Marine mammals are facing many threats that impact their ability to obtain energy to survive and reproduce. Much remains unknown about marine mammal bioenergetics, which hinders real-world applications to conservation and management. We surveyed the bioenergetic community to identify ‘key’ unanswered questions to help guide future marine mammal research efforts.
Abstract
Bioenergetic approaches are increasingly used to understand how marine mammal populations could be affected by a changing and disturbed aquatic environment. There remain considerable gaps in our knowledge of marine mammal bioenergetics, which hinder the application of bioenergetic studies to inform policy decisions. We conducted a priority-setting exercise to identify high-priority unanswered questions in marine mammal bioenergetics, with an emphasis on questions relevant to conservation and management. Electronic communication and a virtual workshop were used to solicit and collate potential research questions from the marine mammal bioenergetic community. From a final list of 39 questions, 11 were identified as ‘key’ questions because they received votes from at least 50% of survey participants. Key questions included those related to energy intake (prey landscapes, exposure to human activities) and expenditure (field metabolic rate, exposure to human activities, lactation, time-activity budgets), energy allocation priorities, metrics of body condition and relationships with survival and reproductive success and extrapolation of data from one species to another. Existing tools to address key questions include labelled water, animal-borne sensors, mark-resight data from long-term research programs, environmental DNA and unmanned vehicles. Further validation of existing approaches and development of new methodologies are needed to comprehensively address some key questions, particularly for cetaceans. The identification of these key questions can provide a guiding framework to set research priorities, which ultimately may yield more accurate information to inform policies and better conserve marine mammal populations.
Introduction
Bioenergetics is the study of the acquisition and allocation of energy by individuals to support maintenance, activity, growth and reproduction (Lavigne et al., 1982). It is an integral component of conservation physiology, which aims to understand and predict how organisms, populations and ultimately ecosystems respond to environmental variation and stressors (Cooke et al., 2013). Many of the conservation challenges facing marine mammals today revolve around bioenergetics, such as environmental variability, climate change, fisheries interactions, predation risk, offshore development, and noise pollution (Wirsing et al., 2008; Davidson et al., 2012; Kovacs et al., 2012; Avila et al., 2018). For example, climate change may alter an individual’s energy intake through changes in prey distribution, abundance, and energy density (von Biela et al., 2019; Gallagher et al., 2022). It can also affect energy expenditure through changes in habitat availability (Pagano and Williams, 2021) or thermal landscapes (Cunningham et al., 2021). Evidence is mounting that the inability to obtain sufficient energy is affecting individual growth, reproduction, and survival of marine mammals (Stirling and Derocher, 2012; Ferguson et al., 2017; Christiansen et al., 2020, 2021; Stewart et al., 2021a). If a sufficiently large number of individuals are affected, this can ultimately affect population growth rates (New et al., 2014; Baylis et al., 2015), as illustrated by the Population Consequences of Disturbance (PCoD) framework (Pirotta et al., 2018a).
The first comprehensive review of marine mammal bioenergetics occurred at the Mammals in the Seas conference organized by the United Nations Food and Agriculture Organization in 1976 (Lavigne et al., 1982). The impetus for this effort was an interest in quantifying the impacts of marine mammals on commercially valuable fish populations. This review stimulated studies that examined the general patterns of energetics at the population level (Lockyer, 1981; Lavigne, 1982), feeding rates of marine mammals (Innes et al., 1987), and a review of metabolic rates (Lavigne et al., 1986). Many of these papers challenged early hypotheses that marine mammals have higher metabolic rates than would be predicted for similarly sized terrestrial mammals (Scholander et al., 1950; Kanwisher and Ridgway, 1983), a topic still debated today (Nagy et al., 1999; Hunter et al., 2000; Williams and Yeates, 2004; Costa and Maresh, 2017; Williams et al., 2020). A symposium in 1985 highlighted advances in our understanding of the energetics of marine mammals and technological developments (Huntley et al., 1987). Our knowledge of energetics has continued to grow, as have the implications and applications to conservation and management issues. Recently, there has been a resurgence in the use of bioenergetic modelling approaches to quantify predator–prey interactions (e.g. Fortune et al., 2013; Chasco et al., 2017; McHuron et al., 2020; Acevedo and Urbán, 2021) and predict the individual- and population-level effects of altered environments on marine mammals (e.g. Christiansen and Lusseau, 2015; Udevitz et al., 2017; Nabe-Nielsen et al., 2018; Farmer et al., 2018b; Pirotta et al., 2019; Gallagher et al., 2020; Silva et al., 2020; Gavrilchuk et al., 2021). Technological and analytical advances have furthered these empirical and modelling methodologies (Pirotta, 2022).
Despite recent advances, the field is still hindered by many of the same uncertainties and data deficiencies that existed nearly four decades ago, especially for cetaceans. As it is not feasible to address these deficiencies for all 127 extant marine mammal species, research priorities must be identified based on the perceived needs of the bioenergetics community. To achieve this, increased communication within the bioenergetic community is needed to align data needs and data generation. Our aim was to identify ‘key’ outstanding questions in the field of marine mammal bioenergetics. The intent is to stimulate and focus research that will most effectively lead to an increased understanding of the ecology and population biology of marine mammals, ultimately facilitating conservation and management efforts. This effort was conducted in tandem with a bioenergetics workshop to discuss and review the current state of knowledge on marine mammal bioenergetics.
Methods
We invited 62 scientists with experience in marine mammal physiology, bioenergetics, trophic ecology and population dynamics, to identify unanswered questions in marine mammal bioenergetics. The participant list was generated by the workshop organizers with additional suggestions from invited participants. Identification of participants by workshop organizers was based on personal knowledge of participants’ research and queries of the relevant bioenergetic and marine mammal literature. Participants were asked to submit no more than 10 questions each with the following guidelines. Questions (i) could not be answered with a simple ‘yes’, ‘no’ or ‘depends’; (ii) could be related to any aspect of bioenergetics but should be applicable to methods used in marine mammal management and conservation; (iii) could be unanswerable or infeasible given current methods; and (iv) could be either species-specific or broadly relevant to a taxonomic group (e.g. pinnipeds, cetaceans). Questions were collated and revised over email discussions and during the bioenergetic workshop (Fig. 1). Questions that were not directly related to bioenergetics were removed from further consideration.
Figure 1.
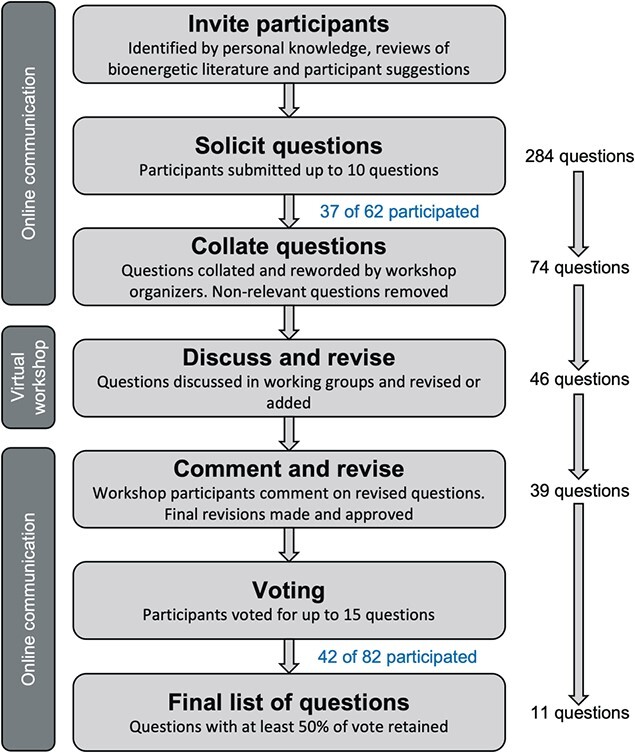
Process for identifying key bioenergetic questions. Figure after Sutherland et al. (2022). The number of participants compared with invitees is shown in blue text for the initial question submission and the voting on final questions. The number of invitees increased from 62 to 82 due to additional participant suggestions by co-authors.
The final list of questions was circulated for voting to the original 62 survey invitees and 20 additional individuals identified by the co-authors of this paper during the process. Participants voted for no more than 15 questions each, with the guidance that a question need not be applicable to all marine mammals (i.e. it might only be important or unanswered for certain species, taxonomic groups or species with similar life history strategies). Voting occurred virtually via a Google Forms survey (25 August–27 September 2021). Survey participants were also asked to provide information on their expertise in marine mammal physiology and whether they had experience developing bioenergetic models (each on a scale of 1–10, from least to most experience). This was to help inform whether any key questions were deemed important by individuals with greater expertise in one discipline over the other (physiologists vs. bioenergetic modellers).
Results and discussion
A total of 284 initial questions were submitted by 37 of the 62 original invited participants. They ranged from detailed physiological questions to broad overarching questions about the impacts of climate change, anthropogenic disturbance and fisheries on marine mammal populations. Questions were revised by co-authors and other bioenergetic workshop participants (Fig. 1) such that detailed physiological questions fell under the umbrella of more general questions. This ensured that final questions were not too narrow in scope. Broad overarching questions were not explicitly included in the final question list because numerous research priorities were needed to address such broad questions, and there was often overlap in research priorities among these questions (i.e. the same research priorities were needed to address a diversity of broad questions). Instead, the final list of questions reflected these individual research priorities. Collation resulted in 39 final questions (Fig. 1). A complete list of the initial and final questions can be found in Tables S1 and S2.
Forty-two of the 82 (62 original +20 additional) invited participants voted in the final survey. Most of the voting participants had current jobs in academia (78%), followed by non-profits (7.1%), government agencies (4.8%), a combination of academia and consulting (2.4%), the private sector (2.4%) or were self-employed (2.4%; Fig. 2). One participant declined to include their affiliation. Self-assigned scores for physiological and bioenergetic modelling knowledge (on a scale of 1–10) ranged from 3–10 (average of 7.3) and 2–10 (average of 6.8), respectively. There were 11 questions that at least 50% of the participants voted for, 16 questions that 25–50% of the participants voted for and 12 questions that received <25% of votes (Fig. 2). We selected those questions that received ≥50% of votes as key questions. Average expertise scores for key questions were similar (absolute differences of 0.06–1.0 on a scale of 1–10), suggesting broad agreement within the community about research priorities.
Figure 2.
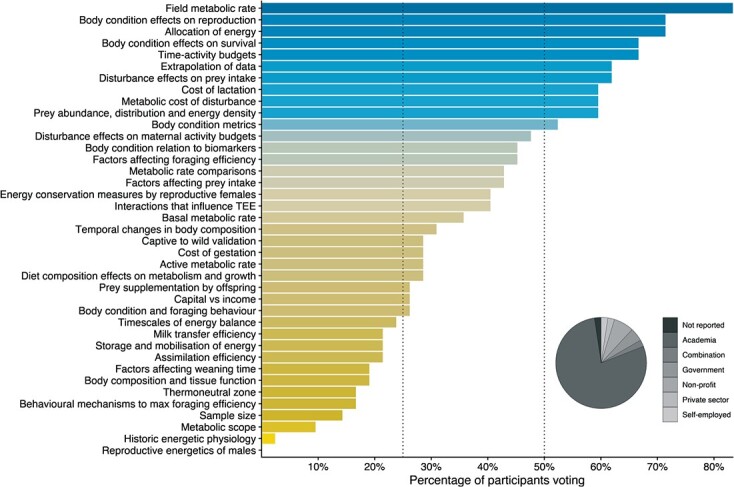
The percentage of survey participants that voted for each of the 39 questions, ordered (and coloured) from least to greatest number of votes. Key questions were defined as those voted by at least 50% of participants. The inset pie graph shows the self-reported employment type of participants that voted in the final survey. The full phrasing of each question can be found in Fig. 3 and Table S2.
