Abstract
Background
Inflammatory dental diseases that occur during pregnancy can cause preterm labor and/or intrauterine growth restriction. Therefore, proactive treatment of dental diseases is necessary during pregnancy. Dexmedetomidine (DEX) is a widely used sedative in the dental field, but research on the effect of DEX on pregnancy is currently insufficient. In this study, we investigated the effects of co-treatment with DEX and lipopolysaccharide (LPS) on inflammatory responses in human amnion-derived WISH cells.
Methods
Human amnion-derived WISH cells were treated with 0.001, 0.01, 0.1, and 1 µg/mL DEX with 1 µg/mL LPS for 24 h. Cytotoxicity of WISH cells was evaluated by 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) assay. The protein expression of cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), p38, and nuclear factor kappa B (NF-κB) was examined by western blot analysis. The mRNA expression of pro-inflammatory cytokines such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α was analyzed by real-time quantitative polymerase chain reaction.
Results
Co-treatment with DEX and LPS showed no cytotoxicity in the WISH cells. The mRNA expression of IL-1β and TNF-α decreased after co-treatment with DEX and LPS. DEX and LPS co-treatment decreased the protein expression of COX-2, PGE2, phospho-p38, and phospho-NF-κB in WISH cells.
Conclusion
Co-treatment with DEX and LPS suppressed the expression of COX-2 and PGE2, as well as pro-inflammatory cytokines such as IL-1β and TNF-α in WISH cells. In addition, the anti-inflammatory effect of DEX and LPS co-treatment was mediated by the inhibition of p38/NF-κB activation.
Keywords: Cyclooxygenase 2 Inhibitors, Dexmedetomidine, Lipopolysaccharides, Prostaglandin E2, WISH Cells
INTRODUCTION
Various physical and hormonal changes occur in pregnant women, which can cause dental diseases such as gingivitis, benign gingival lesions, tooth erosion, periodontitis, and dental caries. Morning sickness in the first trimester of pregnancy increases dental caries as the pH in the mouth becomes more acidic [1]. In particular, progesterone has an affinity for the fundamental constituent of the connective tissue that makes up the gingiva and increases the osmotic pressure of capillaries distributed in the gingiva, which makes the gingiva more vulnerable to inflammation [2]. In addition, physical fatigue and emotional sensitivity in pregnant women can easily lead to neglect of oral hygiene, which also leads to an increased risk of dental disease [3]. Some studies have reported that maternal periapical infections and untreated dental caries can cause preterm birth and/or intrauterine growth restriction (IUGR) [4,5]. Therefore, it is necessary to actively treat dental diseases in pregnant women.
Preterm labor is caused by various pathological conditions including intrauterine infection, uterine ischemia, uterine overdistention, abnormal allergic reactions, cervical disease, and endocrine disorders [6]. Among them, intrauterine infection and/or inflammation are known to be the main risk factors related to spontaneous preterm labor, accounting for approximately 40% of cases [7,8]. In these vulnerable states, the human amniotic epithelial cells are easily activated by external stimuli, such as hormones, mechanical traction, and lipopolysaccharide (LPS), and subsequently secrete pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [9,10]. These pro-inflammatory cytokines increase the production of prostaglandins (PG) through various pathways and stimulate cyclooxygenase-2 (COX-2), which is indispensable for PG synthesis [6,11]. To maintain a normal pregnancy, suppression of the inflammatory response continues during the pregnancy period, and spontaneous delivery occurs as the increase of PG, the main inflammatory substance [12]. Bacterial endotoxins and various inflammatory stimuli induce COX-2 activation, resulting in increased synthesis of PGs leading to uterine contraction and cervical ripening [6,13]. Among PGs, prostaglandin E2 (PGE2) has been found to be the main prostanoid produced by the amnion [6,14].
Even in pregnant women, dental surgery may be require sedation or general anesthesia when abscesses (due to increased inflammation), fractures (due to trauma), and oral cancer occur. Dexmedetomidine (DEX), a selective α-2 agonist, is a preferred sedative in the dental field because of its low respiratory depression [15,16]. In pregnant women, DEX can be used as an analgesic during labor when epidural or spinal anesthesia is rejected or to complement inadequate epidural analgesia [17]. Some studies have investigated the effects of DEX on uterine contractions. Karamon et al. [18] demonstrated that DEX increased spontaneous contractions in the gravid rat myometrium. Sia et al. [19] reported that DEX increased uterine contractility at plasma concentrations of 1 × 10-9 g/mL in the pregnant human myometrium. However, research on the effect of DEX on uterine contractions, especially on the expression of inflammatory substances that can cause uterine contractions is still lacking.
In this study, we explored the effect of DEX and LPS co-treatment on the production of inflammatory substances using human amnion-derived WISH cells, which are a good model for the analysis of PGE2 release induced by various agents [14]. In addition, we investigated the signaling pathways associated with the effect of DEX and LPS co-treatment on the inflammatory response in WISH cells.
