Abstract
Background
The aim of this research was to evaluate clinical and low-cost genetic determinants of treatment outcome in EGFR mutation positive advanced lung adenocarcinoma patients.
Material and Methods
EGFR mutation testing and EGFR 181946C>T genotyping were performed in 101 advanced lung adenocarcinoma patients using qRT-PCR and PCR-RFLP, respectively. Progression-free survival was defined as the time from the start of TKI therapy to date of progression, and overall survival as the time from diagnosis to death from any cause. Pain level was evaluated using a Numerical Rating Scale and the Verbal Descriptor Scale. Statistical significance was considered for P < .05.
Results
Patients were treated with EGFR-TKIs for a period of 1–39months (median 9), with a median PFS of 12.0 months (10.4-13.6, CI 95%), and a median OS of 19.0 months (15.1-22.7, CI 95%). The presence of pain was significantly correlated with the existence of bone (P < .001) and adrenal glands metastases (P = .029). Genetic factors did not have a direct impact on pain management but had a significant effect on the response to TKIs leading to pain alleviation.
Conclusions
EGFR mutation subtype and the EGFR 181946 C>T SNP had a significant effect on the response to TKI inducing an indirect anti-dolorous effect.
Keywords: EGFR, lung cancer, pain, tyrosine kinase inhibitors
Introduction
Lung cancer (LC) prevention measures are not sufficiently effective even in economically developed countries. Specific clinical symptoms are absent in early stages of lung cancer and uptake of screening is inadequate even in countries which perform them, often not including individuals without smoking history.1,2 Thus, around 75% of LC cases are diagnosed in advanced stages when the currative success rate is very low due to high tumor burden and the presence of cancer-related pain is higher. 3 As a temporary stop/slowing down of LC screening programs and diagnostic procedures worldwide during the COVID-19 pandemic has been reported,4,5 concerns have been raised that lung cancer-related pain can origin from direct effects of the tumor, its regional or distant spread, as well as from anti-cancer treatment. 6 Pharmacotherapy is the standard approach for the treatment of cancer pain, but a combination of pharmacological and non-pharmacological measures should be applied when needed.7-9 Bone metastases represent one of the most common sites of metastatic spread of LC and they are usually associated with shorter survival. 10 Radiation therapy (RT) remains the golden standard of palliative management of painful bone metastases and it is performed to relieve pain, prevent pathologic fractures, spinal cord compression as well as to maintain or improve patients’ quality of life. 11 Optimal medical analgesic therapy is essential alongside RT. 12 An individual approach to each patient is necessary, as well as constant monitoring by a multidisciplinary team which includes a pain management specialist.
Serbia was among the highest ranked countries in the world in 2018 when age-standardized rate of lung cancer occurrence was taken into account, 13 mostly due to the combined effect of very low tobacco cost and the lack of a structured national lung cancer screening program. As a higher percentage of Serbian lung cancer patients are diagnosed in advanced stages, around 20–30% exhibit chest pain at diagnosis, and around 6–13% bone pain. 14 Recently, we and others have made investigator-initiated efforts to explore genetic risk factors for LC in Serbia, in an effort to decipher the mechanisms driving its carcinogenesis, reduce the national mortality rate, and lower the number of patients who are diagnosed with advance stage lung cancer.15-18 Advancement in diagnosis and treatment have also been introduced, but the accessibility of new molecular techniques and drugs still needs improvement.19,20 Although many discrepancies are present between various countries in availability of innovative drugs, EGFR mutation testing is regularly applied as companion diagnostics for tyrosine kinase inhibitors (TKIs) in advanced stages of lung cancer. 21 In Serbia, EGFR mutation testing of lung cancer patients was initiated in 2011 from tumor tissue and in 2016 from liquid biopsy.19,22 Patients with confirmed EGFR mutations are treated with first- and second-generation EGFR-TKIs until progression, at which point they are tested again for the presence of a resistant EGFR T790 M mutation which renders them candidates for third generation EGFR-TKIs. 23 All symptoms of the disease, including the pain level, as well as adverse effects and patient performance status are evaluated at diagnosis and closely monitored and managed during this entire TKI treatment course, contributing to the overall patients’ quality of life.
The aim of this research was to detect the possible clinical and low-cost genetic determinants of treatment outcome in EGFR mutation positive advanced lung adenocarcinoma patients treated at our Institute, in an effort to provide data from the Balkan area, which is usually underrepresented in larger meta-analyses.
