Abstract
Background:
Multiple sclerosis (MS) is the most common immune-mediated demyelinating disease in younger adults. Patients with MS (PwMS) are vulnerable to the presence of potential drug–drug interactions (pDDIs) and potential drug–food interactions (pDFIs) as they take numerous medications to treat MS, associated symptoms and comorbidities. Knowledge about pDDIs and pDFIs can increase treatment success and reduce side effects.
Objective:
We aimed at determining the frequency and severity of pDDIs and pDFIs in PwMS, with regard to polypharmacy.
Methods:
In the cross-sectional study, we analysed pDDIs and pDFIs of 627 PwMS aged ⩾18 years. Data collection was performed through patient record reviews, clinical examinations and structured patient interviews. pDDIs and pDFIs were identified using two DDI databases: Drugs.com Interactions Checker and Stockley’s Interactions Checker.
Results:
We identified 2587 pDDIs (counted with repetitions). Of 627 PwMS, 408 (65.1%) had ⩾ 1 pDDI. Polypharmacy (concomitant use of ⩾ 5 drugs) was found for 334 patients (53.3%). Patients with polypharmacy (Pw/P) were found to have a 15-fold higher likelihood of having ⩾ 1 severe pDDI compared with patients without polypharmacy (Pw/oP) (OR: 14.920, p < 0.001). The most frequently recorded severe pDDI was between citalopram and fingolimod. Regarding pDFIs, ibuprofen and alcohol was the most frequent severe pDFI.
Conclusion:
Pw/P were particularly at risk of severe pDDIs. Age and educational level were found to be factors associated with the occurrence of pDDIs, independent of the number of medications taken. Screening for pDDIs/pDFIs should be routinely done by the clinical physician to increase drug safety and reduce side effects.
Keywords: multiple sclerosis, over-the-counter drugs, polypharmacy, potential drug–drug interactions, potential drug–food interactions
Introduction
Potential drug–drug interactions (pDDIs) occur when the pharmacodynamics or pharmacokinetics of an active substance are affected by the intake of other drugs. Changes in drug metabolism such as induction or inhibition of CYP enzymes may be observed due to pDDIs. As a result, pDDIs lead to adverse drug effects that may have serious consequences for the patients. It is estimated that 200,000 to 1 million patients are seriously affected by pDDIs each year in Germany alone. 1 The number of aged and multimorbid patients is increasing rapidly, and consequently, the number of prescribed medications, leading to an exponential increase in the number of pDDIs. 1 Older age typically implies taking a greater number of medications prescribed by different healthcare providers, which increases the risk for clinically relevant pDDIs. 2 pDDIs are responsible for 1–5% of hospitalisations. 3 Moura et al. 4 focused on the economic and clinical problems and they demonstrated that pDDIs are associated with prolonged hospitalisations (15 versus 8 days) as well as additional costs to the health care system (US$192 or more per hospitalisation). In a US study, the burden of pDDIs on the health care system was reported to be between $30 and $180 billion annually.5,6 As a leading cause of increased morbidity and mortality, 770,000 deaths per year can be attributed to pDDIs, which contribute to about 20% of all reported adverse drug events. 7
Potential drug–food interactions (pDFIs) are another cause of adverse drug reactions. Food can regulate the metabolism of drugs, for example, via CYP enzymes and lead to altered drug levels, resulting in increased or decreased drug effects. To improve therapeutic outcomes, it is important for pharmacists and prescribing physicians to identify efficacy-influencing food, ingredients beverages and dietary/lifestyle habits. 8
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the central nervous system associated with inflammation and degeneration. 9 Worldwide, over 2 million people are affected by MS, with an increasing trend (1990 versus 2016: + 10.4%).10,11 MS can occur in different disease courses: primary progressive MS (PPMS), relapsing-remitting MS (RRMS) or secondary progressive MS (SPMS). A clinically isolated syndrome (CIS) often characterises the initial stage of the disease.12,13 As a multifaceted disease, MS can cause a variety of symptoms such as spasticity, bladder dysfunction, visual problems or cognitive and psychological changes. 14 The drug therapy of MS is divided into relapse therapy, disease-modifying therapy and medication for symptom reduction (e.g. antispasmodics like baclofen or cannabinoids).15–18 Disease-modifying drugs (DMDs) are used for immunomodulating treatment.19–21 This is supplemented by symptomatic therapies and comorbidity drugs.14,22 To maintain quality of life and improve functional outcomes, many patients seek additional help in the use of complementary and alternative medicines (CAM) such as dietary supplements or herbal drugs.23–25 It was reported that 67% to 80% of MS patients use CAM and half of them even as an alternative to conventional therapies.26,27 For example, vitamin D supplementation is often part of a nutritional health plan because low cholecalciferol levels in serum have been associated with a higher risk of relapses. 28
The combined use of DMDs, symptomatic therapeutics, comorbidity drugs and CAM increases the risk of polypharmacy.29,30 According to the most common definition, polypharmacy means taking five or more drugs. 31 In a systematic review of seven studies, we found a polypharmacy rate of 15–59% in patients with MS. 32 In a previous study, we also analysed pDDIs in a cohort of women of childbearing age with MS (N = 131). Clinically relevant pDDIs were six times more frequent in women with polypharmacy than in women without polypharmacy. 33
In the present study, we captured the full spectrum of pDDIs in a large cohort of patients with MS. By identifying frequently interacting drugs and common pDDIs, we aimed to raise awareness of avoidable drug combinations and potentially serious consequences, especially in patients affected by polypharmacy. We also evaluated the severity of pDFIs to assess their clinical relevance and provide recommendations for optimising pharmacotherapy in MS.
Materials and methods
Study population
The data for this cross-sectional study were collected from March 2017 to May 2020 at the neurological department (neuroimmunology section) of the Rostock University Medical Centre and at the neurological department of the Ecumenical Hainich Hospital Mühlhausen (Germany). Patients younger than 18 years and subjects without the diagnosis of MS or CIS according to the revised McDonald criteria were excluded. 34 For data collection, we asked inpatients during their hospital stay and outpatients in the waiting period before their routine examination to voluntarily participate in our study. With informed written consent, we acquired data from a total of 627 participants. This study thus included more patients than our previous study on MS (n = 131) and comparable studies on the analysis of pDDIs in other disease contexts (up to n = 481).33,35–38 Approval for this study was granted by the ethics committees of the Thuringia Medical Association and the Rostock University Medical Centre (approval numbers A 2014-0089 and A 2019-0048). The study was conducted in accordance with the Declaration of Helsinki.
Data acquisition
Clinical, pharmaceutical and sociodemographic data of the included patients were collected based on a structured interview, supplemented by anamnesis, a review of patient records and a clinical examination. We considered prescription drugs (Rx), over-the-counter drugs (OTC), dietary supplements (e.g. vitamins and minerals) and CAM in order not to miss any drug intake outside the doctor’s ‘radar’.
The data were divided into three categories:
Sociodemographic data: We obtained data on age, sex, partnership, employment status, school years (without training or university studies), level of education, number of children and siblings as well as place of residence (<5000 residents: rural community, 5000–19,999: provincial town, 20,000–99,999: medium-sized town, ⩾100,000: city).
Clinical data: This category comprised disease duration, disease course (CIS, RRMS, PPMS or SPMS), the number and types of comorbidities and the degree of disability according to the Expanded Disability Status Scale (EDSS). 39
Pharmaceutical data: The data collected included all medications taken per patient with the corresponding names of active ingredients, the trade names of the drugs, the types of application and dosages.
Classification of drugs
The medicines were divided into three categories:
Therapy goal: We distinguished DMDs, symptomatic drugs and comorbidity drugs. DMDs are immunomodulatory drugs for the therapy of MS. Methylprednisolone was included as DMD because it was used for relapse therapy or as repeated pulse therapy for progressive courses. Symptomatic drugs are used to relieve the various symptoms of MS. Comorbidity drugs are medications to treat comorbidities not related to MS.
Period of drug intake: We differentiated between long-term drugs (taken daily or at regular intervals) and on-demand drugs (pro re nata, PRN) which are used sporadically or acutely.
Access: We distinguished drugs that are available only as OTC or on prescription (Rx). OTC drugs also include preparations that are sold in small doses without a prescription, but require a prescription in higher doses (e.g. ibuprofen).
Following the most frequently used definition, polypharmacy was present if five or more medications were taken by the patient. 31
Identification of drug–drug and drug–food interactions
For the determination of pDDIs and pDFIs, we used two online drug interaction databases: Drugs.com Drug Interactions Checker and Stockley’s Interactions Checker. The database search was performed from May 2020 to October 2020 using either the trade names or the active ingredients of the drugs as appropriate.
Drugs.com is a free online database, which provides information on more than 24,000 prescription and OTC medicines as well as herbal pharmaceuticals for patients and medical professionals. In Drugs.com , pDDIs are distinguished according to three levels of severity: major (highly clinically significant), moderate (moderately clinically significant) and minor (minimally clinically significant).
Stockley’s Interactions Checker, maintained by the Royal Pharmaceutical Society, is a subscription-based tool for identifying pDDIs. It contains over 85,000 interactions and is aimed at healthcare professionals. It provides drug interactions with food, beverages and smoking as well as interactions between drugs and herbs. Stockley’s Interactions Checker also classifies the severity of pDDIs into three groups: severe (high clinical relevance), moderate (moderate clinical relevance) and minor (minimal clinical relevance).
