Abstract
The burden of irreversible vision loss from Glaucoma continues to rise. While the disease pathogenesis is not well understood, intraocular pressure (IOP) is the only modifiable risk factor identified to prevent glaucomatous vision loss. Medical management remains the first-line of treatment in most adult glaucomas and the evolution of medical therapy for glaucoma has followed an exponential curve. This review tracks the rapid development of new medications and drug delivery systems in the recent years. Introduction of Rho kinase inhibitors with an entirely new mechanism of action from that of the currently used anti glaucoma medications has been a significant milestone. Latanoprostene Bunod is a novel, single molecule which provides two active metabolites that work through two different pathways for reducing intra ocular pressure. Bimatoprost implants and travoprost punctum plugs attempt to ease chronic medication use in glaucoma patients. Nanotechnology is an evolving route of drug delivery. Role of cannabinoids in medical management of glaucoma remain equivocal. The relatively short term effect on IOP, the risks of developing tolerance and side effects impacting patients’ neurocognitive health greatly outweigh the potential benefit. Research on Latrunculin B, Adenosine receptor agonists, Specific gene silencing and Stem cell therapy are poised to make an impact on glaucoma treatment. While there is some evidence to support the role of Brimonidine in neuroprotection, further research is needed to clarify the role of Memantine and Neurotrophins. Evidence for benefit from dietary supplementation with Alpha lipoic acid, Forskolin , and Ginko Biloba is limited
Keywords: Adenosine receptor agonists, bimatroprost ring, cannabinoids, drug delivery systems, glaucoma, latanoprostene bunod, latrunculins, liposomes, medical management, nano particles, prostaglandin analogue, punctal plug, rho kinase inhibitors, surgical implants, sustained release
The global burden of irreversible vision loss from glaucoma continues to rise as the population ages. It was estimated to affect 64.3 million people aged 40–80 years in 2013, 76 million in 2020 with estimates increasing to 111.8 million in 2040.[1] While the disease pathogenesis is not well understood, intraocular pressure (IOP) is the only modifiable risk factor identified to prevent glaucomatous vision loss. Medical management remains the first-line of treatment in most adult glaucomas which has been helped by the rapid development of unique agents in the last 50 years.
This paper provides an extensive review of the newer glaucoma medications available on the market today, as well as newer methods of drug delivery and drugs that may potentially be available in the future.
Rho Kinase Inhibitors
In 1993, researchers discovered the role of cytoskeletally active agents, such as Rho kinase, in regulating trabecular outflow.[2] Rho A, Rho B and Rho C are a family of G proteins which are active when bound to guanosisne triphosphate (GTP) and inactive when bound to guanosine diphosphate (GDP). The two Rho kinase isoforms (ROCK 1 and ROCK 2) are the effectors of the Rho family.[3]
Rho kinase inhibitors increase aqueous outflow and decrease outflow resistance by increasing the ability of the Schlemm’s canal endothelial cells to form pores. Another hypothesis is that Rho kinase inhibitors cause relaxation of the smooth muscle fibers in the trabecular meshwork and thereby increase outflow.[4] Experimental evidence also supports changes in Schlemm’s canal cytoskeleton causing decrease in focal adhesions in the juxtacanalicular meshwork.[5]
Ripasudil and netarsudil are the two commercially available formulations of Rho kinase inhibitors, both of which work on ROCK1 and ROCK 2 receptors. Ripasudil hydrochloride hydrate (Glanatec®) 0.4% reduced IOP by 2.6 mm of Hg at trough and 3.7 mm of Hg at peak in patients with primary open angle glaucoma (POAG) and ocular hypertension (OHT).[6] The most commonly reported adverse events included conjunctival hyperemia (76%), blepharitis (21%), and allergic conjunctivitis (20%). Additive IOP lowering effect of ripasudil 0.4% with timolol maleate 0.5% was found to be 1.6 mm of Hg at peak and 0.9 mm of Hg at trough.[7] The additive effect with latanoprost 0.005% was a reduction of 1.4 mm of Hg at peak but no significant reduction in IOP at trough,[7]
Netarsudil (Rhopressa®) is a Rho kinase inhibitor and nor-epinephrine transporter inhibitor which decreases IOP by decreasing the outflow resistance. In addition, netarsudil caused reduction in aqueous humor production in animal studies and decrease in episcleral venous pressure in animal and human studies.[8,9] This has been linked to the nor-epinephrine transporter inhibitor activity of netarsudil.
A double-masked randomized controlled trial (RCT) compared the efficacy of netarsudil 0.01%, netarsudil 0.02%, and latanoprost 0.005%, all dosed once daily, in reducing IOP in patients with OHT and POAG with IOP ≥24 and <36 mmHg. Reduction in IOP on day 28 was 5.5, 5.7, and 6.8 mmHg in the netarsudil 0.01%, netarsudil 0.02%, and latanoprost 0.005% groups, respectively.[10] Both concentrations of netarsudil did not meet the noninferiority criteria compared to latanoprost. When the subgroup of patients with IOP ≤26 mmHg was analyzed separately, netarsudil 0.02% was statistically noninferior to latanoprost 0.005%. Netarsudil was, therefore, thought to be more effective in patients with lower baseline IOP. The most commonly reported adverse event was conjunctival hyperemia, which was more common with netarsudil compared to latanoprost.
Following this, two RCTs evaluated the noninferiority of netarsudil 0.02% with timolol maleate 0.5% in patients with a lower baseline IOP of <27 mm of Hg after washout.[11] Both the studies found netarsudil to be statistically noninferior to timolol 0.05% in the subgroup of patients with IOP <25 mm of Hg. The same was not true for the entire cohort. Conjunctival hyperemia was again the most common adverse event reported at 50–53% with netarsudil dosed daily, 59% with netarsudil dosed twice daily, and 8–10% with timolol dosed twice daily. Perilimbal conjunctival micro hemorrhages were reported in 13–17% patients, and cornea verticillata were reported in 9–15% of the patients on netarsudil versus less than 1% patients in the timolol only group. All the adverse effects reversed after cessation of medications. Two recent case series reported a reticular pattern of corneal edema in patients on netarsudil, causing decrease in visual acuity, unlike the earlier reported adverse events.[12,13] Most of these, though not all, have been in eyes with decompensated corneas and resolved on cessation of the drug. The mechanism of this is not yet fully understood.
Mercury -1 and 2 trials reported on the efficacy of a fixed drug combination of netarsudil 0.02% and latanoprost 0.005% (FCNL) dosed once daily, compared to monotherapy with netarsudil 0.02% or latanoprost 0.005%.[14] The mean baseline IOP was 23.6, 23.6, and 23.5 mmHg in the FCNL, netarsudil, and latanoprost groups, respectively. Both studies showed that the FCNL provided higher mean IOP reduction compared with monotherapy. The most common adverse events were conjunctival hyperemia, cornea verticillata, and subconjunctival hemorrhage. The rate of conjunctival hyperaemia was noted to be 58.7, 47.0, and 22.1% in the FCNL, netarsudil, and latanoprost groups, respectively. In the follow up period, 5% of patients in both the FCNL and Netarsudil groups, and 0.2% participants in the latanoprost group discontinued treatment due to conjunctival hyperaemia. The incidence of cornea verticillata was 15.4 and 11.6% in patients who received FCNL and netarsudil, respectively. There were no reports of cornea verticillata in patients receiving latanoprost. Less than 1% participants discontinued treatment because of cornea verticillata. Subconjunctival hemorrhage was found in 10.8, 14.5, and 1.0% in the FCNL, netarsudil, and latanoprost groups, respectively. A Phase 3 clinical trial comparing the efficacy of FCNL to the fixed drug combination of bimatoprost 0.03% and timolol 0.5% is ongoing.[15]
Finally, fasudil is a newer Rho kinase inhibitor which has been studied in a few eyes with end-stage glaucoma with promising results.[16] Table 1 summarizes the major clinical trials on Rho kinase inhibitors.
