Abstract
Objectives
Sarcopenia is a syndrome characterized by reduced muscle mass and function. It is well-recognized as a complication in chronic diseases such as chronic obstructive pulmonary disease. However, little is known about sarcopenia in patients with idiopathic pulmonary fibrosis (IPF). This study aimed to investigate the clinical characteristics of sarcopenia and the association between quality of life and sarcopenia in patients with IPF.
Methods
In this pilot cross-sectional study, 56 Japanese outpatients with IPF (49 men) were enrolled prospectively. Sarcopenia was diagnosed according to the criteria of the Asian Working Group for Sarcopenia 2019. Its associations with clinical parameters including age, pulmonary functions, physical performance, and patient-reported outcomes (PROs) were examined.
Results
The frequency of sarcopenia was 39.3% (n = 22) in this cohort. There were significant differences in St George’s Respiratory Questionnaire (p = .005), modified Medical Research Council score (p = .004), and Hospital and Anxiety Depression Scale depression score (p = .030) between the sarcopenic and non-sarcopenic groups. On multivariate regression analysis, 6-min walk distance (6MWD) was an independent factor associated with sarcopenia (odds ratio 1.241, 95% confidence interval 1.016–1.515, p = .034).
Conclusion
Sarcopenia was associated with PROs and physical performance in patients with IPF.
Keywords: sarcopenia, idiopathic pulmonary fibrosis, patient-reported outcome, 6-min walk test, appendicular skeletal muscle index, bioimpedance analysis, quality of life, depression
Introduction
Idiopathic pulmonary fibrosis (IPF) is a fibrotic and progressive pulmonary disease that may lead to death in most patients. 1 Currently, two antifibrotic drugs (nintedanib and pirfenidone) are approved for IPF, 2 but they can slow, but not stop, the progression of fibrosis.3,4 Aging is one of the critical risk factors for IPF, and epidemiological data suggest that IPF is an aging-related disease.1,5,6 This disease is characterized by reduced lung volumes, decreased gas exchange, symptoms of progressive dyspnea and cough, and reduced exercise capacity. Several known prognostic factors for IPF are: dyspnea score, 7 pulmonary function,8-10 gender, age, and physiology (GAP) index,5,11 functional exercise capacity,12,13 and fibrotic changes as observed through high-resolution computed tomography. 14
Sarcopenia is an age-related syndrome characterized by a progressive and generalized loss of systemic skeletal muscle mass and strength. Moreover, it carries the risk of poor outcomes such as physical disability, poor quality of life, and death.15,16 Diagnosis and treatment of sarcopenia were defined using the Asians in the Asian Working Group for Sarcopenia (AWGS) 2014 consensus and updated by the AWGS 2019 consensus.16 The diagnosis of sarcopenia is based on loss of muscle mass, low muscle strength, and/or low physical performance. The appendicular skeletal muscle index (ASMI) via dual-energy X-ray absorptiometry (DXA) or multi-frequency bioimpedance analysis (BIA) is used to assess the loss of skeletal muscles. 16
There have been reports of sarcopenia in chronic obstructive pulmonary disease (COPD). The prevalence of sarcopenia in patients with COPD was reported to be 15.5% 17 and 21.6% 18 in meta-analyses. Regarding interstitial lung disease (ILD), it is reported that sarcopenia was identified in 32.1% of Japanese patients with ILD. 19 In patients with IPF, the reported muscle assessments were the fat-free mass index, 20 ASMI, 21 and cross-sectional area of the erector spinae muscles.22-26 A lower skeletal muscle mass is associated with lower quality of life and worse prognosis. However, to the best of our knowledge, there is only one report that defined sarcopenia using the ASMI in a cohort of patients with IPF. 27
We aimed to investigate the clinical characteristics of sarcopenia and the association between quality of life and sarcopenia in patients with IPF. In this cross-sectional study, sarcopenia was diagnosed according to the algorithm and criteria of the AWGS 2019. 16
Methods
Ethics
This single-centre, cross-sectional study was conducted in accordance with the amended Declaration of Helsinki and was approved by the ethics review board of Nagoya City University Hospital (approval number 60–20–0190). Written informed consent was obtained from all participants.
Patients
IPF was diagnosed through multidisciplinary discussions according to the 2018 international guidelines. 28 The following inclusion criteria were applied: provided written informed consent for this study, and able to perform the 6-min walk test (6MWT) in order to assess the exercise capacity of IPF patients. The following exclusion criteria were also applied: home oxygen treatment at rest, active cancer, and unable to understand patient-reported outcome questionnaires. Stable IPF outpatients were screened, and 69 patients were identified between April 2021 and March 2022 at Nagoya City University Hospital (Nagoya, Japan). Newly diagnosed patients were also enrolled in this study. A total of 56 outpatients were finally enrolled—four patients were receiving long-term oxygen therapy at rest and therefore would have difficulty performing the 6MWT without oxygen, one patient was unable to perform 6MWT because of unstable angina, three patients were unable to perform 6MWT because of wheelchair use, and five patients declined to participate in the study. Patients who had been hospitalized due to acute exacerbations of IPF or pneumonia had been provided with a rehabilitation program. Other patients with stable IPF had not received rehabilitation.
