Abstract
Background
Salvianolic acid B (Sal B) is a representative component of phenolic acids derived from the dried root and rhizome of Salvia miltiorrhiza Bge. (Labiatae), which promotes angiogenesis in myocardial infarction and diabetic cardiomyopathy. However, whether Sal B has a neuroprotective function in ischemic stroke by promoting angiogenesis is still unclear.
Methods
In the present study, ischemic stroke models were induced in rats by middle cerebral artery occlusion (MCAO), and Sal B (10 or 20 mg/kg/d) was intraperitoneally injected according to a previous study. Neurological deficits were evaluated by the modified Longa five-point scale, modified Bederson scores and cerebral infarction sizes by triphenyltetrazolium chloride (TTC) staining. Apoptotic cells were tested by cleaved-caspase3 immunofluorescence staining and an in situ cell death (TUNEL) detection kit. Human umbilical vein endothelial cells (HUVECs) exposed to hypoxia were used to investigate the effects of Sal B on angiogenesis and tube formation in vitro.
Results
Sal B ameliorated the neurological deficits, decreased the cerebral infarction volumes in rats with ischemic stroke, significantly increased the expression of vascular endothelial growth factor receptor 2 (VEGFR2) and VEGFA and promoted angiogenesis both in vivo and in vitro. Furthermore, Sal B increased stanniocalcin 1 (STC1) expression, induced the phosphorylation of protein kinase B (AKT) and mammalian target of rapamycin (mTOR) activity, enhanced cell migration, and activated VEGFR2/VEGFA signaling in endothelial cells.
Conclusions
This study showed that Sal B promoted angiogenesis and alleviated neurological apoptosis in rats with ischemic stroke by promoting STC1.
Keywords: Ischemic stroke, salvianolic acid B (Sal B), angiogenesis, apoptosis, stanniocalcin 1 (STC1)
Introduction
Stroke is a common condition, leading to serious mortality and long-term disability worldwide. Ischemic stroke accounts for approximately 87% of stroke cases (1). No currently known medications are effective for managing acute ischemic stroke in conjunction with vascular recanalization and supportive care (2). Therefore, identifying a drug that is able to alleviate ischemic cerebral apoplexy is vital. Restoring cerebral blood flow and saving dying neurons is considered to be an effective therapy for ischemic brain injury (3). Emerging evidence suggests that angiogenesis promotes survival after ischemic stroke, reduces rodent cerebral infarction volumes and improves neurological function (4,5).
Salvianolic acid B (Sal B) is one of the most abundant water-soluble compounds extracted from Salvia miltiorrhiza (Danshen), and it is widely used for treating cardiovascular vascular diseases (6). Sal B has been found to possess potent antioxidative capabilities due to its polyphenolic structure (7). Recently, intracellular signaling pathways regulated by Sal B have been investigated both in vitro and in vivo in various cardiovascular experiments (7,8). The cardiovascular protection by salvianolic acids is not only because they act as reactive oxygen species scavengers but also because they inhibit inflammation and metalloproteinase expression in aortic smooth muscle cells. Moreover, salvianolic acid has been reported to enhance angiogenesis in vitro, and our previous study found that Sal B could alleviate cardiac fibrosis and remodeling in diabetic cardiomyopathy via fostering angiogenesis (9,10). Therefore, we speculated that Sal B could protect neurons from ischemia via angiogenesis.
Stanniocalcin-1 (STC-1) was first found to be a glycoprotein hormone in the corpuscles of Stannius bony fish. Increasing evidence indicates that STC-1 may be associated with controlling the angiogenic process (11,12). Although previous studies have suggested that STC-1 is highly expressed and associated with the regulation of VEGF, modulating angiogenesis, the impact of STC-1 on neurological injury remains unclear. Herein, we investigated whether Sal B enhances vascular density in ischemic stroke and decreases neurological injury and whether these effects are dependent on STC-1 expression. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4779/rc).
Methods
Animal model and experimental protocol
Male Sprague–Dawley rats (200–230 g) were purchased from Vital River Laboratory Animal Technology Co. (Beijing), housed at a constant temperature (24 ℃), and given a standard diet. Adult animals were subjected to middle cerebral artery occlusion (MCAO) by using the intraluminal suture model. First, after anesthetization with 1% pentobarbital sodium, the right common carotid artery, external carotid artery and internal carotid artery were isolated. A monofilament nylon suture with a silicone-coated tip (L3200, Jialing Biotech, Guangzhou, China) was inserted into the external carotid artery lumen and advanced into the internal carotid artery for approximately 2 cm to block the origin of the middle cerebral artery. Occlusion was performed for 2 h, and then the nylon suture was withdrawn for reperfusion. Sham surgery rats underwent the same procedure but without the suture inserted. Rats were randomly divided into four groups for treatment: sham surgery (sham); MCAO; MCAO with Sal B 10 mg/kg/d (Sal B-L); and MCAO with Sal B 20 mg/kg/d (Sal B-H) according to our previous study (13,14). Sal B was administered every day by intraperitoneal injection for 3 weeks. The sham and MCAO groups received equal volume of saline.
Experiments were performed under a project license (No. DWLL-2021-140) granted by committee board of Qilu hospital, in compliance the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and Shandong University.
Neurological functional assessments
Neurological function was evaluated at 2 and 3 weeks after MCAO surgery in a blinded manner by using the modified Longa five-point scale and modified Bederson score as previously described (15,16). The modified Longa five-point scale was as follows: 0: no apparent neurological deficits; 1: left forelimb flexion; 2: spontaneous left circling; 3: falls to the left; and 4: no spontaneous movement or loss of consciousness. Rats with scores of 1–3 points were used in this study. The modified Bederson scores were as follows: 0, no deficit; 1, lost forelimb flexion; 2, as for 1, but plus decreased resistance to lateral push; 3, unidirectional circling; 4, displayed longitudinal spinning or seizure activity; and 5, showed no movement.
Measurement of brain infarct volumes
3 weeks after surgery, rats were sacrificed under deep anesthesia. After transcardial perfusion with ice-cold phosphate buffer saline (PBS), brains were sliced into 2.0 mm sections and immersed in 2% TTC (2,3,5-triphenyltetrazolium chloride, Sigma-Aldrich, St. Louis, MO, USA) for 30 min. Normal brain tissue appeared red, and infarcted tissue appeared pale gray. Images were taken of the slices, and infarct volumes were calculated by using Image-Pro Plus 6.0 (Media Cybernetics) by a blinded investigator.