Table 1.
Major drug trials on Rho kinase inhibitors in glaucoma
| Author | Study type | Subjects | Sample size | Follow-up | Outcome | Conclusion | ||
|---|---|---|---|---|---|---|---|---|
| SNJ 1656 Inoue et al.[17] |
RCT* | POAG, OHT 22≤IOP ≤31 |
66 | 7 days | Placebo SNJ 0.03% SNJ 0.05% SNJ 0.1% |
Change at trough** - 2.2 (1.9) - 3.8 (2.7) -4.3 (2.3) - 4.0 (2.5) |
Change at peak*** - 1.5 (2.2) - 5.0 (2.4) - 4.4 (2.7) - 4.5 (1.9) |
|
| AR-12286 Williams et al.[18] |
RCT | POAG, OHT 24≤IOP ≤36 |
89 | 7 days | Vehicle AR-12286 0.05% 0.1% 0.25% |
Mean diurnal IOP change QD AM -1.9-4.0-5.0-4.8 |
Mean diurnal IOP change BID - 2.4-4.1-4.4-6.0 |
AR-12286 was well tolerated and provided statistically significant ocular hypotensive efficacy |
| Ripasudil Tanihara et al.[19] | RCT | POAG, OHT 21<IOP <35 |
210 | 8 weeks | Placebo Ripasudi 0.1% Ripasudil 0.25 Ripasudil 0.4% |
Change at trough - 2.2-3.4-3.2-3.5 |
Change at peak - 2.5-3.7-4.2-4.5 |
K-115 0.4% BD selected to be the optimal dose |
| Ripasudil Phase 3 Tanihara et al.[6] |
Non randomized Open label | POAG, OHT, XFG 15<IOP ≤35 |
388 | 1 year | Ripasudil 0.4% BD Ripasudil 0.4% BD + PGA Ripasudil 0.4%BD + BB Ripasudil 0.4% BD + FC PGA and BB |
Change at trough - 2.6-1.4-2.2-1.7 |
Change at peak - 3.7-2.4-2.0-1.7 |
IOP-lowering effect and an acceptable safety profile as monotherapy or additive therapy |
| Ripasudil with timolol Phase 3 Tanihara et al.[7] |
RCT | POAG, OHT IOP ≥18 on timolol |
208 | 8 weeks | Placebo Ripasudil 0.4% BD |
Change at trough - 1.5-2.4 |
Change at peak - 1.3-2.9 |
Additive IOP-lowering effect at trough and peak levels when combined with timolol |
| Ripasudil with latanoprost Tanihara et al.[7] |
RCT | POAG, OHT IOP ≥18 on latanoprost |
205 | 8 weeks | Placebo Ripasudil 0.4% BD |
Change at trough - 1.8-2.2 |
Change at peak - 1.8-3.2 |
Additive IOP lowering at peak when combined with latanoprost |
| Netarsudil Bacharach et al.[10] |
RCT, Double masked | POAG, OHT 24 ≤IOP ≤36 |
224 | 28 days | Netarsudil 0.01% QD Netarsudil 0.02% QD Latanoprost 0.005% QD |
Change at trough - 5.4-5.9-7.6 |
Change at peak - 5.5-5.7-6.8 |
AR-13324 0.02% was less effective than latanoprost by approximately 1 mmHg |
| ROCKET 1 Netarsudil Phase 3 Serle et al.[11] |
RCT, Double masked | POAG, OHT 20 <IOP <27 at 8 AM and 17 <IOP <27 at 10 AM and 4 PM |
411 | 3 months | Netarsudil 0.02% QD Timolol 0.05% BD |
Mean diurnal IOP 22.5 22.3 |
Diurnal range of change from baseline -3.3 to -5.0-3.7 to -5.1 |
Netarsudil 0.02% was found to be effective and well tolerated |
| ROCKET 2 Netarsudil Phase 3 Serle et al.[11] |
RCT, double masked | POAG, OHT 20 <IOP <27 at 8 AM and 17<IOP <27 at 10 AM and 4 PM |
756 | 12 months | Netarsudil 0.02% QD Netarsudil 0.02% BD Timolol 0.5% BD |
Mean diurnal IOP 21.4 21.5 21.5 |
Diurnal range of change from baseline (maximum baseline IOP <25 mmHg) - 3.3 to -4.6-4.1 to -5.4-3.7 to -5.1 |
Netarsudil statistically noninferior to timolol 0.05% in the subgroup of patients with IOP <25 mm of Hg |
| Fixed combination netarsudil + latanoprost Phase 2 Lewis et al.[20] |
RCT, double masked | POAG, OHT 24 ≤IOP <36 at 8 AM and IOP ≥21 at 10 AM and 4 PM |
298 | 28 days | Latanoprost 0.005% + Netarsudil 0.01% QD Latanoprost 0.005% + Netarsudil 0.02% QD Latanoprost QD Netarsudil 0.02% QD |
Mean diurnal IOP baseline 25.1 (2.3) 25.1 (2.4) 26.0 (2.8) 25.4 (2.7) |
Mean diurnal IOP 17.3 (2.8) 16.5 (2.6) 18.4 (2.6) 19.1 (3.2) |
FDC AR-13324 0.02% and latanoprost 0.005% provides statistically superior ocular hypotensive effect when compared to individual components |
| Pooled data Mercury 1-2 fxed drug combination of netarsudil and latanoprost Asrani. S et al.[14] |
RCT | POAG, OHT | 1468 | 3 months | Netarsudi + Latanoprost Netarsudil Latanoprost |
Mean diurnal IOP at baseline 23.6 23.6 23.5 |
Mean diurnal IOP 3 months 15.8 18.4 17.4 |
Once-daily netarsudil/latanoprost FDC**** produced statistically significant reduction in mean IOP |
*RCT – randomized control trial, **Change from baseline at trough, ***Change from baseline at peak, and **** Fixed drug combination
The value of Rho kinase inhibitors as an adjunctive therapy is significant because the mechanism of action is different from that of the currently used medications. The side-effect profile with a relatively high incidence of conjunctival hyperemia and subconjunctival hemorrhages, however, may prove to be a deterrent to long-term compliance with these medications. The safety and efficacy of Rhokinase inhibitors in individuals below 18 years of age, pregnant, and lactating women is not known. Animal studies with systemic administration of the drugs have not demonstrated harmful effects on the fetus.
While most studies have reported on the role of Rho kinase inhibitors in treating patients with OHT or POAG, the role of Rho kinase inhibitors in the treatment of different types of glaucoma needs further investigation. A prospective observational study of ripasudil found statistically significant drop in IOP in patients with POAG, uveitic glaucoma, and steroid induced glaucoma but not neovasular glaucoma.[21] Other potential areas of investigation include the role of Rho kinase in neuroprotection via increased blood flow to the optic nerve and its proposed role in preventing postsurgical scarring by inhibiting TGF-β-mediated activation of fibroblasts.[22,23]
Latanoprostene Bunod
Latanoprostene bunod 0.024% (LBN) [Vyzulta™] is a unique nitric oxide (NO) donating Prostaglandin F2 alpha analogue. LBN metabolizes into the prostaglandin analogue, latanoprost acid, and butanediol mononitrate; butanediol mononitrate further metabolizes into 1,4 butane diol and NO. Latanoprost acid and NOare the two active metabolites.[24] [Fig. 1]
Figure 1.

