Abstract
Sympathetic ophthalmia is a rare, bilateral, granulomatous, panuveitis following penetrating trauma or surgery to one eye. Clinical presentation commonly occurs within the first year of trauma occurrence but can be delayed by several years. It manifests as acute/chronic granulomatous uveitis with yellowish-white choroidal lesions or Dalen–Fuchs nodules. Initially, patients respond rapidly to corticosteroid therapy, but a majority require long-term use of corticosteroid-sparing agents to prevent recurrences. The purpose of this review is to elaborate on the current understanding of the pathophysiology, the importance of multimodal imaging in early diagnosis, and the role of newer immunomodulatory and biological agents in recalcitrant cases.
Keywords: Dalen–Fuchs nodule, granulomatous uveitis, immunosuppression, ocular trauma, panuveitis, sympathetic ophthalmia
Sympathetic ophthalmia (SO) is described as a bilateral, diffuse, granulomatous, panuveitis following an accidental or surgical penetrating injury to an eye (inciting eye).[1,2] Penetrating or surgical injury to the inciting eye can lead to an inflammatory response, not only in the traumatized eye but also in the fellow eye (sympathizing eye). Sympathetic ophthalmia has been associated with open globe injury, cataract surgery, vitreoretinal surgery, glaucoma surgery, cyclodestructive procedures, resection of iridociliary melanoma, proton beam irradiation of choroidal melanoma, and even squint surgery.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16] It can develop as early as 5 days or as late as 66 years following an injury.[8,7,8,9,10,11,12,13,14,14]
Methods
A PubMed search was performed for all articles in English on sympathetic ophthalmia, panuveitis, ocular trauma, Dalen–Fuchs nodule, granulomatous uveitis, and immunosuppression.
Historical Perspective
Description of disease-simulating sympathetic ophthalmia has been reported during the Hippocratic era.[1] In 1840, William Mackenzie noted an association between men at iron works sustaining penetrating eye injuries and development of iritis, photophobia with progression to atrophic bulbi and poor prognosis.[2]
Dalen (1904) described nodules on the inner surface of the choroid, whereas Ernst Fuch (1905) provided an accurate histopathological description of the disease, including non-necrotic granulomas, lymphocytic infiltration, giant cells formation, and pigmented phagocytes.[3,4] In his original description, Mackenzie postulated that the most probable mechanism for the spread of inflammation from one eye to the other was through the optic nerves and chiasma.[2] Elschnig (1910) was the first to postulate the role of autoimmunity in the pathogenesis of SO.[5] Lately, Rao et al.,[6] in their experimental studies, highlighted the role of retinal S antigen and penetrating injuries in providing this auto-antigen access to the periocular lymphatics to trigger the cascade of immunogenic events culminating in SO in the other eye.
Citing poor prognosis of the disease, enucleation of the injured or inciting eye at the earliest following development of symptoms suggestive of SO was recommended.[7] Soon, it was realized that enucleation of the inciting eye was of no use once symptoms appeared in the sympathizing eye.[8]
Epidemiology
The exact incidence and prevalence of SO are difficult to determine due to the rarity of its occurrence. Marak et al.[9] reported the incidence of SO to be 0.1% after intraocular surgery and 0.2%–0.5% following penetrating injury. However, based on a prospective surveillance study by Kilmartin et al.,[10] extrapolated incidence was estimated to be 0.03 in 100,000 and possibly accounted for 1%–2% of the all uveitis. Sympathetic ophthalmia has a bimodal peak in age distribution. The first peak occurs in childhood and young adults, a possible reflection of higher incidence of ocular injuries and the second in the sixth decade largely due to a greater number of intraocular surgeries in elderly patients.[11] Although the disease has been classically associated with penetrating injury, recent years witnessed a rising trend toward post-surgical SO, possibly due to an early and adequate management of open globe injuries.[10] However, reports from large case series from India have shown that penetrating injuries are still the most common cause of SO, accounting for over 75% of all cases.[12,13,14] This might be a reflection of delayed primary care and wound repair in cases of penetrating injuries. Post-traumatic SO has been more often reported in male young children due to their propensity to sustain eye injuries. With an increasing incidence of post-surgical SO, this gender disparity has also reduced.[17] However, male preponderance has still been reported in some case series on post-surgical SO, possibly due to a gender bias in seeking health care particularly in low income countries like India.[12,14,18]
Ocular surgeries reported to be associated with the development of SO are glaucoma filtration surgery, cataract extraction, scleral buckling, pars plana vitrectomy, cyclo-destructive surgeries, and even squint surgeries. Recently, vitrectomy has been reported to be the most common cause of post-surgical SO, with an incidence ranging from 0.01% to 1% in 1152 retinal procedures.[19,20] Various studies have associated the recent advancement to transconjunctival sutureless vitrectomy with the increase in the incidence of SO. This has been attributed to an increased incidence of wound leak and subsequent ocular hypotony as a potential factor predisposing to subclinical uveal incarceration which results in exposure of ocular antigens.[12,14,18,19,20] However, there has not been variation in the incidence of SO between trans-conjunctival sutureless and sutured vitrectomy methods.[14] Multiple vitreoretinal surgeries and repeated intraoperative handling of uveal tissue increases the risk of sympathetic ophthalmia.[18]
