Abstract
Rodent skeletal muscle contains a large store of nitrate that can be augmented by the consumption of dietary nitrate. This muscle nitrate reservoir has been found to be an important source of nitrite and nitric oxide (NO) via its reduction by tissue xanthine oxidoreductase. To explore if this pathway is also active in human skeletal muscle during exercise, and if it is sensitive to local nitrate availability, we assessed exercise-induced changes in muscle nitrate and nitrite concentrations in young healthy humans, under baseline conditions and following dietary nitrate consumption. We found that baseline nitrate and nitrite concentrations were far higher in muscle than in plasma (~4-fold and ~29-fold, respectively), and that the consumption of a single bolus of dietary nitrate (12.8 mmol) significantly elevated nitrate concentration in both plasma (~19-fold) and muscle (~5-fold). Consistent with these observations, and with previous suggestions of active muscle nitrate transport, we present western blot data to show significant expression of the active nitrate/nitrite transporter sialin in human skeletal muscle. Furthermore, we report an exercise-induced reduction in human muscle nitrate concentration (by ~39%), but only in the presence of an increased muscle nitrate store. Our results indicate that human skeletal muscle nitrate stores are sensitive to dietary nitrate intake and may contribute to NO generation during exercise. Together, these findings suggest that skeletal muscle plays an important role in the transport, storage and metabolism of nitrate in humans.
Keywords: exercise, nitrate, nitric oxide, nitrite, skeletal muscle
Introduction
Nitric oxide (NO) is a ubiquitous mammalian signalling molecule intricately involved in processes such as blood flow regulation, neurotransmission, and muscular contractions (Gaskell et al. 1880; Cannon et al. 2001; Sarelius & Pohl, 2010; Copp et al. 2013; Garthwaite, 2019). In mammals, NO is generated by the oxygen (O2)-dependent conversion of arginine to citrulline by the NO synthase (NOS) enzyme family (Förstermann & Münzel, 2006; Förstermann & Sessa, 2012), and via the reduction of nitrite by deoxy-haem proteins, mainly deoxyhaemoglobin and deoxymyoglobin (Cosby et al. 2003; Gladwin et al. 2005; Lundberg et al. 2008; van Faassen et al. 2009) or molybdopterin-containing enzymes, xanthine oxidoreductase (XOR) or aldehyde oxidase (AO) (Li et al. 2008). Nitrite is derived from the reduction of nitrate by commensal bacteria residing in the oral cavity (Govoni et al. 2008; Kapil et al. 2013), and, as only recently shown, by mammalian tissue nitrate reductases (Jansson et al. 2008; Piknova et al. 2016b). Therefore, nitrate, which can be endogenously produced by the oxidation of NOS-derived NO and nitrite (Lundberg et al. 2008), generated directly by NOS enzymes during the ‘futile’ cycle (Stuehr et al. 2004; Haque et al. 2013), or enter the body via the diet (Hord et al. 2009), should be seen as an important source of NO.
Recent research suggests that skeletal muscle may play a central role in the production, storage and metabolism of nitrate. The concentration of nitrate in skeletal muscle has been found to be far greater than in blood or other organs in rodents (Piknova et al. 2015, 2016b) and humans (Nyakayiru et al. 2017). Based on experiments with the NOS1 (neuronal NOS) knock-out mouse and NOS inhibition in rats, a large portion of the basal nitrate reservoir in rodents was found to be produced by NOS (Haque et al. 2013; Piknova et al. 2015). However, it is also clear that the nitrate store is significantly elevated after the consumption of dietary nitrate, in both rodents (Gilliard et al. 2018) and in diabetic older humans (Nyakayiru et al. 2017).
Continuous NO generation is essential in resting and contracting skeletal muscle due to its role in mitochondrial respiration, glucose uptake, force production and functional hyperaemia (Stamler & Meissner, 2001; McConell et al. 2012). Acute oral treatment with nitrate, as a precursor of NO, restores sympatholysis and improves post-exercise hyperaemia in patients with Becker muscular dystrophy (Nelson et al. 2015; for review see Dombernowsky et al. 2018). It was recently discovered that rodent skeletal muscle tissue is capable of nitrate-to-nitrite reduction, in a reaction catalysed predominantly by XOR (Piknova et al. 2015, 2016b). Moreover, exercise-induced reductions in rodent skeletal muscle nitrate (and transient increases in nitrite) indicate that this pathway may be important for NO supply during exercise, a situation in which NOS function is likely impaired by the accompanying fall in pH and O2 availability (Lundberg et al. 2008; Black et al. 2017). The presence of XOR in human skeletal muscle (Hellsten-Westing, 1993) implies that this nitrate–nitrite pathway may be active and important in humans as well as in rats (Piknova et al. 2016b). If present in human tissues, this pathway may be implicated in the positive effects of dietary nitrate consumption on skeletal muscle contractile function (Bailey et al. 2010; Haider & Folland, 2014; Coggan et al. 2015; Coggan & Peterson, 2018) and exercise performance (see Jones et al. 2018, for review).
In the present study, we utilize analytical procedures previously used to measure nitrate and nitrite ions in rodent skeletal muscle tissue (Piknova et al. 2015) to explore skeletal muscle nitrate storage and metabolism in young healthy adult humans. We show that the concentration of nitrate and nitrite is substantially higher in human skeletal muscle than in plasma and that skeletal muscle nitrate stores can be further augmented by dietary nitrate ingestion. We present western blot data to show that sialin, a nitrate transporter known to concentrate nitrate from the blood into salivary glands (Qin et al. 2012), is expressed in human skeletal muscle. Furthermore, we confirm the presence of NOS1 and XOR in human skeletal muscle homogenates and show that a bout of high-intensity exercise lowers muscle nitrate concentration, but only when the exercise bout is initiated after nitrate consumption and therefore in the presence of high muscle nitrate content. These findings suggest that an active nitrate transport system is present in human skeletal muscle, and that muscle nitrate is metabolized during exercise, at least when muscle nitrate concentration is elevated. Considerable inter-individual differences in nitrate storage and the expression of proteins involved in muscle nitrate metabolism are also reported.
Methods
Ethical approval
All procedures employed in this study were approved by the Sport and Health Sciences Ethics Committee of the University of Exeter and were conducted in accordance with the standards set out in the Declaration of Helsinki, except for registration in a database. Prior to their enrolment into the study, and after the experimental procedures, associated risks and potential benefits of participation had been explained to them, participants gave their written informed consent. Studies at the National Institutes of Health were approved by the Human Research Protection Program Office of NIDDK, approval number 17-NIDDK-00091 (project title ‘Dietary nitrate in skeletal muscle as intrinsic source of nitric oxide’) and conducted under Material Transfer Agreement (MTA; DK 17–0169).