Primary cortical neuronal cultures
Primary neuron-enriched cell cultures of cerebral cortex prepared from newborn rats were isolated in a sterile fashion, cut into blocks (1 mm3), and digested in phosphate-buffered saline (PBS) containing 0.125% (w/v) trypsin (Sigma) at 37 ℃ for 15 min. The reaction was terminated by DMEM-HG culture (Dulbecco’s Modified Eagle Medium-High Glucose culture) medium containing 10% (v/v) fetal bovine serum (FBS). A single-cell suspension was prepared and filtered with a 200-mesh screen. Cells were seeded onto 0.1 mg/mL polylysine (Sigma)-pretreated 6-well culture plates at 1×106 cells/L and incubated in 5% CO2 at 37 ℃. DMEM-HG culture medium was replaced by neurobasal medium (Life Technologies, Carlsbad, CA, USA) buffered with 4.8 mM HEPES (Sigma-Aldrich) and supplemented 2 h later with 2% (v/v) B-27 supplement (Life Technologies), 1.2 mM L-glutamine (Sigma-Aldrich), and 25 mg/L gentamicin (Life Technologies). Cytarabine (10 µg/mL; Sigma) was added on the third day for 24 h to inhibit glial cell proliferation. Half of the culture medium was replaced every 3 days. The cultures were maintained for 7 to 9 days before the experiments.
Hematoxylin and eosin (H&E) and Nissl staining
Rats were anesthetized with anesthesia and transcardially perfused with 30 mL PBS followed by 50 mL 4% paraformaldehyde. Cerebral samples were fixed in 4% paraformaldehyde for 24 h and then 5 mm paraffin sections were made for subsequent analysis.
Immunofluorescence staining
Cerebral samples were postfixed with 4% paraformaldehyde for 24 h and then embedded in optimal cutting temperature compound after cryoprotection in 20% and 30% sucrose in 0.1 M PBS (pH 7.2) overnight. Tissue sections were first incubated with the antibodies anti-MAP-2, anti-GFAP, or anti-cleaved-caspase 3 (1:200; Abcam, Cambridge, MA, USA) overnight at 4 ℃, and then incubated with antibodies goat anti-rabbit Alexa Fluor 594 (1:200) and goat anti-mouse Alexa Fluor 488 (1:200) 30 minutes at room temperature. Nuclei were counterstained with 40-6-diamidino-2-phenylindole (DAPI; Invitrogen). Data were analyzed with ImageProPlus 6.0 (Media Cybernetics).
TUNEL staining
Apoptosis of rat cerebral tissue was detected using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Millipore, MA, USA) according to the manufacturer’s protocol. DAPI was used to stain the nuclei. Positive cells were counted from five randomly selected fields in nonconsecutive sections by using confocal microscopy.
Aortic ring angiogenesis assay
Diameter rings (1 mm) were sliced from the aortae of male Sprague–Dawley rats (200–230 g), placed in a 96-well plate coated with Matrigel Basement Membrane Matrix (BD Biosciences, Billerica, MA, USA), and cultured with serum from rats of the four groups. Serum was replaced every 2 days. On Day 10 of culture, explants were visualized under an Olympus SZX16 Research stereo-zoom microscope. The number of sprouts was counted manually. Three independent experiments were used for each treatment.
Western blot analysis
Equal amounts of protein were separated by 10% SDS–PAGE and then transferred to polyvinylidene fluoride membranes. After incubation with the primary antibodies and secondary antibody, the blots were analyzed using enhanced chemiluminescence detection system. The primary antibodies used were anti-STC1 (1:1,000, Santa Cruz, Shanghai, China), anti-VEGFA (1:1,000; Abcam, Shanghai, China), anti-VEGFR2, anti-AKT, anti-phosphorylated (p)-AKT, anti-mTOR, and anti-p-mTOR (1:1,000; Cell Signaling Technology, Shanghai, China).
Cell culture
Human umbilical vein endothelial cells (HUVECs) (ATCC, Manassas, VA, USA) were cultured in endothelial-cell medium supplemented with 5% FBS and 1% endothelial-cell growth supplement. Cells were cultured in a humidified normal air, 5% CO2 incubator or a humidified 5% CO2, 1% O2 hypoxic incubator at 37 ℃. The 'medium was changed every 48 h. si-STC1(5'- GACACAGUCAGCACAAUCATT-3'; 5'- UGAUUGUGCUGACUGUGUCTT-3') and si-NC duplexes (GeneChem, Shanghai, China) were transfected into HUVECs by using Lipofectamine 3000. Then, the cells were exposed to Sal B (50 µg/mL) for 24 h under hypoxic conditions (1% O2, 5% CO2).
Matrigel HUVEC tube formation assay
HUVECs were cultured on a 24 well flat-bottom plates which were coated with 70 µL/well cold Matrigel (BD Biosciences, Billerica, MA, USA) and incubated for 30 min at 37 ℃ to allow gelation. Results was evaluated via calculating the amount of branch points using ImageJ.
Cell migration assay
Cell motility was examined by scratch assays. Cells were seeded in 6-well plates and transfected with STC1 siRNA until the cells reached 100% confluence. An artificial gap was generated by scratching the cells with a 200 µm pipette tip. Images were taken immediately and after Sal B administration under hypoxia for 24 h.
Cell migration was examined in Transwell chambers. The upper chambers were loaded with HUVECs, and 500 µL endothelial-cell medium containing 5% FBS with or without 50 µg/mL Sal B was loaded into the lower chamber for 24 h at 37 ℃ in a normoxic or hypoxic incubator. Cells on the upper surface were removed with a cotton swab, and then the migrated cells were fixed with 4% formaldehyde and stained with 0.1% crystal violet.
Statistical analysis
Data collected from no less than 3 independent experiments are shown as the mean ± standard error of the mean (SD). Statistical analysis involved one-way ANOVA (Analysis of Variance) followed by Tukey’s test with SPSS v16.0 (SPSS, Chicago, IL, USA). Statistical significance was set at P<0.05.
