Abstract
Functional status and quality of life are not routinely assessed after skilled nursing facility (SNF) discharge. We determined feasibility of measuring frailty among adults ≥65 years admitted to SNF after hospitalization, and post-discharge outcomes. We calculated a frailty index (non-frail [≤0.25], mild frailty [0.26–0.35], moderate [0.36–0.45], and severe [>0.45]). After SNF discharge, we conducted serial telephone interviews measuring ability to perform functional activities and Patient Reported Outcome Measurement Information System (PROMIS) scores. Overall of 68 screened patients, 42 were eligible, and 24 (57.1%) eligible patients were enrolled. Of these, 5 (20.8%) were admitted after elective hospitalizations, 17 (70.8%) were female, and 11 (45.8%) had moderate-to-severe frailty. Frailty was measured in all participants in a mean 32.1 minutes. At 90 days, a total of three participants died, and two were lost to follow-up. Post-discharge functional status varied by frailty, with moderate-to-severe frailty having persistent impairment and lower PROMIS scores (worse quality of life) compared to those with no or mild frailty (38.2 [13.7] vs. 47.3 [8.1] p = .04). Measuring frailty and quality of life in older patients admitted to SNF is feasible. Furthermore, measuring frailty may help identify those at particularly high risk of poor recovery and lower quality of life after discharge.
Keywords: frailty, rehabilitation, functional assessment, quality of life
Background
One in five older adults is discharged to a skilled nursing facility (SNF) after acute hospitalization for post-acute care (Medicare Payment Advisory Commission, 2021). Unfortunately, only 60% of older adults admitted to SNFs are discharged home, and many are re-hospitalized, acquire new disabilities, or die (Achterberg et al., 2019; Buurman et al., 2016). Research from post-acute facilities in Europe, support that frailty, a state of vulnerability (Rockwood & Mitnitski, 2007), is a strong predictor of overall health outcomes after hospitalization, (Kerminen et al., 2020; Stuck et al., 2021) including functional recovery. However, measuring frailty during SNF has not previously been done in the complex post-acute care US system.
Rehabilitation is challenged by the interplay of acute medical stressors, chronic diseases, and psychosocial factors.(Buurman et al., 2016) Many standard metrics typically focus on acute illnesses or presenting functional status without considering underlying physical reserve. Additionally, lack of time remains a significant challenge for providers, who may lack the bandwidth to incorporate lengthy assessments. Understanding effective rehabilitation is also hindered by a lack of information about the functional outcomes after discharge. Functional assessments cease after SNF discharge, and other data sources, such as Medicare claims, cannot measure functional status or quality of life (Gell et al., 2017). Accurate assessment of an older patient’s potential for functional recovery could help inform individualized SNF care plans.
This pilot study was conducted to determine the feasibility of assessing frailty among older patients upon SNF admission and measuring patient-centered outcomes following SNF discharge. Ultimately, the pilot data would support the feasibility of a larger study that would identify frail patients admitted to SNF at risk for poor outcomes who can be targeted for tailored rehabilitation.
Methods
Study Population
From 01/08/2020 to 03/12/2020, we enrolled community dwelling adults >65 years, admitted after acute hospitalization to a single skilled nursing facility. The principal investigator conducted all screening, recruitment, and in-person interviews. Patients were excluded if they were non-English speaking or lacked capacity for consent. The Hebrew SeniorLife Institutional Review Board approved this study. All participants provided written informed consent. Although original recruitment was planned for 100, study enrollment terminated early due to the COVID-19 pandemic.
Measurements
Within 48 hours of SNF admission, the interviewer calculated a 49 deficit-accumulation FI (range: 0–1; Searle et al., 2008). This included 27 comorbidities, 7 activities of daily living (ADLs), 7 instrumental activities of daily living (IADLs), as well as 8 Nagi and Rosow-Breslau physical tasks (Nagi, 1976; Rosow & Breslau, 1966). Comorbidities were determined from medical record. All other FI components were determined during in-person assessment. Frailty was categorized as non-frail (FI ≤ 0.25), mild frailty (FI = 0.26–0.35), moderate (FI = 0.36–0.45), and severe (FI > 0.45) using standard cutpoints (Shi et al., 2020a, 2020b). Time to complete assessment was measured in minutes.
