.00, 94.00) | -3.382 | 0.001 |
| PTP concentration (g/L) | 47.28 (43.38, 51.05) | 62.39 (57.18, 66.37) | -8.491 | 0.000 |
BMI: Body mass index; BP: Blood pressure; PTP: Plasma total protein; ERD: Exudative retinal detachment.
mean±SD; M (P25, P75)
The variables (gestational weeks, duration of hypertension, PTP concentration, maximum and minimum systolic and diastolic BP) which had statistical differences between two groups were analyzed by binary logistic regression analysis. The results showed that the maximum systolic BP and PTP concentration were statistically correlated with the occurrence of ERD (Table 2).
Table 2. Logistic regression analysis of variables with statistical difference between two groups.
| Parameters | B | S.E. | Wald | df | P | Exp(B) | 95%CI for Exp(B) |
| Max systolic BP | 0.049 | 0.017 | 8.484 | 1 | 0.004 | 1.050 | 1.016-1.085 |
| PTP concentration | -0.269 | 0.044 | 38.274 | 1 | 0.000 | 0.764 | 0.702-0.832 |
| Constant | 5.456 | 3.158 | 2.985 | 1 | 0.084 | 234.219 |
BP: Blood pressure; PTP: Plasma total protein; S.E.: Standard error; df: Degree of freedom; Exp(B): OR value.
ROC curve analysis showed that the sensitivities of maximum systolic BP, total protein concentration and combined diagnosis were 0.717, 0.870, and 0.870, respectively; the specificities were 0.617, 0.837, and 0.908, respectively; the area under the curve (AUC) was 0.707 (95%CI: 0.622-0.792), 0.917 (95%CI: 0.868-0.967), and 0.933 (95%CI: 0.890-0.975), respectively. The AUC of combined diagnosis was higher than that of single diagnosis (P<0. 01; Table 3, Figure 3).
Table 3. Comparison of the efficacy of maximum systolic BP, PTP concentration and their combination in the diagnosis of ERD.
| Variable | AUC | Sensitivity | Specificity | Cut-off value | 95%CI | P |
| Max systolic BP | 0.707 | 0.717 | 0.617 | 159.5 | 0.622-0.792 | 0.000 |
| PTP concentration | 0.917 | 0.870 | 0.837 | 54.42 | 0.868-0.967 | 0.000 |
| Combined diagnosis | 0.933 | 0.870 | 0.908 | 8.714 | 0.890-0.975 | 0.000 |
BP: Blood pressure; AUC: Area under the curve; ERD: Exudative retinal detachment; PTP: Plasma total protein. The value of the combined diagnosis=(maximum systolic pressur–plasma colloid osmotic pressure)/plasma colloid osmotic pressure. Plasma colloid osmotic pressure (mm Hg)=2.1×PTP concentration (g/dL)+0.16×PTP concentration2(g/dL)+0.009×PTP concentration3(g/dL).
Figure 3. ROC curve of maximum systolic blood pressure, PTP concentration and combined variable in diagnosis of ERD.
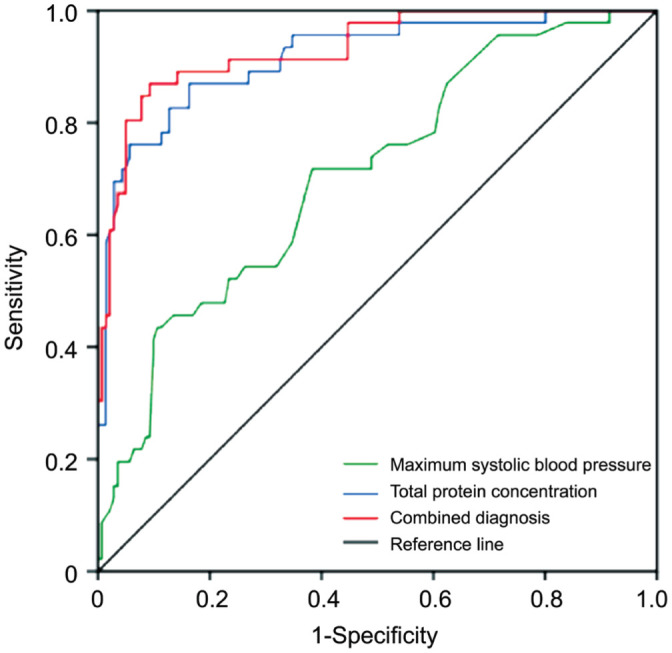
PTP: Plasma total protein; ROC: Receiver operating characteristic; ERD: Exudative retinal detachment.
