Summary
The study of innate immunity and its link to inflammation and host defenses encompasses diverse areas of biology, ranging from genetics and biophysics to signal transduction and physiology. Central to our understanding of these events are the Toll-like Receptors (TLRs), an evolutionarily ancient family of pattern recognition receptors. Herein, we describe the mechanisms and consequences of TLR-mediated signal transduction, with a focus on themes identified in the TLR pathways that also explain the operation of other immune signaling pathways. These themes include the detection of conserved microbial structures to identify infectious agents and the use of supramolecular organizing centers (SMOCs) as signaling organelles that ensure digital cellular responses. Further themes include mechanisms of inducible gene expression, the coordination of gene regulation and metabolism, and the influence of these activities on adaptive immunity. Studies in these areas have informed the development of next-generation therapeutics, thus ensuring a bright future for research in this area.
eTOC blurb – Kagan & Fitzgerald
Kagan & Fitzgerald comprehensively review the functions and mechanisms of the Toll-like Receptor (TLR) family, which are crucial detectors of microbial biomolecules and mediators of cellular immunity. They synthesize the overarching themes that have emerged from TLR signaling but are repeated throughout the pattern recognition receptor (PRR) superfamily.
Introduction
Throughout recorded history, the possibility of infection has posed a significant hindrance to the growth, stability and progress of all societies. Arguably, the greatest benefit that society has experienced from the modern age of biomedical research is derived from efforts to prevent or eliminate infections. The widespread use of vaccines and post-infection anti-microbial therapeutics has enabled children in the developed world to reach adulthood at a much greater rate than experienced by prior generations (Andre et al., 2008). These successes in leveraging scientific discoveries to increase our children’s survival have immeasurable benefits to society and should be embraced by this generation and those that follow. At the foundation of any discussion of infection is a discussion of the immune response that coincides with a microbial encounter. Indeed, many of the clinical symptoms of infection do not necessarily result from actions of the microbe per se, but rather result from the immune response to the microbe (Medzhitov, 2008). Thus, understanding how immune responses are initiated and regulated is a critical aspect of host-microbe interactions. It is with this idea in mind that Toll-like Receptors (TLRs) have emerged as a focal-point of biomedical research, as this small family of proteins serves as one of the earliest determinants of immune activation. In this review, we provide a comprehensive exploration of ways that immunity and host physiology are influenced by TLRs. We highlight several lessons that the study of TLRs has taught us, as many TLR-like activities were subsequently identified in other signaling pathways. These lessons include 1) the mechanisms of microbial detection and signal transduction by TLRs, 2) the means by which the detection of single microbes can lead to the activation of many more than one host cell (i.e. signal amplification), 3) the importance of microbe-inducible protein trafficking pathways to stimulate (or inhibit) inflammatory responses, and 4) the necessity of TLRs for the induction of adaptive immunity. These activities apply not only to the TLR network, but to an increasingly diverse array of receptor families, cells and organisms.
Identification of TLRs as pattern recognition receptors
TLRs were the first family of proteins to fulfill Janeway’s predictions of the defining features of Pattern Recognition Receptors (PRRs) (Janeway, 1989). PRRs were speculated to operate as germline encoded proteins that recognize conserved microbial products (Pathogen Associated Molecular Patterns (PAMPs)), and consequently induce activities that stimulate immunity and host defense. It was further suggested that, since all multicellular organisms face the threat of infection, these putative PRRs would have evolved early in life’s history, and therefore be present in all living beings.
Early studies of the mechanisms used by insects to recognize and fight infections were critical to the discovery of the immune functions of TLRs. Indeed, the identification of evolutionarily conserved NF-κB family transcription factors as regulators of anti-microbial responses in Drosophila melanogaster led to the suggestion that similar defense pathways operate in mammals (Gay and Keith, 1991; Nusslein-Volhard and Wieschaus, 1980; Schneider et al., 1991; Steward, 1987). Subsequent genetic analysis led to the discovery that the Toll protein sits at the apex of the cell-associated factors that drive anti-microbial responses in Drosophila (Lemaitre et al., 1996). The human homologue of the Drosophila Toll protein (known today as TLR4) was subsequently identified as a receptor capable of driving responses in antigen presenting cells that promote inflammation and adaptive immunity. Genetic analysis by three independent groups, first by Poltorak and colleagues (Hoshino et al., 1999; Poltorak et al., 1998; Qureshi et al., 1999), then identified bacterial lipopolysaccharide (LPS) as the microbial stimulus that activates TLR4. These seminal findings unleashed an avalanche of interest in the TLR pathways, with an initial focus on the mechanisms of PAMP detection and receptor signaling. These topics are still under heated investigation today. In the next sections, we will discuss the current knowledge of how TLRs detect ligands and how these detection events lead to changes in cellular behavior that influence downstream inflammatory and adaptive immune responses.
Mechanisms of TLR synthesis and biosynthetic trafficking
All TLRs share a similar domain organization, as each family member is a Type-I transmembrane protein. The N-terminal ectodomain consists of leucine-rich repeats, which is followed by a single transmembrane domain and a cytosolic TIR domain. Genome browsing for proteins that share these features has identified TLR family members in organisms ranging from corals to humans, thus underscoring the idea that PRRs are evolutionarily ancient proteins (O’Neill and Greene, 1998; Rock et al., 1998). All organisms appear to encode a distinct number of TLRs, with humans and mice respectively encoding 10 and 12 TLRs. Drosophila encode 9 Toll receptors and the purple sea urchin encodes 222 TLRs (Imler and Hoffmann, 2002; Satake and Sekiguchi, 2012). The functions of most of these proteins are undefined.
In mammals, TLRs are synthesized in the endoplasmic reticulum (ER) and transported to their ultimate destinations in the cell, which are the plasma or endosomal membranes. Evidence supporting the central role of the ER in TLR synthesis derives from studies of the proteins gp96, PRAT4A and Unc93B1, which are ER-localized proteins (Randow and Seed, 2001; Tabeta et al., 2006; Takahashi et al., 2007). Gp96 is related to the Hsp90 family of proteins that influences the folding and functions of immunoglobulin chains, integrins and TLRs. All TLRs, with the exception of TLR3 are unable to function in macrophages deficient in gp96, an observation consistent with the central role of this protein in folding the entire TLR family (Liu et al., 2010; Yang et al., 2007). Similarly, cells lacking PRAT4A contain no functional TLRs, except TLR3 (Takahashi et al., 2007). This lack of gp96 or PRAT4A requirement for TLR3 signaling may need to be re-examined however, as a PRR distinct from the TLR family (MDA5) has overlapping functions with TLR3 in response to the same chemical ligand (PolyIC) (Gitlin et al., 2006; Kato et al., 2006). Regardless, the central role of ER-localized chaperones in the function of TLRs supports the idea that these PRRs are synthesized in the ER and likely follow the traditional route through the early secretory pathway. Further support of this statement derives from studies demonstrating the sequential glycosylation events of TLRs that is expected to occur as these proteins proceed from the ER to the Golgi complex (reviewed in (Barton and Kagan, 2009)). While the endosomal TLRs are synthetized in the ER along with their plasma membrane-localized counterparts, the former group of receptors are transported to endosomes via a dedicated protein chaperone known as Unc93B1 (Kim et al., 2008). Unc93B1 binds endosomal TLRs in the ER and somehow facilitates their proper folding. The relationship between the folding activities of Unc93B1, gp96 and PRAT4A is undefined. Once folded, Unc93B1 remains bound to its client TLRs and is necessary to maintain TLR stability after synthesis (Pelka et al., 2018). Unc93B1 promotes their incorporation into COPII-coated vesicles that bud off the transitional ER (Lee et al., 2013). After Unc93B1 and its TLR cargo traverse the Golgi complex, the symmetry between the trafficking of all TLRs is lost. TLRs 7,11, 12 and 13 are transported directly from the Golgi into the endosomal network, perhaps via interactions between the TLR and the AP-4 trafficking adaptor (Lee et al., 2013). TLR9, in contrast, is delivered first to the plasma membrane. An AP-2 binding site in Unc93B1 promotes its endocytosis and that of its associated cargo TLR9, leading to their delivery into the endosomal network (Lee et al., 2013). The functional significance of these differential routes to endosomes is unclear, but may underlie the differential phenotypes associated with TLR7 and TLR9 deficiencies in murine models of autoimmunity—discussed later in this manuscript.
Upon delivery to endosomes, TLR7 and Unc93B1 remain associated and Unc93B1 functions to restrict the signaling activities of TLR7. This restriction of signaling is mediated by the cytosolic protein syntenin-1, which promotes exosome formation (Majer et al., 2019). It has been suggested Unc93B1 interactions mediate the internalization of TLR7 into multivesicular bodies, a process that terminates TLR7 signaling (Majer et al., 2019). In the context of TLR9 signaling, Unc93B1 has a different post-trafficking function. TLR9 must be released from Unc93B1 in order to bind CpG DNA and promote downstream signaling events (Majer et al., 2019). Once within endosomes, TLRs 3, 7, 8, 9 and 13 are cleaved by several cathepsins within their leucine-rich repeat containing ectodomains (Ewald et al., 2011; Fukui et al., 2018). The cleaved fragments remain associated with one another and both fragments are important for the inflammatory activities of these receptors (Ohto et al., 2018; Onji et al., 2013). Structural analysis has explained why cleavage of the ectodomains of endosomal TLRs is important for signaling, as the full-length (non-cleaved) proteins are incapable of forming contacts with one another that would be necessary for receptor dimerization (Ohto et al., 2018; Tanji et al., 2016), a prerequisite for signal transduction (Latz et al., 2007). Notably, uncleaved receptors are capable of PAMP interactions (Ohto et al., 2015). Thus, in the case of TLRs in endosomes, interactions with PAMPs are necessary but not sufficient for signal transduction. Functional interactions only occur between cleaved PRRs.
In contrast to the increasingly complex biosynthetic trafficking pathways taken by endosomal TLRs, plasma membrane localized TLRs do not appear to require any dedicated trafficking factors. These receptors include TLRs 1, 2, 4 and 5, which likely reach their ultimate destination via the conventional secretory pathway.
Microbial detection by TLRs
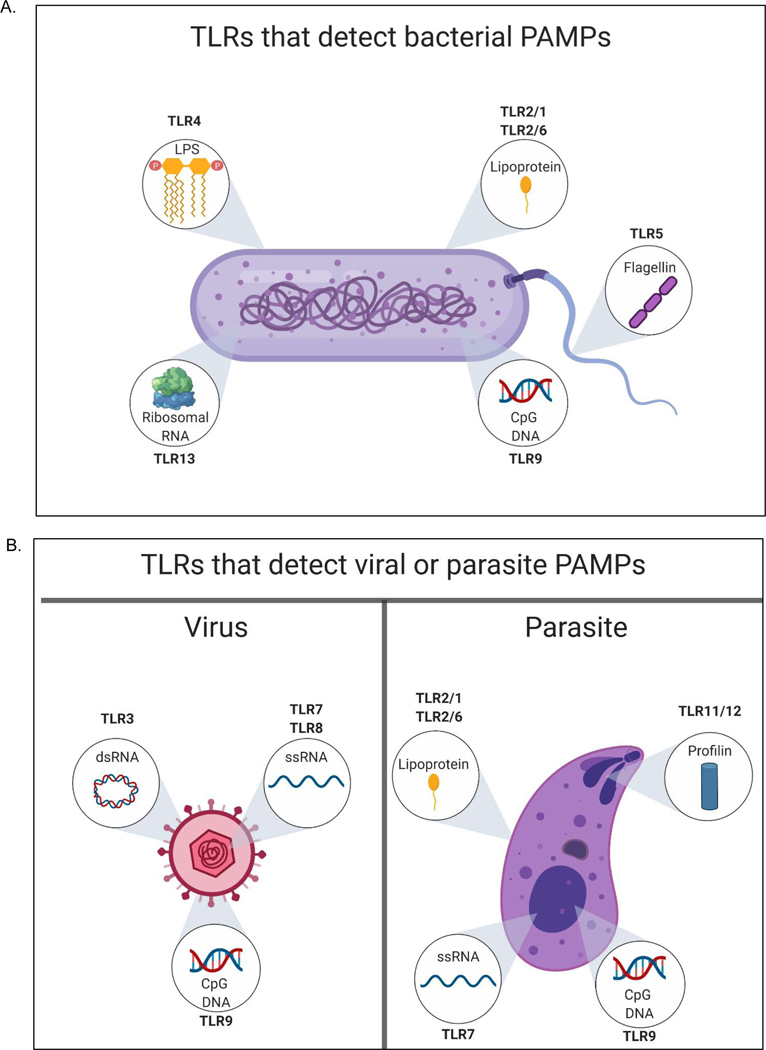
Mammalian TLRs that occupy the plasma membrane include those that detect microbial cell surface components, such as TLR4, which detects LPS (Poltorak et al., 1998; Poltorak et al., 1998), TLR5 (flagellin) (Gewirtz et al., 2001; Hayashi et al., 2001), and TLRs 1, 2 and 6 (bacterial lipoproteins) (Kang et al., 2009; Takeuchi et al., 1999; Takeuchi et al., 2001; Takeuchi et al., 2002). TLRs found in endosomes detect nucleic acids, such as TLR3 (double stranded (ds) RNA) (Alexopoulou et al., 2001), TLR7 and 8 (single stranded (ss) RNA) (Diebold et al., 2004; Greulich et al., 2019; Heil et al., 2004; Hemmi et al., 2002; Kruger et al., 2015), TLR9 (unmethylated CpG containing ssDNA) (Hemmi et al., 2000) and TLR13 (bacterial ribosomal RNA) (Hidmark et al., 2012). In all the above-described examples, direct PAMP-PRR interactions have been identified. Murine TLR11 recognizes the profilin protein from Toxoplasma gondii (Yarovinsky et al., 2005). Ligands for several mammalian TLRs remain undefined. Human TLR10 (which is a pseudogene in mice) does not have a clear ligand yet. This collection of PAMP-PRR interactions allows multiple members of the TLR family to detect individual microbial cells (Figure 1).
Figure 1.
Multiple TLR family members can detect PAMPs on individual microorganisms. A bacterium (A), virus or parasite (B) are depicted. Insets display PAMPs and the TLRs that are responsible for their detection. The TLR names represent those found in mice, as genetic evidence is available to support the importance of each TLR-PAMP interaction indicated.
In insects, the best-characterized TLR is the ancestral Toll Receptor (Anderson et al., 1985a; Anderson et al., 1985b; Lemaitre et al., 1996). This receptor does not detect PAMPs directly, but rather detects the activities of upstream PRRs. In Drosophila, Toll is displayed on the surface of cells in the fat body. Bacterial peptidoglycan (PGN) fragments (e.g. (Lys-type PGN) that enter the open circulatory system of Drosophila bind to the soluble protein PGRP-SA (Gottar et al., 2002). PGN binding by PGRP-SA is facilitated by the actions of the proteins GNBP1 and PGRP-SD, the former of which is a muramidase-like enzyme that hydrolyzes PGN into multiple short ligands for PGRP-SA (Wang et al., 2006). PGN binding by PGRP-SA stimulates a proteolytic cascade that results in the cleavage of the protein pro-Spätzle, which generates the ligand for Toll, Spätzle (Spz). A similar sequence of events occurs during fungal infections, but this process is mediated by a distinct upstream PRR known as GNBP3 (Buchon et al., 2009; Matskevich et al., 2010) which binds to β−1,3-glucans found in the fungal cell wall. Additionally, a latent protease known as persephone can be activated by fungal proteases, leading to Spz production and Toll signaling (Ligoxygakis et al., 2002). While the insect Toll network differs from mammalian TLRs in that a protease cascade links microbial detection by a soluble PRR to downstream defensive responses, a potential benefit of this network was illustrated by the preceding discussion—multiple PRRs can evolve to bind different PAMPs and feed information downstream to a single Toll receptor. Why this similar protease-cascade did not evolve in mammalian TLR networks is unclear, but we speculate that additional network-design distinctions will reveal themselves as other branches on the evolutionary tree are explored. Much of our structural insight derives from studies of mammalian TLR-PAMP interactions. All mammalian TLRs examined form direct contacts with their PAMP ligands through interactions between their leucine rich repeat containing ectodomains and cognate microbial products (Jin and Lee, 2008). In the instances where structural information is available, ligand binding occurs through interactions with a dimer of TLR ectodomains. These ectodomains can be homodimers, in the cases of TLR3, 4, 5, 7, 8 and 9, or as heterodimers of TLRs 1 and 2 or TLRs 2 and 6 (Choe et al., 2005; Jin et al., 2007; Jin and Lee, 2008; Park et al., 2009). The number of PAMPs bound by individual dimers varies. Single molecules of dsRNA bind TLR3 dimers (Choe et al., 2005) and single molecules of di-or tri-acylated lipoproteins bind TLR2-TLR1 or TLR2-TLR6 heterodimers (Jin et al., 2007), respectively. In contrast, TLR9 dimers bind two molecules of CpG containing DNA (Collins and Wilson, 2014 ) and each TLR4 present in a homodimer forms direct or indirect contacts with two molecules of LPS (Park et al., 2009). The affinity of the interactions described above can vary greatly, with some interactions occurring with nanomolar concentrations of ligand (TLR2, 3, 5 and 9) (Leonard et al., 2008; Ohto et al., 2018; Olguin et al., 2013; Vasselon et al., 2004). Other interactions occur at higher concentrations of ligand (TLR4, 7 and 8) (Park et al., 2009; Tanji et al., 2015). High-affinity interactions defined in vitro reflect the behaviors observed within cells, where very low concentrations of ligand are typically sufficient to stimulate inflammatory responses in macrophages. Low affinity (micromolar) interactions, in contrast, do not reflect the behavior of receptor-ligand interactions in cells. In the case of TLR4-LPS interactions, significant mechanistic analysis has explained the disconnect between in vitro affinities and in vivo behaviors. LPS is a complex glycolipid that represents a major constituent of the gram-negative bacterial cell wall. LPS consists of three major components, a hydrophilic solvent-exposed repeating oligosaccharide chain known as O-antigen, a membrane-proximal core oligosaccharide and a membrane-embedded di-glucosamine backbone that is attached to a varying number of acyl chains (Chandler and Ernst, 2017). This membrane-embedded structure is known as Lipid A and represents the specific component of LPS that stimulates TLR4. While TLR4 forms weak and minimal contacts with LPS (Picard et al., 2010), picomolar concentrations of this PAMP are capable of stimulating TLR4-dependent inflammatory responses in macrophages (Gioannini et al., 2004). In order for these high sensitivity interactions to occur, additional LPS-binding proteins must act upstream of TLR4 (Gioanninietal., 2004; Ryuetal., 2017). The extracellular LPS binding protein (LBP) forms direct contacts with the bacterial outer membrane (or micelles of LPS) and alters the outer membrane in a manner that facilitates the extraction of a single molecule of LPS by the protein CD14 (Gioannini et al., 2004). CD14 can either exist as a soluble extracellular protein or a GPI-anchored protein embedded in the outer leaflet of the mammalian cell plasma membrane (Frey et al., 1992; Lee et al., 1993; Tobias et al., 1986; Wright et al., 1990). Regardless of its soluble or membrane-bound positioning, CD14 acts to transfer a single molecule of LPS to the protein MD2 (Gioannini et al., 2004). MD2 is a small protein that interacts stably with the ectodomain of TLR4, forming TLR4-MD2 heterodimers that represent the functional LPS receptor (Schromm et al., 2001; Shimazu et al., 1999). Upon CD14-mediated transfer of LPS to MD2, TLR4 dimerization occurs (Akashi et al., 2000). Structural analysis of this process explained the molecular basis of TLR4 dimerization, as acyl chains within the Lipid A region of LPS interact with distinct regions of two TLR4-MD2 heterodimers (Park et al., 2009). 5 of the 6 acyl chains present in hexacylated Lipid A interact with a hydrophobic pocket present in the MD2 component of a TLR4-MD2 heterodimer. The 6th acyl chain does not interact with the TLR4 component of this heterodimer, but rather interacts with a different TLR4 molecule. LPS structures that contain less than 6 acyl chains have minimal ability to crosslink distinct sets of TLR4-MD2 heterodimers, thus explaining their weakened inflammatory activities (Park et al., 2009).
