Abstract
Background:
Liberation from prolonged tracheostomy ventilation involves ventilator weaning and removal of the tracheal cannula (referred to as decannulation). This study evaluated the incidence, causes, and predictors of unsuccessful decannulation following prolonged weaning.
Methods:
Observational retrospective cohort study of 532 prolonged mechanically ventilated, tracheotomized patients treated at a specialized weaning center between June 2013 and January 2021. We summarized the causes for unsuccessful decannulations and used a binary logistic regression analysis to derive and validate associated predictors.
Results:
Failure to decannulate occurred in 216 patients (41%). The main causes were severe intensive care unit (ICU)-acquired dysphagia (64%), long-term ventilator dependence following weaning failure (41%), excessive respiratory secretions (12%), unconsciousness (4%), and airway obstruction (3%). Predictors of unsuccessful decannulation from any cause were age [odds ratio (OR) = 1.04 year−1; 95% confidence interval (CI), 1.02–1.06; p < 0.01], body mass index [0.96 kg/m2 (0.93–1.00); p = 0.027], Acute Physiology and Chronic Health Evaluation II (APACHE-II) score [1.05 (1.00–1.10); p = 0.036], pre-existing non-invasive home ventilation [3.57 (1.51–8.45); p < 0.01], percutaneous tracheostomies [0.49 (0.30–0.80); p < 0.01], neuromuscular diseases [4.28 (1.21–15.1); p = 0.024], and total mechanical ventilation duration [1.02 day−1 (1.01–1.02); p < 0.01]. Regression models examined in subsets of patients with severe dysphagia and long-term ventilator dependence as the main reason for failure revealed little overlapping among predictors, which even showed opposite effects on the outcome. The application of non-invasive ventilation as a weaning technique contributed to successful decannulation in 96 of 221 (43%) long-term ventilator-dependent patients following weaning failure.
Conclusion:
Failure to decannulate after prolonged weaning occurred in 41%, mainly resulting from persistent ICU-acquired dysphagia and long-term ventilator dependence following weaning failure, each associated with its own set of predictors.
Keywords: dysphagia, mechanical ventilation, non-invasive ventilation, tracheostomy, ventilator weaning
Introduction
Liberation from prolonged tracheostomy ventilation generally involves ventilator weaning and decannulation (tracheal cannula removal), with various approaches to each goal.1–3 Previous studies have examined weaning predictors4–6 and the most effective weaning strategy. 7 Still, only a few have focused on predictors of unsuccessful decannulation3,8–10 or the best approach to decannulation. 1
Failure to decannulate may result from several reasons, such as severe dysphagia, retention of copious secretions requiring invasive airway clearing techniques, or tracheal stenosis, 11 but their frequency following prolonged mechanical ventilation is not known. Moreover, the factors associated with the main reasons for unsuccessful decannulation at an early stage of ventilator weaning have not been examined yet.
The study’s objective was to assess the incidence, causes, and predictors of unsuccessful decannulations among prolonged mechanically ventilated patients.
Methods
We conducted a retrospective, observational cohort study at a specialized weaning center in Germany. Regulatory approval for the project came from the local institutional review board for human studies, which complied with the Declaration of Helsinki (Ethics Committee of the State Chamber of Physicians of Baden-Wuerttemberg, Germany, approval number F–2021–087). As the analyses were retrospective, they were not subject to informed consent.
Patient selection
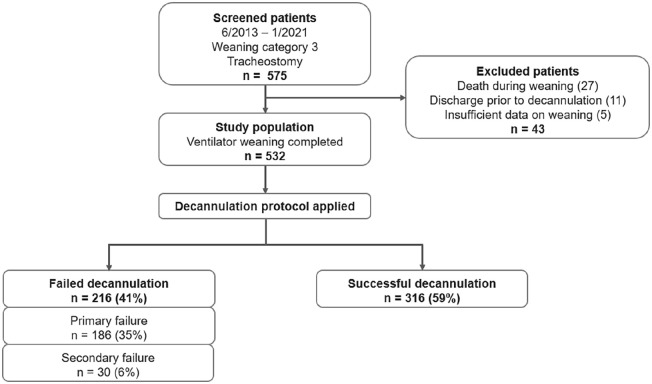
Five hundred thirty-two subjects included in the study had been referred from intensive care units across Germany for weaning from tracheostomy ventilation between June 2013 and January 2021, meeting the prolonged weaning criteria. 12 We excluded patients from the analysis who were discharged or died before the first attempt at decannulation (Figure 1).
Figure 1.
Patient flow diagram. Weaning category 3 refers to the statement from Boles et al. 12
Data collection
Hospitals’ full electronic medical records and charting systems were accessed to collect data (PDMS Metavision ICU, iMDsoft, Tel Aviv, Israel; iMedOne, Telekom Healthcare Solutions, Bonn, Germany). A baseline assessment of patients was performed upon admission to the weaning center, including demographics, the leading cause of mechanical ventilation, and comorbidities. Following ventilator weaning completion, we analyzed each patient’s decannulation attempts and failure rates, including the reasons for failure, and we compared the results of prolonged weaning between patients with successful and unsuccessful decannulations.
Ventilator weaning procedure
Located in the Schillerhoehe Lung Clinic (Gerlingen, Germany), the 12-bed weaning unit was established in 2006. It provides patients with invasive (via tracheal cannulas or endotracheal tubes) and non-invasive ventilatory support (using non-invasive mask ventilation or nasal high-flow cannulas). The multidisciplinary team comprises pulmonologists, respiratory therapists, physiotherapists, speech-language pathologists, and psychologists. Nursing staff and physicians work in rotating shifts of 8 h. Nurse-to-patient ratios are 1:2 during the day and 1:3 at night.
