Abstract
3,4-Methylenedioxypyrovalerone (MDPV) is a selective catecholamine reuptake inhibitor abused for its psychostimulant properties. This study examined if MDPV administration alters impulsive choice measured by delay discounting in rats. Three groups of rats were tested in daily delay discounting sessions to determine the effects of acute cocaine (1.0–30.0 mg/kg), MDPV (0.1–3.0 mg/kg), or saline on mean adjusted delay (MAD). Dose-dependent decreases in MAD were elicited only by acute MDPV, which also suppressed operant responding at the highest dose. Next, rats received post-session injections (30.0 mg/kg cocaine, 3.0 mg/kg MDPV, or saline) every other day for a total of 10 injections. MAD increased during saline treatment, did not change during cocaine treatment, and was reduced during MDPV treatment. In dose-effect re-determinations, no acute drug effects on MAD were observed, but compared to the initial dose-effect determination, MDPV suppressed operant responding in more animals, with zero animals completing trials at the highest dose. All saline and MDPV-treated subjects were sacrificed, and striatal and cortical dopamine levels were quantified by HPLC. These studies indicate that administration of MDPV may increase impulsive choice acutely and persistently. These proimpulsive effects are possibly mediated by increases in striatal dopamine turnover.
Keywords: addiction medicine; bath salts; delay discounting; impulsivity; 3,-methylenedioxypyrovalerone; substance abuse
Introduction
In recent years, novel synthetic drugs have increasingly been found in abuse-ready products, and one of the largest categories are the synthetic cathinone (SC) analogues which are commonly sold as ‘bath salts’. In 2017, the United Nations Office on Drugs and Crime (UNODC) Global Synthetic Drugs Assessment reported that 19% of new psychoactive substances identified to fall under this category (United Nations Office on Drugs and Crime, 2017). This report also indicated that the SC 3,4-methylenedioxypyrovalerone (MDPV) continues to be detected in drug seizures despite international legislative efforts to control its availability. Previous studies have shown that MDPV is a highly potent, catecholamine-selective reuptake inhibitor (Simmler et al., 2013; Iversen et al., 2014; Glennon and Young, 2016) that elicits behavioral effects similar to classical psychostimulants in vitro (Baumann et al., 2013; Kolanos et al., 2013) and in vivo (Fantegrossi et al., 2013; Watterson et al., 2014). Of particular concern are the large number of reports from emergency department visits and calls to poison control centers that reflect incidences of adverse drug events from MDPV use, confirmed by clinical and forensic screens of blood and urine (Spiller et al., 2011; Murray et al., 2012; Wright et al., 2013; Uralets et al., 2014). Acute neurologic and cardiovascular toxicity remain the primary causes of morbidity and mortality associated with MDPV use (Borek and Holstege, 2012; Sivagnanam et al., 2013), but self-harm and accidents through risky behavior have also been documented (Ross et al., 2012; John et al., 2014). Other dopaminergic drugs of abuse, such as cocaine and amphetamine, increase health-risk behaviors that result in unintentional injuries and violence, risky sexual behaviors that lead to sexually transmitted diseases such as HIV infection, additional drug use, as well as unhealthy dietary behaviors and inadequate physical activity (Kalivas and Mcfarland, 2003; Latt et al., 2011; Ersche et al., 2013; Gannon et al., 2014). These changes are more prevalent in chronic users, and the amount of drug abused correlates to the magnitude of increased risk (Harlé et al., 2015; Rodríguez-Cintas et al., 2016).
Increased impulsivity has been demonstrated to be an integral risk factor for health-risk behaviors, including abuse of drugs, and drug use has been found to further increase impulsive choice from baseline levels (Bakhshani, 2014; Grant and Chamberlain, 2014; Gray and Mackillop, 2015; Belin et al., 2016). Mesocorticolimbic dopamine projections mediate trait impulsivity and are involved in reward-motivated behaviors, cognitive control, and emotional responses (Chen et al., 2009; Grant and Chamberlain, 2014; Trifilieff and Martinez, 2014). In drug abuse and addiction, impulsivity and other abuse-related behaviors can be affected by drug-induced modifications to dopamine neurocircuitry that modulates functional interactions between the striatum and other brain regions (Ahmadlou et al., 2013; Cole et al., 2013; Konova et al., 2013). Indeed, there is a large body of literature in both animals (Logue et al., 1992; Paine et al., 2003; Roesch et al., 2007) and humans (Richards et al., 1999; Coffey et al., 2003; García-Marchena et al., 2018) indicating that psychostimulants such as cocaine have proimpulsive effects involving these systems following both acute administration and after prolonged use. However, while it is known that MDPV shares a mechanism of action with cocaine (Baumann et al., 2013), and recent evidence indicates that MDPV downregulates DAT in the striatum and produces lasting alterations in DA signaling (Colon-Perez et al., 2018), proimpulsive effects of MDPV have never before been determined.
Thus, in this studies, we investigated how impulsive choice measured by a delay discounting (DD) task in rats is modulated by either acute, pre-session MDPV administration, or by prolonged exposure to MDPV. In separate rats, identical studies were performed with the mechanistically-similar psychostimulant cocaine, and a saline control group was also used. In a DD task, the subjective value of a reward is discounted if its delivery is delayed, and the amount of delay an organism will tolerate is a commonly-used measure of impulsivity in both humans (reviewed by Reynolds, 2006) and animals (reviewed by Perry and Carroll, 2008), making the DD procedure highly translatable across species (reviewed by Hamilton et al., 2015). Importantly, methodological differences in DD may result in discrepant findings. This can be demonstrated most notably in increasing delay procedures, where the arrangement of prior delays may impact overall estimations of sensitivities to delays (Maguire et al., 2014; Tanno et al., 2014). In this studies, we used an adjusting delay procedure, whereby rats titrated to their own stable delay. The amount by which the delay changes is an important experimental consideration. When large changes (30% increases or decreases) between delays are used, an adjusting delay may never stabilize, such as evidenced in Cardinal et al. (2002). However, Perry et al. (2005), demonstrated that if small, 1 s changes are used, an adjusting delay will reliably stabilize and can predict drug abuse liability behavior like intravenous self-administration. Thus, we used a procedure modified from Perry et al. (2005) in our current experiments.
Following the completion of all phases of the DD experiments, rats from the saline and MDPV groups were sacrificed and monoamine levels were determined in selected brain regions. The major finding of these studies is that acute administration of MDPV to drug-naive rats dose-dependently increased impulsive choice measured by DD, and prolonged MDPV exposure persistently increased impulsive choice even in the absence of the drug, perhaps due to lasting neurochemical changes characterized by increased striatal dopamine turnover.