Participants
Thirteen healthy, recreationally active participants volunteered for this study (8 males, mean ± SD: age 29 ± 12 years, body mass 78.5 ± 11.1 kg, height 1.71 ± 0.23 m, peak pulmonary O2 uptake (), 48.3 ± 8.0 mL kg−1 min−1; and 5 females: age 22 ± 5 years, body mass 71.5 ± 7.8 kg, height 1.70 ± 0.03 m, 41.7 ± 3.7 mL kg−1 min−1). No participant was known to be taking any medication or consuming any dietary supplements, and did not have a history of respiratory, cardiovascular, metabolic or musculoskeletal disease. None of the participants were tobacco smokers.
Pre-experimental tests
Participants made two visits to the laboratory for pre-experimental exercise testing and familiarization. All exercise tests in this study were performed on an electronically braked cycle ergometer (Lode Excalibur Sport, Groningen, The Netherlands). During Visit 1, participants performed a ramp incremental exercise test to task failure for the determination of and gas exchange threshold (GET). After completing 3 min of ‘unloaded’ baseline cycling at 20 W, the work rate was increased linearly by 30 W min−1 until the participant reached volitional exhaustion. The test was terminated when the self-selected pedal rate fell by 10 r.p.m. for more than 5 s, despite strong verbal encouragement. The handlebar and saddle height configuration were recorded and replicated for all subsequent tests. Breath-by-breath pulmonary gas-exchange data were collected and averaged into 10 s bins. was determined as the highest 30 s mean value attained before the test was terminated. The GET was established as described previously (Vanhatalo et al. 2010). The work rate that would require 70% of the difference between the work rate at GET and , plus the work rate at GET (i.e. high-intensity exercise) was subsequently calculated. During Visit 2, participants were familiarized to a high-intensity constant work rate step exercise test (see below for details). All participants were instructed to refrain from strenuous physical activity in the 24 h before these and all other laboratory visits.
Experimental design
Following completion of the pre-experimental visits, participants attended the laboratory on two separate occasions to complete the full experimental protocol (Fig. 1). On the day before each of these visits, participants consumed a standardized low-nitrate diet (containing <25 mg nitrate day−1 and <2 mg nitrite day−1). Participants arrived at the laboratory at 07.45 h, following an overnight fast. Upon arrival, participants were asked to rest in a supine position before a cannula (Insyte-W; Becton-Dickinson, Franklin Lakes, NJ, USA) was inserted into an antecubital vein. In preparation for skeletal muscle biopsy sampling, two incisions (~0.6 cm) were then made through the skin and fascia over the medial part of the vastus lateralis muscle under local anaesthesia (2 mL; 20 mg L−1 lidocaine (lignocaine) without adrenaline) and covered with sterile gauze. A blood sample was then taken, followed immediately by the collection of a muscle tissue sample (‘baseline’). Participants then ingested either 140 mL of concentrated nitrate-rich beetroot juice (NIT; containing 12.8 mmol (794 mg) nitrate) or 140 mL of concentrated nitrate-depleted beetroot juice (placebo, PLA; containing 0.04 mmol (<3 mg) nitrate) in addition to a standardized low nitrate breakfast (72 g porridge oats with 180 mL semi-skimmed milk).
Figure 1. Schematic overview of the experimental protocol.

Participants completed this protocol on two occasions, with nitrate-rich beetroot juice (NIT) or nitrate-depleted beetroot juice (placebo, PLA) supplementation in a randomized manner with repeated measures. Blood and muscle samples were collected at baseline, 120 min post-supplement ingestion, and immediately after a high-intensity bout of exercise (post-exercise).
Following 2 h of supine rest, blood and muscle samples were obtained (‘post-supplement’). Participants then completed a high-intensity ‘step’ exercise test. Step-tests began with 3 min of ‘unloaded’ pedalling at 20 W, followed by a sudden increase to the target work rate. Each bout was continued until task failure as a measure of exercise tolerance. At the point of task failure, blood and muscle samples were again collected (‘post-exercise’). Breath-by-breath pulmonary gas exchange data were collected continuously throughout all step tests. The exercise protocol was initiated 5–10 min after the post-supplement blood and muscle samples were collected.
Sample collection
Blood samples.
All three venous blood samples (i.e. baseline, post-supplement and post-exercise) collected on each visit were drawn into lithium–heparin vacutainers (7.5 mL, Becton-Dickinson) and centrifuged at 3500 g for 6.5 min at 4°C within 30 s of collection. Plasma was immediately extracted into 1.5 mL Eppendorf tubes and stored at −80°C until later determination of plasma nitrate and nitrite concentrations.
Muscle samples.
All three muscle samples on each visit (i.e. baseline, post-supplement and post-exercise) were taken using a Bergström needle modified for manual vacuum (Bergström, 1975). All biopsy samples were placed on sterile gauze, blotted to remove blood and freed from any visible adipose tissue, and then frozen in liquid nitrogen and stored at −80°Cuntil later analysis for muscle nitrate and nitrite determination.
Measurements
Nitrate and nitrite.
Nitrate and nitrite concentrations in plasma and muscle were quantified using a standard gas-phase chemiluminescence technique (Pinder et al. 2008; Piknova & Schechter, 2011). For determination of plasma nitrate and nitrite, samples were first deproteinized using cold methanol precipitation. Specifically, thawed samples were mixed with ice-cold methanol (dilution 1:3 sample: methanol) and then centrifuged at 11,000 g for 5 min at 4°C. Supernatants were collected and immediately used to determine nitrate and nitrite content by chemiluminescence (see further details below). Muscle samples were initially weighed (20–150 mg wet weight), mixed with nitrite preserving solution (K3Fe(CN)6, N-ethylmaleimide, water, Nonidet P-40), as described in Piknova & Schechter (2011), and homogenized using a GentleMacs homogenizer (Miltenyi Biotec Inc., Auburn, CA, USA). Proteins were then precipitated using cold methanol (as described above) before the supernatant was extracted and immediately used for the determination of nitrate and nitrite content by chemiluminescence. The nitrite and nitrate content of the plasma and muscle supernatant was determined by a Sievers gas-phase chemiluminescence NO analyser (Sievers 280i Nitric Oxide Analyser, GE Analytical Instruments, Boulder, CO, USA) as described in Piknova & Schechter (2011) and Piknova et al. (2016a). Nitrate and nitrite data are presented as nanomoles per gram wet weight of tissue.
Sialin, XOR, AO and NOS1 expression.
