Abstract
Objective
An earlier follow-up study from the CogEx rehabilitation trial showed little change in symptoms of depression, anxiety and psychological distress during the first COVID-19 lockdown compared to pre-pandemic measurements. Here, we provide a second follow-up set of behavioral data on the CogEx sample.
Methods
This was an ancillary, longitudinal follow-up study in CogEx, a randomized controlled trial of exercise and cognitive rehabilitation in people with progressive MS involving 11 centres in North America and Europe. Only individuals impaired on the Symbol Digit Modalities Test (SDMT) were included. Participants repeated the COVID Impact survey administered approximately a year later and completed self-report measures of depression, anxiety and MS symptoms that had been obtained at the trial baseline and during the first COVID Impact survey. Participants who completed the second COVID Impact follow-up were included. To identify predictors of the participants’ ratings of their mental and physical well-being, step-wise linear regression was conducted.
Results
Of the 131 participants who completed the first COVID impact survey, 74 participants completed the second follow-up survey (mean age 52 (SD = 6.4) years, 62.2% female, mean disease duration 16.4 (SD = 9.0) years, median EDSS 6.0). Pandemic restrictions prevented data collection from sites in Denmark and England (n = 57). The average time between measurements was 11.4 (SD = 5.56) months. There were no significant differences in age, sex, EDSS, disease course and duration between those who participated in the current follow-up study (n = 74) and the group that could not (n = 57). One participant had COVID in the time between assessments. Participants now took a more negative view of their mental/psychological well-being (p = 0.0001), physical well-being (p = 0.0009) and disease course (p = 0.005) compared to their last assessment. Depression scores increased on the HADS-depression scale (p = 0.01) and now exceeded the clinically significant threshold of ≥ 8.0 for the first time. Anxiety scores on the HADS remained unchanged. Poorer mental well-being was predicted by HADS depression scores (p = 0.012) and a secondary-progressive disease course (p = 0.0004).
Conclusions
A longer follow-up period revealed the later onset of clinically significant depressive symptoms on the HADS and a decline in self-perceptions of mental and physical well-being associated with the COVID-19 pandemic relative to the first follow-up data point.
Trial registration
The trial was registered on September 20th 2018 at www.clinicaltrials.gov having identifier NCT03679468. Registration was performed before recruitment was initiated.
Keywords: COVID-19, Progressive multiple sclerosis, Mental well-being, Longitudinal, Depression, Anxiety
Introduction
Two years into the COVID-19 pandemic, over 407 million people have been infected world-wide with 5,797,099 deaths attributed to the virus [1]. Amongst those more vulnerable to severe infection and potentially, a more compromised outcome, are people with multiple sclerosis who are older, have a progressive disease course, more advanced physical disability and who are on certain immunosuppressive medications [2, 3]. Data also show that early treatment and optimized treatment with disease modifying therapies are being delayed by people with MS canceling visits with neurologists and rehabilitation services, MRI appointments and laboratory tests because of COVID-related fears and restrictions [4, 5].
Another potential source of vulnerability that could compromise the ability of people with MS to manage pandemic-related challenges is their high rate of underlying emotional abnormalities [6]. A number of studies have addressed this concern. Cross sectional data have revealed elevated rates of depression [2, 7] and comorbid anxiety and depression [8–10] and some longitudinal studies have noted increases in anxiety [11] and depression [12] in cohorts assessed before and after the onset of the pandemic. These findings are, however, offset by at least four longitudinal reports of no significant changes in emotion pre and post-pandemic onset [13–16].
One of these four studies comes from our CogEx group [16], a multi-arm, randomized, blinded, sham-controlled trial entailing an international collaboration of researchers in Europe and North America investigating the effects of cognitive rehabilitation and aerobic exercise on primary (processing speed) and secondary (depression, anxiety, motor function) outcome markers in people with progressive MS. We have previously described the challenges of undertaking this study in the midst of a pandemic [17], noting that other clinical trials have faced similar obstacles that center on more difficult recruitment and keeping participants safe from infection [18, 19]. While our initial CogEx findings showed that participants overall became neither more anxious nor depressed once the pandemic started, these data were collected only during the first lockdown period in 2020. CogEx has continued since then during a period of repeated lockdowns thereby presenting an opportunity for us to revisit the question of how our cohort has been faring psychologically after a more prolonged period of restrictions.
