Abstract
Functional immunity (defined here as serum neutralising capacity) critically contributes to conferring protection against SARS-CoV-2 infection and severe COVID-19. This cross-sectional analysis of a prospective, population-based cohort study included 1,894 randomly-selected 16 to 99-year-old participants from two Swiss cantons in March 2022. Of these, 97.6% (95% CI: 96.8–98.2%) had anti-spike IgG antibodies, and neutralising capacity was respectively observed for 94%, 92% and 88% against wild-type SARS-CoV-2, Delta and Omicron variants. Studying functional immunity to inform and monitor vaccination campaigns is crucial.
Keywords: SARS-CoV-2, seroprevalence, neutralising activity, variants of concern
Currently available injectable vaccines confer protective immunity against symptomatic severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection and severe coronavirus disease (COVID-19), however, protection – particularly against infection – wanes over time and is reduced for highly mutated variants such as Omicron (Phylogenetic Assignment of Named Global Outbreak (Pango) lineage: B.1.1.529) [1-4]. Booster vaccinations are important to maintain individual protection against severe disease more than infection [5]. Functional immunity (defined here as neutralising capacity of serum) contributes to protection against infection and severe disease, however, neutralising antibodies in serum wane over time, and are less effective at preventing infection by emerging variants. To the best of our knowledge, evidence is inexistent on functional immunity (defined here as neutralising capacity of serum) in the general population after the Delta (Pango lineage: B.1.617.2) and Omicron waves, and after vaccination and booster campaigns. Immunosurveillance of functional immunity is key to plan vaccination campaigns with respect to both their optimal timing and subgroups to be targeted, and to contemplate other preventive measures to control the burden of disease. Our aims were: (i) to determine the proportion of individuals in the general population with functional immunity against SARS-CoV-2, and (ii) to assess the neutralising activity of antibodies for virus variants of concern.
Study design, sampling, and participants’ characteristics
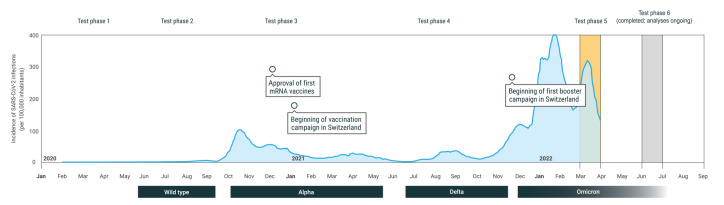
We conducted a cross-sectional analysis of the baseline assessment of a prospective, population-based cohort study, which is part of the Corona Immunitas research programme in Switzerland [6], within which we had completed four phases of seroprevalence studies throughout Switzerland between April 2020 and October 2021 using a standardised protocol. Here we present results from phase five (Figure), for which baseline assessments were done between 1 and 31 March 2022.
Figure.
Evolution of the pandemic, timing of vaccination and booster campaign and test phases of Corona Immunitas, Switzerland, January 2020–April 2022
The interval from 1 to 31 March 2022 is coloured in orange to highlight the epidemiological context where phase 5 of the Corona Immunitas research programme took place. The current study is conducted within this phase 5.
For phase five, we randomly selected individuals from the general population in north-eastern (canton of Zurich) and southern Switzerland (canton of Ticino), two regions that differ across demographic, socio-cultural, linguistic aspects and climate, all of which may impact on the dynamics of the pandemic. The Swiss Federal Office of Statistics provided random samples of the general population in age-stratified (16–29, 30–44, 45–64 and ≥ 65 years) groups, separately for Zurich and for Ticino. We selected these groups after consultation with the Swiss Federal Office of Public Health to adequately account for the potential impact on seroprevalence of social behaviour, adherence to public health measures and vaccination uptake, all of which differ across these age groups. Given a sensitivity of 98% and specificity of 99%, we deemed 200 participants for each stratum to provide precise enough estimates for an expected seroprevalence of 90% or more. The target sample size was thus 200 for each age stratum in the two regions (i.e. total planned sample size of 1,600).
Before the in-person study visit, participants provided information regarding socio-demographics, vaccinations, SARS-CoV-2 infections, hospital and intensive care unit (ICU) admissions and symptoms in case of infections and past medical history, using the secure, web-based Research Electronic Data Capture platform for data collection and management [7,8]. We used questionnaires and data collection procedures identical to the previous phases of Corona Immunitas [6] to allow comparability, with small adaptations to the situation of the pandemic in early 2022. We used medians and interquartile ranges or absolute and relative numbers for the descriptive analysis.
We enrolled overall 1,894 individuals (1,044 from north-eastern and 850 southern Switzerland). Participation rate was 21.4% (1,044/4,879) in north-eastern and 18.9% (850/4,497) in southern Switzerland. Women, individuals aged between 45 and 64 years, and persons with high education and socioeconomic status and vaccination were slightly over- and persons with previous infections underrepresented (Table 1).
