Summary
Over the last decade, mounting evidence has revealed the key roles of gut microbiota in modulating the efficacy and toxicity of anticancer drugs, via mechanisms such as immunomodulation and microbial enzymatic degradation. As such, human microbiota presents as an exciting prospect for developing biomarkers for predicting treatment outcomes and interventional approaches for improving therapeutic effects. In this review, we analyze the current knowledge of the interplays among gut microorganisms, host responses and anticancer therapies (including cytotoxic chemotherapy and targeted therapy), with an emphasis on the immunomodulation function of microbiota which facilitates the efficacy of immune checkpoint inhibitors. Moreover, we propose several microbiota-modulating strategies including fecal microbiota transplantation and probiotics, which can be pursued to optimize the use and development of anticancer treatments. We anticipate that future clinical and preclinical studies will highlight the significance of human microbiome as a promising target towards precision medicine in cancer therapies.
Funding
National Key Research and Development Program of China (2020YFA0907800), Shenzhen Science and Technology Innovation Program (KQTD20200820145822023) and National Natural Science Foundation of China (31900056 and 32000096).
Keywords: Anticancer drug, Microbiota, Immune checkpoint inhibitor, Fecal microbiota transplantation, Probiotics
Introduction
Cancer is a major public health burden and a leading cause of human death worldwide.1 Globally, more than 19.3 million cancer cases are newly diagnosed per annum and over 9.9 million individuals die as a consequence.1 Remarkable advances have been made in tackling cancer in recent years, including early detection, diagnosis, and cancer treatment. A stepwise increase in numbers of anticancer drug approval was recorded from the year 2009 (8 approvals) to the year 2020 (57 approvals).2 These newly approved drugs have largely enriched the therapeutic options, and enhanced the survival and quality of life of cancer patients.
Clinical use of anticancer drugs varies based on tumor location, age, disease stage, metastatic state, genetic heterogeneity, etc.3 Taking colorectal cancer as an example, in patients with high-risk stage II and III colorectal cancer, defined as those with poor prognostic features, adjuvant systemic chemotherapy provides an overall survival benefit. First-line regimens are typically based on various combinations of cytotoxic drugs oxaliplatin, 5-fluorouracil (5-FU), capecitabine, and leucovorin.3 However, prolonged administration, lack of specificity and disparate cytotoxic adverse effects are major limitations in their clinical applications.3 Between 2004 and 2006, three novel monoclonal antibodies (bevacizumab, cetuximab, and panitumumab) as targeted therapies came into use for treating metastatic colorectal cancer, whereas they are only indicated to certain genetic types, and mainly used in combinations with cytotoxic chemotherapies.4 Since 2017, three immune checkpoint inhibitor (ICI) drugs (e.g., pembrolizumab, nivolumab, and ipilimumab) have been clinically utilized for treating specific colorectal cancer subtypes, namely mismatch-repair-deficient (dMMR) microsatellite instability-high (MSI-H) subtypes.5 However, this type only comprises approximately 15% of all colorectal cancer patients, demonstrating that the majority of the patients cannot benefit from ICI therapies.5 For the reasons above, our current armory of effective medications against cancer cells is still limited, therefore, both new agents and predictive biomarkers for selecting beneficial therapies are urgently needed.
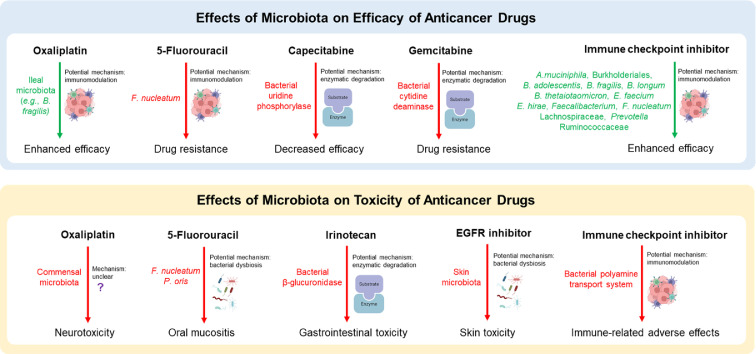
The human colorectums are exposed to and constantly interact with over 3.8 × 1013 microorganisms, which are vital for gastrointestinal (GI), systemic physiology and pathophysiology, including tumorigenesis.6 Human gut microbes may promote, mitigate or have no direct effect on carcinogenesis. Microbes such as Helicobacter pylori, Fusobacterium nucleatum, enterotoxigenic Bacteroides fragilis, and Peptostreptococcus anaerobius have been reported to amplify cancer development and progression via toxins and activation of procarcinogenic signaling pathways.7 On the other hand, Streptococcus thermophilus, Lactobacillus gallinarum, and Lactobacillus reuteri exert suppressing function in carcinogenesis by producing anticancer metabolites.8 Furthermore, mounting evidence suggests that the gut microbiota modulates drug response (including efficacy and toxicity) and prognosis, via mechanisms including immunomodulation, microbial enzymatic degradation, and metabolism of drugs9 (Figure 1). A new discipline, pharmacomicrobiomics, has emerged to exploit the impact of microbiome compositional and functional variations on drug efficacy, toxicity and pharmacokinetics.10 As a promising target for precision medicine in cancer therapies, human microorganisms may be utilized to 1) predict treatment response, and 2) improve efficacy and reduce toxicity of anticancer drugs.
Figure 1.
Effects of microbiota on the efficacy and toxicity of anticancer drugs. Bacterial names are color-coded by their association with drug outcomes (green: increased drug efficacy; red: decreased drug efficacy or increased toxicity). A. muciniphila, Akkermansia muciniphila; B. adolescentis, Bifidobacterium adolescent; B. fragilis, Bacteroides fragilis; B. longum, Bifidobacterium longum; B. thetaiotaomicron, Bacteroides thetaiotaomicron; E. faecium, Enterococcus faecium; E. hirae, Enterococcus hirae; B. obeum, Blautia obeum; F. nucleatum, Fusobacterium nucleatum; EGFR, epidermal growth factor receptor.
Here, we summarize the recent advance of pharmacomicrobiomics of anticancer drugs, with a focus on the impact of gut microbiota on modulating efficacy and toxicity of ICI drugs, and potential applications for improving current therapies by modulating gut microbiota.
