Abstract
Introduction:
This study evaluated the cost-effectiveness of nivolumab plus ipilimumab (NI) versus pemetrexed plus cisplatin/carboplatin (C) as the first-line treatment for unresectable malignant pleural mesothelioma (MPM) from the perspective of US payers.
Methods:
A 10-year partitioned survival model was constructed using survival and safety data from the CheckMate 743 clinical trial. The output metrics of the model included the patient’s lifetime quality-adjusted life years (QALYs), lifetime costs, and incremental cost-effectiveness ratio (ICER). Only direct medical costs were considered. One-way and probabilistic sensitivity analyses were conducted to assess the robustness of the results.
Results:
Among all randomized patients, group NI had an ICER of $475,677/QALY relative to group C. Among patients with epithelioid histology, group NI had an ICER of $760,955/QALY. Among patients with non-epithelioid histology, group NI had an ICER of $418,348/QALY. The ICERs of all three populations exceeded the willingness-to-pay threshold ($150,000). The results of one-way sensitivity analysis revealed that the cost of nivolumab had a great influence on the results. The results of probabilistic sensitivity analysis demonstrated that the possibility of NI being more economical in all randomized patients and in patients with non-epidemiology histology was 0. In patients with epithelioid histology, the probability that NI had an economic advantage was 0.6%.
Conclusions:
From the perspective of US payers, in patients with unresectable MPM, NI has no economic advantage over C.
Keywords: cost-effectiveness, first-line treatment, ipilimumab, malignant pleural mesothelioma, nivolumab
Introduction
Mesotheliomas are rare tumors derived from mesothelial cells in the pleura or other parts, including the peritoneum, pericardium, and testicular tendon sheath; approximately 81% of these tumors are derived from the pleura. 1 Malignant pleural mesothelioma (MPM) is a rare and fatal cancer with high invasiveness and a 5-year survival rate of only approximately 10%. 2 Platinum drugs plus folic acid antimetabolites, such as pemetrexed, were the only approved first-line treatment for MPM since 2004 until October 2020.3,4 However, the long-term survival outcomes with chemotherapy remain poor.5–8 Bevacizumab has been used to treat MPM in recent years, but its use varies by region. 9 A randomized, double-blind phase III clinical trial (CheckMate 743) compared the safety and efficacy of the first-line treatment of unresectable MPM with nivolumab plus ipilimumab (NI) or pemetrexed plus cisplatin/carboplatin (C). The results showed that compared with C, NI could significantly prolong the median overall survival (OS) of patients [14.1 months, 95% confidence interval (CI): 12.4–16.3 months versus 18.1 months, 95% CI: 16.8–21.0 months; hazard ratio = 0.73, 95% CI: 0.61–0.87] with the 3-year OS rate (95% CI) of 15.4% (11.5–19.9) versus 23.2% (18.4–28.2).10,11 The NI regimen can significantly improve the health status of patients with unresectable MPM. The NI regimen has been recommended by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology—Malignant Pleural Mesothelioma (Version 1.2022) as the first-line treatment for MPM. 12
Although the NI regimen has shown good safety and efficacy, it is expensive; in particular, ipilimumab is priced at $160.7030/mg in the United States. 13 The whole course of treatment (one cycle every 6 weeks, with a total treatment time of approximately months) costs approximately $44,800, which is unaffordable for many patients’ families. Our study aims to evaluate the economy of NI versus that of C in the first-line treatment of unresectable MPM from the perspective of US payers.
Materials and methods
Target population and procedures
The population included in this study was consistent with that included in the CheckMate 743 clinical trial. That is, it included eligible patients who were aged 18 years or older with histologically confirmed unresectable MPM that was unamenable to curative therapy (surgery with or without chemotherapy) and an Eastern Cooperative Oncology Group performance status of 0 or 1. 10 In accordance with the design of the CheckMate 743 clinical trial, the NI group received intravenous nivolumab (3 mg/kg) every 2 weeks and ipilimumab (1 mg/kg) every 6 weeks. Treatment was continued until disease progression, unacceptable toxicity, or for 2 years. Patients in group C were intravenously injected with cisplatin (75 mg/m²) or carboplatin (area under the concentration time curve of 5 mg/mL per min) and pemetrexed (500 mg/m²) every 3 weeks for up to six cycles. 10
Model structure
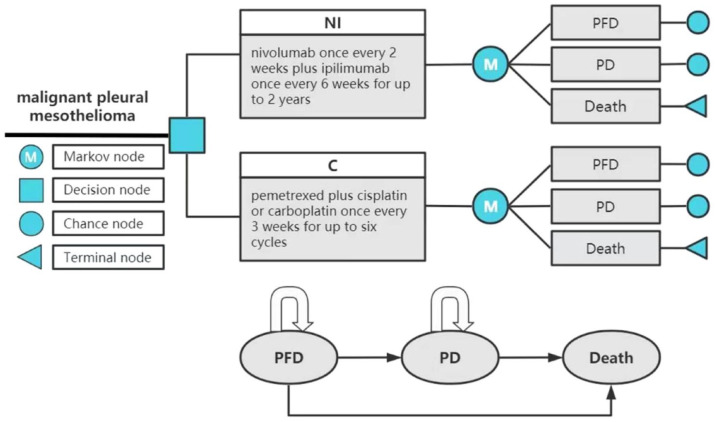
TreeAge Pro 2022 software was used to build the model and conduct statistical analysis. The model included three mutually exclusive health states: progression-free disease (PFD), progressive disease (PD), and death. All patients were assumed to enter the model in the PFD state and to be able to maintain their designated health state or develop into another health state in each cycle (Figure 1). The probability of the PFD state transition to the death state was assumed to be natural mortality.14,15 The relative 5-year survival rate of patients diagnosed with MPM was 10% or less. Thus, the time horizon of the model was set to 10 years. 2 The model period was set to 1 month to facilitate model operation and parameter calculation. The main results of the model output included total cost, quality-adjusted life years (QALY), and incremental cost-effectiveness ratio (ICER). The ICER refers to the additional cost required for each additional QALY. Cost and utility were discounted at the rate of 3%. 16 In this study, $150,000 was used as the willingness-to-pay (WTP) threshold. 17
Figure 1.