METHODS
1. Cell culture
Amnion-derived WISH cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle medium (ATCC) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37℃ in an atmosphere of 5% CO2 in 95% air. The cells were passaged once every three days.
2. LPS stimulation and DEX treatment
A commercially available formulation of dexmedetomidine hydrochloride (KyongBo Pharm, Chungnam, Korea) was used in this study. The formulation was diluted with the culture medium and cells were co-treated with DEX at concentrations of 0.001, 0.01, 0.1, and 1 µg/mL with 1 µg/mL LPS (Sigma, St. Louis, MO, USA) for 24 h (Table 1).
Table 1. The experimental groups in this study.
| Experimental groups | |||||
|---|---|---|---|---|---|
| Control | LPS (1 μg/mL) |
LPS + DEX (0.001 μg/mL) |
LPS + DEX (0.01 μg/mL) |
LPS + DEX (0.1 μg/mL) |
LPS + DEX (1 μg/mL) |
DEX, dexmedetomidine; LPS, lipopolysaccharide.
3. 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) assay
WISH cells (1 × 105 cells/well) were seeded into 24-well plates and cultured for 24 h at 37℃ in an incubator with an atmosphere of 5% CO2. The cells were subsequently exposed to 1 µg/mL LPS and/or DEX at concentrations ranging from 0.001–1 µg/mL for 24 h. Following drug treatment, MTT (Affymetrix Inc. USB, Cleveland, OH, USA) assay was performed by addition of 100 µL MTT solution (5 mg/mL in phosphate buffered saline at pH 7.4) to each well and incubation of the plate at 37℃. The medium was removed after 1 h and 100 µL of dimethyl sulfoxide (DMSO; Biosesang, Seongnam, Korea) was added to each well. The plate was gently rotated on an orbital shaker for 15 min to dissolve the precipitate. Absorbance was measured at 540 nm using a microplate reader (Bio-Rad Model 680; Bio-Rad, Hercules, CA, USA). All experiments were performed at least three times.
4. Quantitative real-time polymerase chain reaction (real-time qPCR)
WISH cells were seeded in 12-well cell culture plates at a density of 5 × 105 cells/well. The cells were treated with LPS alone or with DEX for 24 h. Following drug treatment, total ribonucleic acid (RNA) was isolated using 500 µL TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). One microgram of total messenger RNA (mRNA) per sample was reverse transcribed into cDNA using an oligo (dT) PrimeScript™ 1st strand cDNA Synthesis Kit (TaKaRa Clontech, BD Biosciences, Palo Alto, CA, USA) according to the manufacturer’s instructions. RT-PCR was performed using a SimpliAmp™ thermal cycler (Applied Biosystems, Life Science Technologies, CA, USA). The primers used for PCR were as follows: IL-1β, 5′-CTCGCCAGTGAAATGATGGCT-3′ (forward) and 5′-GTCGGAGATTCGTAGCTGGAT-3′ (reverse); TNF-α, 5′-CCAGGCAGTCAGATCATCTTC-3′ (forward) and 5′-GTTATCTCTCAGCTCCACGC-3′ (reverse); β-actin, 5′-GACCTGACTGACTACCTCATG-3′ (forward) and 5′-CGCTCATTGCCAATGGTGATG-3′ (reverse). The mRNA expression levels were normalized to that of β-actin. IL-1β/β-actin was amplified using 35 cycles of PCR at 95℃ for 30 s, 55℃ for 30 s, and 72℃ for 30 s, and a final extension was performed at 72℃ for 7 min. TNF-α/β-actin was amplified using 35 cycles of PCR at 94℃ for 30 s, 54℃ for 30 s, and 72℃ for 30 s, with a final extension step at 72℃ for 10 min. Real-time qPCR was performed in triplicate. The PCR products were separated on a 1.5% agarose gel. They were assayed using the Gel Doc ImageQuant LAS 500 System (GE Healthcare Biosciences AB, Uppsala, Sweden). The data were analyzed using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
5. Western blot analysis
Proteins were isolated from cells using chilled RIPA buffer (50 mM Tris at pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.5% NP40, 5 mM DTT, 0.2 mM sodium orthovanadate, 100 mM NaF, and 1 mM PMSF) containing 1× protease inhibitor/phosphatase inhibitor cocktail (Cell Signaling Technology). Proteins (25 µg/well) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (GE Healthcare, Chicago, IL, USA). Membranes were blocked with TBS-0.1% Tween-20 (TBST) containing 3% skim milk for 1 h. The membranes were subsequently incubated overnight withα-tubulin (1: 1000; Santa Cruz, CA, USA), PGE synthase 2 (A-2) (1: 1000; Cell Signaling Technology), COX-2 (D5H5) rabbit mAb (1: 1000; Santa Cruz), p38 MAPK (1: 1000; Cell Signaling Technology), phospho-p38 MAP kinase (1: 500; Cell Signaling Technology), NF-κB p65 (1: 1000; Santa Cruz), and phospho-NF-κB p65 27. Ser 536 (1: 500; Santa Cruz) antibodies in TBST with 3% skim milk at 4℃. After washing three times with TBST, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit (1: 1000; Enzo Life Sciences, Plymouth Meeting, PA, USA) or anti-mouse (1: 1000; Santa Cruz Biotechnology) antibodies for 1 h at room temperature. The membranes were subsequently washed three times with TBST, and the protein bands were visualized using enhanced chemiluminescence (ECL) detection reagents (Promega, Madison, WI, USA). Protein expression levels were normalized to those of α-tubulin. The target protein bands were normalized relative to the control band using the ImageJ software.
