Abstract
Patient: Male, 77-year-old
Final Diagnosis: Hepatic portal venous gas
Symptoms: Abdominal pain • constipation • sepsis
Medication: —
Clinical Procedure: Exploratory laparotomy
Specialty: Surgery
Objective:
Rare disease
Background:
Hepatic portal venous gas is a rare and concerning finding occasionally seen on computed tomography (CT) scans, and must be emergently managed, often in the operating room. This condition can present in conjunction with bowel distension, pneumatosis intestinalis, and intestinal ischemia, so care must be taken to examine the imaging closely so as not to miss this dire condition. This report summarizes our experience with a patient who had this problem and how urgent management prevented a lethal outcome.
Case Report:
The patient was a 77-year-old morbidly obese man whose complicated hospital course began with admission for abdominal pain evaluation. This led to a flexible sigmoidoscopy for concerning CT findings suggestive of colitis or malignancy, leading to a perforation at the anterior wall of the sigmoid-rectal junction. Urgent sigmoid colectomy and Hartmann’s procedure were performed along with pelvic drainage. Blood cultures returned positive for Klebsiella. After 10 days, the patient decompensated, and a CT scan showed pneumatosis intestinalis, hepatic portal venous gas, and diffuse small bowel distension. Rectal stump dehiscence had occurred; therefore, 2 repeat abdominal wash-outs were performed with aggressive intensive care. The patient eventually stabilized and was ultimately discharged to a skilled nursing facility 32 days later.
Conclusions:
This case illustrates the importance of prompt imaging, medical management, and, if necessary, surgical exploration in the patient with bowel distension and hepatic portal venous gas on a CT scan. Although uncommon, this finding indicates a potentially poor prognosis and must be addressed emergently to prevent bowel ischemia from progressing in patients with underlying abdominal pathology.
Keywords: Portal Vein, Hepatic Veins, Case Reports
Background
Pneumatosis intestinalis and hepatic portal venous gas represent an abnormal accumulation of gas in the intestinal wall and the portal venous system. In particular, hepatic portal venous gas used to be considered a rare, ominous finding with an estimated mortality rate greater than 80% in the early 1980s [1]. Fortunately, the mortality rate has been down-trending over the past 20 years to approximately 40% in the early 2000s, likely due to its early detection by CT scans and prompt interventions such as surgery before the underlying disease progresses to a fulminant state [2]. Hepatic portal venous gas has been known to occur with numerous biliary and gastrointestinal diseases, and is often associated with alterations of the intestinal wall. Although the exact mechanism is unknown, hepatic portal venous gas is still considered a poor prognostic indicator, as it is most often seen in patients with intestinal ischemia and necrosis [3]. We report a case of hepatic portal venous gas associated with perforated diverticulitis status after Hartmann’s procedure, where a severely edematous small intestine was found during abdominal exploration.
Case Report
A 77-year-old morbidly obese man was initially transferred to our facility from an outside hospital for evaluation of 3 days of constipation and bilateral lower abdominal pain. The CT report from 1 day prior to transport revealed rectal thickening, which was concerning for proctocolitis or potential neoplasm, and the patient was started on metronidazole. Due to the concern for possible malignancy, a flexible sigmoidoscopy was performed on the first day of hospital stay at our facility.
The quality of this study was limited due to the presence of stool in the rectum, but revealed no discrete ulcerations or any evidence of bleeding. In 1 area of concern, mucosal biopsies were taken and the pathology report showed findings consistent with acute diverticulitis with abscess formation extending through muscle to serosa and acute serositis without evidence of malignancy.