Summary score of pDDI and pDFI severity
To combine the information on pDDI severity from Drugs.com and Stockley’s, we assigned scores to each severity level: zero points (no evidence of interaction), one point (minor/mild), two points (moderate) and three points (major/severe). For each pDDI, the sum of the scores of the two databases was then used to define five degrees of pDDI severity (mild, mild-moderate, moderate, moderate-severe and severe). In the case of pDFIs, we adhered to the three-level severity rating from mild to severe, while discrepancies in the information from Drugs.com and Stockley’s were resolved by considering only the higher pDFI severity rating in further analyses.
Statistical analysis
For the statistical analysis of the data, we used IBM SPSS Statistics 27.0 and R version 3.6.0. Descriptive statistics of sociodemographic, clinical and pharmaceutical data as well as pDDI and pDFI data were obtained as means (± standard deviation), medians, ranges, frequencies and percentages. For comparing patients with polypharmacy (Pw/P) and patients without polypharmacy (Pw/oP), we used the following statistical tests: Fisher’s exact test, chi-square test, Mann–Whitney U test and two-sample two-tailed t test as appropriate. The significance level was set at α = 0.05. Binary logistic regression analyses were performed to evaluate the association of sociodemographic, clinical and pharmaceutical data with the presence of at least one pDDI or at least one moderate-severe/severe pDDI. To determine the combined effect of those variables on the occurrence of at least one pDDI, we used multiple logistic regression analysis with forward selection based on likelihood ratio statistics. The figures were created with Microsoft Office Professional Plus 2016, R package corrplot and Cytoscape version 3.9.0.
Results
Characterisation of the study population
In our cohort of 627 patients, the mean age [+standard deviation (SD)] was 48.6 (±13.3) years, and the proportion of female patients was 70.3%. The median EDSS score was 3.5 and the median disease duration was 10 years. Regarding disease course, 415 patients (66.2%) had CIS/RRMS, followed by 154 patients (24.6%) with SPMS and 58 patients (9.3%) with PPMS. Seven patients did not take any medication. The other 620 patients with CIS/MS took 3341 medications in total (counted with repetitions). The median number of medications taken was five. The patients were six times more likely to take long-term medications than on-demand medications (4.6 drugs versus 0.8 drugs on average). On average, 4.2 Rx drugs were taken, compared with an average of 1.1 OTC drugs. Regarding the treatment goal, 82.8% of the MS patients took DMDs. A mean of 2.0 drugs were taken for symptom reduction and an average of 2.5 drugs were taken to treat comorbidities (Table 1).
Table 1.
Sociodemographic, clinical and pharmaceutical data of MS patients with and without pDDIs.
| Parameter | All patients (N = 627) | Patients with ⩾1 pDDI (N = 408) | Patients with no pDDI (N = 219) | p-value | |||
|---|---|---|---|---|---|---|---|
| Sociodemographic data | |||||||
| Sex | 0.927 a | ||||||
| Female | 441 (70.3%) | 286 (70.1%) | 155 (70.8%) | ||||
| Male | 186 (29.7%) | 122 (29.9%) | 64 (29.2%) | ||||
| Age (years) | 19–86 b | 48.6 (13.3) c | 21–86 b | 51.9 (12.6) c | 19–75 b | 42.5 (12.5) c | <0.001 d |
| School years | 6–18 b | 10.5 (1.3) c | 6–18 b | 10.3 (1.2) c | 8–14 b | 10.8 (1.3) c | <0.001 d |
| Educational level | <0.001 e | ||||||
| No training | 19 (3.0%) | 12 (2.9%) | 7 (3.2%) | ||||
| Skilled worker | 398 (63.5%) | 280 (68.6%) | 118 (53.9%) | ||||
| Technical college | 89 (14.2%) | 56 (13.7%) | 33 (15.1%) | ||||
| University | 121 (19.3%) | 60 (14.7%) | 61 (27.9%) | ||||
| Employment status | <0.001 e | ||||||
| In training | 7 (1.1%) | 2 (0.5%) | 5 (2.3%) | ||||
| In studies | 6 (1.0%) | 1 (0.2%) | 5 (2.3%) | ||||
| Employed | 269 (42.9%) | 130 (31.9%) | 139 (63.5%) | ||||
| Unemployed | 25 (4.0%) | 13 (3.2%) | 12 (5.5%) | ||||
| Retired | 304 (48.5%) | 253 (62.0%) | 51 (23.3%) | ||||
| Others | 16 (2.6%) | 9 (2.2%) | 7 (3.2%) | ||||
| Partnership | 0.702 a | ||||||
| No | 162 (25.8%) | 103 (25.2%) | 59 (26.9%) | ||||
| Yes | 465 (74.2%) | 305 (74.8%) | 160 (73.1%) | ||||
| Place of residence | 0.040 e | ||||||
| Rural community | 224 (35.7%) | 150 (36.8%) | 74 (33.8%) | ||||
| Provincial town | 108 (17.2%) | 77 (18.9%) | 31 (14.2%) | ||||
| Medium-sized town | 112 (17.9%) | 77 (18.9%) | 35 (16.0%) | ||||
| City | 183 (29.3%) | 104 (25.5%) | 79 (36.1%) | ||||
| Number of children | 0–4 b | 1 f | 0–4 b | 1 f | 0–4 b | 1 f | 0.003 g |
| 0 | 169 (27.0%) | 91 (22.3%) | 78 (35.6%) | ||||
| 1 | 170 (27.1%) | 118 (28.9%) | 52 (23.7%) | ||||
| ⩾2 | 288 (45.9%) | 199 (48.8%) | 89 (40.6%) | ||||
| Number of siblings | 0–13 b | 1 f | 0–13 b | 1 f | 0–11 b | 1 f | 0.035 g |
| 0 | 71 (11.3%) | 40 (9.8%) | 31 (14.2%) | ||||
| 1 | 305 (48.6%) | 194 (47.5%) | 111 (50.7%) | ||||
| ⩾2 | 251 (40.0%) | 174 (42.6%) | 77 (35.2%) | ||||
| Clinical data | |||||||
| EDSS score | 0–9.0 b | 3.5 f | 0–9.0 b | 4.0 f | 0–7.5 b | 2.0 f | <0.001 g |
| Disease duration (years) | 0–52 b | 10 f | 0–50 b | 12 f | 0–52 b | 9 f | <0.001 g |
| Disease course | <0.001 e | ||||||
| CIS/RRMS | 415 (66.2%) | 223 (54.7%) | 192 (87.7%) | ||||
| SPMS | 154 (24.6%) | 136 (33.3%) | 18 (8.2%) | ||||
| PPMS | 58 (9.3%) | 49 (12.0%) | 9 (4.1%) | ||||
| Comorbidities | 0–9 b | 1 f | 0–9 b | 1 f | 0–7 b | 0 f | <0.001 g |
| No | 184 (29.3%) | 68 (16.7%) | 116 (53.0%) | ||||
| Yes | 443 (70.7%) | 340 (83.3%) | 103 (47.0%) | ||||
| Polypharmacy | <0.001 a | ||||||
| No | 293 (46.7%) | 97 (23.8%) | 196 (89.5%) | ||||
| Yes | 334 (53.3%) | 311 (76.2%) | 23 (10.5%) | ||||
| Pharmaceutical data | |||||||
| Number of drugs taken | 0–19 b | 5 f | 2–19 b | 6 f | 0–9 b | 2 f | <0.001 g |
| 0 | 7 (1.1%) | 0 (0.0%) | 7 (3.2%) | ||||
| 1–4 | 286 (45.6%) | 97 (23.8%) | 189 (86.3%) | ||||
| 5–9 | 261 (41.6%) | 238 (58.3%) | 23 (10.5%) | ||||
| ⩾ 10 | 73 (11.6%) | 73 (17.9%) | 0 (0.0%) | ||||
| Drugs divided by | |||||||
| Period of drug intake | |||||||
| Long-term drugs | 0–16 b | 4.6 (3.1) h | 1–16 b | 5.8 (3.0) h | 0–9 b | 2.2 (1.5) h | <0.001 g |
| PRN drugs | 0–7 b | 0.8 (1.2) h | 0–7 b | 1.0 (1.3) h | 0–5 b | 0.4 (0.8) h | <0.001 g |
| Access | |||||||
| Rx drugs | 0–18 b | 4.2 (3.0) h | 1–18 b | 5.4 (3.0) h | 0–6 b | 1.9 (1.2) h | <0.001 g |
| OTC drugs | 0–8 b | 1.1 (1.3) h | 0–8 b | 1.4 (1.3) h | 0–7 b | 0.7 (1.1) h | <0.001 g |
| Therapy goal | |||||||
| DMDs | 0–2 b | 0.9 (0.4) h | 0–2 b | 0.9 (0.4) h | 0–1 b | 0.7 (0.4) h | <0.001 g |
| Symptomatic drugs | 0–9 b | 2.0 (2.0) h | 0–9 b | 2.6 (2.0) h | 0–8 b | 0.8 (1.1) h | <0.001 g |
| Comorbidity drugs | 0–14 b | 2.5 (2.4) h | 0–14 b | 3.3 (2.6) h | 0–6 b | 1.0 (1.1) h | <0.001 g |
p-value for comparing patients with and without pDDIs (significant differences are indicated in bold). CIS, clinically isolated syndrome; DMD, disease-modifying drug; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; N, number of patients; OTC, over-the-counter; pDDI, potential drug–drug interaction, PPMS, primary progressive MS; PRN, pro re nata; RRMS, relapsing-remitting MS; Rx, prescription; SPMS, secondary progressive MS.
Fisher’s exact test.
Range.
Mean value (standard deviation).
Two-sample two-tailed t test.