Methods
Animals
All experiments were conducted in adult male Sprague-Dawley rats (Charles River Breeding Labs) weighing 220–240 g on delivery. Animals were housed two per cage (23.75 × 45.40 × 17.78 cm3) and were drug-naive prior to beginning the present studies. All animals were housed in a temperature-controlled and humidity-controlled vivarium in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility. Room conditions were maintained at 22 ± 2°C and 45–50% humidity, with lights set to a 12/12 h light/dark cycle. During experimentation, rats were maintained at 85–90% of their free-feed weight based on vendor published growth curves by supplemental feeding (Lab Diet rodent chow Laboratory Rodent Diet no. 5001; PMI Feeds, St Louis, Missouri, USA) 1 h following experimental sessions. Water was available ad libitum in the home cages but was not available during behavioral testing. All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
Drugs
All injections were administered subcutaneously at a volume of 1.0 ml/kg, except for the 10 chronic cocaine injections, which were administered intraperitoneally to reduce the formation of necrotic lesions associated with subcutaneously cocaine administration. ( − )-Cocaine was obtained from the National Institute on Drug Abuse Drug Supply Program, and racemic MDPV was synthesized by one of the authors (K.C.R.) in the Laboratory of Medicinal Chemistry at the National Institute on Drug Abuse (Bethesda, Maryland, USA). All drugs were weighed as HCl salts and dissolved in 0.9% physiological saline. Because cocaine is reliably 10-fold less potent than MDPV in terms of discriminative stimulus effects (Gannon et al., 2016; Berquist and Baker, 2017), reinforcing effects (Schindler et al., 2016; Gannon et al., 2017), aversive effects (Woloshchuk et al., 2016) and locomotor stimulant effects (Marusich et al., 2012; Baumann et al., 2017) in rodents, the dose range tested for cocaine in this studies was 10-fold higher than the dose range tested for MDPV.
Delay discounting
Three groups of rats [n = 12 cocaine and MDPV, n = 11 saline (see Data analysis section below)] were randomly assigned to treatment groups. All animals were trained in custom built operant chambers (model ENV-008-VP; Med Associates) that were enclosed in light-attenuating and sound-attenuating cabinets (model ENV-018M, Med Associates, Fairfax, VT, USA). Each chamber was equipped with two nose-poke keys with integrated stimulus lights, two accessory stimulus lights located above the nose-poke keys, a pump and liquid food dispenser, auditory feedback including a tone and clicker, a house light located near the chamber ceiling on the back wall, and an exhaust fan for air circulation and masking of ambient laboratory noise. The liquid food dispenser was centered between the two nose-poke keys and delivered a palatable liquid reinforcer (evaporated milk diluted 1: 1 with water). All programming and recording were controlled through a MED Associates interface with MED-PC software (V5; Med Associates, Inc, Fairfax, Vermont, USA) by a computer located in an adjacent room.
Nose-poke training
Responses on nose-poke keys were initially reinforced on a fixed ratio 1 (FR1) schedule with 3 s of milk access. Daily sessions lasted for 60 min or until 60 reinforcers were earned, whichever occurred first, and every 20th earned reinforcer increased the FR by one, up to a terminal FR3. The onset of a tone and illumination of house lights signaled reinforcer availability. During training, stimulus lights inside the response keys were illuminated, but only one key was active and delivered reinforcement following completion of the response requirement. Upon successful completion of a daily session on one active key, the active key was switched to the opposite side for the next session. Training in this manner was used to attenuate side bias and resulted in each animal entering the DD experiments with a history of receiving roughly equivalent reinforcement on each of the two nose-poke keys.
Stabilization of mean adjusted delay
Following stable and reliable responding on the nose-poke keys, animals were trained in a mean adjusted delay (MAD) task. Each daily session was preceded by a 10 min timeout period where both the house lights and all stimulus lights were off. Daily sessions lasted for 60 min or until 60 reinforcers were earned, whichever occurred first, and no limited hold was associated with reinforcer availability. An inter-trial interval lasting 10 s where all lights were turned off followed delivery of each reinforcer. Sessions consisted of up to 15 blocks, each containing two forced-choice trials followed by two free choice trials. During forced-choice trials, only one key was active at a time, first the left, then the right, allowing rats to sample reinforcement contingencies across response options. During free choice trials, both keys were active and rats could respond on either key. The left key in the operant chamber always delivered the ‘sooner smaller’ reinforcer of 3 s milk access, presented immediately after completing the response requirement. A ‘larger later’ (LL) reinforcer of four discrete 3 s milk presentations following a variable delay, signaled by the illumination of both accessory stimulus lights located above the response keys, was delivered after responses on the right key. There was no programmed consequence for responses during the delays. Following the completion of response requirements on either key, the house lights were turned on for the duration of reinforcement delivery, and a tone was paired with each 3 s milk presentation. Delay of the LL was initially set at 3 s and was changed in 1 s increments based on free choice key selection across a session. During a free choice block, two sooner smaller key choices decreased the delay by 1 s, two LL key choices increased the delay by 1 s, and one choice on each key resulted in no change to the delay length for the next block. At the end of each session, the delay across each block was averaged and calculated as the MAD for the day, and this value was used as the starting delay on the LL key during the next session. Sessions continued until the MAD stabilized with less than 5 s difference for 5 consecutive days, with a slope between points not statistically different from 0, thus ensuring no increasing or decreasing trends in delay.
Experimental timeline
Following stabilization of the MAD, animals proceeded to test. All test sessions were identical to stabilization sessions, but subjects received various treatments either before or after the experimental session. The first test condition, prior to any drug treatment, was a ‘satiety challenge’ where animals were fed their daily allowance of food 1 h prior to a DD session. This manipulation was performed to possibly assess the role anorectic effects of cocaine or MDPV administration on impulsive choice. Following this, animals underwent 5 days of restabilization prior to a dose-effect determination of either MDPV (0.1, 0.3, 1.0, and 3.0 mg/kg), cocaine (1.0, 3.0, 10.0, and 30.0 mg/kg), or saline (repeated injections of 1 ml/kg) based on their experimental group. One injection was administered each day, consecutively, 10 min prior to the start of experimental sessions, beginning with the lowest dose and increasing in quarter log units. Following dose-effect determinations, MADs were again allowed to stabilize for 5 days without drug or saline administration, then a ‘chronic exposure’ dose regimen was initiated, with animals receiving an alternating injection of the highest dose from the dose-effect determination of their treatment drug (3.0 mg/kg MDPV, 30.0 mg/kg cocaine, or saline) every other day, for a total of 10 drug injections. On nontreatment days, all groups were injected with saline (1 ml/kg). All of these injections were administered 2 h after behavioral testing. After the 10th injection, another 5 days of washout and restabilization were imposed prior to a final acute dose-effect determination repeated exactly the same as described previously.
Brain collection
Following the final DD session, MDPV and saline rats underwent a 10-day drug washout period. After the final day of the washout, animals were anesthetized using inhaled isoflurane and euthanized by decapitation. Brains from all animals were rapidly removed and dissected on ice into sections containing hippocampus, cortex, striatum, and cerebellum, and then flash frozen in liquid nitrogen, and stored at − 80°C until testing. Brain tissue from cocaine animals was collected at a different timepoint following different manipulations, and would not be comparable to the MDPV-treated and saline-treated rats, so neurochemistry data from the cocaine-exposed subjects are not presented here.
Neurochemical profiling
Frozen tissues were weighed then sonically disrupted in 200 μl of 200 mmol/l HClO4 before being centrifuged for 10 min at 13 000 rpm at 4°C to remove cellular debris. Next, the samples were filtered through 0.22 μ Thermo Scientific Titan3 (Waltham, Massachusetts, USA). Cellulose Acetate Syringe Filters. A 100 μl aliquot of the supernatant was placed in a WPS-3000TBSL autosampler maintained at 10°C and 10 μl were injected onto a Thermo Scientific Hypersil BDS C18 column using a Thermo Scientific Dionex Test Phase as the mobile phase (flow rate of 0.5 ml/min). Coulometric detection was accomplished with a Thermo Scientific Dionex 6011RS ultra Coulometric Analytical cell and the signal was analyzed using Thermo Scientific Chromeleon 7.2 Chromatography Data System software. Absolute tissue concentrations (ng/mg) for dopamine, serotonin, and norepinephrine was determined by comparison with external standard curves and corrected for tissue weight. Identical procedures were used to quantify concentrations of the metabolites of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and of serotonin, 5-hydroxyindoleacetic acid (5-HIAA).