Partitioned survival model simulating outcomes for the CheckMate 743 trial. The model considers the transition states of unresectable MPM. All patients start in the PFD state and receive treatment with the two treatment plans. Patients can enter the state of PD and subsequently move to the state of the death.
C, pemetrexed plus cisplatin/carboplatin; MPM, malignant pleural mesothelioma; NI, nivolumab plus ipilimumab; PD, progressed disease; PFD, progression-free disease.
Clinical data
The survival data in this study came from the CheckMate 743 clinical trial, which is a multicenter, randomized, open-label, phase III trial. Eligible participants were randomized to receive NI or C (cisplatin or carboplatin). In the economic evaluation of antitumor drugs, performing parameter distribution fitting on the survival curve to obtain the long-term survival data on patients outside the follow-up period of clinical trials is often necessary due to the limited follow-up times of clinical trials and other factors. 18 The survival data on each arm were digitally extracted from the survival curves of CheckMate 743 using GetData Graph Digitizer software (version 2.26; http://www.getdata-graph-digitizer.com/download.php). In accordance with Guyot et al.’s method, the Kaplan–Meier survival curves were reconstructed using R software (version 3.5.1) to obtain new survival curves. 19 The distribution functions included Weibull, log-logistic, log-normal, Gompertz, exponential, and gamma. 19 Akaike information criterion (AIC), Bayesian information criterion (BIC), and visual simulation methods were used to test the goodness of fit, and distribution functions with low AIC and BIC values and good visual simulation were selected as fitting curves for extrapolation to obtain long-term clinical survival outcomes 20 (Supplemental Table A1).
In the CheckMate 743 trial, the authors only performed a graphical analysis on the progression-free survival (PFS) of the all randomized population (NI versus C = 303 versus 302) with a 3-year minimum follow-up. The authors separately compared all randomized patients (NI versus C = 303 versus 302), patients with epithelioid histology (NI versus C = 229 versus 226), and patients with non-epithelioid histology (NI versus C = 74 versus 76) for OS curve analysis. In our study, log-normal distribution and log-logistic distribution were used to fit the PFS curves of groups NI and C, respectively. Weibull distribution, log-logistic distribution, and exponential distribution were applied to fit the OS curves of different populations in group NI, whereas the OS curves of the three different populations in group C were all fitted with log-logistic distribution. We performed internal model validation. 21 Internal validation demonstrated that the PFS and OS curves were very close to those presented in clinical trials (Supplemental Figures A1–A8). The survival function for each distribution at time t is shown in Supplemental Figure A9. Table 1 presents the key clinical inputs.
Table 1.
Model parameters.
| Variable | Baseline value | Range | Reference | |
|---|---|---|---|---|
| Minimum | Maximum | |||
| NI: Log-normal PFS survival mode | λ = 1.93843, γ = 1.26135 | – | – | Peters et al. 10 |
| C: Log-logistic PFS survival mode | λ = 7.53780, γ = 2.29427 | – | – | Peters et al. 10 |
| NI: OS survival mode | ||||
| NI-A: WeibullPH OS survival mode | λ = 0.0241553, γ = 1.1284343 | – | – | Peters et al. 10 |
| NI-E: Log-logistic OS survival mode | λ = 19.01452, γ = 1.51241 | – | – | Peters et al. 10 |
| NI-N: Exponential OS survival mode | λ = 0.0412831 | – | – | Peters et al. 10 |
| C: OS survival mode | ||||
| C-A: Log-logistic OS survival mode | λ = 14.25065, γ = 1.76236 | – | – | Peters et al. 10 |
| C-E: Log-logistic OS survival mode | λ = 16.70245, γ = 1.70694 | – | – | Peters et al. 10 |
| C-N: Log-logistic OS survival mode | λ = 9.03382, γ = 2.15601 | – | – | Peters et al. 10 |
| NI: Incidence of AEs | ||||
| Asthenia | 0 | – | – | Peters et al. 10 |
| Anemia | 0.003 | 0.0024 | 0.0036 | Peters et al. 10 |
| Neutropenia | 0.007 | 0.0056 | 0.0084 | Peters et al. 10 |
| C: Incidence of AEs | ||||
| Asthenia | 0.042 | 0.0336 | 0.0504 | Peters et al. 10 |
| Anemia | 0.113 | 0.0904 | 0.1356 | Peters et al. 10 |
| Neutropenia | 0.151 | 0.1208 | 0.1812 | Peters et al. 10 |
| Utility | ||||
| PFS | 0.706 | 0.5648 | 0.8472 | Dansk et al. 22 |
| PD | 0.565 | 0.4520 | 0.6780 | Dansk et al. 22 |
| Death | 0 | – | – | Dansk et al. 22 |
| Asthenia | −0.410 | −0.3280 | −0.4920 | Nafees et al. 23 |
| Anemia | −0.073 | −0.0584 | −0.0876 | Wan et al. 24 |
| Neutropenia | −0.460 | −0.3680 | −0.5520 | Nafees et al. 23 |
| Drug cost per mg, 2022 US$ | ||||
| Nivolumab | 29.2450 | 14.6225 | 29.245 | Centers for Medicare and Medicaid Services 13 |
| Ipilimumab | 160.7030 | 80.3515 | 160.703 | Centers for Medicare and Medicaid Services 13 |
| Pemetrexed | 7.6037 | 6.08296 | 9.12444 | Centers for Medicare and Medicaid Services 13 |
| Cisplatin | 0.1864 | 0.14912 | 0.22368 | Centers for Medicare and Medicaid Services 13 |
| Carboplatin | 0.0522 | 0.04176 | 0.06264 | Centers for Medicare and Medicaid Services 13 |
