Abstract
Nanotechnology is a rapidly developing field in the 21st century, and the commercial use of nanomaterials for novel applications is increasing exponentially. To date, the scientific basis for the cytotoxicity and genotoxicity of most manufactured nanomaterials are not understood. The mechanisms underlying the toxicity of nanomaterials have recently been studied intensively. An important mechanism of nanotoxicity is the generation of reactive oxygen species (ROS). Overproduction of ROS can induce oxidative stress, resulting in cells failing to maintain normal physiological redox-regulated functions. This in turn leads to DNA damage, unregulated cell signaling, change in cell motility, cytotoxicity, apoptosis, and cancer initiation. There are critical determinants that can affect the generation of ROS. These critical determinants, discussed briefly here, include: size, shape, particle surface, surface positive charges, surface-containing groups, particle dissolution, metal ion release from nanometals and nanometal oxides, UV light activation, aggregation, mode of interaction with cells, inflammation, and pH of the medium.
Keywords: Lipid peroxidation, Nanotoxicology, Reactive oxygen species
1. Introduction
Nanomaterials are chemical molecules that have surfaces with at least one dimension smaller than 100 nm; engineered nanomaterials have the same specific physicochemical characteristics and are manufactured intentionally [1]. Nanotechnology is a rapidly developing field in the 21st century. The various commercial uses of nanomaterials for novel applications are increasing exponentially. Nanomaterials are extremely small in size and possess a large surface area per unit of volume. These novel physical characteristics of nanomaterials can result in their having drastically different chemical and biological properties compared to the same material in bulk form. The unique chemical and biological properties of nanomaterials make them useful in many products for humans, including some in industry, agriculture, business, medicine, clothing, cosmetics, and food [1–9]. Nanotechnology research and development by industry and governments worldwide have been increasing dramatically. It has been estimated that by 2015, nanoproducts will contribute approximately $1 trillion to the global economy [3,10,11].
Humans may be exposed to nanomaterials through inhalation (respiratory tract), skin contact, ingestion, and injection (blood circulation) [4]. The tiny size of nanomaterials allows them to pass more easily through cell membranes and other biological barriers, therefore, nanomaterials can be easily taken up into living organisms and cause cellular dysfunction [10,11]. In addition, because of their unique properties, including high surface-to-volume ratios, nanomaterials are reactive or catalytic, and thus can be potentially toxic. For the safe development of nanotechnology and the safe use of commercial nanomaterials, investigations regarding the cellular toxicity and phototoxicity of nanomaterials are needed.
Although the unique properties of nanomaterials have resulted in an exponential increase in their use, cytotoxic and genotoxic data for most manufactured nanomaterials have not been published at a correspondingly high rate [1,4–6,12,13]. Many nanomaterials synthesized as commercial products are introduced daily into our lives. For example, zinc oxide nanoparticles (nano-ZnO) are one of the most commonly used nanomaterials, with industrial and commercial applications, including personal skin and hair care products, sunscreens, pigments, coatings, ceramic products, and paints [2,14–16]. Another example is titanium dioxide nanoparticles (nano-TiO2), which are among the top nanomaterials, and are widely used as food additives and drug delivery agents in personal care products, paints, plastics, and cosmetics [6,17,18]. The potential harm of nano-ZnO and nano-TiO2 to human health has attracted public attention. Understandably, the matter of safety and toxicity of nanomaterials has become an issue of interest to the public. Therefore, understanding the interactions of nanomaterials with biological systems is a particularly important scientific issue.
2. Toxicity of nanomaterials
The range of nanotechnology products is wide and they can be classified into several different compound categories, including metals, metal oxides, carbon, silica, and semiconductor nanomaterials [6]. The toxicity of nanomaterials has been studied in different biological systems, both in cell line systems and different organisms, which include rodents, humans, and aquatic species, such as zebrafish [1,19–25], catfish [26], algae [27], and macrophages [28]. Carbon and metallic nanomaterials are among the most widely used types of engineered nanomaterials. Nano-metals, such as nano-gold (nano-Au), nano-silver (nano-Ag), nano-copper, nano-aluminum, nano-nickel, nano-cobalt, and other nano-particles, have also been extensively studied. Metal nano-particles are important industrial materials that are widely used as additives in cosmetics, pharmaceuticals, and food colorants [6]. The skin can be exposed to solid nanoparticles through the application of lotions or creams that contain nano-TiO2 or nano-ZnO as a sunscreen component or fibrous materials coated with nanoscale substances for water or stain-repellent properties. In addition to exposure due to use of consumer products, the manufacture and use of nano-particles inevitably leads to increased occupational and environmental exposure. The toxicity of metal oxide nanoparticles, such as nano-TiO2, nano-ZnO, nano-CuO, nano-CuZn, nano-Fe3O4, and nano-Fe2O3, with nano-TiO2, nano-ZnO in particular, has been reported [6,12,29–31]. As expected, different nanomaterials exhibit different toxic potency. For example, Zhu et al [32] compared the toxicity of three nano-metal oxides, nano-CuO, nano-CdO, and nano-TiO2. Nano-CuO was determined to be the most potent in cytotoxicity and DNA damage, leading to 8-hydroxy-2′-deoxysuanosine (8-OHdG) formation, while nano-TiO2 was the least, without inducing a significant level of 8-OHdG [32].
The production of carbon nanotubes (CNTs) and graphene oxide is becoming commercially important. Under some experimental conditions, investigators have found that CNTs and graphene oxide are toxic [33–38].
The mechanisms underlying the toxicity of nanomaterials have recently been studied intensively. An important mechanism of nanotoxicity is the generation of reactive oxygen species (ROS), resulting in the subsequent formation of oxidative stress in tissues [1].
3. Overproduction of ROS and cell damage
In the mitochondria of cells, ATP is synthesized by reduction of molecular oxygen to water through a sequence of coupled proton and electron transfer reactions. During this process, a small percentage of the oxygen is not reduced completely, resulting in the formation of superoxide anion radicals, and subsequently other oxygen-containing radicals. Thus, ROS are byproducts of cellular oxidative metabolism, much of which occurs in the mitochondria. Biologically relevant ROS include superoxide anion radicals, hydroxyl radicals, singlet oxygen, and hydrogen peroxide (H2O2) [39]. ROS play beneficial physiological roles in cellular signaling systems and induction of mitogenic responses [40,41]. Besides cellular oxidative stress, there are several other biological reactions that can generate ROS in vivo. Transition metals such as copper and iron can also participate in one-electron oxidation–reduction reactions, leading to the formation of ROS [42].
Overproduction of ROS can induce oxidative stress, resulting in cells failing to maintain normal physiological redox-regulated functions [43,44]. The damage in cell function and development includes oxidative modification of proteins to generate protein radicals [45], initiation of lipid peroxidation [46–48], DNA-strand breaks, modification to nucleic acids [49], modulation of gene expression through activation of redox-sensitive transcription factors [50,51], and modulation of inflammatory responses through signal transduction [52], leading to cell death and genotoxic effects [3,53–57].
It has been demonstrated that ROS and oxidative stress are associated with many age-related degenerative diseases [41,45,58,59], including amyotrophic lateral sclerosis, arthritis, cardiovascular disease, inflammation, Alzheimer’s disease, Parkinson’s disease, diabetes, and cancer [8,40,43,52,60–64].
In nuclear and mitochondrial DNA, 8-OHdG is a predominant form of free-radical-induced oxidative lesion. 8-OHdG has been commonly used as a biomarker of oxidative stress, DNA damage, and carcinogenesis, and as a pivotal marker for measuring the effect of oxidative damage to DNA [32].8-OHdG has also been used as a risk factor for many diseases including cancer [65].
Generation of ROS induced by nanomaterials, directly or indirectly, plays a vital role in genotoxicity. Oxidative DNA damage is associated with biological mechanisms involving mutagenesis, carcinogenesis, and aging-related diseases in humans. Oxidative stress is one of several mechanisms leading to nanotoxicity. Some nano-metal oxides can enhance ROS generation, inducing oxidative stress, DNA damage, and unregulated cell signaling, and eventually leading to changes in cell motility, apoptosis, and even carcinogenesis. Therefore, it is imperative that the mechanisms by which nanomaterials mediate and/or promote these adverse events be understood.
