Abstract
Chronic inflammation is a well-established risk factor for several diseases, including cancer. It influences tumor cell biology and the type and density of immune cells in the tumor microenvironment (TME), promoting cancer development. While pro-inflammatory cytokines and chemokines modulate cancer development, emerging evidence has shown that prostaglandin E2 (PGE2) is a known mediator connecting chronic inflammation to cancerization. This review highlights recent advances in our understanding of how the elevation of PGE2 production promotes gastrointestinal cancer initiation, progression, invasion, metastasis, and recurrence, including modulation of immune checkpoint signaling and the type and density of immune cells in the tumor/tissue microenvironment.
Keywords: Inflammation, PGE2, colorectal cancer, gastric cancer, esophageal cancer, hepatocellular cancer, immune evasion, checkpoint inhibition
Introduction:
Inflammation is a complex process triggered by physical or chemical injury or an infectious or autoimmune disease involving numerous types of immune cells, cytokines, bioactive lipids, and chemokines. The inflammatory response is a defense mechanism needed to protect the organism from infection and injury and can be divided into at least two stages, acute and chronic. During acute inflammation, pathogens are eliminated from the host, resulting in tissue healing and repair of the affected site. In most cases, the outcome of this acute inflammation is self-limiting and resolves. However, the inability to achieve resolution leads to a continued, persistent, abnormal inflammatory state, which causes chronic inflammation. Chronic inflammatory processes are more indolent and prominent in auto-immune, neurodegenerative, vascular, and arthritic diseases. During chronic inflammation, the resident immune cells such as lymphocytes and macrophages continuously secrete proinflammatory cytokines and chemokines that chronically recruit, attract, and activate other immune cells (1). In the tumor microenvironment (TME), tumor cell biology is significantly influenced by surrounding fibroblasts, endothelial cells, immune cells, and mediators that they produce.
The immune system in the surrounding TME exhibits a critical influence in creating either a pro-tumorigenic or anti-tumorigenic niche, depending on the mix of cytokines, bioactive lipids, and chemokines present (2). Currently, terms such as “hot” tumors are used to describe TME with increased levels of T-cell infiltration and other components necessary for anti-tumor immunity. In contrast, “cold” tumors are described TMEs with less T-cell infiltration and with massive infiltration of immunosuppressive cells such as myeloid-derived suppressor cells (MDSC) and T regulatory cells (Tregs). While these terms are helpful for tumor characterization, they do little to explain the pro-tumorigenic immune response in cancers, which plays a critical role in the clinical outcome and has been termed “cancer-promoting inflammation”—distinct from acute and chronic forms of inflammation (3). Chronic or tumor-elicited inflammation can affect the TME resulting in tumor evasion, providing tumor-promoting signals that stimulate further tumor growth, progression, and metastatic spread (4).
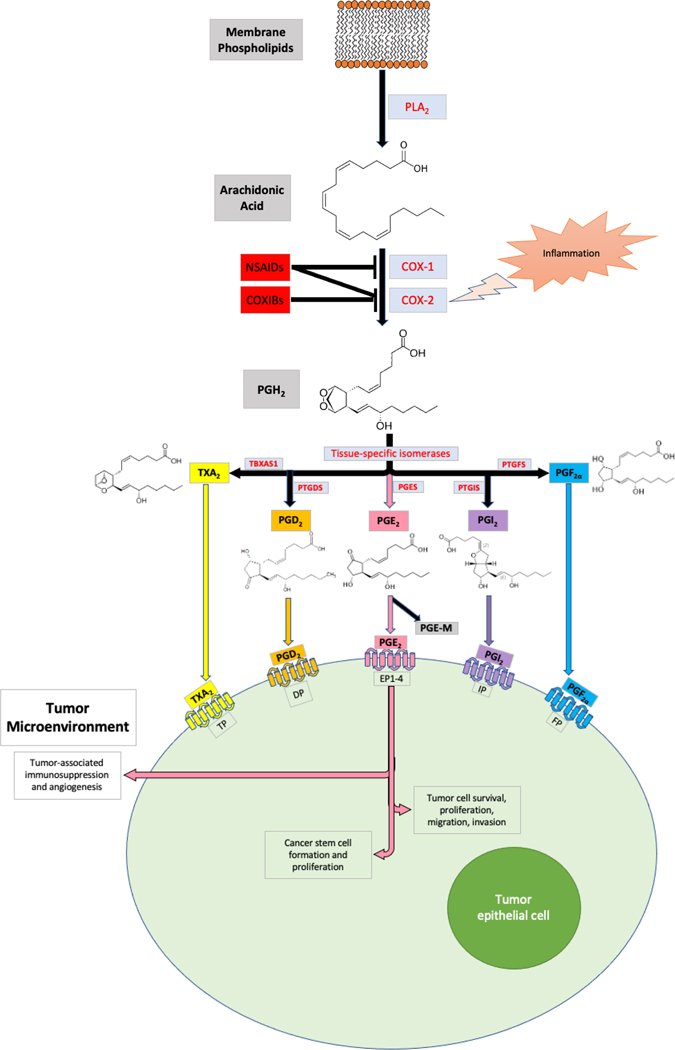
Cyclooxygenase-1 (COX-1), officially named prostaglandin-endoperoxide synthase 1 (PTGS1), and cyclooxygenase-2 (COX-2), formally known as prostaglandin-endoperoxide synthase 2 (PTGS2), are enzymes that convert arachidonic acid into endoperoxide intermediates that are ultimately metabolized to prostaglandins, including PGD2, PGE2, PGF2α, PGI2 (prostacyclin), and thromboxane A2 (TXA2) (1,5) (Figure 1). COX-1 is constitutively expressed in most tissues and is thought to provide basal levels of prostaglandins and thromboxanes for tissue homeostasis and platelet activation. By contrast, COX-2 is rarely expressed in healthy tissues but is highly induced in inflammatory sites and is overexpressed in certain cancers, particularly in over 85% of sporadic colorectal cancers (CRC) and 50% of colorectal adenomas (6–8). Elevated levels of COX-2 were also found in gastric cancer (GC) and esophageal cancer (EC) (9,10). The biological role of COX-2 in inflammation and cancer depends on which prostaglandins are produced in the affected tissue compartment. For example, PGE2 is partly responsible for the cardinal signs of inflammation (1,5), and both PGE2 and PGI2 play critical roles in arthritis and inflammatory bowel diseases (IBD) (11). In addition, PGE2 is the most abundant prostaglandin produced in CRC and GC (11). PGE2 and PGI2 were the main two prostanoids significantly increased in CRC (12). PGE2 exerts cellular effects by binding to cell surface G-protein coupled receptors designated as PGE2 receptors (EP1-EP4).