Methods
Patient Samples
This retrospective study included 101 patients, with inclusion criteria of a minimum age of 18years and clinically and histologically confirmed primary advanced lung adenocarcinoma according to the current WHO classification (stage IIIB/IV, ECOG performance status 0, 1, or 2). 24 According to data that LC risk slowly diminishes and is reduced to 50% after 30years of cessation of active smoking 14 the subjects were divided into 2 groups, non-smoker, that is, never smoker or ex-smoker ≥30years, and smoker, that is, current smoker or ex-smoker <30years, and further into ex-smoker and smoker <15 and ≥15years. The procedures used in this study were approved by the Ethics Committee of the Institute for Oncology and Radiology of Serbia (No. 5665-01) and were in accordance with the Helsinki Declaration of 1975, as revised in 2000. All patients had a minimum age of 18years and signed an informed consent.
Pain Level Assesment and Management
Pain level was evaluated using 2 approaches—a Numerical Rating Scale (NRS) and the Verbal Descriptor Scale (VDS), at baseline and continuously during TKI treatment. For the purpose of this study, a second time-point (after baseline) of official recording of pain level was the time of the first computed tomography (CT) evaluation of the efficacy of TKI treatment, which was after 3months of treatment initiation. 25 Numeric rating scale involved patients' self-reported pain rating from 0 to 10, with 0 representing no pain and 10 representing the worst pain. Mild category of pain had NRS values from 1 to 3, moderate pain had NRS values from 4 to 6, and severe pain had NRS values from 7 to 10. In patients who had difficulty responding to NRS, the VDS was used that included several descriptive phrases that referred to different levels of pain severity or intensity (e.g., “no pain,” “mild pain,” “moderate pain,” “severe pain,” and “the worst pain imaginable”). Pain management was achieved using acetaminophens, non-steroidal anti-inflammatory, and weak and strong opioids 7 (Supplementary Table 1). Single 8-Gy dose radiation therapy and 2 long-course regimens including 20 Gy in 5 fractions and 30 Gy in 10 fractions were used in patients with painful bone metastases as previously described.26,27
EGFR Mutation Testing and TKI Treatment
Formalin-fixed and paraffin-embedded (FFPE) tumor samples were obtained by biopsy/resection and used for routine EGFR (LRG_304, NM_005228.5) mutation testing using the Cobas® EGFR Mutation Test v2 on Cobas® 4800 (Roche Diagnostics). Patients with sensitizing EGFR mutations were treated with EGFR TKIs in the first line until progression or unacceptable toxicity. Progression-free survival (PFS) was defined as the time from the start of TKI therapy to date of progression, and overall survival (OS) as the time from diagnosis to death from any cause. Primary resistance to TKIs was defined as the absence of any response, that is, progression of disease within the first 3months of therapy.
EGFR 181946C>T Genotyping
EGFR 181946C>T (rs2293347, NM_005228.5(EGFR):c.2982 C>T polymorphism was analyzed from FFPE derived DNA using a standard PCR-RFLP approach. 28 A previously sequenced heterozygote sample was used as a control to assure adequate genotyping.
Statistical Analysis
Descriptive methods of statistical analysis (frequencies, percentage, mean, median, and standard deviation/SD/and range) were used to summarize the sample data. The associations between the patients' and tumor characteristics were analyzed using Pearson Chi-Square, Fisher’s exact, t-test, and Mann-Whitney tests and the analyses were performed IBM SPSS Statistics 22 (SPSS Inc, Chicago, IL, USA). Survival analysis was performed using a standard Kaplan–Meier product limit method for graphical presentation; median with corresponding 95% confidence interval (95%CI) was used for description and the Log-rank test for the analysis of difference. Descriptive analyses included genotype and allelic frequencies, and their distribution between groups was tested by Fisher’s exact test; logistic regression was used to calculate the odds ratio (OR) and the 95% confidence interval (CI) to estimate the strength of associations. Two-sided P values <.05 were considered to indicate statistical significance.