On admission, all patients were ventilated in the pressure-controlled, assist-control (A/C) mode. As described previously, a standardized method of ventilator liberation was employed as soon as the criteria for weaning readiness were met.5,6 According to protocol, ventilator weaning starts with a 30-min spontaneous breathing trial (utilizing a T-piece). These weaning trials are conducted once a day, with the duration typically extended by 2–3 h per day, aiming at complete autonomic breathing. Accordingly, weaning success refers to sustained spontaneous breathing over at least seven consecutive days without signs of ventilatory failure (e.g. hypercapnia). These patients remain ventilator-detached on discharge. Conversely, weaning failure is a transition to long-term mechanical ventilation in the outpatient setting (by face mask or tracheostomy tube) due to ventilatory failure. Ventilatory failure describes recurrent hypercapnia during weaning trials (observed on at least two consecutive days), preventing the extension of spontaneous breathing, or sustained hypercapnia (on at least two successive occasions) occurring within 7 days after weaning completion (requiring reinstitution of mechanical ventilation).5,6 Weaning programs always included physiotherapy, nutrition support, and comorbidity treatment.
Non-invasive ventilation for weaning failures
German guidelines on prolonged weaning recommend a strategy of decannulation followed by the employment of (long-term) non-invasive ventilation (NIV) in weaning failure patients with low/medium dependence on ventilators (<8–16 h per day). 13 This approach argues that NIV is equally effective at unloading respiratory muscles and defending alveolar ventilation as invasive mechanical ventilation, 14 which is associated with poor health-related quality of life and high medical costs in the outpatient setting. 15 NIV eligibility criteria were low/medium ventilator dependence (<8–16 h per day), a safe level of interface tolerance (application of a full-face mask), and the ability to self-apply and remove the mask. 13 These patients were started on NIV immediately after decannulation, with intensity and daily application matching their tracheostomy ventilator settings and ventilation times.
In cases of high device dependence (⩾16 h per day), we generally refrain from decannulating patients who need ventilator equipment for life-sustaining mechanical ventilation. 16 This decision was always made within a multidisciplinary team involving the attending physician and respiratory therapist.
Assessment and management of dysphagia
Dysphagia is a common problem in prolonged ventilated patients with tracheostomies that may prevent decannulation. 13 Swallowing function was assessed immediately upon admission to our center, either by a trained respiratory therapist or speech-language pathologist using modified Evan’s blue-dye test 17 and a bedside swallowing examination. Furthermore, we performed a fiberoptic endoscopic evaluation of swallowing (FEES) in selected patients when silent aspiration was still suspected after an inconspicuous blue-dye test, with a penetration-aspiration scale used to classify dysphagia. 18 In case of severe swallowing impairment preventing oral feeding, tests were repeated twice a week throughout weaning (Supplemental Appendix 1: e-Figure 1).
Individualized speech therapy was applied 3–5 times per week in patients with impaired swallowing, consisting of ongoing exercises to improve tongue mobility and strength, the elevation of the larynx, vocal cord closure, and the ability to clear the throat, accompanied by increasing periods of cuff deflation. Referred from intensive care units (ICUs) to our center, most patients received enteral feeding via nasogastric tubes on admission, and we placed percutaneous endoscopic gastrostomy (PEG) feeding tubes when oral food intake was not expected within 2 weeks upon admission. Based on our assessment, we gave nil per os (NPO) instructions and generally refrained from decannulating patients who demonstrated aspirations due to excessive salivation (the inability to protect one’s airways).
Decannulation eligibility criteria
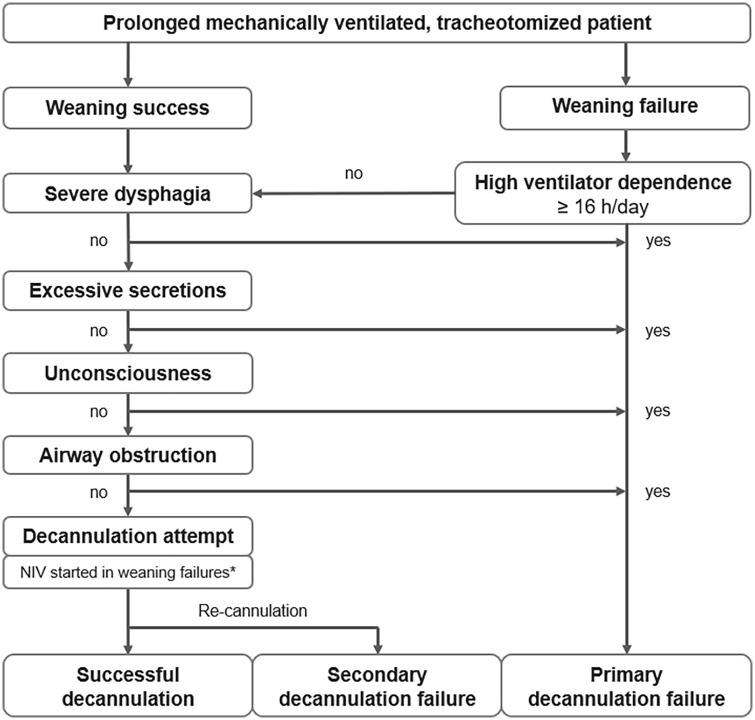
After weaning completion (either successful or unsuccessful), a protocol was used to ensure the main decannulation preconditions were met (Figure 2): (1) severe dysphagia absent (excessive salivation causes aspiration), (2) high dependence on ventilators absent (⩾16 h per day), (3) excessive respiratory secretions absent (generally based on suction frequency), (4) consciousness with preserved airway-protecting reflexes (e.g. coughing), and (5) exclusion of airway obstruction (through flexible endoscopy of the upper airways, subglottic region, and trachea). 11
Figure 2.
Decannulation protocol.
*Refers to patients with low/medium ventilator dependence (⩽8–16 h per day) fulfilling the NIV eligibility criteria (interface tolerance and the ability to self-apply and remove the mask).