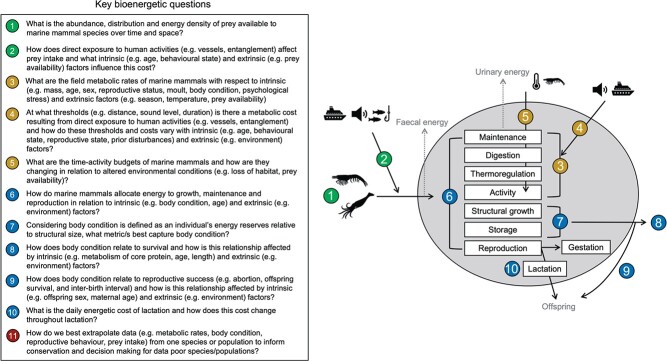
The following sections briefly describe how each question is relevant to bioenergetics and conservation efforts and highlight important data gaps. Additional information on topics covered here, such as bioenergetic models, metabolic rates, body condition and growth and reproductive energetics can be found elsewhere in this special issue (also see reviews by Iverson et al., 2010; Rosen and Worthy, 2018; Watanabe and Goldbogen, 2021). We conclude by discussing some of the existing methodologies available to address key questions and areas where further methodological advancements are needed. The headers below have been paraphrased from the key questions (Fig. 3) and in some cases encompass more than one key question for brevity. As we did not rank key questions in terms of importance, the order of questions below follows the numerical order from Fig. 3.
Figure 3.
The key outstanding bioenergetic questions identified as part of this exercise (left) and a conceptual diagram of where each question (excluding Q11) fits in the energy flow through an animal (grey circle). Questions are coloured based on the general categories of prey intake (green), energy expenditure on self (gold) and energy allocation and storage for other processes, such as reproduction (blue).
What is the abundance, distribution and energy density of prey? (Q1)
Knowledge of the abundance, distribution and energy density of prey in time and space is critical to answering a variety of bioenergetic questions. For example, is there enough prey biomass (of the right type) in the right place, at the right time to support individual and population energy needs? How do factors such as interannual variability, climate change, fisheries and habitat loss affect prey availability? Climate change, which is rapid in some regions, is predicted to have considerable impacts on the distribution, biomass, energy density and body size of prey species (Flores et al., 2012; Yasumiishi et al., 2020; Florko et al., 2021). Such changes have clear bioenergetic implications for marine mammals and the ecosystems they inhabit (Costa, 2008; Laidre et al., 2020; Gallagher et al., 2022). Since the prey landscape is a major driver of the spatiotemporal distribution of marine mammals (Sveegaard et al., 2012; Zerbini et al., 2016; Sigler et al., 2017; Straley et al., 2018; Pendleton et al., 2020), knowledge of prey fields and how they may be changing provides insight into the potential impact of anthropogenic disturbances on energy budgets (Keen et al., 2021). As such, prey fields are critical components of many PCoD models (Nabe-Nielsen et al., 2018; Pirotta et al., 2019; McHuron et al., 2021). At finer temporal and spatial scales (e.g. within a prey patch), measurements of the prey landscape can inform relationships between prey density, foraging effort and energy gain (Bowen et al., 2002; Hazen et al., 2015; Cade et al., 2021). Even in well-studied ecosystems, there are very few (if any) marine mammal species for which the prey landscape (including energy density of prey species) has been sufficiently resolved to predict behaviour and fine-scale spatial distribution through time.
At what thresholds do disturbance from human activities affect energy intake and expenditure? (Q2, Q4)
Direct exposure of marine mammals to human activities can elicit behavioural responses (e.g. changes in foraging behaviour) or cause direct (e.g. injuries from vessels or nets) or indirect (e.g. pollution, physiological effects) physical impacts that have implications for energy balance. For example, marine mammals may spend less time foraging when disturbed (Senigaglia et al., 2016; Harris et al., 2018), which could reduce energy intake. At the same time, a disturbance may alter energy expenditure if it elicits a strong physiological response (Christiansen et al., 2014a; Williams et al., 2017) or causes a switch to activities that have greater energetic costs, such as the increase in surface activity exhibited by some delphinids when exposed to vessels (Coscarella et al., 2003; Lusseau, 2006; Noren et al., 2009, 2012). Traumatic stressors, such as non-lethal entanglement or vessel strikes, can impact energy budgets by altering movement costs, foraging behaviour or energy investment in tissue healing and regrowth (Wells et al., 2008; van der Hoop et al., 2017; Pettis et al., 2017), or cause permanent energetic changes from severe injuries (e.g. amputation). While not a classical disturbance, exposure to contaminants has been linked with metabolic disruptions in grey seals (Halichoerus grypus), leading to reduced weaning mass of pups (Robinson et al., 2018; Bennett et al., 2021). This key question is the logical next step in ongoing research into the behavioural responses of marine mammals to disturbance, as it addresses the thresholds (e.g. duration, severity) that induce behavioural responses that are energetically meaningful to an individual and thus have the potential for population-level consequences. When assessing thresholds, it is important to consider the extent and timescales at which an animal can compensate (Hin et al., 2019; Pirotta et al., 2019; Booth, 2020). Our ability to accurately quantify the energetic implications of disturbance in part relies on addressing other key questions (Q1 on prey landscapes and Q3 on metabolic rates).
What are the field metabolic rates of marine mammals? (Q3)
Field metabolic rates (FMRs) represent an individual’s daily energy expenditure at a given time, which underpins many of the other key questions we identified in this exercise. The costs contained within FMR comprise the majority of an individual’s energy budget (Winship et al., 2002; Rechsteiner et al., 2013; Bejarano et al., 2017; McHuron et al., 2020). Marine mammal FMRs can vary with behavior, season, age class and among species (Arnould et al., 1996b; Costa and Gales, 2000; Mellish et al., 2000; Bowen et al., 2001; Trillmich and Kooyman, 2001; Costa and Gales, 2003; Fowler et al., 2007; Costa, 2008; Villegas-Amtmann et al., 2017a; Jeanniard du Dot et al., 2018; McHuron et al., 2018; Rojano-Donãte et al., 2018; McHuron et al., 2019). Otariids generally have elevated FMRs compared with those of phocids (Costa and Maresh, 2017), and limited data from bottlenose dolphins (Tursiops truncatus) and harbour porpoise (Phocoena phocoena) indicate that FMRs of small odontocetes may align more closely with the high-energy lifestyle of otariids (Costa and Maresh, 2017; Rojano-Donãte et al., 2018). Due to the logistical challenges in measuring FMR in free-ranging marine mammals, most existing measurements are from pinnipeds during lactation. Considerable data gaps remain for many marine mammals, particularly deep-diving beaked (Ziiphidae) and sperm whales (Physeter macrocephalus) and baleen whales. These gaps often result in the use of general allometric equations to estimate metabolic rates in bioenergetic models (e.g. Bejarano et al., 2017; Acevedo and Urbán, 2021). Existing intra- and inter-group-specific differences in marine mammal FMRs, and evidence that there is heterogeneity in allometric scaling factors with body mass and taxonomy (McNab, 2008; Kolokotrones et al., 2010; Hudson et al., 2013), highlight the need for additional FMR data.
What are the time-activity budgets of individuals and how are they changing in response to altered environmental conditions? (Q5)
The amount of time an animal spends engaged in certain activities (e.g. resting, foraging, travelling, breeding/socialising) influences its energy budget through changes in intake, expenditure or both. Climate change and habitat loss alter environmental conditions, forcing (or facilitating) species to either move into new environments or adapt to their existing ones that may be undergoing rapid changes (Silber et al., 2017; Pinsky et al., 2020). Effects of climate change on time-activity budgets have been documented for some marine mammals (Hamilton et al., 2018; Blanchet et al., 2020). While time-activity budgets have been estimated for numerous species, new studies are needed to capture responses to recent environmental changes at varying temporal scales, as environmental variation may influence activity budgets at some scales and not others (Austin et al., 2006). A better understanding of activity-specific metabolic costs is then needed to assess how such changes affect energy expenditure, although this question did not receive enough votes to be classified as a key question in this exercise (Fig. 2). In addition to assessing effects on energy balance, understanding time-activity budgets can also provide insight into a species’ flexibility to respond to environmental perturbations. For example, near-continuous foraging in northern elephant seals (Mirounga angustirostris; Adachi et al., 2021), harbour porpoises (Wisniewska et al., 2016) and sperm whales (Watwood et al., 2006; Farmer et al., 2018a) indicate these species may have little flexibility to adjust to reductions in food availability or interruptions in foraging (but see Hoekendijk et al., 2018; Booth, 2020).
How do marine mammals allocate energy to maintenance, growth and reproduction? (Q6)
Once ingested, energy is directed to a variety of processes, such as digestion, the maintenance of cellular function, production of waste products, thermoregulation, mechanical work/activity, structural growth, reproduction and storage (Fig. 3). Energy allocation must be prioritised when energy intake is insufficient to fuel all these costs (see review by Glazier, 2009). This prioritisation exists at different hierarchical levels, from partitioning among processes (e.g. maintenance vs. growth) to partitioning among organs or tissues within individual compartments. In general, energy allocation to maintenance is prioritised before growth and reproduction (Costa et al., 1989; Soto et al., 2004; Wheatley et al., 2006; Christiansen et al., 2014b, 2018; Kershaw et al., 2021; Smith, 2021). Compensatory mechanisms, such as metabolic depression, may help cope with energy limitation (Markussen et al., 1992; Rosen and Trites, 2002) or periods of high energy demand (Mellish et al., 2000; Shuert et al., 2020), although little is known about the drawbacks of such mechanisms (Halsey, 2021). Understanding these priorities is important as many bioenergetic models use researcher-defined rules regarding allocation when energy intake is insufficient to meet an individual’s needs, such as when foraging may be disrupted by a disturbance (e.g. Villegas-Amtmann et al., 2017b; Farmer et al., 2018b). Reduced allocation to reproduction could lead to changes in offspring body size that persist across an individual’s lifetime, an issue recently highlighted for North Atlantic right whales (Eubalaena glacialis; Stewart et al., 2021b). Understanding when reduced energy allocation to growth may occur, and the magnitude of such reduction, is critical since smaller body size could have wide-ranging impacts on reproductive behaviour and success of many marine mammals.