Western blotting was used to determine the presence of NOS1, XOR, AO and sialin protein in the skeletal muscle tissue of each participant. In short, small tissue samples (5–20 mg wt weight) were first homogenized in radio-immunoprecipitation (RIPA) buffer (Sigma-Aldrich, St. Louis, MO, USA; Cat. no. R0278) containing protease inhibitor cocktail (Calbiochem, La Jolla, CA, USA; 539134) using a GentleMacs tissue dissociator and protein concentration was then determined by bicinchoninic acid (BCA) assay. Denatured samples (50 μg) were run on SDS-PAGE and then transferred to nitrocellulose membrane. The membrane was incubated with primary antibodies (anti-sialin: AlphaDiagnostics, San Antonio, TX, USA; SIAL11-A; anti-XOR: Abcam, Cambridge, UK; ab133268; anti-AO: SantaCruz Biotechnology, Santa Cruz, CA, USA; SC-365291; anti-NOS1: BD Transduction Laboratories, San Diago, CA, USA; 610309; anti-GAPDH: Cell Signalling Technology Inc., Danvers, MA, USA; 97166) overnight at 4°C. Goat-anti-mouse or goat-anti-rabbit antibodies conjugated with horse-radish peroxidase (Jackson ImmunoResearch, West Grove, PA, USA) were used as secondary antibodies and followed by enhanced chemiluminescence (ECL) detection (SuperSignal West Femto maximum sensitivity substrate, Thermo Fisher Scientific, Rockford, IL, USA). Band density was quantified using NIH ImageJ software.
Pulmonary oxygen uptake ().
Pulmonary was measured breath-by-breath using an online gas analyser (Oxycon Pro; Jaeger GmbH, Hoechberg, Germany), as described previously (Wylie et al. 2013). Data were averaged over 10-s periods. End-exercise was defined as the mean value measured over the final 30 s of exercise.
Statistics
A full dataset (n = 13) was not available for all variables due to the failure to obtain a sample (i.e. blood or muscle) at a certain time point. To maximize the available data for each key comparison, independent paired-sample Student’s t tests were employed. Specifically, differences between plasma and muscle concentrations of nitrate and nitrite at baseline, and differences between baseline and post-supplement nitrate and nitrite concentrations in plasma and muscle within the PLA and NIT arms of this study were assessed using independent Student’s t tests. Independent Student’s t tests were also used to assess differences between post-supplement and post-exercise nitrate and nitrite concentrations in plasma and muscle within the PLA and NIT arms. The number of samples (n) for each comparison is presented. Relationships between variables were assessed via Pearson’s product-moment correlation coefficient. Data are presented as means ± standard deviations (SD) unless otherwise stated. Statistical significance was accepted when P < 0.05.
Results
Baseline plasma and muscle nitrate and nitrite concentrations
Baseline concentrations of nitrate and nitrite in plasma and skeletal muscle are presented in Fig. 2. Nitrate (226 ± 213 nmol g−1) and nitrite (5.7 ± 7.4 nmol g−1) concentrations in skeletal muscle were much higher than in plasma (nitrate: 54 ± 27 nmol g−1; nitrite: 0.2 ± 0.2 nmol g−1; both P < 0.01). Muscle nitrite concentration was positively correlated with muscle nitrate concentration (r = 0.77; P < 0.01), but plasma nitrite concentration was not correlated with plasma nitrate concentration (r = −0.02; P > 0.05).
Figure 2. Baseline nitrate (A) and nitrite (B) concentrations were higher in skeletal muscle (filled bars) than in plasma (open bars).
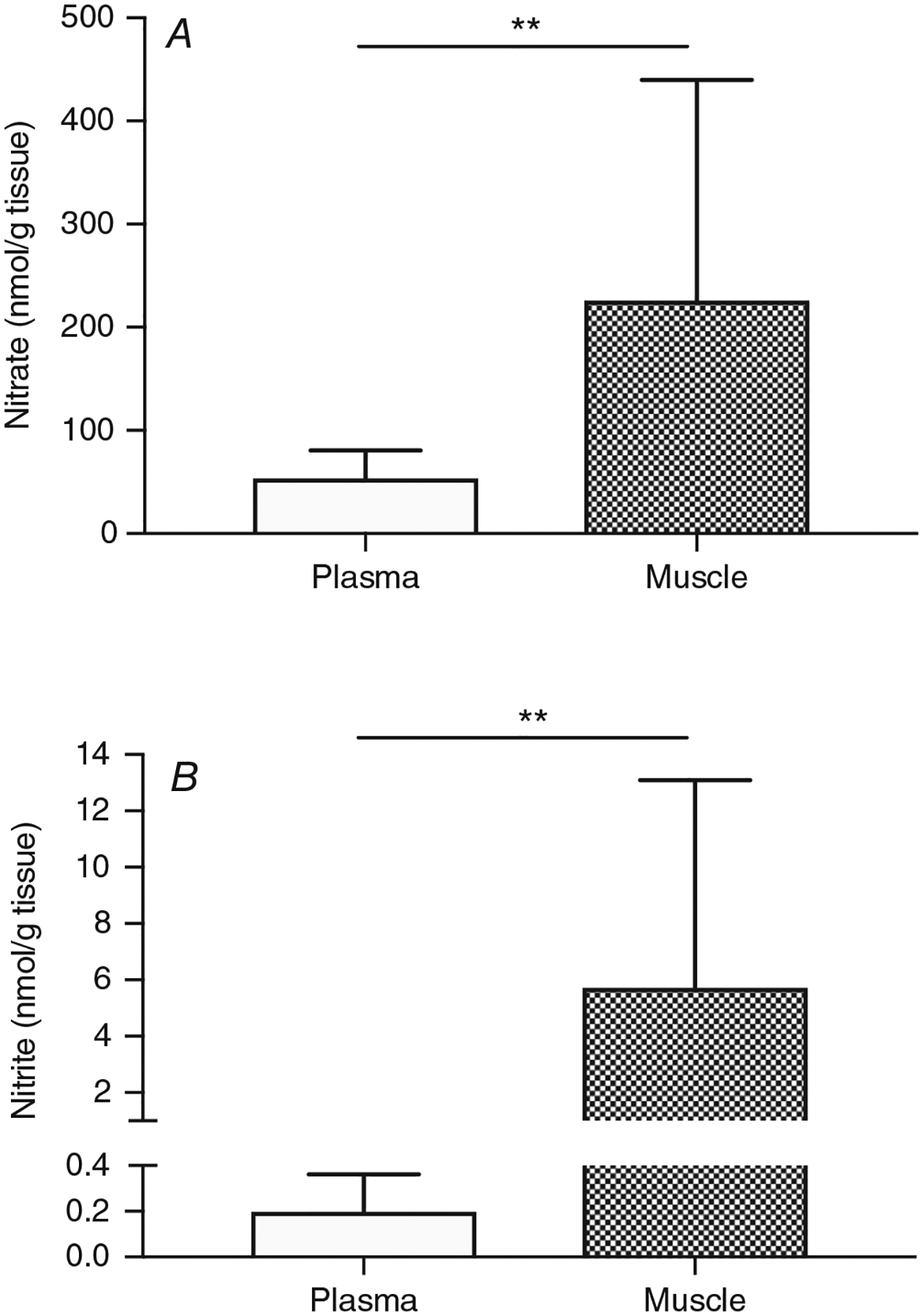
All available baseline data from both PLA and NIT arms were used (n = 24). **Difference of nitrate or nitrite in muscle from plasma values (P < 0.01).