DISCUSSION
As is known to all, there are two kinds of blood retinal barrier in normal retina to keep retina dry and transparent, i.e., inner retinal barrier and outer retinal barrier. The inner retinal barrier is formed by the occlusive zone between the retinal capillary endothelial cells and the pericytes, the outer retinal barrier is composed of the RPE cell and its occlusive zone. When the RPE barrier is damaged, choroidal plasma leaks into the sub-neuroepithelial layer and accumulates in the neuroendothelial layers, then it forms a localized, well-defined, flat discoid retinal detachment, if the RPE barrier is extensively damaged, it may cause wide and highly uplifted ERD[19]. It is common in malignant hypertension[20], Harada disease[21], central serous chorioretinopathy[22], choroidal tumor[23], uveal effusion syndrome[24], posterior scleritis[25]. PIH is associated with severe damage to choroidal circulation, which is generally believed to be caused by extensive destruction of the retinal barrier[10].
ERD is a serious complication of PIH and characterizes by the accumulation of serous or haemorrhagic fluid in the subretinal space secondary to hydrostatic factors. The retina is separated from the RPE beneath it and vision is lost because photoreceptors require contact with the RPE and choroid for metabolic and vascular support. Although most ERD resolves by clinical management after delivery 2 to 12wk[26]–[27], the symptoms caused by ERD, such as blurred vision, dark shadow in front of the eyes, and permanent visual loss may be left in a small number of PIH patients and seriously affected the their normal life. Arab et al[28] found that peripapillary retinal nerve fiber layer reduced in preeclamptic and eclamptic cases as well as in normotensive pregnant women at 2mo postpartum. This reduction was more in the severe forms of PIH. Meanwhile, offspring of mothers with hypertensive disorders during pregnancy have increased risk of cardiovascular disease[29]. Higher maternal BP during pregnancy is associated with persistent microvasculature adaptations in their children. Children of mothers with gestational hypertensive disorders tended to have narrower retinal arteriolar caliber[30]. The destruction of micro-circulation in retina reflects the deterioration of micro-circulation in the other organs. The occurrence of ERD also means the requirement to terminate pregnancy in timely for preventing further damage of multiple organs. Thereby, early prediction of ERD is particularly important and may earn more time for preventing the further damage of the multiple organs before ERD and other hidden troubles occurring in PIH patients.
In the study, independent sample t test and Mann-Whitney U nonparametric test were used to compare gestational weeks, duration of hypertension, maximum and minimum systolic and diastolic BP, and PTP concentration between ERD group and non-ERD group. The results showed significant difference in maximum/minimum systolic and diastolic BP and PTP between the two groups, which suggested they would be involved in ERD. Then, logistic regression analysis was conducted to screen out the independent risk factors of ERD. It was found that BP and PTP concentration were independent predictors of ERD occurrence.
The effective filtration pressure is associated with intra capillary pressure, tissue colloid osmotic pressure, plasma colloid osmotic pressure, and tissue hydraulic pressure. However the level of plasma osmotic pressure depends on the protein content in plasma, some studies have shown that the decrease of PTP or albumin content led to the decrease of plasma colloid osmotic pressure, it weakened the strength of tissue fluid flowing back to capillaries, then choroidal plasma leaked through RPE and its small closure zone which led to ERD[31]–[32], this was consistent with our results. In addition, we think that the increase of systolic BP lead to the rise of capillary internal pressure, which will promote the infiltration of tissue fluid into tissues, and cause damage of choroidal circulation, which will also lead to the occurrence of ERD.
ROC curve was used to analyze the diagnostic value of this algorithm: (maximum systolic pressure–plasma colloid osmotic pressure)/plasma colloid osmotic pressure. The AUC was as high as 0.933, and the cut-off value was 8.714 (Yoden index 0.778), with the corresponding sensitivity of 87.0% and specificity of 90.8%, respectively. Although it still needs a large number of samples to further verification, it seems feasible to predict and early sieve the high-risk PIH patients, its application may reduce stillbirth, small gestational age at delivery, lower birthweight, neonatal mortality, seizures, long-term coronary atherosclerotic heart disease and heart failure, which is great significant for early clinical intervention and improving the short-term and long-term prognosis of patients.