NIV, non-invasive ventilation.
Decannulation procedure
We perform decannulation exclusively in the bronchoscopy unit. In the first step, a bronchoscopy was performed via the tracheal cannula to evaluate the amount of respiratory secretions. Nasal or oral access was used to examine the upper respiratory tract, subglottic region, and upper trachea while temporarily removing the cannula in the next step. The patient remained decannulated when no obstruction of the airway was evident. Contrarily, in case of significant airway obstruction (e.g. due to localized granulation tissue at the internal stoma site), the tracheostomy tube was reinserted. As the last step following removal of the cannula, the stoma was either covered with a sterile dressing or occluded with a button, which would enable access to the airway quickly in case of respiratory distress. Decannulation did not involve downsizing of cannulas, 1 capping trials, or high-flow oxygen therapy. 19
Weaning failure patients eligible for NIV (described above) were started on mask ventilation immediately after decannulation. Typically, these patients were provided with a button to facilitate re-cannulation in case of NIV failure (e.g. due to mask intolerance or development of hypercapnia).
Classification of outcomes
According to our decannulation protocol, we divided the causes of decannulation failure into (1) severe dysphagia, (2) long-term dependence on ventilators (following unsuccessful weaning), (3) excessive respiratory secretions, (4) unconsciousness (with impaired airway-protecting reflexes), and (5) airway obstruction (detected through flexible bronchoscopy). Subjects remaining with a tracheostomy tube due to long-term ventilator dependence following weaning were subdivided into those with high dependency on ventilators (⩾16 h per day) and those unable to establish NIV for other reasons.
Furthermore, decannulation failures were classified as primary and secondary. There was no attempt to remove the tracheal cannula in the case of primary failure, and secondary failure refers to a failed attempt at decannulation that requires re-cannulation. The frequency and the time from decannulation to re-cannulation were assessed as part of the study.
Sample size determination
It was planned to create a model with 5–8 variables readily accessible in clinical practice. With an expected failure rate of 30–40%, 5 we calculated a minimum of 200 patients for the derivation set (assuming at least 10 events per candidate predictor), corresponding to 250 patients total. Almost doubling the sample size was necessary to develop separate models for patients with the most common causes of failed decannulation from a clinical perspective (severe dysphagia and long-term ventilator dependence), occurring in about half of failure patients.
Statistical analysis
Our summary of patients’ demographics and baseline characteristics used descriptive and frequency statistics. A Student’s t-test or Mann–Whitney U-test was used to examine differences in continuous variables depending on the distribution determined by the Kolmogorov–Smirnov normality test. A chi-square or Fisher’s exact test analyzed differences in categorical variables between groups.
We performed a multivariable binary logistic regression analysis to determine whether demographics, clinical characteristics, and comorbidities were associated with decannulation failure. In the first step, based on a random sampling of 80% of the study population, we applied univariate regression analysis to each candidate predictor, with decannulation failure as the dependent variable (Supplemental Appendix 1: e-Tables 1 and 2). Those variables with a p value of less than 0.2 (Wald test) have been utilized as input variables for multivariable model development (utilizing forward selection) in the second step. The remaining 20% of subjects were used as a validation set for determining the model’s discriminatory performance, comparing expected to observed outcomes, with the probability cut-off value for expected failure set at 50%. The models were evaluated for goodness-of-fit using the Hosmer and Lemeshow test and the Nagelkerke R2. Probabilities were expressed as odds ratios (ORs) with confidence intervals (95% CIs).
In addition to developing a predictive model for decannulation failure from any cause, we created separate regression models for the leading reasons for unsuccessful decannulation. Reporting of development and validation of the prediction models followed TRIPOD guidelines. 20 All tests were two-tailed; statistical significance was indicated by p < 0.05. MedCalc® statistical software version 20.106 was used for all analyses (MedCalc Software Ltd, Ostend, Belgium).
Results
Five hundred thirty-two out of 575 patients (92.5%) were considered eligible for the study from June 2013 to January 2021. Twenty-seven patients were excluded because of death during weaning, 11 patients were discharged before the first attempt at decannulation, and 5 patients lacked sufficient data regarding weaning (Figure 1).
In unsuccessful versus successful decannulation, the main differences between admission characteristics were age, disease severity (higher Acute Physiology and Chronic Health Evaluation II (APACHE-II) score and lower albumin concentration), and presence of comorbidities (higher Charlson index). Furthermore, the failure group contained more patients with pre-existing domiciliary NIV, dysphagia, and neuromuscular diseases (Table 1). Concerning prolonged ventilator weaning, 59% of all patients were weaned successfully, demonstrating sustained autonomic breathing at hospital discharge. In the decannulation failure group, weaning success rates were significantly lower (42% versus 70%, p < 0.01) (Table 2).
Table 1.
Clinical characteristics on admission to the weaning center – comparison of patients with unsuccessful and successful decannulation.