6. Statistical analyses
Data are presented as mean ± standard deviation (SD). All experiments were repeated at least three times. Statistical analyses were performed using SigmaPlot v10 software. Statistically significance was set at P < 0.05.
RESULTS
1. Cytotoxicity of DEX and LPS co-treatment in WISH cells
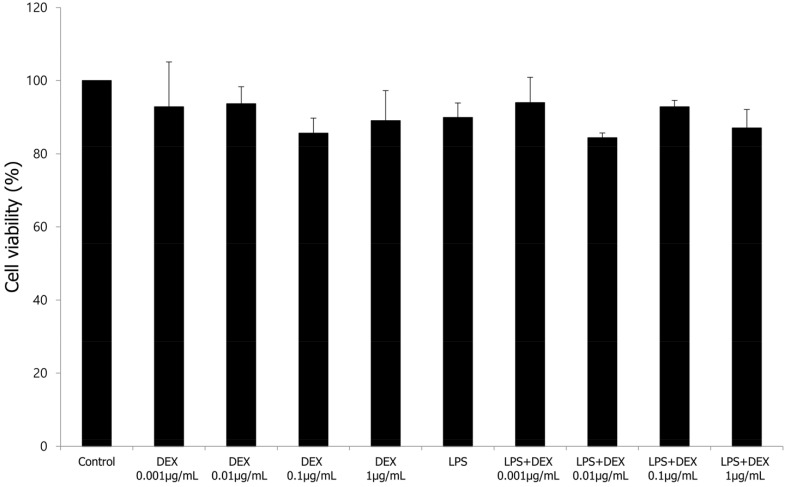
We performed an MTT assay to examine the effects of LPS, DEX, and DEX + LPS co-treatment on the cytotoxicity of WISH cells. WISH cells were treated with 0.001, 0.01, 0.1, and 1 µg/mL DEX, and 1 µg/mL LPS or with DEX and LPS co-treatment. In our study, LPS and DEX showed no cytotoxicity in the WISH cells. In addition, there was no difference in the cell viability following co-treatment with various concentrations of DEX and LPS (Fig. 1).
Fig. 1. Effect of treatment with LPS or co-treatment with DEX and LPS on cytotoxicity in WISH cells was measured by MTT assay. Values are presented as mean ± standard deviation (SD). All experiments were repeated three times. DEX, dexmedetomidine; LPS, lipopolysaccharide; MTT, 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide.
2. Effect of DEX and LPS co-treatment on the expression of pro-inflammatory cytokines (IL-1β and TNF-α) in WISH cells
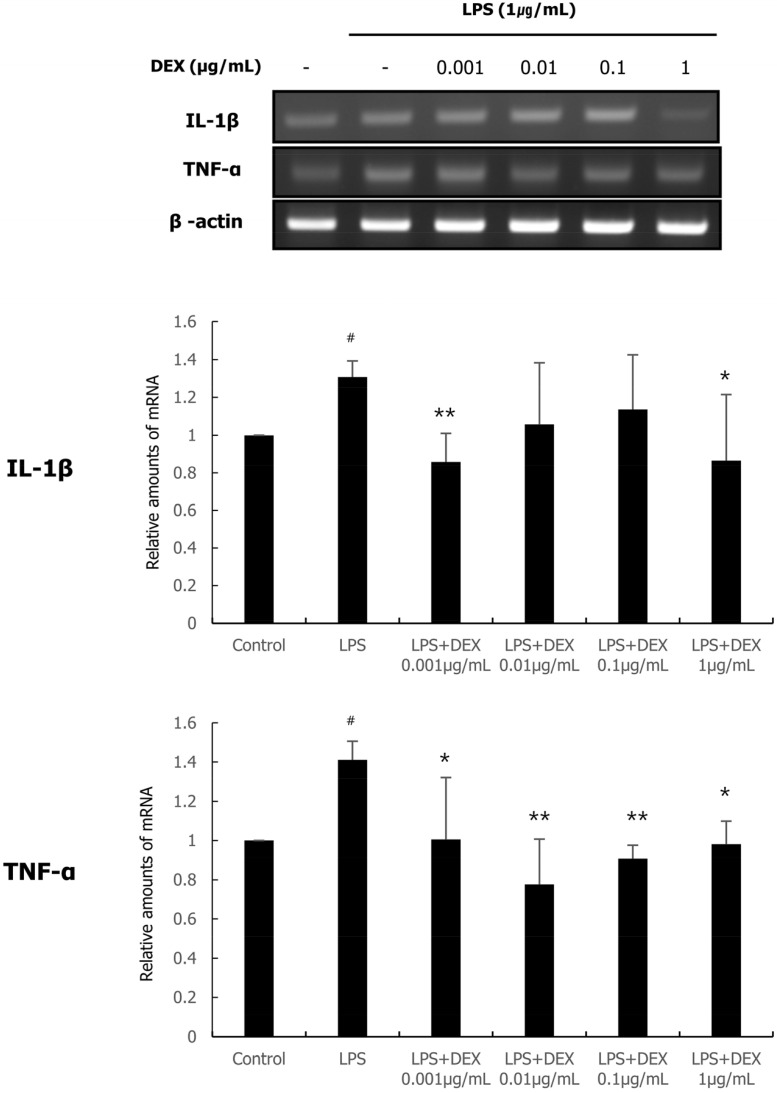