Histopathology
Sympathetic ophthalmia truly represents a panuveitis with histological examinations showing involvement of all the uveal tissue characterized by diffuse thickening of the choroid, the ciliary body, and the iris.[6,21] Interestingly, changes in both the inciting eye and the sympathizing eye are similar. The inflammatory reaction is mostly composed of lymphocytes, epitheloid cells, and giant cells in variable proportions.[6] Classically, SO does not affect the choriocapillaris, although Rathinam et al. showed the presence of lymphocyte infiltration of choriocapillaris in focal areas and areas of necrosis in their series of SO with associated endophthalmitis.[22]
Immunopathological studies suggest an early infiltration of CD4 + helper/inducer T cells followed by an infiltration of CD8 + suppressor/cytotoxic T cells.[23] These findings indicate that there is a dynamic cellular immune response, mediated predominantly by T cells, during the inflammatory process of sympathetic ophthalmia. In addition, a predominance of B cells in uveal infiltrate has been reported, contrary to previously reported T cell predominance but conceivably suggestive of B cell predominance in the final stage of the disease, implying a possible B cell activation of autoreactive T cells.[24] Certain studies have also suggested a genetic predisposition to the development of sympathetic ophthalmia, particularly, human leukocyte antigen (HLA). These include HLA-A11, Cw, DRB1*04, DQB1*04, DR4, and closely related DQw3 and DRw53.[25,26]
Another classical sign found in SO is the formation of Dalen–Fuchs’ nodules seen in approximately 30% of patients.[27] These nodules are histologically composed of lymphocytes, histiocytes, and altered pigment epithelial cells that lie just internal to the Bruch’s membrane. Morphological variation of Dalen–Fuchs’ nodules in SO has been reported. Three types of lesions at the level of the RPE have been reported.[25] The first type includes focal hyperplasia and aggregation of retinal pigment epithelial cells. The second type was classically referred to as Dalen–Fuchs nodules consisting of epithelioid cells and lymphocytes covered by an intact dome of RPE. The third type of lesion was characterized by the degeneration of the overlying RPE leading to disorganization of the Dalen–Fuchs nodules and possible release of their contents into the subretinal space.
Pathogenesis
The exact pathogenesis of SO remains unknown. An important observation is that SO develops after penetrating injury with uveal tissue prolapse or intraocular surgery. None of the animal experimental models have proven the exact etiopathogenesis of SO, but ocular antigen interphotoreceptor retinoid-binding antigen (IRBP) and S antigen have been shown to produce a disease like SO in monkeys.[28]
Rao et al. injected bovine retinal S antigen to the subconjunctival space in a few rabbits, while in others, the same antigen was injected in the anterior chamber.[28,29] The group that received the injection in the anterior chamber showed local tissue reaction in the form of polymorphonuclear leukocytes, but the other eye did not show any SO changes. In contrast, the group that received the injection in the subconjunctival space showed histological features of SO in the other eye as well. The eye is an immune privileged organ with no intraocular lymphatics. As the conjunctiva has extensive lymphatic drainage, the antigens get access to the nearest lymph nodes when inoculated in the subconjunctival space. A similar mechanism occurs at the time of penetrating injury with uveal tissue prolapse, which exposes the uveal antigens to the lymphatics.
Clinical Features
There is a period of latency between penetrating or surgical trauma and the development of sympathetic. The period of latency can range from 2 weeks to many years following trauma or surgery, with 80% of cases manifesting within 3 months and 90% developing within 1 year.[11,30,31,32,33] Patients with SO present with asymmetric bilateral panuveitis, initially exhibiting more severe inflammation in the inciting eye. Clinical features may vary in their severity and onset. Symptoms may range from mild visual disturbance to significant vision loss. The earliest presenting symptom is usually difficulty in near vision due to a change in accommodation. Other manifestations include pain, redness, photophobia, and floaters.[11,13,31,34]
Patients typically present with ciliary injection, mutton-fat keratic precipitates, flare, and inflammatory cells in the anterior chamber.[11,31,35] Thickening of the iris and even iris nodules may be seen. Posterior synechiae are quite common with poorly dilating pupils. Intraocular pressure may be high or low depending on trabeculitis/trabecular meshwork blockage or ciliary body shutdown.[36,37,38] Posterior segment findings include vitritis, vitreous membranes, papillitis, retinal vasculitis, exudative retinal detachment, and choroiditis. Earliest signs include mild anterior segment inflammation, a few retrolental cells, and hyperemia of the disc. The extent of inflammation may be represented from exudative retinal detachment that might vary from small multiple pockets of exudative retinal detachments in the peripapillary area and over the posterior pole to large exudative retinal detachment [Fig. 1]. Small yellow-white lesions at the level of the choroid Dalen–Fuchs nodules are usually seen at the equator and beyond.[11,32,37,38] They tend to undergo atrophy with time.