Table 1. Characteristics of the sample, stratified by canton and age group, Ticino and Zurich, Switzerland, March 2022 (n = 1,894).
| Study site | Ticino | Zurich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age category in years | All | 16–29 | 30–44 | 45–64 | ≥ 65 | All | 16–29 | 30–44 | 45–64 | ≥ 65 | |
| Sample size | 850 | 176 | 208 | 262 | 204 | 1,044 | 183 | 260 | 326 | 275 | |
| Median age in years | 48 | 23 | 38 | 54 | 72 | 50 | 24 | 37 | 55 | 72 | |
| IQR | 32–64 | 19–27 | 34–42 | 49–58 | 68–77 | 34–65 | 21–27 | 33–41 | 51–59 | 68–77 | |
| Age group in years | |||||||||||
| 16–29 | Number | 176 | 176 | 0 | 0 | 0 | 183 | 183 | 0 | 0 | 0 |
| Percentage | 20.7 | 100.0 | 0.0 | 0.0 | 0.0 | 17.5 | 100.0 | 0.0 | 0.0 | 0.0 | |
| 30–44 | Number | 208 | 0 | 208 | 0 | 0 | 260 | 0 | 260 | 0 | 0 |
| Percentage | 24.5 | 0.0 | 100.0 | 0.0 | 0.0 | 24.9 | 0.0 | 100.0 | 0.0 | 0.0 | |
| 45–64 | Number | 262 | 0 | 0 | 262 | 0 | 326 | 0 | 0 | 326 | 0 |
| Percentage | 30.8 | 0.0 | 0.0 | 100.0 | 0.0 | 31.2 | 0.0 | 0.0 | 100.0 | 0.0 | |
| ≥ 65 | Number | 204 | 0 | 0 | 0 | 204 | 275 | 0 | 0 | 0 | 275 |
| Percentage | 24.0 | 0.0 | 0.0 | 0.0 | 100.0 | 26.3 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Sexa | |||||||||||
| Female | Number | 484 | 103 | 125 | 152 | 104 | 567 | 106 | 157 | 169 | 135 |
| Percentage | 56.9 | 58.5 | 60.1 | 58.0 | 51.0 | 54.3 | 57.9 | 60.4 | 51.8 | 49.1 | |
| Educational level | |||||||||||
| Primary | Number | 89 | 39 | 6 | 10 | 34 | 71 | 31 | 7 | 10 | 23 |
| Percentage | 10.5 | 22.2 | 2.9 | 3.8 | 16.7 | 6.8 | 16.9 | 2.7 | 3.1 | 8.4 | |
| Secondary | Number | 475 | 90 | 82 | 180 | 123 | 428 | 79 | 68 | 127 | 154 |
| Percentage | 55.9 | 51.1 | 39.4 | 68.7 | 60.3 | 41.0 | 43.2 | 26.2 | 39.0 | 56.0 | |
| Tertiary | Number | 278 | 46 | 120 | 70 | 42 | 537 | 73 | 181 | 187 | 96 |
| Percentage | 32.7 | 26.1 | 57.7 | 26.7 | 20.6 | 51.4 | 39.9 | 69.6 | 57.4 | 34.9 | |
| Missing | Number | 8 | 1 | 0 | 2 | 5 | 8 | 0 | 4 | 2 | 2 |
| Percentage | 0.9 | 0.6 | 0.0 | 0.8 | 2.5 | 0.8 | 0.0 | 1.5 | 0.6 | 0.7 | |
| Household income (CHF/month) | |||||||||||
| 0–6,000 | Number | 371 | 79 | 64 | 107 | 121 | 353 | 97 | 50 | 62 | 144 |
| Percentage | 43.6 | 44.9 | 30.8 | 40.8 | 59.3 | 33.8 | 53.0 | 19.2 | 19.0 | 52.4 | |
| 6,000–12,000 | Number | 306 | 55 | 100 | 96 | 55 | 392 | 43 | 108 | 143 | 98 |
| Percentage | 36.0 | 31.2 | 48.1 | 36.6 | 27.0 | 37.5 | 23.5 | 41.5 | 43.9 | 35.6 | |
| 12,000–18,000 | Number | 59 | 13 | 20 | 24 | 2 | 173 | 30 | 60 | 70 | 13 |
| Percentage | 6.9 | 7.4 | 9.6 | 9.2 | 1.0 | 16.6 | 16.4 | 23.1 | 21.5 | 4.7 | |
| ≥ 18,000 | Number | 45 | 7 | 15 | 16 | 7 | 75 | 7 | 30 | 36 | 2 |
| Percentage | 5.3 | 4.0 | 7.2 | 6.1 | 3.4 | 7.2 | 3.8 | 11.5 | 11.0 | 0.7 | |
| Missing | Number | 69 | 22 | 9 | 19 | 19 | 51 | 6 | 12 | 15 | 18 |
| Percentage | 8.1 | 12.5 | 4.3 | 7.3 | 9.3 | 4.9 | 3.3 | 4.6 | 4.6 | 6.5 | |
| Employment status | |||||||||||
| Working | Number | 529 | 126 | 183 | 208 | 12 | 731 | 177 | 237 | 292 | 25 |
| Percentage | 62.2 | 71.6 | 88 | 79.4 | 5.9 | 70 | 96.7 | 91.2 | 89.6 | 9.1 | |
| Missing | Number | 5 | 1 | 1 | 1 | 2 | 8 | 0 | 4 | 1 | 3 |
| Percentage | 0.6 | 0.6 | 0.5 | 0.4 | 1.0 | 0.8 | 0.0 | 1.5 | 0.3 | 1.1 | |
| Swiss citizenship | |||||||||||
| Swiss citizen | Number | 677 | 161 | 138 | 212 | 166 | 880 | 157 | 180 | 284 | 259 |