Delirium was assessed using a 3-Minute Diagnostic Assessment for Delirium using the CAM algorithm (3D-CAM; Marcantonio et al., 2014). Cognition was assessed with the Mini-Cog test (Borson et al., 2003; Lorentz et al., 2002). We also measured modified Barthel Index (Shah et al., 1989) grip strength, and gait speed upon SNF admission and discharge. Grip strength was measured in kg with a Jamar dynamometer. A 4-m gait speed was measured in meters per second. If a participant was unable to or refused to complete an assessment, data were treated as missing. We measured quality of life with the Patient Reported Outcome Measurement Information System (PROMIS) Global Health short form v1.2 (Cella et al., 2010; standardized score with mean = 50 and standard deviation [SD] = 10, higher is better). These components were not included in FI, as they do not represent pre-morbid underlying frailty.
After SNF discharge, regardless of discharge disposition, we conducted follow-up telephone interviews at 7, 30, and 90 days. When unable to reach participants by phone, we mailed questionnaires, which included questions about functional status, quality of life and recent health changes. Functional scores were calculated based on the ability to perform 15 daily activities (ADLs) and physical tasks without help (0–15, higher is better). A functional score of 0 was assigned for those who died.
Statistical Analysis
Summative participant demographics are described with means and SD or median and interquartile range (IQR). Feasibility was quantified as the proportion of eligible patients enrolled, the proportion of completed assessments and mean time to complete frailty assessments. Functional scores were summarized by means and graphed by mean functional score, at mean time points from days since hospitalization, by frailty category. PROMIS raw scores were converted into physical and mental health-related quality of life t-scores, compared by mean scores at each follow-up time point. A trend test was performed to compare PROMIS scores between frailty groups at 90 days.
Results
Of 68 screened patients, 42 were eligible, and 24 were enrolled (Supplemental Figure 1). The most common for non-participation was participant refusal (n = 14), followed by lack of capacity (n = 4). The median age was 83 years (IQR = 75–90), and 17 (70.8%) were female (Table 1). One participant was never discharged, leaving a total of 23 participants eligible for follow-up post-SNF discharge.
Table 1.
Baseline Characteristics on Skilled Nursing Facility (SNF) Admission (n = 24).
| Overall cohort n = 24 Median [interquartile range] |
Proportion of available data N(%) | |
|---|---|---|
| Age (median, IQR) | 83 [75–90] | 24 (100) |
| Female sex, n(%) | 17 (70.8) | 24 (100) |
| White race, n(%) | 23 (95.8) | 24 (100) |
| Elective hospitalization, n(%) | 5 (20.8) | 24 (100) |
| Hospitalization length of stay (days) | 5 [4–9] | 24 (100) |
| Living alone, n(%) | 17 (70.8) | 24 (100) |
| Baseline self-reported ADL dependency [0–7] | 0 [0–2] | 24 (100) |
| Baseline frailty index | 0.34 [0.21–0.44] | 24 (100) |
| Non-frail (FI ≤0.25) | 7 (29.2) | 7 (100) |
| Mild frailty (FI 0.26–0.35) | 6 (25.0) | 6 (100) |
| Moderate frailty (FI 0.36–0.45) | 6 (25.0) | 6 (100) |
| Severe frailty (FI >0.45) | 5 (20.8) | 5 (100) |
| SNF admission measurements | ||
| Delirium, n(%) | 4 (16.7) | 24 (100) |
| Number of dependent ADLs (0–7) | 5.0 [5.0–6.0] | 24 (100) |
| Modified barthel index | 57.0 [46.0–71.0] | 24 (100) |
| Grip strength (kg) | 13.7 [11.6–19.5] | 24 (100) |
| Gait speed (m/s) a | 0.4 [0.4–0.5] | 10 (41.7) |
Note. ADL = activities of daily living; FI = Frailty Index; SNF = skilled nursing facility. Baseline Frailty Index calculated from interview within 48 hours of admission based on self-reported comorbidities and functional ability 2 weeks prior to acute hospitalization, categorized by pre-established cutoffs. Hospitalization characteristics including length of stay measured from hospital discharge summary. ADL with a range of 0 to 7, with higher scores indicated more dependency.
