Summary
Background
The relationship between depressive symptoms (DS) and their conversion patterns over time and the new-onset risk of diseases in the middle-aged and elderly population has not been extensively studied.
Methods
Based on The China Health and Retirement Longitudinal Study participants in 2013, we established 13 cohorts involving 12 types of chronic diseases and multimorbidity, who were identified by face-to-face questionnaires. We retrospectively assessed their DS during 2011 and 2013 through the 10-item Center for Epidemiological Studies Depression Scale (CES-D), which were classified into never, newly developed, relieved, and persistent DS, and these participants were followed from 2013 to 2018.
Findings
CES-D scores were new-onset risk factors for 9 diseases. The new-onset risk of diseases increased with higher CES-D scores. When CES-D scores were higher than approximately 6, the hazard ratios (HRs) of emergent diseases were greater than 1. DS was independent new-onset risk factors for 8 diseases, with HRs (95% CI) ranging from 1.2635 (1.0061–1.5867) to 1.5231 (1.0717–2.165). Persistent DS was an independent risk factor for most diseases but might be an independent protective factor for new-onset cancer (HR, 95% CI: 0.276, 0.106–0.723).
Interpretation
DS is closely associated with new-onset risk of chronic diseases and multimorbidity, and awareness of the risk associated with pre-DS status (6<CES-D<12) should be raised. chronic disease risks were almost lower with newly developed and relieved DS than with persistent DS, suggesting the potential benefits of active management of DS to reduce the risk of emergent diseases in middle-aged and elderly population.
Funding
National Natural Science Foundation of China
Keywords: Depressive symptoms, Middle-aged and elderly population, Depressive symptom conversion patterns, Chronic diseases, Multimorbidity
Research in context.
Evidence before this study
We searched the PubMed and Cochrane library databases for studies using the keywords “depressive symptom conversion”, “depression change”, “depression”, “depressive symptom”, “chronic disease risk”, and “multimorbidity”. Recent studies have linked early-stage depression to the risk of new-onset chronic disease. However, no studies have investigated the association between pre-depressive symptom or depressive symptom conversion pattern and new-onset chronic diseases.
Added value of this study
In the current study, our team prospectively conducted the study of the early depressive symptom conversion pattern s in the middle-aged and elderly population over a two-year period and followed up for 5 years. The innovations of this paper are threefold. Firstly, the potential risk of pre- depressive symptom for new-onset chronic diseases was explored in a large cohort for the first time; Secondly, compared with newly developed depressive symptom, relived depressive symptom, and persistent no depressive symptom, persistent depressive symptom displayed an absolute new-onset risk of chronic diseases; Thirdly, this is the first demonstration of a reduction in overall cancer risk associated with relived and persistent depressive symptom.
Meaning of all available evidence
Unlike previous studies, the present study indicated that different depressive symptom conversion patterns harbor a significantly distinct risk of new-onset chronic diseases in the middle-aged and elderly population, providing real-world evidence for the potential value of active depressive symptom management in preventing new-onset chronic diseases.
Alt-text: Unlabelled box
Introduction
Depression has become a public health problem that cannot be ignored among the middle-aged and elderly population. If current trends in depression incidence continue, depression will become one of the top three global disease burdens by 2030, especially in low-income and middle-income countries.1 In 2017, a total of 56.36 million individuals in China suffered from depression, accounting for 21.3% of the world's depressive patients. Depression in China has risen from 15th place in all-cause disability-adjusted life years in 1990 to 10th place in 2017.2
With the acceleration of the aging process in China, the population over 65 years old will reach 400 million in 2050.3 The incidence of age-related chronic diseases, such as cardiovascular and cerebrovascular diseases, endocrine and metabolic diseases, digestive system diseases, and chronic lung diseases, is significantly increasing, and the incidence of chronic lung diseases is significantly increasing.4 A recent epidemiological study in the elderly population in China has demonstrated that up to 75.8% of residents over 60 years old suffer from at least one chronic disease.5
Multimorbidity is common with depression and chronic disorders. Chronic diseases are closely associated with depression, and older adults with depression have higher morbidity and mortality due to serious physical illnesses.6 Patients with cardiovascular disease reported higher rates of depression. Depressive symptoms (DS) are established risk factors for coronary heart disease death.7 Moreover, depression is associated with increased cardiovascular disease-related mortality. Depression is a risk factor for stroke, while more than half of stroke patients reported DS 18 months after the stroke event.8 The prevalence of depression has been estimated to be twofold greater in individuals with diabetes than in those without diabetes,9 and depression may be correlated with diabetes outcomes. In addition, the combined status of depression and chronic diseases, such as kidney disease, asthma, and rheumatism, and its poor prognosis have also been repeatedly reported.
However, DS are also a chronic mental disease that show dynamic changes. In longitudinal datasets of European,10 American,11 and Asian12 populations, there have been studies on the progression of depression repeatedly assessed by Patient Health Questionnaire-9 or telephone interview records. These studies mainly focused on analyzing the risk factors that affect the degree of depression, including individual health status and socioeconomic factors.11,13 However, few studies have explored the impact of conversion of DS on the physical health of the population, that is, the risk of chronic disease. The effects of newly developed, relieved, and persistent DS on new-onset chronic diseases remain unclear. Focusing on the association between conversion status of DS and emergent diseases is of great significance for understanding overall health as a transition from a psychological to physiological disorder. The results of the current study may help determine whether to administer psychological interventions for the middle-aged and elderly population with DS in China.
In the present study, our team aimed to extend the association between changes in DS (newly developed DS, persistent DS, relieved DS, persistent no DS) and 12 emergent common chronic diseases and multimorbidity in the Chinese middle-aged and elderly population using national data from the China Health and Retirement Longitudinal Study (CHARLS). To the best of our knowledge, this study is the first large-scale cohort study to explore the effects of status changes in DS on chronic diseases and multimorbidity based on a Chinese population.
