Abstract
Background:
Intracranial arterial stenosis (ICAS) is a non-marginal cause of stroke/transient ischemic attacks (TIAs) and is associated with high stroke recurrence rate. Some studies have investigated the best secondary prevention ranging from antithrombotic therapy to endovascular treatment (ET). However, no direct comparison between all the possible treatments is currently available especially between single and dual anti-platelet therapies (SAPT and DAPT).
Aim:
To establish whether DAPT is more effective than SAPT in preventing the recurrence of ICAS-related stroke, by means of a network meta-analysis (NMA).
Design:
Systematic review and NMA in accordance to PRISMA guidelines.
Data sources and methods:
We performed a systematic review of trials investigating secondary prevention (SAPT or DAPT, anticoagulant treatment or ET) in patients with symptomatic ICAS available in MEDLINE, Scopus and Web of Science from January 1989 to May 2021. We defined our primary efficacy outcome as the recurrence of ischemic stroke/TIA. We analysed the extracted data with Bayesian NMA approach.
Results:
We identified 815 studies and included 5 trials in the NMA. Sequence generation was adequate in all the selected studies while the allocation concealment method was described in one study. All the included studies reported the pre-specified primary outcomes, and outcome assessment was blinded in all the studies. We used the fixed-effect approach as the heterogeneity was not significant (p > 0.1) according to the Cochran’s Q statistic. DAPT was superior to SAPT and DAPT + ET in preventing stroke/TIA recurrence [respectively, odds ratio (OR), 0.59; confidence interval (CI), 0.39–0.9; and OR, 0.49, CI, 0.26–0.88], while no difference was found between DAPT and oral anticoagulant therapy (OAC). DAPT was safer than OAC (OR, 0.48; CI, 0.26–0.89) and DAPT + ET (OR, 0.50; CI, 0.35–0.71), while no difference was found between DAPT and SAPT.
Conclusion:
DAPT is more effective than SAPT for secondary stroke prevention in patients with symptomatic ICAS, without increasing the risk of haemorrhage.
Registration:
Prospero/CRD42019140033.
Keywords: anti-platelet therapy, arterial stenosis, cerebrovascular disease, DAPT, dual anti-platelet therapy, intracranial atherosclerosis disease, intracranial stenosis, ICAS, intracranial arterial stenosis, meta-analysis, stroke
Introduction
Stroke is a leading cause of death and disability worldwide. Intracranial arterial stenosis (ICAS) is one of the most common causes of stroke.1,2 ICAS is responsible for approximately 5–10% of strokes in White people, 15–29% of transient ischemic attacks (TIAs) or strokes in Black people, and up to 30–50% of strokes in Asian people. 1 Strokes caused by ICAS are associated with a high risk of recurrence compared with other stroke subtypes.1,3,4 Notably, most recurrent strokes occur in the same territory of the first symptomatic event and are often disabling.3,5,6 Concerning the mechanisms underlying ischemic events, hypoperfusion, artery-to-artery embolism and obstruction of small penetrating arteries can all occur in patients with ICAS and can be hypothesized on the basis of the neuroimaging characteristics.1,7
Considering the high rate of recurrence, many therapeutic options for secondary prevention have been investigated, ranging from medical therapy, such as single anti-platelet therapy (SAPT), dual anti-platelet therapy (DAPT) and oral anticoagulation (OAC) therapy with warfarin, to endovascular treatments (ETs).8–12 Trials investigating the efficacy of ETs have been stopped before enrolment was complete because of safety concerns.
Regarding medical therapy, to the best of our knowledge, there are no randomized controlled clinical trials (RCTs) directly comparing the efficacy of DAPT and SAPT in reducing stroke recurrence in patients with symptomatic ICAS.
We aimed to establish whether DAPT is more effective than SAPT in preventing the recurrence of ICAS-related stroke, by means of a network meta-analysis (NMA) of RCTs.
Materials and methods
Search strategy and selection criteria
NMA is a statistical method to summarize information from studies addressing the same question but testing different interventions. Particularly, it allows performing indirect comparisons between treatments (i.e. comparisons not previously addressed directly) and improves the precision for comparisons with few data. 13
This meta-analysis was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-NMA) guidelines. 14
We considered RCTs that included at least two comparison groups with the following treatments: SAPT, DAPT, OAC with warfarin, and ET. We included adult patients (>18 years old) with ischemic stroke or TIA of presumed ICAS origin within 30 days from symptom onset. The diagnosis of ICAS-related arterial stenosis must have been evaluated by digital subtraction angiography, CT angiography, MR angiography or ultrasound. We defined the primary outcome as early recurrence of ischemic stroke or TIA at 90 days and the secondary efficacy outcome as a composite of ischemic stroke or TIA, myocardial infarction, any haemorrhage, and death from any cause. We defined the safety outcome as the occurrence of any major haemorrhage, which includes intracranial and systemic haemorrhage requiring hospitalization.
We adopted Medical Subject Heading (MeSH) terms keywords in the MEDLINE search, Scopus and ISI Web of Science from January 1989 to May 2021. Moreover, we used the same search terms in the Cochrane Central Register of Controlled Trials (CENTRAL). We searched trial registries for ongoing or unpublished trials relevant to our meta-analysis (clinicaltrials.gov and WHO ICTRP). In order not to miss relevant trials and to reduce the likelihood of language bias, we applied no language restrictions for any of the searches.