| Percentage | 79.6 | 91.5 | 66.3 | 80.9 | 81.4 | 84.3 | 85.8 | 69.2 | 87.1 | 94.2 | |
| Missing | Number | 3 | 0 | 0 | 2 | 1 | 6 | 0 | 3 | 1 | 2 |
| Percentage | 0.4 | 0.0 | 0.0 | 0.8 | 0.5 | 0.6 | 0.0 | 1.2 | 0.3 | 0.7 | |
| Lifestyle and conditions | |||||||||||
| Smoking | Number | 178 | 56 | 47 | 52 | 23 | 192 | 37 | 54 | 70 | 31 |
| Percentage | 20.9 | 31.8 | 22.6 | 19.8 | 11.3 | 18.4 | 20.2 | 20.8 | 21.5 | 11.3 | |
| Missing | Number | 3 | 0 | 0 | 1 | 2 | 5 | 0 | 1 | 1 | 3 |
| Percentage | 0.4 | 0.0 | 0.0 | 0.4 | 1.0 | 0.5 | 0.0 | 0.4 | 0.3 | 1.1 | |
| Obese (BMI ≥ 30) | Number | 102 | 8 | 24 | 37 | 33 | 132 | 11 | 27 | 55 | 39 |
| Percentage | 12 | 4.5 | 11.5 | 14.1 | 16.2 | 12.6 | 6 | 10.4 | 16.9 | 14.2 | |
| Missing | Number | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 0 |
| Percentage | 0.1 | 0.0 | 0.0 | 0.4 | 0.0 | 0.2 | 0.0 | 0.4 | 0.3 | 0.0 | |
| ≥ 1 chronic condition | Number | 188 | 14 | 16 | 56 | 102 | 283 | 13 | 30 | 81 | 159 |
| Percentage | 22.1 | 8 | 7.7 | 21.4 | 50 | 27.1 | 7.1 | 11.5 | 24.8 | 57.8 | |
| Missing | Number | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 0 |
| Percentage | 0.1 | 0.0 | 0.0 | 0.0 | 0.5 | 0.2 | 0.0 | 0.4 | 0.3 | 0.0 | |
| Previous SARS-CoV-2 infection | |||||||||||
| Positive test (ever) | Number | 356 | 91 | 112 | 111 | 42 | 339 | 76 | 107 | 100 | 56 |
| Percentage | 41.9 | 51.7 | 53.8 | 42.4 | 20.6 | 32.5 | 41.5 | 41.2 | 30.7 | 20.4 | |
| Missing | Number | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 1 | 1 |
| Percentage | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.4 | 0.3 | 0.4 | |
| Infected recently (NuC antibody positive) | Number | 230 | 64 | 71 | 61 | 34 | 238 | 52 | 73 | 79 | 34 |
| Percentage | 27.1 | 36.4 | 34.1 | 23.3 | 16.7 | 22.8 | 28.4 | 28.1 | 24.2 | 12.4 | |
| Infected recently (NuC antibody positive or positive test 2022) | Number | 318 | 86 | 102 | 92 | 38 | 318 | 71 | 99 | 102 | 46 |
| Percentage | 37.4 | 48.9 | 49 | 35.1 | 18.6 | 30.5 | 38.8 | 38.1 | 31.3 | 16.7 | |
| Past severe infections 2020–2022b | Number | 13 | 0 | 0 | 4 | 9 | 6 | 0 | 0 | 1 | 5 |
| Percentage | 1.5 | 0.0 | 0.0 | 1.5 | 4.4 | 0.6 | 0.0 | 0.0 | 0.3 | 1.8 | |
| Missing | Number | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 |
| Percentage | 0.1 | 0.0 | 0.0 | 0.4 | 0.0 | 0.2 | 0.0 | 0.0 | 0.3 | 0.4 | |
| Vaccination against SARS-CoV-2 | |||||||||||
| Vaccinated (≥ 1 dose) | Number | 765 | 158 | 184 | 237 | 186 | 972 | 169 | 238 | 298 | 267 |
| Percentage | 90 | 89.8 | 88.5 | 90.5 | 91.2 | 93.1 | 92.3 | 91.5 | 91.4 | 97.1 | |
| Missing | Number | 6 | 1 | 0 | 3 | 2 | 4 | 1 | 2 | 1 | 0 |
| Percentage | 0.7 | 0.6 | 0.0 | 1.1 | 1.0 | 0.4 | 0.5 | 0.8 | 0.3 | 0.0 | |
| Booster dose | Number | 494 | 74 | 114 | 164 | 142 | 758 | 122 | 178 | 240 | 218 |
| Percentage | 58.1 | 42 | 54.8 | 62.6 | 69.6 | 72.6 | 66.7 | 68.5 | 73.6 | 79.3 | |
| Missing | Number | 100 | 19 | 24 | 27 | 30 | 79 | 14 | 23 | 30 | 12 |
| Percentage | 11.8 | 10.8 | 11.5 | 10.3 | 14.7 | 7.6 | 7.7 | 8.8 | 9.2 | 4.4 | |
BMI: body mass index; CHF: Swiss francs; ICU: intensive care unit; IQR: interquartile range; NuC: nucleocapsid; SARS-CoV-2: severe acute respiratory coronavirus 2.
a Sex was collected as a binary variable (male; female).
b Severe infections were defined as those requiring a hospital admission (among those three ICU admissions in participants from Ticino and none in Zurich).