Current knowledge on interplays between microbiota and anticancer drugs
Oxaliplatin
Oxaliplatin is the latest platinum-based chemotherapeutic agent and recommended as the first-line regimen for the treatment of advanced cancer of the colon and rectum.11 Oxaliplatin exerts its anticancer effect mainly via forming platinum-DNA abducts to elicit cell death and inhibit DNA replication.12 Moreover, oxaliplatin induces immunogenic cell death by stimulating immune cells such as CD8+ T cells.11
Both efficacy and toxicity of oxaliplatin are affected by the gut microbiota. A recent study on antibiotic-treated mice model suggested that gut microbial metabolites, especially butyrate, improved the chemotherapeutic efficacy of oxaliplatin by regulating CD8+ T cell function, which plays a central role in tumor immunity.13 In addition, cancer patients who responded to oxaliplatin exhibited a higher abundance of serum butyrate compared to non-responding patients.13 Further, the ileal microbiota (e.g., B. fragilis) was found to potentially boost local immune responses (e.g., increase of immune gene transcripts and CD45+ lymphoid cell infiltration) and enhance the antitumor efficacy of oxaliplatin-based chemotherapy.14
Moreover, oxaliplatin could induce extensive adverse events, including peripheral neurotoxicity which affects up to 90% of patients undergoing chemotherapy, and could potentially lead to the discontinuation of treatment.15 Shen et al.15 demonstrated that oxaliplatin-induced hyperalgesia was alleviated in GF mice and antibiotics-treated mice, compared to the control group. Further FMT to GF mice restored the oxaliplatin-induced hyperalgesia, demonstrating the role of gut microbiota in the development of oxaliplatin-induced neurotoxicity. However, the mechanism that links gut microbiota to oxaliplatin-induced hyperalgesia is unclear. Moreover, most of the experiments in abovementioned studies were conducted in mice, which may vary from the situation in the clinic. Therefore, clinical evaluations through a relatively large sample size of patient cohort are necessary for the validation of the above findings from studies in mice.
5-Fluorouracil (5-FU)
5-FU, a fluorinated analog of uracil, is one of the most widely employed antimetabolite chemotherapeutic agents for the treatment of a variety of cancers, including breast cancer, colorectal cancer and gastric cancer, etc. The major mode of action of 5-FU and its active derivatives (e.g., fluoro-deoxyuridine-monophosphate and fluoro-deoxyuridine-triphosphate) is via the inhibition of thymidylate synthase, leading to formation of DNA breaks, disruption of DNA replication and subsequent cell death.16 Other possible mechanisms include incorporation into RNA by replacing uracil and disruption of RNA synthesis.17
5-FU treatment results in a significantly altered gut microbiota, which may be responsible for the side effects including intestinal mucositis.18 In mouse model upon 5-FU treatment, increases of the abundance of Lachnospiraceae, Bacteroides, Blautia and Mucispirillum species, and decreases of Coriobacteria and Deltaproteobacteria were observed.19 In addition, oral mucositis induced by 5-FU treatment is associated with bacterial dysbiotic shifts, including enrichment of Fusobacterium nucleatum and Prevotella oris, and depletion of commensals from the genera Streptococcus, Actinomyces and Veillonella.20
Chemoresistance is a major challenge that limits the clinical utility of 5-FU.17 High abundance of F. nucleatum is associated with 5-FU resistance in colorectal cancer patients.21,22 Zhang et al.21 demonstrated that in colorectal cancer patients who received 5-FU-based adjuvant chemotherapy after radical surgery, high abundance of F. nucleatum correlated with poor recurrence-free survival (RFS), and so did high levels of TLR4 and BIRC3 proteins. The possible mechanisms encompass the upregulation of BIRC3 expression induced by F. nucleatum.21 Furthermore, Yu et al.22 showed that F. nucleatum leads to chemoresistance to 5-FU by orchestrating TLR4-MYD88 innate immune signaling pathway, specific miRNAs (genomic loss of miR-18a* and miR-4802) and autophagy elements (ULK1/ATG7 autophagy network).
The composition of microbiota also modulates the drug efficacy of 5-FU. Yuan et al.19 reported that administration of antibiotics in mouse model attenuated the antitumor efficacy of 5-FU, whereas supplementing probiotics that contains Bifidobacterium and Lactobacillus strains could not improve the efficacy. Moreover, Garcia-Gonzalez et al.23 reported that Escherichia coli OP50 or Comamonas can modulate 5-FU efficacy in Caenorhabditis elegans via bacterial nucleotide metabolism.
Capecitabine
Capecitabine is an orally delivered prodrug of 5-fluorouracil, and commonly applied in combination with platinum-based chemotherapy to treat advanced metastatic breast cancer and colorectal cancer.22 Capecitabine is converted to its cytotoxic form 5-FU by thymidine phosphorylase (mainly in tumor cells and liver), which further exerts antitumor effects as 5-FU.22
Microbiota composition and function are reported to be associated with capecitabine activity, metabolism and chemotoxicity.22 Javdan et al.24 developed a powerful Microbiome-Derived Metabolism (MDM) screening approach and unveiled the role of microbiome-derived enzyme uridine phosphorylase in the deglycosylation of capecitabine, which decreased the chemotherapeutic efficacy. The same group also demonstrated the effect of microbiota on the efficacy of antidiabetic drug acarbose via microbial enzymatic degradation, highlighting this important mechanism by which bacteria influence drug outcome.25 Furthermore, a clinical analysis26 indicated that in 15 patients with breast cancer treated with capecitabine as maintenance chemotherapy, the abundance of specific bacterial species (e.g., Slackia and Blautia obeum) is associated with progression-free survival, and may be employed as microbial markers for predicting capecitabine resistance and prognosis. As a drug for oral administration, capecitabine may experience unveiled interactions with gut microbiota during its absorption and metabolism, and therefore it is important to further investigate the pharmacomicrobiomics of capecitabine for its optimal use.