Chi-squared test.
Median.
Mann–Whitney U test.
Average number of drugs taken per patient (standard deviation).
Comparison of patients with and without polypharmacy
There were 334 patients (53.3%) with polypharmacy (Pw/P) and 293 (46.7%) patients without polypharmacy (Pw/oP). Pw/P were on average 9.4 years older than Pw/oP (p < 0.001, t test). The median EDSS score was 4.5 for Pw/P and 2.0 for Pw/oP. The median disease duration was 3.5 years longer for Pw/P than for Pw/oP. Comorbidities were present in 83.8% of Pw/P compared with 46.8% of Pw/oP (p < 0.001, Mann–Whitney U tests) (Supplementary Table 1).
pDDIs
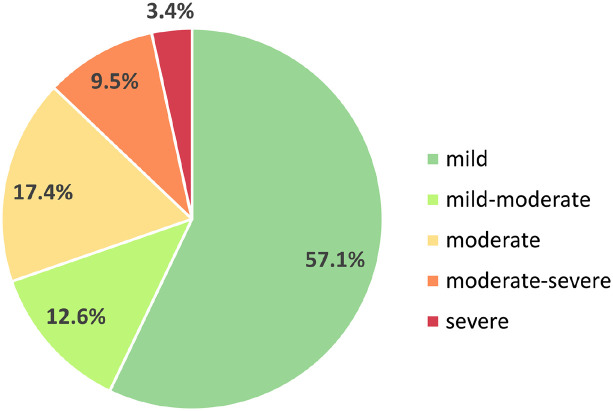
We recorded 2587 pDDIs in the data set (counted with repetitions, 1211 different pDDIs without repetitions, Supplementary Table 2). The majority of pDDIs were mild (57.1%). Moderate-severe and severe interactions together accounted for slightly more than 10% of all pDDIs (9.5% and 3.4%, respectively) (Figure 1).
Figure 1.
Percentage distribution of severity of drug–drug interactions in patients with MS. In this study, 627 MS patients had a total number of 2587 pDDIs. This chart shows the frequencies of the five pDDI severity levels. Most pDDIs were mild (57.1%), while moderate pDDIs had a share of 17.4%. Moderate-severe or severe interactions accounted for 12.9% of all interactions.
MS, multiple sclerosis; pDDIs, potential drug–drug interactions.
In the total population, 408 patients (65.1%) had at least one pDDI. In contrast, we detected no pDDI for 219 patients (34.9%). The patients with pDDIs were on average 9 years older and had a 3 years longer disease duration than the patients without pDDIs. Patients without pDDIs had a median EDSS score of 2.0 whereas patients with at least one pDDI had a median EDSS score of 4.0. The median number of medications taken was 6 in patients with at least one pDDI and 2 in patients without pDDIs. In patients without pDDIs, CIS/RRMS was by far the most common course of MS (87.7% of patients), whereas in patients with at least one pDDI, SPMS and PPMS also accounted for large proportions (CIS/RRMS: 54.7%, SPMS: 33.3%; PPMS: 12.0%) (Table 1). The median number of pDDIs was 4 for Pw/P and 0 for Pw/oP (p < 0.001, Mann–Whitney U test) (Supplementary Table 1). There were 73 patients (11.6%) taking at least 10 drugs (excessive polypharmacy). For those, the median number of pDDIs was 15 (range: 2–55) and 32.9% of them had at least one severe pDDI.
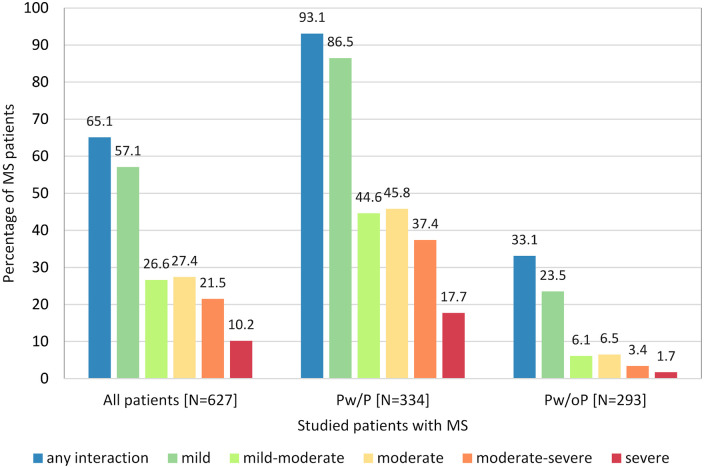
When comparing the prevalence of pDDIs (independently of pDDI severity) Pw/P had clearly more often ⩾1 pDDI as compared with Pw/oP (93.1% versus 33.1%) (Figure 2).
Figure 2.
Comparison of the prevalence of pDDIs of different severity degrees between MS patients with and without polypharmacy. The proportion of patients having pDDIs was significantly higher in Pw/P versus Pw/oP for each degree of severity (Fisher’s exact test p < 0.001). Pw/P were three times more likely to have ⩾1 pDDI than Pw/oP (93.1% versus 33.1%). The distribution of the severity degrees was skewed towards more severe interactions in Pw/P as compared with Pw/oP (chi-square test p = 0.001). Pw/P had a roughly 10-fold higher risk of severe interactions. pDDIs were determined using Stockley’s Interactions Checker and Drugs.com Interactions Checker. Note that the patients could have several pDDIs of different severities at the same time.
MS, multiple sclerosis; pDDIs, potential drug–drug interactions; Pw/oP, patients without polypharmacy; Pw/P, patients with polypharmacy.
Associations of sociodemographic, clinical and pharmaceutical data with the occurrence of pDDIs
Independent associations were found between the following sociodemographic variables and the presence of at least one pDDI: age, school years, educational level, place of residence as well as the number of children and siblings. Older patients with MS were more likely to have one or more pDDIs than younger patients (OR: 1.060 for each one-year increase, 95% CI: 1.045–1.075). More years spent in school were associated with a lower likelihood of having at least one pDDI (OR: 0.771, 95% CI: 0.676–0.879). Further associations with the occurrence of pDDIs in patients with MS were found for degree of disability (EDSS score), disease duration and number of comorbidities (p < 0.001). A one-point increase in the EDSS score led to a 58.6% increase in the probability of having at least one pDDI (OR: 1.586, 95% CI: 1.434–1.754). The odds for the occurrence of at least one pDDI rose with increasing years of disease duration (OR: 1.041, 95% CI: 1.021–1.061) and even doubled with each additional comorbidity (OR: 2.235, 95% CI: 1.893–2.638). Polypharmacy increased the likelihood for the occurrence of pDDIs by 27-fold (OR: 27.322, 95% CI: 16.764–44.529) (Table 2). Multiple logistic regression analysis revealed four associated variables: age (OR: 1.034), educational level (OR: 0.502), number of drugs taken (OR: 2.608) and number of DMDs used (OR: 2.105). The final model had a prediction accuracy of 85.8%. Similar associations were found with regard to the occurrence of moderate-severe/ severe pDDIs. Notably, the risk of moderate-severe or severe pDDIs was increased 15-fold with polypharmacy (OR: 14.920, 95% CI: 8.363–26.619) (Table 2).
Table 2.
Association of sociodemographic, clinical and pharmaceutical parameters with the presence of pDDIs or moderate-severe/severe pDDIs.
| Parameter | ⩾1 pDDI (all severities) | ⩾1 moderate-severe/severe pDDI | ||||
|---|---|---|---|---|---|---|
| OR | 95% confidence interval | p-value a | OR | 95% confidence interval | p-value a | |
| Sociodemographic data | ||||||
| Sex (ref. women) | 1.033 | (0.721–1.481) | 0.859 | 0.938 | (0.630–1.396) | 0.751 |
| Age (in years) | 1.060 | (1.045–1.075) | <0.001 | 1.071 | (1.053–1.089) | <0.001 |
| School years (in years) | 0.771 | (0.676–0.879) | <0.001 | 0.641 | (0.540–0.760) | <0.001 |
| Educational level (ref. no. training) | 0.680 | (0.560–0.827) | <0.001 | 0.678 | (0.534–0.862) | 0.001 |
| Partnership (ref. single) | 1.092 | (0.752–1.585) | 0.644 | 0.825 | (0.551–1.236) | 0.351 |
| Place of residence (ref. rural area) | 0.871 | (0.763–0.995) | 0.041 | 0.959 | (0.829–1.109) | 0.572 |
| Number of children (number) | 1.259 | (1.064–1.489) | 0.007 | 1.430 | (1.191–1.718) | <0.001 |
| Number of siblings (number) | 1.149 | (1.016–1.301) | 0.027 | 1.259 | (1.122–1.413) | <0.001 |
| Clinical data | ||||||
| EDSS score (points) | 1.586 | (1.434–1.754) | <0.001 | 1.479 | (1.346–1.626) | <0.001 |
| Disease duration (in years) | 1.041 | (1.021–1.061) | <0.001 | 1.048 | (1.029–1.068) | <0.001 |
| Comorbidities (number) | 2.235 | (1.893–2.638) | <0.001 | 1.811 | (1.595–2.056) | <0.001 |
| Pharmaceutical data | ||||||
| Number of drugs taken (number) | 2.665 | (2.271–3.127) | <0.001 | 1.616 | (1.487–1.756) | <0.001 |
| Polypharmacy (ref. no. polypharmacy) | 27.322 | (16.764–44.529) | <0.001 | 14.920 | (8.363–26.619) | <0.001 |
| Long-term drugs (number) | 2.306 | (2.006–2.652) | <0.001 | 1.576 | (1.453–1.710) | <0.001 |
| PRN drugs (number) | 1.884 | (1.523–2.332) | <0.001 | 1.482 | (1.276–1.722) | <0.001 |
| Rx drugs (number) | 2.665 | (2.260–3.143) | <0.001 | 1.755 | (1.594–1.932) | <0.001 |
| OTC drugs (number) | 1.743 | (1.463–2.076) | <0.001 | 1.145 | (0.999–1.311) | 0.052 |
| DMD (number) | 2.504 | (1.673–3.748) | <0.001 | 1.324 | (0.836–2.097) | 0.232 |
| Symptomatic drugs (number) | 2.221 | (1.900–2.595) | <0.001 | 1.360 | (1.241–1.491) | <0.001 |
| Comorbidity drugs (number) | 2.187 | (1.876–2.550) | <0.001 | 1.831 | (1.642–2.043) | <0.001 |
ORs and significance values were calculated by binary logistic regression analysis for each parameter. The analysis was based on the data of 627 patients with MS. In the left part of the table, 408 patients with pDDIs were compared with 219 patients without pDDIs. In the right part of the table, 157 patients with ⩾1 moderate-severe or severe pDDI were compared with 470 patients without such pDDI. DMD, disease-modifying drug; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; OR, odds ratio; OTC, over-the-counter; pDDI, potential drug–drug interaction; PRN, pro re nata; ref., reference; Rx, prescription.