Real-time qPCR was performed to evaluate the effect of DEX and LPS co-treatment on the expression of pro-inflammatory cytokines such as IL-1β and TNF-α. As shown in Fig. 2, treatment with 1 µg/mL LPS significantly increased the mRNA expression of IL-1β and TNF-α compared to those in the control. The mRNA expression of IL-1β was significantly decreased by DEX (0.001 and 1 µg/mL) and LPS co-treatment. The mRNA expression of TNF-α was significantly decreased by DEX and LPS co-treatment at all concentrations compared to that in the LPS treatment group.
Fig. 2. The mRNA expression of IL-1β and TNF-α following DEX and LPS co-treatment of WISH cells was measured using quantitative real-time PCR. Relative mRNA level was normalized to that of β-actin and presented as mean ± standard deviation of three independent experiments. #P < 0.05 versus control group; *P < 0.05, **P < 0.01 versus LPS group. DEX, dexmedetomidine; IL, interleukin; LPS, lipopolysaccharide; mRNA, messenger ribonucleic acid; PCR, polymerase chain reaction; TNF, tumor necrosis factor.
3. Effect of DEX and LPS co-treatment on COX-2 and PGE2 expression in WISH cells
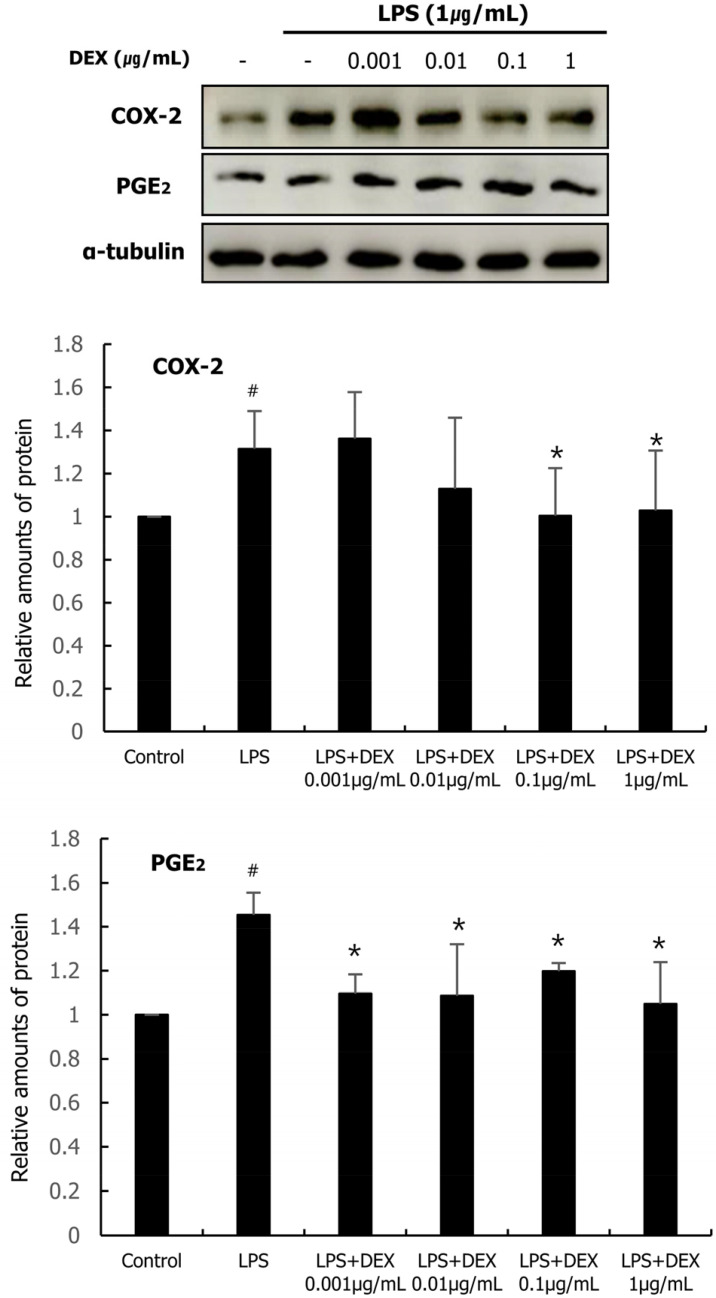
The protein expression levels of COX-2 and PGE2 were analyzed by western blotting. As shown in Fig. 3, LPS treatment increased the protein expression of COX-2 and PGE2 in WISH cells compared to that in the control. DEX (0.1 and 1 µg/mL) and LPS co-treatment significantly decreased the protein expression of COX-2. The protein expression of PGE2 was significantly decreased at all concentrations of DEX compared to that in the LPS treatment group.