DNA is a critical cellular target of ROS. Oxidative DNA damage involves base and sugar lesions, DNA–protein crosslinks, single- and double-strand breaks, and the formation of abasic sites [41]. Highly reactive radicals, such as hydroxyl radicals, can damage DNA quickly in the vicinity; whereas the less-reactive ROS may interact with DNA at a distance.
Superoxide dismutases (SODs), peroxidases, and catalases are some of the prominent antioxidant enzymes that efficiently protect against these harmful biological events. For example, SOD catalyzes the disproportionation of superoxide to H2O2:
Superoxide is a poor oxidant and has a low reactivity toward most biological molecules. Many deleterious effects of superoxide are due to the conversion of superoxide to a more reactive radical, particularly the hydroxyl radical. The conversion of superoxide to hydroxyl (or other powerful oxidants) has been the basis for biological studies [66].
Antioxidants play an important role in preventing, or in most cases, limiting the damage caused by ROS. The hydroxyl radical possesses the highest one-electron reduction potential of all the physiologically relevant ROS, and is extremely reactive with almost every type of biomolecule, including proteins and nucleic acids [43,49,67,68]. There is no known enzymatic reaction that can scavenge the hydroxyl radical in vivo. The only known defense against hydroxyl radicals comes from antioxidants. Antioxidants are essentially reducing agents; they participate in redox reactions by donating electrons or hydrogen atoms. Within limitations, this action allows cells to function normally and avoid the consequences of oxidation of structural and other vital components. This helps prevent premature destruction by malfunctions such as uncontrolled proliferation.
By contrast, compared with normal cells, cancer cells experience increased generation of ROS and oxidative stress, resulting in stimulation of cellular proliferation, mutations, genetic instability, and cell death [69–71]. Thus, chemical species that can scavenge ROS are potential biomedicines for use in cancer chemotherapy.
Chemicals under light irradiation can result in the generation of ROS and the subsequent formation of lipid peroxidation [3,9,53–57,72–76].
4. Mechanism of nanotoxicity generation of ROS
The generation of ROS and the subsequent production of oxidative stress is a predominant mechanism leading to nanotoxicity, including DNA damage, unregulated cell signaling, changes in cell motility, cytotoxicity, apoptosis, and cancer initiation and promotion [10,11,32]. The level of ROS generation by engineered nanomaterials is dependent on the chemical nature of the nanoparticles [1]. Compared to their bulk-size counterparts, engineered nanomaterials possess a small size, high specific surface area, and high surface reactivity, leading to the production of higher levels of ROS, and resulting in cytotoxicity and genotoxicity [5]. A variety of nanomaterials has been found to induce toxicity mediated by ROS in many biological systems, such as human erythrocytes and skin fibroblasts [77].
Winnik and Maysinger [78] demonstrated that quantum dots produced oxidative stress and cell damage mediated by ROS. Akhtar et al [79] reported that silica nanoparticles induced cytotoxicity and resultant oxidative stress in a dose-dependent manner, mediated by the induction of ROS and lipid peroxidation in the cell membrane. Akhtar et al [80] also determined that in mouse embryonic fibroblasts (BALB 3T3), nano-CuO induces cytotoxicity, releasing lactate dehydrogenase (LDH), and causing oxidative stress in a dose-dependent manner, mediated by the induction of ROS and lipid peroxidation. As further substantiation of this mechanism, it has been reported that nano-ZnO induces cytotoxicity that is largely mediated by the induction of ROS, causing oxidative injury, release of mediators for inflammation, resulting in cell death in phagocytic RAW 264.7 cells, and transformation in human bronchial epithelial BEAS-2B cells [11,16]. Similar to nano-ZnO, nano-TiO2 also produces ROS, leading to cytotoxicity and photocytotoxicity [3,18,39,81–87].
Both nano-Au and gold–cobalt nanoalloys have been studied for biomedical applications. Girgis et al [88] determined that in mice, a gold–cobalt nanoalloy induced alteration in the tumor-initiating genes that was associated with an increase of micronuclei formation and the generation of 8-OHdG; while these toxic activities induced by nano-Au were much lower. Girgis et al [88] proposed that these toxic activities are attributed to the increase in oxidative stress.
Hsin et al [89] reported that nano-Ag-induced apoptosis in NIH3T3 cells was mediated by a ROS- and C-Jun-terminal-kinase-dependent mechanism involving the mitochondrial pathway.
Mei et al [90] determined that nano-Ag induced mutation and oxidative stress mediated by ROS formation in mouse lymphoma cells. Kim et al [91] determined that nano-Ag induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. Shvedova et al [92] reported that incubation of high doses of single-walled CNTs in keratinocytes and bronchial epithelial cells, produced ROS, lipid peroxidation, oxidative stress, and mitochondrial dysfunction.
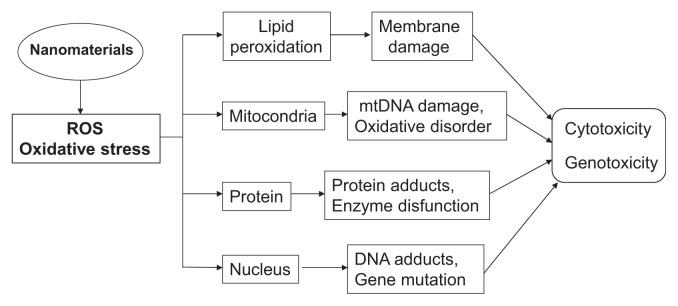
Nano-iron oxide, which is superparamagnetic, is cytotoxic and generates ROS and apoptosis [93]. Wang et al [26] compared the cytotoxicity of four nano-metal oxides, nano-ZnO, nano-TiO2, nano-Co3O4, and nano-CuO, in catfish hepatocytes and human HepG2 cells. In both cell systems, the induced toxicity was in the order of: TiO2 < Co3O4 < ZnO < CuO, and the induced cytotoxicity was due to ROS and damage to the cells and mitochondrial membranes. The cytotoxicity was higher in HepG2 cells than in catfish primary hepatocytes. As shown in Fig. 1, nanomaterial-induced ROS can lead to oxidative stress, cause mitochondrial damage, lipid peroxidation, protein modification, DNA damage, leading to cytotoxicity, genotoxicity, and cancer.
Fig. 1.
Nanomaterial-induced toxicity mediated by ROS generation.
Not all nano-metal-oxide-induced toxicity is mediated by ROS. A good example comes from the study by Karlesson et al [12]. They determined the cytotoxicity, DNA damage, and oxidative stress of different nano-metal oxides (CuO, TiO2, ZnO, CuZnFe2O4, Fe3O4, and Fe2O3), carbon nanoparticles, and multiwalled CNTs in human lung epithelial cell line A549. They determined that nano-CuO was the most potent in inducing cytotoxicity, DNA damage, oxidative lesions, and significantly increasing intracellular ROS. Nano-ZnO showed cytotoxicity and DNA damage. Nano-TiO2, containing both rutile and anatase forms, only caused DNA damage. Nano-Fe3O4 and nano-Fe2O3 exhibited no or low cytotoxicity. Nano-CuZn Fe2O4 was potent in inducing DNA lesions. CNTs led to cytotoxicity and caused DNA damage. These results indicate that nano-CuO exhibits the highest cytotoxicity and genotoxicity, and is the only studied nanomaterial that induces ROS [12].
5. Nanotoxicity—physical and chemical properties of nanomaterials
The production of ROS by nanomaterials may proceed through a variety of mechanisms. The ROS formation from a particular nanomaterial is dependent on the physical and chemical properties of the nanomaterials as well as the testing systems, such as different cell types [94]. The critical chemical and physical structural determinants of the nano-material that lead to the generation of ROS and toxicity include molecular size, shape, oxidation status, surface area, bonded surface species, surface coating, solubility, and degree of aggregation and agglomeration [6,10,11,25,94,95]. Through these intrinsic determinants, certain nanomaterials can stimulate and generate inflammation that can chemically or catalytically convert less toxic oxidants, such as superoxide and H2O2, into more reactive free radicals, such as hydroxyl radicals. In addition, interactions with environmental factors, such as light, are also important factors that determine how nanomaterials interact with biological tissue and affect generation of ROS and the resulting toxicity. Several critical determinants that profoundly determine the nanomaterial-induced toxicity are discussed below.