Irinotecan
Irinotecan is frequently used in the first-line treatment FOLFIRI regimen in combination with 5-FU and folinic acid for treating colorectal cancer.27 As a semisynthetic water-soluble analogue of camptothecin (CPT), it is also known as CPT-11. Irinotecan and its active metabolite (SN-38) inhibits DNA topoisomerase I by forming complex with DNA, and subsequently prevents re-ligation of DNA strands, resulting in DNA breakage, cell cycle arrest and apoptosis.27 Nevertheless, irinotecan causes severe acute or delayed diarrhea in up to 88% of patients, which often leads to dose reduction and even termination of the treatment.9,28
The efficacy and side effects of irinotecan is also swayed by gut microbiota via microbial enzymatic degradation: the gastrointestinal tract (GI) toxicity of irinotecan is mediated by gut bacterial β-glucuronidase (GUS). GUS cleaves the glucuronide from the inactive metabolite of irinotecan (SN-38G), and releases active metabolite (SN-38) in GI tract, which inflicts epithelial damage and diarrhea.28 Microbial GUS can be found in almost all major phyla in gut microbiota: Bacteroidetes, Firmicutes, Verrucomicrobia, and Proteobacteria,29 and multiple studies demonstrated that the combination of irinotecan and GUS inhibitors (e.g., ciprofloxacin, amoxapine and UNC10201652) was able to alleviate irinotecan-induced GI tract damage and the resultant diarrhea in animal models.30, 31, 32 Furthermore, two different studies using rat models suggested that irinotecan increased the abundance of Fusobacteria and Proteobacteria, and the abundance of Clostridium Cluster XI and Enterobacteriaceae.33,34 The altered microbiota itself may also contribute to diarrhea and intestinal inflammation during irinotecan treatment.
Gemcitabine
As a cytidine analogue, gemcitabine (2’,2’-difluoro-2’-deoxycytidine) is one of the first-line treatments for pancreatic ductal adenocarcinoma (PDAC) and many other solid tumors.35 The antitumor mechanisms of gemcitabine involve killing cells with active DNA synthesis during S phase and suppressing cell cycle progression from G1 to S phase.35
Geller et al.36 demonstrated that gemcitabine can be metabolized into its inactive form (2’,2’-difluorodeoxycytidine) by certain bacterial enzymes, resulting in the development of drug resistance. In colon carcinoma models, Mycoplasma hyorhinis and other bacterial species (mainly in Gamma-proteobacteria class) resulted in resistance to gemcitabine, whereas administration of antibiotics abolished this effect. Following experiment elucidated that gemcitabine was metabolized by an isoform of bacterial cytidine deaminase (CDDL), which was mostly expressed in Gamma-proteobacteria. Further, bacterial DNA was detected in 86 out of 113 human PDAC samples (76%), and bacteria cultured from the fresh human PDAC tumors did induce resistance to gemcitabine of human carcinoma cell lines, confirming the presence of intratumor bacteria and their roles in gemcitabine chemoresistance. Similar gemcitabine-metabolizing enzymes were also found in mycoplasma by Voorde et al.37
Epidermal growth factor receptor (EGFR) inhibitor and vascular endothelial growth factor (VEGF) inhibitor
In addition to the traditional chemotherapies, several targeted therapies have become available for treating cancers, including epidermal growth factor receptor (EGFR) inhibitors (e.g., cetuximab or panitumumab) and vascular endothelial growth factor (VEGF) inhibitors (e.g., bevacizumab) for specific types of cancers.
Cetuximab and panitumumab are antibodies that induce EGFR internalization and degradation, which are frequently used for the treatment of non-small cell lung and colorectal cancers.38 However, the skin toxicities (e.g., papulopustular skin rash, pruritus and inflammation) induced by EGFR inhibitors may be aggravated by commensal skin microbiota.38 The skin barrier breakdown caused by anti-EGFR therapy may be permissive for microbiota outgrowth, which exacerbates immune responses (e.g., TNF-α, TH2 cytokines IL-4 and IL-33) and cause inflammation in skin.38
Bevacizumab is a monoclonal antibody acting on the tumor microenvironment, that inhibits VEGF, induces regression of newly formed vessels, and subsequently suppresses angiogenesis and tumor growth.39 Moreover, bevacizumab can facilitate chemotherapeutic effects by affecting the tumor vasculature, therefore it is mainly used in combination with chemotherapy, across a wide range of solid tumor types, including colorectal cancer, cervical cancer and glioblastoma.39 Up to date, the influence of microbiota on the efficacy of bevacizumab has not yet been evaluated, whereas it would be very fascinating to look at the interplay among microbiota, targeted drugs and vasculature in the tumor microenvironment.
CTLA-4 inhibitor
Immune checkpoint inhibitors (ICIs) have revolutionized the strategy of treating advanced-stage cancers, and significantly extended the survival of patients with multiple malignancies, including melanoma, lung cancer, renal cell carcinoma, and gastric cancer.40 Immune checkpoints include cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1) and its ligand PD-L1, which have suppressive functions against activation of anti-tumor T cells. Monoclonal antibodies that inhibit these immune checkpoints can dampen the negative regulation of immune cells and re-activate immune cells (particularly CD8+ cytotoxic T cells) to eliminate tumor cells.40,41
ICIs have become the standard of care for certain types of cancer patients. Taking colorectal cancer for example, patients with mismatch-repair-deficient (dMMR) microsatellite instability-high (MSI-H) colorectal cancer subtypes exerted response rates of 31-55% to ICI therapies.42 However, patients with mismatch-repair-proficient (pMMR) microsatellite stability (MSS) subtypes, which are the vast majority of colorectal cancer (∼85%) is largely unresponsive to current ICIs.5 There is therefore an urgent need to maximize the response rate of colorectal cancer patients to ICIs.
Since 2015, an increasing number of research has demonstrated that the composition of gut microbiota influences the clinical benefit of ICIs in patients with advanced cancers43, 44, 45 (Figure 1), suggesting the potential approach to predicting and improving the efficacy of ICIs by modulating gut microbiome.
CTLA-4 blockade ipilimumab is the first ICI drug targeting T cells to act against cancer, and has been approved to treat melanoma, hepatocellular carcinoma, renal cell carcinoma and colorectal cancer dMMR/MSI-H subtype, etc, since 2011.46 However, compared to those of anti-PD-1 therapy, both efficacy and toxicity of anti-CTLA-4 therapy are suboptimal.47 Therefore, it is important to further understand the mechanism and optimize the clinical use of CTLA-4 blockade.