What metrics best capture body condition? (Q7)
Body condition, defined as the amount of energy reserves relative to structural size, is a physiological unit of considerable interest in conservation-focused bioenergetic studies. As the physical manifestation of energy balance, body condition provides essential information about the health of individuals and populations (Brock et al., 2013; Williams et al., 2013; Christiansen et al., 2020; Raverty et al., 2020; Stewart et al., 2021a). A variety of metrics have been developed to estimate body condition of free-ranging marine mammals, such as those derived from blubber measures, morphometrics, biochemical or chemical markers, body composition and omics (see reviews in Bowen and Northridge, 2010; Castrillon and Bengtson Nash, 2020). A universal metric does not currently exist for marine mammals due to their differences in life -history traits, habitat use, accessibility, body morphology, and the dynamics of energy storage and utilization (Noren and Wells, 2009; Noren et al., 2015, 2021; Kershaw et al., 2017; Castrillon and Bengtson Nash, 2020; Larrat and Lair, 2022). Even within a species, different metrics may be needed depending on the disposition (e.g. free-ranging vs. stranded) and state (e.g. reproductive status) of the animal. Thus, metrics often need to be validated (e.g. Noren et al., 2019) for individual species or groups when possible. Within the context of bioenergetics, metrics that are comparatively inexpensive and non-invasive (i.e. do not require animal handling or tissue sampling) are likely to be the most useful, such as the recent use of unmanned aerial vehicles to estimate body morphology (see What tools do we have to address these key questions?). Such approaches allow body condition data to be collected from many individuals of all age and size classes, reproductive states and body conditions with minimal disturbance.
How does body condition relate to survival and reproductive success? (Q8, Q9)
Behavioural changes are one of the first observable responses of individuals to disturbances or environmental perturbations. The resulting impact of that behavioural change on survival and reproductive success is what drives population dynamics (Pirotta et al., 2018a). As illustrated in the PCoD framework (Pirotta et al., 2018a, Fig. 1), exposure to stressors may affect vital rates through bioenergetic (e.g. changes in energy stores) or (mostly) non-bioenergetic pathways (e.g. immune function, contaminant burden). There also may be feedback between these pathways, such as when a disturbance alters energy balance, which then leads to reduced immune function (or vice versa; Brock et al., 2013; Vera-Massieu et al., 2015). While the focus here is on bioenergetic pathways, relationships between body condition and vital rates may thus incorporate the effects of non-bioenergetic pathways as well. In marine mammals, body size and condition metrics are positively related to foetal growth (Christiansen et al., 2014b), pregnancy rates (Williams et al., 2013; Smout et al., 2020), offspring growth (Christiansen et al., 2018) and survival probability (Hall et al., 2001; Beauplet et al., 2005; Harding et al., 2005; McMahon and Burton, 2005; Bowen et al., 2015; Cheney et al., 2018; Oosthuizen et al., 2018; Stewart et al., 2021a). For most marine mammals, these relationships and thresholds of body condition that equate to failed reproduction or imminent mortality remain largely unknown. In addition to the ability to directly link measured body condition from free-ranging animals with vital rates, these relationships (as well as the upper and lower bounds of body condition) are parameters in many PCoD models (Farmer et al., 2018a; Pirotta et al., 2019; Gallagher et al., 2020). Predictions from such models can be sensitive to parameters associated with relationships between body condition and survival (Pirotta et al., 2018b). Even when predicted behavioural patterns are robust to uncertainty in these relationships, the absolute survival values are not (McHuron et al., 2021).
What is the daily cost of lactation? (Q10)
Lactation is the most costly life -history event that a female mammal will likely experience in her lifetime (Gittleman and Thompson, 1988). Unfavourable environmental conditions (or other changes that affect energy reserves available for lactation) can result in reduced maternal condition or energy delivery to offspring, altered weaning times or longer inter-birth intervals, with different effects depending on reproductive strategy (Trillmich and Limberger, 1985; Arnbom et al., 1997; Noren et al., 2003; Costa, 2008; Chambert et al., 2015; Chinn et al., 2016; Gailey et al., 2020). Knowing how much energy is needed each day to support reproduction is thus key in estimating prey needs and predicting how alterations to energy intake will affect vital rates and population dynamics. The daily cost of lactation has been relatively well studied in pinnipeds using approaches that typically involve repeated handling of individuals, such as combining estimates of milk intake derived from doubly labelled water (DLW) with measurements of milk energy density (e.g. Costa et al., 1986; Iverson et al., 1993; Oftedal et al., 1993; Arnould et al., 1996a; Lydersen et al., 1997; Mellish et al., 1999; Donohue et al., 2002; Wheatley et al., 2006; McDonald et al., 2012). Empirical attempts to estimate lactation costs in most other marine mammals, particularly cetaceans, have been hampered by the inability to quantify milk intake. Relative costs of lactation have been estimated for free-ranging southern right whales (Eubalaena australis) by measuring changes in body size and condition of lactating females relative to the growth of their dependent calves (Christiansen et al., 2018), an approach that requires an estimate of the female’s FMR. Other studies have relied on data derived from captive individuals (Williams et al., 2011) or by summing the estimated costs experienced by a dependent calf (Fortune et al., 2013; Villegas-Amtmann et al., 2015).
How do we best extrapolate data from one species or population to another? (Q11)
Marine mammals are notoriously difficult to study in a natural setting due to logistical challenges that prevent direct measurement. Simple bioenergetic questions, such as ‘how much does an animal weigh?’ or ‘how much prey does an animal consume?’, often require complex or imaginative solutions as most species are either too large to handle or spend most of their time in remote areas underwater. Researchers must extrapolate from the best available biological knowledge when data are missing for their species of interest, with little formal guidance to help inform decisions. Should we look to a closely related species even though they may not share similar body sizes or ecological roles? Should we prioritise habitat use and behavioural similarities over phylogeny? Or should we rely on anecdotal observations from the species of interest even though they may be based on observations from just a few individuals? Choosing the correct input parameters for bioenergetic models has real-world consequences for developing quality management decisions and conservation policy. Several recent meta-analyses have explored variation in metabolic rates (Costa and Maresh, 2017), milk intake (Riek, 2008, 2011, 2021) and lactation strategies (Schulz and Bowen, 2004). Further efforts are needed to understand species groupings across a suite of parameters that are influential on bioenergetic model outputs (Winship et al., 2002; New et al., 2013; Bejarano et al., 2017; Gallagher et al., 2018; McHuron et al., 2020), and whether the appropriate proxy varies depending on the parameter of interest.
What tools do we have to address these key questions?
Many of the methods or tools needed to address these key questions have been around since the inception of the field of marine mammal bioenergetics (Fig. 4). Labelled water, a method that was pioneered in the 1950s (Lifson and McClintock, 1966), remains one of the most direct measures of FMR, milk intake and body composition, and is still widely used for these purposes (e.g. Mellish et al., 1999; Arnould et al., 2003; Fowler et al., 2007; Lang et al., 2011; Pagano et al., 2018). This approach, however, has limited application to cetaceans given logistical constraints surrounding sample collection and potential violation of the assumption that no seawater is ingested during the measurement period (Hui, 1981). Observations of marked individuals throughout their lifetime, such as those obtained from long-term research programs, have provided a wealth of information on reproductive success and survival (e.g. Ford et al., 2010; Schwarz et al., 2013; Wells et al., 2014; Bowen et al., 2015; Le Boeuf et al., 2019). It is difficult to envision how questions that relate bioenergetics to survival and reproductive success could be answered without continued support for such efforts.
Figure 4.
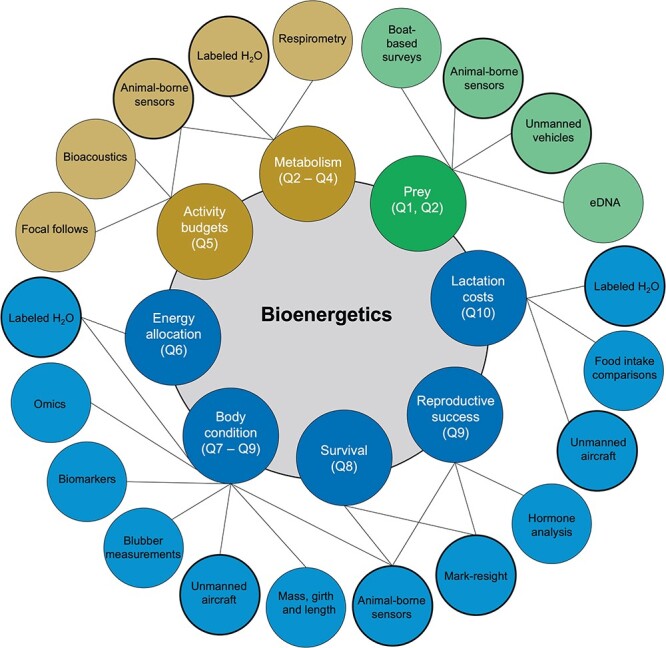
Examples of common and emerging tools or methodologies that can be used to address the key bioenergetic questions identified as part of this exercise. Questions (excluding Q11) are colour-coded as in Fig. 3, with black lines connecting the question to the tool/methodology. Bolded outlines correspond to tools/methodologies that are linked to multiple key questions. In some instances, these represent different tools for collecting the same type of data (e.g. unmanned vehicles and boat-based surveys using acoustic data to characterize prey landscapes), whereas in others they represent different methodologies to address the same question (e.g. eDNA vs. boat-based surveys to characterize prey landscapes).
Emerging technologies and innovative solutions have played a pivotal role in our ability to answer some of these key questions (Fig. 4). For example, animal-borne sensors, and associated statistical approaches for analysing data, have become an invaluable tool for addressing a wide range of questions identified as part of this exercise (Fig. 4). New sensors facilitate data collection on prey capture (e.g. Tennessen et al., 2019; Olivier et al., 2022), drift rates (used to infer body condition; e.g. Biuw, 2003; Biuw et al., 2007) and acceleration, breath rate and heart rate (used to infer FMRs; e.g. Isojunno et al., 2018; Wilson et al., 2020; McDonald et al., 2021). They also provide information on the physical and biological environment that can characterise the prey landscape (Arranz et al., 2011; Goulet et al., 2019; McMahon et al., 2019, 2021). Unmanned systems are currently being applied to marine mammal bioenergetics, particularly the use of aerial systems to estimate body condition and growth (Fearnbach et al., 2018; Lemos et al., 2020; Aoki et al., 2021; Currie et al., 2021; Shero et al., 2021; Stewart et al., 2021a; Christiansen et al., 2022), and surface and underwater systems to survey prey communities (Kuhn et al., 2019; Benoit-Bird et al., 2020). Environmental DNA (eDNA) is a promising emerging tool for addressing questions related to prey landscapes, as it appears able to characterise the distribution and diversity of prey communities (Visser et al., 2021) as well as prey biomass (Rourke et al., 2022). While still in its infancy, eDNA has been used to quantify prey distribution and diversity in areas with critically endangered populations of Yangtze finless porpoise (Neophocaena asiaeorientalis; Qu et al., 2020) and to detect spatiotemporal variability in pelagic forage fish in the Saguenay–St. Lawrence Marine Park, an area used by endangered beluga whales (Delphinapterus leucas; Berger et al., 2020).
