Abstract
Tislelizumab is an anti-programmed death receptor 1 (PD-1) monoclonal immunoglobulin G 4 antibody developed by BeiGene. The structure of tislelizumab has been modified to maximally inhibit the binding of PD-1 to programmed death ligand 1 (PD-L1) and minimize the binding of tislelizumab to Fcγ receptors. In clinical studies, tislelizumab has shown preliminary anti-tumor effects in various solid tumors, such as Hodgkin’s lymphoma, urothelial carcinoma, lung cancer, gastric and esophageal cancer, liver cancer, nasopharyngeal carcinoma, colorectal cancer, and microsatellite instability-high/mismatch repair-deficient tumors. In addition, it also showed new promise in solid tumor treatment in combination with ociperlimab. Due to its satisfactory anti-tumor effects, tislelizumab has received approvals in China for the treatment of classical Hodgkin’s lymphoma, urothelial carcinoma, squamous non-small cell lung cancer, non-squamous non-small cell lung cancer, and hepatocellular carcinoma, and it is now under investigation for a new indication in microsatellite instability-high/mismatch repair-deficient tumors. Moreover, it has been granted orphan designations in hepatocellular carcinoma, esophageal cancer, and gastric cancer, including cancer of the gastroesophageal junction, by the US Food and Drug Administration. Tislelizumab has an acceptable safety profile; the most common adverse effects include fatigue, anemia, and decreased neutrophil count, while the most fatal events have been related to respiratory infection or failure, and hepatic injury. Tislelizumab has an economic advantage compared with other well-studied PD-1/PD-L1 inhibitors; thus, the introduction of it could provide clinical oncologists with an effective weapon against tumors and may alleviate the burden of cancer patients.
Keywords: Tislelizumab, immunotherapy, programmed death receptor 1, programmed death ligand 1, non-small cell lung cancer
Introduction
The development of immunotherapies against malignancies has inaugurated a new era in cancer therapy; among these, antibodies against the programmed death receptor 1 (PD-1) pathway are the most successful. 1 PD-1 and programmed death ligand 1 (PD-L1) proteins are expressed on the surface of many types of cell components of the immune system and tumor cells, and their expressions are regulated at the posttranslational level by both glycosylation and ubiquitination. 2 The activation of the PD-1/PD-L1 pathway by their engagement may cause T cell apoptosis, anergy, exhaustion, and interleukin-10 expression, thus promoting the immune evasion of malignant cells and facilitating the growth of tumors. 3 The underlying mechanism has suggested a clinical utility for PD-1 blockade in anti-tumor therapy.
Anti-PD-1 antibodies have already been applied in clinical practice. To date, measurement of PD-L1 expression by immunohistochemistry is the only approved marker (category 1 recommendation) to select patients who are candidates for PD-1/PD-L1 inhibitors, as was recommended by the NCCN guideline (https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450). There are several anti-PD-1 antibodies, such as nivolumab and pembrolizumab, approved by the US Food and Drug Administration (FDA) for the treatment of cancer. 4 In China, the National Medical Product Administration (NMPA) have also approved several anti-PD-1 antibodies, 4 including tislelizumab, which was approved in December, 2019 for use in patients with relapsed or refractory classical Hodgkin’s lymphoma (r/r cHL) after at least second-line chemotherapy. 5 The second indication for tislelizumab is locally advanced or metastatic urothelial carcinoma (UC) with high PD-L1 expressions after failure of platinum-based chemotherapy, which was approved in April 2020. 6 In 2021, tislelizumab was approved for the treatment of non-small cell lung cancer (NSCLC) in combination with chemotherapy, and hepatocellular carcinoma (HCC) as a single agent.7,8 Meanwhile, applications for a second-line therapy in NSCLC and in microsatellite instability-high (MSI-H)/mismatch repair-deficient (dMMR) solid tumors have been accepted by the NMPA.9,10 This domestic PD-1 antibody has unique features compared to other PD-1 antibodies and has exhibited preliminary anti-tumor effects in various malignant diseases. Here, we summarize the structural features, clinical trials, and safety profiles of tislelizumab, in order to provide an extra option for oncologists and patients in the fight against tumors.
Unique Structural Features of Tislelizumab
Immunoglobulin (Ig) superfamily domains are domains with amino acid sequence and structural similarity to those in Igs, and they are among the most common domain types in humans. PD-1 and its ligands are members of the B7/CD28/CTLA-4 Ig subfamily. PD-1 is a type I transmembrane glycoprotein with a transmembrane domain; its IgV domain locates in the extracellular part and its cytoplasmic domain consists of two tyrosine signaling motifs, the immunoreceptor tyrosine inhibitory motif (ITIM) and the immunoreceptor tyrosine-based switch motif (ITSM).11,12 The extracellular domain of PD-1 is comprised of two β sheets, the front GFCC’ strands and the back ABED strands, and the strands are stabilized by a disulfide bond. The interaction between PD-1 and its ligands mainly involves residues on the front faces of the PD-1 GFCC′ β-sheets, with additional contributions of the FG loops. 2
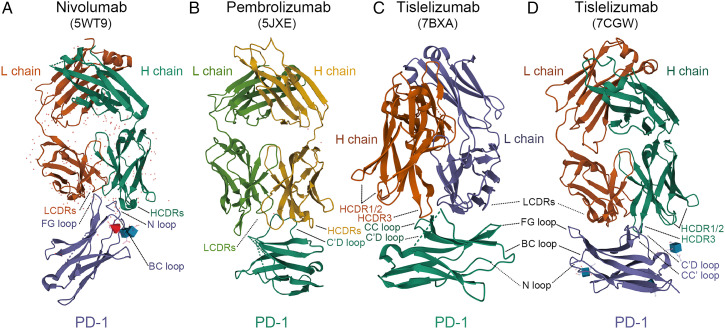
The structural bases of tislelizumab suggests that it may well provide maximum steric abrogation to PD-L1 in its binding to PD-1. The epitope of tislelizumab is formed on the CC′ loop of the front β sheet face of PD-1, which is innovative among anti-PD-1 antibodies; in comparison, other PD-1 antibodies, like nivolumab and pembrolizumab, bind to the N-terminal region and the C’D loop of PD-1, respectively (Figure 1).13-18 The binding of tislelizumab to the front sheet of PD-1 causes a stereospecific hindrance to PD-L1. When the tislelizumab complex was superimposed onto the PD-1/PD-L1 complex, the overlap of tislelizumab to the binding area of PD-L1 to PD-1 was approximately 80%, which is significantly larger than that of pembrolizumab or nivolumab.13,14 The total solvent accessible area covered by the interaction between tislelizumab and PD-1 is 2014 Å2, which was slightly larger than the binding interface of PD-1/PD-L1 (1970 Å2); using surface plasmon resonance, the binding affinity (Kd) of tislelizumab/PD-1 was analyzed as 130 pm, whereas the Kd value of the PD-1/PD-L1 interaction was confirmed as 8.2 mM.13,14,19
Figure 1.
Comparison of the structures of PD-1 in complex with nivolumab-Fab (A) (PDB code: 5WT9), pembrolizumab-Fab (B) (PDB code: 5JXE), and tislelizumab-Fab (C,D) (PDB code: 7BXA, 7CGW). Images were acquired from the Protein Data Bank and relevant research was conducted by Tan S et al, Na Z et al, Lee SH et al, and Hong Y et al, respectively.13-18 L chain, light chain; H chain, heavy chain; LCDRs, complementary determining regions of the light chain; HCDRs, complementary determining regions of the heavy chain; PD-1, programmed death protein 1.
