Summary
Background
We aimed to evaluate overall survival in US patients with amyotrophic lateral sclerosis (ALS) treated with intravenous (IV) edaravone compared with those not treated with IV edaravone in a real-world setting.
Methods
This exploratory retrospective comparative effectiveness observational analysis included patients with ALS who were enrolled in an administrative claims database from 8 August 2017 to 31 March 2020. Propensity score matching identified IV edaravone-treated patients (cases) and non-edaravone-treated patients (controls) matched for covariates: age, race, geographic region, sex, pre-index disease duration, insurance, history of cardiovascular disease, riluzole prescription, gastrostomy tube placement, artificial nutrition, noninvasive ventilation, and all-cause hospitalisation. For cases, the index date was the date of the first claim for IV edaravone. For controls, it was the date IV edaravone was available (8 August 2017). The effect of IV edaravone on all-cause mortality was estimated with shared frailty Cox regression analysis.
Findings
318 cases were matched to 318 controls. In both groups, 208 patients (65.4%) had a history of riluzole prescription. As of 31 March 2021, there were 155 deaths (48.7%) among the cases and 196 among the controls (61.6%). Median overall survival time was 29.5 months with edaravone and 23.5 months without, respectively, and the risk of death was 27% lower in cases than in controls (HR, 0.73; 95% CI, 0.59–0.91; p=0.005).
Interpretation
In this real-world analysis, IV edaravone treatment in a large predominantly riluzole-treated US cohort was associated with prolonged overall survival compared with not using IV edaravone. Data from adequately powered RCTs are needed to support this finding.
Funding
Funded by Mitsubishi Tanabe Pharma America.
Keywords: Amyotrophic lateral sclerosis, Edaravone, Real-world evidence, Survival
Research in context.
Evidence before this study
We performed a search in PubMed in English for studies published from Jan 1, 2017 to Jan 1, 2022 assessing treatment outcomes with edaravone for people with amyotrophic lateral sclerosis (ALS) using the search terms “edaravone” AND “amyotrophic lateral sclerosis” and identified that IV edaravone gained US Food and Drug Administration approval in May 2017 based on results demonstrating slowed loss of function and improved quality of life. However, ALS is a clinically challenging disease to investigate in randomised controlled trial (RCT) settings, especially for evaluation of survival outcomes, due to disease heterogeneity, longer times to diagnosis, and an average life expectancy after symptom development of 2–5 years. Studies using real-world data (RWD) may inform insights beyond those addressed by RCTs and help support regulatory decisions.
Added value of this study
In studies of rare diseases, which are more difficult to evaluate in a clinical trial setting, RWD studies have the potential to provide relevant information to help bridge current knowledge gaps. To better understand long-term survival outcomes in patients with ALS prescribed IV edaravone, we conducted an exploratory propensity score-matched comparative effectiveness cohort study, utilising RWD from a large administrative claims database in which patients with ALS treated with IV edaravone (cases) and non-edaravone-treated patients with ALS (controls) were matched for several covariates. In this study, treatment with IV edaravone was associated with 6-month longer median survival compared with the non-IV edaravone-treated patients (median survival, cases, 29.5 months [95% CI, 25.4–35.9]; controls, 23.5 months [95% CI, 20.0–28.0]).
Implications of all the available evidence
In our analysis, use of IV edaravone treatment compared with not using IV edaravone was associated with improved overall survival in a cohort of commercially insured patients with ALS in the United States. This is the first time that a statistically significant improved survival time was associated with IV edaravone. An adequately powered RCT is needed to support this finding.
Alt-text: Unlabelled box
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterised by motor neuron cell death and progressive muscular weakness that leads to paralysis.1,2 The estimated prevalence rate for 2016 was 5.2 per 100,000 population. ALS prevalence is lowest (0.2 per 100,000 population) in the 18-to-39 age group and highest (17.2 per 100,000 population) in the 70-to-79 age group. Men have a higher overall prevalence rate (7.3 per 100,000 population) than women (3.6 per 100,000 population).1 The prevalence rate in White people is 1.5 times that in Black people, although the disease course is comparable in both groups, with a median survival of 23.3 months from diagnosis in White people and 25.3 months from diagnosis in Black people.1, 2, 3 The clinical trajectories and survival experiences are similar in population- and clinic-based observational studies.2, 3, 4
At the time of the analysis reported here, two disease-modifying treatments, riluzole and intravenous (IV) edaravone, were approved for ALS treatment in the US. Riluzole (Rilutek, Sanofi-Aventis US LLC) received US Food and Drug Administration (FDA) approval in December 1995 and IV edaravone (Radicava, Mitsubishi Tanabe Pharma Corporation) was approved in May 2017 based on results from the pivotal phase 3 clinical trial demonstrating that IV edaravone slowed loss of function, as measured by the ALS Functional Rating Scale-Revised (ALSFRS-R), by 33% (p=0.0013) compared with placebo at 24 weeks.5 These results provided evidence for slower disease progression as measured by the ALSFRS-R, and improved quality of life as measured by the ALS 40-item assessment questionnaire. Long-term survival was not assessed in this clinical trial because of limited study duration.5 In May 2022, edaravone oral suspension was approved by the FDA, supported by a similar bioavailability profile, when compared with IV edaravone.