p: p-value for each regression coefficient (p < 0.05 are indicated in bold).
Interacting active ingredients
The top 20 agents, for which the most pDDIs were counted, ranged from methylprednisolone (pDDI count: 247) to calcium (pDDI count: 73) (Table 3). About 20% of all patients took at least one of these top 20 agents: pantoprazole (N = 178 patients, 28.4%), enoxaparin (N = 127 patients, 20.3%) and methylprednisolone (N = 123 patients, 19.6%). There were significant differences in the use of drugs between patients with and without polypharmacy. For instance, enoxaparin was more often taken by Pw/P than by Pw/oP (Pw/P: 34.1% versus Pw/oP: 4.4%) (p < 0.001, Fisher’s exact test). A listing of all agents involved in pDDIs with the number of total interactions and the distribution of pDDI severity levels is provided in Supplementary Table 3.
Table 3.
The top 20 substances for which the most pDDIs were identified in the cohort of MS patients (N = 627).
| Active ingredient | pDDI count | Degree of pDDI severity, N | Patients, N (%) | p a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Mild-severe | Moderate | Moderate-severe | Severe | Total (N = 627) | Pw/P (N = 334) | Pw/oP (N = 293) | |||
| Methylprednisolone | 247 | 106 | 51 | 63 | 22 | 5 | 123 (19.6%) | 110 (32.9%) | 13 (4.4%) | <0.001 |
| Acetylsalicylic acid | 232 | 83 | 37 | 72 | 33 | 7 | 55 (8.8%) | 48 (14.4%) | 7 (2.4%) | <0.001 |
| Ibuprofen | 211 | 87 | 37 | 28 | 53 | 6 | 105 (16.7%) | 61 (18.3%) | 44 (15.0%) | 0.286 |
| Pantoprazole | 190 | 122 | 6 | 61 | 0 | 1 | 178 (28.4%) | 155 (46.4%) | 23 (7.8%) | <0.001 |
| Baclofen | 189 | 107 | 17 | 58 | 7 | 0 | 78 (12.4%) | 72 (21.6%) | 6 (2.0%) | <0.001 |
| Ramipril | 164 | 80 | 7 | 41 | 31 | 5 | 53 (8.5%) | 41 (12.3%) | 12 (4.1%) | <0.001 |
| Bisoprolol | 151 | 95 | 30 | 18 | 8 | 0 | 51 (8.1%) | 46 (13.8%) | 5 (1.7%) | <0.001 |
| Cannabidiol | 139 | 121 | 3 | 14 | 1 | 0 | 46 (7.3%) | 40 (12.0%) | 6 (2.0%) | <0.001 |
| Dronabinol | 136 | 120 | 5 | 6 | 5 | 0 | 47 (7.5%) | 41 (12.3%) | 6 (2.0%) | <0.001 |
| Torasemide | 127 | 60 | 10 | 54 | 3 | 0 | 22 (3.5%) | 22 (6.6%) | 0 (0.0%) | <0.001 |
| Citalopram | 122 | 36 | 32 | 11 | 16 | 27 | 33 (5.3%) | 25 (7.5%) | 8 (2.7%) | 0.011 |
| Enoxaparin | 112 | 33 | 0 | 6 | 71 | 2 | 127 (20.3%) | 114 (34.1%) | 13 (4.4%) | <0.001 |
| Hydrochlorothiazide | 94 | 42 | 5 | 39 | 6 | 2 | 8 (1.3%) | 7 (2.1%) | 1 (0.3%) | 0.073 |
| Metoprolol | 90 | 53 | 17 | 18 | 2 | 0 | 29 (4.6%) | 25 (7.5%) | 4 (1.4%) | <0.001 |
| Levothyroxine | 90 | 47 | 3 | 37 | 3 | 0 | 82 (13.1%) | 55 (16.5%) | 27 (9.2%) | 0.009 |
| Amlodipine | 86 | 40 | 18 | 25 | 3 | 0 | 25 (4.0%) | 22 (6.6%) | 3 (1.0%) | <0.001 |
| Duloxetine | 84 | 63 | 3 | 5 | 10 | 3 | 21 (3.3%) | 19 (5.7%) | 2 (0.7%) | <0.001 |
| Zopiclone | 83 | 70 | 1 | 10 | 0 | 2 | 65 (10.4%) | 58 (17.4%) | 7 (2.4%) | <0.001 |
| Magnesium | 79 | 76 | 3 | 0 | 0 | 0 | 65 (10.4%) | 49 (14.7%) | 16 (5.5%) | <0.001 |
| Calcium | 73 | 63 | 0 | 9 | 1 | 0 | 33 (5.3%) | 32 (9.6%) | 1 (0.3%) | <0.001 |
The table is sorted by the total number of pDDIs per drug in the data set (pDDI count). In addition, the number of pDDIs broken down by degree of severity and the number of MS patients who received the respective drugs are provided. MS, multiple sclerosis; N, number of patients; pDDI, potential drug–drug interaction; Pw/oP, patients without polypharmacy; Pw/P, patients with polypharmacy.
p: p-value according to Fisher’s exact test for comparing Pw/P and Pw/oP (significant differences are indicated in bold).
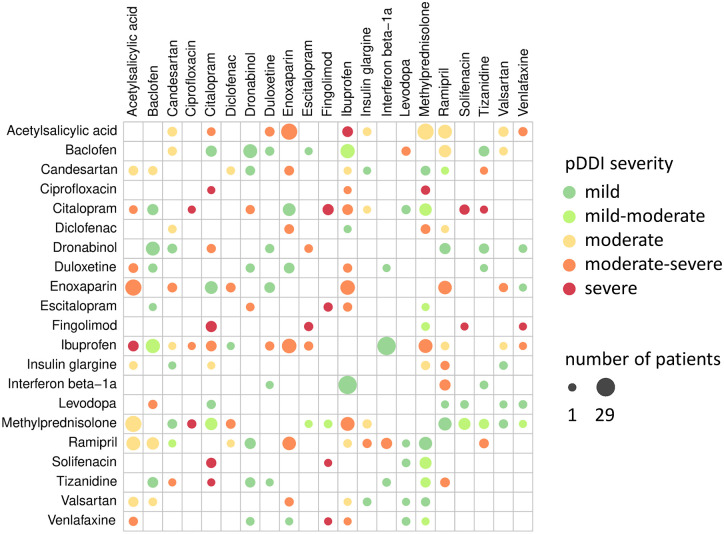
All moderate-severe (N = 18) or severe (N = 5) pDDIs that occurred in at least three of the MS patients studied are shown in Table 4. The most relevant severe pDDIs were found between the following drugs: citalopram ⇔ fingolimod (N = 7 patients) and acetylsalicylic acid ⇔ ibuprofen (N = 6 patients). The moderate-severe pDDIs acetylsalicylic acid ⇔ enoxaparin, ibuprofen ⇔ enoxaparin, methylprednisolone ⇔ ibuprofen, enoxaparin ⇔ ramipril and citalopram ⇔ ibuprofen were significantly more often recorded in Pw/P than in Pw/oP (p < 0.05, Fisher’s exact tests). For those agents involved in the 23 moderate-severe or severe pDDIs that were repeatedly observed and that are listed in Table 4, we visualised the frequency and severity of all pairwise interactions in Figure 3. Among these, the most frequent pDDI was found between interferon beta-1a and ibuprofen (N = 29 patients).
Table 4.