Ascertainment and prevalence of SARS-CoV-2 antibodies
Participants came to onsite visits at a healthcare facility or were offered at-home visits. For each participant, trained personnel collected venous blood samples, according to clinical standards and COVID-19 hygiene measures. We assessed SARS-CoV-2 specific antibodies against the spike and nucleocapsid proteins using Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological (SenASTrIS), a Luminex binding assay [9]. The assay measures binding of IgG antibodies to the trimeric SARS-CoV-2 spike and the nucleocapsid proteins. The test has a high specificity (98%) and sensitivity (99%) and has been validated in samples of the general population and in specific subgroups [9].
We calculated seroprevalence using a Bayesian logistic regression model accounting for the psychometric characteristics of the serological test and applied post-stratification weights based on the target population demographic structure [10]. We conducted all analyses in R, version 4.1.2.
We found that 97.6% (95% credible interval (CI): 96.8–98.2%) had developed IgG antibodies against the spike protein following vaccination and/or infection (Table 2) without relevant differences across age groups and region. Overall, 34% (636/1,894) of the sample originated from people recently infected, based on a self-reported positive laboratory viral test since January 2022, and/or detection of anti-nucleocapsid IgG antibodies.
Table 2. Prevalence of SARS-CoV-2 IgG antibodies and ACE2r-blocking (neutralising capacity) as measured by a virus-free assay, stratified by canton and age group, Ticino and Zurich, Switzerland, March 2022 (n = 1,894).
| Study site | Ticino | Zurich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age groups in years
(total number of individuals) |
All (n = 850) |
16–29 (n = 176) |
30–44 (n = 208) |
45–64 (n = 262) |
≥ 65 (n = 204) |
All (n = 1,044) |
16–29 (n = 183) |
30–44 (n = 260) |
45–64 (n = 326) |
≥ 65 (n = 275) |
|
| Presence of anti-spike IgG antibodies | |||||||||||
| Number | 822 | 172 | 202 | 253 | 195 | 1027 | 179 | 257 | 321 | 270 | |
| % | 96.7 | 97.7 | 97.1 | 96.6 | 95.6 | 98.4 | 97.8 | 98.8 | 98.5 | 98.2 | |
| Level of anti-spike IgG antibodiesa | |||||||||||
| Not detectable | Number | 28 | 4 | 6 | 9 | 9 | 17 | 4 | 3 | 5 | 5 |
| % | 3.3 | 2.3 | 2.9 | 3.4 | 4.4 | 1.6 | 2.2 | 1.2 | 1.5 | 1.8 | |
| Low (≥ 6 − < 12)b | Number | 11 | 6 | 2 | 3 | 0 | 12 | 3 | 4 | 4 | 1 |
| % | 1.3 | 3.4 | 1.0 | 1.1 | 0.0 | 1.1 | 1.6 | 1.5 | 1.2 | 0.4 | |
| Moderate (≥ 12 − < 40)b | Number | 28 | 6 | 10 | 9 | 3 | 20 | 3 | 6 | 7 | 4 |
| % | 3.3 | 3.4 | 4.8 | 3.4 | 1.5 | 1.9 | 1.6 | 2.3 | 2.1 | 1.5 | |
| High (≥ 40)b | Number | 783 | 160 | 190 | 241 | 192 | 995 | 173 | 247 | 310 | 265 |
| % | 92.1 | 90.9 | 91.3 | 92.0 | 94.1 | 95.3 | 94.5 | 95.0 | 95.1 | 96.4 | |
| U/mL according to Elecsys anti-SARS-CoV-2 Sc | Median | 2,511 | 2,443 | 2,624 | 2,534 | 2,453 | 2,637 | 2,637 | 2,707 | 2,605 | 2,637 |
| IQR | 2,139–3,062 | 2,054–3,131 | 2,142–3,175 | 2,140–2,913 | 2,194–2,953 | 2,143–3,051 | 2,202–3,127 | 2,151–3,069 | 2,070–3,012 | 2,139–3,029 | |
| Seroprevalence | |||||||||||
| % | 97.5 | 97.1 | 98.3 | 97.9 | 97.2 | 98.8 | 98.1 | 99.3 | 99.1 | 98.8 | |
| 95% CrI in % | 95.8–99 | 94.3–98.8 | 95.6–99.7 | 95.2–99.6 | 93.9–99.3 | 97.9–99.5 | 96–99.3 | 97.8–99.9 | 97.6–99.8 | 96.8–99.7 | |
| Neutralisation | |||||||||||
| Wild type | Number | 787 | 161 | 190 | 243 | 193 | 996 | 173 | 247 | 312 | 264 |
| % | 92.6 | 91.5 | 91.3 | 92.7 | 94.6 | 95.4 | 94.5 | 95.0 | 95.7 | 96.0 | |
| Delta | Number | 774 | 159 | 188 | 239 | 188 | 978 | 172 | 246 | 305 | 255 |
| % | 91.1 | 90.3 | 90.4 | 91.2 | 92.2 | 93.7 | 94.0 | 94.6 | 93.6 | 92.7 | |
| Omicron | Number | 742 | 154 | 183 | 228 | 177 | 934 | 167 | 233 | 290 | 244 |
| % | 87.3 | 87.5 | 88.0 | 87.0 | 86.8 | 89.5 | 91.3 | 89.6 | 89.0 | 88.7 | |
ACE2r: angiotensin-converting enzyme 2 receptor; CrI: credible interval; IgG: immunglobulin G; IQR: interquartile range; MFI: mean fluorescence intensities; NuC: nucleocapsid.