The detailed search strategies can be found in the previously published NMA Protocol (Prospero, no. CRD42019140033).
Three authors independently reviewed titles and abstracts of the references retrieved from the above-mentioned search strategy and selected all potentially relevant studies. They obtained the full text of potentially relevant publications and the same authors independently reviewed them against the inclusion criteria of the meta-analysis. Different judgements on eligibility were resolved by consensus. We described and documented systematically all choices regarding including or excluding papers.
Data analysis
Two authors independently assessed the risk of bias in the included studies using Cochrane’s ‘Risk of bias’ assessment tool, composed of seven domains. 15 Disagreement was resolved by iteration, discussion and consensus with a third author. The Bayesian NMA was performed using the Markov Chain Monte Carlo engine WinBUGS software (MRC Biostatistics Unit, Cambridge, UK version 1.6.1) and NetMetaXL (Microsoft-Excel-based NMA Tool). 16 For all analyses, we set the number of burn-in iterations for assessing convergence of parameter estimates at 10 000 and the model runs at 10 000.
Evaluation of the homogeneity of similar studies is a key aspect of any systematic review. Standard methods to assess the heterogeneity between studies in pairwise meta-analysis calculations include the Cochran’s Q statistics. If the p value of the Q statistic was less than 0.05, it suggested that there was significant heterogeneity. 17 If significant heterogeneity existed, a random effects model was applied; otherwise, a fixed-effects model was adopted.
Network diagrams and forest plots were performed in order to summarize the results. Network diagram is the graphical representation of a NMA and is composed of edges and nodes. The nodes represent interventions, and the size of the nodes indicates the number of participants. The edges indicate direct comparisons between different interventions and the thickness of the edges represents the number of trials comparing two interventions. 18 The odds ratios (ORs) or risk ratios (RRs) and their 95% confidence intervals (CIs) were reported according to the data availability. The statistical significance level was set to p < 0.05.
Results
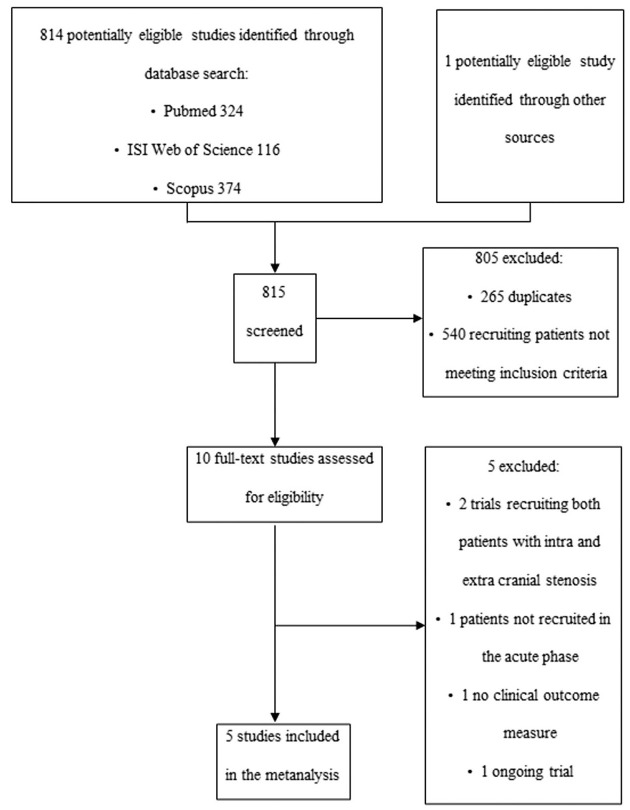
We identified 815 papers through MEDLINE, Scopus, Web of Science, CENTRAL, clinicaltrials.gov and WHO ICTRP, and selected 10 potentially relevant studies. After review, only three RCTs met the inclusion criteria for this meta-analysis.9,10,12 As only two comparisons between treatments were performed in these three trials, specifically DAPT versus DAPT plus ET and OAC versus SAPT, we did not reach the minimum number of comparisons (three) necessary to perform a NMA able to provide information about the efficacy of SAPT versus DAPT. In order to solve this issue, we chose to select from the 10 potentially relevant studies two subgroup analyses of patients with ICAS from RCTs that compared SAPT and DAPT in bigger populations 8,19 (Figure 1). Liu et al. 8 is a subgroup analysis of patients with and without ICAS from the CHANCE trial, and we only considered the ICAS sample. Toyoda et al. 19 is an RCT conducted in patients with high-risk ischemic stroke attributable to both extracranial and intracranial stenosis. However, we included in our NMA only data from the subgroup of patients with ICAS, which were available in the Supplementary Material of the trial. The Toyoda subgroup was used only to evaluate early recurrence of ischemic stroke/TIA because there were no available data regarding the composite and safety outcomes in the ICAS subgroup. We excluded four studies20–23 because the outcome measure was not clinical, 20 the recruited sample included both intracranial and extracranial arterial stenosis,21,23 or because patients were not recruited in the acute phase. 22
Figure 1.
Study selection flowchart.
To evaluate the primary efficacy outcome, which was the early recurrence of ischemic stroke/TIA, we included five studies, three of which were RCTs9,10,12 while the other two were subgroup analyses.8,19 Included studies’ characteristics are summed up in Table 1.
Table 1.