Latanoprost acid (1) and nitric oxide (2) – the two active metabolites of LBN
Latanoprost acid binds to the pProstaglandin F receptor and increases the uveoscleral outflow by matrix metalloproteinases-mediated remodelling of the extracellular matrix of the ciliary muscle.
In the eye, NO synthetases are present in the Schlemm’s canal, trabecular meshwork, and ciliary body. NO causes vasodilation and smooth muscle cell relaxation. It decreases cell contractility and volume, thereby increasing trabecular outflow.
LBN is thus a single molecule that provides two active metabolites that work through two different pathways for reducing intra ocular pressure.
The VOYAGER study compared different concentrations of LBN and latanoprost 0.005% and found that LBN (0.024%) caused a significantly greater reduction in mean diurnal IOP on day 28 with comparable adverse effects.[25] The CONSTELLATION study compared LBN (0.024%) to timolol 0.5% and concluded that LBN caused a statistically significant decrease in both diurnal and nocturnal IOP versus timolol, which caused a significant reduction from baseline in only the diurnal IOP.[26] Subsequently, the APOLLO and LUNAR studies found that LBN 0.024% was noninferior to timolol 0.5%.[27,28,29] Finally, the JUPITER study evaluated long-term safety of LBN with a follow-up period of 52 weeks.[30] Most frequently reported adverse events were conjunctival hyperemia (17%), eye lash growth (16%), eye irritation (11%), eye pain (10%), and increased iris pigmentation (10%). Major trials on LBN are summarized in Table 2.
Table 2.
Major drug trials on Latanoprostene bunod in glaucoma
| Author | Study type | Subjects | Sample size | Follow-up | Outcome | Conclusion | |
|---|---|---|---|---|---|---|---|
| VOYAGER study Weinreb et al.[25] | Randomized, investigator masked, parallel group, dose ranging | POAG, OHT | 413 | 28 days | LBN 0.006% LBN 0.012% LBN 0.024% LBN 0.040% Latanoprost 0.005% |
Reduction in mean diurnal IOP - 7.81-8.26-9.00 8.93-7.77 |
LBN 0.024% once daily was the lower of the two effective concentrations |
| CONSTELLATION Liu et al.[26] | Prospective, open-label randomized crossover trial | Early POAG, OHT | 25 | 28 days | LBN 0.024% Timolol 0.05% |
Decrease in nocturnal IOP from baseline 2.5±3.1 2.3±3.0 |
LBN caused more nocturnal IOP reduction and increase of ocular perfusion pressure than timolol |
| APOLLO study Weinreb et al.[27] | RCT, Double masked | POAG, OHT | 420 | 3 months | LBN 0.024% showed greater IOP lowering than timolol 0.5% BID through the day | ||
| LUNAR study Medeiros et al.[28] | RCT, Double masked | POAG, OHT | 387 | 3 months | LBN 0.024% was noninferior to timolol 0.5% over 3 months, with significantly greater IOP lowering at all but the earliest time point evaluated | ||
| Pooled analysis of APOLLO and LUNAR Weinreb et al.[29] | RCT | POAG, OHT | 840 | 12 months | LBN 0.024% provided greater IOP-lowering compared with timolol 0.5% and maintained lowered IOP through 12 months | ||
| JUPITER study Kawase et al.[30] | Single arm, open label | POAG, OHT | 130 | LBN 0.024% was safe and well tolerated in Japanese subjects with OAG or OHT when used for up to 1 year | |||
More evidence is awaited on the role of LBN as adjunctive therapy. A recent retrospective study on patients on netarsudil and LBN as adjuvant therapy concluded that both showed similar efficacy as when used in monotherapy.[31]
LBN is currently dosed once daily at bedtime. The safety profile of the drug in pregnancy and lactation has not yet been established. Animal studies with systemic administration during embryogenesis have shown adverse effects.[32]
Newer Drug Delivery Systems
Medication noncompliance is a significant challenge for glaucoma patients who commonly complain of difficulty while instilling drops and difficulty in adhering to complex eye drop administration schedules. In an attempt to ease chronic medication use, new sustained drug delivery systems have been developed in the past two decades.
Ocusert was the first sustained pilocarpine implant introduced in 1975, but the product was soon taken off the market because of poor medication tolerability.
The bimatoprost implant (Durysta™) is a sustained release, biodegradable implant that uses the NOVADUR drug delivery system for intracameral use. The implant is administered into anterior chamber using a 28 gauge, single-use, prefilled applicator. The drug delivery system is made of biodegradable polymers that disintegrate by hydrolysis into carbon dioxide and water. Artemis 1 trial showed that both concentrations of durysta (10 and 15 mcg) were noninferior to timolol 0.5%.[33] In this trial, subjects with POAG and OHT received the implant 3 times at 16 week intervals, and after the third administration, 82.1% in the 10 mcg group and 87.8% in the 15 mcg group did not require additional IOP lowering medications for 1 year.
There were no adverse events related to eye lash growth, skin hyperpigmentation, or periorbital fat atrophy. The main concern was the drop in corneal endothelial cell density (CECD). Greater than 20% decrease in CECD was noted at 20 months in 10.2 and 20.8% of the 10 and 15 mcg group, respectively. In phase 3 trials, 3.6% of eyes in the 10 mcg group and 10.3% eyes in the 15 mcg group needed implant removal to correct corneal edema and further loss of corneal endothelial cells. Interestingly, phase 1 and 2 trials of the same drug concentrations in which the drug administration was further spaced apart and provided at unfixed intervals showed lesser corneal endothelial cell loss and did not require implant removal.[34] Ongoing trials are now evaluating new regimens of administration that will prevent significant corneal endothelial cell loss.[35]
Another sustained release application is the bimatoprost ocular ring (BIM ring) which is a silicone and polypropylene ring impregnated with bimatoprost, available in diameters ranging from 24 to 29 mm, designed for insertion between the upper and lower fornices. It continuously elutes bimatoprost for a period of 6 months, after which it needs to be replaced. The rate of drug elution decreases with time, ranging from 35 µg per day on the day of insertion to 6 µg per day at 6 months. IOP control over 6 months was found to be comparable to 0.03% bimatoprost topical drops with the main adverse effect being mucinous discharge from the eye in some patients.[36] Phase 3 trials are awaited and the device is not currently FDA approved for clinical use. Similar to the concept of the ring, contact lenses are an attractive option for drug delivery due to patient familiarity and long hours of use. The use of micelle-laden contact lenses for delivery of glaucoma medications are currently undergoing animal studies and while initial results are promising, the inherent risks of long-term contact lens use need to be considered.[37]
Travoprost punctum plugs (OTX-TP, Ocular Therapeutix, Inc.) is an investigational device undergoing phase 2 clinical trials. Travoprost impregnated in polyethelene glycol resorbable hydrogel rod is inserted into the upper or lower punctum. Within the hydrogel rod, travoprost particles are encapsulated in polyactic acid microparticles, which hydrolyze with time to provide a sustained delivery of travoprost over 90 days.[38] The rod is also impregnated with fluorescein to aid visualization. When OTX-TP was compared to twice daily administration of timolol 0.5%, both the groups showed significant IOP lowering, 4.5–5.7 mmHg for the OTX-TP group and 6.4–7.6 mmHg for the timolol group. The timolol group, interestingly, showed more IOP reduction than expected, which was attributed to the longer contact time of the drug with the ocular surface due to the presence of the placebo punctal plug. A major concern stated in the study was the retention of the plugs. The retention rates were 91, 88, and 48% at days 60, 75, and 90, respectively. The major adverse events reported were foreign body sensation (38.5%), itchiness (15.4%), and epiphora (3.8%). The tolerability of the implant improved with time.
