Abstract
Background
Obesity has been described as a risk factor for COVID‐19 severity and mortality. Previous studies report a linear association between BMI and adverse outcomes, meanwhile in other critical illness, excessive fat tissue is related to improved survival. Whether different BMI is related with the survival of patients with severe COVID‐19 deserves further analysis.
Objective
To determine the mortality rate among hospitalized patients with severe COVID‐19 stratified according to BMI.
Methods
The clinical files of all patients hospitalized from March to December 2020 with a positive PCR test for SARS‐CoV‐2 discharged due to improvement or death, were analyzed. A mixed effects logistic regression was carried out to determine which clinical and biochemical characteristics and comorbidities were associated with in‐hospital mortality.
Results
The cohort consisted of 608 patients with a median age of 59 years (interquartile ranges, IQR 46–69 years), median BMI of 28.7 kg/m2 (IQR 25.4–32.4 kg/m2), 65.5% were male. In‐hospital mortality rate was 43.4%. Of the cohort 0.8% had low weight, 20.9% normal weight, 36.0% overweight, 26.5% obesity grade I, 10.2% obesity grade II and 5.6% obesity grade III. Mortality rate was highest in patients with low weight (80%), followed by patients with obesity grade III (58.8%) and grade II (50.0%). Overweight and underweight/obesity grade III were associated with higher mortality (OR of 9.75 [1.01–1.10] and OR 4.08 [1.64–10.14]), after adjusting by sex and age.
Conclusions
The patients in the underweight/overweight and grade 3 obesity categories are at higher risk of COVID‐19 related mortality, compared to those with grade I or II obesity.
Keywords: body weight, COVID‐19, mortality, obesity, SARS‐CoV‐2
1. INTRODUCTION
Since March 2020, the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) as a pandemic. As of October 2021, the disease had spread to 221 countries, affecting more than 233 million people and causing 4,777,503 deaths. 1 In Mexico, the first COVID‐19 case was reported in late February 2020. By April 2020 the country rapidly reached a community transmission stage. Currently, Mexico is one of the most affected countries globally, surpassed only by the United States, Brazil, and India. 2 Despite many factors could explain the high burden of the COVID‐19 epidemic in Mexico, the high prevalence of overweight (39.1%) and obesity (36.1%) have a fundamental role for the development of adverse clinical outcomes associated with COVID‐19. 3
Obesity is a major risk factor for severe COVID‐19 infection. However, some reports suggest that patients with obesity admitted to the intensive care units (ICU) due other causes of sepsis have an increased survival when compared to normal weight subjects. 4 , 5 This “obesity paradox” has been mentioned in some publications regarding COVID‐19, but there are insufficient data to clarify this association. 5 , 6 Furthermore, low weight and malnourishment are barely considered. 7
Weight extremes show a non‐linear dose‐response to all‐cause mortality, 8 but the information in COVID‐19 patients is still controversial and incomplete. For example, a prospective, community‐based cohort of 6.9 million people in England showed a linear association between increasing BMI and admission to ICU due to COVID‐19, with a higher risk for people with a BMI of 20 kg/m2 or less and an increased mortality risk for people with a BMI higher than 28 kg/m2. This study proposed that the risk of severe COVID‐19 outcomes was attributable to excess weight due metabolic impairment of organ functioning, leading to insulin resistance meanwhile those with low BMI had increased frailty. 9 In Mexico, Coss‐Rovirosa et al. found that patients with obesity had no significant risk of requiring intubation and invasive mechanical ventilation compared with lean subjects. 10 A possible explanation for the discordance observed in those studies is that overweight and obesity were determined using the BMI. Recent evidence suggests that disproportionate adipose tissue distribution, and particularly increased visceral adipose mass predicted an increased risk of adverse outcomes in COVID‐19 patients. 11 However, this parameter is difficult to evaluate in the clinical setting.
The aim of the study was to determine the effect caused by adipose tissue accumulation thought BMI categorization on hospitalized COVID‐19 mortality rates in the center of reference at Mexico City. The hypothesis was that extreme BMI categories (underweight and extreme obesity) could represent a non‐linear risk for COVID‐19 mortality.
2. MATERIALS AND METHODS
2.1. Study design
This was a retrospective cohort of patients admitted to a major tertiary care referral center in Mexico, from March 2020 to February 2021. The study included patients hospitalized due severe COVID‐19 infection. COVID‐19 severity was defined using international guidelines: presence of dyspnea, a respiratory rate of 30 or more breaths per minute, a blood oxygen saturation of 93% or less, a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2:FiO2) of less than 300 mmHg, or chest CT scan showing pneumonia involving more than 50% of the lungs. 12 , 13 Of the large number of patients needing acute, specialized medical care, only those that required invasive mechanical ventilation (IMV) were admitted in the intensive care unit (ICU management); most were transferred to a unit with available beds. The decisions for hospitalization an ICU transfer were made by the treating physicians.
The exclusion criteria were an absent or negative PCR test, patients in whom BMI was not assessed or reported, and those in whom the final outcome could not be clearly ascertained due lack of register or transference to another hospital.