Results
Sal B attenuates ischemic brain injury in MCAO rats
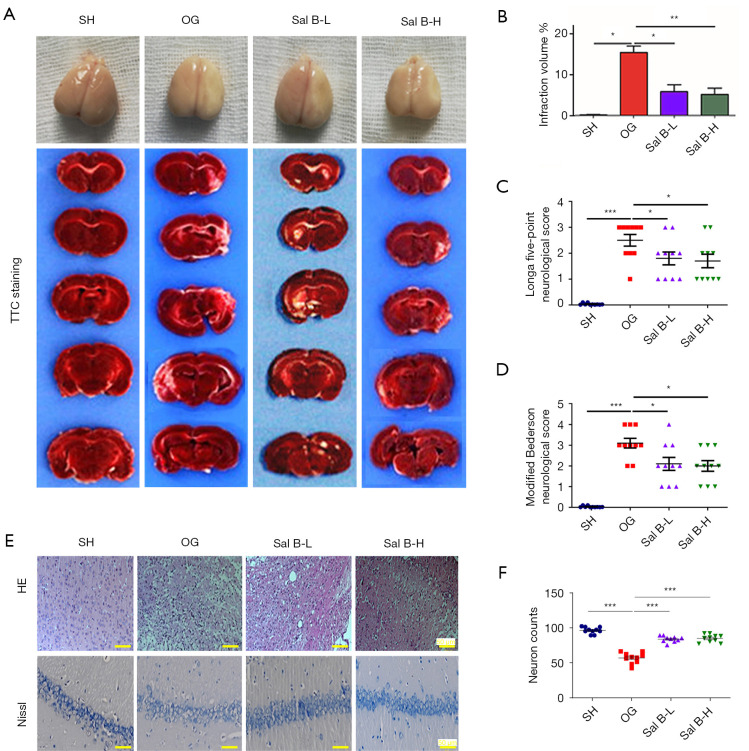
To ascertain the role of Sal B in ischemic stroke, we detected infarct volumes and neurological deficit scores in rats 3 weeks after surgery. TTC staining showed significantly extensive infarct volumes in the MCAO group compared with the sham group, while Sal B at low and high doses significantly reduced the area of infarction (Figure 1A,1B). Moreover, using the Longa five-point neurological and the modified Bederson neurological score system, we found that Sal B administration could significantly improve behavioral motor deficits compared with the MCAO group (Figure 1C,1D). H&E staining in the cerebral cortex region and Nissl staining in the hippocampus region were performed to observe the neuroprotective function of Sal B. Compared with normal nerve cells in sham rats, nerve cells in the infarct area were extensively damaged in MCAO rats, and the surviving neuron number was decreased significantly. However, Sal B administration alleviated the damage (Figure 1E,1F).
Figure 1.
Sal B attenuates ischemic brain injury in MCAO rats. (A) Representative images of serial brain sections showing ischemic infarct in white as determined by TTC staining in the four rat groups: SH; OG; Sal B-L (10 mg/kg/d); Sal B-H (20 mg/kg/d). (B) Quantitative analysis of the infarction volume. (C) Quantitative analysis of neurological outcomes of MCAO rats as measured with a 5-point NSS scale. (D) Quantitative analysis of neurological outcomes of MCAO rats as measured with modified Bederson scores. (E) Representative images of the cerebral cortex region with H&E staining (top row). Representative images of the hippocampal region with Nissl staining (bottom row) (n=6 per group, scale bar: 50 µm). (F) Quantification of neuron counts were shown using mean ± SD. *, P<0.05, **, P<0.01, ***, P<0.001. Sal B, salvianolic acid B; MCAO, middle cerebral artery occlusion; TTC, triphenyltetrazolium chloride; SH, sham operation rats intraperitoneally injected with saline; OG, rats with MCAO intraperitoneally injected with saline; Sal B-L, MCAO rats intraperitoneally injected with a low (L) dose of Sal B; Sal B-H, MCAO rats intraperitoneally injected with a high (H) dose of Sal B; NSS, neurological severity score.
Sal B alleviates neuronal apoptosis in MCAO rats
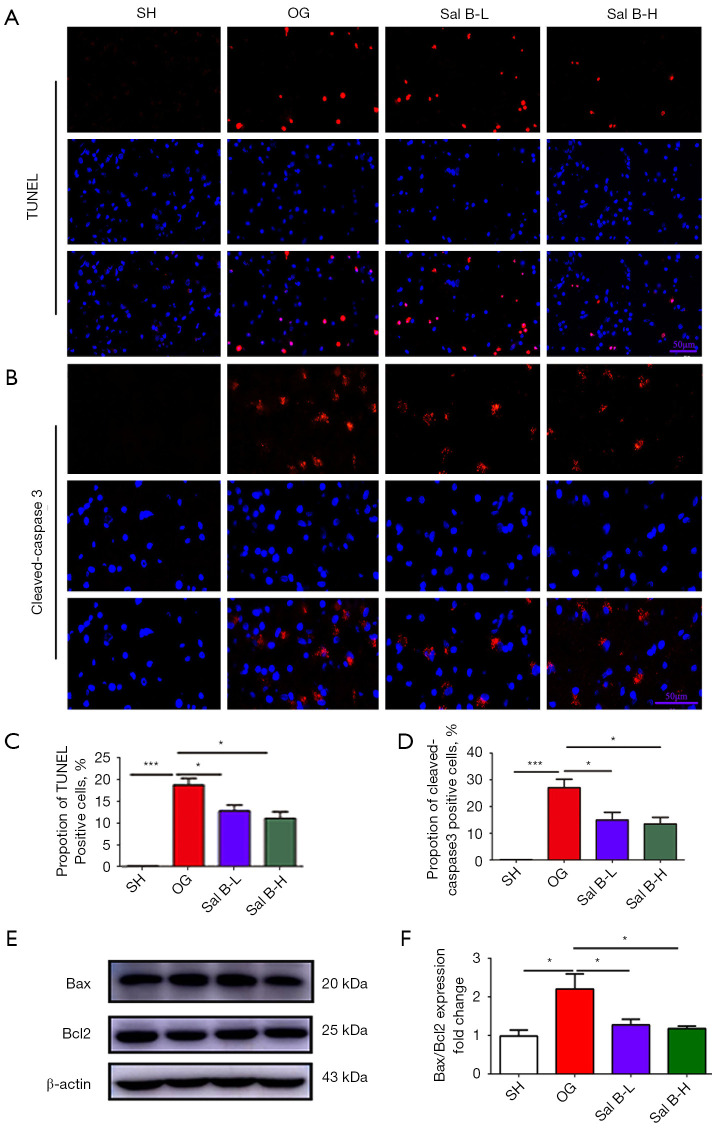
To assess the role of Sal B in neuronal apoptosis, we quantified neuronal cell death by cleaved-caspase3 immunohistochemistry and TUNEL staining. Apoptotic neurons were increased in number in the cerebral cortex region of MCAO rats compared with sham rats, and Sal B treatment markedly reduced the number of apoptotic neurons (Figure 2A-2D). Additionally, western blot analysis (Figure 2E,2F) further confirmed an increase in the apoptosis proteins Bax and Bcl2 in MACO rats, which was attenuated by Sal B administration.
Figure 2.