| Clinical characteristics | All patients (n = 532) | Decannulation failure (n = 216) | Decannulation success (n = 316) | p value a |
|---|---|---|---|---|
| Age (years) | 70 (62–77) | 73 (66–78) | 68 (60–75) | <0.01 b |
| Male gender | 332 (62.4) | 137 (63.4) | 195 (61.7) | 0.688 c |
| Body mass index (kg/m2) | 27.3 (± 6.6) | 26.3 (± 5.8) | 27.9 (± 7.1) | <0.01 b |
| Obesity (defined as BMI ⩾30 kg/m2) | 142 (26.7) | 51 (23.6) | 91 (28.8) | 0.057 c |
| Smoking history | 243 (45.7) | 94 (43.5) | 149 (47.2) | 0.409 c |
| APACHE-II (points) | 17 (13–20) | 17 (14–20) | 16 (13–19) | <0.01 b |
| Albumin (g/dl) | 2.2 (± 0.6) | 2.1 (± 0.6) | 2.2 (± 0.5) | <0.01 b |
| Pre-existing domiciliary NIV | 32 (6.0) | 19 (8.8) | 13 (4.1) | 0.026 c |
| Ventilator days on admission | 22 (15–33) | 21 (14–33) | 23 (16–33) | 0.227 b |
| Intubation to tracheostomy (days) | 10 (6–14) | 9 (6–14) | 10 (7–15) | 0.119 b |
| Percutaneous tracheostomy | 391 (73.5) | 150 (69.4) | 241 (76.3) | 0.080 c |
| ECLA | 35 (6.6) | 7 (3.2) | 28 (8.9) | 0.010 c |
| Reason for mechanical ventilation | ||||
| Pneumonia | 182 (34.2) | 71 (32.9) | 111 (35.1) | 0.591 c |
| Surgery | 122 (22.9) | 48 (22.2) | 74 (23.4) | 0.748 c |
| Acute exacerbation of COPD | 57 (10.7) | 26 (12.0) | 31 (9.8) | 0.415 c |
| Sepsis (including septic shock) | 53 (10.0) | 21 (9.7) | 32 (10.1) | 0.879 c |
| Cardiopulmonary resuscitation | 37 (7.0) | 13 (6.0) | 24 (7.6) | 0.483 c |
| Acute heart failure | 18 (3.4) | 10 (4.6) | 8 (2.5) | 0.189 c |
| Other | 63 (11.8) | 27 (12.5) | 36 (11.4) | 0.698 c |
| Comorbidities | ||||
| Charlson comorbidity index (points) | 6 (4–7) | 6 (5–8) | 5 (4–7) | <0.01 b |
| Diabetes mellitus | 161 (30.3) | 64 (29.6) | 97 (30.7) | 0.793 c |
| Coronary artery disease | 151 (28.4) | 60 (27.8) | 91 (28.8) | 0.798 c |
| Renal insufficiency | 147 (27.6) | 62 (28.7) | 85 (26.9) | 0.648 c |
| Hemodialysis on admission | 71 (13.3) | 32 (14.8) | 39 (12.3) | 0.320 c |
| COPD | 140 (26.3) | 58 (26.9) | 82 (25.9) | 0.817 c |
| Immunosuppression | 86 (16.2) | 26 (12.0) | 60 (19.0) | 0.033 c |
| Chronic heart failure | 84 (15.8) | 33 (15.3) | 51 (16.1) | 0.789 c |
| Malignancy | 51 (9.6) | 19 (8.8) | 32 (10.1) | 0.609 c |
| Hepatopathy | 35 (6.6) | 13 (6.0) | 22 (7.0) | 0.667 c |
| Interstitial lung diseases | 32 (6.0) | 8 (3.7) | 24 (7.6) | 0.064 c |
| Neuromuscular diseases | 19 (3.6) | 15 (6.9) | 4 (1.3) | <0.01 c |
| Pre-existing dysphagia | 13 (2.4) | 11 (5.1) | 2 (0.6) | <0.01 c |
APACHE-II, Acute Physiology and Chronic Health Evaluation II score; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ECLA, extracorporeal lung assistance; NIV, non-invasive ventilation.
Continuous variables are presented as median (– interquartile range [IQR]) or arithmetic means (± standard deviation); categorical variables are presented as numbers (%).
p value for differences between patients with unsuccessful and successful decannulation. Significant values are in bold.
Mann–Whitney U-test.
Chi-square test.
Table 2.
Results of prolonged weaning – comparison of patients with unsuccessful and successful decannulation.
| Results of prolonged weaning | All patients (n = 532) | Decannulation failure (n = 216) | Decannulation success (n = 316) | p value a |
|---|---|---|---|---|
| Weaning completion to decannulation (days) | 5 (3–11) | – | 5 (3–11) | – |
| Decannulation to discharge (days) | 13 (8–21) | – | 13 (8–21) | – |
| Secondary decannulation failures | 30 (5.6) | 30 (13.9) | – | – |
| Decannulation to re-cannulation (days) | 2 (1–4) | 2 (1–4) | – | – |
| Weaning success | 311 (58.5) | 91 (42.1) | 220 (69.6) | < 0.01 b |
| Weaning failure | 221 (41.5) | 125 (57.9) | 96 (30.4) | < 0.01 b |
| Home mechanical ventilation – NIV | 96 (18.0) | – | 96 (30.4) | – |
| Home mechanical ventilation – IMV | 125 (23.5) | 125 (57.9) | – | – |
| Duration of mechanical ventilation (days)* | 44 (32–57) | 47 (33–66) | 41 (31–54) | <0.01 c |
| PEG feeding tube placement | 280 (52.6) | 165 (76.4) | 115 (36.4) | <0.01 b |
| NPO recommendation on discharge | 198 (37.2) | 152 (70.4) | 46 (14.6) | <0.01 b |
IMV, invasive mechanical ventilation; NIV, non-invasive mechanical ventilation; NPO, nil per os; PEG, percutaneous endoscopic gastrostomy.
Continuous variables are presented as median (– interquartile range [IQR]); categorical variables are presented as numbers (%).
p value for differences between patients with unsuccessful and successful decannulation. Significant values are in bold.
Chi-square test.
Mann–Whitney U-test.
Time spent on invasive ventilation, defined as the period between intubation and prolonged ventilator weaning completion.
Decannulation procedure
The median time between weaning completion and the first attempted decannulation was 5 days [interquartile range (IQR), 3–11 days], and the median time from successful decannulation to hospital discharge was 13 days (8–21 days), respectively (Table 2). A button was placed in 204 successfully decannulated patients (65%), compared to 25 patients (83%) with secondary failures, with a median duration of 3 days (2–3 days) and 2 days (1–3 days), respectively.
