Abstract
Background:
Gemogenovatucel-T (Vigil) is a triple-function autologous tumor cell immunotherapy which expresses granulocyte-macrophage colony-stimulating factor and decreases expression of furin and downstream TGF-β1 and TGF-β2. Vigil has suggested survival benefit in frontline maintenance ovarian cancer patients who are BRCA-wt. In addition, Vigil demonstrates relapse-free and overall survival advantage in homologous recombination-proficient patients with OC. Further evidence of clinical benefit and safety has been demonstrated in combination with atezolizumab.
Methods:
In this pilot study (NCT02725489), the concurrent combination of the programmed death-ligand 1 (PD-L1) inhibitor durvalumab and Vigil was explored in advanced BRCA-wt relapsed triple-negative breast cancer (TNBC) patients and stage III-IV recurrent/refractory OC patients. Patients received the combination regimen of Vigil (1 × 10e6-10e7 cells/dose intradermally, up to 12 doses) and durvalumab (1500 mg/dose intravenous infusion, up to 12 months) once every 4 weeks. The primary objective was to evaluate safety of this combination. The study included 13 BRCA-wt patients (TNBC, n = 8; OC, n = 5).
Results:
The most common treatment-emergent adverse events (⩾20%) in all patients included injection-site reaction (92.3%), myalgia (38.5%), bruise at injection site (23.1%), and pruritus (23.1%). Three grade 3 treatment-related adverse events were observed and related to durvalumab. There were no grade 4/5 treatment-related adverse events. Median progression-free survival was 7.1 months and the median overall survival was not reached. Prolonged progression-free survival was improved in patients with PD-L1+ tumors (n = 8, hazard ratio = 0.304, 95% confidence interval, 0.0593-1.56, 1-sided P = .04715) compared with those with PD-L1− tumors.
Conclusions:
Vigil plus durvalumab was well tolerated and showed promising clinical activity in advanced BRCA-wt TNBC and stage III-IV recurrent/refractory OC patients.
Keywords: Ovarian cancer, triple-negative breast cancer, Vigil, durvalumab, checkpoint inhibitor
Introduction
Women’s cancers consist of a mixture of aggressive malignancies that are associated with poor prognosis and clinical outcomes. Breast cancer (BC) is the most prevalent cancer in women 1 and is divided into clinical subtypes, of which triple-negative breast cancer (TNBC: HER2−, ER−, PR−) accounts for nearly 15%.2 -5 Ovarian cancer (OC) is the fifth leading cause of cancer deaths and accounts for 2.3% of all cancer deaths. 6 Five to 10% of all BCs and up to 15% to 25% of all OCs are associated with inheritable genetic mutations.7 -9 Approximately 20% of TNBC10,11 and 15% OC patients are BRCA mutant (BRCA-m), whereas >80% of patients are BRCA wild type (BRCA-wt). 12 Despite significant advantages with checkpoint inhibitor (CI) therapies, poly(ADP)-ribose polymerase (PARP) inhibitors, or vascular endothelial growth factor inhibitors, minimal clinical benefit to these targeted therapies has been shown in BRCA-wt TNBC and OC.10,13 -16 The prognosis remains poor, with 5-year overall survival (OS) estimates of 52% 10 and 25% 16 in TNBC and OC, respectively.
Chemotherapy has been associated with short progression-free survival (PFS) and poor response rates in these TNBC patients 17 ; however, it remains a part of the standard care of treatment for previously treated TNBC. 18 It is possible that immunotherapy might be beneficial in TNBC. 19 Capecitabine maintenance showed improved disease-free survival compared with observation (hazard ratio [HR] = 0.64; 95% CI, 0.42-0.95, P = .03); however, no benefit was seen in 5-year OS (HR = 0.75; 95% CI, 0.47-1.19, P = .22). 20 In relapsed OC, treatment response rates remain low with short progression-free intervals.21,22 Poly(ADP)-ribose polymerase inhibitors have been Food and Drug Administration–approved for the treatment and maintenance of relapsed OC.23 -26 However, the use of PARP inhibitors is less effective in BRCA-wt OC patients compared with those with BRCA-m tumors. In platinum-sensitive relapsed OC survival, advantages to placebo, however, were demonstrated in those who are BRCA-m with olaparib (study 19) (BRCA-m, HR = 0.18; compared with BRCA-wt, HR = 0.54), 27 as well as SOLO-2 with a median PFS (mPFS) of 19.3 months olaparib versus 5.5 months placebo (BRCA-m, HR = 0.33). 28 The NOVA study of niraparib in platinum-sensitive relapsed OC (gBRCA-m, HR = 0.27; non-gBRCA-m [BRCA-wt], HR = 0.45) did not show any OS advantage.23,29
Checkpoint inhibitors as single agent have not demonstrated treatment benefit in relapsed OC thus far. 30 Tumor cell–expressed programmed death-ligand 1 ligand (PD-L1) binds PD-1 receptors to inactivate T cells and evade immune-mediated response. 31 Expression of PD-L1 positively correlates with the presence of tumor-infiltrating lymphocytes (TILs) and expression of both is higher in TNBC tumors,32,33 with PD-L1 found in 20% to 30% of TNBCs. 34 Blockade of the PD-1/PD-L1 axis has been shown to restore effector T-cell activity in a variety of cancers including TNBC. 35
Beyond the firstline setting, treatment options in TNBC include the anti-trophoblast cell surface antigen 2 (Trop-2) antibody-drug conjugate sacituzumab govitecan36,37 and the PD-L1 inhibitor pembrolizumab in combination with chemotherapy.18,38,39 In study of 468 patients, sacituzumab govitecan revealed improved PFS (for all patients 4.8 versus 1.7 months, HR = 0.43, P < .001) and OS (for patients without brain metastases 12.1 versus 6.7 months, HR = 0.48, P < .001) compared with chemotherapy of physician’s choice. 37 In 566 PD-L1 + TNBC patients (KEYNOTE-355), mPFS was extended 4.1 months with the addition of pembrolizumab to chemotherapy (mPFS 9.7 months) versus chemotherapy alone (mPFS 5.6 months) (HR = 0.65, 1-sided P = .0012) but fatal (2.5% of patients) and serious (~1/3 of patients) treatment-related adverse reactions were reported.37 -39 Moreover, the immunotherapy combination of niraparib and pembrolizumab (TOPACIO) in advanced TNBC patients resulted in higher responses in those with tumor BRCA-m (mPFS 8.3 months) versus those with BRCA-wt (mPFS of only 2.1 months), 40 leaving BRCA-wt TNBC patient treatment needs unmet.