Methods
The full CogEx protocol and the methodology in our first CogEx-COVID study has been published previously [20]. To summarize, CogEx (“Improving cognition in people with progressive MS: a multi-arm, randomized, blinded, sham-controlled trial of cognitive rehabilitation and aerobic exercise study”) is an international, 11-centre MS dual-intervention trial funded by the MS Society of Canada. The countries involved are Canada, United States of America, United Kingdom, Italy, Denmark and Belgium. People with primary or secondary MS are randomized to one of four groups: Cognitive Rehabilitation (CR) and Aerobic Exercise (AE), CR and sham AE, sham CR and AE and sham CR and sham AE. The interventions are twice a week for 12 weeks, with three assessments undertaken at baseline, 12 weeks and 6 months. The primary outcome measure in the CogEx study is processing speed as measured by the Symbol Digit Modalities Test (SDMT) and the study has been powered accordingly.
When we first began the CogEx trial, we could not have anticipated that a pandemic would occur and affect our trial. However, the unexpected onset of the pandemic afforded our group the opportunity to assess the effects of the restrictions and lockdowns on the emotional and physical well-being of our sample. As such, we have conducted two add-on studies as it were, unrelated to processing speed and the SDMT, utilizing those participants already enrolled in CogEx, for this purpose.
Our first COVID follow-up study collected emotional and behavioral data by telephone or email during the first pandemic lockdown period in 2020 (February to September). Our second follow-up study, presented here, entails collecting the same data by telephone or email 11.4 (SD = 5.6) months later between June and November 2021.
Participants
The participants who completed our first follow-up study from 11 study sites in six countries (n = 131) were invited by email and/or telephone call to complete a second follow-up COVID survey. Pandemic restrictions prevented further follow-up data collection from sites in Denmark and the United Kingdom, reducing our sample size to a maximum of 74 participants.
Assessments
The COVID Impact Interview was developed by the study team specifically for use in this study in an effort to evaluate the impact of the COVID-19 pandemic and lockdown orders on individuals with progressive MS across the participating centers. It consists of 22 questions related to self and family exposure to COVID-19, length of time under lockdown orders, activities during lockdown, disease symptomatology and interactions with healthcare providers. A set of questions assessing the impact of the pandemic on psychological, financial and physical well-being were included with responses recorded on an integer scale (0–10, with 0 being no impact and 10 being maximal impact). The survey was administered in the individual’s native language. Results were examined in response to each specific question.
The Hospital Anxiety Depression Scale (HADS) is widely used to assess psychological distress in non-psychiatric patients. It consists of two subscales, measured via 14 items, seven items for the anxiety subscale (HADS-Anxiety) and seven for the Depression (HADS-Depression) subscale [21]. Overall, it has demonstrated satisfactory psychometric properties in several different populations, including MS [22, 23]. Each item is scored on a response scale with four alternatives ranging between 0 and 3 and a higher score indicates greater anxiety or depression. The HADS-depression cutoff for clinical depression was defined as scores ≥ 8.0 [24].
The Beck Depression Inventory -II (BDI-II)[25] is an easily administered, 21-item scale that assesses various aspects of depression, useful in determining the presence and severity of depressive symptoms. Each item is concerned with a specific aspect of depression (mood, motivation, appetite) and contains four statements of graded severity expressing how a person might think or feel about that particular aspect of depression. The total score is the sum of all statements endorsed by the participant. A higher score indicates greater depression.
The Multiple Sclerosis Impact Scale (MSIS-29) is a disease specific measure of the impact of MS. It consists of 29 items, 20 associated with a physical scale and 9 associated with a psychological scale, where the sum of each scale is transformed to a scale of 0–100 and higher scores indicating worse health [26]. Items ask about the impact of MS on day-to-day life in the past 2 weeks rated on a 5-point Likert scale. The MSIS-29 has strong reliability and validity in MS samples [26], with existing evidence supporting its responsiveness in rehabilitation trials [27].
Analyses
Descriptive statistics were used to summarize the participant demographic and clinical characteristics using means (SD) for continuous variables, median (25%, 75%) for ordinal variables, and frequencies (%) for categorical variables. Associations between mental health and physical health and disease duration were examined using Spearman correlations. Differences over time in the assessments available at baseline, the first COVID lockdown and follow-up (BDI, HADS, MSIS-29) were evaluated using a mixed model to account for the repeated measures over time. Effect sizes were calculated using the mean difference between two time points from a paired t-test. Differences between the first and second COVID impact survey questions were evaluated using paired t-test, McNemar’s test or Wilcoxon signed rank test, as appropriate. Analyses were conducted in SAS v9.4. Given that the study was an ad on to the original CogEx protocol, there was no priori power calculation to determine optimum sample size for the Covid-related data. The significance level was set at 0.05.
