1 Introduction
These guidelines deal with the diagnosis and treatment of subcutaneous varicose veins and intrafascial varicose veins; Association of the Scientific Medical Societies in Germany (AWMF) register number 037-018.
2 Abstract
2.1 Participating professional associations and organisations
These guidelines for the diagnosis and treatment of varicose veins were prepared under the guidance of the Deutsche Gesellschaft für Phlebologie e. V. (DGP) in cooperation with the Deutsche Gesellschaft für Gefäßchirurgie und Gefäßmedizin—Gesellschaft für operative, endovaskuläre und präventive Gefäßmedizin e. V. (DGG), the Deutschen Gesellschaft für Angiologie, Gesellschaft für Gefäßmedizin e. V. (DGA), the Deutsche Dermatologischen Gesellschaft (DDG), the Deutsche Gesellschaft für Dermatochirurgie e. V. (DGDC), the Berufsverband der Phlebologen e. V. (BVP), and the Arbeitsgemeinschaft der niedergelassenen Gefäßchirurgen Deutschlands e. V. (ANG). This updated 2018/2019 version is based on the guidelines agreed and drafted by the same associations in 2004 and 2009, and it was adopted by the Boards of the participating professional associations on 30 April 2019.
2.2 Development stage of the guidelines
These guidelines are based on a structured consensus process, drawing on published data to create consensus-based guidelines at development stage S2k.
2.3 Delegates of the professional associations
See: https://www.awmf.org/uploads/tx_szleitlinien/037-018l_S2k_Varikose_Diagnostik-Therapie_2019-07.pdf.
2.4 Selected literature
The recommendations are based on the same publications used in previous versions and a systematic literature review carried out on 21 July 2016 in the German Institute for Vascular Public Health Research (DIGG). The review included randomised studies, meta-analyses, and controlled studies. The literature search was carried out in the Medline and PubMed databases with the following search fields in German and/or English: sclerotherapy, endovenous thermal ablation, mechanochemical ablation, cyanoacrylate glue, surgical procedures (stripping), and diagnosis, prognosis, and postoperative care of varicose veins. A manual search was carried out for later publications up to December 2018.
2.5 Recommendations
The strengths of the consensus-based recommendations are based on the recommendations of the AWMF. The following levels are used:
Shall/shall not: strong recommendation
Should/should not: recommendation
Can be considered/can be omitted: open recommendation
3 Method
See guideline report: https://www.awmf.org/uploads/tx_szleitlinien/037-018l_S2k_Varikose_Diagnostik-Therapie_2019-07.pdf.
4 General
4.1 Classification of the varicose vein
Varicose vein disease (varicose vein disease, primary varicose vein) is a degenerative disease of the vein wall in the superficial vein system of the legs in which, under the influence of a range of factors (e.g., pregnancy, orthostatic stress), more or less pronounced and severe varicose veins (varices) develop over the course of the patient’s life [1]. A varicose vein is a lifelong, progressive disease that can have a decisive negative impact on the patient’s quality of life. Nevertheless, a primary varicose vein is not in itself a life-changing disease. This fact shall always be borne in mind in all decisions taken with respect to varicose vein disease.
In the presence of a primary varicose vein, it is essential to distinguish the (primary) varices from those that may develop as a result of obliterate processes in the deep vein system as epifascial collateral veins (secondary varices).
The following basic types of varicose veins can be distinguished, based on topographical and/or morphological criteria:
Varicose saphenous veins, including varicose accessory saphenous veins
Varicose tributary veins
Varicose perforator veins
Pelvic varices
Reticular varices
Spider veins
Veins should be indicated anatomically and topographically using the terms defined in the nomenclature developed in a transatlantic consensus document [2]. According to Hach, haemodynamically significant saphenous vein incompetence can be classified into different degrees (classes) in the refluxing segment [3]; however, these classes do not cover every variation of varicose veins. In the case of incompetence in the junction region of a saphenous vein, the incompetence class is defined by the length of the refluxing segment to the distal reflux point.
Other forms of haemodynamically significant varicose veins exist [4], such as the following:
Incomplete saphenous varicose vein (proximal reflux source in a perforator vein or in another region of the saphenous vein)
Ascending varicose vein without primary incompetence in the junction region
Isolated varicose tributary and perforator vein
Special forms of varicose veins, e.g., pudendal, gluteal, and pelvic varicose veins [5, 6]
The region of the saphenofemoral junction presents a multitude of variants, with or without terminal valve incompetence and with or without incompetence of the preterminal valve [7]. These result in different forms of saphenofemoral incompetence. Similar behaviour is found in the region of the saphenopopliteal junction.
4.2 Natural development of the varicose vein
A large number of epidemiological studies have shown that primary varicose veins are a very frequent disease [8–10]. In the Bonn Vein Study of 2003, one man in six and one woman in five presented chronic venous insufficiency (CVI); however, severe expressions of the disease had diminished in comparison with older epidemiological studies. In this study, 12.4% of men and 15.8% of women presented varicose veins without signs of CVI, and 11.6% of men and 14.9% of women presented venous oedema. Advanced CVI (CEAP classification: C4–C6) was found in 3.8% of men and 3.4% of women [11].
Basic risk factors for varicose veins are advanced age, female sex, pregnancies, and positive family history [12, 13].
Primary varicose vein disease may appear in childhood, and its prevalence increases with age [12, 14]. A genetic disposition is assumed for primary varicose veins [12].
The varicose veins may appear with or without symptoms (C2) or with oedema and/or skin alterations in the context of CVI (C3–C6) [15, 16]. If it is not treated, a medically significant varicose vein, particularly an incompetent saphenous or perforator vein, frequently leads to complications (chronic oedema, trophic skin alterations, venous leg ulcer, deep vein incompetence, varicophlebitis) [13, 17, 18]. An important pathogenetic factor is disturbance of venous haemodynamics, with the development of ambulatory venous hypertension. This leads to increased risk of deep leg vein thrombosis, especially with the simultaneous presence of superficial vein thrombosis [19]. In the Basel study, it was found that individuals with serious or painful varicose veins suffered between nine and 20 times more complications of the venous system, depending on the degree of severity, than individuals of the same age who were free of varicose vein disease [20]. Epidemiological and prospective studies have also confirmed that the presence of a symptomatic varicose vein causes quality of life to deteriorate [21, 22].
Varicose vein disease is progressive over the course of life. In the Edinburgh Vein Study, after 13.4 years, 57.8% of patients with a varicose saphenous vein or chronic venous incompetence presented progression (4.3%/year) [18]. Labropoulos showed that after 19 months, 14.7% of varicose vein patients presented a longer refluxing segment, and 11.2% a progression of the clinical alterations [23]. Engelhorn showed that young patients with early stages of varicose vein disease often present ascending progression of the varicose vein [24]. In a population of 304 patients on the waiting list for an operation on a varicose saphenous vein in England, after 4 years Brewster found progression of the disease in 64% of cases: 5.2% developed a superficial vein thrombus, 22% developed skin alterations, and 12% developed a venous leg ulcer [25]. In the Bonn vein study, after 6.7 years, 19.8% of the patients with varicose nonsaphenous veins and 31.8% of patients with varicose saphenous veins, all in CEAP stage C2, developed progression to CVI [13].
4.3 Indications for varicose vein treatment and referral to a specialist
The objective of varicose vein treatment consists of
normalisation or improvement of vein haemodynamics,
improvement or elimination of congestion pain (heaviness, tension, heat, pain) and/or persistent oedema,
healing, or reduction of the recurrence rate, of venous ulcers and other forms of trophic disturbances, and
prevention of further complications, e.g., superficial vein thrombosis, deep vein incompetence, arthrogenic congestion syndrome, variceal bleeding [26, 27].
Varicose veins may be asymptomatic or symptomatic (painful symptoms). They may lead to the development of oedema and/or skin alterations up to and including venous ulcers. Furthermore, multiple complications may appear, such as superficial vein thrombosis, vein inflammation, or variceal bleeding.
Recommendation 1
In the presence of a symptomatic or significant varicose vein, a special examination should be carried out to plan further procedures. To this end, the patient should be referred to a vein specialist who has sufficient knowledge about the full spectrum of diagnosis, including duplex ultrasound, and the range of possible treatments and/or can make appropriate recommendations.
Recommendation 2
Any patient with variceal bleeding, superficial vein thrombosis, varices during pregnancy, or a venous leg ulcer shall be referred to a vein specialist for further treatment planning.
Recommendation 3
The treatment of a varicose vein and its complications can be conservative or invasive. Choice of treatment is dictated by the will of the patient, the severity and location of the pathological alterations to the superficial and deep vein systems, and the patient’s general state of health.
Recommendation 4
For patients with varicose veins and signs of CVI (venous oedema to venous leg ulcer), a haemodynamically effective treatment shall be sought.
Recommendation 5
In the presence of varicose vein complications (variceal bleeding, superficial vein thrombosis, venous leg ulcer), prompt, appropriate treatment shall be sought.
4.4 Classification and diagnosis
Characterisation of the varicose vein and its effects shall/should be developed jointly with diagnosis of a state or class using a recognised classification system. The CEAP classification for the description of chronic vein diseases [28] has been internationally established. The clinical classification according to CEAP is shown in Table 1.
Table 1.
Clinical classification (C) according to CEAP
| Class | Clinical signs |
|---|---|
| C0 | No visible or palpable signs of venous incompetence |
| C1 | Spider veins and/or reticular varices |
| C2 | Varicose veins |
| C3 | Oedema |
| C4a | Pigmentation, eczema |
| C4b | Atrophie blanche, dermatoliposclerosis |
| C5 | Cured venous leg ulcer |
| C6 | Active venous leg ulcer |
Any C class can present without or with subjective symptoms such as pain or sensations of heaviness, tension, or swelling. In symptomatic patients, a subscript s is added (e.g., C2s = symptomatic varicose vein). Chronic venous incompetence (CVI) is defined as C class C3–C6.
In addition, the CEAP classification can be complemented with aetiological (E), anatomical (A), and pathophysiological (P) criteria as needed. The CEAP classification does not indicate the severity of the varicose vein. The intention of the initiators and developers of the CEAP classification was that it should provide a clinical classification valid for all types of chronic venous diseases.
Recommendation 6
The CEAP classification should be used for classifying a varicose vein.
Apart from the CEAP system, other validated classification systems exist, used principally in the context of scientific studies. The degree of severity of vein disease can be indicated with the Venous Clinical Severity Score (VCSS) [29], among other systems. The impacts of vein disease on quality of life can be measured and described, for example, with the following score systems: the Venous Insufficiency Epidemiologic and Economic Study of Quality of Life (VEINES-QOL/Sym) [30], the Chronic Venous Insufficiency Qualify of Life Questionnaire (CIVIQ) [31], the Aberdeen Varicose Vein Questionnaire (AVVQ) [32], and the Freiburger Questionnaire of Quality of Life (FLQA) [33]. Shortened versions of many of these systems have been described for use in clinical practice.
Recommendation 7
Validated clinical scores and quality-of-life scores can be used to indicate the severity of a varicose vein.
4.5 Patients’ expectations, and explanations given to the patient
The patients’ expectations of planned treatments can be very variable, ranging from cosmetic aspirations through symptom relief and healing of complications or an ulcer to halting progression of the disease. Many patients are motivated by anxiety or fear about the invasiveness and the side effects of treatment measures [34].
Thorough, comprehensive explanation of the many methods available, the advantages and disadvantages of a treatment, possible complications and prospects for success, etc., as well as complete, traceable documentation, are indispensable for the legal validity of a declaration of consent. In Germany, the entire complex of explanation, consent, and documentation has been governed by a special law since 2013 (Law for the Improvement of Patients’ Rights, §§ 630c, d, e, f BGB), known as the Patients’ Rights Law.
Recommendation 8
The patient’s wishes shall be taken into account in the explanations and the selection of the treatment method. They form an important part of the treatment decision.
Recommendation 9
The explanatory conversation shall discuss the type, severity, possible complications, and prognosis of the clinical picture. The different treatment options shall be explained, with their advantages and potential risks.
Recommendation 10
Patients shall be informed of the principles and the effectiveness and side effects of the suggested treatment, and of the possibilities of coverage by health insurance.
Recommendation 11
The explanation should include the fact that the course of vein diseases is in principle progressive and that, as a rule, a long-term, conservative, symptom-oriented treatment shall be applied.
5 Diagnosis
This chapter does not describe the diagnostic procedures as such, since each is the subject of its own guideline. Here only the validity of the procedure is indicated, in conjunction with the treatment decision in the case of a varicose vein.
Before varicose vein treatment can be planned, the disease shall be recognised, with its severity and differential diagnosis to distinguish it from other diseases. Table 2 shows a classification of the objects of diagnosis.
Table 2.
Objects of diagnosis of varicose veins
| The objects of diagnosis of a varicose vein disease are as follows: |
|---|
| Discovery and classification of the haemodynamic disturbance (duplex ultrasound) |
| Classification of the medical significance (medical record, inspection, duplex ultrasound, light reflection rheography [LRR], venous occlusion plethysmography [VOP]) |
| Differentiation of a primary from a secondary varicose vein (duplex ultrasound, occasionally other imaging techniques, LRR, VOP) |
| Discovery and classification of the deep vein system (duplex ultrasound, phlebography, other imaging techniques, LRR, VOP) |
| Follow-up for quality control after the intervention (medical record, inspection, duplex ultrasound, LRR, VOP) |
| Analysis of recurrence (cause, extent) (duplex ultrasound, inspection) |
Objects of diagnosis with accompanying trophic disturbances or skin alterations such as oedema, as well as pain:
Exclusion or confirmation and description of an oedema and clarification of the cause, particularly diagnosis to differentiate oedema caused by an internal ailment—lymphoedema, lipoedema, obesity oedema—from venous oedema (inspection, medical record, recommendation for further investigation)
Differential diagnosis of skin alterations present, if any (medical record, inspection)
Differential diagnosis of painful symptoms present, particularly for differential diagnostic clarification of orthopaedically caused symptoms (inspection, physical examination, medical record, consultation with a neurologist/orthopaedic specialist)
The following are required before a treatment can be indicated:
Exclusion of accompanying peripheral arterial occlusion disease (medical record, inspection, Doppler [ABI], duplex ultrasound)
Exclusion of acute thrombotic event (ultrasound if there is clinical suspicion)
Exclusion of infection (medical record, inspection, laboratory test if necessary)
Recommendation 12
Investigation of the patient’s medical record and clinical examination shall form the basis of the decision for a diagnosis on which further action is planned.
Treatment planning, particularly the choice of the best treatment that will minimise complications and recurrence, is possible only after haemodynamic analysis of the varicose vein. This includes recording and describing the refluxive segments of the saphenous veins and tributaries, the sources of reflux from the deep vein system, and the reentry points of the recirculating blood, as well as analysis of the flow patterns in the deep vein system. The decision of when to start further treatment in addition to compression is also subject to quantification of the haemodynamic effects of the disease, for example by PPG or VOP, particularly in combination with the necessary progression check-up with comparative control.
Recommendation 13
An imaging technique shall be used in the context of standardised phlebological diagnosis. The first-choice method shall be duplex ultrasound. If necessary, vein function shall also be measured (e.g., PPG/LRR or VOP).
Recommendation 15
Complete, traceable documentation of the findings shall be kept.
Recommendation 16
Pathological findings from technical examinations should be tested for clinical significance.
The methods listed below are regarded as standard procedures in clarifying and evaluating vein disease. They are not to be considered as competing alternatives. On the contrary, the combination of their different diagnostic powers and their assessment of different functional and morphological criteria will enable the sum of the results to achieve maximum diagnostic accuracy and reliability.
5.1. Diagnostic instruments
5.1.1 Medical record
Indication and recommendations for application
The patient’s medical record shall be consulted at the time of the first examination. It contains information on the patient’s prior history (how long he or she has suffered varicose veins and which symptoms are present, especially swellings, skin alterations, and itching) and which measures relieve the symptoms (e.g., compression). At the same time, the doctor can also discover the record of drugs prescribed and inquire about thrombosis in the patient’s own record and that of his or her family, as well as ulcers and other previous ailments.
Further enquiry will reveal how the symptoms have altered and how good the patient’s adherence to compression is.
This information will give indications about the severity of the symptoms and thus about a possible line of treatment. The most common symptoms with a varicose vein are a sensation of heaviness, swelling, itching, and occasional pain after standing or sitting for long periods [16].
Documentation
The facts from the patient’s medical history shall be extracted traceably from the documentary record to allow follow-up of the progression of the symptoms and to note changes in the symptoms after the application of treatment.
Recommendation 17
In cases of venous symptoms, the medical record shall be the basis for diagnosis and differential diagnosis.
5.1.2 Clinical examination
Indication
The clinical examination shall be the indispensable prerequisite for phlebological diagnosis. During the inspection, the doctor should look not only for visible varicose veins in the thigh and lower leg—especially the preferential areas in the lower leg—but also for oedema, hyperpigmentation, eczema, and (healed) ulcers, which may be related to chronic venous incompetence. In palpation, look out for stringy subcutaneous hardness, which may indicate acute or past superficial vein thrombosis. Larger areas of subcutaneous hardness may be an indication of (lipo)dermatosclerosis.
Execution
In a mobile patient, the clinical examination should be carried out with the patient standing up.
Documentation recommendations
The clinical expression of a varicose vein should be documented using the CEAP classification [28, 35]. Further information to help assess the evolution of the condition, especially after therapeutic interventions, can be recorded using the Venous Clinical Severity Score (VCSS) [36].
Recommendation 18
The clinical examination shall be taken into the treatment decision consideration as appropriate, as it reflects the severity of the disease.
5.1.3 Global measurement techniques (photoplethysmography, also known as light reflection rheography, and venous occlusion plethysmography)
5.1.3.1 Photoplethysmography (PPG).
Photoplethysmography (also called light reflection rheography, LRR) is described in a specific guideline [37].
Indication
Photoplethysmography or LRR can be used to control the evolution of the disease and can therefore be used on first contact with the patient and in subsequent controls, whether or not invasive treatment has been carried out.
If refilling time improves after application of a tourniquet to prevent reflux in the superficial vein system (e.g., above an incompetent perforator vein), this will enable the examining doctor to judge whether an invasive therapeutic measure in the superficial veins will produce a beneficial effect when there is a simultaneous pathology of the deep leg veins.
Results
Photoplethysmography correlates with C class (CEAP) and VCSS, two clinical severity scores, and the diameter of the great saphenous vein (GSV) [38, 39]. In study groups, the mean value has been found to improve after rehabilitation of the haemodynamic disturbance of the varicose vein [40–44].
No undesired effects are to be expected with this procedure. The interpretability is limited if the muscle pump effects evacuation of less than 3%. The procedure is sensitive to disturbances; false negatives may be recorded if the room temperature falls or if the patient has not rested before the examination.
Recommendation 19
Photoplethysmography (PPG, LRR) can be used to quantify venous function as a screening method and/or to control evolution.
Recommendation 20
An indication for invasive treatment shall not be based exclusively on PPG examination.
5.1.3.2 Venous occlusion plethysmography (VOP).
Venous occlusion plethysmography (VOP) is a procedure for measuring pressure-dependent venous capacity, venous flow, and active volume evacuation [45], thus allowing conclusions to be drawn about the functioning of the deep leg veins.
Recommendation 21
Venous occlusion plethysmography can allow conclusions to be drawn about the functioning of the deep leg veins.
5.1.4 Continuous wave (CW) Doppler ultrasound
Doppler ultrasound converts the blood flow in the vessels into an acoustic signal that can be displayed graphically. All blood flows within the cone of the Doppler beam are captured; it is not possible to distinguish between one vessel and another lying behind it.
Continuous wave (CW) Doppler ultrasound can be used in the initial screening if duplex is not available. Before invasive treatment, the varicose vein diagnosis should be confirmed with duplex ultrasound [46, 47].
Continuous wave Doppler ultrasound can also be used in the initial diagnosis of peripheral arterial occlusion disease [48].
This diagnosis is important for the treatment of varicose veins before compression treatment or the execution of surgery if peripheral arterial occlusion disease is suspected.
Please see the guidelines for the treatment of peripheral arterial occlusion disease for recommendations on how to measure occlusion pressure, including the ankle-brachial index (ABI).
Recommendation 22
An indication for invasive treatment shall not be based exclusively on CW Doppler ultrasound examination.
5.1.5 Duplex ultrasound
Indications
Duplex ultrasound (DUS) is a noninvasive method of examination to detect the underlying haemodynamics of the varicose vein [46, 49]. It also provides information on pathologies of the deep vein system. Duplex ultrasound provides information on vein morphology, the anatomical classification of the pathological findings, and the diameter, occlusion, valve competence, and direction of flow in all three vein types. This information should be obtained for symptomatic varicose veins before advice is given on the need for treatment and its extent and type [46].
Recommendation 23
Duplex ultrasound shall be used as the basis for differentiated indications for the treatment of varicose veins. It should also be used for check-ups after invasive treatment of varicose veins.
Recommendation 24
Duplex ultrasound shall be used for parallel diagnosis during the execution of endovenous varicose vein treatment.
Duplex ultrasound is used to ascertain the cause of chronic venous incompetence in the initial diagnosis of a varicose vein.
Control of the evolution of the disease is recommended if there is clinically visible progress, as well as subsequently depending on the clinical findings.
After an intervention or operation, an early initial check-up is recommended, depending on the clinical course and the type of intervention. Further check-ups should be carried out after 1–3 months to document the early outcomes of the intervention and to detect early recurrence. Further controls will depend on the clinical evolution [46, 49, 50].
Recommendations for execution
Duplex ultrasound should be carried out with a linear probe at frequencies suitable for superficial areas. Information can be obtained in B scan, colour-coded duplex ultrasound and pulsed wave (PW) Doppler mode [49, 50].
Recommendation 25
Clinical examination of varicose veins and duplex ultrasound evaluation of the vein system should be carried out with the patient standing up.
Physical manoeuvres should be used, such as the Valsalva manoeuvre, or, preferably, manual compression of the calf in the standing patient, as well as dynamic manoeuvres [49, 51–53].
Duplex ultrasound should be documented by drawings or text, accompanied by diagnostic ultrasound images. Reflux shall be documented by an image of the flow curve along the time axis, and by the PW flow curve.
Results
The result of the examination is an understanding of the recirculation circuit, including the differentiated findings of the state of the deep vein system [54]. The particular anatomy of certain regions and the variant junctions of the small saphenous vein (SSV) in the popliteal fossa [55] or the variant courses of the GSV shall be shown traceably [54].
Recommendation 26
The proximal reflux source of the varicose vein shall be documented.
Differential examination of terminal and preterminal valves of the GSV allows different types of reflux to be identified [7, 56, 57]. Measuring the diameter of the GSV in the thigh (15 cm distal to the groin) can provide valuable information about the severity of the varicose vein [38, 39] and the risk of recurrence [58, 59].
Undesired effects
Duplex examination with dynamic manoeuvres presents no known risks. Extended examination of the standing patient and use of the Valsalva manoeuvre can lead to temporary vasovagal reactions up to and including syncope.
Limited interpretability
The interpretability of the examination depends not only on the experience of the examiner but also on the condition of the patient (e.g., bedridden, obese).
5.1.6 Phlebography
Phlebography by vein X‑ray using contrast medium was the gold standard for vein diagnosis by imaging for decades. With the wide availability of duplex ultrasound, phlebography has disappeared from routine diagnosis.
Indication
Phlebography can be used as a complementary examination method if the duplex findings are unclear, or to exclude the suspicion of special conditions such as angiodysplasia, pudendal varicose vein, pelvic congestion, or doubts over collateral functions with postthrombotic syndrome. Phlebography is no longer universally available.
Recommendations for execution
Please refer to Hach’s recommendations for the execution of ascending phlebography [60, 61].
Undesired effects/limited interpretability
Invasiveness of the method
Radiation exposure
Possible allergic reactions to the X‑ray contrast medium
The examination cannot be repeated ad lib
No conclusions can be drawn for differential diagnosis
An advantage is the ease of documenting the findings without reliance on an observer.
Recommendation 27
Phlebography shall not be used in the primary diagnosis of a varicose vein.
5.1.7 Other imaging procedures (endovenous)
Computed tomography (CT)
A common indication for computed tomography (CT) is to clarify veins in the trunk (iliac vein, inferior and superior cava veins, and veins in the pectoral girdle) in the context of a lung embolism diagnosis.
(In-)direct CT phlebography and magnetic resonance phlebography
(In-)direct CT phlebography and magnetic resonance (MR) phlebography are used to view the deep veins and may be indicated in special cases (venous malformations). Computed tomography phlebography should be indicated only after critical consideration because of the radiation exposure involved.
Recommendation 28
Computed tomography and MR phlebography shall not be used for the primary diagnosis before treatment of varicose veins.
5.1.8 Other procedures
Phlebodynamometry (PD) is a method for measuring the blood pressure in the peripheral veins and pressure changes in manoeuvre tests and under standardised loads [62]. It is a verifiable method that is highly predictive of venous function. Because it is an invasive procedure (puncture of a vein in the back of the foot), it is not used in everyday routine but is reserved for cases of special doubt.
Recommendation 29
Phlebodynamometry should be reserved for special indications.
6 Varicose vein treatment
Which treatment strategy is most suitable for each individual case will depend not only on the individual findings but also on the patient’s preference. Patients shall therefore be fully informed about the different options available.
Recommendation 30
In cases of symptomatic chronic vein disease, the treatment options should always be selected on an individual basis. Invasive procedures, compression treatment, and treatment with drugs are not competing but complementary options; if necessary, a combination of these procedures may be a good course.
Recommendation 31
The effectiveness of the treatment selected shall be checked regularly against an appropriate parameter (e.g., quality-of-life questionnaire, severity score).
Recommendation 32
The treating doctor shall distinguish between procedures in which the varicose vein is eliminated and those in which this does not occur.
Possible treatments include the following:
- Conservative measures
- Compression treatment
- Physical measures
- Treatment with drugs
- Operative procedures
- Saphenous vein ablation procedures
- Procedures in which the saphenous vein is retained
- Endovenous thermal procedures
- Endovenous laser treatment
- Endovenous radio-frequency treatment
- Endovenous superheated steam treatment
- Endovenous chemical procedures
- Sclerosis treatment
- Cyanoacrylate glue
The choice of treatment procedure(s) shall be decided case by case [63]. As a rule, a combination of different measures is recommended; the preferred result is healing of the diseased vein segment [22, 64]. The different methods can be staggered over time or applied in a single session.
Recommendation 33
The law of minimum invasiveness shall be observed in all measures/interventions. To achieve the object of minimising the invasiveness, a sensible procedure may be a combination of an operation with subsequent sclerosis of tributaries, for example.
6.1 Conservative treatment
Recommendation 34
Conservative treatment can be considered in all stages of the disease.
It shall be remembered that the effectiveness of conservative measures is limited in certain situations (e.g., in old, multimorbid patients). None of the conservative measures mentioned below can eliminate varicose veins or prevent them from developing; however, they can reduce both the symptoms of the disease and the risk of its evolution and complications.
Conservative treatment includes the following:
Different types of compression stockings and bandages
Instrumental intermittent compression/intermittent pneumatic compression (IIC/IPC)
- Other physical decongestion measures, such as
- Manual lymphatic drainage
- Balneotherapy
- Exercise for vascular disease
Drugs
In addition to putting the leg up and activating the muscle pump in the ankle region by adequate exercise, the basic treatment consists of compression bandages and medical compression stockings; these are designed to improve the venous haemodynamics of the diseased leg.
6.1.1 Compression treatment
6.1.1.1 Indications.
Recommendation 35
Compression treatment can be applied in all states of varicose veins and chronic venous incompetence. It can be applied alone or in combination with other procedures.
Recommendation 36
Clinically significant peripheral arterial occlusion disease and/or advanced peripheral neuropathy (e.g., in cases of diabetes mellitus) impose special demands on the technical execution of compression treatment.
Recommendation 37
Selection of the appropriate materials should be guided not only by the indication but also by any comorbidity and the patient’s wishes.
For further details on the different materials, please also see the guideline “Medical Compression Therapy of the Limbs with Medical Compression Stockings (MCS), Phlebological Compression Bandage Systems (PCB) and Medical Adaptive Compression Systems (MAC).”
6.1.1.2 Execution.
A summary of the materials
The available treatments are compression bandages, medical compression stockings, and instrumental intermittent compression.
6.1.1.2.1 Phlebological compression bandage systems (PCB)
Bandage systems using a single component (e.g., only short-stretch bandages) shall be distinguished from systems using several components (e.g., padding plus short-stretch, short-stretch plus long-stretch). The different materials can be combined individually, or a ready-made system can be used. If different materials are combined, care shall be taken because the general properties of the resulting system, such as the rest and work pressures and the elasticity and stiffness of the material, may alter and will not necessarily be the sum of the individual components. According to the law of Laplace, the pressure under the bandage increases over small radii and can—especially without appropriate inner padding—lead to pressure damage in severe cases [65].
Recommendation 38
A compression bandage can be used with inner padding to reduce the risk of severe side effects.
6.1.1.2.2 Medical compression stockings (MCS)
A distinction shall be made between medical compression stockings (MCS) and ulcer compression stockings (UCS). Medical compression stockings are classified by compression class (1–4) (RAL 2008), the type of knit (circular or flat), the elasticity of the material (elastic or rigid), and the type (calf stocking, thigh stocking, etc.). They may be mass-produced or made to measure. Because the international classification of compression classes is not universal, when international studies are evaluated, attention should be paid to the absolute pressure rather than to the compression class.
Ulcer compression stockings are also made on the basis of the RAL standard for MCS (RAL 2009); however, they consist of an inner and an outer stocking, which together give a rest pressure of compression class III. The inner stocking can be worn 24 h a day and provides a sliding surface to allow the outer stocking to be pulled on. The outer stocking should be taken off at night. Depending on the supplier, UCS can also be mass-produced or made to measure, and in the latter case fitted with accessories as required.
Recommendation 39
For treatment with MCS, the different compression classes and materials should be selected according to the individual needs of the patient.
6.1.1.2.3 Medical Adaptive Compression Systems (MAC)
In the last few years, a new type of compression system has become available, which should minimise the donning problems suffered by individual patients with the compression systems available previously [66, 67]. These compression systems are used in the decongestion phase. Like short-stretch bandages, medical adaptive compression systems (MACs) provide a high work pressure and a low rest pressure. In contrast to bandaging systems, loss of pressure can be corrected by adjusting the clips during use, which helps in the remission of oedemas. Because their application is significantly simpler, these systems require less time to apply, and the probability of making a mistake is lower than with more elaborate compression bandaging systems [12]. Patients who are still sufficiently mobile, or whose household members are able, can often apply the MAC themselves after a short introduction. This also improves adherence. Such systems, with their clips, can be taken off and put on, and adjusted as the oedema reduces, relatively easily and independently by sufficiently mobile patients. They are available in different sizes, so MACs can be matched to the patient’s measurements in advance as well as being adjusted at the time of application. There are different systems for treating different indications, such as lymphoedemas, phlebological oedemas, or venous ulcers. Commercially available materials are characterised by relatively high stiffness, which can contribute substantially to their effectiveness. A MAC can be used alone or in combination with MCS. Patients can handle these systems themselves, as they have reproducible pressures and generally a high level of stiffness.
Recommendation 40
In the initial decongestion phase with lymphoedema and pronounced venous oedema, as well as venous leg ulcer, a MAC can be applied as an alternative to bandaging.
6.1.1.2.4 Intermittent pneumatic compression (IPC)
Intermittent pneumatic compression (IPC) systems consist of a compressor/control unit and leg or trouser cuffs for the varicose vein. The compressor is programmed with the treatment time, desired work pressure, and the inflation, plateau, and deflation times, depending on the type of equipment. An IPC system is a listed aid and can be used by both bedridden and ambulatory patients in the clinic and/or at home. A test phase is recommended before equipment is ordered for home use.
For further details about IPC, please see the corresponding guidelines (intermittent pneumatic compression [IPC, IIC]).
To date there are no mandatory treatment protocols for cycle design, treatment time, treatment pressure, or treatment frequency. The data available on the use of IPC for venous oedemas and ulcers are inconsistent. The following recommendation is based on the available literature and the consensus of experts.
Recommendation 41
Intermittent pneumatic compression can be used in patients with venous oedemas and/or dermatoliposclerosis to reduce painful venous symptoms.
6.1.1.3 Use.
Compression treatment can improve venous symptoms and alterations such as venous oedema [68]. The strongest evidence and the broadest basis for compression treatment exists for advanced CVI, especially CEAP states C5 and C6 [69].
6.1.1.3.1 Venous symptoms
Compression treatment reduces venous symptoms such as the feeling of heaviness, paraesthesia, and tendency to swelling (especially after sitting or standing for a long period), even with a low rest pressure of < 20 mm Hg [68, 70–73].
Recommendation 42
All patients with venous symptoms (CEAP classes C1s–C6) shall receive compression treatment to relieve the symptoms.
6.1.1.3.2 Venous oedema
Compression treatment reduces venous oedema in patients with a varicose vein/CVI [74]. The higher the resting pressure and the firmer the material, the more effective the treatment is in reducing the oedema. However, even so-called placebo stockings with a pressure of < 10 mm Hg have been shown to be able to reduce or prevent oedema. Compression treatment with low (10–20 mm Hg) or medium pressure (20–30 mm Hg) can be applied as a prophylactic against venous oedema [75]. Patients with very pronounced oedemas have used compression bandages for initial decongestion, either with different single components or in combination.
Recommendation 43
Compression treatment should be applied to the leg with the oedema in two phases (decongestion phase and maintenance treatment).
Recommendation 44
Slight oedemas can be treated by compression directly with medical compression stockings.
6.1.1.3.3 Skin alterations
Compression treatment also reduces lipodermatosclerotic skin alterations in patients with varicose veins/CVI [76].
6.1.1.3.4 Venous leg ulcer
There is abundant evidence for compression treatment in the treatment and prophylaxis of venous leg ulcers. Compression treatment speeds up the healing of a venous leg ulcer (UCV) or a mixed leg ulcer [69, 77–79]. It also extends the period before recurrence and decreases the frequency of recurrence [69, 78]. Compression treatment with high pressure (at least MCS class 2) appears to be more effective both for treatment and as prophylaxis against recurrence than compression treatment with a lower pressure [78, 80]. Nevertheless, even a low rest pressure (equivalent to compression class [CCL] 1, 18–21 mm Hg) leads to healing and reduced recurrence of UCV [77]. There is, however, a lack of studies comparing no compression with compression treatment as a prophylactic against recurrence [81].
The effectiveness and value of a compression bandage depend on the ability and experience of the person applying it every time it is applied [75]. A well and expertly applied compression bandage is still a very effective measure, even today [75]. In everyday practice, however, we often come across very poorly applied compression bandages, which very quickly lose their initial rest pressure, slip, and produce side effects.
In comparative studies, UCS have proved better than compression bandages with short-stretch bandages for pain reduction, ulcer healing, and frequency of recurrence [82, 83]. For multiple component bandages, however, no significant differences were found between bandages and UCS [84]. A UCS system with a thigh-length inner stocking presents advantages over a system with a lower leg stocking in terms of volume reduction and venous haemodynamics [85].
In a literature review, the healing rates of venous leg ulcers with properly applied compression bandages were comparable to those with UCS. Everyday handling of UCS (other than in studies) is often easier, and incorrect application is less likely to occur. The use of IPC in addition to static compression treatment has proved helpful in venous leg ulcers (see IPC guidelines).
Recommendation 45
Compression bandages shall be applied by properly trained persons.
6.1.1.3.5 Prophylaxis against CVI
Chronic venous insufficiency (CVI) is known to be a chronic-progressive disease, the causes of which have not yet been fully explained. On the basis of current knowledge, therefore, the optimum goal of treatment is not to cure the disease but to reduce its painful symptoms and prevent complications.
As noted previously, there are solid data in the literature that show that compression treatment produces good results in relieving symptoms, curing venous ulcers, and preventing their recurrence. However, there are insufficient data to prove that the use of compression treatment prevents the progression of CVI.
6.1.1.3.6 Postinterventional compression treatment
6.1.1.3.6.1 Indications
Postinterventional compression treatment comprises treatment after stripping operations, tributary removal, endovenous thermal procedures, and both foam and liquid sclerotherapy. The object of postinterventional compression treatment is to reduce treatment-associated symptoms such as pain, oedema, haematoma, and numbness and to improve the clinical outcome.