| Vinorelbine | 0.8624 | 0.68992 | 1.03488 | Centers for Medicare and Medicaid Services 13 |
| Gemcitabine | 0.0192 | 0.01536 | 0.02304 | Centers for Medicare and Medicaid Services 13 |
| Drug administration and follow-up, cost per cycle, 2022 US$ | ||||
| Administration IV, first hour | 142.22 | 113.776 | 170.664 | Centers for Medicare and Medicaid Services 25 |
| Administration IV, additional hour | 30.68 | 24.544 | 36.816 | Centers for Medicare and Medicaid Services 25 |
| Outpatient follow-up visit | 52.33 | 41.864 | 62.796 | Centers for Medicare and Medicaid Services 25 |
| AEs cost per 1-month cycle, 2020 US$ | ||||
| Asthenia | 1065.44 | 852.352 | 1278.528 | Courtney et al. 26 |
| Anemia | 5243.47 | 4194.776 | 6292.164 | Courtney et al. 26 |
| Neutropenia | 16,857.15 | 13,485.720 | 20,228.580 | Courtney et al. 26 |
| Body area surface/m2 | 1.8 | 1.44 | 2.16 | Goulart and Ramsey 27 |
| Weight/kg | 70 | 56 | 84 | Goulart and Ramsey 27 |
| Creatinine clearance/mL/min | 70 | – | – | Goulart and Ramsey 27 |
| Discount rate | 0.03 | 0 | 0.06 | Bousmah et al. 16 |
A, all randomized patients; AEs, adverse events; C, pemetrexed plus cisplatin/carboplatin; E, patients with epithelioid histology; IV, intravenous injection; N, patients with non-epithelioid histology; NI, nivolumab plus ipilimumab; OS, overall survival; PD, progressed disease; PFS, progression-free survival.
Cost and utility
This study was based on the US payer perspective, and we only considered direct medical costs, including drug procurement, follow-up, administration, best supportive care, and adverse events (AEs) management costs. Through the comparison of the AEs of groups NI and C, only three AEs [asthenia (0% versus 4.2%), anemia (0.3% versus 11.3%), and neutropenia (0.7% versus 15.1%)] were included in this study. In reference to the CheckMate 743 clinical trial, first-line treatment was continued until disease progression, unacceptable toxicity, or the prescribed maximum medication time. In accordance with the experimental results, our study considered that the treatment duration of the NI group was 6 months [median = 5.6 months, interquartile range (IQR): 2.0–11.4 months] and that of the C group was 4 months (median = 3.5 month, IQR: 2.7–3.7 months). Moreover, we assumed that the probability of using cisplatin or carboplatin in group C was 0–1. In accordance with the NCCN Guidelines for MPM (version 1.2022), 12 second-line therapy in the NI arm was pemetrexed (500 mg/m2, intravenously every 3 weeks) plus cisplatin (75 mg/m²) or carboplatin (area under the concentration time curve of 5 mg/mL per min). We assumed the same probability range (0%–100%) for carboplatin and cisplatin. Group C was treated with nivolumab (3 mg/kg intravenously once every 2 weeks), vinorelbine (25 mg/m2 intravenously on days 1 and 8 of a 3-week cycle), or gemcitabine (1000 mg/m2 intravenously on days 1 and 8 of a 3-week cycle) monotherapy. 28 The probability of second-line treatment with nivolumab in group C patients was 0.40, and under the assumption that the probability of using vinorelbine or gemcitabine was equal (both were equal to 0.30). All patients were assumed to receive second-line treatment until they progressed, and only the costs of drug acquisition and follow-up for second-line treatment were considered. The drug costs were obtained from the average sales price of Medicare part B drugs provided by the Centers for Medicare & Medicaid Services, and the administration costs were obtained from the Medicare Physician Fee Schedule.13,25 In reference to the median age in the CheckMate 743 trial, the initial model patients had the following characteristics: age of 69 years, mean body weight of 70 kg, surface area of 1.8 m2, and creatinine clearance of 70 mL/min.24,27 Other costs are shown in Table 1.
The utility value represents the health-related quality of life for each health state. The CheckMate 743 trial did not address health utility. Therefore, the utility values and treatment costs for AEs in our model were obtained from other published literature.23,24,26 We assumed that AEs occurred only in the first cycle. Precise utility scores were not available in the original or previous MPM literature. Therefore, the utility scores in our analysis were referenced to published values for non-small-cell lung cancer (NSCLC) 22 under the assumption that the same health status was similar in both groups with 0.706 for the PFS state, 0.565 for the PD state, and 0 for death. All utility values are shown in Table 1.
Sensitivity analysis
One-way sensitivity analysis was performed to account for the effect of the parameters on the model by varying one parameter within the ±20% range (the current price of nivolumab and ipilimumab fluctuated downward by 50% as the value range) of its baseline value, whereas the other parameters were fixed. The discount rate was 0%–6%. 16 Probabilistic sensitivity analyses were performed through Monte-Carlo simulation and were repeated 1000 times, and the results were presented in the form of cost-effectiveness acceptability curves and incremental cost-effectiveness scatter plots.