Figure 1. Prostanoid Synthesis Overview.
Free cellular AA is released after PLA2-mediated conversion of membrane phospholipids. AA can be converted into PGH2 via COX-1 and −2. COX-2 is upregulated only in inflamed tissues. Both COX-1 and −2 are inhibited by non-specific NSAIDs, whereas COXIB compounds may selectively target COX-2. PGH2 may be converted into all five prostanoids via tissue-specific isomerases: TXA2 by TBXAS1; PGD2 by PTGDS; PGE2 by PGES; PGI2 by PTGIS; and PGF2α by PTGFS. Each prostanoid may bind to its respective GPCR on the tumor epithelial cell to exert intracellular effects. Most notably, PGE2 promotes cancer development and progression through numerous mechanisms, as outlined by the text and figures 2 and 3.
Elevation of PGE2 in tumor tissues results from COX-2 and PGE2 synthase induction and marked reduction of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) levels. 15-PGDH converts PGE2 to an “inactive” 15-keto PGE2 which is further metabolized to a stable end urinary PGE2 metabolite (PGE-M). As 15-PGDH levels decrease substantially in GC, CRC, and EC (13–16), PGE-M may serve as a valuable biomarker for predicting cancer risk and prognosis. Several studies have revealed that urinary PGE-M levels are significantly increased in CRC and GC and are associated with worse patient outcomes (17–19). The daily use of aspirin results in lower urinary PGE-M levels in healthy and cancer patients (20). Similarly, low-dose daily aspirin (100 mg per day for seven days) also led to a 46% decrease in PGE2 levels in human colorectal mucosa (21).
In this review, we highlight the role of the COX-2-PGE2 pathway in gastrointestinal (GI) cancers. We also explain why this pathway may serve as valuable targets for cancer prevention and treatment.
COX-2 and PGE2 in gastrointestinal cancers:
Chronic inflammation is a well-known predisposing factor for various GI cancers. For instance, chronic viral infections such as hepatitis B or C viruses are strongly associated with hepatocellular carcinoma (HCC), and human papillomaviruses (HPV) are associated with a three-fold higher risk of esophageal squamous cell carcinoma (22,23). Importantly, it is well established that all cervical cancer and 70% of oropharyngeal cancers are caused by HPV. In addition, chronic bacterial infections such as Helicobacter pylori in the gastric fundus and pylorus result in increased risk and rates of GC (24). Interestingly, some changes in the gut microbiome leading to dysbiosis have been shown to increase the risk of CRC as well (25).
Furthermore, IBD caused by immune system dysfunction increases the risk for CRC (26). The observation that nonsteroidal anti-inflammatory drugs (NSAIDs) have beneficial effects on reducing the incidence, metastasis, and mortality of various solid tumors (11), including GI cancer, supports the concept that COX-2 derived prostaglandins promote cancer development because the anti-tumor effects of these agents are due, in part, to inhibition of cyclooxygenase (COX) activity. Indeed, COX-2 levels are associated with shortened survival in patients with CRC, GC, and EC (27–30). In addition, high COX-2 expression in HCC was strongly associated with decreased survival rates, enhanced lymphatic/vascular invasion, and advanced TNM stages (31).
Colorectal cancer
The effect of NSAIDs, including COX-2 selective inhibitors, on clinical outcomes has been extensively investigated in patients with CRC. In a prospective cohort study of 82,911 women over 20 years, sporadic CRC rates were shown to decrease significantly with regular, long-term NSAID use (32). A randomized controlled trial (RCT) supported the effect of sulindac on adenoma regression in FAP patients (33). In addition, an RCT revealed that treatment of a COX-2 selective inhibitor, celecoxib, significantly reduced polyp burden in FAP patients (34). In addition, three double-blind RCTs, including the Adenoma Prevention with celecoxib (APC), the Adenomatous polyp Prevention on Vioxx (APPROVe) trial, and the Prevention of Colorectal Sporadic Adenomatous Polyps (PreSAP) trials, demonstrated significant reductions in adenoma recurrence following treatment with selective COX-2 inhibitors in patients with a history of sporadic CRC (35–37). Furthermore, several clinical trials revealed that short-term aspirin treatment minimized adenoma recurrence in patients with a history of CRC (38–40). Another RCT showed a 24% decrease in CRC incidence and a 35% decrease in CRC mortality after 20 years of daily aspirin use (41).