Diagnosis of sarcopenia
The algorithm and criteria of the AWGS 2019 16 were used to define sarcopenia in this study. Accordingly, sarcopenia was diagnosed if the patient had low muscle mass, low muscle strength, and/or low physical performance. In addition, severe sarcopenia was defined as the presence of all three—low muscle mass, low muscle strength, and low physical performance. The detailed methods and criteria are described as follows. Muscle mass was assessed through the ASMI (height squared-adjusted, kg/m2), which was assessed with bioelectrical impedance analyser (BIA), using a direct segmental multi-frequency BIA (InBody 720; InBody Japan, Tokyo, Japan). The cut-off criterion for low muscle mass was <7.0 kg/m2 for men and <5.7 kg/m2 for women. Muscle strength was assessed through the handgrip strength, which was measured in the standing position with full elbow extension using an electronic dynamometer (HG-251; N-Force, Wakayama, Japan). Two trials were completed with each hand, and the highest of four values was used in the analysis. Handgrip strength measurement is the recommended method for detecting low muscle strength. The cut-off criterion for low muscle strength was defined as < 28.0 kg for men and <18.0 kg for women. Physical performance was evaluated through gait speed. It was calculated by measuring the time it takes for study participants to walk across a 10-m corridor. The participants were instructed to walk down the corridor at their usual speed. The cut-off criterion for low physical performance was defined as < 1.0 m/s for both men and women.
Pulmonary function tests and 6MWT
All patients completed pulmonary function tests using a spirometer (CHESTAC-8900; Chest, Tokyo, Japan) according to the ATS/ERS criteria. 29 The diffusion capacity for carbon monoxide (DLco) was also measured (CHESTAC-8900). Percent predicted forced vital capacity (% FVC), percent predicted forced expiratory volume in 1.0 s (%FEV1), and percent predicted DLco (%DLco) were calculated based on the patients’ height, age, and sex, according to the Japanese standardized methods. 30 The GAP index was calculated according to the formula proposed by Ley et al. 5 6MWT was conducted without supplemental oxygen in accordance with ATS guidelines. 31
Patient-reported outcomes
Patient-reported outcomes (PROs) were based on the assessment of breathlessness, symptoms, health-related quality of life, and psychological status. Breathlessness was evaluated using the 5-grade modified Medical Research Council dyspnoea scale (MRC). Symptoms and health-related quality of life were evaluated using the St George’s Respiratory Questionnaire (SGRQ). 32 Psychological evaluation was done using the Japanese version of the Hospital and Anxiety Depression Scale (HADS). 33
Strength, assistance in walking, rising from a chair, climbing stairs, and falls (SARC-F) questionnaire was used to measure probable sarcopenia. This questionnaire is composed of five items: strength, assistance in walking, rising from a chair, climbing stairs, and falls. 34
Statistical analyses
Continuous variables are presented as means (±standard deviations) or medians [interquartile ranges]. Categorical variables are presented as numbers and percentages. Differences between the sarcopenic and non-sarcopenic groups were analyzed using Student’s t-test (with normal distribution) or the Mann–Whitney U test (without normal distribution) for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables, as appropriate. Multiple linear regression analysis was performed for detecting the relevance of complication of sarcopenia with PROs. To assess multicollinearity, the variance inflation factor (VIF) values were computed. Effects of factors associated with sarcopenia were evaluated through univariate and multivariate logistic regression analyses. Multivariate analysis was performed including the significant variables from the univariate analysis. Some of the highly correlated variables were excluded on the multivariate analysis if they had Spearman’s or Pearson’s correlation coefficients >0.7 to avoid multicollinearity. Receiver operating characteristic (ROC) curves were constructed to determine the optimal cut-off value for detecting sarcopenia. Statistical significance was set at p < .05. Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 28 (IBM Corp., Armonk, NY, USA).
Results
Patients’ characteristics and frequency of sarcopenia
The study population consisted of 56 Japanese outpatients with IPF, of whom 39.3% (n = 22) has sarcopenia. The patients’ characteristics are shown in Table 1. The mean age was 73.1 ± 7.7 years and mean %FVC was 80.4 ± 15.5% in this cohort. The presence of severe sarcopenia was 16.1% (n = 9) (Supplementary Figure S1). In addition, the distribution of low muscle mass, low handgrip strength, and low gait speed is shown in Supplementary Figure S2. Ten patients were treated with corticosteroids—nine were on corticosteroid taper after acute exacerbations, and the remaining patient treated with corticosteroid for another disease (skin disease).
Table 1.