Tislelizumab was also engineered to minimize binding to the Fcγ receptor (FcγR)—it is incapable of binding to FcγRI as well as to other FcγRs. 20 FcγRs on macrophages induce phagocytosis, a mechanism of T-cell clearance and potential resistance to anti-PD-1 therapy. 5 For IgG antibodies, the interaction of their Fc portion with FcγRs has been found to be crucial for their therapeutic activities, which are induced through antibody-dependent cellular cytotoxicity by type I FcγR (FcγRI)-expressing effector cells, such as natural killer cells and macrophages. 21 However, anti-PD-1 antibodies have been proved to be FcγR-independent in vivo, and the presence of FcγR-binding capacity compromised their anti-tumor activity. 21 The majority of anti-PD-1 antibodies, such as nivolumab and pembrolizumab, are of the IgG4S228P format and are thus able to easily bind to FcγRI.5,20 In contrast, there are several mutations in the Fc-hinge region of tislelizumab, making it unable to bind to FcγRs. 20 In reality, no antibody-dependent cell-mediated or complement-dependent cytotoxicity has been observed in activated T cells treated with high concentrations of tislelizumab, up to 100 μg/mL in vitro. 22
Pharmacological Characteristics of Tislelizumab
Tislelizumab is able to bind to human PD-1 with high specificity and affinity, with the disassociation constant being .15 nmol/L. 5 At the 5 mg/kg dose, PD-1 receptor occupancy is >90%. 23 In clinical trial NCT02407990, the emergence of anti-drug antibodies occurred in 18.7% of patients across the study, with one patient (.3%) positive for neutralizing antibodies; the half-life of tislelizumab was 16.8±5.5 days. 23 The volume of distribution is 4.41 L after a single infusion of tislelizumab 200 mg, and 5.247 L at steady state. 5 Following a single dose of tislelizumab 200 mg, the clearance of tislelizumab was .247 L/day and the half-life was 13.3 days. 5 In ex vivo tumor spheroids, tumor-infiltrating lymphocytes were potently activated by tislelizumab, as evaluated by increased IFN-γ production and tumor-infiltrating lymphocyte proliferation. 24 In colorectal cancer (CRC) patients, tislelizumab treatment at concentration levels of .1, 1, and 10 μg/mL could also lead to higher IFN-γ production than in the nivolumab or pembrolizumab treated groups. 25 Based on the above preclinical data and preliminary pharmacological tests, tislelizumab was tested in therapeutic clinical trials.
Therapeutic Clinical Trials
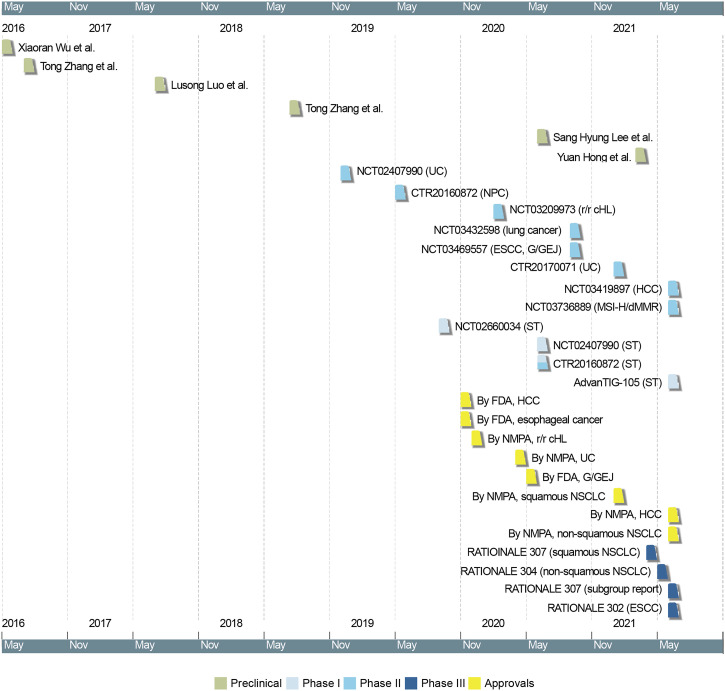
There are 16 published clinical studies on tislelizumab, 4 phase I studies, 8 phase II studies, and 4 phase III study (Figure 2), with the primary endpoints being safety and tolerability for the phase I studies, overall response rate (ORR) for the phase II studies, and progression-free survival (PFS) or overall survival (OS) for the phase III studies.23,26-40 In the dose-escalation phase and fixed-dose expansion phase of the first-in-human study (NCT02407990), the recommended dose and schedule of tislelizumab was determined to be 200 mg every 3 weeks (q3w). 23 Based on the verified dosage, the indication expansion phase of NCT02407990 and CTR20160872 were carried out in patients with solid tumors. In that phase, patients with solid tumors, including melanoma, renal cell cancer, nasopharyngeal carcinoma (NPC), HCC, UC and NSCLC, experienced ORRs ranging from 9.5% to 42.9%.23,27
Figure 2.
Timeline of preclinical and clinical studies of tislelizumab. Dates indicate the time when the latest study results were available online. UC, urothelial carcinoma; NPC, nasopharyngeal carcinoma; r/r cHL, relapsed or refractory classical Hodgkin’s lymphoma; ESCC, esophageal squamous cell carcinoma; G/GEJ, gastric/gastroesophageal junction cancer; HCC, hepatocellular carcinoma; MSI-H/dMMR, microsatellite instability-high/mismatch repair-deficient tumors; ST, solid tumors; FDA, Food and Drug Administration; NMPA, National Medical Product Administration; NSCLC, non small cell lung cancer.
The recommended phase II dose for the combination of tislelizumab and pamiparib, an oral PARP 1/2 inhibitor, was tislelizumab 200 mg q3w plus pamiparib 40 mg twice daily. The combination plan was generally well tolerated in patients with solid tumors and had clinical benefits. In the phase Ia part of NCT02660034, tislelizumab was used in combination with pamiparib in 49 patients with advanced solid tumors. 26 The 49 patients had tumors of the ovary, fallopian tube, pancreas, prostate, and breast, and were divided into 5 cohorts treated with different doses. The combination plan resulted in a 20.4% (10/49) ORR, with 100% treatment-emergent adverse events and no grade 5 adverse events among all included patients. 26 The clinical efficacy of tislelizumab in published studies is summarized in Table 1 and illustrated as follows.
Table 1.
Clinical efficacy of tislelizumab in prospective clinical studies.
| Clinical studies | CTR20160872 | NCT02407990 | NCT02407990 | NCT04004221/CTR20170071 | NCT03209973 | CTR20160872 | NCT03419897 | NCT03736889 | NCT02660034 | NCT03469557 | RATIONALE 302/NCT03430843 | NCT03432598 | RATIONALE 307/NCT03594747 | RATIONALE 307 (≥65 years) |
RATIONALE 304/NCT03663205 | AdvanTIG-105/ NCT04047862 |
| Races | Chinese | 64.3% Caucasian, 28.8% Asian | Caucasian | 96% Chinese, 4% Korean | Chinese | Chinese | Global | Chinese | 89.8% Caucasian, 10.2% Asian | Chinese | Global | Chinese | Chinese | Chinese | Chinese | Global |
| Tumor type | ST | ST | UC | UC | r/r cHL | NPC | HCC | MSI-H/dMMR | ST | ESCC, G/GEJ | ESCC | NSCLC, SCLC | sq NSCLC | sq NSCLC | nsq NSCLC | ST |
| Numbers of cases | 300 | 451 | 17 | 113 | 70 | 20 | 249 | 80 | 49 | 15, 15 | 256, 256 | 37, 17 | 120, 118, 117 | 39, 52, 36 | 223, 111 | 24 |
| Therapeutic regimen | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | tislelizumab plus pamiparib | tislelizumab plus chemotherapy | tislelizumab, chemotherapy | tislelizumab plus chemotherapy | arm A, arm B, arm C | arm A, arm B, arm C | group A, group B | tislelizumab plus ociperlimab |
| ORR | 18.4% (41/223) | 13.3% (60/451) | 29.4% (5/17) | 24% (25/104) | 87.1% (16/70) | 20.0% (3/15) | 12.2% (31/249) | 45.9% (34/74) | 20.4% (10/49) | 46.7 (7/15), 46.7 (7/15) | 20.3%, 9.8% | 62.2% (23/37), 76.5% (13/17) | 60.0%(72/120),63.6%(75/118),51.3%(60/117) | 69.2%, 75.0%, 50.0% | 57.4%(128/223), 36.9%(41/111) | 1 PR |
| DCR | - | 44.60% | 47.1% (8/17) | 38.50% | 92.9% (65/70) | 80.0% (12/15) | NA | 71.6% (53/74) | 53.10% | 80.0% (12/15), | NA | 91.9% (34/37), | 72.5%(87/120),77.1%(91/118),68.4%(80/117) | NA | 89.2% (199/223), 81.1% (90/111) | 9 SD |
| (201/451) | (40/104) | (26/49) | 66.7% (10/15) | 88.2% (15/17) | ||||||||||||
| OS | 11.5 m | 10.3 m | NA | 9.8 m | — | — | 12.4 m | NA | 388 d | NA | 8.6 m, 6.3 m | NA, 15.6 m | NA, NA, NA | NA | NA, NA | NA |
| PFS | 2.6 m | 2.1 m | NA | 2.1 m | 31.5 m | — | 2.7 m | NA | 92 d | 10.4 m, 6.1 m | NA | -, | 7.6 m, 7.6 m, 5.5 m | 9.7 m, 9.7 m, 5.2 m | 9.7 m, 7.6 m | NA |
| Grade 1-5 TRAEs | - | 96.7% (436/451) | NA | 93.8% (106/113) | 97.1% (68/70) | 46.7% (7/15) | NA | NA | 100%(49/49) | 100% (30/30) | NA | 100% (54/54) | 100%(120/120), 99.2%(117/118), 100%(117/117) | NA | 100%(222/222), 99.1%(109/110) | 83.3% (20/24) |
| Grade 3-5 TRAEs | 39.7% (119/300) | 47.0% (212/451) | NA | 44.2% (50/113) | 31.4% (22/70) | 0 | NA | 47.5% (38/80) | — | 76.7% (23/30) | 19%, 56% | 79.6% (43/54) | 85.8%(103/120), 83.9% (99/118), 80.3% (94/117) | 84.6% (33/39), 84.6% (44/52), 82.4% (28/34) | 67.6%(150/222), 53.6% (59/110) | 8.3% (2/24) |
| Grade 5 TRAEs | 4.0% (12/300) | 3.1% (14/451) | NA | 2.7% (3/113) | 0 | 0 | 0.8% (2/249) | 0 | 0 | 3.3% (1/30) | 14%, 12% | 1.9%(1/54) | 0.8% (1/120), 1.7% (2/118), 2.6% (3/117) | NA | 1.4% (3/222), 0.9% (1/110) | 0 |
Abbreviations: ORR, overall survival rate; DCR, disease control rate; OS, overall survival; PFS, progression-free survival; TRAEs, treatment-related adverse effects; ST, solid tumors; UC, urothelial carcinoma; NA, not available; r/r cHL, relapsed or refractory classical Hodgkin lymphoma; NPC, nasopharyngeal carcinoma; HCC, hepatocellular carcinoma; MSI-H/dMMR, microsatellite instability-high/mismatch repair-deficient tumor; ESCC, esophageal squamous cell carcinoma; G/GEJ, gastric/gastroesophageal junction cancer; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; sq, squamous; nsq, non-squamous; arm A, patients receiving tislelizumab plus paclitaxel, carboplatin; arm B, patients receiving tislelizumab plus nab-paclitaxel, carboplatin; arm C, patients receiving placebo plus paclitaxel, carboplatin; group A, tislelizumab plus chemotherapy; group B, chemotherapy alone; PR, partial remission; SD, stable disease.