There are three benefits to the cascade of LPS transfer from LBP to CD14 to MD2-TLR4. First, this sequence of events allows TLR4 dimerization to occur at picomolar concentrations of LPS, whereas removal of any upstream LPS receptor renders higher concentrations necessary for TLR signaling. Second, it has been estimated that thousands of molecules of LPS can be extracted from a single gram-negative bacterium (Gioannini and Weiss, 2007). These thousands of LPS-CD14 complexes can be present on (and stimulate TLR4-MD2 dimers on) thousands of different macrophages (Gioannini and Weiss, 2007). Thus, the LPS extracted from a single bacterium can amplify the number of macrophages participating in a subsequent inflammatory response. This signal amplification strategy can be considered analogous to the PRR-mediated protease cascade that occurs upon PAMP detection in insects, leading to Toll receptor activation. Like CD14 actions, many molecules of Spz can be generated upon detection of individual bacterial or fungal cells, rendering numerous Toll-expressing fat body cells active participants in the immune response.
The third benefit of the LPS transfer cascade relates to the existence of a soluble (not GPI-anchored) population of CD14 (Frey et al., 1992; Moreno et al., 2004). Several cell types express TLR4 and MD2, yet do not express CD14. Endothelial cells are one such example. These cells are weak responders to LPS, as compared to cells that express CD14. Soluble CD14 present in the bloodstream may deliver LPS to TLR4-MD2 on endothelia, increasing the sensitivity of these cells to bacterial infections (Haziot et al., 1993). Additionally, soluble CD14 is present in milk (Labeta et al., 2000). This pool of CD14 can be transported across the intestinal epithelium and delivered to the bloodstream of newborn mice (Ward et al., 2014). This delivery system is so efficient that the LPS unresponsiveness of CD14-deficient mice can be complemented by nursing these animals on wild type mothers. Overall, the presence of an LPS transfer cascade mediated by soluble-and membrane-bound receptors enables a diverse repertoire of cells to alter their responses to bacterial infection. This flexibility-of-activity may enable context-dependent immune responses to be induced.
The mechanistically distinct signal amplification strategies upstream of insect Toll and mammalian TLR4 likely evolved independently of one another. While the benefits of such strategies may explain their existence, the question arises as to why other TLRs do not utilize similar signal amplification approaches. It is possible that other TLRs do use such approaches, but mechanisms have not yet been defined. For example, the scavenger receptors CD36 (Triantafilou et al., 2006), mannose binding lectin (MBL) (Ip et al., 2008), as well as CD14, somehow promote delivery of PAMPs to TLR2, and the DNA-binding proteins HMGB1 and RAGE can promote CpG DNA delivery to endosome-localized TLR9 (Tian et al., 2007). Notably, the genetic requirement for these proteins is most easily revealed when whole bacteria (as opposed to pure ligands) are used to stimulate macrophages. This observation is consistent with the idea that a rate-limiting step in TLR activation is the extraction of PAMPs from the microbial cell, but whether these activities also permit amplification of the number of cells signaling via TLR2 is unknown.
Recent studies of how TLRs 7, 8 and 9 detect their nucleic acid ligands have illustrated ligand-binding mechanisms that are more complex than were initially considered. TLR7 dimerization is most efficient in the presence of ssRNA and free guanosine molecules (Shibata et al., 2016; Zhang et al., 2016). Similarly, TLR8 dimers form most efficiently in the presence of ssRNA and free uridines (Greulich et al., 2019; Kruger et al., 2015; Tanji et al., 2015). TLR9 dimers form best in the presence of CpG-containing DNA and free cytosines, although cytosines that are positioned 5’ to the CpG motif in a single strand of DNA also maximize TLR9 dimerization (Ohto et al., 2018; Pohar et al., 2017). Structural analysis explained these observations, as distinct regions of the TLR7, 8 and 9 ectodomains interact with the free nucleoside and the DNA sequence (Ohto et al., 2018). Free nucleoside binding occurs within the dimerization interface, whereas ssRNA or ssDNA binding is thought to reinforce these interactions and stabilize dimer formation. Why two distinct sites within TLR ectodomains must bind nucleic acids for dimerization to be stabilized is unclear, but it may relate to the nature of the ligands these PRRs detect. Nucleic acids are not uniquely microbial and therefore PRRs that detect nucleic acids as PAMPs have the potential to detect self-molecules and cause autoinflammatory or autoimmune disorders. Indeed, PRRs that detect nucleic acids are commonly implicated as causal factors in murine models of autoinflammatory and autoimmune disease (Sharma et al., 2015). TLRs that detect microbial cell surface components are rarely implicated in autoimmunity. Mechanisms must therefore be in place to maximize the distinction between self and non-self nucleic acids. One such mechanism may relate to the need for receptors to bind ligand within endosomes and not recognize RNA or DNA ligands in the extracellular space.
The importance of spatially restricting TLR recognition of nucleic acids to endosomes was revealed by studies that artificially positioned TLR9 at the cell surface (Barton et al., 2006). Under these conditions, inflammatory responses are induced upon cellular exposure to extracellular DNA, leading to lethal autoinflammatory responses in mice (Mouchess et al., 2011). The process by which dual ligands (nucleic acid sequences and free nucleosides) are produced to maximally dimerize TLRs may occur only in endosomes through the actions of acid-dependent nucleases present in these organelles. Indeed, cells lacking the lysosomal enzyme DNaseII are defective for TLR9 signaling (Chan et al., 2015b) and cells lacking the lysosomal RNase T2 are defective for TLR8 signaling (Greulich et al., 2019). Additionally, at least in the case of TLR3, the affinity of PRR for PAMP is strongest at acidic pH (de Bouteiller et al., 2005). Several mechanisms are therefore in place to ensure that nucleic acid-mediated inflammatory responses only occur from an endosomal location. These mechanisms include 1) the necessary cleavage of TLRs by endosomal proteases to generate functional PRRs, 2) the necessary processing of nucleic acids by endosomal nucleases to generate functional PAMPs, and 3) at least in the case TLR3, the necessary presence of PAMP and PRR in an acidic environment for high affinity receptor-ligand interactions. None of these regulatory steps are important for the detection of bacterial cell wall components, an observation that suggests the critical cost of using nucleic acids as PAMPs that stimulate host immune responses. Numerous regulatory processes must operate to allow efficient distinction between self and non-self when the molecules in question are nucleic acids. From an evolutionary perspective, it is notable that while the risk of self-detection appears high, the PRRs that detect nucleic acids are found in virtually all multicellular organisms that have been examined. Indeed, nucleic acid sensing TLRs are relatively easy to identify in genomes far-ranging on the evolutionary tree. In contrast, although much attention has been paid to the LPS detection systems in mammals, plants display no evidence of PRRs that detect Lipid A (Kutschera et al., 2019), and the CD14-TLR4-MD2 network is missing from the genomes of all fish (for example (Kutschera et al., 2019). Thus, despite the risks associated with using nucleic acids as PAMPs, life as we know it appears to have invested heavily in such a strategy of microbial detection. Even in the case of bacteria-host interactions, where TLRs that detect cell wall components are well known, at least one TLR is uniquely designed to detect bacterial nucleic acids. Murine TLR13 is activated by a specific sequence present in bacterial ribosomal RNA (Hidmarketal., 2012; Oldenburgetal., 2012). This same sequence is the target of the antibiotic erythromycin, and mutation of this RNA sequence renders TLR13 detection impossible (Oldenburg et al., 2012) . Sensing of bacterial ribosomal RNA may be most important during encounters with microbes whose cell walls are modified in ways that render them poorly immunostimulatory. An example of such a microbe is group B Streptococcus, where TLR13 is the near-exclusive driver of inflammatory responses (Hafner et al., 2019; Hidmark et al., 2012). In the next sections, we will shift focus to the downstream consequences of PAMP detection by TLRs.
Mechanisms of TLR signal transduction
The PAMP-mediated dimerization of TLR ectodomains is a fundamental feature of the signaling process, as ectodomain dimerization results in the coordinate dimerization of the cytosolic TIR domains of these PRRs (Latz et al., 2007). Whether present at endosomes or the plasma membrane, TIR domain dimerization results in the activation of massive changes in cellular activities, which include changes in gene transcription, splicing, translation efficiency, autophagy, glycolysis and oxidative phosphorylation (Figure 2). The downstream mechanisms that link dimerized TLRs to these responses are under investigation, but all initiating signals appear to emanate from a dimerized TIR domain. As such, our attention will begin at this level. Upon microbial detection, dimerized receptor TIR domains are detected by the receptor-proximal membrane proteins TIRAP (also known as MAL) (Fitzgerald et al., 2001; Horng et al., 2001) and TRAM (Fitzgerald et al., 2003b; Yamamoto et al., 2003b). TIRAP/MAL is a peripheral membrane protein that surveys the inner leaflets of the plasma and endosomal membranes through the actions of an N-terminal phosphoinositide binding domain (Kagan and Medzhitov, 2006). At its C-terminus, TIRAP/MAL contains a TIR domain, which recognizes dimerized TIR domains of most TLRs and is necessary for signaling by TLRs 2, 4, 7 and 9. Of note, the requirement of TIRAP/MAL for TLR signaling is most easily observed when natural PAMPs are utilized as immunostimulatory agents. The use of unnatural stimuli (e.g. DNA containing phosphorothioate linkages) or high concentrations of natural stimuli can bypass the genetic requirement for TIRAP/MAL in TLR signaling (Bonham et al., 2014; Horng et al., 2002). Upon PAMP-recognition by a TLR, TIRAP/MAL detects the dimerized TIR domain and stimulates the assembly of a large oligomeric scaffold of cytosolic proteins known as a supramolecular organizing center (SMOC) (Kagan et al., 2014; Lin et al., 2010; Motshwene et al., 2009). SMOCs operate in multiple innate immune pathways and are considered the principal subcellular sites of signals that drive cellular responses to microbial detection. SMOCs are not present in resting cells, but are assembled within minutes of TLR dimerization. The SMOCs that govern TLR signaling include the myddosome, which is seeded by TIRAP/MAL and the putative triffosome (Figure 3), which may be seeded by a related protein known as TRAM (described below). The myddosome was first defined in cell-free systems that measured the stoichiometry of its minimal components (Motshwene et al., 2009). Subsequent structural studies illustrated the helical nature of the myddosome, which is a theme that extends to SMOCs that regulate other innate immune signaling pathways (Lin et al., 2010). The core of the myddosome contains multiple copies of the protein MyD88 and members of the IRAK family of serine threonine kinases. Specifically, in cell free systems using recombinant proteins, 6–8 molecules of MyD88 interact with 4 molecules each of IRAK4 and IRAK2. While it is unclear if these stoichiometries represent features of natural myddosomes, the endogenous components of this SMOC can be isolated as a stable complex from cells stimulated with TLR ligands (Bonham et al., 2014). Moreover, the TLR4-induced assembly of myddosomes containing GFP-MyD88 variants have been visualized at the plasma membrane in living cells (Latty et al., 2018). Mechanistically, it appears that the myddosome components are engineered for oligomerization, as the TIR domains of TLRs and TIRAP/MAL function to interact with the C-terminal TIR domain of MyD88. The N-terminal Death domain in MyD88 then recruits IRAKs (which also contain Death domains) (Cao et al., 1996a; Li et al., 2002). This scaffold of TIR and Death domain containing proteins represents the core of the myddosome. IRAK1 is also likely present in myddosomes, although the relative importance of IRAK1 and IRAK2 may differ in humans and mice (Sun et al., 2016). The tight packing of the IRAKs within the myddosome activates their latent kinase activity, driving autophosphorylation and the subsequent recruitment of the E3 ubiquitin ligase TRAF6 (Cao et al., 1996b; Ferrao et al., 2014; Lomaga et al., 1999).
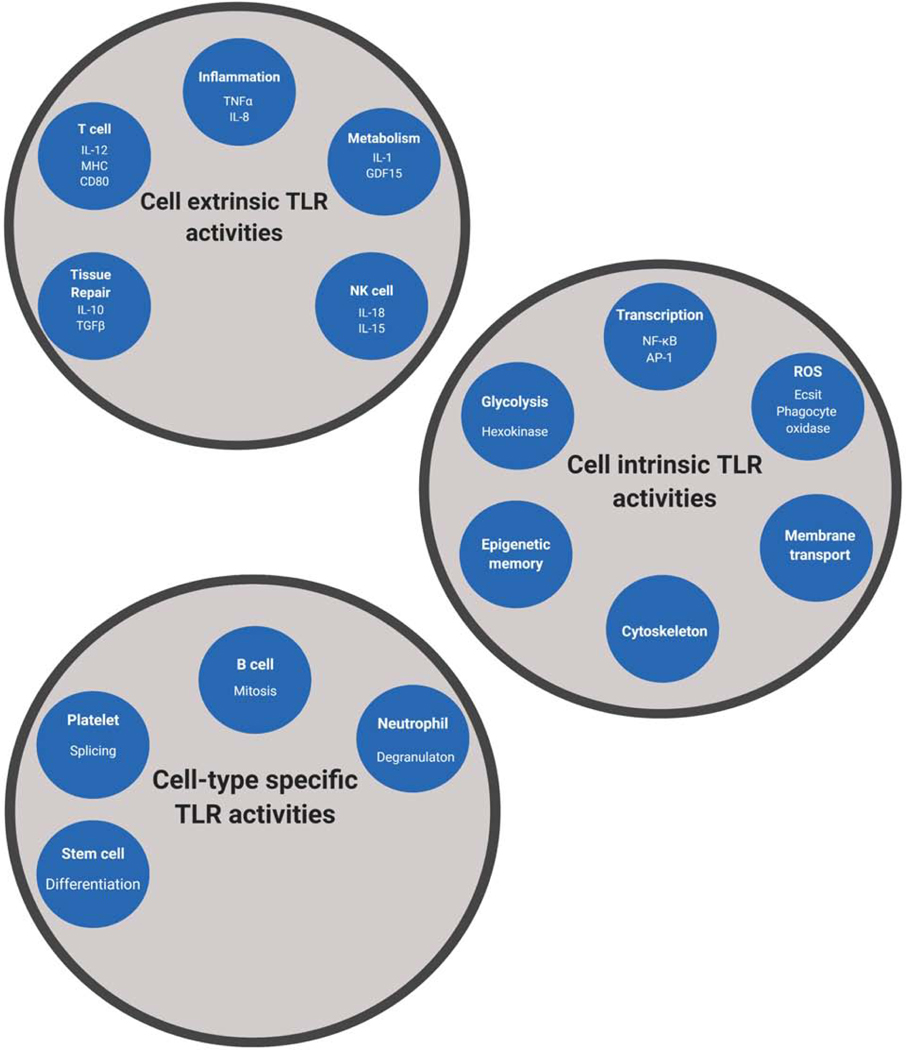
Figure 2.