Results
Basic case analysis
The time horizon was set to 10 years. Combined with the results of the model running, most of the patients died within 10 years, and our model basically simulated the lifelong outcome of the disease. Refer to Table 2 for the basic analysis results. Compared with C, NI could provide higher health benefits to all randomized patients (1.45 QALYs versus 1.19 QALYs), but also had higher total cost ($295,988 versus $168,111). Compared with that of C, the ICER value of NI was $475,677/QALY, which exceeded the threshold of WTP. In patients with epithelioid histology, NI also had higher health effectiveness than C (1.48 QALYs versus 1.34 QALYs) with a higher total cost ($313,857 versus $205,508). Compared with that that of C, the ICER value of NI was $760,955/QALY, which exceeded the WTP. Among patients with non-epithelioid histology, the patients in group NI had a longer QALY than those in group C (1.25 QALYs versus 0.79 QALYs) and at the same time, had higher total cost ($268,724 versus $72,783). The ICER value of NI relative to that of C was $418,348/QALY, which also exceeded the WTP threshold.
Table 2.
Cost-effectiveness analysis.
| Strategies | Life years | QALYs | Total costs (US$) | ICER (US$/QALY) (NI versus C) |
|---|---|---|---|---|
| In randomized patients | ||||
| NI | 2.33 | 1.45 | 295,988 | 475,677 |
| C | 1.87 | 1.19 | 168,111 | |
| Incremental (NI versus C) | 0.46 | 0.27 | 127,877 | |
| In patients with epithelioid histology | ||||
| NI | 2.38 | 1.48 | 313,857 | 760,955 |
| C | 2.15 | 1.34 | 205,508 | |
| Incremental (NI versus C) | 0.23 | 0.14 | 108,349 | |
| In patients with non-epithelioid histology | ||||
| NI | 1.97 | 1.25 | 268,724 | 418,348 |
| C | 1.17 | 0.79 | 72,783 | |
| Incremental (NI versus C) | 0.81 | 0.47 | 195,941 | |
C, pemetrexed plus cisplatin/carboplatin; ICER, incremental cost-effectiveness ratio; NI, nivolumab plus ipilimumab; QALYs, quality-adjusted life years.
Sensitivity analysis
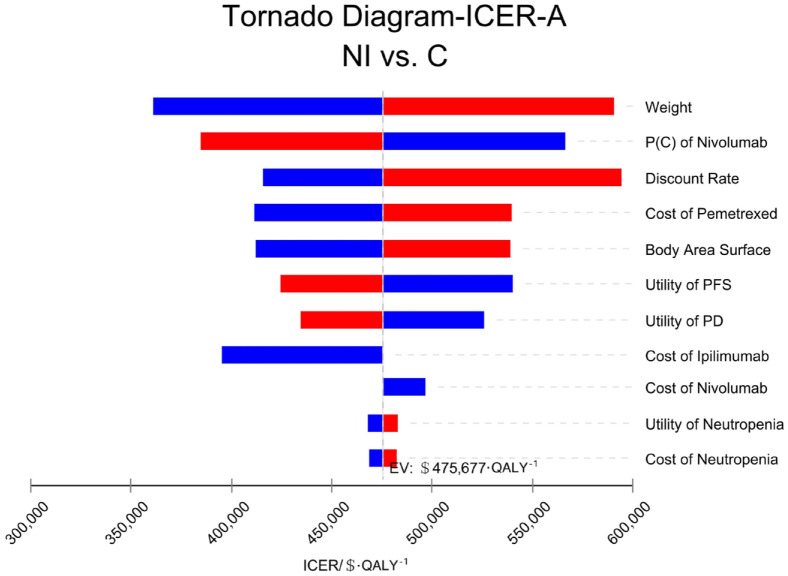
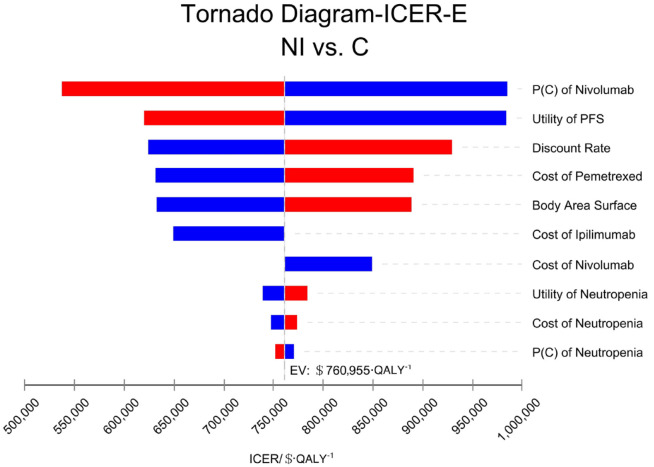
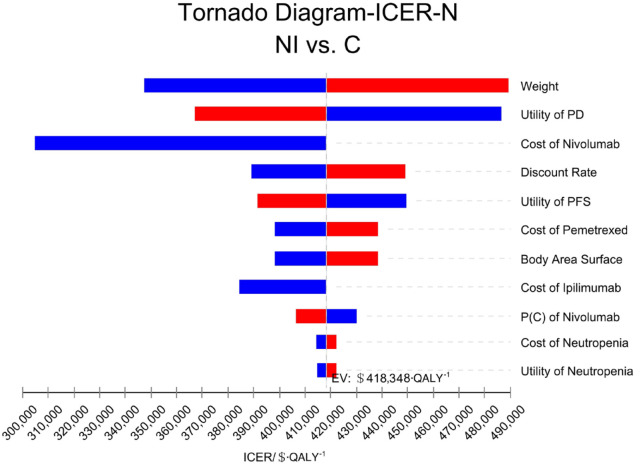
The patient’s weight, the probability of second-line nivolumab treatment in the group C, and the cost of pemetrexed had a great effect on the results of all randomized patients. The tornado diagram of one-way sensitivity analysis is shown in Figure 2. The probability of second-line treatment with nivolumab in group C, the utility value of PFS status, and the discount rate had great influence on the results of the patients with epithelioid histology. The tornado chart of single-factor sensitivity analysis is shown in Figure 3. The patient’s weight, the utility value of PD status, and the price of nivolumab had a strong effect on the results of the patients with non-epithelioid histology. The tornado plot of univariate sensitivity analysis is shown in Figure 4. The results of the one-way sensitivity analysis of the three populations all revealed that the ICER value could not fall below the WTP threshold no matter how all variables changed individually.