In ALS, disease heterogeneity makes it a clinically challenging disease to research and evaluate in clinical trials. Research studies analysing real-world data (RWD) may be able to provide additional information to the application of evidence from randomised controlled trials (RCTs) and inform insights beyond those addressed by RCTs.6,7
Recently, a framework for evaluating the use of real-world evidence (RWE) to support evidence generation and the regulatory approval process was created (US Food and Drug Administration. Framework for FDA's real-world evidence program. December 2018). Whereas RWE was previously used only to inform postmarketing drug safety decisions, RWD studies are now being considered to generate RWE of both safety and effectiveness, and to support regulatory decisions about drug products.8 For example, the indication expansion of palbociclib from women with advanced or metastatic breast cancer and specific tumour types to include men with metastatic breast cancer was supported primarily by RWE derived from electronic health records comparing the outcomes of men receiving palbociclib with men receiving endocrine therapy without palbociclib.9
The objective of our current study was to use RWD collected in a large US administrative claims database to assess overall survival in a cohort of patients with ALS treated with IV edaravone compared with a matched group of patients with ALS not treated with IV edaravone.
Methods
Data source
This is a retrospective, observational, propensity score-matched comparative effectiveness cohort study using health claims data from Optum's Clinformatics Data Mart (CDM) (Optum, Inc. Eden Prairie, MN, US). The CDM is derived from a US-based database of administrative health claims spanning all 50 states for members with commercial and Medicare Advantage health plans. The CDM is statistically de-identified under the expert determination method consistent with the Health Insurance Portability and Accountability Act and managed according to Optum customer data use agreements, and, hence, does not constitute human patient research as defined in 45 CFR 46.102 and does not require an institutional review board assessment or approval for secondary analysis. Optum CDM data include patient-level enrolment information derived from claims submitted for all medical and pharmacy health care services, with information related to health care costs and resource utilisation. The database includes approximately 17 million–19 million annual covered lives for a total of >65 million unique lives over a 13-year period (January 2007 through December 2020).
Study population
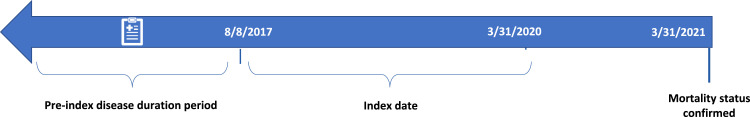
Our reported comparative effectiveness observational analysis includes patients with ALS enrolled in the CDM aged ≥18 years who had a diagnosis of ALS (International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] code G12.21 or ICD-9-CM code 335.20) on any claim and in any inpatient or outpatient setting. Cases included patients with ALS treated with IV edaravone from 8 August 2017–31 March 2020 as indicated by a claim for IV edaravone using Healthcare Common Procedure Coding System (HCPCS) codes J1301, J3490, or C9493, or the National Drug Code 70510-2171-xx. The index date (ie, the start date for survival analysis) was the date of the first claim for IV edaravone. Patients may or may not have continued IV edaravone treatment until death or censoring. For patients not treated with IV edaravone (ie, with no history of IV edaravone prescription), the index date was the date of IV edaravone availability in the US market (8 August 2017) (details displayed in Figure 1). Patients may or may not have received treatment with riluzole. Patients without an ALS diagnosis (ICD-10-CM code G12.21 or ICD-9-CM code 335.20) were excluded.
Figure 1.
Optum CDM retrospective study timeline.
ALS diagnosis indicated by ICD-10-CM code G12·21; ICD-9-CM: 335·20 on at least one claim in any setting (outpatient or inpatient). Additionally captured: claims for gastrostomy tube placement, artificial nutrition, noninvasive ventilation, all-cause hospitalisation.
Pre-index disease duration period: IV edaravone-treated cases—date from ALS diagnosis to date of first claim for IV edaravone. Non-IV edaravone-treated controls—date from ALS diagnosis to date that IV edaravone was commercially available (8 August 2017).
Index date: IV edaravone-treated cases: Date of the first claim for IV edaravone.
Non-IV edaravone-treated controls: Date that IV edaravone was commercially available (8 August 2017).
ALS, amyotrophic lateral sclerosis. CDM, Clinformatics Data Mart. ICD-10-CM, International Classification of Diseases Tenth Revision, Clinical Modification. IV, intravenous.
Patient demographic and clinical characteristics collected in the pre-index disease duration period
The pre-index disease duration period is defined as the period between the date of first claim for ALS diagnosis and the first claim for IV edaravone or the date that IV edaravone was available on the market (8 August 2017) for ALS patients with no IV edaravone treatment.
Demographic characteristics included age, sex, race, region of residence, and insurance coverage (commercial or Medicare Advantage). Clinical characteristics including pre-index disease duration, ALS-related symptoms and procedures (gastrostomy tube placement, artificial nutrition, noninvasive ventilation), history of cardiovascular disease, riluzole prescription, and all-cause hospitalisation were identified by claims using ICD-9-CM and ICD-10-CM, Current Procedural Terminology, or HCPCS codes that describe specific items and services.