Diverse cellular responses induced by TLRs upon microbial detection.
Cell-extrinsic responses induced by TLR signaling are indicated (A). These responses include activities that influence the local (or systemic) environment surrounding the cell that detected a PAMP. Cell-intrinsic responses induced by TLR signaling are indicated (B). These responses occur within the cell that detected a PAMP and contribute to the activities indicates in panel A. Cell type-specific responses induced by TLR signaling are indicated (C). These responses occur uniquely in the cell type indicated, but are mediated by TLR-PAMP interactions. The underlying mechanisms that explain cell type-specific TLR responses are poorly defined.
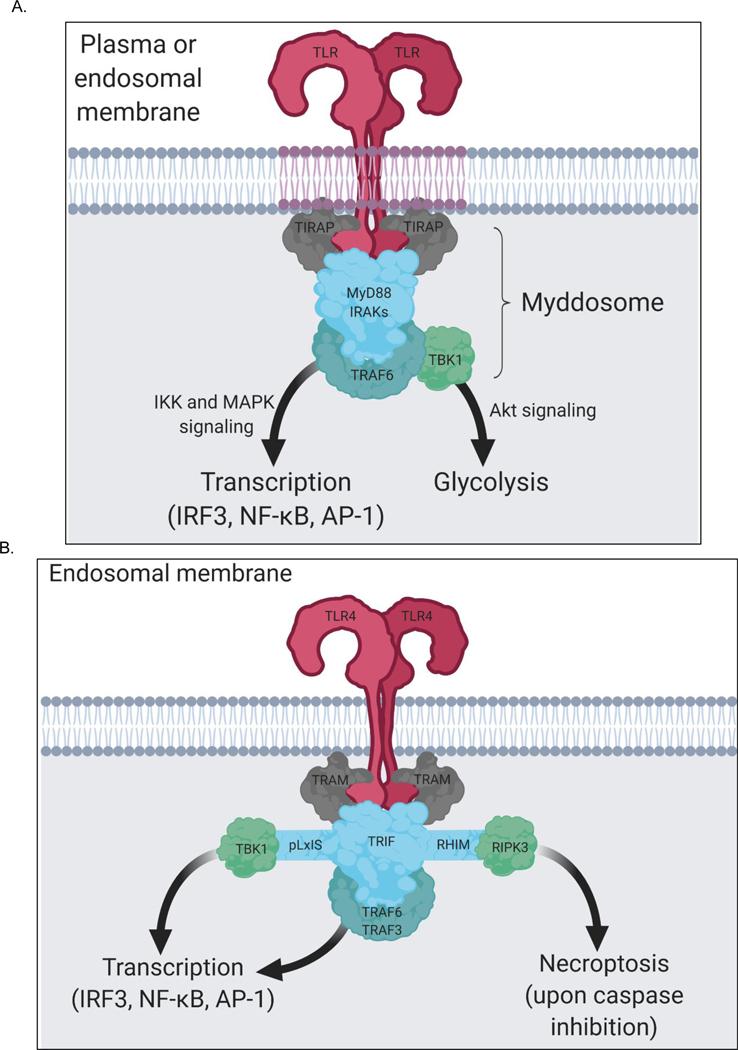
Figure 3.
TLR signaling is mediated by two SMOCs—the myddosome and triffosome
(A) All TLRs, except TLR3, induce the assembly of a supramolecular organizing center (SMOC) called the myddosome upon PAMP detection. Myddosome assembly occurs around the cytosolic tail of dimerized TLRs present at the plasma membrane or endosomes. The enzyme TRAF6 is present in the myddosome. TRAF6 functions to stimulate myddosome-associated TBK1 to drive metabolic changes in the cell and functions to stimulate IKK-and MAPK-dependent transcription factors. The collection of these activities promotes inflammation and host defense. (B) On endosomes TLR4 and TLR3 have the capacity to engage a SMOC called the triffosome. This protein complex is poorly defined, but is believed to be organized and operate as depicted here. TRIF contains a pLxIS motif that promotes TBK1-dependent gene expression and a RHIM domain to promote RIPK3-dependent necroptosis. This latter activity only occurs upon conditions of caspase-inhibition.
Within the myddosome, TRAF6 serves two functions (Figure 3). This enzyme activates the kinase TAK1, which stimulates IκB kinase (IKK)-mediated NF-κB and Mitogen Activated Protein Kinase (MAPK)-mediated AP-1 transcriptional responses (Emmerich et al., 2013; Wang et al., 2001). The activities of these and other TLR-induced transcription factors will be discussed in detail below. In addition, TRAF6 mediates the recruitment of the IKK-related kinase TBK1 to the myddosome (Tan and Kagan, 2019). Myddosome-associated TBK1 functions to stimulate the rapid induction of glycolysis that occurs within minutes of TLR activation. This process is mediated by the kinase AKT (a substrate of TBK1) and results in the phosphorylation of hexokinase, a master regulator of glycolytic metabolism (Everts et al., 2014). The consequences of this early glycolytic shift in the cell are unclear, but it may serve as the first step in the long-term metabolic changes that occur. These long-term changes include an inhibition of oxidative phosphorylation within mitochondria and the consequential enhanced production of acetyl-CoA moieties that fuel the need for histone modifications associated with durable transcriptional activities in the nucleus (Langston et al., 2019). Additional metabolic effects include an increase in glucose utilization to fuel the emphasis on glycolysis in TLR stimulated cells, the downstream consequences of which include increases in ATP and lipid production, which may bolster protein synthesis and secretory activities associated with TLR signaling (Everts et al., 2014; O’Neill et al., 2016). While TBK1 (and its close homologue IKKε) promote rapid glycolysis, other factors contribute to these metabolic effects, including phosphatidylinositol 3-kinase (PI3K) and its upstream TIR domain containing regulator BCAP (Ni et al., 2012; Troutman et al., 2012). The relationship between the BCAP-PI3K-AKT pathway and the TBK1-AKT pathway is undefined. The means by which TRAF6 coordinates myddosome activities that lead to transcription or glycolysis are unclear. In fact, much remains mysterious regarding TRAF6 function. While this protein is well-recognized to operate as a ubiquitin ligase, its activities in the myddosome overlap with those of the E3 ubiquitin ligases pellino-1 and −2 (Moynagh, 2009; Schauvliege et al., 2007). Cells lacking all three of these enzymes are defective for IL-1 induced ubiquitination of myddosome component proteins, whereas cells lacking TRAF6 alone are not defective for these responses (Strickson et al., 2017). In addition, TRAF6 mutants that lack enzymatic activity retain the ability to mediate rapid myddosome-directed transcriptional responses, but these responses cannot be sustained (Strickson et al., 2017). The relative importance of TRAF6 to pellino-1 and −2 in TLR signaling remain to be defined. Similarly, additional studies are necessary to understand the importance of the enzymatic activities verses scaffolding activities of TRAF6 for myddosome signaling. In summary, while the myddosome has emerged as a central mediator of TLR signaling, several questions regarding its operation and regulation cannot yet be answered.
In addition to the myddosome, the putative triffosome operates as a SMOC in the TLR pathways (Figure 3). Genetic deficiencies of MyD88 (or other myddosome components) ablate signaling by all TLRs except TLR4 and TLR3 (Akira and Hoshino, 2003). Downstream of TLR4 and TLR3, MyD88-independent cellular responses have been defined. The first response identified was the expression of genes encoding interferons (IFNs) and IFN-stimulated genes (ISGs) (Kawai et al., 2001; Navarro and David, 1999; Toshchakov et al., 2002). In macrophages and conventional dendritic cells (DCs), TLR3-or TLR4-induced IFN expression is not driven by the myddosome, but rather depends on the proteins TRAM, TRIF and TRAF3 (Fitzgerald et al., 2003b; Hoebe et al., 2003; Yamamoto et al., 2003b). Evidence supports the idea that these respective proteins operate in a manner analogous to TIRAP, MyD88 and TRAF6 within the myddosome. Like TIRAP, TRAM is a peripheral membrane protein that surveys the plasma and endosomal membranes for dimerized TLRs. TRAM accomplishes this task through the actions of a bipartite localization domain consisting of an N-terminal myristoylation motif (Rowe et al., 2006), which is adjacent to a phosphoinositide-binding motif (Kagan et al., 2008). Upon detection of dimerized TLR4 in endosomes, TRAM is thought to interact with TRIF and promote TRAF3-dependent activation of the kinase TBK1. TBK1 then drives the induction of IFN and ISG expression (Fitzgerald et al., 2003a; McWhirter et al., 2004; Sharma et al., 2003), and may also induced similar glycolytic responses that occur when TBK1 is activated within myddosomes (Tan and Kagan, 2019). An explanation for why TRIF (but not MyD88) can promote TBK1-mediated IFN responses was offered by the identification of a 39 amino acids pLxIS motif present within TRIF (Liu et al., 2015). When phosphorylated by TBK1, this motif interacts with the IFN-inducing transcription factor IRF3, which is also a TBK1 substrate. TRIF, not MyD88, therefore has the ability to recruit the TBK1-IRF3 enzyme-substrate pair, leading to IRF3 activation and IFN expression. The activities of TRAM to drive TRIF (triffosome)-dependent responses is restricted to TLR4. TRAM has no ability to interact with TLR3 nor does this protein regulate TLR3 signaling (Yamamoto et al., 2003b). Whether a TRAM-like or TIRAP/MAL-like sorting adaptor operates to link TLR3 to TRIF is unclear.
The above-described sequences of events are commonly associated with signaling by all TLRs in all mammalian cell types examined (Figure 3). Thus, fundamental features of TLR biology include the following: 1) PAMP-mediated TLR dimerization, 2) TLR dimer-mediated SMOC assembly and 3) SMOC-mediated activation of kinases that drive transcription and glycolysis. This latter point highlights the “organizing” nature of these organizing centers, as diverse upstream stimuli feed into SMOCs to generate diverse downstream cellular responses. The location in the cell where SMOC assembly occurs has significant influence over the nature of the downstream effector responses induced by TLRs. An example of location-dependent signaling was illustrated by studies of TLR4. Whereas MD2-TLR4 interacts with LPS at the cell surface, this receptor must translocate (in a CD14-dependent manner) to plasma membrane subdomains known as lipid rafts (Triantafilou et al., 2002). Rafts are enriched in phosphoinositides, in particular phosphatidylinositol 4,5-bisphosphate PI(4,5)P2, which forms high affinity interactions with the localization domain of TIRAP/MAL (Kagan and Medzhitov, 2006). Translocation of dimerized TLR4 to plasma membrane rafts therefore positions ligand bound TLR4 dimers in proximity to TIRAP/MAL. These events promote the TIRAP/MAL-dependent assembly of myddosomes from this subcellular location (Bonham et al., 2014). TLR4 is then thought to undergo endocytosis and it is from endosomes that dimerized TLR4 engages TRAM to initiate TRIF-dependent signaling (Kagan et al., 2008; Zanoni et al., 2011). Notably, the process of TLR4 endocytosis is not mediated by TLR4 itself; rather TLR4 is cargo for an LPS-induced endocytosis response mediated by CD14 (Tan et al., 2015; Zanoni et al., 2011). In macrophages, CD14 is constitutively (but slowly) internalized into endosomes and degraded in lysosomes (Tan et al., 2015). Upon LPS-binding, CD14 endocytosis is greatly accelerated, leading to the co-incident endocytosis of TLR4. LPS-induced CD14 endocytosis proceeds normally in TLR4-deficient cells, but TLR4 endocytosis is strictly dependent on CD14. Moreover, TLR4 mutants containing signaling-incompetent TIR domains are as capable of LPS-induced endocytosis as wild type TLR4 (Tan et al., 2015), thus establishing a cellular response to LPS that is not mediated by TLR4 signaling. The process by which TLR4 is selected as cargo for endocytosis is not only dependent on CD14, it is also dependent on the actions of MD2 (Tan et al., 2015). MD2 mutants that bind TLR4 but cannot dimerize TLR4 are unable to promote LPS-induced endocytosis. This finding indicates that dimerized TLR4 pairs are selectively internalized into cells. Thus, the extracellular domain of TLR4 is not simply a ligand binding domain, it is also a motif that promotes endocytosis upon ligand binding. Direct evidence supporting this statement derives from studies demonstrating that TLR4 mutants lacking their entire cytosolic domains retain the ability to be selected as cargo for CD14-and MD2-dependent endocytosis (Tan et al., 2015). Thus, unlike other well-defined endocytosis-promoting motifs, which are typically contained in the cytosolic tail of transmembrane receptors (McMahon and Boucrot, 2011), the extracellular domain of TLR4 represents its endocytosis motif.
Trans-acting factors that promote CD14-dependent TLR4 endocytosis remain poorly defined, but several have been implicated. One such biosynthetic regulator is the enzyme α1,6 fucosyltransferase (Fut8) (Iijima et al., 2017), which promotes the core fucosylation of CD14. In the absence of Fut8, the ability of CD14 to promote TLR4 endocytosis and signaling is defective, although these phenotypes are only observed in fibroblasts (not phagocytes). In macrophages, LPS stimulates CD14-dependent fluxes in intracellular calcium that are necessary for TLR4 endocytosis. Calcium fluxes may derive from multiple sources, as the chanzyme TRPM7 and inositol 1,4,5-trisphosphate (IP(3)) are implicated in these regulatory events (Chiang et al ., 2012; Schappe et al ., 2018) . ITAM-containing receptors and the tyrosine kinase Syk have also been implicated in TLR4 endocytosis and TRIF-dependent IFN expression in these cells (Lin et al., 2013; Miller et al., 2012; Roy et al., 2014; Zanoni et al., 2011).
This separation of the sites of PAMP detection from signaling observed in the TLR4 pathway extends to TLR2, TLR7 and TLR9. Bacterial lipoproteins have recently been found to promote the TLR2-dependent expression of a subset of ISGs (Nilsen et al., 2015; Salazar et al., 2009; Stack et al., 2014), and viral glycoproteins can drive TLR2-dependent expression of IFNs and ISGs in inflammatory monocytes (Barbalat et al., 2009). The studies that reported these findings differ in the signaling mechanisms proposed, but all concluded that the Type I IFN responses induced by TLR2 require endocytosis. All studies also reported a requirement of MyD88 for the Type I IFN responses induced by TLR2, but there remains no consensus on how MyD88 can induce such responses. The finding that these TLR2-dependent Type I IFN responses occur after endocytosis suggests that myddosomes at the plasma membrane do not have the capacity to induce Type I IFN expression, whereas myddosomes from endosomes may be uniquely endowed with this capacity. Studies of TLR7 and 9 signaling underscore this statement.
TLR7 and 9 signaling depends on MyD88 (Honda et al., 2004; Kawai et al., 2004), and when natural ligands such as viruses are used as stimuli, TIRAP/MAL is also required (Bonham et al., 2014). However, depending on the ligand used to stimulate these receptors, different MyD88-dependent responses are induced. Certain sequences of CpG DNA oligonucleotides induce transcriptional responses that are either heavily biased towards inflammatory cytokine expression or cytokine and IFN expression (Honda et al., 2005a). DNAs that induce these different responses accumulate in different endosomal compartments, with IFN-inducing DNAs accumulating in early endosomes and cytokine-inducing DNAs accumulating in later endosomal compartments (Honda et al., 2005a). Additionally, in plasmacytoid DCs (pDCs), genetic disruption of the adaptor protein (AP)-3 complex lead to a dissociation of cytokine and IFN expression (Sasai et al., 2010). These findings lead to the model whereby TLR7 and 9 induce myddosome assembly and signaling from two different locations within the endosomal network. While the conclusion that IFN-inducing activity is restricted to endosomal TLRs is supported by studies from several laboratories, this theme appears to only apply to Type I IFNs. Type III IFNs (also known as IFNλ) can be induced by TLRs located at the plasma membrane or endosomes in human monocytes (Odendall et al., 2017). Type III IFNs are induced by a process dependent on MyD88 and appear to be co-regulated with inflammatory cytokines and chemokines, as opposed to Type I IFNs. For example, blocking endocytosis in human monocytes prevents TLR4-induced Type I IFN expression, but it does not prevent TLR4-induce Type III IFN or cytokine expression. The co-regulation of cytokines and Type III IFNs by the TLR pathway is probably most important at barrier surfaces, as the receptor for this IFN family is highly expressed by epithelial cells that line the intestine, lung, liver and skin. Recent studies have indicated that Type III IFNs promote the reinforcement of epithelial barrier functions, a process central to host defense (Lazear et al., 2015; Odendall et al., 2017). Like the process of inflammation, barrier integrity may be considered a central feature of immunity, regardless of the nature of the infection. This suggestion may explain why TLR signaling coordinates cytokine and Type III IFN expression. It remains mysterious as to how myddosomes can induce IFN expression in response to some ligands in some cell types and not others, especially because studies in pDCs identified canonical myddosome components (e.g. IRAK4, IRAK1 and TRAF6) in these responses (Kim et al., 2007; Uematsu et al., 2005). Notably, the IFN response induced in pDCs is typified by the expression of IFNα, as opposed to IFNβ, which characterizes myddosome-based IFN responses in other cell types. IFNα expression in pDCs depends highly on IRF7 whereas IFNβ responses in other cells are dominated by IRF3-dependent events downstream of TBK1 (Honda et al., 2005b). Another myddosome response that is cell type dependent occurs specifically in B cells, where TLRs drive the mitosis of these cells (Krieg et al., 1995). Other cell types do not link TLR signaling to cell division. Like IFN expression in pDCs, the mitotic response to TLR ligands in B cells is dependent on canonical myddosome components, which makes these observations all-the-more curious. TRIF signaling downstream of TLR4 also displays cell type (or species-specific) activities. For example, LPS signaling promotes the release of IL-1β from human and porcine monocytes. This process is mediated by CD14, TLR4, TRIF and the inflammasome regulator NLRP3 (described later in this manuscript (Gaidt et al., 2016; Vigano et al., 2015)). Human monocyte-derived macrophages and murine macrophages are unable to link TLR4-TRIF signaling to the release of IL-1β. Cell type and/or species-specific regulators may exist that endow these SMOCs with the ability to diversify effector responses that TLRs can induce (Figure 2).