5.1. Size and shape
The tiny size and high surface-to-volume ratio are the unique physical and chemical properties of nanomaterials that determine nanomaterial-induced toxicity.
As noted previously, because of the tiny size of nano-materials, they can easily penetrate cell membranes and other biological barriers into living organisms and cause cellular dysfunction [10,11]. The amount of cellular uptake decreases with the increase in particle size [96–98]. The smaller the particles, the greater the tendency to enter subcellular organelles based solely on their sizes [99]. Compared with their bulk counterparts, nanomaterials, with such a small size, can reach into the lung, leading to several side effects [5]. Yoshida et al [100] demonstrated that particle size was a critical determinant of the intracellular distribution of amorphous silica and its induced ROS formation, leading to DNA damage in human skin HaCaT cells. Moreover, bactericidal or toxic effects increase as the size of nanoparticles decreases [101].
Li et al [102] determined that the wire-shaped nanomaterial, alpha-MnO2 nanowire-induced cytotoxicity, DNA oxidative damage, and apoptosis in HeLa cells were mediated by ROS and oxidative stress. Long nanowires in cultured fi-broblasts cause failed cell division, DNA damage, and increased ROS; while vertical nanowire arrays induce cell motility and proliferation rate [36]. Nano-TiO2 exposed in cultured WISH cells induced cytotoxicity, morphological alterations, generation of intracellular ROS, and DNA damage [103]. Jiang and colleagues found that for a fixed total surface area, an S-shaped curve dependence for ROS generation per unit surface area occurred as a function of particle size (4–195 nm with the same crystal phase) [104]. Sohaebuddin et al [28] determined the effects of the chemical composition of nano-TiO2, nano-SiO2, and multi-wall CNTs on their toxicity in 3T3 fibroblasts, RAW 264.7 macrophages, and telomerase-immortalized bronchiolar epithelial cells. The results indicated that the composition, molecular size, and target cell type, are all critical determinants of intracellular responses, degree of cytotoxicity, and potential mechanisms of toxicity. Moreover, these nanomaterials induced cell specific responses, resulting in variable toxicity and subsequent cell damage.
Yin et al [39] studied the photocytotoxicity of nano-TiO2 of four different sizes (<25 nm, 31 nm, <100 nm, and 325 nm) and two different crystal forms (anatase and rutile) in human skin keratinocytes. Upon UVA irradiation, all nano-TiO2 particles induced photocytotoxicity and cell membrane damage in a light-dose- and nano-TiO2-dose-dependent manner. The nano-TiO2with a smaller particle size, induced greater cell damage. The anatase form of nano-TiO2 induced higher photocytotoxicity than the rutile form. All the induced photocytotoxic damage was mediated by ROS, generated during UVA irradiation [18].
For silica–titania hollow nanoparticles of different sizes, each with uniform diameters of 25 nm, 50 nm, 75 nm, 100 nm, and 125 nm, Oh et al [105] determined that the 50-nm silica–titania hollow nanoparticles exerted the greatest toxicity on macrophages.
Shape is not always a critical determinant of nanomaterial-induced toxicity. Ray and coworkers determined that a set of nano-Au particles with different shapes had similar cytotoxicity [25]. However, it is still worthwhile to consider it in the process of engineering and application. For instance, ZnO with snowflake particles seems to be the most active among available morphologies (whisker, snowflake, and spherical shapes) [106]. Hexagonal plate-like ZnO nanocrystals were also reported to display significantly higher activity than rod-shaped crystals [107]. In addition, external morphology may directly influence uptake into cells in the following order: rods/spheres > cylinders > cubes [108–110]. Moreover, changes in the aspect ratio of nanorods tend to affect total cell uptake [108]. The larger the contact area of nanoparticles, the higher biocompatibility they reveal. The aforementioned properties may endow upon respective nanoparticles huge differences in interactions with biological systems. For instance, higher dendritic aggregate toxicity was observed for Ni nanoparticles to zebrafish embryos, compared to spherical ones [111]. For the engineering process, asymmetrical properties may provide vital information for new designs.
5.2. Particle surface, surface positive charges, and surface containing groups
Iron oxide magnetic nanoparticles (nano-Fe3O4) have been utilized for biomedical applications. Nano-Fe3O4 coated with poly(ethylenimine) (PEI) and poly(ethylene glycol) (PEG) possess different surface positive charges. Hoskins et al [112] determined that nano-Fe3O4-PEI, which had a higher surface charge than nano-Fe3O4-PEI-PEG, exhibited greater cytotoxicity and ROS formation in human SH-SY5Y, MCF-7, and U937 cell lines, for the formation of ROS and lipid peroxidation. Wang et al [113] demonstrated that hematite nano-Fe2O3 and maghemitenano-Fe2O3, which have different surface structures, induced hydroxyl radicals at different levels.
In the field of biomedical applications used in drug delivery systems, labeling, and tissue engineering, mesoporous silica is one of the most studied nanomaterials [114]. Mesoporous silica and colloidal silica, which have different pore architectures, including specific surface area and pore volume, possess different nanotoxicity in terms of cellular uptake and immune response.
Shi et al [115] demonstrated that copper nanoparticles modified with different surface ligands, for example, 8-mercaptooctanoic acid, 12-mercaptododecanoic acid, and 16-mercaptohexadecanoic acid, exhibited different surface oxidation reactivity resulting in generation of ROS at different levels.
Maccormack et al [116] determined that nano-Si, nano-Au, and nano-CdSe inhibited LDH activity and the inhibition was dependent on particle core and surface-functional-group composition. These nanomaterials bound to abundant proteins nonspecifically via a charge interaction [116]. Oh et al [105] determined that among the silica–titania hollow nanoparticles with different sizes and surface functionalities, cationic silica–titania hollow nanoparticles exhibited the greatest toxicity on J774A.1 macrophages and the highest uptake efficiency. These results illustrate size-dependent and surface-functionality-dependent nanotoxicity and uptake of silica–titania hollow nanoparticles, and are useful for the application of silica–titania hollow nanoparticles as drug delivery and imaging probes [105].
The effect of the composition of quantum dots on quantum-dot-induced toxicity has been well studied [78,117–121]. Winnik and Maysinger [78] determined that quantum-dot-induced toxicity in vitro and in vivo is dependent on the composition, size, and surface-capping materials of quantum dots. Hauck et al [117] studied the toxicity of quantum dots and found that the composition (PbS vs. CdS), size, shape (spheres vs. rods), and surface chemistry (amino vs. carboxylic groups) were critical determinants in quantum-dot-induced toxicity and biodistribution. Zhu et al [121] showed that both the particle size and the chemical structure of quantum dots determined the stability of the monolayer surface coating of quantum dots.
Nanomaterials possess a large specific surface area that can potentially absorb transition metals, such as Fe, Ni, Cu and Cr, onto the surface. These absorbed transition metals can catalyze Fenton reactions, Fenton-like reactions, and Haber–Weiss reactions to generate hydroxyl radicals that can directly attack DNA [122]. Limbach et al [123] found that nanosilica, doped with transition metals in A549 cells, generated high levels of ROS formation.
Besides transition metals, the large surface area of carbon black nanoparticles can also absorb other chemicals, such as polycyclic aromatic hydrocarbons (PAHs). Upon biotransformation, PAHs can be oxidized to PAH quinones, which are redox-active and can be reduced to PAH semiquinones and superoxide anion radicals. Superoxide anion radicals can be dismutated into H2O2, which upon reacting with transition metals produce hydroxyl radicals [122].
Among the fullerenes is C60, which has a spherical shape. Water-soluble, monodisperse, or colloidal fullerene aggregates induce superoxide anions and lipid peroxidation, resulting in cytotoxicity [10]. Different functional groups attached to the surface of fullerenes are critical determinants of fullerene-induced toxicity [10]. Thus, fullerenes with structural and surface modifications have many potential applications based on their capabilities in performing pro-oxidative (generation of free radicals) or antioxidative (scavenging free radicals) activities. Fullerenes can also be phototoxic upon visible or UV light irradiation to excite the fullerene surface. The excited triplet-state fullerenes can transfer energy to molecular oxygen to form singlet oxygen, and transfer an electron to induce superoxide anion radicals, resulting in lipid peroxidation, leading to cytotoxicity [10]. Modification of the fullerene surface by attachment of one or more malonyl groups yields derived fullerenes possessing antioxidant activity [10].