Propensity score matching (PSM) for selection of IV edaravone–treated ALS patients (cases) and non-IV edaravone-treated ALS patients (controls)
IV edaravone-treated cases were matched 1:1 to non-IV edaravone-treated controls using the PSM method. PSM using the nearest-neighbour method with a caliper width equal to 0.1 of the SD of the logit scores was performed,10 accounting for several covariates including age, race, region, sex, insurance, history of cardiovascular disease,11 and history of riluzole prescription. Additionally, to ensure that cases and controls were at similar disease duration, pre-index disease duration was calculated. Since ALSFRS-R and forced vital capacity (FVC) scores, which clinically measure ALS disease severity and progression, are not captured in the CDM, surrogates were used to assess ALS disease severity and included claims for gastrostomy tube placement, artificial nutrition, noninvasive ventilation, and all-cause hospitalisation.12, 13, 14, 15 The balance in baseline characteristics achieved by PSM was evaluated by calculating standardised mean differences (SMDs) between treated and matched control patients in the matched and overall populations. SMDs of less than 10% were considered negligible imbalances.10
Confirmation of ALS in IV edaravone and non–IV edaravone-treated patients
Administrative claims for ALS-related symptoms and procedures identifying respiratory-, nerve-, limb-, and bulbar-related symptoms were tabulated in both IV edaravone and non-IV edaravone-treated ALS patients and compared with the proportion of these symptoms in the two groups. The proportion of ALS-related symptom claims in these patients were contrasted with non-ALS patients according to an established algorithm to confirm that the patients conform to the claims profile of ALS-patients compared with non-ALS patients.16
Outcome: all-cause mortality
The Optum De-identified CDM Database-Date of Death (DOD) table was used to confirm mortality status of IV edaravone-treated cases and non-IV edaravone-treated controls as of 31 March 2021. The DOD table is sourced from the Death Master File maintained by the US Social Security Administration, which provides the month and year of death, enabling the identification of patients’ mortality status. Other sources of death information include the Centers for Medicare & Medicaid Services based on claims indicating member discontinuation or facility discharge due to death.
Statistical analysis
Demographics and clinical variables were assessed descriptively using counts and percentages for categorical variables and measures of central tendency (mean/median/SD/interquartile range) for continuous variables. Differences in survival between IV edaravone-treated cases and non-IV edaravone-treated controls were examined using Kaplan-Meier survival curves. The log-rank test was applied to detect significant differences in survival parameters between the Kaplan-Meier curves. Shared frailty Cox regression analysis was performed to estimate the benefit of IV edaravone by accounting for unobserved heterogeneity between the matched groups. Conditional and marginal estimates of parameters were obtained based on the frailty method.17
Two sensitivity analyses were constructed. The first was a principled sensitivity analysis based on the imputation of missing failure times using a bootstrap approach18 to examine the assumption of noninformative censoring. The second sensitivity analysis was built on inverse probability weighting (IPW).19 The IPW-based method generates upper and lower bounds for the causal effect under fixed values of two sensitivity parameters that characterise the error in the estimated propensity score due to uncontrolled confounding and its correlation with the potential outcome.
The analysis was conducted using R (version 4.0.3, R Core Team, 2020). Propensity score estimating and matching were done using the MatchIt (version: 4.1.0, 2011) package. The “survival” (version 3.2-7, 2021) package was used to perform survival analysis, and the “survminer” (version 0.4.8, 2017) package was used to create the Kaplan-Meier curves.
Role of the funding source
This study was performed by a collaborative work group composed of an academic organisation, a pharmaceutical company, and a statistical company, and funded by Mitsubishi Tanabe Pharma America (MTPA) who developed the protocol and statistical plan prior to the formal calculations presented in this paper. MTPA contributed to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. All authors had access to the data and were responsible for the decision to submit for publication.
Results
Study population for survival analysis: matching, balancing of other ALS-specific claims in cases and controls, pre-index disease treatment, edaravone treatment
Propensity score matching
Patient demographic and ALS-related clinical characteristics before matching are shown in the supplementary table. After matching, characteristics were balanced in the IV edaravone-treated cases and non-IV edaravone-treated controls as indicated by SMD of <0.1 in Table 1 and the supplementary figure.
Table 1.
Demographic and clinical characteristics in IV edaravone-treated and non-IV edaravone-treated patients with ALS after matching.
| IV edaravone-treated cases | Non-IV edaravone-treated controls | Difference | Difference 95% CI | |
|---|---|---|---|---|
| N | 318 | 318 | ||
| Age, mean (SD) | 62.9 (10.1) | 62.7 (10.2) | –0.1981 | –1.7815, 1.3853 |
| Sex, no. (%) | ||||
| Female | 134 (42.1) | 134 (42.1) | 0 | –0.0543, 0.0543 |
| Male | 184 (57.9) | 184 (57.9) | ||
| Medicare Advantage, no. (%) | 189 (59.4) | 189 (59.4) | 0 | –0.0540, 0.0540 |
| Race, no. (%) | ||||
| White | 226 (71.1) | 236 (74.2) | –0.0314 | –0.0804, 0.0175 |
| Black | 28 (8.8) | 18 (5.7) | 0.0314 | –0.0030, 0.0599 |
| Other | 35 (11.0) | 33 (10.4) | 0.0063 | –0.0277, 0.0403 |
| Unknown | 29 (9.1) | 31 (9.7) | –0.0063 | –0.0384, 0.0258 |
| Region, no. (%) | ||||
| Midwest | 64 (20.1) | 86 (27.0) | –0.0692 | –0.1157, 0.0227 |
| Northeast | 51 (16.0) | 59 (18.6) | –0.0252 | –0.0667, 0.0164 |
| South | 131 (41.2) | 101 (31.8) | 0.0943 | –0.0417, 0.1470 |
| West | 72 (22.6) | 71 (22.3) | 0.0031 | –0.0427, 0.0490 |
| Unknown | 0 (0.0) | 1 (0.3) | –0.0031 | –0.0075, 0.0012 |
| Pre-index disease duration (days), mean (SD) | 211.8 (187.6) | 203.7 (285.5) | –8.0440 | –45.6752, 29.5871 |
| Riluzole prescription, no. (%) | 208 (65.4) | 208 (65.4) | 0 | –0.0523, 0.0523 |
| Pre-index cardiovascular disease, no. (%) | 29 (9.1) | 29 (9.1) | 0 | –0.0316, 0.0316 |
| Pre-index gastrostomy tube, no. (%) | 24 (7.5) | 24 (7.5) | 0 | –0.0290, 0.0290 |
| Pre-index artificial nutrition, no. (%) | 43 (13.5) | 38 (11.9) | 0.0157 | –0.0209, 0.0524 |
| Pre-index noninvasive ventilation, no. (%) | 48 (15.1) | 54 (17.0) | –0.0189 | –0.0592, 0.0215 |
| Pre-index hospitalisation, no. (%) | 73 (23.0) | 73 (23.0) | 0 | –0.0462, 0.0462 |
ALS, amyotrophic lateral sclerosis. IV, intravenous.