Influence of dietary nitrate ingestion on plasma and muscle nitrate and nitrite concentrations
The influence of ingesting a single dietary nitrate dose, 12.8 mmol (794 mg), or a placebo (containing negligible nitrate) bolus on plasma and muscle nitrate and nitrite concentrations is shown in Fig. 3. Two hours after dietary nitrate consumption, the concentration of nitrate in plasma increased ~19-fold to 1082 ± 731 nmol g−1 (n = 11; Fig. 3A), and the concentration of nitrate in muscle increased ~5-fold to 1139 ± 894 nmol g−1 (n = 13; Fig. 3B), compared to baseline (P < 0.01).
Figure 3. The concentration of nitrate and nitrite in plasma (A and C, respectively) and muscle (B and D, respectively), measured pre-supplement (Pre) and 2 h after (Post) ingestion of dietary nitrate (NIT) or a placebo (PLA) supplement.
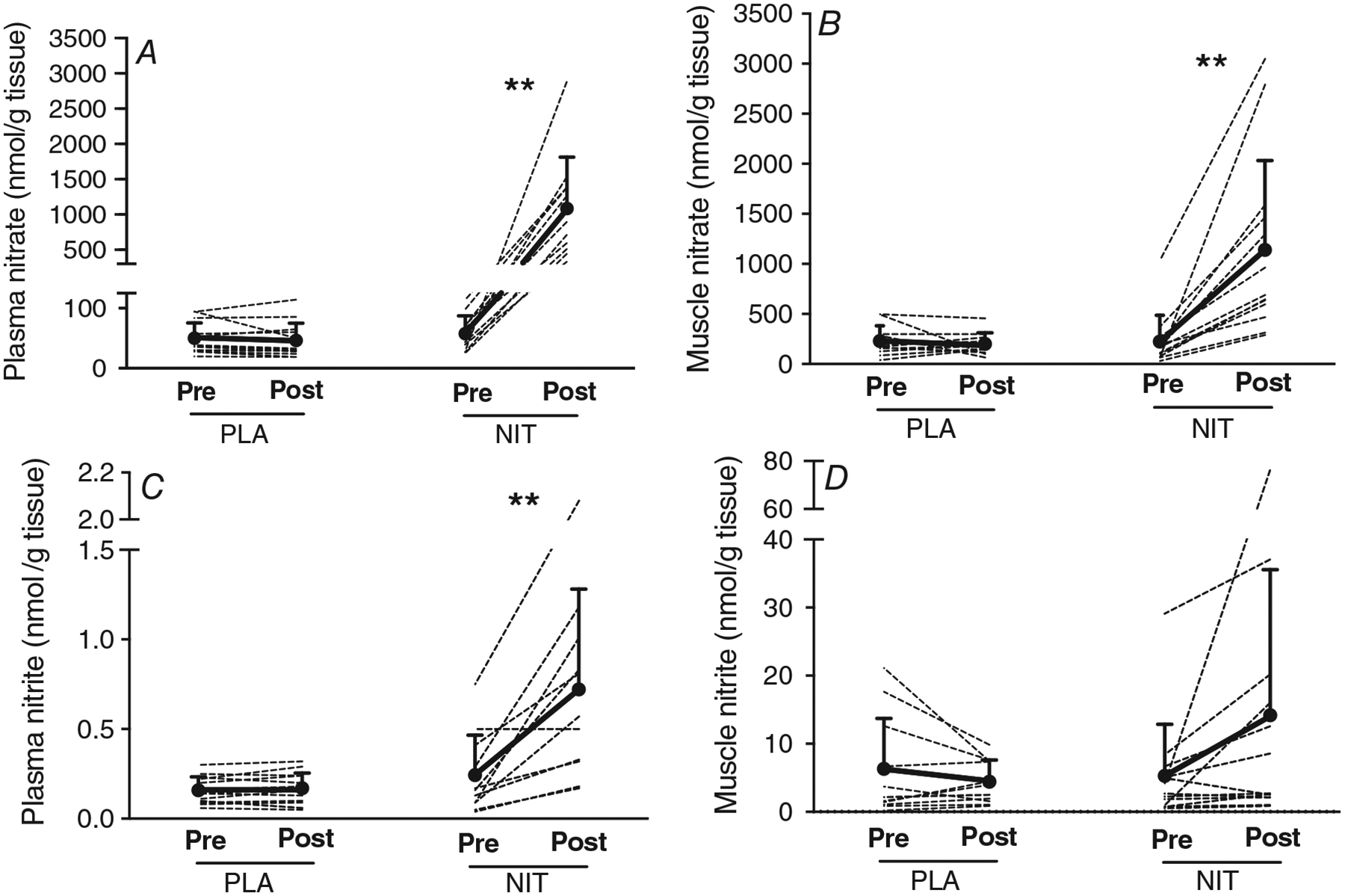
Ingestion of nitrate significantly elevated the concentrations of nitrate in plasma and muscle, and the concentrations of nitrite in plasma. **Difference from pre-supplement (P < 0.01).
After dietary nitrate ingestion, the concentration of nitrite in plasma increased ~3-fold to 0.7 ± 0.6 nmol g−1, compared to baseline (P < 0.01; n = 11; Fig. 3C). Nitrite concentration in muscle also increased ~3-fold to 14.2 ± 21.4 nmol g−1, although this difference was not statistically significant (P = 0.13; n = 13; Fig. 3D). As expected, the consumption of a placebo supplement did not alter plasma nitrate (n = 13) and nitrite (n = 13), or muscle nitrate (n = 11) and nitrite (n = 11) concentrations (all P > 0.05; Fig. 3).
Influence of high-intensity exercise on plasma and muscle nitrate and nitrite concentrations
Two hours after the ingestion of a placebo or dietary nitrate supplement, participants exercised at a high-intensity work rate until they reached task failure. One participant did not reach the criteria for a maximal effort on one of the experimental trials and so these data were not included in the analyses related to the exercise bout. The time to task failure was similar in the placebo and dietary nitrate conditions (PLA: 396 ± 102 s vs. NIT: 389 ± 110 s; n = 12; P > 0.05) and the reached at the end of the exercise bout was also similar in the placebo (3.43 ± 0.63 L min−1) and nitrate (3.43 ± 0.63 L min−1; n = 12; P > 0.05) conditions.
Figure 4 shows the nitrate and nitrite concentrations in plasma and muscle immediately before (i.e. post-supplement) and immediately after the exercise bout (i.e. post-exercise) in the placebo and dietary nitrate conditions. Because the consumption of the placebo supplement had no impact on plasma and muscle nitrate and nitrite, the exercise bout in the placebo condition was initiated in the presence of baseline plasma and muscle nitrate and nitrite concentrations. Under these conditions, the high-intensity exercise bout did not significantly influence plasma nitrate (n = 13) and nitrite (n = 13), or muscle nitrate (n = 10) and nitrite (n = 10) concentrations (all P > 0.05; Fig. 4).
Figure 4. Nitrate concentration in plasma (A) and muscle (B), and nitrite concentration in plasma (C) and muscle (D), measured immediately before (Pre) and after (Post) a high-intensity exercise bout, following the consumption of either a placebo (PLA) or dietary nitrate (NIT) supplement.

