Abstract
Background
Pulmonary hyalinizing granuloma (PHG) is a very rare pulmonary disease characterized by multiple fibrosclerotic inflammatory lung nodules. The disease is supposedly caused by an unusual immune response.
Case presentation
We present a case involving a 53-year-old female with a history of lumpectomy surgery due to invasive ductal carcinoma who was admitted for slowly progressive pulmonary nodules. The patient's elevated serum IgG4 level and the pathological findings obtained in surgical biopsy indicated IgG4-related lung disease. The nodules continued to enlarge despite administration of corticosteroid therapy, and we performed a second surgical biopsy to obtain a correct diagnosis. The pathological findings obtained in the second biopsy were different and consistent with the features of PHG.
Conclusions
In this report, the radiological follow-up data obtained after lumpectomy surgery demonstrate the very early stage of PHG and the following radiological changes over a decade, and the two surgical biopsies support us to realize the pathological change from previous diagnosed disease before PHG.
Keywords: Pulmonary hyalinizing granuloma, IgG4-related lung disease, Interstitial pneumonia
1. Background
Pulmonary hyalinizing granuloma (PHG) is a rare pulmonary disease characterized by multiple benign nodules. The pathological findings in PHG include hyalinized lamellar collagen bundles around bronchovascular bundles with plasma cells and lymphocytes. The pathological findings are essential for diagnosis and in most cases, surgical biopsies are needed. These pulmonary lesions are supposed to be associated with abnormal immune response, leading to fibrosis [1]; however, their pathophysiology remains unclear. The clinical course corresponding to the radiological findings is not well understood either.
We report a case of PHG that had been previously diagnosed as clinically definite IgG4-related lung disease. The findings in this case report can improve our understanding of the pathological and radiological changes in PHG.
2. Case presentation
A 53-year old never smoking female was referred to our center for clarification of asymptomatic radiological abnormalities in computed tomography (CT) in 2013. She had previously undergone lumpectomy due to invasive ductal carcinoma in 2004. Since then, routine monitoring chest CT had been performed every year, and diffuse centrilobular pulmonary nodules began to appear in 2008. Physical examination and vital signs revealed no specific disease. Laboratory tests did not indicate elevated tumor maker levels or autoimmune antibodies, but the findings showed polyclonal immune globulin production (the serum levels of IgG, IgM, IgA, and IgG4 were, respectively, 5926 mg/dl, 220 mg/dl, 595 mg/dl, and 645 mg/dl). A pulmonary function test also revealed no abnormality. Chest CT showed bilateral diffuse centrilobular pulmonary nodules (Fig. 1). However, these nodules did not accumulate fluorodeoxyglucose in positron emission tomography (PET)-CT and were therefore not compatible with recurrent ductal carcinoma. We administered antibiotics for several weeks, but the shadows in chest CT did not show any response to the antibiotic treatment.
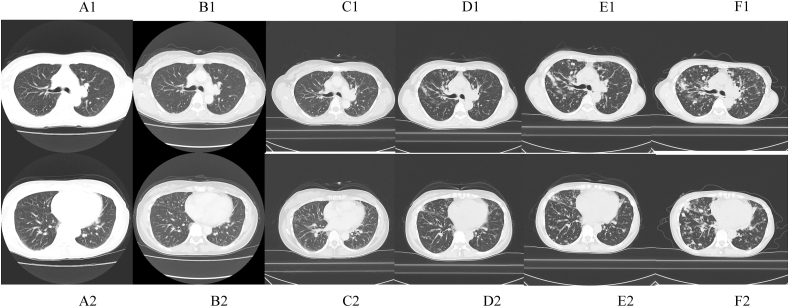
Fig. 1.
Chest computed tomography scans in the upper and lower lobes showing slowly progressive diffuse centrilobular nodules. (A, B, C, D, E and F were obtained in 2008, 2010, 2012, 2014, 2016, and 2018, respectively. 1: Upper area of the lung, 2: Lower area of the lung).