To identify predictors of the participants’ ratings of their mental and physical well-being, step-wise linear regression using both forward and backward elimination, to validate the selected model predictors, was conducted. The predictor variables entered were baseline age, sex, marital status, living alone, employment, EDSS and type of MS. Additional predictors were HADS depression and anxiety scores and change in employment status during the follow-up period.
Consent
Informed consent was obtained from all participants. Ethics permission for the study was obtained by each research centre. Data collection was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Results
Demographic and disease-related data
The mean age of the current sample was 52 years (SD = 6.4) with a mean disease duration of 16.4 (SD = 9.0) years and a median EDSS of 6.0. Forty six (62.2%) participants were female. Disease course comprised 21 (28.4%) people with primary and 53 (71.6) people with secondary progressive MS. The majority of participants were from Italy (68.9%) followed by Canada (17.6%), England (6.8%), USA (5.4%) and Belgium (1.4%).
Demographic and disease-related comparisons were undertaken between the group that participated in the current follow-up study (n = 74) and the group who could not (n = 57). There were no differences between these groups in age (p = 0.71), sex (p = 0.6), disease course (p = 0.98) duration of MS (p = 0.44), and EDSS (p = 0.6).
Longitudinal changes on the patient reported outcomes (PROs)
Mean scores for the outcome measures are shown in Table 1. There was no significant change in depression scores on the BDI-II, but depression scores increased on the HADS from lockdown to follow-up (p = 0.017). The mean HADS depression score at follow up now exceeded the clinically significant threshold of ≥ 8.0 for the first time. The HADS depression score at follow-up was also higher than the baseline score for this cohort that predated the pandemic (p = 0.001; n = 74). Anxiety scores on the HADS remained unchanged as did scores of the MSIS-29, both for the physical and mental subscales, when compared to the first follow-up period and at baseline. There were no sex differences in terms of changes in HADS depression scores over time (p = 0.11).
Table 1.
Longitu dinal changes on the Patient Reported Outcome measures
| Baseline n = 74 |
Initial (June to Sept 2020) n = 74 |
Follow-up (June to Nov 2021) n = 74 |
Effect size* | P value** | |
|---|---|---|---|---|---|
| BDI | 11.1 (7.5) | 12.3 (9.3) | 10.9(6.7) |
0.137 0.195 |
0.19 0.12 |
| HADS-depression | 6.2 (3.9) | 6.9(4.8) | 8.2(5.0) |
0.167 0.312 |
0.186 0.017 |
| HADS-anxiety | 5.7(4.4) | 6.1(4.2) | 6.2(4.1) |
0.08 0.03 |
0.44 0.79 |
| MSIS-29 physical | 46.7 (21.4) | 48.0(22.5) | 49.2(26.2) |
0.101 0.013 |
0.37 0.79 |
| MSIS-29 Mental | 32.6 (21.8) | 34.1(22.4) | 33.4(25.3) |
0.045 0.022 |
0.72 0.98 |
*The first effect size refers to the mean difference between baseline and the initial lockdown and the second effect size to the mean difference between the initial lockdown and the follow-up
**The first p values refers to mean difference between baseline and the initial lockdown and the second p value to the mean difference between the initial lockdown and the follow-up
COVID impact questionnaire
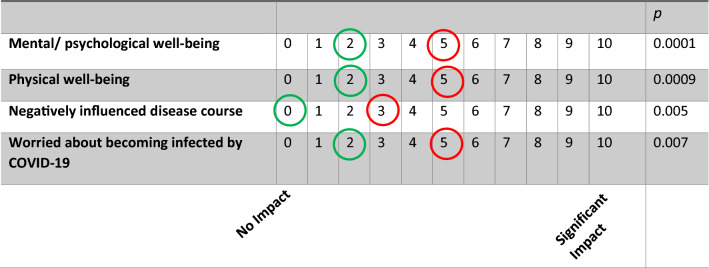
One additional participant acquired COVID during the follow-up period bringing the overall total of participants infected to 4. There was a nominal increase in relatives of participants to have acquired COVID in the intervening period between studies (9(12.5%) vs 13(17.6%), p = 0.058). Participants (n = 74) now took a more negative view of their mental/psychological well-being (p = 0.0001), physical well-being (p = 0.0009) and disease course (p = 0.005) compared to their first assessment (see Fig. 1). They also reported an increase in worry that they might become infected by COVID (p = 0.007, see Fig. 1).
Fig. 1.