Gait speed missing for 14 (58.3%) of participants.
At 7, 30, and 90 days post-SNF discharge, 2 (8.7%), 3 (13.4%), and 1 (4.3%) were lost to follow up and 0 (0%), 1 (4.3%), and 3 (13.4%) had died, respectively. By frailty status, follow-up completion for non-frail or mild frailty at 7, 30, and 90 days post discharge was 12/12 (100%), 10/12 (83.3%), and 11/11 (100%), compared to those with moderate-or-severe frailty 11/11 (100%), 8/9 (88.9%), and 6/8 (75.0%). While non-frail and mildly frail participants completed 30 out of 31 (96.8%) follow-ups themselves, for those with moderate-to-severe frailty only 22 out of 30 (73.3%) were completed directly by the participants.
Frailty assessment was completed in all enrolled participants, with a mean time of 32.1 minutes (SD = 23.2) and a median of 24 minutes (IQR = 20–36). Gait speed on admission could not be ascertained for 14 (58.3%) participants due to medical limitations. The baseline median self-reported ADL dependency was 0 (0–2). Median FI was 0.34 (IQR 0.21–0.44), with 6 (25.0%) having moderate and 5 (20.8%) severe frailty.
The median length of stay at SNF was 14 days (IQR 10–20.5), mean of 21.1 days. Altogether 15 (66.2%) of participants discharged home, 5 (21.7%) discharged to an assisted living setting, 1 (4.4%) discharged to long-term care SNF, and 2 (8.7%) were re-hospitalized (Table 2).
Table 2.
Clinical Outcomes Among the 23 Participants Discharged From Skilled Nursing Facility.
| Overall xohort at discharge (n = 23) Median (interquartile range) |
|
|---|---|
| SNF length of stay, mean days (SD) | 21.1 (27.2) |
| Immediate SNF discharge disposition | |
| Independent living, n(%) | 15 (66.7) |
| Assisted living, n(%) | 5 (20.8) |
| Re-hospitalized, n(%) | 1 (4.2) |
| Long term care nursing home, n(%) | 2 (8.3) |
| 90-day adverse events | |
| Hospitalization | 6 (26.1) |
| Fall | 8 (34.8) |
| Hired additional help | 4 (17.4) |
| Moved residences | 3 (13.0) |
| Death | 3 (13.0) |
| SNF discharge measurements | |
| Number of dependent ADLs (0–7), median [IQR] | 2.0 [1.0–3.5] |
| Modified Barthel index, median [IQR] | 83 [76–95] |
| Grip strength (kg), median [IQR] | 15.0 [10.0–23.0] |
| Gait speed a (m/s), median [IQR] | 0.5 [0.3–0.6] |
Note. ADL = activities of daily living; SNF = skilled nursing facility; SD = standard deviation; IQR = interquartile range. Immediate discharge disposition determined from discharge summaries. A 90-day adverse events determined from follow up interviews with participants or health care proxies. Of 24 enrolled participants, 1 remained in SNF and was censored at end of study and therefore did not contribute data to this table.
Gait speed missing for 8 (33.3%) participants, including those with acute re-hospitalization who could not be tested prior to discharge from SNF.
Post-discharge functional status varied by frailty (Figure 1), with those with moderate-to-severe frailty having persistent impairment. Among those with moderate-to-severe frailty post-discharge physical health-related PROMIS mean scores were lower (38.2 [SD = 13.7]) than those with no or mild frailty (47.3 [SD = 8.1], p = .04). Mental health-related scores were also lower but the difference did not reach statistical significance (40.9 [SD = 8.8] vs. 49.0 [SD = 9.0], p = .09).