6.1.1.3.6.2 Liquid sclerotherapy
Randomised controlled studies have shown that the use of MCS over a period of 3 weeks significantly improves the effectiveness of liquid sclerotherapy of spider veins and reticular varices [86–88]. There are indications that compression can reduce the frequency of side effects, especially hyperpigmentation [88].
Recommendation 46
Compression treatment should be applied after liquid sclerotherapy of spider veins and reticular varices, as it improves the clinical response and reduces hyperpigmentation. The duration of compression treatment can be established individually.
6.1.1.3.6.2 Foam sclerotherapy
The existing recommendations to apply compression treatment after foam sclerotherapy have grown up on a largely historical and regionally specific basis [89]. Painful inflammatory reactions and superficial vein thrombi often occur in tributaries after sclerotherapy. So far there is no sufficient evidence of the effectiveness of compression treatment against complications, which affect tributaries in particular. Whether compression treatment improves the response to the treatment has not yet been investigated.
Recommendation 47
Compression treatment shall be applied after foam sclerotherapy of varicose saphenous veins and tributaries. The type and duration of the treatment can be established individually.
6.1.1.3.6.3 Open operative treatment of the varicose vein
The outcome of a meta-analysis of the duration of compression treatment after surgical vein treatment was that there was no difference in postoperative pain, leg volumes, frequency of complications, or length of sick leave between the short-term compression group (3–10 days) and the long-term group (3–6 weeks) [90, 91].
On the assumption that higher local pressures have a positive effect on postoperative pain, Benigni et al. investigated the effect of a compression pad under a compression stocking after GSV stripping. Postoperative pain was significantly reduced in the group with a compression pad [92]. Similar results were also reported by Lugli et al. after endovenous laser treatment [93].
Reich-Schupke et al. showed that after crossectomy and stripping, compression stockings with a pressure of 23–32 mm Hg were significantly superior to stockings of 18–21 mm Hg in the regression of oedemas, pain, and feelings of tension and discomfort in the leg in the first few weeks [94]. With respect to the choice of compression material, Mariani et al. showed in an investigation that patients who were supplied with compression stockings after an operation presented significant regression of oedemas and better acceptance and quality of life compared with patients who received compression bandages [95].
The current recommendations of the Society for Vascular Surgery and the American Venous Forum support postoperative compression treatment after a stripping operation. This should be continued for at least 1 week [96].
6.1.1.3.6.4 Endovenous thermal procedures
A systematic review of compression treatment after endovenous ablation showed that the strategies and recommendations for postoperative compression treatment after endovenous ablation are very variable and not based on evidence but rather on the authors’ experience [97]. A randomised controlled study published by Ayo et al. showed that postoperative compression treatment with 30–40 mm Hg for 7 days did not produce differences either in the clinical outcome or in patient reports compared with a control group without any compression [98].
Bakker et al., on the other hand, came to the opposite conclusion. The authors investigated to what extent postoperative compression with 32 mm Hg after isolated laser ablation of the GSV, applied for more than 48 h, produced an advantage in quality of life and postoperative morbidity. They showed that after 1 week, the group who wore compression for the whole week presented significantly less pain and significantly better functionality and vitality than the group who wore compression only for 48 h. This difference was no longer detected after 6 weeks [99].
Lugli et al. showed that eccentric compression after endovenous laser ablation significantly reduced postoperative pain in the first week of treatment (p < 0.001) [93].
The current recommendations of the Society for Vascular Surgery and the American Venous Forum support postoperative compression treatment for at least 1 week after endovenous laser ablation [96].
Recommendation 48
After an operation or endovenous thermal treatment of the superficial vein system, initial postoperative/postinterventional compression treatment should be applied. The type and duration of the treatment can be established individually. Eccentric compression in the thigh can be applied for pain reduction after GSV interventions.
6.1.1.3.6.5 Long-term compression after invasive varicose vein treatment
There are no randomised controlled studies that show improvement in the response to treatment as a result of long-term compression treatment.
The greatest lack is in study design and in the length of the posttreatment observation period. Individual studies have not yet confirmed the advantage of long-term compression [89, 93, 100–102].
If symptomatic venous disease persists despite invasive treatment, further compression treatment is indicated [103]. Compression should also be continued if a venous functional deficit persists [104].
Recommendation 49
Patients in whom residual symptoms of chronic venous incompetence persist despite invasive treatment should receive continued compression treatment.
Long-term compression treatment is not recommended to improve the clinical response after invasive vein treatment.
6.1.1.3.7 Selection of material
It is known that the effect of compression even at low pressures (class I, 18–21 mm Hg) helps to reduce or eliminate oedemas [72, 105], improves venous ejection [106], allows venous ulcers to heal [69], and reduces the recurrence rate of new ulcers [78].
Patient adherence falls as the resting pressure of the compression treatment increases, regardless of the indication that led to the compression treatment [81, 94]. To date there is little evidence for special compression pressures to meet different indications. Whenever possible, therefore, the lowest medically justifiable resting pressure should be selected.
The working pressure appears to be the most important parameter for the venous ejection fraction and thus for the effectiveness of compression treatment. This may be influenced on the one hand by the resting pressure and on the other by the firmness and elasticity of the material [77, 106]. It has been shown that the working pressure of the compression material can be achieved not only by increasing the resting pressure but also by reducing the elasticity of the material.
Recommendation 50
To improve patient adherence, the compression treatment shall be applied using the lowest medically justifiable resting pressure. The prescribed resting pressure is established on an individual basis.
6.1.1.4 Aids.
Handling their own compression equipment may cause patients difficulties; they may even find it impossible, depending on their comorbidities [107]. To ensure correct treatment, these patients will need people to help them (e.g., relatives, nursing staff) or will need aids [107, 108].
6.1.1.4.1 Aids for MCS
Aids for putting on and taking off medical compression stockings include rubber gloves, slip-socks, frames, and unrolling aids. These make handling demonstrably easier, especially for elderly and obese patients [108]. Aids for putting on and taking off medical compression stockings are ordered as a special prescription. It is helpful to provide the justification and the indication.
Recommendation 51
Patients should be offered aids when they are prescribed MCS.
Recommendation 52
If the patient cannot put on or take off the MCS by herself/himself even with these aids, the help of another person(s) should be incorporated. The person(s) should be trained in compression treatment practices.
6.1.1.4.2 Aids for bandages
There are no aids to allow patients to put on their own compression bandages. In principle, it is possible to teach patients to put on their own bandages; however, applying a properly wrapped compression bandage to one’s own leg is very difficult, even for a trained individual.
6.1.1.5 Improving adherence.
Patient adherence to compression treatment is often less than optimal. In cases of a severe indication, e.g., venous leg ulcer or postthrombotic syndrome, consistent and complete application of the treatment is essential. There are different measures available to help patients adhere to their treatment. These include programmes such as leg ulcer clubs, nurses to teach self-management, and educational materials for patients in video or text form. However, there is no clear, up-to-date assessment of the effects of these measures on the healing and recurrence rates of venous leg ulcers. Further studies are necessary to evaluate the effects of these measures [109]. Full explanations and repeated recommendations of compression treatment by doctors appear to have a positive impact on adherence to compression treatment by patients with CVI [90].
6.1.1.6 Side effects.
Correctly indicated and executed compression treatment is a safe and effective measure. Dryness and scaling of the skin can be expected on a regular basis as a consequence of the drying effect of compression treatment; these conditions can be relieved with suitably frequent skin care, which should form a part of any compression treatment.
Recommendation 53
All patients with compression treatment shall be recommended to apply skin care, however long the treatment period and whatever the treatment indication.
Allergic reactions to compression stockings are rare [110]. If clinical suspicion arises of a contact allergy in CVI patients, a patch test applied by an allergy specialist is recommended. In allergic contact eczema, recognition of the allergen is decisive, as the allergy can be cured only after the allergen is removed. Apart from the standardised test series, it is useful to consider any textile tests brought in by patients. The most significant factors, apart from isolated textile allergies, are natural latex, textile additives (e.g., formaldehyde), and dyes [111].
Other possibilities, mostly involving mishandling or poor skin care, are nerve damage, circulation disorders, erosion and ulcers, itching, reddening of the skin, eczema, paraesthesia, feeling hot or cold, sweating, constriction, and thrombosis.
6.1.2 Treatment with drugs
6.1.2.1 Indication.
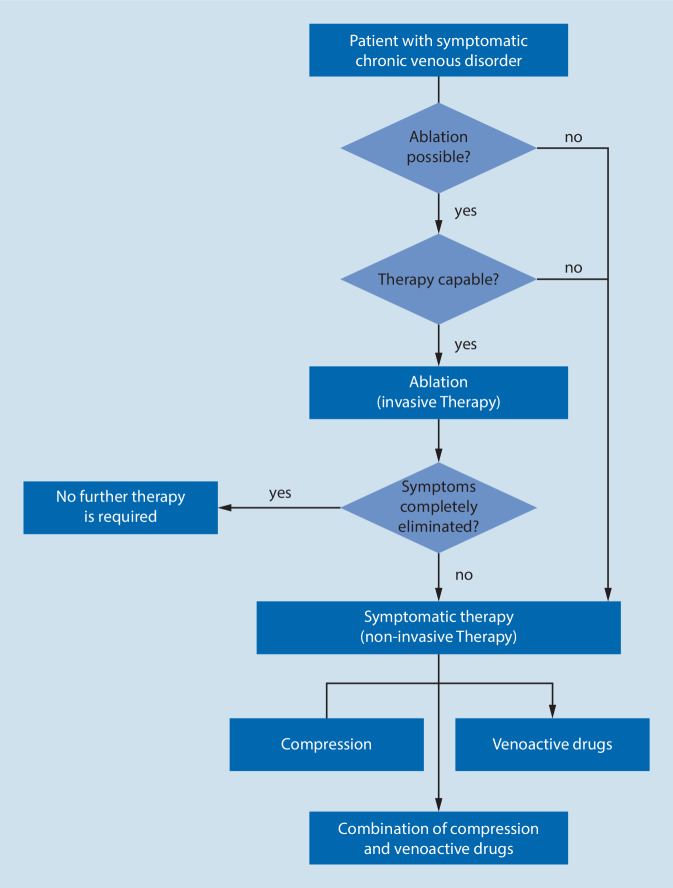
If it is impossible or considered undesirable to treat a symptomatic varicose vein, or if swelling and feelings of heaviness persist even after invasive treatment, the prescription of oral vein treatment drugs of proven effectiveness can be considered ([96, 103]; Fig. 1).
Fig. 1.
The treatment or combination of treatments for symptomatic chronic vein disease shall be selected on an individual basis, determined by different factors (from [103])
Recommendation 54
If it is impossible or considered undesirable to treat symptomatic varicose veins, or if a symptomatic venous picture persists even after invasive treatment, conservative treatment with effective venous drugs can be applied.
Recommendation 55
In cases of chronic disease and persistent symptoms, long-term conservative treatment should be applied.
The different conservative principles complement one another: While the venous reflux is controlled by the external pressure of the compression elements, plant-based vein drugs work on the vessel walls and reduce their permeability. Combining the two procedures appears to strengthen these effects by synergy [112, 113].
Recommendation 56
If single treatment procedures do not produce the desired relief, a combination of the different treatment principles (drug treatment, varicose operation, compression treatment) can be considered.
6.1.2.2 Guidelines for application.
In Germany, the following drugs with evidence-based effectiveness are available for oral treatment: standardised red vine leaf extract (AS 195), standardised horse chestnut extract, and oxerutin. Other drugs of proven effectiveness (e.g., certain ruscus-containing vein tonics, troxerutin–coumarin combinations) are currently not approved in Germany (drug information system [AMIS] of the German drug authorisation agency Arzneimittelzulassungsbehörde 2015). Plant-based vein drugs reach their maximum effectiveness only after a certain period of continuous administration of 2–4 weeks. This should be explained to the patient [10, 74, 112, 114–125].
Recommendation 57
It should be explained to the patient that the clinical impact of the full effectiveness of plant-based vein drugs can generally be assessed only after a certain period of continuous administration of 2–4 weeks.
6.1.2.3 Results.
Controlled studies are available for the substances approved for use in Germany, namely standardised red vine leaf extract (AS 195), standardised horse chestnut extract, and oxerutin. They document both the regression of oedemas and relief of subjective painful symptoms: pain, feelings of heaviness, and tension. The clinical effects are based on the fact that the active ingredients of the drug have an anti-inflammatory effect and normalise the permeability of venous vessels [10].
The symptomatic effectiveness is documented with good evidence for different products [10, 74, 112, 114–118, 120–126]. Standardised red vine leaf extract (AS 195), standardised horse chestnut extract, and oxerutin significantly reduce oedemas. Furthermore, red vine leaf extract and oxerutin have also been shown to produce significant improvement in symptoms [115–117, 120–122, 127, 128].
Table 3 summarises the evidence for the vein drugs approved in Germany. Apart from randomised studies, which formed the source for the above criteria, supporting data have also been provided by small or short studies.
Table 3.
Summary of data for the vein drugs approved in Germany (from [103])
| Active principle | Dose | Data from robust RCTsa | Supporting datab | |
|---|---|---|---|---|
| Significant reduction of oedema | Significant improvement in symptoms | |||
| Standardised red vine leaf extractc |
Once per day 360–720 mg |
Kiesewetter et al. (2000) [120] | Kiesewetter et al. (2000) [120] | Kalus et al. (2004) [123] |
| Rabe et al. (2011) [121] | Rabe et al. (2011) [121] | |||
| Standardised horse chestnut extractd |
Twice per day 50 mg (aescin) |
Diehm et al. (1996) [74] | n. i. | Neiss et al. (1976) [124] |
| Cloarec (1992) [117] | ||||
| Oxerutin |
Twice per day 500 mg |
Unkauf et al. (1996)e [112] | n. s.e | Cloarec et al. (1996) [125] |
| Diebschlag et al. (1994) [122] | Diebschlag et al. (1994) [122] | Grossmann et al. (1997) [118] | ||
| Petruzzellis et al. (2002) [114] | ||||
n. i. not investigated, n. s. not significant, RCT randomised controlled trial
aCarried out in accordance with the guidelines of the German Society of Phlebology (DGP). Vanscheidt et al. (2000) [119]
bSmall and/or short RCTs, inadequate measurement of the reduction of oedemas, etc.
cProportion of active principle red vine leaf extract (RVLE); 4–6:1
dProportion of active principle horse chestnut extract (HCSE); 4.5–5.5:1
eCompression therapy in both treatment groups
The study findings for plant-based drugs shall always be strictly limited to the drugs actually tested. This is true of both red vine leaf extracts and horse chestnut extracts. Standardised production procedures shall be demanded for plant extracts [103].
6.1.2.4 Side effects.
Recommendation 58
Drugs shall not be used in patients who have a known hypersensitivity (allergy) to the drug in question.
Recommendation 59
Attention shall be paid to the following side effects: The principal side effects described for oral administration of the drugs mentioned above are intestinal irritation and skin reactions. If these effects occur, a change to one of the other two evidence-based vein drugs should be considered.
6.1.3 Physiotherapy
6.1.3.1 Manual lymphatic drainage.
Some of the lymph collectors in the leg lie close to the saphenous veins [129]. Lymphatic incompetence is not infrequently induced by venous incompetence; it may improve after treatment of the varicose vein [130]. There are case reports stating that manual lymphatic drainage can reduce treatment-resistant oedemas associated with a varicose vein.
Recommendation 60
Manual lymphatic drainage can be considered in cases of venous oedema associated with a varicose vein, if other treatment options such as compression treatment, invasive treatment of the varicose veins, and treatment with drugs do not sufficiently reduce the venous oedema.
6.2 Invasive varicose vein treatment
Incompetent saphenous veins and accessory veins, varicose tributaries, and incompetent perforator veins can be treated either by open surgery or endovenously.
In most cases, a saphenous varicose vein does not occur in isolation but in combination with a varicose tributary. After ablation of the varicose saphenous vein, the diseased tributary may persist or disappear.
Recommendation 61
In treatment planning, it shall be considered that the incompetence of the tributary may resolve itself after removal of the saphenous vein.
Recommendation 62
Removal of a diseased tributary can be carried out simultaneously with treatment of the saphenous vein, or subsequently.
Anaesthesia is required with most methods. The spectrum ranges from general anaesthesia through peridural anaesthesia to local anaesthesia. In many cases, tumescence, and particularly tumescent local anaesthesia, plays a significant role or is even an integral constituent of the method. Some particularities shall be observed.
Tumescent local anaesthesia in varicose vein treatment
In endovenous thermal varicose vein ablation, the strongest recommendation is for the introduction of a perivasal liquid depot to allow vein ablation with minimal collateral damage and avoidance of burns.
Tumescent local anaesthesia (TLA) can be used concurrently to ensure sufficient analgesia. It involves the subcutaneous infiltration of a very diluted local anaesthetic in a buffered solution with added adrenalin [131, 132]. Omitting any individual component should be avoided due to alterations to the pharmacokinetics [133].
Recommendation 63
In invasive procedures that require the use of a tumescent solution, the combined application of a local anaesthetic can avoid a second anaesthetic procedure.
Recommendation 64
In operations, TLA can be used alone or together with other anaesthetic procedures.
Recommendation 65
Omitting any individual component of the TLA solution should be avoided because of alterations to the pharmacokinetics.
In addition to postoperative maintenance analgesia, TLA can be advantageous for intraoperative mobilisation (hydrodissection) of the vein to be ablated and for reducing the formation of postoperative haematomas [134].
When TLA is used, the recommended limit doses of the local anaesthetics used—although they are not strict limits—are knowingly exceeded [135, 136].
Discussion about the off-label use of TLA has developed in the absence of clear authorisation of the maximum joint dosage in TLA [137]. It should be noted that preparation of the TLA solution requires the production of at least an infusion solution and possibly also a drug [138]. The pertinent hygiene provisions shall be observed. Even with small amounts, the status of off-label use is subject to legal discussion.
Recommendation 66
The use of TLA involves off-label use. This shall be explained to the patient.
6.2.1 Open operative procedure
6.2.1.1 Bases.
Different strategies are available for open operative treatment of varicose veins. In addition to vein removal procedures (e.g., crossectomy, stripping, phlebectomy), vein-conserving concepts also exist (e.g., the CHIVA treatment, extraluminal valvuloplasty). In the presence of trophic disorders, special surgical procedures (e.g., shave treatment, fascia surgery) may be necessary (see the guidelines for the treatment of venous leg ulcer) [139–144].
Recommendation 67
Depending on the individual indication, the following open operative procedures shall be applied: vein removal methods (e.g., crossectomy, stripping, phlebectomy) and vein-conserving concepts (e.g., CHIVA, extraluminal valvuloplasty).
Recommendation 68
In the presence of trophic disorders, special surgical procedures (e.g., shave treatment, fascia surgery) can be necessary.
6.2.1.1.1 Indications
In principle, all forms of varicose vein (except spider veins) characterised by reflux detectable by duplex ultrasound can be treated with an open operational procedure.
The reflux source shall be identified unequivocally before the operation. It may be in the region of the saphenofemoral/saphenopopliteal junction (SFJ/SPJ), a perforator vein, or the pelvic region. The treatment concept shall focus on the reflux source.
A special indication for the operation can result from complications of the varicose vein [145, 146]. These include superficial vein thrombosis, including ascending varicophlebitis [19, 147–151] and variceal bleeding [152].
In cases of a secondary varicose vein or angiodysplastic alterations, the ablation of incompetent superficial veins may be needed to improve venous haemodynamics. Definition of the indication assumes proper diagnosis, excluding a collateral function of the vein to be treated [153, 154].
Recommendation 69
The following indications for varicose vein operations shall be followed: saphenous varicose veins, accessory varicose veins or tributaries, recurrent varicose veins, varicose veins with venous angiodysplasia, superficial vein thrombosis, variceal bleeding.
Recommendation 70
The treatment concept of the varicose vein operation shall focus on the proximal reflux source.
6.2.1.1.2 Contraindications for elective operations on superficial varicose veins
The following absolute and relative contraindications apply to varicose vein operations [155–158].
Absolute contraindications:
Acute thrombosis of the deep leg vein and/or iliac vein
Peripheral arterial occlusion disease from Fontaine stage III (except by special indication)
Known pregnancy
Moribund patient (ASA score 5)
Recommendation 71
The following absolute contraindications shall be observed in open varicose vein operations: acute thrombosis of the deep leg vein/iliac vein, peripheral arterial occlusion disease from Fontaine stage III (except by special indication), known pregnancy, and moribund patient (from American Society of Anesthesiology [ASA] score 5).
Relative contraindications:
Peripheral arterial occlusion disease from Fontaine stage IIb
Serious disturbance of haemostasis
Severe lymphoedema
Very severe general disease (from ASA score 4)
6.2.1.2 Operations to remove varicose veins.
The principle of the classic operative treatments of varicose saphenous veins consists of interrupting the reflux at the proximal and distal reflux sources, selectively removing incompetent sections of the superficial vein system (interrupting the recirculation circuit according to Hach), and thus achieving the longest-lasting normalisation possible of the venous haemodynamic [159–163]. The literature shows that operative removal shall be dependent on the existing state [160, 163–167].
The varicose vein operation can consist of several components in combination, depending on the expression in the findings [151, 157, 159, 168–172].
Recommendation 72
Operative removal should be limited to the diseased vein segments (depending on the existing state).
6.2.1.2.1 Recommendations for execution of surgical procedures to remove varicose veins
The success of operative measures in the superficial vein system is significantly conditioned by preoperative planning with duplex ultrasound support to mark the incompetent veins (mapping). Mapping helps to reveal any anatomical variations and to define the treatment strategy; it should preferably be carried out personally by the operating surgeon.
Recommendation 73
Before vein surgery, the incompetent vein segments should be identified in duplex ultrasound and marked on the skin (mapping).
6.2.1.2.2 Interrupting the incompetent transfascial communication(s)
Flush ligation of the saphenofemoral junction (crossectomy) of the GSV
Flush ligation (or as near to the saphenopopliteal junction as possible) of the small saphenous vein (SSV)
Ablation of incompetent perforator veins
6.2.1.2.2.1 Crossectomy of the great saphenous vein (GSV)
Crossectomy is the flush ligation of the great saphenous vein at the saphenofemoral junction (SFJ), interrupting all the tributary veins that drain into the GSV in the junction region with resection of the GSV itself near the SFJ [159, 165, 173–195].
The use of absorbable or nonabsorbable suture is controversial and is discussed in the literature [173, 196]. At all events, the use of absorbable materials for ligation seems to be associated with more frequent appearance of veins carrying reflux at the ablation point in the junction region [197]. In the past, the use of nonabsorbable suture was normal in up to two-thirds of interventions, as a survey among varicose vein surgeons in Germany, Austria, and Switzerland 20 years ago showed [198]. No disadvantages are known to result from this practice. Nonabsorbable ligation material can therefore be recommended for the SFJ region as the simplest (and cheapest) solution to prevent recurrence [199, 200].
The tributaries that flow into the deep vein in the SFJ region should be interrupted separately [175, 178, 179, 201, 202].
Recommendation 74
To prevent recurrence in the groin region, the crossectomy shall be free of technical imperfections; the use of nonabsorbable suture can be recommended.
6.2.1.2.2.2 Ligation of the small saphenous vein (SSV) at the saphenopopliteal junction (SPJ)
The SPJ region is extremely variable [203–207] and may be difficult to display; flush ligation is very often possible, but not always [155, 175]. To reduce the frequency of saphenopopliteal recurrence, the vein should be interrupted as close as possible to the junction [208–212].
The question as to whether muscle veins that flow into the SPJ should also be interrupted is not yet clear. To prevent hernias, every effort should be made to close the fascia.
Recommendation 75
To reduce the frequency of saphenopopliteal recurrence, the vein should be interrupted as close as possible to the saphenopopliteal junction.
6.2.1.2.2.3 Ablation of perforator veins
In the context of surgical ablation of varicose tributary veins, in individual cases it may be necessary also to interrupt a perforator vein [213–217]. The ablation of incompetent transfascial connecting veins can help prevent or cure trophic skin damage [172, 218–220].
Recommendation 76
Perforator veins should be eliminated when they form the proximal reflux source.
Recommendation 77
In cases of clinical/haemodynamic importance with an incompetent or obstructed deep vein system, the interruption of incompetent perforator veins can be considered.
The following operation techniques are available:
Direct epifascial or subfascial interruption
Subfascial endoscopic perforating vein surgery (SEPS) [141, 221–226].
Subfascial endoscopic perforating vein surgery (SEPS) should be indicated only very reluctantly, and never in cases of ulcer surgery because of the high morbidity rate [221, 227].
Recommendation 78
When indicated, the perforator veins should be interrupted by epifascial or subfascial intervention.
Recommendation 79
Subfascial endoscopic perforating vein surgery shall be used only in individual cases and not carried out routinely.
6.2.1.2.2.4 Ablation of diseased segments of saphenous vein
The saphenous veins (GSV and/or SSV) can be eliminated either completely or partially, depending on where the proximal and distal reflux sources are [4, 157, 228–233]. In incomplete forms of varicose GSV, where the proximal reflux source lies distal to the SFJ region, partial elimination is generally sufficient [234]. Healthy vein segments should be retained [155, 156, 165, 235, 236].
Elimination of a saphenous vein down to the ankle region is associated with a high incidence of sensory nerve disturbances [167, 237–239].
There are various operative methods for removing a diseased segment of saphenous vein (e.g., conventional stripping, invaginated stripping, cryostripping, extraluminal stripping, phlebectomy). The direction of stripping can be from distal to proximal or from proximal to distal. Sensory nerve lesions are less likely with the frequently used proximal-to-distal invaginated stripping method [240]. The literature does not indicate that any particular method is superior to the others [241–248].
Recommendation 80
Various methods can be used for stripping the diseased vein segment, e.g., conventional stripping, invaginated stripping, cryostripping, extraluminal stripping, phlebectomy.
Recommendation 81
Carrying out the stripping manoeuvre from proximal to distal may be preferable, as fewer sensory nerve lesions are observed.
Recommendation 82
During postoperative care, the outcome of the operation (removal of the diseased saphenous vein) should be confirmed by duplex ultrasound.
6.2.1.2.2.5 Ablation of diseased tributaries
Tributary ablation is carried out through very small incisions in the skin with fine hooks or specially made instruments [157, 249, 250].
Recommendation 83
In cases of isolated tributary reflux, phlebectomy can be used as the only method.
Recommendation 84
Combining phlebectomy with simultaneous or subsequent sclerotherapy can be a satisfactory treatment.
6.2.1.2.3 Undesired effects and complications
If correctly carried out, open operative treatment of varicose veins is a safe form of treatment with few side effects. The following undesired effects and complications are rare; however, they may sometimes occur and be observed [155, 163, 251–260].
6.2.1.2.3.1 Intraoperative complications:
Recommendation 85
Special attention shall be paid to the following possible intraoperative complications in open operative treatment of varicose veins: bleeding, injury to large vessels, nerve damage.
6.2.1.2.3.2 Postoperative complications:
Secondary bleeding, haematoma: 0.06%–2.0% [159, 258, 265, 276, 277]
Pigmentation disturbances: individual cases [279]
Superficial vein thrombosis: 0.2%–0.3%
Lymphatic fistula, lymphatic cyst, lymphoedema: 0.02%–1.82% [159, 257, 258, 280]
Deep leg vein thrombosis and/or lung embolism: 0.01%–0.24% [251, 257, 258, 265, 272, 277, 281]
Occurrence of spider veins, matting: individual cases
Pathological scar formation: individual cases
Compartment syndrome: individual cases
Recommendation 86
Special attention shall be paid to the following possible postoperative complications in open operative treatment of varicose veins: secondary bleeding/haematoma, wound infection/healing disturbances, lymph vessel disturbances, superficial/deep leg vein thrombosis, lung embolism.
Death has been reported in isolated cases: 0.004%–0.023% [258, 277, 282].
The frequencies of the individual complications in over 150,000 interventions are shown in Table 4.
Table 4.
Combined total of complications/side effects of open operative treatment of varicose veins (absolute/%): 151,720 legs operated, nine studies, 1983–2013
| Author- | All | Helmig | Balzer | Hagmüller | Nüllen | Frings | Critchley | Hofer | Noppeney | Papapostolou | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | – | 1983 [258] | 1983 [282] | 1992 [257] | 1995 [283] | 1995 [284] | 1997 [265] | 2001 [259] | 2005 [153] | 2013 [252] | |||||||||
| Number of “cases” | – | – | 25,457 | – | – | 47,057 | – | – | – | – | |||||||||
| Patients | – | 13,024 | – | – | 1683 | – | 599 | 1094 | 36,323 | 841 | |||||||||
| Legs | – | 20,353 | – | 3300 | 1981 | – | 973 | 1590 | 49,939 | 1070 | |||||||||
| – | % | n | % | n | % | n | % | n | % | nn | % | nn | % | nn | % | nn | % | nn | % |
| Intraoperative/intraprocedural | |||||||||||||||||||
| Bleeding/Shock | 0.01–0.1 | 1 | 0.005 | 4 | 0.015 | – | – | 13 | 0.66 | – | – | – | – | – | – | – | – | – | – |
| Transfusion | 0.05 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 23 | 0.046 | – | – |
| Injury to large arteries | 0.01 | 1a | 0.005 | 3 | 0.012 | – | – | – | – | 1b | 0.002 | 1b | 0.10 | – | – | 1 | 0.002 | – | – |
| Injury to large veins | 0.01–0.1 | 10 | 0.049 | 2 | 0.008 | 1 | 0.03 | – | – | 4 | 0.009 | – | – | – | – | 21 | 0.042 | – | – |
|
Nerve damage – Motor |
0.01–0.1 | 2c | 0.010 | – | – | 1 | 0.03 | – | – | 3c | 0.006 | 1c | 0.10 | – | – | 6 | 0.012 | – | – |
| – Sensory | 0.2–6.6 | 43 | 0.21 | – | – | – | – | 95 | 4.8 | – | – | 64 | 6.58 | 18 | 1.13 | 48 | 0.096 | 34 | 3.18 |
| Postoperative/postprocedural | |||||||||||||||||||
| Secondary bleeding | 0.06–0.1 | – | – | 15 | 0.059 | – | – | 2 | 0.1 | – | – | – | – | – | – | – | – | – | – |
| Requiring revision | 0.13 | 26 | 0.13 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Requiring transfusion | 0.06–0.21 | 42 | 0.21 | – | – | – | – | – | – | – | – | – | – | – | – | 32 | 0.06 | – | – |
| Haematoma | 0.6–2.0 | 130 | 0.64 | – | – | – | – | 35 | 1.77 | – | – | – | – | 30 | 1.89 | 585 | 1.17 | 6 | 0.56 |
| Puncture/surgical haematoma removal | 0.1 | 25 | 0.12 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 0.093 |
| Suffusion/ecchymosis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Seroma | 1.4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 15 | 1.4 |
| Wound infection | 0.1–2.8 | 25 | 0.12 | 13 | 0.051 | – | – | 1 | 0.05 | 72 | 0.153 | 27 | 2.77 | 21 | 1.32 | 387 | 0.78 | 23 | 2.15 |
| With revision | 0.22 | – | – | – | – | – | – | – | – | – | – | 2 | 0.21 | – | – | 115 | 0.23 | – | – |
| Wound healing disturbance | 0.05–1.38 | 232 | 1.14 | – | – | – | – | 1 | 0.05 | – | – | – | – | 22 | 1.38 | – | – | – | – |
| Pigmentation disturbance | 0.2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 0.18 |
| Lymphatic fistula/cyst/oedema | 0.02–1.82 | 112 | 0.55 | 16 | 0.063 | – | – | 3 | 0.15 | – | – | 8 | 0.82 | 29 | 1.82 | – | – | – | – |
| Superficial vein thrombosis | 0.2–0.3 | – | – | – | – | – | – | – | – | – | 0.049 | 2 | 0.21 | – | – | 146 | 0.29 | 2 | 0.18 |
| Deep leg vein thrombosis | 0.03–0.24 | 11 | 0.05 | 7 | 0.027 | 8 | 0.24 | 2 | 0.1 | 23 | – | 3 | 0.31 | 2 | 0.13 | 39 | 0.08 | 1 | 0.093 |
| Lung embolism | 0.01–0.13 | 2 | 0.01 | 5 | 0.020 | 2 | 0.06 | – | – | 6 | 0.013 | 1 | 0.10 | 2 | 0.13 | 8 | 0.02 | – | – |
| Other complicationsd | 0.1–4.2 | 22 | 0.11 | – | – | – | – | 83 | 4.19 | – | – | 4 | 0.41 | – | – | 56 | 0.11 | 1 | 0.093 |
| Deathse | 0.004–0.023 | 2 | 0.005 | 1 | 0.004 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Injury to large arteries/veins:
aVery thin female patient
bDuring revision of saphenofemoral junction (SFJ) treatment
cDuring saphenopopliteal junction (SPJ) operation, some with course of the nerve in the operation region described as complicated
dOther complications: Helmig: increase of a preexisting swelling (during diagnosis of possible deep vein thrombosis [DVT]); Nüllen: severe oedema; Papapostolou: suture granuloma; Critchley: blisters due to compression bandage, breast infection (?); Noppeney: other intraoperative complications
eDeaths: Balzer: lung embolism; Helmig: 1 × ventricular fibrillation under general anaesthesia, 1 × warfarin bleeding after 9 months (due to thrombus)
6.2.1.2.4 Outcomes
All the currently available studies show a clear improvement in postoperative quality of life after open varicose vein surgery, in both general and disease-specific terms. This effect persists for more than 5 years [185]. The recurrence rate over time is an important parameter for the outcome in terms of postoperative quality of life [285].
Outcome studies shall distinguish between technical (duplex, reflux finding) and clinical recurrence (visible varicose veins, painful symptoms, quality of life) [185, 186, 195, 286]. The period elapsed since the intervention shall also be noted: “Short term” refers to around 1 year, and “long term” to 5 years and longer.
Recommendation 87
Assessment of the recurrence rate should distinguish between technical (duplex, reflux finding) and clinical recurrence (visible varicose veins, painful symptoms, quality of life). Clinical recurrence may arise from a technical recurrence.
Recurrences originating in the SFJ and SPJ regions are particularly significant, since as a rule they can lead to a haemodynamically important recurrence of a varicose vein [287].
A retrospective long-term study of 830 operated legs showed a recurrence rate of less than 3% in the junction region on duplex ultrasound up to 20 years after the operation [200]. The technical recurrence rates in the groin region reported in prospective studies with modern operating techniques (reflux on duplex ultrasound) are around 3% to 5% after 5 years [174, 185, 252]. In the largest multicentre study published in Germany, including over 1000 legs (LaVaCro), the recurrence rate in the SFJ (duplex ultrasound) was 2.6% after 2 years [184]. The often higher recurrence rates reported in the literature, associated with the concepts of high ligation, saphenofemoral ligation, and flush ligation, shall be interpreted with care, as the operations thus described are not necessarily the same as the crossectomy procedure described above [288–290]. The clinical recurrence rate is 20%–45% after 5 years [174, 185, 186, 194, 200, 287]; it includes both recurrence in the course of the treated vein and new varicose veins in other regions. This is due not only to the method used but also to the progression of the chronic varicose condition [3, 158, 187, 291–293].
Recommendation 88
Varicose vein treatment by open operative procedures offers the possibility of good postoperative outcomes with a clear improvement in quality of life. After technically correct crossectomy of the GSV, very low recurrence rates in the SFJ are reported: less than 5%, even in the medium to long term (5 years to more than 10 years). This should be taken into consideration, since recurrence in the SFJ as a rule also leads to haemodynamically important recurrence of the varicose vein.
6.2.1.2.5 Prophylactic measures against recurrence
The following are known to cause recurrence:
Leaving a stump of GSV, incomplete crossectomy
Failure to close the reflux source, e.g., reflux from the pelvic region
Neovascularisation
Modern operating tactics should therefore include the following to prevent recurrence, in addition to correct execution of the crossectomy:
Exact preoperative mapping with duplex ultrasound
Use of nonabsorbable ligation material/suture at the SFJ [198–200]
Techniques to isolate the free endothelium from the GSV stump (coagulation of the free endothelium [294], stitching over and depression of the stump border [196, 295])
Barrier operations with suture/plastic closure of the cribriform fascia [294, 296] and [297, 298]
Recommendation 89
Various measures can be included as prophylactics against recurrence in varicose vein surgery by the open operative procedure. These measures include preoperative mapping by duplex ultrasound, use of nonabsorbable suture for ligation of the SFJ, and techniques for closing the free endothelium of the SFJ stump and for closing the saphenous opening.