Methods
Population
The China Health and Retirement Longitudinal Study(CHARLS) is a survey that covered the population of 450 villages in 28 provinces, 150 counties, and districts across the country. The respondents mainly consisted of those 45 years of age and above. The first interview was conducted in 2011 (wave 1), and then respondents were followed up every two years. At present, the data of 2011 (wave 1), 2013 (wave 2), 2015 (wave 3), and 2018 (wave 4) have been publicly released, including the assessment of participants' health status, living habits, socioeconomics, and other aspects.
CHARLS was approved by the Ethics Committee of Peking University Health Science Center, and informed consent was obtained from all participants.
We chose the population data released in 2013 as the baseline and retroactively traced these people back to 2011. The population with data regarding depression conditions (Center for Epidemiological Studies Depression Scale (CES-D) scores) both in 2011 and 2013 was considered the target population.
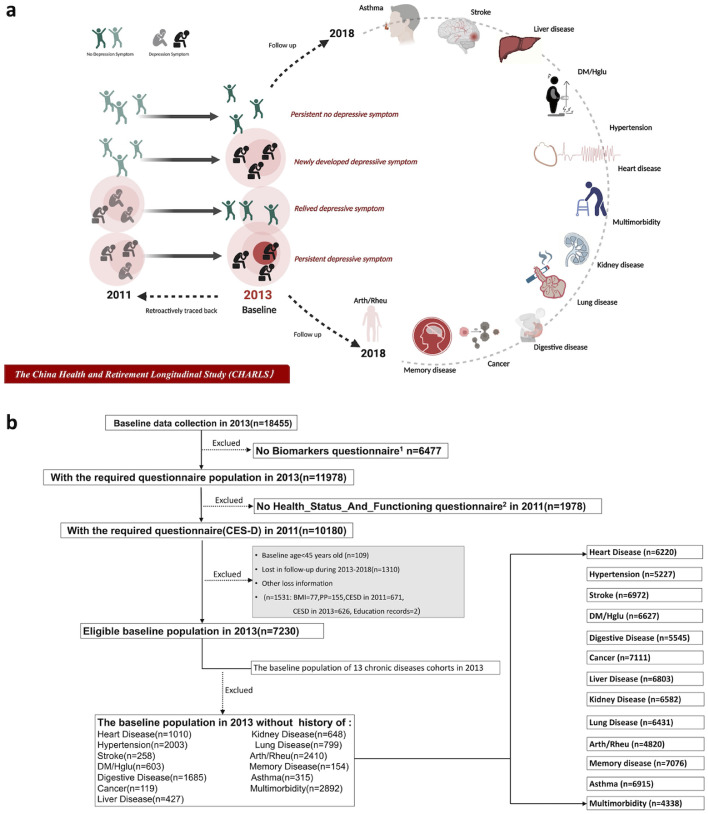
We established different cohorts with 12 types of chronic diseases and multimorbidity in 2013, excluding the population with a disease history at baseline, and then followed these participants until 2018 to obtain data regarding the new incidence of those diseases. The flowchart details of the study design are shown in Figure 1.
Figure 1.
The flowchart of the study design.
a. The study design and concept map.
b. The screening flowchart for study cohorts.
1Dataset of “Biomarker” in 2013 includes information of participants’ blood pressure, weight, height, etc.
2Dataset of “Health_status_and_function” in 2011 includes records of participants’ CES-D.
Abbreviations could be seen in Table 1.
Ascertainment of twelve chronic diseases and multimorbidity
Respondents' illness status was evaluated by the face-to-face answers to question DA007 (Have you been diagnosed with… by a doctor?). A respondent with no less than two chronic diseases was considered to have multimorbidity.14 The time of the initial disease attack was acquired by the answer to question DA009 (When was the condition first diagnosed or known by yourself?). The medical history of the disease was considered either an incident of disease at baseline or a new-onset disease incident during follow-up when the diseases occurred no later than 2013 or later than 2013, respectively. New-onset chronic diseases during follow-up were considered to be endpoints in this study.
Assessment of depressive symptoms (DS)
The 10-item CES-D, which has been verified to have excellent reliability and validity among the old Chinese community dwelling population, was used to assess DS.15 Based on different frequencies of some feeling or behavior, specifically, answers to the questions DC009, DC010, DC011, DC012, DC013, DC014, DC015, DC017, DC018 were assigned a score of 0 to 4 points; the higher the frequency was, the higher the score: scores of 0, 1, 2, and 3 were assigned to “rarely or none of the time”, “some or a little of the time”, “occasionally or a moderate amount of the time”, and “most or all of the time” categories, respectively. For questions DC013 and DC016, which were reversed scored, the higher the frequency was, the lower the score.
The total scores of the 10 items were summed, and the presence of DS was determined by a point total equal to or larger than 12.16
Assessment of conversion patterns of DS
Combined the DS in 2011 and 2013, there were four potential conversion patterns of DS during that time:
-
1.
NoDS-NoDS: never DS (no DS both in 2011 and in 2013).
-
2.
NoDS-DS: newly developed DS (no DS in 2011 and developed DS in 2013).
-
3.
DS-NoDS relieved DS (DS in 2011 and relieved to no DS in 2013).
-
4.
DS-DS: persistent DS (DS both in 2011 and in 2013).
Covariates
Age, sex, systolic blood pressure, diastolic blood pressure, pulse pressure, and body mass index were included in the study. Napping duration and nighttime sleep duration were answers to the question regarding the length of naps (minutes) and the length of a night's sleep (hours) in the past month, respectively. Functional status was measured by activities of daily living (ADL; score of 0–6) and instrumental activities of daily living (IADL; score of 0–5) scales. Scores were assigned to different frequencies of activities (almost daily=3, almost every week=2, not regularly=1), and the total scores for each item were calculated to assess the intensity of social (including interacting with friends, going to clubs, taking part in organizations, and engaging in volunteer activities) and intellectual activities (including playing Ma-Jong, attending courses, doing stock investment and using the internet). A history of smoking (yes/no) and drinking (often/sometimes/never) were recorded in the study. According to the educational records of the interviewed population (BD001:" What is the highest level of education you have attained? "), we divided the population into four groups: illiterate, elementary school, middle school, and college.
More detailed information about the CHARLS and all indicators is provided in the supplementary material(Appendix1).