Several fullerenes, fullerenols, and endohedral metallofullerenols have been demonstrated to be free-radical scavengers [8]. Yin et al [8] studied the ROS-scavenging capability of [Gd@C82 (OH)22]n nanoparticles and compared them with other functionalized fullerenes, for example, functionalized C60-fullerenols and C60-carboxyfullerenes. Electron Spin Resonance (ESR) spin trap measurements in vitro demonstrated that Gd@C82(OH)22, (C60(OH)22) (a fullerenol), and C60(C(COOH)2)2 (a carboxyfullerene) all efficiently scavenged different types of free radicals, including superoxide anion radicals, hydroxyl radicals, and singlet oxygen [8]. In human lung adenocarcinoma A549 cells and rat brain capillary endothelial cells, these fullerene derivatives reduced H2O2-induced cytotoxicity, free radical formation, and mitochondrial damage. The cellular protective effects of these fullerene derivatives correlated well with their ability to scavenge intracellular ROS following relative potencies: Gd@C82(OH)22 ≥ C60(OH)22 > C60(C(COOH)2)2 [8]. These observed different free-radical-scavenging capabilities suggest that both chemical properties, such as surface chemistry (induced different electron affinity), and physical properties, such as degree of aggregation, influence the biological and biomedical activities of functionalized fullerenes [8].
Yang and Watts [31] determined that particle surface played an important role in the phytotoxicity of alumina nanoparticles. Yang et al [124] studied the mechanism of nano-Ag-induced toxicity and found that both dissolved silver and surface coating in Caenorhabditis elegans are important factors that affect toxicity.
5.3. Solubility and particle dissolution
Studer et al [125] determined that intercellular solubility is a determinant affecting nanoparticle-induced cytotoxicity. The finding is that soluble copper metal is less toxic than copper oxide nanoparticles. A similar solubility-dependent effect was subsequently reported by Shaligram and Campbell [94]. The toxicity of copper salts is dependent on the solubility profile and cell type tested.
Shen et al [126] determined that there is a good correlation between the nano-ZnO-induced cytotoxicity and the free intracellular zinc concentration in human immune cells. They also found that both nano-ZnO dissolution and contact to the cells are required to elicit cytotoxicity. The levels of nano-ZnO-induced ROS also correlated with cytotoxicity and intracellular free zinc. These results indicate that intracellular dissolution of zinc nanomaterials was required in order to elicit cytotoxicity. Although antioxidants reduced ROS, nano-ZnO-induced cytotoxicity was not affected. These results suggested that nano-ZnO-induced cytotoxicity was not all caused by ROS.
Mahto et al [127] reported that quantum dot core/shell (CdSe/ZnSe) dispersed in aqueous media, induced ROS formation and released cadmium, leading to toxicity.
Nano-ZnO suspended in Dulbecco’s modified Eagle’s medium (DMEM) induced dose-dependent cytotoxicity and damaged cell membranes in human A549 cells, HepG2 cells, human skin fibroblast cells, human skin keratinocytes, and rat primary neuronal cells. The relative sensitivity to nano-ZnO cytotoxicity in these cell systems was in the order: newborn rat forebrain primary cells > human skin fibroblasts > human skin HaCaT keratinocytes >human bronchoalveolar carcinoma A549 cells >human hepatocellular carcinoma HepG2 cells [3].
The cytotoxicity of nano-ZnO in serum-free DMEM and DMEM supplemented with 10% fetal bovine serum (FBS) was also compared. Although nano-ZnO in DMEM supplemented with 10% FBS induced cytotoxicity at a level similar to that without supplemented with 10% FBS, cytotoxicity was not mediated by ROS. These results indicate that the mechanism of cytotoxicity is medium dependent, implying that cellular growth conditions play a significant role in the induction of cytotoxicity by nano-ZnO [3]. These results also illustrate that not all nanomaterial-induced cell death is mediated by ROS.
5.4. Metal ions released from metal and metal oxide nanoparticles
Beer et al [128] studied the role of the silver ion fraction of nano-Ag suspensions on the nano-Ag-induced cytotoxicity in human A549 lung cells. They found that at high silver ion fractions, the nano-Ag did not induce measurable levels of toxicity to the nano-Ag suspension. However, when the silver ion fraction was 2.6% or lower, nano-Ag suspensions contributed more toxic effects than their supernatant.
Faisal et al [129] determined that nickel oxide nano-particles (nano-NiO) induced cellular ROS, oxidative stress, mitochondrial dysfunction, apoptosis/necrosis, and lipid peroxidation in tomato seedling roots. The activity of antioxidative enzymes, including catalase, glutathione, and SOD, was all enhanced. They concluded that the dissolution of Ni ions from nano-NiO was responsible for the induction of cell death, through triggering the mitochondrion-dependent intrinsic apoptotic pathway.
Foldbjerg et al [130] investigated the effects of well-characterized polyvinyl pyrrolidone (PVP)-coated nano-Ag and silver ions (Ag+) in the human alveolar cell line A549. Both PVP-coated nano-Ag and Ag+ induced cytotoxicity in a dose-dependent manner. The cytotoxicity of both PVP-coated nano-Ag and Ag+ was inhibited by the antioxidant, N-acetylcysteine. A strong correlation between the levels of ROS formation and mitochondrial damage and early apoptosis was observed. DNA damage induced by ROS was also found. The level of bulky DNA-adduct formation was well correlated with the cellular ROS levels and was inhibited by pretreatment with N-acetylcysteine. These results suggest that nano-Ag induced genotoxicity, mediated by ROS [130].
When nanomaterials are in a suspending medium or a biological system where particle dissolution can take place, the ionic species generated from nanomaterial dissolution can also elicit toxicity [131]. Franklin et al [131] observed that Zn+2 ions released from the dissolved nano-ZnO were highly toxic to aquatic organisms. Thus, nano-ZnO dissociation caused disruption of cellular zinc homeostasis, lysosomal and mitochondrial damage, and cell death. Subsequently, Xia et al [11] also determined that dissolution plays an important role in ZnO-induced cytotoxicity.
It has been determined that Ag+ can be released from the surface of nano-Ag by surface oxidation of nano-Ag in the presence of water. The rate of ion release is dependent upon the size of the nano-Ag particles, the sulfur concentrations contained in the nano-Ag, temperature, oxygen, pH, and light. The reaction of Ag+ ions with molecular oxygen generates superoxide radicals and other ROS, resulting in apoptosis and the expression of stress-response-related genes [123,132]. Based on experimental results, it is suggested that the Ag+ ion is the reactive species leading to toxicity of nano-Ag.
Zhu et al [32] studied the toxicity of nano-CuO, nano-CdO, and nano-TiO2. CuO nanoparticles were found to possess the most potent cytotoxicity and to cause the most DNA damage. Nano-CdO showed similar activity, only to a lesser extent than nano-CuO. Nano-TiO2 showed low cytotoxicity, with no increased 8-OHdG levels.
Nano-metals and nano-metal oxides with redox characteristic properties can enhance the formation of ROS, serving as catalysts in ROS production through Fenton reactions, Fenton-like reactions, or the Haber–Weiss cycle reaction, all yielding hydroxyl radicals [1,23,113]. The Haber–Weiss reaction generates hydroxyl radicals (•OH) from H2O2 and superoxide anion radicals ( ).
Fenton reaction
Fenton-like reaction
Haber–Weiss cycle reaction
5.5. Light activation
Many nanomaterials have semiconductor properties. The reactivity of nanomaterials can be dramatically altered by exposure to UV and visible light. Light-induced changes in reactivity may affect the stability of a product containing these nanomaterials. Upon light irradiation, nanomaterials can be excited and react with molecular oxygen to generate ROS and cytotoxicity (i.e., photocytotoxicity). The two commercially important products, nano-TiO2 and nano-ZnO are good examples. The bonding in these materials gives rise to valence bands and conduction bands whose energy difference is similar to that in UV light (for nano-TiO2 and nano-ZnO, this is energy in the UVA spectral region). When these semiconductors are photoexcited, electrons are excited from the valence to the conduction band, giving rise to excitons or electron–hole pairs. These electron–hole pairs can recombine, giving rise to no net chemical transformation. Alternatively, electrons can combine with surface-bound molecules (such as oxygen) to form a radical (such as a superoxide anion radical). Similarly, holes, which are powerful oxidants, can react with surface-bound molecules (such as water) and give rise to radicals (such as the hydroxyl radical). Additional intermediate radical reactions may result in the formation of other ROS including singlet oxygen [17,133–138].