6.2.1.3 Surgical strategies that preserve the saphenous vein.
As an alternative to established procedures, which are based on the removal or destruction of the saphenous vein, the reflux can be stopped using strategies and techniques to recover valve function.
A vein-preserving treatment may be indicated, such as when the presence of atherogenic risk factors suggests that arteriosclerotic circulation disturbances may arise in the future, with the GSV possibly being needed as replacement vessel material.
The feasibility of vein-preserving procedures is dependent on certain conditions. In cases of advanced disease with existing damage to the vein valves and sections of wall, for example due to thrombosis, vein-preserving treatment is generally no longer possible.
The following procedures are available in Germany, although they are relatively seldom used:
Extraluminal valvuloplasty
CHIVA (Cure Conservatrice et Hémodynamique de l’Insuffisance Veineuse en Ambulatoire, i.e., conservative haemodynamic ambulatory treatment of venous incompetence)
6.2.1.3.1 Extraluminal valvuloplasty (EVP)
Introduction
In extraluminal valvuloplasty (EVP), the dilated GSV is reduced to its physiological diameter in the region of the SFJ by placing a restrictive cuff around the outside of the vein. The cuff is made of a piece of alloplastic material measuring 4 cm× 2 cm. The venous valve wings, which did not close due to the stretching of the vein, are thus brought back into contact and can carry out their function of closing the vein [299, 300].
Recommendation 90
Extraluminal valvuloplasty can be used as a procedure to preserve the saphenous vein in suitable patients. Its object is to reestablish valve function in the SFJ region. This can lead to an improvement in saphenous vein function.
6.2.1.3.1.1 Indications
An essential precondition for EVP is ultrasound proof that the wings of the terminal valve of the GSV are intact and mobile. The vein diameter measured at the height of the terminal valve in the standing patient should not exceed 10 mm in women or 12 mm in men [300].
6.2.1.3.1.2 Recommendations for execution
The operative approach is the same as for crossectomy, with additional viewing of the deep vein. The cuff of alloplastic material (polyester, polytetrafluorethylene, or polyurethane) is applied to reduce the diameter of the dilated vein to around 6 mm. The proximal ends of the cuff are fixed to the deep vein. The success of the intervention can be checked intraoperatively with clinical tests or ultrasound. Complementary treatment of the varicose tributary vein can be carried out in the same session or after an interval by various methods.
6.2.1.3.1.3 Undesired effects
Postoperative thrombus formation in the GSV occurs in approximately 2% of cases [301–303]. Wound infections occur with the same frequency observed after crossectomy operations. If deep wound infection occurs (frequency < 1%), the alloplastic material shall be removed [300].
6.2.1.3.1.4 Outcomes
Long-term recovery of valve function was achieved in 59% to 96% of cases in studies with follow-up periods of 5–10 years. Patients in whom the treatment failed were reoperated with crossectomy and stripping in around 3.7% to 15.6% [300].
6.2.1.3.2 CHIVA procedure
The CHIVA procedure is a strategy for treating venous incompetence while preserving the saphenous veins and the draining perforator veins [304].
After analysis of the recirculation with duplex ultrasound, the patient is classified on the basis of the reflux source and the drainage paths, and treatment is planned [305, 306]. After the intervention, the saphenous vein recovers its tone because of the volume relief. In 20% of cases, two sessions are required for the procedure.
6.2.1.3.2.1 Indication
Recommendation 91
The CHIVA procedure can be used in all clinical stages of primary varicose veins. It is not recommended in cases of postphlebitic alterations to the saphenous vein or reflux in very thin saphenous veins, as calibre reduction is not possible in these cases.
6.2.1.3.2.2 Recommendations for execution
The following technical steps are used in the CHIVA strategy, either alone or in combination [305]:
Closure of the saphenofemoral and/or saphenopopliteal junctions by ligation [307, 308]. Nonabsorbable sutures shall be used in surgical crossectomy as part of a CHIVA treatment (preserving the saphenous vein);
Perforator vein interruption (in the rare cases in which a perforator vein is the highest reflux source);
Tributary ligation in combination with 1 and 2;
Tributary interruption in isolation to restore circulation in the refluxing saphenous vein. It is carried out through a sufficiently large incision to allow flush ligation of the tributary at the saphenous vein [305, 306];
The saphenofemoral and saphenopopliteal junctions and some perforator veins can be closed with endoluminal procedures [43, 309].
6.2.1.3.2.3 Prophylactic measures against recurrence
Exact preoperative mapping
Nonabsorbable sutures at the junction
6.2.1.3.2.4 Undesired effects
General surgical complications are as described above. Superficial thrombosis may occur after the intervention in the preserved saphenous vein (1.3%–8%).
6.2.1.3.2.5 Outcomes
The overall recurrence rate detected by duplex ultrasound (reflux in superficial leg veins of over 1 s in duration) is between 18% and 22% after 5–10 years [304, 307, 308].
This breaks down as follows: 2.9% in the SFJ/SPJ, 1.4% from vessels in the pelvic region, and 18.5% reflux from the preserved GSV into distal tributaries without reflux from the deep leg veins, but from competent, more proximal tributaries.
6.2.1.4 Flanking measures and posttreatment.
Preoperative measures may be necessary to decongest oedemas and cure skin alterations caused by congestion or infection. Perioperative antibiotic prophylaxis should not be administered routinely, but it may be necessary in certain cases [310]. Some authors describe the use of blood evacuation during varicose vein surgery to reduce haematomas [139, 262, 276, 311–313]. No definitive assessment of this practice is possible due to the lack of studies to date.
Compression treatment shall be applied with bandages or stockings immediately after the operation. The recommendations of the guidelines on medical compression treatment of the limbs with medical compression stockings (MCS), phlebological compression bandages (PCB), and medical adaptive compression systems (MACs) apply. The patient shall be mobilised immediately.
Thromboembolic drug prophylaxis is not indicated in principle; however, it may be applied depending on the individual risk profile, number of invasive measures carried out, type of general anaesthetic, and duration of the intervention and postoperative immobilisation [276]. The recommendations of the thromboembolic prophylaxis guidelines apply.
Routine postoperative measures include the following:
Early mobilisation [172]
Compression treatment for at least 1 week [101, 172, 314, 315]. Longer compression treatment after an operation on an uncomplicated varicose vein is not unanimously supported by the literature
Wound monitoring, changing of bandages, and removal of stitches if used. Complications such as wound infections, haematomas, or new swellings are recognised and treated in this context
It is a good idea to check the success of the treatment postoperatively by duplex ultrasound
Further examinations are indicated if new varicose veins appear or if progression of the chronic venous incompetence continues.
Recommendation 92
Immediately after varicose vein operations, compression treatment shall be applied for at least 1 week. The patient shall be mobilised immediately.
Recommendation 93
Thromboembolic drug prophylaxis is not indicated in principle; however, it may be applied depending on the individual risk profile and the characteristics of the intervention (extent, duration, etc.).
Recommendation 94
To ensure the success of the treatment, a postoperative examination should be carried out by duplex ultrasound.
6.2.1.5 Recurrent varicose veins.
Recommendation 95
Any varicose veins that appear in a previously treated region shall be considered recurrent varicose veins, independent of the type of previous treatment.
Recurrent veins after treatment can be divided into duplex ultrasound recurrence and clinical recurrence. Duplex ultrasound recurrence as a surrogate parameter frequently precedes clinical recurrence [14, 316, 317].
Recurrence is a socioeconomic problem, since up to 20% of all interventions are for cases of recurrence [153, 318–320]. Varicose vein recurrence occurs most frequently in the regions around the saphenofemoral junction and the saphenopopliteal junction and in perforator veins [321]. In many cases, symptoms of varicose vein recurrence first appear some 7–8 years after initial treatment [322]. Multiple pregnancies and high body mass index are considered risk factors for varicose vein recurrence [323, 324]. Pelvic incompetence can also lead to varicose vein recurrence [325].
The following are currently considered to cause varicose vein recurrence [326, 327]:
Tactical and technical errors in the initial operation and/or recanalisation or nonclosure in an endovenous procedure
Neovascularisation
Progression of the underlying disease
These causes are currently the focus of intensive research. There is insufficient evidence for a reliable, evidence-based assessment as to which procedure results in the lowest number of clinical recurrences [174, 185, 186, 328, 329]. Duplex ultrasound detection of recurrence in the junction region 5 years after treatment is significantly less frequent after crossectomy and stripping or endovenous thermal ablation than after ultrasound-guided foam sclerotherapy [330, 331]. Moreover, duplex ultrasound detection of recurrence in the junction region 5 years after treatment is significantly less frequent after crossectomy and stripping than after endovenous thermal ablation [330, 332].
To date, there is no unified definition of varicose vein recurrence in most studies, so the published causes and recurrence rates are frequently not comparable.
Recommendation 96
In clinical practice, varicose vein recurrence should be divided into clinically significant recurrence and recurrence diagnosed only by duplex ultrasound.
Recommendation 97
Indications for the treatment of recurrent varicose veins should be based on the same principles as those for primary treatment.
Care should be taken because the complication rates of interventions on recurrent varicose veins are higher than for primary interventions.
The whole range of treatments can be considered for recurrent varicose veins. Various barrier techniques are recommended for avoiding postoperative neovascularisation [296, 333–335].
The few published studies that recommend one procedure or another do not satisfy the criteria of evidence-based medicine.
The complication rates for interventions on recurrent varicose veins are higher than for primary interventions [256, 296, 326, 336, 337].
6.2.2 Endovenous procedures
In 1999, Boné reported for the first time the closure of an incompetent saphenous vein using endovenously applied laser light [338]. Radio-frequency treatment of a varicose saphenous vein was described at the same time [339]. Since then, endovenous thermal treatment has developed into a standard treatment method. An integral component of all endovenous procedures is ultrasound before, during, and after treatment [340]. Good knowledge of the ultrasound technique and venous anatomy is necessary.
Recommendation 98
To carry out endovenous thermal treatments, the operator shall have very good knowledge and extensive experience in ultrasound representation of the peripheral veins.
6.2.2.1 Endovenous thermal ablation procedures (EVTA).
6.2.2.1.1 Indications and contraindications
Definite indications for endovenous thermal ablation (EVTA) procedures are varicose GSV and SSV [340, 341]. Applications in the anterior accessory saphenous vein (AASV) and posterior accessory saphenous vein (PASV), incompetent perforator veins, and venous malformations have been described, with good outcomes [88, 342–344].
In addition to the general contraindications for elective operational interventions in the vein system, acute superficial vein thrombosis of the saphenous vein to be treated is also a contraindication.
Recommendation 99
Indications for EVTA should be incompetent GSV, SSV, or anterior accessory saphenous vein (AASV). Incompetence of the posterior accessory saphenous vein (PASV), perforator veins, long varicose segments in cases of venous malformation, and varicose vein recurrence may also be indications.
6.2.2.1.2 General recommendations for execution of endovenous thermal ablation procedures
In the endovenous thermal ablation procedure (EVTA), the vein to be treated is either opened or, preferably, punctured, as a rule at the distal reflux point. A treatment probe is introduced into the vein through a special peripheral venous catheter or a lock (Seldinger technique). In thermal ablation of the SSV, choosing a puncture site in the middle of the calf rather than the supramalleolar region is associated with a reduction in postoperative paraesthesia [345].
Recommendation 100
To avoid sensory nerve damage, it may be a good idea not to treat the SSV with thermal techniques below the mid-calf.
The intravasal position of the endovenous catheter in the treated vein shall be controlled by ultrasound throughout the length of the vein. The probe tip shall be pushed up to the proximal reflux source close to the junction with the deep vein and positioned under ultrasound control. The final position of the probe tip shall be documented with an ultrasound image. Thermal damage of the deep vein shall be avoided. At the same time, occlusion shall be achieved from close to the junction. Leaving a long stump should be avoided to minimise the risk of recurrence of the reflux [322, 330, 346].
Recommendation 101
The correct intravasal position of the endovenous catheter and the position of the probe tip shall be controlled and documented by ultrasound.
Thermal ablation is usually carried out after infusion of a perivenous fluid heat sink (tumescent solution) [347]. To avoid damage to perivenous tissue and to improve contact between the treatment probe and the vein wall, the tumescent solution is applied intrafascially [341, 348–353].
For the legal prerequisites for tumescent local anaesthesia (TLA), see Sect. 6.2.
Recommendation 102
Endovenous thermal ablation shall be carried out under the protection of a perivenous fluid heat sink (tumescent solution).
Endovenous thermal ablation is usually carried out in either the horizontal or the Trendelenburg position [354].
Simultaneous treatment of varicose tributaries during EVTA of the GSV is linked to lower frequency of postoperative follow-up interventions, significantly better quality of life, and significantly lower severity of the disease than EVTA alone [355–357].
Recommendation 103
If varicose tributaries are present, they should be treated simultaneously with EVTA of the saphenous vein.
Postoperative compression treatment is used wherever possible after EVTA procedures [97], for a period that may vary between 2 days and 6 weeks. Different means of compression may be used. Some prospective randomised studies have shown advantages with respect to painful symptoms, use of analgesics, and quality of life in patients who received compression treatment after laser ablation [93, 99, 358].
Recommendation 104
Compression treatment should be applied after endovenous thermal ablation. The duration of compression treatment can be established individually.
For postoperative thrombosis prophylaxis, please see the current S3 guidelines on venous thromboembolism (VTE) prophylaxis [359]. No systematic clinical studies have yet been published on the subject of routine postoperative thrombosis prophylaxis after EVTA procedures.
Recommendation 105
After endovenous thermal ablation, venous thromboembolism (VTE) prophylactic drugs should be administered in accordance with the thrombosis prophylaxis guidelines.
One advantage of EVTA is that it can be carried out safely and effectively without interrupting therapeutic anticoagulation [360]. Patients who were receiving anticoagulants presented no more bleeding than patients with no such treatment, as long as no varicose tributaries were removed [361].
Recommendation 106
Endovenous thermal ablation can be carried out in patients receiving therapeutic anticoagulation without interrupting anticoagulation.
6.2.2.2 Endovenous laser ablation (EVLA).
In endovenous laser ablation (EVLA), laser generators emitting wavelengths of 810–1940 nm are used. With shorter wavelengths (810–980 nm), most of the laser energy is absorbed by the haemoglobin in the erythrocytes, whereas longer wavelengths (≥ 1320 nm) are absorbed mainly by water.
The probe tip may be a simple glass fibre (bare fibre) or a modified laser-emitting fibre (e.g., radial tip, tulip tip, jacket tip). Bare tips emit the laser beam axially. Tulip tips and jacket tips are used to centre the bare fibre in the lumen of the vein. Radial tips emit laser towards the vein wall in a ring through an optical prism. The laser energy causes thermal damage to the vein wall (photon absorption in the tissue). This results in variably expressed collagen denaturation and shrivelling of the treated vein, and finally thrombotic vein closure [362].
Recommendation 107
To optimise treatment outcomes, improvements in the technologies/wavelengths/probe tips should be taken into account in the treatment decision.
6.2.2.2.1 Special recommendations for execution of EVLA
Because of the risk of perforation, the sharp bare fibre tip should be introduced and positioned only using a special guide catheter. The pilot beam alone is insufficient for exact positioning of the laser tip [340].
The laser energy is applied during continuous or stepped withdrawal of the laser probe. The energy is emitted in pulsed or continuous laser mode.
Recommendation 108
A bare fibre tip shall be introduced and positioned only with a special guide catheter.
Recommendation 109
Treatment protocols with continuous withdrawal should be preferred for EVLA. The speed of withdrawal depends on the desired energy density.
Recommendation 110
In EVLA, the energy density, power in watts, and speed of withdrawal in millimetres per second should be adapted to the laser wavelength used and to the vein lumen.
Recommendation 111
The type of application, laser tip, and wavelength used, as well as the tumescent solution composition and volume, shall be documented. This documentation shall include the power in watts, the total energy in joules, the length of vein treated in centimetres, the laser mode, and, if appropriate, the pulse protocol.
The energy density in the treated vein is decisive for the effectiveness of EVLA. Most studies are based on either the linear endovenous energy density (LEED) in J/cm or the endovenous fluence equivalent (EFE) in J/cm2, which takes into account the diameter of the treated vein.
Recommendation 112
The laser energy density (LEED) applied should be in the range of 60–100 J/cm of vein. The energy density should be adapted to the vein diameter.
The success of EVLA depends on the laser power, the vein diameter, and the withdrawal speed (continuous laser emission) or the pulse duration and interval between pulses (pulse mode). There are indications of a negative correlation between the long-term effectiveness, measured by the occlusion rate or the recanalisation rate, and increasing vein diameter [58, 363].
Recommendation 113
When indicating treatment and/or selecting endothermic laser ablation, it may be useful to consider the vein diameter, although a clear threshold value cannot currently be defined.
6.2.2.2.2 Undesired effects
Undesired effects are summarised in Table 5.
Table 5.
Complications and side effects of endovenous thermal treatment of varicose veins
| EVLA 1a | EVLA 2b | RFA (bRFA) | RSTA (sRFA) | EVSA | |
|---|---|---|---|---|---|
| Intraoperative/intraprocedural | |||||
| Bleeding | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Requiring transfusion | – | – | – | – | – |
| Injury to large blood vessels | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Arterial | – | – | – | – | – |
| Venous | – | – | – | – | – |
| Arteriovenous fistula | Individual cases | – | – | Individual cases | – |
| Damage to lymph vessels | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Nerve damage | – | – | – | – | – |
| Motor nerve | Individual cases | 0 (not reported) | 0 (not reported) | Individual cases | 0 (not reported) |
| Sensory nerve | 0–17 | 0–9 | 2–13 | 2–12 | 1–10 |
| Other | – | – | 0 | – | – |
| Other neurological reactions/events | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Allergic reaction | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Burns | 0–3 | 0–0.5 | 0 | 0–1 | 0 |
| Probe errors/punctures/injections | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 |
| Postoperative/postprocedural | |||||
| Secondary bleeding | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Requiring revision | – | – | – | – | – |
| Requiring transfusion | – | – | – | – | – |
| Haematoma | 0–10 | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Puncture/surgical haematoma removal | ? | – | – | – | – |
| (Suffusion) better: Ecchymosis | 10–92 | 2–64 | 0.3–3 | 0–51 | 3–47 |
| Infection | 0–3 | 0–0.8 | 0 | 0–6 | 0 |
| With wound revision | ? | – | – | – | – |
| With (systemic) antibiosis | ? | – | – | – | – |
| Healing disturbances | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| (Skin) necrosis | 0 (cf. burns) | 0 (cf. burns) | – | 0 (cf. burns) | 0 |
| Pigmentation disturbances | 0–43 | 0–4 | 2 | 2–9 | 5–8 |
| Pathological scar formation | 0 (not reported) | 0 (not reported) | 0 | 0 (not reported) | 0 |
| Lymphatic fistula/lymphatic cyst/lymphoedema | 0–9 | 0 (not reported) | 0 | 0–3 | 0 |
| Superficial thrombophlebitis | 0–22 | 0–14 | 0–2 | 0–14 | 8–9 |
| Deep vein thrombosis | 0.6 (including PASTE) | 0–2 | 0 | 0–3 | 0 |
| Lung embolism | 0 (not reported) | 0 (not reported) | 0 (not reported) | Individual cases | 0 (not reported) |
| Occurrence of spider veins/matting | 0–13 | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Compartment syndrome | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
| Deaths | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) | 0 (not reported) |
EVLA endovenous laser ablation, RFA radio-frequency ablation, bRFA bipolar radio-frequency ablation, sRFA segmental radio-frequency ablation, EVSA endovenous steam ablation, PASTE postablation superficial thrombus extension
aEVLA with short wavelengths (810–980 nm) and bare fibre
bEVLA with longer wavelengths (1320 nm, 1470 nm, 1500 nm, 1920 nm, or 1940 nm) and modified laser tips
6.2.2.2.2.1 Endovenous laser ablation (EVLA) with short wavelengths (810–980 nm) and bare fibre
Postoperative pain is reported with frequencies between 21% and 75% [286, 364–368] and maximum intensity between 2 and 6 (visual analogue scale [VAS]0–10) [93, 99, 365, 368–381], diminishing considerably in the first 7 to 10 days post procedure [369, 371–373, 376, 378, 380]. Significantly more intense postoperative pain after EVLA with short wavelengths (810–980 nm) and bare fibre was reported in comparison with the following:
Laser emissions with the same wavelength and modified probe (jacket tip) [382]
Laser with longer wavelengths and modified probe (1470 nm plus radial tip) [385]
Radio-frequency ablation (bipolar, segmental) [369, 373, 376, 380, 383, 386, 387]
Steam ablation [375]
Postoperative internal bleeding in the form of usually moderate ecchymosis is reported after EVLA with short wavelengths (810–980 nm) and bare fibre, with an average frequency of 31% (10%–92%) [93, 286, 364, 365, 367–369, 371, 376, 377, 379, 380, 385, 387–390]. The use of longer wavelengths (980 nm as opposed to 810 nm) is associated with less severe postoperative side effects (ecchymosis, pain, phlebitic reactions) [391]. Haematomas occur considerably less frequently after EVLA, at a frequency of 0–10% [372, 373, 392, 393]. In addition to laser probe perforations, vessels may also be punctured during TLA infusion. In comparison with other thermal ablation procedures, it has been shown that a reduction can be achieved in postoperative ecchymosis by the use of laser at longer wavelengths and/or with a modified probe (radial tip, jacket tip) [382, 384, 385] and by the use of radio-frequency procedures and steam ablation [369, 376, 380, 394].
The compression bandaging technique also has an influence on the rate of postoperative ecchymosis and pain; both side effects are significantly reduced by eccentric compression along the course of the GSV [93].
Burns and/or necrosis after EVLA with short wavelengths (810–980 nm) and bare fibre have been described in individual cases (0–2.6%) [286, 364, 365, 368, 369, 375, 377, 379, 384, 385, 387, 390, 393, 395–397].
Sensory nerve damage (paraesthesia, dysaesthesia) has been reported after EVLA with short wavelengths (810–980 nm) and bare fibre, with an average frequency of 2.4% (0–17%) [286, 364–373, 375–378, 384, 385, 387–389, 393–396, 398–404]. The frequency has been decreasing over time [174, 186, 392]. Studies have shown that EVLA in the SSV appears to be associated with a lower rate of sensory nerve damage than in the GSV [372, 405]. A randomised controlled trial (RCT) reported a significantly lower rate of sensory complications with the use of a 1470-nm radial tip compared with a 980-nm bare fibre [385]. No differences in sensory complications have been reported in comparison with radio-frequency ablation (RFA) procedures [369, 373, 376, 387].
Motor nerve damage has been described only in individual cases for thermal ablation of the SSV and/or the vein of Giacomini [406].
Lymphoedemas after EVLA with short wavelengths (810–980 nm) and bare fibre have only been reported by one work group in an RCT, in this case with a 9.2% frequency 2 months after the intervention [366].
Hyperpigmentation after EVLA with short wavelengths (810–980 nm) and bare fibre is reported with frequencies of 0–43%, with an average rate of 31.3% [286, 364, 366–368, 373, 378, 387, 389, 393, 395, 396, 398–400]. Five years after the operation, the frequency drops to 0–4% [186, 392]. No differences have been reported in comparison with RFA procedures [373, 387].
Spider veins as an undesired side effect have only been reported by one work group in an RCT, with a 13.2% frequency 6 months post procedure [286, 364].
Superficial vein thromboses and periphlebitic tissue reactions are reported after EVLA with short wavelengths (810–980 nm) and bare fibre, with an average frequency of 6.5% (0–22%) [93, 365, 368, 369, 371–373, 375–379, 387, 389, 393, 395, 396, 398–401, 403, 404].
No significant differences in the frequency of postoperative phlebitis could be established in comparison with other thermal ablation procedures [386, 389].
Recommendation 114
Due to the less than optimal side effects profile of EVLA with short wavelengths (810–980 nm) and bare fibre, a procedure with longer wavelengths and/or a modified probe, or else a new-generation radio-frequency procedure, should be preferred for the treatment of saphenous varicose veins.
Infections after EVLA with short wavelengths (810–980 nm) and bare fibre seldom occur; they may also be associated with phlebectomies carried out simultaneously. Frequencies of 0–3%, with an average of 0.4%, are reported [286, 364, 365, 367–369, 371, 373, 375–379, 384, 389, 392, 393, 398–400]. No differences have been found in comparison with other thermal ablation procedures [386, 389].
Thromboembolic complications can be divided into ablation thrombosis, deep leg vein thrombosis (DVT), and lung embolism.
If a thrombus forms postoperatively at the proximal junction of the treated vein, it is called endovenous heat-induced thrombosis (EHIT) in the international literature; if it extends into the deep vein system, it is called postablation superficial thrombus extension (PASTE) [407–409]. Schäffer et al. indicate that this clinical constellation is independent of the procedure used and can appear even after nonthermal procedures; furthermore, it refers to a thrombus extension in the deep and not the superficial vein system. They therefore suggest the term postablation thrombus extension (PATE) [410].
A Cochrane analysis reported a frequency of thromboembolic events after EVLA with short wavelengths (810–980 nm) and bare fibres of 0.6% (DVT and PATE) [389]. A current meta-analysis, including 16,398 patients treated by endovenous thermal procedures, presents differing frequencies of 1.3% (DVT and PATE), 0.2% (DVT), and 0.1% (lung embolism) after EVLA of the GSV [409].
There are indications from multivariate analyses that a vein diameter > 8 mm (GSV) or > 6 mm (SSV), a previous record of deep vein thrombosis, and male sex are factors that increase the risk of a thrombus extension after thermal ablation [411–414]. The therapeutic procedure depends on the extent of the thrombus extension; however, it is not supported by prospective examinations because of the infrequency of the condition. Different treatment protocols or suggestions are reported in the literature, with or without therapeutic anticoagulation [411–414].
There are various recommendations for graduating the subsequent risks of EHIT and PASTE (classification according to Dexter et al.) [407, 408]. Schäffer et al. suggest the classification presented in Table 6 [410]. The stage-dependent treatment recommendation in each case is based on a consensus of experts.
Table 6.
Postablation thrombus extension (PATE): classification and treatment recommendations
| PATE | Anatomical location | Procedure/treatment |
|---|---|---|
| 0 | Extension of the thrombus as far as the deep vein (= flush closure = desired treatment outcome) | No specific measures required |
| I | Extension of the thrombus a few millimetres into the deep vein with obstruction of the lumen of the deep vein up to 25% | Duplex ultrasound control (every 1–2 weeks) until regression of the thrombus (level 0) |
| Consider anticoagulation in prophylactic dosage | ||
| II | Extension into the deep vein with obstruction of the lumen ≤ 50% | Duplex ultrasound control (every 1–2 weeks) until regression of the thrombus (level 0) |
| III | Constriction of the deep vein > 50% without complete occlusion of the deep vein | Therapeutic anticoagulation until regression of the thrombus to level 0 |
| IV | Complete occlusion of the deep vein | Therapeutic anticoagulation analogous to the treatment of deep leg vein thrombosis |
| Regular duplex ultrasound control | ||
| After regression of the thrombus (level 0), suspension of therapeutic anticoagulation can be considered |
6.2.2.2.2.2 Endovenous laser ablation (EVLA) with longer wavelengths (1320–1940 nm) and modified probes
In recent years, several clinical examinations have been published in which EVLA was carried out with longer wavelengths (1320–1940 nm) and modified probes. These include special tips to centre the bare fibre in the lumen (tulip tip), a metal sheath (jacket tip), and radial emission probe heads [382, 384, 385, 415–435].
Experimental examinations indicate that EVLA with longer wavelengths (1470 nm) and radial fibres in continuous withdrawal (1–2 mm/s) lead to controllable and reproducible thermal effects on the tissue. Overaccentuated tissue damage such as carbonisation or vein wall perforations, observed with shorter wavelengths and bare fibre [436], occurs less frequently [437]. Due to the greater absorption of laser in water and/or the cytoplasm of the vein wall cells, the use of a longer wavelength allows the same thermal effect on the vein wall to be achieved with lower power or energy density than with shorter wavelengths and higher energy density [384].
A controlled clinical comparative study shows significantly lower undesired effects such as ecchymosis, paraesthesia, and pain in EVLA with longer wavelengths (1470 nm) and a radial probe compared with EVLA with shorter wavelengths (980 nm) and bare fibre [385]. Apart from the use of longer wavelengths, the clinical outcome can also be improved by the use of modified laser emission probes, reducing undesired effects such as ecchymosis, paraesthesia, and pain. This was shown in clinical and experimental examinations with the use of a probe head to centre the bare fibre in the lumen (tulip tip) [427] and a bare fibre surrounded by a metal sheath (jacket tip) [382, 438]. The use of a radial emission probe (radial tip) has also been observed to offer advantages over bare fibre, with reduced ecchymosis and pain and no negative impact on the effectiveness of vein occlusion up to 5 years post procedure [415, 425, 433].
All in all, the risk profile of EVLA with longer wavelengths and a radial tip can be regarded as low. Serious complications are seldom observed (see table with summary of complications/side effects). None of the comparative studies or cohort studies indicated a higher risk of complications or undesired effects with the use of longer wavelengths and modified probes compared with the control groups.
The significance of the laser wavelengths and probes used and their specific influence on the reduction of undesired effects is currently under experimental and clinical investigation [382].
Recommendation 115
To reduce undesired side effects such as ecchymosis, postoperative pain, and paraesthesia, modified probes, such as radial emission heads, and longer wavelengths should be preferred.
6.2.2.2.3 Outcomes
Since the introduction of endovenous laser ablation 20 years ago, a large number of randomised controlled studies (RCTs) have been carried out. The most extensive data report on first-generation laser systems, particularly short wavelengths (810–980 nm) and bare fibre probes [439]. The majority of RCTs and current case control studies report on the treatment of GSV incompetence, while considerably less information is available about endothermal ablation of the SSV.
There are countless different treatment protocols using different laser generators and/or wavelengths and probes, as well as different power settings, withdrawal speeds, and energy densities.
To date, long-term data from the highest level of evidence (RCTs) according to the Union Internationale de Phlébologie (UIP) definition [46] are available almost exclusively for laser with short wavelengths (810–980 nm) and bare fibre.
6.2.2.2.3.1 Endovenous laser ablation (EVLA) with short wavelengths (810–980 nm) and bare fibre probe
Varicose saphenous veins can be treated effectively by endovenous laser ablation [386, 389, 439–441]. The quality and effectiveness of the treatment can be detected in the short term by the occlusion rate, the side effects spectrum, and the convalescence period; in the medium to the long term, they are shown by their impact on quality of life, postoperative evolution of the disease, and the frequency of recurrence, which may be manifested on duplex ultrasound and/or clinically.
Postoperative quality of life
On completion of the immediate postoperative phase up to a period of 5 years after EVLA, there is a significant improvement in quality of life both in relation to the disease and generally [185, 186, 188, 194, 286, 355, 356, 364, 365, 367–369, 371–376, 378, 392, 398–400, 405, 442, 443]. No significant differences have been found in the postoperative quality of life as compared with other thermal ablation procedures [386, 389, 441].
Postoperative evolution of disease severity
A significant improvement in disease severity has been shown for up to 5 years after EVLA [185, 186, 194, 286, 355, 356, 364, 365, 368, 371–373, 375, 376, 378, 385, 387, 392, 400, 401, 405, 442, 443]. Prospective studies, meta-analyses, and systematic reviews show similar values for EVLA with short wavelengths (810–980 nm) and bare fibre as compared with segmental radio-frequency thermal ablation (sRFA) and endovenous steam ablation (EVSA) [375, 386, 389, 441, 444]. Both EVLA with 1470 nm and a radial probe [385] and sRFA [376] were shown to be superior to EVLA with short wavelengths (810–980 nm) and bare fibre, although only up to 1 month after the interventions.
Convalescence
In a comparison of different treatment methods for GSV ablation, EVLA with short wavelengths (810–980 nm) and bare fibre was shown to be inferior to EVLA with a radial probe [385] and to EVSA [375], but it was similar to radio-frequency ablation (sRFA, bipolar radio-frequency ablation [bRFA]) [369, 373, 387].
Technical–anatomical success (duplex ultrasound)
Studies consider the treatment to be immediately effective, with provable occlusion rates of 95% to 100% on duplex ultrasound [355, 365, 368, 369, 372, 374, 376, 377, 380, 385, 393–396, 400, 401, 403]. After 4–5 years, between 85% and 88% of the treated saphenous veins are still occluded [330, 440]. Compared with other thermal ablation procedures (EVLA with longer wavelengths, sRFA, bRFA, EVSA), EVLA with short wavelengths (810–980 nm) and bare fibre appears to present similar anatomical treatment success rates [369, 375, 380, 384, 385].
There are indications in the literature that the risk of recurrence after EVLA with short wavelengths (810–980 nm) and bare fibre and/or reopening of the ablated vein with progressive vein symptoms (C3 and C4 vs. C2) [445] correlates with the vein diameter [393, 396, 397, 401, 403]; different studies give different critical values for maximum GSV diameter of 8 mm, 9 mm, or 12 mm [393, 396, 401]. In another study, no such correlation could be shown [404]. A further factor affecting the occlusion rate with EVLA is the energy density, which with the use of LEED (linear endovenous energy density) should be at least 60 J/cm [393, 404, 446].
Clinical recurrence/progression of the disease
Clinical recurrence, in the sense of all new postoperative varicose veins, regardless of their location and origin, occurs after EVLA of the GSV with short wavelengths (810–980 nm) and bare fibre in 4% of cases after 1 year, in 16% to 26% of cases after 2 years, and in 45% to 47% of cases after 5 years [185, 186, 368, 400, 443]. Clinical recurrence with origin in the region of the previously operated veins, particularly the SFJ region, is reported at frequencies between 8% and 33% at 5 years after EVLA of the GSV with short wavelengths (810–980 nm) and bare fibre [174, 186, 392]. Due to the use of nonunified study variables, no assertions can be made at present about the long-term effectiveness against clinical recurrence in comparison with other treatment procedures.
6.2.2.2.3.2 Endovenous laser ablation (EVLA) with longer wavelengths (1320–1940 nm) and modified probes
To assess the outcomes of EVLA with long wavelengths (1320–1940 nm) and modified probes, randomised controlled studies [384, 385, 416, 417, 420, 426, 427, 430, 431], prospective comparative studies [415, 418, 419, 422, 425], retrospective comparative studies [429, 433], and prospective cohort studies [382, 421, 424, 428, 434] are available. The long-term outcomes up to 5 years are reported.
The long-term effectiveness of EVLA with longer wavelengths and radial probes can also be deemed to be high. A prospective comparative study with the longest observation period reported to date documented an occlusion rate of 96.7% after 5 years [415]. If the results of all the available studies are taken together, they show occlusion rates of between 87.5% and 100% after observation periods of 3 months to 5 years.
The pain levels and requirement for analgesics are low in all clinical studies of EVLA with longer wavelengths and modified probes. The convalescence is short. The average interval from the operation to resumption of normal physical activity is reported as between 0 and 2 days.
6.2.2.3 Endovenous radio-frequency ablation (RFA).
Two types of radio-frequency treatment are currently authorised in Germany. One is segmental thermal ablation using radio frequency (sRFA) at 120 °C; the other, called radio frequency–induced thermal therapy or bipolar radio-frequency ablation (bRFA), delivers the heat directly to the vein wall through a bipolar probe head, heating it to between 60 °C and 100 °C [447].
In RFA in general, the thermal energy causes homogeneous damage to all the layers of the vein wall, with contraction of the collagen fibres in the vein wall resulting in occlusion of the vein. Homogeneous thermal damage of the vein wall was shown ex vivo in a cow’s foot model [436], and occlusion of the vein by fibrosis in an animal experiment [448]. Postablation fibrosis of the vein wall by bRFA has also been shown in vivo and in an ex vivo experiment [449, 450].
6.2.2.3.1 Special recommendations for execution (method and technique)
In sRFA, the probe head (7 cm or 3 cm) is heated to 120 °C by radio-frequency energy. The energy produced by the generator is delivered to the probe head by a reverse-coupling mechanism, keeping it continuously at 120 °C. Thermal ablation shall be carried out twice in the junction segment and once in the remaining vein segments.