Efficacy of Tislelizumab in Hodgkin’s Lymphoma
Tislelizumab has exhibited a satisfactory anti-tumor effect in patients with r/r cHL. 5 In a phase II, single-arm, multicenter study (NCT03209973), 70 patients with r/r cHL that experienced at least second-line chemotherapy and were ineligible for or not responsive to autologous stem cell transplantation were included and received tislelizumab 200 mg intravenously q3w. 28 The independently assessed ORR, defined as the sum of complete response and partial response, was 87.1% (61/70). 28 The efficacies of nivolumab and pembrolizumab in r/r cHL patients were tested in the trials CHECKMATE-205 and KEYNOTE-087, respectively.41,42 In the 2 studies, the ORRs for r/r cHL patients treated with nivolumab (3 mg/kg, q2w) and pembrolizumab (200 mg, q3w) were 69.1% (168/243) and 69.0% (145/210), respectively.41,42 Moreover, the complete response rate of tislelizumab (62.9%, 44/70) seemed much higher than nivolumab (8.8%, 7/80) or pembrolizumab (22.4%, 47/210). The satisfying anti-tumor effects of tislelizumab in r/r cHL patients contributed to its first approval by the NMPA in December, 2019. 43 In the 3-year follow-up of NCT03209973, PFS of 31.5 months was observed. 44 In addition, a multicenter, open-label, randomized controlled phase III study NCT04486391, that aims to further compare the benefit of tislelizumab to chemotherapy in r/r cHL patients, is ongoing. 45 These studies have shown the bright prospect of tislelizumab in r/r cHL therapy.
Efficacy of Tislelizumab in Urothelial Carcinoma
The anti-tumor effect of tislelizumab in UC patients has been verified, and the indication has been approved. In two phase I studies, the ORRs of tislelizumab monotherapy in UC were 29.4% (5/17) in NCT02407990 and 13.6% (3/22) in CTR20160872.23,27,38 The difference of ORRs between the 2 studies might be attributed to random fluctuation, since the sample sizes are small, difference in patient races, since all patients in the UC cohort of NCT02407990 are caucasian, or the patients’ treatment status, since eligible patients have received previous treatment in NCT02407990, while enrolled patients must have failed in all available standard treatments or be intolerant of them in CTR20160872. Nevertheless, based on the preliminary results, research of tislelizumab in UC progressed to phase II NCT04004221/CTR20170071, in which 113 patients with locally advanced or metastatic PD-L1+ UC who progressed during/following a platinum-based chemotherapy were treated with tislelizumab; the ORR achieved by tislelizumab monotherapy was 24.0% (25/104), and the disease control rate (DCR) was 38.5% (40/104), with the median PFS and median OS being 2.1 months and 9.8 months, respectively. 30 In CHECKMATE-032, 78 patients with metastatic UC were enrolled and treated with nivolumab at 3 mg/kg q2w; the confirmed ORR was shown to be 24.4% (19/78), while the DCR was 52.6% (41/78), with the median PFS and median OS being 2.8 months and 9.7 months, respectively. 46 In KEYNOTE-052, 370 treatment-naive patients with locally advanced/unresectable or metastatic UC were treated with 200 mg pembrolizumab intravenously q3w until confirmed disease progression, intolerable toxicity, physician/patient decision to withdraw, or completion of 24 months of treatment; the achieved ORR was 28.6% (106/370), the DCR was 46.8% (173/370), the median PFS was 2.2 months, and the median OS was 11.3 months. 47 The study populations and treatment status in these clinical trials are different. Patients in KEYNOTE-052 have not received prior systemic chemotherapy, while patients in NCT04004221 have received previous chemotherapy. The satisfactory anti-tumor effects of tislelizumab shown in UC have led to its second approval in April, 2020. 6
Efficacy of Tislelizumab in Lung Cancer
Tislelizumab monotherapy has exhibited preliminary anti-tumor effects in NSCLC and the foundation for further studies was laid. In the indication expansion part of NCT02407990, the ORR of tislelizumab monotherapy in previously treated or untreated NSCLC patients was 12.2% (6/49), 23 and in the phase II part of CTR20160872, tislelizumab monotherapy resulted in an ORR of 17.9% (10/56) in previously treated NSCLC patients. 27 In comparison, in a phase II study CHECKMATE-063, nivolumab monotherapy achieved an ORR of 14.5% (17/117) in treated NSCLC patients, and in a phase I study KEYNOTE-001, whose primary endpoint is ORR, pembrolizumab achieved an ORR of 40.6% (41/101) in treatment-naive NSCLC patients, and an ORR of 22.7% (102/449) in previously treated NSCLC patients, respectively.48,49 The patients in CHECKMATE-063 and NCT02407990 were mainly caucasian (84.6% vs. 64.3%), the patients in CTR20160872 were Chinese, while the race of patients in KEYNOTE-001 were not mentioned. Nevertheless, based on the 2 studies of tislelizumab, the NMPA accepted the listing application of tislelizumab monotherapy in NSCLC as a second-line or further therapy, and phase III trials on NSCLC have been conducted. 10
Tislelizumab plus chemotherapy has conferred a better clinical benefit than chemotherapy alone in NSCLC and has received two approvals. In a multicenter, open-label, phase II study (NCT03432598), tislelizumab in combination with platinum-based chemotherapy was tested in 54 patients with advanced lung cancer and showed preliminary anti-tumor efficacy. 29 In the study, the ORR in the NSCLC patients was 62.2% (23/37), and that in small cell lung cancer was 76.5% (13/17). 29 Later, in a phase III study NCT03594747/RATIONALE 307, the addition of tislelizumab to chemotherapy was related to an improved PFS compared to chemotherapy alone in patients with squamous NSCLC, which laid a foundation for its approval in this disease in January, 2021.7,32 In RATIONALE 307, 360 patients with locally advanced (stage IIIB) or metastatic (stage IV) squamous NSCLC were randomly assigned to receive 1 of the following regimens intravenously every 3 weeks: tislelizumab (200 mg, day 1) plus paclitaxel (175 mg/m2, day 1) and carboplatin (area under the concentration [AUC] of 5, day 1) (arm A, n = 120); tislelizumab (200 mg, day 1) plus nab-paclitaxel (100 mg/m2, days 1, 8, and 15) and carboplatin (AUC of 5, day 1) (arm B, n = 119); or paclitaxel (175 mg/m2, day 1) and carboplatin (AUC of 5, day 1) (arm C, n = 121). 32 The median PFS of arm A (7.6 months) and arm B (7.6 months) were significantly longer than that of arm C (5.5 months), with an HR of .524 between arms A and C (P < .001), and an HR of .478 between arms B and C (P < .001). 32 In patients aged ≥65 years, the median PFS achieved in arm A (9.7 months) or arm B (9.7 months) was also longer than arm C (5.2 months), with an HR of .602 between arms A and C (95% confidence interval .309-1.175), and an HR of .564 (95% confidence interval .302-1.052) between arms B and C. 37 Recently, the result of another phase III trial NCT03663205/RATIONALE 304 has been released, showing that PFS was significantly longer with tislelizumab plus chemotherapy compared to chemotherapy alone in advanced or metastatic non-squamous NSCLC patients (patient numbers: 222 vs. 110; median PFS: 9.7 vs. 7.6 months, HR = .645, P = .0044) and was the basis for the fourth indication of tislelizumab.8,33 In RATIONALE 304, non-squamous NSCLC patients in arm A received tislelizumab 200 mg plus platinum-based chemotherapy (carboplatin area under the curve 5 or cisplatin 75 mg/m2 in combination with pemetrexed 500 mg/m2) once every 3 weeks intravenously for four to six cycles (at investigator’s discretion) during induction treatment, followed by maintenance tislelizumab plus pemetrexed treatment; patients in arm B received platinum-based chemotherapy (carboplatin area under the curve 5 or cisplatin 75 mg/m2 in combination with pemetrexed 500 mg/m2) every 3 weeks for four to six cycles (at investigator’s discretion) during induction treatment, followed by maintenance pemetrexed treatment. 33