Results
In the period from 2011 to 2020, 5650 EGFR mutation analyses were performed, and 10% of EGFR mutated samples were detected, 18 which is in good accordance with literature data for the Caucasian population. 29
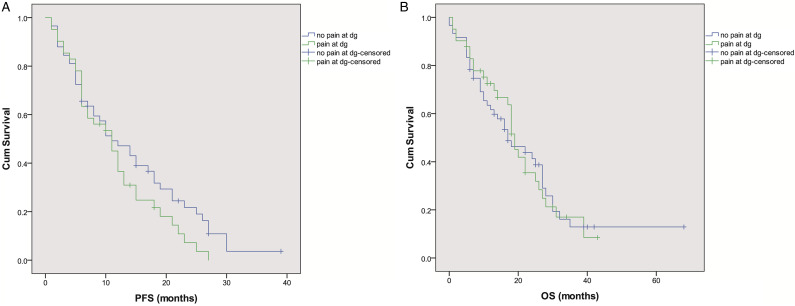
Patients were treated with first-line EGFR-TKIs for a period of 1–39months (median 9months). Median PFS in the whole group was 12.0months (10.4-13.6, CI 95%), with 33% patients still alive at the time of data analysis. Patients who presented with pain at diagnosis did not have a significantly lower PFS compared to those without pain (11.0 (6.8-15.2 95%CI) vs 11.0 (6.3-15.7 95%CI), P = .153) (Figure 1A). Median OS in the whole group was 19.0 months (15.1-22.7, CI 95%). Median OS for patients who presented with pain at diagnosis was 19.0 months (16.7-21.3, 95%CI) and 17.0months (9.2-24.8, 95%CI) for patients without pain, and the difference was not statistically significant (P = .906) (Figure 1B). Primary resistance to TKIs was detected in 15% of patients, and it was not correlated with the presence of pain at diagnosis (P =.904). Smoking status had no significant effect on the occurrence of primary resistance to TKIs (<30 vs ≥30years, P = .752; <15 vs ≥15years, P = .173). AEs were detected in 72% of TKI-treated patients with 97% of them presenting toxicity of a maximum grade 2. The presence of pain at diagnosis was not correlated with higher probability of AEs (P = .557). Smoking status had no significant effect on the occurrence of AEs (<30 vs ≥30years, P = .918; <15 vs ≥15years, P = .240).
Figure 1.
(a) Progression free survival (PFS) and (b) overal survival (OS) curves of patients with and without pain at diagnosis.
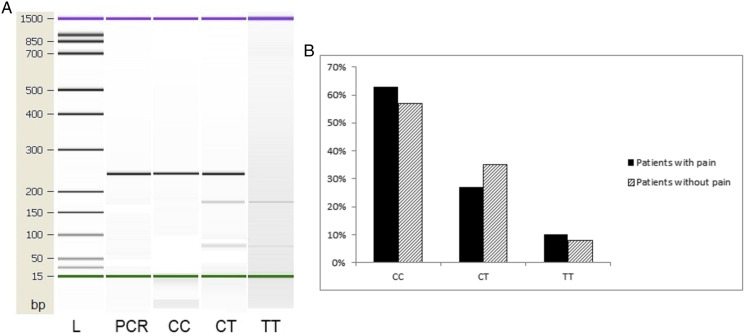
At diagnosis, pain was detected in 41 (41%) patients, and it was significantly correlated with the presence of metastases (P < .001) (Table 1). Upon analysis of the presence of various metastatic sites, it was found that pain was significantly more common in patients with bone and adrenal gland metastases (Table 2). EGFR mutation type was not associated with the presence of pain at baseline when most common EGFR mutations (exon 19 deletion, L858 R point mutation in exon 21) and rare mutations (L861Q, G719X, exon 20 insertion, S768I, double mutants) were compared (P = .351). The frequency of the EGFR 181946T allele was .25 in the whole group, that is, .23 in the group of patients that exhibited pain at diagnosis and .26 in the group without pain (Figure 2). The EGFR 181946 T allele was not associated with the presence of pain at baseline in both the dominant (CC vs CT/TT) [P = .657; OR (95%CI) = 1.31 (.58-2.98)], and the recessive models (CC/CT vs TT) [P = .846; OR (95%CI) = .87 (.21-3.47)].
Table 1.
The Presence of Pain in EGFR Mutated Patients at Diagnosis.
| Characteristics at Diagnosis | No Pain N (%) | With Pain N (%) | P value | |
|---|---|---|---|---|
| Age (mean) | 63 | 60 | .109 | |
| Gender | Male | 18 (30) | 17 (41.5) | .234 |
| Female | 42 (70) | 24 (58.5) | ||
| Performance status (PS) | ECOG 0 | 5 (8.3) | 0 (0) | .080 |
| ECOG 1 | 48 (80) | 33 (80.5) | ||
| ECOG 2 | 4 (6.7) | 5 (12.2) | ||
| ECOG 3 | 2 (3.3) | 3 (7.3) | ||
| ECOG 4 | 1 (1.7) | 0 (0) | ||
| Smoking status | Non-smoker | 30 (53.6) | 15 (39.5) | .179 |
| Smoker | 26 (46.4) | 23 (60.5) | ||
| TNM staging | IIIB | 8 (13.3) | 2 (4.9) | .195 |
| IV | 52 (86.7) | 39 (95.1) | ||
| Presence of metastases | Without mets | 8 (13.3) | 2 (4.9) | <.001 |
| M1a | 29 (48.3) | 9 (22.0) | ||
| M1b | 14 (23.3) | 14 (34.1) | ||
| M1c | 9 (15) | 16 (39) |
M1a - tumor in contralateral lung or pleural/pericardial nodule/malignant effusion. M1b – single extrathoracic metastasis including single non-regional lymph node, M1c – multiple extrathoracic metastases in one or more organs. Statistically significant values are presented in bold.