The exercise bout significantly reduced muscle nitrate and plasma nitrite concentrations. *Difference from pre-exercise (P < 0.05). **Difference from pre-exercise (P < 0.01).
In contrast, the exercise bout in the dietary nitrate condition (NIT) was initiated with elevated concentrations of nitrate and nitrite in plasma and muscle (Fig. 4). Under these conditions, muscle nitrate concentration was reduced by ~39% (n = 11; P < 0.05; Fig. 4B), and plasma nitrite concentration was reduced by ~34% (n = 10; P < 0.01; Fig. 4C) over the course of the exercise bout. Neither the change in muscle nitrate nor the change in plasma nitrite following exercise was correlated with the change in time to task failure from PLA to NIT (both P > 0.05). Plasma nitrate (n = 10) and muscle nitrite (n = 11) concentrations were not influenced by the exercise bout (both P > 0.05; Fig. 4A and D).
Skeletal muscle sialin, NOS1, AO and XOR expression
The presence of XOR, NOS1, AO and sialin in skeletal muscle tissue was assessed using Western blots. As can be seen in Fig. 5, NOS1, XOR, AO and sialin were expressed in the skeletal muscle tissue of all participants. However, the expression of XOR and sialin, and to a lesser extent, NOS1 and AO, appears to vary substantially between individuals.
Figure 5. Western blots of the neuronal nitic oxide synthase (NOS-1), xanthine oxidoreductase (XOR), aldehyde oxidase (AO) and the nitrate transporter, sialin, in human skeletal muscle tissue of each of thirteen individual participants.
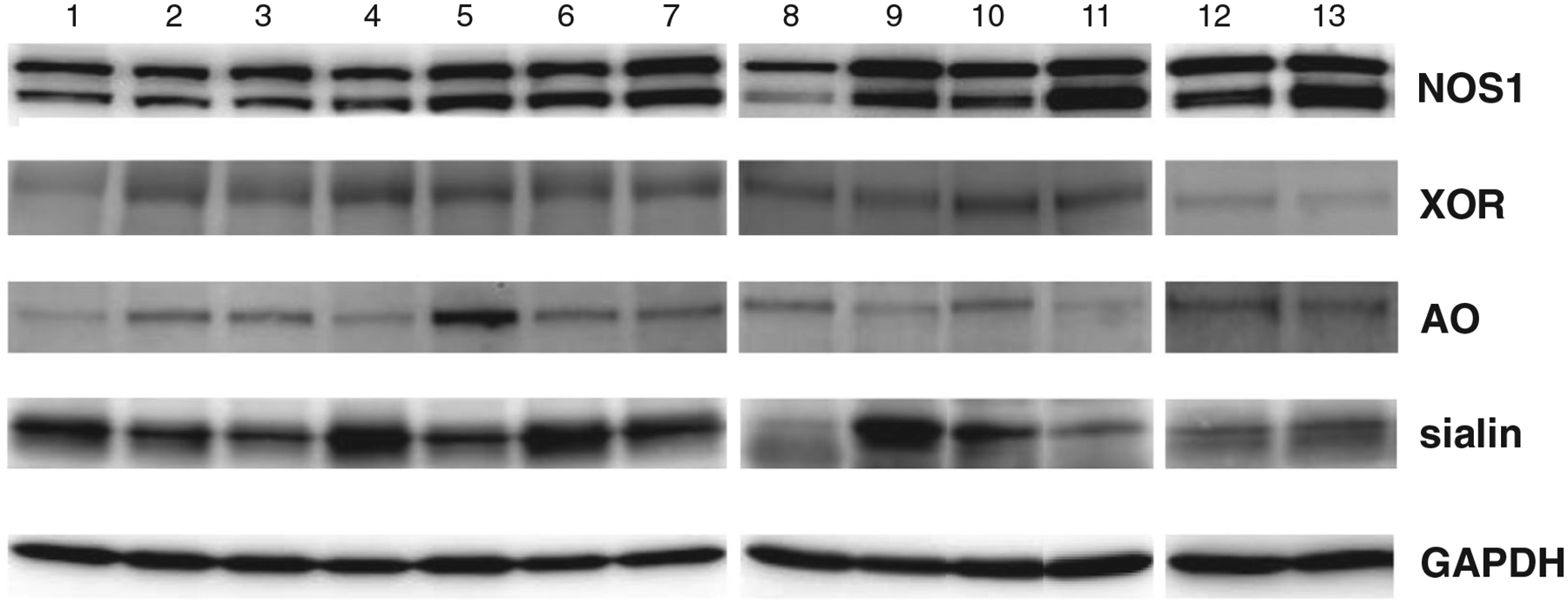
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard. Note that all proteins were present in human skeletal muscle, and that there was large inter-individual variation in all four proteins, most notably in sialin and XOR.
Discussion
In the present study, we adapted sample processing and analytical procedures used previously to measure nitrate and nitrite ions in rodent skeletal muscle (Piknova et al. 2015) to examine the influence of dietary nitrate consumption and acute exercise on human skeletal muscle nitrate storage and metabolism. We show that nitrate and nitrite are present in human skeletal muscle at far higher concentrations than are observed in plasma, and that the concentration of nitrite and particularly nitrate increase after the consumption of dietary nitrate. We confirm the presence of sialin, a known active nitrate/nitrite ion transporter (Qin et al. 2012), in human muscle homogenates, consistent with the existence of an operational active transport system in human skeletal muscle. Further, we report that the concentration of nitrate in skeletal muscle tissue is reduced following acute exercise, suggesting that nitrate may contribute to local NO generation. However, in contrast with our previous finding in rats (Piknova et al. 2016b), this occurs only when the muscle nitrate store is elevated above baseline, i.e. following dietary nitrate consumption.
Baseline plasma and skeletal muscle nitrate and nitrite concentrations
We showed that the baseline concentration of nitrate was 4-fold higher in skeletal muscle than in plasma (Fig. 2). This is consistent with previous observations in rodents (Piknova et al. 2015, 2016b; Gilliard et al. 2018) and older diabetic humans (Nyakayiru et al. 2017). We confirmed robust NOS1 expression in human skeletal muscle tissue (Fig. 5), consistent with previous findings in rodents (Piknova et al. 2015). It is likely that oxymyoglobin-driven oxidation of excess NO produced by this enzyme (and to a lesser extent, endothelial NOS (NOS3); J.W. Park, unpublished observations; Möller & Sylvén, 1981; Sylvén et al. 1984), and the direct production of nitrate from NOS via its ‘futile’ cycle (Stuehr et al. 2004; Li & Poulos, 2005; Tejero et al. 2009) contribute to this human muscle nitrate reservoir. However, the increase in muscle nitrate observed after dietary nitrate consumption presented here (Fig. 3), and the presence of the active nitrate transporter, sialin (Fig. 5), suggests that active uptake of nitrate from the bloodstream is likely also important.
