Abstract
Titanium dioxide nanoparticles (TiO2 NPs) are one of the most widely used nanomaterials that have been manufactured worldwide and applied in different commercial realms. The well-recognized ability of TiO2 to promote the formation of reactive oxygen species (ROS) has been extensively studied as one of the important mechanisms underlying TiO2 NPs toxicity. As the “gold standard” method to quantify and identify ROS, electron spin resonance (ESR) spectroscopy has been employed in many studies aimed at evaluating TiO2 NPs safety. This review aims to provide a thorough discussion of current studies using ESR as the primary method to unravel the mechanism of TiO2 NPs toxicity. ESR spin label oximetry and immune-spin trapping techniques are also briefly introduced, because the combination of spin trapping/labeling techniques offers a promising tool for studying the oxidative damage caused by TiO2 NPs.
Keywords: Electron spin resonance, Reactive oxygen species, Titanium dioxide nanoparticles
1. Introduction
Titanium dioxide nanoparticles (TiO2 NPs) have been widely applied as a coloring agent to provide whiteness and/or opacity in paints and personal care products, as well as being used as a food additive and a drug delivery agent. Moreover, due to their excellent UV absorbance and deflecting properties, TiO2 NPs are a commonly used functional ingredient in cosmetics or skincare products to provide protection against sunlight. In environmental engineering, TiO2 nanocomposites have been employed as a photocatalyst in water pollutant purification and hazardous chemical detoxification. When exposed to UV light, TiO2 NPs absorb photons having an energy equal to or higher than its band gap (>3.0 eV), exciting electrons in the valence band to the conduction band. Photo-excitation, therefore, results in an increased number of conduction band electrons and consequently increased valence band holes. Electrons in the conduction band can reduce substrates in the chemical environment, for example, reduction of oxygen results in the formation of superoxide radical anions. Holes in the valence band can oxidize substrates such as water or hydroxide ions and generate hydroxyl radicals (•OH) [1,2]. Photocatalyzed chemical decomposition usually involves formation of reactive oxygen species (ROS), including superoxide radicals (O2−•) and singlet oxygen (1O2) [3], as well as other intermediate species such as H2O2 or O2 [1]. Because of those highly reactive free radicals generated during UV irradiation, engineered TiO2 NPs have also been recognized for their light-induced biocidal effects against a broad range of harmful microorganisms, including bacteria such as Escherichia coli [4], molds such as Aspergillus niger [5], as well as protozoa such as Giardia and Acanthamoeba species [6].
Exposure to ROS derived from photoexcited TiO2 NPs has raised concerns because ROS are believed to play an important role in many inflammatory skin disorders, skin aging, and cancer formation [7]. Due to the ability of nano-TiO2 to induce ROS generation when irradiated, tremendous efforts have been focused on investigating potential risks associated with human exposure to TiO2 NPs. In addition to direct exposure through consumption of products containing TiO2 NPs, inhalation of NPs in the workplace, or through other environmental sources are possible exposure routes (e.g., emitted nanomaterials that reach the land can potentially contaminate soil and migrate to water systems) [8]. To date, various nanomaterial studies have linked toxicity to the production of ROS. It is well known that the generation of intercellular ROS can lead to oxidative stress, resulting in inflammation, immune response, cellular damage, and genotoxicity [9].
Free radicals, including ROS, are very short-lived entities, making them very difficult to detect when evaluating toxicity associated with oxidative stress. Electron spin resonance (ESR, also known as EPR, electron paramagnetic resonance) has been recognized as a “gold standard” and state-of-the-art tool for detecting and quantifying ROS in chemical and biological systems. Another ESR technique, ESR oximetry, has been used to monitor lipid peroxidation induced by highly reactive radicals. This review summarizes the advantages and recent developments using ESR as a tool to unravel the mechanism of nano-TiO2-induced cytotoxicity and phototoxicity. In addition, immuno-spin trapping, another methodology based on the spin trapping technique to detect protein or DNA radicals, is briefly introduced. The combination of immuno-spin trapping with ESR spin trapping and ESR oximetry can provide a deep insight into the mechanism of ROS generation triggered by nanomaterials, as well as the subsequent oxidative damage to proteins, DNA, and lipids.
2. Electron spin resonance
2.1. ESR spin trapping
ESR is a spectroscopic technique used to detect chemical species with unpaired electrons. ESR has been recognized as the least ambiguous method for characterizing free radicals. Due to its high sensitivity and the ability to identify the generation of radicals in situ, the ESR spin trapping technique is commonly employed in nanoscience research to evaluate both the ROS scavenging capability of nanomaterials with regard to their potential applications in health promotion and cancer chemotherapy [10] and to investigate toxicities related to ROS generation. ESR spectroscopy has also been used for the validation of results obtained using other methods. For instance, the data from ESR spectroscopy using different spin probes – 1-hydroxy-3-carboxypyrrolidine and 4-phosphonooxy-2,2,6,6-tetramethylpiperidine-N-hydroxyl – showed good agreement with the data from confocal fluorescence imaging using different dyes, including 2′,7′-dichlorodihydrofluorescein diacetate, MitoSOX, and Mito-Tracker red CM-H2XRos [11]. Moreover, the development of nontoxic spin traps makes it possible for the detection of free radicals both in vivo [12] and ex vivo [13].
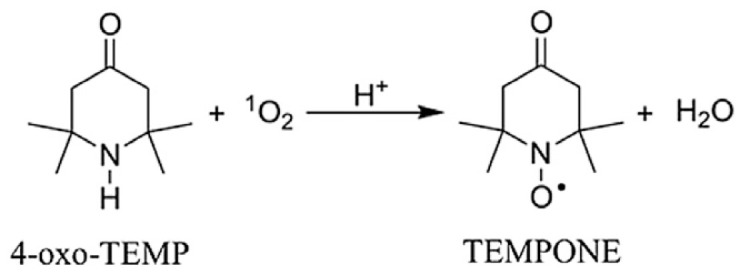
ROS are low-level and short-lived free radicals, which are difficult to determine in chemical and biological systems. Spin trapping agents are therefore employed to intercept the target free radical and to form a relatively stable and distinguishable spin adduct that can be quantified and identified by ESR spectroscopy [10]. Based on their characteristic structures, spin traps can be divided into two groups: nitroso and nitrone. Nitroso spin traps are less readily used in biological studies because of high reactivity of their C-nitroso group [14]. The most commonly used nitrone spin traps include 5,5-dimethyl-1-pyrroline N-oxide (DMPO), α-phenyl-N-tert-butylnitrone (PBN) α-(4-pyridyl-1-oxide)-N-tert-butylnitrone (POBN), and 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DEPMPO). The spin label probes 2,2,6,6-tetramethyl-4-piperidone (TEMP) and 4-oxo-2,2,6,6-tetramethyl-2-piperidone (4-oxo-TEMP) have been employed to detect singlet oxygen [15]. The reaction of 1O2 with 4-oxo-TEMP leads to the formation of a nitroxide radical 4-oxo-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPONE) that exhibits a stable triplet ESR spectrum, Equation (1):
 |
(1) |
In comparison with other nitrone spin traps, DMPO is generally preferable because of its low redox activity and the ability to yield ESR spectra that are highly dependent on the radical species. However, a major drawback of DMPO is that the decomposition of DMPO/•OOH to DMPO/•OH makes it difficult to distinguish between the formation of •OOH and •OH [16]. By contrast, 5-methyl-1-pyrroline N-oxide (BMPO) provides an ideal solution to this problem because of the formation of a more stable BMPO/•OOH adduct that does not decompose to BMPO/•OH [16]. BMPO/•OH has an ESR spectrum similar to that of DMPO/•OH, exhibiting a characteristic set of four lines (1:2:2:1) [2]. Another method to further distinguish whether the signal is from DMPO/•OH or DMPO/O2− • spin adduct is the effects of superoxide dismutase (SOD) [17] or mannitol on the ESR spectrum [18]. Because the former only scavenges O2−•, whereas the latter only reacts with •OH, the predominant species (•OH or O2−•) in the system can be determined by observing changes of the ESR spectra.