Acknowledgments
Conflicts of Interest: Hu L, None; Li DH, None; Wang SY, None.
REFERENCES
- 1.Chen HB, Tao F, Fang XD, Wang XT. Association of hypoproteinemia in preeclampsia with maternal and perinatal outcomes: a retrospective analysis of high-risk women. J Res Med Sci. 2016;21:98. doi: 10.4103/1735-1995.193170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Fu QQ. A meta-analysis of the prevalence of hypertensive disorders during pregnancy in China. Maternal & Child Health Care of China. 2019;34(14):3378–3381. [Google Scholar]
- 3.Rosenthal JM, Johnson MW. Management of retinal diseases in pregnant patients. J Ophthalmic Vis Res. 2018;13(1):62–65. doi: 10.4103/jovr.jovr_195_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan J, Yang M, Liao Y, Qi YN, Ren Y, Liu CR, Huang S, Thabane L, Liu XH, Sun X. PIH1 development and validation of a prediction MODEL on severe maternal outcomes among pregnant women with PRE-eclampsia: a 10-year cohort study. Value Health Reg Issues. 2020;22:S44. doi: 10.1038/s41598-020-72527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato T, Takeuchi M. Pregnancy-induced hypertension-related chorioretinitis resembling uveal effusion syndrome: a case report. Medicine (Baltimore) 2018;97(30):e11572. doi: 10.1097/MD.0000000000011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang LN, Liu ZY, Hei LN, et al. The image characteristics of multispectral scanning laser imaging and optical coherence tomography in patients with pregnancy induced hypertension syndrome. Chin J Ocul Fundus Dis. 2020;36(1):29–32. [Google Scholar]
- 7.Komoto S, Maruyama K, Hashida N, Koh S, Nishida K. Bilateral serous retinal detachment associated with subretinal fibrin-like material in a case of pregnancy-induced hypertension. Am J Ophthalmol Case Rep. 2019;16:100572. doi: 10.1016/j.ajoc.2019.100572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakhda RN. Clinical study of fundus findings in pregnancy induced hypertension. J Family Med Prim Care. 2016;5(2):424–429. doi: 10.4103/2249-4863.192364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Păun VA, Ionescu ZR, Voinea L, Cîrstoiu M, Baroş A, Pricopie Ş, Ciuluvică R. Ocular posterior pole pathological modifications related to complicated pregnancy. A review. Rom J Ophthalmol. 2017;61(2):83–89. doi: 10.22336/rjo.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16(5):308–314. doi: 10.1097/01.icu.0000179803.42218.cc. [DOI] [PubMed] [Google Scholar]
- 11.Laaribi N, Houba A, Bouayad G, Belfaoza S, Debbabi Y, Chatoui S, Zerrouk R, Reda K, Oubaaz A. Retinal detachment secondary to pre-eclampsia: report of two cases. J Fr Ophtalmol. 2019;42(8):e385–e389. doi: 10.1016/j.jfo.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Raposo JTBV, Melo B, Maciel NFBB, Leite SD, Rebelo ÓRC, Lima AMF. Serous retinal detachment in pre-eclampsia: case report and literature review. Rev Bras Ginecol Obstet. 2020;42(11):772–773. doi: 10.1055/s-0040-1718448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otero-Marquez O, Chung H, Lee CS, Choi EY, Ledesma-Gil G, Alauddin S, Lee M, Bhuiyan A, Smith RT. Subretinal deposits in pre-eclampsia and malignant hypertension. Ophthalmol Retina. 2021;5(8):750–760. doi: 10.1016/j.oret.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Duffy J, Cairns AE, Richards-Doran D, et al. International Collaboration to Harmonise Outcomes for Pre-eclampsia (iHOPE) A core outcome set for pre-eclampsia research: an international consensus development study. BJOG. 2020;127(12):1516–1526. doi: 10.1111/1471-0528.16319. [DOI] [PubMed] [Google Scholar]