This diversity of effector functions induced by myddosomes can not only be demonstrated by dissecting the behavior in natural contexts, but can be demonstrated through the use of synthetic biology. For example, myddosomes have been engineered to contain the aforementioned IFN-inducing pLxIS motif appended onto MyD88. When introduced into cells, these synthetic myddosomes induce potent IFN responses to natural upstream stimuli (Tan and Kagan, 2019). Similarly, myddosomes can be engineered to link TLR signaling to the induction of necroptosis, a rapid cell death pathway that proceeds independently of caspases. The finding that myddosomes display natural and programmable signaling flexibility likely explains the common design principles that operate in other SMOCs in the innate immune system. Indeed, inflammasomes have also be engineered to induce unnatural cellular responses using strategies similar to those used to engineer novel myddosome activities (Tan and Kagan, 2019). In addition to the TLR pathways and the receptors that stimulate inflammasome activities, the TNF Receptor pathways, RIG-I like Receptor (RLR) pathways and cGAS-STING pathways (all of which promote inflammation) utilize SMOCs as signaling organelles. Specifically, proteins that sit at the apex of each of these pathways seed the assembly of a receptor-proximal oligomeric signaling platform (Kagan et al., 2014). One possible explanation for common use of SMOCs in innate immune signal transduction has been proposed. The oligomeric nature of these signaling organelles is thought to increase the threshold for effector enzyme activation, thus creating the possibility of all-or-nothing cellular responses to receptor activation. Real-time imaging and systems-based transcriptional analyses of cells stimulated with TLR ligands underscore the likelihood that this hypothesis is correct. For example, single cell analysis of TLR-induced NF-κB translocation into the nucleus demonstrated that increasing the dose of PAMP does not change the rate of transcription factor translocation (Sung et al., 2014). Rather, increasing the dose changes the percent of cells that permit NF-κB translocation. Single cell RNA sequencing analysis reinforced this idea, as increasing the dose of LPS used to stimulate macrophages did not change the amount of cytokine transcripts in each cell (Fischer et al., 2019). Rather, changing the dose of LPS altered the percent of cells that induced cytokine mRNAs. It therefore appears that the cellular responses to TLR ligands are naturally designed for an all-ornothing response to microbial encounters. This type of response may be facilitated by oligomerizing activities that allow a small number of dimerized TLRs (perhaps only a pair of TLRs) to induce myddosome assembly and signaling.
TLR-associated transcription factor activity in nucleus
Following the assembly of the myddosome and triffosome complexes, several transcription factors are activated to facilitate the inducible expression of inflammatory and IFN genes. These include NF-κB, AP-1 and members of the IRF family. Myddosome assembly recruits TRAF6 which activates the transforming growth factor β (TGF-β)-activated kinase 1 (TAK1), leading to activation of the IκB kinase complex consisting of the IKKα and IKKβ kinases scaffolded by IKKγ, also called NEMO (Mitchelletal., 2016; Wangetal., 2001). This kinase complex phosphorylates IκBα on serines 32 and 36, events which tag IκBα for polyubiquitination and subsequent degradation by the proteasome. NF-κB heterodimers, typically consisting of RelA (p65) and p50 then translocate to the nucleus and bind to NF-κB target sites in the promoters and enhancers of immune response genes. These events lead to robust transcriptional responses and expression of cytokines, chemokines and an array of additional immune genes. With slightly delayed kinetics compared to the myddosome, the TRIF pathway also activates NF-κB (Yamamoto et al., 2003a). Upon TLR4 or TLR3 dimerization, the RIP homotypic interaction motif (RHIM) domain of TRIF binds to receptor-interacting serine/threonine-protein kinase 1 (RIPK1), which activates TAK1, leading to IKK activation (Cusson-Hermance et al., 2005; Meylan et al., 2004). While RelA/p65 and p50 heterodimers represent the major NF-κB heterodimer activated, c-Rel, a related family member is also activated downstream of myddosome and triffosome complexes. c-Rel is unique in its specificity for induction of the interleukin-12b (IL-12p40) gene, a key component of the heterodimeric cytokine IL-12 (Sanjabi et al., 2000). Caspase-8 has recently been shown to regulate c-Rel and IL12p40 gene induction (DeLaney et al., 2019). In addition, a cytosolic factor, Cellular Nucleic Acid binding protein (CNBP) coordinates this IL-12p40 response. CNBP is engaged downstream of TAK1 and facilitates c-Rel nuclear translocation and DNA binding to the IL-12b promoter to drive IL-12p40 gene expression (Chen et al., 2018). CNBP controls IL-12 downstream of multiple TLRs including those that signal via MyD88 as well as TRIF (TLR3). TAK1 is a member of the MAPK kinase kinase family activated by TLRs as well as by the pro-inflammatory cytokines interleukin-1 (IL-1) and tumor necrosis factor α (TNFα) (Wang et al., 2001). TAK1 is also an essential intermediate for activation of MAPK cascades. TAK1 activates MAPK kinases which in turn phosphorylate and activate the MAPKs. There are three MAPKs; extracellular signal-regulated kinase kinases (ERK), c-Jun N-terminal kinase (JNK) and p38. These MAPKs phosphorylate AP-1 transcription factors comprised of a heterodimer of Fos and Jun subunits. Regulation of AP-1 occurs through specific interactions controlled by dimer-composition, transcriptional and post-translational events, and interaction with accessory proteins.
The third major transcription factor engaged downstream of TLRs is IRF3 (Kawai et al., 2001; Navarro and David, 1999; Toshchakov et al., 2002). IRF3 is a member of a larger family of proteins initially described for their role in regulating transcription of the IFNβ gene (Sato et al., 1998). IRF3 is a latent factor expressed in multiple cell types including myeloid cells. IRF3 is recruited to TRIF and then regulated by phosphorylation on a C-terminal serine rich cluster (Fitzgerald et al., 2003b). The IKK related kinases TBK1 and IKKε phosphorylate IRF3 (Fitzgerald et al., 2003a; Sharma et al., 2003). This leads to conformational changes and rearrangement of IRF3 monomers to dimers. Dimeric IRF3 then translocates to the nucleus to bind to IRF binding elements in the promoter of IFNβ as well as other anti-viral genes to regulate their expression. Induction of the IFNβ gene occurs through collaborative interactions between NF-κB heterodimers, IRF3 dimers as well as ATF-2/c-Jun heterodimers phosphorylated by MAPK to form the IFNβ enhanceosome (Maniatis, 1986). Newly synthesized IFNβ released from cells signals through autocrine and paracrine means by binding to and activating a heterodimeric receptor complex consisting of IFN-α receptor 1 (IFNAR1) and IFNAR2. IFNAR1/2 engage the JAK/STAT signaling pathway to promote transcription of several IFN Stimulated Genes (ISGs), the products of which prevent viral replication, assembly and release. IFNAR signaling also promotes the restriction of bacterial infections, as ISGs such as IL15 are important for the ability of LPS-stimulated DCs to stimulate IFNγ production from NK cells (Zanoni et al., 2013). Myddosomes also induce IFN expression in response to TLR 7, 8 and 9 ligands in pDCs and the canonical myddosome components IRAK4, IRAK1 and TRAF6 are essential for this response (Honda et al., 2005a; Honda et al., 2004; Honda et al., 2005b). This TLR7/8/9 driven IFN response in pDCs leads to IFNα secretion. pDCs express IRF7 at high levels and phosphorylation of IRF7 by IKKα controls IRF7 homodimers driving IFNα expression. In addition to IRF3 and 7, IRF1 and IRF5 contribute to gene expression in the TLR pathways. In some circumstances, IRF1 is also activated by TLR9 to control IFNβ gene expression (Negishi et al., 2006). IRF5 is activated by myddosome complexes and controls proinflammatory cytokine, but not IFN-α production (Schoenemeyer et al., 2005; Takaoka et al., 2005). IRF5 is phosphorylated by IKKβ on Ser462 (Lopez-Pelaez et al., 2014; Ren et al., 2014) TLRs and innate immune training
Until recently the ability to generate immunological memory was thought to be restricted to the adaptive immune system. Over the last few years, both myeloid (monocytes, macrophages and dendritic cells) as well as lymphoid (natural killer cells and innate lymphoid cells) innate immune cells have been shown to alter their functional capabilities through metabolic and epigenetic programming. As a consequence, innate cells can exhibit heightened or reduced responsiveness to a secondary stimulus. This process which is termed innate immune memory or innate immune training is thought to explain the well validated finding that immunization with BCG, the Tuberculosis vaccine decreased childhood mortality rates by protecting against sepsis and respiratory infections for extended periods (reviewed in (Kleinnijenhuis et al., 2015)). Further, it was known that immunization with BCG or other mycobacterial preparations induced heterologous protection to an array of microbial pathogens, as well as protection against multiple tumor types that persisted for a period of months. Investigations into the mechanism of this heterologous protection afforded by BCG revealed that initial exposure to BCG resulted in significantly more pro-inflammatory cytokine production when stimulated with LPS than cells from unvaccinated subjects, an effect that lasted at least 3 months after vaccination (Kleinnijenhuis et al., 2012; Quintin et al., 2012). Subsequent work showed that monocytes stimulated in vitro with BCG and then rested for several days, produced more TNFα and IL-6 following treatment with TLR2 and TLR4 ligands(Blok et al., 2015). This effect in monocytes was subsequently shown to be a consequence of altered chromatin state and a shift in the metabolism of the cell. Trained cells exhibited increased trimethylation of histone 3 at lysine 4 (H3K4me3) marks at the promoters of pro-inflammatory and metabolic genes including mTOR(Arts et al., 2016). Trained monocytes required a shift in their cell metabolism, switching from oxidative phosphorylation to aerobic glycolysis, and the hyper-inflammatory response to TLR ligands could be blocked by the addition of the mTOR inhibitor rapamycin during the initial BCG stimulus(Arts et al., 2016; Cheng et al., 2014). Taken together, these findings demonstrated that innate immune training is a cell-intrinsic mechanism where monocytes are reprogrammed to a hyper-inflammatory state by means of altered metabolism and chromatin state. While the term trained immunity is a recent addition to the immunological lexicon, the alteration of TLR signaling by prior environmental exposures likely relates to the long-standing appreciation of the process of LPS tolerance. Prior exposures of subjects (or cells) to LPS results in a decreased subsequent responsiveness to TLR4 ligands. Much work has been done on this topic (reviewed in (Dobrovolskaia and Vogel, 2002)), and several mechanisms have been proposed to explain LPS tolerance. A commonality between tolerance and training mechanisms is that both appear to be (in part) regulated at the level of the cytokine genes, with notable changes in histone modifications having been identified that influence these events (Dobrovolskaia and Vogel, 2002). It remains unclear how receptor proximal signaling events are influenced under conditions of innate immune training or tolerance, and the causes of the metabolic changes that occur within trained cells need to be further explored.
TLR-induced ncRNA functions
While much research has focused on the transcription factors that coordinate the inflammatory response, recent evidence has also revealed how non-coding RNAs including microRNAs and lncRNAs modulate these responses. Each subtype of ncRNAs differs in biogenesis, length, and mechanisms to accomplish their biological function. MicroRNAs (miRs) are small non-coding RNAs that are 19–24 nucleotides in length (Rinn and Chang, 2012; Ulitsky and Bartel, 2013)(Rinn and Chang, 2012; Ulitsky and Bartel, 2013). miRNAs act as a guide by base pairing with target mRNAs to initiate translational repression, mRNA deadenylation, and mRNA decay. Multiple miRs are induced or repressed following ligation of distinct TLRs and these miR’s in turn target MyD88, TRIF, IRAKs, TRAF6 as well as the key transcription factors IRF3, NF-κB and AP-1 (reviewed in (O’Neill et al., 2011) (Figure 5).
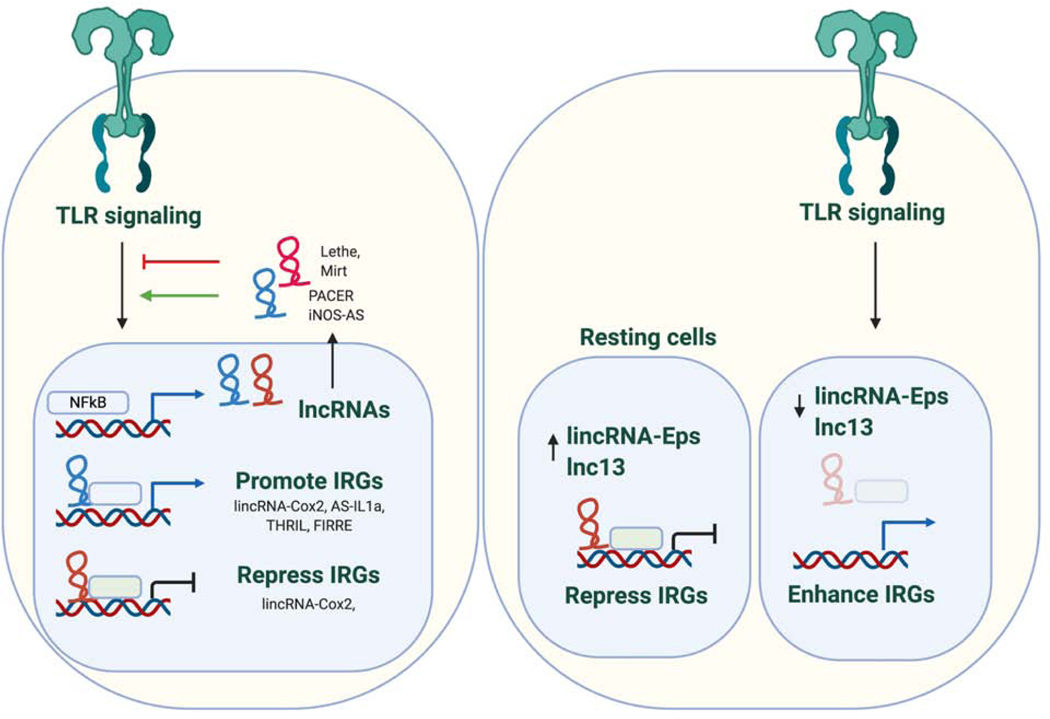
Figure 5.
LncRNAs in TLR signaling.
TLR stimulation leads to temporally regulated changes in the expression of a number of lncRNAs which in turn function to regulate the innate immune response. lncRNAs can function in the cytosol or nucleus by binding proteins to either promote or restrain responses. For example, the lincRNA-Cox2 interacts with the SWI/SNF chromatin remodeling complex to regulate expression of immune genes that require chromatin remodeling for their expression. In addition, this same RNA can interact with A2/B1 to downregulate chemokine expression. iNOS-AS functions as a positive regulator of immune gene expression. The iNOS antisense transcript localizes to the cytoplasm where it promotes the stability and subsequent translation of iNOS mRNA through direct base-pair complementation. Lethe and Mirt are induced lncRNAs that inhibit NF B function. Lethe binds RelA to sequester NF B while Mirt2 acts indirectly by reducing Lys63 (K63)-linked ubiquitination of TRAF6 a key regulator of NF B activation. In addition to these inducible RNAs, some lncRNAs are expressed in myeloid cells in the absence of stimulation and can be downregulated upon TLR activation. These lncRNAs can restrain immune gene expression by regulating chromatin accessibility (eg. lnc13 or lincRNA-Eps). It is likely that additional lncRNAs will be identified that modulate IRF signaling and IRF regulated gene expression downstream of TLRs to modulate the entire TLR inducible program.