a Unit for levels of anti-spike IgG antibodies is the MFI as measured by the Luminex binding assay Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological (SenASTrIS) [9].
b Low: from threshold of test positivity (MFI ≥ 6) to < 3 standard deviations above this threshold (MFI < 12); moderate: ≥ 3 standard deviations above positivity (MFI ≥ 12) threshold but unlikely to provide neutralisation (MFI < 40); high: neutralising capacity likely (MFI ≥ 40).
c For interpretation of quantitative results MFI values were converted to U/mL as measured by the Elecsys Anti-SARS-CoV-2 immunoassay produced by Roche. Roche anti-S IgG = 10^(−0.6108069 + 2.0072882 × log10(MFI + 1)) as developed by the Department of Clinical Immunology & Allergy of the University Hospital of Lausanne based on population-based samples.
Ascertainment and prevalence of neutralising capacity against wild-type SARS-CoV-2 as well as Delta and Omicron variants
We also assessed the presence of SARS-CoV-2 neutralising antibodies using a cell- and virus-free assay [11]. This assay measures the proportion of antibodies that block the interaction of the angiotensin-converting enzyme 2 receptor (ACE2r) with the receptor-binding domain of the trimer spike protein of the wild type and variants of concern.
The proportion of individuals whose antibodies showed ACE2r-blocking capacity in this virus-free assay was high against the wild-type SARS-CoV-2 (1,783/1,894; 94%), and Delta variant (1,752/1,894; 93%) and appeared only slightly lower for the Omicron (1,676/1,894; 88%), with no relevant differences across the age groups, but slightly higher proportions in north-eastern compared with southern Switzerland (Table 2). When stratified for recent infection, we found that more participants with anti-nucleocapsid IgG antibodies seemed to show ACE2r-blocking capacity against Omicron than those without (96% (221/230) vs 84% (521/620) in Ticino and 93% (222/238) vs 88% (712/806) in Zurich). In contrast, for wild-type SARS-CoV-2 and Delta variant, ACE2r-blocking capacities against Omicron remained similar, whether anti-nucleocapsid IgG antibodies were present or not. The proportions of participants with anti-nucleocapsid IgG antibodies also appeared to decrease across groups with increasing age (Table 1).
Discussion
The introduction of vaccines against SARS-CoV-2 and the circulation of highly infectious but less virulent variants of concern, including Omicron, have considerably contributed to reducing the burden of COVID-19 on individuals and health services. Infection spreading is still substantial, but hospital and ICU admissions, and mortality rates have steadily decreased in many countries, including in Switzerland, since late December 2021 [12,13]. It is plausible that seroprevalence (i.e. the proportion of individuals with anti-spike SARS-CoV-2 antibodies) exceeds 90% for the adult population in countries that were considerably exposed to natural infection and attained high vaccination coverage at the same time [14].
Indeed, by March 2022, almost the entire population in the current study developed antibodies against SARS-CoV-2, irrespective of age and region of residence in Switzerland. The vast majority of individuals also developed antibodies with neutralising capacity against the wild type virus, as well as the Delta and Omicron variants. Neutralising antibodies are critical for protection against infection and play an important role in protection against severe disease [15,16]. Our findings additionally suggest that a substantial part of the general population in Switzerland developed functional hybrid immunity as a result of infection and vaccination. Of note and as suggested by an apparent differential proportion of anti-nucleocapsid IgG antibodies across age groups (Table 1), a smaller proportion of the elderly population might have had hybrid functional immunity potentially widening the immunity gap over time.
To the best of our knowledge, no population-based seroprevalence studies conducted in European countries in 2022 have been published to date. However, our findings on seroprevalence nearing 100% are expected, as in line with the increase already reported in European [14] and non-European countries [17,18]. A remarkable result of this study is the high proportion of the population whose antibodies showed neutralising activity against different variants of SARS-CoV-2, including Omicron. This may be due to the booster campaign offered in late autumn of 2021, but it is also likely ascribable to the high incidence of infections caused by both Delta and Omicron variants in late 2021 and early 2022 (Figure), when more stringent public health measures were progressively relaxed. The combination of infections and booster vaccinations likely explains the high prevalence of functional immunity at present.