Data analysis
Delay discounting
Results of the DD experiments were expressed either as the mean (±SEM) percent of MAD during stability or as the mean (± SEM) of the raw MAD in seconds. Percent stability MAD was determined for each animal, then averaged, and was calculated by the formula:
A Grubbs’ test (ESD method) was performed within each treatment group to detect and exclude any outliers present. To do this, the total %stability MAD for each animal during all experimental conditions following stability was summed and processed through the GraphPad QuickCalcs Grubbs’ test. One of the 12 rats from the saline group was found to be a significant outlier (i.e. > 2 SD from the global mean of all rats in all groups) and was thus excluded from the study, resulting in an n of 11 for this control group. Sessions to stability, as well as the MAD during stability, were each analyzed between treatment groups using a one-way analysis of variance (ANOVA) followed by a Tukey’s multiple comparisons test. Differences between MAD during stability and following the satiety challenge were analyzed using paired t-tests within each treatment group. MAD values obtained during the prolonged treatment phase, composed of intermittent repeated exposure, were analyzed with linear regression analysis, followed by a one-way ANOVA and Tukey’s multiple comparisons to detect differences between slopes. The initial saline injections, which were used as controls for acute drug administration, were compared using one-way ANOVAs and Tukey’s multiple comparisons test. Dose-effect curves were analyzed by performing a linear regression of %stability MAD, ending with the highest completed dose (e.g. animals that showed suppressed responding at 3 mg/kg MDPV had linear regressions run from 0.1 to 1.0 mg/kg). Results are displayed as the mean slope. If the slope was negative, this was interpreted as dose-dependent changes to the MAD. Suppression of operant responding was defined as animals not completing all 15 blocks within a session. Difference scores between groups were calculated by subtracting the %stability MAD of acute saline administration when drug-naive, from the %stability MAD following repeated administration of their assigned treatment. Difference scores were analyzed by a one-way ANOVA followed by a Tukey’s multiple comparisons test.
Neurochemical profiling
All ex-vivo results are expressed as mean (± SEM) density displayed as ng of parent monamine over mg of tissue, or as or as the mean (± SEM) ratio of metabolite over the parent compound. Analysis of all ex-vivo results was carried out using unpaired t-tests.
Statistical significance was declared at P value less than 0.05 for all statistical analyses. All statistical analyses and creation of figures were performed using GraphPad Prism software (V7.03; GraphPad Software Inc., La Jolla, California, USA).
Results
Delay discounting
The number of sessions required to reach stability criteria was not significantly different between groups [F(2, 32) = 2.08, P > 0.05] (Table 1, left section) and no significant differences in MAD values during stability were observed among groups [F(2, 32) = 1.09, P > 0.05]. A wide range of MADs was observed in animals within each group (Supplementary Fig. 1, Supplemental digital content 1, http://links.lww.com/BPHARM/A28). The average MAD following the satiety challenge was found to be significantly reduced compared to the average MAD at stability for the saline [t(10) = 3.146, P = 0.0104], cocaine [t(11) = 3.32, P = 0.0068], and MDPV [t(11) = 5.588, P = 0.0002] groups (see Table 1, right side, for the average MAD at stability and the average MAD during the satiety challenge). Figure 1 displays that the MAD decreases in most animals following the satiety challenge (black circles), compared to the MAD measured at stability (white circles). Despite the reductions in the MAD observed during the satiety challenge, all rats earned all available reinforcers during the test, and MAD rapidly returned to ~ 100% of the %stability MAD during a 5-day recovery for each group following the satiety challenge.
Table 1.
Average number of days ( ± SEM) to reach stability criteria, and the average mean adjusted delay ( ± SEM) at stability and during the satiety challenge for all treatment groups
| Groups | Days to stability ( ± SEM) | Average mean adjusted delay: stability ( ± SEM) | Average mean adjusted delay: satiety challenge ( ± SEM) |
|---|---|---|---|
| Saline | 42.36 ( ± 3.80) | 12.07 ( ± 0.74) | 9.66 ( ± 1.13)* |
| Cocaine | 39.50 ( ± 0.38) | 14.59 ( ± 1.55) | 12.09 ( ± 1.58)* |
| 3,4-Methylenedioxypyrovalerone | 44.66 ( ± 1.57) | 10.97 ( ± 1.30) | 7.61 ( ± 1.17)* |
No significant differences were detected either between groups in the days to reach stability, or in the mean adjusted delay values during stability between groups.
Significant differences were detected when comparing the mean adjusted delay during stability and the satiety challenge within a group.
P< 0.05 compared to mean adjusted delay during stability.
Fig. 1.
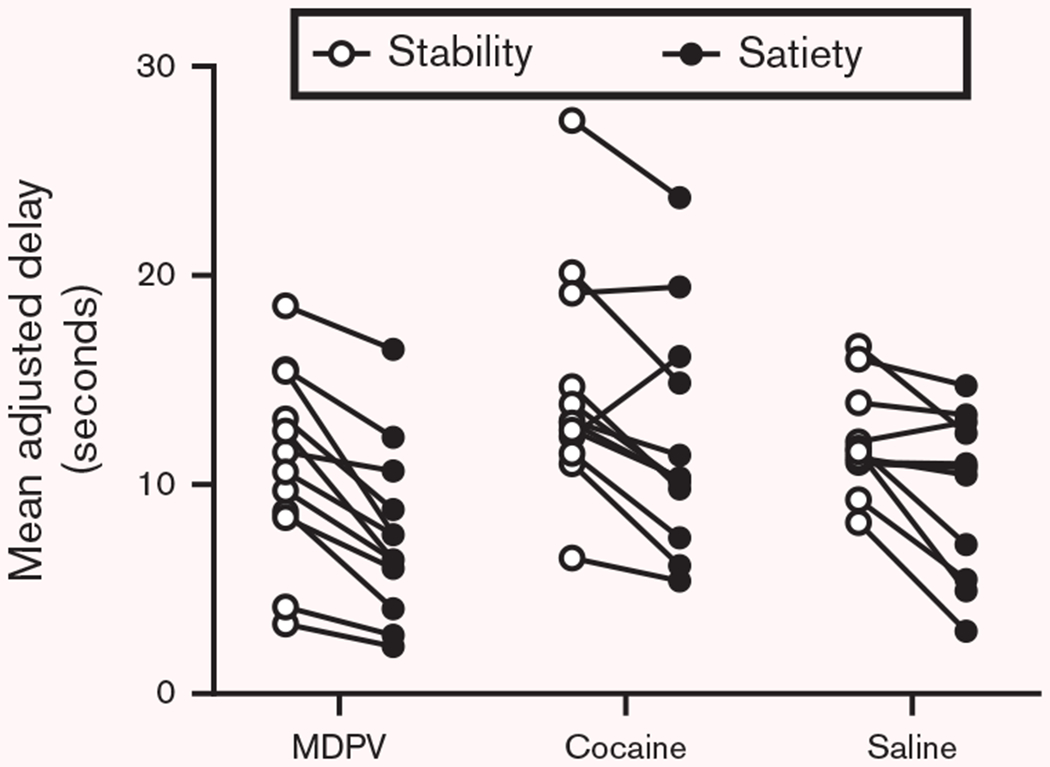
Effects of satiation (black circles) on MAD compared to MAD during stability (white circles). Abscissa: treatment group. Ordinate: raw MAD displayed in seconds. For most animals assessed within each group, the satiety challenge decreased the MAD compared to stability, and the group mean MAD following the satiety challenge was significantly different than the MAD during stability within each treatment group (see Results). MDPV, 3,4-methylenedioxypyrovalerone.