The information regarding comorbidities and the results of laboratory tests performed upon hospital admission was obtained from the electronic medical records. Only internal medicine specialists oversaw the initial evaluation of the patients at the respiratory triage and together with pneumologists and intensivists were responsible for the management of patients during hospitalization.
Registered cardiovascular comorbidities included hypertension, coronary artery disease, arrhythmia, valvular heart disease and heart failure; respiratory comorbidities included chronic obstructive pulmonary disease, asthma and sleep apnea; metabolic comorbidities included abnormalities in glucose metabolism (diabetes, impaired fasting glucose and glucose intolerance), dyslipidemias (hypercholesterolemia, hypertriglyceridemia, hypoalphalipoproteinemia). Other comorbidities registered in the database were kidney and liver disease as well as the presence of malignancies. Diabetes, obesity and hypertension were considered as major comorbidities for COVID‐19 complications and death. Significant comorbidities were defined as any other diseases associated with those outcomes.
Anthropometric and clinical measurements were determined at the time of hospital admission.
2.2. Biochemical determinations
The tests for glucose, total cholesterol (TC), high density lipoprotein cholesterol (HDL‐c), triglycerides (TG), creatinine, fibrinogen, D‐dimer, procalcitonin, albumin, lactic dehydrogenase (LDH), ultrasensitive C‐reactive‐protein (CRP), ferritin, INR, uric acid and vitamin D were analyzed with commercially available kits on an automated platform. Complete blood count was performed using an automated hematological analyzer. Glycated hemoglobin (HbA1c) was evaluated enzymatically using the Atellica CH analyzer. Low‐density lipoprotein cholesterol (LDL‐c) was calculated with Friedewald formula: LDL‐c (mg/dl) = TC mg/dl–(HDL‐c mg/dl + TG mg/dl/5) if TG were <400 mg/dl. Tests were done after at least 8 h fasting, when possible. In all cases, tests were performed within the first 24 h from arrival, before additional therapies were initiated.
2.3. Ethics approval and consent to participate
The study was approved by the ethics committee of our institution. The procedures were in compliance with local ethical regulations, the Mexican Law of General Health regarding Health Research, the institutional regulations, and the Declaration of Helsinki of 1975 and its amendments. It was also in compliance with the currently approved international codes and norms regarding Good Clinical Practice in Clinical Investigation. The protocol and the aim of the study were fully explained to the subjects or their families, who sign the corresponding informed consent.
2.4. Statistical analysis
Categorical variables are shown as frequencies and proportions. Continuous variables are shown as median and interquartile ranges (IQR). The clinical and biochemical characteristics of patients with severe COVID‐19 that were discharged or died were assessed using a chi‐squared test for categorical variables or Mann–Whitney's U test for continuous variables. The risk of death at follow‐up was evaluated using a Cox Proportional Hazard Regression Model.
A p‐value <0.05 was considered to be statistically significant. Statistical software consisted of SPSS version 25.
3. RESULTS
3.1. Study population
From 1100 patients initially included, 492 were excluded due mild or moderate COVID‐19 (Figure 1) with a final sample composed of 608 patients. The general characteristics of the group were: 65.5% male, with a median age of 59 years (IQR 46–69 years), 56.7% had been discharged due to improvement of their condition and 43.4% died. The most frequent comorbidities were diabetes (43.1%), obesity (41.7%), hypertension (40.5%), dyslipidemia (17.4%), kidney disease (16.3%), cardiopathy (13.3%), cancer (11.4%) and pneumopathy (8.1%). The median BMI was 28.7 kg/m2 (IQR 25.4–32.4 kg/m2). Patients with underweight represented 0.8%; 20.9% had normal weight, 36% were overweight, 26.5% had grade I obesity, 10.2% had grade II obesity and 5.6% had grade III obesity. Five patients had a BMI higher than 50 kg/m2, which is sometimes classified as super obesity.
FIGURE 1.
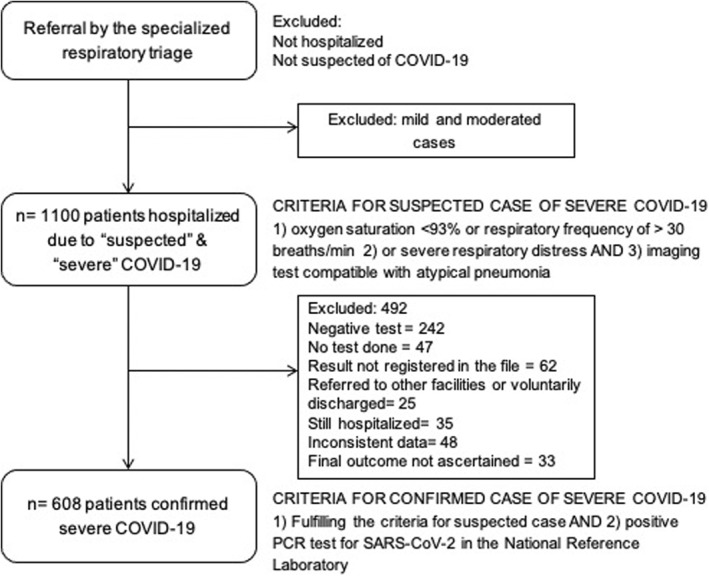
Patient selection flowchart
The patients were hospitalized for a median of 10 days (IQR 6–16 days). Table 1 shows the differences between the patients who improved versus the ones who died. The comorbidities significantly associated with death were diabetes mellitus, hypertension and kidney disease. Other biochemical differences among groups are shown in Table 2. Twenty‐five percent of the patients had overweight and obesity. Obesity was more frequent in women than in men (53.6% vs. 35.9%, p < 0.001), but the highest BMIs and severe forms of obesity were observed in men.