2.2. ESR spin label oximetry
As aforementioned, ESR spectroscopy detects molecules with unpaired electrons. Theoretically, the direct detection of molecular oxygen by ESR is possible because molecular oxygen is a triplet radical that possesses two unpaired electrons. However, the broadening of lines in the spectrum of oxygen in biological systems makes oxygen undetectable by ESR. This problem has been solved using a stable free radical (usually nontoxic) as a spin label in ESR oximetry [19]. ESR oximetry is based on the changes observed in the ESR spectrum of the spin label produced by collisions with molecular oxygen. ESR oximetry allows the monitoring of oxygen consumption/formation in a dynamic system. Collision of the spin label with O2 produces a spin exchange, resulting in shorter relaxation times (both T1 and T2) and ESR signals with broader line widths and decreasing peak height. Line broadening is caused by Heisenberg exchange between the spin label and molecular oxygen dissolved in solution [19]. Measurements that depend on T1 and T2 can both offer a direct indication of the O2 concentration. Because it is more easily measured experimentally, investigators primarily examine changes in T2-sensitive line width rather than T1-sensitive saturation recovery for ESR oximetry [20].
ESR spin label oximetry has been extensively applied to study biological processes involving the participation of oxygen, including measuring cellular respiration rate [21], studying O2 concentration across the cell plasma [22], and the detection of lipid peroxidation. It has been suggested that this method is more sensitive than the traditional thiobarbituric acid assay, especially in very early stages of lipid peroxidation [23]. It is well recognized that lipid peroxidation proceeds as a chain reaction involving continuous depletion of O2. Thus, oxygen consumption by such mechanisms can be a direct indicator of the peroxidation rate and can be observed as a reduction in line widths and increase in peak height for the spin label. Because the area beneath the signal intensity versus the magnetic field curve remains constant, the narrowing of the ESR signal is necessarily accompanied by an increase in the peak height of the ESR spectrum [16]. Therefore, the value of oxygen concentration can be obtained from a calibrated curve of the ESR line width versus the oxygen concentration [2].
3. TiO2 NPs and reactive oxygen species
3.1. Photogeneration of ROS
ROS are a group of highly reactive molecules that are intermediate products of cellular oxidative metabolism. Biologically relevant ROS include singlet oxygen (1O2), peroxides (e.g., hydrogen peroxide), and free radicals [e.g., superoxide radical ( ), peroxyl radical (•OOR), and hydroxyl radical (•OH)]. ROS play critical roles in a variety of physiological processes in plants and animals with regard to regulation of the immune system and the development of the inflammatory response, activation of transcription factors and gene expression, and modulation of programmed cell death (i.e., apoptosis) [24,25]. Excessive levels of ROS can oxidize cell constituents such as lipids, proteins, and DNA, and consequently pose a threat to cell integrity [26]. Mitochondria are one of the main sources of ROS in cells. In the mitochondrial respiratory chain, electrons are continuously transferred to molecular oxygen, producing ROS as a byproduct of oxidative phosphorylation [27]. With the presence of TiO2 NPs (10 μg/mL) and UVA, alteration of mitochondrial function was observed for HaCaT cells, accompanied by a 14-fold increase in mitochondrial DNA damage, indicated by mitochondrial “common deletion” [28]. Several studies have identified the intercellular oxidative stress caused by ROS as an important factor for genotoxicity [2], cytotoxicity [29–31], as well as tissue damage and inflammation [32–34].
3.1.1. Mechanism of ROS generation
Generation of ROS during photoexcited TiO2 was first discovered in the early 20th century. A study by Goodeve and Kitchener [35] in 1938 described photobleaching of dyes by TiO2. Photobleaching was attributed to the generation of active oxygen species on the surface of photoexcited TiO2. TiO2 absorbs light in the UVA (320–400 nm) and UVB (290–320 nm) spectral regions of the terrestrial solar spectrum. When TiO2 absorbs photons with energy equal to or higher than its band gap (3.0 eV for rutile and 3.2 eV for anatase phase), electrons are excited from the valence band of TiO2 to its conduction band, resulting in the formation of an electron–hole pair (e−•/h+) [1]. The holes (h+) in the valence band are highly oxidizing and can react with H2O or hydroxide ions to produce hydroxyl radicals (OH), and the electrons in the conduction band can reduce O2 to produce superoxide radical anions (O2−•) [36]. This reduction–oxidation (redox) potential of TiO2 has a significant impact on biological systems. The fundamental process of ROS production involving photo-induced electrons and holes can be expressed as follows [37,38]:
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Equation (2) describes the absorption of a photon. Equations (3–5) are photocatalytic redox pathways involved in the generation of a superoxide radical anion and a hydroxyl radical. Equation (6) and Equation (7) show the possible generation of hydrogen peroxide by reductive or oxidative pathways, respectively. Electrons and holes generated during photoexcitation are localized at different defect sites on the surface and in the bulk material [1]. ESR is usually used when identifying the charge trapping center formed by UV irradiation of the catalyst [39]. The results show that electrons are trapped as Ti(III) centers, whereas the holes are trapped as oxygen-centered radicals, such as •OH, covalently linked to surface titanium atoms [40,41]. However, it remains unclear whether the production of ROS occurs on the surface of the TiO2 or in the solution [38].