Moderate-severe and severe pDDIs that were recorded in at least three patients with MS.
| Potential drug–drug interaction | All patients (N = 627) | Pw/P (N = 334) | Pw/oP (N = 293) | p a |
|---|---|---|---|---|
| Severe | ||||
| Citalopram ⇔ Fingolimod | 7 (1.1%) | 5 (1.5%) | 2 (0.7%) | 0.458 |
| Acetylsalicylic acid ⇔ Ibuprofen | 6 (1.0%) | 6 (1.8%) | 0 (0.0%) | 0.033 |
| Citalopram ⇔ Solifenacin | 5 (0.8%) | 4 (1.2%) | 1 (0.3%) | 0.378 |
| Ciprofloxacin ⇔ Methylprednisolone | 3 (0.5%) | 3 (0.9%) | 0 (0.0%) | 0.252 |
| Escitalopram ⇔ Fingolimod | 3 (0.5%) | 2 (0.6%) | 1 (0.3%) | 1.000 |
| Moderate-severe | ||||
| Acetylsalicylic acid ⇔ Enoxaparin | 21 (3.3%) | 20 (6.0%) | 1 (0.3%) | <0.001 |
| Enoxaparin ⇔ Ibuprofen | 16 (2.6%) | 14 (4.2%) | 2 (0.7%) | 0.005 |
| Ibuprofen ⇔ Methylprednisolone | 14 (2.2%) | 13 (3.9%) | 1 (0.3%) | 0.002 |
| Enoxaparin ⇔ Ramipril | 13 (2.1%) | 13 (3.9%) | 0 (0.0%) | <0.001 |
| Interferon beta-1a ⇔ Ramipril | 7 (1.1%) | 5 (1.5%) | 2 (0.7%) | 0.458 |
| Citalopram ⇔ Ibuprofen | 6 (1.0%) | 6 (1.8%) | 0 (0.0%) | 0.033 |
| Diclofenac ⇔ Enoxaparin | 4 (0.6%) | 4 (1.2%) | 0 (0.0%) | 0.127 |
| Diclofenac ⇔ Methylprednisolone | 4 (0.6%) | 4 (1.2%) | 0 (0.0%) | 0.127 |
| Acetylsalicylic acid ⇔ Duloxetine | 4 (0.6%) | 4 (1.2%) | 0 (0.0%) | 0.127 |
| Ramipril ⇔ Tizanidine | 4 (0.6%) | 4 (1.2%) | 0 (0.0%) | 0.127 |
| Candesartan ⇔ Tizanidine | 4 (0.6%) | 4 (1.2%) | 0 (0.0%) | 0.127 |
| Acetylsalicylic acid ⇔ Venlafaxine | 3 (0.5%) | 2 (0.6%) | 1 (0.3%) | 1.000 |
| Enoxaparin ⇔ Valsartan | 3 (0.5%) | 3 (0.9%) | 0 (0.0%) | 0.252 |
| Baclofen ⇔ Levodopa | 3 (0.5%) | 3 (0.9%) | 0 (0.0%) | 0.252 |
| Duloxetine ⇔ Ibuprofen | 3 (0.5%) | 2 (0.6%) | 1 (0.3%) | 1.000 |
| Insulin glargine ⇔ Ramipril | 3 (0.5%) | 3 (0.9%) | 0 (0.0%) | 0.252 |
| Citalopram ⇔ Dronabinol | 3 (0.5%) | 3 (0.9%) | 0 (0.0%) | 0.252 |
| Escitalopram ⇔ Ibuprofen | 3 (0.5%) | 3 (0.9%) | 0 (0.0%) | 0.252 |
The table is sorted by pDDI severity and prevalence. It is also indicated how often a particular pDDI was counted in the groups of patients with polypharmacy (Pw/P) and without polypharmacy (Pw/oP), respectively. MS, multiple sclerosis; N, number of patients; pDDIs, potential drug–drug interactions; Pw/oP, patients without polypharmacy; Pw/P, patients with polypharmacy.
p: p-value according to Fisher’s exact test for comparing Pw/P and Pw/oP (significant differences are indicated in bold).
Figure 3.
Interaction heatmap of drugs for which moderate-severe or severe pDDIs have been repeatedly noted in patients with MS. Shown is the frequency and severity of pDDIs between drugs involved in moderate-severe or severe pDDIs that were identified in at least three patients with MS (see also Table 4). The active ingredients are listed in alphabetical order. The size of the dots represents the frequency of pDDIs in the patient cohort (N = 627). The colour of the dots indicates the severity of the pDDI. The most common interaction has been recorded between interferon beta-1a and ibuprofen (29 patients).
MS, multiple sclerosis; pDDIs, potential drug–drug interactions.
Potential drug–food interactions
In the analysis of pDFIs, 254 drugs were found to be involved in pDFIs in our study population. Of these, 34 drugs belong to at least one severe pDFI, with alcohol being responsible for 21 severe pDFIs (e.g. acetaminophen ⇔ alcohol) (Supplementary Table 4). The pDFIs with the 20 active ingredients most frequently involved in pDDIs are listed in Table 5 and visualised in Figure 4. The only severe pDFI in this subset was found for ibuprofen ⇔ alcohol. A total of 105 patients (16.7%) may be at risk of this pDFI as they took ibuprofen. Three pDFIs were found for dronabinol, which may affect 47 patients (7.5%) (Table 5).
Table 5.
Drug–food interactions for the top 20 substances for which the most pDDIs were identified.
| Active ingredient | Patients, N (%) | Degree of drug–food interaction severity | ||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Methylprednisolone | 123 (19.6%) | – | Grapefruit, tobacco | – |
| Acetylsalicylic acid | 55 (8.8%) | Alcohol, food | – | – |
| Ibuprofen | 105 (16.7%) | – | – | Alcohol |
| Pantoprazole | 178 (28.4%) | – | – | – |
| Baclofen | 78 (12.4%) | – | Alcohol | – |
| Ramipril | 53 (8.5%) | Alcohol | Food (potassium-containing) | – |
| Bisoprolol | 51 (8.1%) | Alcohol, tobacco | – | – |
| Cannabidiol | 46 (7.3%) | – | Food (high-fat meal), grapefruit | – |
| Dronabinol | 47 (7.5%) | Grapefruit | Alcohol, food (high-fat meal) | – |
| Torasemide | 22 (3.5%) | – | – | – |
| Citalopram | 33 (5.3%) | – | Alcohol | – |
| Enoxaparin | 127 (20.3%) | – | – | – |
| Hydrochlorothiazide | 8 (1.3%) | – | – | – |
| Metoprolol | 29 (4.6%) | Alcohol, tobacco | Food | – |
| Levothyroxine | 82 (13.1%) | – | Food a , grapefruit | – |
| Amlodipine | 25 (4.0%) | Grapefruit | Alcohol | – |
| Duloxetine | 21 (3.3%) | Tobacco | Alcohol | – |
| Zopiclone | 65 (10.4%) | – | Alcohol, food (high-fat/heavy meal) | – |
| Magnesium | 65 (10.4%) | – | – | – |
| Calcium | 33 (5.3%) | – | Food b | – |
pDFI databases often only indicate ‘food’ as an interaction partner of a drug. This usually refers to the timing of the food intake or a certain food composition such as food high in fat or potassium-containing food. Food: The timing of food intake is a factor influencing the absorption of ingested medicines. Patients, N (%): number of MS patients who have received the respective drug. pDDIs, potential drug–drug interactions; pDFI, potential drug–food interaction.
Dietary fibre, milk, soy products, coffee, nuts and seeds.
Foods high in oxalic acid (e.g. spinach or rhubarb) or phytic acid (e.g. bran and whole grains).
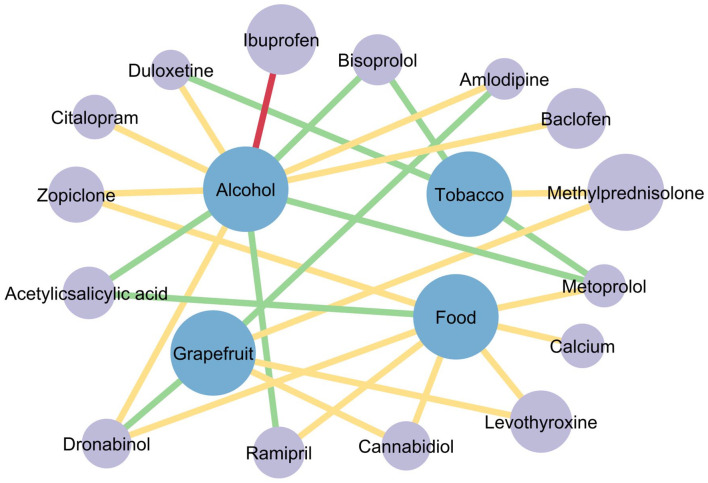
Figure 4.
Network of pDFIs for the top 20 drugs for which the most pDDIs were recorded. Grey dots stand for medications and blue dots represent other substances. The size of the grey dots shows the number of patients taking this drug (e.g. methylprednisolone was taken by 123 patients). The line colour indicates the severity of the interaction: green – mild interaction, yellow – moderate interaction and red – severe interaction. A total of 28 pDFIs were found between the top 20 drugs for which the most pDDIs were identified (Tables 3 and 5). Between those, there are 100 different pDDIs, which are not shown here for simplicity. A severe pDFI was found between ibuprofen and alcohol. Among the top 20 drugs, pantoprazole, torasemide, enoxaparin, hydrochlorothiazide and magnesium showed no interaction with alcohol, food or tobacco smoke.
pDDIs, potential drug–drug interactions; pDFIs, potential drug–food interactions.
Discussion
This study focused on the prevalence and severity of pDDIs in patients with MS. Therefore, the medication schedules of 627 patients were checked. Our study serves the purpose of showing health professionals which patients may have a high likelihood of having pDDIs and which drugs may be most frequently involved. A special feature of this study represents the analysis of pDFIs of the drugs that were taken by our patient cohort.