TABLE 1.
Main comorbidities and outcomes between the patients with severe COVID‐19 that improved or died
| Variable | Follow‐up | ||
|---|---|---|---|
| Discharged n = 344 | Death n = 264 | p* | |
| Age (years) | 51 (41–60.7) | 62 (52–71) | <0.001 |
| Male sex (%) | 220 (64.0) | 178 (67.4) | 0.372 |
| Hypertension (%) | 113 (32.8) | 133 (50.3) | <0.001 |
| Years with hypertension | 3 (1–12) | 10 (3–15) | 0.294 |
| Diabetes (%) | 131 (38.1) | 131 (49.6) | 0.004 |
| Dyslipidemia (%) | 63 (18.3) | 43 (16.3) | 0.514 |
| Kidney disease (%) | 40 (11.6) | 59 (22.3) | <0.001 |
| Cardiopathy (%) | 40 (11.7) | 41 (15.5) | 0.165 |
| Lung disease (%) | 25 (7.3) | 24 (9.1) | 0.413 |
| Neoplasm (%) | 28 (8.1) | 39 (14.8) | 0.010 |
| BMI, kg/m2 | 28.4 (25.4–31.3) | 29.7 (26.0–34.7) | 0.305 |
| Obesity (BMI > 30 kg/m2, %) | 140 (59.2) | 114 (50.4) | 0.480 |
| Disease outcomes | |||
| Admission to the ICU (%) | 54 (15.6) | 86 (32.5) | <0.001 |
| Invasive mechanical ventilation (%) | 49 (14.2) | 132 (50) | <0.001 |
| Days hospitalized (number, IQR) | 10 (6–15) | 9 (4–15.3) | 0.027 |
Note: Results are expressed in medians (interquartile ranges) or percentages.
Abbreviations: BMI, body mass index; ICU, intensive care unit.
*Statistically different among groups using Mann–Whitney U or squared chi tests.
TABLE 2.
Biochemical characteristics of hospitalized patients with severe COVID‐19, comparing those that improved or died
| Variable | Follow‐up | ||
|---|---|---|---|
| Discharged n = 344 | Death n = 264 | p* | |
| Saturation without supplementary oxygen at admission (%) | 80 (70–90) | 65 (52–81) | <0.001 |
| Blood count | |||
| Leukocytes, ×103/ml | 8.1 (6.3–11.9) | 10.8 (7.4–15.6) | 0.010 |
| Neutrophils, ×103/ml | 6.9 (4.8–10.2) | 9.4 (6.4–13.9) | 0.009 |
| Lymphocytes, ×103/ml | 0.81 (0.59–1.15) | 0.72 (0.50–1.15) | 0.225 |
| Hemoglobin, g/L | 14.8 (13.2–16.1) | 14.1 (11.8–15.7) | 0.485 |
| Sepsis & severity biomarkers | |||
| Fibrinogen, mg/dl | 666 (536–773) | 750 (598–798) | 0.170 |
| D‐dimer, μg/ml | 1.1 (0.72–2.39) | 2.1 (1.1–2.4) | <0.001 |
| Procalcitonin, ng/ml | 0.21 (0.09–0.48) | 0.90 (0.28–3.12) | <0.001 |
| Albumin, g/L | 3.3 (3.0–3.7) | 3.0 (2.7–3.4) | 0.002 |
| LDH, U/L | 389 (288–494) | 519 (390–703) | <0.001 |
| Reactive C protein, mg/dl | 9.41 (3.68–17.30) | 18.52 (11.17–26.35) | <0.001 |
| Ferritin, ng/ml | 922 (498–1489) | 1047 (557–1659) | 0.303 |
| INR | 1.10 (1.04–1.19) | 1.17 (1.09–1.27) | 0.001 |
| Nutritional and metabolic parameters | |||
| FPG, mg/dl | 108 (87–145) | 125 (95–185) | 0.008 |
| HbA1c, % | 6.4 (5.8–9.0) | 6.5 (6.0–8.4) | 0.741 |
| Total cholesterol, mg/dl | 143 (124–174) | 125 (103–156) | 0.003 |
| HDL‐c, mg/dl | 28 (22–35) | 23 (16–30) | 0.036 |
| LDL‐c, mg/dl | 79 (63–108) | 62 (47–85) | 0.008 |
| Triglycerides, mg/dl | 155 (119–221) | 181 (124–277) | 0.458 |
| Creatinine, mg/dl | 0.81 (0.70–1.01) | 0.98 (0.78–1.89) | 0.027 |
| Uric acid, mg/dl | 4.3 (3.1–5.7) | 5.2 (3.4–7.5) | 0.129 |
| 25‐OH vitamin D, ng/dl | 16.1 (11.2–20.5) | 12.5 (9.1–18.2) | 0.845 |
Note: Results are expressed in medians (interquartile ranges) or percentages.