Although the molecular mechanisms underlying the anti-tumor effects of NSAIDs, especially aspirin, have not been fully understood, compelling evidence demonstrates that these agents’ inhibitory effects are partly due to the reduction of PGE2 production by inhibiting COX-1 and COX-2. Indeed, cohort studies showed that regular aspirin use significantly reduced tumor recurrence in patients whose sporadic CRC expressed elevated levels of COX-2 (42), and its use after the diagnosis of CRC at stages I, II, and III improved overall survival, especially among individuals whose tumors overexpress COX-2 (43). However, a 2018 ASPREE trial showed that cancer-related mortality was 3.1% in the aspirin group compared to 2.3% in the placebo group (44) in a 5-year study. Since participants in the ASPREE had only been followed for a median of approximately five years, it has been noted that they will need to be studied for a more extended period to understand better the potential effect of aspirin on cancer diagnoses and deaths. Further studies are required to determine whether aspirin’s impact on cancer in older adults is quite different from other-age adults.
Crohn’s disease and ulcerative colitis predispose affected individuals to higher risk for CRC. Prolonged use of aminosalicylate in patients with ulcerative colitis has been shown to reduce CRC risk (45,46) significantly. Likewise, the use of etoricoxib in a double-blinded RCT study showed protection against CRC in IBD patients (45). Furthermore, a recent case-control study showed that long-term use of 5-aminosalicylate resulted in a dramatic reduction in CRC rates in IBD patients (46). However, there is ongoing concern about the chronic use of NSAIDs in patients with IBD, as this can cause disease flares that are sometimes quite severe.
Obesity is a risk factor for several comorbidities and cancer, and its association with CRC has been widely studied. Unlike IBD-associated CRC studies, obesity-associated CRC studies have been less conclusive regarding NSAID effects. An epidemiologic study recently demonstrated that long-term aspirin use resulted in lower colorectal adenoma recurrence rates in overweight individuals compared to normal-weight individuals (47). However, two prospective cohort studies demonstrated that the effect of aspirin on CRC risk is independent of the body mass index (48). Further studies are needed to determine whether aspirin reduces CRC risk in obese individuals.
Evidence of colorectal tumor promotion by PGE2 was initially demonstrated in mouse models of sporadic CRC and FAP. PGE2 treatment dramatically increased both small and large intestinal adenoma burden in ApcMin/+ mice and significantly enhanced azoxymethane (AOM)-induced colon tumor incidence and multiplicity (49,50). PGE2 also reverses the anti-tumor effects of NSAIDs in the ApcMin/+ mouse model (51). Likewise, the elevation of endogenous PGE2 by genetically deleting 15-PGDH increased tumor burden in ApcMin/+ and AOM treated mice (52). In contrast, genetic deletion of microsomal PGE2 synthase 1 (mPGES-1) in ApcMin/+ and AOM mice decreased tumor formation in the small and large intestines (53). Moreover, one study revealed that loss of EP2 in ApcΔ716 mice resulted in reduced intestinal tumor burden, while lack of EP1 and EP3 did not (54). Similarly, deletion of EP1 or EP4, but not EP3, attenuates AOM-induced aberrant crypt foci (55,56). Interestingly, one report indicated that loss of EP3 promoted colon tumor development in AOM-treated mice (57). In addition, loss of EP2 in a colitis-associated CRC mouse model resulted in a decrease in colonic tumor formation, while loss of EP1 and EP3 increased tumor numbers (58). These studies concluded that the pro-tumorigenic effects of PGE2 are mainly mediated through its activation of the EP2 and EP4 receptors and probably not EP1 or EP3.
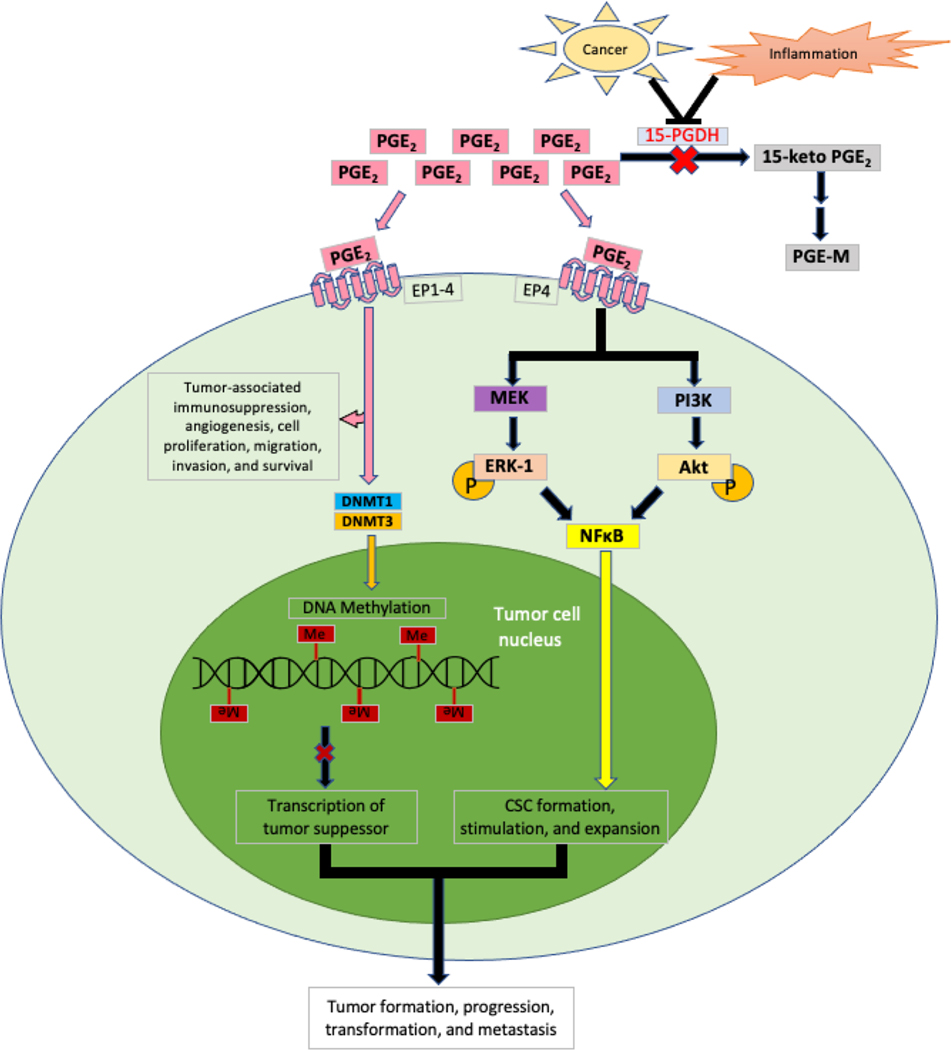