3.1.2. Hydroxyl and superoxide radicals
The photocatalytic mechanisms and formation of ROS of the photoirradiated TiO2 NPs have been intensively studied using ESR spectroscopy. It has been suggested that, in general, TiO2 photocatalytic reactions mainly proceed from the hydroxyl radicals (•OH) by the oxidation of water and superoxide radicals (O2−•) produced by the reduction of oxygen [42]. In aqueous solutions, the formation of •OH spin adducts have been observed using different spin traps, including DMPO, POBN, DEPMPO [38], and BMPO [2]. Hydroxyl radical has been recognized as the most important cause for the photogenotoxicity of TiO2 NPs [18]. By contrast, only a few studies reported positive results on the formation of superoxide radicals [43], whereas other studies using ESR spectroscopy observed no evidence of O2−• generation [2,18,38,44].
3.1.3. Singlet oxygen
Besides •OH and O2−•, several studies also found singlet oxygen generation during photoexcitation of TiO2. The mechanism is rather complicated and unequivocal evidence is suggested by different studies. Sterically hindered cyclic amines, including 2,2,6,6-tetramethyl-4-piperidinol (TMPol) and TEMP, can be used to detect 1O2, because they do not react with other oxygen radicals such as •OH and O2−• (or •OOH) [15,45]. The reaction between these spin traps and singlet oxygen yields stable nitroxide radicals that can be monitored by ESR signals. When using TMPol as the spin trap, Reeves et al [18] observed that no singlet oxygen was formed on UVA irradiated TiO2 NPs in aqueous solutions. Another study by the same group of researchers reported similar results [38] and argued that false positive results may have been obtained because those amines can be oxidized by other ROS such as •OH [46]. Interestingly, in a study conducted by Nosaka et al [3], electrochemical measurements revealed that sterically hindered cyclic amine 4-hydroxy-2,2,6,6-tetramethylpiperidine can be directly oxidized with holes (h+) in photoexcited TiO2 to produce the 4-hydroxyl-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) radical. The possibility of other processes, such as reactions with singlet molecular oxygen, superoxide radical, and hydroxyl radical, was excluded from the reaction mechanism in that study.
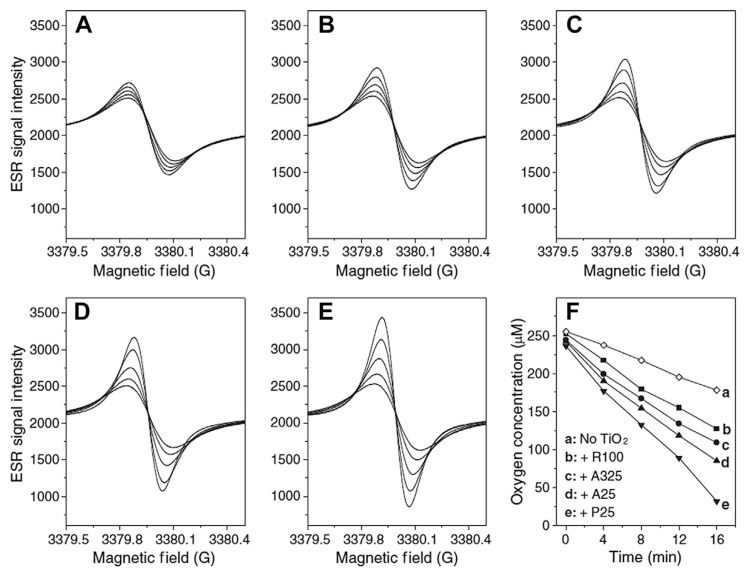
Konaka et al [47] provided evidence for the direct production of singlet oxygen during photoexcitation of TiO2 using ESR spectroscopy. It was noted that the generation of 1O2 originated from direct production rather than a sequential reaction involving , based on the fact that the addition of DMPO to the reaction mixture amplified the signal of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO), a reaction product of spin probe TEMP and 1O2. However, a completely different result has been found in a later study by Lipovsky et al [42]. The ESR signal of singlet oxygen (using TEMP as the spin probe) was found to disappear with the addition of DMPO to the suspension of TiO2 NPs. Under similar experimental conditions (i.e., aerated aqueous TiO2 NPs suspension), Daimon et al [43] confirmed the generation of 1O2 using the luminol chemiluminescence probe method. It was suggested that the production of 1O2 could be due to an electron transfer process involving . A two-step mechanism was thus proposed for 1O2 formation. The first step is a reduction of O2 to using conduction band electrons (e−), with the second being the oxidation of to 1O2 using valence band holes or trapped holes (h+). Using ESR spectroscopy with TEMP as the spin probe, Yin et al [2] also observed similarresults and successfully detected the formation of 1O2 [2]. Using BMPO as spin trap, only BMPO/•OH adduct was observed and no ESR signal for was found, suggested by the fact that the ESR spectrum of the BMPO spin adduct did not perceptibly change when SOD was added (Fig. 1C and D). However, this study suggested that part of 1O2 formation proceeds via a superoxide-dependent mechanism, whereas •OH formation is not formed via superoxide. This prediction is supported by the observation that addition of SOD leads to a noticeable reduction of the 1O2 signal without effects on the •OH-dependent ESR signal (Fig. 1).
Fig. 1.
Effect of SOD on the generation of hydroxyl radicals and singlet oxygen by P25 during photoexcitation with UVA light. ESR spectra were recorded at room temperature 2 minutes after the UV light was turned on. Samples containing 25 mM BMPO and (A) without TiO2, (B) with 0.1 mg/mL R100, (C) 0.1 mg/mL A325, (D) 0.1 mg/mL A25, (E) 0.1 mg/mL P25, and (F) same as (E) but with the addition of 20% DMSO. The symbol + indicates the ESR signal of the BMPO/•CH3 adduct. Instrumental settings: microwave power, 10 mW; modulation frequency, 100 kHz; modulation amplitude, 1 G; scan range, 100 G. DMSO = dimethyl sulfoxide; ESR = electron spin resonance; BMPO = 5-methyl-1-pyrroline N-oxide; SOD =superoxide dismutase; TiO2 = titanium dioxide. Note. From “Phototoxicity of nanotitanium dioxides in HaCaT keratinocytes – Generation of reactive oxygen species and cell damage,” by J.J. Yi, J. Liu, M. Ehrenshaft, et al, 2012, Toxicol Appl Pharmacol, 263, p. 81–8. Copyright 2012, Toxicology and Applied Pharmacology. Reprinted with permission.