Regardless, some key questions are unlikely to be comprehensively addressed without further technological advancements or validation. In particular, the question about FMR, which was the most agreed-upon key question by survey participants, is one area where both validation and advancements are needed. For example, there remains uncertainty in how the approaches commonly used to estimate, or infer, cetacean FMRs (e.g. breathing rates) compare with methods that provide a more direct measure of FMR (e.g. DLW) and the level of uncertainty around estimates given the assumptions of such approaches (Fahlman et al., 2016 and associated responses). New methods that provide estimates or broad scale proxies of FMR from tissue samples (e.g. Chung et al., 2019) would be extremely valuable in furthering our understanding of FMRs in marine mammals, particularly baleen whales and deep-diving odontocetes. Non-invasive sensing technology using near-infrared spectroscopy that continuously measures the rate of O2 consumption is being developed, which provides a new avenue to understand energetic regulation in marine mammals (Ruesch et al., 2022). Other areas where advancements are needed include continuing efforts to improve animal-borne sensors (e.g. miniaturisation, data transmission and processing) and validation of existing and development of new approaches for estimating body condition and lactation costs of cetaceans.
Concluding remarks
Marine mammals tend to live in spatially and temporally variable environments that are changing rapidly with recent climate changes. At the same time, they are also facing increasing exposure to human activities in the marine environment. Such conditions will continue to influence individuals and populations through bioenergetic pathways, which can have cascading impacts on the ecosystems they inhabit through consumptive or non-consumptive mechanisms (Kiszka et al., 2015; Estes et al., 2016; Savoca et al., 2021). Changing environmental conditions may not always negatively impact species, and in some cases may facilitate range expansion, which could alter consumptive pressure on prey populations and create new management challenges. Here we have identified 11 key questions that may help guide research priorities to further our understanding of these pathways. While comprehensive, this list of key questions is certainly not exhaustive and does not necessarily imply that questions that were not included are unimportant or should not be addressed. Instead, they represent the questions that most participants agreed were important gaps, indicating that addressing these questions might have the broadest application across different disciplines, species and approaches.
The end goal of many marine mammal bioenergetic studies is to provide information that can be used to inform policies to better conserve populations by minimising or mitigating risks from human activities in the context of ecosystem management. For example, research on energy requirements, prey intake and body condition of Southern Resident killer whales has contributed to management decisions aiming to ensure adequate Chinook salmon (Oncorhynchus tshawytscha) availability to aid in killer whale population recovery (Ford et al., 2010; Noren, 2011; Williams et al., 2011; Chasco et al., 2017; Wasser et al., 2017; Stewart et al., 2021a). Similarly, PCoD modelling of sperm whales (Farmer et al., 2018a) was used to inform NOAA’s Biological Opinion on federal oil and gas program activities in the Gulf of Mexico. In addition to providing information about the health of marine mammal populations and insights into factors that may be affecting population trajectories, the data collected to address the key questions identified in this exercise will help refine and verify the values of the parameters used in bioenergetic models. This will ensure more accurate predictions of energy needs and the consequences of anthropogenetic and environmental impacts on marine mammal populations.
Funding
This work was funded by the Marine Mammal Commission (MMC19-173). The Office of Naval Research funded the bioenergetic workshop (N000142012392) that provided support for this work.
Data availability
The original submitted questions and final collated questions are included in supplemental material. There are no other data associated with this article.
Supplementary Material
Acknowledgements
We thank all the people that made this exercise possible by participating in the surveys and bioenergetic workshop.
Conflict of interest
The authors declare there are no conflicts of interest.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Contributor Information
Elizabeth A McHuron, Cooperative Institute for Climate, Ocean, and Ecosystem Studies, University of Washington, Seattle, WA, 98195, USA.
Stephanie Adamczak, Ecology and Evolutionary Biology Department, University of California Santa Cruz, Santa Cruz, CA, 95064, USA.
John P Y Arnould, School of Life and Environmental Sciences, Deakin University, Burwood, VIC 3125, Australia.
Erin Ashe, Oceans Initiative, Seattle, WA, 98102, USA.
Cormac Booth, SMRU Consulting, Scottish Oceans Institute, University of St. Andrews, St. Andrews KY16 8LB, UK.
W Don Bowen, Biology Department, Dalhousie University, Halifax, NS B3H 4R2, Canada; Population Ecology Division, Bedford Institute of Oceanography, Dartmouth, NS B2Y 4A2, Canada.
Fredrik Christiansen, Aarhus Institute of Advanced Studies, 8000 Aarhus C, Denmark; Zoophysiology, Department of Biology, Aarhus University, 8000 Aarhus C, Denmark; Center for Sustainable Aquatic Ecosystems, Harry Butler Institute, Murdoch, Murdoch University, WA 6150, Australia.
Magda Chudzinska, SMRU Consulting, Scottish Oceans Institute, University of St. Andrews, St. Andrews KY16 8LB, UK; Sea Mammal Research Unit, Scottish Oceans Institute, University of St. Andrews, St. Andrews KY16 9XL, UK.
Daniel P Costa, Ecology and Evolutionary Biology Department, University of California Santa Cruz, Santa Cruz, CA, 95064, USA.
Andreas Fahlman, Fundación Oceanogràfic de la Comunitat Valenciana, 46005 Valencia, Spain; Kolmården Wildlife Park, 618 92 Kolmården, Sweden.
Nicholas A Farmer, NOAA/National Marine Fisheries Service, Southeast Regional Office, St. Petersburg, FL, 33701, USA.
Sarah M E Fortune, Department of Oceanography, Dalhousie University, Halifax, NS B3H 4R2, Canada.
Cara A Gallagher, Plant Ecology and Nature Conservation, University of Potsdam, 14476 Potsdam, Germany.
Kelly A Keen, Ecology and Evolutionary Biology Department, University of California Santa Cruz, Santa Cruz, CA, 95064, USA.
Peter T Madsen, Zoophysiology, Department of Biology, Aarhus University, 8000 Aarhus C, Denmark.
Clive R McMahon, IMOS Animal Tagging, Sydney Institute of Marine Science, Mosman, NSW 2088, Australia.
Jacob Nabe-Nielsen, Department of Ecoscience, Aarhus University, 4000 Roskilde, Denmark.
Dawn P Noren, Conservation Biology Division, Northwest Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, Seattle, WA, 98112, USA.
Shawn R Noren, Institute of Marine Science, University of California Santa Cruz, Santa Cruz, CA, 95060, USA.
Enrico Pirotta, Centre for Research into Ecological and Environmental Modelling, University of St. Andrews, St. Andrews KY16 9LZ, UK.
David A S Rosen, Institute for Oceans and Fisheries, University of British Columbia, Vancouver, BC V6T 1ZA, Canada.
Cassie N Speakman, School of Life and Environmental Sciences, Deakin University, Burwood, VIC 3125, Australia.
Stella Villegas-Amtmann, Ecology and Evolutionary Biology Department, University of California Santa Cruz, Santa Cruz, CA, 95064, USA.
Rob Williams, Oceans Initiative, Seattle, WA, 98102, USA.
References
- Acevedo J, Urbán J (2021) Estimates of Fuegian sprat consumption by humpback whales in the Magellan Strait feeding area as predicted by a bioenergetic model. Mar Ecol Prog Ser 657: 223–239. [Google Scholar]
- Adachi T, Takahashi A, Costa DP, Robinson PW, Hückstädt LA, Peterson SH, Holser RR, Beltran RS, Keates TR, Naito Y (2021) Forced into an ecological corner: round-the-clock deep foraging on small prey by elephant seals. Sci Adv 7: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Isojunno S, Bellot C, Iwata T, Kershaw J, Akiyama Y, Martín López LM, Ramp C, Biuw M, Swift Ret al. (2021) Aerial photogrammetry and tag-derived tissue density reveal patterns of lipid-store body condition of humpback whales on their feeding grounds. Proc R Soc B Biol Sci 288: 20202307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnbom T, Fedak MA, Boyd IL (1997) Factors affecting maternal expenditure in southern elephant seals during lactation. Ecology 78: 471–483. [Google Scholar]
- Arnould J, Boyd IL, Socha DG (1996a) Milk consumption and growth efficiency in Antarctic fur seal (Arctocephalus gazella) pups. Can J Zool 74: 254–266. [Google Scholar]
- Arnould J, Boyd IL, Speakman JR (1996b) The relationship between foraging behavior and energy expenditure in Antarctic fur seals. J Zool Soc London 239: 769–782. [Google Scholar]
- Arnould JPY, Luque SP, Guinet C, Costa DP, Kingston J, Shaffer SA (2003) The comparative energetics and growth strategies of sympatric Antarctic and subantarctic fur seal pups at Îles Crozet. J Exp Biol 206: 4497–4506. [DOI] [PubMed] [Google Scholar]
- Arranz P, Soto NA, Madsen PT, Brito A, Bordes F, Johnson MP (2011) Following a foraging fish-finder: diel habitat use of Blainville’s beaked whales revealed by echolocation. PLoS One 6: e28353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin D, Bowen WD, McMillan JI, Iverson SJ (2006) Linking movement, diving, and habitat to foraging success in a large marine predator. Ecology 87: 3095–3108. [DOI] [PubMed] [Google Scholar]
- Avila IC, Kaschner K, Dormann CF (2018) Current global risks to marine mammals: taking stock of the threats. Biol Conserv 221: 44–58. [Google Scholar]
- Baylis AMM, Orben RA, Arnould JPY, Christiansen F, Hays GC, Staniland IJ (2015) Disentangling the cause of a catastrophic population decline in a large marine mammal. Ecology 96: 2834–2847. [DOI] [PubMed] [Google Scholar]
- Beauplet G, Barbraud C, Chambellant M, Guinet C (2005) Interannual variation in the post-weaning and juvenile survival of subantarctic fur seals: influence of pup sex, growth rate and oceanographic conditions. J Anim Ecol 74: 1160–1172. [Google Scholar]
- Bejarano AC, Wells RS, Costa DP (2017) Development of a bioenergetic model for estimating energy requirements and prey biomass consumption of the bottlenose dolphin Tursiops truncatus. Ecol Model 356: 162–172. [Google Scholar]