Sal B alleviates neuronal apoptosis in MCAO rats. (A) Representative images of TUNEL staining (red) in the cerebral cortex region. Scale bars =50 µm. (B) Representative images of immunofluorescence staining for cleaved-caspase3 (red) expression in the cerebral cortex region. Scale bars =50 µm. (C,D) Quantitative analysis of the number of TUNEL- and cleaved-caspase3-positive neurons. (E,F) Western blot analysis of Bax and Bcl2 expression and the ratio of Bax to Bcl2 in rats. (n=6 per group). Data are mean ± SD. *, P<0.05, ***, P<0.001. Sal B, salvianolic acid B; MCAO, middle cerebral artery occlusion; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling; SH, sham operation rats intraperitoneally injected with saline; OG, rats with middle cerebral artery occlusion intraperitoneally injected with saline; Sal B-L, MCAO rats intraperitoneally injected with a low (L) dose of Sal B; Sal B-H, MCAO rats intraperitoneally injected with a high (H) dose of Sal B.
Sal B alleviates primary cortical neuronal apoptosis
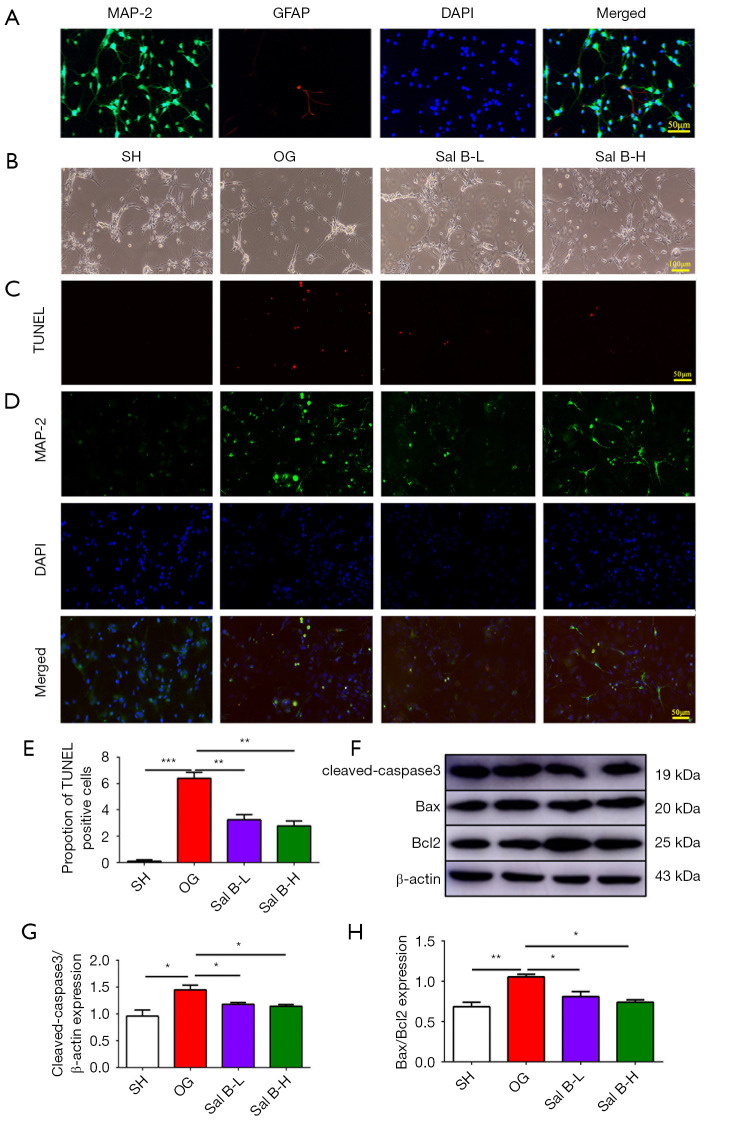
To investigate the protective effect of Sal B on neurons specifically, not glial cells, which are both major cellular components of brain tissue, we subjected primary cortical neurons to serum of rats from the four groups. Primary cortical neurons were identified by MAP-2 immunofluorescence staining, distinguished from glial cells identified by GFAP (Figure 3A). Then, we assessed primary neuronal cell apoptosis by co-staining for the neuronal marker MAP-2 and TUNEL. Consistent with our expected results, neurons exposed to hypoxia and serum from MCAO rats for 24 h showed higher levels of apoptosis compared with neurons from rats exposed to normoxia and serum from sham rats. Treatment with Sal B at low and high doses reduced the number of apoptotic neurons (Figure 3B-3D). We examined the activity of cleaved caspase-3, Bax and Bcl2 by western blot analysis. Similar to the results in vivo, in vitro, increased neuronal apoptosis under hypoxia and serum from MCAO rats could be attenuated by Sal B administration (Figure 3E-3H).
Figure 3.
Serum containing Sal B alleviates primary cortical neuronal apoptosis. (A) Double immunofluorescence staining with antibodies against MAP-2 (green) and GFAP (red). Scale bars =50 µm. (B) Morphological changes in primary cortical neurons after rat serum treatment. Scale bars =100 µm. (C,D) Immunofluorescence staining with TUNEL staining (red, C) and antibodies against MAP-2 (green, D). Scale bars =50 µm. (E) Quantitative analysis of the number of TUNEL-positive neurons. (F) Western blot analysis of cleaved caspase-3, Bax and Bcl2 expression. Quantification of cleaved-caspase3 protein expression (G) and the ratio of Bax to Bcl2 (H) in rats. (n=6 per group). Data are mean ± SD. *, P<0.05, **, P<0.01, ***, P<0.001. Sal B, salvianolic acid B; GFAP, glial fibrillary acidic protein; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling; SH, sham operation rats intraperitoneally injected with saline; OG, rats with middle cerebral artery occlusion intraperitoneally injected with saline; Sal B-L, MCAO rats intraperitoneally injected with a low (L) dose of Sal B; Sal B-H, MCAO rats intraperitoneally injected with a high (H) dose of Sal B.
Sal B promotes angiogenesis in vivo and in vitro
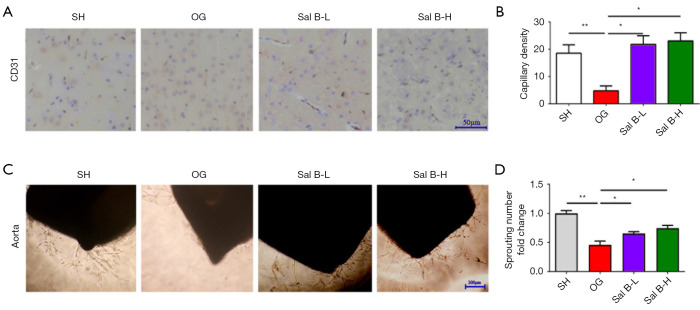
Neurogenesis is linked to angiogenesis in the ischemic brain. New blood vessels in the ischemic brain provide scaffolds to draw neuroblasts to the ischemic boundary region. Additionally, activated cerebral endothelial cells in angiogenic vessels secrete cytokines to attract neuroblasts to the region. The blockage of stroke-induced angiogenesis reduces neurogenesis (4,5). To evaluate the effect of Sal B on microcirculation in MCAO rats, we evaluated capillary density in vivo by CD31 immunohistochemistry. Ischemic stroke in rats substantially diminished vessel density at 3 weeks after surgery; however, Sal B administration increased the vessel density (Figure 4A,4B). Meanwhile, an aortic ring angiogenesis assay was performed to further assess the pro-angiogenesis effect of Sal B. The number of sprouts of aortic rings cultured with serum from MCAO rats was significantly inhibited under hypoxia, while serum from Sal B increased sprouts of aortic rings under hypoxia (Figure 4C,4D).