Statistical methods
Comparisons of differences among DS conversion groups were analyzed by ANOVA and Kruskal–Wallis tests for continuous variables in line with normal and nonnormal distributions, respectively, and by chi-square tests for categorical variables. Descriptive statistics are presented for the proportion of new-onset chronic disease across the DS groups and based on the change in DS in the 13 chronic disease cohorts. Hazard ratios (HRs) and 95% confidence intervals (CIs) of CES-D scores in 2013, DS in 2013, and DS conversion group during 2011–2013 for the risk of new-onset chronic diseases were estimated by Cox regression models and adjusted by covariates(including information of demographic background, individual biometric values, living and activity habits, and baseline medical history). Kaplan–Meier analyses with log-rank tests were performed to separately assess cumulative incidence rates in the DS group in 2013 to 5-year new-onset chronic diseases in the 13 cohorts. We explored linear and potential nonlinear trends between CES-D scores in 2013 and HRs (95% CI) for new-onset chronic diseases with restricted cubic spline (RCS) regression with 3 knots and obtained the possible cutoff value of CES-D scores for the risk of those diseases. Produce forest plots to visualize the different new-onset risks to diseases of CES-D score or DS in 2013. And display the risks to chronic diseases across three conversion patterns of DS during 2011–2013 compared with the persistent No-DS pattern in the same way. All calculations were performed and graphics were created using R (version 3.6.3-Mac OS X 10.11). Two-tailed p values were used throughout the study, and p<0.05 was considered statistically significant.
Role of funding source
This study was supported by the National Natural Science Foundation of China to W.L.(Grant No. 81974113).
Results
Characteristics of the study cohort stratified by conversion patterns of DS from 2011 to 2013
The subjects in this study were grouped based on their DS in 2011 and 2013. A total of 1916 persons of 7230 participants experienced DS in 2011. In 2013, 1644 participants were diagnosed with DS (Appendix 2a). A total of 918, 4588, 726, and 998 participants were enrolled in the persistent DS (DS-DS), persistent no DS (NoDS-NoDS), newly developed DS (NoDS-DS), and relieved DS (DS-NoDS) groups, respectively, according to the DS status of participants in 2011 and 2013. Almost all included variables significantly differed between populations with different conversion patterns of DS, except for pulse pressure (p=0.625). In general, compared with the other three groups, the DS-DS group had the lowest proportion of males (%, 29.2 vs. 38.2 vs. 41.0 vs. 53.4, p<0.001), the lowest durations of night sleep (hours, 5.22 vs. 5.65 vs. 5.90 vs. 6.49, p<0.001) and naps (minutes, 27.93 vs. 35.03 vs. 37.08 vs. 40.34, p<0.001), and the lowest scores for intellectual activities (0.28 vs. 0.35 vs. 0.40 vs. 0.64, p<0.001) and social activities (1.05 vs. 1.16 vs. 1.23 vs. 1.37, p<0.001). In terms of medical history, except for hypertension, this population had the highest proportion of the 11 other chronic diseases and multimorbidity. At the same time, some indicators, such as the BMI (kg/m2, 23.39 vs. 23.60 vs. 23.78 vs. 24.14, p<0.001), systolic blood pressure (mmHg, 127.49 vs. 130.22 vs. 130.40 vs. 130.73, p=0.002), diastolic blood pressure (mmHg, 74.68 vs. 76.48 vs. 76.52 vs. 76.74, p<0.001), and proportions of smoking (%, 31.2 vs. 35.5 vs. 39.6 vs. 45.6, p<0.001) and heavy drinking (%, 14.6 vs. 23.1 vs. 25.1 vs. 30.4, p<0.001) among the people with persistent DS, were significantly lower than those among the other three DS conversion patterns (Table 1).
Table 1.
Basic characteristics of the participants at baseline stratified by conversion types of depression symptoms from 2011 to 2013.a
| Stratified by conversion types of depression symptoms from 2011 to 2013 |
||||||
|---|---|---|---|---|---|---|
| NoDS-NoDS | NoDS-DS | DS-NoDS | DS-DS | Pb | SMD | |
| n | 4588 | 726 | 998 | 918 | ||
| Age(years), mean ± SD | 59.65 (8.59) | 59.19 (8.46) | 61.11 (8.77) | 60.65 (8.36) | <0.001 | 0.131 |
| Sex=male (%) | 2450 (53.4) | 277 (38.2) | 409 (41.0) | 268 (29.2) | <0.001 | 0.261 |
| BMI (Kg/m2), mean ± SD | 24.14 (3.78) | 23.78 (3.75) | 23.60 (3.69) | 23.39 (3.77) | <0.001 | 0.108 |