On the second day of hospitalization, the patient developed worsening abdominal pain exacerbated by palpation, tachycardia greater than 120 bpm (Table 1), and an elevated white blood cell count at 13.9×109/L, up from 7.4×109/L the day before (Table 2). Blood cultures were sent, and a repeat abdominal CT was performed, which showed moderate intra-abdominal free air consistent with perforation, likely secondary to the recent flexible sigmoidoscopy and/or diverticulitis. The patient was emergently taken to the operating room for exploratory laparotomy, which revealed a significantly dilated small bowel without evidence of injury and non-purulent fluid containing fecal matter in the pelvis secondary to a 3×7 cm perforation on the anterior wall of the sigmoid-rectal junction. The entire colon was dilated and full of soft, formed stool. Sigmoid colectomy and Hartmann’s procedure were performed, resulting in a rectal stump and a descending colostomy. In 1 area of concern in the sigmoid colon, surgical specimens were sampled, and the histopathological study showed findings consistent with acute diverticulitis with abscess formation extending through muscle to serosa and acute serositis without evidence of malignancy. The peritoneal cavity was vigorously irrigated and cleansed with warm solutions of vancomycin/gentamicin and Dakin’s solution before closure. A Blake drain was left in the pelvis, and a nasogastric tube was placed for bowel rest. In the initial days after surgery, the patient showed signs of improvement. Intravenous antibiotics, including vancomycin, piperacillin/tazobactam, and metronidazole, were continued as a prophylactic measure but subsequently tapered as appropriate. By the seventh postoperative day, copious liquid stool output from the ostomy was noted, and the patient slowly improved. As a result, the nasogastric tube was removed.
Table 1.
Vital signs at admission and during hospital stay. SIRS criteria – must meet 2+ of the following: body temperature <36°C or >38°C, tachycardia at >90 BPM, tachypneic at >20/minute, WBC <4000/mm3 OR >12 000/mm3 OR >10% bands.
| Vital signs on admission | |||||
| BMI 37.5 |
T 36.5°C |
BP 138/81 mmHg |
HR 92 BPM |
RR 19/min |
SpO2 % 98% room air |
| Vital signs on day 2 of hospital stay | |||||
| T 37.6°C |
BP 131/80 mmHg |
HR 130 BPM |
RR 22/min |
SpO2% 92% room air |
|
| Vital signs on day 9 of hospital stay | |||||
| T 36.3°C |
BP 125/78 mmHg |
HR 101 BPM |
RR 17/min |
SpO2% 96% room air |
|
| Vital signs on day 10 of hospital stay (postoperative day 8) | |||||
| T 36.8 °C |
BP 69/43 mmHg |
HR 125 BPM |
RR 20/min |
SpO2% 93% room air |
|
T – temperature; BP – blood pressure; HR – heart rate; RR – respiratory rate; SpO2% – pulse oximeter reading.
Table 2.
Lab values at admission and during stay.
| Lab finding | Admission (Day 1) | Day 2 | Day 9 | Day 10 |
|---|---|---|---|---|
| Sodium | 139 mmol/L | 137 mmol/L | 148 mmol/L | 144 mmol/L |
| Potassium | 3.9 mmol/L | 3.6 mmol/L | 3.7 mmol/L | 3.9 mmol/L |
| Chloride | 109 mmol/L | 106 mmol/L | 111 mmol/L | 106 mmol/L |
| Carbon dioxide | 25 mmol/L | 23 mmol/L | 27 mmol/L | 28 mmol/L |
| Anion gap | 5 mmol/L | 8 mmol/L | 10 mmol/L | 10 mmol/L |
| Glucose | 119 mmol/L | 161 mg/dL | 132 mg/dL | 203 mg/dL |
| BUN | 10 mmol/L | 9 mg/dL | 17 mg/dL | 20 mg/dL |
| Creatinine | 0.7 mmol/L | 0.9 mg/dL | 0.8 mg/dL | 1.0 mg/dL |
| GFR | 140 mL/min/1.73 m2 | 105 mL/min/1.73 m2 | 121 mL/min/1.73 m2 | 93 mL/min/1.73 m2 |
| AST | 21 unit(s)/L | – | – | – |
| ALT | 24 unit(s)/L | – | – | – |
| Alkaline phosphatase | 78 unit(s)/L | – | – | – |
| Bilirubin, total | 0.4 mg/dL | – | – | – |
| Bilirubin, cConj | <0.1 mg/dL | – | – | – |
| Protein, total | 6.4 g/dL | – | – | – |
| Albumin | 2.9 g/dL | 2.6 g/dL | 1.9 g/dL | 2.1 g/dL |
| Phosphate | 3.4 mg/dL | 3.5 mg/dL | 4.1 mg/dL | 4.1 mg/dL |
| Calcium | 8.9 mg/dL | 8.4 mg/dL | 8.5 mg/dL | 8.9 mg/dL |
| Magnesium | 1.9 mg/dL | – | 2.0 mg/dL | 2.4 mg/dL |
| WBC | 7.4×109/L | 13.9×109/L | 10.7×109/L | 18.2×109/L |
| RBC | 4.0×1012/L | 4.7×1012/L | 4.7×1012/L | 4.9×1012/L |
| Hemoglobin | 12.2 g/dL | 14.2 g/dL | 13.9 g/dL | 14.8 g/dL |
| HCT | 38.5% | 44.2% | 43.8% | 45.7% |
| MCV | 96.5 fL | 94 fL | 93.8 fL | 93.6 fL |
| PLT | 237×109/L | 252×109/L | 379×109/L | 461×109/L |
WBC – white blood cell; BUN – blood urea nitrogen; GFR – glomerular filtration rate; AST – aspartate aminotransferase; ALT – alanine aminotransferase; RBC – red blood cell; MCV – mean corpuscular volume; PLT – platelet; HCT – hematocrit.