Frequency and causes of decannulation failure
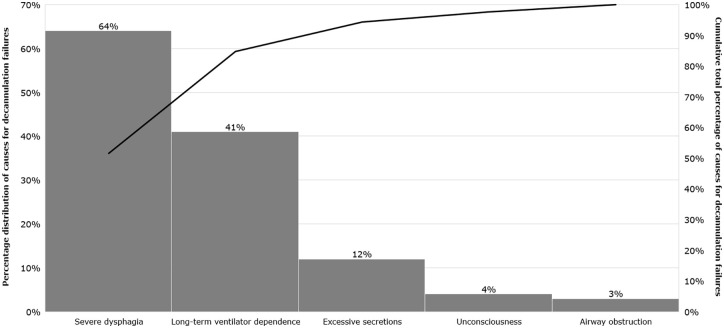
Unsuccessful decannulation from any cause occurred in 216 patients (41%), of whom 30 patients (6%) had secondary failures (Figure 1). In contrast, only 91 out of 311 (29%) successfully weaned patients failed decannulation. Among all patients, the main reasons for failure were severe dysphagia (64%) and long-term dependence on ventilators (41%; 28% were highly ventilator-dependent, and 13% failed to establish NIV for other reasons). The less common causes were excessive respiratory secretions (12%), unconsciousness (4%), or airway obstructions (3%) due to tracheal stenosis or bilateral paralysis of the vocal cords. In 48 patients (22%) who failed to decannulate, more than one cause was noted, and six patients (3%) showed more than two reasons (Figure 3).
Figure 3.
Causes of unsuccessful decannulation following prolonged weaning. Pareto diagram showing the percentage distribution of causes for decannulation failures on the left (histogram) and the cumulative total percentage on the right (black line).
Among the 30 patients (6%) with secondary failures (re-cannulations), the median time to re-cannulation was 2 days (1–4 days) (Table 2). Re-cannulations were mainly caused by hypercapnia despite the application of NIV (43%) and retained respiratory secretions (53%).
Non-invasive ventilation for weaning failures
NIV as a weaning technique was successfully performed on 96 of 221 (43%) weaning failure patients, effectively facilitating successful decannulation (Table 2). A variety of factors contributed to the failure of NIV employment in 29 of 216 patients (13%) with failed decannulations, including mask intolerance (11 patients, 3 had psychiatric diseases), severe critical illness neuropathy that prevented self-employment of the mask (5 patients), and secondary decannulation failures due to hypercapnia occurring during the NIV attempt (13 patients).
In the analysis of the 13 patients with failed attempts at NIV (secondary failures requiring re-cannulation), the median number of hours per day finally spent on tracheostomy ventilation at discharge was 10 h (8–12 h per day), compared with 8 h per day (6–10 h per day) in the 96 patients successfully transitioned to NIV (p < 0.01). In contrast, among 60 of 216 patients (28%) not decannulated due to high ventilator dependence (primary decannulation failure), mechanical ventilation was required for a median of 24 h per day (23–24 h per day) at discharge.
Assessment and management of dysphagia
Severe dysphagia accounted for decannulation failures in 138 patients (64%). There were significantly more patients with PEG feeding tubes placed during weaning (76% versus 36%) and NPO recommendations at discharge from the hospital (70% versus 15%) in the decannulation failure group (Table 2). In addition, when comparing patients with and without severe dysphagia as the primary cause of failure, 89% versus 38% had PEG tubes placed, and 96% versus 17% had NPO instructions at discharge, respectively (p < 0.01).
Predictors of decannulation failure
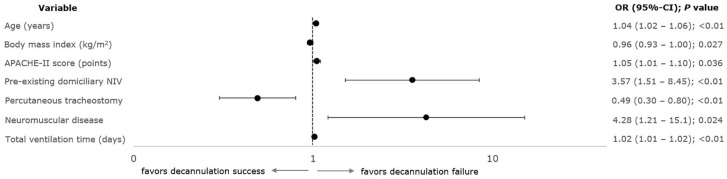
Based on the multivariate binary logistic regression analysis, age [OR = 1.04 year−1; 95% CI = (1.02–1.06)], body mass index [0.96 kg/m2 (0.93–1.00)], APACHE-II score [1.05 (1.00–1.10); p = 0.036], pre-existing domiciliary NIV [3.57 (1.51–8.45)], percutaneous tracheostomies [0.49 (0.30–0.80)], neuromuscular diseases [4.28 (1.21–15.1)], and total duration of (invasive) mechanical ventilation [1.02 day−1 (1.01–1.02)] were independently related to decannulation failure from any cause in 426 derivation cohort subjects (Figure 4; Supplemental Appendix 1: e-Table 3). Validation in the remaining 106 patients revealed similar model performance with reasonable specificity [82% (70–91)] but poor sensitivity [39% (24–54)] and accuracy overall [62% (52–71)] (Supplemental Appendix 1: e-Table 4).
Figure 4.
Variables associated with decannulation failure – results of multivariable binary logistic regression analysis (derivation set). Forest plot of variables independently associated with decannulation failure. One hundred and sixty-seven out of 426 patients (39.2%) in the derivation set failed decannulation. Multivariable regression models included candidate predictors with p values less than 0.2 in the univariable analysis.
APACHE-II, Acute Physiology and Chronic Health Evaluation II score; 95% CI, 95% confidence interval; OR, odds ratio.
Predictors hardly overlapped in the regression models for dysphagia-related failed decannulations and long-term dependence on ventilators. In addition, male gender and chronic obstructive pulmonary disease (COPD) showed opposite correlations with these outcomes, resulting in exclusion from the final model for all types of unsuccessful decannulations (Supplemental Appendix 1: e-Tables 5–7).
Discussion
Study results can be summarized as follows: Prolonged mechanically ventilated patients treated at a specialized weaning center failed decannulation in 41%, mainly due to persistent ICU-acquired dysphagia and long-term dependence on ventilators, each associated with its own set of predictors. Other factors, such as excessive respiratory secretions, unconsciousness, or airway obstruction, contributed to decannulation failure.