This study has three major strengths: (i) we did a cross-sectional analysis of a prospective, population-based study, whereas population-based studies on SARS-CoV-2 neutralising antibodies are almost inexistent in Europe [19]; (ii) we adopted a standardised protocol and antibody test, across sites and time, since the beginning of the pandemic; (iii) the current study was timely in March 2022, which was a few weeks after very high incidence of infections due to the Delta and Omicron BA.1 and BA.2 subvariants (Figure). Limitations include the low participation rate and overrepresentation of people with higher education and socioeconomic status. However, while vaccination uptake may be higher, infection rates may have been lower in this group compared with the entire population. Other limitations include the limited scope of immune function assessed (e.g. no T-cell function) and the possibility that (future) variants may evade neutralisation as assessed here. Future immuno-epidemiological studies may also assess mucosal IgA and tissue resident cellular immunity, which are not induced by current injectable vaccines, but increasingly recognised as important for consideration in future vaccines [20].
Conclusion
In conclusion, antibody response and neutralising capacity are both very high in the Swiss population after the booster campaign in late 2021, and after high rates of infections due to the Delta and Omicron variants of SARS-CoV-2. This results in robust protective immunity. The temporal trajectory of protective immunity must be monitored to determine if, when and to whom booster vaccinations should be offered.
Ethical statement
The ethics committees of the cantons of Zurich (BASEC Registration No 2020-01247), and Ticino (BASEC Registration No 2020-01514) authorised the study, and all participants provided written informed consent.
Funding statement
This study is part of the Corona Immunitas research network, coordinated by the Swiss School of Public Health (SSPH+), and funded by fundraising of SSPH+ that includes funds of the Swiss Federal Office of Public Health and private funders (ethical guidelines for funding stated by SSPH+ were respected), by funds of the cantons of Switzerland (Vaud, Zurich, and Basel), and by institutional funds of the universities. Additional funding, specific to this study, was provided by the Federal Office of Public Health, Switzerland. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability statement
Data are available on request.
Acknowledgements
CORONA IMMUNITAS RESEARCH GROUP – 25.03.2022
Emiliano Albanese, MD, PhD (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Rebecca Amati, PhD (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland) Antonio Amendola, Msc (Department of Business Economics, Health and Social Care (DEASS),, University of Applied Sciences & Arts of Southern Switzerland (SUPSI), Switzerland; Alexia Anagnostopoulos, MD MPH (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Daniela Anker, PhD (Population Health Laboratory (#PopHealthLab), University of Fribourg, Switzerland; Institute of Primary Health Care (BIHAM), University of Bern, Switzerland); Anna Maria Annoni, Msc (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Hélène Aschmann, PhD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Andrew Azman, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Institute of Global Health, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Antoine Bal, MSc (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Tala Ballouz, MD MPH (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Hélène Baysson, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Kleona Bezani, Msc (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Annette Blattmann (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Patrick Bleich (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Murielle Bochud, MD, PhD (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Patrick Bodenmann, MD, Msc (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Gaëlle Bryand Rumley, MSc (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Peter Buttaroni, Msc (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Audrey Butty, MD (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Anne Linda Camerini, PhD (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Arnaud Chiolero, MD, PhD (Population Health Laboratory (#PopHealthLab), University of Fribourg, Switzerland; Institute of Primary Health Care (BIHAM), University of Bern, Switzerland; Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montréal, Canada); Patricia Orializ Chocano-Bedoya, MD, PhD (Institute of Primary Health Care (BIHAM), University of Bern; Population Health Laboratory (#PopHealthLab), University of Fribourg, Switzerland); Prune Collombet (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Laurie Corna, PhD (Department of Business Economics, Health and Social Care (DEASS),, University of Applied Sciences & Arts of Southern Switzerland (SUPSI), Switzerland); Luca Crivelli, PhD (Department of Business Economics, Health and Social Care (DEASS), University of Applied Sciences & Arts of Southern Switzerland (SUPSI), Switzerland); Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Stéphane Cullati, PhD (Population Health Laboratory (#PopHealthLab), University of Fribourg, Switzerland; Department of Readaptation and Geriatrics, University of Geneva, Switzerland); Valérie D'Acremont, MD, PhD (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland; Swiss Tropical and Public Health Institute, Basel, Switzerland); Diana Sofia Da Costa Santos (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Agathe Deschamps (Cantonal Medical Service Neuchâtel); Paola D’Ippolito (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Anja Domenghino, Dr. med. (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Richard Dubos, MSc (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Roxane Dumont, MSc (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Olivier Duperrex, MD,MSc (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Julien Dupraz, MD, MAS (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Malik Egger (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Emna El-May, MSc (Population Health Laboratory (#PopHealthLab), University of Fribourg, Switzerland); Nacira El Merjani (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Nathalie Engler (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Adina Mihaela Epure, MD (Population Health Laboratory (#PopHealthLab), University of Fribourg, Switzerland); Lukas Erksam (Institute of Primary Health Care (BIHAM), University of Bern, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Sandrine Estoppey (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Marta Fadda, PhD (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Vincent Faivre (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Jan Fehr, MD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Andrea Felappi (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Craig Fenwick, PhD (Service of Immunology and Allergy, Department of Medicine, Lausanne University Hospital and University of Lausanne, 1011 Lausanne, Switzerland); Maddalena Fiordelli, PhD (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Antoine Flahault, MD, PhD (Institute of Global Health, Faculty of Medicine, University of Geneva, Geneva, Switzerland; Division of Tropical and Humanitarian Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Luc Fornerod, MAS (Observatoire valaisan de la santé (OVS), Sion, Switzerland); Cristina Fragoso Corti, PhD (Department of environment construction and design (DACD, University of Applied Sciences & Arts of Southern Switzerland (SUPSI), Switzerland); Natalie Francioli (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Marion Frangville, MSc (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Irène Frank, PhD (Luzerner Kantonsspital, Spitalstrasse, 6000 Luzern 16); Giovanni Franscella, Msc (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Anja