Contact between the treatment probe and the vein wall can be improved by external compression and positioning [354].
Recommendation 116
Radio-frequency ablation can be carried out in the Trendelenburg position. Contact between the treatment probe and the vein wall can be improved by external compression to optimise treatment results.
6.2.2.3.2 Undesired effects
Undesired effects are summarised in Table 5.
6.2.2.3.2.1 Radio frequency–induced segmental thermal ablation/segmental radio-frequency ablation (sRFA)
The following complications and undesired effects have been described:
Haematomas [451]
Pigmentation [376]
PATE [409, 410, 412, 453], c.f. Sect. 6.2.2.2.2.1 PATE after EVLA
Burns [454]
In individual cases: lymphocele, wound infection, arteriovenous fistula [349, 386]
The incidence of paraesthesia after sRFA in the European cohort study was 3.4% in the first postoperative week, and then it fell to 0.4% and remained at that value throughout the 5‑year observation period [451, 456]. This represents a clear improvement over previous treatment procedures [351].
Ecchymosis, generally caused by the application of tumescence, is more frequently described in the perioperative context, with a reported incidence between 5.8% [451] and 33.3% [376].
Induration and shortening of the treated saphenous vein arise due to thermal alteration. Thin patients feel this process, and the thickened vein can be felt under the skin. After around 4–6 weeks it regresses, and the vein can no longer be felt. There are no robust figures in the literature on this side effect and its regression.
Pigmentation as an undesired effect is described in the European multicentre study as occurring perioperatively in 2.4% of cases [451], with persistence after 5 years in 0.4% [456].
The incidence of accompanying superficial vein thrombosis (phlebitis) was 1.0% in the European multicentre study [451] and 8.2% in a randomised multicentre study [444].
Skin burns are rare (0–1.3%) [369, 456]. They occur less frequently with the consistent application of tumescent solution, which was not always done when the method was first developed [457].
Thromboembolic complications occur with a frequency of 0–3.4% [373, 456, 458, 459]. The current meta-analysis cited above showed differing frequencies of 1.4% (DVT and PATE), 0.5% (DVT), and 0.1% (lung embolism) after RFA of the GSV [409].
The reported incidence of PATE after sRFA was 3% in SSV treatment [412] and 1.2% to 2.4% after GSV ablation [409, 453]. More recent works have shown that there is a higher risk of PATE when tributary extirpation is carried out in addition to RFA and when the patient has a prior history of DVT [460]. In addition, vein diameter > 10 mm and intervention time > 40 min are described as independent risk factors for the occurrence of a PATE [461]. For treatment of a PATE, see Table 6.
6.2.2.3.2.2 Radio frequency–induced thermal treatment/bipolar radio frequency ablation (bRFA)
There is little literature on bRFA. Ten articles have been published since 2007, including one randomised controlled study [380], one prospective nonrandomised study [387], four prospective cohort studies [447, 450, 462, 463], two prospective studies [464, 465], one retrospective study [466], and one review [354]. The observation periods range between 3 months and 12 months; no long-term studies have yet been published.
Postoperative pain after bRFA is reported with frequencies of none to 74%, with an intensity of 0–2 on the visual analogue scale (VAS) of 0–10 [380, 387, 447, 462, 463].
Postoperative internal bleeding is generally rare, reported with a frequency of 0.9–3% [380, 387, 463, 465].
Burns are not described.
Sensory nerve damage (paraesthesia, dysaesthesia) is reported as the predominant side effect by several authors, with incidence of 1.7%–12.5%; this shows a statistically significant tendency to occur after treatment of the SSV [387, 462, 463].
There are no reports in the literature to date of motor nerve damage, lymphoedemas, or spider veins after bRFA treatment.
Postoperative hyperpigmentation occurs fairly rarely, in 0–1.5% [380, 387, 463, 465].
Superficial vein thrombosis (thrombophlebitic symptoms) is described by only one work group, with a frequency of 2.4% [463].
Serious complications such as deep leg vein thrombosis or lung-artery embolism are not described in the current literature.
6.2.2.3.3 Outcomes
Technical success (feasibility)
With proper patient selection (preoperative duplex ultrasound of the course of the saphenous vein), probing and thermal treatment of the saphenous vein is possible in over 95% of patients [451].
6.2.2.3.2.1 Radio frequency–induced segmental thermal thermal ablation/segmental radio frequency ablation (sRFA)
The data availability for outcomes after treatment of superficial vein incompetence is good. There are numerous retrospective analyses. The data from a prospective multicentre cohort study were published up to 5 years post procedure [456], and there are several prospective randomised controlled studies of sRFA vs. EVLA [369, 373, 376] and of sRFA vs. crossectomy/stripping vs. EVLA vs. foam sclerotherapy [331, 444], as well as comparative studies on improvements in quality of life and venous symptoms. There are also meta-analyses comparing the different treatment procedures with one another [386, 389].
The rate of perioperative painful symptoms is very low and sometimes better than the rate for EVLA [376, 444]. As a rule, the resumption of everyday activities occurs sooner than after EVLA [444].
In a Danish prospective randomised multicentre study, the rate of reflux in the GSV 1 year after sRFA was found to be 4.8%. The rate for EVLA was 5.8% [444]. The outcomes were constant over 5 years. The occlusion rate in the GSV 5 years after the sRFA intervention was 94.2% [331].
The occlusion rate in a prospective clinical multicentre cohort study 60 months post procedure was 91.9 ± 1.8%. A significant improvement in the VCSS over the whole study period of 60 months was also shown in the European cohort study, as well as a permanent improvement in the C state [456].
Some prospective randomised studies show significant improvement in quality of life after sRFA up to 60 months post procedure [376, 415, 467].
6.2.2.3.2.2 Radio frequency–induced thermal treatment/bipolar radio frequency ablation (bRFA)
Because of the short observation periods, the published studies of bRFA can make only limited assertions on postoperative quality of life and the postoperative evolution of the severity of the disease after treatment by bRFA. One work group reports an improvement in the VCSS of 79.3% on the original values after 12 months and a highly significant improvement in the digital photoplethysmography values after 3 months and 12 months [387].
In this study, convalescence and return to normal activity after bRFA is given as being within 3 days for 92.4% of patients.
Technical–anatomical success (duplex ultrasound)
Six studies of bRFA have been published since 2009, showing occlusion rates of 74%–98% after 3–12 months [380, 387, 447, 462, 463, 465]; however, they are comparable only up to a point because different treatment protocols were used. The power applied ranged from 18 W to 26 W and the impedance-guided withdrawal speed from 0.5 cm/s to 2 cm/s. A slower withdrawal speed is associated with a higher occlusion rate, as it results in higher energy delivered. The majority of authors recommend power of 18 W and withdrawal speed of 0.5–0.7 cm/s [380, 387, 462, 463, 465].
The execution varies considerably in individual studies, so no clear recommendations can be given [387, 462, 463].
6.2.2.4 Endovenous steam ablation (EVSA) and other procedures. 6.2.2.4.1 Indication
The steam ablation treatment method (also known as steam vein sclerosis [SVSTM] or endovenous steam ablation [EVSA]) was authorised in Germany in 2009. It is indicated for the treatment of GSV/SSV incompetence and large lumen varicose tributaries.
6.2.2.4.2 Recommendations for execution
Steam is generated from a few microlitres of sterile water under high pressure (over 200 bar) and is injected into the vein through the catheter tip at a temperature of approximately 120 °C. Sixty joules of heat energy are generated with every burst of steam, spread through up to 10 cm of the treated vein.
6.2.2.4.3 Undesired effects
Undesired effects include nerve lesion/paraesthesia in 0.9%–9.6% [375, 468], ecchymosis/haematoma in 3.1%–47.3% [375, 469], phlebitis distal to the treated segment in 8.5% [375], pain in the treated vein after the control exploration in 35% [470], and hyperpigmentation in 4.6%–7.6% [375, 468].
6.2.2.4.4 Outcomes
Clinical outcomes of steam ablation have been published in three small prospective case series [469–471], one nonrandomised study in comparison with crossectomy and stripping [468], and one randomised controlled study in comparison with 980-nm laser ablation [375]. The recanalisation rate after 6–12 months post intervention is between 4% and 10% [375, 468–471].
6.2.2.5 Other endovenous thermal procedures.
Endovenous microwave ablation (EMA) of varicose veins as an alternative endothermal treatment method has so far been investigated only in two studies [394, 472]. In the randomised study by Yang comparing it with the stripping operation, 10% of the patients presented skin burns after EMA (p < 0.01) [472].
Recommendation 117
Because of the lack of data and the high number of skin burns, microwave ablation of varicose veins cannot currently be recommended outside clinical studies.
6.2.2.6 Reflux recurrence from the saphenofemoral region.
From previous studies of varicose vein recurrence after crossectomy and stripping (C/S), we know that leaving a long stump of the GSV and/or the SSV can be a source of recurrence of the varicose vein, sometimes through the AASV [302, 473–475]. With endovenous thermal treatments, the ablation generally ends a few millimetres to a few centimetres below the junction of the GSV or SSV with the deep vein system. As a result, a stump of vein is left behind. Preexisting reflux in the stump can vanish, persist, or reappear at a later date.
We know from studies with an observation period of 5 years or more that reflux in the saphenous vein stump occurs more often after endovenous thermal treatment than after C/S [174, 186, 330, 476]. Most of the data refer to EVLA treatments, while this issue has been investigated only occasionally after RFA or cyanoacrylate treatment [477].
The clinical consequences of this reflux are controversial. In the meta-analysis of Hamann et al., the clinical outcomes after C/S and EVLA are comparable, despite frequent saphenofemoral reflux after EVLA [330]. In the work by Rass and Flessenkämper, reflux in the junction region was more common after EVLA than after crossectomy and stripping; however, clinical recurrence of varicose veins in the treated leg was equally frequent overall [186, 476]. Clinical recurrence originating in the treated junction is more common after EVLA with short wavelengths (810–980 nm) and bare fibre. The significance of this finding cannot yet be judged.
There are few long-term outcomes from comparative studies for the new methods with longer wavelengths and radial emission probes [415, 478, 479], and reflux in the SFJ/SPJ or AASV was not always an endpoint in these studies. Investigation of recurrence from the SFJ/SPJ shall await further studies, particularly RCTs and/or large case control studies.
6.2.2.7 General overview of endovenous thermal ablation procedures (EVTA).
Endovenous laser ablation is a safe, established treatment procedure for the treatment of saphenous vein incompetence of the GSV and SSV; the intrafascial course of the accessory veins of the GSV, such as the AASV and PASV; and the femoropopliteal vein. In technical terms, it appears to remain open to further development. Endovenous laser ablation with first-generation laser using short wavelengths (810–980 nm) and bare fibre has proved to have a worse side effects profile than lasers with longer wavelengths (1320–1940 nm) and modified probes, and also RFA [376, 380]. Compared with crossectomy and stripping, duplex ultrasound recurrence of inguinal reflux and clinical recurrence in general from the junction regions are evidence of poorer long-term effectiveness (studies with observation period of 5 years). Nevertheless, the overall clinical recurrence rate, the improvement in quality of life, and the reduction in symptoms after crossectomy and stripping do not differ significantly from the outcomes after EVLA and RFA [186, 415, 476, 480].
Endovenous laser ablation with laser at longer wavelengths (1320–1940 nm) and modified probes is the current state of the art. Studies show minor side effects and quick postoperative convalescence, as well as good effectiveness with high occlusion rates. However, valid long-term studies and controlled comparative studies with open operation are still lacking for these new procedures. Studies are also lacking that would allow assertions to be made, based on stratification, about individualised indications justified by prognosis of presumably significant parameters (e.g., vein diameter, CEAP classification).
The radio frequency (RFA) treatments currently available, i.e. segmental thermal ablation using radio frequency (segmental radio frequency ablation [sRFA]) and radio frequency-induced thermal therapy (bipolar radio frequency ablation [bRFA]), have also become established as safe and effective treatment procedures for eliminating intrafascial venous reflux. They offer perioperative advantages in terms of pain, and quick resumption of everyday activities. Because of lack of data for bRFA, only limited assertions can be made about the medium and long-term outcomes.
Endovenous laser ablation and sRFA have shown significant improvements in quality of life and the clinical severity (VCSS) of CVI in clinical multicentre comparative randomised studies and cohort studies.
Recommendation 118
Endovenous laser ablation and radio frequency ablation with modern procedures to eliminate epifascial reflux in cases of saphenous vein incompetence should be offered to varicose vein patients as alternatives to other treatment procedures.
6.2.3 Chemical procedures
Procedures for vein occlusion by other means than heat include sclerotherapy procedures and nonthermal catheter procedures, particularly mechanochemical endovenous ablation (MOCATM) and vein gluing.
6.2.3.1 Sclerotherapy.
Sclerotherapy refers to tactical elimination of a vein segment by targeted injection of a sclerosing agent. In Germany, polidocanol is authorised as a premixed sclerosing agent for varicose vein elimination. Common salt solution is not authorised for use as a sclerosing agent, and there are no scientific data on its use.
6.2.3.1.1 Indications
Recommendation 119
Sclerotherapy can be used for all forms of varicose veins.
Sclerotherapy is suitable for all forms of varicose veins, including veins of very varying diameters. Periulcerous varices, genital varicose veins, and spider veins are all clear indications for sclerotherapy. Vascular malformations and seroma after varicose vein surgery can be treated effectively with sclerotherapy. Literature overview: saphenous veins (GSV and SSV) [406, 444, 481–486], tributaries [486–488], perforators [487, 489, 490], reticular varices and spider veins [491–494], remaining and recurrent varicose veins after previous interventions [494–499], pudendal or pelvic varicose veins [494, 500, 501], peri-ulcer varicose veins [502–505], and varicose veins in venous malformations [506–508].
6.2.3.1.2 Contraindications
There are few absolute contraindications for sclerotherapy [509–513].
Recommendation 120
The absolute and relative contraindications for sclerotherapy shall be observed.
Recommendation 121
Absolute contraindications are known allergies to the sclerosant, acute venous thromboembolism, and local infection in the region to be treated or a severe generalised infection.
Recommendation 122
For foam sclerotherapy, known symptomatic right-to-left shunt is also an absolute contraindication.
Recommendation 123
In patients with known thrombophilia and a high risk for thrombus formation, sclerotherapy should be carried out under additional prophylactic drugs to guard against thrombosis.
6.2.3.1.2.1 Absolute contraindications
Known allergy to the sclerosing agent
Acute venous thromboembolism
Local infection in the region of the sclerotherapy or severe generalised infection
For foam sclerotherapy:
Known symptomatic right-to-left shunt (e.g., symptomatic patent foramen ovale)
6.2.3.1.2.1 Relative contraindications (individual risk–benefit assessment is obligatory)
Pregnancy
Lactation (if the indication is urgent, interrupt lactation for 2–3 days)
Severe peripheral arterial occlusive disease
Poor general state of health
High risk for thromboembolism (e.g., known history of thromboembolic events, known severe thrombophilia, active cancer)
Long-term immobility or bed- patient
For foam sclerotherapy:
Neurological disorders, including migraine, after previous foam sclerotherapy
In cases of relative contraindications, sclerotherapy (especially foam sclerotherapy) can be carried out—if strongly indicated—under special safety considerations.
6.2.3.1.3 Execution
Recommendation 124
Sclerotherapy of spider veins and reticular varices (C1) should be applied with the limbs in a horizontal position and using a low-friction syringe.
Recommendation 125
The sclerosing agent should be applied with the patient lying down.
Recommendation 126
Sclerotherapy of varicose tributaries that are not visible percutaneously, or incompetent saphenous veins or perforator veins, should be carried out under ultrasound control (B mode).
Recommendation 127
Direct puncture of a perforator vein should be avoided.
The basis of sclerotherapy is the intravenous application of a sclerosing agent. Sclerotherapy is usually planned and applied in sequence from proximal to distal reflux sources and from larger to smaller varicose veins.
Polidocanol (Lauromacrogol 400) is the only sclerosing agent authorised in Germany. It is available in solutions at concentrations of 0.25%, 0.5%, 1%, 2%, or 3%. The concentration and the dosage of the sclerosing agent are selected in accordance with the calibre of the varicose vein to be treated (see Tables 7 and 8). A maximum dose of 2 mg polidocanol/kg body weight should not be exceeded in any treatment session (e.g., 140 mg polidocanol for 70 kg body weight).
Table 7.
Recommended amounts per injection for polidocanol in liquid sclerotherapy with single injections [514]
| Indication | Volume/injection point |
|---|---|
| Spider veins (C1) | Up to 0.2 ml |
| Reticular varices (C1) | Up to 0.5 ml |
| Varicose veins (C2) | Up to 2.0 ml |
Table 8.
Recommended concentrations in liquid sclerotherapy with polidocanol [514]
| Indication | Concentration (%) |
|---|---|
| Spider veins | 0.25–1.0 |
| Reticular varices | 0.5–1.0 |
| Small varicose veins | 1.0 |
| Medium-sized varicose veins | 2.0–3.0 |
| Large varicose veins | 3.0 |
6.2.3.1.3.1 Liquid sclerotherapy
Recommendation 128
The following recommendations on concentrations and amounts per injection for liquid sclerotherapy should be observed (Tables 7 and 8). Concentrations and amounts are reference values and may be adapted according to the therapist’s assessment.
6.2.3.1.3.2 Sclerotherapy with foam sclerosant
Numerous studies have shown that foam sclerosants are safe and effective. Foam sclerotherapy can therefore be regarded as an established procedure for the treatment of varicose veins. The application of sclerosant as a foam (polidocanol with ambient air) has been authorised by the German Federal Institute for Drugs and Medical Devices (BfArM) since 2009 [514].
6.2.3.1.3.2.1 Foam production
Recommendation 129
For all indications, a three-way stopcock (Tessari method) or a two-way connector (double-syringe method), or a similar appropriate method, should be used for production of the sclerotherapy foam.
Recommendation 130
For all indications, the foam should be prepared by mixing with ambient air; alternatively, O2/CO2 can be used.
Detergent-type sclerosants, such as polidocanol, can be converted into a fine-bubble foam by special techniques. Ambient air or other gases (CO2) can be used for this purpose.
In Tessari’s method, the foam is produced by mixing liquid and air in two syringes, which are connected by a three-way stopcock. The best mixture ratio of sclerosant to air is between 1:3 and 1:4 [515, 516]. In the double-syringe system, polidocanol is mixed with air by turbulent mixing in two syringes connected by a special two-way connector [515, 516].
6.2.3.1.3.2.2 Recommendations for execution of foam sclerotherapy
Recommendation 131
In routine foam sclerotherapy, no more than 10 ml of foam should be injected per day/session. Larger volumes of foam may be injected after individual risk–benefit assessment.
Recommendation 132
The following concentrations should be observed in proportion to the diameter of the treated vein segment. The suggested concentrations and amounts are reference values and may be adapted according to the therapist’s assessment.
In view of its effectiveness, relatively low volumes of sclerosant are recommended in comparison with liquid sclerotherapy (see Table 9; [444, 486, 488, 490–494, 496–498, 502–508, 512, 517–523]). The foam sclerosant can be applied to the vein to be treated through a cannula or a short or long catheter.
Table 9.
Recommended concentration of foam sclerosants
| Indication | Polidocanol concentration (%) |
|---|---|
| Spider veins | Up to 0.5 |
| Reticular varices | Up to 1.0 |
| Varicose tributary veins | Up to 2.0 |
| Great saphenous vein, small saphenous vein | |
| < 4 mm | 1.0 |
| ≥ 4 to ≤ 8 mm | 1.0–3.0 |
| > 8 mm | 3.0 |
| Incompetent perforator veins | 1.0–3.0 |
| Recurrent varicose veins | 1.0–3.0 |
| Venous malformations | 1.0–3.0 |
The following additional safety measures apply to foam sclerotherapy: Use a maximum of 10 ml foam, avoid Valsalva manoeuvres, and have the patient remain lying down for a few minutes after application.
6.2.3.1.4 Outcomes
Recommendation 133
With spider veins and reticular varices (C1), the success of treatment can be assessed from the clinical outcome in the control after sclerotherapy. With varicose veins (C2) and venous malformations, clinical and ultrasound examinations shall be carried out.
The success of sclerotherapy in spider veins, reticular varices, and visible tributaries can be controlled by clinical examination. For other indications, success of the treatment should be controlled by duplex ultrasound. This will allow the clinician to establish whether the treated vein is occluded completely, partially, or not at all. If partial occlusion has occurred, the examination will show whether the segment in question presents antegrade, retrograde, or no flow.
6.2.3.1.5 Complications and side effects
If correctly carried out, sclerotherapy is a safe treatment with few side effects. Undesired effects or complications seldom occur [521, 524]. However, undesired effects may be observed (see Tables 10 and 11).
Table 10.
Serious complications after sclerotherapy
| Side effect | Liquid sclerotherapy | Foam sclerotherapy |
|---|---|---|
| Allergic reactions/anaphylaxis |
Individual cases < 0.01% |
Individual cases < 0.01% |
| Skin necrosis |
Individual cases < 0.01% |
Individual cases < 0.01% |
| Neurological reactions/stroke/TIA |
Individual cases < 0.01% |
Individual cases < 0.01% |
| Distal deep thrombosis |
Rare ≥ 0.01 to < 0.1% |
Occasional ≥ 0.1 to < 1% |
| Proximal deep thrombosis |
Very rare < 0.01% |
Very rare < 0.01% |
| Lung embolism |
Individual cases < 0.01% |
Individual cases < 0.01% |
| Damage to motor nerves |
Individual cases < 0.01% |
Individual cases < 0.01% |
Table 11.
Minor side effects after sclerotherapy
| Side effect | Liquid sclerotherapy | Foam sclerotherapy |
|---|---|---|
| Vision disorders |
Very rare < 0.01% |
Occasional ≥ 0.1 to < 1% |
| Migraine, headache |
Very rare < 0.01% |
Occasional ≥ 0.1 to < 1% |
| Sensory nerve damage |
Individual cases < 0.01% |
Rare ≥ 0.01–< 0.1% |
| Dry cough |
Rare ≥ 0.01–< 0.1% |
Very rare < 0.01% |
| Superficial phlebitis | Unclear | Unclear |
| Matting |
Frequent ≥ 1–< 10% |
Frequent ≥ 1–< 10% |
| Hyperpigmentation |
Frequent ≥ 1 to < 10% |
Frequent ≥ 1 to < 10% |
| Small areas of skin necrosis |
Rare ≥ 0.01–< 0.1% |
Very rare < 0.01% |
Recommendation 134
Attention shall be paid to the side effects of sclerotherapy shown in Tables 10 and 11.
6.2.3.1.6 Mechanochemical endovenous ablation (MOCA)
Recommendation 135
Mechanochemical endovenous ablation (MOCA) can be used as an alternative to other sclerotherapy methods for saphenous vein sclerotherapy.
The principle of MOCA is based on a combination of mechanical and chemical alteration of the endothelium [525–527]. The rotating wire at the point of the catheter provokes vasoconstriction of the vein.
6.2.3.1.6.1 Indications
Based on study data, the indications for the procedure to treat incompetent GSV or SSV of diameter up to 12 mm (level A) [528–530] are the same as for treatment using other catheter-supported or operative procedures [531–533]. Mechanochemical endovenous ablation can be combined with other surgical and endovenous procedures.
6.2.3.1.6.2 Limitations and contraindications
The impossibility of probing the vein to be treated is a limitation for the procedure, and a vein diameter > 12 mm is a relative contraindication.
Because of the use of a sclerosant, the procedure is subject to the same relative and absolute contraindications that are applicable to sclerotherapy [534–540].
6.2.3.1.6.3 Recommendations for execution
The treatment catheter is introduced into the vein and positioned 2 cm below the saphenofemoral or saphenopopliteal junction (according to the manufacturer’s instructions) under ultrasound control. Depending on the vein diameter, liquid polidocanol at 1%–3% [528–530] can be injected up to the maximum dose of 2 mg/kg body weight per day [514]. The target vein can be probed both antegrade and retrograde [533, 541].
6.2.3.1.6.4 Outcomes
Studies published to date report occlusion rates for the GSV and VSP after 6 months and 12 months of 96.7 and 94% [529, 542–544], respectively, with a complication rate described as low and little perioperative pain [545, 546]. The 24-month data for the GSV showed an obliteration rate of 94%–95% with significant improvement in vein-related quality of life [547, 548]. The published data refer to vein diameters of less than 12 mm.
6.2.3.2 Cyanoacrylate.
The vein glue used in Germany is N‑butyl 2-cyanoacrylate.
Only one cyanoacrylate treatment is authorised for varicose vein treatment.
Recommendation 136
Saphenous varicose veins can alternatively be treated with cyanoacrylate glue.
6.2.3.2.1 Indication
The use of a vein gluing procedure, like other catheter-supported procedures, is indicated for treating saphenous vein incompetence.
6.2.3.2.2 Recommendations for execution
The vein is accessed by puncture under ultrasound control (B mode). In the vein gluing procedure, the vein is closed (i.e., glued) by polymerisation after intravasal application of the glue. Use of the vein gluing procedure allows treatment without tumescent local anaesthesia or general anaesthetic. If the glue catheter is pushed forward too close to the deep vein, the glue can enter the deep vein system, leading to severe complications.
6.2.3.2.3 Outcomes
The first studies showed saphenous vein occlusion rates of 99% after 3 months [370, 549] and 97.2% or 93% after 1 year [550, 551]. The first long-term outcomes show an occlusion rate of 95.3% after 24 months and 94.4% after 36 months [552, 553].
6.2.3.2.4 Limitations and undesired effects
In the cyanoacrylate procedure, the vein diameter appears to lead to limitations: Diameters in excess of 8–10 mm present high levels of recanalisation [554]. After treatment of the GSV, phlebitic reactions occur with a frequency of approximately 11%–20%, appearing a few days postoperatively and persisting for up to 1 month [550, 551]. This reaction has not been reported to date in the SSV.
No definite recommendation can yet be given because of the lack of data.
7 Varicose veins in pregnancy
Pregnancy has an impact on the vein system of the lower limbs because of both functional alterations and the structural and hormonal changes that occur. Up to 40% of all pregnant women present a new or progressing varicose vein [357].
Recommendation 137
Any varicose vein that appears during pregnancy shall be diagnosed by a vein specialist.
Various univariate studies, but not multivariate analyses, have shown that pregnancy is an independent significant risk factor for the appearance or progression of a varicose vein [555, 556].
Women with a history of three or more pregnancies frequently present a varicose vein [557–559].
According to the available data, pregnancy is one of the many risk factors for recurrence of reflux after high ligation and stripping of the saphenous vein. The probability of recurrence in women who become pregnant after varicose vein treatment is increased by a factor of 2.69 compared with women who do not become pregnant [558].
The subjective risk evaluation for the development of venous incompetence [560] and the perception of painful symptoms is variable and age dependent [561].
7.1 Treatment
Recommendation 138
Patients with varicose veins in pregnancy should be informed about the physiological changes and their course, as well as the risks.
The prophylactic effect of compression treatment to prevent a varicose vein during pregnancy has not been proved [357], but compression can improve the symptoms [357].
No study data are available on operative or interventional measures to treat a varicose vein in pregnancy, since pregnancy is established as an exclusion criterion in all studies.
The use of polidocanol for liquid or foam sclerotherapy in pregnancy has not been shown to be safe by study data from trials in humans [514].
Recommendation 139
Invasive treatment of varicose veins during pregnancy should be indicated only in exceptional cases.
Acknowledgments
Leitung
Felizitas Pannier, PD Dr. med., Praxis für Dermatologie und Phlebologie, Bonn; Thomas Noppeney, PD Dr. med., Klinik für Gefäß- und endovaskuläre Chirurgie, Nürnberg.
Steuerungsgruppe
Breu Franz Xaver, Dr. med., Praxis für Gefäßerkrankungen, Gmund; Kluess Holger, Dr. med. Cutaris, München; Mumme Achim, Prof. Dr. med., Klinik für Gefäßchirurgie, St. Josef Hospital, Bochum; Schmedt Claus Georg, PD Dr. med., Diakonissenkrankenhaus, Schwäbisch Hall.
Delegierte DGP
Alm Jens, Dr. med. Dermatologikum, Hamburg; Breu Franz Xaver, Dr. med., Praxis für Gefäßerkrankungen, Gmund; Flessenkämper Ingo, Dr. med., Klinikum rechts der Isar, München; Hartmann Karsten, Dr. med., Praxis für Gefäßmedizin, Freiburg; Kahle Birgit, Prof. Dr. med., Klinik für Dermatologie, UKSH, Campus Lübeck, Lübeck; Kluess Holger, Dr. med. Cutaris, München; Mendoza Erika, Dr. med., Venenpraxis, Wunstorf; Mühlberger Dominic, Dr. med. Dr. med. univ., Klinik für Gefäßchirurgie, Bochum Mumme Achim, Prof. Dr. med., Klinik für Gefäßchirurgie, St. Josef Hospital, Bochum; Pannier, Felizitas, PD Dr. med., Praxis für Dermatologie und Phlebologie, Bonn Noppeney, Thomas, PD Dr. med., Klinik für Gefäß- und endovaskuläre Chirurgie, Nürnberg; Rass Knuth, PD Dr. med., Krankenhaus St. Brigida, Simmerath; Reich-Schupke, Prof. Dr. med., Venenzentrum Bochum, Bochum; Stenger Dietmar, Dr. med., Praxis für Gefäßmedizin, Saarlouis; Stücker Markus, Prof. Dr. med., Venenzentrum Bochum, Bochum; Tesmann Jens, Dr. med., Hautzentrum Innenstadt, Stuttgart.
Delegierte DGA
Schwarz Thomas, Dr. med., Praxis für Gefäßmedizin, Freiburg; Werth Sebastian, Universitätsklinikum, Dresden.
Delegierte DGG
Nüllen Helmut, Praxis für Gefäßmedizin, Mönchengladbach; Schmedt Claus Georg, PD Dr. med., Diakonissenkrankenhaus, Schwäbisch Hall.
Delegierte ANG
Noppeney Thomas, PD, Noppeney, Thomas, PD Dr. med., Klinik für Gefäß- und endovaskuläre Chirurgie, Nürnberg.
Delegierte DDG
Valesky Eva, Prof. Dr. med., Universitätsklinikum, Klinik für Dermatologie, Frankfurt.
Delegierte DGDC
Bruning Guido, Dr. med., Tabea Krankenhaus, Hamburg.
Delegierte BVP
Gerlach Horst, Dr. med., Viernheim.
Declarations
Conflict of interest
F. Pannier, T. Noppeney, J. Alm, F.X. Breu, G. Bruning, I. Flessenkämper, H. Gerlach, K. Hartmann, B. Kahle, H. Kluess, E. Mendoza, D. Mühlberger, A. Mumme, H. Nüllen, K. Rass, S. Reich-Schupke, D. Stenger, M. Stücker, C.G. Schmedt, T. Schwarz, J. Tesmann, J. Teßarek, S. Werth, and E. Valesky declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
The supplement containing this article is not sponsored by industry.
Footnotes
This S2k guideline was published online by the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V, AWMF) in early 2019, with registration number 037-018. The guideline is valid until 30 March 2024.