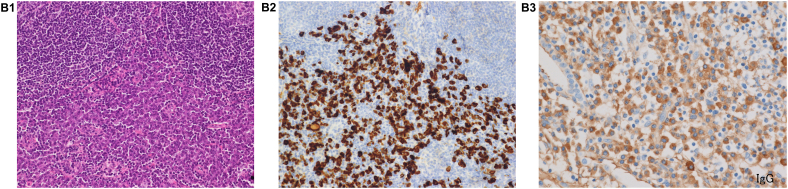
We performed a video-assisted thoracic surgery biopsy (VATS) wedge resection, which showed no evidence of a tumor and excessive lymphoplasmacyte infiltration into the interstitium, peribronchovascular sheath, and subpleura. Pathological assessment of the resected lymph nodules also revealed 200 IgG4-positive plasma cells/high-power field (HPF) and an IgG4/IgG-ratio of 50% (Fig. 2). Pathological findings also showed a lack of monoclonality of cells infiltration. These pathological findings and the elevated serum IgG4 level supported the diagnosis of IgG4-related lung disease. We administered corticosteroid therapy at an initial dose of 30 mg/day to the patient for several months, but the nodules did not respond to the therapy. After corticosteroid therapy, she underwent regular follow-up without any medications and began to complain of dyspnea on exertion in 2016. The pulmonary nodules of chest CT continued to enlarge slowly. In the pulmonary function test, the forced expiratory volume 1.0 had decreased to 1.67 L/s from 2.34 L/s over 3 years, while laboratory tests did not show any new abnormalities. To obtain a correct diagnosis, we performed a second VATS wedge resection, which showed different pathological characteristics from the first assessments; this sample showed hypocellular lamellar collagen bundles around bronchovascular bundles with associated plasma cells and lymphocytes. Furthermore, there was no epithelioid cell granuloma in the second biopsy (Fig. 2). The pathological findings were consistent with the features of pulmonary hyalinizing granuloma (PHG). In a few cases, corticosteroid therapy has been reported to be of benefit to patients with PHG, but the lack of effectiveness of corticosteroids in improving pulmonary nodules had already been proven in this case. The patient has currently not received any treatment, and the pulmonary nodules have been growing in size on chest CT (Fig. 1).
Fig. 2.
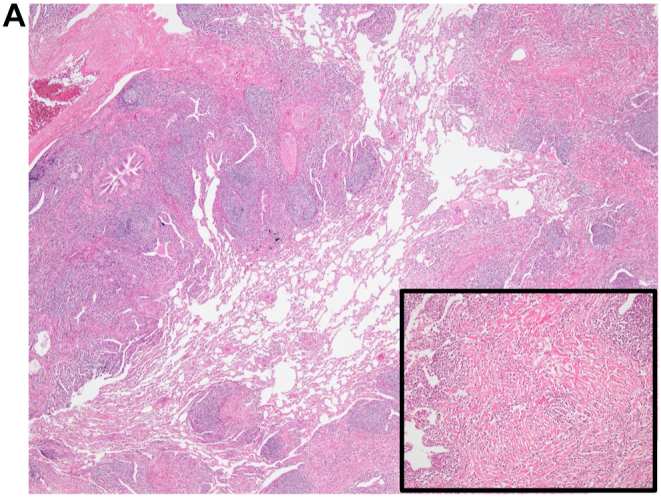
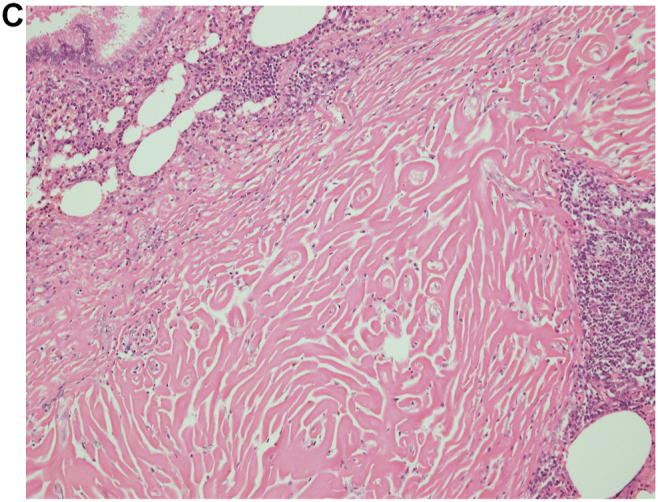
(A), View of the first surgical biopsy in 2013, which shows lymphoplasmacyte infiltration into the interstitium, peribronchovascular sheath, and subpleura (20x). Inset: High-power view showing storiform fibrosis (100x). (B-1), HE staining in the first surgical biopsy (x200). (B-2), IgG4 staining in the first surgical biopsy (x200). (B-3), IgG staining in the first surgical biopsy (x200). An IgG4/IgG-ratio of 50%. (C), View of the second surgical biopsy in 2016, which shows lamellar hyalinized collagen bundles around bronchovascular bundles with an associated dense rim of plasma cells and lymphocytes (100x).