Durvalumab is an anti-PD-L1 antibody approved in lung cancers 41 but specifically has not shown benefit in BRCA-wt TNBC patients. 42 In BRCA-wt platinum-sensitive relapsed OC patients, the triple combination of bevacizumab/olaparib/durvalumab (MEDIOLA) suggested prolonged progression-free survival (mPFS for triple combination bevacizumab/olaparib/durvalumab 14/7 months versus doublet olaparib/durvalumab mPFS 5.5 months) supporting further exploration in an ongoing phase 3 study (DUO-O). 43
Vigil is an autologous cellular immunotherapy comprising irradiated tumor cells that encompass the full matrix of the patient’s tumor-associated antigens.44,45 Genetic modifications of the autologous tumor cell product are made to optimize tumor-specific antigen presentation, dendritic cell activation (increasing GM-CSF), and tolerance escape (blocking TGF-β1 activation). 45 Recently, efficacy involving relapse-free survival and OS was suggested in BRCA-wt OC patients who received Vigil in the frontline maintenance setting (VITAL study), as well as homologous recombination proficient (HRP) OCs (HR = 0.386) when compared with placebo.46,47 Another pilot study of Vigil administered first in sequence with combination atezolizumab demonstrated safety and preliminary survival advantage in BRCA-wt relapsed OC patients. 48
This proof-of-principle study evaluated the safety of Vigil combined with the anti-PD-L1 antibody durvalumab together in relapsed BRCA-wt TNBC and OC patients.
Materials and Methods
Trial design and treatments
This article evaluates the phase 2 study “Pilot Study of Durvalumab and Vigil in Advanced Women’s Cancers” (NCT02725489). This study originally contained 2 parts, the first part of which is reported in the article. This part was a safety run-in to evaluate the Vigil dose (either cohort 1: 1 × 106 or cohort 2: 1 × 107 cells). We determined that both dose levels were safe. Part 2 of the study was not initiated, due to the inability of the single enrollment site to identify further eligible patients. However, further phase 2/3 clinical trials are under consideration.
This pilot study was conducted at Mary Crowley Cancer Research Centers. For Vigil construction, BC or OC tissue was collected during standard of care surgical procedure. Tissue and peripheral blood mononuclear cell samples were collected and analyzed for BRCA1/2 molecular profiling using a cell quality of 40 and a minimum allele depth of 5 (Ocean Ridge Biosciences, Deerfield Beach, Florida). Tumor tissue was sent for homologous recombination deficiency testing using myChoice CDx (Myriad, Inc., Salt Lake City, Utah). Per assay guidelines, a score of ⩾42 was used to identify patients who were homologous recombination deficient (HRD), and <42 who were HRP. PD-L1 status was determined by NeoGenomics Laboratories (Fort Myers, Florida) with monoclonal rabbit anti-PD-L1 antibody, clone 28-8 for 4 patients or ProPath (Dallas, Texas) with anti-PD-L1 antibody E1L3N for 9 patients. PD-L1 positivity was based on a score ⩾1% assessed by immunohistochemistry, PD-L1 negativity <1%. Patients received Vigil (1 × 10e6-10e7 cells/dose intradermally) and durvalumab (1500 mg/dose intravenous infusion) once every 4 weeks for up to 12 doses. Informed consent was obtained prior to procurement and prior to main study registration. Written documentation of full institutional review board approval of the protocol and consent documents were obtained before initiation of the study. The trial is registered with clinicaltrials.gov, NCT02725489.
Patients
Women who had histologically confirmed diagnosis of TNBC or OC (histologies included high-grade serous carcinoma [n = 2], serous [n = 2], and papillary serous carcinoma [n = 1]) who had failed at least one prior line of standard of care (SOC) therapy were eligible for the trial. Subjects were required to have at least 4 vials of Vigil-manufactured, Eastern Cooperative Oncology Group (ECOG)-performance status (PS) ⩽ 1, and normal organ and marrow function as defined per-protocol. In addition, absolute neutrophil count ⩾ 1500/mm3, platelets ⩾ 100 000/mm3, hemoglobin ⩾ 5.59 mmol/L, serum bilirubin ⩽ 1.5× institutional upper limit of normal, aspartate transaminase/alanine transaminase (AST/ALT) ⩽ 2.5× institutional upper limit of normal, creatinine > 50 mL/min, thyroid-stimulating hormone within institutional limits were required for enrollment.
Tumor procurement and manufacturing
Gradalis, Inc. (Carrollton, Texas) manufactured Vigil from the harvested tumor tissue. Manufacturing was a 2-day process. The equivalent of a “golf ball size” mass (10-30 g tissue, cumulative) was required for Vigil manufacturing. Lesions extending into the bowel lumen were excluded due to the risk of bacterial contamination. Vigil plasmid construction, cGMP manufacturing, tissue processing, and transfection were performed as previously described.6,45,47,49 Briefly, a tumor cell suspension is transfected with Vigil plasmid. Following transfection, cells are irradiated at 10 000 cGy and aliquoted at 1 × 107 cells per vial. Vials are frozen until administration.