List of included studies and characteristics.
| Population | Study type | Dates | Interventions | Group 1 | Group 2 | Primary outcome | Primary outcome occurrence in group 1 versus group 2 | |
|---|---|---|---|---|---|---|---|---|
| Chimowitz et al.
9
WASID |
TIA/stroke (mRS ⩽ 3) within 90 days, with 50–99% stenosis of major intracranial artery (with age of at least 40 years) | Double-blind, multicenter clinical trial | February 1999 and July 2003 | Aspirin versus Warfarin | 289 (Warfarin) | 280 (Aspirin) | Ischemic stroke, brain haemorrhage or death from vascular causes others than stroke | 21.8% versus 22.1% p = 0.83 |
| Chimowitz et al.
10
SAMMPRIS |
Recent TIA/stroke attributed to stenosis of 70–99% of the diameter of a major intracranial artery | Investigator-initiated, randomized, clinical trial | November 2008–April 2011 | Aggressive medical management versus PTAS + aggressive medical management | 224 (ET + DAPT + aggressive medical management) | 227 (DAPT for 3 months + aggressive medical management) | Stroke in the territory of the qualifying artery or death during the follow-up period | 20.5% versus 11.5% p = 0.009 |
| Zaidat et al.
12
VISSIT |
18–85 years of age; and had symptomatic intracranial stenosis (70%-99%)involving the internal carotid, middle cerebral, intracranial vertebral or basilar arteries with a hard transient ischemic attack (TIA) or stroke attributable to the territory of the target lesion within the past 30 days. | Randomized multicenter clinical trial | January 2009–June 2012 | Medical therapy alone versus balloon-expandable stent plus medical therapy | 58 (ET + DAPT) | 53 (DAPT for 3 months) | Stroke or hard TIA in the territory of the qualifying artery within 12 months of randomization | 36.2% versus 15.1% p = 0.02 |
| Toyoda et al.
19
(Intracranial Subgroup analysis) |
Clinical diagnosis of noncardioembolic stroke that developed between 8 and 180 days before the start of the protocol treatment aged 20–85 years | Multicentre, open-label, randomized controlled trial | 13 December 2013, to 31 March 2017 | SAPT versus DAPT (with cilostozol) | 272 (SAPT) | 275 (DAPT with cilostazol for at least 6 months) | Ischemic stroke during the follow-up period | 9.2% versus 4% p < 0.001 |
| Wang et al.
24
CHANCE (ICAS subgroup analysis) |
Minor stroke (with ICAS) | Randomized, double-blind, placebo-controlled clinical trial | October 2009 and July 2012 | SAPT versus DAPT | 250 (SAPT) | 231 (DAPT for 21 days) | Stroke event (ischemic or haemorrhagic) at 90 days | 13.6% versus 11.3% p < 0.001 |
DAPT, dual anti-platelet therapy; ET, endovascular treatment; ICAS, intracranial arterial stenosis, PTAS, percutaneous transluminal angioplasty and stenting; SAPT, single anti-platelet therapy.
According to our previously published NMA protocol (Prospero, no. CRD42019140033), our goal was to evaluate the early recurrence of stroke or TIA. However, the duration of the follow-up greatly differed among the included studies for all the considered outcomes, namely, the recurrence of stroke/TIA, the secondary efficacy outcome and the safety. Therefore, we chose to assess each efficacy and safety outcome taking into account the follow-up time reported by each trial.
In this NMA, we used the fixed-effect approach as the heterogeneity was not significant (p > 0.1) according to the Cochran’s Q statistic.
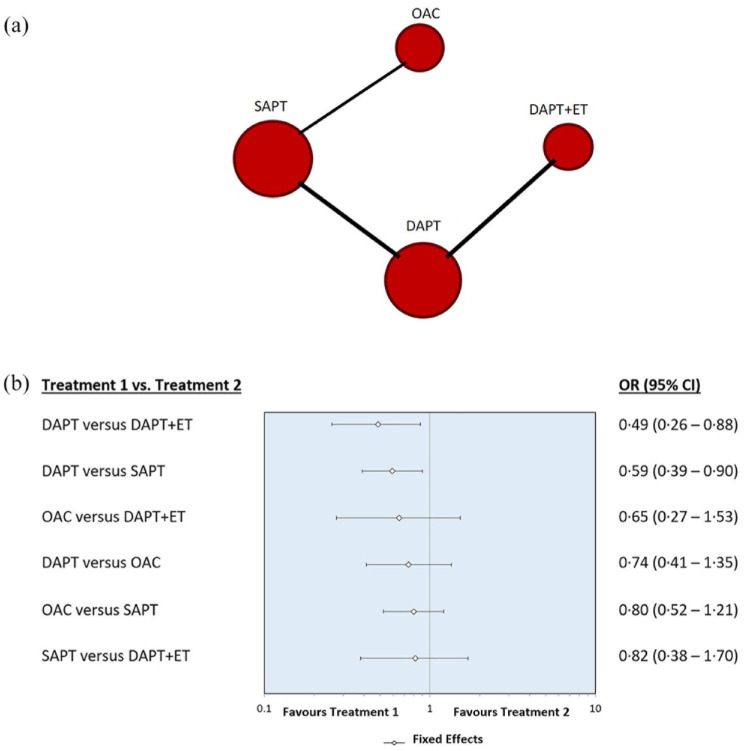
The primary efficacy outcome (ischemic stroke/TIA recurrence) was evaluated at a mean follow-up time of 8.2 months. Specifically, it was assessed at a mean follow-up time of 1.8 years in the WASID trial, at 30 days in the SAMMPRIS trial, at 30 days in the VISSIT trial, at 90 days in the CHANCE subgroup analysis and at a mean follow-up time of 1.4 years in the Toyoda et al. trial. The NMA that we performed confirmed that the use of DAPT alone is more effective than DAPT plus ET (OR, 0.49 with 95% CI, 0.26–0.88). In addition, it showed that the use of DAPT compared with SAPT as secondary prevention is more effective in lowering the risk of recurrence of ischemic stroke/TIA (OR, 0.59, 0.39–0.90). There was no statistically significant difference regarding our primary efficacy outcome in the comparison between the use of OAC and DAPT + ET (OR, 0.65, 0.27–1.53), DAPT and OAC (OR, 0.74, 0.41–1.35), OAC and SAPT (OR, 0.80, 0.52–1.21), SAPT and DAPT + ET (OR, 0.82, 0.38–1.70). Figure 2 shows network diagram and forest plot for the primary outcome.