Figure 4.
Sal B increases angiogenesis. (A) Immunochemical staining with antibodies against CD31 in cerebral tissue sections 3 weeks after surgery from all rat groups. Scale bars =50 µm. (B) Evaluation of capillary density. (C) Vessel outgrowth in the aortic ring assay. Scale bars =200 µm. (D) Quantification of vessel growth from aortic rings. Data are mean ± SD. *, P<0.05, **, P<0.01. Sal B, salvianolic acid B; SH, sham operation rats intraperitoneally injected with saline; OG, rats with MCAO intraperitoneally injected with saline. Sal B-L, MCAO rats intraperitoneally injected with a low (L) dose of Sal B; Sal B-H, MCAO rats intraperitoneally injected with a high (H) dose of Sal B.
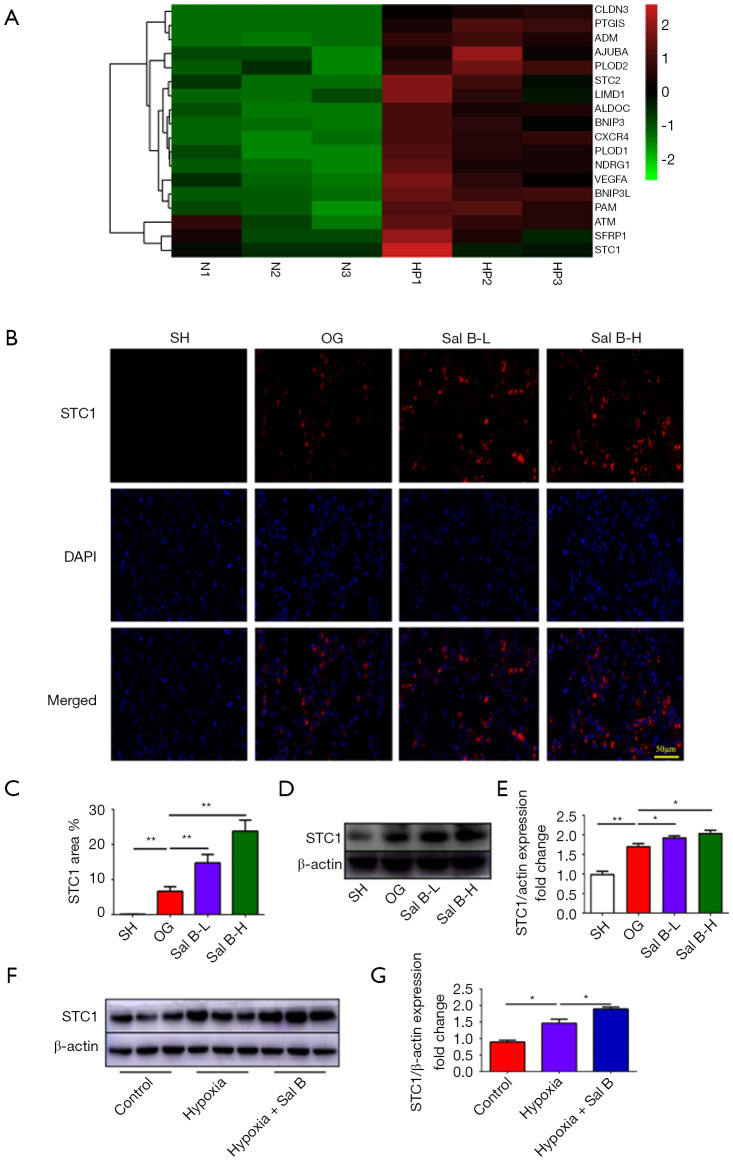
Sal B promotes angiogenesis in ischemic stroke rats by upregulating STC1 activity
The heatmap shows that STC1 was identified as one of several hypoxia-responsive genes related to hypoxia-driven angiogenesis (Figure 5A). Several reports found that STC1 could increase vascular density and is associated with angiogenesis (12,17-19). To investigate whether Sal B promotes angiogenesis by activating STC1, we evaluated the expression of STC1 by immunohistochemical staining and western blot analysis in vivo and in vitro. Immunohistochemical staining showed increased STC1 expression in MCAO rats compared with sham rats, and Sal B treatment further increased STC1 expression (Figure 5B,5C). Western blot analysis (Figure 5D,5E) confirmed an increase in STC1 expression in MCAO rats that was further increased by low and high doses of Sal B. Similarly, Sal B administration increased STC1 levels of HUVECs compared with the group exposed to normoxia and hypoxia (Figure 5F,5G). The aboce results indicated that Sal B administration enhances angiogenesis in ischemic stroke via targeting STC1.
Figure 5.
Sal B increases cerebral STC1 activity. (A) Heatmap showing the differentially expressed genes in the hypoxia-treated group compared with the control group. (B) Representative STC1 immunohistochemistry staining of cerebral tissue sections. Scale bars =50 µm. (C) Assessment of the STC1 area in all rat groups. Western blot analysis of STC1 expression (D,E), in vivo and (F,G), in HUVECs. (n=6 per group). Data are mean ± SD. *, P<0.05, **, P<0.01. Sal B, salvianolic acid B; STC1, Sal B increased stanniocalcin 1; HUVEC, human umbilical vein endothelial cell; SH, sham operation rats intraperitoneally injected with saline; OG, rats with middle cerebral artery occlusion intraperitoneally injected with saline; Sal B-L, MCAO rats intraperitoneally injected with a low (L) dose of Sal B; Sal B-H, MCAO rats intraperitoneally injected with a high (H) dose of Sal B.