3.2. Effects of intrinsic properties on ROS generation
3.2.1. Particle size and crystal phase
The photoreactivity of TiO2 NPs is largely dependent on their inherent material properties, such as their particle size, shape, surface characteristics, and crystal structure [31,48]. Generally, nanomaterials exhibit higher reactivity compared with the corresponding bulk material due to the increased surface area. From this standpoint, TiO2 NPs with smaller size have been found to promote more ROS formation when photoexcited, which consequently may elicit more oxidative stress to biological systems [2,3,49]. Anatase and amorphous forms of nano-TiO2 show higher phototoxicity and cytotoxicity than its rutile form [49,50]. Furthermore, the arrangement and coordination of the surface atoms on the different crystal facets largely influence the photocatalytic activity of TiO2 nanocrystals. Several studies have suggested that the anatase {001} face is associated with higher photocatalytic efficiency due to its highly active titanium and oxygen centers [51], whereas others found that the anatase {101} surface exhibited enhanced activity [52].
Sayes et al [53] conducted a study on toxicity of TiO2 NPs using human dermal fibroblasts and human lung epithelial cells. The aim was to correlate the crystal structure of nano-TiO2 with their ability to elicit cytotoxicity and inflammatory responses. Their results suggested that the phase composition of nano-TiO2 was strongly correlated with cytotoxicity as well as ROS generation. Anatase TiO2 showed 100 times more cytotoxicity than rutile TiO2 at the equivalent treatment level. In their study, ROS generation was quantified by measuring azo dye photodegradation. Using a more direct and nondestructive method, ESR spectroscopy, Yin et al [2] observed similar results for ROS generation in TiO2 NPs suspensions. After UVA irradiation, the intensity of •OH generation for different crystal forms at the same concentration (0.1 mg/mL) followed the trend: P25 (31 nm, anatase/rutile) >A25 (<25 nm, anatase) >A235 (325 mesh, anatase), whereas R100 TiO2 (<100 nm, rutile) in the same aqueous solution did not show hydroxyl radical production [2]. In their study, the relative efficiency of ROS generation by these four nano-TiO2 particles was in agreement with the phototoxicity experiment using human HaCaT keratinocytes, a transformed epidermal human cell line, further confirming the hypothesis that ROS production was most likely involved in the phototoxic mechanism.
3.2.2. Synthesis method and surface coating
Synthesis method and surface coating have also been demonstrated to be critical in determining the toxicity of nano-TiO2. Nagaveni et al [54] noted that under identical UV exposure, combustion-synthesized nano-TiO2 resulted in a two times higher initial degradation rate of phenol compared with commercial P25 TiO2. This superior photocatalytic activity can be attributed to crystallinity, higher surface area, more surface hydroxyl groups, and improved optical absorption at higher wavelengths (570 nm and 467 nm, corresponding band gap energies of 2.18 eV and 2.65 eV). Using an in situ sol-gel method, Kavitha et al [55] synthesized anatase phase titania–chitosan NPs with spherical and irregular morphology (4.5–10.5 nm). Having tunable biocompatibility with human gastric adenocarcinoma cells and efficient antibacterial activity against Staphylococcus aureus, this nano-TiO2 material might be a promising biomaterial for orthopedic and tissue engineering applications.
Using ESR spectroscopy, Sawada et al [56] demonstrated that TiO2 NPs coated with fluoridated apatite (FAp-TiO2, 100 nm) promoted ROS via photocatalysis and exhibited antifungal activity towards Candida albicans. In a study by Carlotti et al [57], the oxidation of linoleic acid and porcine ear skin induced by UV irradiation was investigated in the presence of different uncoated and coated titanium powders. They noted that surface characteristics largely influenced the observed oxidative damage. In their study, two types of coated TiO2 specimens were used, namely PW Covasil S-1 and Tego Sun TS plus. Whereas the former showed a high photocatalytic activity towards the peroxidation of linoleic acid, the latter displayed a marked protective effect [57].
In addition, nano-silver, the most widely employed anti-microbial nanomaterial, has been deposited onto TiO2 NPs, a common strategy for synthesizing nanocomposites. The strong antibacterial activity of TiO2@Ag NPs against different bacterial species has been reported under UV light [58], visible light illumination [59], as well as in the dark [60]. TiO2@Ag NPs reduced the viability of Leishmania tropica, and Leishmania infantum promastigotes 3- and 10-fold in the dark, respectively, whereas these rates diminished approximately 20-fold for each species in the presence of visible light. Nonvisible light-exposed TiO2@Ag NPs were more effective against L. infantum parasites, whereas visible light-exposed TiO2@AgNPs exhibited nearly the same antileishmanial effect against both species [60]. A decrease in pH was discovered during photocatalysis using silver-modified TiO2 NPs. And such a pH change has been attributed to silver ion reduction [61].
3.3. Effects of environmental conditions on ROS generation
Environmental conditions, including light illumination, pH, solution composition, and biological media, also influence the generation of ROS by nano-TiO2.
3.3.1. UV irradiation and light illumination
Previous studies have suggested that, without UV exposure, nano-TiO2 show little cytotoxicity [62]. It is generally agreed that ROS production of TiO2 nanomaterials is initiated by UV irradiation. To date, there is no conclusion whether ROS can be generated on the TiO2 surface in an aqueous condition without illumination. For TiO2 NPs of different crystal forms and size, no ROS promotion was detected by ESR without exposure to light [2]. Wamer and Yin [44] conducted a study using human dermal fibroblasts to evaluate the toxicity of TiO2 in tattoo inks. The results suggested that anatase TiO2 was phototoxic but not cytotoxic, whereas the sample that only contains rutile TiO2 was neither phototoxic nor cytotoxic.
However, Lipovsky et al [42] investigated visible light-induced reactions of a suspension of TiO2 NPs in water using the ESR spin trapping technique, and their results suggested that, without light illumination, formation of both •OH and were detected for TiO2 rutile and anatase phases (50 nm NPs), but singlet oxygen was not detected in aqueous suspensions of TiO2 NPs for either of these two crystal forms. When exposed to light in the blue part of visible spectrum (400–500 nm), increased levels of both •OH and O2−• were detected. Singlet oxygen formation was observed with rutile NPs during irradiation [42].
Without light exposure, long-term exposure to TiO2 NPs has been proven to lead to significant alterations in the expression of various genes and has promoted production of ROS and peroxidation of lipids, proteins, and DNA in mouse lung tissue [63]. Exposure of zebrafish embryos to TiO2 NPs produced malformation and death only when the fish were also illuminated (light source: 250 W blue spectrum metal halide lamp) [64]. A similar result was obtained using a fish cell viability assay. TiO2 alone (0.1–1000 μg/mL) had limited effect on gold-fish skin cells, whereas co-exposure with UVA (0.5–2.0 kJ/m−2) caused a significant dose-dependent decrease in cell viability, which was dependent on both the concentration of TiO2 and the dose of UVA administered [18]. Using the comet assay, the same group of researchers also found that TiO2 NPs are in fact genotoxic without UV irradiation, as all concentrations tested produced a significant increase in the level of Fpg-sensitive sites, which suggests that 8-hydroxyguanine is probably a major product of TiO2-induced oxidative stress linked to genotoxicity. ROS promotion was also observed in toxicity studies using animal models. In a study where mice were injected with TiO2 NPs for 45 days consecutively, accumulation of TiO2 NPs and ROS were found in mouse spleen, accompanied by the development of congestion and lymph nodule proliferation of spleen tissue [34].