IV edaravone-treated ALS patients and non-IV edaravone-treated ALS patients were matched for riluzole prescription [65.4%; 65.4%], cardiovascular disease [9.1%, 9.1%], gastrostomy tube placement [7.5%, 7.5%], artificial nutrition [13.5%, 11.9%], noninvasive ventilation [15.1%, 17.0%], and all-cause hospitalisation [23.0%, 23.0%].
Confirmation that ALS patients had claims characteristics distinguished from non-ALS patients
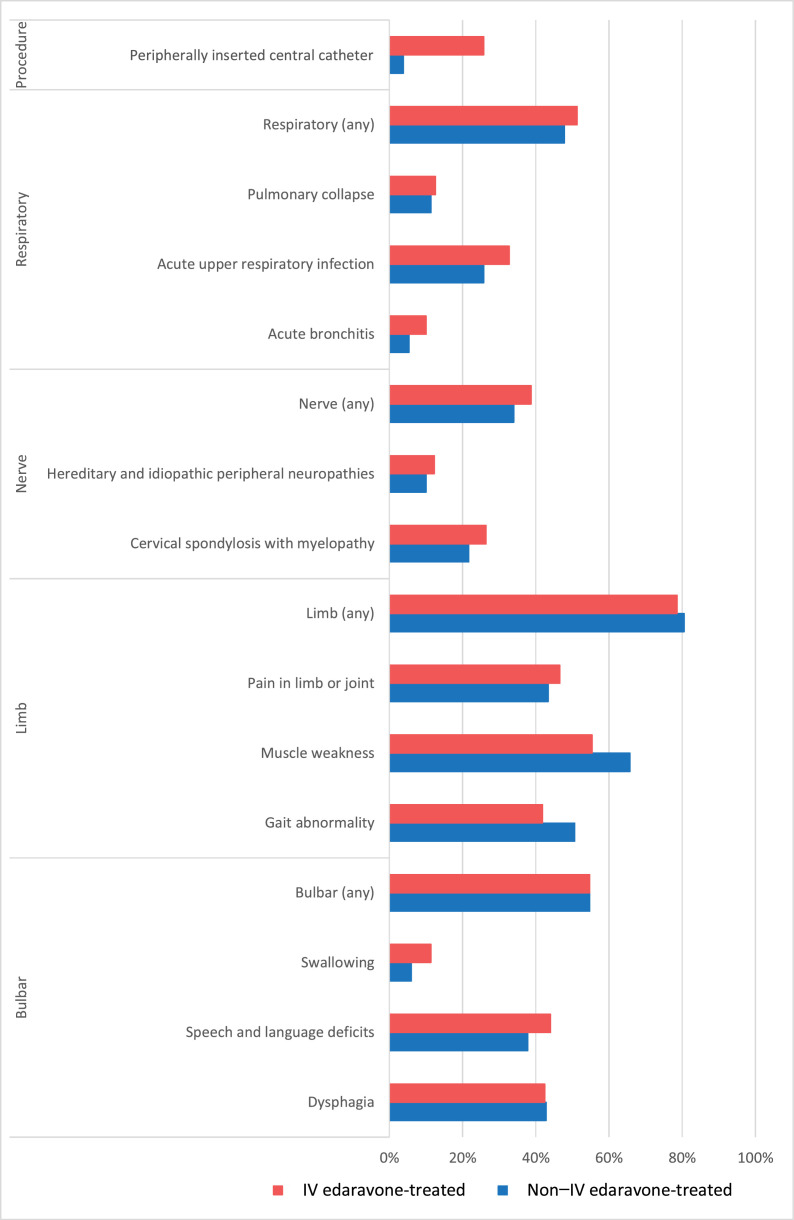
Although not considered as confounding factors, we identified claims for ALS-related symptoms and procedures in the two groups. The proportion of claims for respiratory-, nerve-, limb-, and bulbar-related symptoms identifying patients with ALS are shown in Figure 2. The most common symptoms for both IV edaravone-treated and non-IV edaravone-treated patients with ALS were limb related. The proportion of claims for bulbar-, limb-, nerve-, and respiratory-related symptoms were similar between the groups; however, the IV edaravone-treated patients with ALS are characterised by more common insertion of an IV catheter.
Figure 2.
ALS-related symptoms and procedures at baseline (pre-index period*) in commercially insured patients with ALS treated with IV edaravone and non-IV edaravone-treated patients with ALS.
*Defined as the period between the date for first claim for ALS diagnosis and the first claim of IV edaravone for IV edaravone-treated patients or the date IV edaravone was available on the market (8 August 2017) for patients with no IV edaravone treatment.
ALS, amyotrophic lateral sclerosis. IV, intravenous.
Pre-index disease duration
The pre-index disease duration period was 211.8 days (SD, 187.6) for IV edaravone-treated cases vs 203.7 (SD, 285.5) for non-IV edaravone-treated controls, which amounts to approximately 7 months for each group.