PGE2 binds to its receptors initiating intracellular signaling pathways that cause tumor-associated immunosuppression, angiogenesis, cell proliferation, migration, invasion, and survival (59,60) (Figure 2). For example, PGE2 promotes intestinal adenoma formation and growth by silencing tumor suppressor genes via induction of DNA methyltransferases DNMT1/DNMT3B expression (61). In addition, PGE2 promotes colonic cancer stem cell (CSC) formation and expansion by activation of NF-κB via the EP4-PI3K/MEK/MAPK pathway (62,63). A recent report showed that treatment of ApcMin/+ mice with celecoxib reduces PD-L1 expression in premalignant adenomas accompanied by an influx of CD8+ T cells (64). Moreover, another study showed that aspirin reduced tumor growth accompanied by reduction of PD-1 in CD8+ T cells and macrophages via increasing pro-resolving mediators in a mouse model of colitis-associated tumorigenesis (65). An in vitro study revealed that the COX-2-PGE2 pathway mediated the effect of bladder tumor cells on induction of PD-L1 in bone marrow-derived macrophages and MDSCs in a co-culture system (66). These PD-L1 positive cells were immunosuppressive (66). Moreover, PGE2 also inhibited alveolar macrophage phagocytosis against bacteria (67). In addition, recent studies revealed that combined inhibition of PGE2 signaling and PD-1 increased CTL proliferation in vitro (68), and EP4 antagonists enhanced antitumor efficacy of PD-1 in a syngeneic mouse model of CRC (69) and a mouse model of colitis-associated tumorigenesis (70).
Figure 2. Regulation of tumor formation by PGE2.
Acting through EP1–4, PGE2 promotes tumor-associated immunosuppression, angiogenesis, and cell proliferation, as well as migration, invasion, and survival. In addition, it activates DNA methyltransferases (i.e., DNMT1/DNMT3B) to methylate and silence transcription tumor suppressor genes. Acting through EP4, PGE2 promotes CSC formation, stimulation, and expansion via activation of NFκB through the MEK/MAPK and PI3K/AKT pathways. Overall, these processes enhance tumor formation, progression, transformation, and metastasis.
Gastric cancer
Like CRC, population-based case-control and cohort have illustrated that regular and long-term use of NSAIDs, including aspirin, significantly decreased GC malignancy incidence and mortality (71,72). In vivo studies showed that simultaneous overexpression of both COX-2 and mPGES-1 in gastric cells was sufficient to induce hyperplastic gastric lesions (73), which progressed to tumors with simultaneous activation of Wnt signaling (74). The cooperative activation of Wnt and COX-2-PGE2 pathways could induce CD44+ slow-cycling tumor growth and expansion in vivo (75), indicating that PGE2 enhances gastric CSC expansion. PGE2 also promoted gastric tumor formation and growth by silencing tumor suppressor genes (i.e., MGMT and CNR1) via induction of DNMT3B expression in vivo (76). In addition, activation of the COX-2/PGE2 pathway may induce IL-11, CXCL1, CXCL2, and CXCL5 expression in human and mouse gastric tumors (77) and modulate the type and density of immune cells in the tumor microenvironment.
Esophageal cancer
Esophageal cancer (EC) in the upper two-thirds of the esophagus is most commonly characterized by the presence of squamous cell carcinomas (SCC) that are usually due to long-term alcohol and smoke exposure. In contrast, esophageal adenocarcinomas (EAC) in the lower one-third are generally associated with the metaplastic changes of Barrett’s esophagus after prolonged gastroesophageal reflux disease (GERD) (78). These risk factors usually correlate with COX-2 elevation in these cancers (79). One meta-analysis study found that a particular COX-2 polymorphism is associated with the esophageal SCC and EAC formation in Asian populations, whereas another COX-2 polymorphism is only associated with EAC formation in Caucasian populations (80). Another recent meta-analysis revealed that aspirin use reduced incidence rates of both SCC and EAC (81). Two other studies showed that the use of aspirin and other COX-2 selective inhibitors reduced the risk of EAC (82,83). COX-2 is thought to drive Barret’s esophagus (BE) and EAC formation by increasing TXA2 (84). Interestingly, one study demonstrated that esophageal SCC patients with higher COX-2 levels in tumor tissues had lower response rates to neoadjuvant chemoradiotherapy (85).
An in vivo study revealed that treatment with a COX-2 inhibitor (JTE-522) significantly inhibited NMBA-induced esophageal SCC tumorigenesis (86). In addition, a selective COX-2 inhibitor attenuated GERD-induced esophagitis and BE and inhibited EAC formation accompanied by reduction of PGE2 levels in vivo (87). In studies of a surgical mouse model of esophagoduodenostomy, aspirin has been shown to inhibit BE and EAC development (84). An in vitro study demonstrated that a novel quinoline derivative, 83b1, has anti-cancer effects on esophageal SCC cells accompanied by downregulation of COX-2 mRNA and PGE2 (88).