3.3.2. pH and solvents
It is notable that, when employing ESR to evaluate the ability of materials to generate/scavenge ROS, different experimental conditions, such as type of spin trap, pH, and composition of solvent, may lead to different results [65]. In an early study, Jaeger and Bard [66] observed that ESR spectra were consistent with the formation of both •OH and following absorption of UV radiation by TiO2 (anatase). However, Wamer et al [67], using DMPO as a spin trap, observed, during UV irradiation of TiO2, an ESR signal characteristic of DMPO/•OH spin adduct alone. Dodd and Jha [38] also reported the formation of •OH adduct in UVA irradiated nano-TiO2 (aerated aqueous suspension) using different spin traps including POBN, DMPO, and DEPMPO, and also found no evidence of . Their research also showed that in the absence of O2, no ESR signal was observed for PBN, DMPO, or DEPMPO. Only POBN/•OH adduct was observed under hypoxic conditions [38]. In a recent study to determine the phototoxicity of TiO2 NPs with different crystal forms and molecular sizes, the generation of •OH in aqueous suspension was observed using BMPO spin trap, and no characteristic was observed [2].
Hydrogen peroxide plays an important role in the regulation of a wide variety of biological processes. It has been demonstrated that with the addition of H2O2 into aqueous suspension containing TiO2, the concentration of increased with a small amount of H2O2 and slightly decreased at a certain concentration, and then became almost unchanged at a higher H2O2 concentration [43]. The authors suggested that the first increase in could be attributed to the oxidation of H2O2 with h+ [equation (8)]
| (8) |
At the same time, the consumption of the valence band hole (h+) hinders the (e−)–(h+) recombination, and consequently accelerates the reduction of O2 to generate [Equation (2)]. When the concentration of H2O2 is above 0.2 mM, decreases with the further increase of H2O2, which might be explained by desorption of O2 from the TiO2 surface, resulting from the adsorption of H2O2 [43]. This competition of adsorption on the TiO2 surface was also suggested in the same study using ethanol [43]. Here, the addition of a small amount ethanol resulted in the increased formation of , yet subsequently decreased with further addition of ethanol to the suspension. However, singlet oxygen decreased monotonically with additional ethanol.
4. TiO2 NPs result in cellular oxidative damage
4.1. Lipid peroxidation
Lipid peroxidation is an oxidative damage that leads to a change of the integrity and functionality of cell membranes. It happens when free radicals abstract electrons from the lipid molecules. Nanomaterials can disrupt normal cellular function through lipid peroxidation, and ROS have been proven to be responsible for membrane damage that eventually leads to the degeneration of cells [53]. In vivo studies have suggested that lipid oxidation is involved with pathogenesis of various aging related diseases, including coronary heart disease, Parkinson’s disease, and cancer [68–70]. Wang et al [29] reported a toxicity study in which rat synovial cells were treated with different concentrations of TiO2 NPs (0 mg/mL, 3 mg/mL, 30 mg/mL, and 300 mg/mL). ROS were overproduced especially in cells exposed to 30 mg/mL and 300 mg/mL TiO2 NPs. They also observed the lipid peroxidation product, malondialdehyde, and oxidative damage in cells, as well as a significant decrease in activity of the endogenous antioxidant enzymes including SOD and catalase.
TiO2 NPs have been demonstrated to lead to skin peroxidation in animal models, for example, porcine skin, which is a well-accepted and readily available model for estimating damage to human skin [57]. Oxidative stress has also been found to occur during TiO2 NPs dermal application on rats. In a 14 consecutive day toxicity study, different doses of TiO2 NPs (20 nm; 14 mg/kg, 28 mg/kg, 42 mg/kg, and 56 mg/kg) were applied to rat skin. The results of this study suggested that exposure to TiO2 NPs increased peroxidation of lipids and confirmed that nano-TiO2 toxicity is associated with oxidant generation and resultant oxidative stress to cells [71]. The results of these studies suggest that when investigating TiO2 NPs toxicity, lipid peroxidation is a critical factor due to its impact on cell membranes. These studies are particularly relevant because of the application of TiO2 NPs in many skincare and cosmetic products.
Egg PC liposome suspension was prepared as a lipid membrane model to study possible oxidative damage resulting from ROS generated during exposure of TiO2 NPs to UV light. By measuringthe hyperfine structural changes of the ESR spectrum with 15N-PDT as the spin label, it has been demonstrated that ROS can produce a time-dependent peroxidation [2], and the peroxidation rate is P25 > A25 > A325 > R100, which follows the same trend of ROS production in TiO2 NPs suspension (Fig. 2).
Fig. 2.
Effect of different TiO2 samples on lipid peroxidation in liposomes. Oxygen consumption was measured in a closed chamber using liposome suspensions and the spin label 15N-PDT. The liposome samples contained 30 mg/mL Egg PC and 0.1 mM 15N-PDT spin label mixed with (A) no TiO2, (B) 0.03 mg/mL of R100, (C) 0.03 mg/mL of A325, (D) 0.03 mg/mL of A25, and (E) 0.03 mg/mL of P25. Lipid peroxidation was initiated by UV (340 nm) irradiation. The ESR spectra were recorded with the low field line of the 15N-PDT spin label every 4 minutes after the sample was sealed in a quartz capillary tube. The spectra were obtained with 0.5 mW incident microwave power and with 0.05 G field modulation at ambient temperature. The progressive increases in peak-to-peak signal intensity (and accompanying progressive narrowing of the line width) in each panel are due to time-dependent oxygen consumption resulting from lipid peroxidation, as shown in (F). The enhancement effects of different TiO2 nanoparticles on lipid peroxidation may be seen as bigger changes in the peak-to-peak signal intensities seen in (B–E) compared with (A). Note. From “Phototoxicity of nanotitanium dioxides in HaCaT keratinocytes – Generation of reactive oxygen species and cell damage,” by J.J. Yi, J. Liu, M. Ehrenshaft, et al, 2012, Toxicol Appl Pharmacol, 263, p. 81–8. Copyright 2012, Toxicology and Applied Pharmacology. Reprinted, with permission. ESR = electron spin resonance; TiO2 = titanium dioxide.