- 15.Benschop L, Schalekamp-Timmermans S, Roeters van Lennep JE, et al. Gestational hypertensive disorders and retinal microvasculature: the Generation R Study. BMC Med. 2017;15(1):153. doi: 10.1186/s12916-017-0917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang ZZ, Lu JM, Qin XH. Case report: optical coherence tomography can find typical features in pregnancy-induced hypertension with retinopathy. Optom Vis Sci. 2019;96(5):372–375. doi: 10.1097/OPX.0000000000001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amer R, Nalcı H, Yalçındağ N. Exudative retinal detachment. Surv Ophthalmol. 2017;62(6):723–769. doi: 10.1016/j.survophthal.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Zeng YM, Deng XM, Li WZ. Critical Care Medicine. Beijing: People's Medical Publishing House; 2006. pp. 28–29. [Google Scholar]
- 19.de Venecia G, Jampol LM. The eye in accelerated hypertension. II. Localized serous detachments of the retina in patients. Arch Ophthalmol. 1984;102(1):68–73. doi: 10.1001/archopht.1984.01040030052033. [DOI] [PubMed] [Google Scholar]
- 20.Kapoor A, Kumar A, Chawla R. Bilateral exudative retinal detachment and choroidopathy as the presenting signs of malignant hypertension. BMJ Case Rep. 2021;14(4):e242413. doi: 10.1136/bcr-2021-242413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu El-Asrar AM, Van Damme J, Struyf S, Opdenakker G. New perspectives on the immunopathogenesis and treatment of uveitis associated with vogt-koyanagi-harada disease. Front Med (Lausanne) 2021;8:705796. doi: 10.3389/fmed.2021.705796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, Jaisser F, Behar-Cohen F. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Feng KM, Chen YH, Chen JT, Lin LF, Tsai WC, Chen CL. A pulmonary pleomorphic carcinoma patient with exudative retinal detachment secondary to choroid metastasis as initial presentation-a case report. Medicina(Kaunas) 2021;57(6):539. doi: 10.3390/medicina57060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markan A, Moharana B, Dogra M, Singh R. Multimodal imaging to aid in diagnosis of uveal effusion syndrome type 3. BMJ Case Rep. 2021;14(3):e239556. doi: 10.1136/bcr-2020-239556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Zhao W, Tao QQ, Lin S, Li XR, Zhang XM. Comparison of the clinical features between posterior scleritis with exudative retinal detachment and Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2022;42(2):479–488. doi: 10.1007/s10792-021-02064-w. [DOI] [PubMed] [Google Scholar]
- 26.AlTalbishi A, Khateb S, Amer R. Elschnig's spots in the acute and remission stages in preeclampsia: spectral-domain optical coherence tomographic features. Eur J Ophthalmol. 2015;25(5):e84–e87. doi: 10.5301/ejo.5000586. [DOI] [PubMed] [Google Scholar]
- 27.Vigil-De Gracia P, Ortega-Paz L. Retinal detachment in association with pre-eclampsia, eclampsia, and HELLP syndrome. Int J Gynecol Obstet. 2011;114(3):223–225. doi: 10.1016/j.ijgo.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Arab M, Entezari M, Ghamary H, Ramezani A, Ashori A, Mowlazadeh A, Yaseri M. Peripapillary retinal nerve fiber layer thickness in preeclampsia and eclampsia. Int Ophthalmol. 2018;38(6):2289–2294. doi: 10.1007/s10792-017-0718-9. [DOI] [PubMed] [Google Scholar]
- 29.Thoulass JC, Robertson L, Denadai L, et al. Hypertensive disorders of pregnancy and adult offspring cardiometabolic outcomes: a systematic review of the literature and meta-analysis. J Epidemiol Community Health. 2016;70(4):414–422. doi: 10.1136/jech-2015-205483. [DOI] [PubMed] [Google Scholar]
- 30.Yesil GD, Gishti O, Felix JF, et al. Influence of maternal gestational hypertensive disorders on microvasculature in school-age children: the generation R study. Am J Epidemiol. 2016;184(9):605–615. doi: 10.1093/aje/kww059. [DOI] [PubMed] [Google Scholar]
- 31.Fujioka S, Karashima K, Nishikawa N, Saito Y. Optic disk manifestation in diabetic eyes with low serum albumin: late fluorescein staining and high blood flow velocities in the optic disk. Jpn J Ophthalmol. 2004;48(1):59–64. doi: 10.1007/s10384-003-0005-3. [DOI] [PubMed] [Google Scholar]
- 32.Sun DF, Wang YL, Wang B, Xu CL, Zhang G, Li J, Zhang XM. Predictive risk factors for exudative retinal detachment after vitrectomy for proliferative diabetic retinopathy. Medicine. 2019;98(8):e14603. doi: 10.1097/MD.0000000000014603. [DOI] [PMC free article] [PubMed] [Google Scholar]