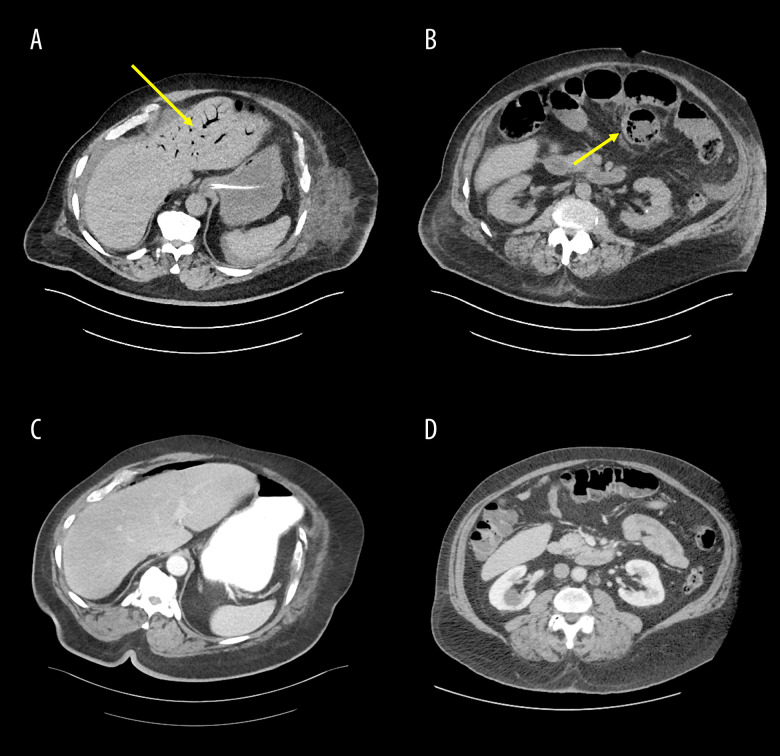
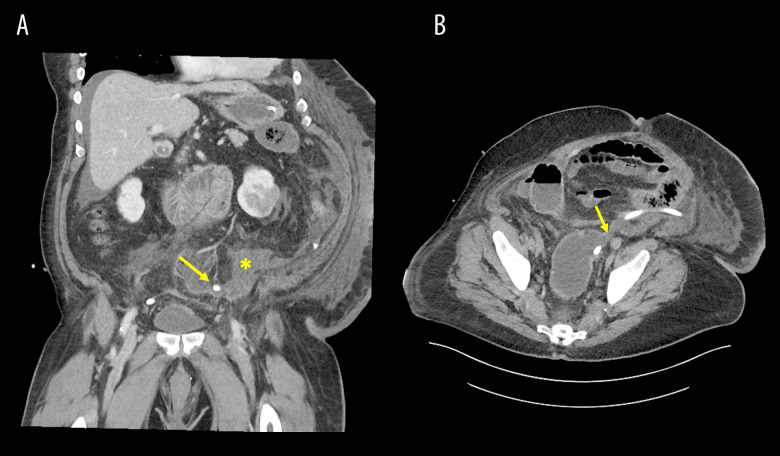
On postoperative day 8, the patient reported an episode of vomiting and was ill-appearing. His white blood cell count increased to 18.2×109/L from 10.7×109/L the day before (Table 2), and blood pressure subsequently fell to 69/46 mmHg (Table 1) causing concern for septicemia. A CT scan showed diffuse dilation of the small bowel with pneumatosis and evidence of hepatic portal venous gas (Figure 1A, 1B), which had not been evident on previous scanning (Figure 1C, 1D). Apparent dehiscence of the Hartmann’s pouch with adjacent free fluid within the lower abdomen and pelvis was also noted on the CT scan (Figure 2). The patient was emergently returned to the operating room for exploration. The small bowel was noted to be markedly distended and edematous with crepitus, but was judged to be viable without evidence of leakage. Of note, the jejunum was grossly thickened. The rectal stump was grossly dehisced, leaking non-purulent mucus into the pelvis and overwhelming the originally placed Blake drain. The mucus was thoroughly removed and cultured (Table 3) and the Blake drain was repositioned. Further, given that the patient was critically ill and requiring multiple vasopressors in addition to his edematous small bowel, an ABThera abdominal dressing system was placed after copious irrigation with warm solutions of vancomycin/gentamicin and Dakin’s solution. Intraoperative cultures were obtained and subsequently grew Klebsiella pneumoniae as well as Enterobacter cloacae complex and Candida albicans.
Figure 1.
CT scans of hepatic portal venous gas and pneumatosis. A and B show the hepatic portal venous gas and the pneumatosis on the tenth day of the patient’s hospital stay (postoperative day 8) in the transverse view. The yellow arrow in A shows hepatic portal venous gas, and the yellow arrow in B shows pneumatosis. C and D show the same transverse view of the patient’s abdomen as A and B, but were taken 1 day prior to his hospitalization and show no hepatic portal venous gas or pneumatosis.