Patients’ characteristics.
| Variable | Total (n = 56) | Sarcopenic (n = 22) | Non-sarcopenic (n = 34) | p-value |
|---|---|---|---|---|
| Age, years | 73.1 ± 7.7 | 76.9 ± 5.1 | 70.6 ± 8.1 | <.001 |
| Gender, men, n (%) | 49 (87.5) | 19 (86.4) | 30 (88.2) | .572 |
| BMI, kg/m2 | 22.3 ± 3.1 | 20.7 ± 2.8 | 23.3 ± 3.0 | .002 |
| Smoking status | ||||
| Current or ex-smoker, n (%) | 46 (82.1%) | 18 (81.8%) | 28 (82.4%) | .613 |
| Smoking history, pack-years | 30 [9–46] | 24 [5–41] | 38 [17–48] | .235 |
| Histological diagnosis, n (%) | 18 (32.1) | 3 (13.6) | 15 (44.1) | .017 |
| Duration from diagnosis, days | 728 [356–1789] | 1015 [162–2218] | 686 [380–1283] | .887 |
| Pulmonary function test | ||||
| FVC, % predicted | 80.1 ± 16.2 | 74.6 ± 14.8 | 83.7 ± 16.3 | .037 |
| FEV1, % predicted | 81.4 ± 15.1 | 77.7 ± 13.8 | 83.8 ± 15.6 | .143 |
| FEV1/FVC, % | 81.6 ± 8.7 | 82.5 ± 9.3 | 81.0 ± 8.3 | .535 |
| DLCO, % predicted | 66.4 ± 18.5 | 62.9 ± 19.2 | 68.5 ± 18.0 | .281 |
| GAP score | 3 [3–4] | 4 [3–5] | 3 [3–4] | .018 |
| 6MWT | ||||
| 6MWD, m | 403 ± 96 | 327 ± 88 | 453 ± 64 | <.001 |
| Lowest SpO2, % | 89 [85–92] | 86 [83–92] | 90 [85–92] | .207 |
| Modified MRC score | 1 [0–2] | 2 [1–3] | 1 [0–1] | .004 |
| SGRQ score | ||||
| Symptom | 38.9 [19.7–65.7] | 50.5 [25.4–76.4] | 29.4 [13.8–58.4] | .117 |
| Activity | 41.5 [19.3–66.2] | 66.3 [38.8–77.5] | 30.4 [18.5–48.0] | .001 |
| Impact | 17.6 [7.9–34.8] | 31.2 [13.6–49.3] | 13.9 [7.3–26.8] | .014 |
| Total | 28.8 [15.2–46.0] | 46.2 [30.6–58.4] | 24.4 [14.4–34.5] | .005 |
| HADS score | ||||
| Anxiety | 4 [1–7] | 4 [1–9] | 3 [1–5] | .385 |
| Depression | 6 [3–8] | 7 [5–11] | 4 [3–8] | .030 |
| SARC-F score | 2 [1–4] | 4 [2–5] | 1 [1–2] | <.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 31 (55.4) | 15 (68.2) | 16 (47.1) | .120 |
| Dyslipidemia | 20 (35.7) | 8 (36.4) | 12 (35.3) | .935 |
| Diabetes mellitus | 15 (26.8) | 6 (27.3) | 9 (26.5) | .947 |
| Coronary artery disease | 5 (8.9) | 1 (4.5) | 4 (11.8) | .340 |
| COPD | 3 (5.4) | 2 (9.1) | 1 (2.9) | .555 |
| Chronic kidney disease | 2 (3.6) | 1 (4.5) | 1 (3.2) | .636 |
| Previous malignancy | 14 (25.0) | 8 (36.4) | 6 (17.6) | .114 |
| History of acute exacerbation, n (%) | 8 (14.3) | 4 (18.2) | 4 (11.8) | .698 |
| Treatment, n (%) | ||||
| Pirfenidone | 22 (39.3) | 7 (31.8) | 15 (44.1) | .357 |
| Nintedanib | 18 (32.1) | 6 (27.3) | 12 (14.7) | .606 |
| Corticosteroid | 10 (17.9) | 5 (22.7) | 5 (16.1) | .337 |
| ASMI, kg/m2 | ||||
| Men | 7.1 [6.4–7.5] | 6.2 [6.0–6.5] | 7.3 [7.2–7.8] | <.001 |
| Women | 5.3 [5.0–5.7] | 5.1 [5.0–5.2] | 5.7 [5.4–5.9] | .400 |
| Gait speed, m/s | 1.1 ± 0.3 | 0.8 ± 0.2 | 1.2 ± 0.2 | <.001 |
| Handgrip strength, kg | ||||
| Men | 32.8 [27.2–38.0] | 26.1 [23.0–28.5] | 37.6 [32.8–40.5] | <.001 |
| Women | 17.0 [14.2–19.9] | 15.8 [13.8–16.4] | 19.9 [17.9–21.3] | .229 |
Data are presented as means (± standard deviation), medians [interquartile range], or numbers (%). Abbreviations: BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1.0 s; DLCO, diffusing capacity of the lung for carbon monoxide; GAP, gender, age and physiology; 6MWT, 6-min walk test; 6MWD, 6-min walk distance; SpO2, oxygen saturation by pulse oximetry; MRC, Medical Research Council; SGRQ, St. George’s Respiratory Questionnaire; HADS, Hospital Anxiety and Depression Scale; SARC-F, strength, assistance in walking, rising from a chair, climbing stairs, and falls; COPD, chronic obstructive pulmonary disease; ASMI, appendicular skeletal muscle index.