Vetizou et al.44 demonstrated gut microbiota, particularly members in Bacteroidales, played a key role in the immunomodulatory effects of CTLA-4 inhibitor. In mice and patients with metastatic melanoma, increased levels of Bacteroides thetaiotaomicron, B. fragilis and Burkholderiales were associated with T cell responses and the efficacy of CTLA-4 blockade. Antitumor effects of CTLA-4 blockade were compromised in antibiotic-treated or GF mice, whereas FMT from a specific cluster of melanoma patients with relatively more Bacteroides spp. to recipient mice restored the level of antitumor effects.44 B. fragilis, B. thetaiotaomicron and Burkholderiales induced IL-12-dependent TH1 immune responses in tumor draining lymph nodes, which facilitated the antitumor effects of CTLA-4 blockade in mice and patients while maintaining intestinal integrity.44 Another clinical study48 demonstrated that patients whose baseline gut microbiota was enriched with Faecalibacterium and other Firmicutes had superior clinical responses to ipilimumab, including longer progression-free survival and overall survival.
A major hurdle for ICI therapy in the clinic is immune-related adverse effects (irAEs), with 90% of patients developing irAEs in any grade, most commonly colitis.47 Dubin et al.49 characterized the intestinal microbiota of patients for predicting the development of intestinal inflammation and colitis following ipilimumab treatment. The findings suggested that colitis-free patients possessed higher abundance of the Bacteroidetes phylum, compared to patients progressed to colitis. Also, microbiota-associated modules for bacterial polyamine transport system are associated with an increased risk of colitis.49
PD-1/PD-L1 inhibitor
Multiple PD-1 blockers (e.g., nivolumab and pembrolizumab) and PD-L1 blockers (e.g., atezolizumab, avelumab and durvalumab) are approved for the treatment of various types of cancers including solid tumors, such as melanoma, lung cancer, head and neck cancer, and colorectal cancer dMMR/MSI-H subtype as well as hematological tumors such as Hodgkin's lymphoma.46
Similar to CTLA-4 inhibitors, the outcome of PD-1 blockades in both mice and patients is markedly influenced by the gut microbiota. Early in 2015, Sivan et al.45 demonstrated that oral gavage of commensal Bifidobacterium into mice enhanced the efficacy of anti-PD-1 therapy. Transcriptomic profiling suggested that host dendritic cells were activated by Bifidobacterium-derived signals, and further boosted effector function of CD8+ T cells. Routy et al.43 profiled fecal samples from lung and kidney cancer patients undergoing anti-PD-1 therapy, and segregated responding patients from non-responding patients based on the profiles of fecal microbiome. In responding patients, Akkermansia muciniphila and Enterococcus hirae showed a higher level of abundance in fecal microbiome. Further, FMT from the responders (but not from the non-responders) into antibiotic-treated or GF mice improved the therapeutic effects of anti-PD-1 therapy. Moreover, oral administration of A. muciniphila alone or in combination with E. hirae into mice after FMT with the non-responder feces restored the antitumor activity. Immunological changes elicited by A. muciniphila and E. hirae in mice include the promotion of the IL-12 secretion by dendritic cells, and the accumulation of CCR9+ CXCR3+ CD4+ T cells in the tumor microenvironment.43 Matson et al.50 compared gut microbiota composition in metastatic melanoma patients with different clinical responses and found Bifidobacterium longum, Bifidobacterium adolescentis, and Enterococcus faecium were significantly enriched in patients who responded to anti-PD-1 therapies. Fecal transfer to GF mice from responders and non-responders, paralleled the patient clinical responses, respectively. Similarly, Gopalakrishnan et al.51 studied the oral and gut microbiome of melanoma patients who received anti-PD-1 therapy. Fecal samples of responding patients showed higher alpha diversity and enrichment of the Ruminococcaceae family and the Faecalibacterium genus, compared to those of non-responders. Metagenomic analysis revealed the enrichment of anabolic functions in responders and the enrichment of catabolic functions in non-responders. GF mice transplanted with responder feces had a better response to immunotherapy compared to those transplanted with non-responder feces, and a higher level of CD8+ T cells in the tumor microenvironment as well as in the gut, which were in line with the clinical observation.51 The association between gut microbiota and clinical responses in patients with GI cancer that received anti-PD-1/L1 treatment has also been investigated.52 The 16S and metagenomic analyses revealed that several factors are related to the favorable clinical outcomes, including1 increased ratio of Prevotella and Bacteroides2; enrichment of Ruminococcaceae and Lachnospiraceae3; enrichment of short-chain fatty acid (SCFA) producing bacteria, e.g., Eubacterium, Lactobacillus and Streptococcus.52 More recently, Fusobacterium nucleatum has been reported to promote the therapeutic effects of PD-L1 blockade in mice and patient-derived organoid models with colorectal cancer, by activating PD-L1 expression and upregulating the accumulation of IFN-γ+ CD8+ tumor-infiltrating lymphocytes.53
Interestingly, the bacterial species that were found enriched in the responders to anti-PD-1 therapy by different studies are very diverse across the global population, which may be related to the differences of geographic location, sequencing method, analytical pipeline, etc. The detailed interplays between gut microbiota and tumor immune microenvironment remain largely unknown and to be further explored. Nevertheless, these studies provide a strong rationale for the development of microbiota-centered approach to predicting and enhancing the therapeutic effects of ICIs in patients. We anticipate that a multiparameter model incorporating pharmacomicrobiomics to be applied in the near future to prognosticate which patients are likely to benefit from immunotherapies.