Figure 2.
(a) Network diagram and (b) forest plot for the primary efficacy outcome.
The secondary composite outcome (composite of ischemic stroke or TIA, myocardial infarction, any haemorrhage, death from any cause) was evaluated at a mean follow-up time of 1.5 years. Specifically, it was assessed at a mean follow-up time of 1.8 years in the WASID trial, at 32.4 months in the SAMMPRIS trial, at 1 year in the VISSIT trial and at 90 days in the CHANCE subgroup analysis.
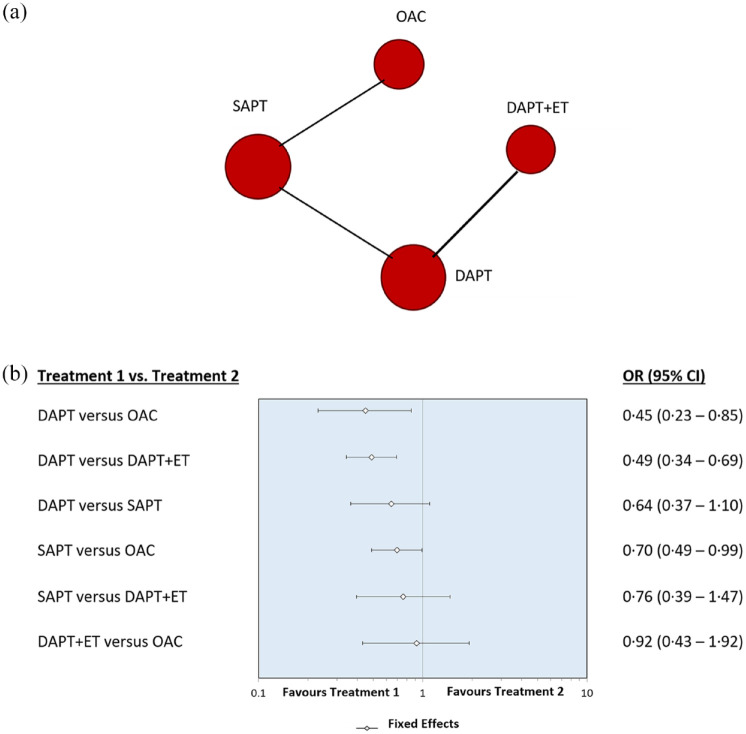
The use of DAPT was more effective than anticoagulation therapy with OAC (OR, 0.45, 0.23–0.85) and DAPT plus ET (OR, 0.49, 0.34–0.69) in reducing the composite secondary outcome. The use of SAPT compared with OAC is more effective in lowering the risk of the composite outcome but with a low statistical significance level (OR, 0.70, 0.49–0.99). No statistically significant difference was observed in the comparison between the use of DAPT and SAPT (OR, 0.64, 0.37–1.10).
There was also no statistically significant difference between the use of SAPT and DAPT plus ET (OR, 0.76, 0.39–1.47), and DAPT plus ET and OAC (OR, 0.92, 0.43–1.92). Figure 3 shows network diagram and forest plot for the secondary composite outcome.
Figure 3.
(a) Network diagram and (b) forest plot for the secondary efficacy outcome.
The safety outcome (any major haemorrhage) was evaluated at a mean follow-up time of 1.5 years. Specifically, it was assessed at a mean follow-up time of 1.8 years in the WASID trial, at 32.4 months in the SAMMPRIS trial, at 1 year in the VISSIT trial and at 90 days in the CHANCE subgroup analysis.