Efficacy of Tislelizumab in Gastric and Esophageal Cancer
Tislelizumab in combination with chemotherapy was effective in gastric/gastroesophageal junction cancer (G/GEJ). In phase I/II studies, tislelizumab achieved ORRs of 13.0% (7/54) in a caucasian-predominant population and 16.7% (4/24) in Chinese patients with gastric cancer.23,27 In a single-arm, multicenter, phase II study (NCT03469557), 30 patients with esophageal squamous cell carcinoma (ESCC) or G/GEJ were given tislelizumab plus chemotherapy. 31 Patients with G/GEJ experienced an ORR of 46.7% (7/15 patients) and a DCR of 66.7% (10/15) after treatment with tislelizumab, oxaliplatin and capecitabine. 31 In contrast, nivolumab plus capecitabine and oxaliplatin contributed to an ORR of 76.5% (13/17), while pembrolizumab plus cisplatin, and 5-fluorouracil or capecitabine achieved an ORR of 60.0% (15/25), and a DCR of 80.0% (20/25).50,51 The results of tislelizumab have contributed to an orphan designation by the FDA for the treatment of G/GEJ in May 2020. 52 In addition, the effect of adding tislelizumab in chemotherapy is being confirmed in a phase III study (NCT03777657). 45
The efficacy of tislelizumab monotherapy or a combination plan in ESCC is promising. In the phase II part of CTR20160872, tislelizumab monotherapy in Chinese ESCC patients that had failed all available standard treatments resulted in an ORR of 7.7% (2/26) and a DCR of 34.6% (9/26). 27 In phase II NCT03469557, treatment naive Chinese ESCC patients with ESCC had an ORR of 46.7% (7/15) and a DCR of 80.0% (12/15) after treatment with tislelizumab plus cisplatin and 5-FU. 31 In comparison, the ORR for pembrolizumab monotherapy in ESCC patients after 2 or more lines of therapy was 9.9% (12/121). 53 The differences might be partially attributed to the different races and treatment histories of the patients. In any case, tislelizumab monotherapy was granted an orphan designation in esophageal cancer by the FDA in June 2019. 52 Later, in a phase III study (RATIONALE 302/NCT03430843), tislelizumab monotherapy was proved to have improved the OS of ESCC patients compared to chemotherapy alone (patient numbers: 256 vs. 256; median OS 8.6 vs 6.3 months; HR .70, P < .001). 35 In similar studies, nivolumab improved the OS of ESCC patients with previous treatment history (patient numbers: 210 vs. 209; median OS 10.9 vs. 8.4 months; HR .77, P = .019), as did pembrolizumab (patient numbers: 198 vs. 203; median OS 8.2 vs. 7.1 months; HR .78, P = .0095).54,55 Based on the superior clinical benefit conferred by tislelizumab to chemotherapy, the Biologics License Application for tislelizumab in ESCC has been accepted by the U.S. FDA. 56 Moreover, there are ongoing phase III studies. For example, RATIONALE 311/NCT03957590 is comparing the effect of tislelizumab or placebo plus concurrent chemoradiotherapy in Chinese ESCC patients; NCT03783442 is comparing the effect of tislelizumab plus chemotherapy vs. chemotherapy alone in global ESCC patients.57,58 With the support of these studies, the future of tislelizumab in ESCC is worth anticipating.
Efficacy of tislelizumab in CRC and colorectal cancer and microsatellite instability-high/mismatch repair-deficient tumors
Tislelizumab showed a satisfactory effect on CRC and MSI-H/dMMR tumors. Microsatellite instability (MSI) is caused by the deficiency of the DNA mismatch repair (MMR) system, resulting from a germline mutation in MMR genes or an epigenetic extinction of the MLH1 gene. 59 Approximately 5% of metastatic CRCs are MSI-H/dMMR, and the dMMR subset of CRC showed upregulation of at least five targetable checkpoint molecules.59,60 Tumors with MSI/dMMR are characterized by a high tumor mutational burden, with highly infiltrated immune cells, and the MMR status in tumors could predict the clinical benefit of immune checkpoint blockade with PD-1 inhibitors.59,61 In a caucasian-predominant population with treatment-refractory diseases, pembrolizumab achieved an ORR of 52.9% (9/17) and a DCR of 82.4% (14/17) in MSI-H/dMMR solid tumors, and an ORR of 40.0% (4/10) in MSI-H/dMMR CRC. 61 The efficacy of tislelizumab in MSI-H/dMMR tumors has also been investigated. The initial ORR achieved by tislelizumab in previously treated MSI-H/dMMR tumors was 18.8% (3/16) and that in CRC (5 of 21 were MSI-H) was 14.3% (3/21).23,27 In phase II NCT03736889, the efficacy of tislelizumab was investigated in 80 Chinese patients with previously treated, locally advanced, unresectable or metastatic histologically confirmed MSI-H/dMMR solid tumors; the ORR determined by an independent review committee was 45.9% (34/74), and the DCR was 71.6% (53/74); the ORR was 39.1% (n = 18/46) in CRC and 57.1% (n=16/28) in non-CRC patients, and all evaluated patients were MSI-H/dMMR. 40 Due to the satisfactory anti-tumor effect, the NMPA has accepted the listing application of tislelizumab for the treatment of previously-treated, unresectable or metastatic MSI-H/dMMR tumors. 9
Efficacy of Tislelizumab in Hepatocellular Carcinoma
Tislelizumab has proved to be effective in HCC patients, a population with a continuously high unmet medical need. Previous studies of nivolumab and pembrolizumab in global populations have shown ORRs of 19.6% (42/214) and 17.3% (18/104), respectively, in previously treated HCC patients.62,63 Exploration of the therapeutic effects of tislelizumab in global HCC patients who had received at least one prior line of systemic therapy has yielded a result indicating an ORR of 12.4% (31/249). 34 Thus, the treatment of tislelizumab in HCC was approved by the NMPA in June 2021, and by the FDA in November 2019.8,52 In addition, a large, global phase III study comparing tislelizumab as a first-line treatment in patients with inoperable HCC (NCT03412773) is currently ongoing. 45
Efficacy of Tislelizumab in Nasopharyngeal Carcinoma
Tislelizumab produced an ORR of 20.0% in the treatment of NPC. As of May 11, 2018, 20 Chinese NPC patients who have received prior radiotherapy (20/20) and ≥1 line of systemic treatment (19/20) were included and given tislelizumab 200 mg intravenously q3w until unacceptable toxicity, consent withdrawal, or no evidence of continued clinical benefit. 36 Of the 15 evaluable participants, 3 achieved complete remission or partial remission. 36 In comparison, the ORR for nivolumab monotherapy in previously treated NPC patients was 20.5% (9/44), and that for pembrolizumab monotherapy was 26.3% (5/19).64,65
Efficacy of Tislelizumab in Other Settings
Tislelizumab has shown preliminary anti-tumor effects in solid tumors when combined with ociperlimab, a novel, humanized, monoclonal antibody that binds to T cell Ig and ITIM domain with high affinity and specificity. In a phase I study, the combination of tislelizumab and ociperlimab resulted in an ORR of 4.2% (1/24) and a DCR of 41.7% (10/24) in previously treated patients, without causing grade ≥4 treatment-related adverse events (TRAEs). 39 Besides, several trials investigating the effect of this combination are ongoing,66-69 the results of which are worth expecting.