Our previous studies are, to our knowledge, the only studies on pDDIs in patients with MS in the literature.33,40 We found in a smaller study of 131 women in childbearing age that the prevalence of having at least one pDDI of average danger was significantly higher in Pw/P than in Pw/oP (31.5% versus 5.2%, p < 0.001). 33 There were also significant associations between polypharmacy and higher age, higher degree of disability (EDSS score) and higher number of comorbidities. 33 In our recently published study, we found significantly higher pDDI prevalence rates for MS patients with cardiovascular, neurological, psychiatric and orthopaedic comorbidities. 40 The present study focused on the analysis of pDDIs and their severity by incorporating information from Drugs.com. We determined sociodemographic and clinical factors that are associated with an increased likelihood of (severe) pDDIs in patients with MS.
The relatively high proportion of MS patients with at least one pDDI detected in our study is a main consequence of the drug-intensive treatment to reduce disease activity and to alleviate MS-related symptoms but is also related to the presence of comorbidities, especially older age. However, only slightly more than 10% of all recorded pDDIs were moderate-severe or severe pDDIs. Due to the lack of studies on pDDIs in MS patients, we looked at the prevalence of pDDIs in other medical disciplines. Doan et al. 37 demonstrated that the likelihood of at least one pDDI in hospitalised patients aged 65 or older depends on the number of drugs taken (e.g. 50% for persons taking 5–9 drugs). In a study of outpatients taking oral anticancer drugs, a proportion of 263 patients (89.4%) had at least one pDDI. 38 Ismail et al. reported an overall prevalence of pDDIs of 78% in 678 patients receiving chemotherapy. A large proportion of those (39.2%) had only one to two pDDIs, and severe interactions accounted for the majority of pDDIs (67.3%). 41 However, the results are difficult to compare because different patient inclusion criteria and different pDDI databases were used in these studies.
Although the association between polypharmacy and pDDIs is well known, our study described for the first time that polypharmacy led to a 15-fold (OR: 14.920) increase in the likelihood of severe or moderate-severe pDDIs in patients with MS. In our study, we found an age difference between patients with and without pDDIs of almost 10 years. The association between the occurrence of pDDIs and age is consistent with previous studies. Janchawee et al. 42 found that the odds of having at least one pDDI increased with an age difference of 20 years by a factor of 1.8. Bjerrum et al. 2 could also relate the presence of pDDIs to higher age and a higher number of medications taken. The increase in multimorbidity with age and the use of multiple medications to treat comorbidities significantly contribute to polypharmacy and the risk of pDDIs.
Methylprednisolone was the active substance with the most interactions in our data set (247 pDDIs). Of these, most pDDIs were mild interactions (n = 106). On the one hand, relapse therapy with high-dose methylprednisolone is carried out as standard. 43 On the other hand repeated pulse therapy (e.g. every 3 months) is also occasional used by patients with SPMS or PPMS, although convincing class I evidence is lacking. 44 During the period of our data collection, many patients with PPMS or SPMS have been treated in this way.45,46 Acetylsalicylic acid and ibuprofen ranked second and third among the agents with the highest pDDI counts. This puts two common OTC agents among the top triggers of pDDIs in patients with MS. Ibuprofen, as a non-steroidal anti-inflammatory drug (NSAID), influences inflammatory processes, acts as an analgesic and is one of the therapeutic strategies for treatment-related pain. 47 For instance, the early phase of interferon beta therapy can lead to flu-like symptoms and myalgias, while ibuprofen (as well as acetaminophen) can help to relieve these.48–50 Of note, only a few pDDIs were recorded for vitamin supplements (vitamin C, D and E), and none of them were moderate-severe or severe.
Particularly severe pDDIs are clinically relevant due to their potentially serious consequences (including death). The most frequent moderate-severe pDDIs were acetylsalicylic acid ⇔ enoxaparin (N = 21 patients, 3.3%) and enoxaparin ⇔ ibuprofen (N = 16, 2.6%). Those pDDIs may lead to an increased risk of bleeding. For this reason, careful clinical laboratory monitoring is indicated in patients taking acetylsalicylic acid or enoxaparin. 51 The most common severe pDDI occurred between citalopram and fingolimod (N = 7 patients, 1.1%). Citalopram accounted for most of the severe interactions (N = 27) in our study. As a selective serotonin reuptake inhibitor (SSRI), citalopram is often prescribed to patients with anxiety disorders or depression. A side effect of citalopram may cause prolongation of the QT interval, which may lead to ventricular arrhythmias or sudden cardiac dead. 52 Fingolimod is used for the treatment of RRMS, and administration of the first dose may also prolong the QT interval, especially when given concomitantly with SSRIs. 53 Thus, citalopram should be avoided within the first days after the start of fingolimod therapy, but afterwards there are no safety concerns so far, so that the actual severity of this pDDI strongly depends on the timing.53–55 Although some pDDIs can only be explained theoretically and have not been proven in studies, an assessment of the individual risk factors should still be performed.
Taking into account all degrees of severity, the most common pDDI was a mild interaction between cannabidiol (CBD) and dronabinol (=tetrahydrocannabidiol, THC) (n = 41, 6.5%). CBD and THC are components of Cannabis sativa, which is contained in Nabiximols (Sativex®).56–58 Cannabis sativa is used in MS to improve the symptoms of moderate to severe spasticity and as an off-label treatment for urge incontinence.59,60 It was found that both agents can be substrates as well as inhibitors of cytochrome P450 enzymes and thus interact with other medications. 61 Conversely, a change in the activity of the enzymes can lead to higher or lower CBD/THC levels. Due to impaired attention and altered psychomotor abilities, patients taking cannabis should be advised not to engage in safety-related activities requiring full concentration and motor skills, e.g. driving motor vehicles. 62
The consideration of pDFIs is important to increase the success of treatments. Pharmacists and clinical staff should therefore pay attention on frequently used drugs that are associated with pDFIs. Foods beverages and lifestyle factors that can interfere with the effect of medicines include for example alcoholic drinks, grapefruit juice and tobacco smoking. In our study population, we were able to detect 34 severe pDFIs. The most frequent severe pDFI was between ibuprofen and alcohol (n = 105 patients). It has been shown that regular ibuprofen users who drink alcoholic beverages have a 2.7-fold higher risk of upper gastrointestinal bleeding compared with nonusers. 63 For methylprednisolone, we detected moderate pDFIs with grapefruit (juice) and tobacco. Grapefruit juice can increase the bioavailability of oral methylprednisolone in plasma by 75% but does not significantly affect cortisol plasma concentrations. 64 Although clinical relevance is low, the effect of methylprednisolone may be enhanced in individuals who ingest a high amount of grapefruit juice. 64 For dronabinol, a moderate pDFI is described when combined with high-fat food. With regard to bioavailability, an increase in the maximum concentration (in plasma) by a factor of one to three can be observed for dronabinol (administered as a spray) when a high-fat diet is taken. 65 According to Stott et al. 65 this interaction seems to be clinically less relevant due to interindividual variability. Nevertheless, the doctor should recommend taking dronabinol-containing drugs outside mealtimes in order to avoid possible fluctuations in effect.
Our study cohort well resembled data from the German MS registry (18,030 registered patients) in terms of age (on average, 46.3 years), sex (72% female), median EDSS score (3.0) and disease course distribution.66,67 Thelen et al. 68 reported a similar range of patients meeting the criteria for polypharmacy (15–65% of MS patients). An Italian study by Patti et al. 35 reported a polypharmacy rate of 32.3% in MS patients aged 41–50 years and of 41.2% in patients aged over 50 years. In our previous study of women of childbearing age with MS, the proportion of patients with polypharmacy was 41.2%. 33
Some limitations of this study should be mentioned. From the structured interviews and the patient records, there is no claim to completeness of the data regarding the number and type of medications used. There is a possibility of a wrongly low/high number of recorded medications as patients often do not exactly know their own medication, or they take additional OTC drugs or CAMs that they do not mention exactly. For instance, patients often fail to mention the use of NSAIDs to their physicians. 69 Furthermore, adverse reactions because of a pDDI do not necessarily have to occur in a patient, but there is an increased probability. In this study, we did not record adverse drug reactions that actually occurred in the patients. Further limitations are the unknown adherence of drug intake and the unmeasured individual metabolism characteristics of the patients (e.g. CYP enzyme expression).70–72 Our study did not assess the patients’ actual dietary pattern, time of food intake or cigarette and alcohol consumption. In further studies, one might explicitly ask MS patients about drug side effects in the following after an initial check of the medication schedules for pDDIs or, if applicable, measure drug levels in the blood to detect pDDIs and pDFIs that actually occur. In the future, deep learning algorithms could improve the prediction of pDDIs and pDFIs. 73
Conclusion
In our study of 627 patients with MS, we found at least one pDDI in 408 patients (65.1%). Patients with at least one pDDI were on average 9.4 years older and had 3 years longer disease duration than patients without pDDIs. According to our data, Pw/P are 15 times more likely to have a severe pDDI than Pw/oP. Age and educational level were identified as factors associated with the presence of pDDIs. The most frequent severe pDDI was citalopram ⇔ fingolimod. Therefore, caution is advised when initiating fingolimod therapy in patients using citalopram. Methylprednisolone, acetylsalicylic acid and ibuprofen had the highest pDDI count. This underlines an increased risk of pDDIs from the use of OTC preparations (e.g. acetylsalicylic acid and ibuprofen). In our analysis of pDFIs, 34 severe pDFIs were identified. We found that the combination of ibuprofen and alcohol was the most frequent severe pDFI. Subsequent studies should address dietary habits as well as alcohol and cigarette consumption via questionnaires, or, if possible, be substantiated by laboratory tests. This would allow a better assessment of the actual risk of pDFIs to optimise the medication plan of individual patients.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221108391 for Associated factors of potential drug-drug interactions and drug–food interactions in patients with multiple sclerosis by Jane Louisa Debus, Paula Bachmann, Niklas Frahm, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker and Uwe Klaus Zettl in Therapeutic Advances in Chronic Disease
Supplemental material, sj-xlsx-2-taj-10.1177_20406223221108391 for Associated factors of potential drug-drug interactions and drug–food interactions in patients with multiple sclerosis by Jane Louisa Debus, Paula Bachmann, Niklas Frahm, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker and Uwe Klaus Zettl in Therapeutic Advances in Chronic Disease
Supplemental material, sj-xlsx-3-taj-10.1177_20406223221108391 for Associated factors of potential drug-drug interactions and drug–food interactions in patients with multiple sclerosis by Jane Louisa Debus, Paula Bachmann, Niklas Frahm, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker and Uwe Klaus Zettl in Therapeutic Advances in Chronic Disease
Supplemental material, sj-xlsx-4-taj-10.1177_20406223221108391 for Associated factors of potential drug-drug interactions and drug–food interactions in patients with multiple sclerosis by Jane Louisa Debus, Paula Bachmann, Niklas Frahm, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker and Uwe Klaus Zettl in Therapeutic Advances in Chronic Disease
Acknowledgments
Special thanks go to the nurses and physicians of the outpatient and inpatient MS wards of the neurological clinics at the Rostock University Centre and the Ecumenical Hainich Hospital Mühlhausen for their support during data collection.