Abbreviations: 25‐OH vitamin D, 25‐hydroxy vitamin D; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; INR, international normalized ratio; LDH, lactic dehydrogenase.
*Statistically different among groups using Mann–Whitney U or squared chi tests.
3.2. Differences in metabolic and comorbid characteristics stratified by BMI categories
The frequency of hypertension was similar among BMI groups (p = 0.199), as were the frequencies of diabetes (p = 0.998) and cardiopathy (p = 0.443). There were significant differences in kidney disease (p = 0.018), the patients with low weight had higher frequency of kidney disease (60%) in comparison with those in other BMI categories (18.9% for normal weight, 17.4% for overweight, 9.9% in grade I obesity, 21% in grade II and 14.7% in grade III). Also, neoplasms were more common in the lower weight group (40%), while normal weight had a frequency of 15.7%, 11.9% in overweight patients, 8.7% in grade I obesity, 4.8% in grade II and 5.9% in grade III (p = 0.041). On the other hand, the probability of a lung disease increased with BMI with 0% in the low weight group, 9% in the normal weight, 6% in overweight, 6% in grade I obesity, 13% in grade II obesity and 24% in grade III obesity (p = 0.006) (Table 3).
TABLE 3.
Clinical and biochemical characteristics of patients according their BMI
| Variable | BMI classification | ||||||
|---|---|---|---|---|---|---|---|
| <18.5 kg/m2 | 18.5–24.9 kg/m2 | 25–29.9 kg/m2 | 30–34.9 kg/m2 | 35–39.9 kg/m2 | >40 kg/m2 | p* | |
| Age (years) | 39 (30–67) | 64 (47–73) | 61 (48–68) | 57 (48–68) | 53 (46–69) | 52 (40–66) | 0.028 |
| BMI (kg/m2) | 17.57 (15.05–19.72) | 23.73 (22.22–24.39) | 27.39 (26.21–28.5) | 31.55 (30.63–33.04) | 36.33 (35.63–37.46) | 43.19 (40.60–49.17) | <0.001 |
| Male sex (%) | 20 | 31 | 27 | 43 | 42 | 50 | 0.003 |
| Hypertension (%) | 60 | 36 | 49 | 45 | 51 | 57 | 0.199 |
| Diabetes (%) | 60 | 63 | 65 | 64 | 79 | 71 | 0.311 |
| Dyslipidemia (%) | 20 | 17 | 17 | 14 | 29 | 12 | 0.174 |
| Kidney disease (%) | 60 | 19 | 17 | 10 | 21 | 15 | 0.018 |
| Cardiopathy (%) | 20 | 18 | 11 | 11 | 16 | 18 | 0.443 |
| Lung disease (%) | 0 | 9 | 6 | 6 | 13 | 24 | 0.006 |
| Neoplasm (%) | 40 | 16 | 12 | 9 | 5 | 6 | 0.041 |
| Disease outcomes | |||||||
| Admission to the ICU (%) | 0 | 16 | 27 | 21 | 26 | 32 | 0.205 |
| Invasive mechanical ventilation (%) | 20 | 26 | 28 | 29 | 39 | 44 | 0.210 |
| Days hospitalized (number, IQR) | 7 (2–30) | 10 (6–18) | 10 (6–16) | 10 (6–15) | 10 (6–16) | 10 (6–14) | 0.973 |
| Saturation without supplementary oxygen at admission (%) | 80 | 72 (60–84) | 80 (61–88) | 78 (61–89) | 74 (58–85) | 65 (57–79) | 0.204 |
| Leukocytes, ×103/ml | 6.8 (2.6–10.7) | 9.6 (6.5–13.6) | 9.3 (6.6–13) | 9.6 (6.7–13.3) | 8.8 (6.8–12.4) | 9.2 (6.3–15.4) | 0.915 |
| Neutrophils, ×103/ml | 5.6 (5.1–5.6) | 8.3 (5.4–12.3) | 8.0 (5.4–11.6) | 8.2 (5.3–11.6) | 7.3 (5.5–10.9) | 8.3 (4.8–13.2) | 0.998 |
| Lymphocytes, ×103/ml | 0.71 (0.15–0.71) | 0.69 (0.49–0.93) | 0.74 (0.51–0.98) | 0.79 (0.56–1.18) | 0.98 (0.67–1.23) | 0.85 (0.61–1.14) | 0.003 |
| Hemoglobin, g/L | 10.3 (7.1–12.7) | 13.9 (12–15.4) | 14.5 (12.7–15.9) | 14.7 (13.1–16) | 14.8 (13.2–16.1) | 15.3 (13.3–17) | 0.008 |
| Fibrinogen, mg/dl | 530 (435–530) | 673 (558–786) | 713 (587–797) | 668 (547–770) | 686 (596–783) | 707 (464–841) | 0.360 |
| D‐dimer, μg/ml | 2.23 (0.66–2.23) | 1.97 (1.14–4.5) | 1.58 (0.83–3.6) | 1.25 (0.73–2.5) | 1.22 (0.77–2.4) | 1.21 (0.79–2.3) | 0.012 |