In addition to the above-mentioned studies, tislelizumab is also undergoing clinical trials on various tumors. 45 Most of them (135/147) are located in China. The studied tumors included HCC, urologic neoplasms, CRC, and lymphoma; in these trials, tislelizumab was combined with different therapeutic regimens, like ablation, bacillus calmette-guerin, surufatinib, and lenalidomide. 45 It is hopeful that these clinical trials may bring about more indications for tislelizumab.
Safety Profiles of Tislelizumab
Although tislelizumab has shown preliminary efficacy in the treatment of various tumors, it has inevitable adverse effects. The criteria of adverse events across the studies were based on the Common Terminology Criteria for Adverse Events from the National Cancer Institute and included 5 grades, referring to mild, moderate, severe, life-threatening adverse events, or death, in an ascending order. 70 Across the studies, the incidence of TRAEs for tislelizumab monotherapy ranges from 46.7% to 97.1%, and the treatment-related death rate varies from 0 to 4%; the incidence of TRAE-related death was uniquely high for RATIONALE 302, but there were only incomplete results, which are not eligible for detailed analysis (Table 1).23,26-36
Overall, the safety profile of tislelizumab is not as good as other well-studied checkpoint inhibitors but is acceptable. For checkpoint inhibitors, the incidence of TRAEs varies between 54% and 76%. 71 The incidence of grade 5 TRAEs for PD-1 inhibitors and PD-L1 inhibitors was .361% (33/9136) and .63% (12/3164), respectively, in a pooled analysis. 72 In the phase I part of CTR20160872, no dose-limiting toxicity (DLT) was observed; grade 3 or higher AEs were observed in 39.7% (119/300) patients across the study, with 4.0% (12/300) death. 27 In NCT02407990, one DLT was observed during dose escalation (grade 3 colitis in the 5 mg/kg q2w cohort); grade 3 or higher AEs were observed in 43.9% (198/451) patients, with 3.1% (14/451) of deaths. 23 In the first reported phase I study of nivolumab, TRAEs were observed in 69.9% (207/296) patients, and grade 3 or 4 TRAEs occurred in 13.9% (41/296) patients, with 1.0% (3/296) of drug-related deaths. 73 In a phase I study of pembrolizumab, the data were 70.9% (351/495), 9.5% (47/495), and .2% (1/495), respectively. 49
Blood routine abnormalities and fatigue are important and frequent TRAEs for tislelizumab. In the analyses of the most common TRAEs across 9 prospective clinical studies with complete results, fatigue is the most common TRAE in tislelizumab monotherapy, and anemia is the most common TRAE in combination therapy; for grade 3 or higher TRAEs, anemia is the most frequent in tislelizumab monotherapy, and decreased neutrophil count ranks the first among all TRAEs in combination therapy (Table 2 and Table 3).23,26-33 In comprehensive meta-analyses, the most common all grade TRAE and grade 3 or higher TRAE for PD-1/PD-L1 monotherapy is fatigue, the most common all-grade TRAE for PD-1/PD-L1 plus chemotherapy is anemia, and the most common grade 3 or higher TRAE is neutropenia.74,75 Anemia ranks the second in grade 3 or higher TRAEs for PD-1/PD-L1 monotherapy. 74 The results indicate that fatigue and blood routine abnormalities are frequent adverse events in immunotherapy and should be monitored on a regular basis.
Table 2.
Top 20 all grade treatment-related adverse events for tislelizumab monotherapy (left columns) and combination therapies (right columns) across 9 prospective clinical studies with complete results on tislelizumab.
| All grade TRAEs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials |
NCT02407990 (ST, n=451) |
CTR20160872 (ST, N=300) |
CTR20170071 (UC, N=113) |
NCT03209973 (r/r cHL, N=70) |
Sum | Trials |
NCT02660034 (ST, N=49) |
RATIONALE 304 (NSCLC, Group A, n=222) |
RATIONALE 307 (NSCLC, arm A, n = 120) |
RATIONALE 307 (NSCLC, arm B, n = 118) |
NCT03432598 (NSCLC, N=54) |
NCT03469557 (ESCC/GEJ, N=30) |
Sum |
| Treatment | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Treatment | Tislelizumab plus pamiparib | Tislelizumab plus chemotherapy | Tislelizumab plus chemotherapy | Tislelizumab plus chemotherapy | Tislelizumab plus chemotherapy | Tislelizumab plus chemotherapy | ||
| Fatigue | 28.4% | 7.7% | 6.0% | 7.1% | 163 | Anemia | 24.0% | 86.1% | 88.3% | 93.2% | 79.6% | 60.0% | 480 |
| Decreased appetite | 20.4% | 13.0% | 19.0% | 0.0% | 152 | Decreased neutrophil count | 0.0% | 82.0% | 63.3% | 61.0% | 72.2% | 40.0% | 381 |
| Anemia | 9.1% | 23.0% | 27.0% | 10.0% | 148 | Decreased white blood cell count | 0.0% | 82.4% | 53.3% | 57.6% | 74.1% | 43.3% | 368 |
| Nausea | 24.8% | 7.0% | 8.0% | 5.7% | 146 | Decreased platelet count | 10.0% | 69.9% | 34.2% | 44.1% | 42.6% | 40.0% | 288 |
| Increased AST | 5.0% | 23.0% | 17.0% | 10.0% | 118 | Nausea | 67.0% | 43.7% | 30.0% | 43.2% | 33.3% | 53.3% | 251 |
| Rash | 13.5% | 10.7% | 13.0% | 12.9% | 117 | Increased ALT | 24.0% | 48.6% | 41.7% | 34.7% | 37.0% | 30.0% | 240 |
| Diarrhea | 18.2% | 6.3% | 6.0% | 10.0% | 115 | Increased AST | 20.0% | 43.3% | 35.8% | 33.9% | 37.0% | 33.3% | 219 |
| Increased ALT | 5.6% | 19.0% | 16.0% | 12.9% | 109 | Decreased appetite | 0.0% | 33.8% | 43.3% | 44.1% | 35.2% | 56.7% | 215 |
| Vomiting | 14.7% | 6.0% | 8.0% | 5.7% | 97 | Fatigue | 57.0% | 38.3% | 24.2% | 17.8% | 35.2% | 50.0% | 197 |
| Constipation | 17.6% | 0.0% | 15.0% | 0.0% | 96 | Alopecia | 0.0% | 0.0% | 64.2% | 69.5% | 24.1% | 0.0% | 172 |
| Cough | 13.7% | 6.3% | 0.0% | 17.1% | 93 | Vomiting | 35.0% | 27.1% | 23.3% | 22.9% | 25.9% | 43.3% | 159 |
| Decreased weight | 8.9% | 10.7% | 0.0% | 8.6% | 78 | Constipation | 18.0% | 23.9% | 30.0% | 28.0% | 0.0% | 0.0% | 131 |
| Abdominal pain | 15.3% | 0.0% | 5.0% | 0.0% | 75 | Rash | 0.0% | 21.2% | 20.8% | 22.0% | 13.3% | 0.0% | 105 |
| Pyrexia | 0.0% | 5.0% | 19.0% | 54.3% | 74 | Pyrexia | 20.0% | 0.0% | 20.0% | 20.3% | 0.0% | 30.0% | 67 |
| Back pain | 14.8% | 0.0% | 0.0% | 5.7% | 71 | Pain in extremity | 10.0% | 0.0% | 33.3% | 14.4% | 0.0% | 0.0% | 62 |
| Increased gamma-glutamyl transferase | 0.0% | 14.3% | 6.0% | 0.0% | 50 | Hypoalbuminemia | 0.0% | 0.0% | 22.5% | 17.8% | 0.0% | 33.3% | 58 |
| Hypothyroidism | 0.0% | 5.0% | 10.0% | 32.9% | 49 | Musculoskeletal pain | 0.0% | 24.8% | 0.0% | 0.0% | 0.0% | 0.0% | 55 |
| Dyspnea | 9.9% | 0.0% | 0.0% | 0.0% | 45 | Arthralgia | 12.0% | 0.0% | 20.8% | 17.8% | 0.0% | 0.0% | 52 |
| Decreased neutrophil count | 0.0% | 9.0% | 5.0% | 14.3% | 43 | Hypoproteinemia | 0.0% | 23.0% | 0.0% | 0.0% | 0.0% | 0.0% | 51 |
| Decreased platelet count | 0.0% | 10.0% | 0.0% | 11.4% | 38 | Increased blood bilirubin level | 0.0% | 0.0% | 22.5% | 12.7% | 0.0% | 30.0% | 51 |
Abbreviations: TRAEs, treatment-related adverse effects; AST, aspartate aminotransferase; ALT, alanine transaminase; ST, solid tumors; UC, urothelial carcinoma; r/r cHL, relapsed or refractory classical Hodgkin lymphoma; NSCLC, non-small cell lung cancer; ESCC, esophageal squamous cell carcinoma; GEJ, gastric/gastroesophageal junction cancer.