Potential microbiome-modulating strategies for improving cancer therapeutic effects
Fecal microbiota transplantation (FMT)
FMT is an efficient approach to remodeling gut microbiota of patients and has been widely applied to treat recurrent Clostridioides difficile infections, inflammatory bowel diseases and metabolic syndrome, etc.54,55 The therapeutic role of FMT in cancer treatment is being actively investigated. Two papers published in 2021 both demonstrated that FMT promoted the efficacy of anti-PD-1 therapy in melanoma patients.54,55 In the two studies, three out of ten (30%) and six out of fifteen (40%) patients who were initially immunotherapy-refractory showed clinical responses upon receiving responder-derived FMT together with anti-PD-1 therapy. These findings demonstrated that the combination of FMT and anti-PD-1 rebuilt the gut microbiota and modified the tumor microenvironment, including increased CD8+ T cell activation and altered gene expression. These encouraging results indicate a potential strategy to improve anti-PD-1 treatment by modulating gut microbiota using FMT. Currently, a number of clinical trials, including NCT04116775, NCT04521075, NCT04758507, and NCT04130763 (Table 1), are being undertaken to evaluate the safety and efficacy of this strategy in other cancer types (e.g., lung cancer, gastric cancer, kidney cancer and prostate cancer), and to further evaluate the underlying mode of action of the combined use of FMT and ICIs.
Table 1.
Ongoing clinical trials on microbiota-regulating approaches for improving therapeutic effects of anticancer drugs.
| Approach | Anticancer drug | Cancer type | Study aim | Trial identifier |
|---|---|---|---|---|
| Fecal microbiota transplantation (FMT) | Pembrolizumab, enzalutamide | Prostate cancer | FMT and pembrolizumab for men with metastatic prostate cancer | NCT04116775 |
| FMT | Nivolumab | Melanoma, unresectable melanoma, non-small cell lung cancer | To evaluate the safety and efficacy of FMT and nivolumab in subjects with metastatic or inoperable melanoma, MSI-H, dMMR or non-small cell lung cancer | NCT04521075 |
| FMT | Immune checkpoint inhibitors (ICIs, not specified) | Renal cell carcinoma | FMT to improve efficacy of ICIs in renal cell carcinoma | NCT04758507 |
| FMT | Anti-PD-1 (not specified) | Gastrointestinal system cancer | FMT capsule for improving the efficacy of anti-PD-1 | NCT04130763 |
| FMT | Pembrolizumab, nivolumab | Metastatic colorectal adenocarcinoma, metastatic small intestinal adenocarcinoma, stage IV colorectal cancer | FMT and reintroduction of anti-PD-1 therapy for the treatment of metastatic colorectal cancer in anti-PD-1 non-responders | NCT04729322 |
| FMT | Anti-PD-1 (not specified) | Lung cancer | FMT capsule to enhance the efficacy of anti-PD-1 in advanced lung cancer treated with immunotherapy | NCT04924374 |
| FMT | Anti-PD-1/L1 (not specified) | Lung cancer | Study of how human microbiota trans-plantation affect the efficacy of PD-1 immunotherapy in patients with non-small cell lung cancer | ChiCTR2100043472 |
| Probiotics VE800 (a consortium of 11 human commensal bacterial strains) | Nivolumab | Melanoma, gastric cancer | Study of probiotics VE800 and nivolumab in treating patients with selected types of advanced or metastatic cancer | NCT04208958 |
| Probiotic B. fragilis bf-839 | Anti-PD-1 (not specified) | Non-small cell lung cancer | Probiotic (B. fragilis bf-839) to enhance the efficacy and reduce side effects of anti-PD-1 antibody in the treatment of lung cancer | ChiCTR2100054558 |
| Probiotic Clostridium butyricum CBM588 | Anti-PD-1 anti-CTLA-4 combination | Kidney cancer | To evaluate the effect of CBM588 on the efficacy of the nivolumab/ipilimumab combination | NCT03829111 |
| Traditional Chinese Medicine (ginseng polysaccharides) | Anti-PD-1 (not specified) | Lung cancer | To examine the sensitization effect of ginseng polysaccharides on lung cancer patients to anti-PD-1 immunotherapy | Not found |
As the ability to restore the reduced diversity and dysbiosis of gut microbiota, FMT is able to alleviate adverse effects of cancer treatments. Wang et al.56 reported that the first clinical study of ICI-related colitis successfully treated by FMT, accompanied by a reshaped gut microbiome and increased proportion of regulatory T cells within the colonic mucosa. Furthermore, in cancer patients treated with cytotoxic chemotherapy, FMT also serves as a highly effective and safe treatment against chemotherapy-induced C. difficile infection.57 One of the major challenges in FMT is fecal donor selection and screening, as a precise donor inclusion and exclusion criteria is critical for the success of this strategy.
Probiotics
Probiotics, including natural probiotics and genetically engineered ones, showed great potential as live biotherapeutic products (LBPs) for both improving the efficacy and limiting the side effects of cancer chemotherapy drugs.58
As discussed in Section 2.7 and 2.8, multiple bacterial species may enhance the efficacy of ICIs, including Akkermansia muciniphila,43 Bifidobacterium spp.,45 B. fragilis44 and Lactobacillus rhamnosus Probio-M9.59 Tanoue et al.60 reported that a defined commensal consortium of 11 strains induced interferon-γ-producing CD8+ T cells in the intestine and enhanced the therapeutic efficacy of ICIs in mouse models. The 11-strain mixture is under Phase 1 clinical trial as an orally administered treatment combined with anti-PD-1 drug nivolumab (ClinicalTrials.gov identifier: NCT04208958) (Table 1). Moreover, Anker et al.61 isolated a patient-derived prostate-specific immunomodulatory bacterial strain, E. coli CP1, which increased the survival of prostate cancer mice in combination with anti-PD-1. Most recently, a randomized phase 1 trial (ClinicalTrials.gov identifier: NCT03829111) reported that a bifidogenic live biotherapeutic product Clostridium butyricum CBM588 enhanced the efficacy of nivolumab-ipilimumab combination in patients with kidney cancer.62
Administration of probiotics can be used for the amelioration of adverse effects of anticancer drugs as well. Wang et al.63 demonstrated that immune-related adverse effects (e.g., colitis) of CTLA-4 inhibitor was ameliorated by the administration of probiotic Bifidobacterium strains in mice.