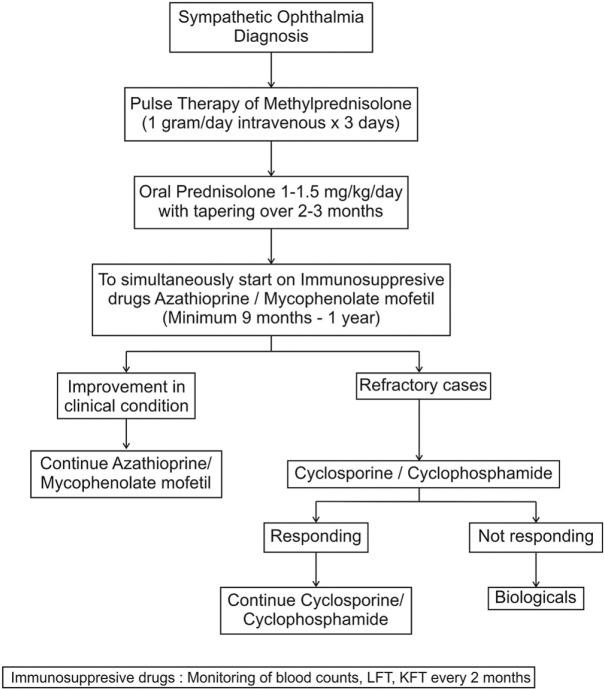
Figure 1.
Color fundus photograph of a case of sympathetic ophthalmia with multiple neurosensory detachments (black arrows) (a), which resolved after systemic steroid and immunomodulators (b)
Sunset glow appearance of the fundus, a classical feature seen in late stages, is due to depigmentation of the choroid as well as changes in RPE[32,39] [Fig. 2]. Recurrent anterior segment inflammation with sunset glow fundus is important feature of SO. The sequelae of inflammation noted in SO are quite variable, depending on the severity of ocular inflammation and whether therapy has been initiated. Secondary glaucoma as well as cataract are commonly seen either due to the disease itself or as a side effect of steroid therapy. In addition, retinal and optic atrophy may occur in association with retinal detachment, subretinal fibrosis, and underlying choroidal atrophy.[40] Choroidal neovascularization and phthisis bulbi are rare and usually related to delayed diagnosis and inappropriate treatment.
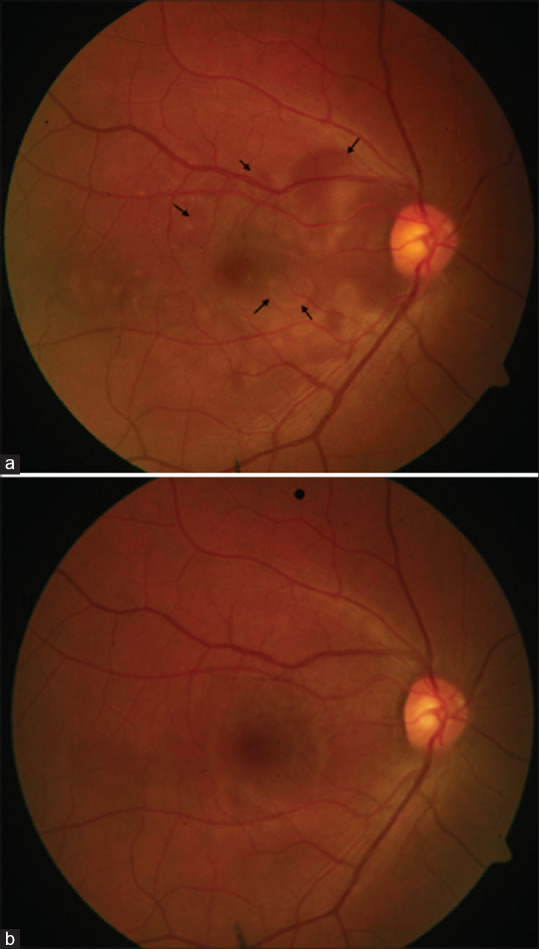
Figure 2.