Fig. 3. Western blot analysis was conducted to analyze the protein expression of COX-2 and PGE2, in WISH cells were treated with DEX (0.001 – 1 µg/mL) and/or 1 µg/mL LPS. The effect of DEX and LPS co-treatment on the protein expression of COX-2 and PGE2 were evaluated. Relative density analysis was performed using NIH Image program and normalized to that of α-tubulin. Data are presented as mean ± standard deviation of three independent experiments. #P < 0.05 versus control group; *P < 0.05 versus LPS group. COX, cyclooxygenase; DEX, dexmedetomidine; LPS, lipopolysaccharide; PGE, prostaglandin. DEX, dexmedetomidine; LPS, lipopolysaccharide.
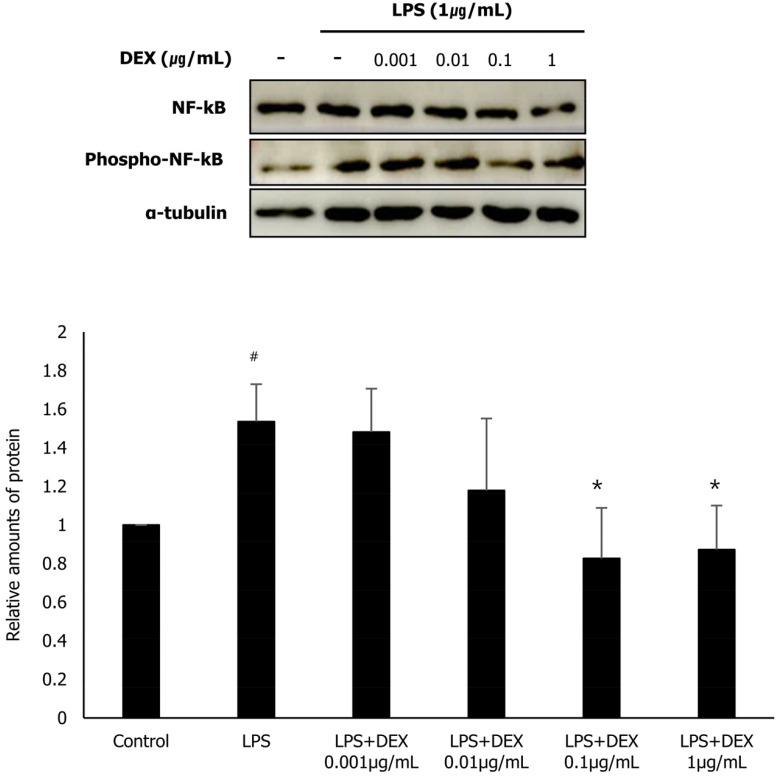
4. Effect of DEX and LPS co-treatment on the phosphorylation of p38 and NF-κB in WISH cells
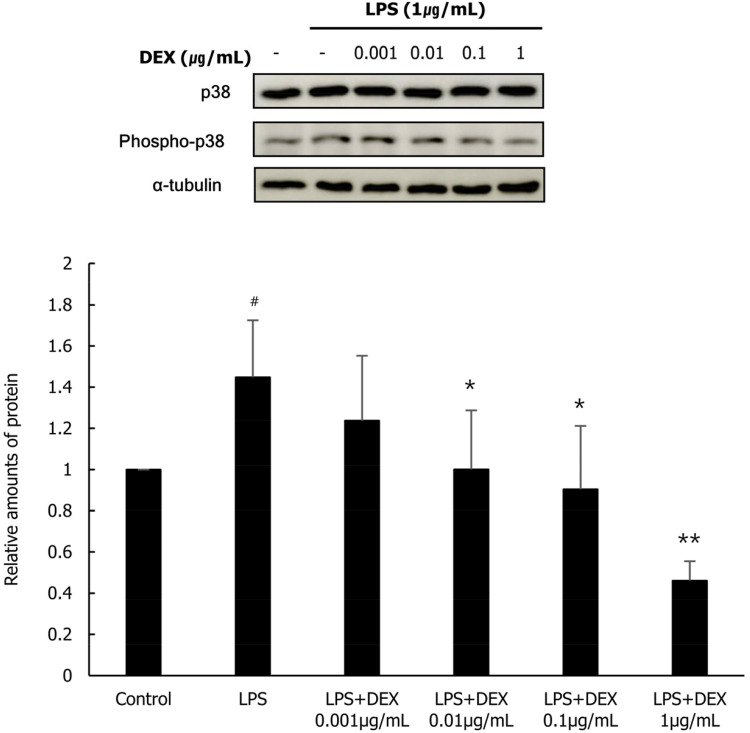
To evaluate the role of MAPK p38 and NF-κB in the inhibition of the expression of inflammatory substances following DEX and LPS co-treatment in WISH cells, we investigated the protein expression of p38 and NF-κB, and their activated forms phospho-p38 and phospho-NF-κB, respectively using western blot analysis. LPS treatment significantly increased the protein expression of phospho-p38 and phospho-NF-κB in WISH cells compared to that in the control. The protein expression of phospho-p38 in the DEX (0.01, 0.1, and 1 µg/mL) and LPS co-treatment group was significantly reduced compared to that in the LPS treatment group (Fig. 4). In addition, the protein expression of phospho-NF-κB was significantly decreased following DEX (0.1, and 1 µg/mL) and LPS co-treatment compared to that in the LPS treatment group (Fig. 5). Our results suggest that the inhibitory effect of DEX and LPS on the expression of inflammatory substances in WISH cells is mediated via the suppression of p38/NF-κB activation.
Fig. 4. Phosphorylation of p38 following co-treatment of WISH cells with DEX and LPS was evaluated using western blot analysis. Relative amounts of phospho-p38 protein were analyzed in triplicate experiments and normalized to that of p38. #P < 0.05 versus control group; *P < 0.05 versus LPS group. DEX, dexmedetomidine; LPS, lipopolysaccharide.
Fig. 5. Phosphorylation of NF-κB following co-treatment of WISH cells with DEX and LPS was evaluated using western blot analysis. Relative amounts of phospho-NF-κB protein were analyzed by triplicate experiments and normalized to that of NF-κB. #P < 0.05 versus control group; *P < 0.05, **P < 0.01 versus LPS group. DEX, dexmedetomidine; LPS, lipopolysaccharide; NF-κB, nuclear factor kappa B.
DISCUSSION