Disease evaluation and efficacy assessments
Subjects remained on treatment until disease progression, death, product toxic effect, or until Vigil dose exhaustion. Disease progression was determined radiographically by local investigators using the Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1). Disease was assessed at baseline, at cycle 3, every 2 cycles thereafter, and at the end of treatment.
The data cutoff date for analysis was February 10, 2021. The primary endpoint was to evaluate and characterize the tolerability and safety profile of Vigil combined with durvalumab. Investigators assessed and reported adverse events. After study completion, post hoc analysis of PFS and OS was assessed in (1) all patients, intent-to-treat (ITT), (2) TNBC patients, and (3) OC patients. The PFS and OS were estimated using the Kaplan-Meier (KM) method and assessed in GraphPad PRISM (San Diego, California). Hazard ratio was assessed by log-rank analysis and 1-sided log-rank P values. The proportional hazards assumption was assessed by the Grambsch and Therneau test.
Results
Patient population
From August 2, 2016 through January 22, 2019, 13 patients were registered onto the study, of which 8 were TNBC and 5 OC (Figure 1). All patients were BRCA-wt. Nine of the 13 patients had tissue available for HR testing, 6 patients were HRP, 3 were HRD. Eight of the 13 patients were PD-L1+ (Figure 1). All patients failed at least one prior systemic therapy (median 4, range 1-6). Patient demographics are summarized in Table 1. The median follow-up time was 14.7 months.
Figure 1.
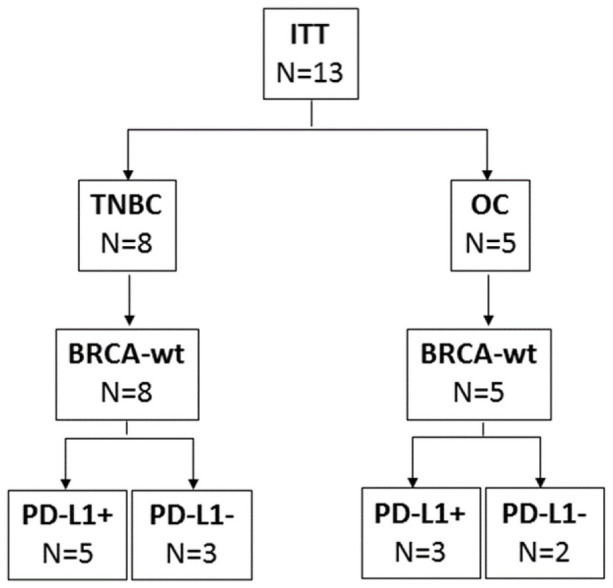
Consort diagram. Thirteen patients consented and registered onto trial, of which 8 were TNBC and 5 OC. All 13 patients were BRCA-wt. Five of the 8 TNBC patients were PD-L1+, and 3 of the 5 OC patients were PD-L1+.
ITT indicates intent-to-treat; OC, ovarian cancer; PD-L1, programmed death-ligand 1; TNBC, triple-negative breast cancer.
Table 1.
Patient characteristics at baseline.
| Characteristic | All patients (n = 13) | TNBC patients (n = 8) | OC patients (n = 5) |
|---|---|---|---|
| Age, median (range) | 50 (39-74) | 48 (39-55) | 66 (55-74) |
| ECOG performance status, No. (%) | |||
| 0 | 8 (61.5) | 5 (62.5) | 3 (60) |
| 1 | 5 (38.5) | 3 (37.5) | 2 (40) |
| Race, No. (%) | |||
| Asian | 1 (7.7) | 1 (12.5) | 0 (0) |
| Black or African American | 1 (7.7) | 0 (0) | 1 (20) |
| Caucasian or White | 10 (76.9) | 7 (87.5) | 3 (60) |
| Unknown | 1 (7.7) | 0 (0) | 1 (20) |
| Primary cancer, No. (%) | |||
| Breast | 8 (61.5) | 8 (100) | 0 (0) |
| Ovarian | 4 (30.8) | 0 (0) | 4 (80) |
| Peritoneal | 1 (7.7) | 0 (0) | 1 (20) |
| Tumor stage at diagnosis | |||
| I | 0 (0) | 0 (0) | 0 (0) |
| II | 5 (38.4) | 5 (62.5) | 0 (0) |
| III | 6 (46.15) | 1 (12.5) | 5 (100) |
| IV | 1 (7.6) | 1 (12.5) | 0 (0) |
| Unknown | 1 (7.6) | 1 (12.5) | 0 (0) |
| N stage at diagnosis | |||
| 0 | 0 (0) | 0 (0) | 1 (20) |
| 1 | 4 (30.8) | 3 (37.5) | 4 (80) |
| 2 | 2 (15.4) | 2 (25) | 0 (0) |
| 3 | 2 (15.4) | 2 (25) | 0 (0) |
| Unknown | 1 (7.6) | 1 (12.5) | 0 (0) |
| M stage at diagnosis | |||
| 0 | 6 (46.15) | 4 (50) | 2 (40) |
| 1 | 3 (23.1) | 2 (25) | 1 (20) |
| Unknown | 4 (30.8) | 2 (25) | 2 (40) |
| Prior lines of therapy, median (range) | 4 (1-6) | 5 (1-6) | 1 (1-6) |
| BRCA status, No. (%) a | |||
| g/sBRCA1/2wt | 13 (100) | 8 (100) | 5 (100) |
| g/sBRCA1/2m | 0 (0) | 0 (0) | 0 (0) |
| Homologous recombination status, No. (%) b | |||
| HRP | 6 (46) | 3 (37.5) | 3 (60) |
| HRD | 3 (23) | 2 (25) | 1 (20) |
| NA | 4 (31) | 3 (37.5) | 1 (20) |
| PD-L1 status, No. (%) c | |||
| Positive | 8 (61.5) | 5 (62.5) | 3 (60) |
| Negative | 5 (38.5) | 3 (37.5) | 2 (40) |
| No. of total cycles, median (range) | 6 (1-12) | 5 (1-12) | 11 (2-12) |
| Dose of Vigil received, % | |||
| 1 × 10e6 cells/mL | 6 (46.2) | 6 (75) | 0 (0) |
| 1 × 10e7 cells/mL | 7 (53.8) | 2 (25) | 5 (100) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HRD, homologous recombination deficient; HRP, homologous recombination proficient; NA, not available or not done; OC, ovarian cancer; PD-L1, programmed death-ligand 1; TNBC, triple-negative breast cancer.