A 32-year-old male presented with corneoscleral tear and uveal tissue prolapse (a). He underwent primary wound repair. Four months after injury he developed sympathetic ophthalmia in the left eye. He was managed on systemic steroids and azathioprine. At 1 year follow-up, the left eye had sunset glow fundus with nummular scars in the periphery (white arrow) (b)
Differential Diagnosis
The diagnosis of sympathetic ophthalmia (SO) is based on clinical findings and a peculiar history. The appearance of inflammation in the sympathizing eye following trauma to the fellow eye is a classic clinical setting for SO. However, posttraumatic infections and lens-induced inflammations need to be ruled out before making a diagnosis of SO. Furthermore, diagnosis is more difficult in post-surgical cases, particularly when both eyes have undergone surgeries.
The presentation of Vogt–Koyanagi–Harada (VKH) syndrome, with granulomatous panuveitis and multiple pockets of subretinal fluid, is strikingly similar to that of SO.[39] It is generally believed that integumentary signs such as poliosis, vitiligo, alopecia, and associated features of hearing disturbances, meningismus, and CSF pleocytosis are more frequently noted in cases of VKH as compared to SO. However, recent studies point out that CSF pleocytosis and meningeal symptoms are also noted in cases of SO. More importantly, these features should not solely be used to distinguish SO from VKH.[41] Although quite similar in clinical presentations, the distinct history of trauma or ocular surgery to the fellow eye clinches the diagnosis of sympathetic Ophthalmia apart from VKH [Table 1].
Table 1.
Differentiating feature between sympathetic ophthalmia and Vogt–Koyanagi–Harada syndrome
| Sympathetic Ophthalmia | Vogt–Koyanagi–Harada Syndrome | |
|---|---|---|
| Age | All ages | 20-50 years of age |
| Penetrating/surgical trauma | Almost always present | Absent |
| Skin changes | Uncommon or unrelated | Common (60-90%) |
| CNS findings | Uncommon | Common (85%) |
| Hearing dysfunction | Uncommon | Common (75%) |
| Exudative Retinal Detachment | Common | Frequently seen |
| CSF findings | Usually normal | Pleocytosis (84%) |
Early stages of SO may present only with posterior segment inflammation. Early detection at this stage is important as it carries a relatively good visual prognosis.[13] Other causes of granulomatous uveitis such as sarcoidosis, tuberculosis, and infectious uveitis also need to be ruled out.[36,38] Chronic central serous chorioretinopathy (CSCR), in a similar clinical setting, can present a challenge to distinguish inflammatory from non-inflammatory cases.[42]
Rarely, at extremes of age, the presence of multi-focal infiltrates in the subretinal and sub-RPE spaces increases the possibility of detecting intraocular lymphoma. Neuro-imaging and a subretinal biopsy from the lesions are required for a diagnosis of lymphoma.[43] Atypical presentations such as progressive subretinal fibrosis with multifocal granulomatous chorioretinitis have also been reported in SO.[44]
Investigations
Critical criteria for a diagnosis of sympathetic ophthalmia (SO) include bilateral uveitis with a history of unilateral ocular trauma or surgery and an anterior chamber and vitreous inflammation or panuveitis with choroidal involvement.[13] Although a clinical diagnosis, various imaging modalities aid in diagnosing sympathetic ophthalmia and help monitor the response to treatment and disease progression. Subsequent ancillary investigations can aid a clinician in managing a case of sympathetic ophthalmia.
B-scan ultrasonography
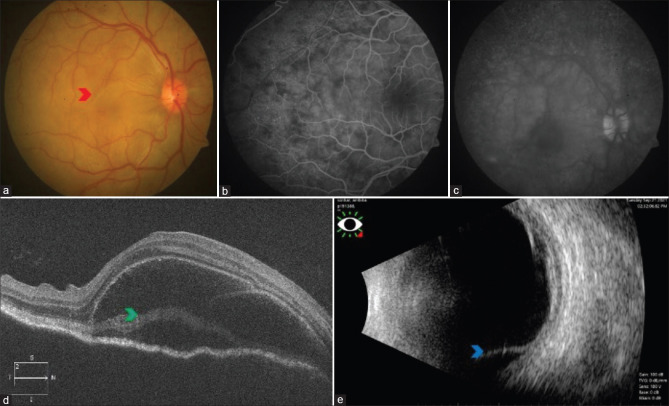
Although choroidal thickness is better measured using enhanced-depth imaging (EDI) OCT, a B-scan ultrasound can help measure choroidal thickness in patients with poor media visibility due to cataract, posterior synechiae, or vitritis. Studies have shown diffuse choroidal thickening and shallow retinal detachment at the macula in patients with sympathetic ophthalmia [Fig. 3].[45,46]
Figure 3.