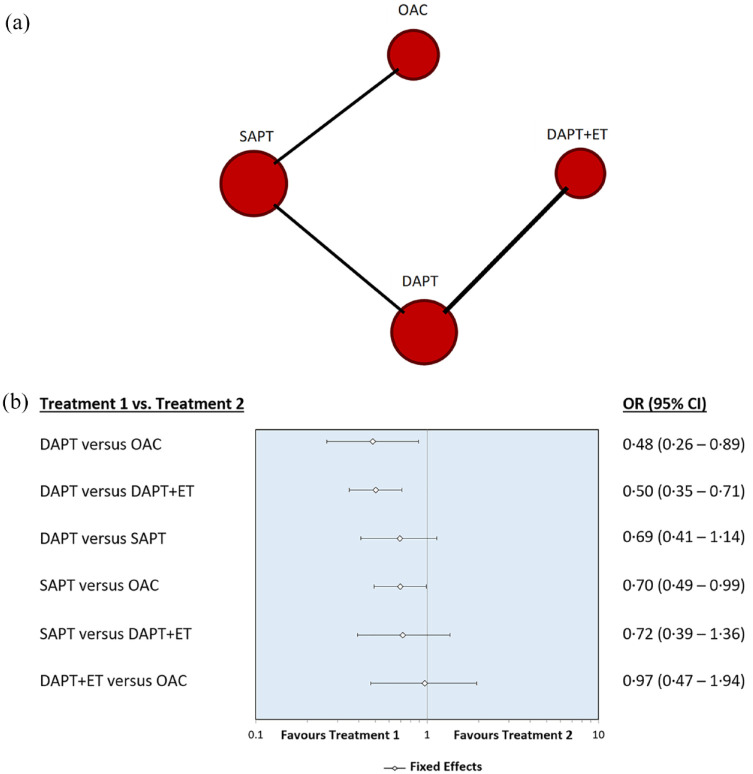
Our analysis showed that the use of DAPT was safer than anticoagulation therapy with OAC (OR, 0.48, 0.26–0.89) and DAPT plus ET (OR, 0.50, 0.35–0.71).
The use of SAPT was safer than OAC but with a low statistical significance level (OR, 0.70, 0.49–0.99). There was also no statistically significant difference in terms of safety between the use of DAPT and SAPT (OR 0.69, 0.41–1.14).
There was no statistically significant difference regarding the risk of haemorrhage in the comparison between the use of SAPT and DAPT plus ET (OR, 0.72, 0.39–1.36), and DAPT plus ET and OAC (OR, 0.97, 0.47–1.94). Figure 4 shows network diagram and forest plot for the safety outcome. In the Supplementary Materials, we summarize the results obtained for each outcome after having excluded the WASID trial from the analysis to focus exclusively on anti-platelet therapy and found similar results (Supplementary Materials Figures 1, 2 and 3).
Figure 4.
(a) Network diagram and (b) forest plot for the safety outcome.
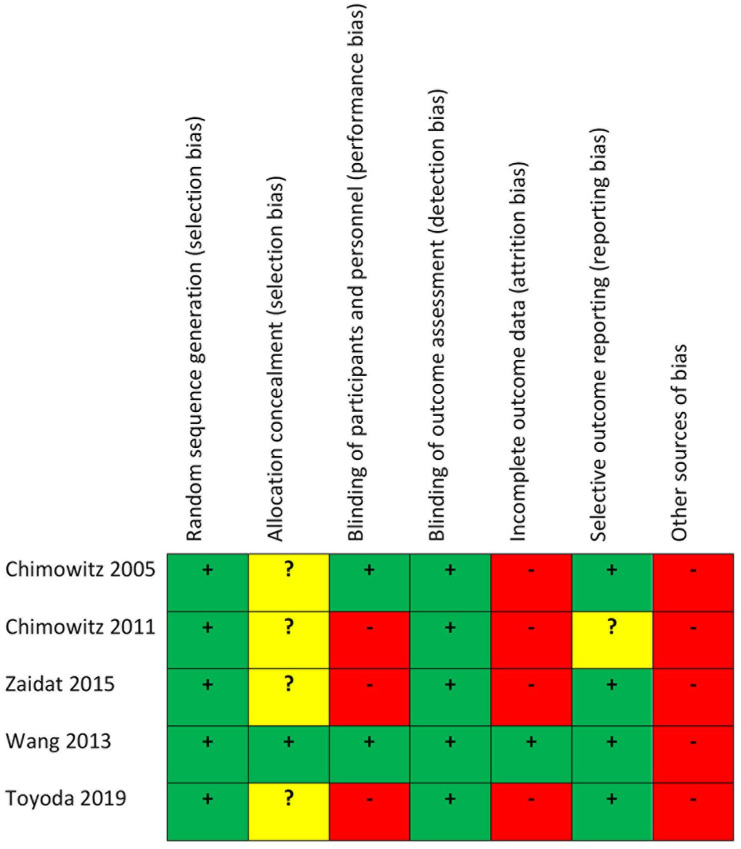
We summarize the risk of bias of the included studies in Figure 5.
Figure 5.
Risk of bias of the included studies.
Sequence generation was adequate in all the reported studies.8–12,19,24–29 The allocation concealment method was described in CHANCE, but was not reported in WASID, SAMMPRIS, VISSIT and Toyoda. Regarding treatment, two of the selected studies were double-blinded (WASID, CHANCE), while the remaining three were not (Toyoda, SAMMPRIS, VISSIT). The choice not to blind participants depended on the use of invasive angiography for SAMMPRIS and VISSIT. Outcome assessment was blinded in all the selected studies. WASID reported that 1.8% and 2.8% of the patients in the SAPT and OAC groups, respectively, were lost to follow-up. SAMMPRIS reported that 5% and 3% of the patients in the DAPT and percutaneous transluminal angioplasty and stenting (PTAS) groups were lost to follow-up. VISSIT had 11.3% and 2% of patients lost to follow-up, respectively, in the DAPT and ET groups. Toyoda et al. reported a loss to follow-up rate of 8.8% and 5.3% for the SAPT and the DAPT groups. In the CHANCE trial, 0.7% of patients were lost to follow-up. All the included studies reported the pre-specified primary outcomes. However, we considered the subgroup of patients with ICAS for CHANCE and Toyoda, this being a potential source of bias. A possible bias in four of the included studies comes from incomplete recruitment of patients. WASID and SAMMPRIS prematurely stopped patients’ enrolment because of safety concerns. VISSIT was stopped soon after SAMMPRIS publication. Toyoda did not reach the planned sample size because of slow recruitment. Finally, we considered ICAS subgroups of CHANCE and Toyoda trials; therefore, the sample size was not calculated specifically to evaluate treatment effects in the ICAS sample.