Preterm labor and birth increase neonatal mortality and morbidity in children under the age of five, which not only impacts the family affected but also presents considerable socioeconomic burden [7]. Therefore, studies on the inhibition of inflammatory substances that have the greatest influence on preterm labor are valuable in the medical and social sciences. In this study, we found that co-treatment with DEX and LPS inhibited the expression of inflammatory substances in amnion-derived WISH cells. This result is consistent with that of a previous study that showed that pretreatment with DEX inhibited the LPS-induced increase in inflammatory substances in WISH cells [20]. Based on these findings, DEX inhibits the production of inflammatory substances that increase the risk of preterm labor when used for sedation and general anesthesia during pregnancy.
Our results showed that co-treatment of WISH cells with DEX and LPS resulted in a decrease in the expression of IL-1β and TNF-α compared to treatment with LPS alone. IL-1β is one of the most important members of the IL-1 family and is the first pro-inflammatory cytokine known to be involved in preterm labor [6]. Previous studies have reported that IL-1β induces premature contractility of the myometrium in mice and pregnant rhesus monkeys, and systemic administration of IL-1β significantly increases the production of other pro-inflammatory cytokines including IL-6, TNF-α, and PGs [21,22]. It is also known that IL-1β activates COX-2 [23]. TNF-α is a pro-inflammatory cytokine belonging to the TNF superfamily and is involved in the regulation and stimulation of PG synthesis during preterm labor, like IL-1β [24]. In addition, an increase in TNF-α in the amniotic fluid activates the NF-κB pathway, leading to the amplification of the inflammatory processes related to preterm birth [25]. TNF-α stimulates cervical ripening and uterine contraction [6,26].
Many studies on the anti-inflammatory effects of DEX have recently been reported. Xu et al. [27] showed that DEX treatment decreased the serum concentrations of TNF-α and IL-6 in mice with sepsis. Several recent randomized controlled studies have reported that DEX effectively reduced the level of serum inflammatory factors in patients undergoing intestinal surgery and that of C-reactive protein and procalcitonin, the most common inflammatory biomarkers in patients with sepsis [28,29]. However, because these studies did not examine the production of inflammatory substances in the amniotic membrane, it is difficult to correlate their results to the results of our study. Several studies on the effects of DEX on uterine contractions have reported that DEX increases uterine contractions [30,31]. However, as these studies also did not investigate the effect of DEX on the production of inflammatory substances in the uterus or amniotic membrane, further studies on the effect of DEX on the inflammatory response in the amniotic membrane should be conducted.