Hepatocellular carcinoma
The influence of the COX-2-PGE2 pathway on hepatocellular cancer (HCC) has become a topic of interest. COX-2 expression was elevated in HCC compared to normal tissues, and patients with high COX-2 expression in HCC tissues experienced a worse 5-year overall survival (31). Observational studies revealed that aspirin use significantly reduced the risk of HCC (89–91). One of these meta-analyses further showed that aspirin use improved liver-related mortality (91). Another that used aspirin or non-aspirin NSAIDs also reduced the risk of HCC recurrence (91). One randomized controlled trial showed that a COX-2 inhibitor significantly improved disease-free survival in liver patients without viral hepatitis after initial curative treatment (92), indicating that COX-2 inhibitors may prevent liver cancer recurrence.
Knockdown of COX-2 using RNAi reduced tumorigenicity in a xenograft model of HCCs accompanied by reduced PGE2 levels and inhibited HCC cell proliferation in vitro (93). Multiple in vitro studies have demonstrated that the COX-2/PGE2 axis regulates HCC cell proliferation, apoptosis, migration, invasion, and epithelial-mesenchymal transitions (EMTs) via various signaling pathways. For example, celecoxib was shown to inhibit HCC cell proliferation and induce HCC apoptosis (31). In the same study, celecoxib also suppressed HCC migration and invasion by inducing E-cadherin via targeting the COX-2-PGE2-EP2-Akt/ERK pathways (31). Another in vitro study revealed that a selective COX-2 inhibitor, meloxicam, inhibited HCC cell proliferation and migration, whereas PGE2 reversed the effect of meloxicam on HCC cells via the β-catenin signaling pathway (94). Another in vitro study indicated that TGFβ induced EMT in HCC via COX-2 and Akt pathways (95). Under hypoxic conditions, COX-2/PGE2 induced HIF2α expression in vitro and in vivo (96). These pathways need to be evaluated further in spontaneous mouse models of liver cancer.
Summary:
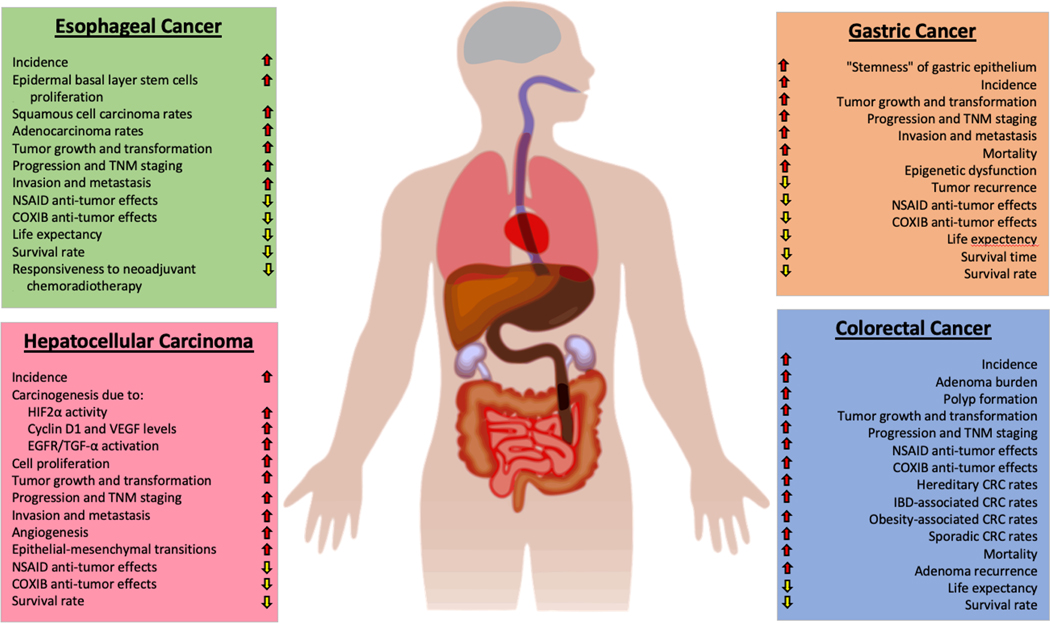
The role of the COX-2-PGE2 pathway in GI cancers, including CRC, GC, EC, and HCC, has been extensively investigated. Mounting evidence reveals that this pathway promotes GI tumor initiation, growth, progression, metastases by multiple signal pathways (Figure 3). Although long-term daily use of NSAIDs, including aspirin and COX-2 selective inhibitors, reduces the incidence and development of GI cancers, cardiovascular (except aspirin) and gastrointestinal side effects of NSAIDs have dampened enthusiasm for their use as chemopreventive agents. Targeting PGE2 signaling at the EP receptor level alone may be efficacious in CRC prevention and treatment and avoid NSAIDs’ unwanted side effects. Further studies are being undertaken to evaluate the efficacy of EP2 and EP4 antagonists in GI cancers, along with their long-term toxicities and impact on the immune system’s ability to attack tumor cells directly. For example, one report showed that an EP4 antagonist (E7046) was safe in patients with advanced solid tumors, including GI cancers, in a phase I trial (97). Other clinical trials of EP antagonists are currently recruiting cancer patients (NCT04344795 and NCT03658772), and it will be essential to examine the safety profile once these studies have been completed. There is great interest in understanding the role of PGE2 in modulating immune checkpoint signaling and the type and density of immune cells that reside in the tumor microenvironment. It has not escaped our attention that inhibitors of the PGE2 signaling pathway, when combined with checkpoint inhibition, could help revert tumor cell immune evasion, enhance responsiveness to treatment, and possibly overcome resistance to therapy.
Figure 3. General effects of PGE2 on four different gastrointestinal cancers.