There were significant differences in age (p < .001), body mass index (BMI) (p = .002), %FVC (p = .037), GAP score (p = .018), 6-min walk distance (6MWD) (p < .001), and SARC-F score (p < .001) between the sarcopenic and non-sarcopenic groups. On the other hand, there were no significant differences in comorbidities or treatments (antifibrotic drugs or corticosteroids) between the groups. Similarly, there were no significant differences regarding durations from diagnosis to enrollment between the groups (Table 1). The groups using and not using corticosteroids were also compared, and significant differences in the respective parameters between them were not found (Supplementary Table S1).
PROs between the sarcopenic and non-sarcopenic groups
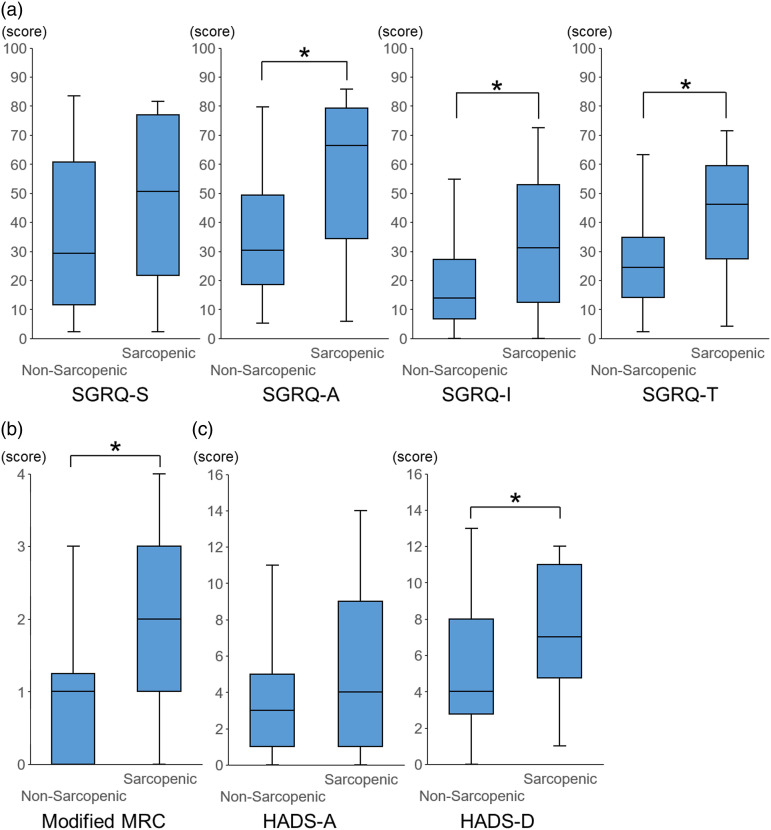
The comparison of PROs between the sarcopenic and non-sarcopenic patients with IPF showed that there was a significant difference in the SGRQ-total score (p = .005), SGRQ-activity score (p = .001), and SGRQ-impact score (p = .014) (Figure 1(a). Similarly, there was a difference in the modified MRC score (p = .004) and HADS-depression score (p = .030) between the two groups (Figure 1(b), 1(c). On the other hand, there was no difference in the HADS-anxiety score (Figure 1(c).
Figure 1.
Comparisons of the SGRQ score, modified MRC score, and HADS score between those with and without sarcopenia. Comparisons of the SGRQ score (a), modified MRC score (b), and HADS score (c) between IPF patients with and without sarcopenia are shown. * p < .05. Abbreviations: SGRQ, St. George’s Respiratory Questionnaire; MRC, Medical Research Councils; IPF, idiopathic pulmonary fibrosis; SGRQ-S, St. George’s Respiratory Questionnaire-Symptom score; SGRQ-A, St. George’s Respiratory Questionnaire-Activity score; SGRQ-I, St. George’s Respiratory Questionnaire-Impact score; SGRQ-T, St. George’s Respiratory Questionnaire-Total score; HADS-A, Hospital Anxiety and Depression Scale-Anxiety; HADS-D, Hospital Anxiety and Depression Scale-Depression.
Multiple linear regression analysis was performed for detecting the association of the complication of sarcopenia with PROs (Table 2). The diagnosis of sarcopenia was an independent factor contributing to SGRQ-activity score (p = .028) and HADS- depression score (p = .014) (Table 2). The VIF values of diagnosis of sarcopenia, age, %FVC, and %DLCO were 1.36, 1.29, 1.41, and 1.29, respectively. All VIF values were <10, and there was no multicollinearity among these factors.
Table 2.