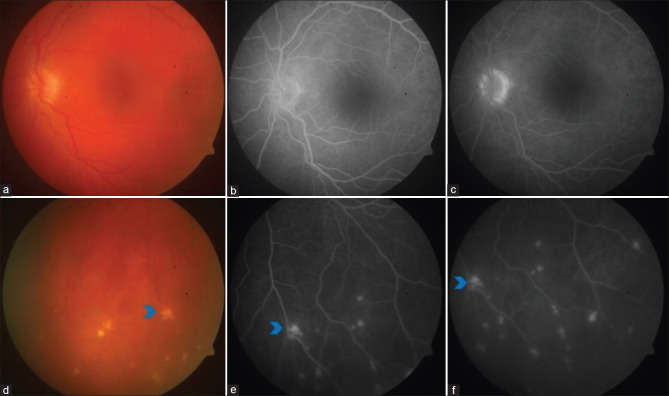
Right eye color fundus photograph of a case of sympathetic ophthalmia with disc hyperemia and multiple pockets of exudative retinal detachment (red arrowhead) (a). FFA showing pinpoint hyperfluorescence in the early phase (b), followed by the pooling of dye within the area of neurosensory detachment in the late phase (c). SD-OCT showing neurosensory detachment with the split of photoreceptors at the level of myloid (green arrowhead) (d). B-scan showing retinochoroid thickening and exudative retinal detachment inferiorly (blue arrowhead) (e)
Fundus fluorescein angiography (FFA)
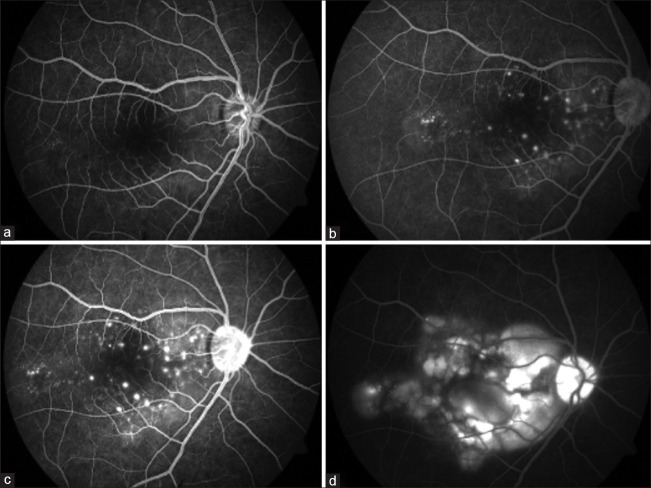
During the acute stages of the disease, two distinct angiographic findings have been described. The most common angiographic finding is the presence of multiple pinpoint hyperfluorescent leaks during the early phase with dye pooling in the later phase[47] [Fig. 4]. This is attributed to the disruption of RPE cell junctions, which causes the dye to pool in the subretinal space.[46] This angiographic finding is similar to what has been classically described in acute VKH.
Figure 4.
FFA showing multiple hyperfluorescent leaks in the early phase (a, b, and c), followed by the pooling of dye within the area of neurosensory detachment in the late phase (d)
The second, less common type resembles a pattern observed in acute posterior multifocal placoid pigment epitheliopathy (APMPPE), wherein hypo-fluorescent spots are observed in the early phase that become hyper-fluorescent in the later phase[13] [Fig. 5]. These lesions have a mottled appearance with slight elevation as compared to APMPPE lesions.[48] The hypofluorescence is attributed to either presence of Dalen–Fuchs nodules causing obscuration of the underlying choroid or due to the obliteration of choriocapillaris secondary to choroidal stromal inflammation.[49]
Figure 5.
Fundus picture showing exudative retinal detachment in the peripapillary area and macula (a). FFA showing hypofluorescent spots (yellow arrows) along with multiple hyperfluorescent leaks in the early phase (b and c), followed by leakage and pooling in the late phase (d) (yellow arrows)
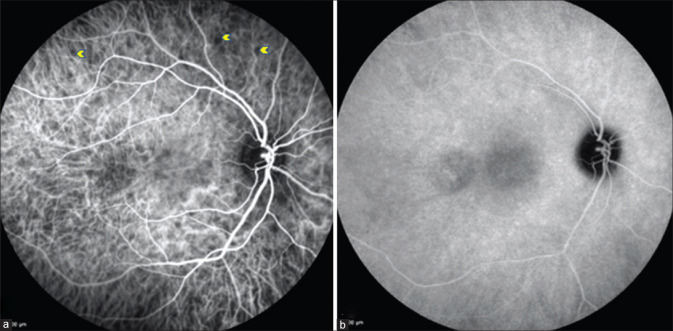
During the chronic stage, variable angiographic features can be seen owing to the presence of features such as chorioretinal nummular scarring (window defects) [Figs. 6 and 7], subretinal fibrosis (hyper fluorescence with staining), or choroidal neovascular membrane (hyper fluorescence with leakage).[13]
Figure 6.