Figure 2 displays the %stability MAD for acute testing sessions wherein animals were drug-naive (left panel), or following repeated exposure (right panel). Initial %stability MAD values for all groups were not statistically different [F(2, 32)=0.3134, P > 0.05] when tested with acute saline injection, with all groups exhibiting %stability MAD values at ~ 100% of control (Fig. 2, left panel, first SAL point for all groups). Further acute injections of saline did not alter impulsive choice (Fig. 2, left panel, white circles), as saline-treated animals maintained stable MAD values at ~ 100% of control across repeated injections with a slope of ~ 0.60 as a function of injection number. Acute administration of various doses of cocaine (Fig. 3, left panel, grey squares) also did not alter impulsive choice across the doses tested, with a calculated slope of − 0.56. However, injections of various doses of MDPV (Fig. 2, left panel, black triangles) decreased %stability MAD, with a calculated slope of −18.86, and suppressed operant responding in 75% of the animals at the highest dose. Once again, each group’s MAD returned to ~ 100% of the %stability MAD during a 5-day recovery period.
Fig. 2.
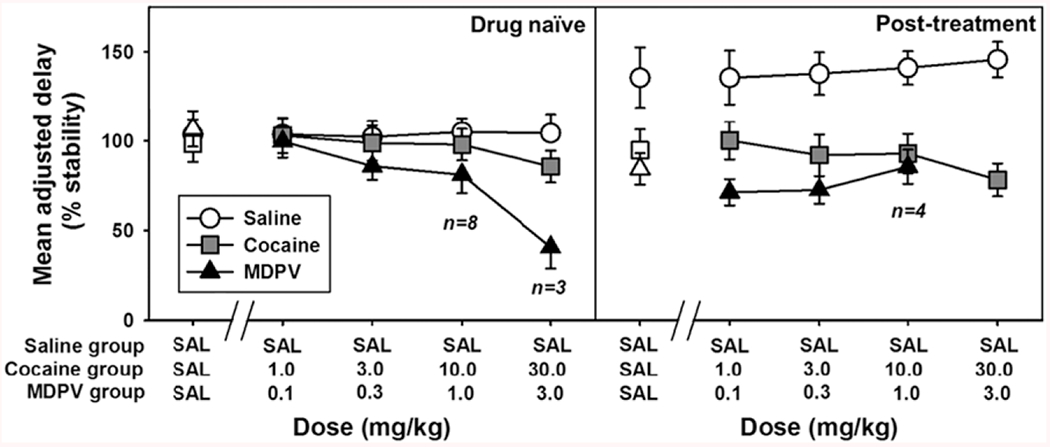
Effects of daily, acute administration of escalating doses of cocaine or MDPV, or repeated saline injection, on the MAD of rats when drug-naïve (left) or following prolonged treatment of 3.0 mg/kg MDPV or 30.0 mg/kg cocaine, or saline (right). Abscissa: dose of drug (mg/kg), or saline (ml/kg), based on treatment group assigned. Ordinate: MAD expressed as a percent of the stability criteria. Group sizes below points indicate the number of subjects in the MDPV group that completed the session, at that dose. MDPV, 3,4-methylenedioxypyrovalerone.
Fig. 3.
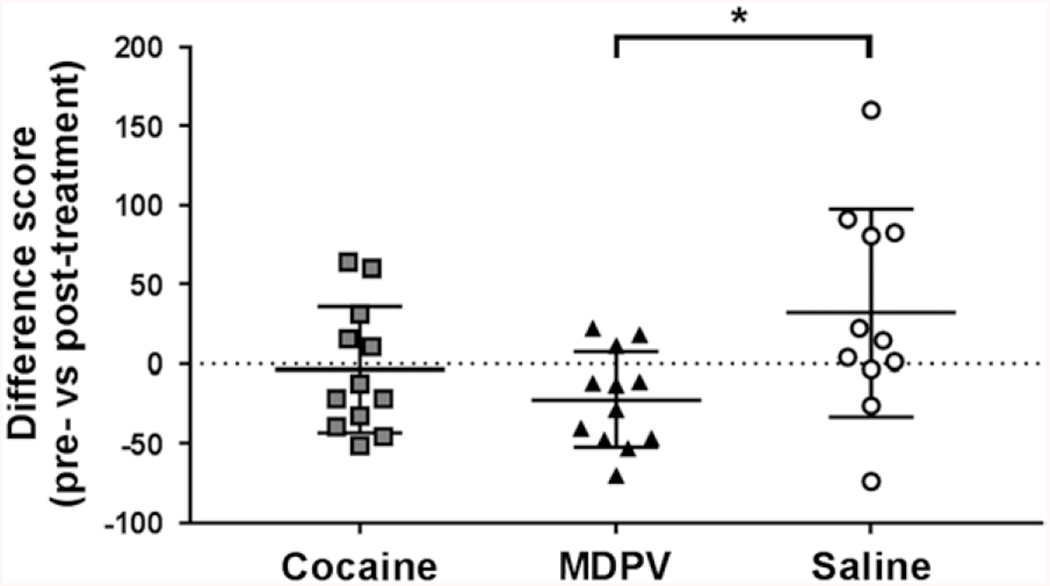
Difference scores between the % stability MAD of animals following acute saline when drug-naïve, or after prolonged administration of their assigned treatment. Abscissa: treatment group. Ordinate: Difference score measured as pre-treatment (drug-naïve) acute saline % stability MAD subtracted from the post-treatment (group assigned) acute saline % stability MAD. Significant differences were found between the MDPV and saline groups, but no differences were seen among either the MDPV and cocaine group, or the cocaine and saline group. *P < 0.05 compared to saline. MDPV, 3,4-methylenedioxypyrovalerone.
MAD was recorded during the chronic treatment (see Supplementary Fig. 2, Supplemental digital content 1, http://links.lww.com/BPHARM/A28, for %stability MAD during the chronic treatment phase and the subsequent 5 day washout), where trends in %stability MAD were observed as evidenced by statistically significant differences in the slopes (saline estimated slope = 0.65; cocaine estimated slope = 1.13; MDPV estimated slope = − 1.19) [F(2, 48) = 11, P = 0.0001]. These data were measured from the first post-session drug treatment to the final treatment (19 days). The slope of the MDPV group was negative and found to be statistically different from the saline group [q(32) = 4.862, P = 0.0046] and the cocaine group [q(32) = 6.273, P = 0.0003], but there was no difference found between the cocaine and saline groups [q(32) = 1.273, P > 0.05]. Following the repeated, post-session injections of saline, 30.0 mg/kg cocaine or 3.0 mg/kg MDPV, differences emerged between the treatment groups when tested with acute saline injection. Compared to the initial dose-effect determination, the %stability MAD was increased in the saline control group (Fig. 2, right panel, first white circle), remained relatively unchanged in the cocaine group (Fig. 2, right panel, white square), and was reduced in the MDPV group (Fig. 2, right panel, white triangle). These differences were found to be statistically significant [F(2, 32) = 1.257, P = 0.0194] between the saline and MDPV groups [q(32) = 4.024, P = 0.0204], but not between the saline and cocaine groups [q(32) = 3.204, P > 0.05], or the cocaine and MDPV groups [q(32) = 0.8392, P > 0.05].