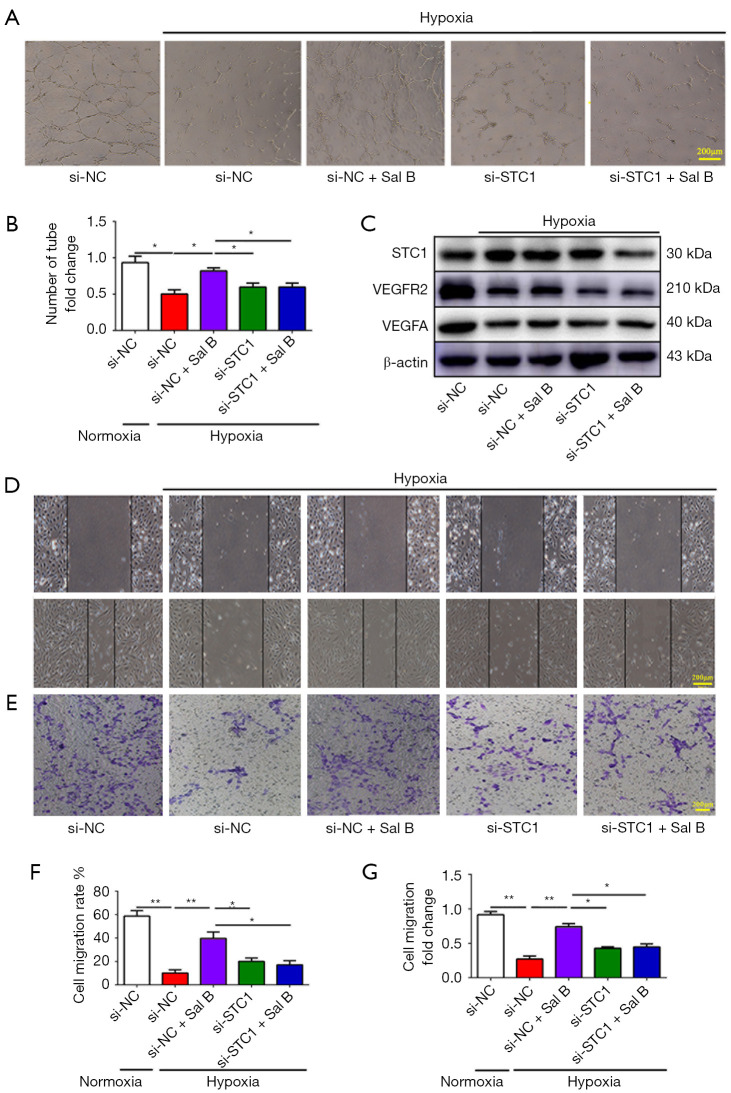
To investigate how STC1 regulates angiogenesis, we investigated the role of STC1 in vessel formation. In the Matrigel tube-formation assay, hypoxia significantly blocked tube formation by HUVECs, whereas supplementing the media with Sal B significantly increased tube formation and branching, and siRNA-mediated inhibition of STC1 remarkably enhanced the ability of Sal B to constitute capillary-like structures (Figure 6A,6B). Sal B upregulated VEGFA and VEGFR2, but siRNA knockdown of STC1 expression reduced VEGFA and VEGFR2 expression (Figure 6C). To reduce STC1 expression, STC1 siRNA was transfected into HUVECs. Sal B contributed to HUVEC migration, which was counteracted by STC1 siRNA knockdown (Figure 6D-6G). Similar results were obtained by Transwell chamber assays, which indicated that knockdown of STC1 expression could inhibit the invasion of HUVECs. These results suggested that blocking STC1 potentially attenuated angiogenesis under hypoxia.
Figure 6.
Sal B promotes angiogenesis by upregulating STC1 expression. (A) Representative HUVEC tube-like structures in all treatment groups: si-NC, HUVECs under normoxia; si-NC+Hypoxia, HUVECs under hypoxia; si-NC+Hypoxia+Sal B, HUVECs treated with Sal B (50 µg/mL) under hypoxia; si-STC1+Hypoxia, HUVECs treated with siRNA to knockdown STC1 under hypoxia, si-STC1 + Hypoxia + Sal B, HUVECs treated with siRNA to knockdown STC1 and Sal B (50 µg/mL) under hypoxia. Scale bars =200 µm. (B) Quantification of tube-like structures. (C) Western blot analysis of STC1, VEGFR2, and VEGFA expression. (D,E) Representative micrograph of the scratch assay and Transwell chamber assay. (F,G) Evaluation of cell migration on the basis of the scratch assay and Transwell chamber assay results (n=3). Data are mean ± SD. *, P<0.05, **, P<0.01. Sal B, salvianolic acid B; STC1, Sal B increased stanniocalcin 1; HUVEC, human umbilical vein endothelial cell.
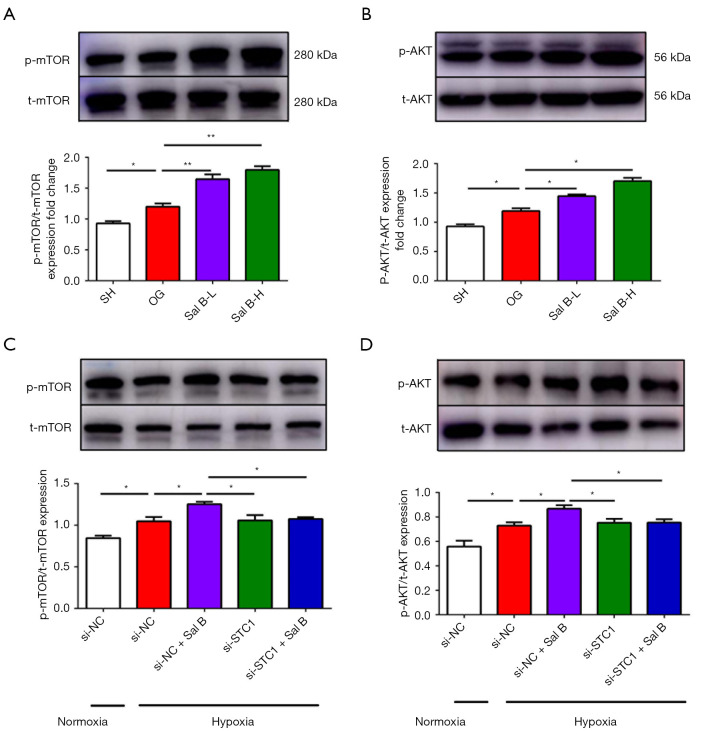
Sal B induces AKT/mTOR phosphorylation by upregulating STC1
The AKT/mTOR signaling pathway is involved in cell migration and angiogenesis. In particular, accumulating evidence has shown that the AKT/mTOR signaling pathway plays an important role in stroke-induced angiogenesis (5,20). Thus, we evaluated the phosphorylation of AKT and mTOR in HUVECs and the infarct area of rats. Low and high doses of Sal B evidently enhanced the phosphorylation of mTOR and AKT in rats compared with sham and MCAO rats (Figure 7A,7B). Similarly, Sal B treatment increased p-mTOR levels and phosphorylated (p)-AKT in HUVECs (Figure 7C,7D) and STC1 inhibition reversed the upregulating effects of Sal B on p-AKT and p-mTOR levels.
Figure 7.