- Bennett KA, Robinson KJ, Armstrong HC, Moss SEW, Scholl G, Tranganida A, Eppe G, Thomé JP, Debier C, Hall AJ (2021) Predicting consequences of POP-induced disruption of blubber glucose uptake, mass gain rate and thyroid hormone levels for weaning mass in grey seal pups. Environ Int 152: 106506. [DOI] [PubMed] [Google Scholar]
- Benoit-Bird KJ, Southall BL, Moline MA, Claridge DE, Dunn CA, Dolan KA, Moretti DJ (2020) Critical threshold identified in the functional relationship between beaked whales and their prey. Mar Ecol Prog Ser 654: 1–16. [Google Scholar]
- Berger CS, Bougas B, Turgeon S, Ferchiou S, Ménard N, Bernatchez L (2020) Groundtruthing of pelagic forage fish detected by hydroacoustics in a whale feeding area using environmental DNA. Environ DNA 2: 477–492. [Google Scholar]
- Biela V, Arimitsu ML, Piatt JF, Heflin BM, Schoen S (2019) Extreme reduction in condition of a key forage fish during the Pacific marine heatwave of 2014–2016. Mar Ecol Prog Ser 613: 171–182. [Google Scholar]
- Biuw M (2003) Blubber and buoyancy: monitoring the body condition of free-ranging seals using simple dive characteristics. J Exp Biol 206: 3405–3423. [DOI] [PubMed] [Google Scholar]
- Biuw M, Boehme L, Guinet C, Hindell M, Costa D, Charrassin J, Roquet F, Bailleul F, Meredith M, Thorpe Set al. (2007) Variations in behavior and condition of a Southern Ocean top predator in relation to in situ oceanographic conditions. PNAS 104: 13705–13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet MA, Aars J, Andersen M, Routti H (2020) Space-use strategy affects energy requirements in Barents Sea polar bears. Mar Ecol Prog Ser 639: 1–19. [Google Scholar]
- Booth CG (2020) Food for thought: harbor porpoise foraging behavior and diet inform vulnerability to disturbance. Mar Mamm Sci 36: 195–208. [Google Scholar]
- Bowen WD, Tully D, Boness DJ, Bulheier BM, Marshall GJ (2002) Prey-dependent foraging tactics and prey profitability in a marine mammal. Mar Ecol Prog Ser 244: 235–245. [Google Scholar]
- Bowen WD, Heyer CE, Mcmillan JI, Iverson SJ (2015) Offspring size at weaning affects survival to recruitment and reproductive performance of primiparous gray seals. Ecol Evol 5: 1412–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WD, Iverson SJ, Boness DJ, Oftedal OT (2001) Foraging effort, food intake and lactation performance depend on maternal mass in a small phocid seal. Funct Ecol 15: 325–334. [Google Scholar]
- Bowen WD, Northridge S (2010) Morphometrics, age estimation, and growth. In Boyd IL, Bowen WD, Iverson SJ, eds, Marine Mammal Ecology and Conservation: A Handbook of Techniques. Oxford University Press, Oxford. [Google Scholar]
- Brock PM, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K (2013) Immune activity, body condition and human-associated environmental impacts in a wild marine mammal. PLoS One 8: e67132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade DE, Seakamela SM, Findlay KP, Fukunaga J, Kahane-Rapport SR, Warren JD, Calambokidis J, Fahlbusch JA, Friedlaender AS, Hazen ELet al. (2021) Predator-scale spatial analysis of intra-patch prey distribution reveals the energetic drivers of rorqual whale super-group formation. Funct Ecol 35: 894–908. [Google Scholar]
- Castrillon J, Bengtson Nash S (2020) Evaluating cetacean body condition; a review of traditional approaches and new developments. Ecol Evol 10: 6144–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambert T, Rotella JJ, Garrott RA (2015) Female Weddell seals show flexible strategies of colony attendance related to varying environmental conditions. Ecology 96: 479–488. [DOI] [PubMed] [Google Scholar]
- Chasco B, Kaplan IC, Thomas A, Acevedo-Gutiérrez A, Noren D, Ford MJ, Hanson MB, Scordino J, Jeffries S, Pearson Set al. (2017) Estimates of Chinook salmon consumption in Washington State inland waters by four marine mammal predators from 1970 to 2015. Can J Fish Aquat Sci 74: 1173–1194. [Google Scholar]
- Cheney B, Wells RS, Barton TR, Thompson PM (2018) Laser photogrammetry reveals variation in growth and early survival in free-ranging bottlenose dolphins. Anim Conserv 21: 252–261. [Google Scholar]
- Chinn SM, Miller MA, Tinker MT, Staedler MM, Batac FI, Dodd EM, Henkel LA (2016) The high cost of motherhood: end-lactation syndrome in southern sea otters (Enhydra lutris nereis) on the Central California Coast, USA. J Wildl Dis 52: 307–318. [DOI] [PubMed] [Google Scholar]
- Christiansen F, Bejder L, Burnell S, Ward R, Charlton C (2022) Estimating the cost of growth in southern right whales from drone photogrammetry data and long-term sighting histories. Mar Ecol Prog Ser 687: 173–194. [Google Scholar]
- Christiansen F, Dawson SM, Durban JW, Fearnbach H, Miller CA, Bejder L, Uhart M, Sironi M, Corkeron P, Rayment Wet al. (2020) Population comparison of right whale body condition reveals poor state of the North Atlantic right whale. Mar Ecol Prog Ser 640: 1–16. [Google Scholar]
- Christiansen F, Lusseau D (2015) Linking behavior to vital rates to measure the effects of non-lethal disturbance on wildlife. Conserv Lett 8: 424–431. [Google Scholar]
- Christiansen F, Rasmussen MH, Lusseau D (2014a) Inferring energy expenditure from respiration rates in minke whales to measure the effects of whale watching boat interactions. J Exp Mar Bio Ecol 459: 96–104. [Google Scholar]
- Christiansen F, Rodríguez-González F, Martínez-Aguilar S, Urbán J, Swartz S, Warick H, Vivier F, Bejder L (2021) Poor body condition associated with an unusual mortality event in gray whales. Mar Ecol Prog Ser 658: 237–252. [Google Scholar]
- Christiansen F, Víkingsson GA, Rasmussen MH, Lusseau D (2014b) Female body condition affects foetal growth in a capital breeding mysticete. Funct Ecol 28: 579–588. [Google Scholar]
- Christiansen F, Vivier F, Charlton C, Ward R, Amerson A, Burnell S, Bejder L (2018) Maternal body size and condition determine calf growth rates in southern right whales. Mar Ecol Prog Ser 592: 267–281. [Google Scholar]
- Chung MT, Trueman CN, Godiksen JA, Holmstrup ME, Grønkjær P (2019) Field metabolic rates of teleost fishes are recorded in otolith carbonate. Commun Biol 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscarella MA, Dans SL, Crespo EA, Pedraza SN (2003) Potential impact of dolphin watching unregulated activities in Patagonia. The Journal of Cetacean Research and Management 5: 77–84. [Google Scholar]
- Costa D, Le Boeuf BJ, Huntley AC, Ortiz C (1986) The energetics of lactation in the northern elephant seal, Mirounga angustirostris. J Zool 209: 21–33. [Google Scholar]
- Costa DP (2008) A conceptual model of the variation in parental attendance in response to environmental fluctuation: foraging energetics of lactating sea lions and fur seals. Aquat Conserv Mar Freshw Ecosyst 17: S44–S52. [Google Scholar]
- Costa DP, Croxall JP, Duck C (1989) Foraging energetics of Antarctic fur seals in relation to changes in prey availability. Ecology 70: 596–606. [Google Scholar]
- Costa DP, Gales NJ (2000) Foraging energetics and diving behavior of lactating New Zealand sea lions, Phocarctos hookeri. J Exp Biol 203: 3655–3665. [DOI] [PubMed] [Google Scholar]
- Costa DP, Gales NJ (2003) Energetics of a benthic diver: seasonal foraging ecology of the Australian sea lion, Neophoca cinerea. Ecol Monogr 73: 27–43. [Google Scholar]
- Costa DP, Maresh JL (2017) Energetics. In Wursig B, Thewissen J, Kovacs KM, eds, Encyclopedia of Marine Mammals. Academic Press, Cambridge, MA: pp. 329–335 [Google Scholar]
- Cunningham SJ, Gardner JL, Martin RO (2021) Opportunity costs and the response of birds and mammals to climate warming. Front Ecol Environ 19: 300–307. [Google Scholar]
- Currie JJ, Aswegen M, Stack SH, West KL, Vivier F, Bejder L (2021) Rapid weight loss in free ranging pygmy killer whales (Feresa attenuata) and the implications for anthropogenic disturbance of odontocetes. Sci Rep 11: 8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AD, Boyer AG, Kim H, Pompa-Mansilla S, Hamilton MJ, Costa DP, Ceballos G, Brown JH (2012) Drivers and hotspots of extinction risk in marine mammals. PNAS 109: 3395–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MJ, Costa DP, Goebel E, Antonelis GA, Baker JD (2002) Milk intake and energy expenditure of free-ranging northern fur seal, Callorhinus ursinus, pups. Physiol Biochem Zool 75: 3–18. [DOI] [PubMed] [Google Scholar]
- Estes JA, Heithaus M, McCauley DJ, Rasher DB, Worm B (2016) Megafaunal impacts on structure and function of ocean ecosystems. Annu Rev Env Resour 41: 83–116. [Google Scholar]
- Fahlman A, Van Hoop J, Der MMJ, Levine G, Rocho-Levine J, Brodsky M (2016) Estimating energetics in cetaceans from respiratory frequency: why we need to understand physiology. Biol Open 5: 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer NA, Baker K, Zeddies DG, Denes SL, Noren DP, Garrison LP, Machernis A, Fougères EM, Zykov M (2018a) Population consequences of disturbance by offshore oil and gas activity for endangered sperm whales (Physeter macrocephalus). Biol Conserv 227: 189–204. [Google Scholar]
- Farmer NA, Noren DP, Fougères EM, Machernis A, Baker K (2018b) Resilience of the endangered sperm whale Physeter macrocephalus to foraging disturbance in the Gulf of Mexico, USA: a bioenergetic approach. Mar Ecol Prog Ser 589: 241–261. [Google Scholar]
- Fearnbach H, Durban JW, Ellifrit DK, Balcomb KC (2018) Using aerial photogrammetry to detect changes in body condition of endangered southern resident killer whales. Endanger Species Res 35: 175–180. [Google Scholar]
- Ferguson SH, Young BG, Yurkowski DJ, Anderson R, Willing C, Nielsen O (2017) Demographic, ecological, and physiological responses of ringed seals to an abrupt decline in sea ice availability. PeerJ 5: e2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H, Atkinson A, Kawaguchi S, Krafft BA, Milinevsky G, Nicol S, Reiss C, Tarling GA, Werner R, Bravo Rebolledo Eet al. (2012) Impact of climate change on Antarctic krill. Mar Ecol Prog Ser 458: 1–19. [Google Scholar]
- Florko KRN, Tai TC, Cheung WWL, Ferguson SH, Sumaila UR, Yurkowski DJ, Auger-Méthé M (2021) Predicting how climate change threatens the prey base of Arctic marine predators. Ecol Lett 24: 2563–2575. [DOI] [PubMed] [Google Scholar]
- Ford JKB, Ellis GM, Olesiuk PF, Balcomb KC (2010) Linking killer whale survival and prey abundance: food limitation in the oceans’ apex predator? Biol Lett 6: 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune S, Trites A, Mayo C, Rosen D, Hamilton P (2013) Energetic requirements of North Atlantic right whales and the implications for species recovery. Mar Ecol Prog Ser 478: 253–272. [Google Scholar]