Color fundus photograph showing healed nummular scars (a), which appear hyperfluorescent (window defects) on FFA in early phase (b), and minimal increase in hyperflourescence (c) in the late phase
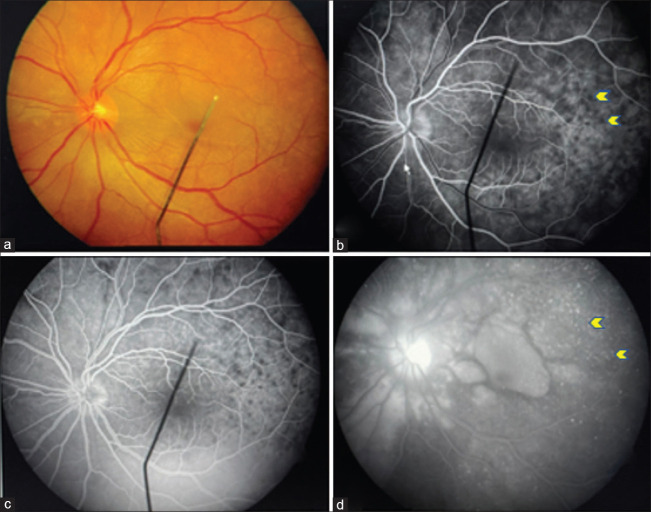
Figure 7.
Left eye fundus picture showing sunset glow fundus (a) and Dalen–Fuchs spots (blue arrowhead) in inferior retina (d). FFA showing disc staining ( b and c) with Dalen–Fuchs spots appearing hyperfluorescent spots (blue arrowhead) both in the early and late phases (window defect) (e and f)
Apart from diagnosing the disease, FFA helps monitor the disease’s progression and response as the changes seen in the acute phase resolve with treatment.
Indocyanine green angiography (ICGA)
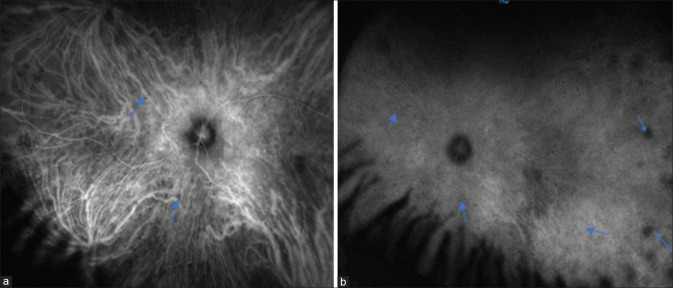
Indocyanine green angiography helps in the study of choroidal vasculature and creates better understanding of disease pathogenesis as SO is primarily a choroidal pathology. Studies have shown the presence of hypocyanescent spots in the early phase of ICGA, which remain hypocyanescent [Fig. 8] or become isocyanescent [Fig. 9] in the later phase during the acute stage of SO.[50,51] These hypocyanescent lesions are attributed to the presence of Dalen–Fuchs’ nodules or cellular infiltration of the choroid. Bernasconi et al.[51] have attributed the persistence and fading of hypocyanescence in the later phase to cicatricial and active lesions, respectively. These hypocyanescent lesions disappear following corticosteroid therapy, which is associated with clinical improvement.[46]
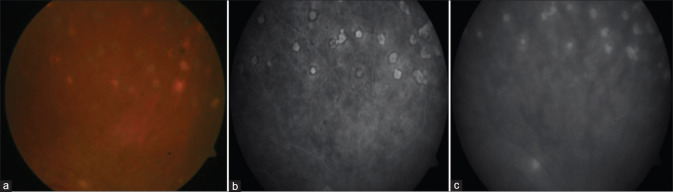
Figure 8.
Widefield ICGA showing hypocyanescent spots (blue arrows) in the early phase (a) which persists in the late phase (b)
Figure 9.
ICGA showing hypocyanescent spots (yellow arrows) (a) that become isocyanescent during the late phase (b)
Fundus Autofluorescence (FAF)
Active disease is associated with hyper autofluorescence signals corresponding to areas of neurosensory detachment. As the condition heals and leads to RPE changes, FAF shows stippled hyper autofluorescence similar to leopard spots.[46,52] Thus, AF can be used to study the health of RPE. A hyper autofluorescence signal suggests a metabolically active RPE, and a hypo autofluorescence signal indicates RPE loss. However, SO being primary choroidal stromal pathology, FAF does not play a significant role in the diagnosis and management of the disease.
Optical coherence tomography (OCT)
The advent of enhanced depth imaging OCT (EDI-OCT) and swept-source OCT has allowed high-resolution images of retinal and choroidal layers. Studies have shown multiple serous detachments along with hyperreflective septa in some cases[53,54] [Fig. 10]. These hyperreflective septa are now regarded as a bacillary layer detachment, as seen in VKH and other posterior segment pathologies. It is considered a split in the photoreceptor layer at the inner segment myoid, which resolves rapidly with corticosteroid treatment.[55] Apart from this, hyperreflective lesions at the level of RPE with disruption of the inner segment-outer segment junction can be visualized, which corresponds to Dalen–Fuchs nodules.[52] A study by Gupta et al.[53] has shown the resolution of OCT features such as disruption in RPE–Bruch’s membrane complex and inner segment/outer segment (IS/OS) junction, with treatment during the acute phase of the disease.