Table 3.
Top 20 grade 3-5 treatment-related adverse events for tislelizumab monotherapy (left columns) and combination therapies (right columns) across 9 prospective clinical studies with complete results on tislelizumab.
| Grade 3-5 TRAEs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials |
NCT02407990 (ST, n=451) |
CTR20160872 (ST, N=300) |
CTR20170071 (UC, N=113) |
NCT03209973 (r/r cHL, N=70) |
Sum | Trials |
NCT02660034 (ST, N=49) |
RATIONALE 304 (NSCLC, Group A, n=222) |
RATIONALE 307 (NSCLC, arm A, n = 120) |
RATIONALE 307 (NSCLC, arm B, n = 118) |
NCT03432598 (NSCLC, N=54) |
NCT03469557 (ESCC/GEJ, N=30) |
Sum |
| Treatment | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Tislelizumab monotherapy | Treatment | Tislelizumab plus pamiparib | Tislelizumab plus chemotherapy | Tislelizumab plus chemotherapy | Tislelizumab plus chemotherapy | Tislelizumab plus chemotherapy | Tislelizumab plus chemotherapy | ||
| Anemia | 4.9% | 3.0% | 7.0% | 0.0% | 39 | Decreased neutrophil count | 0.0% | 44.6% | 51.7% | 45.8% | 48.1% | 3.3% | 242 |
| Lower respiratory tract infection | 5.1% | 0.0% | 0.0% | 0.0% | 23 | Decreased white blood cell count | 0.0% | 21.6% | 22.5% | 27.1% | 13.0% | 6.7% | 116 |
| Increased AST | 1.5% | 3.0% | 2.0% | 0.0% | 18 | Anemia | 12.0% | 14.9% | 7.5% | 22.9% | 18.5% | 6.7% | 87 |
| Increased ALT | 1.8% | 1.0% | 4.0% | 0.0% | 16 | Decreased platelet count | 0.0% | 19.4% | 4.2% | 13.6% | 13.0% | 3.3% | 72 |
| Decreased neutrophil count | 0.0% | 4.0% | 0.0% | 1.4% | 13 | Increased ALT | 6.0% | 3.6% | 1.7% | 1.7% | 5.6% | 3.3% | 19 |
| Nausea | 1.3% | 2.0% | 0.0% | 0.0% | 12 | Increased AST | 6.0% | 2.3% | 0.0% | 0.8% | 1.9% | 6.7% | 12 |
| Fatigue | 1.8% | 0.7% | 1.0% | 0.0% | 11 | Vomiting | 4.0% | 0.5% | 0.8% | 0.0% | 1.9% | 16.7% | 10 |
| Diarrhea | 1.8% | 0.3% | 2.0% | 0.0% | 11 | Fatigue | 4.0% | 1.4% | 0.0% | 0.0% | 1.9% | 3.3% | 7 |
| Abdominal pain | 1.6% | 0.0% | 2.0% | 0.0% | 9 | Rash | 0.0% | 0.5% | 3.3% | 1.7% | 0.0% | 0.0% | 7 |
| Hypokalemia | 2.0% | 0.0% | 0.0% | 0.0% | 9 | Decreased appetite | 0.0% | 1.4% | 0.8% | 0.8% | 0.0% | 6.7% | 7 |
| Hyponatremia | 0.7% | 0.0% | 5.0% | 0.0% | 9 | Nausea | 4.0% | 0.5% | 0.0% | 0.0% | 1.9% | 0.0% | 4 |
| Vomiting | 1.8% | 0.0% | 0.0% | 0.0% | 8 | Hyponatremia | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 13.3% | 4 |
| Dysphagia | 1.6% | 0.0% | 0.0% | 0.0% | 7 | Pain in extremity | 0.0% | 0.0% | 2.5% | 0.0% | 0.0% | 0.0% | 3 |
| Increased blood bilirubin level | 0.6% | 0.3% | 3.0% | 0.0% | 7 | Diarrhea | 4.0% | 0.0% | 0.0% | 0.0% | 0.0% | 3.3% | 3 |
| Back pain | 1.3% | 0.0% | 0.0% | 1.4% | 7 | Increased gamma-glutamyl transferase | 6.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 3 |
| Hyperglycemia | 1.1% | 0.0% | 1.0% | 0.0% | 6 | Autoimmune hepatitis | 6.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 3 |
| Pleural effusion | 1.3% | 0.0% | 0.0% | 0.0% | 6 | Hypokalemia | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 6.7% | 2 |
| Hypertension | 1.3% | 0.0% | 0.0% | 0.0% | 6 | Decreased weight | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 6.7% | 2 |
| Upper respiratory tract infection | 0.0% | 0.0% | 3.0% | 2.9% | 5 | Small intestinal obstruction | 4.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 2 |
| Decreased weight | 0.7% | 0.7% | 0.0% | 0.0% | 5 | Hepatitis | 4.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 2 |
Abbreviations: TRAEs, treatment-related adverse effects; AST, aspartate aminotransferase; ALT, alanine transaminase; ST, solid tumors; UC, urothelial carcinoma; r/r cHL, relapsed or refractory classical Hodgkin lymphoma; NSCLC, non-small cell lung cancer; ESCC, esophageal squamous cell carcinoma; GEJ, gastric/gastroesophageal junction cancer.
Respiratory and hepatic events are the most frequent causes of treatment-related death with tislelizumab. Among the 9 studies, fatal TRAEs occurred in 7.23,26-33 In total, there were 12 fatal events that are considered to be related to tislelizumab monotherapy or tislelizumab combination therapy.23,27,29-33 In most of the cases (9/12), the event was related to respiratory failure/infection or hepatic injury; in the other 3 cases, the event was related to brain edema, hydrocephalus, and “death”.23,29-33 This is consistent with a previous meta-analysis on fatal events related to immunotherapy. 76 In that analysis, anti-PD-1/PD-L1-related fatalities were often caused by pneumonitis, hepatitis, and neurotoxic effects. 76 The results indicate that patients with related underlying diseases should be treated with caution and carefully monitored.
Discussion
Tislelizumab is a domestic PD-1 antibody in China with a different antibody structure from traditional PD-1 antibodies, which may optimize its anti-tumor effect.13,14,20 It was approved for the treatment of r/r cHL, UC, squamous NSCLC, non-squamous NSCLC, and HCC by the NMPA, and for the treatment of esophageal cancer, HCC, and G/GEJ by the FDA.6-8,43,52 In addition, the preliminary effects of tislelizumab have been investigated in several malignant tumors, such as NPC, ESCC, CRC, and MSI-H/dMMR tumors, with acceptable adverse effects.23,26-36 The studies with available data are listed in Figure 2 and summarized in Table 1. Otherwise, ongoing trials may provide references for new indications in the future. 45
The flaw of this study is evident: comparisons between studies could not be made directly because of the differences in patients’ baseline characteristics. Patients in the tislelizumab studies mainly included local Chinese, while those of nivolumab and pembrolizumab involved other races. There has also been no direct head-to-head comparisons yet with other anti-PD-1 antibodies, like nivolumab and pembrolizumab. Thus, no conclusions can be drawn in terms of the superiority or inferiority in anti-tumor effects among these drugs.