Long non-coding RNAs are larger molecules defined as 200 nucleotides or greater in length (Guo et al., 2010). lncRNAs are RNA polymerase II transcripts and are polyadenylated. lncRNAs can function through a variety of mechanisms to regulate gene expression acting either in the nucleus to control transcription or post-transcriptionally by partnering with RNA, DNA, proteins or a combination of these molecules. Numerous studies have shown that lncRNA expression is upregulated or downregulated following TLR engagement. lncRNAs are frequently amongst the most dynamically regulated genes in TLR activated cells (Carpenter et al., 2013). For example lincRNA-Cox2 (Carpenter et al., 2013; Hu et al., 2016), Mirt2 (Du et al., 2017) and AS-IL-1a (Chan et al., 2015a) are all induced in macrophages exposed to LPS. These RNAs then modulate distinct aspects of the TLR-induced program functioning as positive or negative regulators of TLR induced responses. For example, lincRNA-Cox2 one of the first lncRNAs identified in immune cells, is induced downstream of multiple TLRs. This lincRNA can associate with the SWI/SNF chromatin remodeling complex to promote the expression of inflammatory genes or with hnRNPA2/B1 to repress chemokine gene transcription (Carpenter et al., 2013; Hu et al., 2016). Several TLR regulated lncRNAs modulate NF-kB signaling. Mirt2 isn induced by LPS and functions as a negative regulator of the TLR induced response by binding with, and reducing Lys63 (K63)-linked ubiquitination of TRAF6 a key regulator of NF-κB and MAPK pathways (Du et al., 2017). Lethe, another inducible gene, binds to the NF-kB RelA subunit and prevents RelA induced gene expression (Rapicavoli et al., 2013). Several lncRNAs have also been identified which restrain immune gene expression in the absence of stimulation, often by altering chromatin state at regulatory regions of immune gene (eg. lincRNA-Eps, lnc13)(Atianand et al., 2016; Castellanos-Rubio et al., 2016). Although the evidence for lncRNA-dependent regulation of the TLR response is expanding, the fact that so few lincRNAs are characterized in detail highlights the potential for a greater role for these RNAs in innate immunity. There is also growing evidence that annotated lncRNAs are in fact protein coding. Recently, two TLR regulated lncRNAs were found to encode short proteins or peptides. A putative lncRNA 1810058I24Rik was downregulated in both human and murine myeloid cells exposed to LPS. This transcript was found to be localized in the cytosol and was in fact translated leading to production of a 47 amino acid micropeptide that was localized on the mitochondrion and shown to be important for inflammasome activation. Indeed, another study has revealed that a large number of transcripts currently annotated as lncRNAs are associated with ribosomes and in some cases are translated. Among these, a potently regulated transcript, Aw112010 was found to be encoded from a non-canonical ORF and using a genetic approach in mice was shown to be important in Salmonella typhimurium infection and intestinal inflammation (Jackson et al., 2018). These examples highlight the importance of careful evaluation of protein coding potential of these RNAs.
TLR-mediated regulation of inflammasomes and necroptosomes
Most cytokines and immune response genes induced by TLR signaling are regulated at the level of RNA stability or transcription. Some cytokines such as interleukin-1β (IL-1β) and the related cytokine IL-18 however, require new transcription and proteolytic maturation of induced precursors to generate mature biologically active cytokines. NF-κB is a key regulator of pro-IL1β gene transcription. In addition, IL-1β is sensitive to alterations in the metabolic state of macrophages. A prominent feature of macrophage activation in response to TLRs is an increase in glycolytic activity, with a concomitant reduction of mitochondrial respiration and inhibition of the tricarboxylic acid (TCA) cycle (O’Neill and Pearce, 2016). The elevated glycolytic flux provides biosynthetic intermediates and ATP, while the reduced reliance on the TCA cycle sends citrate and succinate out of the mitochondria. In the cytosol, succinate blocks prolyl hydroxylases that normally target hypoxia-inducible factor 1α (HIF-1α) for proteasomal degradation. This results in stabilization of HIF-1α, which then drives transcription of pro-IL-1β (Corcoran and O’Neill, 2016). Interleukin-1β(IL-1β) converting enzyme (ICE), later named caspase-1, is the principal enzyme responsible for the proteolytic maturation of newly induced IL-1β (Kostura et al., 1989). Caspase-1 is activated within SMOCs called inflammasomes (Martinon et al., 2002). Inflammasomes come in (at least) two distinct flavors containing either an NLR (Nucleotide-binding domain and leucine rich repeat containing) or an AIM2 like Receptor (ALR). The human and mouse genome encode multiple NLRs, including Nlrp1, Nlrp3, Nlrc4, Nlrp12. These factors, along with the proteins Pyrin, seed the assembly of inflammasomes in response to a range of ligands of microbial, environmental or endogenous origin (Martinon and Tschopp, 2007; Schattgen and Fitzgerald, 2011). The ALRs are DNA binding proteins which bind dsDNA ligands directly. Upon activation, ALR or NLRs oligomerize and trigger the helical fibrillar assembly of an adapter protein ASC via pyrin domain (PYD)–PYD interactions. ASC fibrils assemble into large structures, called ASC specks and recruit pro-caspase-1, leading to its autoproteolytic activation. Activated caspase-1 cleaves pro-IL-1β and pro-IL-18 to form the mature cytokines. These cytokines lack a leader sequence and until recently the unconventional means by which they were released from cells was unknown. Now we understand that caspase-1 cleaves the cytoplasmic protein gasdermin-D (Kayagaki et al., 2015; Shi et al., 2015). The N-terminal fragment of cleaved gasdermin-D multimerizes into a large pore that inserts itself into the plasma membrane of activated cells. This pore serves as a conduit for the secretion of mature IL-1β and IL-18 (reviewed in (Orning et al., 2019).
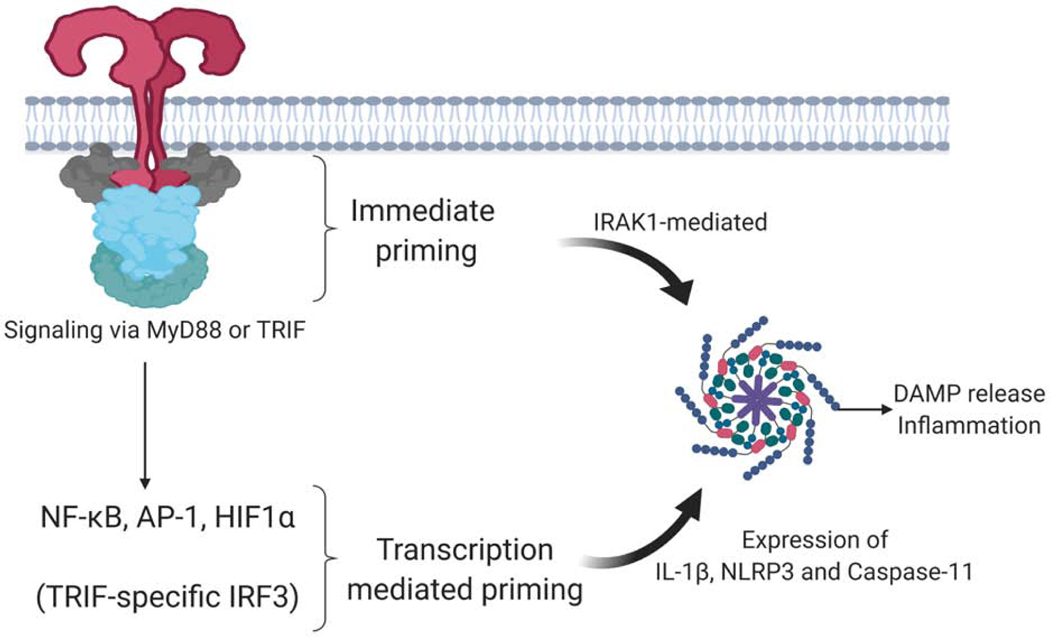
TLRs are instrumental in controlling inflammasome activity, particularly those comprised of NLRP3 (Figure 4). The protein level of NLRP3 is low in resting macrophages, serving as a bottleneck to inflammasome formation. NLRP3 must be induced and a threshold concentration reached to support the formation of the inflammasome. Cells can acquire this priming signal from signaling receptors that activate NF-κB, the best studied of which are the TLRs. TLR signaling leads to new transcription of Nlrp3 and new synthesis of pro-IL1β (Bauernfeind et al., 2009; Franchi et al., 2009) (Figure 4). Transcription of Nlrp3 is dependent on NF-κB activation. Simultaneous engagement of TLRs and NLRP3 can also lead to assembly of the NLRP3 inflammasome in a manner that does not require new protein synthesis. This rapid response pathway is dependent on IRAK1 which directly links TLR ligation to rapid NLRP3 inflammasome assembly (Fernandes-Alnemri et al., 2013; Lin et al., 2014) (Figure 4). TLRs also modulate inflammasome activation through post-translational modifications. NLRP3 can be phosphorylated on both serine and tyrosine residues. Phosphorylation at S3 inhibits NLRP3 activation, while phosphorylation at S194, mediated by JUN N-terminal kinase 1 (JNK1) promotes NLRP3 activation. NLRP3 is also phosphorylated on tyrosine residues and dephoshorylation by the protein tyrosine phosphatase PTPN22 enables NLRP3 activation (Song et al., 2017).
Figure 4.
TLR-mediated regulation of inflammasomes
TLR signaling can prime cells for an enhanced responsiveness to stimuli that drive the assembly and activity of inflammasomes. Simultaneous exposure of cells to TLR ligands and stimuli that promote inflammasome assembly lead to an immediate pyroptosis. This immediate pyroptosis is dependent on the myddosome component IRAK1, but is independent of any transcriptional response in the cell. In cells that have been pre-exposed to TLR ligands, the transcriptional upregulation of inflammasome regulators NLRP3, Caspase-11 and IL-1β lead to a more efficient assembly and activity of DAMPs such as IL-1β. Other examples of TLR-mediated influence on inflammasome activities are described in the text.
Ubiquitination of NLRP3 also regulates the activity of inflammasomes. TLR stimulation increases expression of F-box only protein 3 (FBXO3), which degrades F-box/LRR-repeat protein 2 (FBXL2), an E3 ligase that would otherwise modify NLRP3 with K48-linked ubiquitin chains and promote its degradation by the proteasome (Han et al., 2015). Downregulation of FBXL2 stabilizes NLRP3, enhancing its activity. TRIM31 triggered by TLR activation, also causes K48 linked ubiquitination of NLRP3 resulting in its degradation (Song et al., 2016). BRCC3, activated by priming signals, removes these chains, thereby enabling its oligomerization (Py et al., 2013). The phosphorylation state of NLRP3 can impact its ubiquitylation state, highlighting the complexity of these regulatory checkpoints. TLRs can also impact non-canonical inflammasome activation. TLR signaling via TRIF promotes expression of caspase-11, a caspase-1 like protease that also leads to limited proteolysis of gasdermin D, facilitating IL-1 release and pyroptotic cell death (Broz et al., 2012; Rathinam et al., 2012). All of these observations indicate that TLRs regulate NLRP3 and caspase-11 inflammasome activity, serving as priming, licensing and limiting signals that alter the activation status of these pathways.
As described above, the cross-talk of TLR signaling with inflammasome responses is coupled to the cleavage of gasdermin-D. Cleaved gasdermin-D undergoes oligomerization and membrane insertion eventually leading to plasma membrane rupture and cell lysis (Kayagaki et al., 2015; Shi et al., 2015). This process releases endogenous inflammatory activators, some of which also activate TLRs. In addition, TLR signaling can be coupled to necroptosis, a caspase-independent form of lytic cell death. The serine threonine kinase RIPK1 is recruited to activated TLR complexes promoting pro-survival and NF-κB signaling (Cusson-Hermance et al., 2005; Meylan et al., 2004). The kinase activity of RIPK1 is dispensable for this response. Rather the RIPK1 protein acts as a critical scaffold modified by linear ubiquitin and K63-linked polyubiquitin chains which recruit TAK1/TAB2/TAB3 complex to activate the IKK and MAPK pathways. RIPK1 also regulates caspase-8-mediated apoptosis and when caspase-8 activity is blocked, RIPK3-dependent programmed necrosis or necroptosis ensues. In this latter pathway, RIPK1 and RIPK3 undergo auto-and trans phosphorylation resulting in RIPK3-mediated phosphorylation and activation of mixed lineage kinase domain-like protein (MLKL), a pore forming protein that promotes necroptotic death (reviewed in (Weinlich et al., 2017)). Necroptotic death is a lytic inflammatory cell death due to the release of intracellular molecules such as HMGB1 and IL-1α, which amplify inflammatory cascades. In the TLR3 and TLR4 pathways, TRIF interacts with RIPK1 and RIPK3 via RHIMs present in both proteins (Cusson-Hermance et al., 2005). When caspase-8 is blocked, TRIF engages RIPK1/RIPK3 complexes to stimulate the MLKL-dependent necroptotic death pathway. This pathway is analogous to the RIPK1-RIPK3-MLKL pathway engaged downstream of TNFR1 signaling. Multiple TLRs can trigger necroptosis. Those using the adapter protein MyD88 trigger RIPK1–RIPK3 activation indirectly by inducing intermediate TNF production that triggers necroptosis via TNFR1, whereas TLR3 and TLR4 engage RIPK1/RIPK3 directly via RHIM domain interactions between these proteins and TRIF. Constitutive signaling by type I IFNs also contributes to necroptosis as it induces expression of MLKL and other pathway components (Sarhan et al., 2019).
While several examples of TLR signaling influences inflammasome or necrosome activities, there are many unanswered questions related to these topics. Chief among these questions relates to the dynamics and regulation of these events. Specific mutations can be made in genes that skew TLR signaling to induce specific death pathways (e.g. caspase-8), but whether the natural regulation of caspase-8 expression influences the propensity for a cell death pathway to occur is unclear. Additionally, while oft-discussed, the triffosome remains a putative protein complex. No detailed understanding of the biochemical events that regulate triffosome assembly or activity exists. Finally, the rapid transition from events that activate gasdermin-D and MLKL to actual cell death has hampered our ability to dissect the molecular events that influence the activities of these pore forming proteins. In the next sections, we will focus on how the effector responses elicited in response to TLRs play a critical role at the interface of the innate and adaptive immune systems.
Intrinsic and pathogen-directed negative regulation of TLR signaling
Several regulatory mechanisms influence TLR pathway activities. Some of these mechanisms prime the TLR pathways for more robust responses, with the best-recognized being IFNγ. By processes that remain poorly defined, IFNγ-treated cells permit TLR-dependent inflammatory cytokine production to a greater extent than cells that were not treated with IFNγ. It has been suggested that this enhanced activity relates to the functions of IRF1, an IFNγ-inducible transcription factor that forms a complex with MyD88 (Negishi et al., 2006). Anti-inflammatory cytokines also regulate TLR signaling, as treatments of macrophages with exogenous IL-10 and TGFβ interferes with the ability of TLR ligands to stimulate robust gene expression (Hacker et al., 2006). This negative regulatory mechanism can also operate in an autocrine fashion, as IL-10 production from TLR ligand stimulated cells limits the magnitude of inflammatory cytokine production (Hacker et al., 2006). Macrophages deficient in IL-10 will produce high amounts of cytokines in response to TLR ligands than their wild type counterparts, and this enhancement of inflammatory activity influences the progression of murine models of colitis (Asseman et al., 1999; Fiorentino et al., 1991; Shouval et al., 2016).
At the level of myddosome assembly, the pro-inflammatory ubiquitin-based activities o f TRAF6 are subject to negative regulation. The ubiquitin-specific proteases A20 and CYLD counteract TRAF6 (and perhaps pellino) activities in the myddosome, acting to remove ubiquitin chains that have been attached to specific components of this SMOC (Heyninck and Beyaert, 1999). Accordingly, cells deficient for A20 display enhanced TLR-dependent inflammatory responses, which can result in life-threatening autoinflammatory phenotypes in animals. Mice dually deficient in A20 and MyD88 do not suffer from lethal disease, thus indicating the importance of A20 in limiting myddosome activities in vivo (Turer et al., 2008). CYLD-deficient mice do not exhibit as severe a phenotype (in terms of mouse viability) as A20-deficient mice, yet several auto-inflammatory symptoms result from the absence of CYLD, such as a susceptibility to colitis (Zhang et al., 2006).
TLR4 endocytosis, which is essential for TRIF pathway activation, is subject to negative regulation by factors induced by TLR4 signaling. For example, TLR4 stimulates the production and release of prostaglandin E2 (PGE2) and the stimulation of the PGE2 receptor EP4. EP4 signaling interferes with the extent of LPS-induced TLR4 endocytosis, thereby limiting TRIF pathway activation (Perkins et al., 2018). Blocking EP4 activity in vitro results in enhanced TRIF signaling and an increased susceptibility to inflammation driven lethality during bacterial infection of mice.
Bacterial and viral pathogens also utilize strategies of minimize TLR-induced inflammatory responses. The strategies used by pathogens to block TLRs are numerous but can be divided into two categories. One category represents strategies that target receptor-proximal signaling stages that are unique to the TLR pathway. The second category represents strategies that disrupt signaling stages that are not unique to TLRs, such as those that prevent NF-κB or MAPK activation. The following discussion will focus on the former category, as only this category can be considered as strategies when pathogenic intent is clear—the prevention of TLR signaling. An increasing variety of host-adapted bacteria modify Lipid A structure of LPS into a form that differs from the hexa-acylated, di-phosphorylated structure that best stimulate CD14-MD2-TLR4 dependent inflammatory responses. Among others, examples of such Lipid A alterations include those that occur in pathogenic Yersinia and Francisella, where these bacteria contain Lipid A structures that contain tetra-acylated Lipid A that cannot efficiently crosslink TLR4 and therefore cannot efficiently promote inflammation (Montminy et al., 2006; Shaffer et al., 2007). Commensal bacteria utilize similar strategies of TLR evasion, which may be important for non-inflammatory colonization of the intestinal lumen. For example, Bacteroides species commonly contain penta-acylated and mono-phosphorylated Lipid A structures as the dominant component of LPS (Cullen et al., 2015). These structures weakly activate TLR4-dependent inflammatory responses. Mutant Bacteroides thetaiotaomicron that contain di-phosphorylated Lipid A induce strong TLR4-dependent inflammatory responses, thus underscoring the importance of de-phosphorylation in the evasion of TLR4 signaling (Tan et al., 2015). Consistent with the idea that minimizing TLR4 signaling is an important aspect of commensalism, the vast majority of Bacteroides present in the human gut encode the enzyme LpxF, which is responsible for generating monophosphorylated Lipid A (Cullen et al., 2015).