Figure 10.
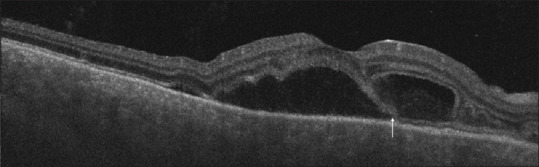
SS-OCT showing neurosensory detachment associated with the split of photoreceptors at the level of myloid (white arrow) resulting in bacillary layer detachment
Apart from changes in the outer retina, changes in the choroid during the acute phase of the disease include diffuse choroidal thickening, choroidal folds, and loss of choroidal architecture [Fig. 11].[52,54] Choroidal thickening is regarded as a critical OCT biomarker to monitor disease activity. Choroidal vascularity index (CVI) is a recently introduced OCT biomarker for monitoring disease activity in SO. Choroidal vascularity index is regarded as a stable index and is less influenced by physiological factors.[56]
Figure 11.
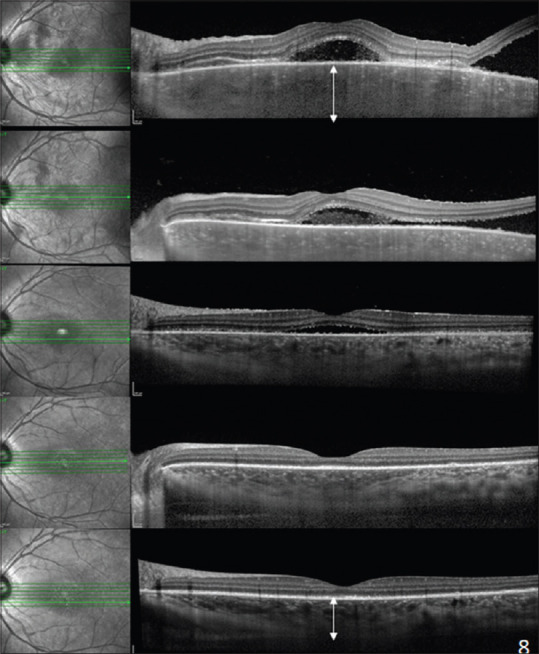
EDI-SD-OCT showing multiple neurosensory retinal detachments associated with diffuse choroidal thickening and loss of choroidal architecture. Subsequent scans show a reduction in choroidal thickness by restoring choroidal architecture (white arrows) after starting immunomodulatory treatment
Optical coherence tomography angiography (OCTA)
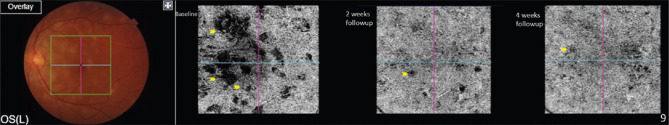
Multiple small areas of choriocapillaris flow voids that likely correspond to areas of choriocapillaris ischemia, which decrease in size and disappear with treatment, have been observed in OCTA [Fig. 12].[57] Choroidal neovascular membrane can also be better visualized with the help of OCTA.[58]
Figure 12.
OCTA showing flow voids (yellow arrows) in choriocapillaris slab, which reduce and disappear with treatment on subsequent follow-up
While FFA and ICGA provide two-dimensional images, OCTA offers three-dimensional images based on a volumetric dataset. Choriocapillaris ischemia detected on OCTA as flow voids correlate with the hypocyanescent lesions of ICGA in various inflammatory choroidal pathologies such as VKH and SO.[59] Although OCTA is a noninvasive alternative to ICGA for pathologies involving choriocapillaris, it cannot visualize medium and large choroidal vessels very well. The flow voids need to be differentiated from loss of signal transmission and artifacts making its role in managing SO unclear.[60]
Diagnosis of SO is majorly based on the history of trauma and clinical features. Multimodal imaging helps in monitoring disease progression, detecting any recurrences, and monitoring response to therapy. Newer OCT have come up as useful noninvasive tools for assessing retinochoroidal microstructural and microvascular abnormalities, respectively, in patients with choroidal stromal pathologies such as SO and VKH. Optical coherence tomography angiography is a promising tool, whose use in choroidal pathologies is gaining increasing popularity.