Figure 1.
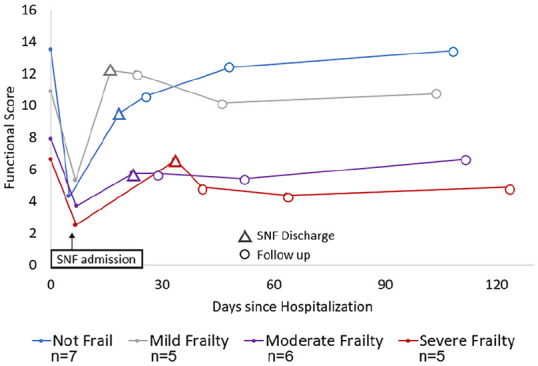
Mean function after post-acute skilled nursing facility admission by baseline frailty (n = 23).
Note. SNF = skilled nursing facility. Frailty measured by self-reported functional status and comorbidities 2 weeks prior to acute hospitalization. Functional score is the sum of independent ADLs and physical tasks (0–16; higher is better, death assigned a score of 0). Triangles denote SNF discharge time, and open circles denote follow up times.
Discussion
In this study, we demonstrated feasibility of calculating frailty in 100% of SNF admission in an average time of 32.1 minutes. We were able to complete 58/63 (92.1%) of all possible follow-up assessments via telephone for up to 90 days post SNF discharge, with 100% of participants completing at least one assessment, 22/23 (95.7%) completing two assessments, and 19/23 (82.6%) completing all assessments. Furthermore, follow-ups were completed despite limitations posed by the COVID-19 pandemic. Frailty and outcome measurements were generally feasible during and after post-acute care, respectively. However, many participants with moderate or severe frailty at baseline died or were lost to follow-up. Overall, our pilot data suggests a trend toward worse quality of life after SNF discharge for those with frailty.
The COVID-19 pandemic posed unique challenges to the recruitment and follow-up of the post-acute SNF population. Although study enrollment was terminated early, we enrolled 24 (57.1%) of 42 eligible participants. The 1-week follow-up survey had the highest responses overall; however frail participants were lost to follow-up more often. This was partly due to the ongoing COVID-19 pandemic, as some participants re-located or were hospitalized due to illness. Many frail participants were reached through their provided secondary contacts or health care proxies. This highlights the importance of engaging caregivers early on in research involving community dwelling frail older adults, and early (e.g., within 1 week) follow up assessments.
The impact on frailty in functional recovery in the post-acute SNF setting is poorly understood, challenged by differences in frailty measurement in previous studies (Roberts et al., 2018). However measurement of frailty is critical to understanding how baseline vulnerability impacts functional recovery for older adults in post-acute SNF care. Here, we demonstrate feasibility of measuring an FI based on comprehensive geriatrics assessment within 48 hours of SNF admission. An advantage of this approach is that it does not rely on physical measurements, which may be difficult to obtain due to acute limitations. For example, over half of our participants could not participate in gait speed measurement due to medical limitations such as weight-bearing status or safety concerns.
Our study is limited by small sample size and enrollment at a single SNF. The primary barrier to enrollment was refusal by participant. It compares favorably to larger studies in the nursing home setting,(Lenze et al., 2019) with good follow-up rates overall. Importantly, we were able to directly measure quality of life using validated scales, demonstrating that not only functional outcomes but mental and physical health related quality of life can be followed over time. Future work should include additional SNFs to improve enrollment, and also expand racial/ethnic diversity of the population, as our study sample was >90% White. Since we aimed to assess the time to complete an assessment, the frailty assessment was done by a researcher, not a clinician. However, during the COVID-19 pandemic, frailty was measurable in 100% of SNF patients using routine data collected from clinical provider notes(Shi et al., 2021). Furthermore, frailty can also be operationalized in the future through electronic health records (Callahan et al., 2021) and claims-based measurements (Kim et al., 2019).