Upon UVA irradiation, nano-ZnO induced dose-dependent cytotoxicity, LDH release, lipid peroxidation, and DNA damage in a light dose and substrate dose response manner in human keratinocytes. The level of photocytotoxicity was mainly dependent on the level of ROS production [86]. Both the hydroxyl radical and the superoxide anion radical were formed. Nano-ZnO also induced single-strand DNA breaks in supercoiled ΦX174 plasmid DNA. Under visible light illumination, nano-ZnO induced the LDH leakage, hydroxyl radical generation, and 8-OHdG formation in a dose-dependent manner. Collectively, these results demonstrate the photocytotoxic and photogenotoxic effects of nano-ZnO on human skin keratinocytes in vitro.
A mechanism was proposed [86]. Upon receiving UVA irradiation, the resulting photoexcited nano-ZnO favorably extracted electrons from water or hydroxide ions to generate hydroxyl radicals. The pathways of transferring energy to molecular oxygen to form singlet oxygen, or transferring an electron to molecular oxygen to generate superoxide radical anions are not favored. The overall results indicate that photoirradiation of nano-ZnO-generated ROS (mainly hydroxyl radicals) cause DNA cleavage, induce lipid peroxidation, and generate endogenous DNA adducts (8-OHdG) [86].
Zhao et al [139] studied the phototoxicity of BiOCl single-crystalline nanosheets with different surface structures under UV light irradiation. It was determined that the generation of ROS is surface structure dependent.
5.6. Aggregation and mode of interaction with cells
Graphene oxide and graphene are two-dimensional carbon-based nanomaterials and are potential candidates for biomedical applications including sensors, cell labeling, bacterial inhibition, and drug delivery [140]. Liao et al [140] found that the toxicity of graphene and graphene oxide depended on whether or not aggregation occurred and on the mode of interaction with cells (i.e., suspension versus adherent cell types).
Ekstrand-Hammarström et al [141] determined that crystal structure, surface area, and degree of aggregation and agglomeration determined the level of ROS and cytotoxicity elicited by TiO2. Kim et al [91] discussed the physiochemical characteristics that can determine nano-Ag-induced toxicity, which include size, release of Ag+ ions, and agglomeration/aggregation.
5.7. Inflammation leading to ROS formation
McCarthy et al [142] found that amorphous nano-SiO2 can cause an inflammatory effect in the lungs. They also observed that the amorphous nano-SiO2 induced inflammation in submucosal cells, and generated ROS, leading to apoptosis and decreased cell survival.
5.8. pH of the system
Fe3O4 magnetic nanoparticles exhibited an intrinsic peroxidase-like activity under acidic conditions and a catalase-like activity at neutral pH [113]. The authors proposed that this pH-dependent dual enzyme activity can be utilized for cancer treatment through producing ROS in tumor tissues [113].
He et al [23] determined that in the presence of H2O2, nano-Ag can induce hydroxyl radicals through a Fenton-like mechanism, and the level of hydroxyl radical formation is dependent on experimental pH circumstances. The level of hydroxyl radical formation was in the order: pH 1.2 > pH > 3.6 > 4.6. When the pH was at 7.4, there was no significant formation of hydroxyl radicals. Wang et al [113] also determined that both hematite nano-Fe2O3 and maghemitenano-Fe2O3 induced hydroxyl radicals, with intensity following the order pH 1.2 > 4.2 > 7.2. Apparently, all the above-described results indicate that lower pH conditions facilitate Fenton and Fenton-like reactions to generate hydroxyl radicals.
6. Difficulties in determination of the mechanism of nanotoxicity in cells and in vivo
Most nanomaterial-induced toxicity is through free-radical mechanisms, among which, generation of oxidative stress mediated by ROS formation is most important. Essentially, free-radical mechanisms are difficult to elucidate at the molecular level because free radicals are highly reactive and, in most cases, have an extremely short half-life. Most biologically relevant free radicals are short-lived, making them difficult to be directly detected. ROS have a short half-life, in the range of about 10−9 seconds, and thus, without derivatization, are difficult to be isolated directly for structural identification. Thus, ESR spin trapping techniques to identify the formation of ROS from chemical reactions are commonly used [18].
Biochemical and cell-based methods are commonly used to elucidate the acute and chronic toxicity elicited by nano-materials. The benefits of using cultured cell lines are many, including reproducibility, ease of treatment with test material, and easy measurement of cytotoxicity and other toxicological endpoints. It has been demonstrated that cultured cells have significantly high sensitivity to the effects of nanomaterials [10]. Nevertheless, compared with chemical reactions, it is more challenging to determine mechanisms of free radicals formed in vivo and in cells.
Alternatively, the correlation of results from ESR studies with the cell-based results will provide a profile for each nanomaterial that integrates chemical and biochemical approaches. ESR spin trapping is the only approach that provides direct evidence of the existence of free radicals and a means to identify them. This makes it possible to study the dynamic effects of free radicals in different systems. ESR is widely used in free radical research, and is increasingly used in mechanistic studies of free-radical generation in the presence of nanomaterials.
Therefore, mechanistic studies frequently use ESR techniques for detection of free-radical formation. For example, Yin et al [39] determined the photocytotoxicity of nano-TiO2 in several different cell systems and found that the resulting toxicity was mediated by ROS. The results also indicated that the singlet oxygen was formed partially via a superoxide-dependent mechanism, while the hydroxyl radical formation was not via superoxide. Citing another example, ESR measurements revealed that nano-ZnO, following photoexcitation by UVA, extracted electrons from water or hydroxide ions to generate hydroxyl radicals; it did not transfer energy to molecular oxygen to generate singlet oxygen, nor transfer an electron to molecular oxygen to generate superoxide radical anions [86]. The ESR study also revealed that hydroxyl radical formation is not via superoxide.
Nevertheless, free radical reactions proceed so rapidly that the original formation of specific free radicals is difficult to determine. Taking hydroxyl radical formation as an example, it can be generated directly: (1) from a photoexcited nano-particle extracting electrons from water or hydroxide ions; (2) through the Haber–Weiss reaction from the reaction of a redoxed-type nanoparticle initially with superoxide, and subsequently with H2O2; and (3) many other pathways. It is even more challenging to determine the free-radical mechanism of nanomaterial-induced toxicity in a biological system, particularly in vivo.
7. Perspectives
Production of engineered nanomaterials for commercial use has been increasing exponentially, therefore, the safety and toxicity of nanomaterials has become an issue of interest to the public. Due to the complication of free-radical formation and reaction, the elucidation of a detailed biochemical mechanism of nanomaterial-induced ROS formation leading to toxicity is challenging. Understanding the mechanism of nanomaterial-induced toxicity is the first defense for hazard prevention. Reaching this ultimate goal will enable us to avoid synthesizing toxic nanomaterials for commercial use.
In all the physiologically relevant ROS, the hydroxyl radical possesses the highest one-electron reduction potential and is reactive with almost all types of biomolecules including lipids, proteins, and nucleic acids [49,67]. As a result of their reactivity and ability to damage biological targets, hydroxyl radicals can serve as an ideal representative ROS for investigation. Consequently, if a nanomaterial induces ROS, it is important to determine the formation of hydroxyl radicals.
Enzymatic defenses have evolved to protect against harmful biological oxidants. SODs, peroxidases and catalases are some of the prominent and extensively studied antioxidant enzymes. Antioxidants also play an important role in preventing/limiting the damage caused by ROS. The hydroxyl radical is the most powerful ROS in causing biological damage. Although there are no antioxidant enzymes that can destroy hydroxyl radical, some endogenous and dietary antioxidants are effective in scavenging hydroxyl radicals. Dietary antioxidants may play an important role in limiting oxidative damage and reducing the risk of numerous chronic diseases related to advancing age [143,144]. Consequently, the development of dietary antioxidants to scavenge hydroxyl radicals formed from nanomaterials may serve as one of the strategies for prevention of nanomaterial-induced toxicity.
Acknowledgments
We thank Mr. Winfred G. Aker for technical review of this manuscript.