The inherent value of this study is obvious. Since cancer is a major health issue in China and worldwide, ranking the second in the causes of death globally, there is a hugely unmet need for anticancer drugs. 77 Although the PD-1/PD-L1 inhibitors pembrolizumab, nivolumab, atezolizumab, and durvalumab have been approved in China, the economic burden is heavy. 78 Recently, the NMPA has included tislelizumab in the medical insurance catalogue and lowered its cost. 79 The overall costs of tislelizumab may differ slightly in different regions but is overall lower than those of the other 4 drugs mentioned above with the patient assistant program. 80 The cost-effectiveness of tislelizumab in various tumors has not yet been analyzed. However, due to its preliminary efficacy in various solid tumors and its relatively low price in the market, this drug could provide tumor patients with a new weapon and alleviate the economic burden. Tislelizumab also has advantages compared with other domestic PD-1 antibodies: some studies involved patients from other countries, which makes the result of them more applicable to other races.23,26,31 As the studies continue, tislelizumab could be expected to be approved in more settings and allowed to achieve globalization.
Conclusion
As a modified PD-1 inhibitor, tislelizumab has shown therapeutic effects in the treatment of various cancers and has been generally well-tolerated. While monotherapy exhibited preliminary anti-tumor effects, combination therapies also showed new promise and remain to be further investigated. The introduction of tislelizumab could provide clinical oncologists with a relatively economical choice in the battle against tumors, and may alleviate the burden of tumor patients.
Footnotes
Author contributions: All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Qing Geng, MD, PhD https://orcid.org/0000-0003-4515-5084
References
- 1.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367(6477):x182. doi: 10.1126/science.aax0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 pathway. Sci Adv. 2020;6(38):eabd2712. doi: 10.1126/sciadv.abd2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):324r-328r. doi: 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin X, Lu X, Luo G, Xiang H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur J Med Chem. 2020;186:111876. doi: 10.1016/j.ejmech.2019.111876 [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Keam SJ. Tislelizumab: First Approval. Drugs. 2020;80(6):617-624. doi: 10.1007/s40265-020-01286-z [DOI] [PubMed] [Google Scholar]
- 6.National Medical Product Administration . Release Notice of Drug Approvals on April 13, 2020; 2020. https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20200413134101928.html. Accessed February 24, 2022. [Google Scholar]
- 7.National Medical Product Administration . Release Notice of Drug Approvals on January 18, 2021; 2021. https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20210118091934189.html Accessed February 24, 2022. [Google Scholar]
- 8.National Medical Product Administration . Release Notice of Drug Approvals on June 22, 2021; 2021. https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20210622215839168.html Accessed February 24, 2022. [Google Scholar]
- 9.National Medical Product Administration . Mailing Details of Drug Receipts on June 22, 2021; 2021. https://www.nmpa.gov.cn/zwfw/zwfwjfxx/jfsjcx/20210630085841110.html Accessed February 24, 2022. [Google Scholar]
- 10.National Medical Product Administration . Mailing Details of Drug Receipts on March 18, 2021; 2021. https://www.nmpa.gov.cn/zwfw/zwfwjfxx/jfsjcx/20210329150000118.html Accessed February 24, 2022. [Google Scholar]
- 11.Wang J, Yang T, Xu J. Therapeutic development of immune checkpoint inhibitors. Adv Exp Med Biol. 2020;1248:619-649. doi: 10.1007/978-981-15-3266-5_23 [DOI] [PubMed] [Google Scholar]
- 12.Kong X. Discovery of new immune checkpoints: family grows up. Adv Exp Med Biol. 2020;1248:61-82. doi: 10.1007/978-981-15-3266-5_4 [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Lee HT, Lim H, Kim Y, Park UB, Heo Y. Crystal structure of PD-1 in complex with an antibody-drug tislelizumab used in tumor immune checkpoint therapy. Biochem Biophys Res Commun. 2020;527(1):226-231. doi: 10.1016/j.bbrc.2020.04.121 [DOI] [PubMed] [Google Scholar]
- 14.Hong Y, Feng Y, Sun H, et al. Tislelizumab uniquely binds to the CC' loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio. 2021;11(3):782-792. doi: 10.1002/2211-5463.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burley SK, Bhikadiya C, Bi C, et al. RCSB Protein Data Bank: powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021;49(D1):D437-D451. doi: 10.1093/nar/gkaa1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehnal D, Bittrich S, Deshpande M, et al. Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021;49(W1):W431-W437. doi: 10.1093/nar/gkab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan S, Zhang H, Chai Y, et al. An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat Commun. 2017;8:14369. doi: 10.1038/ncomms14369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Na Z, Yeo SP, Bharath SR, et al. Structural basis for blocking PD-1-mediated immune suppression by therapeutic antibody pembrolizumab. Cell Res. 2017;27(1):147-150. doi: 10.1038/cr.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown ME, Bedinger D, Lilov A, et al. Assessing the binding properties of the anti-PD-1 antibody landscape using label-free biosensors. PLoS One. 2020;15(3):e229206. doi: 10.1371/journal.pone.0229206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079-1090. doi: 10.1007/s00262-018-2160-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell. 2015;28(3):285-295. doi: 10.1016/j.ccell.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Song J, Li Y, et al. Abstract 2226: Anti-human PD-1 antibody BGB-A317 exhibits potent immune cell activation. Cancer Res. 2016;76(14 suppl ment):2226. doi: 10.1158/1538-7445.AM2016-2226 [DOI] [Google Scholar]
- 23.Desai J, Deva S, Lee JS, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2019;8(1):e453. doi: 10.1136/jitc-2019-000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Zhang T, Fu C, et al. Activation of tumor infiltrating lymphocytes from colorectal cancer and colorectal liver metastasis patients by anti-human PD-1 Antibody BGB-A317 in a 3D spheroid system. J Clin Oncol. 2016;34(15_suppl l):e14560. doi: 10.1200/JCO.2016.34.15_suppl.e14560 [DOI] [Google Scholar]
- 25.Luo L, Wu X, Zhang T, et al. Abstract 5626: Investigation of T cell activation by anti-human PD-1 antibodies Nivolumab, Pembrolizumab and BGB-A317 using tumor-infiltrating lymphocytes (TILs) from colorectal cancer and colorectal liver metastasis patients. Cancer Res. 2017;77(13 suppl):5626. doi: 10.1158/1538-7445.AM2017-5626 [DOI] [Google Scholar]
- 26.Friedlander M, Meniawy T, Markman B, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(9):1306-1315. doi: 10.1016/S1470-2045(19)30396-1 [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Guo J, Zhang Q, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. 2020;8(1):e437. doi: 10.1136/jitc-2019-000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y, Gao Q, Zhang H, et al. Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia. 2020;34(2):533-542. doi: 10.1038/s41375-019-0545-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Zhao J, Ma Z, et al. A phase 2 study of tislelizumab in combination with platinum-based chemotherapy as first-line treatment for advanced lung cancer in chinese patients. Lung Cancer. 2020;147:259-268. doi: 10.1016/j.lungcan.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 30.Ye D, Liu J, Zhou A, et al. Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Sci. 2021;112(1):305-313. doi: 10.1111/cas.14681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Bai Y, Xu N, et al. Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2020;26(17):4542-4550. doi: 10.1158/1078-0432.CCR-19-3561 [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Lu S, Yu X, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer. JAMA Oncol. 2021;7(5):709-717. doi: 10.1001/jamaoncol.2021.0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu S, Wang J, Yu Y, et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J Thorac Oncol. 2021;16(9):1512-1522. doi: 10.1016/j.jtho.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 34.Ducreux M, Abou-Alfa G, Ren Z, et al. O-1 Results from a global phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with unresectable hepatocellular carcinoma. Ann Oncol. 2021;32:S217. doi: 10.1016/j.annonc.2021.05.005 [DOI] [Google Scholar]