Edaravone treatment
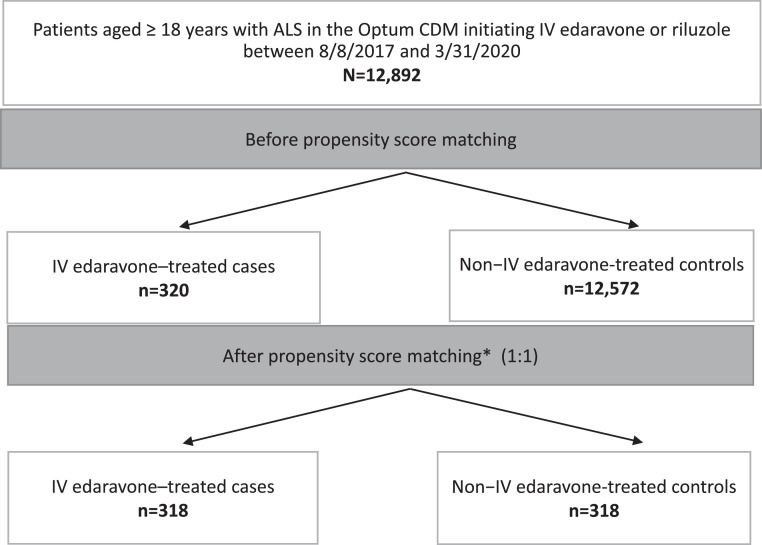
Between 8 August 2017 and 31 March 2020, 12,892 patients with ALS had initiated IV edaravone, riluzole, or no treatment, and of those patients with ALS, 320 were treated with IV edaravone vs 12,572 who were not (Figure 3). After applying the PSM method, 318 IV edaravone-treated cases were matched to 318 non-IV edaravone-treated controls and were included in the analysis. Median IV edaravone treatment duration was 8.6 months (interquartile range, 3.5–14.8). Of 197 patients who stopped IV edaravone treatment early, 112 remained in the database, and of those 112 patients, 26 were still alive until the last claim. In total, 64 out of 121 patients who continued IV edaravone throughout the study period left the database (ie, had no claim for 30 days after the last claim up to 31 March 2021) while still on edaravone treatment, and 60 patients in the control group left the database during the study period.
Figure 3.
Patient disposition.
*Matching on age, race, region, sex, pre-index disease duration, insurance, pre-index claims for cardiovascular disease and riluzole prescription, gastrostomy tube placement, artificial nutrition, noninvasive ventilation, and all-cause hospitalisation.
ALS, amyotrophic lateral sclerosis. CDM, Clinformatics Data Mart. IV, intravenous.
Survival in IV edaravone-treated patients compared with non-IV edaravone-treated patients
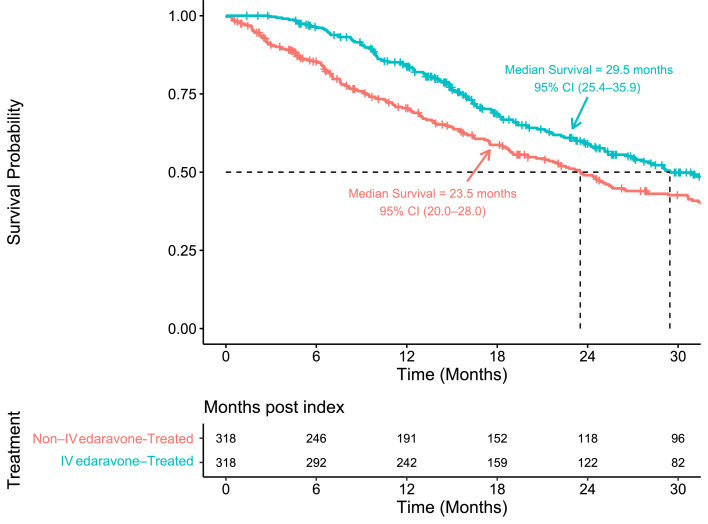
Analysis of the CDM DOD table confirmed 155 all-cause deaths (48.7%) recorded among the IV edaravone-treated cases compared with 196 all-cause deaths (61.6%) among the non-IV edaravone-treated controls on 31 March 2021.
Median survival was 29.5 months (95% CI, 25.4–35.9) for the IV edaravone-treated cases compared with 23.5 months (95% CI, 20.0–28.0) for the non-IV edaravone-treated controls (Figure 4). Treatment with IV edaravone was associated with a 6-month longer median survival compared with the non-IV edaravone-treated patients with ALS. Table 2 shows the estimated probability of survival at 12, 18, 24, and 30 months. The risk of death was 27% lower for IV edaravone-treated cases than for non-treated controls (HR, 0.73; 95% CI, 0.59–0.91; p=0.005). The first sensitivity analysis, based on the imputation of missing failure times using a bootstrap approach, confirmed the assumption of noninformative censoring. The second sensitivity analysis, using IPW to examine the effect of uncontrolled confounding using nonrandomised data, confirmed the statistically significant effect of IV edaravone treatment on survival (HR, 0.65; 95% CI, 0.53–0.79; p<0.0001) and was consistent with the PSM estimate in terms of direction (HR <1.0 [protective]).
Figure 4.
Overall survival analysis.
IV, intravenous.
Table 2.
Estimated survival probability at a specific time (months) by propensity score method.a
| IV edaravone-treated cases | Non-IV edaravone-treated controls | |
|---|---|---|
| 12 months | 0.837 (0.797–0.880) | 0.704 (0.654–0.759) |
| 18 months | 0.685 (0.632–0ּ.743) | 0.587 (0.532–0.648) |
| 24 months | 0.591 (0.533–0.655) | 0.490 (0.434–0.554) |
| 30 months | 0.499 (0.438–0.569) | 0.426 (0.370–0.491) |
The estimated probability of survival at the different time points is not used to determine significance in overall survival; therefore, any significant differences in survival probability are derived from differences in overall survival. (HR, 0.73; 95% CI, 0.59–0.91; p=0.005).