Longitudinal change in perception of psychological and physical well-being impacted by the pandemic
Interactions with friends and family declined, but not significantly (8 [5, 10] vs. 6 [4, 10], p = 0.17) while online social interactions remained unchanged (8 [5, 10] vs. 8 [6, 10], p = 0.27). There was a significant increase sense of solitude (13.9% vs. 25.7%, p = 0.049). Participants did not report any significant changes in their level of physical activities. When it came to cognitive activities, there was an increase in participants engaging in jigsaw puzzles (p = 0.04), but not in other activities (see Table 2).
Table 2.
Longitdinal changes on COVID Impact Questionnaire
| Lockdown n = 74 |
Follow up n = 74 |
p value | |
|---|---|---|---|
| Scale: 1–10: 1 being the least and 10 being the most markedly negative impact of COVID | |||
| Interaction with family and friends | 8 [5, 10] | 6 [4, 10] | 0.17 |
| Virtual interaction with family and friends | 8 [5, 10] | 8 [6, 10] | 0.27 |
| Percentage % of YES responses | |||
| Sense of solitude | 10 (13.9%) | 19 (25.7%) | 0.049 |
| Cognitively active | 9 (12.5%) | 12 (16.2%) | 0.79 |
| Online courses | 6 (8.3%) | 3 (4.1%) | 0.51 |
| Reading books | 25 (34.7%) | 27 (36.5%) | 0.83 |
| Jigsaw puzzles | 8 (11.1%) | 17 (23.0%) | 0.04 |
| Reading news | 34 (47.2%) | 36 (48.6%) | 1.00 |
| Word games | 27 (37.5%) | 19 (25.7%) | 0.17 |
| Other activities | 22 (30.6%) | 14 (18.9%) | 0.17 |
| Physical activity | 0.82 | ||
| Not at all | 25 (35.7%) | 3 (44.3%) | |
| Non-aerobic activities (e.g., yoga) | 21 (30.0%) | 15 (21.4%) | |
| Aerobic activities less than 30 min per day | 13 (18.6%) | 6 (8.6%) | |
| Aerobic activities at least 30 min per day | 11 (15.7%) | 18 (25.7%) | |
There was a significant correlation in follow-up scores between people reporting a decline in mental health and a subjective worsening in physical health (r = 0.367, p = 0.002) and disease course (r = 0.572, p = 0.0001).
Predictors of mental and physical decline
Two significant variables predicted negative subjective perceptions of mental well-being, namely HADS depression scores (p = 0.012) and secondary progressive disease course (p = 0.0004). In relation to the latter, there were no differences in EDSS (p = 0.25) or HADS depression scores (p = 0.11) between those with primary and secondary MS.
No predictors were identified for subjective physical well-being.
Discussion
Our data are notable for revealing a worsening in depression on the HADS in people with progressive MS during the Covid-19 pandemic. With an extended period of follow-up, approximately one year on from our first follow-up point, we observed a statistically significant decline in psychological well-being in our sample. In addition, participants reported a subjective deterioration in physical well-being, a heightened concern about their disease course and increased worry about acquiring the SARS-CoV-2 virus.
This study should be seen alongside a small literature describing the effects of Covid-19 on clinical trials that were overtaken by the pandemic [18, 19]. Facility closures, reluctance on the part of participants to volunteers, the introduction of, and challenges posed by, protective equipment and the ethical considerations of balancing the importance of keeping a trail running versus the attendant risks in doing so [28], are factors that were common to CogEx as well, and which we have described previously [17].
One of the factors that set our results apart from most other longitudinal MS studies reporting well-being is that our findings come from a third time point. Data were collected on average almost one year after our last follow-up study which compared psychological and physical symptoms during the first lockdown in 2020 to baseline data collected before the pandemic’s onset. These earlier follow-up data showed little, if any symptom deterioration. Now, with a third time point added, a fundamentally different picture emerges, characterized by a widespread perception on the part of people with progressive MS that their emotional and physical health have deteriorated. By way of comparison, we were only able to find one other study, albeit in a heterogeneous sample of people with neuroinflammatory disease, with three data points too. However, data collection only began following the onset of the pandemic and the duration of overall follow up was relatively short, i.e., nine months compared to our 20-months timespan. No significant change in depression over time was reported [29]. A recent meta-analysis of studies examining the psychological impact of the Covid-19 pandemic on people with MS reached the same conclusion [30]. Our findings, therefore, place in perspective those MS studies, both longitudinal and cross-sectional, that found no increase in depression and emotional distress during the pandemic [16]. This suggests that results from the studies undertaken thus far should be considered provisional given the demonstrated potential for the later onset of deterioration in well-being with the passage of time.