Scan QR code & read article online
References
- 1.Leu H, Vogt M, Pfrunder H, Odermatt B. Phlebosclerosis: disorder or disease? Vasa. 1991;20:230–236. [PubMed] [Google Scholar]
- 2.Eklof B, Perrin M, Delis KT, Rutherford RB, Gloviczki P. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49(2):498–501. doi: 10.1016/j.jvs.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Hach W. Spezielle Diagnostik Der Primären Varikose. Gräfelfing: Demeter; 1981. [Google Scholar]
- 4.Pichot O, Sessa C, Bosson J-L. Duplex imaging analysis of the long saphenous vein reflux: basis for strategy of endovenous obliteration treatment. Int Angiol. 2002;21(4):333–336. [PubMed] [Google Scholar]
- 5.Abu-Own A, Scurr JH, Smith PDC. Saphenous vein reflux without incompetence at the saphenofemoral junction. br J Surg. 1994;81(10):1452–1454. doi: 10.1002/bjs.1800811016. [DOI] [PubMed] [Google Scholar]
- 6.Fratila A. Surgical treatment of primary varicosis. In: Ratz J, Geronemus R, Goldman M, Maloney M, Padilla R, editors. Textbook of dermatologic surgery. Philadelphia: Lippincott-Raven; 1998. pp. 593–620. [Google Scholar]
- 7.Stücker M, Moritz R, Altmeyer P, Reich-Schupke S. New concept: different types of insufficiency of the saphenofemoral junction identified by duplex as a chance for a more differentiated therapy of the great saphenous vein. Phlebology. 2013;28(5):268–274. doi: 10.1177/0268355513476215. [DOI] [PubMed] [Google Scholar]
- 8.Evans C, Fowkes F, Ruckley C, et al. Edinburgh vein study: methods and response in a survey of venous disease in the general population. Phlebology. 1997;12:127–135. doi: 10.1177/026835559701200403. [DOI] [Google Scholar]
- 9.Fischer H. Venenleiden – Eine Repräsentative Untersuchung in Der Bevölkerung Der Bundesrepublik Deutschland (Tübinger Studie) Urban & Schwarzenberg; 1981. [Google Scholar]
- 10.Rabe E, Guex JJ, Morrison N, et al. Treatment of chronic venous disease with flavonoids: Recommendations for treatment and further studies. Phlebology. 2013;28(6):308–319. doi: 10.1177/0268355512471929. [DOI] [PubMed] [Google Scholar]
- 11.Rabe E, Pannier-Fischer F, Bromen K, et al. Bonner Venenstudie der Deutschen Gesellschaft für Phlebologie. Phlebologie. 2003;32:1–14. doi: 10.1055/s-0037-1617353. [DOI] [Google Scholar]
- 12.Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15(3):175–184. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Pannier F, Rabe E. Progression in venous pathology. Phlebology. 2015;30(1_suppl):95–97. doi: 10.1177/0268355514568847. [DOI] [PubMed] [Google Scholar]
- 14.Schultz-Ehrenburg U. Prospective epidemiological study on the beginning of varicose veins—Bochum Study I–IV. Phlebologie. 2009;38:17–25. doi: 10.1055/s-0037-1622252. [DOI] [Google Scholar]
- 15.Perrin M, Eklof B, Rij VANA, et al. Venous symptoms: the SYM Vein Consensus statement developed under the auspices of the European Venous Forum. Int Angiol. 2016;35(4):374–398. [PubMed] [Google Scholar]
- 16.Wrona M, Jöckel KH, Pannier F, Bock E, Hoffmann B, Rabe E. Association of venous disorders with leg symptoms: results from the Bonn vein study 1. Eur J Vasc Endovasc Surg. 2015;50(3):360–367. doi: 10.1016/j.ejvs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Pannier F, Rabe E. The relevance of the natural history of varicose veins and refunded care. Phlebology. 2012;27(Suppl 1):23–26. doi: 10.1258/phleb.2012.012S23. [DOI] [PubMed] [Google Scholar]
- 18.Lee AJ, Robertson LA, Boghossian SM, et al. Progression of varicose veins and chronic venous insufficiency in the general population in the Edinburgh Vein Study. J Vasc Surg Venous Lymphat Disord. 2015;3(1):18–26. doi: 10.1016/j.jvsv.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Guex J. Thrombotic complications of varicose veins. A literature review of the role of superficial venous thrombosis. dermatol Surg. 1996;22:378–382. [PubMed] [Google Scholar]
- 20.Widmer L, Stähelin H, Nissen C, Da Silva A. Venen‑, Arterien-Krankheiten, Koronare Herzkrankheit Bei Berufstätigen. Prospektiv-Epidemiologische Untersuchung. Basler Studien I–III, 1995–1978. Bern: Huber; 1981. [Google Scholar]
- 21.Kurz X, Lamping DL, Kahn SR, et al. Do varicose veins affect quality of life? Results of an international population-based study. J Vasc Surg. 2001;34(4):641–648. doi: 10.1067/mva.2001.117333. [DOI] [PubMed] [Google Scholar]
- 22.MacKenzie RK, Paisley A, Allan PL, Lee AJ, Ruckley CV, Bradbury AW. The effect of long saphenous vein stripping on quality of life. J Vasc Surg. 2002;35(6):1197–1203. doi: 10.1067/mva.2002.121985. [DOI] [PubMed] [Google Scholar]
- 23.Labropoulos N, Leon L, Kwon S, et al. Study of the venous reflux progression. J Vasc Surg. 2005;41(2):291–295. doi: 10.1016/j.jvs.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Engelhorn CA, Manetti R, Baviera MM, et al. Progression of reflux patterns in saphenous veins of women with chronic venous valvular insufficiency. Phlebology. 2012;27(1):25–32. doi: 10.1258/phleb.2011.010077. [DOI] [PubMed] [Google Scholar]
- 25.Brewster SF, Nicholson S, Farndon JR. The varicose vein waiting list: results of a validation exercise. Ann R Coll Surg Engl. 1991;73(4):223–226. [PMC free article] [PubMed] [Google Scholar]
- 26.Schmeller W. Das Arthrogene Stauungssyndrom. Sprunggelenksveränderungen Bei Chronischer Veneninsuffizienz. Berlin: Diesbach; 1990. [Google Scholar]
- 27.Püschel K, Koops E, Lignitz E. Fatale Blutungen aus Unterschenkelvarizen. Phlebol Proktol. 1987;16:162–165. [Google Scholar]
- 28.Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248–1252. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Vasquez MA, Rabe E, McLafferty RB, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American venous forum Ad hoc outcomes working group. J Vasc Surg. 2010;52(5):1387–1396. doi: 10.1016/j.jvs.2010.06.161. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Dumville JC, Ashby RL, et al. Validation of the VEINES-QOL quality of life instrument in venous leg ulcers: repeatability and validity study embedded in a randomised clinical trial. BMC Cardiovasc Disord. 2015;15(1):85. doi: 10.1186/s12872-015-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in Chronic Lower Limb Venous Insufficiency (CIVIQ) Qual Life Res. 1996;5(6):539–554. doi: 10.1007/BF00439228. [DOI] [PubMed] [Google Scholar]
- 32.Staniszewska A, Tambyraja A, Afolabi E, Bachoo P, Brittenden J. The aberdeen varicose vein questionnaire, patient factors and referral for treatment. Eur J Vasc Endovasc Surg. 2013;46(6):715–718. doi: 10.1016/j.ejvs.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Augustin M, Lange S, Wenninger K, Seidenglanz K, Amon U, Zschocke I. Validation of a comprehensive Freiburg Life Quality Assessment (FLQA) core questionnaire and development of a threshold system. Eur J Dermatol. 2004;14(2):107–113. [PubMed] [Google Scholar]
- 34.Marsden G, Perry M, Kelley K, Davies AH. Diagnosis and management of varicose veins in the legs: summary of NICE guidance. BMJ. 2013;347:f4279–f4279. doi: 10.1136/bmj.f4279. [DOI] [PubMed] [Google Scholar]
- 35.Lattimer C, Kalodiki E, Azzam M, Geroulakos G. An international survey on the interpretation of pigmentation using the C class oft he clinical, etiological, anatomical, patophysiological classification. Veins Lymphat. 2013;2:e15. doi: 10.4081/vl.2013.e15. [DOI] [Google Scholar]
- 36.Passman MA, McLafferty RB, Lentz MF, et al. Validation of Venous Clinical Severity Score (VCSS) with other venous severity assessment tools from the American Venous Forum, National Venous Screening Program. J Vasc Surg. 2011;54(6 Suppl):2S–9S. doi: 10.1016/j.jvs.2011.05.117. [DOI] [PubMed] [Google Scholar]
- 37.Pannier F, Gerlach H, Stücker M, Schimmelpfennig L, Rabe E. Leitlinie: Venöse Diagnostik mit der Licht-Reflexions-Rheographie/Photoplethysmographie der Deutschen Gesellschaft für Phlebologie. Phlebologie. 2012;41:229–289. doi: 10.1055/s-0037-1621825. [DOI] [Google Scholar]
- 38.Mendoza E, Blättler W, Amsler F. Great saphenous vein diameter at the saphenofemoral junction and proximal thigh as parameters of venous disease class. Eur J Vasc Endovasc Surg. 2013;45(1):76–83. doi: 10.1016/j.ejvs.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Mendoza E, Amsler F, Kalodiki E. Correlation between GSV diameter and varicose clinics. Phlebologie. 2016;45(01):29–35. doi: 10.12687/phleb2291-1-2016. [DOI] [Google Scholar]
- 40.Wienert V, Blazek V, Mayer O. Nichtinvasive hämodynamische Untersuchungen vor und nach Babcock-Stripping. Phlebol. Proktol. 1986;15:72–74. [Google Scholar]
- 41.Mendoza E, Berger V, Zollmann C, Bomhoff M, Amsler F. Diameter-reduction of the great saphenous vein and common femoral vein after CHIVA. Phlebologie. 2011;40:73–78. doi: 10.1055/s-0037-1621759. [DOI] [Google Scholar]
- 42.Mendoza E. Diameter reduction of the great saphenous vein and the common femoral vein after CHIVA: long-term results. Phlebologie. 2013;42(2):65–69. doi: 10.12687/phleb2127_2_2013. [DOI] [Google Scholar]
- 43.Mendoza E, Amsler F. CHIVA with endoluminal procedures: Laser versus VNUS. Phlebologie. 2017;46(01):5–12. doi: 10.12687/phleb2346-1-2017. [DOI] [Google Scholar]
- 44.Brunken A, Rabe E, Pannier F. Changes in venous function after foam sclerotherapy of varicose veins. Phlebology. 2009;24(4):145–150. doi: 10.1258/phleb.2009.008068. [DOI] [PubMed] [Google Scholar]
- 45.Gerlach H, Partsch H, Rabe E, Gallenkemper G, Jünger M. Leitlinie zur venösen Diagnostik mit der Venenverschlußplethysmographie mittels Dehnungsmessstreifen. Phlebologie. 1999;28:68–69. [Google Scholar]
- 46.De Maeseneer M, Pichot O, Cavezzi A, et al. Duplex ultrasound investigation of the veins of the lower limbs after treatment for varicose veins—UIP consensus document. Eur J Vasc Endovasc Surg. 2011;42(1):89–102. doi: 10.1016/j.ejvs.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Lee BB, Nicolaides AN, Myers K, et al. Venous hemodynamic changes in lower limb venous disease: the UIP consensus according to scientific evidence. Int Angiol. 2016 doi: 10.1002/14651858.CD010508.pub2.Corresponding. [DOI] [PubMed] [Google Scholar]
- 48.Geier B, Brach A, Freis H. Diagnostik der peripheren arteriellen Verschlusskrankheit (PAVK) Phlebologie. 2016;45(4):181–288. [Google Scholar]
- 49.Coleridge-Smith P, Labropoulos N, Partsch H, Myers K, Nicolaides A, Cavezzi A. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part I. basic principles. Eur J Vasc Endovasc Surg. 2006;31(1):83–92. doi: 10.1016/j.ejvs.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 50.Mendoza E. Duplexsonographie Der Oberflächlichen Beinvenen. 2. Berlin Heidelberg: Springer; 2013. [Google Scholar]
- 51.Mendoza E. Provokationsmanöver für die duplexsonographische Diagnostik der Varikose. In: Mendoza E, editor. Duplexsonographie Der Oberflächlichen Beinvenen. 2. Berlin, Heidelberg: Springer; 2013. pp. 103–115. [Google Scholar]
- 52.Habenicht M, Rabe E, Amsler F, Mendoza E. Toe elevation manoeuvre to assess venous reflux in comparison to manual calf compression and release. Vasa. 2016;45(4):299–304. doi: 10.1024/0301-1526/a000541. [DOI] [PubMed] [Google Scholar]
- 53.Lattimer CR, Azzam M, Kalodiki E, Geroulakos G. Venous filling time using air-plethysmography correlates highly with great saphenous vein reflux time using duplex. Phlebology. 2014;29(2):90–97. doi: 10.1258/phleb.2012.012042. [DOI] [PubMed] [Google Scholar]
- 54.Caggiati A, Bergan JJ, Gloviczki P, Eklof B, Allegra C, Partsch H. Nomenclature of the veins of the lower limb: extensions, refinements, and clinical application. J Vasc Surg. 2005;41(4):719–724. doi: 10.1016/j.jvs.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Georgiev M, Myers KA, Belcaro G. The thigh extension of the lesser saphenous vein: from Giacomini’s observations to ultrasound scan imaging. J Vasc Surg. 2003;37(3):558–563. doi: 10.1067/mva.2003.77. [DOI] [PubMed] [Google Scholar]
- 56.Mendoza E, Stücker M. Duplex-ultrasound assessment of the saphenofemoral junction. Phlebol Rev. 2015;3:78–85. doi: 10.5114/pr.2015.57468. [DOI] [Google Scholar]
- 57.Zollmann P, Zollmann C, Zollmann P, et al. Determining the origin of superficial venous reflux in the groin with duplex ultrasound and implications for varicose vein surgery. J Vasc Surg Venous Lymphat Disord. 2017;5(1):82–86. doi: 10.1016/j.jvsv.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Van der Velden SK, Lawaetz M, De Maeseneer MGR, et al. Predictors of recanalization of the great saphenous vein in randomized controlled trials 1 year after endovenous thermal ablation. Eur J Vasc Endovasc Surg. 2016;52(2):234–241. doi: 10.1016/j.ejvs.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Nayman A, Yildiz I, Koca N, Deniz S, Koplay M, Oguzkurt L. Risk factors associated with recanalization of incompetent saphenous veins treated with radiofrequency ablation catheter. Diagn Interv Imaging. 2017;98(1):29–36. doi: 10.1016/j.diii.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Hach W. Phlebographie Der Bein- Und Beckenvenen. Konstanz: Schnetztor; 1985. [Google Scholar]
- 61.Weber J. Phlebographie. Bonn: Rabe; 2010. [Google Scholar]
- 62.Gerlach H, Partsch H, Rabe E, Gallenkemper G, Jünger M. Leitlinie zur venösen Diagnostik mit der Phlebodynamometrie. Phlebologie. 1999;28:66–67. [Google Scholar]
- 63.Bergan J. Ambulatory surgery of varicose veins. In: Goldmann M, Bergan J, editors. Ambulatory treatment of venous disease: an illustrative guide. St. Louis: Mosby; 1996. pp. 149–154. [Google Scholar]
- 64.Simonian S. Recurrence following combined surgery and postoperative sclerotherapy of varicose veins. In: Negus D, Jantet G, Coleridge-Smith P, editors. Phlebology ’95. Berlin: Springer; 1995. pp. 474–476. [Google Scholar]
- 65.Flour M, Clark M, Partsch H, et al. Dogmas and controversies in compression therapy: report of an International Compression Club (ICC) meeting, Brussels, May 2011. Int Wound J. 2013;10(5):516–526. doi: 10.1111/j.1742-481X.2012.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams A. A review of the evidence for adjustable compression wrap devices. J Wound Care. 2016;25(5):242–247. doi: 10.12968/jowc.2016.25.5.242. [DOI] [PubMed] [Google Scholar]
- 67.Damstra RJ, Partsch H. Prospective, randomized, controlled trial comparing the effectiveness of adjustable compression Velcro wraps versus inelastic multicomponent compression bandages in the initial treatment of leg lymphedema. J Vasc Surg. 2013;1(1):13–19. doi: 10.1016/j.jvsv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Rabe E, Partsch H, Hafner J, et al. Indications for medical compression stockings in venous and lymphatic disorders: an evidence-based consensus statement. Phlebology. 2018;33(3):163–184. doi: 10.1177/0268355516689631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD000265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vayssairat M, Ziani E, Houot B. Placebo controlled efficacy of class 1 elastic stockings in chronic venous insufficiency of the lower limbs. J Mal Vasc. 2000;25(4):256–262. [PubMed] [Google Scholar]
- 71.Benigni JP, Sadoun S, Allaert FA, Vin F. Efficacy of Class 1 elastic compression stockings in the early stages of chronic venous disease. A comparative study. Int Angiol. 2003;22(4):383–392. [PubMed] [Google Scholar]
- 72.Blättler W, Thomae H-J, Amsler F. Venous leg symptoms in healthy subjects assessed during prolonged standing. J Vasc Surg. 2016;4(4):455–462. doi: 10.1016/j.jvsv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Özdemir ÖC, Sevim S, Duygu E, Tuğral A, Bakar Y. The effects of short-term use of compression stockings on health related quality of life in patients with chronic venous insufficiency. J Phys Ther Sci. 2016;28(7):1988–1992. doi: 10.1589/jpts.28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diehm C, Trampisch HJ, Lange S, Schmidt C. Comparison of leg compression stocking and oral horse-chestnut seed extract therapy in patients with chronic venous insufficiency. Lancet. 1996;347(8997):292–294. doi: 10.1016/S0140-6736(96)90467-5. [DOI] [PubMed] [Google Scholar]
- 75.Partsch H. Intermittent pneumatic compression in immobile patients. Int Wound J. 2008;5(3):389–397. doi: 10.1111/j.1742-481X.2008.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vandongen YK, Stacey MC. Graduated compression elastic stockings reduce lipodermatosclerosis and ulcer recurrence. Phlebology. 2000;15(1):33–37. doi: 10.1007/s005230070035. [DOI] [Google Scholar]
- 77.Mosti G, Mattaliano V, Partsch H. Inelastic compression increases venous ejection fraction more than elastic bandages in patients with superficial venous reflux. Phlebol J Venous Dis. 2008;23(6):287–294. doi: 10.1258/phleb.2008.008009. [DOI] [PubMed] [Google Scholar]
- 78.Clarke-Moloney M, Keane N, O’Connor V, et al. Randomised controlled trial comparing European standard class 1 to class 2 compression stockings for ulcer recurrence and patient compliance. Int Wound J. 2014;11(4):404–408. doi: 10.1111/j.1742-481X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ladwig A, Haase H, Bichel J, Schuren J, Jünger M. Compression therapy of leg ulcers with PAOD. Phlebology. 2014;29(1 suppl):7–12. doi: 10.1177/0268355514529507. [DOI] [PubMed] [Google Scholar]
- 80.Nelson EA, Cullum N, Jones J (2004) Venous leg ulcers. Clin Evid (12):2774–2792 (http://www.ncbi.nlm.nih.gov/pubmed/15865821) [PubMed]
- 81.Nelson EA, Bell-Syer SE, Cullum NA, Webster J. Compression for preventing recurrence of venous ulcers. Nelson EA, ed. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD002303. [DOI] [PubMed] [Google Scholar]
- 82.Amsler F, Willenberg T, Blättler W. In search of optimal compression therapy for venous leg ulcers: a meta-analysis of studies comparing divers bandages with specifically designed stockings. J Vasc Surg. 2009;50(3):668–674. doi: 10.1016/j.jvs.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 83.Jünger M, Wollina U, Kohnen R, Rabe E. Efficacy and tolerability of an ulcer compression stocking for therapy of chronic venous ulcer compared with a below-knee compression bandage: results from a prospective, randomized, multicentre trial. Curr Med Res Opin. 2004;20(10):1613–1623. doi: 10.1185/030079904X4086. [DOI] [PubMed] [Google Scholar]
- 84.Ashby RL, Gabe R, Ali S, et al. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): A randomised controlled trial. Lancet. 2014;383(9920):871–879. doi: 10.1016/S0140-6736(13)62368-5. [DOI] [PubMed] [Google Scholar]
- 85.Konschake W, Riebe H, Pediaditi P, Haase H, Jünger M, Lutze S. Compression in the treatment of chronic venous insufficiency: Efficacy depending on the length of the stocking. Jung F, Gori T, eds. Clin Hemorheol Microcirc. 2016;64(3):425–434. doi: 10.3233/CH-168122. [DOI] [PubMed] [Google Scholar]
- 86.Kern P, Ramelet AA, Wütschert R, Hayoz D. Compression after sclerotherapy for telangiectasias and reticular leg veins: a randomized controlled study. J Vasc Surg. 2007;45(6):1212–1216. doi: 10.1016/j.jvs.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 87.Nootheti PK, Cadag KM, Magpantay A, Goldman MP. Efficacy of graduated compression stockings for an additional 3 weeks after sclerotherapy treatment of reticular and telangiectatic leg veins. Dermatol Surg. 2009;35(1):53–57. doi: 10.1111/j.1524-4725.2008.34382.x. [DOI] [PubMed] [Google Scholar]
- 88.Weiss RA, Munavalli G. Endovenous ablation of truncal veins. Semin Cutan Med Surg. 2005;24(4):193–199. doi: 10.1016/j.sder.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Hamel-Desnos CM, Guias BJ, Desnos PR, Mesgard A. Foam sclerotherapy of the saphenous veins: randomised controlled trial with or without compression. Eur J Vasc Endovasc Surg. 2010;39(4):500–507. doi: 10.1016/j.ejvs.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 90.Uhl J-FF, Benigni J-PP, Chahim M, Fréderic D. Prospective randomized controlled study of patient compliance in using a compression stocking: Importance of recommendations of the practitioner as a factor for better compliance. Phlebology. 2018;33(1):36–43. doi: 10.1177/0268355516682886. [DOI] [PubMed] [Google Scholar]
- 91.Huang T-W, Chen S-L, Bai C-H, Wu C-H, Tam K-W. The optimal duration of compression therapy following varicose vein surgery: a meta-analysis of randomized controlled trials. Eur J Vasc Endovasc Surg. 2013;45(4):397–402. doi: 10.1016/j.ejvs.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 92.Benigni JP, Allaert FA, Desoutter P, Cohen-Solal G, Stalnikiewicz X. The efficiency of pain control using a thigh pad under the elastic stocking in patients following venous stripping: results of a case-control study. Perspect Vasc Surg Endovasc Ther. 2011;23(4):238–243. doi: 10.1177/1531003511431737. [DOI] [PubMed] [Google Scholar]
- 93.Lugli M, Cogo A, Guerzoni S, Petti A, Maleti O. Effects of eccentric compression by a crossed-tape technique after endovenous laser ablation of the great saphenous vein: a randomized study. Phlebology. 2009;24(4):151–156. doi: 10.1258/phleb.2008.008045. [DOI] [PubMed] [Google Scholar]
- 94.Reich-Schupke S, Feldhaus F, Altmeyer P, Mumme A, Stücker M. Efficacy and comfort of medical compression stockings with low and moderate pressure six weeks after vein surgery. Phlebology. 2014;29(6):358–366. doi: 10.1177/0268355513484142. [DOI] [PubMed] [Google Scholar]
- 95.Mariani F, Marone EM, Gasbarro V, et al. Multicenter randomized trial comparing compression with elastic stocking versus bandage after surgery for varicose veins. J Vasc Surg. 2011;53(1):115–122. doi: 10.1016/j.jvs.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 96.Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5):2S–48S. doi: 10.1016/j.jvs.2011.01.079. [DOI] [PubMed] [Google Scholar]
- 97.El-Sheikha J, Nandhra S, Carradice D, et al. Compression regimes after endovenous ablation for superficial venous insufficiency—A survey of members of the Vascular Society of Great Britain and Ireland. Phlebology. 2016;31(1):16–22. doi: 10.1177/0268355514567732. [DOI] [PubMed] [Google Scholar]
- 98.Ayo D, Blumberg SN, Rockman CR, et al. Compression versus no compression after endovenous ablation of the great saphenous vein: a randomized controlled trial. Ann Vasc Surg. 2017;38:72–77. doi: 10.1016/j.avsg.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 99.Bakker NA, Schieven LW, Bruins RMGG, van den Berg M, Hissink RJ. Compression stockings after endovenous laser ablation of the great saphenous vein: a prospective randomized controlled trial. Eur J Vasc Endovasc Surg. 2013;46(5):588–592. doi: 10.1016/j.ejvs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 100.Houtermans-Auckel JP, van Rossum E, Teijink JAW, et al. To wear or not to wear compression stockings after varicose vein stripping: a randomised controlled trial. Eur J Vasc Endovasc Surg. 2009;38(3):387–391. doi: 10.1016/j.ejvs.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 101.Raraty M, Greaney M, Blair S. There is no benefit from 6 week’s postoperative compression after varicose vein surgery: a prospective randomised trial. Phlebology. 1999;14:21–25. [Google Scholar]
- 102.Rodrigus I, Bleyn J. For how long do we have to advise elastic support after varicose vein surgery? A prospective randomized study. Phlebology. 1991;6(2):95–98. doi: 10.1177/026835559100600207. [DOI] [Google Scholar]
- 103.Stücker M, Debus ES, Hoffmann J, et al. Consensus statement on the symptom-based treatment of chronic venous diseases. JDDG. 2016;14(6):575–583. doi: 10.1111/ddg.13006. [DOI] [PubMed] [Google Scholar]
- 104.Blättler W, Zimmet SE. Compression therapy in venous disease. Phlebology. 2008;23(5):203–205. doi: 10.1258/phleb.2008.081004. [DOI] [PubMed] [Google Scholar]
- 105.Blazek C, Amsler F, Blaettler W, Keo HH, Baumgartner I, Willenberg T. Compression hosiery for occupational leg symptoms and leg volume: a randomized crossover trial in a cohort of hairdressers. Phlebology. 2013;28(5):239–247. doi: 10.1258/phleb.2011.011108. [DOI] [PubMed] [Google Scholar]
- 106.Mosti G, Partsch H. Is low compression pressure able to improve venous pumping function in patients with venous insufficiency? Phlebology. 2010;25(3):145–150. doi: 10.1258/phleb.2009.009023. [DOI] [PubMed] [Google Scholar]
- 107.Reich-Schupke S, Murmann F, Altmeyer P, Stücker M. Compression therapy in elderly and overweight patients. Vasa. 2012;41(2):125–131. doi: 10.1024/0301-1526/a000175. [DOI] [PubMed] [Google Scholar]
- 108.Sippel K, Seifert B, Hafner J. Donning devices (foot slips and frames) enable elderly people with severe chronic venous insufficiency to put on compression stockings. Eur J Vasc Endovasc Surg. 2015;49(2):221–229. doi: 10.1016/j.ejvs.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 109.Weller CD, Buchbinder R, Johnston RV. Interventions for helping people adhere to compression treatments for venous leg ulceration. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD008378.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valesky EM, Kaufmann R, Meissner M. Contact allergy to compression stockings: Is this possible? Phlebologie. 2014;43(03):140–143. doi: 10.12687/phleb2190-3-2014. [DOI] [Google Scholar]
- 111.Lazarov A. European Standard Series patch test results from a contact dermatitis clinic in Israel during the 7-year period from 1998 to 2004. Contact Derm. 2006;55(2):73–76. doi: 10.1111/j.0105-1873.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 112.Unkauf M, Rehn D, Klinger J, de la Motte S, Grossmann K. Investigation of the efficacy of oxerutins compared to placebo in patients with chronic venous insufficiency treated with compression stockings. Arzneimittelforschung. 1996;46(5):478–482. [PubMed] [Google Scholar]
- 113.Anderson JH, Geraghty JG, Wilson YT, Murray GD, Mcardle CS, Anderson JR. Paroven and graduated compression hosiery for superficial venous insufficiency. Phlebology. 1990;5(4):271–276. doi: 10.1177/026835559000500408. [DOI] [Google Scholar]
- 114.Petruzzellis V, Troccoli T, Candiani C, et al. Oxerutins (Venoruton®): efficacy in chronic venous insufficiency—a double-blind, randomized, controlled study. Angiology. 2002;53(3):257–263. doi: 10.1177/000331970205300302. [DOI] [PubMed] [Google Scholar]
- 115.Ramelet AA, Boisseau MR, Allegra C, et al. Veno-active drugs in the management of chronic venous disease. An international consensus statement: current medical position, prospective views and final resolution. Clin Hemorheol Microcirc. 2005;33(4):309–319. [PubMed] [Google Scholar]
- 116.Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011;41(1):117–125. doi: 10.1016/j.ejvs.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 117.Cloarec M Study on the effect of a new vasoprotective Venostasin administered over a period of 2 months in chronic venous insufficiency of the lower limb (data from 1992). Unpublished data
- 118.Großmann K. Vergleich der Wirksamkeit einer kombinierten Therapie mit Kompressionsstrümpfen und Oxerutin versus Kompressionsstrümpfe und Placebo bei Patienten mit CVI. Phlebologie. 1997;26:105–110. [Google Scholar]
- 119.Vanscheidt W, Heidrich H, Jünger M, et al. Guidelines for testing drugs for chronic venous insufficiency. Vasa. 2000;29(4):274–278. doi: 10.1024/0301-1526.29.4.274. [DOI] [PubMed] [Google Scholar]
- 120.Kiesewetter H, Koscielny J, Kalus U, et al. Efficacy of orally administered extract of red vine leaf AS 195 (folia vitis viniferae) in chronic venous insufficiency (stages I–II). A randomized, double-blind, placebo-controlled trial. Arzneimittelforschung. 2000;50(2):109–117. doi: 10.1055/s-0031-1300174. [DOI] [PubMed] [Google Scholar]
- 121.Rabe E, Stücker M, Esperester A, Schäfer E, Ottillinger B. Efficacy and tolerability of a red-vine-leaf extract in patients suffering from chronic venous insufficiency—Results of a double-blind placebo-controlled study. Eur J Vasc Endovasc Surg. 2011;41(4):540–547. doi: 10.1016/j.ejvs.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 122.Diebschlag W, Nocker W, Lehamacher W. A clinical comparison of two doses of O-(P-Hydroxyethyl)-rutosides (oxerutins) in patients with chronic venous insufficiency. j Pharm Med. 1994;4:7–14. [Google Scholar]
- 123.Kalus U, Koscielny J, Grigorov A, Schaefer E, Peil H, Kiesewetter H. Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by orally administered extract of red vine leaves AS 195: A randomised, double-blind, placebo-controlled, crossover study. Drugs R D. 2004;5(2):63–71. doi: 10.2165/00126839-200405020-00001. [DOI] [PubMed] [Google Scholar]
- 124.Neiss A, Böhm C. Demonstration of the effectiveness of the horse-chestnut-seed extract in the varicose syndrome complex. MMW Munch Med Wochenschr. 1976;118(7):213–216. [PubMed] [Google Scholar]
- 125.Cloarec M, Clément R, Griton P. A double-blind clinical trial of hydroxyethylrutosides in the treatment of the symptoms and signs of chronic venous insufficiency. Phlebology. 1996;11(2):76–82. doi: 10.1177/026835559601100210. [DOI] [Google Scholar]
- 126.Martinez-Zapata MJ, Vernooij RW, Uriona Tuma SM, et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD003229.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brunner F, Hoffmann C, Schuller-Petrovic S. Responsiveness of human varicose saphenous veins to vasoactive agents. Br J Clin Pharmacol. 2001;51(3):219–224. doi: 10.1046/j.1365-2125.2001.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Allegra C. Chronic venous insufficiency: the effects of health-care reforms on the cost of treatment and hospitalisation—an Italian perspective. Curr Med Res Opin. 2003;19(8):761–769. doi: 10.1185/030079903125002559. [DOI] [PubMed] [Google Scholar]
- 129.Schacht V, Luedemann W, Abels C, Von Rautenfeld DB. Anatomy of the subcutaneous lymph vascular network of the human leg in relation to the great saphenous vein. Anat Rec. 2009;292(1):87–93. doi: 10.1002/ar.20765. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka H, Yamamoto N, Suzuki M, et al. Insufficient lymph drainage causes abnormal lipid accumulation and vein wall degeneration. Ann Vasc Dis. 2016;9(4):277–284. doi: 10.3400/avd.oa.16-00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bruning G, Teichler A, Standl T, Diederich A, Moll I. Prilocaine pharmacokinetics and the influence of vitamin C on methaemoglobin concentrations in tumescent anaesthesia. Phlebologie. 2007;36(03):145–150. doi: 10.1055/s-0037-1622178. [DOI] [Google Scholar]
- 132.Bruning G, Rasmussen H, Teichler A, Standl T, Moll I. Pharmakokinetik von Articain in der Tumeszenz lokalanästhesie. Phlebologie. 2010;39(04):218–225. doi: 10.1055/s-0037-1622315. [DOI] [Google Scholar]
- 133.Sagoo KS, Inoue K, Winker W, Salfeld K. Pharmakokinetische Untersuchungen bei der Tumeszenz-Lokalanästhesie mit Prilocain in der Varizenchirurgie. Phlebologie. 2000;29(06):154–162. doi: 10.1055/s-0037-1617342. [DOI] [Google Scholar]
- 134.Schöpf E, Augustin M, Sommer B, Sattler G. Tumeszenz-Lokalanästhesie: Ein neues Verfahren der Lokalanästhesie. Dtsch Arztebl. 2001;98:A545–A548. [Google Scholar]
- 135.Klein JA, Jeske DR. Estimated maximal safe dosages of tumescent Lidocaine. Anesth Analg. 2016;122(5):1350–1359. doi: 10.1213/ANE.0000000000001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Freitag B (2011) Repetitorium Anästhesiologie, Schmerztherapie und Notfallmedizin in Berlin vom 19–26. Februar 2011. Deutsche Akademie für anästhesiologische Fortbildung
- 137.Frings N, Glowacki P, Greiner A, Rass K. Off-label use of tumescent local anaesthesia in endovascular and surgical techniques. Phlebologie. 2016;45(04):234–238. doi: 10.12687/phleb2316-4-2016. [DOI] [Google Scholar]
- 138.Tumeszenz-Lokalanästhesie. Stellungnahme des wissenschaftlichen Arbeitskreises Regionalanästhesie der DGAI. Anästh Intensivmed. 2000;41:114–115.