Figure 2.
CT scans of rectal stump dehiscence with free fluid. A shows free fluid accumulation in the pelvis secondary to the rectal stump dehiscence in the coronal view. The placement of the Blake drain is indicated by the yellow arrow, and the collection of free fluid is indicated by the yellow star. B shows the rectal stump dehiscence in the transverse view. The rectal stump dehiscence is indicated by the yellow arrow.
Table 3.
Microbiology results.
| Culture source | Day 1 | Day 2 | Day 10 |
|---|---|---|---|
| Blood | – | – | Klebsiella pneumoniae |
| Urine | No growth | – | No growth |
| Peritoneal fluid | – | No growth, no organisms, many WBCs | – |
| Abscess | – | – | Klebsiella pneumoniae, Enterobacter cloacae complex, Candida albicans |
| Tissue | – | – | Klebsiella pneumoniae, Enterobacter cloacae complex |
WBC – white blood cell.
Sensitivities were obtained and the patient received vancomycin, piperacillin/tazobactam, and metronidazole. Candida albicans was most likely caused by sample contamination, given the rare growth on only 1 of the 2 samples for abdominal abscess, and treatment was deferred. Repeat cultures 2 days later showed persistence of gram-negative rods, so pharmacotherapy was changed to meropenem. This same day, on the twelfth day of hospital stay, as the patient’s condition stabilized and he was taken off vasopressors, he was brought back to the operating room for attempted formal abdominal closure.
Mucus was again discovered coming from the rectal stump; however, the small bowel was healthy and less edematous, so a second Blake drain was placed in the pelvis. A Malecot drain was placed into the rectum for improved mucus drainage, and the abdominal cavity was closed. Afterwards, the patient rapidly improved. Once the colostomy was again productive, his diet was advanced, and in a short time thereafter, he was able to be discharged to a skilled nursing facility. Total hospitalization was 32 days.