Only a few studies have focused on decannulation procedures in prolonged ventilated patients,3,10,21–23 reporting failure rates between 5% and 65%,3,21,22 with considerable heterogeneity in patient characteristics (e.g. neurological versus medical patients), study designs (e.g. excluding patients with weaning failure), and prediction variables analyzed. As part of our methodology, we first determined the major reasons for failure and then analyzed the associated factors, providing a differentiated perspective on decannulation. That’s also why we chose not to exclude weaning failure patients. The present cohort revealed several predictors, such as higher age, lower body mass index, higher comorbidity burden (as measured by the Charlson index), and disease severity (indicated by APACHE-II score), consistent with prior studies conducted in medical and neurological ICU patients.3,10,23 A percutaneous tracheostomy prevented failures, most probably due to physicians preferably referring patients to surgery who are likely to keep their tracheostomies a priori. 10
Logistic regression models’ discriminatory performance for failure from any cause revealed poor accuracy (along with reasonable specificity) since we included predictors associated with the principal causes of decannulation failure rather than the causes themselves. Moreover, predictors for subsets of dysphagia-related failures and long-term ventilator dependence hardly overlapped, contributing to this finding. Nevertheless, the advantage of this approach is that it may identify high-risk patients early during their ICU stay (at the expense of less accurate prognostication), allowing specific interventions to prevent failures (e.g. a timely evaluation of swallowing coupled with tailored speech therapy).
ICU-acquired dysphagia 24 occurs in up to 84% of intubated patients, with incidence varying remarkably between studies25,26 due to differences in swallowing assessment and outcome definitions. 27 Patients with tracheostomies most frequently experience swallowing disorders, with the tracheal cannula itself being a risk factor for dysphagia persistence.28,29 Thus, studies relying exclusively on extubated patients may not reflect the true incidence of dysphagia in intensive care. Specifically, we focused on patients with severe dysphagia preventing decannulation, present in 64% of failures. Still, attenuated diseases contribute to even higher incidence rates across the entire study population. Accordingly, 53% of all patients (whether decannulated or not) underwent PEG tube feedings, and the proportion of patients with NPO recommendations on discharge was 37%, about twice as many as reported after successful extubation (18%). 26 Swallowing impairments in our cohort were independently related to a higher age and total duration of mechanical ventilation, matching prior observations in non-neurologically critically ill patients.25,30 Interestingly, high body mass indexes and COPD presence seem to protect against swallowing dysfunction, though no thorough explanation can be offered.
Our study’s factors independently associated with long-term dependence on ventilators correlated well with predictors identified in previous research.5,31 NIV effectively facilitated decannulation in 96 of 221 weaning failure patients (43%) with low dependence on ventilators. Considering reports on invasively ventilated patients in outpatient settings, widespread adoption of this strategy may help prevent health-related quality of life impairments and medical care costs following unsuccessful weaning. 15 So far, the literature provides little information regarding the role of NIV in decannulating prolonged ventilated patients with tracheostomies. While most articles report the percentage of patients who had NIV following prolonged ventilation, they rarely provide details regarding the decannulation protocol or the intensity and duration of NIV.2,32,33 Ceriana et al. 2 proposed a protocol for implementing NIV in tracheotomized patients developing hypercapnia after weaning completion. Approximately 10% of patients assigned to the decannulation protocol failed the NIV attempt, matching our results, considering 13 failed attempts from 109 patients (11.9%) following unsuccessful weaning. Similarly, they received NIV for an average of 6–8 h a day. Thus, higher ventilation times in patients who failed to switch to NIV probably indicate a threshold for safe NIV employment.
When determining whether a patient should undergo decannulation, clinicians consider both the amount of respiratory secretions and their level of consciousness to be essential factors, 8 which accounted for 16% of failures in the present study. Despite the difficulty of quantifying, assessing the frequency of suctioning (the way it is also applied in our center) is a commonly used method of evaluating secretions.19,34 This approach has also been superior to capping trials before decannulation (simulating tracheostomy tube removal), which significantly delayed decannulation and increased failure rates in a large randomized controlled trial. 19 In the present analysis, despite its significance, unconsciousness (associated with impaired airway-protecting reflexes) was only a minor contributing factor in failed decannulations. These observations are most likely because patients treated in centers specializing in prolonged ventilator weaning (usually referred by medical and surgical ICUs) differ significantly from those in neuro-intensive care and neuro-rehabilitation facilities, where central nervous system diseases are generally the leading cause of mechanical ventilation. 10 Airway obstruction following prolonged weaning is typically a consequence of granulation tissue forming at the site of the internal stoma.11,35 Interventional bronchoscopy proved safe and effective in treating such tracheal stenoses, 11 explaining why airway obstruction accounted for only a small number of failed decannulations in the present analysis.
Although this is the largest study to provide a comprehensive overview of causes and predictors of unsuccessful decannulations in patients who have been ventilated for prolonged periods, this analysis has limitations. First, due to the single-center nature of the study, conducted at a reference center for difficult weaning patients, the results are not necessarily generalizable, even though only minimal exclusion criteria were applied. Furthermore, since this was a retrospective analysis, we may have missed confounding factors causing critical covariates to be imbalanced among groups, which could have biased the results. Second, although we used a standardized swallowing assessment, future prospective studies should evaluate dysphagia severity exclusively based on fiberoptic swallowing evaluations and validated dysphagia scores, allowing more accurate comparison of swallowing impairments between subjects. Third, since patients were only observed until discharge from the hospital without further follow-up, information about subsequent re-cannulations is lacking. However, the observed time between successful decannulation and hospital discharge (2 weeks in median) seems sufficient for thoroughly evaluating a patient’s airway-protecting and spontaneous breathing abilities, the main determinants of decannulation failure or success in the present analysis.