BRCA status determined by Ocean Ridge Biosciences.
Homologous recombination status determined by Myriad myChoice CDx.
PD-L1 status determined by NeoGenomics Laboratories or ProPath; positivity was based on a score ⩾1% assessed by immunohistochemistry.
Safety
All reported treatment-related adverse events in ITT, TNBC, and OC patients are shown in Tables 2 to 4, respectively. There were 51 treatment-related adverse events, 42 (82.3%) were grade 1, 6 (11.8%) were grade 2, and 3 (5.9%) were grade 3. No grade 3 treatment-related AEs were Vigil-related. No grade 4 or 5 treatment-related adverse events occurred. The most common treatment-related adverse events of any grade were injection-site reaction. This occurred in 12 of 13 patients (92.3%), myalgia in 5 (38.5%), bruising at injection site in 3 (23.1%), and pruritus in 3 (23.1%). In total, there were 25 (49%) durvalumab-related grade 1-3 adverse events and 26 (51%) Vigil-related grade 1-3 adverse events.
Table 2.
Treatment-related adverse events in all patients.
| ITT (n = 13), % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
| Adrenal insufficiency | 0 | (0) | 1 | (7.7) | 0 | (0) | 0 | (0) |
| Arthralgia | 2 | (15.4) | 1 | (7.7) | 0 | (0) | 0 | (0) |
| Arthritis | 1 | (7.7) | 0 | (0) | 0 | (0) | 0 | (0) |
| Elevated ALT | 0 | (0) | 0 | (0) | 1 | (7.7) | 0 | (0) |
| Elevated AST | 0 | (0) | 0 | (0) | 1 | (7.7) | 0 | (0) |
| Bleeding at injection site | 2 | (15.4) | 0 | (0) | 0 | (0) | 0 | (0) |
| Bone pain | 2 | (15.4) | 0 | (0) | 0 | (0) | 0 | (0) |
| Bruise at injection site | 3 | (23.1) | 0 | (0) | 0 | (0) | 0 | (0) |
| Chills | 1 | (7.7) | 0 | (0) | 0 | (0) | 0 | (0) |
| Diarrhea | 1 | (7.7) | 0 | (0) | 0 | (0) | 0 | (0) |
| Dry mouth | 1 | (7.7) | 0 | (0) | 0 | (0) | 0 | (0) |
| Erythema at chest wall disease | 2 | (15.4) | 0 | (0) | 0 | (0) | 0 | (0) |
| Erythematous pruritic rash | 1 | (7.7) | 0 | (0) | 0 | (0) | 0 | (0) |
| Fatigue | 0 | (0) | 0 | (0) | 1 | (7.7) | 0 | (0) |
| Fever | 2 | (15.4) | 0 | (0) | 0 | (0) | 0 | (0) |
| Headache | 2 | (15.4) | 0 | (0) | 0 | (0) | 0 | (0) |
| Hypothyroidism | 0 | (0) | 2 | (15.4) | 0 | (0) | 0 | (0) |
| Infusion-related reaction | 0 | (0) | 1 | (7.7) | 0 | (0) | 0 | (0) |
| Injection-site reaction | 12 | (92.3) | 1 | (7.7) | 0 | (0) | 0 | (0) |
| Myalgia | 5 | (38.5) | 0 | (0) | 0 | (0) | 0 | (0) |
| Pruritus | 3 | (23.1) | 0 | (0) | 0 | (0) | 0 | (0) |
| Swelling at chest wall disease | 2 | (15.4) | 0 | (0) | 0 | (0) | 0 | (0) |
| Total | 42 | (82.3) | 6 | (11.8) | 3 | (5.9) | 0 | (0) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; ITT, intent-to-treat.
Table 4.
Treatment-related adverse events in ovarian cancer patients.
| Ovarian cancer (n = 5), % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
| Adrenal insufficiency | 0 | (0) | 1 | (20) | 0 | (0) | 0 | (0) |
| Bleeding at injection site | 2 | (40) | 0 | (0) | 0 | (0) | 0 | (0) |
| Bruise at injection site | 1 | (20) | 0 | (0) | 0 | (0) | 0 | (0) |
| Chills | 1 | (20) | 0 | (0) | 0 | (0) | 0 | (0) |
| Diarrhea | 1 | (20) | 0 | (0) | 0 | (0) | 0 | (0) |
| Injection-site reaction | 5 | (100) | 0 | (0) | 0 | (0) | 0 | (0) |
| Myalgia | 1 | (20) | 0 | (0) | 0 | (0) | 0 | (0) |
| Pruritus | 1 | (20) | 0 | (0) | 0 | (0) | 0 | (0) |
| Total | 12 | (92.3) | 1 | (7.7) | 0 | (0) | 0 | (0) |
Table 3.