The ESR spin trap technique has also been employed for in vivo determination of lipid radicals, the products of lipid peroxidation, and to evaluate oxidative damage [72]. Here, intact Navicula sp. algae were suspended in 40 mM PBN stock solution with the addition of spin trap POBN (130 mM) prior to detection. The authors acknowledged the possible drawbacks for employing ESR including probe instability, interference with tissue metabolism, and lack of spin specificity. However they found this technique useful, especially when combined with other biochemical strategies.
4.2. Nucleic acid damage
The specificity of the reactions between nitrone spin traps with free radicals has already made spin trapping with ESR detection the most universal tool for the detection of free radicals in biological systems [73]. Based on this concept, the immuno-spin trapping technique has been developed and extensively studied by Mason [73] to detect DNA or protein radicals. By trapping those radicals with DMPO, stable nitrone adducts can be formed and easily detected using an anti-DMPO serum with enzyme-linked immunosorbent assay (ELISA) and Western blot assay [73]. Using the method described by Mason et al [74,75], a significant increase in human serum albumin protein radical was observed using DMPO as the spin trap when human serum albumin protein was treated with P25 TiO2 NPs and UV irradiation [2].
5. Concluding remarks
Concomitant with the growing uses of nanomaterials is the need to better define their safety. As the size of particles decreases, so too does their surface area for the same quantity of material increase. This leads to many dramatic changes in the properties for nanomaterials. This change is of particular interest for nanomaterials capable of generating highly ROS. However, detection of such short-lived radicals remains technically challenging. ESR spectroscopy, a sensitive, nondestructive, in situ approach, is capable of unraveling the mechanisms of ROS-related toxicity of nanomaterials. The formation of ROS (including 1O2, •OH, and
) could be detected using different spin traps and depends on the intrinsic properties of the nanomaterials, including size, crystal phase, and surface characteristics, as well as environmental conditions. In particular, ESR spin label oximetry that detects changes in O2 levels in a system is a reliable and effective method to monitor lipid peroxidation rate. Such an oxygen-monitoring ability is of critical importance when it comes to assessing cellular oxidative damage due to ROS that could lead to subsequent adverse health effects. Moreover, the knowledge generated from ESR studies on TiO2 NPs could form a solid base for evaluating risk associated with nanomaterials.
Acknowledgments
The authors thank the Chinese Scholarship Council for providing a scholarship to M.Li for her PhD studies at the University of Maryland, College Park, MD, USA.
Footnotes
The views presented in this paper are those of the authors and do not necessarily reflect official positions or policies of the US Food and Drug Administration.
REFERENCES
- 1. Chen X, Mao SS. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev. 2007;107:2891–9. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- 2. Yin JJ, Liu J, Ehrenshaft M, et al. Phototoxicity of nanotitanium dioxides in HaCaT keratinocytes – Generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol. 2012;263:81–8. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nosaka Y, Daimon T, Nosaka AY, et al. Singlet oxygen formation in photocatalytic TiO2 aqueous suspension. J Phys Chem B. 2004;6:2917–8. [Google Scholar]
- 4. Wu P, Xie R, Imlay K, Shang JK. Visible-light-induced bactericidal activity of titanium dioxide codoped with nitrogen and silver. Environ Sci Technol. 2010;44:6992–7. doi: 10.1021/es101343c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu KP, Huang YT, Yang SC. The antifungal efficacy of nano-metals supported TiO2 and ozone on the resistant Aspergillus niger spore. J Hazard Mater. 2013;261:155–62. doi: 10.1016/j.jhazmat.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 6. Sökmen M, Değerli S, Aslan A. Photocatalytic disinfection of Giardia intestinalis and Acanthamoeba castellani cysts in water. Exp Parasitol. 2008;119:44–8. doi: 10.1016/j.exppara.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 7. He YY, Huang JL, Block ML, et al. Role of phagocyte oxidase in UVA-induced oxidative stress and apoptosis in keratinocytes. J Invest Dermatol. 2005;125:560–6. doi: 10.1111/j.0022-202X.2005.23851.x. [DOI] [PubMed] [Google Scholar]
- 8. Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tournebize J, Sapin-Minet A, Bartosz G, et al. Pitfalls of assays devoted to evaluation of oxidative stress induced by inorganic nanoparticles. Talanta. 2013;116:753–63. doi: 10.1016/j.talanta.2013.07.077. [DOI] [PubMed] [Google Scholar]
- 10.Yin JJ, Fu PP. Application of electron spin resonance to study food antioxidative and prooxidative activities. In: Gudjonsdottir M, Belton P, Webb G, editors. Magnetic resonance in food science: challenges in a changing world. Cambridge, UK: The Royal Society of Chemistry; 2009. p. 213. [Google Scholar]
- 11. Kuznetsov AV, Kehrer I, Kozlov AV, et al. Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal Bioanal Chem. 2011;400:2383–90. doi: 10.1007/s00216-011-4764-2. [DOI] [PubMed] [Google Scholar]
- 12. Tada M, Yokoyama H, Ito O, et al. Evaluation of the hepatic reduction of a nitroxide radical in rats receiving ascorbic acid, glutathione or ascorbic acid oxidase by in vivo electron spin resonance study. J Gastroenterol Hepatol. 2004;19:99–105. doi: 10.1111/j.1440-1746.2004.03201.x. [DOI] [PubMed] [Google Scholar]
- 13. Guarini S, Bazzani C, Ricigliano G, et al. Influence of ACTH1–24 on free radical levels in the blood of haemorrhage-shocked rats: direct ex vivo detection by electron spin resonance spectrometry. Br J Pharmacol. 1996;119:29–34. doi: 10.1111/j.1476-5381.1996.tb15673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalyanaraman B, Perez-Reyes E, Mason RP. The reduction of nitroso-spin traps in chemical and biological systems. A cautionary note. Tetrahedron Lett. 1979;20:4809–12. [Google Scholar]
- 15. Lion Y, Delmelle M, van de Vorst A. New method of detecting singlet oxygen production. Nature. 1976;263:442–3. doi: 10.1038/263442a0. [DOI] [PubMed] [Google Scholar]
- 16.Yin JJ, Zhao B, Xia Q, et al. Electron spin resonance spectroscopy for studying the generation and scavenging of reactive oxygen species by nanomaterials in nanopharmaceutics: the potential application of nanomaterials. Singapore: World Scientific Publishing Co; 2012. pp. 375–400. [Google Scholar]