Discussion
Our analysis indicates that the use of DAPT is more effective than SAPT in reducing the risk of recurrence of ischemic stroke at a mean follow-up of 8 months. However, the use of DAPT was not superior to SAPT in lowering the risk of the composite of secondary outcomes, at a mean follow-up time of 1.5 years. In addition, we found that DAPT did not increase the risk of haemorrhage at a mean follow-up time of 1.5 years. The significant difference between the time points at which the primary and secondary outcomes have been evaluated could explain the lack of effect of DAPT in reducing the occurrence of the composite secondary outcome. Indeed, ICAS patients are likely to have a systemic atherosclerotic disease which entails an increased risk of cardiovascular morbidity and death. 30 Moreover, in minor stroke, regardless of the occurrence of ICAS, the effect of DAPT in reducing ischemic stroke/TIA is mostly observed within the first 10 days. 31 As a consequence, considering that the secondary outcome has been evaluated at a mean follow-up time of 1.5 years, the occurrence of the composite outcome could be similar between SAPT and DAPT groups, even though the risk of haemorrhage was not increased by DAPT. Our results confirm that DAPT is more effective than DAPT plus ET in lowering both the risk of recurrence of ischemic stroke or TIA and the incidence of the composite of secondary outcomes. Moreover, DAPT proves to be safer than DAPT plus ET. The use of DAPT also appears more effective than OAC in reducing the risk of incidence of secondary outcomes. Finally, OAC is associated to a higher risk of bleeding than both DAPT and SAPT. The reported evidence is actually stronger than what can arise from the available individual trials or subgroups analyses. In fact, the use of a combination of clopidogrel and aspirin for 90 days followed by aspirin alone is currently suggested as secondary prevention for patients with a recent ischemic event (within 30 days) and a 70–99% ICAS. 1 The recommendation comes from the results of SAMMPRIS that showed that the 30-day rate of ischemic stroke and death in the medical treatment group (DAPT and intensive risk factor management) was considerably lower than in both the aspirin and OAC arms of the WASID trial. This was also supported by the CLAIR trial, showing that DAPT was more effective than SAPT in reducing micro-embolic signals detected by transcranial Doppler on day 2 and 7 after randomization in patients with recently (<7 days) symptomatic ICAS. 20 However, the above-mentioned recommendation 1 was more an experts’ opinion than an actual comparison of quantitative data. Therefore, the purpose of this analysis was to offer an objective statistical comparison of all the data currently available on this topic. As stated in the protocol, our goal was to perform a NMA with data obtained only from RCTs. However, that could not be achieved. In fact, only three RCTs met the inclusion criteria for this NMA, and only two comparisons between two groups of treatments were performed, specifically DAPT versus DAPT plus ET and OAC versus SAPT. These data were not sufficient to perform a NMA able to provide information about the direct and indirect comparisons of the four considered treatments. In fact, at least three different comparisons between these treatments were required. Therefore, we chose to include two other studies wherein a third comparison was performed, specifically SAPT versus DAPT.8,19 Liu et al. 8 is a subgroup analysis of patients with and without ICAS from the CHANCE trial, while Toyoda et al. 19 is an RCT conducted in patients with high-risk ischemic stroke attributable to both extracranial and intracranial stenosis. However, our NMA only included data from the subgroup of patients with ICAS. We acknowledge that, as a result of the inclusion of these two study subgroups, the level of the evidence is lower than a NMA of RCTs because of the inherent risk of bias due to subgroup analyses.
Another issue is the difference between the time points at which the outcomes had been evaluated in the included studies. According to the NMA protocol, we wanted to evaluate the primary and secondary efficacy outcomes and the safety outcome at 90 days, but the length of the follow-up time differed among the included studies, and only the CHANCE subgroup analysis reported the outcomes at 90 days. For this reason, we evaluated the outcomes at a time point that is the average of the follow-up times of all the included studies for every considered outcome. Furthermore, patients were not administered the same kind and dose of DAPT in all the included trials, and even the duration of DAPT differed significantly among the studies. Specifically, in the DAPT arm of the Toyoda trial, a combination of cilostazol with aspirin or clopidogrel was used, while patients in the other three trials wherein DAPT was used were all administered aspirin in association with clopidogrel, although at a slightly different dose. Consequently, it can be argued that a different composition of the DAPT could have an impact on the results obtained with this NMA. However, a previous trial 32 designed to compare the efficacy of two different combinations of DAPT administered for 7 months, namely aspirin and clopidogrel versus aspirin and cilostazol, in patients with symptomatic ICAS, did not show any significant difference in terms of progression of the arterial stenosis, recurrence of ischemic stroke and occurrence of major haemorrhagic complications between these treatments. Therefore, it is reasonable to assume that our results were not influenced by the use of DAPT with cilostazol in the Toyoda trial.