Interestingly, we found that in humans nitrite is present in muscle at far higher concentrations than is observed in plasma (Fig. 2B). To the best of our knowledge, this study is the first to measure nitrite in human skeletal muscle tissue. The concentration of nitrite measured in human skeletal muscle (5.7 nmol g tissue−1) is consistent with that observed in the skeletal muscle of rats (5 nmol g tissue−1; Gilliard et al. 2018). Both on-site oxidation of excess NOS-derived NO and active uptake of nitrite from the bloodstream likely contribute to this nitrite pool. However, the presence of XOR in human skeletal muscle (Fig. 5), the reduction in muscle nitrate during acute exercise (see below), and the positive correlation between basal muscle nitrate and nitrite concentrations suggest that intramuscular nitrate reduction also contributes to this nitrite pool. Irrespective of the source, the presence of several nitrite–NO pathways in skeletal muscle involving, for example, deoxymyoglobin (Shiva et al. 2007), mitochondrial enzymes (Kozlov et al. 1999) and XOR (Piknova et al. 2015), suggests that this nitrite pool may be an important source of NO generation in human skeletal muscle, particularly in hypoxic and acidic conditions.
While serving to support local NO bioavailability in skeletal muscle (see below), the presence of a muscle-to-blood nitrate gradient and a nitrate transporter (sialin) implies that human skeletal muscle may also be capable of distributing this ion into the bloodstream via passive diffusion, where it can then be reduced to nitrite in the oral cavity (Govoni et al. 2008) or in other organs with nitrate reductase activity, such as the liver (Jansson et al. 2008). This suggests that skeletal muscle has the potential to play a role in the regulation of whole-body NO bioavailability. Additional work is required to test this hypothesis.
Influence of nitrate ingestion on muscle nitrate and nitrite concentrations
To assess the impact of dietary nitrate ingestion on human muscle nitrate and nitrite stores, we measured the concentrations of these ions in samples collected 2 h after the consumption of a single bolus of nitrate-rich beetroot juice. Nitrate consumption significantly increased plasma nitrate (Fig. 3A) and muscle nitrate concentrations (Fig. 3B). The observed increase of plasma and muscle nitrate is in line with previous findings from our group (Bailey et al. 2010; Wylie et al. 2013) and others (e.g. Nyakayiru et al. 2017). Together, these findings confirm that the skeletal muscle of young and older adults effectively sequesters nitrate from the bloodstream.
Both the muscle-to-blood nitrate gradient under basal conditions (Fig. 2), and the rise in muscle nitrate after dietary nitrate consumption (Fig. 3B), suggests that human skeletal muscle possesses active nitrate transport machinery. Sialin is an active nitrate transporter, and is known to be responsible for concentrating nitrate from blood into salivary glands in humans (Qin et al. 2012), an important first step for the conversion of nitrate to nitrite in the oral cavity (Lundberg et al. 2008). Qin et al. (2012) reported the presence of low levels (relative to salivary gland, liver and brain) of sialin in the skeletal muscle of miniature pigs. We report here that sialin is also present in human skeletal muscle tissue (Fig. 5), which suggests that it may be involved in skeletal muscle nitrate uptake. To the best of our knowledge, this is the first report that sialin is present in human skeletal muscle, and, as such, it will require more detailed characterization in the future. It is also important to note that other transport systems capable of nitrate transport, such as chloride anion transporter 1 (CLC1), an isoform present exclusively in the skeletal muscle (Zifarelli & Pusch, 2009), may also be involved in active skeletal muscle nitrate uptake. The expression of sialin and to a lesser extent also other proteins of interest (i.e. NOS1, XOR and AO) is highly variable between individuals (Fig. 5), which likely contributes to the higher variability of nitrate and nitrite in human tissue compared to rats.
Two hours after the consumption of dietary nitrate, we observed a 3-fold increase in muscle nitrite concentration. While this difference did not reach statistical significance, due to large variability between individuals, the concentration of muscle nitrite increased in all but two of the participants assessed (Fig. 3D). As reported previously (e.g. Wylie et al. 2013) and as shown in Fig. 3C, a significant increase in plasma nitrite occurs after the consumption of dietary nitrate, an effect that is mostly dependent on nitrate-to-nitrite reduction by commensal bacteria in the oral cavity (Govoni et al. 2008). Uptake of this nitrite by skeletal muscle results in an increase in muscle nitrite after dietary nitrate consumption (Fig. 3D). This supports the assumption that improvements in skeletal muscle contractile force or fatigue resistance following dietary nitrate consumption (Bailey et al. 2010; Fulford et al. 2013; Haider & Folland, 2014; Coggan et al. 2015; Coggan & Peterson, 2018) are mediated, at least in part, by increased muscle nitrite concentration and NO bioavailability.
A further important finding from the present study is the substantial inter-individual difference in both baseline muscle nitrate concentration (range: 30–1036 nmol g tissue−1) and in the increase in muscle nitrate concentration after consumption of dietary nitrate (range: 247–2723 nmol g tissue−1). Baseline muscle nitrate concentration likely reflects the balance between nitrate transport into the muscle from the bloodstream, endogenous nitrate synthesis within the muscle (i.e. oxidation of NOS-derived NO and nitrate production by NOS1) and nitrate reduction by XOR, AO or other mammalian nitrate reductases. It is therefore possible that at least part of the large inter-individual variability in baseline muscle nitrate concentration is related to the variable expression of NOS1, XOR, AO and sialin in muscle. Moreover, assuming that sialin is involved in muscle nitrate uptake, the large inter-individual differences in the expression of this protein (Fig. 5) may also be mediating the variable increase in muscle nitrate concentration observed after nitrate consumption (Fig. 3B). However, it is likely that other factors also contribute, including dietary nitrate intake, physical activity levels, muscle fibre type and capillarization.
Influence of exercise on skeletal muscle nitrate and nitrite concentrations
During skeletal muscle contraction, an increase in NO supply occurs to support the elevated rate of mitochondrial respiration, glucose uptake and other processes, including functional hyperaemia (Stamler & Meissner, 2001). Recent observations that acute exercise in rodents causes a significant decline in muscle nitrate and a transient increase in muscle nitrite have raised the possibility that nitrate stored in skeletal muscle may be a significant source of NO (via a nitrite intermediate) during exercise. Consistent with this hypothesis, in vitro experiments have revealed the presence of nitrate-to-nitrite and nitrite-to-NO reduction in rodent skeletal muscle homogenates, a process mediated (principally) by the mammalian nitrate reductase XOR, and facilitated by contraction-induced hypoxia and acidosis (Piknova et al. 2015, 2016b). The presence of XOR and AO in human skeletal muscle tissue (Fig. 5) suggests that this pathway may also be functional in humans.