| SBP (mmHg), mean ± SD | 130.22 (20.23) | 130.73 (21.45) | 130.40 (20.74) | 127.49 (22.07) | 0.002 | 0.077 |
| DBP (mmHg), mean ± SD | 76.74 (13.11) | 76.52 (11.91) | 76.48 (13.83) | 74.68 (13.17) | <0.001 | 0.080 |
| PP (mmHg), mean ± SD | 74.09 (10.58) | 73.45 (10.09) | 74.06 (10.43) | 74.48 (10.48) | 0.265 | 0.050 |
| CESD in 2011, medians (IQR) | 5.00 [2.00, 7.00] | 7.00 [4.00, 9.00] | 15.00 [13.00, 18.00] | 17.00 [14.00, 21.00] | <0.001 | 2.179 |
| CESD in 2013, medians (IQR) | 5.00 [3.00, 7.00] | 14.00 [13.00, 17.00] | 7.00 [5.00, 9.00] | 17.00 [14.00, 20.00] | <0.001 | 2.213 |
| IADL, mean ± SD | 0.16 (0.53) | 0.52 (0.95) | 0.40 (0.87) | 0.81 (1.28) | <0.001 | 0.372 |
| ADL, mean ± SD | 2.47 (1.45) | 2.16 (1.26) | 2.22 (1.30) | 2.31 (1.25) | <0.001 | 0.125 |
| NapTime(minutes), mean ± SD | 40.34 (45.06) | 35.03 (44.61) | 37.08 (47.01) | 27.93 (41.66) | <0.001 | 0.148 |
| NightTime(hours), mean ± SD | 6.49 (1.61) | 5.65 (1.98) | 5.90 (1.89) | 5.22 (2.04) | <0.001 | 0.366 |
| Social Activity, mean ± SD | 1.37 (1.71) | 1.16 (1.60) | 1.23 (1.64) | 1.05 (1.53) | <0.001 | 0.107 |
| Intellectual Activity, mean ± SD | 0.64 (1.20) | 0.35 (0.82) | 0.40 (0.92) | 0.28 (0.79) | <0.001 | 0.191 |
| Education=illiterate (%) | 921 (20.1) | 208 (28.7) | 301 (30.2) | 304 (33.1) | <0.001 | 0.280 |
| =elementary school(%) | 1889 (41.2) | 305 (42.0) | 458 (45.9) | 448 (48.8) | ||
| =middle school(%) | 1672 (36.4) | 210 (28.9) | 235 (23.5) | 161 (17.5) | ||
| =college (%) | 106 (2.3) | 3 (0.4) | 4 (0.4) | 5 (0.5) | ||
| Smoke = yes (%) | 2093 (45.6) | 258 (35.5) | 395 (39.6) | 286 (31.2) | <0.001 | 0.164 |
| Drink =often (%) | 1396 (30.4) | 182 (25.1) | 231 (23.1) | 134 (14.6) | <0.001 | 0.210 |
| =sometimes (%) | 376 (8.2) | 52 (7.2) | 65 (6.5) | 76 (8.3) | ||
| =never (%) | 2816 (61.4) | 492 (67.8) | 702 (70.3) | 708 (77.1) | ||
| Number of Diseases in 2013 | 1.20 (1.23) | 1.69 (1.42) | 1.67 (1.43) | 2.21 (1.72) | <0.001 | 0.348 |
| Medical history= | ||||||
| Heart disease (%) | 523 (11.4) | 118 (16.3) | 151 (15.1) | 218 (23.7) | <0.001 | 0.170 |
| Hypertension (%) | 1185 (25.8) | 234 (32.2) | 293 (29.4) | 291 (31.7) | <0.001 | 0.079 |
| Stroke (%) | 138 (3.0) | 23 (3.2) | 46 (4.6) | 51 (5.6) | <0.001 | 0.076 |
| DM/Hglu (%) | 338 (7.4) | 74 (10.2) | 84 (8.4) | 107 (11.7) | <0.001 | 0.084 |
| Digestive disease (%) | 865 (18.9) | 197 (27.1) | 277 (27.8) | 346 (37.7) | <0.001 | 0.215 |
| Cancer (%) | 61 (1.3) | 15 (2.1) | 20 (2.0) | 23 (2.5) | 0.035 | 0.044 |
| Liver disease (%) | 229 (5.0) | 47 (6.5) | 62 (6.2) | 89 (9.7) | <0.001 | 0.093 |
| Kidney disease (%) | 305 (6.6) | 79 (10.9) | 112 (11.2) | 152 (16.6) | <0.001 | 0.159 |
| Lung disease (%) | 388 (8.5) | 95 (13.1) | 138 (13.8) | 178 (19.4) | <0.001 | 0.164 |
| Arth/Rheu (%) | 1270 (27.7) | 282 (38.8) | 402 (40.3) | 456 (49.7) | <0.001 | 0.235 |
| Memory disease (%) | 73 (1.6) | 18 (2.5) | 23 (2.3) | 40 (4.4) | <0.001 | 0.085 |
| Asthma (%) | 129 (2.8) | 42 (5.8) | 62 (6.2) | 82 (8.9) | <0.001 | 0.136 |
| Multimorbidity (%) | 1502 (32.7) | 349 (47.9) | 482 (48.3) | 560 (61.0) | <0.001 | 0.292 |
Divided the population into four conversion patterns of depressive symptoms according to their depressive symptom assessed by CES-D in 2011 and 2013 separately.
Quantitative data of normal and non-normal distribution were expressed as mean (SD) and medians with interquartile range (IQR). Categorical data were presented as amounts(percentages), respectively. Comparisons of differences among groups were respectively analyzed by ANOVA test and Kruskal-Wallis test for continuous variables in line with normal and non-normal distribution, and by Chi-square test for categorical variables.
Abbreviations: DS Depressive symptom, NoDS No Depressive symptom, BMI Body Mass Index, SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure, PP Pulse Pressure, CESD-10 The 10-item Center for Epidemiological Studies, IADL Instrumental Activities of Daily Living, ADL Activities of Daily Living, DM/Hglu diabetes or high blood sugar, Arth/Rheu Arthritis or Rheumatism.
Altogether, 7230 participants were followed up for 5 years. The longitudinal cohort study was conducted based on these 4 groups, and the new onset of 12 chronic diseases and multimorbidity were tracked for 5 years (Appendix 2b).