Conclusions
In conclusion, we demonstrated the feasibility of frailty measurement and functional follow-up in SNF patients. Our work suggests that recovery patterns after post-acute SNF care and quality of life may differ by baseline frailty status. However, follow-up in this vulnerable population is challenged by a high mortality rate and drop-out rate. Measuring and understanding these patient-centered outcomes are critically important to informing care for vulnerable frail patients and their caregivers, particularly during the complex post-acute period. This is the first step to informing future work targeting frailty with SNF-based rehabilitation programs. Future work should consider leveraging technologies and including health care proxies and care partners to improve follow-up.
Supplemental Material
Supplemental material, sj-docx-1-ggm-10.1177_23337214221116978 for Feasibility of Measuring Frailty and Patient-Reported Outcomes During and After Post-Acute Skilled Nursing Facility Rehabilitation by Sandra Shi, Ellen P. McCarthy, Susan L. Mitchell and Dae Hyun Kim in Gerontology and Geriatric Medicine
Footnotes
Author Note: A preliminary version of this work was presented at the American Geriatrics Society 2021 annual scientific meeting.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Kim is a consultant to Alosa Health and VillageMD. The other authors declare no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (Grant T32 AG023480).
Institutional Review Board (IRB): The HSL IRB office approved this study under 45 CFR 46.110, Expedited Category 4, 5, and 7 (IRB-2019-38).
ORCID iD: Sandra Shi  https://orcid.org/0000-0001-6801-5602
https://orcid.org/0000-0001-6801-5602
Supplemental Material: Supplemental material for this article is available online.
References
- Achterberg W. P., Cameron I. D., Bauer J. M., Schols J. M. (2019). Geriatric rehabilitation—State of the art and future priorities. Journal of the American Medical Directors Association, 20(4), 396–398. 10.1016/j.jamda.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Borson S., Scanlan J. M., Chen P., Ganguli M. (2003). The mini-cog as a screen for dementia: Validation in a population-based sample. Journal of the American Geriatrics Society, 51(10), 1451–1454. 10.1046/j.1532-5415.2003.51465.x [DOI] [PubMed] [Google Scholar]
- Buurman B. M., Han L., Murphy T. E., Gahbauer E. A., Leo-Summers L., Allore H. G., Gill T. M. (2016). Trajectories of disability among older persons before and after a hospitalization leading to a skilled nursing facility admission. Journal of the American Medical Directors Association, 17(3), 225–231. 10.1016/j.jamda.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan K. E., Clark C. J., Edwards A. F., Harwood T. N., Williamson J. D., Moses A. W., Willard J. J., Cristiano J. A., Meadows K., Hurie J., High K. P., Meredith J. W., Pajewski N. M. (2021). Automated frailty screening at-scale for pre-operative risk stratification using the electronic frailty index. Journal of the American Geriatrics Society, 69(5), 1357–1362. 10.1111/jgs.17027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., Amtmann D., Bode R., Buysse D., Choi S., Cook K., Devellis R., Dewalt D., Fries J. F., Gershon R., Hahn E. A., Lai J. S., Pilkonis P., Revicki D., . . . Hays R. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gell N. M., Mroz T. M., Patel K. V. (2017). Rehabilitation services use and patient-reported outcomes among older adults in the United States. Archives of Physical Medicine and Rehabilitation, 98(11), 2221–2227.E3. 10.1016/j.apmr.2017.02.027 [DOI] [PubMed] [Google Scholar]