Table 2.
Localisation of metastases associated with the presence of pain at baseline.
| Metastatic Site | No Pain N (%) | With Pain N (%) | P value | |
|---|---|---|---|---|
| Liver | No liver mets | 46 (76.7) | 35 (85.4) | .281 |
| Liver mets | 14 (23.3) | 6 (14.6) | ||
| Bone | No bone mets | 55 (91.7) | 19 (46.3) | <.001 |
| Bone mets | 5 (8.3) | 22 (53.7) | ||
| Non-regional lymph nodes (ln) | No ln mets | 58 (96.7) | 38 (92.7) | .393 |
| Ln mets | 2 (3.3) | 3 (7.3) | ||
| Pericard | No pericard mets | 53 (88.3) | 36 (87.8) | 1.000 |
| Pericard mets | 7 (11.7) | 5 (12.2) | ||
| Brain | No brain mets | 53 (88.3) | 38 (92.7) | .736 |
| Brain mets | 7 (11.7) | 3 (7.3) | ||
| Lung | No lung mets | 34 (56.7) | 24 (58.5) | .852 |
| Lung mets | 26 (43.3) | 17 (41.5) | ||
| Adrenal gland | No adr.gl.mets | 58 (96.7) | 34 (82.9) | .029 |
| adr.gl. Mets | 2 (3.3) | 7 (17.1) | ||
| Malignant pleural effusion | No pleural eff | 33 (55) | 21 (51.2) | .708 |
| Pleural eff | 27 (45) | 20 (48.8) |
Statistically significant values are presented in bold.
Figure 2.
(a) PCR and RFLP results and (b) genotype distribution of EGFR 181946 CT polymorphic variants in patients with and without pain at diagnosis. Column 1: 244 bp PCR product. Column 2: CC (wild type), Column 3: CT (heterozygot, Column 4: TT (recessive homozygote). L – High-sensitivity DNA ladder (Agilent Technologies). 1500 bp upper and 15 bp lower marker are present in each column.
Patients with pain at baseline were treated with analgesics (and radiation in 21 patients with bone metastases) (Supplemental Table 1 and 2) and good pain management was achieved. At the second time-point of official recording of pain level (3 months after the initiation of TKI treatment and the first CT evaluation of its efficacy), 20% of patients manifested pain which was statistically lower compared to baseline (P = .011), and there was a statistically significant improvement of the pain level (P < .001) (Table 3).
Table 3.
Pain intensity according to the Numerical Rating Scale (NRS) and the Verbal Descriptor Scale (VDS).
| Pain Intensity | Baseline (N, %) | After 3 months (N, %) |
|---|---|---|
| 1–3 (NRS) or mild (VDS) | 13 (31.7) | 17 (65.4) |
| 4–7 (NRS) or moderate (VDS) | 22 (53.7) | 7 (26.9) |
| 8–10 (NRS) or severe (VDS) | 6 (14.6) | 2 (7.7) |
| Total | 41 | 26 |
Of the 41 patients that presented with pain at diagnosis, 20 patients had a worsening of pain after having achieved good pain control initially, probably due to disease progression (14 cases of progression of the primary lesion in the first three months). Additional 6 patients who did not present with pain at diagnosis developed pain, again mostly due to disease progression despite TKI treatment. The remaining 21 patients achieved optimal pain control throughout the treatment course, although 17 of them eventually progressed (6 cases of a new lesion and 11 cases of progression of the primary lesion). There was no significant difference in mPFS of patients who had a worsening of pain after having achieved good pain control initially and those who achieved optimal pain control throughout the treatment course (P = .779).