IV, intravenous.
Discussion
In our analysis, IV edaravone treatment compared with not using IV edaravone in a predominantly riluzole-treated cohort was associated with improved overall survival in a cohort of commercially insured patients with ALS. These findings, in a larger, more diverse group of treated patients with ALS compared with those who were included in the original RCTs, are promising for the hypothesis that beneficial treatment effects on rate of functional change in short-term clinical trials may be translated into increased survival, which should be tested in an adequately powered RCT.
Riluzole was shown to increase survival among patients with ALS in clinical trials and in a real-world setting.20,21 Based on a systematic review of all evidence from clinical trial data, treatment with riluzole resulted in significantly greater survival rates than placebo in two of four studies (HR, 0.80; 95% CI, 0.64–0.99; p=0.042), with a survival advantage with riluzole 100 mg seen at 12 months, but not at 18 months.21 In the RCT, riluzole increased median survival by about 3 months compared with placebo,21 whereas RWE has indicated that treatment with riluzole extended survival by 6–19 months.20
Prior studies investigating the post-marketing, real-world experience of edaravone have employed differing methods and shown mixed results.22, 23, 24 The previous VA study evaluating the real-world use and safety of IV edaravone for the treatment of ALS within the US VA health care system reported lower death rates per 100 patient-years with IV edaravone treatment (18.0 for IV edaravone vs 29.3 for riluzole only [HR, 0.77; 95% CI, 0.43–1.18]), which compared HRs of the edaravone group to riluzole-only subgroups and utilised Bonferroni-corrected CIs.22 This difference did not reach statistical significance,22 unlike results from our study, which showed a statistically significant effect of IV edaravone on survival (HR, 0.73; 95% CI, 0.59–0.91; p=0.005). A pragmatic single-arm observational study evaluating patients receiving edaravone treatment in Italian ALS centres compared with matched historical controls from the Pooled Resource Open-Access ALS Clinical Trials (PROACT) database did not show a difference in time to event clinical outcomes assessed as time to dropping to 24 on the ALSFRS-R or time to reaching 60% FVC.23 In the Italian study, a multivariable Cox proportional hazards regression model was used to analyse the time to event effect, adjusted for by age, site of onset, diagnostic delay, and disease progression rate. However, the Italian study did not include concurrent controls treated in the same manner, while our study employed PSM to match for age, race, region, sex, insurance, history of cardiovascular disease, history of riluzole prescription, and five surrogate markers for disease severity. An additional pragmatic single-arm observational study from the German Motor Neuron Disease Network (MND-NET) did not find a survival benefit with a median 11.4 months of follow-up (interquartile range 6.6–18.9 months).24 Different from our study, the MND-NET study did not include contemporaneous controls and the PSM only accounted for 4 covariates: site of disease onset, age at onset, disease duration, and baseline ALSFRS-R score.
Patients in the MND-NET study who died while on edaravone had significantly worse baseline prognostic factors (faster disease progression, older age at disease onset, lower ALSFRS-R score) compared with those who survived while on edaravone.24 In the VA study, despite a propensity score-matched cohort evaluation, residual confounding or baseline differences likely existed between the two cohorts; this may have been because cases and controls were not at similar stages of disease progression for which we corrected with our case definition and by calculating a pre-index disease duration to match IV edaravone-treated cases and non-IV edaravone-treated controls.22 Additionally, the control group in the VA study included patients treated with riluzole only who continued to receive treatment longer because of differential censoring. In the study reported here, patients with ALS with no treatment also were included, and this may have contributed to the difference in reaching statistical significance between cases and controls. Further, while both studies included patients who discontinued edaravone, our study follows patients who continued on edaravone for a longer period of time; in the VA study, 151/369 (40.9%) VA IV edaravone-treated ALS patients received treatment over 23 months,22 while in our study 121/318 (38.1%) patients were treated with IV edaravone over 31 months.
The strengths of this retrospective analysis are the large sample of US patients with ALS who were treated with IV edaravone since FDA approval and its use of an administrative claims database, as well as the length of follow-up observation time. Due to the heterogeneity of ALS, patients can progress at different rates, and this may present a challenge to matching cases and controls; we used claims for gastrostomy tube placement, artificial nutrition, noninvasive ventilation, and all-cause hospitalisation to assess disease severity, and compared pre-index disease duration to ensure balance. The pre-index disease duration period indicates that matching of cases and controls occurred during the first two years after diagnosis when the mortality risk has been shown to be the highest.25 Initiating riluzole treatment early in this period has been demonstrated to increase its effect on survival26,27 and function as measured by Kings ALS Stage transition.28,29 Additional strengths include the consistency between our matched variables and those previously denoted as separating ALS cases from non-ALS cases in a Medicare claims database,16 as well as the variables included in a clinical prediction model for survival in patients with ALS.27
Although the Optum CDM database included a large cohort of ALS patients to draw from in a similar time frame (2017 and beyond) compared with other claims databases, 12,892 compared with 4086 in the VA Data Warehouse and 1047 in the Answer ALS clinical registry, several limitations of our analysis related to selection bias should be noted.29,30 The current study included only patients with ALS who have commercial health coverage or Medicare Advantage plans. Consequently, results of this analysis may not be generalisable to patients with ALS with other insurance plans or without health insurance coverage. This study relied on administrative claims data, which are subject to coding limitations and entry error. The possibility of underdiagnosis of ALS may have led to a selection bias and/or smaller sample sizes, as patients with ALS who were untreated, who did not have a relevant diagnosis recorded on their medical claims, or who were no longer enrolled in the Optum CDM database during the post-index period were excluded from the analysis. While differences between cases and controls were controlled for by PSM, adjustment was limited to those characteristics that can be measured from administrative claims. Therefore, the study population may appear to have been healthier than the total population of patients with ALS.