| Procalcitonin, ng/ml | 2.83 | 0.51 (0.18–2.21) | 0.27 (0.11–0.82) | 0.42 (0.11–1.17) | 0.24 (0.12–1.81) | 0.85 (0.15–1.34) | 0.110 |
| Albumin, g/L | 3.0 (2.4–3.6) | 3.1 (2.7–3.4) | 3.2 (2.9–3.5) | 3.2 (2.8–3.6) | 3.3 (3–3.6) | 3.1 (2.8–3.6) | 0.054 |
| LDH, U/L | 421 (380–421) | 420 (330–559) | 420 (312–578) | 430 (295–618) | 473 (387–683) | 457 (345–610) | 0.373 |
| Reactive C protein, mg/dl | 11.2 (6.0–22) | 12.3 (5.9–22) | 12.8 (4.9–21.8) | 12.3 (5.6–19.6) | 13.6 (5.5–23.4) | 16.1 (9.6–21.8) | 0.495 |
| Ferritin, ng/ml | 70.1 | 922 (568–1661) | 1025 (600–1728) | 908 (466–1530) | 892 (311–1756) | 853 (447–1156) | 0.069 |
| INR | 1.16 (1.02–1.16) | 1.18 (1.07–1.26) | 1.13 (1.05–1.23) | 1.11 (1.04–1.19) | 1.14 (1.07–1.19) | 1.15 (1.04–1.27) | 0.146 |
| FPG, mg/dl | 108 (76–141) | 113 (87–145) | 110 (89–158) | 117 (90–164) | 131 (99–217) | 120 (93–165) | 0.086 |
| HbA1c, % | 5.4 (4.4–5.4) | 5.9 (5.3–7.8) | 6.6 (6.0–9.8) | 6.3 (5.8–8.3) | 6.6 (6.2–8.3) | 7.8 (6–9.4) | 0.039 |
| Total cholesterol, mg/dl | 121 (99–121) | 131 (106–161) | 134 (114–169) | 138 (117–167) | 150 (124–179) | 124 (115–170) | 0.188 |
| HDL‐c, mg/dl | 39 (25–39) | 26 (21–34) | 26 (18–33) | 25 (20–33) | 24 (17–33) | 26 (20–32) | 0.411 |
| LDL‐c, mg/dl | 44 (18–44) | 69 (53–93) | 73 (53–98) | 72 (59–102) | 76 (58–94) | 65 (55–103) | 0.328 |
| Triglycerides, mg/dl | 148 (112–148) | 161 (108–230) | 157 (119–239) | 163 (123–248) | 214 (139–333) | 161 (137–224) | 0.073 |
| Creatinine, mg/dl | 9.67 (2.7–17.6) | 0.90 (0.70–1.27) | 0.88 (0.73–1.25) | 0.82 (0.71–1.04) | 0.87 (0.71–1.27) | 0.97 (0.71–1.66) | 0.454 |
| Uric acid, mg/dl | 7.5 | 4.2 (2.9–6.3) | 4.5 (3.2–6.3) | 4.7 (3.0–6) | 4.8 (3.2–7) | 6.3 (3.7–7.7) | 0.609 |
| 25‐OH vitamin D, ng/dl | 5.72 | 13.1 (10–19.3) | 16.5 (11.3–20.5) | 13.7 (11–20.4) | 13.3 (7.3–17.8) | 9.7 (7.3–20.2) | 0.123 |
Note: Results are expressed in medians (interquartile ranges) or percentages.
Abbreviations: 25‐OH vitamin D, 25‐hydroxy vitamin D; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; INR, international normalized ratio; LDH, lactic dehydrogenase.
*Statistically different among groups using squared chi test or Kruskal–Wallis test, accordingly.
Patients with any degree of obesity had higher lymphocyte counts (p = 0.003), higher hemoglobin (p = 0.008) and glycated hemoglobin (p = 0.039) and lower D‐dimer (p = 0.012) than the patients with low or normal weight and overweight. Other clinical and biochemical characteristics grouped by BMI are depicted in Table 3. The patients in the extreme weights (grouped as underweight and grade III obesity) did not show any significant difference in the laboratory tests compared to the patients in other BMI categories, except for higher uric acid concentrations (6.3 vs. 4.5 mg/dl, p = 0.008).
Further analysis revealed that 30.8% of the patients with neumopathy had been diagnosed with the disease in the last month before hospitalization or during it, with a median time from diagnosis for the whole cohort of 3.5 years. For nephropathy, 53.8% were diagnosed during their hospitalization for COVID, the patients with previous diagnosis had a median of 5.8 years with the disease. Finally, for neoplasms, 36.7% had been diagnosed within the previous 6 months from hospitalization and 13.3% were diagnosed during it. Weight loss before hospitalization was reported by 80% of the patients with neoplasms.