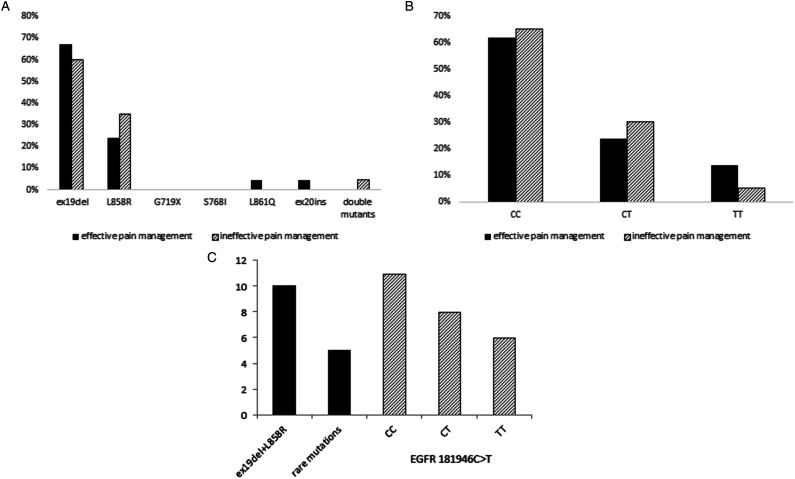
EGFR mutation subtype (Figure 3A) and the type of the EGFR 181946 CT polymorphic variant (Figure 3B) did not have a significantly impact on pain management (P = .837), but had a significant effect on response to TKI treatment (Figure 3C). Patients with most frequent mutations (ex19del and L858 R) had a median PFS of 10 months, while patients with rare mutations (L861Q, G719X, exon 20 insertion, S768I, and double mutants) had a median PFS of 5months (P = .016). Similarly, the presence of the EGFR 181946 T allele influenced the response to TKIs, as carriers of the wild type CC genotype had a median PFS of 11months, CT carriers of 8 months, and teratesla carriers of 6months.
Figure 3.
The effect of (a) EGFR mutation types and (b) EGFR 181946 CT polymorphic variants on pain management and (c) response to TKI treatment.
Discussion
Underlying genetic variations in the EGFR gene (mutations and small nucletide polymorphisms) are known to alter protein function and affect the biology of the tumor, inducing a different symptom presentation in patients and altering the therapeutic efficacy of EGFR-TKIs.30-32 We analyzed the effect of the most common EGFR mutations in exons 18-21 and the polymorphism rs2293347 (181946G>A) on pain occurrence and combined analgesic/TKI treatment efficacy. EGFR mutation subtype and the type of the EGFR 181946 CT polymorphic variant did not have a direct impact on pain occurrence and management, but had a significant effect on response to TKI indirectly affecting patients’ symptoms. Thus, the presence of specific EGFR genetic changes induces a different response to TKIs which ultimately led to a decrease in the disease burden and general pain alleviation during treatment.
Cancer-related pain is very common in lung cancer patients as majority of cases are diagnosed in advanced stages especially in countries like Serbia without appropriate lung screening programs and smoking cessation measures. As early palliative care and symptom management have proven effects on the improvement of the quality of life and longer survival they are an essential part of lung cancer patients’ care. 33 Although other countries have performed multicenter prospective studies, 34 no detailed studies that evaluate the factors associated with pain in EGFR mutated advanced lung adenocarcinoma patients treated with EGFR-TKIs have been performed in our region so far.
Most commonly described causes of pain in patients with advanced LC are related to skeletal and adrenal metastases and pancoast tumors. 35 In our study, the presence of pain at diagnosis was significantly correlated with the presence of metastases, especially in the bones and adrenal glands, which is in accordance with previously published data.10,36 The World Health Organization (WHO) recommends use of Three-Step Analgesic Ladder model for adequate pain management in cancer patients. 37 Paracetamol is the mainstay of the first two steps of the WHO Analgesic Ladder in many countries as well as in our study. 38 Taking into account the well-known side effects of long-term use of NSAIDs they were not used regularly in our study except as a single intermittent therapy or in combination with paracetamol for mild pain relief. Since some authors suggested replacing weak opioids with low doses of oral morphine thereby eliminating step 2 of the Ladder, the cornerstone of analgesic therapy in our study was oral morphine. 39 Nevertheless, 10% of our patients had adequate pain relief control with tramadol as a mild opioid of step 2 in maximum used dose 200 mg per day. Oxycodone or hydromorphone, in both immediate-release and slow-release formulations for oral administration are effective alternatives to oral morphine.40,41 In this study, oral methadone was used only in a small set of patients (2%) with severe pain resistant to previous pharmacotherapy. Transdermal fentanyl was used as an efficient option for patients with stable opioid requirements, as well as for patients that had problems with oral administration of drugs or patients. Adjuvant analgesics that were used in this study were pregabaline, gabapentin, and dexamethasone. Overall, good pain management was achieved, with a significant decrease of patients who still had pain during treatment, and with a statistically lower pain level. In patients who developed a new lesion or had progression of the primary tumor during TKI treatment, we also observed worsening of pain despite having previously achieved good pain control.