PGE2 can promote tumor initiation, progression, angiogenesis, transformation, metastasis, and recurrence in EC, GC, HCC, and CRC by activating numerous intracellular signaling pathways, as described in detail herein. In addition to blunting responsiveness to neoadjuvant chemoradiotherapy and NSAID/COXIB treatments, PGE2 upregulation substantially worsens the overall prognosis. It also enhances epigenetic dysfunction due to DNA methylation of various tumor suppressor genes, as shown in Figure 2.
Acknowledgments:
We thank the National Colorectal Cancer Research Alliance (NCCRA) for its generous past support (R.N.D.). Special thanks to Dr. Dingzhi Wang for the extensive editing of this manuscript.
Financial support: R.N. DuBois: Hollings Cancer Center CCSG grant, Medical University of South Carolina (P30 CA138313).
Footnotes
Conflicts of interest:
The authors declare no potential conflicts of interest.
References
- 1.Yao C, Narumiya S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br J Pharmacol 2019;176(3):337–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019;51(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24(5):541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res 2019;79(18):4557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011;31(5):986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994;107(4):1183–8. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 2001;1(1):11–21. [DOI] [PubMed] [Google Scholar]
- 8.Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol 2002;42:55–80. [DOI] [PubMed] [Google Scholar]
- 9.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997;57(7):1276–80. [PubMed] [Google Scholar]
- 10.Yu HP, Xu SQ, Liu L, Shi LY, Cai XK, Lu WH, et al. Cyclooxygenase-2 expression in squamous dysplasia and squamous cell carcinoma of the esophagus. Cancer Lett 2003;198(2):193–201. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, DuBois RN. Role of prostanoids in gastrointestinal cancer. J Clin Invest 2018;128(7):2732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mal M, Koh PK, Cheah PY, Chan EC. Ultra-pressure liquid chromatography/tandem mass spectrometry targeted profiling of arachidonic acid and eicosanoids in human colorectal cancer. Rapid Commun Mass Spectrom 2011;25(6):755–64. [DOI] [PubMed] [Google Scholar]
- 13.Seo SH, Kang MS, Kim KH, An MS, Ha TK, Bae KB, et al. Correlation of 15-prostagladin dehydrogenase expression with clinicopathological factors and survival rate in gastric adenocarcinoma. Int J Surg 2015;13:96–101. [DOI] [PubMed] [Google Scholar]
- 14.Yang GT, Wang J, Xu TZ, Sun XF, Luan ZY. Expression of PGDH correlates with cell growth in both esophageal squamous cell carcinoma and adenocarcinoma. Asian Pac J Cancer Prev 2015;16(3):997–1000. [DOI] [PubMed] [Google Scholar]
- 15.Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A 2004;101(50):17468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem 2005;280(5):3217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JC, Schmidt CR, Shrubsole MJ, Billheimer DD, Joshi PR, Morrow JD, et al. Urine PGE-M: A metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol 2006;4(11):1358–65. [DOI] [PubMed] [Google Scholar]
- 18.Shrubsole MJ, Cai Q, Wen W, Milne G, Smalley WE, Chen Z, et al. Urinary prostaglandin E2 metabolite and risk for colorectal adenoma. Cancer Prev Res (Phila) 2012;5(2):336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong LM, Shu XO, Gao YT, Milne G, Ji BT, Yang G, et al. Urinary prostaglandin E2 metabolite and gastric cancer risk in the Shanghai women’s health study. Cancer Epidemiol Biomarkers Prev 2009;18(11):3075–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Taylor JA, Milne GL, Sandler DP. Association between urinary prostaglandin E2 metabolite and breast cancer risk: a prospective, case-cohort study of postmenopausal women. Cancer Prev Res (Phila) 2013;6(6):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrignani P, Sacco A, Sostres C, Bruno A, Dovizio M, Piazuelo E, et al. Low-Dose Aspirin Acetylates Cyclooxygenase-1 in Human Colorectal Mucosa: Implications for the Chemoprevention of Colorectal Cancer. Clin Pharmacol Ther 2017;102(1):52–61. [DOI] [PubMed] [Google Scholar]
- 22.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol 2016;64(1 Suppl):S84–S101. [DOI] [PubMed] [Google Scholar]
- 23.El-Zimaity H, Di Pilato V, Novella Ringressi M, Brcic I, Rajendra S, Langer R, et al. Risk factors for esophageal cancer: emphasis on infectious agents. Ann N Y Acad Sci 2018;1434(1):319–32. [DOI] [PubMed] [Google Scholar]
- 24.Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 2016;150(1):64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song M, Chan AT, Sun J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020;158(2):322–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stidham RW, Higgins PDR. Colorectal Cancer in Inflammatory Bowel Disease. Clin Colon Rectal Surg 2018;31(3):168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogino S, Kirkner GJ, Nosho K, Irahara N, Kure S, Shima K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res 2008;14(24):8221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Z, Yang Y, Zhao Y, Huang Y. The prognostic value of cyclooxygenase-2 expression in patients with esophageal cancer: evidence from a meta-analysis. Oncotargets and Therapy 2017;10:2893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J, Su H, Zhou Y-y, Guo L-l. Cyclooxygenase-2 Expression is associated with poor overall survival of patients with gastric cancer: a meta-analysis. Digestive Diseases and Sciences 2014;59:436–45. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Liu J, Sui X. Correlation of COX-2 and MMP-13 expressions with gastric cancer and their effects on prognosis. J BUON 2019;24(1):187–93. [PubMed] [Google Scholar]