Treatment-related adverse events in breast cancer patients.
| Breast cancer (n = 8), % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
| Arthralgia | 2 | (25) | 1 | (12.5) | 0 | (0) | 0 | (0) |
| Arthritis | 1 | (12.5) | 0 | (0) | 0 | (0) | 0 | (0) |
| Elevated ALT | 0 | (0) | 0 | (0) | 1 | (12.5) | 0 | (0) |
| Elevated AST | 0 | (0) | 0 | (0) | 1 | (12.5) | 0 | (0) |
| Bone pain | 2 | (25) | 0 | (0) | 0 | (0) | 0 | (0) |
| Bruise at injection site | 2 | (25) | 0 | (0) | 0 | (0) | 0 | (0) |
| Dry mouth | 1 | (12.5) | 0 | (0) | 0 | (0) | 0 | (0) |
| Erythema at chest wall disease | 2 | (25) | 0 | (0) | 0 | (0) | 0 | (0) |
| Erythematous pruritic rash | 1 | (12.5) | 0 | (0) | 0 | (0) | 0 | (0) |
| Fatigue | 0 | (0) | 0 | (0) | 1 | (12.5) | 0 | (0) |
| Fever | 2 | (25) | 0 | (0) | 0 | (0) | 0 | (0) |
| Headache | 2 | (25) | 0 | (0) | 0 | (0) | 0 | (0) |
| Hypothyroidism | 0 | (0) | 2 | (25) | 0 | (0) | 0 | (0) |
| Infusion-related reaction | 0 | (0) | 1 | (12.5) | 0 | (0) | 0 | (0) |
| Injection-site reaction | 7 | (87.5) | 1 | (12.5) | 0 | (0) | 0 | (0) |
| Myalgia | 4 | (50) | 0 | (0) | 0 | (0) | 0 | (0) |
| Pruritus | 2 | (25) | 0 | (0) | 0 | (0) | 0 | (0) |
| Swelling at chest wall disease | 2 | (25) | 0 | (0) | 0 | (0) | 0 | (0) |
| Total | 30 | (78.9) | 5 | (13.2) | 3 | (7.9) | 0 | (0) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase.
The most common treatment-related adverse events in TNBC patients (n = 8) were injection-site reaction (n = 7 [87.5%]) and myalgia (n = 4 [50%]) and in OC patients (n = 5) was injection-site reaction (n = 5 [100%]) and bleeding at the injection site (n = 2 [40%]). Three grade 3 treatment-related adverse events in TNBC patients were reported to be related to durvalumab and were elevated AST (n = 1 [12.5%]), elevated ALT (n = 1 [12.5%]), and fatigue (n = 1 [12.5%]). One TNBC patient discontinued durvalumab treatment after 3 combination cycles due to elevated liver enzyme levels and continued with 3 cycles of Vigil single agent until disease progression.
No treatment-related adverse events ⩾grade 3 occurred in OC patients.
Efficacy
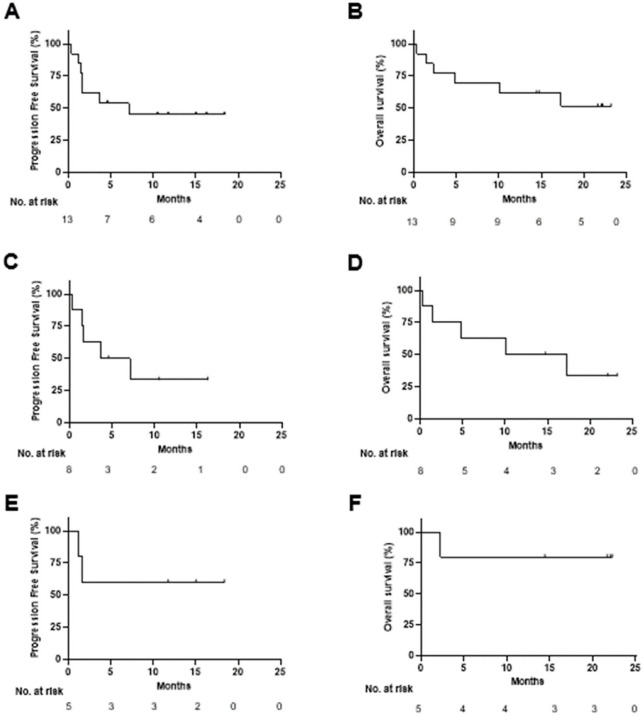
The PFS and OS KM analysis of all patients (ITT) is shown in Figure 2A and B. The mPFS was 7.1 months and the median OS (mOS) was not reached. Subgroup analysis shows a mPFS of 5.4 and mOS of 13.7 months in TNBC patients (Figure 2C and D). In OC patients, both mPFS and mOS were not reached (Figure 2E and F).
Figure 2.
Efficacy of Vigil in combination with durvalumab. (A) PFS of all study subjects, (B) OS of all study subjects, (C) PFS of TNBC subjects, (D) OS of TNBC subjects, (E) PFS of OC subjects, (F) OS of OC subjects.
OC indicates ovarian cancer; OS, overall survival; PFS, progression-free survival; TNBC, triple-negative breast cancer.