Considering the available data and especially the different duration of DAPT in the trials we chose to consider, it is not possible to establish with certainty how long DAPT should be administered in ICAS patients. About this issue, we have to consider that the pooled meta-analysis of CHANCE and POINT trials demonstrated that in minor stroke, regardless of the presence of ICAS, DAPT reduces ischemic events mostly within the first 10 days after the index event without increasing the risk of major haemorrhage. On the contrary, from day 11 to day 90, DAPT does not reduce the risk of major ischemic events, while it does not increase the risk of major haemorrhages, but only that of minor haemorrhages. 31 According to these results, for patients with minor stroke, regardless of the presence of ICAS, DAPT is currently recommended for 21 days. However, it is well established that patients with ischemic stroke caused by ICAS have a significantly higher risk of recurrence compared with that of patients with other stroke subtypes1,3,4 and that a new ischemic event is often disabling.3,5,6 Our NMA shows that DAPT is more effective than SAPT in symptomatic ICAS and that the risk of haemorrhage is not increased. Therefore, considering the high risk of recurrence of ischemic stroke in ICAS patients1,3,4 and taking into account the results from the pooled meta-analysis of CHANCE and POINT trials, 31 we think that it could be reasonable to administer DAPT for at least 3 months in patients with symptomatic ICAS.
Conclusion
This NMA suggests that DAPT is more effective than SAPT for secondary stroke prevention in patients with ICAS, without increasing the risk of haemorrhage.
Supplemental Material
Supplemental material, sj-tiff-1-tan-10.1177_17562864221114716 for Dual anti-platelet therapy for secondary prevention in intracranial atherosclerotic disease: a network meta-analysis by Giuseppe Reale, Aurelia Zauli, Giuseppe La Torre, Alice Mannocci, Michael V. Mazya, Marialuisa Zedde, Silvia Giovannini, Marco Moci, Chiara Iacovelli and Pietro Caliandro in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tiff-2-tan-10.1177_17562864221114716 for Dual anti-platelet therapy for secondary prevention in intracranial atherosclerotic disease: a network meta-analysis by Giuseppe Reale, Aurelia Zauli, Giuseppe La Torre, Alice Mannocci, Michael V. Mazya, Marialuisa Zedde, Silvia Giovannini, Marco Moci, Chiara Iacovelli and Pietro Caliandro in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tiff-3-tan-10.1177_17562864221114716 for Dual anti-platelet therapy for secondary prevention in intracranial atherosclerotic disease: a network meta-analysis by Giuseppe Reale, Aurelia Zauli, Giuseppe La Torre, Alice Mannocci, Michael V. Mazya, Marialuisa Zedde, Silvia Giovannini, Marco Moci, Chiara Iacovelli and Pietro Caliandro in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
ORCID iD: Pietro Caliandro  https://orcid.org/0000-0002-1190-4879
https://orcid.org/0000-0002-1190-4879
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Giuseppe Reale, Dipartimento di Scienze dell’Invecchiamento, Neurologiche, Ortopediche e della Testa-Collo, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Dipartimento di Neuroscienze, Università Cattolica del Sacro Cuore, Rome, Italy.
Aurelia Zauli, Dipartimento di Neuroscienze, Università Cattolica del Sacro Cuore, Rome, Italy.
Giuseppe La Torre, Department of Public Health and Infectious Disease, Sapienza/Policlinico Umberto I, Rome, Italy.
Alice Mannocci, Faculty of Economics, Universitas Mercatorum, Rome, Italy.
Michael V. Mazya, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden Department of Neurology, Karolinska University Hospital, Stockholm, Sweden.
Marialuisa Zedde, Neurology Unit, Stroke Unit, Azienda Unità Sanitaria Locale-IRCCS di Reggio Emilia, Via Giovanni Amendola, 2, Reggio Emilia 42122, Italy.
Silvia Giovannini, Dipartimento di Scienze dell’Invecchiamento, Neurologiche, Ortopediche e della Testa-Collo, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Marco Moci, Dipartimento di Neuroscienze, Università Cattolica del Sacro Cuore, Rome, Italy.
Chiara Iacovelli, Dipartimento di Scienze dell’Invecchiamento, Neurologiche, Ortopediche e della Testa-Collo, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Pietro Caliandro, Dipartimento di Scienze dell’Invecchiamento, Neurologiche, Ortopediche e della Testa-Collo, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Giuseppe Reale: Conceptualization; Investigation; Validation; Writing – original draft.
Aurelia Zauli: Investigation; Writing – original draft.
Giuseppe La Torre: Investigation; Methodology.
Alice Mannocci: Data curation; Formal analysis; Investigation; Software.
Michael V. Mazya: Investigation; Visualization; Writing – review & editing.
Marialuisa Zedde: Project administration; Writing – review & editing.
Silvia Giovannini: Investigation; Writing – review & editing.
Marco Moci: Investigation; Visualization; Writing – review & editing.
Chiara Iacovelli: Data curation; Formal analysis; Investigation.