Cox regression analysis of CES-D scores for new-onset risk assessment of 13 disease conditions
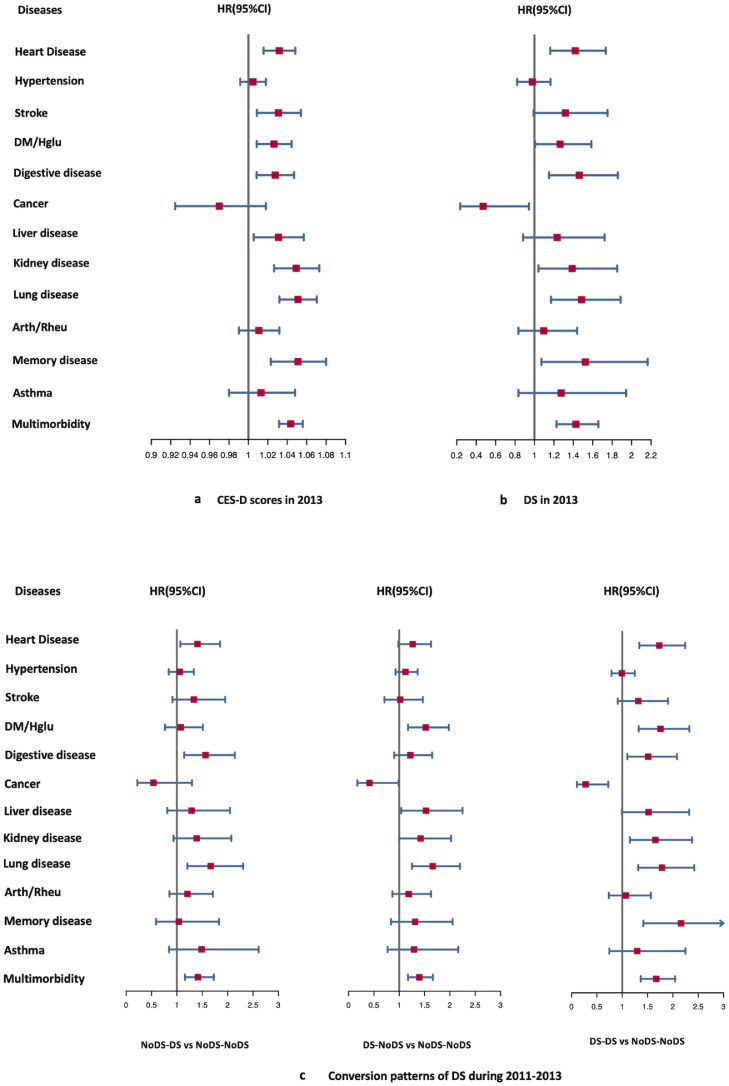
CES-D scores were independent new-onset risk factors for heart disease, stroke, diabetes mellitus (DM)/high glucose (Hglu), digestive disease, liver disease, kidney disease, lung disease, memory disease, and multimorbidity by Cox regression analysis adjusted for covariates (Appendix 2c). The HRs(95%CIs) were visualized in form of forest plots (Figure 2a). There was a generally positive and linear association between CES-D scores and the risk of the above diseases by RCS regression (Figure 3). The risk of participants developing new diseases increased with higher CES-D scores. CES-D scores were positively and linearly associated with the new-onset risk of 9 diseases (P-nonlinear>0.05), and the CES-D score with an HR=1 for the above 9 types of chronic diseases was approximately 6. However, CES-D scores were not an independent new-onset risk factor for hypertension, cancer, arthritis/rheumatoid arthritis (arth/rheu), or asthma (Appendix 2c model 2; Figure 3 b, f, j, and l).
Figure 2.
Forest plots including HRs(95%CI) for the relationship between new-onset risks of 13 chronic diseases and DS conditions.
a. Forest plots for the HRs(95%CIs) of CES-D scores in 2013 for new-onset risks of 13 chronic diseases.
b. Forest plots for the HRs(95%CIs) of DS in 2013 for new-onset risks of 13 chronic diseases.
c. Forest plots for the HRs(95%CIs) of three conversion patterns of DS (NoDS-DS: newly DS, DS-NoDS: relieved DS, DS-DS: persistent DS) on the new-onset risk of 13 chronic diseases compared with never DS (NoDS-NoDS).
Models were all adjusted by age, sex, BMI, SBP, DBP, PP, smoke, drink, IADL, ADL, Naptime, Nighttime, Education, Social Activity, and intellectual Activity. In addition, considering the interrelationships between chronic diseases, we included 11 chronic diseases history as covariates (except for multimorbidity and cohort outcome event disease itself) in our subgroup analysis for each disease cohort to control for the effect of baseline medical history on outcome events when analyzing the risk of CES-D on the new-onset chronic diseases. Specific covariate information for each model is available in Appendix 2c.
Abbreviations could be seen in Table 1.
Figure 3.
The combined graphs of restricted cubic splines with 3 knots to flexibly model the association between 2013 CES-D and HRs (95% CI) for new-onset diseases and distribution density maps of 2013 CES-D in each chronic disease subgroup cohort.
a. Heart disease, b. Hypertension, c. Stroke, d. DM/Hglu, e. Digestive disease, f. Cancer, g. Liver disease, h. Kidney disease, I. Lung disease, j. Arth/Rheu, k. Memory disease, l. Asthma, and m. Multimorbidity
The vertical axis (left) represents the Hazard ratio for diseases in the restricted cubic splines; the vertical axis (right) represents the 2013 CES-D distribution fraction of the population in density maps. The cutoff refers to the value of 2013 CES-D when hazard ratios for almost all new-onset chronic diseases equal 1.
Models were all adjusted by age, sex, BMI, SBP, DBP, PP, smoke, drink, IADL, ADL, Naptime, Nighttime, Education, Social Activity, and intellectual Activity. In addition, considering the interrelationships between chronic diseases, we included 11 chronic diseases history as covariates (except for multimorbidity and cohort outcome event disease itself) in our subgroup analysis for each disease cohort to control for the effect of baseline medical history on outcome events when analyzing the risk of CES-D on the new-onset chronic diseases. Specific covariate information for each model is available in Appendix 2c.
Abbreviations could be seen in Table 1.
Longitudinal associations between DS and new-onset risk of chronic disease conditions
Cox regression analysis adjusted for covariates revealed that DS were significant new-onset risks for heart disease (HR, 95% CI: 1.4196, 1.162–1.7341,p=0.0006), DM/Hglu (HR, 95% CI: 1.2635, 1.0061–1.5867,p=0.0442), digestive disease (HR, 95% CI: 1.4619, 1.1503–1.8579,p=0.0019), kidney disease (HR, 95% CI: 1.3881, 1.0406–1.8515,p=0.0257), lung disease (HR, 95% CI: 1.4861, 1.1708–1.8863,p=0.0011), memory disease (HR, 95% CI: 1.523, 1.0717–2.165,p=0.0190) and multimorbidity (HR, 95% CI: 1.4857, 1.2685–1.7401,p<0.0001). In particular, DS were a significant protective factor for cancer (HR, 95% CI: 0.4710, 0.2352–0.9432,p=0.0336) (Appendix 2d). The HRs(95%CIs) were displayed by forest plots (Figure 2b).The cumulative chronic diseases and multimorbidity incidence plots among the people with and without DS at baseline during the 5-year follow-up are shown in Figure 4.