Discussion
Hepatic portal venous gas was first described in 1955 in the pediatric population, where 6 infants developed this gas in their liver vasculature secondary to necrotizing enterocolitis; unfortunately, all died [4]. In 1960, the first adult case was described, in which the patient had small bowel gangrene secondary to superior mesenteric artery thrombosis [5]. Since that time, most hepatic portal venous gas cases have been associated with bowel necrosis, the majority with fatal outcomes. As more experience with this condition has been obtained, the introduction of more sensitive imaging studies, such as the CT scan, has allowed for early detection of intestinal wall involvement. This advance has decreased the mortality rate to 40% (from its earlier rate of over 80%) estimated by a study in 2000, making pneumatosis and hepatic portal venous gas comparatively less ominous findings [6]. The condition of the present patients deteriorated on the tenth day of his hospital stay, and his vital signs (Table 1) and leukocytosis met the systemic inflammatory response syndrome (SIRS) criteria for septic shock [7]. Given his recent Hartmann’s pouch procedure, the complete clinical picture raised a concern for potential abscess formation near the procedural site or a rectal stump dehiscence. A repeat CT scan was performed and revealed hepatic portal venous gas and small bowel pneumatosis with free fluid in the pelvis. A subsequent exploratory laparotomy confirmed an edematous but viable and intact intestine. Appropriate and aggressive surgical interventions, such as draining the dehiscence of the rectal stump and removing accumulated mucus, were performed together with continued intravenous antibiotics, antifungal agents, vasopressors, and fluid resuscitation. Early diagnostic findings of hepatic portal venous gas by CT scan and prompt surgical and medical interventions, as demonstrated in our patient, can prevent the progression to septic shock and necrotizing enterocolitis.
The exact pathogenesis of hepatic portal venous gas is not entirely understood. Anatomically, the hepatic portal vein drains blood from the intestine to the liver, and it is believed that hepatic portal vein gas is preceded by intestinal wall alteration, which could be secondary to bowel distension and sepsis [6]. Bowel distension mechanically disrupts the intestine’s mucosa and allows intraluminal gas to become intravascular. Our patient presented with severe constipation, complicated by diverticular ulceration and perforation. During the first exploratory laparotomy, a significantly dilated small bowel was noted. The mechanical stress caused by the distension likely damaged the integrity of the mucosa and possibly allowed intraluminal gas to become intravascular and travel to the liver. Direct intraluminal bacterial invasion also likely occurred from this patient’s weakened intestinal wall integrity.
CT imaging has now become the criterion standard for diagnosing hepatic portal venous gas [6,8]. The diagnostic feature of hepatic portal venous gas is a branching lucency pattern caused by the accumulation of gas in the portal veins, predominantly in the anterior-superior aspect of the left lobe, which was true for our patient [9]. Moussa et al suggested that hepatic portal venous gas involving >3 hepatic segments with pneumatosis intestinalis is associated with a poor prognosis, whereas hepatic portal venous gas involving ≤2 hepatic segments without pneumatosis intestinalis suggests a better outcome [8]. It is worth mentioning that hepatic portal venous gas can be confused with pneumobilia, a condition where gas accumulates in the biliary ducts. However, in hepatic portal venous gas the accumulation of gas can extend to the liver capsule, whereas in pneumobilia the gas does not extend toward the capsule to the same extent [6]. The proposed mechanism for this difference in gas distribution between these 2 conditions is that the direction of blood flow works synergistically with the gas diffusion in hepatic portal venous gas, allowing gas to travel further toward the liver capsule. This is in contrast to pneumobilia, in which the direction of the bile flow is opposite to the direction of gas diffusion, preventing the gas from migrating peripherally [9]. Little et al reported that ultrasound could be used as an alternative for detecting hepatic portal venous gas, as echogenic gas can be seen flowing in the hepatic portal veins [10].