- 35.Ajani J, El Hajbi F, Cunningham D, et al. O-15 Randomized, phase 3 study of second-line tislelizumab vs chemotherapy in advanced or metastatic esophageal squamous cell carcinoma (RATIONALE 302) in the overall population and Europe/North America subgroup. Ann Oncol. 2021;32:S225. doi: 10.1016/j.annonc.2021.05.807 [DOI] [Google Scholar]
- 36.Wang S, Huang X, Bai Y, et al. Preliminary results with tislelizumab, an investigational anti-PD-1 antibody, in Chinese patients with nasopharyngeal cancer (NPC). J Clin Oncol. 2019;37(15_suppl l):2556. doi: 10.1200/JCO.2019.37.15_suppl.255631283408 [DOI] [Google Scholar]
- 37.Wang J, Lu S, Yu X, et al. RATIONALE-307: Tislelizumab plus chemotherapy versus chemotherapy alone as first-line treatment for advanced squamous NSCLC in patients aged ≥ 65. J Clin Oncol. 2021;39(15_suppl l):9102. doi: 10.1200/JCO.2021.39.15_suppl.9102 [DOI] [Google Scholar]
- 38.Sandhu S, Hill A, Gan H, et al. 285 - Tislelizumab, an anti-PD-1 antibody, in patients with urothelial carcinoma (UC): Results from an ongoing phase I/II study. Ann Oncol. 2018;29(suppl l_10):x24. doi: 10.1093/annonc/mdy487 [DOI] [Google Scholar]
- 39.Frentzas S, Meniawy T, Kao SC, et al. AdvanTIG-105: Phase 1 dose-escalation study of anti-TIGIT monoclonal antibody ociperlimab (BGB-A1217) in combination with tislelizumab in patients with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl l):2583. doi: 10.1200/JCO.2021.39.15_suppl.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Xu Y, Zang A, et al. A phase 2 study of tislelizumab monotherapy in patients with previously treated, locally advanced unresectable ormetastatic microsatellite instability-high/mismatch repair deficient solid tumors. J Clin Oncol. 2021;39(15_suppl l):2569. doi: 10.1200/JCO.2021.39.15_suppl.2569 [DOI] [Google Scholar]
- 41.Armand P, Engert A, Younes A, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol. 2018;36(14):1428-1439. doi: 10.1200/JCO.2017.76.0793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic hodgkin lymphoma. J Clin Oncol. 2017;35(19):2125-2132. doi: 10.1200/JCO.2016.72.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Medical Product Administration . Release Notice of Drug Approvals on December 31; 2019. https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20191231145701966.html Accessed February 24, 2022. [Google Scholar]
- 44.Song Y, Gao Q, Zhang H, et al. Tislelizumab for relapsed/refractory classical Hodgkin lymphoma: 3-year follow-up and correlative biomarker analysis. Clin Cancer Res. 2021;28:2021-2023. doi: 10.1158/1078-0432.CCR-21-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. National Library of Medicine . Clinical trials on tislelizumab. 2021. https://clinicaltrials.gov/ct2/results?recrs=&cond=&term=tislelizumab&cntry=&state=&city=&dist= Accessed February 24, 2022. [Google Scholar]
- 46.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590-1598. doi: 10.1016/S1470-2045(16)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265. doi: 10.1016/S1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 50.Bang Y, Kang Y, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22(4):828-837. doi: 10.1007/s10120-018-00909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30(2):250-258. doi: 10.1093/annonc/mdy540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.U.S. Food & Drug Administration . Search Orphan Drug Designations and Approvals; 2021. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/listResult.cfm. Accessed February 24, 2022. [Google Scholar]
- 53.Shah MA, Kojima T, Hochhauser D, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus. JAMA Oncol. 2019;5(4):546-550. doi: 10.1001/jamaoncol.2018.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506-1517. doi: 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 55.Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138-4148. doi: 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 56.Beigene news . BeiGene Announces U.S. FDA Acceptance of Biologics License Application for Tislelizumab in Esophageal Squamous Cell Carcinoma. 2021. https://ir.beigene.com/news-details/?id=5c97c078-8ee7-4a4e-aba3-19d5a9dac715 Accessed February 24, 2022. [Google Scholar]
- 57.Yu R, Wang W, Li T, et al. RATIONALE 311: tislelizumab plus concurrent chemoradiotherapy for localized esophageal squamous cell carcinoma. Future Oncol. 2021;17(31):4081-4089. doi: 10.2217/fon-2021-0632 [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Kato K, Hubner R, et al. A randomized, placebo-controlled, phase III trial-in-progress to evaluate the efficacy and safety of tislelizumab plus chemotherapy as first-line treatment for unresectable, locally advanced recurrent/metastatic esophageal squamous cell carcinoma (ESCC). J Clin Oncol. 2019;37(15_suppl l):S2656. doi: 10.1200/JCO.2019.37.15_suppl.TPS2656 [DOI] [Google Scholar]
- 59.Cohen R, Rousseau B, Vidal J, Colle R, Diaz LA, André T. Immune Checkpoint Inhibition in Colorectal Cancer: Microsatellite Instability and Beyond. Targeted Oncol. 2020;15(1):11-24. doi: 10.1007/s11523-019-00690-0 [DOI] [PubMed] [Google Scholar]
- 60.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43-51. doi: 10.1158/2159-8290.CD-14-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 64.Ma B, Lim WT, Goh BC, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol. 2018;36(14):1412-1418. doi: 10.1200/JCO.2017.77.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu C, Lee SH, Ejadi S, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol. 2017;35(36):4050-4056. doi: 10.1200/JCO.2017.73.3675 [DOI] [PubMed] [Google Scholar]
- 66.Wu L, Wang P, Hsiao S, et al. AdvanTIG-202: A phase 2 study investigating anti-TIGIT monoclonal antibody ociperlimab plus anti-PD-1 monoclonal antibody tislelizumab in patients with previously treated recurrent or metastatic cervical cancer. J Clin Oncol. 2021;39(15_suppl l):S5595. doi: 10.1200/JCO.2021.39.15_suppl.TPS5595 [DOI] [Google Scholar]
- 67.Socinski MA, Spira AI, Paz-Ares LG, et al. AdvanTIG-302: Anti-TIGIT monoclonal antibody (mAb) ociperlimab (OCI) plus tislelizumab (TIS) versus pembrolizumab (PEM) in programmed death ligand-1 (PD-L1) selected, previously untreated, locally advanced, unresectable or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39(15_suppl l):S9128. doi: 10.1200/JCO.2021.39.15_suppl.TPS9128 [DOI] [Google Scholar]
- 68.Xu R, Kim S, Tougeron D, et al. AdvanTIG-203: A randomized phase 2 study comparing anti-TIGIT ociperlimab plus tislelizumab versus tislelizumab plus placebo as second-line treatment in patients with advanced or recurrent esophageal squamous cell carcinoma (ESCC) expressing programmed death-ligand 1 (PD-L1). J Clin Oncol. 2021;39(15_suppl l):S4150. doi: 10.1200/JCO.2021.39.15_suppl.TPS4150 [DOI] [Google Scholar]
- 69.U.S. National Library of Medicine . Search Results for: Tislelizumab and Ociperlimab; 2021. https://clinicaltrials.gov/ct2/results?cond=&term=tislelizumab+and+ociperlimab&cntry=&state=&city=&dist= Accessed February 24, 2022. [Google Scholar]
- 70.National Institute of Health . Common Terminology Criteria for Adverse Events (CTCAE). 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Accessed February 24, 2022.
- 71.Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi: 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Zhou S, Yang F, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials. JAMA Oncol. 2019;5(7):1008-1019. doi: 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou X, Yao Z, Bai H, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22(9):1265-1274. doi: 10.1016/S1470-2045(21)00333-8 [DOI] [PubMed] [Google Scholar]
- 76.Wang DY, Salem J, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors. JAMA Oncol. 2018;4(12):1721-1728. doi: 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. GBD 2016 Causes of Death CollaboratorsLancet. 2017;390(10100):1151-1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo L, Wei R, Lin Y, Kwok HF. Clinical and Recent Patents Applications of PD-1/PD-L1 Targeting Immunotherapy in Cancer Treatment-Current Progress, Strategy, and Future Perspective. Front Immunol. 2020;11:1508. doi: 10.3389/fimmu.2020.01508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.National Healthcare Security Administration . Public consultation of Work plan for the adjustment of the national insurance drug list in 2020 (Draft for Comments). 2020. http://www.nhsa.gov.cn/art/2020/8/3/art_48_3394.html?from=timeline Accessed February 24, 2022. [Google Scholar]
- 80.National Healthcare Security Administration . (2020). The notice of issuing <National Drug Catalogue for Basic Medical Insurance, Work-related Injury Insurance and Maternity Insurance (2020)>, by National Health Security Administration and Ministry of Human Resouraces and Social Security of the People's Republic of China. http://www.nhsa.gov.cn/art/2020/12/28/art_37_4220.html [Accessed February 24, 2022]. [Google Scholar]