The current study demonstrated that the anti-inflammatory effect of DEX and LPS co-treatment on WISH cells was mediated via the suppression of p38/NF-κB activation. NF-κB is a transcription factor that plays an important role in the transcription of pro-inflammatory molecules such as COX-2, IL-1β, and TNF-α [32]. COX-2 is an established NF-κB-dependent gene, and PGs produced by COX-2 induce cervical ripening, and its increase in activated amnion is known to play a critical role in labor initiation [33]. p38 MAPK is a family of serine/threonine kinases related to pro-inflammatory signaling cascades in several cell types [34]. p38 MAPK signaling regulates the transcriptional activity of NF-κB. Previous studies have reported that inhibition of p38 with potent and specific inhibitors leads to the attenuation of the transcriptional activity of NF-κB in various cell types [35,36]. Our results suggest that co-treatment of WISH cells with DEX and LPS suppresses the transcriptional activity of NF-κB through inhibition of p38 activation, which reduces the expression of COX-2 and consequently reduces the expression of PGs.
This study has some limitations. In this study we evaluated the effect of DEX on the inflammatory response in WISH cells in vitro. Further in vivo studies are required to verify this conclusion. In addition, other signaling pathways related to the anti-inflammatory effects of DEX and LPS co-treatment need to be investigated. As we only examined the effects of DEX and LPS co-treatment on the activation of p38 and NF-κB, additional experiments on the activation of other transcription factors or MAPKs involved in the expression of inflammatory factors are also required.
In conclusion, we demonstrated that DEX and LPS co-treatment suppressed the expression of COX-2 and PGE2, as well as pro-inflammatory cytokines, including IL-1β and TNF-α, in WISH cells. In addition, we showed that co-treatment with DEX and LPS mediates anti-inflammatory effects in WISH cells via the inhibition of p38/NF-κB activation. Although further research on the anti-inflammatory effect and mechanism of DEX is required, this study is significant in that it suggests that DEX reduces the expression of inflammatory substances that increase the risk of preterm labor.
ACKNOWLEDGMENTS
This work was supported by a 2-Year Research grant from the Pusan National University.
Footnotes
- Tae-Sung Kim: Project administration, Writing – original draft, Writing – review & editing.
- Ji-Young Yoon: Methodology, Writing – review & editing.
- Cheul-Hong Kim: Conceptualization, Methodology.
- Eun-Ji Choi: Supervision.
- Yeon Ha Kim: Investigation, Methodology, Software.
- Eun-Jung Kim: Conceptualization, Supervision, Writing – original draft.
COMPETING INTERESTS: The authors declare that no competing interests exist.
References
- 1.Gajendra S, Kumar JV. Oral health and pregnancy: a review. N Y State Dent J. 2004;70:40–44. [PubMed] [Google Scholar]
- 2.Jang KA, Kim KO, Lee SO. Comparing oral health care awareness and practice in pregnant women with and without oral health education experience. J Korean Soc Matern Child Health. 2016;20:169–177. [Google Scholar]
- 3.Park JH, Lee KS. Comparison of oral care patterns before and during the pregnancy. J Dent Hyg Sci. 2011;11:273–278. [Google Scholar]
- 4.Saraiva MC, Bettiol H, Barbieri MA, Silva AA. Are intrauterine growth restriction and preterm birth associated with dental caries? Community Dent Oral Epidemiol. 2007;35:364–376. doi: 10.1111/j.1600-0528.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 5.Harjunmaa U, Jarnstedt J, Alho L, Dewey KG, Cheung YB, Deitchler M, et al. Association between maternal dental periapical infections and pregnancy outcomes: results from a cross-sectional study in Malawi. Trop Med Int Health. 2015;20:1549–1558. doi: 10.1111/tmi.12579. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillaux C, Méhats C, Vaiman D, Cabrol D, Breuiller-Fouché M. Functional screening of TLRs in human amniotic epithelial cells. J Immunol. 2011;187:2766–2774. doi: 10.4049/jimmunol.1100217. [DOI] [PubMed] [Google Scholar]
- 10.Abe Y, Komatsubara M, Saito M, Toda M, Shinozaki H, Tamura T, et al. Activin A is stimulated by tumor necrosis factor-alpha and modulates collagen gene expression in human amniotic cells. J Endocrinol Invest. 2013;36:515–520. doi: 10.3275/8816. [DOI] [PubMed] [Google Scholar]
- 11.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Nancy P, Erlebacher A. T cell behavior at the maternal-fetal interface. Int J Dev Biol. 2014;58:189–198. doi: 10.1387/ijdb.140054ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Premyslova M, Li W, Alfaidy N, Bocking AD, Campbell K, Gibb W, et al. Differential expression and regulation of microsomal prostaglandin E(2) synthase in human fetal membranes and placenta with infection and in cultured trophoblast cells. J Clin Endocrinol Metab. 2003;88:6040–6047. doi: 10.1210/jc.2003-030618. [DOI] [PubMed] [Google Scholar]