Figure 4.
Kaplan-Meier plots of cumulative incidence rate to new-onset chronic diseases by DS and NoDS in 2013 in various disease cohorts with the log-rank test.
a. Heart disease, b. Hypertension, c. Stroke, d. DM/Hglu, e. Digestive disease, f. Cancer, g. Liver disease, h. Kidney disease, I. Lung disease, j. Arth/Rheu, k. Memory disease, l. Asthma, and m. Multimorbidity.
Abbreviations could be seen in Table 1.
Longitudinal associations between conversion patterns of DS during 2011 and 2013 and new-onset risk of 13 chronic disease conditions at follow-up from 2013 to 2018
Cox regression was used to evaluate the associations between persistent DS, persistent no DS, relieved DS, newly developed DS, and new-onset risk for 13 diseases (Appendix 2e). Newly developed DS were independent risk factors for four chronic diseases: heart disease (HR, 95% CI: 1.405, 1.067–1.849,p=0.0153), digestive disease (HR, 95% CI: 1.564, 1.143–2.141,p=0.005), lung disease (HR, 95% CI: 1.667, 1.205–2.305,p=0.002), and multimorbidity (HR, 95% CI: 1.462, 1.176–1.816,p=0.0007). Relieved DS were also independent risk factors for four chronic diseases: DM/Hglu (HR, 95% CI: 1.523, 1.173–1.979,p=0.002), liver disease (HR, 95% CI: 1.529, 1.039–2.249,p=0.031), lung disease (HR, 95% CI: 1.660, 1.252–2.200,p<0.001), multimorbidity (HR, 95% CI: 1.670, 1.390–2.007,p=0.0002). Persistent DS was associated with a higher new-onset risk of 8 chronic diseases, all except hypertension, stroke, liver disease, arth/rheu, and asthma. In particular, relieved DS (HR, 95% CI: 0.414, 0.173–0.986,p=0.0460) and persistent DS (HR, 95% CI: 0.276, 0.106–0.723,p=0.0090) were both significant protective factors for cancer. Figure 2c visualized the HRs(95%CIs) above.
Discussion
This study demonstrated the risk associations of pre-DS status and DS conversion patterns to new-onset chronic diseases. The main results of this study are as follows: (a). The study expands the current understanding of DS and new-onset chronic disease risk: CES-D scores were independent new-onset risk factors for 9 chronic diseases (all except for hypertension, cancer, arth/rheu, and asthma). The risk of developing chronic diseases increased with high CES-D scores. The risk threshold was approximately 6, indicating that DS (CES-D>12) has an absolute risk for new-onset chronic diseases, while the pre-DS status (6<CES-D<12) had potential risks for new-onset chronic diseases. (b). DS was independent new-onset risk factors for 7 new chronic diseases (all except for hypertension, stroke, liver disease, arth/rheu, and asthma), with HRs (95% CI) ranging from 1.2635 (1.0061–1.5867) to 1.5231 (1.0717–2.165). (c). The effect of short-term changes in DS on the risk of developing new-onset chronic diseases among middle-aged and elderly individuals has been rarely reported. Compared with other conversion patterns of DS, persistent DS were an independent risk factor for most emergent chronic diseases (all except for hypertension, stroke, liver disease, arth/rheu, and asthma) and almost exerted the highest new-onset risk of diseases. (d) Relieved DS (HR, 95% CI: 0.414, 0.173–0.986) and persistent DS (HR, 95% CI: 0.276, 0.106–0.723) may be related to decreased cancer incidence.
Depression is strongly associated with the risk of developing cardiovascular disease. A large meta-analysis study found that depressed patients without cardiovascular disease already had autonomic dysregulation and high levels of inflammatory immune markers.17 This study found that these potential risk markers might be increasing in those with pre-DS status (7<CES-D<12). As CES-D scores increased to the threshold meeting a depression diagnosis, the risk of new-onset disease greatly increased. This study further showed that individuals with newly developed DS, and persistent DS had a significantly increasing risk of new-onset heart disease compared with individuals who persistently showed no DS. The current study proposed the potential value of cognitive-behavioral therapy in reducing cardiovascular disease risk through biological effects.18
The association between DS and hypertension has remained controversial in most epidemiological investigations. A multicenter prospective cohort study showed that DS could predict the risk of developing hypertension in a younger population, especially blacks (23–35 years). Depressed youth had 2.84 times higher risk of hypertension than normal youth.19 Another study from the National Health and Nutrition Examination I Epidemiologic Follow-up Study found that major depression was an independent predictor of hypertension in blacks and whites.20 However, another study from the CARDIA cohort showed that depression was not a significant risk factor for hypertension at 10 years in the study population (OR:1.32,95%CI: 0.92–1.90; risk RR for blood pressure:1.15,95% CI:0.75–1.76). No significant association between depression and hypertension was found in Korean community residents over 65 years of age.21 A study of veterans in the National Health and Nutrition Examination Survey showed that major DS were protective factors for well-controlled blood pressure (OR: 0.28, 95% CI: 0.09–0.85 in the National Health and Nutrition Examination Survey).22 This study confirmed that neither baseline CES-D scores nor conversion of DS was significantly associated with new-onset hypertension in the middle-aged and elderly population. Blood pressure is regulated by multiple factors, such as genetic background, living habits, and psychological factors; anger and hostility are personality traits related to hypertension,23 which contrast with the depression, fatigue, and lethargy that are more likely to occur in depressed patients; depression may involve a physiological imbalance in the norepinephrine system, and there is a mediating role of neuropeptide Y22 between depression and blood pressure control. Although influenced by a variety of factors, there may be no significant correlation between depressive conditions and new-onset risk of hypertension.