- 139.Hermanns H-J. Varizenchirurgie und Adipositas per magna – Erfahrungen mit der neuen Boazul-Manschette bis 90 cm Beinumfang. zentralbl Chir. 2008;133(04):363–366. doi: 10.1055/s-2008-1076861. [DOI] [PubMed] [Google Scholar]
- 140.Hach W. Wie es zur prätibialen Fasziotomie kam. Phlebologie. 2004;33:110–114. doi: 10.1055/s-0037-1617282. [DOI] [Google Scholar]
- 141.Langer C, Fuhrmann J, Grimm H, Vorpahl U. Orthostatische Kompartmentdruckmessung nach endoskopischer Fasziotomie. Phlebologie. 1995;24:163–167. [Google Scholar]
- 142.Pflug J. Operative Behandlung des supramalleolären medialen Konstriktionssyndroms bei nicht oder schlecht heilenden Ulcera cruris venosa. Phlebologie. 1995;24:36–43. [Google Scholar]
- 143.Schmeller W, Roszinski S. Shave therapy for surgical treatment of persistent venous ulcer with large superficial dermatoliposclerosis. Hautarzt. 1996;47(9):676–681. doi: 10.1007/s001050050488. [DOI] [PubMed] [Google Scholar]
- 144.Schwahn-Schreiber C, Schmeller W, Gaber Y. Langzeitergebnisse (7 Jahre) nach Shave-Therapie bzw. kruraler Fasziektomie bei persistierenden venösen Ulcera. Phlebologie. 2006;35:89–91. doi: 10.1055/s-0037-1622134. [DOI] [Google Scholar]
- 145.Blättler W, Frick E. Complications of superficial thrombophlebitis. Schweiz Med Wochenschr. 1993;123(6):223–228. [PubMed] [Google Scholar]
- 146.Blättler W, Bulling B, Hertel T, Rabe E. Leitlinien zur Diagnostik und Therapie der Thrombophlebitis superficialis. Phlebologie. 1998;27(02):58–59. doi: 10.1055/s-0037-1617220. [DOI] [Google Scholar]
- 147.Hach W, Hach-Wunderle V. Chirurgische und konservative Behandlung einer transfaszial progredierenden Varikophlebitis der Stammvenen und der Perforansvenen. Gefässchirurgie. 1996;1:172–176. [Google Scholar]
- 148.Jorgensen JO, Hanel KC, Morgan AM, Hunt JM. The incidence of deep venous thrombosis in patients with superficial thrombophlebitis of the lower limbs. J Vasc Surg. 1993;18(1):70–73. doi: 10.1067/mva.1993.42072. [DOI] [PubMed] [Google Scholar]
- 149.Marshall M, Schwahn-Schreiber C. Die oberflächliche Thrombophlebitits, ein Nicht der Rede wert Krankheitsbild. Phlebologie. 2008;37:122–129. doi: 10.1055/s-0037-1622221. [DOI] [Google Scholar]
- 150.Nüllen H. Ist die Krampfadererkrankung ein Risikofaktor für das Auftreten einer tiefen Beinvenenthrombose und ergibt sich daraus ggf. eine Indikation zur prophylaktischen Operation einer Krampfadererkrankung? Gefässchirurgie. 1996;1:118–119. [Google Scholar]
- 151.Salzmann G. Peripheral venous diseases: the surgical approach. In: Lancer P, Topol E, editors. Panvascular Medicine. Berlin: Springer; 2002. pp. 1526–1538. [Google Scholar]
- 152.McCarthy W, Dann CR, Pearce W, Yao J. Management of sudden profuse bleeding from varicose veins. Surgery. 1993;113:178–183. [PubMed] [Google Scholar]
- 153.Noppeney T, Nüllen H. Die Rezidivvarikose – Was ist das? Gefäßchirurgie. 2005;10:424–427. doi: 10.1007/s00772-005-0425-0. [DOI] [Google Scholar]
- 154.Raju S, Easterwood L, Fountain T, Fredericks RK, Neglen PN, Devidas M. Saphenectomy in the presence of chronic venous obstruction. Surgery. 1998;123(6):637–644. doi: 10.1016/S0039-6060(98)70212-0. [DOI] [PubMed] [Google Scholar]
- 155.May R. Primäre Varikosis. In: Heberer G, van Dongen R, editors. Gefäßchirurgie: Kirschnersche Allgemeine Und Spezielle Operationslehre. Berlin: Springer; 1993. [Google Scholar]
- 156.Tibbs D. Varicose veins and related disorders. Oxford: Butterworth-Heinemann; 1992. [Google Scholar]
- 157.Noppeney T, Nüllen H, editors. Varikose – Diagnostik, Therapie Und Begutachtung. Heidelberg: Springer; 2010. [Google Scholar]
- 158.Hach W, Mumme A, Hach-Wunderle V. VenenChirurgie. Operative, interventionelle und konservative Aspekte. Stuttgart: Schattauer; 2012. [Google Scholar]
- 159.Bergan J. Surgical management of primary and recurrent varicose veins. In: Gloviczki P, Yao J, editors. Handbook of venous disorders. 2. London: Arnold; 2001. pp. 289–302. [Google Scholar]
- 160.Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: Five-year results of a randomized trial. J Vasc Surg. 1999;29(4):589–592. doi: 10.1016/S0741-5214(99)70302-2. [DOI] [PubMed] [Google Scholar]
- 161.Hach W, Hach-Wunderle V. Evolution of theoretical concepts in surgery of trunk varicosis from the nineteenth century to the present. Gefässchirurgie. 2001;6(2):111–118. doi: 10.1007/s007720100132. [DOI] [Google Scholar]
- 162.Recek C, Karisch E, Gruber J. Veränderungen der Perforansvenen und tiefen Unterschenkelvenen nach Beseitigung des Saphena-Refluxes. Phlebologie. 2000;29:37–40. doi: 10.1055/s-0037-1617324. [DOI] [Google Scholar]
- 163.Wigger P. Die chirurgische Therapie der primären Varikose. Schweiz Med Wochenschr. 1998;128:1781–1788. [PubMed] [Google Scholar]
- 164.Brunner U. Einige operationsbezogene Stichworte rund um die Beinvarikose. Vasomed. 1995;7:463–468. [Google Scholar]
- 165.Hach W. Die Erhaltung eines transplantationswürdigen Venensegmentes bei der partiellen Saphenaresektion als Operationsmethode der Stammvarikose. Phlebol Proktol. 1981;10:171–173. [Google Scholar]
- 166.Hach W, Hach-Wunderle V. Das Stripping und die Konkurrenzverfahren zur chirurgischen Behandlung der Stammvarikose. Gefässchirurgie. 2000;5(1):56–61. doi: 10.1007/PL00010580. [DOI] [Google Scholar]
- 167.Kaspar S, Zbynek V. Long-term results of the selective varicose vein surgery. In: Raymond-Martimbeau P, Prescott R, Zummo M, editors. Phlebology ’92. Paris: John Libbey Eurotext; 1992. pp. 156–158. [Google Scholar]
- 168.Biedermann H, Fraedrich G. Endoskopische und minimal invasive Varizenchirurgie. Acta Chir Austriaca. 2000;32:19–21. doi: 10.1007/BF02950348. [DOI] [Google Scholar]
- 169.Fischer R. Die operative Behandlung der primären Varikose. In: Marshall M, Breu F, editors. Handbuch Der Angiologie. Arterien‑, Venen- Und Lymphgefäßerkrankungen in Klinik Und Praxis. Landsberg: Ecomed; 1999. [Google Scholar]
- 170.Fratila A, Rabe E. The differentiated surgical treatment of primary varicosis. Semin Dermatol. 1993;12(2):102–116. [PubMed] [Google Scholar]
- 171.Hobbs J. Operations for varicose veins. In: De Weese J, editor. Rob and Smith’s operative surgery. London: Butterworth; 1983. [Google Scholar]
- 172.Loeprecht H. Varicose veins-surgical therapy. Chirurg. 1997;68(10):1048–1052. doi: 10.1007/s001040050321. [DOI] [PubMed] [Google Scholar]
- 173.Frings N, Glowacki P, Nelle A, Van-Thanh-Phuong T. Prospektive Studie zur Verhinderung der Neoangiogenese nach Magna-Crossektomie* – Erste Ergebnisse. Zentralbl Chir. 2001;126(7):528–530. doi: 10.1055/s-2001-16271. [DOI] [PubMed] [Google Scholar]
- 174.Gauw SA, Lawson JA, Van Vlijmen-Van Keulen CJ, et al. Five-year follow-up of a randomized, controlled trial comparing saphenofemoral ligation and stripping of the great saphenous vein with endovenous laser ablation (980 nm) using local tumescent anesthesia. J Vasc Surg. 2016;63(2):420–428. doi: 10.1016/j.jvs.2015.08.084. [DOI] [PubMed] [Google Scholar]
- 175.Hobbs J. Can we prevent recurrence of varicose veins? In: Grennhalgh R, Jamieson C, Nicolaides A, editors. Vascular surgery. Issues in current practice. London: Grune Stratton; 1986. pp. 355–375. [Google Scholar]
- 176.Jiang P, Van Rij AM, Christie R, Hill G, Solomon C, Thomson I. Recurrent varicose veins: patterns of reflux and clinical severity. Vascular. 1999;7(3):332–339. doi: 10.1177/096721099900700311. [DOI] [PubMed] [Google Scholar]
- 177.Kluess H, Rabe E, Gallenkemper G, Mulkens P, Kreysel H. The anatomy of the saphenofemoral junction—A vascular network with therapeutic implications. In: Negus D, Jantet G, Coleridge-Smith P, editors. Phlebology 95, Suppl. 1. Berlin: Springer; 1995. pp. 45–47. [Google Scholar]
- 178.Kluess H, Mulkens P, Rabe E, Kreysel H. Die Magna-Krossektomie: Qualitätsanspruch an Planung und Durchführung. In: Dummer R, Panizzon R, Burg G, editors. Operative Und Konservative Dermatoonkologie Im Interdisziplinären Grenzbereich. Fortschritte Der Operativen Und Onkologischen Dermatologie. Berlin: Blackwell; 1996. pp. 272–274. [Google Scholar]
- 179.Lefebvre-Vilardebo M. The sapheno-femoral area: anatomic study and concepts for the prevention of varicose recurrences. J Mal Vasc. 1991;16(4):355–358. [PubMed] [Google Scholar]
- 180.Lemasle P, Uhl J, Lefevbre-Vilardebo M, Baud J, Gillot C. Veines lympho-ganglionnaires inguinales – Aspectes anatomiques et échographiques – conséquences sur la définition de la néogenèse – conséquences thérapeutiques. Phlebologie. 1999;52:263–269. [Google Scholar]
- 181.Leu H, Brunner U. Überbrückung der unterbrochenen Vena saphena magna durch multiple, kleinkalibrige, neugebildete Venen. In: Brunner U, editor. Die Leiste. Diagnostische und therapeutische Aspekte der Arteriologie, Phlebologie und Lymphologie. Bern: Huber; 1979. pp. 167–171. [Google Scholar]
- 182.Lofgren EP, Lofgren KA. Recurrence of varicose veins after the stripping operation. arch Surg. 1971;102(2):111–114. doi: 10.1001/archsurg.1971.01350020021006. [DOI] [PubMed] [Google Scholar]
- 183.Agrifoglio G, et al. Results of surgical treatment of varicose veins: one- to fourteen-year follow-up study of 416 patients. JAMA. 1961;178(9):906–911. doi: 10.1001/jama.1961.03040480036007. [DOI] [PubMed] [Google Scholar]
- 184.Papastopolou G, Altenkämper H, Bernheim C, et al. Die LaVaCro-Studie: Langzeitergebnisse der Varizenoperation mit Crossektomie und Stripping der V. saphena magna. Phlebologie. 2013;42(5):253–260. doi: 10.12687/phleb2139-5-2013. [DOI] [Google Scholar]
- 185.Rasmussen L, Lawaetz M, Bjoern L, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years. J Vasc Surg. 2013;58(2):421–426. doi: 10.1016/j.jvs.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 186.Rass K, Frings N, Glowacki P, Gräber S, Tilgen W, Vogt T. Same site recurrence is more frequent after endovenous laser ablation compared with high ligation and stripping of the great saphenous vein: 5 year results of a randomized clinical trial (RELACS Study) Eur J Vasc Endovasc Surg. 2015;50(5):648–656. doi: 10.1016/j.ejvs.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 187.Rivlin S. The surgical cure of primary varicose veins. br J Surg. 1975;62(11):913–917. doi: 10.1002/bjs.1800621114. [DOI] [PubMed] [Google Scholar]
- 188.Van Der Velden SK, Biemans AAM, De Maeseneer MGR, et al. Five-year results of a randomized clinical trial of conventional surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy in patients with great saphenous varicose veins. br J Surg. 2015;102(10):1184–1194. doi: 10.1002/bjs.9867. [DOI] [PubMed] [Google Scholar]
- 189.Brunner U, Pouliadis G, Thürlemann A. Zur Vermeidung von Rezidivvarikosen ab der Leiste. In: Brunner U, editor. Die Leiste. Diagnostische und therapeutische Aspekte der Arteriologie, Phlebologie und Lymphologie. Aktuelle Probleme in der Angiologie. Bern: Huber; 1979. pp. 172–181. [Google Scholar]
- 190.Brunner U. Suprainguinaler Zugang zur Krossektomie. In: Brunner U, editor. Die Leiste. Diagnostische und therapeutische Aspekte der Arteriologie, Phlebologie und Lymphologie. Aktuelle Probleme in der Angiologie. Bern: Huber; 1979. pp. 142–147. [Google Scholar]
- 191.Chandler JG, Pichot O, Sessa C, Schuller-Petrović S, Osse FJ, Bergan JJ. Defining the role of extended saphenofemoral junction ligation: A prospective comparative study. J Vasc Surg. 2000;32(5):941–953. doi: 10.1067/mva.2000.110348. [DOI] [PubMed] [Google Scholar]
- 192.Couffinhal J, Bouchereau R. Angionenèse et insuffisance veineuse superficielle „la maladie induite“. Phlebologie. 1990;43:609–613. [PubMed] [Google Scholar]
- 193.De Maeseneer MG, Ongena KP, Van den Brande F, Van Schil PE, De Hert SG, Eyskens EJ. Duplex ultrasound assessment of neovascularization after saphenofemoral or sapheno-popliteal junction ligation. Phlebology. 1997;12(2):64–68. doi: 10.1177/026835559701200205. [DOI] [Google Scholar]
- 194.Disselhoff BCVM, der Kinderen DJ, Kelder JC, Moll FL. Five-year results of a randomised clinical trial of endovenous laser ablation of the great saphenous vein with and without ligation of the saphenofemoral junction. Eur J Vasc Endovasc Surg. 2011;41(5):685–690. doi: 10.1016/j.ejvs.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 195.Flessenkamper I, Anastasiadou Z, Roll S, Stenger D, Hartmann M, Augustin M. Krankheitsspezifische Lebensqualität im Vergleich dreier operativer Therapien der V.-saphena-magna-Varikose. Gefaesschirurgie. 2014;19(5):451–458. doi: 10.1007/s00772-014-1371-5. [DOI] [Google Scholar]
- 196.Heim D, Negri M, Schlegel U, De Maeseneer M. Resecting the great saphenous stump with endothelial inversion decreases neither neovascularization nor thigh varicosity recurrence. J Vasc Surg. 2008;47(5):1028–1032. doi: 10.1016/j.jvs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 197.Kluess H, Gallenkemper G, Mulkens P, Rabe E. Die modifizierte Crossektomie der Vena saphena magna – farbduplexsonographische Kontrolle nach zweieinhalb Jahren. Vasomed. 1997;9(Suppl):5. [Google Scholar]
- 198.Kluess H, Rabe E, Mulkens P. Welche Bedeutung hat das Nahtmaterial in der Varizenchirurgie? (Ergebnisse einer Umfrage zu 19.000 Stammveneneingriffen) Vasomed. 1998;10(Suppl):24. [Google Scholar]
- 199.Kluess H, Drosner M, Hanauske U, Pettke-Rank C, Schmoeckel I. Strategie zur Vermeidung von Rezidivkrossenvarikosen der V. saphena magna: Erste Ergebnisse nach konsequenter Verwendung eines nicht resorbierbaren Ligarturfadens. Phlebologie. 2003;32:A22. doi: 10.1055/s-0037-1621449. [DOI] [Google Scholar]
- 200.Rafi-Stenger L. Retrospektive Langzeitstudie zur Inzidenz der Neovaskularisations‑/Rezidivrate nach Vena saphena magna Crossektomie mittels Ultraschallmonitoring. Vasa. 2016;45:1–105. [Google Scholar]
- 201.Gillies T, Ruckley C. Surgery for recurrent varicose veins. Curr Pract Surg. 1996;8:22–27. [Google Scholar]
- 202.Nabatoff RA. Reasons for major recurrence following operations for varicose veins. Surg Gynecol Obstet. 1969;128(2):275–278. [PubMed] [Google Scholar]
- 203.Burihan E, Baptista-Silva J. Anatomicall study of the small saphenous vein (saphena parva): types of termination. In: Negus D, Jantet G, Coleridge-Smith P, editors. Phlebology ’95. Berlin Heidelberg: Springer; 1995. pp. 57–60. [Google Scholar]
- 204.Cavezzi A, Tabarini C, Collura M, Sigismondi G, Barboni M, Carigi V. Hemodynamique de la junction sapheno-poplitee: evaluation par echo-doppler couleur. Phlebologie. 2002;55:309–316. [Google Scholar]
- 205.Cavezzi A, Labropoulos N, Partsch H, et al. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part II. anatomy. Eur J Vasc Endovasc Surg. 2006;31(3):288–299. doi: 10.1016/j.ejvs.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 206.Kandel R. Surgical anatomical peculiartities in the treatment of the varicose short saphenous vein. Zentralbl Phlebol. 1967;6(2):313–324. [PubMed] [Google Scholar]
- 207.Ramelet A, Buchheim G, Landry M. Vena Saphena Parva: Klassifikation und therapeutische Strategie. Vasomed. 1992;4:692–695. [Google Scholar]
- 208.Creton D. 125 reinterventions for recurrent popliteal varicose veins after excision of the short saphenous vein. Anatomical and physiological hypotheses of the mechanism of recurrence. J Mal Vasc. 1999;24(1):30–36. [PubMed] [Google Scholar]
- 209.Fischer R. Wo in der Fossa poplitea soll man die Vena saphena parva beim Stripping ligieren? Phlebol Proktol. 1985;14:129–132. [Google Scholar]
- 210.Fischer R. Die Resultate der Strippingoperation bei der Vena saphena parva. Vasa. 1987;16:349–351. [PubMed] [Google Scholar]
- 211.Mildner A, Hilbe G. Parvarezidive nach subfaszialer Ligatur. Phlebologie. 1997;26:35–39. [Google Scholar]
- 212.Mulkens P. Funktionsanalysen nach Krossektomie der V.saphena parva. 1996. [Google Scholar]
- 213.Bassi G. Klinische Bedeutung insuffizienter Vv. Perforantes. In: May R, Partsch H, Staubesand J, editors. Venae Perforantes. München: Urban Schwarzenberg; 1981. pp. 110–113. [Google Scholar]
- 214.Bello M, Scriven M, Hartshorne T, Bell PRF, Naylor AR, London NJM. Role of superficial venous surgery in the treatment of venous ulceration. br J Surg. 1999;86(6):755–759. doi: 10.1046/j.1365-2168.1999.01155.x. [DOI] [PubMed] [Google Scholar]
- 215.Bjordal R. Die Zirkulation in insuffizienten Vv. perforantes des Wade bei venösen Störungen. In: May R, Partsch H, editors. Venae Perforantes. München, Wien, Baltimore: Urban Schwarzenberg; 1981. pp. 71–88. [Google Scholar]
- 216.Bjordal R. Klinischen und therapeutische Konsequnezen der beobachteten hämodynamischen Strömungsmuster. In: May R, Partsch H, editors. Venae Perforantes. Urban Schwarzenberg; 1981. pp. 89–93. [Google Scholar]
- 217.O’Donnell TF. The present status of surgery of the superficial venous system in the management of venous ulcer and the evidence for the role of perforator interruption. J Vasc Surg. 2008;48(4):1044–1052. doi: 10.1016/j.jvs.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 218.Fitridge RA, Dunlop C, Raptis S, Thompson MM, Leppard P, Quigley F. A prospective randomized trial evaluating the haemodynamic role of incompetent calf perforating veins. Aust N Z J Surg. 1999;69(3):214–216. doi: 10.1046/j.1440-1622.1999.01525.x. [DOI] [PubMed] [Google Scholar]
- 219.Hach W, Hach-Wunderle V, Nestle W. Pathology and treatment of insufficiency of Cockett’s perforating veins. Gefässchirurgie. 2000;5(2):130–137. doi: 10.1007/s007720050193. [DOI] [Google Scholar]
- 220.Haeger K. The problem of radicality in surgery for venous incompetence of the leg. Vasa. 1972;1:127–134. [PubMed] [Google Scholar]
- 221.Fischer R, Schwahn-Schreiber C, Sattler G. Conclusions of a consensus conference on subfascial endoscopy of perforating veins in the medial lower leg. Vasc Surg. 1998;32(4):339–346. doi: 10.1177/153857449803200407. [DOI] [Google Scholar]
- 222.Hauer G. Operationstechnik der endoskopischen subfascialen Discision der Perforansvenen. Chirurg. 1987;58:172–175. [PubMed] [Google Scholar]
- 223.Hauer G, Nasralla F, Wisser I, Schneidemann G. Zur endoskopischen Perforansdissektion und Fasziotomie. Gefässchirurgie. 1997;2:222–226. doi: 10.1007/s007720050041. [DOI] [Google Scholar]
- 224.Kianifard B, Holdstock J, Allen C, Smith C, Price B, Whiteley MS. Randomized clinical trial of the effect of adding subfascial endoscopic perforator surgery to standard great saphenous vein stripping. br J Surg. 2007;94(9):1075–1080. doi: 10.1002/bjs.5945. [DOI] [PubMed] [Google Scholar]
- 225.Pierik EG, van Urk H, Hop WC, Wittens CH. Endoscopic versus open subfascial division of incompetent perforating veins in the treatment of venous leg ulceration: a randomized trial. J Vasc Surg. 1997;26(6):1049–1054. doi: 10.1016/S0741-5214(97)70019-3. [DOI] [PubMed] [Google Scholar]
- 226.Vashist MG, Malik V, Singhal N. Role of Subfascial endoscopic perforator surgery (SEPS) in management of perforator incompetence in varicose veins: a prospective randomised study. Indian J Surg. 2014;76(2):117–123. doi: 10.1007/s12262-012-0675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gloviczki P, Bergan JJ, Rhodes JM, et al. Mid-term results of endoscopic perforator vein interruption for chronic venous insufficiency: lessons learned from the North American Subfascial Endoscopic Perforator Surgery registry. J Vasc Surg. 1999;29(3):489–502. doi: 10.1016/S0741-5214(99)70278-8. [DOI] [PubMed] [Google Scholar]
- 228.Babcock W. A new operation for exstirpation of varicose veins of the leg. NY Med J. 1907;86:153e6. [Google Scholar]
- 229.Boersma D, Kornmann VNN, van Eekeren RRJP, et al. Treatment modalities for small Saphenous vein insufficiency. j Endovasc Ther. 2016;23(1):199–211. doi: 10.1177/1526602815616375. [DOI] [PubMed] [Google Scholar]
- 230.Campanello M, Hammarsten J, Forsberg C, Bernland P, Henrikson O, Jensen J. Standard stripping versus long Saphenous vein-saving surgery for primary varicose veins: a prospective, randomized study with the patients as their own controls. Phlebology. 1996;11(2):45–49. doi: 10.1177/026835559601100202. [DOI] [Google Scholar]
- 231.Durkin MT, Turton EPL, Scott DJA, Berridge DC. A prospective randomised trial of PIN versus conventional stripping in varicose vein surgery. Ann R Coll Surg Engl. 1999;81(3):171–174. [PMC free article] [PubMed] [Google Scholar]
- 232.Gawrychowski J, Zbronski R. The results of limited stripping of long saphenous vein connected with the local varicotomy and perforators ligation: comparison with a group after Babcock procedure. In: Raymond-Martimbeau P, Prescott R, Zummo M, editors. Phlebology ’92. Paris: John Libbey Eurotext; 1992. pp. 1141–1142. [Google Scholar]
- 233.Hach W, Hach-Wunderle V. Die Rezirkulationskreise Der Primären Varikose. Berlin, Heidelberg: Springer; 1994. [Google Scholar]
- 234.Schweiger H, Schnell O, Sturm J. Das Schicksal der Restsaphena nach stadiengerechter Varizenoperation. Gefäßchirurgie. 2002;7:13–16. doi: 10.1007/s00772-001-0188-1. [DOI] [Google Scholar]
- 235.Friedell ML, Samson RH, Cohen MJ, et al. High ligation of the greater Saphenous vein for treatment of lower extremity varicosities: the fate of the vein and therapeutic results. Ann Vasc Surg. 1992;6(1):5–8. doi: 10.1007/BF02000659. [DOI] [PubMed] [Google Scholar]
- 236.Rutherford RB, Sawyer JD, Jones DN. The fate of residual saphenous vein after partial removal or ligation. J Vasc Surg. 1990;12(4):422–428. doi: 10.1016/0741-5214(90)90044-B. [DOI] [PubMed] [Google Scholar]
- 237.Holme JB, Skajaa K, Holme K. Incidence of lesions of the saphenous nerve after partial or complete stripping of the long saphenous vein. Acta Chir Scand. 1990;156(2):145–148. [PubMed] [Google Scholar]
- 238.Koyano K, Sakaguchi S. Selective stripping operation based on Doppler ultrasonic findings for primary varicose veins of the lower extremities. Surgery. 1988;103(6):615–619. [PubMed] [Google Scholar]
- 239.Ramasastry SS, Dick GO, Futrell JW. Anatomy of the saphenous nerve: relevance to saphenous vein stripping. Am Surg. 1987;53(5):274–277. [PubMed] [Google Scholar]
- 240.Jaworucka-Kaczorowska A, Oszkinis G, Huber J, Wiertel-Krawczuk A, Gabor E, Kaczorowski P. Saphenous vein stripping surgical technique and frequency of saphenous nerve injury. Phlebology. 2015;30(3):210–216. doi: 10.1177/0268355514539316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Corbett CRR, Harries WJ. Which vein stripper, metal or plastic disposable? Phlebology. 1991;6(3):149–151. doi: 10.1177/026835559100600301. [DOI] [Google Scholar]
- 242.Fischer R. Universal stripper for varicose vein surgery. Chirurg. 1996;67(3):280–282. [PubMed] [Google Scholar]
- 243.Klem TMAL, Schnater JM, Schütte PR, Hop W, van der Ham AC, Wittens CHA. A randomized trial of cryo stripping versus conventional stripping of the great saphenous vein. J Vasc Surg. 2009;49(2):403–409. doi: 10.1016/j.jvs.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 244.Klöpper M. Die Kryovariektomie – Methode und Ergebnisse 1988–1996. Ambul Oper. 1996;4:132–134. [Google Scholar]
- 245.Lacroix H, Nevelsteen A, Suy R. Invaginating versus classic stripping of the long Saphenous vein: a randomized prospective study. Acta Chir Belg. 1999;99(1):22–25. doi: 10.1080/00015458.1999.12098435. [DOI] [PubMed] [Google Scholar]
- 246.Manner K. Geelhaar, Stickel. Kryotechnik in der Varizenchirurgie. Chirurg. 1999;70(1):79–84. doi: 10.1007/s001040050611. [DOI] [PubMed] [Google Scholar]
- 247.Tyrell M, Rocker M, Maisey N, Marshall R, Taylor P, Negus D. A randomised trial to compare standard and invagination stripping of the long saphenous vein in the thigh. In: Negus D, Jantet G, Coleridge-Smith P, editors. Phlebology ’95. Berlin: Springer; 1995. pp. 451–453. [Google Scholar]
- 248.Wilson S, Pryke S, Scott R, Walsh M, Barker SGE. ‘Inversion’ stripping of the long Saphenous vein. Phlebology. 1997;12(3):91–95. doi: 10.1177/026835559701200304. [DOI] [Google Scholar]
- 249.Large J. Surgical treatment of saphenous varices, with preservation of the main great saphenous trunk. J Vasc Surg. 1985;2(6):886–891. doi: 10.1016/0741-5214(85)90139-9. [DOI] [PubMed] [Google Scholar]
- 250.Oesch A. PIN-Stripping. Phlebologie. 1996;25:177–182. [Google Scholar]
- 251.Miller GV, Lewis WG, Sainsbury JRC, Macdonald RC. Morbidity of varicose vein surgery: auditing the benefit of changing clinical practice. Ann R Coll Surg Engl. 1996;78(4):345–349. doi: 10.1016/j.pec.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Papapostolou G, Altenkämper H, Bernheim C, et al. The LaVaCro-Study: Long-term results following saphenofemoral ligation and stripping of the great saphenous vein. First year results. Phlebologie. 2013;42:253–260. doi: 10.12687/phleb2139-5-2013. [DOI] [Google Scholar]
- 253.Denck H, Hugeneck J, Garaguly G. Folgenschwere Fehler bei Varizenoperationen speziell in der Leiste. In: Brunner U, editor. Die Leiste. Diagnostische und therapeutische Aspekte der Arteriologie, Phlebologie und Lymphologie. Aktuelle Probleme in der Angiologie. Bern: Huber; 1979. pp. 148–159. [Google Scholar]
- 254.Denck H. Schwere und leichte Komplikationen bei der Operation einer Stammvarikose der Vena saphena magna. Medwelt. 1989;40:40–42. [Google Scholar]
- 255.Frings N, Van-Thann T, Glowacki P, Subasinghe C. Komplikationen in der Varizenchirurgie und Strategien zu ihrer Vermeidung. Phlebologie. 2002;31(01):26–37. doi: 10.1055/s-0037-1617249. [DOI] [Google Scholar]
- 256.Hafez H, Jarvis S, Harvey A, Corbett R. Get it right first: the results of primary operations for varicose veins are better than for recurrence. In: Negus D, Jantet G, Coleridge-Smith P, editors. Phlebology 95. Berlin Heidelberg: Springer; 1995. pp. 400–403. [Google Scholar]
- 257.Hagmüller G. Komplikationen bei der Chirurgie der Varikose. Langenbecks Arch Chir. 1992;470(Suppl):4. [PubMed] [Google Scholar]
- 258.Helmig L. Häufigkeit von Frühkomplikationen bei 13.024 Krampfaderoperationen. Phlebol Proktol. 1983;12:184–195. [Google Scholar]
- 259.Hofer T. Komplikationen nach varizenchirurgischen eingriffen. Phlebologie. 2001;30(1):26–30. doi: 10.1055/s-0037-1617266. [DOI] [Google Scholar]
- 260.Mildner A, Hilbe G. Komplikationen bei der Varizenchirurgie. zentralbl Chir. 2001;126(7):543–545. doi: 10.1055/s-2001-16268. [DOI] [PubMed] [Google Scholar]
- 261.Lees T, Singh S, Beard J, Spencer P, Rigby C. Prospective audit of surgery for varicose veins. br J Surg. 1997;84(1):44–46. doi: 10.1046/j.1365-2168.1997.02475.x. [DOI] [PubMed] [Google Scholar]
- 262.Robinson J, Macierewicz J, Beard JD. Using the Boazul cuff to reduce blood loss in varicose vein surgery. Eur J Vasc Endovasc Surg. 2000;20(4):390–393. doi: 10.1053/ejvs.2000.1189. [DOI] [PubMed] [Google Scholar]
- 263.Simonetti S, Bianchi S, Martinoli C. Neurophysiological and ultrasound findings in sural nerve lesions following stripping of the small saphenous vein. Muscle Nerve. 1999;22(12):1724–1726. doi: 10.1002/(SICI)1097-4598(199912)22:12<1724::AID-MUS18>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 264.Cox SJ, Wellwood JM, Martin A. Saphenous nerve injury caused by stripping of the long Saphenous vein. BMJ. 1974;1(5905):415–417. doi: 10.1136/bmj.1.5905.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Critchley G, Handa A, Maw A, Harvey A, Harvey MR, Corbett CRR. Complications of varicose vein surgery. Ann R Coll Surg Engl. 1997;79(2):105–110. [PMC free article] [PubMed] [Google Scholar]
- 266.Gorny P, Chahine D, Blanchemaison P, Renaudin J. Crossectomie, eveinage court ou ling ? Revue de la littérature et discussion. Phlebologie. 1996;49:87–91. [Google Scholar]
- 267.Hach W, Hach-Wunderle V. Nervenläsionen in der Chirurgie der primären Varikose. Gefässchirurgie. 2002;7(2):97–102. doi: 10.1007/s00772-002-0200-4. [DOI] [Google Scholar]
- 268.Hellerer O, Brückner W, Aigner R, Kleinschmidt J, Heltzel W. Klinik und morphologisches Korrelat der sensiblen Störungen nach Exhairese der Vena saphena magna. In: Hach W, Salzmann G, editors. Die Chirurgie Der Venen. Ergebnisse Der Angiologie. Stuttgart: Schattauer; 1982. pp. 219–223. [Google Scholar]
- 269.Ellinghaus E, Ellinghaus D, Krusche P, et al. Genome-wide association analysis for chronic venous disease identifies EFEMP1 and KCNH8 as susceptibility loci. Sci Rep. 2017;7:45652. doi: 10.1038/srep45652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Brunner U. Zur Vermeidung von sekundären Lymphödemen. Phlebol Proktol. 1975;4:266–272. [Google Scholar]
- 271.Weissleder H, Schuchhardt C. Iatrogene Schäden des Lymphgefäßsystems. Vasomed. 1993;5:342–348. [Google Scholar]
- 272.Frings N, Glowacki P, Subasinghe C. Major-Komplikationen in der Varizenchirurgie – Lassen sich die existierenden Studien vergleichen? Phlebologie. 2001;30(02):31–35. doi: 10.1055/s-0037-1617267. [DOI] [Google Scholar]
- 273.Helmig L, Stelzer G, Ehresmann U, Salzmann P. Verletzungen der tiefen Venen bei Krampfaderoperationen. Chirurg. 1983;54:118–123. [PubMed] [Google Scholar]
- 274.Largiader J, Blattler W, Gloor B. Combination therapy of venous thrombosis with local thrombolysis and surgical thrombectomy. Kongressbd Dtsch Ges Chir Kongr. 2001;118:479–481. [PubMed] [Google Scholar]
- 275.Natali J, Benhamou AC. Iatrogenic vascular injuries. A review of 125 cases (excluding angiographic injuries) J Cardiovasc Surg. 1979;20(2):169–176. [PubMed] [Google Scholar]
- 276.Mildner A, Hilbe G. Blutungskomplikationen bei der Varizenchirurgie. Abhängigkeit von der perioperativen Thromboseprophylaxe mit NMH sowie der Verwendung einer intraoperativen Blutleere. Phlebologie. 2000;29:163–166. doi: 10.1055/s-0037-1617339. [DOI] [Google Scholar]
- 277.Natali J. Surgical treatment of varices. Enquiry into 87.000 cases. J Cardiovasc Surg. 1964;5:713–721. [PubMed] [Google Scholar]
- 278.Vorpahl U, Stahl J, Langer C. Fasciitis necroticans. Klinischer Verlauf – Diagnostik – Therapie. Phlebologie. 1996;25:69–72. [Google Scholar]
- 279.Horsch S. Surgery of varicose veins. Surgical errors and complications. Langenbecks Arch Chir. 1988;2(Suppl):153–156. [PubMed] [Google Scholar]
- 280.Hauser J, Brunner U. Das sekundäre Lymphödem durch Blockade in der Leistengegend. In: Brunner U, editor. Die Leiste. Diagnostische und therapeutische Aspekte der Arteriologie, Phlebologie und Lymphologie. Bern: Huber; 1979. pp. 216–220. [Google Scholar]
- 281.Verlato F, Zucchetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the thigh. J Vasc Surg. 1999;30(6):1113–1115. doi: 10.1016/S0741-5214(99)70051-0. [DOI] [PubMed] [Google Scholar]
- 282.Balzer B. Venen. In: Carstensen G, editor. Intra- Und Postoperative Komplikationen. Berlin Heidelberg: Springer; 1983. pp. 107–115. [Google Scholar]
- 283.Nüllen H, Reese von Ohlen C. Ambulante Varizenchirurgie in der Praxis. In: Imig H, Schröder A, editors. Varizen, Popliteal-Aneurysmen. Darmstadt: Steinkopf; 1995. pp. 73–79. [Google Scholar]
- 284.Frings N, Wagner B, Brus M. Varicose vein surgery under local anaesthesia. Complications of n = 47057 operations. Phlebology. 1995;10:465–467. doi: 10.1007/978-1-4471-3095-6_197. [DOI] [Google Scholar]
- 285.Faubel R, Schäfer I, Augustin M, Bruning G. Langzeitergebnisse und Analysen von Zusammenhängen 5 Jahre nach Varizenstripping. Phlebologie. 2010;39(5):263–269. doi: 10.1055/s-0037-1622322. [DOI] [Google Scholar]
- 286.Brittenden J, Cotton SC, Elders A, et al. A randomized trial comparing treatments for varicose veins. N Engl J Med. 2014;371(13):1218–1227. doi: 10.1056/NEJMoa1400781. [DOI] [PubMed] [Google Scholar]
- 287.Flessenkamper I, Hartmann M, Hartmann K, Stenger D, Roll S. Endovenous laser ablation with and without high ligation compared to high ligation and stripping for treatment of great saphenous varicose veins: Results of a multicentre randomised controlled trial with up to 6 years follow-up 303 336. Phlebology. 2016;31(1):23–33. doi: 10.1177/0268355514555547. [DOI] [PubMed] [Google Scholar]
- 288.Perala J, Rautio T, Biancari F, et al. Radiofrequency endovenous obliteration versus stripping of the long saphenous vein in the management of primary varicose veins: 3-year outcome of a randomized study. Ann Vasc Surg. 2005;19(5):669–672. doi: 10.1007/s10016-005-6613-2. [DOI] [PubMed] [Google Scholar]
- 289.Pronk P, Gauw SA, Mooij MC, et al. Randomised controlled trial comparing sapheno-femoral ligation and stripping of the great saphenous vein with endovenous laser ablation (980 nm) using local tumescent anaesthesia: one year results89317. Eur J Vasc Endovasc Surg. 2010;40(5):649–656. doi: 10.1016/j.ejvs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 290.Subramonia S, Lees T. Randomized clinical trial of radiofrequency ablation or conventional high ligation and stripping for great saphenous varicose veins. br J Surg. 2010;97(3):328–336. doi: 10.1002/bjs.6867. [DOI] [PubMed] [Google Scholar]
- 291.Hach W, Hach-Wunderle V. Das theoretische Verständnis der „Rezidivvarikose nach Operation“. Gefässchirurgie. 1998;3(1):42–46. doi: 10.1007/PL00010496. [DOI] [Google Scholar]
- 292.Perrin MR, Guex JJ, Ruckley CV, et al. Recurrent varices after surgery (REVAS), a consensus document. REVAS group. Cardiovasc Surg. 2000;8(4):233–245. doi: 10.1016/S0967-2109(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 293.Salzmann G. Rezidivvarikose nach chirurgischer Primärtherapie. In: Hach W, Salzmann G, editors. Die Chirurgie der Venen. Ergebnisse der Angiologie. 1982. pp. 225–227. [Google Scholar]
- 294.Schnyder S, Gabler S, Meier TO, et al. Successful reduction of clinical relevant neovascularization with a modified crossectomy combined with a barrier technique after 10-year follow-up 389. Phlebology. 2012;27(8):404–408. doi: 10.1258/phleb.2011.011065. [DOI] [PubMed] [Google Scholar]
- 295.Winterborn RJ, Foy C, Heather BP, Earnshaw JJ. Randomised trial of flush Saphenofemoral ligation for primary great Saphenous varicose veins. Eur J Vasc Endovasc Surg. 2008;36(4):477–484. doi: 10.1016/j.ejvs.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 296.Freis H, Geier B, Mumme A, Strohmann B, Stücker M, Hummel T. Barrier patch implantation during redo surgery for varicose vein recurrences in the groin: 1-year results. Ann Vasc Surg. 2016;35:98–103. doi: 10.1016/j.avsg.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 297.van Rij AM, Jones GT, Hill BG, et al. Mechanical inhibition of angiogenesis at the saphenofemoral junction in the surgical treatment of varicose veins: early results of a blinded randomized controlled trial. Circulation. 2008;118(1):66–74. doi: 10.1161/CIRCULATIONAHA.107.726869. [DOI] [PubMed] [Google Scholar]