In this exploratory RWD propensity score-matched analysis, patients with ALS prescribed IV edaravone and who continued edaravone over 31 months after the index date survived longer than those not prescribed IV edaravone in a real-world setting. Combined with the phase 3 pivotal trial demonstrating that IV edaravone slowed the rate of functional decline by 33% (p=0.0013) compared with placebo as measured by the ALSFRS-R at 24 weeks,5 these results provide further support that IV edaravone may have both functional and real-world survival benefit in patients with ALS. Although RWD are not intended to replace the prospective clinical trial, in studies of rare diseases, which are more difficult to evaluate in a clinical trial setting, RWD studies have the potential to provide pertinent relevant information to payers, health care providers, patients, and caregivers in a cost- and time-effective manner with minimal burden to the patient, until a RCT can be conducted.
Contributors
MH, BRB, JDB, YL, and JZ participated in the conceptualisation and design of the manuscript. BRB, JDB, MC, YL, GSZ, JZ, and MH were involved with investigation and the acquisition, curation, formal analysis, and interpretation of study data. BRB, YL, and MH verified the underlying data. BRB, JDB, MC, YL, GSZ, JZ, and MH participated in the writing – original draft, and all authors participated in the writing – review & editing of the manuscript. BRB, JDB, MC, YL, GSZ, JZ, and MH participated in the critical revision of the manuscript for important intellectual content. YL and JZ performed the statistical analysis and validation of the study. MH was responsible for project administration, technical, or material support, as well as supervision of the study. All authors had full access to all data in the study, and all authors critically reviewed and approved the final version of the manuscript.
Data sharing statement
Data used in the conduct of this study were licensed to Mitsubishi by Optum and are available from the corresponding author upon reasonable request (malgorzata_ciepielewska@mt-pharma-us.com).
Declaration of interests
BRB has served as a consultant for Mitsubishi Tanabe Pharma America (MTPA), ITF Pharma, and MediciNova, on scientific advisory or data safety monitoring boards for AB Science, Biogen, and Ionis, and as a speakers bureau member for MTPA, Cytokinetics, the Muscular Dystrophy Association (MDA), ITF Pharma, and Biohaven. He has received research support from Orion, Alexion, MTPA, Biohaven, MediciNova, MDA, Amyotrophic Lateral Sclerosis (ALS) Association, and the National ALS Registry of the Centers for Disease Control Agency for Toxic Substances and Disease Registry. He is a noncompensated member of the ALS Quality Measures Subcommittee of the American Academy of Neurology, and member, Medical Advisory Committee, ALS Association, North Carolina Chapter. JDB reports research funding from Genentech, Alexion, Anelixis, Biogen, MTPA, Brainstorm Cell Therapeutics, Amylyx Pharmaceuticals, nQ Medical, MT Pharma Holdings of America (MTPHA), Transposon Therapeutics, RAPA Therapeutics, National Institute of Neurological Disorders and Stroke, MDA, ALS Finding a Cure, ALS Association, and ALS One. He has served as a member of scientific advisory boards for Biogen, Clene Nanomedicine, Janssen, MTPA, MTPHA, Sawai and Regeneron; holds equity in ReactNeuro; and has been an educational speaker for Projects in Knowledge, Inc. MC and GSZ are employees of MTPA. YL and JZ are employees of Princeton Pharmatech. MH was an employee of MTPA at time of study.
Acknowledgements
This study was funded by Mitsubishi Tanabe Pharma America (MTPA). Medical writing assistance was provided by Reem Berro, PhD, and Leandra Dang, PharmD, of Cadent, a Syneos Health® group company, and was supported by Mitsubishi Tanabe Pharma America.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101590.