Sal B increases mTOR and protein kinase B (AKT) signaling activation by upregulating STC1 expression. Western blot analysis of (A) phosphorylated (p)-mTOR and total (t)-mTOR expression; (B) p-AKT and t-AKT expression in rats; (C) p-mTOR and t-mTOR expression; and (D) p-AKT and t-AKT expression in HUVECs. (n=3). Data are mean ± SD. *, P<0.05, **, P<0.01. mTOR, mammalian target of rapamycin; Sal B, salvianolic acid B; STC1, Sal B increased stanniocalcin 1; HUVEC, human umbilical vein endothelial cell; SH, sham operation rats intraperitoneally injected with saline; OG, rats with middle cerebral artery occlusion intraperitoneally injected with saline; Sal B-L, MCAO rats intraperitoneally injected with a low (L) dose of Sal B; Sal B-H, MCAO rats intraperitoneally injected with a high (H) dose of Sal B.
Discussion
In this study, we investigated the effect of Sal B on ischemic stroke in rats. Our results suggested that Sal B could attenuate the neurological injury of MCAO rats by alleviating infarct volume and neuronal apoptosis. Notably, we revealed the detailed mechanism in which Sal B-promoted cerebral angiogenesis by enhancing STC1 activity.
Hypoxia and ischemia induced by MCAO surgery leads to ischemic cell death in neurons and organ dysfunction. Fortunately, neuronal damage could be limited and recovered through the activation of angiogenesis and vasculogenesis (21,22). Therefore, discovering a drug that can alleviate nerve injury will have a significant impact on clinical practice. Sal B is the most abundant and bioactive constituent of S. miltiorrhizae, the dried root of S. miltiorrhiza Bge., a popular traditional Chinese herbal medicine used to treat various diseases (10,20,23,24). A previous study showed that Sal B reduces the infarct size and improves the neurobehavioral function against I/R-induced brain injury in mice by increasing the viability of microglia and astrocytes, inhibiting inflammation and apoptosis and enhancing blood-brain barrier integrity. Furthermore, Sal B promotes angiogenesis and improves microcirculation in the ischemic myocardium and cerebrum (10,25,26). The current study found that Sal B strengthens angiogenesis and attenuate neurological injury in rats with ischemic stroke by enhancing STC1. Furthermore, we found that Sal B attenuated ischemic stroke-associated neurological injury via increasing the expression of STC1 and promoting angiogenesis by stimulating the VEGFR2 and VEGFA expression in vivo and in vitro.
STC1 is a secreted multifunctional protein. It acts as a hormone regulating calcium and phosphate homoeostasis that was originally discovered in bony fish and later found in humans (11,27). STC1 is expressed in a wide variety of tissues, such as the intestine, heart, lung, kidney, and cerebral neurons (12). Recently, increasing evidence has shown that STC1 plays a cellular protective function in several fundamental biological processes, such as angiogenesis, inflammation, carcinogenesis and wound repair (12). STC1 is expressed in a wide variety of tissues but is not detected in the circulation under normal circumstances. STC1 is upregulated in in vitro models of angiogenesis and at sites of pathological angiogenesis (27). Furthermore, STC1 overexpression can increase the vascular density (28). Regulation of STC1 may be a key feature of the angiogenic response since it is one of several hypoxia-responsive genes coupled to hypoxia-driven angiogenesis (29). It has been reported that STC-1 also has antioxidant effects, which may play a role by inducing mitochondrial uncoupling protein (UCP), which in turn may reduce the formation of ROS (30). The expression of STC-1 is upregulated in the ischemic penumbra of human and rat cerebral infarction (31), which is important for poststroke function. STC-1 hormone has a neuroprotective effect on cerebral ischemia–reperfusion injury. These effects are achieved by reducing brain edema and inhibiting the permeability of the blood-brain barrier. In the hippocampus of rats, STC-1 also attenuates cerebral ischemia–reperfusion injury by preventing oxidative damage to lipids and proteins (32).
To elucidate the mechanism by which Sal B alleviates neurological injury via enhancing angiogenesis, we explored the function of Sal B in HUVECs under hypoxic conditions, the main pathological factor of ischemic stroke. Inhibition of STC1 by siRNA evidently abolished the Sal B-upregulated VEGFR2 and VEGFA and decreased HUVEC tube formation. Furthermore, Sal B enhanced HUVEC migration, and siRNA knockdown of STC1 reversed this trend but could not inhibit the proliferation of HUVECs, which is consistent with a previous study. In conclusion, our results support the role of Sal B in improving cerebral angiogenesis by targeting STC1 activity.
Angiogenesis plays a vital role in the regeneration progress of post-ischemic tissue. Angiogenic vessels provide neurotrophic support to neurons, improve cerebral microcirculation perfusion and reduce infarct volumes (25,26). Additionally, neuroblasts have been found to be concentrated around blood vessels after stroke (24). Hence, restoring local blood perfusion in ischemic brain tissue plays a key role in tissue repair and functional recovery, especially in the later stages of stroke (5). However, previous studies reported that post-ischemic angiogenesis follows a temporal course (33). Endothelial cells begin mitosis as early as 1 day after mouse cerebral reperfusion after 30 min of MCAO, and the number of vessels begins to significantly increase on the third day before peaking at 7 days after stroke. The level of VEGF is also increased but decreases to near the pre-ischemic level or even lower at 7 to 10 days after stroke (10). Our study showed that Sal B increases VEGF as well as VEGFR2 expression thus promoting angiogenesis via inhibiting STC1 and ameliorating microcirculatory disruption in ischemic stroke after MCAO.
In summary, Sal B may protect against neuronal apoptosis and attenuate neurological injury in ischemic rats by enhancing angiogenesis, which may be involved in the upregulation of VEGF and VEGFR2 by targeting STC1. These findings provide a novel therapeutic approach for ischemic stroke by taking advantage of Sal B.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (#91839301 and #81970251), the National Key R & D Program of China (#2017YFC0908700, 2017YFC0908703), the Taishan Scholar Project of Shandong Province of China (No. ts20190972), the Key Program for Technologies R&D of Shandong Province (No. 2020SFXGFY06), and Key Issues of Jinan Science and Technology Bureau (#20170409).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. DWLL-2021-140) granted by committee board of Qilu hospital, in compliance the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and Shandong University.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4779/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4779/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4779/coif). The authors have no conflicts of interest to declare.