Management of a patient who develops hepatic portal venous gas can be broadly categorized as surgical or non-surgical. In a study published in 2003, 26 patients with hepatic portal venous gas were studied retrospectively. Eleven underwent surgery, and 3 of these patients died (73% surgical survival). These 3 patients demonstrated significant bowel ischemia during their operations. Of the 15 patients in this study who did not undergo surgery, 6 died and all were poor surgical candidates (60% non-surgical survival) [2]. As these statistics suggest, the difference in clinical outcomes between surgical and non-surgical management was not apparent. It was concluded that clinical correlation is essential for determining hepatic portal venous gas management. There have been radiologic and clinical criteria proposed to help identify patients with hepatic portal venous gas who would benefit from surgery. A retrospective study from 2020 using analysis of similarities among non-surgical cases with poor outcomes proposed a scoring system to identify those critical enough for surgical management. Having 2 or more of the following findings were suggestive of poor outcomes with nonoperative management: ascites on CT, peritoneal irritation on physical exam, and unstable vital signs indicating shock [11]. As presented in another retrospective study from 2020, other prognostic factors that have been proposed to specifically indicate bowel ischemia prompting surgical management include base excess, lactate, neutrophil-lymphocyte ratio, and CRP [12], In our patient, the CT scan on the day he became hypotensive raised a clinical suspicion for rectal stump dehiscence, making exploratory laparotomy the reasonable choice for management. Intravenous fluid resuscitation, vasopressors, and antibiotics were also indicated given his bacteremia and presumed septic status. The advancement of interdisciplinary medical care, as well as rigorous clinical testing of proposed treatment stratification algorithms, will likely further decrease the mortality rate of patients with hepatic portal venous gas. We believe that such interdisciplinary care contributed greatly to the positive outcome of our patient and his ultimate survival.
Conclusions
Hepatic portal venous gas is historically an ominous CT imaging finding associated with high mortality rates; however, the best approach for managing hepatic portal venous gas has not been formulated. Our case report illustrates the importance of prompt imaging per clinical correlation, medical management, and, if necessary, surgical exploration in patients with bowel distension and hepatic portal venous gas on a CT scan. Though uncommon, this finding indicates a potentially poor prognosis and must be addressed emergently to prevent bowel ischemia from progressing in patients with underlying abdominal pathology. In our case, the competent and cooperative interdisciplinary medical care from the nursing, surgical and medical teams benefited the patient and prevented a lethal outcome.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Cambria RP, Margolies MN. Hepatic portal venous gas in diverticulitis: Survival in a steroid-treated patient. Arch Surg. 1982;117:834–35. doi: 10.1001/archsurg.1982.01380300074015. [DOI] [PubMed] [Google Scholar]
- 2.Iannitti DA, Gregg SC, Mayo-Smith WW, et al. Portal venous gas detected by computed tomography: Is surgery imperative? Dig Surg. 2003;20:306–15. doi: 10.1159/000071756. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Su Y, Wang X, et al. Hepatic portal venous gas associated with colon cancer. Medicine (Baltimore) 2017;96(50):e9352. doi: 10.1097/MD.0000000000009352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe JN, Evans WA. Gas in the portal veins of the liver in infants; A roentgenographic demonstration with postmortem anatomical correlation. Am J Roentgenol Radium Ther Nucl Med. 1955;74:486–88. [PubMed] [Google Scholar]
- 5.Susman N, Senturia HR. Gas embolization of the portal venous system. Am J Roentgenol Radium Ther Nucl Med. 1960;83:847–50. [PubMed] [Google Scholar]
- 6.Alqahtani S, Coffin CS, Burak K, et al. Hepatic portal venous gas: A Report of two cases and a review of the epidemiology, pathogenesis, diagnosis and approach to management. Can J Gastroenterol. 2007;21(5):309–13. doi: 10.1155/2007/934908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–74. [PubMed] [Google Scholar]
- 8.Moussa M, Marzouk I, Abdelmoula K, et al. Role of computed tomography in predicting prognosis of hepatic portal venous gas. Int J Surg Case Rep. 2017;30:177–82. doi: 10.1016/j.ijscr.2016.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebastia C, Quiroga S, Espin E, et al. Portomesenteric vein gas: Pathologic mechanisms, CT findings, and prognosis. Radiographics. 2000;20:1213–24. doi: 10.1148/radiographics.20.5.g00se011213. ; discussion 1224–26. [DOI] [PubMed] [Google Scholar]
- 10.Little AF, Ellis SJ. ‘Benign’ hepatic portal venous gas. Australas Radiol. 2003;47:309–12. doi: 10.1046/j.1440-1673.2003.01184.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonda M, Osuga T, Ikura Y, et al. Optimal treatment strategies for hepatic portal venous gas: A retrospective assessment. World J Gastroenterol. 2020;26(14):1628–37. doi: 10.3748/wjg.v26.i14.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii M, Yamashita S, Tanaka M, et al. Clinical features of patients with hepatic portal venous gas. BMC Surg. 2020;20(1):300. doi: 10.1186/s12893-020-00973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]