- Fowler SL, Costa DP, Arnould JPY, Gales NJ, Burns JM (2007) Ontogeny of oxygen stores and physiological diving capability in Australian sea lions. Funct Ecol 21: 922–935. [Google Scholar]
- Gailey G, Sychenko O, Tyurneva O, Yakovlev Y, Vertyankin V, Van Der (2020) Effects of sea ice on growth rates of an endangered population of gray whales. Sci Rep 10: 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CA, Chimienti M, Grimm V, Nabe-Nielsen J (2022) Energy-mediated responses to changing prey size and distribution in marine top predator movements and population dynamics. J Anim Ecol. 91: 241–254. [DOI] [PubMed] [Google Scholar]
- Gallagher CA, Grimm V, Kyhn LA, Kinze CC, Nabe-Nielsen J (2020) Movement and seasonal energetics mediate vulnerability to disturbance in marine mammal populations. Am Nat 197: 296–311. [DOI] [PubMed] [Google Scholar]
- Gallagher CA, Stern SJ, Hines E (2018) The metabolic cost of swimming and reproduction in harbor porpoises (Phocoena phocoena) as predicted by a bioenergetic model. Mar Mamm Sci 34: 875–900. [Google Scholar]
- Gavrilchuk K, Lesage V, Fortune SME, Trites AW, Plourde S (2021) Foraging habitat of North Atlantic right whales has declined in the Gulf of St. Lawrence, Canada, and may be insufficient for successful reproduction. Endanger Species Res 44: 113–136. [Google Scholar]
- Gittleman JL, Thompson D (1988) Energy allocation in mammalian reproduction. Am Zool 28: 863–875. [Google Scholar]
- Glazier D (2009) Resource Allocation Patterns. In Rauw WM, ed, Resource Allocation Theory Applied to Farm Animal Production. CAB International, Wallingford, pp. 22–43. [Google Scholar]
- Goulet P, Guinet C, Swift R, Madsen PT, Johnson M (2019) A miniature biomimetic sonar and movement tag to study the biotic environment and predator-prey interactions in aquatic animals. Deep Res Part I Oceanogr Res Pap 148: 1–11. [Google Scholar]
- Hall AJ, McConnell BJ, Barker RJ (2001) Factors affecting first-year survival in grey seals and their implications for life history strategies. J Anim Ecol 70: 138–149. [Google Scholar]
- Halsey LG (2021) The mystery of energy compensation. Physiol Biochem Zool 94: 380–393. [DOI] [PubMed] [Google Scholar]
- Hamilton CD, Kovacs KM, Ims RA, Lydersen C (2018) Haul-out behaviour of Arctic ringed seals (Pusa hispida): inter-annual patterns and impacts of current environmental change. Polar Biol 41: 1063–1082. [Google Scholar]
- Harding K, Fujiwara M, Axberg Y, Harkönen T (2005) Mass dependent energetics and survival in harbour seal pups. Funct Ecol 19: 129–135. [Google Scholar]
- Harris CM, Thomas L, Falcone EA, Hildebrand J, Houser D, Kvadsheim PH, Lam FPA, Miller PJO, Moretti DJ, Read AJet al. (2018) Marine mammals and sonar: dose-response studies, the risk-disturbance hypothesis and the role of exposure context. J Appl Ecol 55: 396–404. [Google Scholar]
- Hazen EL, Friedlaender AS, Goldbogen JA (2015) Blue whales (Balaenoptera musculus) optimize foraging efficiency by balancing oxygen use and energy gain as a function of prey density. Sci Adv 1: e1500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hin V, Harwood J, de Roos AS (2019) Bio-energetic modeling of medium-sized cetaceans shows high sensitivity to disturbance in seasons of low resource supply Ecol App 29: e01903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekendijk JPA, Spitz J, Read AJ, Leopold MF, Fontaine MC (2018) Resilience of harbor porpoises to anthropogenic disturbance: must they really feed continuously? Mar Mamm Sci 34: 258–264. [Google Scholar]
- Hoop J, Corkeron P, Moore M (2017) Entanglement is a costly life-history stage in large whales. Ecol Evol 7: 92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LN, Isaac NJB, Reuman DC (2013) The relationship between body mass and field metabolic rate among individual birds and mammals. J Anim Ecol 82: 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CA (1981) Seawater consumption and water flux in the common dolphin Delphinus delphis. Physiol Zool 54: 430–440. [Google Scholar]
- Hunter AMJ, Trites AW, Pauly D (2000) Estimates of basal metabolic and feeding rates for marine mammals from measurements of maximum body length. Proc Comp Nutr Soc 103–106. [Google Scholar]
- Huntley AC, Costa DP, Worthy GAJ, Castellini MA (1987) Approaches to Marine Mammal Energetics. The Society for Marine Mammalogy, Lawrence, KS [Google Scholar]
- Innes S, Lavigne DM, Earle WM, Kovacs KM (1987) Feeding rates of seals and whales. J Anim Ecol 56: 115–130. [Google Scholar]
- Isojunno S, Aoki K, Curé C, Kvadsheim PH, O’Malley Miller PJ (2018) Breathing patterns indicate cost of exercise during diving and response to experimental sound exposures in long-finned pilot whales. Front Physiol 9: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson SJ, Bowen W, Boness DJ, Oftedal OT (1993) The effect of maternal size and milk energy output on pup growth in grey seals (Halichoerus grypus). Physiol Zool 66: 61–88. [Google Scholar]
- Iverson SJ, Sparling CE, Williams TM, Lang SLC, Bowen WD (2010) Measurement of individual and population energetics of marine mammals. In IL Boyd, WD Bowen, SJ Iverson, eds, Marine Mammal Ecology and Conservation: A Handbook of Techniques. Oxford University Press, Oxford, pp. 165–190. [Google Scholar]
- Jeanniard du Dot T, Trites AW, Arnould JPY, Speakman JR, Guinet C (2018) Trade-offs between foraging efficiency and pup feeding rate of lactating northern fur seals in a declining population. Mar Ecol Prog Ser 600: 207–222. [Google Scholar]
- Kanwisher JW, Ridgway SH (1983) The physiological ecology of whales and porpoises. Sci Am 248: 110–121. [Google Scholar]
- Keen KA, Beltran RS, Pirotta E, Costa DP (2021) Emerging themes in population consequences of disturbance models. Proc R Soc B Biol Sci 288: 20210325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw JL, Ramp CA, Sears R, Plourde S, Brosset P, Miller PJO, Hall AJ (2021) Declining reproductive success in the Gulf of St. Lawrence’s humpback whales (Megaptera novaeangliae) reflects ecosystem shifts on their feeding grounds. Glob Chang Biol 27: 1027–1041. [DOI] [PubMed] [Google Scholar]
- Kershaw JL, Sherrill M, Davison NJ, Brownlow A, Hall AJ (2017) Evaluating morphometric and metabolic markers of body condition in a small cetacean, the harbor porpoise (Phocoena phocoena). Ecol Evol 7: 3494–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiszka JJ, Heithaus MR, Wirsing AJ (2015) Behavioural drivers of the ecological roles and importance of marine mammals. Mar Ecol Prog Ser 523: 267–281. [Google Scholar]
- Kolokotrones T, Savage V, Deeds EJ, Fontana W (2010) Curvature in metabolic scaling. Nature 464: 753–756. [DOI] [PubMed] [Google Scholar]
- Kovacs KM, Aguilar A, Aurioles D, Burkanov V, Campagna C, Gales N, Gelatt T, Goldsworthy SD, Goodman SJ, Hofmeyr GJGet al. (2012) Global threats to pinnipeds. Mar Mamm Sci 28: 414–436. [Google Scholar]
- Kuhn C, De Robertis A, Sterling J, Mordy C, Meinig C, Lawrence-Slavas N, Cokelet E, Levine M, Tabisola H, Jenkins Ret al. (2019) Test of unmanned surface vehicles to conduct remote focal follow studies of a marine predator. Mar Ecol Prog Ser 635: 1–7. [Google Scholar]
- Laidre KL, Atkinson S, Regehr EV, Stern HL, Born EW, Wiig Ø, Lunn NJ, Dyck M (2020) Interrelated ecological impacts of climate change on an apex predator. Ecol Appl 30: e02071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SLC, Iverson SJ, Bowen WD (2011) The influence of reproductive experience on milk energy output and lactation performance in the grey seal (Halichoerus grypus). PLoS One 6: e19487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrat S, Lair S (2022) Body condition index in beluga whale (Delphinapterus leucas) carcasses derived from morphometric measurements. Mar Mamm Sci 38: 274–287. [Google Scholar]
- Lavigne DM (1982) Similarity in energy budgets of animal populations. J Anim Ecol 51: 195–206. [Google Scholar]
- Lavigne DM, Barchard W, Innes S, Oritsland NA (1982) Pinniped bioenergetics. In Mammals in the Seas - Small Cetaceans, Seals, Sirenians, and Otters. Food and Agriculture Organization of the United Nations, Fisheries Series 5, Rome, pp. 191–235. [Google Scholar]
- Lavigne DM, Innes S, Worthy GAJ, Kovacs KM, Schmitz OJ, Hickie JP (1986) Metabolic rates of seals and whales. Can J Zool 64: 279–284. [Google Scholar]
- Le Boeuf B, Condit R, Reiter J (2019) Lifetime reproductive success of northern elephant seals (Mirounga angustirostris). Can J Zool 97: 1203–1217. [Google Scholar]
- Lemos LS, Burnett JD, Chandler TE, Sumich JL, Torres LG (2020) Intra- and inter-annual variation in gray whale body condition on a foraging ground. Ecosphere 11: e03094. [Google Scholar]
- Lifson N, McClintock R (1966) Theory of use of the turnover rates of body water for measuring energy and material balance. J Theor Biol 12: 46–74. [DOI] [PubMed] [Google Scholar]
- Lockyer C (1981) Growth and energy budgets of large baleen whales from the Southern Hemisphere. Mamm Seas 3, FAO Fish Ser no 5: 379–487. [Google Scholar]
- Lusseau D (2006) The short-term behavioral reactions of bottlenose dolphins to interactions with boats in Doubtful Sound, New Zealand. Mar Mamm Sci 22: 802–818. [Google Scholar]
- Lydersen C, Kovacs KM, Hammill MO (1997) Energetics during nursing and early postweaning fasting in hooded seal (Cystophora cristata) pups from the Gulf of St Lawrence, Canada. J Comp Physiol B Biochem Syst Environ Physiol 167: 81–88. [DOI] [PubMed] [Google Scholar]
- Markussen NH, Ryg M, Oritsland NA (1992) Metabolic rate and body composition of harbour seals, Phoca vitulina, during starvation and refeeding. Can J Zool 70: 220–224. [Google Scholar]
- McDonald BI, Elmegaard SL, Johnson M, Wisniewska DM, Rojano-Doñate L, Galatius A, Siebert U, Teilmann J, Madsen PT (2021) High heart rates in hunting harbour porpoises. Proc R Soc B Biol Sci 288: 20211596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BI, Goebel ME, Crocker DE, Costa DP (2012) Biological and environmental drivers of energy allocation in a dependent mammal, the Antarctic fur seal pup. Physiol Biochem Zool 85: 134–147. [DOI] [PubMed] [Google Scholar]
- McHuron EA, Aerts L, Gailey G, Sychenko O, Costa DP, Mangel M, Schwarz LK (2021) Predicting the population consequences of acoustic disturbance, with application to an endangered gray whale population. Ecol Appl 31: e02440. [DOI] [PubMed] [Google Scholar]