Figure 2.
One-way sensitivity analysis in all randomized patients (A).
C, pemetrexed plus cisplatin/carboplatin; ICER, incremental cost-effectiveness ratio; NI, nivolumab plus ipilimumab; P, probability; PD, progressive disease; PFS, progression-free survival.
Figure 3.
One-way sensitivity analysis in patients with epithelioid histology (E).
C, pemetrexed plus cisplatin/carboplatin; ICER, incremental cost-effectiveness ratio; NI, nivolumab plus ipilimumab; P, probability; PFS, progression-free survival.
Figure 4.
One-way sensitivity analysis in patients with non-epithelioid histology (N).
C, pemetrexed plus cisplatin/carboplatin; ICER, incremental cost-effectiveness ratio; NI, nivolumab plus ipilimumab; P, probability; PD, progressive disease; PFS, progression-free survival.
The results of probabilistic sensitivity analysis are presented in the appendix (Supplemental Figure A10–A12). In all randomized patients and in patients with non-epithelioid histology, all points were above the WTP threshold ($150,000) line, indicating that the probability that NI was economical was 0 under this WTP threshold. In patients with epithelioid histology, only six points were below the threshold line of WTP. This result indicated that under this threshold line of WTP, the probability that NI had economic advantages was very low.
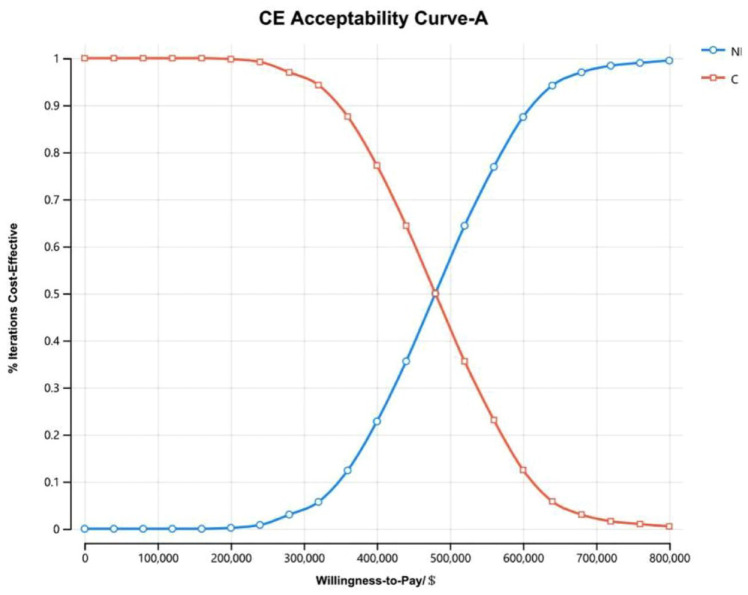
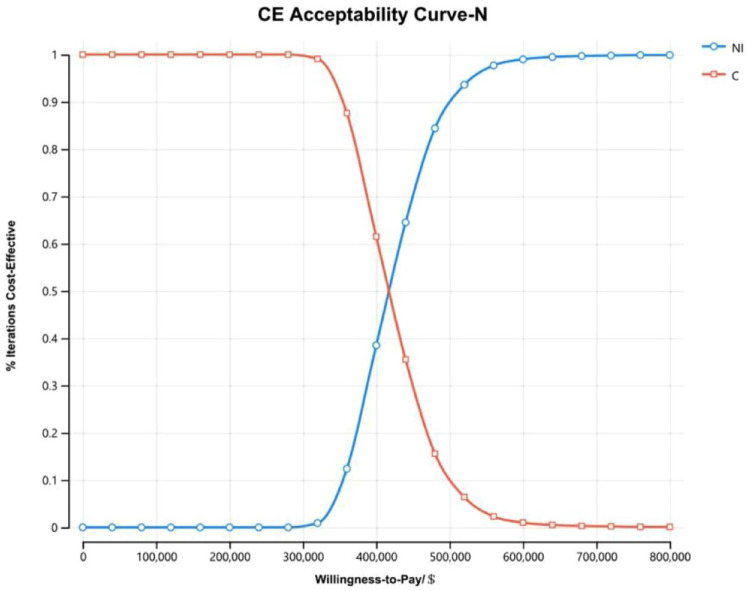
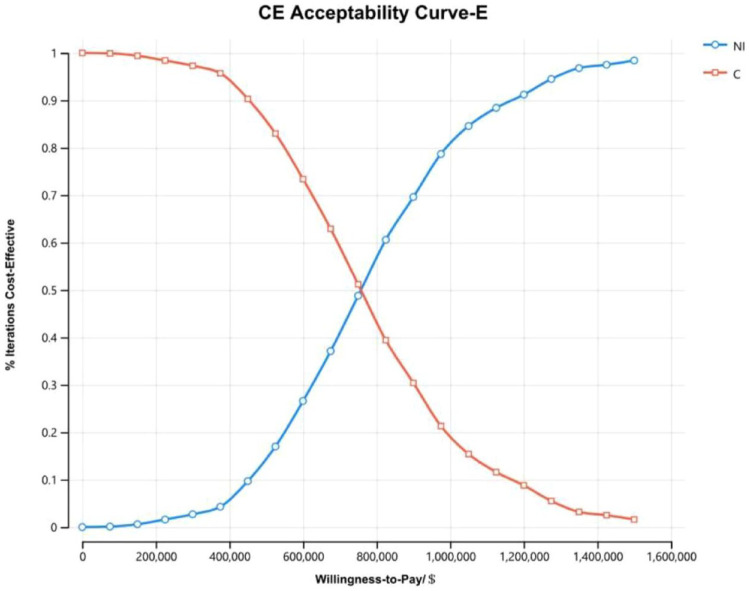
The cost-effectiveness acceptability curves of all randomized patients (Figure 5) and patients with non-epithelioid histology (Figure 6) showed that as the WTP threshold increased, the probability that NI was economical increased. However, when the WTP threshold was $150,000, the probability that NI was economical was 0. The cost-effectiveness acceptability curves of patients with epithelioid histology (Figure 7) illustrated that when the WTP fluctuated within the range of $0/QALY to $1,600,000/QALY, the probability that NI was economical increased with the increase in the WTP threshold but that when the WTP threshold was $150,000, the probability that the NI group was economical was only 0.6%.
Figure 5.
The cost-effectiveness acceptability curves of all randomized patients (A).
C, pemetrexed plus cisplatin/carboplatin; CE, cost-effective; NI, nivolumab plus ipilimumab.
Figure 6.
The cost-effectiveness acceptability curves of patients with non-epithelioid histology (N).
C, pemetrexed plus cisplatin/carboplatin; CE, cost-effective; NI, nivolumab plus ipilimumab.
Figure 7.
The cost-effectiveness acceptability curves of patients with epithelioid histology (E).
C, pemetrexed plus cisplatin/carboplatin; CE, cost-effective; NI, nivolumab plus ipilimumab.
Discussion
In contrast to conventional chemotherapy, NI is effective in prolonging survival in patients with epithelioid histology and in patients without epithelioid histology and improves the duration of response (DOR). The median (95% CI) DOR was 11.6 months (8.2–16.8 months) in the NI group versus 6.7 months (5.6–7.1 months) in the chemotherapy group. Among responders, the 3-year DOR rate was 28% versus 0%. Therefore, evaluating the cost-effectiveness of NI is necessary. In this study, we assessed for the first time the cost-effectiveness of NI for the treatment of MPM by building an economic model method and synthesizing the latest evidence.