An increase in depression in our study was detected on the Hospital Anxiety and Depression Scale (HADS), but not the Beck Depression Inventory (BDI-II). One reason for this might be found in the difference in phenomenology between these two measures. The HADS has been developed specifically for detecting depression in people with a physical illness and has been validated for use in people with MS [24]. The same is not true for the BDI-II. Potential somatic confounders of depression such as disturbances in sleep, appetite, weight and interest in sex that may arise directly as a consequence of a medical illness have been removed in the HADS, but not the BDI. This leaves the focus of the HADS on the core features of mood disturbance with questions pertaining to enjoyment, laughter, cheerfulness, and looking forward to things. Our data show that it is in relation to these symptoms that people with progressive MS were faring more poorly as the pandemic progressed. Furthermore, the deterioration in depression seen on the HADS was notable for a mean score that now exceeds the threshold of equal or greater to 8.0 indicating clinically significant depression. At the same time, the mean BDI-II score fell below the threshold of 13 indicative of mild depression. A concomitant increase in anxiety did not accompany the change in depression on the HADS.
Our data also revealed people with a secondary progressive disease course rated their psychological well-being lower than individuals with primary progressive MS. Non-Covid-related data support this observation. A study comparing behavioral symptoms in 40 people with SPMS and 30 with PPMS showed that the former had significantly more depression in addition to more emotional distress in general as elicited on certain indices of the Symptom Checklist-90 [31]. Allied to these findings are longitudinal data from a study limited to people with PPMS showing that depression scores never changed over a 12-months period, even in the 25% of participants with evidence of disease activity [32]. This relative stability in mentation in people with PPMS, at least over the short term, contrasts with worsening depression and a decline in quality of life in people with people who transition from RRMS to SPMS [33]. In addition, data from over 4000 people in the UK MS Registry show that the highest rates of depression are in people with SPMS, irrespective of gender [34].
Another notable finding to emerge from our data was that participants reported a significantly greater sense of solitude/loneliness as the pandemic lingered. Somewhat surprisingly, this variable did not predict subjective mental well-being, unlike in another MS-Covid study where it emerged as the most robust predictor of depression [29]. The reasons for these discrepant findings are unclear, but may relate to differences in sample composition and sample size.
The CogEx protocol requires participants to attend interventions twice a week for 12 weeks, thus bringing them into frequent contact with the research assistants. This interaction did not seem to prevent a deterioration in their emotional well-being over time. At the time of writing, our study-blinding is still in effect so we do not know which participants were assigned to the exercise protocol. Exercise is, however, thought to ameliorate depression [35] and enhance well-being [36] in people with MS during the pandemic. A corollary to this is that depression and perceptions of poorer mental and physical health in pwMS during the pandemic have been linked to a reduced physical level in general [37]. While these collective findings are concordant, the cross-sectional nature of all these studies demands a note of caution when it comes to draw firm conclusions.
Our study has certain limitations. COVID restrictions in certain centres, namely Denmark and England, meant a reduced sample size compared to our first follow-up assessment. While this could have introduced an element of bias into our current data, this was offset by the absence of demographic and disease-related differences between participants who took part in this second follow-up study and those who did not. We also did not have in-person physical examinations because of virtual patient care and as such could not corroborate whether subjective perceptions of physical decline were confirmed by objective neurological assessments. Finally, the add-on nature of the study, as described in the method section, meant that there was no a priori sample size calculation for the Covid-related subjective emotional and physical well-being data reported here.
In summary, the results of a second follow-up assessment undertaken on average 20 months into the pandemic revealed people with progressive MS becoming more depressed, feeling more distressed emotionally and rating their physical health more poorly with the passage of time. The subjective decline in emotional and physical health, not apparent at an earlier follow-up assessment, highlights the deleterious effects of pandemic longevity on the health of people with MS and speaks to the need for health care professionals to remain vigilant in detecting and managing the delayed onset of mental health difficulties.
Funding
Supported by the MS Society of Canada (Grant # EGID3185).
Data availability
To promote data transparency, anonymized data will be available upon reasonable request.