- 298.Winterborn RJ, Earnshaw JJ. Randomised trial of polytetrafluoroethylene patch insertion for recurrent great Saphenous varicose veins. Eur J Vasc Endovasc Surg. 2007;34(3):367–373. doi: 10.1016/j.ejvs.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 299.Lane RJ, Cuzzilla ML, Coroneos JC, Phillips MN, Platt JT. Recurrence rates following external valvular stenting of the Saphenofemoral junction: a comparison with simultaneous contralateral stripping of the great Saphenous vein. Eur J Vasc Endovasc Surg. 2007;34(5):595–603. doi: 10.1016/j.ejvs.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 300.Mumme A, Stücker M, Hummel T. Die extraluminale Valvuloplastie der Vena saphena magna: Reparative Therapie der Stammvarikose. Gefässchirurgie. 2014;19(7):637–642. doi: 10.1007/s00772-014-1353-7. [DOI] [Google Scholar]
- 301.Belcaro G, Agus G, Errichi BM, et al. Gore external valve support for superficial saphenous vein incompetence: a 10-year, follow-up registry. Panminerva Med. 2011;53(3 Suppl):35–42. [PubMed] [Google Scholar]
- 302.Geier B, Voigt I, Marpe B, et al. External valvuloplasty in the treatment of greater saphenous vein insufficiency: a five-year follow up. Phlebology. 2003;18(3):137–142. doi: 10.1258/026835503322381342. [DOI] [Google Scholar]
- 303.Kim ID, Boong LB, Bergan JJ. Venous hemodynamic changes after external banding valvuloplasty with varicosectomy in the treatment of primary varicose veins. J Cardiovasc Surg. 1999;40(4):567–570. [PubMed] [Google Scholar]
- 304.Bellmunt-Montoya S, Escribano JM, Dilme J, Martinez-Zapata MJ. CHIVA method for the treatment of chronic venous insufficiency. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD009648.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Franceschi C, Cappelli M, Ermini S, et al. CHIVA: hemodynamic concept, strategy and results. Int Angiol. 2016;35(1):8–30. [PubMed] [Google Scholar]
- 306.Zamboni P, Gianesini S, Menegatti E, Tacconi G, Palazzo A, Liboni A. Great saphenous varicose vein surgery without saphenofemoral junction disconnection. Br J Surg. 2010;97(6):820–825. doi: 10.1002/bjs.7022. [DOI] [PubMed] [Google Scholar]
- 307.Parés JO, Juan J, Tellez R, et al. Varicose vein surgery: stripping versus the chiva method: a randomized controlled trial. ann Surg. 2010;251(4):624–631. doi: 10.1097/SLA.0b013e3181d0d0a3. [DOI] [PubMed] [Google Scholar]
- 308.Carandina S, Mari C, De Palma M, et al. Varicose vein stripping vs haemodynamic correction (CHIVA): a long term randomised trial. Eur J Vasc Endovasc Surg. 2008;35(2):230–237. doi: 10.1016/j.ejvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 309.Gianesini S, Menegatti E, Malagoni AM, Occhionorelli S, Zamboni P. Mini-invasive high-tie by clip apposition versus crossectomy by ligature: long-term outcomes and review of the available therapeutic options. Phlebology. 2017;32(4):249–255. doi: 10.1177/0268355516648066. [DOI] [PubMed] [Google Scholar]
- 310.Salzmann G, Kirschner P, Hoffmann O, Vanderpuye R. Perioperative Antibiotikaprophylaxe bei der paratibialen Fasziotomie. Phlebologie. 1995;24:44–47. [Google Scholar]
- 311.Fischer R. Erfahrungen mit der Blutleere oder Blutsperre bei der Varizenoperation. Phlebologie. 1994;23:1–6. [PubMed] [Google Scholar]
- 312.Rigby KA, Palfreyman SS, Beverley C, Michaels JA. Surgery for varicose veins: use of tourniquet. In: Rigby KA, editor. Cochrane Database of Systematic Reviews. Chichester: John Wiley Sons; 2002. [DOI] [PubMed] [Google Scholar]
- 313.Sykes TCF, Brookes P, Hickey NC. A prospective randomised trial of tourniquet in varicose vein surgery. Ann R Coll Surg Engl. 2000;82(4):280–282. [PMC free article] [PubMed] [Google Scholar]
- 314.Travers J, Makin G. Reduction of varicose vein recurrence by use of postoperative compression stockings. Phlebology. 1994;9:104–107. doi: 10.1177/026835559400900304. [DOI] [Google Scholar]
- 315.Biswas S, Clark A, Shields DA. Randomised clinical trial of the duration of compression therapy after varicose vein surgery. Eur J Vasc Endovasc Surg. 2007;33(5):631–637. doi: 10.1016/j.ejvs.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 316.De Maeseneer MG, Vandenbroeck CP, Hendriks JM, Lauwers PR, Van Schil PE. Accuracy of duplex evaluation one year after varicose vein surgery to predict recurrence at the sapheno-femoral junction after five years. Eur J Vasc Endovasc Surg. 2005;29(3):308–312. doi: 10.1016/j.ejvs.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 317.Robertson LA, Evans CJ, Lee AJ, Allan PL, Ruckley CV, Fowkes FGR. Incidence and risk factors for venous reflux in the general population: Edinburgh vein study. Eur J Vasc Endovasc Surg. 2014;48(2):208–214. doi: 10.1016/j.ejvs.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 318.Darke SG. The morphology of recurrent varicose veins. Eur J Vasc Surg. 1992;6(5):512–517. doi: 10.1016/S0950-821X(05)80626-7. [DOI] [PubMed] [Google Scholar]
- 319.Mumme A, Olbrich S, Barbera L, Stücker M. Saphenofemorales Leistenrezidiv nach Stripping der Vena saphena magna: technischer Fehler oder Neovaskularisation? Phlebologie. 2002;31(02):38–41. doi: 10.1055/s-0037-1617254. [DOI] [Google Scholar]
- 320.Noppeney T, Storck M, Nullen H, et al. Perioperative quality assessment of varicose vein surgery : commission for quality assessment of the German Society for Vascular Surgery. Langenbecks Arch Surg. 2016;401(3):375–380. doi: 10.1007/s00423-016-1387-2. [DOI] [PubMed] [Google Scholar]
- 321.Perrin MR, Labropoulos N, Leon LR. Presentation of the patient with recurrent varices after surgery (REVAS) J Vasc Surg. 2006;43(2):327–334. doi: 10.1016/j.jvs.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 322.Geier B, Stücker M, Hummel T, et al. Residual stumps associated with inguinal varicose vein recurrences: a multicenter study. Eur J Vasc Endovasc Surg. 2008;36(2):207–210. doi: 10.1016/j.ejvs.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 323.Fischer R, Chandler JG, Stenger D, Puhan MA, De Maeseneer MG, Schimmelpfennig L. Patient characteristics and physician-determined variables affecting saphenofemoral reflux recurrence after ligation and stripping of the great saphenous vein. J Vasc Surg. 2006;43(1):81–87. doi: 10.1016/j.jvs.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 324.Hartmann K, Klode J, Pfister R, et al. Recurrent varicose veins: Sonography-based re-examination of 210 patients 14 years after ligation and saphenous vein stripping. Vasa. 2006;35(1):21–26. doi: 10.1024/0301-1526.35.1.21. [DOI] [PubMed] [Google Scholar]
- 325.Geier B, Mumme A, Asciutto G, Marpe B, Köster O, Barbera L. Pelvine Insuffizienz und Varikosis der unteren Extremität. Phlebologie. 2006;35(05):243–248. doi: 10.1055/s-0037-1622149. [DOI] [Google Scholar]
- 326.Kostas T, Ioannou CV, Touloupakis E, et al. Recurrent varicose veins after surgery: a new appraisal of a common and complex problem in vascular surgery. Eur J Vasc Endovasc Surg. 2004;27(3):275–282. doi: 10.1016/j.ejvs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 327.Noppeney T, Nüllen H. REVAT (Recurrent Varices After Treatment) Phlebologie. 2009;38(06):271–274. doi: 10.1055/s-0037-1622280. [DOI] [Google Scholar]
- 328.Theivacumar NS, Darwood R, Gough MJ. Neovascularisation and recurrence 2 years after varicose vein treatment for Sapheno-femoral and great Saphenous vein reflux: a comparison of surgery and Endovenous laser ablation. Eur J Vasc Endovasc Surg. 2009;38(2):203–207. doi: 10.1016/j.ejvs.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 329.Rasmussen L, Lawaetz M, Serup J, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy, and surgical stripping for great saphenous varicose veins with 3-year follow-up. J Vasc Surg Venous Lymphat Disord. 2013;1(4):349–356. doi: 10.1016/j.jvsv.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 330.Hamann SAS, Giang J, De Maeseneer MGR, Nijsten TEC, van den Bos RR. Editor’s choice—five year results of great Saphenous vein treatment: a meta-analysis. Eur J Vasc Endovasc Surg. 2017;54(6):760–770. doi: 10.1016/j.ejvs.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 331.Lawaetz M, Serup J, Lawaetz B, et al. Comparison of endovenous ablation techniques, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Extended 5-year follow-up of a RCT. Int Angiol. 2017;36(3):281–288. doi: 10.23736/S0392-9590.17.03827-5. [DOI] [PubMed] [Google Scholar]
- 332.Flessenkamper I, Hartmann M, Hartmann K, Stenger D, Roll S. Endovenous laser ablation with and without high ligation compared to high ligation and stripping for treatment of great saphenous varicose veins: Results of a multicentre randomised controlled trial with up to 6 years follow-up. Phlebology. 2016;31(1):23–33. doi: 10.1177/0268355514555547. [DOI] [PubMed] [Google Scholar]
- 333.De Maeseneer MG, Vandenbroeck CP, Van Schil PE. Silicone patch saphenoplasty to prevent repeat recurrence after surgery to treat recurrent saphenofemoral incompetence: long-term follow-up study. J Vasc Surg. 2004;40(1):98–105. doi: 10.1016/j.jvs.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 334.Frings N, Tran P, Nelle A, Köhler L. Freies Endothel des Magna-Krossenstumpfes und Neoreflux/Neoangiogenese. Phlebologie. 2004;33(05):156–159. doi: 10.1055/s-0037-1621555. [DOI] [Google Scholar]
- 335.Gerontopoulou SA, Kath W, Rass K. Short-term efficacy of inguinal reoperation for recurrent Saphenofemoral incompetence using the stump suture technique. Ann Vasc Surg. 2018;53:197–204. doi: 10.1016/j.avsg.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 336.De Maeseneer M. Surgery for recurrent varicose veins: toward a less-invasive approach? Perspect Vasc Surg Endovasc Ther. 2011;23(4):244–249. doi: 10.1177/1531003511408338. [DOI] [PubMed] [Google Scholar]
- 337.Frings N, Glowacki P. Tran P. II. und III. Krossenrezidiv der Vena saphena magna/Vena saphena parva. Phlebologie. 2006;35:1–6. [Google Scholar]
- 338.Boné C. Tratamiento endoluminal de las varices con laser de Diodo. Estudio preliminary. Rev Patol Vasc. 1999;5:35–46. [Google Scholar]
- 339.Fassiadis N, Kiarnifard B, Holdstock J, Whiteley M. No recurrences of reflux following radiofrequency ablation of the long saphenous vein (VNUS closure) at one year. Br J Surg. 2001;88(Suppl 1):49–50. [Google Scholar]
- 340.Proebstle TM, Pannier FM, Schuller-Petrovic S, Offermann M, Hohenleutner U, Rabe E. Konsensus zur endovenösen Lasertherapie der Varikose. Phlebologie. 2004;33(03):106–109. doi: 10.1055/s-0037-1617279. [DOI] [Google Scholar]
- 341.Chandler JG, Pichot O, Sessa C, Schuller-Petrovicć S, Kabnick LS, Bergan JJ. Treatment of primary venous insufficiency by endovenous Saphenous vein obliteration. Vasc Surg. 2000;34(3):201–214. doi: 10.1177/153857440003400303. [DOI] [Google Scholar]
- 342.Gibson K, Khilnani N, Schul M, Meissner M. American College of Phlebology Guidelines—treatment of refluxing accessory saphenous veins. Phlebology. 2017;32(7):448–452. doi: 10.1177/0268355516671624. [DOI] [PubMed] [Google Scholar]
- 343.Peden E, Lumsden A. Radiofrequency ablation of incompetent perforator veins. Perspect Vasc Surg Endovasc Ther. 2007;19(1):73–77. doi: 10.1177/1531003507299478. [DOI] [PubMed] [Google Scholar]
- 344.Zerweck C. Nonoperative Therapy in the Treatment of Varicose Vein Disease. Z Gefäßmed. 2016;13:12–19. [Google Scholar]
- 345.Doganci S, Yildirim V, Demirkilic U. Does puncture site affect the rate of nerve injuries following endovenous laser ablation of the small Saphenous veins? Eur J Vasc Endovasc Surg. 2011;41(3):400–405. doi: 10.1016/j.ejvs.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 346.Rits J, Maurins U, Präve S, et al. Endovenöse Lasertherapie der Vena saphena magna: Drei-Jahresergebnisse einer randomisierten prospektiven Studie mit 0 vs 2 cm Abstand zur tiefen Vene. Vasomed. 2018;30:15. [Google Scholar]
- 347.van den Bos RR, Kockaert MA, Neumann HAM, Nijsten T. Technical review of endovenous laser therapy for varicose veins. Eur J Vasc Endovasc Surg. 2008;35(1):88–95. doi: 10.1016/j.ejvs.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 348.Lahl W, Hofmann B, Jelonek M, Nagel T. Die endovenöse Lasertherapie der Varicosis – echte Innovation oder teure Spielerei? Zentralbl Chir. 2006;131(1):45–50. doi: 10.1055/s-2006-921389. [DOI] [PubMed] [Google Scholar]
- 349.Luebke T, Gawenda M, Heckenkamp J, Brunkwall J. Meta-analysis of endovenous radiofrequency obliteration of the great Saphenous vein in primary varicosis. j Endovasc Ther. 2008;15(2):213–223. doi: 10.1583/07-2287.1. [DOI] [PubMed] [Google Scholar]
- 350.Merchant RF, Pichot O. Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency. J Vasc Surg. 2005;42(3):502–510. doi: 10.1016/j.jvs.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 351.Noppeney T, Noppeney J, Winkler M. Update der Ergebnisse nach Radiofrequenzobliteration zur Ausschaltung der Varikose. Gefässchirurgie. 2008;13(4):258–264. doi: 10.1007/s00772-008-0602-z. [DOI] [Google Scholar]
- 352.Sybrandy JEM, Wittens CHA. Initial experiences in endovenous treatment of saphenous vein reflux. J Vasc Surg. 2002;36(6):1207–1212. doi: 10.1067/mva.2002.128936. [DOI] [PubMed] [Google Scholar]
- 353.Zimmet SE, Min RJ. Temperature changes in perivenous tissue during endovenous laser treatment in a swine model. J Vasc Interv Radiol. 2003;14(7):911–915. doi: 10.1097/01.RVI.0000082811.60648.A4. [DOI] [PubMed] [Google Scholar]
- 354.Goodyear SJ, Nyamekye IK. Radiofrequency ablation of varicose veins: best practice techniques and evidence. Phlebology. 2015;30(2):9–17. doi: 10.1177/0268355515592771. [DOI] [PubMed] [Google Scholar]
- 355.Carradice D, Mekako AI, Hatfield J, Chetter IC. Randomized clinical trial of concomitant or sequential phlebectomy after endovenous laser therapy for varicose veins. br J Surg. 2009;96(4):369–375. doi: 10.1002/bjs.6556. [DOI] [PubMed] [Google Scholar]
- 356.El-Sheikha J, Nandhra S, Carradice D, et al. Clinical outcomes and quality of life 5 years after a randomized trial of concomitant or sequential phlebectomy following endovenous laser ablation for varicose veins. br J Surg. 2014;101(9):1093–1097. doi: 10.1002/bjs.9565. [DOI] [PubMed] [Google Scholar]
- 357.NICE (2013) National Institute for Health and Care Excellence. Varicose veins in the legs—the diagnosis and management of varicose veins. http://guidance.nice.org.uk/CG168 [PubMed]
- 358.Elderman JH, Krasznai AG, Voogd AC, Hulsewé KWE, Sikkink CJJM. Role of compression stockings after endovenous laser therapy for primary varicosis. J Vasc Surg. 2014;2(3):289–296. doi: 10.1016/j.jvsv.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 359.Encke A, Haas S, Kopp I. The prophylaxis of venous thromboembolism. Dtsch Aerztebl Online. 2016;113(31–32):532–538. doi: 10.3238/arztebl.2016.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Theivacumar NS, Gough MJ. Influence of warfarin on the success of endovenous laser ablation (EVLA) of the great Saphenous vein (GSV) Eur J Vasc Endovasc Surg. 2009;38(4):506–510. doi: 10.1016/j.ejvs.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 361.Sharifi M, Mehdipour M, Bay C, Emrani F, Sharifi J. Effect of anticoagulation on endothermal ablation of the great saphenous vein. J Vasc Surg. 2011;53(1):147–149. doi: 10.1016/j.jvs.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 362.Fan CM, Rox-Anderson R. Endovenous laser ablation: mechanism of action. Phlebology. 2008;23(5):206–213. doi: 10.1258/phleb.2008.008049. [DOI] [PubMed] [Google Scholar]
- 363.Spreafico G, Piccioli A, Bernardi E, et al. Endovenous laser ablation of great and small saphenous vein incompetence with a 1470-nm laser and radial fiber. J Vasc Surg Venous Lymphat Disord. 2014;2(4):403–410. doi: 10.1016/j.jvsv.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 364.Brittenden J, Cotton SC, Elders A, et al. Clinical effectiveness and cost-effectiveness of foam sclerotherapy, endovenous laser ablation and surgery for varicose veins: results from the Comparison of LAser, Surgery and foam Sclerotherapy (CLASS) randomised controlled trial. Health Technol Assess. 2015;19(27):1–342. doi: 10.3310/hta19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Disselhoff BC, der Kinderen DJ, Kelder JC, Moll FL. Randomized clinical trial comparing endovenous laser with cryostripping for great saphenous varicose veins. Br J Surg. 2008;95(10):1232–1238. doi: 10.1002/bjs.6351. [DOI] [PubMed] [Google Scholar]
- 366.Flessenkamper I, Hartmann M, Stenger D, Roll S. Endovenous laser ablation with and without high ligation compared with high ligation and stripping in the treatment of great saphenous varicose veins: initial results of a multicentre randomized controlled trial. Phlebology. 2013;28(1):16–23. doi: 10.1258/phleb.2011.011147. [DOI] [PubMed] [Google Scholar]
- 367.Mozafar M, Atqiaee K, Haghighatkhah H, Taheri MS, Tabatabaey A, Lotfollahzadeh S. Endovenous laser ablation of the great saphenous vein versus high ligation: long-term results. Lasers Med Sci. 2014;29(2):765–771. doi: 10.1007/s10103-013-1389-z. [DOI] [PubMed] [Google Scholar]
- 368.Rass K, Frings N, Glowacki P, et al. Comparable effectiveness of endovenous laser ablation and high ligation with stripping of the great saphenous vein: two-year results of a randomized clinical trial (RELACS study) Arch Dermatol. 2012;148(1):49–58. doi: 10.1001/archdermatol.2011.272. [DOI] [PubMed] [Google Scholar]
- 369.Nordon IM, Hinchliffe RJ, Brar R, et al. A prospective double-blind randomized controlled trial of radiofrequency versus laser treatment of the great saphenous vein in patients with varicose veins. Ann Surg. 2011;254(6):876–881. doi: 10.1097/SLA.0b013e318230af5a. [DOI] [PubMed] [Google Scholar]
- 370.Pronk P, Gauw SAA, Mooij MCC, et al. Randomised controlled trial comparing sapheno-femoral ligation and stripping of the great saphenous vein with endovenous laser ablation (980 nm) using local tumescent anaesthesia: one year results. Eur J Vasc Endovasc Surg. 2010;40(5):649–656. doi: 10.1016/j.ejvs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 371.Rasmussen LH, Bjoern L, Lawaetz M, Blemings A, Lawaetz B, Eklof B. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short-term results. J Vasc Surg. 2007;46(2):308–315. doi: 10.1016/j.jvs.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 372.Samuel N, Carradice D, Wallace T, Mekako A, Hatfield J, Chetter I. Randomized clinical trial of endovenous laser ablation versus conventional surgery for small saphenous varicose veins. Ann Surg. 2013;257(3):419–426. doi: 10.1097/SLA.0b013e318275f4e4. [DOI] [PubMed] [Google Scholar]
- 373.Shepherd AC, Gohel MS, Brown LC, Metcalfe MJ, Hamish M, Davies AH. Randomized clinical trial of VNUS®ClosureFASTTM radiofrequency ablation versus laser for varicose veins. Br J Surg. 2010;97(6):810–818. doi: 10.1002/bjs.7091. [DOI] [PubMed] [Google Scholar]
- 374.Theivacumar NS, Dellagrammaticas D, Mavor AID, Gough MJ. Endovenous laser ablation: Does standard above-knee great saphenous vein ablation provide optimum results in patients with both above- and below-knee reflux? A randomized controlled trial. J Vasc Surg. 2008;48(1):173–178. doi: 10.1016/j.jvs.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 375.Van Den Bos RR, Malskat WSJ, De Maeseneer MGR, et al. Randomized clinical trial of endovenous laser ablation versus steam ablation (LAST trial) for great saphenous varicose veins. Br J Surg. 2014;101(9):1077–1083. doi: 10.1002/bjs.9580. [DOI] [PubMed] [Google Scholar]
- 376.Almeida JI, Kaufman J, Göckeritz O, et al. Radiofrequency endovenous closureFAST versus laser ablation for the treatment of great Saphenous reflux: a multicenter, single-blinded, randomized study (RECOVERY study) J Vasc Interv Radiol. 2009;20(6):752–759. doi: 10.1016/j.jvir.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 377.Christenson JT, Gueddi S, Gemayel G, Bounameaux H. Prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up. J Vasc Surg. 2010;52(5):1234–1241. doi: 10.1016/j.jvs.2010.06.104. [DOI] [PubMed] [Google Scholar]
- 378.Darwood RJ, Theivacumar N, Dellagrammaticas D, Mavor AI, Gough MJ. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. Br J Surg. 2008;95(3):294–301. doi: 10.1002/bjs.6101. [DOI] [PubMed] [Google Scholar]
- 379.Disselhoff BC, der Kinderen DJ, Kelder JC, Moll FL. Randomized clinical trial comparing endovenous laser ablation of the great Saphenous vein with and without ligation of the sapheno-femoral junction: 2-year results. Eur J Vasc Endovasc Surg. 2008;36(6):713–718. doi: 10.1016/j.ejvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 380.Goode SD, Chowdhury A, Crockett M, et al. Laser and radiofrequency ablation study (LARA study): a randomised study comparing radiofrequency ablation and endovenous laser ablation (810 nm) Eur J Vasc Endovasc Surg. 2010;40(2):246–253. doi: 10.1016/j.ejvs.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 381.Memetoglu ME, Kurtcan S, Kalkan A, Ozel D. Combination technique of tumescent anesthesia during endovenous laser therapy of saphenous vein insufficiency. Interact Cardiovasc Thorac Surg. 2010;11(6):774–778. doi: 10.1510/icvts.2010.240762. [DOI] [PubMed] [Google Scholar]
- 382.Kabnick LS, Sadek M. Fiber type as compared to wavelength may contribute more to improving postoperative recovery following endovenous laser ablation. J Vasc Surg Venous Lymphat Disord. 2016;4(3):286–292. doi: 10.1016/j.jvsv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 383.Hamel-Desnos C, Desnos P, Allaert F-AA, Kern P, “Thermal group” for the French Society of Phlebology and the Swiss Society of Phlebology Thermal ablation of saphenous veins is feasible and safe in patients older than 75 years: A prospective study (EVTA study) Phlebology. 2015;30(8):525–532. doi: 10.1177/0268355514540882. [DOI] [PubMed] [Google Scholar]
- 384.Vuylsteke M, De Bo TH, Dompe G, Di Crisci D, Abbad CM, Mordon S. Endovenous laser treatment: Is there a clinical difference between using a 1500 nm and a 980 nm diode laser? A multicenter randomised clinical trial. Int Angiol. 2011;30(4):327–334. [PubMed] [Google Scholar]
- 385.Doganci S, Demirkilic U. Comparison of 980 nm laser and bare-tip fibre with 1470 nm laser and radial fibre in the treatment of great saphenous vein varicosities: a prospective randomised clinical trial. Eur J Vasc Endovasc Surg. 2010;40(2):254–259. doi: 10.1016/j.ejvs.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 386.Siribumrungwong B, Noorit P, Wilasrusmee C, Attia J, Thakkinstian A. A systematic review and meta-analysis of randomised controlled trials comparing endovenous ablation and surgical intervention in patients with varicose vein. Eur J Vasc Endovasc Surg. 2012;44(2):214–223. doi: 10.1016/j.ejvs.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 387.Tesmann JP, Thierbach H, Dietrich A, Grimme H, Vogt T, Rass K. Radiofrequency induced thermotherapy (RFITT) of varicose veins compared to endovenous laser treatment (EVLT): a non-randomized prospective study concentrating on occlusion rates, side-effects and clinical outcome. Eur J Dermatol. 2011;21(6):945–951. doi: 10.1684/ejd.2011.1515. [DOI] [PubMed] [Google Scholar]
- 388.Flessenkämper I, Stenger D, Hartmann M, Roll S. Endoluminale Lasertherapie vs. Crossektomie/Stripping bei Vena saphena magna-Varicosis.Klinische und sonographische Ergebnisse. Phlebologie. 2013;42(1):7–11. doi: 10.12687/phleb2128_1_2013. [DOI] [Google Scholar]
- 389.Nesbitt C, Bedenis R, Bhattacharya V, Stansby G. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus open surgery for great saphenous vein varices. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD005624.pub3. [DOI] [PubMed] [Google Scholar]
- 390.Park SW, Hwang JJ, Yun IJ, et al. Randomized clinical trial comparing two methods for endovenous laser ablation of incompetent perforator veins in thigh and great saphenous vein without evidence of saphenofemoral reflux. Dermatol Surg. 2012;38(4):640–646. doi: 10.1111/j.1524-4725.2011.02261.x. [DOI] [PubMed] [Google Scholar]
- 391.Kabnick LS. Outcome of different endovenous laser wavelengths for great saphenous vein ablation. J Vasc Surg. 2006;43(1):88–93. doi: 10.1016/j.jvs.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 392.Samuel N, Wallace T, Carradice D, Mazari FA, Chetter IC. Comparison of 12-w versus 14-w endovenous laser ablation in the treatment of great saphenous varicose veins: 5-year outcomes from a randomized controlled trial. Vasc Endovascular Surg. 2013;47(5):346–352. doi: 10.1177/1538574413487265. [DOI] [PubMed] [Google Scholar]
- 393.Vuylsteke M, Liekens K, Moons P, Mordon S. Endovenous laser treatment of saphenous vein reflux: how much energy do we need to prevent recanalizations? Vasc Endovascular Surg. 2008;42(2):141–149. doi: 10.1177/1538574407311107. [DOI] [PubMed] [Google Scholar]
- 394.Mao J, Zhang C, Wang Z, Gan S, Li K. A retrospective study comparing endovenous laser ablation and microwave ablation for great saphenous varicose veins. Eur Rev Med Pharmacol Sci. 2012;16(7):873–877. [PubMed] [Google Scholar]
- 395.Agus GB, Mancini S, Magi G, et al. The first 1000 cases of Italian Endovenous-laser Working Group (IEWG). Rationale, and long-term outcomes for the 1999–2003 period. Int Angiol. 2006;25(2):209–215. [PubMed] [Google Scholar]
- 396.Desmyttère J, Grard C, Wassmer B, Mordon S. Endovenous 980-nm laser treatment of saphenous veins in a series of 500 patients. J Vasc Surg. 2007;46(6):1242–1247. doi: 10.1016/j.jvs.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 397.Pannier F, Rabe E. Mid-term results following endovenous laser ablation (EVLA) of saphenous veins with a 980 nm diode laser. Int Angiol. 2008;27(6):475–481. [PubMed] [Google Scholar]
- 398.Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. br J Surg. 2011;98(4):501–510. doi: 10.1002/bjs.7394. [DOI] [PubMed] [Google Scholar]
- 399.Biemans AA, Kockaert M, Akkersdijk GP, et al. Comparing endovenous laser ablation, foam sclerotherapy, and conventional surgery for great saphenous varicose veins. J Vasc Surg. 2013;58(3):727–734. doi: 10.1016/j.jvs.2012.12.074. [DOI] [PubMed] [Google Scholar]
- 400.Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Clinical and technical outcomes from a randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. br J Surg. 2011;98(8):1117–1123. doi: 10.1002/bjs.7615. [DOI] [PubMed] [Google Scholar]
- 401.Gonzalez-Zeh R, Armisen R, Barahona S. Endovenous laser and echo-guided foam ablation in great saphenous vein reflux: one-year follow-up results. J Vasc Surg. 2008;48(4):940–946. doi: 10.1016/j.jvs.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 402.Kalteis M, Adelsgruber P, Messie-Werndl S, Gangl O, Berger I. Five-year results of a randomized controlled trial comparing high ligation combined with endovenous laser ablation and stripping of the great saphenous vein. Dermatol Surg. 2015;41(5):579–586. doi: 10.1097/DSS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 403.Park SJ, Bin Yim S, Cha DW, Kim SC, Lee SH. Endovenous laser treatment of the small saphenous vein with a 980-nm diode laser: early results. Dermatol Surg. 2008;34(4):517–524. doi: 10.1111/j.1524-4725.2007.34097.x. [DOI] [PubMed] [Google Scholar]
- 404.Theivacumar NS, Dellagrammaticas D, Beale RJ, Mavor AID, Gough MJ. Factors influencing the effectiveness of endovenous laser ablation (EVLA) in the treatment of great Saphenous vein reflux. Eur J Vasc Endovasc Surg. 2008;35(1):119–123. doi: 10.1016/j.ejvs.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 405.Nandhra S, El-sheikha J, Carradice D, et al. A randomized clinical trial of endovenous laser ablation versus conventional surgery for small saphenous varicose veins. J Vasc Surg. 2015;61(3):741–746. doi: 10.1016/j.jvs.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 406.Shadid N, Ceulen R, Nelemans P, et al. Randomized clinical trial of ultrasound-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein. Br J Surg. 2012;99(8):1062–1070. doi: 10.1002/bjs.8781. [DOI] [PubMed] [Google Scholar]
- 407.Dexter D, Kabnick L, Berland T, et al. Complications of endovenous lasers. Phlebology. 2012;27(1_suppl):40–45. doi: 10.1258/phleb.2012.012s18. [DOI] [PubMed] [Google Scholar]
- 408.Pavlović MD, Schuller-Petrović S, Pichot O, et al. Guidelines of the first international consensus conference on endovenous thermal ablation for varicose vein disease—ETAV consensus meeting 2012. Phlebology. 2015;30(4):257–273. doi: 10.1177/0268355514524568. [DOI] [PubMed] [Google Scholar]
- 409.Healy DA, Kimura S, Power D, et al. A systematic review and meta-analysis of thrombotic events following endovenous thermal ablation of the great Saphenous vein. Eur J Vasc Endovasc Surg. 2018;56(3):410–424. doi: 10.1016/j.ejvs.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 410.Schäffer N, Weingard I, Kiderlen M, et al. Appositionsthrombus als Komplikation endovenöser Katheterverfahren (Post ablation thrombus extension [PATE]) Phlebologie. 2018 doi: 10.12687/phleb2417-2-2018. [DOI] [Google Scholar]
- 411.Harlander-Locke M, Jimenez JC, Lawrence PF, Derubertis BG, Rigberg DA, Gelabert HA. Endovenous ablation with concomitant phlebectomy is a safe and effective method of treatment for symptomatic patients with axial reflux and large incompetent tributaries. J Vasc Surg. 2013;58(1):166–172. doi: 10.1016/j.jvs.2012.12.054. [DOI] [PubMed] [Google Scholar]
- 412.Harlander-Locke M, Jimenez JC, Lawrence PF, et al. Management of endovenous heat-induced thrombus using a classification system and treatment algorithm following segmental thermal ablation of the small saphenous vein. J Vasc Surg. 2013;58(2):427–431. doi: 10.1016/j.jvs.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 413.Lawrence PF, Chandra A, Wu M, et al. Classification of proximal endovenous closure levels and treatment algorithm. J Vasc Surg. 2010;52(2):388–393. doi: 10.1016/j.jvs.2010.02.263. [DOI] [PubMed] [Google Scholar]
- 414.Rhee SJ, Cantelmo NL, Conrad MF, Stoughton J. Factors influencing the incidence of endovenous heat-induced thrombosis (EHIT) Vasc Endovascular Surg. 2013;47(3):207–212. doi: 10.1177/1538574413478494. [DOI] [PubMed] [Google Scholar]
- 415.Lawson JA, Gauw SA, van Vlijmen CJ, et al. Prospective comparative cohort study evaluating incompetent great saphenous vein closure using radiofrequency-powered segmental ablation or 1470-nm endovenous laser ablation with radial-tip fibers (Varico 2 study) J Vasc Surg Venous Lymphat Disord. 2018;6(1):31–40. doi: 10.1016/j.jvsv.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 416.Malskat WSJ, Giang J, De Maeseneer MGR, Nijsten TEC, Van Den Bos RR. Randomized clinical trial of 940- versus 1470-nm endovenous laser ablation for great saphenous vein incompetence. Br J Surg. 2016;103(3):192–198. doi: 10.1002/bjs.10035. [DOI] [PubMed] [Google Scholar]
- 417.Maurins U, Rabe E, Pannier F. Does laser power influence the results of endovenous laser ablation (EVLA) of incompetent saphenous veins with the 1 470-nm diode laser? A prospective randomized study comparing 15 and 25 W. Int Angiol. 2009;28(1):32–37. [PubMed] [Google Scholar]
- 418.Mendes-Pinto D, Bastianetto P, Cavalcanti Bragalyra L, Kikuchi R, Kabnick L. Endovenous laser ablation of the great saphenous vein comparing 1920-nm and 1470-nm diode laser. Int Angiol. 2016;35(6):599–604. [PubMed] [Google Scholar]
- 419.Mese B, Bozoglan O, Eroglu E, et al. A comparison of 1,470-nm endovenous laser ablation and radiofrequency ablation in the treatment of great Saphenous veins 10 mm or more in size. Ann Vasc Surg. 2015;29(7):1368–1372. doi: 10.1016/j.avsg.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 420.Pannier F, Rabe E, Maurins U. 1470 nm diode laser for endovenous ablation (EVLA) of incompetent saphenous veins—a prospective randomized pilot study comparing warm and cold tumescence anaesthesia. Vasa. 2010;39(3):249–255. doi: 10.1024/0301-1526/a000037. [DOI] [PubMed] [Google Scholar]
- 421.Pannier F, Rabe E, Rits J, Kadiss A, Maurins U. Endovenous laser ablation of great saphenous veins using a 1470 nm diode laser and the radial fibre—follow-up after six months. Phlebology. 2011;26(1):35–39. doi: 10.1258/phleb.2010.009096. [DOI] [PubMed] [Google Scholar]
- 422.Proebstle TM, Moehler T, Gul D, Herdemann S. Endovenous treatment of the great saphenous vein using a 1,320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31(12):1678–1683. doi: 10.2310/6350.2005.31308. [DOI] [PubMed] [Google Scholar]
- 423.Schmedt C, Esipova A, Dikic S, Demhasaj S, Comsa F, Sroka R. Erste klinische Ergebnisse der Endovenösen Lasertherapie (ELT) mit Thulium (Tm) Laser (1940 nm) und radialer Lichtapplikation. Vasomed. 2014;26:294. [Google Scholar]
- 424.Schmedt C-G, Esipova A, Dikic S, et al. Endovenous laser therapy (ELT) of Saphenous vein reflux using thulium laser (tm, 1940 nm) with radial fiber—one year results. Eur J Vasc Endovasc Surg. 2016;52(3):413–414. doi: 10.1016/j.ejvs.2016.07.071. [DOI] [Google Scholar]
- 425.Schwarz T, Von HE, Furtwangler C, Rastan A, Zeller T, Neumann FJ. Endovenous laser ablation of varicose veins with the 1470-nm diode laser. J Vasc Surg. 2010;51(6):1474–1478. doi: 10.1016/j.jvs.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 426.Venermo M, Saarinen J, Eskelinen E, et al. Randomized clinical trial comparing surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy for the treatment of great saphenous varicose veins. Br J Surg. 2016;103(11):1438–1444. doi: 10.1002/bjs.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Vuylsteke ME, Thomis S, Mahieu P, Mordon S, Fourneau I. Endovenous laser ablation of the great saphenous vein using a bare fibre versus a tulip fibre: a randomised clinical trial. Eur J Vasc Endovasc Surg. 2012;44(6):587–592. doi: 10.1016/j.ejvs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 428.Yang C, Chou H, Lo Y. Incompetent great Saphenous veins treated with endovenous 1,320-nm laser: results for 71 legs and morphologic evolvement study. Dermatol Surg. 2006;32(12):1453–1457. doi: 10.1111/j.1524-4725.2006.32355.x. [DOI] [PubMed] [Google Scholar]