- 31.Tai Y, Zhang LH, Gao JH, Zhao C, Tong H, Ye C, et al. Suppressing growth and invasion of human hepatocellular carcinoma cells by celecoxib through inhibition of cyclooxygenase-2. Cancer Manag Res 2019;11:2831–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 2005;294(8):914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993;328(18):1313–6. [DOI] [PubMed] [Google Scholar]
- 34.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000;342(26):1946–52. [DOI] [PubMed] [Google Scholar]
- 35.Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology 2006;131(6):1674–82. [DOI] [PubMed] [Google Scholar]
- 36.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 2006;355(9):885–95. [DOI] [PubMed] [Google Scholar]
- 37.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 2006;355(9):873–84. [DOI] [PubMed] [Google Scholar]
- 38.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR, uk CAPTG. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 2008;134(1):29–38. [DOI] [PubMed] [Google Scholar]
- 39.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003;348(10):891–9. [DOI] [PubMed] [Google Scholar]
- 40.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 2003;348(10):883–90. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376(9754):1741–50. [DOI] [PubMed] [Google Scholar]
- 42.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 2007;356(21):2131–42. [DOI] [PubMed] [Google Scholar]
- 43.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA 2009;302(6):649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. The New England journal of medicine 2018;379(16):1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Miedany Y, Youssef S, Ahmed I, El Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol 2006;101(2):311–7. [DOI] [PubMed] [Google Scholar]
- 46.Bernstein CN, Eaden J, Steinhart AH, Munkholm P, Gordon PH. Cancer prevention in inflammatory bowel disease and the chemoprophylactic potential of 5-aminosalicylic acid. Inflamm Bowel Dis 2002;8(5):356–61. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Baron JA, Mott LA, Burke CA, Church TR, McKeown-Eyssen GE, et al. Aspirin may be more effective in preventing colorectal adenomas in patients with higher BMI (United States). Cancer Causes Control 2006;17(10):1299–304. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Smith-Warner SA, Chan AT, Wu K, Spiegelman D, Fuchs CS, et al. Aspirin use, body mass index, physical activity, plasma C-peptide, and colon cancer risk in US health professionals. Am J Epidemiol 2011;174(4):459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis 2003;24(5):985–90. [DOI] [PubMed] [Google Scholar]
- 50.Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell 2004;6(3):285–95. [DOI] [PubMed] [Google Scholar]
- 51.Hansen-Petrik MB, McEntee MF, Jull B, Shi H, Zemel MB, Whelan J. Prostaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) mice. Cancer Res 2002;62(2):403–8. [PubMed] [Google Scholar]
- 52.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A 2006;103(32):12098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res 2008;68(9):3251–9. [DOI] [PubMed] [Google Scholar]
- 54.Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med 2001;7(9):1048–51. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res 1999;59:5093–6. [PubMed] [Google Scholar]
- 56.Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, et al. Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Res 2002;62(1):28–32. [PubMed] [Google Scholar]
- 57.Shoji Y, Takahashi M, Kitamura T, Watanabe K, Kawamori T, Maruyama T, et al. Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut 2004;53(8):1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma X, Aoki T, Tsuruyama T, Narumiya S. Definition of Prostaglandin E2-EP2 Signals in the Colon Tumor Microenvironment That Amplify Inflammation and Tumor Growth. Cancer Res 2015;75(14):2822–32. [DOI] [PubMed] [Google Scholar]
- 59.Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer 2010;10(3):181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D, Cabalag CS, Clemons NJ, DuBois RN. Cyclooxygenases and Prostaglandins in Tumor Immunology and Microenvironment of Gastrointestinal Cancer. Gastroenterology 2021;161(6):1813–29. [DOI] [PubMed] [Google Scholar]
- 61.Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med 2012;18(2):224–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology 2015;149(7):1884–95 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellamkonda K, Chandrashekar NK, Osman J, Selvanesan BC, Savari S, Sjolander A. The eicosanoids leukotriene D4 and prostaglandin E2 promote the tumorigenicity of colon cancer-initiating cells in a xenograft mouse model. BMC Cancer 2016;16:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cecil DL, Gad EA, Corulli LR, Drovetto N, Lubet RA, Disis ML. COX-2 inhibitors decrease expression of PD-L1 in colon tumors and increase the influx of Type I tumor infiltrating lymphocytes. Cancer Prev Res (Phila) 2022. doi 10.1158/1940-6207.CAPR-21-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Matteis R, Flak MB, Gonzalez-Nunez M, Austin-Williams S, Palmas F, Colas RA, et al. Aspirin activates resolution pathways to reprogram T cell and macrophage responses in colitis-associated colorectal cancer. Sci Adv 2022;8(5):eabl5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A 2017;114(5):1117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 2004;173(1):559–65. [DOI] [PubMed] [Google Scholar]
- 68.Miao J, Lu X, Hu Y, Piao C, Wu X, Liu X, et al. Prostaglandin E2 and PD-1 mediated inhibition of antitumor CTL responses in the human tumor microenvironment. Oncotarget 2017;8(52):89802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Cui L, Georgiev P, Singh L, Zheng Y, Yu Y, et al. Combination of EP4 antagonist MF-766 and anti-PD-1 promotes anti-tumor efficacy by modulating both lymphocytes and myeloid cells. Oncoimmunology 2021;10(1):1896643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu W, Yu W, He J, Liu W, Yang J, Lin X, et al. Reprogramming immunosuppressive myeloid cells facilitates immunotherapy for colorectal cancer. EMBO Mol Med 2021;13(1):e12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akre K, Ekstrom AM, Signorello LB, Hansson LE, Nyren O. Aspirin and risk for gastric cancer: a population-based case-control study in Sweden. Br J Cancer 2001;84(7):965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer 2009;100(3):551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J 2004;23(7):1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology 2006;131(4):1086–95. [DOI] [PubMed] [Google Scholar]