Following the chronic treatment phase, successive injections of saline (to control for repeated handling and injections in the cocaine and MDPV dose-effect redeterminations) for the saline treatment group did not systematically increase or decrease MAD values (Fig. 2, right panel, white circles), indicated by a calculated slope of 1.70. Discrete administrations of 1.0, 3.0 and 10.0 mg/kg cocaine doses did not alter %stability MAD lower than 90% of control, although injection of 30.0 mg/kg cocaine reduced MAD to 78.21 ± 9.13% (Fig. 3, right panel, grey squares). However, the slope of the linear regression calculated from cocaine data was ~ 0 (− 0.66). Across increasing doses of MDPV, the group %stability MAD was relatively unchanged compared to the initial saline injection (Fig. 2, right panel, black triangles), with a slope calculated at 1.65, but the suppression of operant responding elicited by MDPV appeared to be potentiated, such that only four of the 12 animals tested met criteria for a completed session at 1.0 mg/kg acute MDPV, and no animals completed the session at the highest dose tested.
Figure 3 displays the difference scores of discrete saline injection challenges for each group, calculated from the %stability MAD between animals within a group when drug-naive, or following repeated injections of their assigned treatment. The saline treatment group (Fig. 3, white circles) had an average positive difference score, indicating an increase in the %stability MAD in animals following the prolonged treatment phase. The cocaine group (Fig. 3, gray squares) had a difference score of ~ 0, indicating no change between measurements, while the MDPV group (Fig. 3, black triangles) had a negative difference score, indicating that even in the absence of acute MDPV, impulsive choice was persistently increased following prolonged treatment with 3.0 mg/kg MDPV. These differences were significant among groups [F(2, 32) = 1.789, P = 0.0279], with the MDPV difference score is significantly less than the saline difference score [q(32) = 3.958, P = 0.0228], while no significant differences were found between cocaine and either saline [q(32) = 2.586, P>0.05], or MDPV [q(32) = 1.403, P > 0.05] groups.
Figure 4 displays the concentration of different neurochemicals in striatal (top) and cortical (bottom) tissue from rats in the MDPV and saline groups after a 10-day washout period. Dopamine concentrations (Fig. 4, top left panel) were not significantly different between the saline (3.11 ± 0.25 ng/mg) and MDPV (2.76 ± 0.16) [t(21) = 1.209, P > 0.05] groups, though significant increases were found in DOPAC concentrations (Fig. 4, top middle panel) [t(21)=4.933, P < 0.0001] in the MDPV group. When looking at the total dopamine turnover in striatal tissues, expressed as DOPAC/DA (Fig. 4, top right panel), a significant increase in turnover was noted in the MDPV group [t(21) = 7.434, P < 0.0001]. While no differences were found between norepinephrine, 5-HT, or 5-HIAA levels in striatal tissues between groups (data not shown), a significantly higher serotonin turnover (5-HIAA/5-HT) was also observed in tissue drawn from MDPV-treated rats (1.64 ± 0.07) compared to that observed in the saline-treated animals (1.35 ± 0.04) [t(21) = 3.363, P = 0.0029]. No differences were found between MDPV and saline in cortical dopamine (Fig. 4, bottom left panel) [t(20) = 0.983, P > 0.05], DOPAC (Fig. 4, bottom middle panel) [t(21) = 1.802, P > 0.05], or DOPAC/DA (Fig. 4, bottom right panel) [t(20) = 0.326, P > 0.05] levels.
Fig. 4.
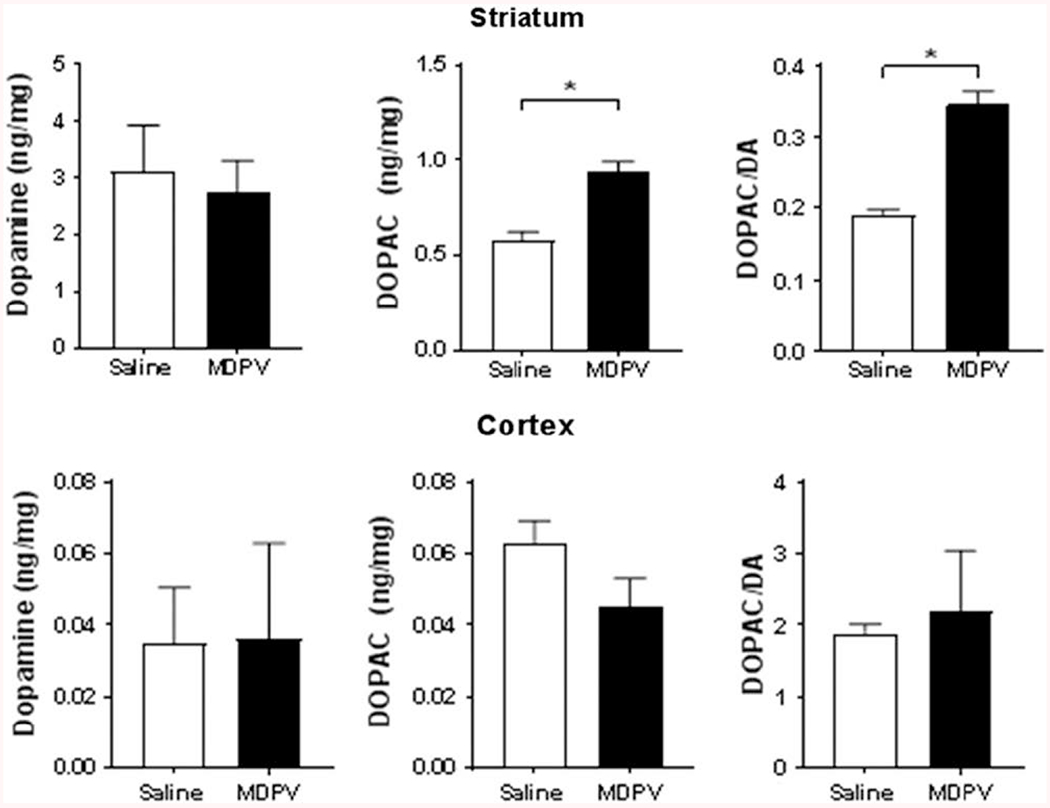
Results from striatal tissue HPLC analysis for DA and DOPAC. Abscissa: treatment group. Ordinate: neurochemical measured, expressed as ng of neurotransmitter per mg of total protein amount. *P < 0.05 compared to saline. MDPV, 3,4-methylenedioxypyrovalerone.
Discussion
Psychostimulants have the capacity to produce proimpulsive effects in non-human animals (Logue et al., 1992; Paine et al., 2003; Roesch et al., 2007) and in humans (Richards et al., 1999; Coffey et al., 2003). Case reports and clinical toxicological studies have revealed that MDPV may exhibit proimpulsive effects similar to those of established psychostimulants, which may potentially contribute to MDPV’s adverse health outcomes (Ross et al., 2012; John et al., 2014). In this study, we used an adjusting delay DD procedure to measure both the acute and long-term effects of MDPV administration on impulsive choice. Following stabilization of MAD, a satiety challenge was conducted prior to drug testing to establish a baseline to compare any acute anorectic effects of MDPV or cocaine to our adjusting delay procedure. We found that rats fed their entire daily allotment of food 1 h prior to the start of the session still earned all available reinforcers, but with a significantly reduced MAD. The use of a milk reinforcer (as opposed to food pellets) may have contributed to this finding.