To gain insight into whether the human skeletal muscle reservoir is utilized for NO production in humans, we assessed changes in muscle nitrate and nitrite concentrations after a short bout (~6.5 min) of high-intensity exercise. Moreover, to determine if this pathway is influenced by the amount of available nitrate, we tested these potential exercise-related changes under basal conditions (i.e. PLA condition) and after dietary nitrate consumption (i.e. NIT condition). We found a reduction in muscle nitrate when the exercise bout was initiated after nitrate consumption and, therefore, in the presence of an increased muscle nitrate store (Fig. 4B), but not after exercise under basal conditions. These findings differ from our previous study in rats, in which nitrate reduction was observed under non-supplemented conditions (Piknova et al. 2016b), and suggests that the muscle nitrate reduction pathway in humans may be particularly active when local nitrate availability is high. The apparent contrast with rats may be explained by the high variability in the level of necessary enzymes in humans and the fact that, contrary to rodents, humans individualize their diets. Moreover, it is possible that the participants in the present study were nitrate deficient upon arrival at the laboratory due to the prescribed low-nitrate diet in the 24 h preceding each testing visit. This is likely important given that diet history influences the ability of skeletal muscle to store and possibly use nitrate, at least in rodents (Gilliard et al. 2018). It is also possible that the shorter exercise duration (6.5 min in the present study compared to 1 h in the rodent study; Piknova et al. 2016b) limited muscle nitrate reduction under basal conditions to an undetectable level.
In rodents, a reduction in muscle nitrate during exercise was accompanied by a transient increase in muscle nitrite (Piknova et al. 2016b). Conversely, in the present study, the exercise-induced lowering of muscle nitrate after nitrate consumption occurred alongside a non-significant ~40% decline in muscle nitrite. We speculate that the observed decline in muscle nitrite concentration is due to the rate of nitrite-to-NO reduction exceeding that of nitrate-to-nitrite reduction. Indeed, there is a larger array of known nitrite–NO reduction pathways than nitrate–nitrite pathways in skeletal muscle (Kozlov et al. 1999; Shiva et al. 2007; van Faassen et al. 2009). Moreover, the higher exercise intensity administered in the present study, compared to that in rodents (Piknova et al. 2016b), would likely have resulted in relatively greater muscle acidosis and hypoxia, further augmenting nitrite-to-NO reduction. Further studies are necessary to dissect the influence of exercise duration and intensity on muscle (and blood) nitrate and nitrite dynamics, both with and without preceding dietary nitrate supplementation.
In the present study, acute dietary nitrate supplementation did not alter the time to task failure during short-duration (~6–7 min), high-intensity cycle exercise. Acute nitrate supplementation has been reported to be ergogenic in some (e.g. Lansley et al. 2011; Shannon et al. 2017) but not all (e.g. Kelly et al. 2014) previous studies using a similar design. The physiological and other factors that may influence the ergogenicity of nitrate supplementation are debated but it is clear that positive effects on exercise performance are more often reported following longer-duration (≥3–7 days) dietary supplementation (Jones et al. 2018). It should also be acknowledged that variability in exercise performance may be increased when invasive experimental techniques, particularly muscle biopsy procedures, are employed. Nevertheless, on the basis of the results of the present study, in which there was no significant benefit to exercise performance, the functional advantage of the increase in muscle nitrate concentration following dietary nitrate supplementation and of the reduction of muscle nitrate during exercise, remains unclear. Additional studies with a larger sample size are required to determine if intramuscular nitrate reduction is necessary for optimal human skeletal muscle function and/or is involved in mediating the improvements in exercise performance that may be observed following dietary nitrate consumption (McMahon et al. 2017; Jones et al. 2018).
Conclusions
We show for the first time in humans that baseline concentrations of both nitrate and nitrite are far greater in skeletal muscle than in plasma. We also report that the active nitrate transporter sialin is present in human skeletal muscle and propose that this transporter may be responsible for the increase in muscle nitrate concentration observed following the consumption of dietary nitrate. Although further work is required, these findings are consistent with the hypothesis that skeletal muscle may act as a nitrate and nitrite ‘reservoir’, supporting whole body NO homeostasis via diffusion-driven distribution of these ions into the bloodstream (Piknova et al. 2015, 2016b). We also report an exercise-induced reduction in muscle nitrate concentration following nitrate ingestion. Although exercise performance was not enhanced, this finding provides the first indication that, in these circumstances, human skeletal muscle NO generation during contraction may be supported by the reduction of local nitrate stores. Further research is required to extend our understanding of the functional importance of skeletal muscle in the mammalian nitrate–nitrite–NO cycle.
Key points.
Nitric oxide (NO), a potent vasodilator and a regulator of many physiological processes, is produced in mammals both enzymatically and by reduction of nitrite and nitrate ions.
We have previously reported that, in rodents, skeletal muscle serves as a nitrate reservoir, with nitrate levels greatly exceeding those in blood or other internal organs, and with nitrate being reduced to NO during exercise.
In the current study, we show that nitrate concentration is substantially greater in skeletal muscle than in blood and is elevated further by dietary nitrate ingestion in human volunteers. We also show that high-intensity exercise results in a reduction in the skeletal muscle nitrate store following supplementation, likely as a consequence of its reduction to nitrite and NO.
We also report the presence of sialin, a nitrate transporter, and xanthine oxidoreductase in human skeletalmuscle, indicating thatmuscle has the necessary apparatus for nitrate transport, storage and metabolism.
Funding
No external funding was received for this work.
Biography

Lee J. Wylie is a Lecturer in Human Physiology at the University of Exeter. His research focuses on nitric oxide biology and specifically the role of dietary nitrate supplementation in enhancing indices of cardiovascular and metabolic health and exercise performance.
Footnotes
Competing interests
Alan N. Schechter is listed as a co-inventor on several patents issued to the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases. He receives royalties based on NIH licensing of these patents for clinical development but no other compensation. The other authors declare that they have no conflicts of interest.