Radiotherapy has an important role in bone pain management, treatment of metastatic spinal cord compression and palliative treatment of pathological bone fractures that cannot be surgically stabilized.11,42 Various palliative radiotherapy regimens are recommended for this indication, but an 8 Gy single dose should be considered as a regimen of choice for patients with painful bone metastases according to newest ESMO Clinical Practice Guideline for management of pain in adult cancer patients. 38 In this study, the most commonly used regimens for palliative antidolorous radiation therapy were short-course 8 Gy single fraction as well as two long-course regimens including 20 Gy in 5 fractions and 30 Gy in 10 fractions. 27 Although the presence of painful bone metastases is usually associated with shorter survival, 43 in our study no difference in PFS or OS was observed in patients who presented with bone metastases-related pain at diagnosis.
Conclusions: EGFR mutation subtype and the EGFR 181946 C>T SNP had a significant effect on the response to TKI inducing an indirect anti-dolorous effect and might be useful for accessible, low-cost prediction of treatment outcome in advanced NSCLC, especially in low-income countries.
Supplemental Material
Supplemental Material for Evaluation of Clinical and Genetic Determinants of Treatment OutCome In EGFR Mutation Positive Advanced Lung Adenocarcinoma by Vera Jokic, Katarina Savic-Vujovic, Jelena Spasic, Zoran Bukumiric, Mladen Marinkovic, Davorin Radosavljevic, and Milena Cavic in Dose-Response
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant agreement No. 451-03-68/2022-14/200043) and the LungCARD - MSCA-RISE (Horizon 2020 - Research and Innovation Framework 337 Programme, European Commision, Grant agreement No. 734790). JS and MC are supported by the Science Fund of the Republic of Serbia (PROMIS TRACEPIGEN Project No. 6060876).
Ethics Approval: The procedures used in this study were approved by the Ethics Committee of the Institute for Oncology and Radiology of Serbia and were in accordance with the Helsinki Declaration of 1964 and its later amendments or comparable ethical standards. All patients signed an informed consent.
Data Availability: Data The data that support the findings of this study are not publicly available due to ethics restrictions, and can be obtained from the corresponding author upon reasonable request.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Katarina Savic-Vujovic https://orcid.org/0000-0002-4701-6291
Jelena Spasic https://orcid.org/0000-0002-1386-9114
Milena Cavic https://orcid.org/0000-0002-7604-9295
References
- 1.Kerpel-Fronius A, Tammemägi M, Cavic M, et al. Screening for lung cancer in individuals who never smoked: an international association for the study of lung cancer early detection and screening committee report. J Thorac Oncol. 2022;17(1):56-66. doi: 10.1016/j.jtho.2021.07.031 [DOI] [PubMed] [Google Scholar]
- 2.Kerpel-Fronius A, Tammemägi MC, Tammemägi MC, et al. Lung cancer screening in persons who never smoked has to be evaluated—a response to letter to the editor. J Thorac Oncol. 2022;17(2):e20-e21. doi: 10.1016/J.JTHO.2021.12.009 [DOI] [PubMed] [Google Scholar]
- 3.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(suppl 4):iv192-iv237. doi: 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 4.Huber RM, Cavic M, Kerpel-Fronius A, et al. Lung cancer screening considerations during respiratory infection outbreaks, epidemics or pandemics: an international association for the study of lung cancer early detection and screening committee report. J Thorac Oncol. 2022;17(2):228-238. doi: 10.1016/J.JTHO.2021.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavic M, Krivokuca A, Boljevic I, et al. Exploring the real-world effect of the SARS-CoV-2 pandemic on the molecular diagnostics for cancer patients and high-risk individuals. Expert Rev Mol Diagn. 2021;21(1):101-107. doi: 10.1080/14737159.2021.1860760 [DOI] [PubMed] [Google Scholar]
- 6.Simmons CPL, Macleod N, Laird BJA. Clinical management of pain in advanced lung cancer. Clin Med Insights Oncol. 2012;6:331-346. doi: 10.4137/CMO.S8360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swarm RA, Paice JA, Anghelescu DL, et al. Adult cancer pain, version 3.2019, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17(8):977-1007. doi: 10.6004/jnccn.2019.0038 [DOI] [PubMed] [Google Scholar]