At present, the economic research on MPM is very limited. In 2017, a Markov model was established to compare the cost-effectiveness of adding bevacizumab versus pemetrexed plus cisplatin from the perspective of Chinese payers. Model calculations showed that using bevacizumab as a part of first-line and maintenance therapy provided an additional 0.112 QALYs at the additional cost of $81,447. That is, compared with chemotherapy alone, the ICER of chemotherapy plus bevacizumab was $727,203/QALY, which was well above the accepted WTP threshold of three times the gross domestic product per capita of China ($23,970 per QALY). 29 Most of the existing economic studies on MPM focused on chemotherapy.30–32 In 2012, Woods et al., on the basis of the results of the EORTC08983 trial, used an indirect comparison method to evaluate the relative efficacy of raltitrexed combined with cisplatin and pemetrexed combined with cisplatin. They concluded that raltitrexed in combination with cisplatin was an economical first-line treatment for patients with MPM. However, no cost-effectiveness studies related to immunotherapy for MPM currently exist. In recent years, tumor immunotherapy has developed rapidly, and many new therapeutic targets have been discovered. However, programmed death 1 (PD-1) and its ligand programmed cell death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) remain the therapeutic targets that have been most investigated in detail. Immunotherapy has made great progress in the treatment of malignant tumors and is superior to traditional chemotherapy and radiotherapy. However, the drugs that are currently used for immunotherapy, such as ipilimumab, which inhibits CTLA-4, and nivolumab, which inhibits the interaction of PD-1 with PD-L1, are relatively expensive. Therefore, whether immunotherapy has economic advantages over traditional chemotherapy is a major concern of numerous researchers worldwide.
Despite the promising results of the Checkmate 743 trial, our health economics analysis showed that from the US payers’ point of view, the immunotherapy of NI is not a cost-effective alternative to traditional chemotherapy for the first-line treatment of unresectable MPM. Among all randomized patients, group NI had 0.27 QALYs more than group C, resulting in an increase in cost of $127,877 and an ICER of $475,677/QALY. Among patients with epithelioid histology, group NI had 0.14 QALYs more than group C, resulting in an increase in cost of $108,349 and an ICER of $760,955/QALY. Among patients with non-epithelioid histology, group NI had 0.46 QALYs more than group C, resulting in an increase in cost of $195,941 and an ICER of $418,348/QALY. In the three populations, the ICER value was all above the WTP threshold, that is, NI was not cost-effective. However at the same time, patients with epithelioid histology receiving NI therapy had a higher QALY (1.25 versus 1.48) than the patients with non-epithelioid histology. This result indicated that NI therapy has a better effect on patients with epithelioid histology than on those without. However, the ICER of the patients with non-epithelioid histology was approximately half that of the patients with epithelioid histology. This situation indicated that the use of NI therapy in patients with non-epithelioid histology was more economical than in other patients.