3. Discussion and conclusion
PHG is a rare pulmonary disease that was first described in 1977 [2]. According to one review, 135 cases of PHG were identified before 2015, in which the mean age at diagnosis was 44.6 years, and there was no predominance of sex and races [1]. No apparent symptoms were seen in 27.8% of patients with PHG [1]. PHG may be associated with abnormal immune responses including autoimmune disease, infection, and tumor [1,3]. However, the exact pathophysiology remains unknown.
The radiological findings in PHG are characterized by the presence of solitary or multiple benign pulmonary nodules, which are sometimes misdiagnosed as metastatic lung cancers [[3], [4], [5]]. The size of the nodules is variable, and most nodules were reported to be between 1.5 and 5 cm in size [1]. Accumulation of FDG in PET-CT was shown in 60% of the cases of PHG, which suggests that it was difficult to differentiate PHG from metastatic lung cancers [1]. Pathological examinations, especially for surgical biopsy, are essential for the diagnosis of PHG, which is composed of homogenous, pink, hyalinized lamellar collagen with associated plasma cells and lymphocytes that are located around the bronchovascular bundles [3]. On the basis of pathological findings, PHG should be distinguished from nodular amyloidosis, pulmonary lymphoma, fungal infections, and myofibroblastic tumors [3,6]. The radiological and pathological findings in PHG are speculated to represent an end stage of inflammatory and fibrotic disease, which means that patients with PHG must have background disease, even though it may not be detectable in some cases [1,3,7].
Infection, especially mycobacterial and fungal infection, may induce PHG and various autoimmune diseases such as rheumatoid arthritis; Sjögren syndrome and sarcoidosis have been reported to be associated with this disease [1,8,9]. In our case, however, there was no indication of these diseases, except for IgG4-related lung disease. Lymphoma and amyloidosis were important differential diseases in this case. In the present case, there was no lymphadenopathy in the whole body and sIL-2R was only mildly elevated. The histopathology also showed no findings suggestive of lymphoma.
Unfortunately, no characteristic staining is performed for amyloidosis. However, the morphological features of the pathology were negative for amyloidosis and there were no findings suggestive of amyloidosis in the general physical examination.
IgG4-related disease is a newly recognized systemic disorder characterized by elevated serum IgG4 levels and infiltration of IgG4-positive plasma cells into various organs. It has been reported that the respiratory system could be a target of IgG4-related disease, and Matsui S et al. proposed acceptable diagnostic criteria for it [10,11]. On the basis of these criteria, the pathological findings of the first VATS in 2013 and other clinical manifestations met the criteria for definite diagnosis of IgG4-related lung disease. The radiological findings are divided into 4 categories, of which the bronchovascular type is very similar to the findings in our case [10]. A case report of PHG in a patient with a history of sarcoidosis showed IgG4-positive plasma cells in the pathology of sarcoidosis, but this case did not mention the above-mentioned criteria for IgG4-related lung disease [12]. Without any immune suppressive agents, the nodules worsen in 32.6% of cases with PHG [1]. There is no established treatment for PHG, but some cases have been shown to respond to corticosteroid therapy [1,13], whereas our case did not.
In conclusion, we describe a case of PHG induced by IgG4-related lung disease, which included two meaningful points for insights into PHG. First, the two rounds of surgical biopsy outlined the process of progressive fibrosis triggered by IgG4-related lung disease. Second, the routine chest CT examinations after lumpectomy surgery revealed the findings for the very early stage of PHG and the subsequent radiological changes over a decade. These findings can improve the current understanding of the pathophysiology and long-term natural course of PHG.
Ethics approval and consent to participate
This study is exempt from ethics committee approval because it is a case report at the National Hospital Organization Kyoto Medical Center.