- McHuron EA, Luxa K, Pelland NA, Holsman K, Ream R, Zeppelin T, Sterling JT (2020) Practical application of a bioenergetic model to inform management of a declining fur seal population and their commercially important prey. Front Mar Sci 7: 597973. [Google Scholar]
- McHuron EA, Peterson SH, Hückstädt LA, Melin SR, Harris JD, Costa DP (2018) The energetic consequences of behavioral variation in a marine carnivore. Ecol Evol 8: 4340–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHuron EA, Sterling JT, Costa DP, Goebel ME (2019) Factors affecting energy expenditure in a declining fur seal population. Conserv Physiol 7: coz103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CR, Burton HR (2005) Climate change and seal survival: evidence for environmentally mediated changes in elephant seal, Mirounga leonina, pup survival. Proc R Soc B Biol Sci 272: 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CR, Hindell MA, Charrassin JB, Corney S, Guinet C, Harcourt R, Jonsen I, Trebilco R, Williams G, Bestley S (2019) Finding mesopelagic prey in a changing Southern Ocean. Sci Rep 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CR, Roquet F, Baudel S, Belbeoch M, Bestley S, Blight C, Boehme L, Carse F, Costa DP, Fedak MAet al. (2021) Animal Borne Ocean sensors—AniBOS—an essential component of the Global Ocean observing system. Front Mar Sci 8: 1–21.35685121 [Google Scholar]
- McNab BK (2008) An analysis of the factors that influence the level and scaling of mammalian BMR. Comp Biochem Physiol A Mol Integr Physiol 151: 5–28. [DOI] [PubMed] [Google Scholar]
- Mellish JAE, Iverson SJ, Bowen WD (2000) Metabolic compensation during high energy output in fasting, lactating grey seals (Halichoerus grypus): metabolic ceilings revisited. Proc R Soc B Biol Sci 267: 1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellish JE, Iverson SJ, Bowen WD, Hammill MO (1999) Fat transfer and energetics during lactation in the hooded seal: the roles of tissue lipoprotein lipase in milk fat secretion and pup blubber deposition. J Comp Physiol B 169: 377–390. [DOI] [PubMed] [Google Scholar]
- Nabe-Nielsen J, Van BFM, Grimm V, Teilmann J, Thompson PM (2018) Predicting the impacts of anthropogenic disturbances on marine populations. Conserv Lett 11: e12563. [Google Scholar]
- Nagy KA, Girard IA, Brown TK (1999) Energetics of free-ranging mammals, reptiles, and birds. Annu Rev Nutr 19: 247–277. [DOI] [PubMed] [Google Scholar]
- New LF, Clark JS, Costa DP, Fleishman E, Hindell MA, Klanjšček T, Lusseau D, Kraus S, McMahon CR, Robinson PWet al. (2014) Using short-term measures of behaviour to estimate long-term fitness of southern elephant seals. Mar Ecol Prog Ser 496: 99–108. [Google Scholar]
- New LF, Moretti DJ, Hooker SK, Costa DP, Simmons SE (2013) Using energetic models to investigate the survival and reproduction of beaked whales (family Ziphiidae). PLoS One 8: e68725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren DP (2011) Estimated field metabolic rates and prey requirements of resident killer whales. Mar Mamm Sci 27: 60–77. [Google Scholar]
- Noren DP, Crocker DE, Williams TM, Costa DP (2003) Energy reserve utilization in northern elephant seal (Mirounga angustirostris) pups during the postweaning fast: size does matter. J Comp Physiol B 173: 443–454. [DOI] [PubMed] [Google Scholar]
- Noren DP, Dunkin RC, Williams TM, Holt MM (2012) Energetic costs of behaviors performed in response to vessel disturbance: one link in the population consequences of acoustic disturbance model. In Popper A, Hawkins A, eds, The Effects of Noise on Aquatic Life. Advances in Experimental Medicine and Biology, Vol 730. Springer, New York, NY, pp. 427–430 [DOI] [PubMed] [Google Scholar]
- Noren DP, Johnson AH, Rehder D, Larson A (2009) Close approaches by vessels elicit surface active behaviors by southern resident killer whales. Endanger Species Res 8: 179–192. [Google Scholar]
- Noren SR, Schwarz L, Chase K, Aldrich K, Van OKMM, Leger JS (2019) Validation of the photogrammetric method to assess body condition of an odontocete, the short-finned pilot whale Globicephala macrorhynchus. Mar Ecol Prog Ser 620: 185–200. [Google Scholar]
- Noren SR, Schwarz L, Robeck TR (2021) Topographic variations in mobilization of blubber in relation to changes in body mass in short-finned pilot whales (Globicephala macrorhynchus). Physiol Biochem Zool 94: 228–240. [DOI] [PubMed] [Google Scholar]
- Noren SR, Udevitz MS, Triggs L, Paschke J, Oland L, Jay CV (2015) Identifying a reliable blubber measurement site to assess body condition in a marine mammal with topographically variable blubber, the Pacific walrus. Mar Mamm Sci 31: 658–676. [Google Scholar]
- Noren SR, Wells RS (2009) Blubber deposition during ontogeny in free-ranging bottlenose dolphins: balancing disparate roles of insulation and locomotion. J Mammal 90: 629–637. [Google Scholar]
- Oftedal OT, Bowen WD, Boness DJ (1993) Energy transfer by lactating hooded seals and nutrient deposition in their pups during the four days from birth to weaning. Physiol Biochem Zool 66: 412–436. [Google Scholar]
- Olivier PA, Andrews R, Burkanov V, Davis RW (2022) Diving behavior, foraging strategies, and energetics of female Steller sea lions during early lactation. J Exp Mar Bio Ecol 550: 151707. [Google Scholar]
- Oosthuizen WC, Altwegg R, Nevoux M, Bester MN, Bruyn PJN (2018) Phenotypic selection and covariation in the life-history traits of elephant seals: heavier offspring gain a double selective advantage. Oikos 127: 875–889. [Google Scholar]
- Pagano AM, Durner GM, Rode KD, Atwood TC, Atkinson SN, Peacock E, Costa DP, Owen MA, Williams TM (2018) High-energy, high-fat lifestyle challenges an Arctic apex predator, the polar bear. Science (80-) 359: 568–572. [DOI] [PubMed] [Google Scholar]
- Pagano AM, Williams TM (2021) Physiological consequences of Arctic sea ice loss on large marine carnivores: unique responses by polar bears and narwhals. J Exp Biol 224: jeb228049. [DOI] [PubMed] [Google Scholar]
- Pendleton DE, Holmes EE, Redfern J, Zhang J (2020) Using modelled prey to predict the distribution of a highly mobile marine mammal. Divers Distrib 26: 1612–1626. [Google Scholar]
- Pettis HM, Rolland RM, Hamilton PK, Knowlton AR, Burgess EA, Kraus SD (2017) Body condition changes arising from natural factors and fishing gear entanglements in North Atlantic right whales Eubalaena glacialis. Endanger Species Res 32: 237–249. [Google Scholar]
- Pinsky ML, Selden RL, Kitchel ZJ (2020) Climate-driven shifts in marine species ranges: scaling from organisms to communities. Ann Rev Mar Sci 12: 153–179. [DOI] [PubMed] [Google Scholar]
- Pirotta E (2022) A review of bioenergetic modelling for marine mammal populations. Conserv Physiol 10: coac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta E, Booth CG, Costa DP, Fleishman E, Kraus S, Lusseau D, Moretti D, New LF, Schick RS, Schwarz LKet al. (2018a) Understanding the population consequences of disturbance. Ecol Evol 8: 9934–9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta E, Mangel M, Costa DP, Goldbogen J, Harwood J, Hin V, Irvine LM, Mate BR, McHuron EA, Palacios DMet al. (2019) Anthropogenic disturbance in a changing environment: modelling lifetime reproductive success to predict the consequences of multiple stressors on a migratory population. Oikos 128: 1340–1357. [Google Scholar]
- Pirotta E, Mangel M, Costa DP, Mate BR, Goldbogen JA, Palacios DM, Hückstädt LA, McHuron E, Schwarz LK, New LF (2018b) A dynamic state model of migratory behavior and physiology to assess the consequences of environmental variation and anthropogenic disturbance on marine vertebrates. Am Nat 191: E40–E56. [DOI] [PubMed] [Google Scholar]
- Qu C, Stewart KA, Clemente-Carvalho R, Zheng J, Wang Y, Gong C, Ma L, Zhao J, Lougheed SC (2020) Comparing fish prey diversity for a critically endangered aquatic mammal in a reserve and the wild using eDNA metabarcoding. Sci Rep 10: 16715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raverty S, Leger JS, Noren DP, Huntington KB, Rotstein DS, Gulland FMD, Ford JKB, Bradley Hanson M, Lambourn DM, Huggins Jet al. (2020) Pathology findings and correlation with body condition index in stranded killer whales (Orcinus orca) in the northeastern Pacific and Hawaii from 2004 to 2013. PLoS One. 15: e0242505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner EU, Rosen DAS, Trites AW (2013) Energy requirements of Pacific white-sided dolphins (Lagenorhynchus obliquidens) as predicted by a bioenergetic model. J Mammal 94: 820–832. [Google Scholar]
- Riek A (2008) Relationship between milk energy intake and growth rate in suckling mammalian young at peak lactation: an updated meta-analysis. J Zool 274: 160–170. [Google Scholar]
- Riek A (2011) Allometry of milk intake at peak lactation. Mamm Biol 76: 3–11. [Google Scholar]
- Riek A (2021) Comparative phylogenetic analysis of milk output at peak lactation. Comp Biochem Physiol Part A 257: 110976. [DOI] [PubMed] [Google Scholar]
- Robinson KJ, Hall AJ, Debier C, Eppe G, Thomé JP, Bennett KA (2018) Persistent prganic pollutant burden, experimental POP exposure, and tissue properties affect metabolic profiles of blubber from gray seal pups. Environ Sci Technol 52: 13523–13534. [DOI] [PubMed] [Google Scholar]
- Rojano-Donãte L, McDonald BI, Wisniewska DM, Johnson M, Teilmann J, Wahlberg M, Højer-Kristensen J, Madsen PT (2018) High field metabolic rates of wild harbour porpoises. J Exp Biol 221: jeb185827. [DOI] [PubMed] [Google Scholar]
- Rosen DAS, Trites AW (2002) Changes in metabolism in response to fasting and food restriction in the Steller sea lion (Eumetopias jubatus). Comp Biochem Physiol Part B 132: 389–399. [DOI] [PubMed] [Google Scholar]
- Rosen DAS, Worthy GAJ (2018) Nutrition and energetics. In CRC Handbook of Marine Mammal Medicine. CRC Press, Boca Raton, FL, pp. 695–738. [Google Scholar]
- Rourke ML, Fowler AM, Hughes JM, Broadhurst MK, DiBattista JD, Fielder S, Wilkes Walburn J, Furlan EM (2022) Environmental DNA (eDNA) as a tool for assessing fish biomass: a review of approaches and future considerations for resource surveys. Environ DNA 4: 9–33. [Google Scholar]