Acute administration of MDPV to drug-naive animals revealed dose-dependent decreases in MAD, and also suppressed responding in nine of the 12 animals at the highest dose. As described in the methods, we defined suppressed responding as any sessions where animals did not complete all 15 blocks. Further, following chronic administration of MDPV, animals appeared sensitized to the response-suppressing effects of MDPV, with no animals completing all trials after the second exposure to 3.0 mg/kg. This may account for the apparent absence of dose-dependent effects of MDPV on MAD following chronic MDPV treatment. Indeed, the largest effects on MAD elicited by MDPV during the initial dose-effect determination occurred following administration of 3.0 mg/kg, but we were unable to re-assess proimpulsive effects of this dose after chronic treatment due to total suppression of responding in all subjects. However, due to the differences in reinforcers earned between the satiety challenge and high-doses of acute MDPV, it seems unlikely that the suppression of responding was due to any anorectic effects of MDPV. Rather, it is possible that 3.0 mg/kg MDPV produced prolonged periods of stereotyped behavior, which animals became sensitized to, following repeated exposure. Previous studies have demonstrated MDPV-elicited sensitization to motor stimulant effects occurs in Sprague-Dawley rats following 7 days of 0.5 mg/kg intraperitoneally MDPV (Berquist et al., 2016), or in Sprague-Dawley rats administered five injections of 1.0 or 5.0 mg/kg intraperitoneally at 48 h intervals (Watterson et al., 2016). It is interesting to note that Watterson et al. (2016) also performed injections of the same MDPV doses at 24 h intervals, but saw no sensitized increases in locomotor activity in those animals, indicating that the 48 h interval, which is similar to our chronic drug phase, is more effective at eliciting sensitization to motor stimulant effects. This lack of sensitization following 24 h repeated injections of MDPV is also the reason we used daily acute administration of MDPV during acute dose-effect determinations, as opposed to injecting animals every other day. In contrast to MDPV, no doses of cocaine tested noticeably decreased MAD regardless of whether animals were drug-naive or had a history of prolonged cocaine administration, and calculation of dose–response curve slopes revealed no significant dose-dependent effects. Further, the pattern of sensitization to suppression of operant responding observed during the second acute dose-effect determination of MDPV following the chronic drug treatment was not observed in the cocaine group. Thus, the present findings indicate that acute administration of cocaine does not increase impulsivity using this adjusting delay DD procedure, which is consistent with previous rat DD data (Smethells and Carroll, 2015). This is likely because adjusting delay procedures are relatively insensitive to acute drug effects, which may be better detected using an increasing delay procedure (Stein and Madden 2013; Craig et al., 2014). Despite the apparent insensitivity of this procedure to acute drug effects, differences in MAD following acute MDPV were still observed, indicating that MDPV may be more effective at increase impulsive choice in the acute setting than cocaine. Importantly, this difference between MDPV and cocaine is not likely to be a simple function of dose, as numerous studies have previously demonstrated that MDPV is reliably 10-fold more potent than cocaine in terms of discriminative stimulus effects (Gannon et al., 2016; Berquist and Baker, 2017), reinforcing effects (Schindler et al., 2016; Gannon et al., 2017), aversive effects (Woloshchuk et al., 2016) and locomotor stimulant effects (Marusich et al., 2012; Baumann et al., 2017) in rodents. The dose range tested for cocaine in the present studies was accordingly 10-fold higher than the dose range tested for MDPV in the present experiments to compensate for this potency difference.
Training in DD procedures is critically important to subsequent performance, and here we observed that the MAD of the saline group increased over the duration of these studies, whereas that of the cocaine group remained relatively stable. One possible explanation for the increased %stability MAD observed among saline-treated rats is that they progressively habituated to the task and their performance increased with continued practice afforded by exposure to the task. This interpretation may be supported when considering the similarities between DD and differential reinforcement of low rates of responding (DRL) tasks. In both tasks, animals are trained to withhold responding for a set delay in order to obtain reinforcement. Under a DRL schedule, short delays (i.e. 5 s) are possible to train in only a few days (Kirshenbaum et al., 2008), while longer delays (i.e. 70 s) require upwards of 50 sessions (Stoffel and Cunnigham, 2008). Training up to these delays under a DRL schedule requires slowly incrementing the delay until subjects reliably respond at the desired delay, and there may be similarities here to the prolonged exposure to MAD sessions employed in the present study. Thus, while significant differences were not detected between the cocaine and saline groups in most of the measures in this experiment, the fact that the MAD of the cocaine group did not increase over time may be cautiously interpreted as cocaine disrupting the expected increase in MAD commensurate with practice, as observed in the saline control group. Further, we found that the MAD for MDPV actually decreased over time, once again indicating increased effectiveness in the capacity of MDPV to increase choice impulsivity.
In this regard it is noteworthy that MDPV, but not cocaine, produced persistent increases in choice impulsivity even during drug abstinence. Cocaine is the most widely studied psychostimulant in studies investigating the chronic effects of drugs on impulsive choice in animals, but the factors involved in mediating impulsive choice remain imperfectly understood (Setlow et al., 2009). It is possible that the duration of cocaine exposure, or the total cumulative amount of drug administered, determines the magnitude of changes in impulsive choice. For example, Paine et al. (2003) and Winstanley et al. (2007) used similar fixed-delay procedures, however, only Paine et al. (2003) found an altered impulsive choice in cocaine-treated animals. One reason for these discordant findings may be that Paine et al. (2003) administered three daily injections of cocaine (15 mg/kg, intraperitoneally) for 14 days, whereas Winstanley et al. (2007) administered two daily injections of cocaine (15 mg/kg, intraperitoneally) for 21 days. Further, studies by Dandy and Gatch (2009) and by Mitchell et al. (2014) have specifically investigated the importance of total cocaine exposure on changes to impulsivity. The findings of the present study indicate that MDPV may facilitate proimpulsive effects more strongly or more quickly than cocaine, but further study is required to determine if these differences are pharmacodynamic (i.e. due to increased selectivity and effectiveness of MDPV at DAT and NET), pharmacokinetic (i.e. related to MDPV’s longer duration of action), or behavioral (i.e. modulated by parameters of the DD procedure).