Funding
No funding was received for this case report.
Authors' contributions
KF was responsible for literature review, data collection, and manuscript writing. MO, TI, YY, SS and TM cared for the patient. KM performed the pathological analyses of this case. All authors contributed to the manuscript review before submission and approved the final version of the manuscript.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Consent for publication
Written informed consent was obtained from the patient for publication of the detailed of his medical case.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
We thank Drs. Osamu Kanai and Koichi Nakatani for their assistance of patient care.
Abbreviations
- PHG
pulmonary hyalinizing granuloma
- CT
computed tomography
- PET-CT
positron emission tomography – computed tomography
- VATS
video-assisted thoracic surgery
- HPF
high power field
References
- 1.Lhote R., Haroche J., Duron L., et al. Pulmonary hyalinizing granuloma: a multicenter study of 5 new cases and review of the 135 cases of the literature. Immunol. Res. 2017 Feb;65(1):375–385. doi: 10.1007/s12026-016-8852-4. [DOI] [PubMed] [Google Scholar]
- 2.Engleman P., Liebow A.A., Gmelich J., et al. Pulmonary hyalinizing granuloma. Am. Rev. Respir. Dis. 1977;115:997–1008. doi: 10.1164/arrd.1977.115.6.997. [DOI] [PubMed] [Google Scholar]
- 3.Ussavarungsi K., Khoor A., Jolles H.I., et al. A 40-year-old woman with multiple pulmonary nodules. Pulmonary hyalinizing granuloma. Chest. 2014 Dec;146(6):e198–e203. doi: 10.1378/chest.14-0796. [DOI] [PubMed] [Google Scholar]
- 4.Düzgün N., Kurtipek E., Esme H., et al. Pulmonary hyalinizing granuloma mimicking metastatic lung cancer. Case Rep. Pulmonol. 2015;2015 doi: 10.1155/2015/610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Na K.J., (1), Song S.Y., Kim J.H., et al. Subpleural pulmonary hyalinizing granuloma presenting as a solitary pulmonary nodule. J. Thorac. Oncol. 2007 Aug;2(8):777–779. doi: 10.1097/JTO.0b013e3180ebe9b8. [DOI] [PubMed] [Google Scholar]
- 6.Düzgün N., Kurtipek E., Esme H., et al. Pulmonary hyalinizing granuloma mimicking metastatic lung cancer. Case Rep. Pulmonol. 2015;2015 doi: 10.1155/2015/610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandão V., Marchiori E., Zanetti G., et al. Hyalinizing granuloma: an unusual case of a pulmonary mass. Case Rep. Med. 2010;2010 doi: 10.1155/2010/984765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinckard J.K., Rosenbluth D.B., Patel K., et al. Pulmonary hyalinizing granuloma associated with Aspergillus infection. Int. J. Surg. Pathol. 2003 Jan;11(1):39–42. doi: 10.1177/106689690301100112. [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka K., Imanishi N., Matsuoka T., et al. Pulmonary hyalinizing granuloma detected in a family member after confirmation of tuberculosis in his father. Ann. Thorac. Cardiovasc. Surg. 2014;20(Suppl):632–634. doi: 10.5761/atcs.cr.13-00076. [DOI] [PubMed] [Google Scholar]
- 10.Inoue D., Zen Y., Abo H., et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology. 2009 Apr;251(1):260–270. doi: 10.1148/radiol.2511080965. [DOI] [PubMed] [Google Scholar]
- 11.Matsui S., Yamamoto H., Minamoto S., et al. Proposed diagnostic criteria for IgG4-related respiratory disease. Respir. Invest. 2016 Mar;54(2):130–132. doi: 10.1016/j.resinv.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Chapman E.M., Gown A., Mazziotta R., et al. Pulmonary hyalinizing granuloma with associated elevation in serum and tissue IgG4 occurring in a patient with a history of sarcoidosis. Am. J. Surg. Pathol. 2012 May;36(5):774–778. doi: 10.1097/PAS.0b013e318248713d. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto S., Fujii W., Takahashi T., et al. Pulmonary hyalinizing granuloma with hydronephrosis. Intern. Med. 2002 Jun;41(6):463–466. doi: 10.2169/internalmedicine.41.463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.