Multiple linear regression analysis for the scores of patient-reported outcomes (PROs).
| B | 95%CI | β | p-value | |
|---|---|---|---|---|
| Modified MRC score | ||||
| diagnosis of sarcopenia | 0.500 | −0.173-1.172 | 0.198 | .142 |
| Age | 0.035 | −0.007-0.0076 | 0.216 | .101 |
| FVC, % predicted | −0.011 | −0.032-0.011 | −0.136 | .319 |
| DLCO, % predicted | −0.025 | −0.043-−0.008 | −0.380 | .005 |
| SGRQ-symptom score | ||||
| diagnosis of sarcopenia | 3.166 | −13.717-20.048 | 0.058 | .708 |
| Age | 0.745 | −0.300-1.791 | 0.216 | .158 |
| FVC, % predicted | −0.306 | −0.849-0.236 | −0.178 | .262 |
| DLCO, % predicted | −0.179 | −0.616-0.259 | −0.124 | .416 |
| SGRQ-activity score | ||||
| diagnosis of sarcopenia | 15.157 | 1.742-28.572 | 0.295 | .028 |
| Age | 0.387 | −0.444-1.218 | 0.119 | .354 |
| FVC, % predicted | −0.354 | −0.785-0.078 | −0.218 | .106 |
| DLCO, % predicted | −0.415 | −0.762-−0.067 | −0.304 | .020 |
| SGRQ-impact score | ||||
| diagnosis of sarcopenia | 9.375 | −1.779-20.529 | 0.243 | .098 |
| Age | 0.302 | −0.389-0.993 | 0.123 | .384 |
| FVC, % predicted | −0.332 | −0.691-0.026 | −0.273 | .069 |
| DLCO, % predicted | −0.101 | −0.390-0.188 | −0.098 | .486 |
| SGRQ-total score | ||||
| diagnosis of sarcopenia | 10.049 | −0.808-20.905 | 0.254 | .069 |
| Age | 0.395 | −0.278-1.067 | 0.157 | .244 |
| FVC, % predicted | −0.331 | −0.680-0.018 | −0.265 | .063 |
| DLCO, % predicted | −0.207 | −0.488-0.075 | −0.196 | .146 |
| HADS-anxiety score | ||||
| diagnosis of sarcopenia | 1.247 | −1.382-3.876 | 0.155 | .345 |
| Age | −0.004 | −0.167-0.159 | −0.008 | .962 |
| FVC, % predicted | 0.007 | −0.077-0.092 | 0.028 | .867 |
| DLCO, % predicted | <0.001 | −0.068-0.068 | 0.001 | .993 |
| HADS-depression score | ||||
| diagnosis of sarcopenia | 2.824 | 0.588-5.060 | 0.392 | .014 |
| Age | −0.085 | −0.224-0.053 | −0.187 | .222 |
| FVC, % predicted | 0.040 | −0.032-0.112 | 1.110 | .272 |
| DLCO, % predicted | −0.009 | −0.067-0.049 | −0.049 | .749 |
Abbreviations: CI, confidence interval; MRC, Medical Research Council; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; SGRQ, St. George’s Respiratory Questionnaire; HADS, Hospital Anxiety and Depression Scale.
Factors associated with sarcopenia in patients with IPF
Comparisons of the risk factors between sarcopenic and non-sarcopenic patients with IPF through univariate regression analysis are shown in Table 3. Age (odds ratio (OR) 1.149, 95% confidence interval (CI) 1.043–1.266, p = .005), BMI (OR 0.725, 95% CI 0.576–0.912, p = .006), %FVC (OR 0.963, 95% CI 0.928–0.999, p = .044), 6MWD (OR 0.977, 95% CI 0.965–0.989, p < .001), and SARC-F score (OR 1.992, 95% CI 1.333–2.976, p < .001) were associated with sarcopenia. Multivariate regression analysis showed that 6MWD was an independent factor associated with sarcopenia (OR 0.978, 95% CI 0.964–0.992, p = .002) (Table 4).
Table 3.
Factors associated with sarcopenia on univariate analyses.
| Variables | OR | 95%CI | p-value |
|---|---|---|---|
| Age | 1.149 | 1.043-1.266 | .005 |
| Gender, men | 0.844 | 0.170-4.197 | .836 |
| BMI | 0.725 | 0.576-0.912 | .006 |
| FVC, % predicted | 0.963 | 0.928-0.999 | .044 |
| FEV1, % predicted | 0.972 | 0.934-1.010 | .146 |
| DLco, % predicted | 0.983 | 0.953-1.014 | .278 |
| 6MWD | 0.977 | 0.965-0.989 | <.001 |
| Lowest SpO2 in 6MWT | 0.944 | 0.863-1.031 | .198 |
| SARC-F score | 1.992 | 1.333-2.976 | <.001 |
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1.0 s; DLCO, diffusing capacity of the lung for carbon monoxide; 6MWD, 6-min walk distance; SpO2, oxygen saturation by pulse oximetry; 6MWT, 6-min walk test; SARC-F, strength, assistance in walking, rising from a chair, climbing stairs, and falls.
Table 4.
Factors associated with sarcopenia on multivariate analysis.
| Variable | OR | 95%CI | p-value |
|---|---|---|---|
| Age | 1.168 | 0.968-1.409 | .105 |
| Body mass index | 0.731 | 0.499-1.070 | .107 |
| FVC, % predicted | 1.002 | 0.934-1.075 | .953 |
| 6MWD | 0.978 | 0.962-0.994 | .007 |
| SARC-F score | 1.091 | 0.583-2.040 | .786 |
Abbreviations: OR, odds ratio; CI, confidence interval; FVC, forced vital capacity; 6MWD, 6-min walk distance; SARC-F, strength, assistance in walking, rising from a chair, climbing stairs, and falls.