Nanotechnology is another novel route of drug delivery that is fast evolving. Nanoparticles range from 1 to 100 nm in size and medications piggybacked on to various nanoparticles have the ability to bypass biological barriers rendering the drug directly at the target site.[39] Subconjunctival injection of dorzolamide-loaded polymer microparticles, supraciliary injection of brimonidine-laden microspheres, and intravitreal injection of brimonidine, travoprost, and bimatoprost-laden nanosponges have completed successful animal studies.[40]
Investigational Glaucoma Medications
Cannabinoids
Cannabinoids are derived from the cannabis plant (phytocannabinoids) or are artificially produced (synthetic cannabinoids). They interact with cannabinoid receptors 1 and 2 in the human body (CB1 and CB2), which are the natural receptors for endocannabinoids which modulate pain, memory, and appetite. CB1 and CB2 are expressed in the human retina, ciliary body, iris, Schlemm’s canal, trabecular meshwork, and the retinal pigment epithelium.[41,42]
The neuroprotective effect of cannabinoids is linked to the inhibition of glutamate release. Hommer et al.[43] reported a significant increase in the optic nerve head blood flow with 5 mg oral Dronabinol in 24 subjects, when compared to a placebo. Many animal studies also support better retinal ganglion cell survival with the use of cannabinoids.
Oral cannabinoid Delta 9 tetrahydrocannabinol (THC) was reported to demonstrate IOP reduction 30 min after administration. However, on long-term use, over 9 months, drug doses had to be increased due to development of tachyphylaxis. Most patients discontinued the study due to side effects including dizziness, confusion, sleepiness, anxiety, and depression.[44,45]
Palmitoyl ethanolamide (PEA) is a congener of the endogenous cannabinoid, anandamide (AEA) that is cosynthesized with AEA in many human cells. It prolongs the action of AEA by competing with fatty acid amide hydrolase involved in the hydrolysis of AEA. The use of PEA in glaucoma was first reported by Gagliano et al.[46] in a cross-over study with a reduction in IOP of 6.2% after 2 months of treatment. Oral PEA was also effective in reducing the IOP spike post yttrium aluminum garnet laser iridotomy.[47]
Inhalational cannabinoids reportedly caused a 2.1 mm of Hg drop in IOP from baseline 80 min after administration of cigarettes containing 12 mg Delta 9 THC, but the IOP lowering effect was found to be linked with tolerance.[48] Inhalational administration of Delta 9 THC led to higher IOP reduction compared to oral administration. However, the IOP reduction was noted to be short term with a significant decrease in IOP (4.1 ± 1.5 mmHg) at 30 min that peaked at 90 min (6.6 ± 1.5 mmHg). The most common side effect was a significant decrease in systolic and diastolic blood pressures resulting in postural hypotension.[49]
Topical cannabinoids have failed to demonstrate a significant effect on IOP in clinical trials. The challenge with topical administration is the lipophilic nature of cannabinoids. Mineral oil, needed as vehicle for topical formulations, leads to poor penetration of the drug, lid inflammation, and conjunctival hyperemia.[50] Topical formulations of THC with cyclodextrins, which are cyclic oligosaccharides with a central cavity that is hydrophobic to hold the drug molecule and an outer surface that is hydrophilic so as to allow water solubility, are undergoing animal studies.[51]
Albumin solubilized, intravenous Delta 9 THC caused a dose-dependent peak IOP lowering of 60%, but the effect was short lived.[52,53] Hypotension and presyncopal episodes were the most commonly reported side effects.
Despite extensive research, the role of cannabinoids in medical management of glaucoma remains equivocal. The relatively short-term effect on IOP, the risks of developing tachyphylaxis, and serious side effects impacting patients’ general and neurocognitive health greatly outweigh the potential benefit at this time. Future research may provide stronger evidence for their use in neuroprotection with tolerable side effects.
Adenosine receptor agonists
Adenosine is a nucleoside that activates the G protein linked to adenosine receptors, A1, A2A, A2B, and A23. It increases the conventional outflow facility by shrinkage of cell volume and remodeling of the extracellular matrix in human trabecular meshwork cells. A1, A2A, and A3 agonists are currently undergoing Phase 1 and 2 trials. Phase 2 trials of trabodenoson, a selective A1 agonist, showed clinically and statistically significant IOP reduction with no serious adverse events.[54,55]
Prostanoid receptor agonist
Omidenepag isopropyl (OMDI) is a nonprostaglandin, selective, prostanoid EP2 receptor agonist, known to decrease IOP by increasing the conventional and uveoscleral outflow. Phase 1 trials of OMDI showed clinically significant IOP reductions and the drug was well tolerated.[56] Recently published Phase 3 trials from Japan established noninferiority of OMDI 0.002% when compared with latanoprost 0.005% in reducing IOP in POAG and OHT over 4 weeks.[57] The common adverse events reported were conjunctival hyperaemia (24.5%), increased corneal thickness (11.7%), and photophobia (4.3%). A mean increase of 15 µm (2.7%) in central corneal thickness was found in patients on OMDI, the mechanism of which is not well understood. There were no reports of corneal edema or drop in visual acuity, further research on the effect of the drug on corneal health including corneal endothelial count may be warranted. This drug is currently approved for use in Japan.
Small interference RNA
RNA interference is the cutting-edge technology of specific gene silencing, using small bits of RNA called small interference RNA (siRNA).[58] SYL040012 is a siRNA developed to specifically silence the Beta 2 adrenergic receptor (ADRB2) at the ciliary body, thereby reducing the aqueous humor production. In vitro and in vivo studies in animal models of SYL040012 have shown significant IOP reduction and good safety profile.
Neuroprotection
Neuroprotection is the holy grail of glaucoma care. Glaucoma is known to be a neurodegenerative disease which causes chronic progressive RGC death, and glaucoma treatment remains restricted to reduction in IOP at this time. Lowering IOP removes a stressor for neuropathy and arguably is a form of neuroprotection. The search for non-IOP-dependent neuroprotection is ongoing. Though a consensus on the actual cause of glaucomatous optic neuropathy is awaited, the cellular processes that cause RGC death include exposure to neurotoxic substances like NO and glutamate, deprivation of internal trophic factors, loss of cellular self-repair process, and intracellular destructive process.[59] [Fig. 2].
Figure 2.