3.3. COVID‐19 mortality risk
The patients with at least one of the major comorbidities (obesity, diabetes or hypertension) had significantly higher risk of in‐hospital death (OR 1.786, 95% CI 1.165–2.738, p < 0.001). The patients with extreme BMIs (low weight or grade III obesity) had higher risk of mortality with an OR 2.193 (CI 95% 1.127–4.270, p = 0.018). Other comorbidities were not significant to predict death except for kidney disease (OR 2.187, CI 95% 1.410–3.392, p < 0.001) and neoplasm (OR 1.956, CI 95% 1.169–3.273, p = 0.010).
A model including age, neoplasia, kidney disease and extreme BMIs was significant (p = 0.024) but individual factors other than extreme BMI were not individually significant. Furthermore, obesity was associated with a higher probability of having any of the 3 major comorbidities (OR 2.168, 95% CI 1.954–2.407, p < 0.001), but not the other significant comorbidities (nephropathy p = 0.066, neumopathy p = 0.103).
These high mortality rates among underweight and patients with severe obesity resulted in a J‐shaped curve (Figure 2). The fixed Cox Proportional Hazard Regression model showed that for mortality due COVID‐19, the hazard ratio (HR) for normal weight was 1.33 (0.45–3.94, p = 0.597), for overweight was 0.64 (0.38–1.08, p = 0.095), for grade I obesity was 0.60 (0.37–0.98, p = 0.043), for grade II obesity was 0.54 (0.32–0.91, p = 0.022) and for grade III obesity was 0.76 (0.43–1.33, p = 0.340). No differences were found in the HR for mechanical ventilation requirements nor for UCI admission by BMI category.
FIGURE 2.
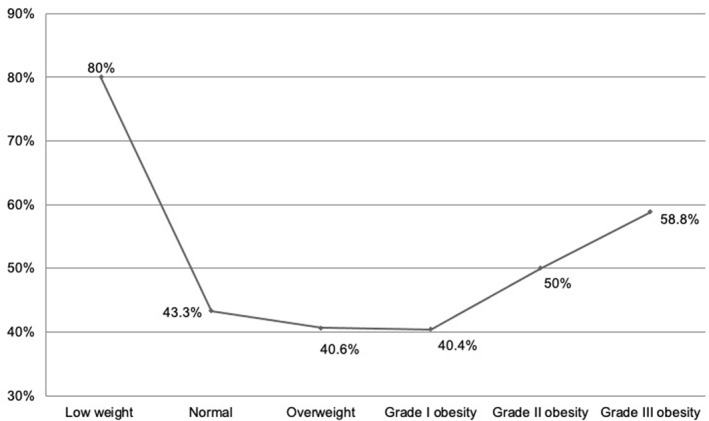
J‐shaped curve depicting the frequency of mortality in each BMI group. The leaner and heaviest patients had the highest mortality rates
Those patients with extreme BMIs (underweight/grade III obesity) had a HR for COVID‐19 mortality of 1.45 (1.07–1.95, p = 0.014) and a HR for requirement of mechanical ventilation of 1.71 (1.023–2.874, p = 0.041). The HR for ICU requirement was not significant in this group (HR 1.31, 0.705–2.43, p = 0.392). Regarding mortality due COVID‐19 or requirement of mechanical ventilation, no differences were observed using an interaction analysis with the major comorbidities, nor age or HbA1c (Figure 3).
FIGURE 3.
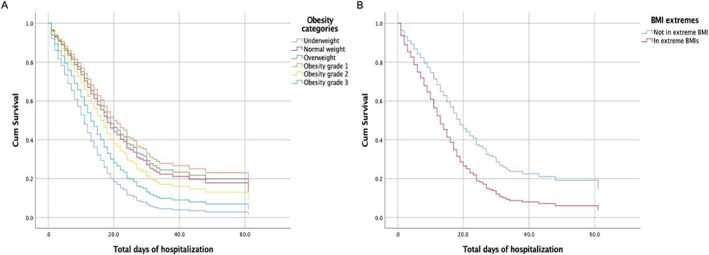
Cox Proportional Hazard Ratio for COVID‐19 mortality. (A) Patients with grade I and II obesity had the lowest HR. (B) Patients with underweight and grade III obesity (extremes BMI) had the higher HR
4. DISCUSSION
The mortality rates in the current study are higher than those reported previously, 14 especially for the patients with underweight and grade III obesity. The patients within extremes of the BMI classifications were more prone to require mechanical ventilation or had worse outcomes than those with normal weight or grade I or II obesity. Even when some patients with excess weight survived, the concept of the “obesity paradox” cannot be generalized for all degrees of obesity, particularly if other comorbidities are present in the context of severe COVID‐19. The patients in this cohort seem to be different in other important parameters compared with other publications: they are younger, the frequency of metabolic comorbidities is high, and these were corroborated by laboratory and imaging testing. 15
The elevated mortality rates in this cohort are multifactorial. These patients represent only one extreme of the disease spectrum, since only patients fulfilling the severity criteria and required hospitalization were included. This referral center is equipped with diagnostic tests and specialized treatments not available in other general hospitals, which makes it more likely to have patients with multiple and severe comorbidities that require a multidisciplinary attention. Despite the available resources, many of them are chronically or terminally ill and had worse outcomes than the general population.