- 17. Wang CC, Wang S, Xia Q, et al. Phototoxicity of zinc oxide nanoparticles in HaCaT keratinocytes – Generation of oxidative DNA damage during UVA and visible light irradiation. J Nanosci Nanotechnol. 2013;13:3880–8. doi: 10.1166/jnn.2013.7177. [DOI] [PubMed] [Google Scholar]
- 18. Reeves JF, Davies SJ, Dodd NJ, et al. Hydroxyl radicals (•OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res. 2008;640:113–22. doi: 10.1016/j.mrfmmm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 19. Gallez B, Baudelet C, Jordan BF. Assessment of tumor oxygenation by electron paramagnetic resonance: principles and applications. NMR Biomed. 2004;17:240–62. doi: 10.1002/nbm.900. [DOI] [PubMed] [Google Scholar]
- 20. Altenbach C, Flitsch SL, Khorana HG, et al. Structural studies on transmembrane proteins. 2. Spin labeling of bacteriorhodopsin mutants at unique cysteines. Biochemistry. 1989;28:7806–12. doi: 10.1021/bi00445a042. [DOI] [PubMed] [Google Scholar]
- 21. James PE, Jackson SK, Grinberg OY, et al. The effects of endotoxin on oxygen consumption of various cell types in vitro: an EPR oximetry study. Free Radic Biol Med. 1995;18:641–7. doi: 10.1016/0891-5849(94)00179-n. [DOI] [PubMed] [Google Scholar]
- 22. Yin JJ, Mossoba MM, Kramer JK, et al. Effects of conjugated linoleic acid on oxygen diffusion-concentration product and depletion in membranes by using electron spin resonance spin-label oximetry. Lipids. 1999;34:1017–23. doi: 10.1007/s11745-999-0452-y. [DOI] [PubMed] [Google Scholar]
- 23.Hyde JS, Subczynski WK. Spin label oximetry Biological magnetic resonance. In: Berliner LJ, Reuben J, editors. Spin labeling: theory and applicationsvol. Vol. 8. New York: Plenum Press; 1989. pp. 399–425. [Google Scholar]
- 24. Brieger K, Schiavone S, Miller FJ, Jr, et al. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;17:142. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 25. Hancock JT, Neill SJ, Wilson ID. Nitric oxide and ABA in the control of plant function. Plant Sci. 2011;181:555–9. doi: 10.1016/j.plantsci.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 26. Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–8. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 27. Guzun R, Karu-Varikmaa M, Gonzalez-Granillo M, et al. Mitochondria–cytoskeleton interaction: distribution of β-tubulins in cardiomyocytes and HL-1 cells. Biochim Biophys Acta. 2011;1807:458–69. doi: 10.1016/j.bbabio.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 28. Jaeger A, Weiss DG, Jonas L. Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology. 2012;296:27–36. doi: 10.1016/j.tox.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Ma J, Dong L, et al. Effect of anatase TiO2 nanoparticles on the growth of RSC-364 rat synovial cell. J Nanosci Nanotechnol. 2013;13:3874–9. doi: 10.1166/jnn.2013.7145. [DOI] [PubMed] [Google Scholar]
- 30. Sarkar A, Das J, Manna P, et al. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology. 2011;290:208–17. doi: 10.1016/j.tox.2011.09.086. [DOI] [PubMed] [Google Scholar]
- 31. Liu S, Xu L, Zhang T, et al. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology. 2010;267:172–7. doi: 10.1016/j.tox.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 32. Cui Y, Liu H, Zhou M, et al. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J Biomed Mater Res A. 2011;96:221–9. doi: 10.1002/jbm.a.32976. [DOI] [PubMed] [Google Scholar]
- 33. Sun Q, Tan D, Ze Y, et al. Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. J Hazard Mater. 2012;235–236:47–53. doi: 10.1016/j.jhazmat.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 34. Li N, Duan Y, Hong M, et al. Spleen injury and apoptotic pathway in mice caused by titanium dioxide nanoparticles. Toxicol Lett. 2010;195:161–8. doi: 10.1016/j.toxlet.2010.03.1116. [DOI] [PubMed] [Google Scholar]
- 35. Goodeve CF, Kitchener JA. The mechanism of photosensitisation by solids. Trans Farad Soc. 1938;34:902–8. [Google Scholar]
- 36. Maness PC, Smolinski S, Blake DM, et al. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65:4094–8. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hashimoto K, Irie H, Fujishima A. TiO2 ~ photocatalysis: a historical overview and future prospects. Jpn J Appl Phys. 2005;44:8269–85. [Google Scholar]
- 38. Dodd NJ, Jha AN. Photoexcitation of aqueous suspensions of titanium dioxide nanoparticles: an electron spin resonance spin trapping study of potentially oxidative reactions. Photochem Photobiol. 2011;87:632–40. doi: 10.1111/j.1751-1097.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- 39. Gopal NO, Lo HH, Ke SC, et al. A potential site for trapping photogenerated holes on rutile TiO2 surface as revealed by EPR spectroscopy: an avenue for enhancing photocatalytic activity. J Am Chem Soc. 2005;132:10982–3. doi: 10.1021/ja909901f. [DOI] [PubMed] [Google Scholar]
- 40. D’Arienzo M, Carbajo J, Bahamonde A, et al. Photogenerated defects in shape-controlled TiO2 anatase nanocrystals: a probe to evaluate the role of crystal facets in photocatalytic processes. J Am Chem Soc. 2011;133:17652–61. doi: 10.1021/ja204838s. [DOI] [PubMed] [Google Scholar]
- 41. Hurum DC, Gray KA, Rajh T, et al. Recombination pathways in the Degussa P25 formulation of TiO2: surface versus lattice mechanisms. J Phys Chem B. 2005;109:977–80. doi: 10.1021/jp045395d. [DOI] [PubMed] [Google Scholar]
- 42. Lipovsky A, Levitski L, Tzitrinovich Z, et al. The different behavior of rutile and anatase nanoparticles in forming oxy radicals upon illumination with visible light: an EPR study. Photochem Photobiol. 2012;88:14–20. doi: 10.1111/j.1751-1097.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 43. Daimon T, Hirakawa T, Kitazawa M, et al. Formation of singlet molecular oxygen associated with the formation of superoxide radicals in aqueous suspensions of TiO2 photocatalysts. Appl Catal A Gen. 2008;340:169–75. [Google Scholar]
- 44. Wamer WG, Yin JJ. Photocytotoxicity in human dermal fibroblasts elicited by permanent makeup inks containing titanium dioxide. J Cosmet Sci. 2011;62:535–47. [PubMed] [Google Scholar]
- 45. Dzwigaj S, Pezerat H. Singlet oxygen-trapping reaction as a method of 1O2 detection: role of some reducing agents. Free Radic Res. 1995;23:103–15. doi: 10.3109/10715769509064025. [DOI] [PubMed] [Google Scholar]