Furthermore, several engineered microbes were developed for improving cancer treatment.64 Canale et al.64 developed an engineered E. coli Nissle 1917 strain that locally increased intratumoral L-arginine levels, a key determinant of anti-tumor effectiveness. Intratumorally injection of this strain synergizes with PD-L1 inhibitor in promoting tumor control and the survival in tumor-bearing mice.64 Another engineered E. coli Nissle 1917 strain with augmented myrosinase which catalyzes dietary glucosinolate as a prodrug into an anticancer molecule sulforaphane, displayed significant tumor inhibitive effects.65 Moreover, a Salmonella enterica strain engineered with a synchronized lysis circuit (SLC) that can lyse and release anti-tumor toxin synchronously.66 The combination administration of this strain and the chemotherapeutic drug 5-FU in mice exhibited notable increased anti-tumor effect than either therapy alone.66 In the future, the dose of probiotics supplemented to the patients should be determined through preclinical and clinical trials to maximize the functionality of boosting drug efficacy.
Bacteriophage
Phage-guided modulation of gut microbiota can achieve high selectivity in limiting specific bacteria that contribute to chemoresistance and side effects of cancer chemotherapy.67 Zheng et al.67 isolated a phage against Fusobacterium nucleatum, and showed oral administration of the phage specifically eliminated F. nucleatum in mice gut as well as F. nucleatum-induced chemoresistance to irinotecan.67 This study emphasis bacteriophages as a promising strategy for microbiome-editing and enhancing the efficacy of anticancer therapies, whereas the safety and tolerability endpoints are needed to be examined in the use of phage-drug combinations in the clinic.
Minimizing the use of antibiotics
Although some members in gut microbiota are accountable for certain negative effects of certain anti-tumor drugs, bacterial ablation with antibiotics dampens the efficacy of multiple anticancer drugs, including cyclophosphamide,68 platinum salts (e.g., oxaliplatin and cisplatin), anti-CTLA-4 and anti-PD-1/L1 antibodies.69 Viaud et al.68 discovered that treatment of antibiotics led to the therapeutic failure of cyclophosphamide in tumor-bearing mice, as the gut microbiota stimulate specific immune responses which are critical for the anticancer effect of cyclophosphamide. Moreover, a prospective, multicenter clinical study69 demonstrated that the use of broad-spectrum antibiotic is associated with the poorer outcome following ICI treatment. Worse overall survival (2 months for prior antibiotic therapy vs 26 months for no prior antibiotic therapy) was observed in patients with lung cancer (n = 119), melanoma (n = 38), and other tumor types (n = 39).69 As such, the use of antibiotics in cancer patients should be minimized for optimal responses to cancer therapy.
Other microbiota-modulating agents
Traditional Chinese medicines (TCMs) and dietary interventions have also been studied for their effects in modulating gut microbiome and drug outcome.70 Using germ-free mouse models, Huang et al.70 demonstrated that ginseng polysaccharides (the main active substance in TCM ginseng) sensitize lung cancer tumor-bearing mice to anti-PD-1 immunotherapy, by modulating both microbial composition (e.g., Parabacteroides distasonis and Bacteroides vulgatus) and microbial metabolites (e.g., valeric acid and L-kynurenine). Similarly, a classical TCM formula Gegen Qinlian decoction has been reported to enhance the effect of PD-1 inhibitor by reshaping the gut microbiota and the tumor microenvironment.71 Further, as multiple TCMs have been reported to modulate gut microbiota and at the same time exert anti-tumor effects, their capacity of improving anticancer drug efficacy via governing microbiota would be interesting to investigate.
Furthermore, Rizvi et al.72 reported that high-salt diet (HSD), which can activate NK cells and gut microbiota (e.g., increasing abundance of Bifidobacterium), is able to enhance anti-PD-1 therapy and tumor regression in mice. Given that a number of dietary interventions may sensitize multiple cancer cell types to anticancer therapies, such as fasting, ketogenic diet (which provides glucose restriction) and supplementation of histidine,73,74 future studies may further highlight the effects of microbiome on the synergistic effects of anticancer drugs and dietary interventions.
Outstanding questions
The gut microbiota acts as an “invisible organ” to modulate the efficacy and toxicity of multiple antitumor drugs against various cancer types. As a promising step towards precision and personalized medicine, microbiota may become an important biomarker or interventional target to improve future cancer treatment.
Multiple observational studies have revealed associations between the gut microbiota composition and anticancer drug outcome. However, the causative role of microbiota in drug efficacy and toxicity should be further investigated with animal models or clinical trials with large cohorts. Moreover, differences between humans and animal models in interacting with microbiota and drugs should be cognizant of in terms of translating results from animal models to humans.
A very diversified pool of bacterial species which may enhance efficacy of immune checkpoint blockade has been identified through different research groups across the globe. This is not very surprising because the composition of human gut microbiota is influenced by many geographic factors such as ethnic groups and lifestyles.75 Thus, it would be valuable to carry out studies in diverse geographic locations, especially in under-represented locations. In addition, the observed results may be interfered by different sample collection, sequencing and data analytical methods. Therefore, a standardized and consistent pipeline for studying pharmacomicrobiomics is required to be utilized.
Certain bacterial species exerted opposite effects on modulating efficacy of different drugs. For example, Fusobacterium nucleatum strains, which have been recognized as pathobionts involved in colorectal cancer progression,7 may abate the anticancer efficacy of 5-FU while promoting the efficacy of PD-L1 blockade.22,53 As such, a compressive evaluation of benefits and risks is needed before applying interventions with certain microbial species. Moreover, same microbiota-manipulating intervention (e.g., FMT or probiotics) may result in heterogeneous responses among various populations. This may be due to the heterogeneity of native gut microbiota patterns and functions prior to these interventions. Therefore, associations between native gut microbiota and intervention effectiveness need to be investigated further, and predictive algorithms are required for more precise and effective microbiota-modulating medicine.
Other outstanding questions for future research include more detailed mechanisms, applicability of cancer types, safety and efficacy of each microbiota-modulating approach, as well as the discovery of biomarkers based on microbiome for predicting drug responses. Further understanding of the tripartite interplays among microbiota, host responses and anticancer drugs will lead to more precise, personalized and optimized therapeutics for cancer patients.