- 75.Ishimoto T, Oshima H, Oshima M, Kai K, Torii R, Masuko T, et al. CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci 2010;101(3):673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong CC, Kang W, Xu J, Qian Y, Luk STY, Chen H, et al. Prostaglandin E2 induces DNA hypermethylation in gastric cancer in vitro and in vivo. Theranostics 2019;9(21):6256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci 2016;107(4):391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015;21(26):7933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moon H, White AC, Borowsky AD. New insights into the functions of Cox-2 in skin and esophageal malignancies. Exp Mol Med 2020;52(4):538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang Y, Liu JL, Wu Y, Zhang ZY, Wu R. Cyclooxygenase-2 polymorphisms and susceptibility to esophageal cancer: a meta-analysis. Tohoku J Exp Med 2011;223(2):137–44. [DOI] [PubMed] [Google Scholar]
- 81.Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2020;31(5):558–68. [DOI] [PubMed] [Google Scholar]
- 82.Zhang S, Zhang XQ, Ding XW, Yang RK, Huang SL, Kastelein F, et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta-analysis. British journal of cancer 2014;110(9):2378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sivarasan N, Smith G. Role of aspirin in chemoprevention of esophageal adenocarcinoma: a meta-analysis. J Dig Dis 2013;14(5):222–30. [DOI] [PubMed] [Google Scholar]
- 84.Zhang T, Wang Q, Ma WY, Wang K, Chang X, Johnson ML, et al. Targeting the COX1/2-Driven thromboxane A2 pathway suppresses Barrett’s esophagus and esophageal adenocarcinoma development. EBioMedicine 2019;49:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jalalabadi Y, Shirazi A, Ghavam-Nasiri MR, Aledavood SA, Sardari D, Memar B, et al. Evaluating the expression of cyclooxygenase-2 enzyme by immunohistochemistry in normal and tumoral tissue before and after neoadjuvant chemoradiotherapy in patients with esophageal cancer in Khorasan Province. J Cancer Res Ther 2018;14(3):509–15. [DOI] [PubMed] [Google Scholar]
- 86.Li Z, Shimada Y, Kawabe A, Sato F, Maeda M, Komoto I, et al. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by JTE-522, a selective COX-2 inhibitor. Carcinogenesis 2001;22(4):547–51. [DOI] [PubMed] [Google Scholar]
- 87.Oyama K, Fujimura T, Ninomiya I, Miyashita T, Kinami S, Fushida S, et al. [Cyclooxygenase (COX)-2 expression in a rat duodenoesophageal reflux model and chemoprevention of adenocarcinoma by the selective COX-2 inhibitor nimesulide]. Nihon Shokakibyo Gakkai Zasshi 2007;104(8):1183–91. [PubMed] [Google Scholar]
- 88.Pun IH, Chan D, Chan SH, Chung PY, Zhou YY, Law S, et al. Anti-cancer Effects of a Novel Quinoline Derivative 83b1 on Human Esophageal Squamous Cell Carcinoma through Down-Regulation of COX-2 mRNA and PGE2. Cancer Res Treat 2017;49(1):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang S, Yu Y, Ryan PM, Dang M, Clark C, Kontogiannis V, et al. Association of aspirin therapy with risk of hepatocellular carcinoma: A systematic review and dose-response analysis of cohort studies with 2.5 million participants. Pharmacol Res 2020;151:104585. [DOI] [PubMed] [Google Scholar]
- 90.Memel ZN, Arvind A, Moninuola O, Philpotts L, Chung RT, Corey KE, et al. Aspirin Use Is Associated with a Reduced Incidence of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Hepatol Commun 2021;5(1):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan RZH, Lockart I, Abdel Shaheed C, Danta M. Systematic review with meta-analysis: The effects of non-steroidal anti-inflammatory drugs and anti-platelet therapy on the incidence and recurrence of hepatocellular carcinoma. Aliment Pharmacol Ther 2021;54(4):356–67. [DOI] [PubMed] [Google Scholar]
- 92.Takami Y, Eguchi S, Tateishi M, Ryu T, Mikagi K, Wada Y, et al. A randomised controlled trial of meloxicam, a Cox-2 inhibitor, to prevent hepatocellular carcinoma recurrence after initial curative treatment. Hepatol Int 2016;10(5):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lv X, Chen Z, Li S, Xie H. Knockdown of cyclooxygenase-2 leads to growth inhibition and cell cycle arrest in hepatocellular carcinoma cells. Onco Targets Ther 2019;12:4341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li T, Zhong J, Dong X, Xiu P, Wang F, Wei H, et al. Meloxicam suppresses hepatocellular carcinoma cell proliferation and migration by targeting COX-2/PGE2-regulated activation of the beta-catenin signaling pathway. Oncol Rep 2016;35(6):3614–22. [DOI] [PubMed] [Google Scholar]
- 95.Ogunwobi OO, Wang T, Zhang L, Liu C. Cyclooxygenase-2 and Akt mediate multiple growth-factor-induced epithelial-mesenchymal transition in human hepatocellular carcinoma. J Gastroenterol Hepatol 2012;27(3):566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dong XF, Liu TQ, Zhi XT, Zou J, Zhong JT, Li T, et al. COX-2/PGE2 Axis Regulates HIF2alpha Activity to Promote Hepatocellular Carcinoma Hypoxic Response and Reduce the Sensitivity of Sorafenib Treatment. Clin Cancer Res 2018;24(13):3204–16. [DOI] [PubMed] [Google Scholar]
- 97.Hong DS, Parikh A, Shapiro GI, Varga A, Naing A, Meric-Bernstam F, et al. First-in-human phase I study of immunomodulatory E7046, an antagonist of PGE2-receptor-E-type 4 (EP4), in patients with advanced cancers. Journal for Immunotherapy of Cancer 2020;8:e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]