Declarations
Conflicts of interest
Anthony Feinstein is on Advisory Boards for Akili Interactive and Roche, and reports grants from the MS Society of Canada, book royalties from Johns Hopkins University Press, Cambridge University Press, Amadeus Press and Glitterati Editions, and speaker’s honoraria from Novartis, Biogen, Roche and Sanofi-Genzyme. Maria Pia Amato received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, and Teva Pharmaceutical Industries; and received research support from Biogen Idec, Merck-Serono, Roche, Pharmaceutical Industries and Fondazione Italiana Sclerosi Multiplav. Giampaolo Brichetto has been awarded and received research support from Coloplast, Novartis, Roche, Fondazione Italiana Sclerosi Multipla, ARSEP, H2020 EU Call, Italian Ministry of Health. Jeremy Chataway has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society and the Rosetrees Trust. He is supported in part by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre, London, UK. He has been a local principal investigator for commercial trials funded by: Actelion, Biogen, Novartis and Roche; has received an investigator grant from Novartis; and has taken part in advisory boards/consultancy for Azadyne, Biogen, Celgene, MedDay, Merck and Roche. Nancy D. Chiaravalloti is on an Advisory Board for Akili Interactive and is a member of the Editorial Boards of Multiple Sclerosis Journal and Frontiers in NeuroTrauma. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. John DeLuca is an Associate Editor of the Archives of Physical Medicine and Rehabilitation, received compensation for consulting services and/or speaking activities from Biogen Idec, Bristol Myers Squibb, Celgene, MedRhythms, Jansssen Roche and Novartis; and receives research support from Biogen Idec, National Multiple Sclerosis Society, Consortium of Multiple Sclerosis Centers, National Academy of Neuropsychology and National Institutes of Health. Cecilia Meza has no disclosures to report. Peter Feys is editorial board member of NNR and MSJ, provides consultancy to NeuroCompass and was board of advisory board meetings for BIOGEN. Massimo Filippi is Editor-in-Chief of the Journal of Neurology and Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology, received compensation for consulting services and/or speaking activities from Alexion, Almirall, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries, and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, the Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). Jennifer Freeman has been awarded research grants from the NIHR, UK. Matilde Inglese is Co-Editor for Controversies for Multiple Sclerosis Journal; received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme; and received research support from NIH, NMSS, the MS Society of Canada, the Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, H2020 EU Call. Robert W. Motl has no disclosures to report. Maria Assunta Rocca received speaker honoraria from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva and research support from the Canadian MS Society and Fondazione Italiana Sclerosi Multipla. Brian Sandroff has no disclosures to report. Gary Cutter is a member of Data and Safety Monitoring Boards for Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Mapi Pharmaceuticals LTD, Merck, Merck/Pfizer, Opko Biologics, OncoImmune, Neurim, Novartis, Ophazyme, Sanofi-Aventis, Reata Pharmaceuticals, Teva pharmaceuticals, VielaBio Inc, Vivus, NHLBI (Protocol Review Committee), NICHD (OPRU oversight committee). He is on Consulting or Advisory Boards for Biodelivery Sciences International, Biogen, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Neurogenesis LTD, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Roche, TG Therapeutics. Dr. Cutter is employed by the University of Alabama at Birmingham and President of Pythagoras, Inc. a private consulting company located in Birmingham AL. Amber Salter is a statistical editor for Circulation: Cardiovascular Imaging.
Ethical standard
The study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre, affiliated with the University of Toronto. Signed consent was obtained from all participants at all study sites.
References
- 1.Hopkins J (2020) COVID-19 Map—Johns Hopkins Coronavirus Resource Center. p. 1. https://coronavirus.jhu.edu/map.html