- 46. Rosenthal IC, Murali K, Yang GC, et al. A new approach for EPR detection of hydroxyl radicals by reaction with sterically hindered cyclic amines and oxygen. FEBS Lett. 1987;222:75–8. doi: 10.1016/0014-5793(87)80194-1. [DOI] [PubMed] [Google Scholar]
- 47. Konaka R, Kasahara E, Dunlap WC, et al. Irradiation of titanium dioxide generates both singlet oxygen and superoxide anion. Free Radic Biol Med. 1999;27:294–300. doi: 10.1016/s0891-5849(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 48. Sharma VK. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment – A review. J Environ Sci Health A. 2009;44:1485–95. doi: 10.1080/10934520903263231. [DOI] [PubMed] [Google Scholar]
- 49. Xue C, Wu J, Lan F, et al. Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J Nanosci Nanotechnol. 2010;10:8500–7. doi: 10.1166/jnn.2010.2682. [DOI] [PubMed] [Google Scholar]
- 50. Sanders K, Degn LL, Mundy WR, et al. In vitro phototoxicity and hazard identification of nano-scale titanium dioxide. Toxicol Appl Pharmacol. 2012;258:226–36. doi: 10.1016/j.taap.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 51. Ma XY, Chen ZG, Hartono SB, et al. Fabrication of uniform anatase TiO2 particles exposed by {001} facets. Chem Commun. 2010;35:6608–10. doi: 10.1039/c0cc01473g. [DOI] [PubMed] [Google Scholar]
- 52. Wu N, Wang J, Tafen DN, et al. Shape-enhanced photocatalytic activity of single-crystalline anatase TiO2 (101) nanobelts. J Am Chem Soc. 2010;132:6679–85. doi: 10.1021/ja909456f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sayes CM, Wahi R, Kurian PA, et al. Correlating nanoscale titania structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006;92:174–85. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- 54. Nagaveni K, Sivalingam G, Hegde MS, et al. Photocatalytic degradation of organic compounds over combustion-synthesized nano-TiO2. Environ Sci Technol. 2004;38:1600–4. doi: 10.1021/es034696i. [DOI] [PubMed] [Google Scholar]
- 55. Kavitha K, Sutha S, Prabhu M, et al. In situ synthesized novel biocompatible titania–chitosan nanocomposites with high surface area and antibacterial activity. Carbohydr Polym. 2013;93:731–9. doi: 10.1016/j.carbpol.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 56. Sawada T, Yoshino F, Kimoto K, et al. ESR detection of ROS generated by TiO2 coated with fluoridated apatite. J Dent Res. 2010;89:848–53. doi: 10.1177/0022034510370806. [DOI] [PubMed] [Google Scholar]
- 57. Carlotti ME, Ugazio E, Gastaldi L, et al. Specific effects of single antioxidants in the lipid peroxidation caused by nano-titania used in sunscreen lotions. J Photochem Photobiol B. 2009;96:130–5. doi: 10.1016/j.jphotobiol.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 58. He C, Yu Y, Hu X, et al. Influence of silver doping on the photocatalytic activity of titania films. Appl Surf Sci. 2002;200:239–47. [Google Scholar]
- 59. Yuan Y, Ding J, Xu J, et al. TiO2 nanoparticles co-doped with silver and nitrogen for antibacterial application. J Nanosci Nanotechnol. 2010;10:4868–74. doi: 10.1166/jnn.2010.2225. [DOI] [PubMed] [Google Scholar]
- 60. Allahverdiyev AM, Abamor ES, Bagirova M, et al. Investigation of antileishmanial activities of TiO2@Ag nanoparticles on biological properties of L. tropica and L. infantum parasites in vitro. Exp Parasitol. 2013;135:55–63. doi: 10.1016/j.exppara.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 61. Vamathevan V, Amal R, Beydoun D, et al. Photocatalytic oxidation of organics in water using pure and silver-modified titanium dioxide particles. J Photochem Photobiol A. 2002;148:233–45. [Google Scholar]
- 62. Rehn B, Seiler F, Rehn S, et al. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicol Appl Pharmacol. 2003;189:84–95. doi: 10.1016/s0041-008x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 63. Li B, Ze Y, Sun Q, et al. Molecular mechanisms of nanosized titanium dioxide-induced pulmonary injury in mice. PLoS ONE. 2013;8:e55563. doi: 10.1371/journal.pone.0055563. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64. Bar-Ilan O, Louis KM, Yang SP, et al. Titanium dioxide nanoparticles produce phototoxicity in the developing zebrafish. Nanotoxicology. 2012;6:670–9. doi: 10.3109/17435390.2011.604438. [DOI] [PubMed] [Google Scholar]
- 65. Finkelstein E, Rosen GM, Rauckman EJ. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980;200:1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- 66. Jaeger CD, Bard AJ. Spin trapping and electron spin resonance detection of radical intermediates in photodecomposition of water at TiO2 particulate systems. J Phys Chem. 1979;83:3146–52. [Google Scholar]
- 67. Wamer WG, Yin JJ, Wei RR. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic Biol Med. 1997;23:851–8. doi: 10.1016/s0891-5849(97)00068-3. [DOI] [PubMed] [Google Scholar]
- 68. Agil A, Duran R, Barrero F, et al. Plasma lipid peroxidation in sporadic Parkinson’s disease. Role of the L-dopa. J Neurol Sci. 2006;240:31–6. doi: 10.1016/j.jns.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 69. Gago-Dominguez M, Castelao JE. Role of lipid peroxidation and oxidative stress in the association between thyroid diseases and breast cancer. Crit Rev Oncol Hematol. 2008;68:107–14. doi: 10.1016/j.critrevonc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 70. Regnström J, Nilsson J. Lipid oxidation and inflammation-induced intimal fibrosis in coronary heart disease. J Lab Clin Med. 1994;124:162–8. [PubMed] [Google Scholar]
- 71. Unnithan J, Rehman MU, Ahmad FJ, et al. Aqueous synthesis and concentration-dependent dermal toxicity of TiO2 nanoparticles in Wistar rats. Biol Trace Elem Res. 2011;143:1682–94. doi: 10.1007/s12011-011-9010-4. [DOI] [PubMed] [Google Scholar]
- 72. Gonzalez PM, Aguiar MB, Malanga G. Electronic paramagnetic resonance (EPR) for the study of ascorbyl radical and lipid radicals in marine organisms. Comp Biochem Physiol A Physiol. 2013;165:439–47. doi: 10.1016/j.cbpa.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 73. Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med. 2004;36:1214–23. doi: 10.1016/j.freeradbiomed.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 74. Ehrenshaft M, Mason RP. Protein radical formation on thyroid peroxidase during turnover as detected by immuno-spin trapping. Free Radic Biol Med. 2006;41:422–30. doi: 10.1016/j.freeradbiomed.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 75. Ranguelova K, Bonini MG, Mason RP. (Bi)sulfite oxidation by copper, zinc-superoxide dismutase: sulfite-derived, radical-initiated protein radical formation. Environ Health Perspect. 2010;118:970–5. doi: 10.1289/ehp.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]