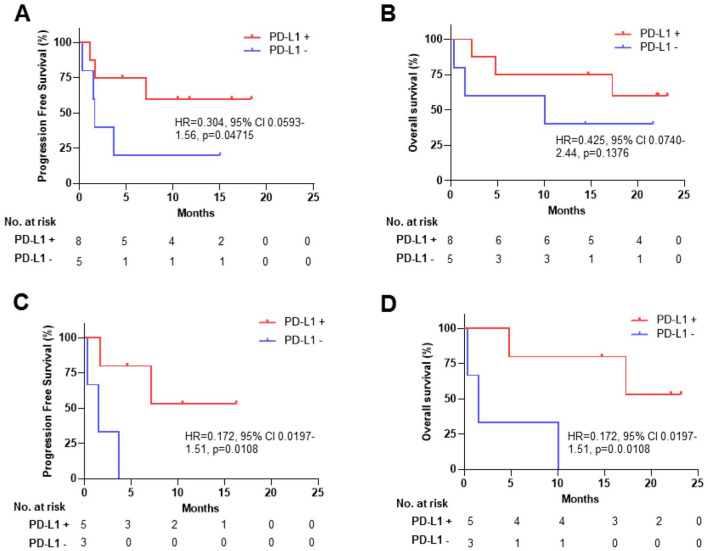
Eight (61.5%) of the 13 patients were PD-L1+ tumors (TNBC, n = 5; OC, n = 3). In the ITT population including both TNBC and OC patients, mPFS in PD-L1+ patients was not reached versus 1.61 months in PD-L1− patients (HR = 0.304, 95% CI, 0.0593-1.56, 1-sided P = .04715) (Figure 3A). In TNBC patients, mPFS in PD-L1+ was not reached versus 1.51 months in PD-L1− patients (HR = 0.172, 95% CI, 0.0197-1.51, 1-sided P = .0108). The proportional hazards assumption was assessed by the Grambsch and Therneau test. The proportionality assumptions for PFS of all subjects, OS of all subjects, PFS of TNBC subjects, and OS of TNBC were all satisfied (P = .75, .37, .82, and .82, respectively). Number of OC patients was too small for comparison of PD-L1+ versus PD-L1− mPFS and OS assessment. Number of patients was also too small for comparison of PFS or OS in the HRP and HRD subset population.
Figure 3.
Efficacy stratified by PD-L1 score. Red, PD-L1+; blue, PD-L1−. (A) PFS of all study subjects, (B) OS of all study subjects, (C) PFS of TNBC subjects, (D) OS of TNBC subjects.
CI indicates confidence interval; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; TNBC, triple-negative breast cancer.
Discussion
Results of this proof-of-principle study show favorable safety with concurrent combination of Vigil and durvalumab and support evidence of benefit in TNBC despite small sample size of this clinical trial. Preclinical studies, however, suggest tumor-specific vaccine administration prior to checkpoint (PD-1/PD-L1) blockade enhances anticancer responses by priming and increasing the abundance of neoantigen-specific CD8+ T cells. In a colorectal mouse model, tumor-specific vaccine was administered first followed by anti-PD-1 therapy. Results demonstrated that neoantigen identification and stimulation of targeting CD8+ cells prior to checkpoint inhibition enhanced clinical benefit with combination therapy. 50 The combination of a cell-based, GM-CSF-secreting vaccine (GVAX) before anti-CTLA-4 inhibitor also showed increased tumor responses in prostate cancer models associated with generation of increased tumor neoantigen–specific CD8+ T cells in circulation. 51 Moreover, clinical comparison of Vigil prior to CIs versus concurrent with CIs demonstrated reduced CI-related ⩾grade 3 toxic effect. These results suggest early targeting of CD8+ cells will enhance direct antitumor immune attack and will reduce off target toxic-related activity.
While poorly immunogenic tumors are not as sensitive to CI therapy alone, tumor-specific vaccine prior to CI therapy turns “cold tumors hot” by increasing the number of infiltrating tumor-specific T cells which enhances CI efficacy.52,53 Both TIL and CD8+ T-cell priming through vaccine therapy induce gIFN and subsequently the expression of PD-L1 on tumor cells which facilitates the efficiency of CI therapy post tumor-specific vaccine. 54 Vigil has demonstrated induction of circulating mononuclear cell increase and gIFN production to autologous tumor via ELISPOT study.55 -57 Moreover, increase in circulating CD8+ cells has been observed following Vigil therapy. 57
CI monotherapy has shown limited benefit in both TNBC and OC, also known as more poorly immunogenic tumor types. Recent study indicates that the combination of checkpoint therapy with pegylated liposomal doxorubicin achieves better overall response rates (ORR 13.3%); however, OS was not significantly improved, while mPFS was 3.7 months. 58 The combination of PARP inhibitor olaparib and CI durvalumab in recurrent OC showed modest clinical activity with improved PFS associated with increased gIFN production. 59 In TNBC, a recent study SAFIR02-BREAST IMMUNO showed that durvalumab in maintenance did not improve PFS (HR = 1.40, 95% CI, 1.00-1.96; P = .047) or OS (HR = 0.84, 95% CI, 0.54-1.29; P = .423) compared with chemotherapy. 60 Recent results of the phase 3 study (IMagyn050) atezolizumab in combination with bevacizumab and frontline chemotherapy in women with advanced OC did not meet the primary endpoint of PFS. 61 The global phase 3 study (Javelin ovarian 100) of avelumab in combination with frontline chemotherapy involving 998 patients with advanced OC was stopped by the data safety monitoring board as efficacy results did not support the use of avelumab in the frontline setting.62,63 Mathematical computationally based algorithms may improve response to personalized therapeutics including CI.64,65
Consistent with recent double-blind randomized controlled results of Vigil in newly diagnosed OC patients, 46 molecular expression of BRCA-wt in malignant tissue has been associated with increased immunogenicity measured by increased abundance of immune cells and higher clonal neoantigen expression as compared with BRCA-m tumors. 66 The abundance of clonal tumor neoantigens in the Vigil vaccine of BRCA-wt TNBC and OC patients may improve CI responses. This study is limited by small sample sizes.