- 2.Vercellino M, Bosa C, Alteno A, Schillaci V, Petracca M, Marasciulo S, et al. Impact of COVID-19 lockdown on progressive multiple sclerosis patients. Neurol Sci. 2022;1:3. doi: 10.1007/s10072-022-05909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portaccio E, Fonderico M, Hemmer B, Derfuss T, Stankoff B, Selmaj K, et al. Impact of COVID-19 on multiple sclerosis care and management: results from the European Committee for Treatment and Research in Multiple Sclerosis survey. Mult Scler J. 2021 doi: 10.1177/13524585211005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateen FJ, Rezaei S, Alakel N, Gazdag B, Kumar AR, Vogel A. Impact of COVID-19 on U.S. and Canadian neurologists’ therapeutic approach to multiple sclerosis: a survey of knowledge, attitudes, and practices. J Neurol. 2020;267:3467–3475. doi: 10.1007/s00415-020-10045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koc ER, Demir AB, Topaloglu E, Turan OF, Ozkaya G. Effects of quarantine applied during the COVID-19 pandemic on mental health and quality of life in patients with multiple sclerosis and healthy controls. Neurol Sci. 2022;43(4):2263–2269. doi: 10.1007/s10072-022-05901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motolese F, Rossi M, Albergo G, Stelitano D, Villanova M, Di Lazzaro V et al (2020) The psychological impact of COVID-19 pandemic on people with multiple sclerosis. Front Neurol 11:580507. www.frontiersin.org [DOI] [PMC free article] [PubMed]
- 8.Ramezani N, Ashtari F, Bastami EA, Ghaderi K, Hosseini SM, Naeini MK, et al. Fear and anxiety in patients with multiple sclerosis during COVID-19 pandemic; report of an Iranian population. Mult Scler Relat Disord. 2021;1(50):102798. doi: 10.1016/j.msard.2021.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alirezaei M, Eskandarieh S, Sahraian MA, Naser MA. Depression, anxiety, and fear of COVID-19 in patients with multiple sclerosis in pandemic era: a cross-sectional study. Neurol Sci. 2022;43(1):59–66. doi: 10.1007/s10072-021-05612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broche-Pérez Y, Jiménez-Morales RM, Monasterio-Ramos LO, Vázquez-Gómez LA, Fernández-Fleites Z. Fear of COVID-19, problems accessing medical appointments, and subjective experience of disease progression, predict anxiety and depression reactions in patients with Multiple Sclerosis. Mult Scler Relat Disord. 2021;1(53):103070. doi: 10.1016/j.msard.2021.103070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stojanov A, Malobabic M, Milosevic V, Stojanov J, Vojinovic S, Stanojevic G, et al. Psychological status of patients with relapsing-remitting multiple sclerosis during coronavirus disease-2019 outbreak. Mult Scler Relat Disord. 2020;1(45):102407. doi: 10.1016/j.msard.2020.102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strober L, Weber E, Lequerica A, Chiaravalloti N. Surviving a global pandemic: the experience of depression, anxiety, and loneliness among individuals with multiple sclerosis. Mult Scler Relat Disord. 2022 doi: 10.1016/j.msard.2022.103497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manuela A, Rocco C, Alvino B, Alessandro D, Daniela B, Gioacchino T et al (2022) The psychological impact of Covid-19 pandemic on people with Multiple Sclerosis: a meta-analysis. Mult Scler Relat Disord 103774. https://linkinghub.elsevier.com/retrieve/pii/S2211034822002899 [DOI] [PMC free article] [PubMed]
- 14.Becker H, Stuifbergen AK, Lim S, Kesler SR (2022) Health promotion, functional abilities, and quality of life before and during COVID-19 in people with multiple sclerosis. Nurs Res 71(2):84–89. https://pubmed.ncbi.nlm.nih.gov/34967826/ [DOI] [PubMed]
- 15.Sbragia E, Colombo E, Pollio C, Cellerino M, Lapucci C, Inglese M, et al. Embracing resilience in multiple sclerosis: a new perspective from COVID-19 pandemic. Psychol Heal Med. 2022;27(2):352–360. doi: 10.1080/13548506.2021.1916964. [DOI] [PubMed] [Google Scholar]
- 16.Chiaravalloti ND, Amato MP, Brichetto G, Chataway J, Dalgas U, DeLuca J, et al. The emotional impact of the COVID-19 pandemic on individuals with progressive multiple sclerosis. J Neurol. 2021;268(5):1598–1607. doi: 10.1007/s00415-020-10160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinstein A, Amato MP, Brichetto G, Chataway J, Chiaravalloti ND, Cutter G et al (2022) The impact of the COVID-19 pandemic on an international rehabilitation study in MS: the CogEx experience. J Neurol 269(4):1758–1763. https://pubmed.ncbi.nlm.nih.gov/34741240/ [DOI] [PMC free article] [PubMed]
- 18.Weber MS, Nicholas JA, Yeaman MR (2021) Balancing potential benefits and risks of Bruton tyrosine kinase inhibitor therapies in multiple sclerosis during the COVID-19 Pandemic. Neurol - Neuroimmunol Neuroinflamm 8(6). https://nn.neurology.org/content/8/6/e1067 [DOI] [PMC free article] [PubMed]
- 19.Lowe R, Barlow C, Lloyd B, Latchem-Hastings J, Poile V, Scoble C et al (2021) Lifestyle, exercise and activity package for people living with progressive multiple sclerosis (LEAP-MS): adaptions during the COVID-19 pandemic and remote delivery for improved efficiency. Trials 22(1). https://pubmed.ncbi.nlm.nih.gov/33863342/ [DOI] [PMC free article] [PubMed]