A previous study in Mexico showed that people living with obesity, diabetes and hypertension had a 1.4‐fold, 1.8‐fold and 1.7‐fold higher odds of developing severe COVID‐19 on admission. 16 This is similar to these results, where the extreme BMIs (low weight or grade III obesity) had 1.45‐fold higher odds of COVID‐19 mortality. To our knowledge this is the first study that evaluated obesity but also low weight as risk for severe outcomes in COVID‐19; however, it only included the more severe cases and thus results cannot be extrapolated to milder cases. Also, another study reported that the addition of obesity to any number of comorbidities significantly increased the risk of fatality due to COVID‐19. 17 In the current study, the high mortality rate for patients in the extreme BMIs and the low rate for the first two obesity grades, were not affected by the presence of diabetes or dyslipidemia. This could be related with the high prevalence of those diseases in all the BMI categories in this study and agrees with the high incidence reported in Mexico. 3
Obesity has been related to worse COVID‐19 outcomes through different physiopathological pathways, such as the fact that the accumulation of fat tissue, especially visceral and white tissue, is related to chronic inflammation. 18 Another mechanism is the increased expression of ACE receptors in patients with hypertension, kidney disease and obesity, which may facilitate the access of the virus to susceptible tissues. 19 It is not currently known if the combination of these comorbidities increases this expression even further. Moreover, the influence of obesity seems to be paradoxical even after COVID‐19 infection. In a retrospective analysis of a prospective, observational registry in the USA, a BMI higher than 35 kg/m2 was associated with adverse cardiovascular, renal, pulmonary and mental health after acute COVID‐19, the postacute sequelae of COVID‐19 (PASC). However, nervous system, hepatic, endocrine or hematological complications as well as mortality rates were not different among groups. The authors suggest that these results could be related to obesity‐induced hyperinflammation, immune dysfunction and co‐morbidities. 20
A single determination of BMI was not a reliable biomarker to predict the outcomes in this group. The patients that were underweight or actively losing weight before the diagnosis of COVID‐19 were more likely to have neoplasms, uncontrolled diabetes or kidney disease, as well as other comorbidities associated with weight loss. These results suggest that the weight history in the few months before hospitalization should also be assessed.
Additionally, some differences in biochemical and hematological evaluation were found among BMI categories. Those patients in the lowest categories had lower HbA1c levels. However, this has to be taken cautiously, since patients with underweight and some with normal weight had lower hemoglobin levels. Also, lymphocytes were lower in the patients with the lowest BMI. Lymphopenia has been associated with disease severity and mortality. 21 , 22 However, despite statistical differences, the lymphocyte counts were below the normal ranges in the whole group (1–3.7 × 103/ml), which reflects the severity of the disease.
The obesity paradox in patients with obesity may be related with a certain protection mainly to the patients with a stable weight and enough reserves to fight an acute and severe illness, which means that the weight classification is not enough to predict mortality in these patients. It would be desirable to know the weight history for our patients in the weeks or months before hospitalization and to consider different comorbidities (known or unknown) according to their evolution, something that may prove to be difficult for the healthcare staffs in the middle of a pandemic. However, our results show that the patients in the lower weights or that have been actively losing weight may benefit from additional efforts to evaluate kidney or neoplastic comorbidities, while the patients in the higher weights may benefit from screening from lung and kidney diseases. All the population needs to be cautiously and continuously evaluated for metabolic diseases during their hospital stay such as hypertension or diabetes, and additional attention should be paid to the evolution of these diseases after the acute process and long term follow up.
A limitation of this study is that we used BMI as the only biomarker for obesity. This parameter may not be enough to determine the full metabolic state, where people actively losing or gaining muscle or fat, may behave differently than the patients with stable weight even when they are all in the same classification by BMI. 23 However, patients are not routinely measured for waist or hip circumference and body composition tests were not available in the COVID‐19 area in our center. Also, the sample size of patients with underweight is small, and a correct interpretation of the results in them should be done cautiously. These results should not be interpreted as a recommendation to increase body weight in order to improve the chances to survive COVID‐19. The studies in open population show that the patients in healthy body weights have less probabilities of having symptomatic COVID‐19 or developing complications or requiring hospitalizations. 24 Population based interventions should be clear about the need to keep a healthy lifestyle to prevent complications.
Another limitation is that the follow‐up of these patients to their hospital discharge could be not enough to determine the overall effect of the disease, since it was not possible to evaluate the long‐term mortality for the survivors. However, this study shows that the published data for obesity as a risk factor for adverse outcomes in severe COVID‐19 requires further analysis in each population.
5. CONCLUSION
The mortality rate of patients with severe COVID‐19 varies within BMI classifications. In this cohort, patients with grade I or II obesity had lesser risk of death meanwhile those in the lowest or highest BMI groups had the highest mortalities, describing a J‐shaped curve.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Alejandra Albarrán‐Sánchez, Claudia Ramírez‐Rentería, Juan C. Anda‐Garay, Maura E. Noyola‐García, Paolo Alberti‐Minutti, Guillermo Flores‐Padilla, Luis A. Guizar‐García and Carlos E. Contreras‐García participated in the collection and analysis of data and helped to draft the manuscript; Daniel Marrero‐Rodríguez, Keiko Taniguchi‐Ponciano, Moises Mercado and Aldo Ferreira‐Hermosillo participated in the analysis of data and helped to draft the manuscript. All the authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The research project was not supported by extramural funding.