Frei, PhD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Marco Geigges, PhD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Semira Gonseth Nusslé, MD, MSc (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Clément Graindorge, MD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Idris Guessous, MD, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Erika Harju, PhD (Department of Health Sciences and Medicine, University of Lucerne, Frohburgstrasse 3, 6002 Lucerne); Séverine Harnal (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Medea Imboden, PhD (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Emilie Jendly (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Ayoung Jeong, PhD (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Christian R Kahlert, MD (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland; Children's Hospital of Eastern Switzerland, Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Laurent Kaiser, MD, PhD (Geneva Center for Emerging Viral Diseases and Laboratory of Virology, Geneva University Hospitals, Geneva, Switzerland; Division of Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland; Department of Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Laurent Kaufmann (Service de La Santé Publique, Canton de Neuchâtel, Neuchâtel, Switzerland); Marco Kaufmann PhD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Dirk Keidel, MSc (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Simone Kessler (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Philipp Kohler, MD, MPH (Cantonal Hospital St. Gallen, Clinic for Infectious Diseases and Hospital Epidemiology, St. Gallen, Switzerland); Christine Krähenbühl (Luzerner Kantonsspital, Spitalstrasse, 6000 Luzern 16); Susi Kriemler, MD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Julien Lamour (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Sara Levati, PhD (Department of Business Economics, Health and Social Care (DEASS), University of Applied Sciences & Arts of Southern Switzerland (SUPSI), Switzerland); Pierre Lescuyer, PhD (Division of Laboratory Medicine, Geneva University Hospitals, Geneva, Switzerland); Andrea Loizeau, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Elsa Lorthe, RM, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Chantal Luedi (Department Health Sciences and Medicine, University of Lucerne, Frohburgstrasse 3, 6002 Lucerne); Jean-Luc Magnin, PhD (Laboratory, HFR-Fribourg, Fribourg, Switzerland); Chantal Martinez (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Eric Masserey (Cantonal Medical Office, General Health Department, Canton of Vaud, Switzerland); Dominik Menges, MD MPH (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Gisela Michel, PhD (Department of Health Sciences and Medicine, University of Lucerne, Frohburgstrasse 3, 6002 Lucerne); Rosalba Morese, PhD (Faculty of Communication, Culture and Society, Università della Svizzera italiana, Lugano, Switzerland; Faculty of Biomedical Sciences, Università della Svizzera italiana, Lugano, Switzerland); Nicolai Mösli (Swiss TPH, Basel, Switzerland; University of Basel, Basel, Swtizerland); Natacha Noël (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Giuseppe Pantaleo, MD (Service of Immunology and Allergy, Department of Medicine, Lausanne University Hospital, University of Lausanne and Swiss Vaccine Research Institute, Lausanne University Hospital, University of Lausanne, Switzerland; Daniel Henry Paris, MD PhD (Swiss TPH, Basel, Switzerland; University of Basel, Basel, Swtizerland); Jérôme Pasquier, PhD (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Céline Pellaton, PhD (Service of Immunology and Allergy, Department of Medicine, Lausanne University Hospital, and University of Lausanne, Switzerland); Francesco Pennacchio, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Stefan Pfister, PhD (Laboratory, HFR-Fribourg, Fribourg, Switzerland); Giovanni Piumatti, PhD (Fondazione Agnelli, Turin, Italy); Géraldine Poulain (Division of Laboratory Medicine, Geneva University Hospitals, Geneva, Switzerland); Nicole Probst-Hensch, Dr. phil.II, PhD, MPH (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Caroline Pugin (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Milo Puhan, MD, PhD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Nick Pullen, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Thomas Radtke, PhD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Manuela Rasi, MScN (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Aude Richard (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Institute of Global Health, University of Geneva, Switzerland); Viviane Richard, MSc (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Claude-François Robert (Cantonal Medical Service Neuchâtel); Pierre-Yves Rodondi, MD (Institute of Family Medicine, University of Fribourg, Fribourg, Switzerland); Nicolas Rodondi, MD, MAS (Institute of Primary Health Care (BIHAM), University of Bern; Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Serena Sabatini, PhD (Institute of Public Health (IPH), Università della Svizzera italiana, Lugano, Switzerland); Khadija Samir (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Javier Sanchis Zozaya, MD (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Virginie Schlüter, MAS (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Alexia Schmid, MSc (Institute of Family Medicine, University of Fribourg, Fribourg, Switzerland); Valentine Schneider (Cantonal Medical Service Neuchâtel); Maria Schüpbach (Institute of Primary Health Care (BIHAM), University of Bern, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Nathalie Schwab (Institute of Primary Health Care (BIHAM), University of Bern, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Claire Semaani (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Alexandre Speierer (Institute of Primary Health Care (BIHAM), University of Bern; Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Amélie Steiner-Dubuis (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Silvia Stringhini, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Department of Health and Community Medicine, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Stefano Tancredi, MD (Population Health Laboratory (#PopHealthLab), University of Fribourg, Switzerland); Stéphanie Testini (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Julien Thabard (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Mauro Tonolla, PD PhD (Department of environment construction and design (DACD, University of Applied Sciences & Arts of Southern Switzerland (SUPSI), Switzerland); Nicolas Troillet, MD, MSc (Office du médecin cantonal, Sion, Switzerland); Agne Ulyte, MD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Sophie Vassaux (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland); Thomas Vermes, MSc (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Switzerland); Jennifer Villers, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Viktor von Wyl (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Cornelia Wagner, MSc (Population Health Laboratory (#PopHealthLab), University of Fribourg, Switzerland); Rylana Wenger (Institute of Primary Health Care (BIHAM), University of Bern, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern); Erin West, PhD (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Ania Wisniak, MD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland; Institute of Global Health, Faculty of Medicine, University of Geneva, Geneva, Switzerland); Melissa Witzig, Msc (Swiss Tropical and Public Health Institute, Department of Epidemiology and Public Health, Basel, Switzerland; University of Basel, Basel, Swtizerland); María-Eugenia Zaballa, PhD (Unit of Population Epidemiology, Division of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland); Kyra Zens, PhD, MPH (Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland); Claire Zuppinger (Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland)
Conflict of interest: None declared.