Conclusion
Forty percent of prolonged ventilated patients failed decannulation following weaning, mainly due to persistent, severe ICU-acquired dysphagia and long-term dependence on ventilators, each associated with its own set of predictors, suggesting a more critical view of the decannulation process is warranted (e.g. when planning future studies). Based on these results, a timely evaluation of patients at high risk for dysphagia coupled with a tailored speech therapy program and widespread adoption of NIV as a weaning technique may help prevent decannulation failures.
Authors’ note
This work was performed at the Schillerhoehe Lung Clinic, Solitudestrasse 18, 70839 Gerlingen (Germany).
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221109655 for Incidence, causes, and predictors of unsuccessful decannulation following prolonged weaning by Alessandro Ghiani, Konstantinos Tsitouras, Joanna Paderewska, Katrin Milger, Swenja Walcher, Mareike Weiffenbach, Claus Neurohr and Nikolaus Kneidinger in Therapeutic Advances in Chronic Disease
Acknowledgments
Not applicable.
Footnotes
ORCID iDs: Alessandro Ghiani  https://orcid.org/0000-0002-4137-5373
https://orcid.org/0000-0002-4137-5373
Nikolaus Kneidinger  https://orcid.org/0000-0001-7583-0453
https://orcid.org/0000-0001-7583-0453
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alessandro Ghiani, Lung Center Stuttgart – Schillerhoehe Lung Clinic, Department of Pulmonology and Respiratory Medicine, Affiliated to the Robert-Bosch-Hospital GmbH, Auerbachstr. 110, 70376 Stuttgart, Germany.
Konstantinos Tsitouras, Lung Center Stuttgart – Schillerhoehe Lung Clinic, Department of Pulmonology and Respiratory Medicine, Affiliated to the Robert-Bosch-Hospital GmbH, Stuttgart, Germany.
Joanna Paderewska, Lung Center Stuttgart – Schillerhoehe Lung Clinic, Department of Pulmonology and Respiratory Medicine, Affiliated to the Robert-Bosch-Hospital GmbH, Stuttgart, Germany.
Katrin Milger, Department of Internal Medicine V (Pulmonology), Ludwig-Maximilians-University (LMU) of Munich, Munich, Germany; Comprehensive Pneumology Center (CPC-M), German Center for Lung Research (DZL), Munich, Germany.
Swenja Walcher, Lung Center Stuttgart – Schillerhoehe Lung Clinic, Department of Pulmonology and Respiratory Medicine, Affiliated to the Robert-Bosch-Hospital GmbH, Stuttgart, Germany.
Mareike Weiffenbach, Department of Acute Geriatrics and Geriatric Rehabilitation, Robert-Bosch-Hospital GmbH, Stuttgart, Germany.
Claus Neurohr, Lung Center Stuttgart – Schillerhoehe Lung Clinic, Department of Pulmonology and Respiratory Medicine, Affiliated to the Robert-Bosch-Hospital GmbH, Stuttgart, Germany; Comprehensive Pneumology Center (CPC-M), German Center for Lung Research (DZL), Munich, Germany.
Nikolaus Kneidinger, Department of Internal Medicine V (Pulmonology), Ludwig-Maximilians-University (LMU) of Munich, Munich, Germany; Comprehensive Pneumology Center (CPC-M), German Center for Lung Research (DZL), Munich, Germany.
Declarations
Ethics approval and consent to participate: The study was approved by the local institutional review board for human studies (Ethics Committee of the State Chamber of Physicians of Baden-Wuerttemberg, Germany, file number F–2021–087) and performed following the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the analysis.
Consent for publication: Not applicable.
Author contributions: Alessandro Ghiani: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Writing – original draft.
Konstantinos Tsitouras: Data curation; Formal analysis; Writing – review & editing.
Joanna Paderewska: Data curation; Formal analysis; Writing – review & editing.
Katrin Milger: Formal analysis; Writing – review & editing.
Swenja Walcher: Data curation; Formal analysis; Writing – review & editing.
Mareike Weiffenbach: Data curation; Formal analysis; Writing – review & editing.
Claus Neurohr: Formal analysis; Supervision; Writing – review & editing.