The three models established in our study took into account the effect of different second-line treatment drugs on the results. In accordance with the CheckMate 743 trial and NCCN guidelines, we assumed that the patients in the NI group received pemetrexed plus cisplatin/carboplatin for second-line therapy after progression. The probability of receiving nivolumab in group C originated from the CheckMate 743 trial. However, given that the article did not explain the specific chemotherapy plan of this group, we assumed that in addition to nivolumab, the use of vinorelbine and gemcitabine for second-line therapy had the same probability range in accordance with NCCN guidelines. Through one-way sensitivity analysis, we found that in all patients and epithelioid patients, the probability of receiving nivolumab in group C had a great influence on the results. However, no matter how the probability changed within the preset range, the ICER value was always above the WTP threshold, and the NI group showed an absolute cost-effective disadvantage. We suspect that this situation may be related to the higher price of nivolumab than that of other drugs. We directly observed that nivolumab price had a great effect on the outcomes in the non-epithelioid population. This observation verified our conjecture to a certain extent.
The NCCN guidelines did not clearly indicate the use cycle of nivolumab combined with ipilimumab in the treatment of patients with MPM. The specifications of nivolumab issued by the US Food and Drug Administration (FDA) describe the life cycle as follows: in combination with ipilimumab until disease progression, unacceptable toxicity, or up to 2 years in patients without disease progression. That is to say, at present, the FDA also has no clear regulations on the use cycle. However, using 2 years as the usage period is obviously incorrect because from the clinical trial, the PFS was far less than 2 years (the CheckMate 743 trial only provided the PFS curves of all patients). This situation would make our calculated cost become significantly higher than the actual cost (the above analysis showed that the price of nivolumab had a great influence on the results). Therefore, in our study, we assumed that the duration of first-line treatment with nivolumab and ipilimumab in the NI group was equal to the median duration in the CheckMate 743 trial, whereas that of second-line treatment with nivolumab and ipilimumab in group C was until the progression of patients with MPM. The same assumption was made for the cycles with pemetrexed plus cisplatin/carboplatin in the NI group.
This study still has certain limitations. First, although the CheckMate 743 trial provided the OS curves of three different populations, it did not provide the PFS curves of the different populations. Therefore, for all three different populations (all randomized patients, patients with epithelioid histology, and patients with non-epithelioid histology), our study used their respective OS curves but utilized the PFS curve of all random population to fit the survival model. This approach would lead to some bias. Second, only some adverse drug events were included in this study to simplify the model, and the treatment cost data of AEs originated from published relevant literature rather than real-world data. Although this approach may also lead to certain bias, the sensitivity analysis results showed that such parameters had little influence on the results. Moreover, no specific immune-related AEs were included. Although immune-related AEs can lead to serious outcomes in patients, cost has low impact on outcomes. In addition, the conclusion of our study is that the immune group is not economical. Without calculating immune-related AEs, we believe that our results underestimate the economics of the chemotherapy group. That is, calculating immune-related AEs would increase the economy of the results of the chemotherapy group and would not affect the conclusions. Third, the utility value is a key parameter for pharmacoeconomic evaluation. However, given the unavailability of precise utility scores in the original or previous MPM literature, the utility scores in our analysis were taken in reference to the published values for NSCLC. 29 Although the results of one-way sensitivity analysis demonstrated that the utility values of PFS status and PD status played an important role in the results, the tornado diagrams showed that no matter how the utility values of PFS status or PD status changed within the preset range, the ICER values were always above the WTP threshold. Fourth, because AE rates and costs were low in both groups, this portion of the cost was not discounted (costs in 2020 US$).
Conclusion
The pharmacoeconomic evaluation carried out in this study conformed with the standard methodological process. 18 Despite some limitations, the results obtained have high reliability. That is, when $150,000 was used as the threshold of WTP, immunotherapy (NI) had no economic advantage over traditional chemotherapy (C) in the first-line treatment of unresectable MPM.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221116604 for Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as the first-line treatment for unresectable malignant pleural mesothelioma by Liu Yang, Xueqiong Cao, Na Li, Bin Zheng, Maobai Liu and Hongfu Cai in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank all of the people who have contributed to this paper.
Footnotes
ORCID iD: Hongfu Cai  https://orcid.org/0000-0002-4923-4882
https://orcid.org/0000-0002-4923-4882
Supplemental materials: Supplemental material for this article is available online.
Contributor Information
Liu Yang, Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China; School of Pharmacy, Fujian Medical University, Fuzhou, China.
Xueqiong Cao, Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China; School of Pharmacy, Fujian Medical University, Fuzhou, China.
Na Li, Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China; School of Pharmacy, Fujian Medical University, Fuzhou, China.
Bin Zheng, Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China; School of Pharmacy, Fujian Medical University, Fuzhou, China.
Maobai Liu, Department of Pharmacy, Fujian Medical University Union Hospital, Fujian Medical University, Xinquan Road 29, Fuzhou 350100, China; Fujian Medical University, Fuzhou, China; Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China; School of Pharmacy, Fujian Medical University, Fuzhou, China.
Hongfu Cai, Department of Pharmacy, Fujian Medical University Union Hospital, Fujian Medical University, Xinquan Road 29, Fuzhou 350100, China; Fujian Medical University, Fuzhou, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: All authors participated in this study and approved the final version.
Author contribution(s): Liu Yang: Software; Writing – original draft.