- 14.Pavan B, Buzzi M, Ginanni-Corradini F, Ferretti ME, Vesce F, Biondi C. Influence of oxytocin on prostaglandin E2, intracellular calcium, and cyclic adenosine monophosphate in human amnion-derived (WISH) cells. Am J Obstet Gynecol. 2000;183:76–82. [PubMed] [Google Scholar]
- 15.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5:128–133. doi: 10.4103/0259-1162.94750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maze M, Scarfini C, Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin. 2001;17:881–897. doi: 10.1016/s0749-0704(05)70185-8. [DOI] [PubMed] [Google Scholar]
- 17.Mattingly JE, D'Alessio J, Ramanathan J. Effects of obstetric analgesics and anesthetics on the neonate : a review. Paediatr Drugs. 2003;5:615–627. doi: 10.2165/00148581-200305090-00004. [DOI] [PubMed] [Google Scholar]
- 18.Karaman S, Evren V, Firat V, Cankayali I. The effects of dexmedetomidine on spontaneous contractions of isolated gravid rat myometrium. Adv Ther. 2006;23:238–243. doi: 10.1007/BF02850129. [DOI] [PubMed] [Google Scholar]
- 19.Sia AT, Kwek K, Yeo GS. The in vitro effects of clonidine and dexmedetomidine on human myometrium. Int J Obstet Anesth. 2005;14:104–107. doi: 10.1016/j.ijoa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Shin SH, You JC, Ahn JH, Kim YH, Yoon JU, Cho AR, et al. Anti-inflammatory effects of dexmedetomidine on human amnion-derived WISH cells. Int J Med Sci. 2020;17:2496–2504. doi: 10.7150/ijms.49909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadeau-Vallee M, Quiniou C, Palacios J, Hou X, Erfani A, Madaan A, et al. Novel noncompetitive IL-1 receptor-biased ligand prevents infection- and inflammation-induced preterm birth. J Immunol. 2015;195:3402–3415. doi: 10.4049/jimmunol.1500758. [DOI] [PubMed] [Google Scholar]
- 22.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–263. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J Physiol. 1999;520:399–406. doi: 10.1111/j.1469-7793.1999.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-alpha in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol. 2002;187:1159–1162. doi: 10.1067/mob.2002.127457. [DOI] [PubMed] [Google Scholar]
- 26.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Bao H, Si Y, Wang X. Effects of dexmedetomidine on early and late cytokines during polymicrobial sepsis in mice. Inflamm Res. 2013;62:507–514. doi: 10.1007/s00011-013-0604-5. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Kang Z, Wang Y, Zhao J, Li S. The anti-inflammatory effect of dexmedetomidine administration on patients undergoing intestinal surgery: a randomized study. Drugs R D. 2021;21:445–453. doi: 10.1007/s40268-021-00368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohta Y, Miyamoto K, Kawazoe Y, Yamamura H, Morimoto T. Effect of dexmedetomidine on inflammation in patients with sepsis requiring mechanical ventilation: a sub-analysis of a multicenter randomized clinical trial. Crit Care. 2020;24:493–498. doi: 10.1186/s13054-020-03207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sia AT, Kwek K, Yeo GS. The in vitro effects of clonidine and dexmedetomidine on human myometrium. Int J Obstet Anesth. 2005;14:104–107. doi: 10.1016/j.ijoa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Karaman S, Evren V, Firat V, Cankayali I. The effects of dexmedetomidine on spontaneous contractions of isolated gravid rat myometrium. Adv Ther. 2006;23:238–243. doi: 10.1007/BF02850129. [DOI] [PubMed] [Google Scholar]
- 32.Min KJ, Lee JT, Joe EH, Kwon TK. An IκBα phosphorylation inhibitor induces heme oxygenase-1 (HO-1) expression through the activation of reactive oxygen species (ROS)-Nrf2-ARE signaling and ROS-PI3K/Akt signaling in an NF-κB-independent mechanism. Cell Signal. 2011;23:1505–1513. doi: 10.1016/j.cellsig.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Lim S, MacIntyre DA, Lee YS, Khanjani S, Terzidou V, Teoh TG, et al. Nuclear factor kappa B activation occurs in the amnion prior to labour onset and modulates the expression of numerous labour associated genes. PLoS One. 2012;7:e34707. doi: 10.1371/journal.pone.0034707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 35.Bhat NR, Feinstein DL, Shen Q, Bhat AN. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem. 2002;277:29584–29592. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- 36.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, et al. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]