- Kerminen H., Huhtala H., Jäntti P., Valvanne J., Jämsen E. (2020). Frailty index and functional level upon admission predict hospital outcomes: An interRAI-based cohort study of older patients in post-acute care hospitals. BMC Geriatrics, 20(1), 160. 10.1186/s12877-020-01550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Patorno E., Pawar A., Lee H., Schneeweiss S., Glynn R. J. (2019). Measuring frailty in administrative claims data: Comparative performance of four claims-based frailty measures in the United States medicare data. The Journals of Gerontology: Series A, 75(6), 1120–1125. 10.1093/gerona/glz224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze E. J., Lenard E., Bland M., Barco P., Miller J. P., Yingling M., Lang C. E., Morrow-Howell N., Baum C. M., Binder E. F., Rodebaugh T. L. (2019). Effect of enhanced medical rehabilitation on functional recovery in older adults receiving skilled nursing care after acute rehabilitation: A randomized clinical trial. JAMA Network Open, 2(7), e198199. 10.1001/jamanetworkopen.2019.8199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentz W. J., Scanlan J. M., Borson S. (2002). Brief screening tests for dementia. Canadian Journal of Psychiatry, 47(8), 723–733. 10.1177/070674370204700803 [DOI] [PubMed] [Google Scholar]
- Marcantonio E. R., Ngo L. H., O’Connor M., Jones R. N., Crane P. K., Metzger E. D., Inouye S. K. (2014). 3D-CAM: Derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: A cross-sectional diagnostic test study. Annals of Internal Medicine, 161(8), 554–561. 10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicare Payment Advisory Commission. (2021). March 2021 report to the congress: Medicare payment policy. Author. [Google Scholar]
- Nagi S. Z. (1976). An epidemiology of disability among adults in the United States. Milbank Memorial Fund Quarterly. Health & Society, 54(4), 439–467. http://www.ncbi.nlm.nih.gov/pubmed/137366 [PubMed] [Google Scholar]
- Roberts P. S., Goud M., Aronow H. U., Riggs R. V. (2018). Frailty in a post-acute care population: A scoping review. PM & R, 10(11), 1211–1220. 10.1016/j.pmrj.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Rockwood K., Mitnitski A. (2007). Frailty in relation to the accumulation of deficits. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 62(7), 722–727. 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- Rosow I., Breslau N. (1966). A Guttman health scale for the aged. Journal of Gerontology, 21(4), 556–559. http://www.ncbi.nlm.nih.gov/pubmed/5918309 [DOI] [PubMed] [Google Scholar]
- Searle S. D., Mitnitski A., Gahbauer E. A., Gill T. M., Rockwood K. (2008). A standard procedure for creating a frailty index. BMC Geriatrics, 8, 24. 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Vanclay F., Cooper B. (1989). Improving the sensitivity of the barthel index for stroke rehabilitation. Journal of Clinical Epidemiology, 42(8), 703–709. 10.1016/0895-4356(89)90065-6 [DOI] [PubMed] [Google Scholar]
- Shi S. M., Lo O. Y., Newmeyer N., Bakaev I., Kim D. H. (2021). Recovery from coronavirus disease 2019 among older adults in post-acute skilled nursing facilities. Journal of the American Medical Directors Association, 22(6), 1138–1141.E1. 10.1016/j.jamda.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S. M., McCarthy E. P., Mitchell S. L., Kim D. H. (2020. a). Changes in predictive performance of a frailty index with availability of clinical domains. Journal of the American Geriatrics Society, 68(8), 1771–1777. 10.1111/jgs.16436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S. M., McCarthy E. P., Mitchell S. L., Kim D. H. (2020. b). Predicting mortality and adverse outcomes: Comparing the frailty index to general prognostic indices. Journal of General Internal Medicine, 35(5), 1516–1522. 10.1007/s11606-020-05700-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck A. K., Mangold J. M., Wittwer R., Limacher A., Bischoff-Ferrari H. A. (2021). Ability of 3 frailty measures to predict short-term outcomes in older patients admitted for post-acute inpatient rehabilitation. Journal of the American Medical Directors Association, 23(5), 880–884. 10.1016/j.jamda.2021.09.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ggm-10.1177_23337214221116978 for Feasibility of Measuring Frailty and Patient-Reported Outcomes During and After Post-Acute Skilled Nursing Facility Rehabilitation by Sandra Shi, Ellen P. McCarthy, Susan L. Mitchell and Dae Hyun Kim in Gerontology and Geriatric Medicine