Nikolaus Kneidinger: Formal analysis; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Ceriana P, Carlucci A, Navalesi P, et al. Weaning from tracheotomy in long-term mechanically ventilated patients: feasibility of a decisional flowchart and clinical outcome. Intensive Care Med 2003; 29: 845–848. [DOI] [PubMed] [Google Scholar]
- 2. Ceriana P, Nava S, Vitacca M, et al. Noninvasive ventilation during weaning from prolonged mechanical ventilation. Pulmonology 2019; 25: 328–333. [DOI] [PubMed] [Google Scholar]
- 3. Park C, Ko RE, Jung J, et al. Prediction of successful decannulation of tracheostomized patients in medical intensive care units. Respir Res 2021; 22: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jubran A, Grant BJB, Duffner LA, et al. Long-term outcome after prolonged mechanical ventilation. A long-term acute-care hospital study. Am J Respir Crit Care Med 2019; 199: 1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghiani A, Paderewska J, Sainis A, et al. Variables predicting weaning outcome in prolonged mechanically ventilated tracheotomized patients: a retrospective study. J Intensive Care 2020; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghiani A, Paderewska J, Walcher S, et al. Mechanical power normalized to lung-thorax compliance predicts prolonged ventilation weaning failure: a prospective study. BMC Pulm Med 2021; 21: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jubran A, Grant BJB, Duffner LA, et al. Effect of pressure support vs unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation: a randomized trial. JAMA 2013; 309: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stelfox HT, Crimi C, Berra L, et al. Determinants of tracheostomy decannulation: an international survey. Crit Care 2008; 12: R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santus P, Gramegna A, Radovanovic D, et al. A systematic review on tracheostomy decannulation: a proposal of a quantitative semiquantitative clinical score. BMC Pulm Med 2014; 14: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heidler MD, Salzwedel A, Jöbges M, et al. Decannulation of tracheotomized patients after long-term mechanical ventilation – results of a prospective multicentric study in German neurological early rehabilitation hospitals. BMC Anesthesiol 2018; 18: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghiani A, Tsitouras K, Paderewska J, et al. Tracheal stenosis in prolonged mechanically ventilated patients: prevalence, risk factors, and bronchoscopic management. BMC Pulm Med 2022; 22: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29: 1033–1056. [DOI] [PubMed] [Google Scholar]
- 13. Schönhofer B, Geiseler J, Dellweg D, et al. Prolonged weaning: S2k guideline published by the German respiratory society. Respiration 2020; 99: 982–1083. [DOI] [PubMed] [Google Scholar]
- 14. Vitacca M, Ambrosino M, Clini E, et al. Physiological response to pressure support ventilation delivered before and after extubation in patients not capable of totally spontaneous autonomous breathing. Am J Respir Crit Care Med 2001; 164: 638–641. [DOI] [PubMed] [Google Scholar]
- 15. Huttmann SE, Magnet FS, Karagiannidis C, et al. Quality of life and life satisfaction are severely impaired in patients with long-term invasive ventilation following ICU treatment and unsuccessful weaning. Ann Intensive Care 2018; 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Windisch W, Geiseler J, Simon K, et al. German national guideline for treating chronic respiratory failure with invasive and non-invasive ventilation: revised edition 2017 − part 1. Respiration 2018; 96: 66–97. [DOI] [PubMed] [Google Scholar]
- 17. Bechet S, Hill F, Gilheaney O, et al. Diagnostic accuracy of the modified Evan’s blue dye test in detecting aspiration in patients with tracheostomy: a systematic review of the evidence. Dysphagia 2016; 31: 721–729. [DOI] [PubMed] [Google Scholar]
- 18. Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996; 11: 93–98. [DOI] [PubMed] [Google Scholar]
- 19. Hernandez Martinez G, Rodriguez ML, Vaquero MC, et al. High-flow oxygen with capping or suctioning for tracheostomy decannulation. N Engl J Med 2020; 383: 1009–1017. [DOI] [PubMed] [Google Scholar]
- 20. Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol 2015; 68: 134–143. [DOI] [PubMed] [Google Scholar]
- 21. O’Connor HH, Kirby KJ, Terrin N, et al. Decannulation following tracheostomy for prolonged mechanical ventilation. J Intensive Care Med 2009; 24: 187–194. [DOI] [PubMed] [Google Scholar]
- 22. Choate K, Barbetti J, Currey J. Tracheostomy decannulation failure rate following critical illness: a prospective descriptive study. Aust Crit Care 2009; 22: 8–15. [DOI] [PubMed] [Google Scholar]
- 23. Pasqua F, Nardi I, Provenzano A, et al. Weaning from tracheostomy in subjects undergoing pulmonary rehabilitation. Multidiscip Respir Med 2015; 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Macht M, Wimbish T, Bodine C, et al. ICU-acquired swallowing disorders. Crit Care Med 2013; 41: 2396–2405. [DOI] [PubMed] [Google Scholar]
- 25. Macht M, Wimbish T, Clark BJ, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care 2011; 15: R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schefold JC, Berger D, Zürcher P, et al. Dysphagia in mechanically ventilated ICU patients (DYnAMICS): a prospective observational trial. Crit Care Med 2017; 45: 2061–2069. [DOI] [PubMed] [Google Scholar]
- 27. Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest 2010; 137: 665–673. [DOI] [PubMed] [Google Scholar]
- 28. Garuti G, Reverberi C, Briganit A, et al. Swallowing disorders in tracheotomised patients: a multidisciplinary/multiprofessional approach in decannulation protocols. Multidiscip Respir Med 2014; 9: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zielske J, Bohne S, Brunkhorst FM, et al. Acute and long-term dysphagia in critically ill patients with severe sepsis: results of a prospective controlled observational study. Eur Arch Otorhinolaryngol 2014; 271: 3085–3093. [DOI] [PubMed] [Google Scholar]
- 30. Skoretz SA, Yau TM, Ivanov J, et al. Dysphagia and associated risk factors following extubation in cardiovascular surgical patients. Dysphagia 2014; 29: 647–654. [DOI] [PubMed] [Google Scholar]
- 31. Magnet FS, Bleichroth H, Huttmann SE, et al. Clinical evidence for respiratory insufficiency type II predicts weaning failure in long-term ventilated, tracheotomised patients: a retrospective analysis. J Intensive Care 2018; 6: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guia M, Ciobanu LD, Sreedharan JK, et al. The role of non-invasive ventilation in weaning and decannulating critically ill patients with tracheostomy: a narrative review of the literature. Pulmonology 2021; 27: 43–51. [DOI] [PubMed] [Google Scholar]
- 33. Sancho J, Servera E, Jara-Palomares L, et al. Noninvasive ventilation during the weaning process in chronically critically ill patients. ERJ Open Res 2016; 2: 00061-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hernandez G, Ortiz R, Pedrosa A, et al. The indications for tracheotomy conditions the predictors of time to decannulation in critical patients. Med Intensiva 2012; 36: 531–539. [DOI] [PubMed] [Google Scholar]
- 35. Hagmeyer L, Oesterlee U, Treml M, et al. Successful weaning and decannulation after interventional bronchoscopic recanalization of tracheal stenosis. J Crit Care 2014; 29: 695.e9–695.e14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221109655 for Incidence, causes, and predictors of unsuccessful decannulation following prolonged weaning by Alessandro Ghiani, Konstantinos Tsitouras, Joanna Paderewska, Katrin Milger, Swenja Walcher, Mareike Weiffenbach, Claus Neurohr and Nikolaus Kneidinger in Therapeutic Advances in Chronic Disease