- 20.Feinstein A, Amato MP, Brichetto G, Chataway J, Chiaravalloti N, Dalgas U, et al. Study protocol: Improving cognition in people with progressive multiple sclerosis: a multi-arm, randomized, blinded, sham-controlled trial of cognitive rehabilitation and aerobic exercise (COGEx) BMC Neurol. 2020 doi: 10.1186/s12883-020-01772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Vol. 67, Acta psychiatr. scand [DOI] [PubMed]
- 22.Iani L, Lauriola M, Costantini M. A confirmatory bifactor analysis of the hospital anxiety and depression scale in an Italian community sample. Health Qual Life Outcomes. 2014;12(1):1–8. doi: 10.1186/1477-7525-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 24.Honarmand K, Feinstein A. Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler. 2009;15(12):1518–1524. doi: 10.1177/1352458509347150. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA and Brown G (2022) Beck depression inventory-second edition | The National Child Traumatic Stress Network. https://www.nctsn.org/measures/beck-depression-inventory-second-edition
- 26.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A (2001) The Multiple Sclerosis Impact Scale (MSIS-29) a new patient-based outcome measure. Brain 124(5):962–973. https://academic.oup.com/brain/article/124/5/962/309935 [DOI] [PubMed]
- 27.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ (2005) How responsive is the Multiple Sclerosis Impact Scale (MSIS-29)? A comparison with some other self report scales. J Neurol Neurosurg Psychiatry 76(11):1539–1543. https://jnnp.bmj.com/content/76/11/1539 [DOI] [PMC free article] [PubMed]
- 28.McDermott MM, Newman AB. Preserving clinical trial integrity during the coronavirus pandemic. J Am Med Assoc. 2020;323(21):2135–2136. doi: 10.1001/jama.2020.4689. [DOI] [PubMed] [Google Scholar]
- 29.Levit E, Cohen I, Dahl M, Edwards K, Weinstock-Guttman B, Ishikawa T et al (2022) Worsening physical functioning in patients with neuroinflammatory disease during the COVID-19 pandemic. Mult Scler Relat Disord 58. https://pubmed.ncbi.nlm.nih.gov/35016114/ [DOI] [PMC free article] [PubMed]
- 30.Altieri M, Capuano R, Bisecco A, d’Ambrosio A, Buonanno D, Tedeschi G, et al. The psychological impact of Covid-19 pandemic on people with Multiple Sclerosis: a meta-analysis. Mult Scler Relat Disord. 2022;61:103774. doi: 10.1016/j.msard.2022.103774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vleugels L, Pfennings L, Pouwer F, Cohen L, Ketelaer P, Polman C et al (1998) Psychological functioning in primary progressive versus secondary progressive multiple sclerosis. Br J Med Psychol 71(1):99–106. https://pubmed.ncbi.nlm.nih.gov/9561309/ [DOI] [PubMed]
- 32.Pérez-Miralles F, Prefasi D, García-Merino A, Ara JR, Izquierdo G, Meca-Lallana V et al (2021) Short-term data on disease activity, cognition, mood, stigma and employment outcomes in a cohort of patients with primary progressive multiple sclerosis (UPPMS study). Mult Scler Relat Disord 50. https://pubmed.ncbi.nlm.nih.gov/33647591/ [DOI] [PubMed]
- 33.Healy BC, Zurawski J, Chitnis T, Weiner HL, Glanz BI. Patient-reported outcomes associated with transition to secondary progressive multiple sclerosis. Qual Life Res. 2021 doi: 10.1007/s11136-021-03034-6. [DOI] [PubMed] [Google Scholar]
- 34.Jones KH, Ford DV, Jones PA, John A, Middleton RM, Lockhart-Jones H et al (2012) A large-scale study of anxiety and depression in people with multiple sclerosis: A survey via the web portal of the UK MS register. PLoS One 7(7). www.plosone.org [DOI] [PMC free article] [PubMed]
- 35.Carotenuto A, Scandurra C, Costabile T, Lavorgna L, Borriello G, Moiola L, et al. Physical exercise moderates the effects of disability on depression in people with multiple sclerosis during the covid-19 outbreak. J Clin Med. 2021;10(6):1–7. doi: 10.3390/jcm10061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costabile T, Carotenuto A, Lavorgna L, Borriello G, Moiola L, Inglese M, et al. COVID-19 pandemic and mental distress in multiple sclerosis: implications for clinical management. Eur J Neurol. 2021;28(10):3375–3383. doi: 10.1111/ene.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abasıyanık Z, Kurt M, Kahraman T. COVID-19 and physical activity behaviour in people with neurological diseases: a systematic review. J Dev Phys Disabil. 2022 doi: 10.1007/s10882-022-09836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
To promote data transparency, anonymized data will be available upon reasonable request.