References
- Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N & Jones AM (2010). Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985) 109, 135–148. [DOI] [PubMed] [Google Scholar]
- Bergström J (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35, 609–616. [PubMed] [Google Scholar]
- Black MI, Jones AM, Blackwell JR, Bailey SJ, Wylie LJ, McDonagh STJ, Thompson C, Kelly J, Sumners P, Mileva KN, Bowtell JL & Vanhatalo A (2017). Muscle metabolic and neuromuscular determinants of fatigue during cycling in different exercise intensity domains. J Appl Physiol (1985) 122, 446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RO, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH & Gladwin MT (2001). Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest 108, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan AR, Leibowitz JL, Kadkhodayan A, Thomas DP, Ramamurthy S, Spearie CA, Waller S, Farmer M & Peterson LR (2015). Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 48, 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan AR & Peterson LR (2018). Dietary nitrate enhances the contractile properties of human skeletal muscle. Exerc Sport Sci Rev 46, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC & Musch TI (2013). Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol 591, 2885–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO & Gladwin MT (2003). Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Dombernowsky NW, Ölmestig JNE, Witting N & Kruuse C (2018). Role of neuronal nitric oxide synthase (nNOS) in Duchenne and Becker muscular dystrophies – Still a possible treatment modality? Neuromuscul Disord 28,914–926. [DOI] [PubMed] [Google Scholar]
- Förstermann U & Münzel T (2006). Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113, 1708–1714. [DOI] [PubMed] [Google Scholar]
- Förstermann U & Sessa WC (2012). Nitric oxide synthases: regulation and function. Eur Heart J 33, 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford J, Winyard PG, Vanhatalo A, Bailey SJ, Blackwell JR & Jones AM (2013). Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch 465, 517–528. [DOI] [PubMed] [Google Scholar]
- Garthwaite J (2019). NO as a multimodal transmitter in the brain: discovery and current status. Br J Pharmacol 176, 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell WH (1880). On the tonicity of the heart and blood vessels. J Physiol 3, 48–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliard CN, Lam JK, Cassel KS, Park JW, Schechter AN & Piknova B (2018). Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide 75, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Feelisch M & Lundberg JO (2005). The emerging biology of the nitrite anion. Nat Chem Biol 1, 308–314. [DOI] [PubMed] [Google Scholar]
- Govoni M, Jansson EA, Weitzberg E & Lundberg JO (2008). The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19, 333–337. [DOI] [PubMed] [Google Scholar]
- Haider G & Folland JP (2014). Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med Sci Sports Exerc 46, 2234–2243. [DOI] [PubMed] [Google Scholar]
- Haque MM, Tejero J, Bayachou M, Wang Z-Q, Fadlalla M & Stuehr DJ (2013). Thermodynamic characterization of five key kinetic parameters that define neuronal nitric oxide synthase catalysis. FEBS J 280, 4439–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten-Westing Y (1993). Immunohistochemical localization of xanthine oxidase in human cardiac and skeletal muscle. Histochemistry 100, 215–222. [DOI] [PubMed] [Google Scholar]
- Hord NG, Tang Y & Bryan NS (2009). Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 90, 1–10. [DOI] [PubMed] [Google Scholar]
- Jansson EÅ, Huang L, Malkey R, Govoni M, Nihlén C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E & Lundberg JO (2008). A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol 4, 411–417. [DOI] [PubMed] [Google Scholar]
- Jones AM, Thompson C, Wylie LJ & Vanhatalo A (2018). Dietary nitrate and physical performance. Annu Rev Nutr 38, 303–328. [DOI] [PubMed] [Google Scholar]
- Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E & Ahluwalia A (2013). Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Vanhatalo A, Bailey SJ, Wylie LJ, Tucker C, List S, Winyard PG & Jones AM (2014). Dietary nitrate supplementation: effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. Am J Physiol Regul Integr Comp Physiol 307, R920–R930. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Staniek K & Nohl H (1999). Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett 454, 127–130. [DOI] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N & Jones AM (2011). Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc 43, 1125–1131. [DOI] [PubMed] [Google Scholar]
- Li H, Cui H, Kundu TK, Alzawahra W & Zweier JL (2008). Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem 283, 17855–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H & Poulos TL (2005). Structure–function studies on nitric oxide synthases. J Inorg Biochem 99, 293–305. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E & Gladwin MT (2008). The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7, 156–167. [DOI] [PubMed] [Google Scholar]
- McConell GK, Rattigan S, Lee-Young RS, Wadley GD & Merry TL (2012). Skeletal muscle nitric oxide signaling and exercise: a focus on glucose metabolism. Am J Physiol Endocrinol Metab 303, E301–E307. [DOI] [PubMed] [Google Scholar]
- McMahon NF, Leveritt MD & Pavey TG (2017).The effect of dietary nitrate supplementation on endurance exercise performance in healthy adults: a systematic review and meta-analysis. Sports Med 47, 735–756. [DOI] [PubMed] [Google Scholar]
- Möller P & Sylvén C (1981). Myoglobin in human skeletal muscle. Scand J Clin Lab Invest 41, 479–482. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Rosenberry R, Barresi R, Tsimerinov EI, Rader F, Tang X, Mason O, Schwartz A, Stabler T, Shidban S, Mobaligh N, Hogan S, Elashoff R, Allen JD & Victor RG (2015). Sodium nitrate alleviates functional muscle ischaemia in patients with Becker muscular dystrophy. J Physiol 593, 5183–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakayiru J, Kouw IWK, Cermak NM, Senden JM, van Loon LJC & Verdijk LB (2017). Sodium nitrate ingestion increases skeletal muscle nitrate content in humans. J Appl Physiol (1985) 123, 637–644. [DOI] [PubMed] [Google Scholar]
- Piknova B, Park JW, Cassel KS, Gilliard CN & Schechter AN (2016a). Measuring nitrite and nitrate, metabolites in the nitric oxide pathway, in biological materials using the chemiluminescence method. J Vis Exp e54879; DOI: 10.3791/54879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piknova B, Park JW, Kwan Jeff Lam K & Schechter AN (2016b). Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide 55–56, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piknova B, Park JW, Swanson KM, Dey S, Noguchi CT & Schechter AN (2015). Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 47, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piknova B & Schechter AN (2011). Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods Mol Biol 704, 39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder AG, Rogers SC, Khalatbari A, Ingram TE & James PE (2008). The measurement of nitric oxide and its metabolites in biological samples by ozone-based chemiluminescence. Methods Mol Biol 476, 11–28. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Ong HL, Adams D, Gahl WA, Zheng C, Qi S, Jin L, Zhang C, Gu L, He J, Deng D, Ambudkar IS & Wang S (2012). Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci U S A 109, 13434–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarelius I & Pohl U (2010). Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol 199, 349–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon OM, Barlow MJ, Duckworth L, Williams E, Wort G, Woods D, Siervo M & O’Hara JP (2017). Dietary nitrate supplementation enhances short but not longer duration running time-trial performance. Eur J Appl Physiol 117, 775–785. [DOI] [PubMed] [Google Scholar]
- Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM & Gladwin MT (2007). Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100, 654–661. [DOI] [PubMed] [Google Scholar]
- Stamler JS & Meissner G (2001). Physiology of nitric oxide in skeletal muscle. Physiol Rev 81, 209–237. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Santolini J, Wang Z-Q, Wei C-C & Adak S (2004). Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem 279, 36167–36170. [DOI] [PubMed] [Google Scholar]
- Sylvén C, Jansson E & Böök K (1984). Myoglobin content in human skeletal muscle and myocardium: relation to fibre size and oxidative capacity. Cardiovasc Res 18, 443–446. [DOI] [PubMed] [Google Scholar]
- Tejero J, Santolini J & Stuehr DJ (2009). Fast ferrous heme – NO oxidation in nitric oxide synthases. FEBS J 276, 4505–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO & Mason R (2009). Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev 29, 683–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG & Jones AM (2010). Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299, R1121–R1131. [DOI] [PubMed] [Google Scholar]
- Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A & Jones AM (2013). Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115, 325–336. [DOI] [PubMed] [Google Scholar]
- Zifarelli G & Pusch M (2009). Conversion of the 2 Cl−/1 H+ antiporter ClC-5 in a NO3−/H+ antiporter by a single point mutation. EMBO J 28, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]