Search strategy and selection criteria
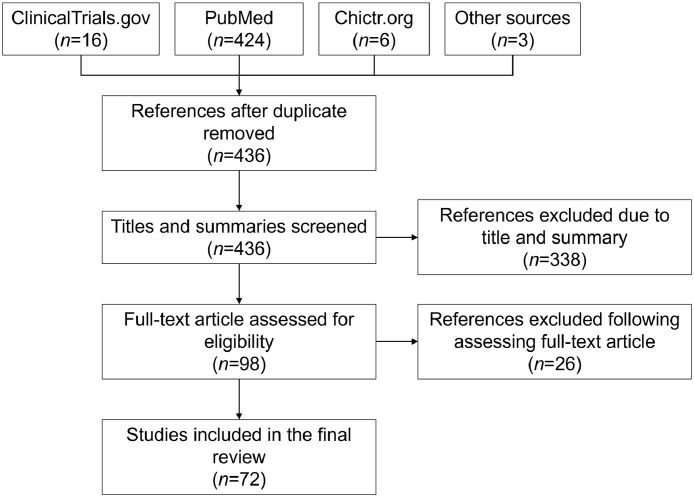
Data for this review were identified via searches within PubMed, ClinicalTrials.gov, Chictr.org, and references from relevant articles using keywords “microbiota”, “anticancer drug”, “chemotherapy”, and “immunotherapy” (Figure 2). Articles published between 1980 and 2022 were included with particular emphasis on those published in the past five years.
Figure 2.
Flow chart of the literature review process.
Contributors
J.H., X.M. and W.Z. conceived this study. J.H., W.L. and W.K. collected data and wrote the manuscript. Y.H. and R.Y. provided significant feedback and edited the manuscript. X.M. and W.Z. revised the manuscript and supervised this study. All authors contributed to the article and approved the submitted version.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
This work is supported by National Key Research and Development Program of China (2020YFA0907800), Shenzhen Science and Technology Innovation Program (KQTD20200820145822023) and National Natural Science Foundation of China (31900056 and 32000096).
Contributor Information
Xiangyu Mou, Email: mouxy5@ms.sysu.edu.cn.
Wenjing Zhao, Email: zhaowj29@ms.sysu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Olivier T, Haslam A, Prasad V. Anticancer drugs approved by the US food and drug administration from 2009 to 2020 according to their mechanism of action. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.38793. e2138793, 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Delavan B, Roberts R, Tong W. Lessons learned from two decades of anticancer drugs. Trends Pharmacol Sci. 2017;38(10):852–872. doi: 10.1016/j.tips.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Translat Med. 2016;8(328) doi: 10.1126/scitranslmed.aad7118. 328rv4,1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, An Y, Qin X, et al. Gut microbiota-derived metabolites in colorectal cancer: the bad and the challenges. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.739648. 739648,1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69(10):1867–1876. doi: 10.1136/gutjnl-2020-321153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16(11):690–704. doi: 10.1038/s41575-019-0209-8. [DOI] [PubMed] [Google Scholar]
- 9.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14(6):356–365. doi: 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- 10.Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018;6(1):92. doi: 10.1186/s40168-018-0483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoch S, Gajewski S, Rothfuss J, Hartwig A, Koberle B. Comparative study of the mode of action of clinically approved platinum-based chemotherapeutics. Int J Mol Sci. 2020;21(18):1–21. doi: 10.3390/ijms21186928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Lian W, Yuan Y, Li M. The synergistic effects of oxaliplatin and piperlongumine on colorectal cancer are mediated by oxidative stress. Cell Death Dis. 2019;10(8):600. doi: 10.1038/s41419-019-1824-6. 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Y, Fu L, Li Y, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33(5):988–1000. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Picard M, Yonekura S, Slowicka K, et al. Ileal immune tonus is a prognosis marker of proximal colon cancer in mice and patients. Cell Death Differ. 2021;28(5):1532–1547. doi: 10.1038/s41418-020-00684-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen S, Lim G, You Z, et al. Gut Microbiota is Critical for The Induction of Chemotherapy-Induced Pain. Nat Neurosci. 2017;20(9):1213–1216. doi: 10.1038/nn.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain S. On a new proposed mechanism of 5-fluorouracil-mediated cytotoxicity. Trends Cancer. 2020;6(5):365–368. doi: 10.1016/j.trecan.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-Fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 2020;206 doi: 10.1016/j.pharmthera.2019.107447. [DOI] [PubMed] [Google Scholar]
- 18.Li HL, Lu L, Wang XS, et al. Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Front Cell Infect Microbiol. 2017;7:455. doi: 10.3389/fcimb.2017.00455. ,1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan L, Zhang S, Li H, et al. The influence of gut microbiota dysbiosis to the efficacy of 5-fluorouracil treatment on colorectal cancer. Biomed Pharmacother. 2018;108:184–193. doi: 10.1016/j.biopha.2018.08.165. [DOI] [PubMed] [Google Scholar]
- 20.Hong BY, Sobue T, Choquette L, et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome. 2019;7(1):66. doi: 10.1186/s40168-019-0679-5. 1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Yang Y, Weng W, et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J Exp Clin Cancer Res. 2019;38(1):1–14. doi: 10.1186/s13046-018-0985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548–563. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Gonzalez AP, Ritter AD, Shrestha S. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell. 2017;169(3):431–441. doi: 10.1016/j.cell.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javdan B, Lopez JG, Chankhamjon P, et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell. 2020;181(7):1661–1679. doi: 10.1016/j.cell.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balaich J, Estrella M, Wu G, et al. The human microbiome encodes resistance to the antidiabetic drug acarbose. Nature. 2021;600(7887):110–115. doi: 10.1038/s41586-021-04091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan X, Ma F, Sun X, et al. Gut microbiota profiling in patients with HER2-negative metastatic breast cancer receiving metronomic chemotherapy of capecitabine compared to those under conventional dosage. Front Oncol. 2020;10:902. doi: 10.3389/fonc.2020.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamseddine AN, Ducreux M, Armand JP, et al. Intestinal bacterial beta-glucuronidase as a possible predictive biomarker of irinotecan-induced diarrhea severity. Pharmacol Ther. 2019;199:1–15. doi: 10.1016/j.pharmthera.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt AP, Pellock SJ, Biernat KA, et al. Targeted inhibition of gut bacterial beta-glucuronidase activity enhances anticancer drug efficacy. Proc Natl Acad Sci USA. 2020;117(13):7374–7381. doi: 10.1073/pnas.1918095117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollet RM, D'Agostino EH, Walton WG, et al. An atlas of beta-glucuronidases in the human intestinal microbiome. Structure. 2017;25(7):967–977. doi: 10.1016/j.str.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt AP, Pellock SJ, Biernat KA, et al. Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proc Natl Acad Sci USA. 2020;117(13):7374–7381. doi: 10.1073/pnas.1918095117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kodawara T, Higashi T, Negoro Y, et al. The inhibitory effect of ciprofloxacin on the beta-glucuronidase-mediated deconjugation of the irinotecan metabolite SN-38-G. Basic Clin Pharmacol Toxicol. 2016;118(5):333–337. doi: 10.1111/bcpt.12511. [DOI] [PubMed] [Google Scholar]