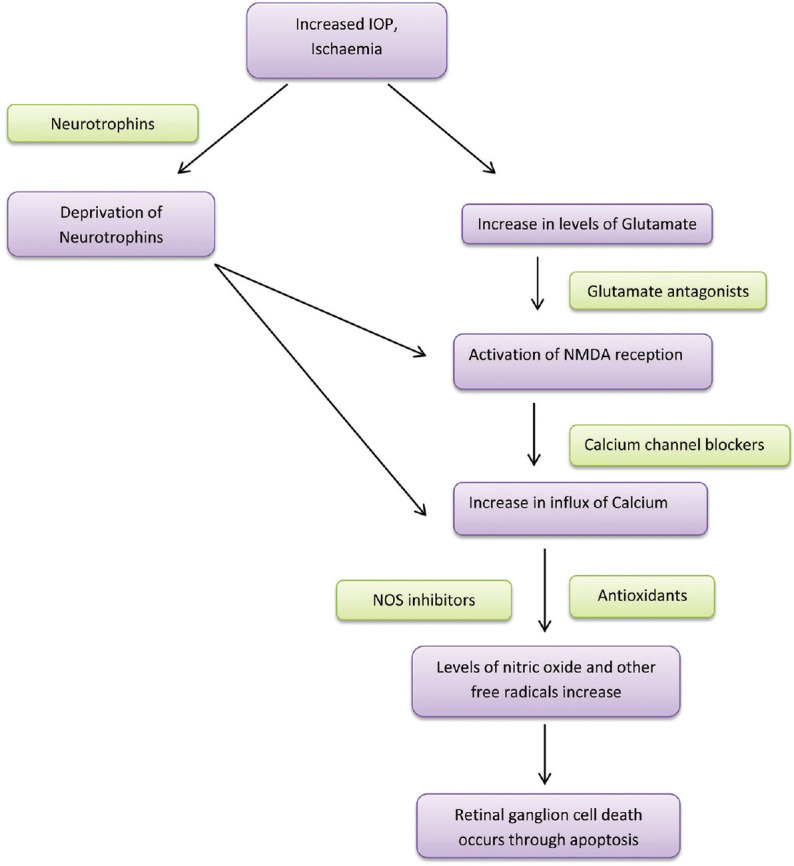
Neuroprotection in glaucoma
The rationale of treatment is that the intervention corrects the imbalance between the cellular death and survival signals, thus, preserving visual function.
Memantine
Elevated levels of glutamate are toxic to retinal ganglion cells and the resulting cell death is mediated by excitotoxicity of the N-methyl-D-aspartate (NMDA) receptor, by causing an excess of intracellular calcium and cell death.[60] Memantine is an NMDA receptor antagonist and can prevent cell death by calcium influx. Four-year follow-up results from two double-masked, placebo-controlled, multicenter RCTs with 2298 patients with POAG showed that memantine at the 10 and 20 mg daily doses did not prevent or decrease progression of glaucoma based on standard automated perimetry and optic disc photography findings.[61]
Brimonidine
The IOP-independent neuroprotective effect of brimonidine, an alpha-2 adrenergic agonist, has been demonstrated in animal models. The proposed mechanism involves upregulation of antiapoptotic factors, modulation of glutamate-induced excitotoxicity, inhibition of NO synthetase, and inhibition of glial activity.[62,63] Studies have demonstrated a significant reduction in retinal nerve fiber layer loss in OHT patients treated with brimonidine compared to those treated with timolol.[63,64]
Neurotrophins
Neurotrophic factors play a key role in cell survival. Brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor, glial cell-line-derived neurotrophic factor and nerve growth factor (NGF) are potential candidates in neuroprotection undergoing preclinical studies. Valproic acid, traditionally used to treat epilepsy, has been demonstrated to induce neuroprotection by stimulating the BDNF–TrkB pathway. Animal studies demonstrated protective effect of topical application of NGF drops on RGCs in a rat model of glaucoma. Topical NGF drops have also been shown to demonstrate improvement in visual fields, contrast sensitivity, and electrofunctional tests in a few patients with advanced glaucoma.[65] Obstacles in the safe administration of these molecules at the intended site of action, poor understanding of pharmacokinetics, and lack of clarity on the long-term effects of these agents remain challenges in the translation to human trials.[66]
Gene therapy
Gene therapy for glaucoma is still in the early stages of research. The large number of chromosome loci responsible for POAG, challenges in gene transfer with final binding at the intended site, and the possibility of mutagenesis have all dampened progress of this mode of treatment.[67]
Aquaporin 1 is a protein in the ciliary body involved in aqueous production by facilitating the transmembrane transport of water. Disruption of Aquaporin 1 by gene therapy with CRISPR-Cas9 RNA has been reported to reduce IOP in animal models.[68] The treatment which targets a gene involved in a physiologic process rather than a specific gene mutation has the potential to be universally applicable.
A unique adeno-associated virus (AAV) gene therapy for glaucoma that targets the BDNF has been validated in mouse models. Intravitreal injection of AAV2 vectors increased the production of BDNF and increased duration of action of BDNF by upregulating tropomyosin-related receptor kinase B.[69]
Stem cell therapy
Traditional glaucoma treatment modalities aim to delay or arrest the progression of glaucoma. Stem cell therapy provides the captivating possibility of regenerating and repopulating RGCs and possibly restoring vision lost from glaucoma. Preclinical studies have validated that mesenchymal stem cells secrete neurotrophins which promote cell survival and can repopulate RGCs in the retina.[70]
Stem cell therapy may also play a role in cell-based functional restoration of the trabecular meshwork. Current evidence shows that there is a population of adult stem cells in the Schwalbe’s ring and the anterior trabecular meshwork.[71] These adult stem cells play a crucial role in tissue repair and may also be expanded in vitro for tissue regeneration. Restoration of aqueous humor flow in mouse models following transplantation of iPSC-derived trabecular meshwork cells has been reported.[72] Further clinical validation of the role of stem cells in glaucoma management is awaited.
Alternative medicine
Dietary supplementation with Alpha lipoic acid has been shown to decrease oxidative stress and improve RGC survival in animal models of glaucoma.[73] The association of Vitamin C with POAG failed to reach statistical significance.[74,75] Though the current evidence is limited by the smaller sample sizes, unpredictability of visual field tests and short follow up, forskolin containing supplements have shown to decrease IOP beyond the reduction achieved by antiglaucoma medications alone.[76] Flavanoids like Gingko biloba have been demonstrated to have a positive impact on ocular blood flow though the impact on the preservation of visual fields remains unclear. Ginko biloba extracts have also demonstrated neuroprotective and antiinflammatory effects on retinal ganglion cells in animal studies.[77]
Nutritional supplementation has a good safety profile, larger, better designed RCTs with longer follow-up are required to evaluate its role in glaucoma.