Appendix. Supplementary materials
References
- 1.Mehta P, Raymond J, Punjani R, et al. Prevalence of amyotrophic lateral sclerosis (ALS), United States, 2016. Amyotroph Lateral Scler Frontotemporal Degener. 2022;23:220–225. doi: 10.1080/21678421.2021.1949021. [DOI] [PubMed] [Google Scholar]
- 2.Raymond J, Oskarsson B, Mehta P, Horton K. Clinical characteristics of a large cohort of US participants enrolled in the National Amyotrophic Lateral Sclerosis (ALS) Registry, 2010–2015. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:413–420. doi: 10.1080/21678421.2019.1612435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand D, Polak M, Glass JD, Fournier CN. Comparison of phenotypic characteristics and prognosis between black and white patients in a tertiary ALS clinic. Neurology. 2021;96:e840–e844. doi: 10.1212/WNL.0000000000011396. [DOI] [PubMed] [Google Scholar]
- 4.Punjani R, Wagner L, Horton K, Kaye W. Atlanta metropolitan area amyotrophic lateral sclerosis (ALS) surveillance: incidence and prevalence 2009–2011 and survival characteristics through 2015. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:123–130. doi: 10.1080/21678421.2019.1682614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Group; Edaravone (MCI-186) ALS Study Group Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:505–512. doi: 10.1016/S1474-4422(17)30115-1. [DOI] [PubMed] [Google Scholar]
- 6.Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special task force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf. 2017;26:1033–1039. doi: 10.1002/pds.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaulieu-Jones BK, Finlayson SG, Yuan W, et al. Examining the use of real-world evidence in the regulatory process. Clin Pharmacol Ther. 2020;107:843–852. doi: 10.1002/cpt.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin JM, Patorno E, Desai RJ, et al. Emulating randomized clinical trials with nonrandomized real-world evidence studies: first results from the RCT DUPLICATE Initiative. Circulation. 2021;143:1002–1013. doi: 10.1161/CIRCULATIONAHA.120.051718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wedam S, Fashoyin-Aje L, Bloomquist E, et al. FDA approval summary: palbociclib for male patients with metastatic breast cancer. Clin Cancer Res. 2020;26:1208–1212. doi: 10.1158/1078-0432.CCR-19-2580. [DOI] [PubMed] [Google Scholar]
- 10.Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66:S84–90.e1. doi: 10.1016/j.jclinepi.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu K, Ji H, Hu N. Cardiovascular comorbidities in amyotrophic lateral sclerosis: a systematic review. J Clin Neurosci. 2022;96:43–49. doi: 10.1016/j.jocn.2021.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Bond L, Ganguly P, Khamankar N, et al. A comprehensive examination of percutaneous endoscopic gastrostomy and its association with amyotrophic lateral sclerosis patient outcomes. Brain Sci. 2019;9:223. doi: 10.3390/brainsci9090223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludolph AC, Dorst J, Dreyhaupt J, et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann Neurol. 2020;87:206–216. doi: 10.1002/ana.25661. [DOI] [PubMed] [Google Scholar]
- 14.Berlowitz DJ, Howard ME, Fiore JF, Jr, et al. Identifying who will benefit from non-invasive ventilation in amyotrophic lateral sclerosis/motor neurone disease in a clinical cohort. J Neurol Neurosurg Psychiatry. 2016;87:280–286. doi: 10.1136/jnnp-2014-310055. [DOI] [PubMed] [Google Scholar]
- 15.Pisa FE, Logroscino G, Giacomelli Battiston P, Barbone F. Hospitalizations due to respiratory failure in patients with amyotrophic lateral sclerosis and their impact on survival: a population-based cohort study. BMC Pulm Med. 2016;16:136. doi: 10.1186/s12890-016-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JR, Fitzhenry D, Grant L, Martyn D, Kerr DA. Diagnosis pathway for patients with amyotrophic lateral sclerosis: retrospective analysis of the US Medicare longitudinal claims database. BMC Neurol. 2013;13:160. doi: 10.1186/1471-2377-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balan TA, Putter H. Nonproportional hazards and unobserved heterogeneity in clustered survival data: when can we tell the difference? Stat Med. 2019;38:3405–3420. doi: 10.1002/sim.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson D, White IR, Seaman S, Evans H, Baisley K, Carpenter J. Relaxing the independent censoring assumption in the Cox proportional hazards model using multiple imputation. Stat Med. 2014;33:4681–4694. doi: 10.1002/sim.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen C, Li X, Li L, Were MC. Sensitivity analysis for causal inference using inverse probability weighting. Biom J. 2011;53:822–837. doi: 10.1002/bimj.201100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews JA, Jackson CE, Heiman-Patterson TD, Bettica P, Brooks BR, Pioro EP. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:509–518. doi: 10.1080/21678421.2020.1771734. [DOI] [PubMed] [Google Scholar]
- 21.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;3 doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vu M, Tortorice K, Zacher J, et al. Assessment of use and safety of edaravone for amyotrophic lateral sclerosis in the Veterans Affairs health care system. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunetta C, Moglia C, Lizio A, et al. The Italian multicenter experience with edaravone in amyotrophic lateral sclerosis. J Neurol. 2020;267:3258–3267. doi: 10.1007/s00415-020-09993-z. [DOI] [PubMed] [Google Scholar]
- 24.Witzel S, Maier A, Steinbach R, et al. Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Netw Open. 2020;3 doi: 10.1001/jamaneurol.2021.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C T-C, Chiu Y-W, Wang K-C, et al. Riluzole and prognostic factors in amyotrophic lateral sclerosis long-term and short-term survival: a population-based study of 1149 cases in Taiwan. J Epidemiol. 2013;23:35–40. doi: 10.2188/jea.JE20120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cetin H, Rath J, Füzi J, et al. Epidemiology of amyotrophic lateral sclerosis and effect of riluzole on disease course. Neuroepidemiology. 2015;44:6–15. doi: 10.1159/000369813. [DOI] [PubMed] [Google Scholar]
- 27.Knibb JA, Keren N, Kulka A, et al. A clinical tool for predicting survival in ALS. J Neurol Neurosurg Psychiatry. 2016;87:1361–1367. doi: 10.1136/jnnp-2015-312908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jongh AD, van Eijk RPA, van den Berg LH. Evidence for a multimodal effect of riluzole in patients with ALS? J Neurol Neurosurg Psychiatry. 2019;90:1183–1184. doi: 10.1136/jnnp-2018-320211. [DOI] [PubMed] [Google Scholar]
- 29.Thakore NJ, Lapin BR, Pioro EP. Pooled resource open-access ALS clinical trials consortium. Stage-specific riluzole effect in amyotrophic lateral sclerosis: a retrospective study. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:140–143. doi: 10.1080/21678421.2019.1655060. [DOI] [PubMed] [Google Scholar]
- 30.Baxi EG, Thompson T, Li J, et al. Answer ALS, a large-scale resource for sporadic and familial ALS combining clinical and multi-omics data from induced pluripotent cell lines. Nat Neurosci. 2022;25:226–237. doi: 10.1038/s41593-021-01006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.