Receiver operator characteristic curve for predicting sarcopenia
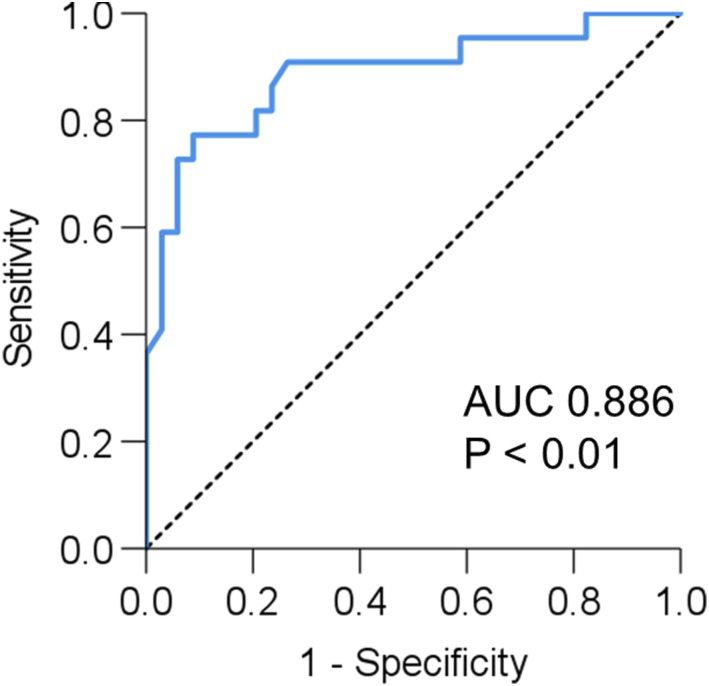
An ROC curve analysis of 6MWD for predicting sarcopenia was performed. Results showed that 6MWD can significantly predict sarcopenia (AUC = 0.886, 95% CI 0.790–0.983, p < .001). The best cut-off value was 370 m (sensitivity: 77.3%, specificity: 91.2%) according to the Youden index, which maximizes the value of ‘sensitivity + specificity-1’. (Figure 2).
Figure 2.
Receiver operating characteristic curves for predicting sarcopenia in patients with IPF. ROC curve of 6MWD for predicting sarcopenia. AUC is 0.873, and the optimal cut-off value is 370 m. Abbreviations: IPF, idiopathic pulmonary fibrosis; ROC, receiver operating characteristic; AUC, area under the curve; 6MWD, 6-min walk distance.
Discussion
In the comparison of PROs between the sarcopenic and non-sarcopenic patients with IPF, there were significant differences in the SGRQ score, modified MRC score, and HADS-depression score. In addition, on multiple linear regression analysis, the complication of sarcopenia was an independent factor contributing to the SGRQ-Activity score and HADS-Depression score. It has been reported that sarcopenic groups felt more subjective activity limitation and worsened quality of life in COPD, 35 and a similar trend was observed in the present cohort of IPF patients. The SGRQ and modified MRC are both indicators of quality of life and prognostic factors for patients with IPF.7,32,36 Similarly, depression significantly affects the quality of life of patients with IPF. 37 The prevalence of depression in patients with sarcopenia was relatively high, and there was a correlation between sarcopenia and depression in a meta-analysis. 38 There are multiple mechanisms that interact with both sarcopenia and depression, including neurotrophins, oxidative stress, inflammation, and the regulation of lifestyle behaviors. 38 Disability and insufficient physical activity due to decreased muscle strength and muscle mass may cause depression. 39 In the present cohort, sarcopenia was significantly more associated with depression score than with age or poor respiratory function, which was an interesting finding. It is widely known that PROs play key roles in the multifaceted symptomatic management of patients with IPF. 40 Therefore, it may be important in clinical practice to note that sarcopenia is associated with PROs.
This study showed that approximately one in three patients with IPF had sarcopenia in our cross-sectional cohort based on the AWGS 2019 criteria. 16 In Japanese community-dwelling older adults, the prevalence of sarcopenia was 11.5% in men (mean age, 77.3 years) and 16.7% in women (mean age, 77.0 years). 41 This suggests that sarcopenia may be more frequent in patients with IPF. It was reported that sarcopenia in 4.6% and sarcopenic obesity in 2.3% of IPF patients in Northern Italy. 27 While this report in Northern Italy assessed sarcopenia at the time of IPF diagnosis, no reports exist examining the prevalence of sarcopenia in IPF patients longitudinally. There were no significant differences regarding durations from diagnosis to enrollment between the sarcopenic and non-sarcopenic groups in the present cohort. A longitudinal survey will be needed.
There were significant differences in age, BMI, %FVC, GAP score, 6MWD and SARC-F score between the sarcopenic and non-sarcopenic groups. In patients with COPD, age, BMI and severity of COPD have been reported to be significant factors associated with sarcopenia. 42 Similarly, in this study’s cohort of patients with IPF patients, age, pulmonary function (%FVC) and their combination (GAP score) were associated with sarcopenia. Loss of skeletal muscle mass is considered to be induced by systemic inflammation, inactivity, malnutrition and enhanced energy expenditure.15,43–45 Impaired pulmonary function, exercise-induced hypoxemia and nutrition status might influence inactivity leading to sarcopenia. It is possible that continuous rehabilitation and nutritional guidance in outpatients with IPF prevent or improve sarcopenia. However, further studies are needed to demonstrate it.