There has been considerable interest in the recent past on the role of YOGA and lifestyle changes in glaucoma. Current literature provides little evidence to support the use of YOGA, relaxation techniques, or special diets for slowing/arresting progression of glaucoma.[78]
Cytidine 5’diphosphocholine or citicoline is an endogenous compound involved in the synthesis of membrane phospholipids. It is known to increase the levels of dopamine, serotonin, and noradrenaline in the central nervous system.[79] Pecori Giraldi et al.[80] first studied the effect of intramuscular (IM) injections of 1 g of citicoline for 10 consecutive days in glaucoma patients and reported an improvement in visual fields by computerized perimetry in 75% of the 34 examined eyes. Another prospective study with 23 participants, who were followed over 10 years, reported better visual field preservation in the subgroup that received 1 g citicoline IM for 15 days repeated every 6 months, in addition to the topical hypotensive medications.[81] Many studies using oral citicoline 500 mg BD over different dosing schedules ranging from 2 weeks to 60 days have demonstrated an improvement in visual function as measured by visually evoked potential and pattern electro retinogram[82,83] Ottobelli et al. studied the effect of the oral solution of citicoline on 41 patients with POAG who were concurrently on topical hypotensive medications and had a documented progression rate of more than -1 dB per year despite maintaining IOP less than 18 mm of Hg. Over 2 years, participants taking the oral solution of citicoline were noted to have a significant reduction in the mean rate of visual field progression.[84] A recent randomized control trial evaluated the effect of citicoline eye drops on the rate of further progression in patients on topical hypotensive medications with documented progression on visual field testing and IOP of less than 18 mm of Hg.[85] RNFL thickness measurements suggested that the citicoline eye drops may slow disease progression in these patients. The study reported 1.86 µm of RNFL loss in 3 years in citicoline group, versus 2.99 µm of RNFL loss in the placebo group (P = 0.02). The study, however, had a small sample size and many patients underwent a change in the treatment regimen or surgery during the follow-up period. A larger prospective trial is needed to elucidate the role of citicoline in glaucoma.
Conclusion
To conclude, the past few decades have opened up multiple new horizons in glaucoma treatment. [Fig. 3]. With the pace and scale of ongoing research, we have reason to look forward to newer medications, delivery systems, and novel therapeutic modalities being available for patient care.
Figure 3.

New glaucoma medications at various stages of development
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040:A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Kopczynski CC, Epstein DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014;30:85–7. doi: 10.1089/jop.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanna AP, Johnson M. Rho Kinase inhibitors as a novel treatment for glaucoma and ocular hypertension. Ophthalmology. 2018;125:1741–56. doi: 10.1016/j.ophtha.2018.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thieme H, Nuskovski M, Nass JU, Pleyer U, Strauss O, Wiederholt M. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Invest Ophthalmol Vis Sci. 2000;41:4240–6. [PubMed] [Google Scholar]
- 5.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–37. [PubMed] [Google Scholar]
- 6.Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Fukushima A, et al. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016;94:e26–34. doi: 10.1111/aos.12829. [DOI] [PubMed] [Google Scholar]
- 7.Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Suganami H, et al. Additive intraocular pressure-lowering effects of the rho kinase inhibitor ripasudil (K-115) combined with timolol or latanoprost:A report of 2 randomized clinical trials. JAMA Ophthalmol. 2015;133:755–61. doi: 10.1001/jamaophthalmol.2015.0525. [DOI] [PubMed] [Google Scholar]
- 8.Wang R-F, Williamson JE, Kopczynski C, Serle JB. Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. J Glaucoma. 2015;24:51–4. doi: 10.1097/IJG.0b013e3182952213. [DOI] [PubMed] [Google Scholar]
- 9.Kiel JW, Kopczynski CC. Effect of AR-13324 on episcleral venous pressure in Dutch belted rabbits. J Ocul Pharmacol Ther. 2015;31:146–51. doi: 10.1089/jop.2014.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacharach J, Dubiner HB, Levy B, Kopczynski CC, Novack GD AR-13324-CS202 Study Group. Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology. 2015;122:302–7. doi: 10.1016/j.ophtha.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Serle JB, Katz LJ, McLaurin E, Heah T, Ramirez-Davis N, Usner DW, et al. Two Phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure:Rho Kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2) Am J Ophthalmol. 2018;186:116–27. doi: 10.1016/j.ajo.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Moumneh K, Sheybani A, Fellman RL, Godfrey DG, Grover DS. Reticular corneal edema or corneal honeycombing in eyes treated with netarsudil:A case series. J Glaucoma. 2020;29:607–10. doi: 10.1097/IJG.0000000000001516. [DOI] [PubMed] [Google Scholar]
- 13.LoBue SA, Moustafa GA, Vu A, Amin M, Nguyen T, Goyal H. Transient reticular cystic corneal epithelial edema with topical netarsudil:A case series and review. Cornea. 2021;40:1048–54. doi: 10.1097/ICO.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 14.Asrani S, Bacharach J, Holland E, McKee H, Sheng H, Lewis RA, et al. Fixed-dose combination of netarsudil and latanoprost in ocular hypertension and open-angle glaucoma:Pooled efficacy/safety analysis of phase 3 MERCURY-1 and -2. Adv Ther. 2020;37:1620–31. doi: 10.1007/s12325-020-01277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aerie Pharmaceuticals Inc. Safety and Efficacy Study of PG324 (Netarsudil/Latanoprost 0.02%/0.005%) ophthalmic solution compared to ganfort®ophthalmic solution in open angle glaucoma or ocular hypertension. Sep 15, 2017. ClinicalTrials.gov:NIH U.S. National Library of Medicine. 2019. Available from:https://clinicaltrials.gov/ct2/show/NCT03284853 .
- 16.Pakravan M, Beni AN, Ghahari E, Varshochian R, Yazdani S, Esfandiari H, et al. The ocular hypotensive efficacy of topical fasudil, a rho-associated protein kinase inhibitor, in patients with end-stage glaucoma. Am J Ther. 2017;24:e676–80. doi: 10.1097/MJT.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Tanihara H, Tokushige H, Araie M. Efficacy and safety of SNJ-1656 in primary open-angle glaucoma or ocular hypertension. Acta Ophthalmol. 2015;93:e393–5. doi: 10.1111/aos.12641. [DOI] [PubMed] [Google Scholar]
- 18.Williams RD, Novack GD, van Haarlem T, Kopczynski C AR-12286 Phase 2A Study Group. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am J Ophthalmol. 2011;152:834–41.e1. doi: 10.1016/j.ajo.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M, et al. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156:731–6. doi: 10.1016/j.ajo.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Lewis RA, Levy B, Ramirez N, Kopczynski CC, Usner DW, Novack GD, et al. Fixed-dose combination of AR-13324 and latanoprost:A double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol. 2016;100:339–44. doi: 10.1136/bjophthalmol-2015-306778. [DOI] [PubMed] [Google Scholar]
- 21.Tanihara H, Kakuda T, Sano T, Kanno T, Gunji R. Safety and efficacy of ripasudil in Japanese patients with glaucoma or ocular hypertension:12-month interim analysis of ROCK-J, a post-marketing surveillance study. BMC Ophthalmol. 2020;20:275. doi: 10.1186/s12886-020-01490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta Y, Takaseki S, Yoshitomi T. Effects of ripasudil hydrochloride hydrate (K-115), a Rho-kinase inhibitor, on ocular blood flow and ciliary artery smooth muscle contraction in rabbits. Jpn J Ophthalmol. 2017;61:423–32. doi: 10.1007/s10384-017-0524-y. [DOI] [PubMed] [Google Scholar]
- 23.Honjo M, Tanihara H, Kameda T, Kawaji T, Yoshimura N, Araie M. Potential role of Rho-associated protein kinase inhibitor Y-27632 in glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2007;48:5549–57. doi: 10.1167/iovs.07-0878. [DOI] [PubMed] [Google Scholar]
- 24.Hoy SM. Latanoprostene bunod ophthalmic solution 0.024%:A review in open-angle glaucoma and ocular hypertension. Drugs. 2018;78:773–80. doi: 10.1007/s40265-018-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinreb RN, Ong T, Scassellati Sforzolini B, Vittitow JL, Singh K, Kaufman PL, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma:The VOYAGER study. Br J Ophthalmol. 2015;99:738–45. doi: 10.1136/bjophthalmol-2014-305908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JHK, Slight JR, Vittitow JL, Scassellati Sforzolini B, Weinreb RN. Efficacy of latanoprostene bunod 0.024% compared with timolol 0.5% in lowering intraocular pressure over 24 hours. Am J Ophthalmol. 2016;169:249–57. doi: 10.1016/j.ajo.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Weinreb RN, Scassellati Sforzolini B, Vittitow J, Liebmann J. Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension:The APOLLO study. Ophthalmology. 2016;123:965–73. doi: 10.1016/j.ophtha.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Martin KR, Peace J, Scassellati Sforzolini B, Vittitow JL, Weinreb RN. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension:The LUNAR study. Am J Ophthalmol. 2016;168:250–9. doi: 10.1016/j.ajo.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Weinreb RN, Liebmann JM, Martin KR, Kaufman PL, Vittitow JL. Latanoprostene bunod 0.024% in subjects with open-angle glaucoma or ocular hypertension:Pooled phase 3 study findings. J Glaucoma. 2018;27:7–15. doi: 10.1097/IJG.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawase K, Vittitow JL, Weinreb RN, Araie M JUPITER study group. Long-term safety and efficacy of latanoprostene bunod 0.024% in japanese subjects with open-angle glaucoma or ocular hypertension:The JUPITER study. Adv Ther. 2016;33:1612–27. doi: 10.1007/s12325-016-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta AA, Kanu LN, Sood-Mendiratta S, Quinones R, Hawkins A, Lehrer RA, et al. Experience with netarsudil 0.02% and latanoprostene bunod 0.024% as adjunctive therapy for glaucoma. Eur J Ophthalmol? 2021 doi: 10.1177/1120672121998913. 1120672121998913. [DOI] [PubMed] [Google Scholar]
- 32.Bausch & Inc. VYZULTA (latanoprostene bunod ophthalmic solution) 0.024%, for topical ophthalmic use:US prescribing information, 2017. Available from:https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-vyzulta .