References
- 1.Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics 2011;8:319-29. 10.1007/s13311-011-0053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krupinski J, Kaluza J, Kumar P, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994;25:1794-8. 10.1161/01.STR.25.9.1794 [DOI] [PubMed] [Google Scholar]
- 3.Zhang P, Yu H, Zhou N, et al. Early exercise improves cerebral blood flow through increased angiogenesis in experimental stroke rat model. J Neuroeng Rehabil 2013;10:43. 10.1186/1743-0003-10-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui X, Chopp M, Zacharek A, et al. Combination treatment of stroke with sub-therapeutic doses of Simvastatin and human umbilical cord blood cells enhances vascular remodeling and improves functional outcome. Neuroscience 2012;227:223-31. 10.1016/j.neuroscience.2012.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Tang G, Zhang Z, et al. Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci Lett 2014;579:46-51. 10.1016/j.neulet.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2005;45:1345-59. 10.1177/0091270005282630 [DOI] [PubMed] [Google Scholar]
- 7.Ho JH, Hong CY. Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. J Biomed Sci 2011;18:30. 10.1186/1423-0127-18-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu CD, Liu NN, Zhang S, et al. Salvianolic acid A prevented cerebrovascular endothelial injury caused by acute ischemic stroke through inhibiting the Src signaling pathway. Acta Pharmacol Sin 2021;42:370-81. 10.1038/s41401-020-00568-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Zhao X, Mao S, et al. Management of SAH with traditional Chinese medicine in China. Neurol Res 2006;28:436-44. 10.1179/016164106X115044 [DOI] [PubMed] [Google Scholar]
- 10.Yang MC, You FL, Wang Z, et al. Salvianolic acid B improves the disruption of high glucose-mediated brain microvascular endothelial cells via the ROS/HIF-1α/VEGF and miR-200b/VEGF signaling pathways. Neurosci Lett 2016;630:233-40. 10.1016/j.neulet.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 11.Chang AC, Jeffrey KJ, Tokutake Y, et al. Human stanniocalcin (STC): genomic structure, chromosomal localization, and the presence of CAG trinucleotide repeats. Genomics 1998;47:393-8. 10.1006/geno.1997.5120 [DOI] [PubMed] [Google Scholar]
- 12.Holmes DI, Zachary IC. Vascular endothelial growth factor regulates stanniocalcin-1 expression via neuropilin-1-dependent regulation of KDR and synergism with fibroblast growth factor-2. Cell Signal 2008;20:569-79. 10.1016/j.cellsig.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 13.Li CL, Liu B, Wang ZY, et al. Salvianolic acid B improves myocardial function in diabetic cardiomyopathy by suppressing IGFBP3. J Mol Cell Cardiol 2020;139:98-112. 10.1016/j.yjmcc.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 14.Jiang P, Guo Y, Dang R, et al. Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: involvement of autophagy and NLRP3 inflammasome. J Neuroinflammation 2017;14:239. 10.1186/s12974-017-1013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 1986;17:472-6. 10.1161/01.STR.17.3.472 [DOI] [PubMed] [Google Scholar]
- 16.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84-91. 10.1161/01.STR.20.1.84 [DOI] [PubMed] [Google Scholar]
- 17.Teng H, Zhang ZG, Wang L, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab 2008;28:764-71. 10.1038/sj.jcbfm.9600573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung HY, Lai KP, Chan HY, et al. Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 2005;146:4951-60. 10.1210/en.2005-0365 [DOI] [PubMed] [Google Scholar]
- 19.Varghese R, Gagliardi AD, Bialek PE, et al. Overexpression of human stanniocalcin affects growth and reproduction in transgenic mice. Endocrinology 2002;143:868-76. 10.1210/endo.143.3.8671 [DOI] [PubMed] [Google Scholar]
- 20.Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006;26:13007-16. 10.1523/JNEUROSCI.4323-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanazawa M, Takahashi T, Ishikawa M, et al. Angiogenesis in the ischemic core: A potential treatment target? J Cereb Blood Flow Metab 2019;39:753-69. 10.1177/0271678X19834158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Guan F, Cui F, et al. Enhanced angiogenesis by the hyaluronic acid hydrogels immobilized with a VEGF mimetic peptide in a traumatic brain injury model in rats. Regen Biomater 2019;6:325-34. 10.1093/rb/rbz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X, Peng Y, Liang H, et al. TSLP/TSLPR promote angiogenesis following ischemic stroke via activation of the PI3K/AKT pathway. Mol Med Rep 2018;17:3411-7. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Zhang X, Cui L, et al. Salvianolic acids enhance cerebral angiogenesis and neurological recovery by activating JAK2/STAT3 signaling pathway after ischemic stroke in mice. J Neurochem 2017;143:87-99. 10.1111/jnc.14140 [DOI] [PubMed] [Google Scholar]
- 25.Deng Y, Yang M, Xu F, et al. Combined Salvianolic Acid B and Ginsenoside Rg1 Exerts Cardioprotection against Ischemia/Reperfusion Injury in Rats. PLoS One 2015;10:e0135435. 10.1371/journal.pone.0135435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo HD, Cui GH, Tian JX, et al. Transplantation of salvianolic acid B pretreated mesenchymal stem cells improves cardiac function in rats with myocardial infarction through angiogenesis and paracrine mechanisms. Int J Cardiol 2014;177:538-42. 10.1016/j.ijcard.2014.08.104 [DOI] [PubMed] [Google Scholar]
- 27.Wu LM, Guo R, Hui L, et al. Stanniocalcin-1 protects bovine intestinal epithelial cells from oxidative stress-induced damage. J Vet Sci 2014;15:475-83. 10.4142/jvs.2014.15.4.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadipoor A, Lee RH, Prockop DJ, et al. Stanniocalcin-1 attenuates ischemic cardiac injury and response of differentiating monocytes/macrophages to inflammatory stimuli. Transl Res 2016;177:127-42. 10.1016/j.trsl.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi X, Wang J, Qin Y. Recombinant adeno-associated virus-delivered hypoxia-inducible stanniocalcin-1 expression effectively inhibits hypoxia-induced cell apoptosis in cardiomyocytes. J Cardiovasc Pharmacol 2014;64:522-9. 10.1097/FJC.0000000000000146 [DOI] [PubMed] [Google Scholar]
- 30.Roddy GW, Rosa RH, Jr, Oh JY, et al. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol Ther 2012;20:788-97. 10.1038/mt.2011.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Kz, Lindsberg PJ, Tatlisumak T, et al. Stanniocalcin: A molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci U S A 2000;97:3637-42. 10.1073/pnas.97.7.3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonfante S, Della Giustina A, Danielski LG, et al. Stanniocalcin-1 ameliorates cerebral ischemia by decrease oxidative stress and blood brain barrier permeability. Microvasc Res 2020;128:103956. 10.1016/j.mvr.2019.103956 [DOI] [PubMed] [Google Scholar]
- 33.Zhang RL, Chopp M, Gregg SR, et al. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J Cereb Blood Flow Metab 2009;29:1240-50. 10.1038/jcbfm.2009.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as