Pietro Caliandro: Conceptualization; Investigation; Project administration; Supervision; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013; 12: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma YH, Leng XY, Dong Y, et al. Risk factors for intracranial atherosclerosis: a systematic review and meta-analysis. Atherosclerosis 2019; 281: 71–77. [DOI] [PubMed] [Google Scholar]
- 3. Famakin BM, Chimowitz MI, Lynn MJ, et al. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke 2009; 40: 1999–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caliandro P, Reale G, Demchuk AM, et al. Symptomatic intracranial atherosclerotic disease: an ultrasound 2-year follow-up pilot study. Neurol Sci 2018; 39: 1955–1959. [DOI] [PubMed] [Google Scholar]
- 5. Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–563. [DOI] [PubMed] [Google Scholar]
- 6. Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011; 69: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung JM, Kang DW, Yu KH, et al. Predictors of recurrent stroke in patients with symptomatic intracranial arterial stenosis. Stroke 2012; 43: 2785–2787. [DOI] [PubMed] [Google Scholar]
- 8. Liu L, Wong KSL, Leng X, et al. Dual antiplatelet therapy in stroke and ICAS. Neurology 2015; 85: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005; 352: 1305–1316. [DOI] [PubMed] [Google Scholar]
- 10. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA: J Am Med Assoc 2015; 313: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 13. Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997; 50: 683–691. [DOI] [PubMed] [Google Scholar]
- 14. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 15. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019; 10: ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown S, Hutton B, Clifford T, et al. A microsoft-excel-based tool for running and critically appraising network meta-analyses – an overview and application of NetMetaXL. Syst Rev 2014; 3: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 18. Catalá-López F, Tobías A, Cameron C, et al. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int 2014; 34: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 19. Toyoda K, Uchiyama S, Yamaguchi T, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol 2019; 18: 539–548. [DOI] [PubMed] [Google Scholar]
- 20. Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010; 9: 489–497. [DOI] [PubMed] [Google Scholar]
- 21. Markus HS, Larsson SC, Kuker W, et al. Stenting for symptomatic vertebral artery stenosis. Neurology 2017; 89: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miao Z, Jiang L, Wu H, et al. Randomized controlled trial of symptomatic middle cerebral artery stenosis: endovascular versus medical therapy in a Chinese population. Stroke 2012; 43: 3284–3290. [DOI] [PubMed] [Google Scholar]
- 23. Compter A, van der Worp HB, Schonewille WJ, et al. Stenting versus medical treatment in patients with symptomatic vertebral artery stenosis: a randomised open-label phase 2 trial. Lancet Neurol 2015; 14: 606–614. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013; 369: 11–19. [DOI] [PubMed] [Google Scholar]
- 25. Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology 2003; 22: 106–117. [DOI] [PubMed] [Google Scholar]
- 26. Chimowitz MI, Lynn MJ, Turan TN, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in Intracranial Stenosis Trial. J Stroke Cerebrovasc Dis 2011; 20: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaidat OO, Castonguay AC, Fitzsimmons BF, et al. Design of the vitesse intracranial stent study for ischemic therapy (VISSIT) trial in symptomatic intracranial stenosis. J Stroke Cerebrovasc Dis 2013; 22: 1131–1139. [DOI] [PubMed] [Google Scholar]
- 28. Wang Y, Johnston SC. and CHANCE Investigators. Rationale and design of a randomized, double-blind trial comparing the effects of a 3-month clopidogrel-aspirin regimen versus aspirin alone for the treatment of high-risk patients with acute nondisabling cerebrovascular event. Am Heart J 2010; 160: 380–386. [DOI] [PubMed] [Google Scholar]
- 29. Toyoda K, Uchiyama S, Hoshino H, et al. Protocol for Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com): a randomized, open-label, parallel-group trial. Int J Stroke 2015; 10: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoshino T, Sissani L, Labreuche J, et al. Prevalence of systemic atherosclerosis burdens and overlapping stroke etiologies and their associations with long-term vascular prognosis in stroke with intracranial atherosclerotic disease. JAMA Neurol 2018; 75: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pan Y, Elm JJ, Li H, et al. Outcomes associated with clopidogrel-aspirin use in minor stroke or transient ischemic attack: a pooled analysis of clopidogrel in high-risk patients with acute non-disabling cerebrovascular events (CHANCE) and platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trials [published correction appears in JAMA Neurol 30 September 2019; Published correction appears in JAMA Neurol 16 August 2021]. JAMA Neurol 2019; 76: 1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwon SU, Hong KS, Kang DW, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke 2011; 42: 2883–2890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tiff-1-tan-10.1177_17562864221114716 for Dual anti-platelet therapy for secondary prevention in intracranial atherosclerotic disease: a network meta-analysis by Giuseppe Reale, Aurelia Zauli, Giuseppe La Torre, Alice Mannocci, Michael V. Mazya, Marialuisa Zedde, Silvia Giovannini, Marco Moci, Chiara Iacovelli and Pietro Caliandro in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tiff-2-tan-10.1177_17562864221114716 for Dual anti-platelet therapy for secondary prevention in intracranial atherosclerotic disease: a network meta-analysis by Giuseppe Reale, Aurelia Zauli, Giuseppe La Torre, Alice Mannocci, Michael V. Mazya, Marialuisa Zedde, Silvia Giovannini, Marco Moci, Chiara Iacovelli and Pietro Caliandro in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tiff-3-tan-10.1177_17562864221114716 for Dual anti-platelet therapy for secondary prevention in intracranial atherosclerotic disease: a network meta-analysis by Giuseppe Reale, Aurelia Zauli, Giuseppe La Torre, Alice Mannocci, Michael V. Mazya, Marialuisa Zedde, Silvia Giovannini, Marco Moci, Chiara Iacovelli and Pietro Caliandro in Therapeutic Advances in Neurological Disorders