In this study, 6MWD was an independent factor associated with sarcopenia as shown by the multivariate analysis. It has been reported that sarcopenic patients with COPD had shorter 6MWD. 46 The loss of muscle mass may lead to a decrease in exercise capacity in patients with IPF. In addition, an ROC curve analysis of 6MWD for predicting sarcopenia was performed, and the results showed that the best cut-off value was 370 m. Since this cut-off value was evaluated in a single-center, small, cross-sectional setting, larger studies are needed. The diagnosis of sarcopenia is not always easy to make at any medical facility, especially when assessing muscle mass by DXA or BIA. Therefore, the use of this cut-off value may help clinicians know when to suspect sarcopenia in patients with IPF.
Ten patients were treated with corticosteroids in this study, and the results showed that there was no significant difference in the presence of sarcopenia. However, the possibility of muscle atrophy, due to the side effects of corticosteroids, affecting sarcopenia cannot be ruled out. Further studies are needed to explore this possibility.
The present study has the following limitations. First, the results were obtained through an analysis of all Japanese patients from a single center with a small sample size, which might have introduced selection bias. Second, most patients were men, thus the results might not be representative of the overall population. Third, this was a cross-sectional study and sarcopenia was not assessed at the time of IPF diagnosis. A longitudinal survey will be needed to clarify the progression or improvement of sarcopenia. Fourth, we excluded patients on home oxygen treatment at rest who were unable to perform the 6MWT in order to assess the exercise capacity of IPF patients. However, the results might not be representative of the overall population of patients with IPF. A large sample-sized, high-quality study is warranted to overcome these issues.
In conclusion, sarcopenia was present in 39.3% of patients with IPF in the present cohort (in which most participants were men). Diagnosing sarcopenia in patients with IPF is important because it may affect physical performance and PROs. The knowledge of the cut-off value (370 m) for 6MWD may help clinicians know when to suspect sarcopenia in patients with IPF. Further studies are needed to demonstrate that continuous rehabilitation and nutritional guidance prevent or improve sarcopenia in outpatients with IPF.
Supplemental Material
Supplemental Material for Frequency and impact on clinical outcomes of sarcopenia in patients with idiopathic pulmonary fibrosis by Kohei Fujita, Hirotsugu Ohkubo, Akiko Nakano, Yuta Mori, Kensuke Fukumitsu, Satoshi Fukuda, Yoshihiro Kanemitsu, Takehiro Uemura, Tomoko Tajiri, Ken Maeno, Yutaka Ito, Tetsuya Oguri, Yoshiyuki Ozawa, Takayuki Murase and Akio Niimi in Chronic Respiratory Disease
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions: KoF and HO contributed equally to this study. KoF and HO drafted the submitted article and take responsibility for the integrity of the data and the accuracy of the data analysis. ANa, YM, KeF, SF, YK, TU, TT, KM, YI, TO, and ANi contributed to the interpretation of the manuscript. YO contributed as a radiologist. TM contributed as a pathologist. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Hirotsugu Ohkubo https://orcid.org/0000-0002-8538-1150
Ken Maeno https://orcid.org/0000-0002-4561-4044
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 4.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: Analysis of pooled data from three multinational phase 3 trials. Eur Respir J 2016; 47: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012; 156: 684–691. [DOI] [PubMed] [Google Scholar]
- 6.Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014; 190: 773–779. [DOI] [PubMed] [Google Scholar]
- 7.Nishiyama O, Taniguchi H, Kondoh Y, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J 2010; 36: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 8.Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 830–836. [DOI] [PubMed] [Google Scholar]
- 9.Sharp C, Adamali HI, Millar AB, et al. A comparison of published multidimensional indices to predict outcome in idiopathic pulmonary fibrosis. ERJ Open Res 2017; 3: 0096–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterniti MO, Bi Y, Rekić D, et al. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2017; 9: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 11.Jo HE, Glaspole I, Grainge C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: Analysis from the Australian idiopathic pulmonary fibrosis registry. Eur Respir J 2017; 49: 1601592. [DOI] [PubMed] [Google Scholar]
- 12.du Bois RM, Albera C, Bradford WZ, et al. 6-minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J 2014; 43: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 13.Vainshelboim B, Oliveira J, Fox BD, et al. The prognostic role of ventilatory inefficiency and exercise capacity in idiopathic pulmonary fibrosis. Respiratory Care 2016; 61: 1100–1109. [DOI] [PubMed] [Google Scholar]
- 14.Sumikawa H, Johkoh T, Colby TV, et al. Computed tomography findings in pathological usual interstitial pneumonia: Relationship to survival. Am J Respir Crit Care Med 2008; 177: 433–439. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Aging 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21: 300–307. [DOI] [PubMed] [Google Scholar]