The neurobiological mechanisms mediating impulsivity, particularly those following repeated drug administration, are incompletely understood (Grant and Chamberlain, 2014), but most sources agree that dopaminergic meso-corticolimbic projections are heavily involved. A recent study revealed that, among other functional changes, a single acute administration of 3 mg/kg MDPV in adult, male Long Evans rats increased DA turnover in the striatum 24 h after the injection (Colon-Perez et al., 2018). Interestingly, this is consistent with the dopaminergic changes observed in the present study, despite differences in a number of MDPV administrations employed and the inclusion of a 10-day drug washout prior to euthanasia and tissue collection. No other studies have detailed monoamine concentrations in the striatum following repeated administration of doses of MDPV larger than 1.0 mg/kg, and further Kohler et al. (2018) observed no changes in the rate of monoamine turnover in several other brain regions after seven days of 1.0 mg/kg MDPV administration in rats. Number of drug administrations and length of washout has been shown to be an important consideration when measuring levels of extracellular DA following cocaine administration (Karoum et al.., 1990; Imperato et al., 1992; Alburges and Wamsley, 1993; Binienda et al., 2002; Puig et al., 2012), but these parameters have not been extensively investigated with MDPV. Moreover, while no differences were detected in either 5-HT or 5-HIAA in this study, turnover rates of serotonin were significantly increased in striatal tissue from MDPV-treated rats. Interestingly, unexpected serotonin syndrome following MDPV intoxication has been described in the clinical literature (Mugele et al., 2012), indicating that, while MDPV does not directly act at SERT, it may have some indirect or modulatory activity on the serotonergic system. While a single case report should always be interpreted cautiously, previous preclinical studies have reported that the hyperthermic effects of MDPV (at a high ambient temperature) are attenuated by the serotonin-selective reuptake inhibitor fluoxetine (Gannon et al., 2018a) and that the 5-HT2C receptor agonist lorcaserin dose-dependently decreased MDPV self-administration (Gannon et al., 2018b). It is worth noting that lorcaserin also decreases the self-administration of other drug reinforcers, including oxycodone (Neelakantan et al., 2017) and nicotine (Fletcher et al., 2018), so while there is some indication that the serotonin system may play a role in mediating in-vivo effects of MDPV, the exact interactions are currently unknown, and these findings warrant future studies exploring possible interactions between serotonergic targets and MDPV. Further, it is probable that neurobiological changes are not limited to the striatum, and previous studies have demonstrated the importance of the prefrontal cortex in modulating impulse control (Dalley et al., 2008; Bari and Robbins, 2013). We investigated monoamine concentrations in cortical tissues but were unable to detect any differences between saline and MDPV groups in any neurochemicals measured, indicating that while dopamine may play a critical role in cognitive processes in the cortex (Seamans and Yang, 2004), changes in impulsivity may not relate to absolute monoamine concentration, but to more subtle factors not measured in the present experiments. One additional consideration is that we were unable to directly compare cocaine and MDPV head-to-head in the present experiments, thus, without a better established positive control to compare MDPV-related changes to, all monoamine data should be interpreted with caution as to how they may inform the behavioral changes here described.
Current drug abuse trends indicate that MDPV and other SCs have remained on the illicit market and continue to be an international concern. The present studies indicate that some of the adverse health-risk outcomes seen following repeated ‘bath salts’ administration in humans may be linked to increases in impulsivity, which was here demonstrated in rats both acutely and following repeated exposure to a common constituent of these products, MDPV. While the exact mechanism for these changes is currently unknown, this study reports that prolonged treatment with MDPV may yield significant neurobiological changes in the striatal dopaminergic system, which is known to be involved in impulsive choice. Thus, further study is warranted to better characterize the long-term behavioral and neurochemical consequences of MDPV in the hopes they may be used to dictate behavioral or pharmacological treatments in the clinic.
Supplementary Material
Acknowledgements
This research was conducted as part of the doctoral dissertation of WSH. These studies were supported in part by the University of Arkansas for Medical Sciences Center for Translational Neuroscience (grant GM110702), the University of Arkansas for Medical Sciences Translational Research Institute (grant RR029884), and the National Institutes of Health National Institute on Drug Abuse (grant DA039195); a training grant from the National Institutes of Health (T32 DA022981); this work was partially supported by the Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA).
This project was supported in part by DA039195, DA040907, NS100512, and the UAMS Center for Translational Neuroscience.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.behaviouralpharm.com.
References
- Ahmadlou M, Ahmadi K, Rezazade M, Azad-Marzabadi E (2013). Global organization of functional brain connectivity in methamphetamine abusers. Clin Neurophysiol 124:1122–1131. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Wamsley JK (1993). Effects on monoamine levels in rat CNS after chronic administration of cocaine. Invest Clin 34:181–192. [PubMed] [Google Scholar]
- Bakhshani NM (2014). Impulsivity: a predisposition toward risky behaviors. Int J High Risk Behav Addict 3:e20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. (2013). Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Heustis MA (2017). Neuropharmacology of 3,4-methylenedioxypyrovalerone (MDPV), its metabolites, and related analogs. Curr Top Behav Neurosci 32:93–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-rauscent A, Everitt BJ, Dalley JW (2016). In search of predictive endophenotypes in addiction: insights from preclinical research. Genes Brain Behav 15:74–88. [DOI] [PubMed] [Google Scholar]
- Berquist MD, Traxler HK, Mahler AM, Baker LE (2016). Sensitization to the locomotor stimulant effects of ‘bath salt’ constituents, 4-methylmethcathinone (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), in male sprague-dawley rats. Drug Alcohol Depend 164:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD II, Baker LE (2017). Characterization of the discriminative stimulus effects of 3,4-methylenedioxypyrovalerone in male Sprague-Dawley rats. Behav Pharmacol 28:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binienda ZK, Pereira F, Alper K, Slikker W Jr, Ali SF (2002). Adaptation to repeated cocaine administration in rats. Ann N Y Acad Sci 965:172–179. [DOI] [PubMed] [Google Scholar]
- Borek HA, Holstege CP (2012). Hyperthermia and multiorgan failure after abuse of ‘bath salts’ containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med 60:103–105. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Daw N, Robbins TW, Everitt BJ (2002). Local analysis of behavior in the adjusting-delay task for assessing choice of delayed reinforcement. Neural Netw 15:617–634. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chen PC, Chiang YC (2009). Molecular mechanisms of psychostimulant addiction. Chang Gung Med J 32:148–154. [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT (2003). Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol 11:18–25. [DOI] [PubMed] [Google Scholar]
- Cole DM, Oei NY, Soeter RP, Both S, van Gerven JM, Rombouts SA, Beckmann CF (2013). Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex 1509–1516. [DOI] [PubMed] [Google Scholar]
- Colon-Perez LM, Pino JA, Saha K, Pompilus M, Kaplitz S, Choudhury N, et al. (2018). Functional connectivity, behavioral and dopaminergic alterations 24 h following acute exposure to synthetic bath salt drug methylenedioxypyrovalerone. Neuropharmacology 137:178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AR, Maxfield AD, Stein JS, Renda CR, Madden GJ (2014). Do the adjusting-delay and increasing-delay tasks measure the same construct: delay discounting? Behav Pharmacol 25:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW (2008). Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–260. [DOI] [PubMed] [Google Scholar]