Xueqiong Cao: Validation; Writing – review & editing.
Na Li: Data curation; Investigation.
Bin Zheng: Formal analysis.
Maobai Liu: Supervision; Visualization.
Hongfu Cai: Methodology; Writing – original draft.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Fujian Provincial Department of Science & Technology (grant no. 2020Y9070, 2018Y9037, and 2021R0053).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: All datasets for this study are included in the article as well as in the supplementary material.
References
- 1. Van Schil P, Van Meerbeeck J. Malignant pleural and peritoneal mesothelioma: clinical update 2018. Transl Lung Cancer Res 2018; 7: 505–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mutti L, Peikert T, Robinson BWS, et al. Scientific advances and new frontiers in mesothelioma therapeutics. J Thorac Oncol 2018; 13: 1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popat S, Baas P, Faivre-Finn C, et al. Malignant pleural mesothelioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2022; 33: 129–142. [DOI] [PubMed] [Google Scholar]
- 4. Opitz I, Scherpereel A, Berghmans T, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 2020; 58: 1–24. [DOI] [PubMed] [Google Scholar]
- 5. Santoro A, O’Brien ME, Stahel RA, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the international expanded access program. J Thorac Oncol 2008; 3: 756–763. [DOI] [PubMed] [Google Scholar]
- 6. Taylor P, Castagneto B, Dark G, et al. Single-agent pemetrexed for chemonaive and pretreated patients with malignant pleural mesothelioma: results of an International Expanded Access Program. J Thorac Oncol 2008; 3: 764–771. [DOI] [PubMed] [Google Scholar]
- 7. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–2644. [DOI] [PubMed] [Google Scholar]
- 8. van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005; 23: 6881–6889. [DOI] [PubMed] [Google Scholar]
- 9. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016; 387: 1405–1414. [DOI] [PubMed] [Google Scholar]
- 10. Peters S, Scherpereel A, Cornelissen R, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol 2022; 33: 488–499. [DOI] [PubMed] [Google Scholar]
- 11. Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021; 397: 375–386. [DOI] [PubMed] [Google Scholar]
- 12. NCCN. NCCN guidelines, (2022). https://www.nccn.org/guidelines/guidelines-detail?category=1id=1442 (accessed 21 March 2022).
- 13. Centers for Medicare and Medicaid Services. ASP drug pricing files, (2022). https://www.cms.gov (accessed 21 March 2022).
- 14. Arias E, Xu J. United States life tables, 2019. Natl Vital Stat Rep 2022; 70: 1–59. [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention, (2022). https://www.cdc.gov/nchs/nvss/life-expectancy.htm#publications (accessed 23 March 2022).
- 16. Bousmah MA, Nishimwe ML, Tovar-Sanchez T, et al. Cost-utility analysis of a dolutegravir-based versus low-dose efavirenz-based regimen for the initial treatment of HIV-infected patients in Cameroon (NAMSAL ANRS 12313 Trial). Pharmacoeconomics 2021; 39: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014; 371: 796–797. [DOI] [PubMed] [Google Scholar]
- 18. Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013; 33: 743–754. [DOI] [PubMed] [Google Scholar]
- 19. Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao F, Languille C, Karzazi K, et al. Efficiency of fine scale and spatial regression in modelling associations between healthcare service spatial accessibility and their utilization. Int J Health Geogr 2021; 20: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol 2015; 33: 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dansk V, Large S, Bertranou E, et al. A review of health state utility values used in UK nice appraisals in advanced NSCLC. Value Health 2016; 19: A745. [Google Scholar]
- 23. Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol 2017; 13: e195–e203. [DOI] [PubMed] [Google Scholar]
- 24. Wan X, Luo X, Tan C, et al. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: a United States-based cost-effectiveness analysis. Cancer 2019; 125: 3526–3534. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Medicare and Medicaid Services. CY 2022 Medicare physician fee schedule (PFS) final rule, (2022). https://www.cms.gov/medicare/medicare-fee-for-service-payment/physicianfeesched (accessed 21 March 2022).
- 26. Courtney PT, Yip AT, Cherry DR, et al. Cost-effectiveness of Nivolumab-Ipilimumab combination therapy for the treatment of advanced non-small cell lung cancer. JAMA Netw Open 2021; 4: e218787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health 2011; 14: 836–845. [DOI] [PubMed] [Google Scholar]
- 28. Zauderer MG, Kass SL, Woo K, et al. Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer 2014; 84: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhan M, Zheng H, Xu T, et al. Cost-effectiveness analysis of additional bevacizumab to pemetrexed plus cisplatin for malignant pleural mesothelioma based on the MAPS trial. Lung Cancer 2017; 110: 1–6. [DOI] [PubMed] [Google Scholar]
- 30. Arrieta O, Munoz-Montano W, Muniz-Hernandez S, et al. Efficacy, safety, and cost-minimization analysis of continuous infusion of low-dose gemcitabine plus cisplatin in patients with unresectable malignant pleural mesothelioma. Front Oncol 2021; 11: 641975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woods B, Paracha N, Scott DA, et al. Raltitrexed plus cisplatin is cost-effective compared with pemetrexed plus cisplatin in patients with malignant pleural mesothelioma. Lung Cancer 2012; 75: 261–267. [DOI] [PubMed] [Google Scholar]
- 32. Dundar Y, Bagust A, Dickson R, et al. Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation. Health Technol Assess 2007; 11: 1–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221116604 for Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as the first-line treatment for unresectable malignant pleural mesothelioma by Liu Yang, Xueqiong Cao, Na Li, Bin Zheng, Maobai Liu and Hongfu Cai in Therapeutic Advances in Medical Oncology