Strategies of PAMP alteration to prevent TLR signaling also apply to TLR2, TLR5 and TLR9. Staphylococcus aureus utilizes the acetyltransferase OatA to alter the structure of its peptidoglycan into a form that is difficult to digest by lysozyme present in phagosomes. OatA-deficient S. aureus is easily digested and are more easily detected by TLR2 (and TLR9) (Shimada et al., 2010). In a thematically similar manner Helicobacter pylori has altered the amino acids that code for flagellin to evade TLR5 detection. This process is thought to be important for the ability of H. pylori to evade inflammatory responses associated with its colonization of the human gastric epithelia, as epithelial cells produce neutrophil chemokines (e.g. IL-8) via TLR5 upon flagellin detection (Gewirtz et al., 2004).
Pathogens also utilize protein-based virulence factors to directly interfere with TLR signaling events. For example, an increasing number of bacteria and viruses encode TIR domain containing proteins that are reported to somehow interact non-productively with myddosome components. The mechanisms underlying these non-productive interactions are not well-defined, but mutant pathogens that lack these TIR domain containing proteins induce strong inflammatory responses and are avirulent (Bowie et al., 2000; Harte et al., 2003). An additional strategy used by pathogens to downregulate TLR signaling is to encode proteases that cleave and inactive key signaling nodes. For example, several viruses encode proteases that cleave TRIF, including Hepatitis C Virus and coxsackievirus B3 (Li et al., 2005; Mukherjee et al., 2011). These cleavage events correlate with an inhibition of TLR3 signaling, which depends on TRIF for its activities. While these examples provide a perspective on the wide range of strategies that can be used by mammalian and microbial cells to regulate (or inactivate) TLR signaling, there significant gaps remain. The most striking knowledge gap derives from the fact that the viral proteases implicated in the regulation of TLR signaling have multiple substrates in infected cells. The relative importance of each substrate in viral infection is poorly defined. An increased emphasis on the study of microbial proteases during infections with mutant pathogens should enable a more-detailed analysis of these important regulatory events.
TLR-mediated stimulation of adaptive immunity
TLRs are instrumental in the control of adaptive immunity impacting both T and B cell responses. Maturation of DCs is a prerequisite for the initiation of antigen specific immune responses (Nussenzweig and Steinman, 1980). Upregulation of co-stimulatory molecules such as CD80 and CD86 on DCs following TLR ligation endows DCs with the capacity to prime naïve T cells (Hoebe et al., 2003; Pasare and Medzhitov, 2004a). In addition, the peptide-MHC complex is also regulated by TLR signals. TLR signaling promotes the acidification of endosomes in DCs, which facilitates peptide loading onto MHC molecules (Blander and Medzhitov, 2006; Trombetta et al., 2003). TLRs also promote the fusion of MHC-I containing endosomes with phagosomes, a process that facilitates cross presentation of exogenous antigens for the stimulation of CD8+ T cell responses (Blander and Medzhitov, 2006). In addition to promoting MHC peptide loading, TLR signals, in particular LPS, promote the redistribution of MHC class I and II molecules to the surface of DCs (Turley et al., 2000). DCs express a collection of different PRRs, some of which function to internalize antigen delivering it into antigen processing and presentation compartments (Edwards et al., 2003; Hornung et al., 2002). Ligands on phagocytic cargo engage PRRs and activate signal transduction pathways. The context of antigen delivery is therefore important. Antigens captured by DCs and presented to T cells at steady state induce tolerance. In contrast, antigens captured by DCs during infection or inflammation where there is induction of co-stimulatory molecules induce prolonged T cell activation (Lanzavecchia and Sallusto, 2001). Activation of PRRs also leads to DC migration from the infected tissue to the draining lymph node, where stimulation of naïve T cells may occur.
Detection of microbial products by DCs is translated into different classes of effector responses. TLR activation provides information about the type of invading pathogen being recognized and instructs lymphocytes to mount the most appropriate effector response to clear the infection. The choice of effector response is dictated by the receptors that are engaged and the cytokines released from these cells. Cytokines produced by activated DCs shape the differentiation of naïve CD4 T cells (reviewed in (Pasare and Medzhitov, 2004a). For example, production of IL-12 and IL-18 downstream of TLRs drives Th1 cell polarization, resulting in Th1 cells that produce IFNγ. MyD88 is critical for the induction of these responses, as Th1 cell differentiation and production of IFNγ is defective in mice lacking MyD88 (Pasare and Medzhitov, 2004a, b). Exactly how IL-18 is released from DCs in this context remains an unanswered question. The role of the inflammasome and the ability of gasdermin D to act as a conduit for IL-18 has not been demonstrated. The production of IL-1, IL-6 and IL-23 all of which are activated downstream of TLRs and C-type lectin receptors act together with TGFβ to promote the development of TH17 cells that produce IL-17 (reviewed in (Gaffen et al., 2014)). The precise mechanisms regulating IL-1 release in these contexts are unclear and need to be resolved. Recent studies indicate that the process of DC-T cell interactions can promote IL-1β release from TLR-stimulated DCs, by a process dependent on TNF superfamily proteins produced by responding T cells (Jain et al., 2020). DCs are functionally heterogeneous and different subsets have been defined that express subset specific PRRs (Edwards et al., 2003; Hornung et al., 2002; Merad et al., 2013). As a result, these cells respond to different stimuli, engage distinct signaling pathways and produce specific cytokines that in turn define the Th cell subset that will be generated (Anderson and Murphy, 2019). For example, CD8α+ DCs which are important for cross-presentation are important for activation of CD8+ T cells. These DCs express TLR3 as well as RLRs and produce Type I IFNs and IL-12 in response to viruses or when endosomal TLR3 recognizes virus-infected cells that have been taken up (Schulz et al., 2005)}. Plasmacytoid DCs are another DC subset, specialized for induction of Type I IFNs and control of anti-viral immunity (Edwards et al., 2003; Hornung et al., 2002; Merad et al., 2013). pDC express endosomal TLRs including TLR7 and 9 that recognize ssRNA and CpG DNA respectively, but these cells do not express TLRs that detect bacterial cell wall components. As such, pDCs can be considered cells that are designed specifically to detect viral infections.
B cell responses and antibody production are also regulated by TLRs (Kawai et al., 1999; Krieg et al., 1995; Leadbetter et al., 2002). B cell responses can be divided into two types, those that are T cell independent such as innate like B cells and those that are dependent on T cells. Innate like B cells, B1a and B1b cells express TLRs and respond to microbiota-derived stimuli (Kreuk et al., 2019). TLRs and BCRs collaborate to induce both canonical and non-canonical NF-κB signaling and natural immunoglobulin IgM antibody production important for protection against bacteria and viruses such as Influenza. Toll-like receptors (TLRs) are critical for shaping the Ig repertoire of B-1a cells and regulating their antibody production. These findings have important implications for steady state B-1a responses to both self and microbiota-derived antigens (Kreuk et al., 2019).
T cell dependent antibody responses require engagement of the B cell receptor (BCR) by the antigen. Macrophages and DCs deliver antigen to naïve B cells in the follicle. While small Ags can go through the conduit system to B cells, larger particulate antigens need to be delivered via subcapsular sinus macrophages. These macrophages do not phagocytose antigens as efficiently as other macrophage subsets but rather act as vehicles to bring Ag to the B cell on their surface. B cells recognize Ag on macrophages. In addition to antigen, these cells need help from follicular helper T cells (Tfh) through CD40L. Further, these cells are dependent on cytokines that induce class switch to a particular heavy chain isotype. Antibody responses especially IgG2 in mice or IgG1 or 3 in humans are regulated by TLRs expressed on B cells(Pasare and Medzhitov, 2005). TLRs in this context provide information about the nature of the antigen and whether this is associated with a PAMP. TLRs on B cells are important in coordinating the antibody response during infection as well as in autoimmune disease, where engagement of the BCR and TLR7 or 9 occurs in response to self-antigens complexed with nucleic acids such as RNA or DNA containing immune complexes (Leadbetter et al., 2002).
Disease phenotypes associated with TLR pathway mutations
Genetic studies in humans have identified rare mutations affecting the MyD88 pathway in humans that present with pyogenic bacterial diseases in childhood. Patients with MyD88 and IRAK4 deficiencies have identical clinical and immunological profiles. Like mice lacking MyD88, the patients with these mutations are highly susceptible to invasive diseases caused by Streptococcus pneumoniae, such as meningitis and septicemia and in some cases to infection with Staphylococcus or Pseudomonas (Maglione et al., 2014; Picard et al., 2010; von Bernuth et al., 2008). Patients with these mutations are compromised for all TLR responses with the exception of TLR3 and a limited TLR4 response. Further, these patients have defective responses to IL-1β, IL-18, and IL-33 all of which signal via MyD88-IRAK4 in all hematopoietic and nonhematopoietic cells. MyD88-deficient mice were also found to be susceptible to this collection of bacterial pathogens. Surprisingly, patients with these mutations are resistant to infection with other bacterial, viral and fungal pathogens revealing a more restricted profile of infectious agents controlled by TLRs in humans than would have been predicted from the heightened susceptibility of MyD88 KO mice to diverse microbes. In addition to MyD88 and IRAK4, autosomal recessive TIRAP deficiency has also been reported in several individuals resulting in life-threatening staphylococcal disease during childhood in one of eight individuals carrying these mutations. All eight patient fibroblasts and leukocytes had impaired responses to TLR1/2, TLR2/6, and TLR4 agonists. However, the whole-blood response to lipoteichoic acid (LTA) a TLR2/6 agonist was abolished only in the index case individual, the only family member lacking LTA-specific antibodies (Abs). This defective response was reversed by anti-LTA monoclonal antibody (mAb). These observations indicate that TIRAP-deficiency can be rescued by antibodies suggesting that the immune system responds despite genetic defects impairing TLR function. This finding could explain the less penetrant phenotypes observed in humans with these mutations (Israel et al., 2017).
Genetic deficiency in TLRs has also been inked to Herpes simplex virus-1 (HSV-1) encephalitis (HSE), the most common form of sporadic viral encephalitis in the developed world. Autosomal recessive deficiency in UNC-93B results in impaired interferon-alpha/beta and -lambda responses (Casrouge et al., 2006). Autosomal dominant TLR3 deficiency also causes HSE by impairing cortical neuron-intrinsic type I IFN immunity to HSV-1 (Zhang et al., 2007). Further, TRAF3 deficiency is also associated with HSE resulting from compromised TLR3-dependent induction of IFNs (Perez de Diego et al., 2010).
Dysfunction or dysregulation of TLR signaling contributes to an array of chronic inflammatory diseases including sepsis, rheumatoid arthritis, asthma, atherosclerosis and systemic lupus erythematosis (reviewed extensively in (Cook et al., 2004). In particular, the development of the autoimmune disease systemic lupus erythematosis (SLE) is associated with dysfunctional TLR7 and 9. These nucleic acid sensing intracellular TLRs are expressed on B cells and pDCs, two cell types implicated in SLE in humans. The direct activation of autoreactive B cells as well as enhanced IFN production by pDCs are important contributors to the pathogenesis of SLE. Elevated type I IFN production is associated with disease severity in SLE (reviewed in (Anders, 2005; Anders et al., 2004). Consistent with these observations, mice that overexpress TLR7 develop a lupus-like disease and deficiency of TLR7 in lupus prone mouse strains are protective (Christensen et al., 2006). Studies in mice indicate that TLR9 plays a more complex role. While TLR9 clearly contributes to the development of anti-dsDNA responses unexpectedly, TLR9 deficient lupus prone mice develop more severe disease (Christensen et al., 2005). Studies in mice lacking both TLR7 and 9 suggest the enhanced disease in TLR9 deficient mice is related to exaggerated TLR7 signaling (Christensen and Shlomchik, 2007; Christensen et al., 2006).
Perspectives
In this review, we offered a detailed discussion of the molecular and cellular mechanisms that influence and are impacted by TLR signaling. We focused on regulatory processes that represent themes that apply to (virtually) the entire superfamily of PRRs. Such themes include the direct recognition of conserved microbial products by PRRs as indicators of potentially infectious encounters and strategies to amplify the downstream inflammatory and defensive responses of the host. Both of these strategies were first discovered by the study of TLRs or the insect Toll Receptor, yet are now recognized to exist in diverse PRR families. For example, the PRR cGAS shares no homology to TLRs, yet this receptor binds microbial products directly (double stranded DNA) and produces a rapidly diffusible cyclic dinucleotide as a second messenger (Sun et al., 2013; Wu et al., 2013). This second messenger (cGAMP) can spread intercellularly to stimulate IFN and inflammatory responses in a wider range of cells than initially detected the infection (Ablasser et al., 2013). Additional themes include the use of SMOCs to create all-or-nothing responses to infection and the importance of microbe-inducible protein trafficking pathways to stimulate (or inhibit) inflammatory responses. These latter two strategies are now recognized to extend to all known PRR signaling pathways (Kagan et al., 2014). Finally, the link between TLR signaling and the induction of adaptive immunity has laid the foundation for the development of novel vaccination approaches that target TLRs and an increasingly wide variety of PRR pathways. Despite these gains in knowledge, large gaps in our understanding of TLRs remains. For example, much of our knowledge is derived from loss of genetic analysis, where it is difficult to define the dynamics of the TLR pathways. As the field develops, an increasing emphasis should be placed on assays that monitor TLR signaling in wild type cells and organisms, with a diverse set of cell types and hosts likely offering unique perspectives on TLR pathway operation. Diversifying cell types and organisms (and life stages of each organism) will likely inform discussions of the evolution of the TLR network, which seems to operate in one way or another in all multicellular organisms. Underlying all these interests is an opportunity to leverage our knowledge of TLRs into novel assays that help diagnose disease and novel treatments of these diseases. The increasing recognition that inflammation influences the symptoms of most human diseases, and the natural process of aging, makes it difficult for us to predict how our basic knowledge of TLR signaling will be used in the future. Only one prediction is certain—TLRs have much more to teach us about ourselves and the world around us.