Footnotes
ORCID iD: Jane Louisa Debus  https://orcid.org/0000-0002-6217-2962
https://orcid.org/0000-0002-6217-2962
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jane Louisa Debus, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Gehlsheimer Str. 20, 18147 Rostock, Germany.
Paula Bachmann, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Rostock, Germany.
Niklas Frahm, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Rostock, Germany.
Pegah Mashhadiakbar, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Rostock, Germany.
Silvan Elias Langhorst, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Rostock, Germany.
Barbara Streckenbach, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Rostock, Germany; Department for Neurology, Ecumenic Hainich Hospital gGmbH, Mühlhausen, Germany.
Julia Baldt, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Rostock, Germany; Department for Neurology, Ecumenic Hainich Hospital gGmbH, Mühlhausen, Germany.
Felicita Heidler, Department for Neurology, Ecumenic Hainich Hospital gGmbH, Mühlhausen, Germany.
Michael Hecker, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Rostock, Germany.
Uwe Klaus Zettl, Neuroimmunology Section, Department of Neurology, Rostock University Medical Centre, Rostock, Germany.
Declarations
Ethics approval and consent to participate: Approval for this study was granted by the ethics committees of the Thuringia Medical Association and the Rostock University Medical Centre (approval numbers A 2014-0089 and A 2019-0048). The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication: Not applicable.
Author contributions: Jane Louisa Debus: Conceptualisation; Data curation; Formal analysis; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Paula Bachmann: Data curation; Writing – review & editing.
Niklas Frahm: Conceptualisation; Data curation; Methodology; Writing – review & editing.
Pegah Mashhadiakbar: Investigation; Writing – review & editing.
Silvan Elias Langhorst: Investigation; Writing – review & editing.
Barbara Streckenbach: Investigation; Writing – review & editing.
Julia Baldt: Investigation; Writing – review & editing.
Felicita Heidler: Data curation; Writing – review & editing.
Michael Hecker: Conceptualisation; Data curation; Formal analysis; Methodology; Visualisation; Writing – review & editing.
Uwe Klaus Zettl: Conceptualisation; Methodology; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JLD, PB, PM, SEL, BS, JB and FH declare no conflict of interest. NF received travel funds for research meetings from Novartis. MH received speaking fees and travel funds from Bayer HealthCare, Biogen, Merck Serono, Novartis and Teva. UKZ received speaking fees, travel support and/or financial support for research activities from Alexion, Almirall, Bayer, Biogen, Janssen, Merck Serono, Novartis, Octapharm, Roche, Sanofi Genzyme, Teva as well as EU, BMBF, BMWi and DFG.
Availability of data and material: The data sets generated and analysed in this study are available from the corresponding author on reasonable request.
References
- 1. Voigt N, Ort K, Sossalla S. Drug-drug interactions you should know! Fortschr Neurol Psychiatr 2019; 87: 320–332. [DOI] [PubMed] [Google Scholar]
- 2. Bjerrum L, Gonzalez Lopez-Valcarcel B, Petersen G. Risk factors for potential drug interactions in general practice. Eur J Gen Pract 2008; 14: 23–29. [DOI] [PubMed] [Google Scholar]
- 3. Dechanont S, Maphanta S, Butthum B, et al. Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2014; 23: 489–497. [DOI] [PubMed] [Google Scholar]
- 4. Moura CS, Acurcio FA, Belo NO. Drug-drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci 2009; 12: 266–272. [DOI] [PubMed] [Google Scholar]
- 5. Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother 2013; 4(Suppl. 1): S73–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc 2001; 41: 192–199. [DOI] [PubMed] [Google Scholar]
- 7. Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin Drug Saf 2012; 11: 83–94. [DOI] [PubMed] [Google Scholar]
- 8. Deng J, Zhu X, Chen Z, et al. A review of food-drug interactions on oral drug absorption. Drugs 2017; 77: 1833–1855. [DOI] [PubMed] [Google Scholar]
- 9. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018; 378: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler 2020; 26: 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zettl UK, Stüve O, Patejdl R. Immune-mediated CNS diseases: a review on nosological classification and clinical features. Autoimmun Rev 2012; 11: 167–173. [DOI] [PubMed] [Google Scholar]
- 14. Rommer PS, Eichstädt K, Ellenberger D, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Mult Scler 2019; 25: 1641–1652. [DOI] [PubMed] [Google Scholar]
- 15. Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med 2020; 133: 1380–1390.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toosy A, Ciccarelli O, Thompson A. Symptomatic treatment and management of multiple sclerosis. Handb Clin Neurol 2014; 122: 513–562. [DOI] [PubMed] [Google Scholar]
- 17. Patejdl R, Penner IK, Noack TK, et al. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev 2016; 15: 210–220. [DOI] [PubMed] [Google Scholar]
- 18. Patejdl R, Zettl UK. Spasticity in multiple sclerosis: contribution of inflammation, autoimmune mediated neuronal damage and therapeutic interventions. Autoimmun Rev 2017; 16: 925–936. [DOI] [PubMed] [Google Scholar]
- 19. Rommer PS, Zettl UK. Managing the side effects of multiple sclerosis therapy: pharmacotherapy options for patients. Expert Opin Pharmacother 2018; 19: 483–498. [DOI] [PubMed] [Google Scholar]
- 20. Rommer P, Zettl UK. Treatment options in multiple sclerosis and neuromyelitis optica spectrum disorders. Curr Pharm Des 2022; 28: 428–436. [DOI] [PubMed] [Google Scholar]
- 21. Wiendl H, Gold R, Berger T, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord 2021; 14: 17562864211039648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marrie RA. Comorbidity in multiple sclerosis: past, present and future. Clin Invest Med 2019; 42: E5–E12. [DOI] [PubMed] [Google Scholar]
- 23. Petersen MJ, Bergien SO, Staerk D. A systematic review of possible interactions for herbal medicines and dietary supplements used concomitantly with disease-modifying or symptom-alleviating multiple sclerosis drugs. Phytother Res 2021; 35: 3610–3631. [DOI] [PubMed] [Google Scholar]
- 24. Rommer PS, König N, Sühnel A, et al. Coping behavior in multiple sclerosis-complementary and alternative medicine: a cross-sectional study. CNS Neurosci Ther 2018; 24: 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kochs L, Wegener S, Sühnel A, et al. The use of complementary and alternative medicine in patients with multiple sclerosis: a longitudinal study. Complement Ther Med 2014; 22: 166–172. [DOI] [PubMed] [Google Scholar]
- 26. Gotta M, Mayer CA, Huebner J. Use of complementary and alternative medicine in patients with multiple sclerosis in Germany. Complement Ther Med 2018; 36: 113–117. [DOI] [PubMed] [Google Scholar]
- 27. Apel A, Greim B, König N, et al. Frequency of current utilisation of complementary and alternative medicine by patients with multiple sclerosis. J Neurol 2006; 253: 1331–1336. [DOI] [PubMed] [Google Scholar]
- 28. Runia TF, Hop WCJ, Rijke YB, et al. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology 2012; 79: 261–266. [DOI] [PubMed] [Google Scholar]
- 29. Frahm N, Hecker M, Zettl UK. Multi-drug use among patients with multiple sclerosis: a cross-sectional study of associations to clinicodemographic factors. Sci Rep 2019; 9: 3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frahm N, Hecker M, Zettl UK. Polypharmacy in chronic neurological diseases: multiple sclerosis, dementia and Parkinson’s disease. Curr Pharm Des 2021; 27: 4008–4016. [DOI] [PubMed] [Google Scholar]
- 31. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr 2017; 17: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frahm N, Hecker M, Zettl UK. Polypharmacy among patients with multiple sclerosis: a qualitative systematic review. Expert Opin Drug Saf 2020; 19: 139–145. [DOI] [PubMed] [Google Scholar]
- 33. Frahm N, Hecker M, Langhorst SE, et al. The risk of polypharmacy, comorbidities and drug-drug interactions in women of childbearing age with multiple sclerosis. Ther Adv Neurol Disord 2020; 13: 1756286420969501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 35. Patti F, Penaherrera JN, Zieger L, et al. Clinical characteristics of middle-aged and older patients with MS treated with interferon beta-1b: post-hoc analysis of a 2-year, prospective, international, observational study. BMC Neurol 2021; 21: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bergien SO, Petersen CM, Lynning M, et al. Use of natural medicine and dietary supplements concomitant with conventional medicine among people with Multiple Sclerosis. Mult Scler Relat Disord 2020; 44: 102197. [DOI] [PubMed] [Google Scholar]