Albarrán‐Sánchez A, Ramírez‐Rentería C, Anda‐Garay JC, et al. Differences in mortality rate among patients hospitalized with severe COVID‐19 according to their body mass index. Obes Sci Pract. 2022;8(4):423‐432. 10.1002/osp4.584
Alejandra Albarrán‐Sánchez and Claudia Ramírez‐Rentería contributed equally to this work.
REFERENCES
- 1. WHO . Weekly Epidemiological Update COVID‐19, 28 September 2021; 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---28-september-2021 [Google Scholar]
- 2. CONACYT – CentroGeo – GeoInt – DataLab . Covid‐19 Mexico. Gobierno de México; 2021. Accessed March, 2021. https://datos.covid-19.conacyt.mx/ [Google Scholar]
- 3. Shamah‐Levy TV‐OE, Heredia‐Hernández O, Romero‐Martínez M, et al. Encuesta Nacional de Salud y Nutrición 2018‐19: Resultados Nacionales. Instituto Nacional de Salud Pública; 2020. [Google Scholar]
- 4. Biscarini S, Colaneri M, Ludovisi S, et al. The obesity paradox: analysis from the SMAtteo COvid‐19 REgistry (SMACORE) Cohort. Nutr Metab Cardiovasc Dis; 2020;30(11):1920‐1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halasz G, Leoni ML, Villani GQ, Nolli M, Villani M. Obesity, overweight and survival in critically ill patients with SARS‐CoV‐2 pneumonia: is there an obesity paradox? Preliminary results from Italy. Eur J Prev Cardiol. 2020:2047487320939675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavie CJ, Coursin DB, Long MT. The obesity paradox in infections and implications for COVID‐19. Mayo Clin Proc. 2021;96(3):518‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li T, Zhang Y, Gong C, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID‐19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non‐linear dose‐response meta‐analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao M, Piernas C, Astbury NM, et al. Associations between body‐mass index and COVID‐19 severity in 6.9 million people in England: a prospective, community‐based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coss‐Rovirosa MF, Aguilar‐Soto M, Cuenca D, et al. Are overweight and obesity risk factors for invasive mechanical ventilation in severe coronavirus disease 2019 pneumonia? Arch Endocrinol Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected ‐ obesity, impaired metabolic health and COVID‐19. Nat Rev Endocrinol. 2021;17(3):135‐149. [DOI] [PubMed] [Google Scholar]
- 12. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698‐710. [DOI] [PubMed] [Google Scholar]
- 13. Berlin DA, Gulick RM, Martinez FJ. Severe Covid‐19. N Engl J Med. 2020;383(25):2451‐2460. [DOI] [PubMed] [Google Scholar]
- 14. Zhang F, Xiong Y, Wei Y, et al. Obesity predisposes to the risk of higher mortality in young COVID‐19 patients. J Med Virol. 2020;92(11):2536‐2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parra‐Bracamonte GM, Lopez‐Villalobos N, Parra‐Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID‐19 in a large data set from Mexico. Ann Epidemiol. 2020;52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Denova‐Gutierrez E, Lopez‐Gatell H, Alomia‐Zegarra JL, et al. The association of obesity, Type 2 diabetes, and hypertension with severe coronavirus disease 2019 on admission among Mexican patients. Obes. 2020;28(10):1826‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bello‐Chavolla OY, Bahena‐Lopez JP, Antonio‐Villa NE, et al. Predicting mortality due to SARS‐CoV‐2: a mechanistic score relating obesity and diabetes to COVID‐19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanchis‐Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and outcomes in COVID‐19: when an epidemic and pandemic Collide. Mayo Clin Proc. 2020;95(7):1445‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan T, Xiao R, Wang N, Shang R, Lin G. Obesity and severe coronavirus disease 2019: molecular mechanisms, paths forward, and therapeutic opportunities. Theranostics. 2021;11(17):8234‐8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aminian A, Bena J, Pantalone KM, Burguera B. Association of obesity with postacute sequelae of COVID‐19. Diabetes Obes Metab. 2021;23(9):2183‐2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang HJ, Qi GQ, Gu X, et al. Lymphocyte blood levels that remain low can predict the death of patients with COVID‐19. Med Baltim. 2021;100(28):e26503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Illg Z, Muller G, Mueller M, Nippert J, Allen B. Analysis of absolute lymphocyte count in patients with COVID‐19. Am J Emerg Med. 2021;46:16‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu P, Ma F, Lou H, Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Publ Health. 2013;13:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang IS, Kong KA. Body mass index and severity/fatality from coronavirus disease 2019: a nationwide epidemiological study in Korea. PLoS One. 2021;16(6):e0253640. [DOI] [PMC free article] [PubMed] [Google Scholar]