- 8.Dans M, Smith T, Back A, et al. NCCN guidelines insights: palliative care, version 2.2017. J Natl Compr Cancer Netw. 2017;15(8):989-997. doi: 10.6004/jnccn.2017.0132 [DOI] [PubMed] [Google Scholar]
- 9.Cavic M, Kovacevic T, Zaric B, et al. Lung cancer in Serbia. J Thorac Oncol. 2022;17:867-872. Published online 2022. [DOI] [PubMed] [Google Scholar]
- 10.Kuchuk M, Kuchuk I, Sabri E, et al. The incidence and clinical impact of bone metastases in non-small cell lung cancer. Lung Cancer. 2015;89(2):197-202. doi: 10.1016/j.lungcan.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 11.De Felice F, Piccioli A, Musio D, Tombolini V. The role of radiation therapy in bone metastases management. Oncotarget. 2017;8(15):25691-25699. doi: 10.18632/oncotarget.14823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz S. The role of radiation therapy in controlling painful bone metastases. Curr Pain Headache Rep. 2012;16(4):300-306. doi: 10.1007/s11916-012-0271-1 [DOI] [PubMed] [Google Scholar]
- 13.World Cancer Research Fund . Lung cancer statistics. 2018. https://www.wcrf.org/dietandcancer/cancer-trends/lung-cancer-statistics. https://www.wcrf.org/dietandcancer/cancer-trends/lung-cancer-statistics Accessed on March 3, 2018
- 14.Serbia M, of H of the R of . National Guidelines of Good Clinical Practice for the Diagnostics and Treatment of Lung Cancer 2012; 2012. http://www.onk.ns.ac.rs/pdf/VodicZaDijagnostikovanjeILlecenjeKarcinomaPluca.pdf Accessed April 14, 2022. [Google Scholar]
- 15.Cavic M, Krivokuca A, Spasic J, et al. The influence of methylenetetrahydrofolate reductase and thymidylate synthetase gene polymorphisms on lung adenocarcinoma occurrence. J BUON. 2014;19(4):1024-1028. https://www.jbuon.com/archive/19-4-1024.pdf [PubMed] [Google Scholar]
- 16.Cavic M, Spasic J, Krivokuca A, et al. TP53 and DNA-repair gene polymorphisms genotyping as a low-cost lung adenocarcinoma screening tool. J Clin Pathol. 2019;72(1):75-80. doi: 10.1136/jclinpath-2018-205553 [DOI] [PubMed] [Google Scholar]
- 17.Velinovic M, Jankovic R, Jovanovic D, et al. Tumor characteristics, expressions of ERCC1, Bax, p53, IGF1R, Bcl2, Bcl2/Bax and prognostic factors for overall survival in patients with lung carcinoid. J BUON. 2019;24(1):256-266. [PubMed] [Google Scholar]
- 18.Jokic V, Savic-Vujovic K, Spasic J, et al. Hematological parameters in EGFR-mutated advanced NSCLC patients treated with TKIs: predicting survival and toxicity. Expert Rev Anticancer Ther. 2021;21(6):673-679. doi: 10.1080/14737140.2021.1893694 [DOI] [PubMed] [Google Scholar]
- 19.Cavic M, Krivokuca A, Boljevic I, et al. Pharmacogenetics in cancer therapy - 8 years of experience at the Institute for Oncology and Radiology of Serbia. J BUON. 2016;21(5):1287-1295. [PubMed] [Google Scholar]
- 20.Cavic M, Krivokuca A, Pavlovic M, et al. EGFR mutation testing from liquid biopsy of non-small cell lung cancer at the Institute for oncology and radiology of Serbia. J BUON. 2020;25(6):2635-2648. https://jbuon.com/archive/25-6-2635.pdf [PubMed] [Google Scholar]
- 21.Jankovic R, J Goncalves H, Cavic M, et al. LungCARD - Report on worldwide research and clinical practices related to lung cancer. J BUON. 2019;24(1):11-19. [PubMed] [Google Scholar]
- 22.Cavic M, Krivokuca A, Jankovic R, Radulovic S. Personalized therapy of malignant diseases. Pirot Zb Arch. 2015;40:69-79. doi: 10.5937/pirotzbor1540069C [DOI] [Google Scholar]
- 23.Jiang T, Su C, Ren S, et al. A consensus on the role of osimertinib in non-small cell lung cancer from the AME lung cancer collaborative group. J Thorac Dis. 2018;10(7):3909-3921. doi: 10.21037/jtd.2018.07.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193-203. doi: 10.1016/j.chest.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 25.Karcioglu O, Topacoglu H, Dikme O, Dikme O. A systematic review of the pain scales in adults: which to use? Am J Emerg Med. 2018;36(4):707-714. doi: 10.1016/j.ajem.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 26.Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888-896. doi: 10.1002/cncr.27616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow R, Hoskin P, Hollenberg D, et al. Efficacy of single fraction conventional radiation therapy for painful uncomplicated bone metastases: a systematic review and meta-analysis. Ann Palliat Med. 2017;6(2):125-142. doi: 10.21037/apm.2016.12.04 [DOI] [PubMed] [Google Scholar]