- 429.Arslan Ü, Çalık E, Tort M, et al. More successful results with less energy in endovenous laser ablation treatment: long-term comparison of bare-tip fiber 980 nm laser and radial-tip fiber 1470 nm laser application. Ann Vasc Surg. 2017;45:166–172. doi: 10.1016/j.avsg.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 430.Bozoglan O, Mese B, Eroglu E, Ekerbiçer HC, Yasim A. Comparison of endovenous laser and radiofrequency ablation in treating varices in the same patient. J Lasers Med Sci. 2017;8(1):13–16. doi: 10.15171/jlms.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Dumantepe M, Uyar I. Comparing cold and warm tumescent anesthesia for pain perception during and after the endovenous laser ablation procedure with 1470 nm diode laser. Phlebology. 2015;30(1):45–51. doi: 10.1177/0268355513512827. [DOI] [PubMed] [Google Scholar]
- 432.Gunes T, Altin F, Kutas B, et al. Less painful tumescent solution for patients undergoing endovenous laser ablation of the saphenous vein. Ann Vasc Surg. 2015;29(6):1123–1127. doi: 10.1016/j.avsg.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 433.Hirokawa M, Kurihara N. Comparison of bare-tip and radial fiber in endovenous laser ablation with 1470 nm diode laser. Ann Vasc Dis. 2014;7(3):239–245. doi: 10.3400/avd.oa.14-00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Jibiki M, Miyata T, Futatsugi S, Iso M, Sakanushi Y. Effect of the wide-spread use of endovenous laser ablation on the treatment of varicose veins in Japan: a large-scale, single institute study) Laser Ther. 2016;25(3):171–177. doi: 10.5978/islsm.16-OR-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Lattimer CR, Kalodiki E, Azzam M, Makris GC, Somiayajulu S, Geroulakos G. Interim results on abolishing reflux alongside a randomized clinical trial on laser ablation with phlebectomies versus foam sclerotherapy. Int Angiol. 2013;32(4):394–403. [PubMed] [Google Scholar]
- 436.Schmedt C-G, Sroka R, Steckmeier S, et al. Investigation on radiofrequency and laser (980 nm) effects after endoluminal treatment of Saphenous vein insufficiency in an ex-vivo model. Eur J Vasc Endovasc Surg. 2006;32(3):318–325. doi: 10.1016/j.ejvs.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 437.Sroka R, Weick K, Sadeghi-Azandaryani M, Steckmeier B, Schmedt CG. Endovenous laser therapy—application studies and latest investigations. J Biophotonics. 2010;3(5–6):269–276. doi: 10.1002/jbio.200900097. [DOI] [PubMed] [Google Scholar]
- 438.Massaki ABMN, Kiripolsky MG, Detwiler SP, Goldman MP. Endoluminal laser delivery mode and wavelength effects on varicose veins in an Ex vivo model. Lasers Surg Med. 2013;45(2):123–129. doi: 10.1002/lsm.22069. [DOI] [PubMed] [Google Scholar]
- 439.Rass K. Current clinical evidence on endovenous laser ablation (EVLA) from randomised trials. Phlebologie. 2016;45(04):201–206. doi: 10.12687/phleb2317-4-2016. [DOI] [Google Scholar]
- 440.Balint R, Farics A, Parti K, et al. Which endovenous ablation method does offer a better long-term technical success in the treatment of the incompetent great saphenous vein? Review. Vascular. 2016;24(6):649–657. doi: 10.1177/1708538116648035. [DOI] [PubMed] [Google Scholar]
- 441.Lynch NP, Clarke M, Fulton GJ. Surgical management of great saphenous vein varicose veins: a meta-analysis. Vascular. 2015;23(3):285–296. doi: 10.1177/1708538114542633. [DOI] [PubMed] [Google Scholar]
- 442.Gale SS, Lee JN, Walsh ME, Wojnarowski DL, Comerota AJ. A randomized, controlled trial of endovenous thermal ablation using the 810-nm wavelength laser and the ClosurePLUS radiofrequency ablation methods for superficial venous insufficiency of the great saphenous vein. J Vasc Surg. 2010;52(3):645–650. doi: 10.1016/j.jvs.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 443.Rasmussen LH, Bjoern L, Lawaetz M, Lawaetz B, Blemings A, Eklof B. Randomised clinical trial comparing endovenous laser ablation with stripping of the great saphenous vein: clinical outcome and recurrence after 2 years86. Eur J Vasc Endovasc Surg. 2010;39(5):630–635. doi: 10.1016/j.ejvs.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 444.Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. br J Surg. 2011;98(8):1079–1087. doi: 10.1002/bjs.7555. [DOI] [PubMed] [Google Scholar]
- 445.Carradice D, Wallace T, Gohil R, Chetter I. A comparison of the effectiveness of treating those with and without the complications of superficial venous insufficiency. ann Surg. 2014;260(2):396–401. doi: 10.1097/SLA.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 446.Cowpland CA, Cleese AL, Whiteley MS. Factors affecting optimal linear endovenous energy density for endovenous laser ablation in incompetent lower limb truncal veins—A review of the clinical evidence. Phlebology. 2017;32(5):299–306. doi: 10.1177/0268355516648067. [DOI] [PubMed] [Google Scholar]
- 447.Camci M, Harnoss B, Akkersdijk G, et al. Effectivness and tolerability of bipolar radiofrequency-induced thermotherapy for the treatment of incompetent saphenous veins. Phlebologie. 2009;38(01):5–11. doi: 10.1055/s-0037-1622251. [DOI] [Google Scholar]
- 448.Weiss RA. Comparison of endovenous radiofrequency versus 810 nm diode laser occlusion of large veins in an animal model. Dermatol Surg. 2002;28(1):56–61. doi: 10.1046/j.1524-4725.2002.01191.x. [DOI] [PubMed] [Google Scholar]
- 449.Strejcek C, Pock L, Fara P. Bipolare Radiofrequenz-induzierte Thermotherapie (RFITT) zur sicheren und effektiven Behandlung von venösen Refluxkrankheiten: histopathologische Studienergebnisse. Phlebologie. 2007;36:A22. [Google Scholar]
- 450.Reich-Schupke S, Mumme A, Stücker M. Histopathological findings in varicose veins following bipolar radiofrequency-induced thermotherapy—Results of an ex vivo experiment. Phlebology. 2011;26(2):69–74. doi: 10.1258/phleb.2010.010004. [DOI] [PubMed] [Google Scholar]
- 451.Proebstle TM, Vago B, Alm J, Gockeritz O, Lebard C, Pichot O. Treatment of the incompetent great saphenous vein by endovenous radiofrequency powered segmental thermal ablation: first clinical experience. J Vasc Surg. 2008;47(1):151–156. doi: 10.1016/j.jvs.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 452.van den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49(1):230–239. doi: 10.1016/j.jvs.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 453.Kurihara N, Hirokawa M, Yamamoto T. Postoperative venous thromboembolism in patients undergoing endovenous laser and radiofrequency ablation of the Saphenous vein. Ann Vasc Dis. 2016;9(4):259–266. doi: 10.1107/S160053680401116X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Dermody M, O’Donnell TF, Balk EM. Complications of endovenous ablation in randomized controlled trials. J Vasc Surg Venous Lymphat Disord. 2013;1(4):427–436. doi: 10.1016/j.jvsv.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 455.O’Donnell TF, Eaddy M, Raju A, Boswell K, Wright D. Assessment of thrombotic adverse events and treatment patterns associated with varicose vein treatment. J Vasc Surg Venous Lymphat Disord. 2015;3(1):27–34. doi: 10.1016/j.jvsv.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 456.Proebstle TM, Alm BJ, Göckeritz O, et al. Five-year results from the prospective European multicentre cohort study on radiofrequency segmental thermal ablation for incompetent great saphenous veins. Br J Surg. 2015;102(3):212–218. doi: 10.1002/bjs.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Noppeney T, Brunner A, Noppeney J. Long-term results after radiofrequency ablation in 1998. Phlebologie. 2014;43(04):197–200. doi: 10.12687/phleb2201-4-2014. [DOI] [Google Scholar]
- 458.Zuniga JMR, Hingorani A, Ascher E, et al. Short-term outcome analysis of radiofrequency ablation using ClosurePlus vs ClosureFast catheters in the treatment of incompetent great saphenous vein. J Vasc Surg. 2012;55(4):1048–1051. doi: 10.1016/j.jvs.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 459.Poder TG, Fisette JF, Bédard SK, Despatis MA. Is radiofrequency ablation of varicose veins a valuable option? A systematic review of the literature with a cost analysis. Can J Surg. 2018;61(2):128–138. doi: 10.1503/cjs.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Hicks CW, DiBrito SR, Magruder JT, Weaver ML, Barenski C, Heller JA. Radiofrequency ablation with concomitant stab phlebectomy increases risk of endovenous heat-induced thrombosis. J Vasc Surg. 2017;5(2):200–209. doi: 10.1016/j.jvsv.2016.10.081. [DOI] [PubMed] [Google Scholar]
- 461.Sermsathanasawadi N, Voravitvet TY, Chinsakchai K, Wongwanit C, Ruangsetakit C, Mutirangura P. Risk factors for endovenous heat-induced thrombosis after endovenous radiofrequency ablation performed in Thailand. Phlebology. 2016;31(8):582–587. doi: 10.1177/0268355515599303. [DOI] [PubMed] [Google Scholar]
- 462.Boon R, Akkersdijk GJM, Nio D. Percutaneus treatment of varicose veins with bipolar radiofrequency ablation. Eur J Radiol. 2010;75(1):43–47. doi: 10.1016/j.ejrad.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 463.Newman JE, Meecham L, Walker RJ, Nyamekye IK. Optimising treatment parameters for radiofrequency induced thermal therapy (RFiTT): a comparison of the manufacturer’s treatment guidance with a locally developed treatment protocol. Eur J Vasc Endovasc Surg. 2014;47(6):664–669. doi: 10.1016/j.ejvs.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 464.Hnátek L, Duben J, Dudesek B, Gatek J. Endoluminal radiofrequency ablation of varices. Rozhl Chir. 2007;86(11):582–586. [PubMed] [Google Scholar]
- 465.Braithwaite B, Hnatek L, Zierau U, et al. Radiofrequency-induced thermal therapy: results of a European multicentre study of resistive ablation of incompetent truncal varicose veins. Phlebology. 2013;28(1):38–46. doi: 10.1258/phleb.2012.012013. [DOI] [PubMed] [Google Scholar]
- 466.Zierau UT, Lahl W. The endovenous RFITT-treatment of varicose veins, a new method of interventional phlebology. Phlebologie. 2009;38(01):12–16. doi: 10.1055/s-0037-1622247. [DOI] [Google Scholar]
- 467.Ahadiat O, Higgins S, Ly A, Nazemi A, Wysong A. Review of endovenous thermal ablation of the great Saphenous vein: endovenous laser therapy versus radiofrequency ablation. Dermatol Surg. 2018;44(5):679–688. doi: 10.1097/DSS.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 468.Woźniak W, Mlosek RK, Ciostek P. Assessment of the efficacy and safety of steam vein sclerosis as compared to classic surgery in lower extremity varicose vein management. Wideochir Inne Tech Maloinwazyjne. 2015;10:15–24. doi: 10.5114/wiitm.2015.48573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.van den Bos RR, Milleret R, Neumann M, Nijsten T. Proof-of-principle study of steam ablation as novel thermal therapy for saphenous varicose veins. J Vasc Surg. 2011;53(1):181–186. doi: 10.1016/j.jvs.2010.06.171. [DOI] [PubMed] [Google Scholar]
- 470.Mlosek RK, WoŹniak W, Gruszecki L, Stapa RZ. The use of a novel method of endovenous steam ablation in treatment of great saphenous vein insufficiency: Own experiences. Phlebology. 2014;29(1):58–65. doi: 10.1258/phleb.2012.012092. [DOI] [PubMed] [Google Scholar]
- 471.Milleret R, Huot L, Nicolini P, et al. Great saphenous vein ablation with steam injection: results of a multicentre study. Eur J Vasc Endovasc Surg. 2013;45(4):391–396. doi: 10.1016/j.ejvs.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 472.Yang L, Wang XP, Su WJ, Zhang Y, Wang Y. Randomized clinical trial of endovenous microwave ablation combined with high ligation versus conventional surgery for varicose veins. Eur J Vasc Endovasc Surg. 2013;46(4):473–479. doi: 10.1016/j.ejvs.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 473.Bush RG, Bush P, Flanagan J, et al. Factors associated with recurrence of varicose veins after thermal ablation: results of the recurrent veins after thermal ablation study. Sci World J. 2014 doi: 10.1155/2014/505843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Garner JP, Heppell PSJ, Leopold PW. The lateral accessory saphenous vein—a common cause of recurrent varicose veins. Ann R Coll Surg Engl. 2003;85(6):389–392. doi: 10.1308/003588403322520744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Stücker M, Netz K, Breuckmann F, Altmeyer P, Mumme A, Chandler JG. Histomorphologic classification of recurrent saphenofemoral reflux. J Vasc Surg. 2004;39(4):816–822. doi: 10.1016/j.jvs.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 476.Flessenkamper IH, Stenger D, Hartmann M, Hartmann K, Roll S. Two-year results of a prospective randomised controlled multicenter trial to compare open operative therapy vs. endoluminal venous laser therapy with and without high ligation for the therapy of varicose greater saphenous veins. zentralbl Chir. 2015;140(1):27–34. doi: 10.1055/s-0033-1360347. [DOI] [PubMed] [Google Scholar]
- 477.Proebstle TM, Möhler T. A longitudinal single-center cohort study on the prevalence and risk of accessory saphenous vein reflux after radiofrequency segmental thermal ablation of great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2015;3(3):265–269. doi: 10.1016/j.jvsv.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 478.Schuler L, Weingard I, Kiderlen M, Theodoridis A, Hartmann K. Endoluminal thermal ablation of varicose great Saphenous vein—a randomized single center application comparison of laser ablation (EVLA 1470nmTM), radio frequency ablation (RFITT and closurefastTM) and Superheated steam with average post-operative Follo. Ann Vasc Med Res. 2018;5(2):1091. [Google Scholar]
- 479.Sporbert F, Zollmann C, Zollmann P, et al. Endoluminal thermal ablation of the great saphenous vein (GSV) insufficiency. Phlebologie. 2016;45(06):357–362. doi: 10.12687/phleb2336-6-2016. [DOI] [Google Scholar]
- 480.Kheirelseid EAH, Crowe G, Sehgal R, et al. Systematic review and meta-analysis of randomized controlled trials evaluating long-term outcomes of endovenous management of lower extremity varicose veins. j Vasc Surg Venous Lymphat Disord. 2018;6(2):256–270. doi: 10.1016/j.jvsv.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 481.Hamel-Desnos C, Desnos P, Wollmann J-C, Ouvry P, Mako S, Allaert F-A. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in Sclerotherapy of the greater Saphenous vein: initial results. Dermatol Surg. 2003;29(12):1170–1175. doi: 10.1111/j.1524-4725.2003.29398.x. [DOI] [PubMed] [Google Scholar]
- 482.Alòs Villacrosa J, Carreño P, López JA, Estadella B, Serra-Prat M, Marinel-lo J. Efficacy and safety of sclerotherapy using polidocanol foam: a controlled clinical trial. Eur J Vasc Endovasc Surg. 2006 doi: 10.1016/j.ejvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 483.Ouvry P, Allaert F-A, Desnos P, Hamel-Desnos C. Efficacy of polidocanol foam versus liquid in sclerotherapy of the great Saphenous vein: a multicentre randomised controlled trial with a 2-year follow-up. Eur J Vasc Endovasc Surg. 2008;36(3):366–370. doi: 10.1016/j.ejvs.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 484.Rabe E, Otto J, Schliephake D, Pannier F. Efficacy and safety of great saphenous vein sclerotherapy using standardised polidocanol foam (ESAF): a randomised controlled multicentre clinical trial. Eur J Vasc Endovasc Surg. 2008;35(2):238–245. doi: 10.1016/j.ejvs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 485.Devereux N, Recke AL, Westermann L, Recke A, Kahle B. Catheter-directed foam sclerotherapy of great Saphenous veins in combination with pre-treatment reduction of the diameter employing the principals of Perivenous tumescent local anesthesia. Eur J Vasc Endovasc Surg. 2014;47(2):187–195. doi: 10.1016/j.ejvs.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 486.Myers KA, Jolley D, Clough A, Kirwan J. Outcome of ultrasound-guided Sclerotherapy for varicose veins: medium-term results assessed by ultrasound surveillance. Eur J Vasc Endovasc Surg. 2007;33(1):116–121. doi: 10.1016/j.ejvs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 487.Zhang J, Jing Z, Schliephake DE, Otto J, Malouf GM, Gu Y-Q. Efficacy and safety of Aethoxysklerol ® (polidocanol) 0.5 %, 1 % and 3 % in comparison with placebo solution for the treatment of varicose veins of the lower extremities in Chinese patients (ESA-China Study) Phlebol J Venous Dis. 2012;27(4):184–190. doi: 10.1258/phleb.2011.010094. [DOI] [PubMed] [Google Scholar]
- 488.Guex JJ. Ultrasound guided sclerotherapy (USGS) for perforating veins (PV) Hawaii Med J. 2000;59(6):261–262. [PubMed] [Google Scholar]
- 489.Masuda EM, Kessler DM, Lurie F, Puggioni A, Kistner RL, Eklof B. The effect of ultrasound-guided sclerotherapy of incompetent perforator veins on venous clinical severity and disability scores. J Vasc Surg. 2006;43(3):551–557. doi: 10.1016/j.jvs.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 490.van Neer P, Veraart JCJM, Neumann H. Posterolateral thigh perforator varicosities in 12 patients: a normal deep venous system and successful treatment with ultrasound-guided sclerotherapy. Dermatologic Surg. 2006;32(11):1346–1352. doi: 10.1111/j.1524-4725.2006.32304.x. [DOI] [PubMed] [Google Scholar]
- 491.Kahle B, Leng K. Efficacy of sclerotherapy in varicose veins—a prospective, blinded, placebo-controlled study. Dermatol Surg. 2004;30(5):723–728. doi: 10.1111/j.1524-4725.2004.30207.x. [DOI] [PubMed] [Google Scholar]
- 492.Norris MJ, Carlin MC, Ratz JL. Treatment of essential telangiectasia: effects of increasing concentrations of polidocanol. J Am Acad Dermatol. 1989;20(4):643–649. doi: 10.1016/S0190-9622(89)70077-3. [DOI] [PubMed] [Google Scholar]
- 493.Kahle B, Denk K, Schliephake D, Recke A. Effektivität der Sklerosierungstherapie in Abhängigkeit vom Alter der Patienten. Phlebologie. 2010;39(04):202–207. doi: 10.1055/s-0037-1622314. [DOI] [Google Scholar]
- 494.Kakkos SK, Bountouroglou DG, Azzam M, Kalodiki E, Daskalopoulos M, Geroulakos G. Effectiveness and safety of ultrasound-guided foam sclerotherapy for recurrent varicose veins: immediate results. j Endovasc Ther. 2006;13(3):357–364. doi: 10.1583/05-1781.1. [DOI] [PubMed] [Google Scholar]
- 495.McDonagh B, Sorenson S, Gray C, et al. Clinical spectrum of recurrent postoperative varicose veins and efficacy of sclerotherapy management using the compass technique. phlebol J Venous Dis. 2003;18(4):173–186. doi: 10.1258/026835503322597992. [DOI] [Google Scholar]
- 496.Smith PC. Chronic venous disease treated by ultrasound guided foam sclerotherapy. Eur J Vasc Endovasc Surg. 2006;32(5):577–583. doi: 10.1016/j.ejvs.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 497.Coleridge Smith P. Sclerotherapy and foam sclerotherapy for varicose veins. Phlebology. 2009;24(6):260–269. doi: 10.1258/phleb.2009.009050. [DOI] [PubMed] [Google Scholar]
- 498.Bradbury AW, Bate G, Pang K, Darvall KA, Adam DJ. Ultrasound-guided foam sclerotherapy is a safe and clinically effective treatment for superficial venous reflux. J Vasc Surg. 2010;52(4):939–945. doi: 10.1016/j.jvs.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 499.Darvall KAL, Bate GR, Adam DJ, Silverman SH, Bradbury AW. Duplex ultrasound outcomes following ultrasound-guided foam sclerotherapy of symptomatic recurrent great Saphenous varicose veins. Eur J Vasc Endovasc Surg. 2011;42(1):107–114. doi: 10.1016/j.ejvs.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 500.Sukovatykh BS, Rodionov OA, Sukovatykh MB, Khodykin SP. Diagnosis and treatment of atypical forms of varicose disease of pelvic veins. Vestn Khir Im I I Grek. 2008;167(3):43–45. [PubMed] [Google Scholar]
- 501.Paraskevas P. Successful ultrasound-guided foam sclerotherapy for vulval and leg varicosities secondary to ovarian vein reflux: a case study. Phlebology. 2011;26(1):29–31. doi: 10.1258/phleb.2009.009086. [DOI] [PubMed] [Google Scholar]
- 502.Stücker M, Reich S, Hermes N, Altmeyer P. Safety and efficiency of perilesional sclerotherapy in leg ulcer patients with postthrombotic syndrome and/or oral anticoagulation with phenprocoumon. J Dtsch Dermatol Ges. 2006;4(9):734–738. doi: 10.1111/j.1610-0387.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- 503.De Waard M, Der Kinderen D. Duplex ultrasonography—guided foam sclerotherapy of incompetent perforator veins in a patient with bilateral venous leg ulcers. Dermatol Surg. 2005;31(5):580–583. doi: 10.1097/00042728-200505000-00018. [DOI] [PubMed] [Google Scholar]
- 504.Hertzman PA, Owens R. Rapid healing of chronic venous ulcers following ultrasound-guided foam sclerotherapy. Phlebology. 2007;22(1):34–39. doi: 10.1258/026835507779700662. [DOI] [PubMed] [Google Scholar]
- 505.Pang KH, Bate GR, Darvall KAL, Adam DJ, Bradbury AW. Healing and recurrence rates following ultrasound-guided foam sclerotherapy of superficial venous reflux in patients with chronic venous ulceration. Eur J Vasc Endovasc Surg. 2010;40(6):790–795. doi: 10.1016/j.ejvs.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 506.Yamaki T, Nozaki M, Sasaki K. Color duplex-guided sclerotherapy for the treatment of venous malformations. Dermatol Surg. 2000;26(4):323–328. doi: 10.1046/j.1524-4725.2000.99248.x. [DOI] [PubMed] [Google Scholar]
- 507.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Prospective randomized efficacy of ultrasound-guided foam sclerotherapy compared with ultrasound-guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J Vasc Surg. 2008;47(3):578–584. doi: 10.1016/j.jvs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 508.Blaise S, Charavin-Cocuzza M, Riom H, et al. Treatment of low-flow vascular malformations by ultrasound-guided sclerotherapy with polidocanol foam: 24 cases and literature review. Eur J Vasc Endovasc Surg. 2011;41(3):412–417. doi: 10.1016/j.ejvs.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 509.Drake L, Guidelines/Outcomes Committee, Force T. Guidelines of care for sclerotherapy treatment of varicose and telangiectatic leg veins. J Am Acad Dermatol. 1996;34(3):523–528. doi: 10.1016/S0190-9622(96)90467-3. [DOI] [PubMed] [Google Scholar]
- 510.Rabe E, Pannier-Fischer F, Gerlach H, Breu FX, Guggenbichler S, Zabel M. Guidelines for sclerotherapy of varicose veins (ICD 10: I83.0, I83.1, I83.2, and I83.9) Dermatol Surg. 2004;30(5):687–693. doi: 10.1111/j.1524-4725.2004.30201.x. [DOI] [PubMed] [Google Scholar]
- 511.Rabe E, Pannier F, Gerlach H, Breu FX, Guggenbichler S, Wollmann JC. Leitlinie: Sklerosierungsbehandlung der Varikose. Phlebologie. 2008;37(1):27–34. doi: 10.1055/s-0037-1622205. [DOI] [Google Scholar]
- 512.Breu FX, Guggenbichler S, Wollmann JC, Second European Consensus Meeting on Foam Sclerotherapy Duplex ultrasound and efficacy criteria in foam sclerotherapy from the 2nd European Consensus Meeting on Foam Sclerotherapy 2006, Tegernsee, Germany. Vasa. 2008;37(1):90–95. doi: 10.1024/0301-1526.37.1.90. [DOI] [PubMed] [Google Scholar]
- 513.Guex J-J. Contra indications of sclerotherapy, update 2005. J Mal Vasc. 2005;30(3):144–149. doi: 10.1016/S0398-0499(05)83831-4. [DOI] [PubMed] [Google Scholar]
- 514.Kreussler (ed) (2009) Fachinformationen Aethoxysklerol 0,25 %/0,5 %/1 %/2 %/3 % Stand Oktober 2009. Chemische Fabrik Kreussler Co
- 515.Wollmann J-C. The history of sclerosing foams. Dermatol Surg. 2004;30(5):694–703. doi: 10.1111/j.1524-4725.2004.30208.x. [DOI] [PubMed] [Google Scholar]
- 516.Wollmann JC. 60 Jahre Sklerosierungsschaum. Phlebologie. 2004;33(2):63–70. doi: 10.1055/s-0037-1621401. [DOI] [Google Scholar]
- 517.Alòs J, Carreño P, López JA, Estadella B, Serra-Prat M, Marinel-lo J. Efficacy and safety of sclerotherapy using polidocanol foam: a controlled clinical trial. Eur J Vasc Endovasc Surg. 2006;31(1):101–107. doi: 10.1016/j.ejvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 518.Hamel-Desnos C, Ouvry P, Benigni J-P, et al. Comparison of 1 % and 3 % polidocanol foam in ultrasound guided sclerotherapy of the great saphenous vein: a randomised, double-blind trial with 2 year-follow-up. “the 3/1 study.”. Eur J Vasc Endovasc Surg. 2007;34(6):723–729. doi: 10.1016/j.ejvs.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 519.Kern P, Ramelet A-A, Wutschert R, Bounameaux H, Hayoz D. Single-blind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30(3):367–372. doi: 10.1111/j.1524-4725.2004.30102.x. [DOI] [PubMed] [Google Scholar]
- 520.Rao J, Wildemore JK, Goldman MP. Double-blind prospective comparative trial between foamed and liquid polidocanol and sodium tetradecyl sulfate in the treatment of varicose and Telangiectatic leg veins. Dermatol Surg. 2005;31(6):631–635. doi: 10.1097/00042728-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 521.Rathbun S, Norris A, Stoner J. Efficacy and safety of endovenous foam sclerotherapy: meta-analysis for treatment of venous disorders. Phlebology. 2012;27(3):105–117. doi: 10.1258/phleb.2011.011111. [DOI] [PubMed] [Google Scholar]
- 522.Ceulen R, Bullens-Goessens Y, Pi-Van De Venne S, Nellemans P, Veraart J, Sommer A. Outcomes and side effects of duplex-guided sclerotherapy in the treatment of great saphenous veins with 1 % versus 3 % polidocanol foam: results of a randomized controlled trial with 1-year follow-up. Dermatol Surg. 2007;33(3):276–281. doi: 10.1111/j.1524-4725.2007.33062.x. [DOI] [PubMed] [Google Scholar]
- 523.Brodersen J, Geismar U. Catheter-assisted vein sclerotherapy: a new approach for sclerotherapy of the greater Saphenous vein with a double-lumen balloon catheter. Dermatol Surg. 2007;33(4):469–475. doi: 10.1111/j.1524-4725.2007.33095.x. [DOI] [PubMed] [Google Scholar]
- 524.Guex J-J. Complications of sclerotherapy: an update. Dermatol Surg. 2010;36(Suppl 2):1056–1063. doi: 10.1111/j.1524-4725.2009.01409.x. [DOI] [PubMed] [Google Scholar]
- 525.Kendler M, Averbeck M, Simon JC, Ziemer M. Histology of saphenous veins after treatment with the ClariVein® device—an ex-vivo experiment. J. Dtsch. Dermatol. Ges. 2013;11(4):348–352. doi: 10.1111/ddg.12022. [DOI] [PubMed] [Google Scholar]
- 526.Whiteley MS, Dos SSJ, Lee CT, J-MM L. Mechanochemical ablation causes endothelial and medial damage to the vein wall resulting in deeper penetration of sclerosant compared with sclerotherapy alone in extrafascial great saphenous vein using an ex vivo model. J Vasc Surg Venous Lymphat Disord. 2017;5(3):370–377. doi: 10.1016/j.jvsv.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 527.van Eekeren RRJP, Hillebrands JL, van der Sloot K, de Vries J-PPM, Zeebregts CJ, Reijnen MMPJ. Histological observations one year after mechanochemical endovenous ablation of the great Saphenous vein. j Endovasc Ther. 2014;21(3):429–433. doi: 10.1583/13-4588MR.1. [DOI] [PubMed] [Google Scholar]
- 528.Mueller RL, Raines JK. Clarivein mechanochemical ablation. Vasc Endovascular Surg. 2013;47(3):195–206. doi: 10.1177/1538574413477216. [DOI] [PubMed] [Google Scholar]
- 529.Elias S, Raines JK. Mechanochemical tumescentless endovenous ablation: final results of the initial clinical trial. Phlebology. 2012;27(2):67–72. doi: 10.1258/phleb.2011.010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Whiteley MS. Glue, steam and Clarivein—best practice techniques and evidence. Phlebology. 2015;30(2 Suppl):24–28. doi: 10.1177/0268355515591447. [DOI] [PubMed] [Google Scholar]
- 531.Wittens C, Davies AH, Baekgaard N, et al. Editor’s choice—management of chronic venous disease: clinical practice guidelines of the European society for vascular surgery (ESVS)349. Eur J Vasc Endovasc Surg. 2015;49(6):678–737. doi: 10.1016/j.ejvs.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 532.NICE (2016) National Institute for Health and Care Excellence. Endovenous mechanochemical ablation for varicose veins. nice.org.uk/guidance/ipg557
- 533.Moore HM, Lane TR, Franklin IJ, Davies AH. Retrograde mechanochemical ablation of the small saphenous vein for the treatment of a venous ulcer. Vascular. 2014;22(5):375–377. doi: 10.1177/1708538113516320. [DOI] [PubMed] [Google Scholar]
- 534.Baccaglini U, Spreafico G, Castoro C, Sorrentino P. Consensus conference on sclerotherapy or varicose veins of the lower limbs. Phlebology. 1997;12(1):2–16. doi: 10.1177/026835559701200102. [DOI] [PubMed] [Google Scholar]
- 535.Rabe E. Grundlagen Der Phlebologie. 3. Köln: Viavital; 2003. [Google Scholar]
- 536.Vin F. Principes de la Sclérothéraphie des Troncs Saphènes Internes. Phlebologie. 1997;50:229–341. [Google Scholar]
- 537.Pannier F, Rabe E, the Guideline Group Results from RCTs in sclerotherapy: European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(1 suppl):39–44. doi: 10.1177/0268355514528133. [DOI] [PubMed] [Google Scholar]
- 538.Villavicencio J, Pfeifer J, Lohr J, Goldman M, Cranley R, Spence R. Sclerotherapy for varicose veins: practice guidelines and sclerotherapy procedures. In: Glovicki P, Yao J, editors. Handbook of venous disorders. London: ChapmannHall Medical; 1996. pp. 337–354. [Google Scholar]
- 539.Hamel-Desnos CM, Gillet J-L, Desnos PR, Allaert FA. Sclerotherapy of varicose veins in patients with documented thrombophilia: a prospective controlled randomized study of 105 cases. Phlebol J Venous Dis. 2009;24(4):176–182. doi: 10.1258/phleb.2009.008081. [DOI] [PubMed] [Google Scholar]
- 540.Guex JJ, Schliephake DE, Otto J, Mako S, Allaert FA. The French polidocanol study on long-term side effects. Dermatol Surg. 2010;36(Sup):993–1003. doi: 10.1111/j.1524-4725.2009.01407.x. [DOI] [PubMed] [Google Scholar]
- 541.Sullivan LP, Quach G, Chapman T. Retrograde mechanico-chemical endovenous ablation of infrageniculate great saphenous vein for persistent venous stasis ulcers. Phlebology. 2014;29(10):654–657. doi: 10.1177/0268355513501301. [DOI] [PubMed] [Google Scholar]
- 542.van Eekeren RRJP, Boersma D, Elias S, et al. Endovenous mechanochemical ablation of great Saphenous vein incompetence using the clarivein device: a safety study. j Endovasc Ther. 2011;18(3):328–334. doi: 10.1583/11-3394.1. [DOI] [PubMed] [Google Scholar]
- 543.Bishawi M, Bernstein R, Boter M, et al. Mechanochemical ablation in patients with chronic venous disease: a prospective multicenter report. Phlebol J Venous Dis. 2014;29(6):397–400. doi: 10.1177/0268355513495830. [DOI] [PubMed] [Google Scholar]
- 544.Boersma D, van Eekeren RRJP, Werson DAB, van der Waal RIF, Reijnen MMJP, de Vries J-PPM. Mechanochemical endovenous ablation of small Saphenous vein insufficiency using the Clarivein® device: one-year results of a prospective series. Eur J Vasc Endovasc Surg. 2013;45(3):299–303. doi: 10.1016/j.ejvs.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 545.Bootun R, Lane TRAA, Dharmarajah B, et al. Intra-procedural pain score in a randomised controlled trial comparing mechanochemical ablation to radiofrequency ablation: The Multicentre VenefitTM versus ClariVein® for varicose veins trial. Phlebology. 2016;31(1):61–65. doi: 10.1177/0268355514551085. [DOI] [PubMed] [Google Scholar]
- 546.Vun SV, Rashid ST, Blest NC, Spark JI. Lower pain and faster treatment with mechanico-chemical endovenous ablation using ClariVein. Phlebology. 2015;30(10):688–692. doi: 10.1177/0268355514553693. [DOI] [PubMed] [Google Scholar]
- 547.Witte ME, Reijnen MMPJ, de Vries J-P, Zeebregts CJ. Mechanochemical endovenous occlusion of varicose veins using the Clarivein® device. Surg Technol Int. 2015;26:219–225. [PubMed] [Google Scholar]
- 548.Ozen Y, Cekmecelioglu D, Sarikaya S, et al. Mechano-chemical endovenous ablation of great Saphenous vein insufficiency:two-year results. Damar Cer Derg. 2014;23(3):176. doi: 10.9739/uvcd.2014-41766. [DOI] [Google Scholar]
- 549.Morrison N, Gibson K, McEnroe S, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose) J Vasc Surg. 2015;61(4):985–994. doi: 10.1016/j.jvs.2014.11.071. [DOI] [PubMed] [Google Scholar]
- 550.Proebstle TM, Alm J, Dimitri S, et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2015;3(1):2–7. doi: 10.1016/j.jvsv.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 551.Morrison N, Gibson K, Vasquez M, et al. VeClose trial 12-month outcomes of cyanoacrylate closure versus radiofrequency ablation for incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2017;5(3):321–330. doi: 10.1016/j.jvsv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 552.Gibson K, Morrison N, Kolluri R, et al. Twenty-four month results from a randomized trial of cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2018;6(5):606–613. doi: 10.1016/j.jvsv.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 553.Morrison N, Kolluri R, Vasquez M, Madsen M, Jones A, Gibson K. Comparison of cyanoacrylate closure and radiofrequency ablation for the treatment of incompetent great saphenous veins: 36-Month outcomes of the VeClose randomized controlled trial. Phlebology. 2018 doi: 10.1177/0268355518810259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Chan YC, Law Y, Cheung GC, Ting AC, Cheng SW. Cyanoacrylate glue used to treat great saphenous reflux: measures of outcome. Phlebology. 2017;32(2):99–106. doi: 10.1177/0268355516638200. [DOI] [PubMed] [Google Scholar]
- 555.Fowkes F, Lee A, Evans C, Allan P, Bradbury A, Ruckley C. Lifestyle risk factors for lower limb venous reflux in the general population: Edinburgh Vein Study. Int J Epidemiol. 2001;30(4):846–852. doi: 10.1093/ije/30.4.846. [DOI] [PubMed] [Google Scholar]
- 556.Mota-Capitão L, Menezes JD, Gouveia-Oliveira A. Clinical predictors of the severity of chronic venous insufficiency of the lower limbs: a multivariate analysis. Phlebol J Venous Dis. 1995;10(4):155–159. doi: 10.1177/026835559501000406. [DOI] [Google Scholar]
- 557.Jukkola TM, Mäkivaara LA, Luukkaala T, Hakama M, Laurikka J. The effects of parity, oral contraceptive use and hormone replacement therapy on the incidence of varicose veins. J Obstet Gynaecol. 2006;26(5):448–451. doi: 10.1080/01443610600747389. [DOI] [PubMed] [Google Scholar]
- 558.Ropacka-Lesiak M, Kasperczak J, Breborowicz GH. Risk factors for the development of venous insufficiency of the lower limbs during pregnancy—part 1. Ginekol Pol. 2012;83(12):939–942. [PubMed] [Google Scholar]
- 559.Fischer R, Chandler JG, Stenger D, Puhan MA, De Maeseneer MG, Schimmelpfennig L. Patient characteristics and physician-determined variables affecting saphenofemoral reflux recurrence after ligation and stripping of the great saphenous vein. J Vasc Surg. 2006;43(1):81–87. doi: 10.1016/j.jvs.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 560.Zubilewicz R, Chmiel-Perzyńska I, Derkacz M, Schabowski J. The women’s span of knowledge about chronic venous disease. Fam Med Prim Care Rev. 2009;11(4):919–922. [Google Scholar]
- 561.Klemetti R, Kurinczuk JJ, Redshaw M. Older women’s pregnancy related symptoms, health and use of antenatal services. Eur J Obstet Gynecol Reprod Biol. 2011;154(2):157–162. doi: 10.1016/j.ejogrb.2010.10.025. [DOI] [PubMed] [Google Scholar]