Acknowledgements
The authors would like to acknowledge Kerstin Nundel (Umass Meedical School) for helpful discussions and funding from the NIH (AI067497 to KAF) and (AI133524, AI093589, AI116550, and P30DK34854 to JCK). JCK holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, and Hornung V. (2013). Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503, 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, and Miyake K. (2000). Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol 164, 3471–3475. [DOI] [PubMed] [Google Scholar]
- Akira S, and Hoshino K. (2003). Myeloid differentiation factor 88-dependent and -independent pathways in toll-like receptor signaling. J Infect Dis 187 Suppl 2, S356–363. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, and Flavell RA (2001). Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413, 732–738. [DOI] [PubMed] [Google Scholar]
- Anders HJ (2005). A Toll for lupus. Lupus 14, 417–422. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Banas B, and Schlondorff D. (2004). Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15, 854–867. [DOI] [PubMed] [Google Scholar]
- Anderson DA 3rd, and Murphy KM (2019). Models of dendritic cell development correlate ontogeny with function. Adv Immunol 143, 99–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KV, Bokla L, and Nusslein-Volhard C. (1985a). Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42, 791–798. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Jurgens G, and Nusslein-Volhard C. (1985b). Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42, 779–789. [DOI] [PubMed] [Google Scholar]
- Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, et al. (2008). Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ 86, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts RJ, Joosten LA, and Netea MG (2016). Immunometabolic circuits in trained immunity. Semin Immunol 28, 425–430. [DOI] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, and Powrie F. (1999). An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atianand MK, Hu W, Satpathy AT, Shen Y, Ricci EP, Alvarez-Dominguez JR, Bhatta A, Schattgen SA, McGowan JD, Blin J, et al. (2016). A Long Noncoding RNA lincRNA-EPS Acts as a Transcriptional Brake to Restrain Inflammation. Cell 165, 1672–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalat R, Lau L, Locksley RM, and Barton GM (2009). Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10, 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, and Kagan JC (2009). A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol 9, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Kagan JC, and Medzhitov R. (2006). Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol 7, 49–56. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183, 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM, and Medzhitov R. (2006). Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812. [DOI] [PubMed] [Google Scholar]
- Blok BA, Arts RJ, van Crevel R, Benn CS, and Netea MG (2015). Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol 98, 347–356. [DOI] [PubMed] [Google Scholar]
- Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W, Iwasaki A, Knipe DM, and Kagan JC (2014). A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 156, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie A, Kiss-Toth E, Symons JA, Smith GL, Dower SK, and O’Neill LA (2000). A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc Natl Acad Sci U S A 97, 10162–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, and Monack DM (2012). Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL, and Lemaitre B. (2009). A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A 106, 12442–12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Henzel WJ, and Gao X. (1996a). IRAK: a kinase associated with the interleukin-1 receptor. Science 271, 1128–1131. [DOI] [PubMed] [Google Scholar]
- Cao Z, Xiong J, Takeuchi M, Kurama T, and Goeddel DV (1996b). TRAF6 is a signal transducer for interleukin-1. Nature 383, 443–446. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB, et al. (2013). A long noncoding RNA mediates both activation and repression of immune response genes. Science 341, 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, et al. (2006). Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314, 308–312. [DOI] [PubMed] [Google Scholar]
- Castellanos-Rubio A, Fernandez-Jimenez N, Kratchmarov R, Luo X, Bhagat G, Green PH, Schneider R, Kiledjian M, Bilbao JR, and Ghosh S. (2016). A long noncoding RNA associated with susceptibility to celiac disease. Science 352, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Atianand M, Jiang Z, Carpenter S, Aiello D, Elling R, Fitzgerald KA, and Caffrey DR (2015a). Cutting Edge: A Natural Antisense Transcript, AS-IL1alpha, Controls Inducible Transcription of the Proinflammatory Cytokine IL-1alpha. J Immunol 195, 1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MP, Onji M, Fukui R, Kawane K, Shibata T, Saitoh S, Ohto U, Shimizu T, Barber GN, and Miyake K. (2015b). DNase II-dependent DNA digestion is required for DNA sensing by TLR9. Nat Commun 6, 5853. [DOI] [PubMed] [Google Scholar]
- Chandler CE, and Ernst RK (2017). Bacterial lipids: powerful modifiers of the innate immune response. F1000Res 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sharma S, Assis PA, Jiang Z, Elling R, Olive AJ, Hang S, Bernier J, Huh JR, Sassetti CM, et al. (2018). CNBP controls IL-12 gene transcription and Th1 immunity. J Exp Med 215, 3136–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, et al. (2014). mTOR-and HIF1-alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CY, Veckman V, Limmer K, and David M. (2012). Phospholipase Cgamma-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation. J Biol Chem 287, 3704–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J, Kelker MS, and Wilson IA (2005). Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science 309, 581–585. [DOI] [PubMed] [Google Scholar]
- Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, and Shlomchik MJ (2005). Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med 202, 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SR, and Shlomchik MJ (2007). Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol 19, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, and Shlomchik MJ (2006). Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428. [DOI] [PubMed] [Google Scholar]
- Collins B, and Wilson IA (2014). Crystal structure of the C-terminal domain of mouse TLR9. Proteins 82, 2874–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Pisetsky DS, and Schwartz DA (2004). Toll-like receptors in the pathogenesis of human disease. Nat Immunol 5, 975–979. [DOI] [PubMed] [Google Scholar]
- Corcoran SE, and O’Neill LA (2016). HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest 126, 3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, and Goodman AL (2015). Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, and Kelliher MA (2005). Rip1 mediates the Trif-dependent toll-like receptor 3-and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem 280, 36560–36566. [DOI] [PubMed] [Google Scholar]
- de Bouteiller O, Merck E, Hasan UA, Hubac S, Benguigui B, Trinchieri G, Bates EE, and Caux C. (2005). Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem 280, 38133–38145. [DOI] [PubMed] [Google Scholar]
- DeLaney AA, Berry CT, Christian DA, Hart A, Bjanes E, Wynosky-Dolfi MA, Li X, Tummers B, Udalova IA, Chen YH, et al. (2019). Caspase-8 promotes c-Rel-dependent inflammatory cytokine expression and resistance against Toxoplasma gondii. Proc Natl Acad Sci U S A 116, 11926–11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, and Reis e Sousa C. (2004). Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, and Vogel SN (2002). Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect 4, 903–914. [DOI] [PubMed] [Google Scholar]
- Du M, Yuan L, Tan X, Huang D, Wang X, Zheng Z, Mao X, Li X, Yang L, Huang K, et al. (2017). The LPS-inducible lncRNA Mirt2 is a negative regulator of inflammation. Nat Commun 8, 2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, and Reis e Sousa C. (2003). Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol 33, 827–833. [DOI] [PubMed] [Google Scholar]
- Emmerich CH, Ordureau A, Strickson S, Arthur JS, Pedrioli PG, Komander D, and Cohen P. (2013). Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci U S A 110, 15247–15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJ, et al. (2014). TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol 15, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald SE, Engel A, Lee J, Wang M, Bogyo M, and Barton GM (2011). Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med 208, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, and Alnemri ES (2013). Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol 191, 3995–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrao R, Zhou H, Shan Y, Liu Q, Li Q, Shaw DE, Li X, and Wu H. (2014). IRAK4 dimerization and trans-autophosphorylation are induced by Myddosome assembly. Mol Cell 55, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, and O’Garra A. (1991). IL-10 inhibits cytokine production by activated macrophages. J Immunol 147, 3815–3822. [PubMed] [Google Scholar]
- Fischer C, Metsger M, Bauch S, Vidal R, Bottcher M, Grote P, Kliem M, and Sauer S. (2019). Signals trigger state-specific transcriptional programs to support diversity and homeostasis in immune cells. Sci Signal 12. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, and Maniatis T. (2003a). IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4, 491–496. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, et al. (2001). Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413, 78–83. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, and Golenbock DT (2003b). LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 198, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, and Nunez G. (2009). Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol 183, 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, Finlay BB, and Wright SD (1992). Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med 176, 1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui R, Yamamoto C, Matsumoto F, Onji M, Shibata T, Murakami Y, Kanno A, Hayashi T, Tanimura N, Yoshida N, et al. (2018). Cleavage of Toll-Like Receptor 9 Ectodomain Is Required for In Vivo Responses to Single Strand DNA. Front Immunol 9, 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg AV, and Cua DJ (2014). The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14, 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, and Hornung V. (2016). Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 44, 833–846. [DOI] [PubMed] [Google Scholar]
- Gay NJ, and Keith FJ (1991). Drosophila Toll and IL-1 receptor. Nature 351, 355–356. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, and Madara JL (2001). Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167, 1882–1885. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Yu Y, Krishna US, Israel DA, Lyons SL, and Peek RM Jr. (2004). Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J Infect Dis 189, 1914–1920. [DOI] [PubMed] [Google Scholar]
- Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, and Weiss JP (2004). Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A 101, 4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioannini TL, and Weiss JP (2007). Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res 39, 249–260. [DOI] [PubMed] [Google Scholar]
- Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, and Colonna M. (2006). Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A 103, 8459–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, Ferrandon D, and Royet J. (2002). The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416, 640–644. [DOI] [PubMed] [Google Scholar]
- Greulich W, Wagner M, Gaidt MM, Stafford C, Cheng Y, Linder A, Carell T, and Hornung V. (2019). TLR8 Is a Sensor of RNase T2 Degradation Products. Cell 179, 1264–1275 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ingolia NT, Weissman JS, and Bartel DP (2010). Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, Kamps MP, Raz E, Wagner H, Hacker G, et al. (2006). Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439, 204–207. [DOI] [PubMed] [Google Scholar]
- Hafner A, Kolbe U, Freund I, Castiglia V, Kovarik P, Poth T, Herster F, Weigand MA, Weber ANR, Dalpke AH, et al. (2019). Crucial Role of Nucleic Acid Sensing via Endosomal Toll-Like Receptors for the Defense of Streptococcus pyogenes in vitro and in vivo. Front Immunol 10, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lear TB, Jerome JA, Rajbhandari S, Snavely CA, Gulick DL, Gibson KF, Zou C, Chen BB, and Mallampalli RK (2015). Lipopolysaccharide Primes the NALP3 Inflammasome by Inhibiting Its Ubiquitination and Degradation Mediated by the SCFFBXL2 E3 Ligase. J Biol Chem 290, 18124–18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MT, Haga IR, Maloney G, Gray P, Reading PC, Bartlett NW, Smith GL, Bowie A, and O’Neill LA (2003). The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med 197, 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, and Aderem A. (2001). The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103. [DOI] [PubMed] [Google Scholar]
- Haziot A, Rong GW, Silver J, and Goyert SM (1993). Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide. J Immunol 151, 1500–1507. [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, and Bauer S. (2004). Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, and Akira S. (2002). Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3, 196–200. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. (2000). A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745. [DOI] [PubMed] [Google Scholar]
- Heyninck K, and Beyaert R. (1999). The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett 442, 147–150. [DOI] [PubMed] [Google Scholar]
- Hidmark A, von Saint Paul A, and Dalpke AH (2012). Cutting edge: TLR13 is a receptor for bacterial RNA. J Immunol 189, 2717–2721. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, and Beutler B. (2003). Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol 4, 1223–1229. [DOI] [PubMed] [Google Scholar]
- Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, and Taniguchi T. (2005a). Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434, 1035–1040. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC, and Taniguchi T. (2004). Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci U S A 101, 15416–15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, et al. (2005b). IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Flavell RA, and Medzhitov R. (2002). The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420, 329–333. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, and Medzhitov R. (2001). TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol 2, 835–841. [DOI] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, and Hartmann G. (2002). Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 168, 4531–4537. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, and Akira S. (1999). Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162, 3749–3752. [PubMed] [Google Scholar]
- Hu G, Gong AY, Wang Y, Ma S, Chen X, Chen J, Su CJ, Shibata A, Strauss-Soukup JK, Drescher KM, et al. (2016). LincRNA-Cox2 Promotes Late Inflammatory Gene Transcription in Macrophages through Modulating SWI/SNF-Mediated Chromatin Remodeling. J Immunol 196, 2799–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima J, Kobayashi S, Kitazume S, Kizuka Y, Fujinawa R, Korekane H, Shibata T, Saitoh SI, Akashi-Takamura S, Miyake K, et al. (2017). Core fucose is critical for CD14-dependent Toll-like receptor 4 signaling. Glycobiology 27, 1006–1015. [DOI] [PubMed] [Google Scholar]
- Imler JL, and Hoffmann JA (2002). Toll receptors in Drosophila: a family of molecules regulating development and immunity. Curr Top Microbiol Immunol 270, 63–79. [DOI] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Moore KJ, Stuart LM, and Ezekowitz RA (2008). Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med 205, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel L, Wang Y, Bulek K, Della Mina E, Zhang Z, Pedergnana V, Chrabieh M, Lemmens NA, Sancho-Shimizu V, Descatoire M, et al. (2017). Human Adaptive Immunity Rescues an Inborn Error of Innate Immunity. Cell 168, 789–800 e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R, Kroehling L, Khitun A, Bailis W, Jarret A, York AG, Khan OM, Brewer JR, Skadow MH, Duizer C, et al. (2018). The translation of non-canonical open reading frames controls mucosal immunity. Nature 564, 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Irizarry-Caro RA, McDaniel MM, Chawla AS, Carroll KR, Overcast GR, Philip NH, Oberst A, Chervonsky AV, Katz JD, et al. (2020). T cells instruct myeloid cells to produce inflammasome-independent IL-1beta and cause autoimmunity. Nat Immunol 21, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA Jr. (1989). Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54 Pt 1, 1–13. [DOI] [PubMed] [Google Scholar]
- Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, and Lee JO (2007). Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082. [DOI] [PubMed] [Google Scholar]
- Jin MS, and Lee JO (2008). Structures of the toll-like receptor family and its ligand complexes. Immunity 29, 182–191. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Magupalli VG, and Wu H. (2014). SMOCs: supramolecular organizing centres that control innate immunity. Nat Rev Immunol 14, 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, and Medzhitov R. (2006). Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125, 943–955. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, and Medzhitov R. (2008). TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, Han SH, Lee H, Paik SG, and Lee JO (2009). Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31, 873–884. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. (2006). Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, and Akira S. (1999). Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122. [DOI] [PubMed] [Google Scholar]
- Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, et al. (2004). Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol 5, 1061–1068. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, and Akira S. (2001). Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol 167, 5887–5894. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671. [DOI] [PubMed] [Google Scholar]
- Kim TW, Staschke K, Bulek K, Yao J, Peters K, Oh KH, Vandenburg Y, Xiao H, Qian W, Hamilton T, et al. (2007). A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med 204, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Brinkmann MM, Paquet ME, and Ploegh HL (2008). UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452, 234–238. [DOI] [PubMed] [Google Scholar]
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, et al. (2012). Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 109, 17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinnijenhuis J, van Crevel R, and Netea MG (2015). Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans R Soc Trop Med Hyg 109, 29–35. [DOI] [PubMed] [Google Scholar]
- Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, and Schmidt JA (1989). Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A 86, 5227–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuk LS, Koch MA, Slayden LC, Lind NA, Chu S, Savage HP, Kantor AB, Baumgarth N, and Barton GM (2019). B cell receptor and Toll-like receptor signaling coordinate to control distinct B-1 responses to both self and the microbiota. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, and Klinman DM (1995). CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549. [DOI] [PubMed] [Google Scholar]
- Kruger A, Oldenburg M, Chebrolu C, Beisser D, Kolter J, Sigmund AM, Steinmann J, Schafer S, Hochrein H, Rahmann S, et al. (2015). Human TLR8 senses UR/URR motifs in bacterial and mitochondrial RNA. EMBO Rep 16, 1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera A, Dawid C, Gisch N, Schmid C, Raasch L, Gerster T, Schaffer M, Smakowska-Luzan E, Belkhadir Y, Vlot AC, et al. (2019). Bacterial medium-chain 3-hydroxy fatty acid metabolites trigger immunity in Arabidopsis plants. Science 364, 178–181. [DOI] [PubMed] [Google Scholar]
- Labeta MO, Vidal K, Nores JE, Arias M, Vita N, Morgan BP, Guillemot JC, Loyaux D, Ferrara P, Schmid D, et al. (2000). Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J Exp Med 191, 1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston PK, Nambu A, Jung J, Shibata M, Aksoylar HI, Lei J, Xu P, Doan MT, Jiang H, MacArthur MR, et al. (2019). Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat Immunol 20, 1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, and Sallusto F. (2001). The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol 13, 291–298. [DOI] [PubMed] [Google Scholar]
- Latty SL, Sakai J, Hopkins L, Verstak B, Paramo T, Berglund NA, Cammarota E, Cicuta P, Gay NJ, Bond PJ, et al. (2018). Activation of Toll-like receptors nucleates assembly of the MyDDosome signaling hub. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, et al. (2007). Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol 8, 772–779. [DOI] [PubMed] [Google Scholar]
- Lazear HM, Nice TJ, and Diamond MS (2015). Interferon-lambda: Immune Functions at Barrier Surfaces and Beyond. Immunity 43, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, and Marshak-Rothstein A. (2002). Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607. [DOI] [PubMed] [Google Scholar]
- Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, and Barton GM (2013). UNC93B1 mediates differential trafficking of endosomal TLRs. Elife 2, e00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Kravchenko V, Kirkland TN, Han J, Mackman N, Moriarty A, Leturcq D, Tobias PS, and Ulevitch RJ (1993). Glycosyl-phosphatidylinositol-anchored or integral membrane forms of CD14 mediate identical cellular responses to endotoxin. Proc Natl Acad Sci U S A 90, 9930–9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, and Hoffmann JA (1996). The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983. [DOI] [PubMed] [Google Scholar]
- Leonard JN, Ghirlando R, Askins J, Bell JK, Margulies DH, Davies DR, and Segal DM (2008). The TLR3 signaling complex forms by cooperative receptor dimerization. Proc Natl Acad Sci U S A 105, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M Jr., and Lemon SM (2005). Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A 102, 2992–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strelow A, Fontana EJ, and Wesche H. (2002). IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci U S A 99, 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, and Reichhart JM (2002). Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297, 114–116. [DOI] [PubMed] [Google Scholar]
- Lin KM, Hu W, Troutman TD, Jennings M, Brewer T, Li X, Nanda S, Cohen P, Thomas JA, and Pasare C. (2014). IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 111, 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Lo YC, and Wu H. (2010). Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Huang DY, Chu CL, Lin YL, and Lin WW (2013). The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci Signal 6, ra71. [DOI] [PubMed] [Google Scholar]
- Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li Y, Wu S, Li Y, Hao B, Bona R, et al. (2010). Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun 1, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, Du F, Ren J, Wu YT, Grishin NV, et al. (2015). Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630. [DOI] [PubMed] [Google Scholar]
- Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, et al. (1999). TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Pelaez M, Lamont DJ, Peggie M, Shpiro N, Gray NS, and Cohen P. (2014). Protein kinase IKKbeta-catalyzed phosphorylation of IRF5 at Ser462 induces its dimerization and nuclear translocation in myeloid cells. Proc Natl Acad Sci U S A 111, 17432–17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione PJ, Simchoni N, Black S, Radigan L, Overbey JR, Bagiella E, Bussel JB, Bossuyt X, Casanova JL, Meyts I, et al. (2014). IRAK-4 and MyD88 deficiencies impair IgM responses against T-independent bacterial antigens. Blood 124, 3561–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer O, Liu B, Woo BJ, Kreuk LSM, Van Dis E, and Barton GM (2019). Release from UNC93B1 reinforces the compartmentalized activation of select TLRs. Nature 575, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T. (1986). Mechanisms of human beta-interferon gene regulation. Harvey Lect 82, 71–104. [PubMed] [Google Scholar]
- Martinon F, Burns K, and Tschopp J. (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10, 417–426. [DOI] [PubMed] [Google Scholar]
- Martinon F, and Tschopp J. (2007). Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 14, 10–22. [DOI] [PubMed] [Google Scholar]
- Matskevich AA, Quintin J, and Ferrandon D. (2010). The Drosophila PRR GNBP3 assembles effector complexes involved in antifungal defenses independently of its Toll-pathway activation function. Eur J Immunol 40, 1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, and Boucrot E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12, 517–533. [DOI] [PubMed] [Google Scholar]
- McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, and Maniatis T. (2004). IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc Natl Acad Sci U S A 101, 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. (2008). Origin and physiological roles of inflammation. Nature 454, 428–435. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, and Mortha A. (2013). The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31, 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, and Tschopp J. (2004). RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol 5, 503–507. [DOI] [PubMed] [Google Scholar]
- Miller YI, Choi SH, Wiesner P, and Bae YS (2012). The SYK side of TLR4: signalling mechanisms in response to LPS and minimally oxidized LDL. Br J Pharmacol 167, 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S, Vargas J, and Hoffmann A. (2016). Signaling via the NFkappaB system. Wiley Interdiscip Rev Syst Biol Med 8, 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, et al. (2006). Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 7, 1066–1073. [DOI] [PubMed] [Google Scholar]
- Moreno C, Merino J, Ramirez N, Echeverria A, Pastor F, and Sanchez-Ibarrola A. (2004). Lipopolysaccharide needs soluble CD14 to interact with TLR4 in human monocytes depleted of membrane CD14. Microbes Infect 6, 990–995. [DOI] [PubMed] [Google Scholar]
- Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock AM, Robinson CV, Latz E, and Gay NJ (2009). An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem 284, 25404–25411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchess ML, Arpaia N, Souza G, Barbalat R, Ewald SE, Lau L, and Barton GM (2011). Transmembrane mutations in Toll-like receptor 9 bypass the requirement for ectodomain proteolysis and induce fatal inflammation. Immunity 35, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynagh PN (2009). The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol 30, 33–42. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Morosky SA, Delorme-Axford E, Dybdahl-Sissoko N, Oberste MS, Wang T, and Coyne CB (2011). The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog 7, e1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, and David M. (1999). p38-dependent activation of interferon regulatory factor 3 by lipopolysaccharide. J Biol Chem 274, 35535–35538. [DOI] [PubMed] [Google Scholar]
- Negishi H, Fujita Y, Yanai H, Sakaguchi S, Ouyang X, Shinohara M, Takayanagi H, Ohba Y, Taniguchi T, and Honda K. (2006). Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc Natl Acad Sci U S A 103, 15136–15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, MacFarlane A.W.t., Toft M, Lowell CA, Campbell KS, and Hamerman JA (2012). B-cell adaptor for PI3K (BCAP) negatively regulates Toll-like receptor signaling through activation of PI3K. Proc Natl Acad Sci U S A 109, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen NJ, Vladimer GI, Stenvik J, Orning MP, Zeid-Kilani MV, Bugge M, Bergstroem B, Conlon J, Husebye H, Hise AG, et al. (2015). A role for the adaptor proteins TRAM and TRIF in toll-like receptor 2 signaling. J Biol Chem 290, 3209–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig MC, and Steinman RM (1980). Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med 151, 1196–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslein-Volhard C, and Wieschaus E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, and Greene C. (1998). Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol 63, 650–657. [PubMed] [Google Scholar]
- O’Neill LA, Kishton RJ, and Rathmell J. (2016). A guide to immunometabolism for immunologists. Nat Rev Immunol 16, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, and Pearce EJ (2016). Immunometabolism governs dendritic cell and macrophage function. J Exp Med 213, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Sheedy FJ, and McCoy CE (2011). MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol 11, 163–175. [DOI] [PubMed] [Google Scholar]
- Odendall C, Voak AA, and Kagan JC (2017). Type III IFNs Are Commonly Induced by Bacteria-Sensing TLRs and Reinforce Epithelial Barriers during Infection. J Immunol 199, 3270–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto U, Ishida H, Shibata T, Sato R, Miyake K, and Shimizu T. (2018). Toll-like Receptor 9 Contains Two DNA Binding Sites that Function Cooperatively to Promote Receptor Dimerization and Activation. Immunity 48, 649–658 e644. [DOI] [PubMed] [Google Scholar]
- Ohto U, Shibata T, Tanji H, Ishida H, Krayukhina E, Uchiyama S, Miyake K, and Shimizu T. (2015). Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 520, 702–705. [DOI] [PubMed] [Google Scholar]
- Oldenburg M, Kruger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, Bathke B, Lauterbach H, Suter M, Dreher S, et al. (2012). TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science 337, 1111–1115. [DOI] [PubMed] [Google Scholar]
- Olguin Y, Villalobos P, Carrascosa LG, Young M, Valdez E, Lechuga L, and Galindo R. (2013). Detection of flagellin by interaction with human recombinant TLR5 immobilized in liposomes. Anal Bioanal Chem 405, 1267–1281. [DOI] [PubMed] [Google Scholar]
- Onji M, Kanno A, Saitoh S, Fukui R, Motoi Y, Shibata T, Matsumoto F, Lamichhane A, Sato S, Kiyono H, et al. (2013). An essential role for the N-terminal fragment of Toll-like receptor 9 in DNA sensing. Nat Commun 4, 1949. [DOI] [PubMed] [Google Scholar]
- Orning P, Lien E, and Fitzgerald KA (2019). Gasdermins and their role in immunity and inflammation. J Exp Med 216, 2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, and Lee JO (2009). The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195. [DOI] [PubMed] [Google Scholar]
- Pasare C, and Medzhitov R. (2004a). Toll-dependent control mechanisms of CD4 T cell activation. Immunity 21, 733–741. [DOI] [PubMed] [Google Scholar]
- Pasare C, and Medzhitov R. (2004b). Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 6, 1382–1387. [DOI] [PubMed] [Google Scholar]
- Pasare C, and Medzhitov R. (2005). Control of B-cell responses by Toll-like receptors. Nature 438, 364–368. [DOI] [PubMed] [Google Scholar]
- Pelka K, Bertheloot D, Reimer E, Phulphagar K, Schmidt SV, Christ A, Stahl R, Watson N, Miyake K, Hacohen N, et al. (2018). The Chaperone UNC93B1 Regulates Toll-like Receptor Stability Independently of Endosomal TLR Transport. Immunity 48, 911–922 e917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, Herman M, Cardon A, Durandy A, Bustamante J, et al. (2010). Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33, 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DJ, Richard K, Hansen AM, Lai W, Nallar S, Koller B, and Vogel SN (2018). Autocrine-paracrine prostaglandin E2 signaling restricts TLR4 internalization and TRIF signaling. Nat Immunol 19, 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, Arkwright PD, McDonald D, Geha RS, Takada H, Krause JC, et al. (2010). Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 89, 403–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohar J, Lainscek D, Ivicak-Kocjan K, Cajnko MM, Jerala R, and Bencina M. (2017). Short single-stranded DNA degradation products augment the activation of Toll-like receptor 9. Nat Commun 8, 15363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. (1998). Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088. [DOI] [PubMed] [Google Scholar]
- Py BF, Kim MS, Vakifahmetoglu-Norberg H, and Yuan J. (2013). Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49, 331–338. [DOI] [PubMed] [Google Scholar]
- Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, et al. (2012). Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, and Malo D. (1999). Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189, 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randow F, and Seed B. (2001). Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol 3, 891–896. [DOI] [PubMed] [Google Scholar]
- Rapicavoli NA, Qu K, Zhang J, Mikhail M, Laberge RM, and Chang HY (2013). A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife 2, e00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, and Fitzgerald KA (2012). TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Chen X, and Chen ZJ (2014). IKKbeta is an IRF5 kinase that instigates inflammation. Proc Natl Acad Sci U S A 111, 17438–17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, and Chang HY (2012). Genome regulation by long noncoding RNAs. Annu Rev Biochem 81, 145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock FL, Hardiman G, Timans JC, Kastelein RA, and Bazan JF (1998). A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A 95, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DC, McGettrick AF, Latz E, Monks BG, Gay NJ, Yamamoto M, Akira S, O’Neill LA, Fitzgerald KA, and Golenbock DT (2006). The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci U S A 103, 6299–6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Karmakar M, and Pearlman E. (2014). CD14 mediates Toll-like receptor 4 (TLR4) endocytosis and spleen tyrosine kinase (Syk) and interferon regulatory transcription factor 3 (IRF3) activation in epithelial cells and impairs neutrophil infiltration and Pseudomonas aeruginosa killing in vivo. J Biol Chem 289, 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Kim SJ, Rah SH, Kang JI, Jung HE, Lee D, Lee HK, Lee JO, Park BS, Yoon TY, et al. (2017). Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 46, 38–50. [DOI] [PubMed] [Google Scholar]
- Salazar JC, Duhnam-Ems S, La Vake C, Cruz AR, Moore MW, Caimano MJ, Velez-Climent L, Shupe J, Krueger W, and Radolf JD (2009). Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta. PLoS Pathog 5, e1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S, Hoffmann A, Liou HC, Baltimore D, and Smale ST (2000). Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci U S A 97, 12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Weindel CG, Smirnova I, Tang AY, Ilyukha V, Sorokin M, Buzdin A, Fitzgerald KA, et al. (2019). Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ 26, 332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai M, Linehan MM, and Iwasaki A. (2010). Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science 329, 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake H, and Sekiguchi T. (2012). Toll-like receptors of deuterostome invertebrates. Front Immunol 3, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Tanaka N, Hata N, Oda E, and Taniguchi T. (1998). Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-beta gene. FEBS Lett 425, 112–116. [DOI] [PubMed] [Google Scholar]
- Schappe MS, Szteyn K, Stremska ME, Mendu SK, Downs TK, Seegren PV, Mahoney MA, Dixit S, Krupa JK, Stipes EJ, et al. (2018). Chanzyme TRPM7 Mediates the Ca(2+) Influx Essential for Lipopolysaccharide-Induced Toll-Like Receptor 4 Endocytosis and Macrophage Activation. Immunity 48, 59–74 e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattgen SA, and Fitzgerald KA (2011). The PYHIN protein family as mediators of host defenses. Immunol Rev 243, 109–118. [DOI] [PubMed] [Google Scholar]
- Schauvliege R, Janssens S, and Beyaert R. (2007). Pellino proteins: novel players in TLR and IL-1R signalling. J Cell Mol Med 11, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DS, Hudson KL, Lin TY, and Anderson KV (1991). Dominant and recessive mutations define functional domains of Toll, a transmembrane protein required for dorsal-ventral polarity in the Drosophila embryo. Genes Dev 5, 797–807. [DOI] [PubMed] [Google Scholar]
- Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, and Golenbock DT (2005). The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem 280, 17005–17012. [DOI] [PubMed] [Google Scholar]
- Schromm AB, Lien E, Henneke P, Chow JC, Yoshimura A, Heine H, Latz E, Monks BG, Schwartz DA, Miyake K, et al. (2001). Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med 194, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, and Reis e Sousa C. (2005). Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892. [DOI] [PubMed] [Google Scholar]
- Shaffer SA, Harvey MD, Goodlett DR, and Ernst RK (2007). Structural heterogeneity and environmentally regulated remodeling of Francisella tularensis subspecies novicida lipid A characterized by tandem mass spectrometry. J Am Soc Mass Spectrom 18, 1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Fitzgerald KA, Cancro MP, and Marshak-Rothstein A. (2015). Nucleic Acid-Sensing Receptors: Rheostats of Autoimmunity and Autoinflammation. J Immunol 195, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, and Hiscott J. (2003). Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. [DOI] [PubMed] [Google Scholar]
- Shibata T, Ohto U, Nomura S, Kibata K, Motoi Y, Zhang Y, Murakami Y, Fukui R, Ishimoto T, Sano S, et al. (2016). Guanosine and its modified derivatives are endogenous ligands for TLR7. Int Immunol 28, 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Gotz F, et al. (2010). Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe 7, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, and Kimoto M. (1999). MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189, 1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval DS, Biswas A, Kang YH, Griffith AE, Konnikova L, Mascanfroni ID, Redhu NS, Frei SM, Field M, Doty AL, et al. (2016). Interleukin 1beta Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 151, 1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Liu B, Huai W, Yu Z, Wang W, Zhao J, Han L, Jiang G, Zhang L, Gao C, et al. (2016). The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat Commun 7, 13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, Huang YJ, Cai H, Zhan XY, et al. (2017). NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell 68, 185–197 e186. [DOI] [PubMed] [Google Scholar]
- Stack J, Doyle SL, Connolly DJ, Reinert LS, O’Keeffe KM, McLoughlin RM, Paludan SR, and Bowie AG (2014). TRAM is required for TLR2 endosomal signaling to type I IFN induction. J Immunol 193, 6090–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R. (1987). Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science 238, 692–694. [DOI] [PubMed] [Google Scholar]
- Strickson S, Emmerich CH, Goh ETH, Zhang J, Kelsall IR, Macartney T, Hastie CJ, Knebel A, Peggie M, Marchesi F, et al. (2017). Roles of the TRAF6 and Pellino E3 ligases in MyD88 and RANKL signaling. Proc Natl Acad Sci U S A 114, E3481–E3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Li N, Oh KS, Dutta B, Vayttaden SJ, Lin B, Ebert TS, De Nardo D, Davis J, Bagirzadeh R, et al. (2016). Comprehensive RNAi-based screening of human and mouse TLR pathways identifies species-specific preferences in signaling protein use. Sci Signal 9, ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, and Chen ZJ (2013). Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung MH, Li N, Lao Q, Gottschalk RA, Hager GL, and Fraser ID (2014). Switching of the relative dominance between feedback mechanisms in lipopolysaccharide-induced NF-kappaB signaling. Sci Signal 7, ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, et al. (2006). The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol 7, 156–164. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Shibata T, Akashi-Takamura S, Kiyokawa T, Wakabayashi Y, Tanimura N, Kobayashi T, Matsumoto F, Fukui R, Kouro T, et al. (2007). A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med 204, 2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, et al. (2005). Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434, 243–249. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, and Akira S. (1999). Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11, 443–451. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, and Akira S. (2001). Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 13, 933–940. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, and Akira S. (2002). Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169, 10–14. [DOI] [PubMed] [Google Scholar]
- Tan Y, and Kagan JC (2019). Innate Immune Signaling Organelles Display Natural and Programmable Signaling Flexibility. Cell 177, 384–398 e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Zanoni I, Cullen TW, Goodman AL, and Kagan JC (2015). Mechanisms of Toll-like Receptor 4 Endocytosis Reveal a Common Immune-Evasion Strategy Used by Pathogenic and Commensal Bacteria. Immunity 43, 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji H, Ohto U, Motoi Y, Shibata T, Miyake K, and Shimizu T. (2016). Autoinhibition and relief mechanism by the proteolytic processing of Toll-like receptor 8. Proc Natl Acad Sci U S A 113, 3012–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji H, Ohto U, Shibata T, Taoka M, Yamauchi Y, Isobe T, Miyake K, and Shimizu T. (2015). Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat Struct Mol Biol 22, 109–115. [DOI] [PubMed] [Google Scholar]
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. (2007). Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8, 487–496. [DOI] [PubMed] [Google Scholar]
- Tobias PS, Soldau K, and Ulevitch RJ (1986). Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med 164, 777–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, et al. (2002). TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol 3, 392–398. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, and Triantafilou K. (2006). Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem 281, 31002–31011. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, and Triantafilou K. (2002). Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci 115, 2603–2611. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Ebersold M, Garrett W, Pypaert M, and Mellman I. (2003). Activation of lysosomal function during dendritic cell maturation. Science 299, 1400–1403. [DOI] [PubMed] [Google Scholar]
- Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, and Pasare C. (2012). Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci U S A 109, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer EE, Tavares RM, Mortier E, Hitotsumatsu O, Advincula R, Lee B, Shifrin N, Malynn BA, and Ma A. (2008). Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J Exp Med 205, 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, and Mellman I. (2000). Transport of peptide-MHC class II complexes in developing dendritic cells. Science 288, 522–527. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, et al. (2005). Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7-and TLR9-mediated interferon-{alpha} induction. J Exp Med 201, 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I, and Bartel DP (2013). lincRNAs: genomics, evolution, and mechanisms. Cell 154, 26–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T, Detmers PA, Charron D, and Haziot A. (2004). TLR2 recognizes a bacterial lipopeptide through direct binding. J Immunol 173, 7401–7405. [DOI] [PubMed] [Google Scholar]
- Vigano E, Diamond CE, Spreafico R, Balachander A, Sobota RM, and Mortellaro A. (2015). Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun 6, 8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, et al. (2008). Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321, 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, and Chen ZJ (2001). TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351. [DOI] [PubMed] [Google Scholar]
- Wang L, Weber AN, Atilano ML, Filipe SR, Gay NJ, and Ligoxygakis P. (2006). Sensing of Gram-positive bacteria in Drosophila: GNBP1 is needed to process and present peptidoglycan to PGRP-SA. EMBO J 25, 5005–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TL, Goto K, and Altosaar I. (2014). Ingested soluble CD14 contributes to the functional pool of circulating sCD14 in mice. Immunobiology 219, 537–546. [DOI] [PubMed] [Google Scholar]
- Weinlich R, Oberst A, Beere HM, and Green DR (2017). Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18, 127–136. [DOI] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, and Mathison JC (1990). CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249, 1431–1433. [DOI] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, and Chen ZJ (2013). Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. (2003a). Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, and Akira S. (2003b). TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 4, 1144–1150. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, and Li Z. (2007). Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, et al. (2005). TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629. [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, and Kagan JC (2011). CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147, 868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Spreafico R, Bodio C, Di Gioia M, Cigni C, Broggi A, Gorletta T, Caccia M, Chirico G, Sironi L, et al. (2013). IL-15 cis presentation is required for optimal NK cell activation in lipopolysaccharide-mediated inflammatory conditions. Cell Rep 4, 1235–1249. [DOI] [PubMed] [Google Scholar]
- Zhang J, Stirling B, Temmerman ST, Ma CA, Fuss IJ, Derry JM, and Jain A. (2006). Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest 116, 3042–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, et al. (2007). TLR3 deficiency in patients with herpes simplex encephalitis. Science 317, 1522–1527. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ohto U, Shibata T, Krayukhina E, Taoka M, Yamauchi Y, Tanji H, Isobe T, Uchiyama S, Miyake K, et al. (2016). Structural Analysis Reveals that Toll-like Receptor 7 Is a Dual Receptor for Guanosine and Single-Stranded RNA. Immunity 45, 737–748. [DOI] [PubMed] [Google Scholar]