- 33.Medeiros FA, Walters TR, Kolko M, Coote M, Bejanian M, Goodkin ML, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1) Ophthalmology. 2020;127:1627–41. doi: 10.1016/j.ophtha.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Craven ER, Walters T, Christie WC, Day DG, Lewis RA, Goodkin ML, et al. 24-Month phase i/ii clinical trial of bimatoprost sustained-release implant (Bimatoprost SR) in glaucoma patients. Drugs. 2020;80:167–79. doi: 10.1007/s40265-019-01248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.18 month prospective efficacy and safety study of bimatoprost intracameral implant (DURYSTA) ClinicalTrials.gov.Identifier. NCT04647214. [Google Scholar]
- 36.Brandt JD, DuBiner HB, Benza R, Sall KN, Walker GA, Semba CP, et al. Long-term safety and efficacy of a sustained-release bimatoprost ocular ring. Ophthalmology. 2017;124:1565–6. doi: 10.1016/j.ophtha.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Xu J, Ge Y, Bu R, Zhang A, Feng S, Wang J, et al. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J Control Release. 2019;305:18–28. doi: 10.1016/j.jconrel.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Perera SA, Ting DS, Nongpiur ME, Chew PT, Aquino MCD, Sng CC, et al. Feasibility study of sustained-release travoprost punctum plug for intraocular pressure reduction in an Asian population. Clin Ophthalmol. 2016;10:757–64. doi: 10.2147/OPTH.S102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Occhiutto ML, Maranhão RC, Costa VP, Konstas AG. Nanotechnology for medical and surgical glaucoma therapy-A Review. Adv Ther. 2020;37:155–99. doi: 10.1007/s12325-019-01163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aref AA. Sustained drug delivery for glaucoma:Current data and future trends. Curr Opin Ophthalmol. 2017;28:169–74. doi: 10.1097/ICU.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 41.Passani A, Posarelli C, Sframeli AT, Perciballi L, Pellegrini M, Guidi G, et al. Cannabinoids in glaucoma patients:The never-ending story. J Clin Med. 2020;9:3978. doi: 10.3390/jcm9123978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plange N, Arend KO, Kaup M, Doehmen B, Adams H, Hendricks S, et al. Dronabinol and retinal hemodynamics in humans. Am J Ophthalmol. 2007;143:173–4. doi: 10.1016/j.ajo.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 43.Hommer N, Kallab M, Szegedi S, Puchner S, Stjepanek K, Bauer M, et al. The effect of orally administered dronabinol on optic nerve head blood flow in healthy subjects-A randomized clinical trial. Clin Pharmacol Ther. 2020;108:155–61. doi: 10.1002/cpt.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flach AJ. Delta-9-tetrahydrocannabinol (THC) in the treatment of end-stage open-angle glaucoma. Trans Am Ophthalmol Soc. 2002;100:215–22. discussion 222-4. [PMC free article] [PubMed] [Google Scholar]
- 45.Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure:A pilot study. J Glaucoma. 2006;15:349–53. doi: 10.1097/01.ijg.0000212260.04488.60. [DOI] [PubMed] [Google Scholar]
- 46.Gagliano C, Ortisi E, Pulvirenti L, Reibaldi M, Scollo D, Amato R, et al. Ocular hypotensive effect of oral palmitoyl-ethanolamide:A clinical trial. Invest Ophthalmol Vis Sci. 2011;52:6096–100. doi: 10.1167/iovs.10-7057. [DOI] [PubMed] [Google Scholar]
- 47.Pescosolido N, Librando A, Puzzono M, Nebbioso M. Palmitoylethanolamide effects on intraocular pressure after Nd:YAG laser iridotomy:An experimental clinical study. J Ocul Pharmacol Ther. 2011;27:629–35. doi: 10.1089/jop.2010.0191. [DOI] [PubMed] [Google Scholar]
- 48.Flom MC, Adams AJ, Jones RT. Marijuana smoking and reduced pressure in human eyes:Drug action or epiphenomenon? Invest Ophthalmol. 1975;14:52–5. [PubMed] [Google Scholar]
- 49.Merritt JC, Crawford WJ, Alexander PC, Anduze AL, Gelbart SS. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology. 1980;87:222–8. doi: 10.1016/s0161-6420(80)35258-5. [DOI] [PubMed] [Google Scholar]
- 50.Jay WM, Green K. Multiple-drop study of topically applied 1% delta 9-tetrahydrocannabinol in human eyes. Arch Ophthalmol. 1983;101:591–3. doi: 10.1001/archopht.1983.01040010591012. [DOI] [PubMed] [Google Scholar]
- 51.Hingorani T, Gul W, Elsohly M, Repka MA, Majumdar S. Effect of ion pairing on in vitro transcorneal permeability of a Δ(9) -tetrahydrocannabinol prodrug:Potential in glaucoma therapy. J Pharm Sci. 2012;101:616–26. doi: 10.1002/jps.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purnell WD, Gregg JM. Delta (9)-tetrahydrocannabinol, euphoria and intraocular pressure in man. Ann Ophthalmol. 1975;7:921–3. [PubMed] [Google Scholar]
- 53.Cooler P, Gregg JM. Effect of delta-9-tetrahydrocannabinol on intraocular pressure in humans. South Med J. 1977;70:951–4. doi: 10.1097/00007611-197708000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin Ophthalmol. 2011;5:667–77. doi: 10.2147/OPTH.S15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myers JS, Sall KN, DuBiner H, Slomowitz N, McVicar W, Rich CC, et al. A dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of 2 and 4 weeks of twice-daily ocular trabodenoson in adults with ocular hypertension or primary open-angle glaucoma. J Ocul Pharmacol Ther. 2016;32:555–62. doi: 10.1089/jop.2015.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aihara M, Lu F, Kawata H, Tanaka Y, Yamamura K, Odani-Kawabata N, et al. Pharmacokinetics, safety, and intraocular pressure-lowering profile of omidenepag isopropyl, a selective, nonprostaglandin, prostanoid ep2 receptor agonist, in healthy japanese and caucasian volunteers (Phase I Study) J Ocul Pharmacol Ther. 2019;35:542–50. doi: 10.1089/jop.2019.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension:The Phase 3 AYAME Study. Am J Ophthalmol. 2020;220:53–63. doi: 10.1016/j.ajo.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Martínez T, González MV, Roehl I, Wright N, Pañeda C, Jiménez AI. In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol Ther. 2014;22:81–91. doi: 10.1038/mt.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz M, Yoles E. Neuroprotection:A new treatment modality for glaucoma? Curr Opin Ophthalmol. 2000;11:107–11. doi: 10.1097/00055735-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–93. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 61.Weinreb RN, Liebmann JM, Cioffi GA, Goldberg I, Brandt JD, Johnson CA, et al. Oral memantine for the treatment of glaucoma:Design and results of 2 randomized, placebo-controlled, phase 3 studies. Ophthalmology. 2018;125:1874–85. doi: 10.1016/j.ophtha.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 62.Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol. 2013;13:12–5. doi: 10.1016/j.coph.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function:results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151:671–81. doi: 10.1016/j.ajo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 64.Sena DF, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst Rev. 2017;1:CD006539. doi: 10.1002/14651858.CD006539.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rocco ML, Soligo M, Manni L, Aloe L. Nerve growth factor:Early studies and recent clinical trials. Curr Neuropharmacol. 2018;16:1455–65. doi: 10.2174/1570159X16666180412092859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimura A, Namekata K, Guo X, Harada C, Harada T. Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. Int J Mol Sci. 2016;17:1584. doi: 10.3390/ijms17091584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson AM, Di Polo A. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2012;19:127–36. doi: 10.1038/gt.2011.142. [DOI] [PubMed] [Google Scholar]
- 68.Wu J, Bell OH, Copland DA, Young A, Pooley JR, Maswood R, et al. Gene therapy for glaucoma by ciliary body aquaporin 1 disruption using CRISPR-Cas9. Mol Ther. 2020;28:820–9. doi: 10.1016/j.ymthe.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osborne A, Khatib TZ, Songra L, Barber AC, Hall K, Kong GYX, et al. Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling. Cell Death Dis. 2018;9:1007. doi: 10.1038/s41419-018-1041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrell CR, Fellabaum C, Arsenijevic A, Markovic BS, Djonov V, Volarevic V. Therapeutic potential of mesenchymal stem cells and their secretome in the treatment of glaucoma. Stem Cells Int. 2019;2019:7869130. doi: 10.1155/2019/7869130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun H, Zhu Q, Guo P, Zhang Y, Tighe S, Zhu Y. Trabecular meshwork cells are a valuable resource for cellular therapy of glaucoma. J Cell Mol Med. 2019;23:1678–86. doi: 10.1111/jcmm.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu W, Jain A, Gramlich OW, Tucker BA, Sheffield VC, Kuehn MH. Restoration of aqueous humor outflow following transplantation of iPSC-derived trabecular meshwork cells in a transgenic mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2017;58:2054–62. doi: 10.1167/iovs.16-20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inman DM, Lambert WS, Calkins DJ, Horner PJ. α-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS One. 2013;8:e65389. doi: 10.1371/journal.pone.0065389. doi:10.1371/journal.pone. 0065389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramdas WD, Schouten JSAG, Webers CAB. The effect of vitamins on glaucoma:A systematic review and meta-analysis. Nutrients. 2018;10:359. doi: 10.3390/nu10030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loskutova E, O'Brien C, Loskutov I, Loughman J. Nutritional supplementation in the treatment of glaucoma:A systematic review. Surv Ophthalmol. 2019;64:195–216. doi: 10.1016/j.survophthal.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Mutolo MG, Albanese G, Rusciano D, Pescosolido N. Oral administration of forskolin, homotaurine, carnosine, and folic acid in patients with primary open angle glaucoma:Changes in intraocular pressure, pattern electroretinogram amplitude, and foveal sensitivity. J Ocul Pharmacol Ther. 2016;32:178–83. doi: 10.1089/jop.2015.0121. [DOI] [PubMed] [Google Scholar]
- 77.Labkovich M, Jacobs EB, Bhargava S, Pasquale LR, Ritch R. Ginkgo Biloba Extract in ophthalmic and systemic disease, with a focus on normal-tension glaucoma. Asia Pac J Ophthalmol (Phila) 2020;9:215–25. doi: 10.1097/APO.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhartiya S, Ichhpujani P. Complementary and alternate management of glaucoma:The verdict so far. J Curr Glaucoma Pract. 2014;8:54–7. doi: 10.5005/jp-journals-10008-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberti G, Tanga L, Michelessi M, Quaranta L, Parisi V, Manni G, et al. Cytidine 5'-diphosphocholine (Citicoline) in glaucoma:Rationale of its use, current evidence and future perspectives. Int J Mol Sci. 2015;16:28401–17. doi: 10.3390/ijms161226099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pecori Giraldi J, Virno M, Covelli G, Grechi G, De Gregorio F. Therapeutic value of citicoline in the treatment of glaucoma (computerized and automated perimetric investigation) Int Ophthalmol. 1989;13:109–12. doi: 10.1007/BF02028649. [DOI] [PubMed] [Google Scholar]
- 81.Virno M, Pecori-Giraldi J, Liguori A, De Gregorio F. The protective effect of citicoline on the progression of the perimetric defects in glaucomatous patients (perimetric study with a 10-year follow-up) Acta Ophthalmol Scand Suppl 2000? 232:56–7. doi: 10.1111/j.1600-0420.2000.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 82.Parisi V. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5'-diphosphocholine (citicoline):A study of 8 years of follow-up. Doc Ophthalmol. 2005;110:91–102. doi: 10.1007/s10633-005-7348-7. [DOI] [PubMed] [Google Scholar]
- 83.Rejdak R, Toczołowski J, Kurkowski J, Kamiński ML, Rejdak K, Stelmasiak Z, et al. Oral citicoline treatment improves visual pathway function in glaucoma. Med Sci Monit. 2003;9:PI24–8. [PubMed] [Google Scholar]
- 84.Ottobelli L, Manni GL, Centofanti M, Iester M, Allevena F, Rossetti L. Citicoline oral solution in glaucoma:Is there a role in slowing disease progression? Ophthalmologica. 2013;229:219–26. doi: 10.1159/000350496. [DOI] [PubMed] [Google Scholar]
- 85.Rossetti L, Iester M, Tranchina L, Ottobelli L, Coco G, Calcatelli E, et al. Can treatment with citicoline eyedrops reduce progression in glaucoma?The results of a randomized placebo-controlled clinical trial. J Glaucoma. 2020;29:513–20. doi: 10.1097/IJG.0000000000001565. [DOI] [PMC free article] [PubMed] [Google Scholar]