Footnotes
This article is not an official guidance or policy statement of the US Food and Drug Administration (FDA). No official support or endorsement by the US FDA is intended or should be inferred.
Conflicts of interest
All authors have no conflicts of interest to declare.
REFERENCES
- 1. Gonzalez L, Lison D, Kirsch-Volders M. Genotoxicity of engineered nanomaterials: a critical review. Nanotoxicology. 2008;2:252–73. [Google Scholar]
- 2. Brar SK, Verma M, Tyagi RD, et al. Engineered nanoparticles in wastewater and wastewater sludge–evidence and impacts. Waste Manag. 2010;30:504–20. doi: 10.1016/j.wasman.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 3. Chiang HM, Xia Q, Zou X, et al. Nanoscale ZnO induces cytotoxicity and DNA damage in human cell lines and rat primary neuronal cells. J Nanosci Nanotechnol. 2012;12:2126–35. doi: 10.1166/jnn.2012.5758. [DOI] [PubMed] [Google Scholar]
- 4. Oberdörster G, Maynard A, Donaldson K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ray PC, Yu H, Fu PP. Nanogold-based sensing of environmental toxins: excitement and challenges. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29:52–89. doi: 10.1080/10590501.2011.551315. [DOI] [PubMed] [Google Scholar]
- 8. Yin JJ, Lao F, Fu PP, et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials. 2009;30:611–21. doi: 10.1016/j.biomaterials.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin JJ, Lao F, Meng J, et al. Inhibition of tumor growth by endohedral metallofullerenol nanoparticles optimized as reactive oxygen species scavenger. Mol Pharmacol. 2008;74:1132–40. doi: 10.1124/mol.108.048348. [DOI] [PubMed] [Google Scholar]
- 10. Nel A, Xia T, Madler L, et al. Toxic potential of materials at the nanolevel. Science. 2006;311:622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 11. Xia T, Kovochich M, Liong M, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2:2121–34. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karlsson HL, Cronholm P, Gustafsson J, et al. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21:1726–32. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 13. Oberdorster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect. 2004;112:1058–62. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blinova I, Ivask A, Heinlaan M, et al. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ Pollut. 2010;158:41–7. doi: 10.1016/j.envpol.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 15. Dechsakulthorn F, Hayes A, Bakand S, et al. In vitro cytotoxicity assessment of selected nanoparticles using human skin fibroblasts. AATEX. 2007;14(Special Issue):397–400. [Google Scholar]
- 16. Fan Z, Lu JG. Zinc oxide nanostructures: synthesis and properties. J Nanosci Nanotechnol. 2005;5:1561–73. doi: 10.1166/jnn.2005.182. [DOI] [PubMed] [Google Scholar]
- 17. Kangwansupamonkon W, Lauruengtana V, Surassmo S, et al. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomedicine. 2009;5:240–9. doi: 10.1016/j.nano.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Yin JJ, Zhao B, Xia Q, et al. Electron spin resonance spectroscopy for studying the generation and scavenging of reactive oxygen species by nanomaterials. In: Liang X-J, editor. Nanopharmaceuticals: the potential application of nanomaterials. Singapore: World Scientific Publishing Company; 2012. pp. 375–400. [Google Scholar]
- 19. Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13608–19. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 20. Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–7. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 21. Goodman CM, McCusker CD, Yilmaz T, et al. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 22. Griffitt RJ, Luo J, Gao J, et al. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem. 2008;27:1972–8. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- 23. He W, Zhou YT, Wamer WG, et al. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials. 2012;33:7547–55. doi: 10.1016/j.biomaterials.2012.06.076. [DOI] [PubMed] [Google Scholar]
- 24. Ozel RE, Alkasir RS, Ray K, et al. Comparative evaluation of intestinal nitric oxide in embryonic zebrafish exposed to metal oxide nanoparticles. Small. 2013;9:4250–61. doi: 10.1002/smll.201301087. [DOI] [PubMed] [Google Scholar]
- 25. Wang S, Lu W, Tovmachenko O, et al. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett. 2008;463:145–9. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Aker WG, Hwang HM, et al. A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci Total Environ. 2011;409:4753–62. doi: 10.1016/j.scitotenv.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Z, Li J, Zhao J, et al. Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter. Environ Sci Technol. 2011;45:6032–40. doi: 10.1021/es2010573. [DOI] [PubMed] [Google Scholar]
- 28. Sohaebuddin SK, Thevenot PT, Baker D, et al. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part Fibre Toxicol. 2010;7(22):1–17. doi: 10.1186/1743-8977-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, et al. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin CY, Zhu BS, Wang XF, et al. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem Res Toxicol. 2008;21:1871–7. doi: 10.1021/tx800179f. [DOI] [PubMed] [Google Scholar]
- 31. Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005;158:122–32. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 32. Zhu X, Hondroulis E, Liu W, et al. Biosensing approaches for rapid genotoxicity and cytotoxicity assays upon nanomaterial exposure. Small. 2013;9:1821–30. doi: 10.1002/smll.201201593. [DOI] [PubMed] [Google Scholar]
- 33. Bottini M, Bruckner S, Nika K, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–6. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 34. Ding L, Stilwell J, Zhang T, et al. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005;5:2448–64. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magrez A, Kasas S, Salicio V, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–5. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 36. Persson H, Kobler C, Molhave K, et al. Fibroblasts cultured on nanowires exhibit low motility, impaired cell division, and DNA damage. Small. 2013;9:4006–16. doi: 10.1002/smll.201300644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang W, Wang C, Li Z, et al. Unraveling stress-induced toxicity properties of graphene oxide and the underlying mechanism. Adv Mater. 2012;24:5391–7. doi: 10.1002/adma.201202678. [DOI] [PubMed] [Google Scholar]
- 38. Zhu L, Chang DW, Dai L, et al. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7:3592–7. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
- 39. Yin JJ, Liu J, Ehrenshaft M, et al. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes – generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol. 2012;263:81–8. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 41. Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 42. Yin J-J, Fu PP, Lutterodt H, et al. Dual role of selected antioxidants found in dietary supplements: crossover between anti-and pro-oxidant activities in the presence of copper. J Agric Food Chem. 2012;60:2554–61. doi: 10.1021/jf204724w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halliwell B, Gutteridge JMC, editors. The chemistry of oxygen radicals and other oxygen-derived species. New York: Oxford University Press; 1989. [Google Scholar]
- 44. Meng H, Xia T, George S, et al. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano. 2009;3:1620–7. doi: 10.1021/nn9005973. [DOI] [PubMed] [Google Scholar]
- 45. Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–94. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- 46. Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–62. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 47. Poli G, Leonarduzzi G, Biasi F, et al. Oxidative stress and cell signalling. Curr Med Chem. 2004;11:1163–82. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 48. Poon HF, Calabrese V, Scapagnini G, et al. Free radicals and brain aging. Clin Geriatr Med. 2004;20:329–59. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 49. Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Crawford DR. Regulation of mammalian gene expression by reactive oxygen species. In: Gilbert DL, Colton CA, editors. Reactive oxygen species in biological systems: an interdisciplinary approach. New York: Kluwer Academic Publishers; 1999. pp. 155–71. [Google Scholar]
- 51. Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic Biol Med. 2004;37:582–93. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Bodamyali T, Stevens CR, Blake DR, et al. Reactive oxygen/nitrogen species and acute inflammation: a physiological process. In: Winyard PG, Blake DR, Evans CH, editors. Free radicals and inflammation. Basel: Springer; 2000. pp. 11–9. [Google Scholar]