- Ruesch A, McKnight JC, Fahlman A, Shinn-Cunningham BG, Kainerstorfer JM (2022) Near-infrared spectroscopy as a tool for marine mammal research and care. Front Physiol 12: 816701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoca MS, Czapanskiy MF, Kahane-Rapport SR, Gough WT, Fahlbusch JA, Bierlich KC, Segre PS, Di Clemente J, Penry GS, Wiley DNet al. (2021) Baleen whale prey consumption based on high resolution foraging measurements. Nature 599: 85–90. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hock R, Walters V, Irving L (1950) Adaptation to cold in Arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic rate. Biol Bull 99: 259–271. [DOI] [PubMed] [Google Scholar]
- Schulz TM, Bowen WD (2004) Pinniped lactation strategies: evaluation of data on maternal and offspring life history traits. Mar Mamm Sci 20: 86–114. [Google Scholar]
- Schwarz LK, Goebel ME, Costa DP, Kilpatrick AM (2013) Top-down and bottom-up influences on demographic rates of Antarctic fur seals Arctocephalus gazella. J Anim Ecol 82: 903–911. [DOI] [PubMed] [Google Scholar]
- Senigaglia V, Christiansen F, Bejder L, Gendron D, Lundquist D, Noren DP, Schaffar A, Smith JC, Williams R, Martinez Eet al. (2016) Meta-analyses of whale-watching impact studies: comparisons of cetacean responses to disturbance. Mar Ecol Prog Ser 542: 251–263. [Google Scholar]
- Shero MR, Dale J, Seymour AC, Hammill MO, Mosnier A, Mongrain S, Johnston DW (2021) Tracking wildlife energy dynamics with unoccupied aircraft systems and 3-dimensional photogrammetry. Methods Ecol Evol. 12: 2458–2472. [Google Scholar]
- Shuert CR, Halsey LG, Pomeroy PP, Twiss SD (2020) Energetic limits: defining the bounds and trade-offs of successful energy management in a capital breeder. J Anim Ecol 89: 2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler MF, Gende SM, Csepp DJ (2017) Association of foraging Steller sea lions with persistent prey hots spots in southeast Alaska. Mar Ecol Prog Ser 571: 233–243. [Google Scholar]
- Silber GK, Lettrich MD, Thomas PO, Baker JD, Baumgartner M, Becker EA, Boveng P, Dick DM, Fiechter J, Forcada Jet al. (2017) Projecting marine mammal distribution in a changing climate. Front Mar Sci 4: 413. [Google Scholar]
- Silva WTAF, Harding KC, Marques GM, Bäcklin BM, Sonne C, Dietz R, Kauhala K, Desforges JP (2020) Life cycle bioenergetics of the gray seal (Halichoerus grypus) in the Baltic Sea: population response to environmental stress. Environ Int 145: 106145. [DOI] [PubMed] [Google Scholar]
- Smith C (2021) A warming Southern Ocean may compromise Antarctic blue whale foetus growth. J Vertebr Biol 70: 20114.1-11. [Google Scholar]
- Smout S, King R, Pomeroy P (2020) Environment-sensitive mass changes influence breeding frequency in a capital breeding marine top predator. J Anim Ecol 89: 384–396. [DOI] [PubMed] [Google Scholar]
- Soto KH, Trites AW, Arias-Schreiber M (2004) The effects of prey availability on pup mortality and the timing of birth of South American sea lions (Otaria flavescens) in Peru. J Zool 264: 419–428. [Google Scholar]
- Stewart JD, Durban JW, Fearnbach H, Barrett-Lennard LG, Casler PK, Ward EJ, Dapp DR (2021a) Survival of the fattest: linking body condition to prey availability and survivorship of killer whales. Ecosphere 12: e03660. [Google Scholar]
- Stewart JD, Durban JW, Knowlton AR, Lynn MS, Fearnbach H, Barbaro J, Perryman WL, Miller CA, Moore MJ (2021b) Decreasing body lengths in North Atlantic right whales. Curr Biol 31: 3174–3179. [DOI] [PubMed] [Google Scholar]
- Stirling I, Derocher AE (2012) Effects of climate warming on polar bears: a review of the evidence. Glob Chang Biol 18: 2694–2706. [DOI] [PubMed] [Google Scholar]
- Straley JM, Moran JR, Boswell KM, Vollenweider JJ, Heintz RA, Quinn TJ, Witteveen BH, Rice SD (2018) Seasonal presence and potential influence of humpback whales on wintering Pacific herring populations in the Gulf of Alaska. Deep Res Part II Top Stud Oceanogr 147: 173–186. [Google Scholar]
- Sutherland WJ, Atkinson PW, Butchart SHM, Capaja M, Dicks LV, Fleishman E, Gaston KJ, Hails RS, Hughes AC, Le Anstey Bet al. (2022) A horizon scan of global biological conservation issues for 2022. Trends Ecol Evol 37: 95–104. [DOI] [PubMed] [Google Scholar]
- Sveegaard S, Nabe-Nielsen J, Stæhr KJ, Jensen TF, Mouritsen KN, Teilmann J (2012) Spatial interactions between marine predators and their prey: herring abundance as a driver for the distributions of mackerel and harbour porpoise. Mar Ecol Prog Ser 468: 245–253. [Google Scholar]
- Tennessen JB, Holt MM, Hanson MB, Emmons CK, Giles DA, Hogan JT (2019) Kinematic signatures of prey capture from archival tags reveal sex differences in killer whale foraging activity. J Exp Biol 222: jeb191874. [DOI] [PubMed] [Google Scholar]
- Trillmich F, Kooyman GL (2001) Field metabolic rate of lactating female Galápagos fur seals (Arctocephalus galapagoensis): the influence of offspring age and environment. Comp Biochem Physiol Part A 129: 741–749. [DOI] [PubMed] [Google Scholar]
- Trillmich F, Limberger D (1985) Drastic effects of El Niño on Galapagos pinnipeds. Oecologia 67: 19–22. [DOI] [PubMed] [Google Scholar]
- Udevitz MS, Jay CV, Taylor RL, Fischbach AS, Beatty WS, Noren SR (2017) Forecasting consequences of changing sea ice availability for Pacific walruses. Ecosphere 8: e02014. [Google Scholar]
- Vera-Massieu C, Brock PM, Godínez-Reyes C, Acevedo-Whitehouse K (2015) Activation of an inflammatory response is context-dependent during early development of the California sea lion. R Soc Open Sci 2: 150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Amtmann S, McDonald BI, Páez-Rosas D, Aurioles-Gamboa D, Costa DP (2017a) Adapted to change: low energy requirements in a low and unpredictable productivity environment, the case of the Galapagos sea lion. Deep Res Part II 140: 94–104. [Google Scholar]
- Villegas-Amtmann S, Schwarz L, Gailey G, Sychenko O, Costa D (2017b) East or west: the energetic cost of being a gray whale and the consequence of losing energy to disturbance. Endanger Species Res 34: 167–183. [Google Scholar]
- Villegas-Amtmann S, Schwarz LK, Sumich JL, Costa DP (2015) A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere 6: 183. [Google Scholar]
- Visser F, Merten VJ, Bayer T, Oudejans MG, De Jonge DSW, Puebla O, Reusch TBH, Fuss J, Hoving HJT (2021) Deep-sea predator niche segregation revealed by combined cetacean biologging and eDNA analysis of cephalopod prey. Sci Adv 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser SK, Lundin JI, Ayres K, Seely E, Giles D, Balcomb K, Hempelmann J, Parsons K, Booth R (2017) Population growth is limited by nutritional impacts on pregnancy success in endangered southern resident killer whales (Orcinus orca). PLoS One 12: e0179824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe YY, Goldbogen JA (2021) Too big to study? The biologging approach to understanding the behavioural energetics of ocean giants. J Exp Biol 224: jeb202747. [DOI] [PubMed] [Google Scholar]
- Watwood SL, Miller PJO, Johnson M, Madsen PT, Tyack PL (2006) Deep-diving foraging behaviour of sperm whales (Physeter macrocephalus). J Anim Ecol 75: 814–825. [DOI] [PubMed] [Google Scholar]
- Wells RS, Allen JB, Hofmann S, Bassos-Hull K, Fauquier DA, Barros NB, DeLynn RE, Sutton G, Socha V, Scott MD (2008) Consequences of injuries on survival and reproduction of common bottlenose dolphins (Tursiops truncatus) along the west coast of Florida. Mar Mamm Sci 24: 774–794. [Google Scholar]
- Wells RS, Smith CR, Sweeney JC, Townsend FI, Fauquier DA, Stone R, Langan J, Schwacke LH, Rowles TK (2014) Fetal survival of common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Aquat Mamm 40: 252–259. [Google Scholar]
- Wheatley KE, Bradshaw CJA, Davis LS, Harcourt RG, Hindell MA (2006) Influence of maternal mass and condition on energy transfer in Weddell seals. J Anim Ecol 75: 724–733. [DOI] [PubMed] [Google Scholar]
- Williams R, Krkošek M, Ashe E, Branch TA, Clark S, Hammond PS, Hoyt E, Noren DP, Rosen D, Winship A (2011) Competing conservation objectives for predators and prey: estimating killer whale prey requirements for Chinook salmon. PLoS One 6: e26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Vikingsson GA, Gislason A, Lockyer C, New L, Thomas L, Hammond PS (2013) Evidence for density-dependent changes in body condition and pregnancy rate of North Atlantic fin whales over four decades of varying environmental conditions. ICES J Mar Sci 70: 1273–1280. [Google Scholar]
- Williams TM, Blackwell SB, Richter B, Sinding MHS, Heide-Jørgensen MP (2017) Paradoxical escape responses by narwhals (Monodon monoceros). Science 358: 1328–1331. [DOI] [PubMed] [Google Scholar]
- Williams TM, Peter-Heide Jørgensen M, Pagano AM, Bryce CM (2020) Hunters versus hunted: New perspectives on the energetic costs of survival at the top of the food chain. Funct Ecol 34: 2015–2029. [Google Scholar]
- Williams TM, Yeates L (2004) The energetics of foraging in large mammals: a comparison of marine and terrestrial predators. Int Congr Ser 1275: 351–358. [Google Scholar]
- Wilson RP, Börger L, Holton MD, Scantlebury DM, Gómez-Laich A, Quintana F, Rosell F, Graf PM, Williams H, Gunner Ret al. (2020) Estimates for energy expenditure in free-living animals using acceleration proxies: a reappraisal. J Anim Ecol 89: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship A, Trites A, Rosen D (2002) A bioenergetic model for estimating the food requirements of Steller sea lions Eumetopias jubatus in Alaska, USA. Mar Ecol Prog Ser 229: 291–312. [Google Scholar]
- Wirsing AJ, Heithaus MR, Frid A, Dill LM (2008) Seascapes of fear: evaluating sublethal predator effects experienced and generated by marine mammals. Mar Mamm Sci 24: 1–15. [Google Scholar]
- Wisniewska DM, Johnson M, Teilmann J, Rojano-Doñate L, Shearer J, Sveegaard S, Miller LA, Siebert U, Madsen PT (2016) Ultra-high foraging rates of harbor porpoises make them vulnerable to anthropogenic disturbance. Curr Biol 26: 1441–1446. [DOI] [PubMed] [Google Scholar]
- Yasumiishi EM, Cieciel K, Andrews AG, Murphy J, Dimond JA (2020) Climate-related changes in the biomass and distribution of small pelagic fishes in the eastern Bering Sea during late summer, 2002–2018. Deep Res Part II Top Stud Oceanogr 181–182: 104907. [Google Scholar]
- Zerbini AN, Friday NA, Palacios DM, Waite JM, Ressler PH, Rone BK, Moore SE, Clapham PJ (2016) Baleen whale abundance and distribution in relation to environmental variables and prey density in the Eastern Bering Sea. Deep Res Part II Top Stud Oceanogr 134: 312–330. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original submitted questions and final collated questions are included in supplemental material. There are no other data associated with this article.