- 37. Doan J, Zakrzewski-Jakubiak H, Roy J, et al. Prevalence and risk of potential cytochrome P450-mediated drug-drug interactions in older hospitalized patients with polypharmacy. Ann Pharmacother 2013; 47: 324–332. [DOI] [PubMed] [Google Scholar]
- 38. Prely H, Herledan C, Caffin AG, et al. Real-life drug-drug and herb-drug interactions in outpatients taking oral anticancer drugs: comparison with databases. J Cancer Res Clin Oncol 2022; 148: 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 40. Bachmann P, Frahm N, Debus JL, et al. Prevalence and severity of potential drug–drug interactions in patients with multiple sclerosis with and without polypharmacy. Pharmaceutics 2022; 14: 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ismail M, Khan S, Khan F, et al. Prevalence and significance of potential drug-drug interactions among cancer patients receiving chemotherapy. BMC Cancer 2020; 20: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Janchawee B, Wongpoowarak W, Owatranporn T, et al. Pharmacoepidemiologic study of potential drug interactions in outpatients of a university hospital in Thailand. J Clin Pharm Ther 2005; 30: 13–20. [DOI] [PubMed] [Google Scholar]
- 43. Galea I, Ward-Abel N, Heesen C. Relapse in multiple sclerosis. BMJ 2015; 350: h1765. [DOI] [PubMed] [Google Scholar]
- 44. Winkelmann A, Loebermann M, Reisinger EC, et al. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol 2016; 12: 217–233. [DOI] [PubMed] [Google Scholar]
- 45. Ellenberger D, Eichstädt K, Flachenecker P, et al. Decreasing longitudinal use of glucocorticosteroids in multiple sclerosis. Mult Scler Relat Disord 2018; 25: 173–174. [DOI] [PubMed] [Google Scholar]
- 46. Rommer PS, Buckow K, Ellenberger D, et al. Patients characteristics influencing the longitudinal utilization of steroids in multiple sclerosis – an observational study. Eur J Clin Invest 2015; 45: 587–593. [DOI] [PubMed] [Google Scholar]
- 47. Pöllmann W, Feneberg W. Current management of pain associated with multiple sclerosis. CNS Drugs 2008; 22: 291–324. [DOI] [PubMed] [Google Scholar]
- 48. Neilley LK, Goodin DS, Goodkin DE, et al. Side effect profile of interferon beta-1b in MS: results of an open label trial. Neurology 1996; 46: 552–554. [DOI] [PubMed] [Google Scholar]
- 49. Reess J, Haas J, Gabriel K, et al. Both paracetamol and ibuprofen are equally effective in managing flu-like symptoms in relapsing-remitting multiple sclerosis patients during interferon beta-1a (AVONEX) therapy. Mult Scler 2002; 8: 15–18. [DOI] [PubMed] [Google Scholar]
- 50. Walther EU, Hohlfeld R. Multiple sclerosis: side effects of interferon beta therapy and their management. Neurology 1999; 53: 1622–1627. [DOI] [PubMed] [Google Scholar]
- 51. Théroux P, Ouimet H, McCans J, et al. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med 1988; 319: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 52. Maljuric NM, Noordam R, Aarts N, et al. Use of selective serotonin re-uptake inhibitors and the heart rate corrected QT interval in a real-life setting: the population-based Rotterdam Study. Br J Clin Pharmacol 2015; 80: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bermel RA, Hashmonay R, Meng X, et al. Fingolimod first-dose effects in patients with relapsing multiple sclerosis concomitantly receiving selective serotonin-reuptake inhibitors. Mult Scler Relat Disord 2015; 4: 273–280. [DOI] [PubMed] [Google Scholar]
- 54. Camm J, Hla T, Bakshi R, et al. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am Heart J 2014; 168: 632–644. [DOI] [PubMed] [Google Scholar]
- 55. Bayas A, Schuh K, Baier M, et al. Combination treatment of fingolimod with antidepressants in relapsing-remitting multiple sclerosis patients with depression: a multicentre, open-label study – REGAIN. Ther Adv Neurol Disord 2016; 9: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 2011; 18: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 57. Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice–results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol 2014; 71: 271–279. [DOI] [PubMed] [Google Scholar]
- 58. Zettl UK, Rommer P, Hipp P, et al. Evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management of patients with spasticity due to multiple sclerosis. Ther Adv Neurol Disord 2016; 9: 9–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Keating GM. Delta-9-tetrahydrocannabinol/cannabidiol oromucosal spray (Sativex®): a review in multiple sclerosis-related spasticity. Drugs 2017; 77: 563–574. [DOI] [PubMed] [Google Scholar]
- 60. Abo Youssef N, Schneider MP, Mordasini L, et al. Cannabinoids for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis: a systematic review and meta-analysis. BJU Int 2017; 119: 515–521. [DOI] [PubMed] [Google Scholar]
- 61. Zendulka O, Dovrtělová G, Nosková K, et al. Cannabinoids and cytochrome p450 interactions. Curr Drug Metab 2016; 17: 206–226. [DOI] [PubMed] [Google Scholar]
- 62. Brubacher JR, Chan H, Staples JA. Cannabis-impaired driving and Canadian youth. Paediatr Child Health 2020; 25(Suppl. 1): S21–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaufman DW, Kelly JP, Wiholm BE, et al. The risk of acute major upper gastrointestinal bleeding among users of aspirin and ibuprofen at various levels of alcohol consumption. Am J Gastroenterol 1999; 94: 3189–3196. [DOI] [PubMed] [Google Scholar]
- 64. Varis T, Kivistö KT, Neuvonen PJ. Grapefruit juice can increase the plasma concentrations of oral methylprednisolone. Eur J Clin Pharmacol 2000; 56: 489–493. [DOI] [PubMed] [Google Scholar]
- 65. Stott CG, White L, Wright S, et al. A phase I study to assess the effect of food on the single dose bioavailability of the THC/CBD oromucosal spray. Eur J Clin Pharmacol 2013; 69: 825–834. [DOI] [PubMed] [Google Scholar]
- 66. Flachenecker P, Eichstädt K, Berger K, et al. Multiple Sklerose in Deutschland: aktualisierte Auswertungen des MS-Registers der DMSG 2014–2018. Fortschr Neurol Psychiatr 2020; 88: 436–450. [DOI] [PubMed] [Google Scholar]
- 67. Ohle L-M, Ellenberger D, Flachenecker P, et al. Chances and challenges of a long-term data repository in multiple sclerosis: 20th birthday of the German MS registry. Sci Rep 2021; 11: 13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thelen J, Zvonarev V, Lam S, et al. Polypharmacy in multiple sclerosis: current knowledge and future directions. Mo Med 2021; 118: 239–245. [PMC free article] [PubMed] [Google Scholar]
- 69. Abdolrasulnia M, Weichold N, Shewchuk R, et al. Agreement between medical record documentation and patient-reported use of nonsteroidal antiinflammatory drugs. Am J Health Syst Pharm 2006; 63: 744–747. [DOI] [PubMed] [Google Scholar]
- 70. Zettl UK, Bauer-Steinhusen U, Glaser T, et al. Adherence to long-term interferon beta-1b injection therapy in patients with multiple sclerosis using an electronic diary. Adv Ther 2016; 33: 834–847. [DOI] [PubMed] [Google Scholar]
- 71. Zettl UK, Bauer-Steinhusen U, Glaser T, et al. Comparative evaluation of patients’ and physicians’ satisfaction with interferon beta-1b therapy. BMC Neurol 2016; 16: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Klauer T, Zettl UK. Compliance, adherence, and the treatment of multiple sclerosis. J Neurol 2008; 255(Suppl. 6): 87–92. [DOI] [PubMed] [Google Scholar]
- 73. Ryu JY, Kim HU, Lee SY. Deep learning improves prediction of drug-drug and drug-food interactions. Proc Natl Acad Sci USA 2018; 115: E4304–E4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221108391 for Associated factors of potential drug-drug interactions and drug–food interactions in patients with multiple sclerosis by Jane Louisa Debus, Paula Bachmann, Niklas Frahm, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker and Uwe Klaus Zettl in Therapeutic Advances in Chronic Disease
Supplemental material, sj-xlsx-2-taj-10.1177_20406223221108391 for Associated factors of potential drug-drug interactions and drug–food interactions in patients with multiple sclerosis by Jane Louisa Debus, Paula Bachmann, Niklas Frahm, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker and Uwe Klaus Zettl in Therapeutic Advances in Chronic Disease
Supplemental material, sj-xlsx-3-taj-10.1177_20406223221108391 for Associated factors of potential drug-drug interactions and drug–food interactions in patients with multiple sclerosis by Jane Louisa Debus, Paula Bachmann, Niklas Frahm, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker and Uwe Klaus Zettl in Therapeutic Advances in Chronic Disease
Supplemental material, sj-xlsx-4-taj-10.1177_20406223221108391 for Associated factors of potential drug-drug interactions and drug–food interactions in patients with multiple sclerosis by Jane Louisa Debus, Paula Bachmann, Niklas Frahm, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker and Uwe Klaus Zettl in Therapeutic Advances in Chronic Disease