- 17.Sepúlveda-Loyola W, Osadnik C, Phu S, et al. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2020; 11: 1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benz E, Trajanoska K, Lahousse L, et al. Sarcopenia in COPD: A systematic review and meta-analysis. Eur Respir Rev 2019; 28: 190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanada M, Sakamoto N, Ishimoto H, et al. A comparative study of the sarcopenia screening in older patients with interstitial lung disease. BMC Pulm Med 2022; 22: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama O, Yamazaki R, Sano H, et al. Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology 2017; 22: 480–485. [DOI] [PubMed] [Google Scholar]
- 21.Ebihara K, Iwanami Y, Yamasaki K, et al. Appendicular skeletal muscle mass correlates with patient-reported outcomes and physical performance in patients with idiopathic pulmonary fibrosis. Tohoku J Exp Med 2021; 253: 61–68. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Yoshimura K, Enomoto Y, et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci Rep 2018; 8: 14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon SW, Choi JS, Lee SH, et al. Thoracic skeletal muscle quantification: Low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res 2019; 20: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano A, Ohkubo H, Taniguchi H, et al. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci Rep 2020; 10: 2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awano N, Inomata M, Kuse N, et al. Quantitative computed tomography measures of skeletal muscle mass in patients with idiopathic pulmonary fibrosis according to a multidisciplinary discussion diagnosis: A retrospective nationwide study in Japan. Respir Investig 2020; 58: 91–101. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Aono Y, Kono M, et al. Cause of mortality and sarcopenia in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy. Respirology 2021; 26: 171–179. [DOI] [PubMed] [Google Scholar]
- 27.Faverio P, Fumagalli A, Conti S, et al. Nutritional assessment in idiopathic pulmonary fibrosis: A prospective multicentre study. ERJ Open Res 2022; 8: 0443–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. [DOI] [PubMed] [Google Scholar]
- 29.Laszlo G. Standardisation of lung function testing: Helpful guidance from the ATS/ERS task force. Thorax 2006; 61: 74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubota M, Kobayashi H, Quanjer PH, et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig 2014; 52: 242–250. [DOI] [PubMed] [Google Scholar]
- 31.ATS committee on proficiency standards for clinical pulmonary function laboratories, ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 32.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. [DOI] [PubMed] [Google Scholar]
- 33.Matsudaira T, Igarashi H, Kikuchi H, et al. Factor structure of the hospital anxiety and depression scale in Japanese psychiatric outpatient and student populations. Health Qual Life Outcomes 2009; 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malmstrom TK, Morley JE. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013; 14: 531–532. [DOI] [PubMed] [Google Scholar]
- 35.Koo HK, Park JH, Park HK, et al. Conflicting role of sarcopenia and obesity in male patients with chronic obstructive pulmonary disease: Korean national health and nutrition examination survey. PLoS One 2014; 9: e110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furukawa T, Taniguchi H, Ando M, et al. The St. George’s respiratory questionnaire as a prognostic factor in IPF. Respir Res 2017; 18: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuda T, Taniguchi H, Ando M, et al. Depression is significantly associated with the health status in patients with idiopathic pulmonary fibrosis. Intern Med 2017; 56: 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Tong X, Ma Y, et al. Prevalence of depression in patients with sarcopenia and correlation between the two diseases: Systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2022; 13: 128–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brach JS, FitzGerald S, Newman AB, et al. Physical activity and functional status in community-dwelling older women: A 14-year prospective study. Arch Intern Med 2003; 163: 2565–2571. [DOI] [PubMed] [Google Scholar]
- 40.Kalluri M, Luppi F, Ferrara G, et al. What patients with idiopathic pulmonary fibrosis and caregivers want: Filling the gaps with patient reported outcomes and experience measures. Am J Med 2020; 133: 281–289. [DOI] [PubMed] [Google Scholar]
- 41.Kitamura A, Seino S, Abe T, et al. Sarcopenia: Prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle 2021; 12: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Limpawattana P, Inthasuwan P, Putraveephong S, et al. Sarcopenia in chronic obstructive pulmonary disease: A study of prevalence and associated factors in the Southeast Asian population. Chron Respir Dis 2018; 15: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans WJ, Morley JE, Argilés J, et al. Cachexia: A new definition. Clin Nutr 2008; 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 44.Morley JE, Tomas DR, Wilson MM, et al. Cachexia: Pathophysiology and clinical relevance. Am J Clin Nutr 2006; 83: 735–743. [DOI] [PubMed] [Google Scholar]
- 45.Durham WJ, Dillon EL, Sheffield-Moore M, et al. Inflammatory burden and amino acid metabolism cancer cachexia. Curr Opin Clin Nutr Metab Care 2009; 12: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byun MK, Cho EN, Chang J, et al. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Frequency and impact on clinical outcomes of sarcopenia in patients with idiopathic pulmonary fibrosis by Kohei Fujita, Hirotsugu Ohkubo, Akiko Nakano, Yuta Mori, Kensuke Fukumitsu, Satoshi Fukuda, Yoshihiro Kanemitsu, Takehiro Uemura, Tomoko Tajiri, Ken Maeno, Yutaka Ito, Tetsuya Oguri, Yoshiyuki Ozawa, Takayuki Murase and Akio Niimi in Chronic Respiratory Disease