Conclusions
Despite the small number of patients evaluated in this trial, combination of Vigil/durvalumab appears well tolerated and is suggestive of benefit at least in the TNBC population which is an encouraging direction for further exploration of Vigil first prior to concurrent Vigil combination durvalumab in phase 2/3 assessment. These data, while preliminary, advance the knowledge of the use of CIs in combination with other immunotherapies (ie, Vigil) in an unmet need group of patients, such as BRCA-wt TNBC and OC. Checkpoint inhibitor responses have been historically low in BRCA-wt TNBC and OC making these results particularly interesting and warrant further investigation in a larger cohort of patients.
Acknowledgments
The authors would like to acknowledge Brenda Marr for her competent and knowledgeable assistance in the preparation of the article.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Gradalis, Inc.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MB contributed to resources; PA, LM, and LS to formal analysis; GW, SH, and EB to data curation; PA and LM to writing original draft preparation; MB, PA, LM, GW, SH, EB, LS, and JN to writing review and editing; LM and JN to conceptualization and supervision.
ORCID iD: John Nemunaitis  https://orcid.org/0000-0002-2234-2381
https://orcid.org/0000-0002-2234-2381
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [DOI] [PubMed] [Google Scholar]
- 2. Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45:27-40 [DOI] [PubMed] [Google Scholar]
- 3. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-752. [DOI] [PubMed] [Google Scholar]
- 4. Spriggs DR, Brady MF, Vaccarello L, et al. Phase III randomized trial of intravenous cisplatin plus a 24- or 96-hour infusion of paclitaxel in epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:4466-4471. [DOI] [PubMed] [Google Scholar]
- 5. Malorni L, Shetty PB, De Angelis C, et al. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat. 2012;136:795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Senzer N, Barve M, Kuhn J, et al. Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol Ther. 2012;20:679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elezaby M, Lees B, Maturen KE, et al. BRCA mutation carriers: breast and ovarian cancer screening guidelines and imaging considerations. Radiology. 2019;291:554-569. [DOI] [PubMed] [Google Scholar]
- 8. Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3-10. [DOI] [PubMed] [Google Scholar]
- 9. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer—epidemiology, risk factors, and genetics. BMJ. 2000;321:624-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenup R, Buchanan A, Lorizio W, et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol. 2013;20:3254-3258. [DOI] [PubMed] [Google Scholar]
- 12. Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bayraktar S, Gutierrez-Barrera AM, Liu D, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat. 2011;130:145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim SI, Lee M, Kim HS, et al. Effect of BRCA mutational status on survival outcome in advanced-stage high-grade serous ovarian cancer. J Ovarian Res. 2019;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yadav S, Ladkany R, Yadav D, et al. Impact of BRCA mutation status on survival of women with triple-negative breast cancer. Clin Breast Cancer. 2018;18:e1229-e1235. [DOI] [PubMed] [Google Scholar]
- 16. Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer (Auckl). 2016;10:25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roebroek AJ, Umans L, Pauli IG, et al. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase furin. Development. 1998;125:4863-4876. [DOI] [PubMed] [Google Scholar]
- 19. Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Wang S-S, Huang H, et al. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA. 2021;325:50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herzog TJ, Monk BJ. Bringing new medicines to women with epithelial ovarian cancer: what is the unmet medical need? Gynecol Oncol Res Pract. 2017;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lahouassa H, Daddacha W, Hofmann H, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012;13:223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghisoli M, Rutledge M, Stephens PJ, et al. Case report: immune-mediated complete response in a patient with recurrent advanced Ewing sarcoma (EWS) after vigil immunotherapy. J Pediatr Hematol Oncol. 2017;39:e183-e186. [DOI] [PubMed] [Google Scholar]
- 24. Barve M, Kuhn J, Lamont J, et al. Follow-up of bi-shRNA furin / GM-CSF engineered autologous tumor cell (EATC) immunotherapy Vigil® in patients with advanced melanoma. Biomed Genet Genom. 2016;1:81-86. [Google Scholar]
- 25. Nemunaitis J, Barve M, Orr D, et al. Summary of bi-shRNA/GM-CSF augmented autologous tumor cell immunotherapy (FANG) in advanced cancer of the liver. Oncology. 2014;87:21-29. [DOI] [PubMed] [Google Scholar]
- 26. Moore KN, Pothuri B, Monk B, Coleman RL. PARP inhibition in recurrent ovarian cancer. Clin Adv Hematol Oncol. 2020;18:647-655. [PubMed] [Google Scholar]
- 27. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852-861. [DOI] [PubMed] [Google Scholar]
- 28. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284. [DOI] [PubMed] [Google Scholar]
- 29. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164. [DOI] [PubMed] [Google Scholar]
- 30. Mittica G, Genta S, Aglietta M, Valabrega G. Immune checkpoint inhibitors: a new opportunity in the treatment of ovarian cancer? Int J Mol Sci. 2016;17:1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8:2171-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488-1493. [DOI] [PubMed] [Google Scholar]