- 28.Choi JE, Park SH, Kim KM, et al. Polymorphisms in the epidermal growth factor receptor gene and the risk of primary lung cancer: a case-control study. BMC Cancer. 2007;7:199. doi: 10.1186/1471-2407-7-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gahr S, Stoehr R, Geissinger E, et al. EGFR mutational status in a large series of Caucasian European NSCLC patients: data from daily practice. Br J Cancer. 2013;109(7):1821-1828. doi: 10.1038/bjc.2013.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellanos E, Feld E, Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12(4):612-623. doi: 10.1016/j.jtho.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 31.Winther-Larsen A, Nissen PH, Jakobsen KR, et al. Genetic polymorphism in the epidermal growth factor receptor gene predicts outcome in advanced non-small cell lung cancer patients treated with erlotinib. Lung Cancer. 2015;90(2):314-320. doi: 10.1016/j.lungcan.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 32.Ma F, Sun T, Shi Y, et al. Polymorphisms of EGFR predict clinical outcome in advanced non-small-cell lung cancer patients treated with Gefitinib. Lung Cancer. 2009;66(1):114-119. doi: 10.1016/j.lungcan.2008.12.025 [DOI] [PubMed] [Google Scholar]
- 33.Irwin KE, Greer JA, Khatib J, Temel JS, Pirl WF. Early palliative care and metastatic non-small cell lung cancer: potential mechanisms of prolonged survival. Chron Respir Dis. 2013;10(1):35-47. doi: 10.1177/1479972312471549 [DOI] [PubMed] [Google Scholar]
- 34.Wei Y-F, Huang W-T, Liu T-C, et al. Factors associated with improvement in symptoms and quality of life for first-line EGFR-tyrosine kinase inhibitor treatment in patients with EGFR-mutated non-small-cell lung cancer - A multicenter prospective SMILE study. J Cancer. 2019;10(17):4151-4158. doi: 10.7150/jca.30507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marulli G, Battistella L, Mammana M, Calabrese F, Rea F. Superior sulcus tumors (Pancoast tumors). Ann Transl Med. 2016;4(12):239. doi: 10.21037/atm.2016.06.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bazhenova L, Newton P, Mason J, et al. Adrenal metastases in lung cancer: clinical implications of a mathematical model. J Thorac Oncol. 2014;9(4):442-446. doi: 10.1097/JTO.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . Cancer Pain Relief with a Guide to Opioid Availability. Second Edition; 1996. https://apps.who.int/medicinedocs/documents/s22085en/s22085en.pdf [Google Scholar]
- 38.Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(suppl 4):iv166-iv191. doi: 10.1093/annonc/mdy152 [DOI] [PubMed] [Google Scholar]
- 39.Bandieri E, Romero M, Ripamonti CI, et al. Randomized trial of low-dose morphine versus weak opioids in moderate cancer Pain. J Clin Oncol. 2016;34(5):436-442. doi: 10.1200/JCO.2015.61.0733 [DOI] [PubMed] [Google Scholar]
- 40.Pigni A, Brunelli C, Caraceni A. The role of hydromorphone in cancer pain treatment: a systematic review. Palliat Med. 2011;25(5):471-477. doi: 10.1177/0269216310387962 [DOI] [PubMed] [Google Scholar]
- 41.King SJ, Reid C, Forbes K, Hanks G. A systematic review of oxycodone in the management of cancer pain. Palliat Med. 2011;25(5):454-470. doi: 10.1177/0269216311401948 [DOI] [PubMed] [Google Scholar]
- 42.Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7(1):4-12. doi: 10.1016/j.prro.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 43.D’Antonio C, Passaro A, Gori B, et al. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol. 2014;6(3):101-114. doi: 10.1177/1758834014521110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Evaluation of Clinical and Genetic Determinants of Treatment OutCome In EGFR Mutation Positive Advanced Lung Adenocarcinoma by Vera Jokic, Katarina Savic-Vujovic, Jelena Spasic, Zoran Bukumiric, Mladen Marinkovic, Davorin Radosavljevic, and Milena Cavic in Dose-Response