Authors’ contributions: MP had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: MP, EA, JF, AF, RA, MK
Acquisition, analysis, or interpretation of data: AF, RA, MK, CP, MP, EA, JF, SS
Drafting of the manuscript: MP
Critical revision of the manuscript for important intellectual content: AF, RA, MK, CP, MP, EA, JF, SS
Statistical analysis: MK
Obtained funding: MP, JF, EA
Administrative, technical, or material support: EA, JF, CP, MP
Supervision: EA, JF, MP
References
- 1.Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA. 2021;326(20):2043-54. 10.1001/jama.2021.19499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021;385(24):e84. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med. 2022;386(13):1207-20. 10.1056/NEJMoa2118691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyke KE, Atmar RL, Islas CD, Posavad CM, Szydlo D, Paul Chourdhury R, et al. DMID 21-0012 Study Group . Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med. 2022;3(7):100679. 10.1016/j.xcrm.2022.100679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J Med Virol. 2022;94(7):2969-76. 10.1002/jmv.27697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West EA, Anker D, Amati R, Richard A, Wisniak A, Butty A, et al. Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int J Public Health. 2020;65(9):1529-48. 10.1007/s00038-020-01494-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenwick C, Croxatto A, Coste AT, Pojer F, André C, Pellaton C, et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol. 2021;95(3):e01828-20. 10.1128/JVI.01828-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313-9. 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenwick C, Turelli P, Pellaton C, Farina A, Campos J, Raclot C, et al. A high-throughput cell- and virus-free assay shows reduced neutralization of SARS-CoV-2 variants by COVID-19 convalescent plasma. Sci Transl Med. 2021;13(605):eabi8452. 10.1126/scitranslmed.abi8452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. OurWorldInData.org. [Accessed 18 May 2022]. Available from: https://ourworldindata.org/covid-hospitalizations#licence.
- 13.European Centre for Disease Prevention and Control (ECDC). Data on hospital and ICU admission rates and current occupancy for COVID-19. Stockholm: ECDC; Jul 2022. Available from: https://www.ecdc.europa.eu/en/publications-data/download-data-hospital-and-icu-admission-rates-and-current-occupancy-covid-19
- 14.Office for National Statistics (ONS). Coronavirus (COVID-19) latest insights: Antibodies 2022. Newport: ONS; Jul 2022. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/antibodies
- 15.Nguyen D, Simmonds P, Steenhuis M, Wouters E, Desmecht D, Garigliany M, et al. SARS-CoV-2 neutralising antibody testing in Europe: towards harmonisation of neutralising antibody titres for better use of convalescent plasma and comparability of trial data. Euro Surveill. 2021;26(27):2100568. 10.2807/1560-7917.ES.2021.26.27.2100568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 17.Clarke KEN, Jones JM, Deng Y, Nycz E, Lee A, Iachan R, et al. Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies - United States, September 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(17):606-8. 10.15585/mmwr.mm7117e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields AM, Faustini SE, Hill HJ, Al-Taei S, Tanner C, Ashford F, et al. increased seroprevalence and improved antibody responses following third primary SARS-CoV-2 immunisation: An update from the COV-AD Study. Front Immunol. 2022;13:912571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aziz NA, Corman VM, Echterhoff AKC, Müller MA, Richter A, Schmandke A, et al. Seroprevalence and correlates of SARS-CoV-2 neutralizing antibodies from a population-based study in Bonn, Germany. Nat Commun. 2021;12(1):2117. 10.1038/s41467-021-22351-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topol EJ, Iwasaki A. Operation Nasal Vaccine—Lightning speed to counter COVID-19. American Association for the Advancement of Science; 2022. p. eadd9947. [DOI] [PubMed] [Google Scholar]