Depression is an independent risk factor for the onset of diabetes.24 Consistent with our results, persistent and relieved DS were independent risk factors for new-onset DM/Hglu, and relieved DS (HR, 95% CI: 1.523, 1.173–1.979) was associated with a lower risk of new-onset DM/Hglu than persistent DS (HR, 95% CI: 1.755, 1.324–2.325). However, newly developed DS were not significantly associated with that. This may indicate that a history of DS and persistent DS promote the development of DM through chronic changes in the body's neuroendocrine axis and immune-inflammatory activity.25 Increased release and enhanced action of the counterregulatory hormone led to sympathoadrenal, hypothalamic-pituitary-adrenocortical hyperactivity, hypothalamic-growth hormone axis activity, altered glucose transport function, and increased immune-inflammatory activity (possibly through proinflammatory cytokines, interleukin (IL)-1, IL-6 and tumor necrosis factor-a (TNF-a) mediation).25 Long-term DS leads to insulin resistance and pancreatic β-cell dysfunction through these pathways and perhaps remission of DS(HR, 95% CI: 1.523, 1.173–1.979) was associated with a lower risk of new-onset DM/Hglu than persistent DS (HR, 95% CI: 1.755, 1.324–2.325), but short-term relief of DS may not fully compensate for these negative changes immediately.25
The bidirectional association between digestive diseases and DS has been repeatedly demonstrated in previous studies, and this bidirectional association may be mediated by activation of the brain-gut axis. Additionally, inflammatory markers, such as zonulin and intestinal fatty acid-binding protein, mediate the links between DS and digestive disorders through bacteria and microbes of the "leaky gut hypothesis".26 Moreover, DS eventually leads to intestinal dysfunction or other intestinal organic diseases through multiple pathways, such as endocrine, neurological, immune, and intestinal microbial homeostasis imbalance.27 These multisystem and multidimensional associations may be responsible for pre-depression pre-DS status, that is, a CES-D score as low as 6, which is associated with an increased risk of new-onset digestive disorders.
Cancer survivors often experience depression. However, the impact of depression on emerging cancer is uncertain. Persistent depression that lasts for at least 6 years may increase the risk of cancer in the population.28 While the Baltimore Epidemiologic Catchment Area Study indicated that major depression was a risk factor for cancer incidence (HR, 95% CI: 1.87, 1.16–3.01), no statistically significant differences were noted in breast, colon, lung, prostate, and skin cancer.29 The incidence in the stomach, colon, rectum, liver, breast, cervix, uterus, prostate, etc., was not increased in hospitalized patients with depression, and the incidences of cervical cancer (HR, 95% CI: 0.70, 0.56–0.85) and rectal cancer (HR, 95% CI: 0.79, 0.68–0.92) were even lower in the depression cohort.30 A Mendelian randomization study31 showed that depression was not causally related to prostate cancer risk. Interestingly, this study found that persistent depression reduced the risk of cancer (HR, 95% CI: 0.3754, 0.1472–0.9572). The higher new-onset risk of cancer in the non-persistent DS group may be because the study population in the non-DS group, compared to the DS group, had more associated well-defined adverse behaviors, such as a higher proportion of smoking (Appendix 2a; 2011: 44.2% vs. 35.5%, 2013: 44.5% vs. 33.1%, respectively) and heavy drinking (Appendix 2a; 2011: 29.7% vs. 19.1%, 2013: 29.1% vs. 19.2%). That is, the high-risk behaviors of individuals without DS increase the risk of tumor incidence, resulting in a relatively lower risk of tumor incidence among people with DS.
A recent meta-analysis showed that there were higher odds of having a depressive disorder with each additional chronic disease compared with the healthy population (OR, 95% CI: 1.45, 1.28–1.64).32 However, some prospective studies have shown that the relationships between depression and multimorbidity are not clear. A study of depression and anxiety from the Netherlands33 found that current major depression increased the incidence of somatic diseases at a 6-year follow-up. A case-control study in the San Francisco Bay Area34 showed that patients with depression had a nearly two-thirds higher risk of physical illness than those without depression. Based on a longitudinal cohort of the Chinese middle-aged and elderly population, this study demonstrated that pre-DS, DS, newly DS, relieved DS, and persistent DS contribute to new-onset multimorbidity. These potential connections may be explained by Beck's developmental model of depression, the activity restriction model of depressed affect, and the psychological and biological pathways model:35 cohorts with depression may have unhealthy lifestyle behaviors (unhealthy diet and sedentary lifestyle) and reduced ability to manage their own health (irregular insulin injections and taking medications without following the doctor's advice). In addition, chronic metabolic abnormalities and immune-inflammatory dysregulation may also mediate the association between depression and multiple comorbidities.
To our knowledge, the current study is the first to explore the differential impact of dynamics in DS on new-onset chronic diseases. This provides an important realistic basis for supposing that actively managing DS can reduce the occurrence of chronic diseases and multimorbidity in the long run. However, there are also some limitations. The health function information used in this study was mainly self-reported, The degree of accuracy was affected by the educational level, cognitive level, and economic and medical level of the interviewee; in particular, the reports of their specific chronic disease onset time may be biased, and the actual disease incidence may have been underestimated. The lack of a subtype of chronic disease requires further validation of our findings in more specific disease cohorts. In addition, the present study included only individuals in the Chinese population, and whether these conclusions can be applied to other ethnic groups needs to be further confirmed.
Contributors
We thank all the researchers who contributed to this article: Conceptualization and Funding Acquisition: Niuniu Hou and WeiLi; Investigation, Methodology, Project Administration: Yaoling Wang, Liping Wang, Minfang Chen, and Kang Yang; Software and Visualization: Yaoling Wang and KaiWen; Art and Drawing Instruction: Yujie Lan; Writing-Original Draft Preparation: Yaoling Wang, Niuniu Hou, and Gege Jiang; Writing-Review & editing: Yaoling Wang and Niuniu Hou.
Data sharing statement
The data that support the findings of this study are available from http://charls.pku.edu.cn/index/zh-cn.html. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of National Development Institute, Peking University.
Declaration of interests
None exist
Acknowledgements
Also, special thanks to all members of the CHARLS research team, all fieldworkers, and every interviewee.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101603.
Contributor Information
Niuniu Hou, Email: hou_neoniu@163.com.
Wei Li, Email: drwli@hust.edu.cn.