- 53. Fu PP, Xia Q, Sun X, et al. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)-light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012;30:1–41. doi: 10.1080/10590501.2012.653887. [DOI] [PubMed] [Google Scholar]
- 54. Xia Q, Boudreau MD, Zhou Y-T, et al. UVB photoirradiation of Aloe vera formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation. J Food Drug Anal. 2011;19:396–402. [Google Scholar]
- 55. Xia Q, Chiang H-M, Zhou Y-T, et al. Phototoxicity of kava – formation of reactive oxygen species leading to lipid peroxidation and DNA damage. Am J Chin Med. 2012;40:1271–88. doi: 10.1142/S0192415X12500942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xia Q, Yin JJ, Cherng SH, et al. UVA photoirradiation of retinyl palmitate –formation of singlet oxygen and superoxide, and their role in induction of lipid peroxidation. Toxicol Lett. 2006;163:30–43. doi: 10.1016/j.toxlet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 57. Xia Q, Yin JJ, Fu PP, et al. Photo-irradiation of Aloe vera by UVA – formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation. Toxicol Lett. 2007;168:165–75. doi: 10.1016/j.toxlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 58. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 59. Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–86. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 60.Butterfield DA. Alzheimer’s α-amyloid peptide and free radical oxidative stress. In: Gilbert DL, Colton CA, editors. Reactive oxygen species in biological systems: an interdisciplinary approach. New York: Kluwer Academic Publishers; 1999. pp. 609–38. [Google Scholar]
- 61.Cohen G. Oxidative stress and Parkinson’s disease. In: Gilbert DL, Colton CA, editors. Reactive oxygen species in biological systems: an interdisciplinary approach. New York: Kluwer Academic Publishers; 1999. pp. 593–608. [Google Scholar]
- 62. Kawanishi S, Hiraku Y, Murata M, et al. The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic Biol Med. 2002;32:822–32. doi: 10.1016/s0891-5849(02)00779-7. [DOI] [PubMed] [Google Scholar]
- 63. Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 64. Sachidanandam K, Fagan SC, Ergul A. Oxidative stress and cardiovascular disease: antioxidants and unresolved issues. Cardiovasc Drug Rev. 2005;23:115–32. doi: 10.1111/j.1527-3466.2005.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 65. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 66. Wardman P, Candeias LP. Fenton chemistry: an introduction. Radiat Res. 1996;145:523–31. [PubMed] [Google Scholar]
- 67. Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145:532–41. [PubMed] [Google Scholar]
- 68. Lubec G. The hydroxyl radical: from chemistry to human disease. J Investig Med. 1996;44:324–6. [PubMed] [Google Scholar]
- 69. Hileman EA, Achanta G, Huang P. Superoxide dismutase: an emerging target for cancer therapeutics. Expert Opin Ther Targets. 2001;5:697–710. doi: 10.1517/14728222.5.6.697. [DOI] [PubMed] [Google Scholar]
- 70. Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 71. Toyokuni S, Okamoto K, Yodoi J, et al. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 72. Cherng SH, Xia Q, Blankenship LR, et al. Photodecomposition of retinyl palmitate in ethanol by UVA light-formation of photodecomposition products, reactive oxygen species, and lipid peroxides. Chem Res Toxicol. 2005;18:129–38. doi: 10.1021/tx049807l. [DOI] [PubMed] [Google Scholar]
- 73. Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 74. Yin JJ, Xia Q, Fu PP. UVA photoirradiation of anhydroretinol – formation of singlet oxygen and superoxide. Toxicol Ind Health. 2007;23:625–31. doi: 10.1177/0748233708090909. [DOI] [PubMed] [Google Scholar]
- 75. Yu H, Xia Q, Yan J, et al. Photoirradiation of polycyclic aromatic hydrocarbons with UVA light – a pathway leading to the generation of reactive oxygen species, lipid peroxidation, and DNA damage. Int J Environ Res Public Health. 2006;3:348–54. doi: 10.3390/ijerph2006030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhao Y, Xia Q, Yin JJ, et al. Photoirradiation of dehydropyrrolizidine alkaloids –formation of reactive oxygen species and induction of lipid peroxidation. Toxicol Lett. 2011;205:302–9. doi: 10.1016/j.toxlet.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 77. Li Y, Yu S, Wu Q, et al. Chronic Al2O3-nanoparticle exposure causes neurotoxic effects on locomotion behaviors by inducing severe ROS production and disruption of ROS defense mechanisms in nematode Caenorhabditis elegans. J Hazard Mater. 2012:219–30. doi: 10.1016/j.jhazmat.2012.03.083. [DOI] [PubMed] [Google Scholar]
- 78. Winnik FM, Maysinger D. Quantum dot cytotoxicity and ways to reduce it. Acc Chem Res. 2013;46:672–80. doi: 10.1021/ar3000585. [DOI] [PubMed] [Google Scholar]
- 79. Akhtar MJ, Ahamed M, Kumar S, et al. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276:95–102. doi: 10.1016/j.tox.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 80. Akhtar MJ, Ahamed M, Fareed M, et al. Protective effect of sulphoraphane against oxidative stress mediated toxicity induced by CuO nanoparticles in mouse embryonic fibroblasts BALB 3T3. J Toxicol Sci. 2012;37:139–48. doi: 10.2131/jts.37.139. [DOI] [PubMed] [Google Scholar]
- 81. Applerot G, Lipovsky A, Dror R, et al. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv Funct Mater. 2009;19:842–52. [Google Scholar]
- 82. Gurr JR, Wang AS, Chen CH, et al. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213:66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 83. Kang SJ, Kim BM, Lee YJ, et al. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- 84. Shukla RK, Kumar A, Pandey AK, et al. Titanium dioxide nanoparticles induce oxidative stress-mediated apoptosis in human keratinocyte cells. J Biomed Nanotechnol. 2011;7:100–1. doi: 10.1166/jbn.2011.1221. [DOI] [PubMed] [Google Scholar]
- 85. Shukla RK, Sharma V, Pandey AK, et al. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro. 2011;25:231–41. doi: 10.1016/j.tiv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 86. Wang C-C, Wang S, Xia Q, et al. Phototoxicity of zinc oxide nanoparticles in HaCaT keratinocytes – generation of oxidative DNA damage during UVA and visible light irradiation. J Nanosci Nanotechnol. 2013;13:3880–8. doi: 10.1166/jnn.2013.7177. [DOI] [PubMed] [Google Scholar]
- 87. Wang JJ, Sanderson BJ, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007;628:99–106. doi: 10.1016/j.mrgentox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 88. Girgis E, Khalil WK, Emam AN, et al. Nanotoxicity of gold and gold-cobalt nanoalloy. Chem Res Toxicol. 2012;25:1086–98. doi: 10.1021/tx300053h. [DOI] [PubMed] [Google Scholar]
- 89. Hsin YH, Chen CF, Huang S, et al. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179:130–9. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 90. Mei N, Zhang Y, Chen Y, et al. Silver nanoparticle-induced mutations and oxidative stress in mouse lymphoma cells. Environ Mol Mutagen. 2012;53:409–19. doi: 10.1002/em.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim S, Ryu DY. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J Appl Toxicol. 2013;33:78–89. doi: 10.1002/jat.2792. [DOI] [PubMed] [Google Scholar]
- 92. Shvedova AA, Castranova V, Kisin ER, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66:1909–26. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 93. Liu Y, Li X, Bao S, et al. Plastic protein microarray to investigate the molecular pathways of magnetic nanoparticle-induced nanotoxicity. Nanotechnology. 2013;24:175501. doi: 10.1088/0957-4484/24/17/175501. [DOI] [PubMed] [Google Scholar]
- 94. Shaligram S, Campbell A. Toxicity of copper salts is dependent on solubility profile and cell type tested. Toxicol In Vitro. 2013;27:844–51. doi: 10.1016/j.tiv.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 95. Lu W, Senapati D, Wang S, et al. Effect of surface coating on the toxicity of silver nanomaterials on human skin keratinocytes. Chem Phys Lett. 2010;487:92–6. doi: 10.1016/j.cplett.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. He C, Hu Y, Yin L, et al. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–66. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 97. Sakai N, Matsui Y, Nakayama A, et al. Functional-dependent and size-dependent uptake of nanoparticles in pc12. J Phys Conf Ser. 2011;304:012049. [Google Scholar]
- 98. Wang S-H, Lee C-W, Chiou A, et al. Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J Nanobiotechnol. 2010;8:33–45. doi: 10.1186/1477-3155-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Boyoglu C, He Q, Willing G, et al. Microscopic studies of various sizes of gold nanoparticles and their cellular localizations. ISRN Nanotechnol. 2013 doi: 10.1155/2013/123838. [DOI] [Google Scholar]