- Dandy KL, Gatch M (2009). The effects of chronic cocaine exposure on impulsivity in rats. Behav Pharmacol 20:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Stochl J, Woodward JM, Fletcher PC (2013). The skinny on cocaine: insights into eating behavior and body weight in cocaine-dependent men. Appetite 71:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC (2013) In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 38:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Li Z, Silenieks LB, MacMillan C, DeLannoy I, Higgins GA (2018). Preclinical evidence for combining the 5-HT2C receptor agonist lorcaserin and varenicline as a treatment for nicotine dependence. Addict Biol 24:376–387. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Reichard EE, Fantegrossi WE (2014). Psychostimulant abuse and HIV infection: cocaine, methamphetamine, and ‘bath salts’ cathinone analogues. Curr Addict Rep 1:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE (2016). Stereoselective effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovaleone in mice: drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther 356:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT (2017). Individual differences in the relative reinforcing effects of 3,4-methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. J Pharmacol Exp Ther 361:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Rice KC, Fantegrossi WE (2018a). Role of monoaminergic systems and ambient temperature in bath salts constituent 3,4-methylenedioxypyrovalerone (MDPV)-elicited hyperthermia and locomotor stimulation in mice. Neuropharmacology 134:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Sulima A, Rice KC, Collins GT (2018b). Inhibition of cocaine and 3,4-methylenedioxypyrovalerone (MDPV) self-administration by lorcaserin is mediated by 5-HT2C receptors in rats. J Pharmacol Exp Ther 364:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marchena N, Ladrón de Guevara-Miranda D, Pedraz M, Araos PF, Rubio G, Ruiz JJ, et al. (2018). Higher impulsivity as a distinctive trait of severe cocaine addiction among individuals treated for cocaine or alcohol use disorders. Front Psychiatry 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R (2016). Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res Bull 126:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR (2014). Impulsive action and impulsive choice across substance and behavioral addictions: cause or consequence?. Addict Behav 39:1632–1639. [DOI] [PubMed] [Google Scholar]
- Gray JC, Mackillop J (2015). Impulsive delayed reward discounting as a genetically-influenced target for drug abuse prevention: a critical evaluation. Front Psychol 6:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Baldois IM, Bickel WK, Fillmore M, et al. (2015). Choice impulsivity: definitions, measurement issues, and clinical implications. Personal Disord 6: 182–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlé KM, Zhang S, Schiff M, Mackey S, Paulus MP, Yu AJ (2015). Altered statistical learning and decision making in methamphetamine dependence: evidence from a two-armed bandit task. Front Psychol 6:1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Mele A, Scrocco MG, Puglisi-Allegra S (1992). Chronic cocaine alters limbic extracellular dopamine. Neurochemical basis for addiction. Eur J Pharmacol 212:299–300. [DOI] [PubMed] [Google Scholar]
- Iversen L, White M, Treble R (2014). Designer psychostimulants: pharmacology and differences. Neuropharmacology 87:59–65. [DOI] [PubMed] [Google Scholar]
- John ME, Thomas-Rozea C, Hahn D (2014). Bath salts abuse leading to new onset psychosis and potential for violence. Clin Schizophr Relat Psychoses 11:120–124. [PubMed] [Google Scholar]
- Kalivas PW, Mcfarland K (2003). Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44–56. [DOI] [PubMed] [Google Scholar]
- Karoum F, Suddath RL, Wyatt RJ (1990). Chronic cocaine and rat brain catecholamines: long-term reduction in hypothalamic and frontal cortex dopamine metabolism. Eur J Pharmacol 186:1–8. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AP, Brown SJ, Hughes DM, Doughty AH (2008). Differential-reinforcement-of-low-rate-schedule performance and nicotine administration: a systematic investigation of dose, dose-regimen, and schedule requirement. Behav Pharmacol 19:683–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler RJ, Perrine SA, Baker LE (2018). Repeated exposure to 3,4-methylenedioxypyrovalerone and cocaine produces locomotor sensitization with minimal effects on brain monoamines. Neuropharmacology 134:22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Solis E Jr, Sakloth F, De Felice LJ, Glennon RA (2013). ‘Deconstruction’ of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. ACS Chem Neurosci 4:1524–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ (2013). Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry 70:857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt N, Jurd S, Tennant C, Lewis J, Macken L, Joseph A, et al. (2011). Alcohol and substance use by patients with psychosis presenting to an emergency department: changing patterns. Australas Psychiatry 19:354–359. [DOI] [PubMed] [Google Scholar]
- Logue AW, Tobin H, Chelonis KK, Wang RY, Geary N, Schacthter S (1992). Cocaine decreases self-control in rats: a preliminary report. Psychopharmacology (Berl) 109:245–247. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Henson C, France CP (2014). Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology 87:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL (2012). Effects of synthetic cathinones contained in ‘bath salts’ on motor behavior and a functional observational battery in mice. Neurotoxicology 33:1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Ouimet DJ, Fuchs RA, Morgan D, Setlow B (2014). Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behav Neurosci 128:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugele K, Nanagas KA, Tormoehlen LM (2012). Serotonin syndrome associated with MDPV use: a case report. Ann Emerg Med 60:100–102. [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC (2012). Death following recreational use of designer drug ‘bath salts’ containing 3,4-methylenedioxypyrovalerone (MDPV). J Med Toxicol 8:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, et al. (2017). Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem Neurosci 8:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC (2003). Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res 147:135–147. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME (2008). The role of impulsive behavior in drug abuse. Psychopharmcology (Berl) 200:1–26. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005). Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 178:193–201. [DOI] [PubMed] [Google Scholar]
- Puig S, Noble F, Benturguia N (2012). Short- and long-lasting behavioral and neurochemical adaptations: relationship with patterns of cocaine administration and expectation of drug effects in rats. Transl Psychiatry 2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B (2006). A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol 17:651–667 [DOI] [PubMed] [Google Scholar]
- Richards JB, SAbol KE, de Wit H (1999). Effects of methamphetamine on the adjusting amount procedure, a model of impulse behavior in rats. Psychopharmacology (Berl) 146:432–439. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cintas L, Daigre C, Grau-López L, Barral C, Pérez-Pazos J, Voltes N, et al. (2016). Impulsivity and addiction severity in cocaine and opioid dependent patients. Addict Behav 58:104–109. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G (2007). Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci 27:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA (2012). Psychoactive ‘bath salts’ intoxication with methylenedioxypyrovalerone. Am J Med 125:854–858. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH (2016). Reinforcing and neurochemical effects of the ‘bath salts’ constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 233:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004). The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74:1–58. [DOI] [PubMed] [Google Scholar]
- Setlow B, Mendez IA, Mitchell MR, Simon NW (2009). Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav Pharmacol 20: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli C, Schramm Y, Dieu LH, Huwyler J, et al. (2013). Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivagnanam K, Chaudari D, Lopez P, Sutherland ME, Ramu VK (2013). ‘Bath salts’ induced severe reversible cardiomyopathy. Am J Case Rep 14:288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethells JR, Carroll ME (2015). Discrepant effects of acute cocaine on impulsive choice (delay discounting) in female rats during an increasing- and adjusting-delay procedure. Psychopharmacology (Berl) 232:2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J (2011). Clinical experience with and analytical confirmation of ‘bath salts’ and ‘legal highs’ (synthetic cathinones) in the United States. Clin Toxicol 49:499–505. [DOI] [PubMed] [Google Scholar]
- Stein JS, Madden GJ (2013). Delay discounting and drug abuse: empirical, conceptual, and methodological considerations. In: MacKillop J, de Wit H, editors. Handbook of addiction psychopharmacology. 1st. Hoboken: Wiley-Blackwell. pp.165–208. [Google Scholar]
- Stoffel EC, Cunnigham KA (2008). The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend 92:69–78. [DOI] [PubMed] [Google Scholar]
- Tanno T, Maguire DR, Henson C, France CP (2014). Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacology (Berl) 231:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D (2014). Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 76 Pt B:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2017). Global synthetic drugs assessment. Vienna: United Nations: 2017 [Google Scholar]
- Uralets V, Rana S, Morgan S, Ross W (2014). Testing for designer stimulants: metabolic profiles of 16 synthetic cathinones excreted free in human urine. J Anal Toxicol 38:233–241. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. (2014). Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Taylor SB, Nemirovsky NE, Olive MF (2016). Sensitization to the motor stimulant effects of 3,4-methylenedioxypyrovalerone (MDPV) and cross-sensitization to methamphetamine in rats. J Drug Alcohol Res 5:235967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, et al. 2007. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci 27:10497–10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshchuk CJ, Nelson KH, Rice KC, Riley AL (2016). Effects of 3,4-methylenedioxypyrovalerone (MDPV) pre-exposure on the aversive effects of MDPV, cocaine and lithium chloride: Implications for abuse vulnerability. Drug Alcohol Depend 167:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TH, Cline-parhamovich K, Lajoie D, Parsons L, Dunn M, Ferslew KE (2013). Deaths involving methylenedioxypyrovalerone (MDPV) in Upper East Tennessee. J Forensic Sci 58:1558–1562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