- 33. Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartkopf AD, Taran FA, Wallwiener M, et al. PD-1 and PD-L1 immune checkpoint blockade to treat breast cancer. Breast Care (Basel). 2016;11:385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yi H, Li Y, Tan Y, Fu S, Tang F, Deng X. Immune checkpoint inhibition for triple-negative breast cancer: current landscape and future perspectives. Front Oncol. 2021;11:648139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741-751. [DOI] [PubMed] [Google Scholar]
- 37. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529-1541. [DOI] [PubMed] [Google Scholar]
- 38. Shimokawa M, Kogawa T, Shimada T, et al. Overall survival and post-progression survival are potent endpoint in phase III trials of second/third-line chemotherapy for advanced or recurrent epithelial ovarian cancer. J Cancer. 2018;9:872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. [DOI] [PubMed] [Google Scholar]
- 40. Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5:1132-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barve M, Adams N, Plato L, et al. Case report: immune checkpoint inhibitor elicited complete response in a heavily pretreated patient with metastatic endometrial carcinoma with a high tumor mutation burden (TMB). Mol Med: Curr Asp. 2017;1:005. [Google Scholar]
- 42. Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21:1155-1164. [DOI] [PubMed] [Google Scholar]
- 43. Drew Y, Penson RT, O’Malley DM, et al. Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol. 2020;31:S551-S589. [Google Scholar]
- 44. Ghisoli M, Barve M, Schneider R, et al. Pilot trial of FANG immunotherapy in Ewing’s sarcoma. Mol Ther. 2015;23:1103-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maples P, Kumar P, Yu Y, et al. FANG vaccine: autologous tumor cell vaccine genetically modified to express GM-CSF and block production of furin. Bioprocess J. 2010;8:4-14. [Google Scholar]
- 46. Rocconi RP, Grosen EA, Ghamande SA, et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020;21:1661-1672. [DOI] [PubMed] [Google Scholar]
- 47. Rocconi RP, Monk BJ, Walter A, et al. Gemogenovatucel-T (Vigil) immunotherapy demonstrates clinical benefit in homologous recombination proficient (HRP) ovarian cancer. Gynecol Oncol. 2021;161:676-680. [DOI] [PubMed] [Google Scholar]
- 48. Rocconi RP, Stevens EE, Bottsford-Miller JN, et al. Proof of principle study of sequential combination atezolizumab and Vigil in relapsed ovarian cancer. Cancer Gene Ther. 2022;29:369-382. [DOI] [PubMed] [Google Scholar]
- 49. Oh J, Barve M, Matthews CM, et al. Phase II study of Vigil(R) DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol Oncol. 2016;143:504-510. [DOI] [PubMed] [Google Scholar]
- 50. Tondini E, Arakelian T, Oosterhuis K, et al. A poly-neoantigen DNA vaccine synergizes with PD-1 blockade to induce T cell-mediated tumor control. Oncoimmunology. 2019;8:1652539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wada S, Jackson CM, Yoshimura K, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. 2013;11:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Curran MA, Glisson BS. New hope for therapeutic cancer vaccines in the era of immune checkpoint modulation. Annu Rev Med. 2019;70:409-424. [DOI] [PubMed] [Google Scholar]
- 53. Karaki S, Anson M, Tran T, et al. Is there still room for cancer vaccines at the era of checkpoint inhibitors. Vaccines (Basel). 2016;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abiko K, Matsumura N, Hamanishi J, et al. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Senzer N, Barve M, Nemunaitis J, et al. Long term follow up: phase I trial of “bi-shRNA furin/GMCSF DNA/autologous tumor cell” immunotherapy (FANG™) in advanced cancer. J Vaccines Vaccin. 2013;4:209. [Google Scholar]
- 56. Oh J, Barve M, Senzer N, et al. Long-term follow-up of phase 2A trial results involving advanced ovarian cancer patients treated with Vigil(R) in frontline maintenance. Gynecol Oncol Rep. 2020;34:100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rocconi RP, Stanbery L, Madeira da, Silva L, et al. Long-term follow-up of gemogenovatucel-T (Vigil) survival and molecular signals of immune response in recurrent ovarian cancer. Vaccines (Basel). 2021;9:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pujade-Laurainea E, Fujiwarab K, Ledermannc JA, et al., Eds. Avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant or refractory epithelial ovarian cancer: primary and biomarker analysis of the phase III JAVELIN Ovarian 200 trial. Paper presented at: 50th Annual Meeting of the Society of Gynecologic Oncology; March 16-19, 2019; Honolulu, HI. [Google Scholar]
- 59. Lampert EJ, Zimmer A, Padget M, et al. Combination of PARP inhibitor olaparib, and PD-L1 inhibitor durvalumab, in recurrent ovarian cancer: a proof-of-concept phase II study. Clin Cancer Res. 2020;26:4268-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dalenc F, Garberis I, Filleron T, et al. Abstract GS3-02: durvalumab compared to maintenance chemotherapy in patients with metastatic breast cancer: results from phase II randomized trial SAFIR02-IMMUNO. Cancer Res. 2020;80:GS3-02-GS3. [Google Scholar]
- 61. Creeden J, Ong S, Gillman C, et al. The role of TGFbeta in clinical cancer response. Clin Oncol Res. 2020;3:1-8.34142081 [Google Scholar]
- 62. Monk BJ, Colombo N, Oza AM, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:1275-1289. [DOI] [PubMed] [Google Scholar]
- 63. Monk BJ, Herzog TJ, Krivak TC, Lewin SN, Moore KN. Considerations for the JAVELIN OVARIAN 100 study. OncLive. May 27, 2020. https://www.onclive.com/view/considerations-for-the-javelin-ovarian-100-study
- 64. Nave O, Elbaz M. Artificial immune system features added to breast cancer clinical data for machine learning (ML) applications. Biosystems. 2021;202:104341. [DOI] [PubMed] [Google Scholar]
- 65. Sliheet E, Robinson M, Morand S, et al. Network based analysis identifies TP53m-BRCA1/2wt-homologous recombination proficient (HRP) population with enhanced susceptibility to Vigil immunotherapy [published online ahead of print November 16, 2021]. Cancer Gene Ther. doi: 10.1038/s41417-021-00400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]