- 32.Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330(6005):831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsgård RA, Marrachelli VG, Korpela K, et al. Chemotherapy-induced gastrointestinal toxicity is associated with changes in serum and urine metabolome and fecal microbiota in male Sprague–Dawley Rats. Cancer Chemother Pharmacol. 2017;80(2):317–332. doi: 10.1007/s00280-017-3364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin XB, Dieleman LA, Ketabi A, et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS One. 2012;7(7):e39764. doi: 10.1371/journal.pone.0039764. ,1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia Y, Xie J. Promising molecular mechanisms responsible for gemcitabine resistance in cancer. Genes Dis. 2015;2(4):299–306. doi: 10.1016/j.gendis.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357(6356):1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vande Voorde J, Sabuncuoğlu S, Noppen S, et al. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J Biol Chem. 2014;289(19):13054–13065. doi: 10.1074/jbc.M114.558924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klufa J, Bauer T, Hanson B, et al. Hair eruption initiates and commensal skin microbiota aggravate adverse events of anti-EGFR therapy. Sci Transl Med. 2019;11(522):1–17. doi: 10.1126/scitranslmed.aax2693. [DOI] [PubMed] [Google Scholar]
- 39.Mori F, Ishida T, Ito A, et al. Potent antitumor effects of bevacizumab in a microenvironment-dependent human lymphoma mouse model. Blood Cancer J. 2012;2(4):e67. doi: 10.1038/bcj.2012.12. ,1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 41.Shergold AL, Millar R, Nibbs RJB. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol Res. 2019;145 doi: 10.1016/j.phrs.2019.104258. , 1-23. [DOI] [PubMed] [Google Scholar]
- 42.Das S, Allen A, Berlin J. Immunotherapy after immunotherapy: response rescue in a patient with microsatellite instability-high colorectal cancer post-pembrolizumab. Clin Colorectal Cancer. 2020;19(2):137–140. doi: 10.1016/j.clcc.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 44.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivan A, Corrales L, Hubert N, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29(1):71–83. doi: 10.1093/annonc/mdx686. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Zheng P. preserving the CTLA-4 checkpoint for safer and more effective cancer immunotherapy. Trends Pharmacol Sci. 2020;41(1):4–12. doi: 10.1016/j.tips.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 49.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng Z, Cheng S, Kou Y, et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol Res. 2020;8(10):1251–1261. doi: 10.1158/2326-6066.CIR-19-1014. [DOI] [PubMed] [Google Scholar]
- 53.Gao Y, Bi D, Xie R, et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct Target Ther. 2021;6(1):398. doi: 10.1038/s41392-021-00795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baruch EN, Youngster I, Ben-Betzalel G, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24(12):1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hefazi M, Patnaik MM, Hogan WJ, Litzow MR, Pardi DS, Khanna S. Safety and efficacy of fecal microbiota transplant for recurrent clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single-institution retrospective case series. Mayo Clin Proc. 2017;92(11):1617–1624. doi: 10.1016/j.mayocp.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 58.O'Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2(17057):1–6. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 59.Gao G, Ma T, Zhang T, et al. Adjunctive probiotic Lactobacillus rhamnosus probio-M9 administration enhances the effect of anti-PD-1 antitumor therapy via restoring antibiotic-disrupted gut microbiota. Front Immunol. 2021;12(772532):1–11. doi: 10.3389/fimmu.2021.772532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanoue T, Morita S, Plichta DR, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 61.Anker JF, Naseem AF, Mok H, Schaeffer AJ, Abdulkadir SA, Thumbikat P. Multi-faceted immunomodulatory and tissue-tropic clinical bacterial isolate potentiates prostate cancer immunotherapy. Nat Commun. 2018;9(1):1591. doi: 10.1038/s41467-018-03900-x. :1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dizman N, Meza L, Bergerot P, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022;28(4):704–712. doi: 10.1038/s41591-022-01694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci USA. 2018;115(1):157–161. doi: 10.1073/pnas.1712901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canale FP, Basso C, Antonini G, et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. 2021;598(7882):662–666. doi: 10.1038/s41586-021-04003-2. [DOI] [PubMed] [Google Scholar]
- 65.Ho CL, Tan HQ, Chua KJ, et al. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat Biomed Eng. 2018;2(1):27–37. doi: 10.1038/s41551-017-0181-y. [DOI] [PubMed] [Google Scholar]
- 66.Din MO, Danino T, Prindle A, et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature. 2016;536(7614):81–85. doi: 10.1038/nature18930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng DW, Dong X, Pan P, et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3(9):717–728. doi: 10.1038/s41551-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 68.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang J, Liu D, Wang Y, et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (Anti-PD-1/PD-L1) Immunotherapy. Gut. 2021;71:734–745. doi: 10.1136/gutjnl-2020-321031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lv J, Jia Y, Li J, et al. Gegen qinlian decoction enhances the effect of pd-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10(6):415. doi: 10.1038/s41419-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rizvi ZA, Dalal R, Sadhu S, et al. High-salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci Adv. 2021;7(37):1–17. doi: 10.1126/sciadv.abg5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature. 2020;579(7800):507–517. doi: 10.1038/s41586-020-2124-0. [DOI] [PubMed] [Google Scholar]
- 75.He Y, Wu W, Zheng HM, et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24(10):1532–1535. doi: 10.1038/s41591-018-0164-x. [DOI] [PubMed] [Google Scholar]