- 100. Yoshida T, Yoshikawa T, Nabeshi H, et al. Relation analysis between intracellular distribution of nanomaterials, ROS generation and DNA damage. Yakugaku Zasshi. 2012;132:295–300. doi: 10.1248/yakushi.132.295. [DOI] [PubMed] [Google Scholar]
- 101. Auffan M, Rose J, Wiesner MR, et al. Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ Pollut. 2009;157:1127–33. doi: 10.1016/j.envpol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 102. Li Y, Tian X, Lu Z, et al. Mechanism for alpha-MnO2 nanowire-induced cytotoxicity in HeLa cells. J Nanosci Nanotechnol. 2010;10:397–404. doi: 10.1166/jnn.2010.1719. [DOI] [PubMed] [Google Scholar]
- 103. Saquib Q, Al-Khedhairy AA, Siddiqui MA, et al. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol In Vitro. 2012;26:351–61. doi: 10.1016/j.tiv.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 104. Braydich-Stolle LK, Schaeublin NM, Murdock RC, et al. Crystal structure mediates mode of cell death in TiO2 nanotoxicity. J Nanopart Res. 2009;11:1361–74. [Google Scholar]
- 105. Oh WK, Kim S, Choi M, et al. Cellular uptake, cytotoxicity, and innate immune response of silica-titania hollow nanoparticles based on size and surface functionality. ACS Nano. 2010;4:5301–13. doi: 10.1021/nn100561e. [DOI] [PubMed] [Google Scholar]
- 106. Przybyszewska M, Zaborski M. The effect of zinc oxide nanoparticle morphology on activity in crosslinking of carboxylated nitrile elastomer. Express Polymer Lett. 2009;3:542–52. [Google Scholar]
- 107. McLaren A, Valdes-Solis T, Li G, et al. Shape and size effects of ZnO nanocrystals on photocatalytic activity. J Am Chem Soc. 2009;131:12540–1. doi: 10.1021/ja9052703. [DOI] [PubMed] [Google Scholar]
- 108. Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 109. Gratton SEA, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105:11613–8. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Qiu Y, Liu Y, Wang L, et al. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials. 2010;31:7606–19. doi: 10.1016/j.biomaterials.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 111. Ispas C, Andreescu D, Patel A, et al. Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Environ Sci Technol. 2009;43:6349–56. doi: 10.1021/es9010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hoskins C, Cuschieri A, Wang L. The cytotoxicity of polycationic iron oxide nanoparticles: common endpoint assays and alternative approaches for improved understanding of cellular response mechanism. J Nanobiotechnol. 2012;10:15. doi: 10.1186/1477-3155-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang B, Yin J-J, Zhou X, et al. Physicochemical origin for free radical generation of iron oxide nanoparticles in biomicroenvironment: catalytic activities mediated by surface chemical states. J Phys Chem C. 2012;117:383–92. [Google Scholar]
- 114. Lee S, Yun HS, Kim SH. The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials. 2011;32:9434–43. doi: 10.1016/j.biomaterials.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 115. Shi M, Kwon HS, Peng Z, et al. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano. 2012;6:2157–64. doi: 10.1021/nn300445d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Maccormack TJ, Clark RJ, Dang MK, et al. Inhibition of enzyme activity by nanomaterials: potential mechanisms and implications for nanotoxicity testing. Nanotoxicology. 2012;6:514–25. doi: 10.3109/17435390.2011.587904. [DOI] [PubMed] [Google Scholar]
- 117. Hauck TS, Anderson RE, Fischer HC, et al. In vivo quantum-dot toxicity assessment. Small. 2010;6:138–44. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- 118. Hoshino A, Hanada S, Yamamoto K. Toxicity of nanocrystal quantum dots: the relevance of surface modifications. Arch Toxicol. 2011;85:707–20. doi: 10.1007/s00204-011-0695-0. [DOI] [PubMed] [Google Scholar]
- 119. Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 120. Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009;238:280–8. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhu Z-J, Yeh Y-C, Tang R, et al. Stability of quantum dots in live cells. Nature Chem. 2011;3:963–8. doi: 10.1038/nchem.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Petersen EJ, Nelson BC. Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal Bioanal Chem. 2010;398:613–50. doi: 10.1007/s00216-010-3881-7. [DOI] [PubMed] [Google Scholar]
- 123. Limbach LK, Wick P, Manser P, et al. Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ Sci Technol. 2007;41:4158–63. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- 124. Yang X, Gondikas AP, Marinakos SM, et al. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ Sci Technol. 2011;46:1119–27. doi: 10.1021/es202417t. [DOI] [PubMed] [Google Scholar]
- 125. Studer AM, Limbach LK, Van Duc L, et al. Nanoparticle cytotoxicity depends on intracellular solubility: comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol Lett. 2010;197:169–74. doi: 10.1016/j.toxlet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 126. Shen C, James SA, Dejonge MD, et al. Relating cytotoxicity, zinc ions and reactive oxygen in ZnO nanoparticle exposed human immune cells. Toxicol Sci. 2013;136:120–30. doi: 10.1093/toxsci/kft187. [DOI] [PubMed] [Google Scholar]
- 127. Mahto SK, Yoon TH, Rhee SW. A new perspective on in vitro assessment method for evaluating quantum dot toxicity by using microfluidics technology. Biomicrofluidics. 2010;4 doi: 10.1063/1.3486610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Beer C, Foldbjerg R, Hayashi Y, et al. Toxicity of silver nanoparticles – nanoparticle or silver ion? Toxicol Lett. 2012;208:286–92. doi: 10.1016/j.toxlet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 129. Faisal M, Saquib Q, Alatar AA, et al. Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J Hazard Mater. 2013;250–251:318–32. doi: 10.1016/j.jhazmat.2013.01.063. [DOI] [PubMed] [Google Scholar]
- 130. Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85:743–50. doi: 10.1007/s00204-010-0545-5. [DOI] [PubMed] [Google Scholar]
- 131. Franklin NM, Rogers NJ, Apte SC, et al. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol. 2007;41:8484–90. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- 132. Lubick N. Nanosilver toxicity: ions, nanoparticleş or both? Environ Sci Technol. 2008;42:8617. doi: 10.1021/es8026314. [DOI] [PubMed] [Google Scholar]
- 133. Daimon T, Nosaka Y. Formation and behavior of singlet molecular oxygen in TiO2 photocatalysis studied by detection of near-infrared phosphorescence. J Phys Chem C. 2007;111:4420–4. [Google Scholar]
- 134. Ghosh M, Bandyopadhyay M, Mukherjee A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere. 2010;81:1253–62. doi: 10.1016/j.chemosphere.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 135. Hirakawa K, Hirano T. Singlet oxygen generation photocatalyzed by TiO2 particles and its contribution to biomolecule damage. Chem Lett. 2006;35:832–3. [Google Scholar]
- 136. Mura GM, Ganadu ML, Lubinu G, et al. Photodegradation of organic waste coupling hydrogenase and titanium dioxide. Ann NY Acad Sci. 1999;879:267–75. doi: 10.1111/j.1749-6632.1999.tb10430.x. [DOI] [PubMed] [Google Scholar]
- 137. Sycheva LP, Zhurkov VS, Iurchenko VV, et al. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutat Res. 2011;726:8–14. doi: 10.1016/j.mrgentox.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 138. Vevers WF, Jha AN. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology. 2008;17:410–20. doi: 10.1007/s10646-008-0226-9. [DOI] [PubMed] [Google Scholar]
- 139. Zhao K, Zhang L, Wang J, et al. Surface structure-dependent molecular oxygen activation of BiOCl single-crystalline nanosheets. J Am Chem Soc. 2013;135:15750–3. doi: 10.1021/ja4092903. [DOI] [PubMed] [Google Scholar]
- 140. Liao KH, Lin YS, Macosko CW, et al. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl Mater Interfaces. 2011;3:2607–15. doi: 10.1021/am200428v. [DOI] [PubMed] [Google Scholar]
- 141. Ekstrand-Hammarstrom B, Akfur CM, Andersson PO, et al. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology. 2012;6:623–34. doi: 10.3109/17435390.2011.598245. [DOI] [PubMed] [Google Scholar]
- 142. McCarthy J, Inkielewicz-Stepniak I, Corbalan JJ, et al. Mechanisms of toxicity of amorphous silica nanoparticles on human lung submucosal cells in vitro: protective effects of fisetin. Chem Res Toxicol. 2012;25:2227–35. doi: 10.1021/tx3002884. [DOI] [PubMed] [Google Scholar]
- 143. Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 144. Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–95. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]