Appendix. Supplementary materials
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren X, Yu S, Dong W, et al. Burden of depression in China, 1990–2017: findings from the global burden of disease study 2017. J Affect Disord. 2020;268:95–101. doi: 10.1016/j.jad.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Zeng Y. Towards deeper research and better policy for healthy aging –using the unique data of Chinese longitudinal healthy longevity survey. China Econ J. 2012;5:131–149. doi: 10.1080/17538963.2013.764677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Yu W, Zhou J, et al. Relationship between the number of noncommunicable diseases and health-related quality of life in Chinese older adults: a cross-sectional survey. Int J Environ Res Public Health. 2020;17:E5150. doi: 10.3390/ijerph17145150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang LM, Chen ZH, Zhang M, et al. [Study of the prevalence and disease burden of chronic disease in the elderly in China] Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi. 2019;40:277–283. doi: 10.3760/cma.j.issn.0254-6450.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Cuijpers P, Vogelzangs N, Twisk J, et al. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171:453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- 7.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 8.Chapman DP, Perry GS, Strine TW. The vital link between chronic disease and depressive disorders. Prev Chronic Dis. 2005;2:A14. [PMC free article] [PubMed] [Google Scholar]
- 9.Ohira T, Iso H, Satoh S, et al. Prospective study of depressive symptoms and risk of stroke among japanese. Stroke. 2001;32:903–908. doi: 10.1161/01.str.32.4.903. [DOI] [PubMed] [Google Scholar]
- 10.Merikangas KR, Zhang H, Avenevoli S, et al. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich cohort study. Arch Gen Psychiatry. 2003;60:993–1000. doi: 10.1001/archpsyc.60.9.993. [DOI] [PubMed] [Google Scholar]
- 11.Beard JR, Tracy M, Vlahov D, et al. Trajectory and socioeconomic predictors of depression in a prospective study of residents of New York city. Ann Epidemiol. 2008;18:235–243. doi: 10.1016/j.annepidem.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Chen C-M, Mullan J, Griffiths D, et al. Trajectories of depression and their relationship with health status and social service use. Arch Gerontol Geriatr. 2011;53:e118–e124. doi: 10.1016/j.archger.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Tuithof M, Ten Have M, van Dorsselaer S, et al. Course of subthreshold depression into a depressive disorder and its risk factors. J Affect Disord. 2018;241:206–215. doi: 10.1016/j.jad.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Yao S-S, Cao G-Y, Han L, et al. Prevalence and patterns of multimorbidity in a nationally representative sample of older Chinese: results from the China health and retirement longitudinal study. J Gerontol A Biol Sci Med Sci. 2020;75:1974–1980. doi: 10.1093/gerona/glz185. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Mui AC. Factorial validity of the center for epidemiologic studies depression scale short form in older population in China. Int Psychogeriatr. 2014;26:49–57. doi: 10.1017/S1041610213001701. [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Bishwajit G, Zhou Y, et al. Intensity, frequency, duration, and volume of physical activity and its association with risk of depression in middle- and older-aged Chinese: evidence from the China health and retirement longitudinal study, 2015. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch C, Wilhelm M, Salzmann S, et al. A meta-analysis of heart rate variability in major depression. Psychol Med. 2019;49:1948–1957. doi: 10.1017/S0033291719001351. [DOI] [PubMed] [Google Scholar]
- 18.Euteneuer F, Neuert M, Salzmann S, Fischer S, Ehlert U, Rief W. Does psychological treatment of major depression reduce cardiac risk biomarkers? An exploratory randomized controlled trial. Psychol Med. 2022:1–15. doi: 10.1017/S0033291722000447. Epub ahead of print. PMID: 35232509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson K, Jonas BS, Dixon KE, et al. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary artery risk development in young adults. Arch Intern Med. 2000;160:1495–1500. doi: 10.1001/archinte.160.10.1495. [DOI] [PubMed] [Google Scholar]
- 20.Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National health and nutrition examination survey I epidemiologic follow-up study. Arch Fam Med. 1997;6:43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Kim J-M, Stewart R, Shin I-S, et al. Vascular disease/risk and late-life depression in a Korean community population. Br J Psychiatry J Ment Sci. 2004;185:102–107. doi: 10.1192/bjp.185.2.102. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Waring ME, Park CL, et al. Do depressive symptoms predict blood pressure control in US veterans? J Gen Intern Med. 2022;37:57–63. doi: 10.1007/s11606-021-06709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psychosocial factors in the development of hypertension - PubMed, https://pubmed.ncbi.nlm.nih.gov/10949069/. Accessed 12 April 2022.
- 24.Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013;74:31–37. doi: 10.4088/JCP.12r07922. [DOI] [PubMed] [Google Scholar]
- 25.Musselman DL, Betan E, Larsen H, et al. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54:317–329. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson L, Gustafsson A, Lavant E, et al. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr Scand. 2019;139:185–193. doi: 10.1111/acps.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The role of the gut-brain axis in depression: endocrine, neural, and immune pathways - PubMed, https://pubmed.ncbi.nlm.nih.gov/32827123/. Accessed 12 April 2022.
- 28.Penninx BW, Guralnik JM, Pahor M, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888–1893. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- 29.Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow-up of the Baltimore epidemiologic catchment area sample. Cancer Causes Control CCC. 2010;21:191–199. doi: 10.1007/s10552-009-9449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalton SO, Mellemkjaer L, Olsen JH, et al. Depression and cancer risk: a register-based study of patients hospitalized with affective disorders, Denmark, 1969–1993. Am J Epidemiol. 2002;155:1088–1095. doi: 10.1093/aje/155.12.1088. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Kong J, Diao X, et al. Depression and prostate cancer risk: a Mendelian randomization study. Cancer Med. 2020;9:9160–9167. doi: 10.1002/cam4.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Read JR, Sharpe L, Modini M, et al. Multimorbidity and depression: a systematic review and meta-analysis. J Affect Disord. 2017;221:36–46. doi: 10.1016/j.jad.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Gaspersz R, Lamers F, Beekman ATF, et al. The impact of depressive disorder symptoms and subtypes on 6-year incidence of somatic diseases. Psychother Psychosom. 2018;87:308–310. doi: 10.1159/000491933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2010;123:222–229. doi: 10.1016/j.jad.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.