Management of Sympathetic Ophthalmia
Medical management
The initial management of sympathetic ophthalmia involves the administration of high-dose systemic corticosteroid therapy based on the clinical presentation, the severity of the disease, and the presenting visual acuity. In 1995, the National Eye Institute provided the benefits of high-dose systemic anti-inflammatory therapy (corticosteroids in the range of 0.5–2 mg/kg/day prednisolone) in salvaging the vision.[31] Intravenous corticosteroid therapy is generally the preferred approach for severe cases with iris nodules, severe granulomatous panuveitis, and serous retinal detachments. Intravenous pulse therapy with methylprednisolone (1 g/day) for 3 days helps in rapid resolution of anatomical abnormalities such as vitritis, multifocal retinal detachments, and anterior chamber inflammation.[31,53,61] In certain situations, the intravenous therapy can be prolonged for 5–7 days based on the ocular response. There can be potential hazards of intravenous pulse therapies such as rapid fluctuations in blood pressure, blood glucose levels, and serious adverse effects such as reactivation of pulmonary tuberculosis. Therefore, care must be taken during the initial aggressive therapies considering the risk–benefit ratio.
Once the initial inflammatory response has been overcome, the systemic corticosteroid therapy (usually started at 1 mg/kg/day prednisolone) can be tapered off over a 2-to-3-month period. A 3-month period usually provides a reasonable time to assess the effectiveness of therapy. However, if the patient needs further surgical interventions, an increase in systemic corticosteroid therapy may be warranted.[62]
Immunosuppressive therapies are usually indicated in patients with sympathetic ophthalmia to decrease the need for prolonged systemic corticosteroid therapy. Typically, the combination of corticosteroid and immunosuppressive therapy is initiated at the onset so that adequate time is given for the action of the immunosuppressive agent to set in. In addition, in situations where there is intolerance to a particular agent, an alternate agent can be initiated. Various agents can be used in the management of sympathetic ophthalmia [Table 2]. Commonly used agents include mycophenolate mofetil, azathioprine, cyclosporine, and cyclophosphamide.[38,62,63,64,65] The latter is used for refractory cases given its severe systemic toxicity [Fig. 13].
Table 2.
Various immunosuppressive drugs used in the treatment of sympathetic ophthalmia[66]
| Class | Generic name | Dose | Expected onset of action | Side effects | Advantage/Disadvantage |
|---|---|---|---|---|---|
| Antimetabolite | Azathioprine | 1-4 mg/kg/day PO | 4-12 weeks | Bone marrow suppression, Gastrointestinal upset, Hepatotoxicity, hypersensitivity | can be given once daily or as divided dose twice daily |
| Methotrexate | 7.5-25 mg/week PO, SC, or IM | 2-12 weeks | Hepatotoxicity, Interstitial pneumonia, cytopenia, oral ulcers, fetal loss | -weekly oral dose - Folic acid 1 mg/day - Regular liver function test/2 months - Non-carcinogenic |
|
| Mycophenolate Mofetil | 500-1500 g PO BD | 2-12 weeks | Diarrhea, Nausea, Myelosuppression, High cost | Possibility of better tolerated than azathioprine | |
| Alkylating group | Cyclophosphamide | 1-3 mg/kg/day PO | 2-8 weeks | Bone marrow suppression, hemorrhagic cystitis, malignancy, infection | Teratogenic Can be given as iv pulse |
| Chlorambucil | 0.1-0.2 mg/kg/day PO | 4-12 weeks | Infertility, bone marrow suppression, teratogenic | Teratogenic | |
| T-cell inhibitor | Cyclosporine | 2.5-10 mg/kg/day PO BD | 2-6 weeks | Renal dysfunction, tremor, hirsutism, hypertension, gum hyperplasia | Monitor for renal toxicity, BP No myelosuppression |
|
| |||||
| Biologicals | |||||
|
| |||||
| TNF inhibitors | Infliximab | IV 3-5 mg/kg loading at weeks 0, 2, and 6, then maintenance 3-10 mg/kg every 4-8 weeks; maximal dose 20 mg/kg in children |
Susceptible to infections, including reactivation of tuberculosis, histoplasmosis, hepatitis B, fungal infection; hypersensitivity reactions, demyelinating disease; lupus-like syndrome; malignancy; thromboembolic events, congestive heart failure | Increased risk of lymphoma | |
| Adalimumab | SC 40 mg every 1-2 weeks (if bodyweight <30 kg; 20 mg every 2 weeks); loading doses of 80-160 mg are recommended |
Susceptible to infections, allergic reaction, increased severity of chickenpox or shingles, drug-induced lupus, skin cancer | Requires monitoring of blood counts and liver function tests | ||
| IL-6 receptor antagonist | Tocilizumab | IV Initial 4 mg/kg every 4 weeks, then increase to 8-12 mg/kg every 2-4 weeks |
Serious infections, hypersensitivity reactions, and gastrointestinal perforation | Do not inject if there is an active infection | |
(PO: per oral, SC: subcutaneous, IM: intramuscular, IV: intravenous, BD: twice a day)
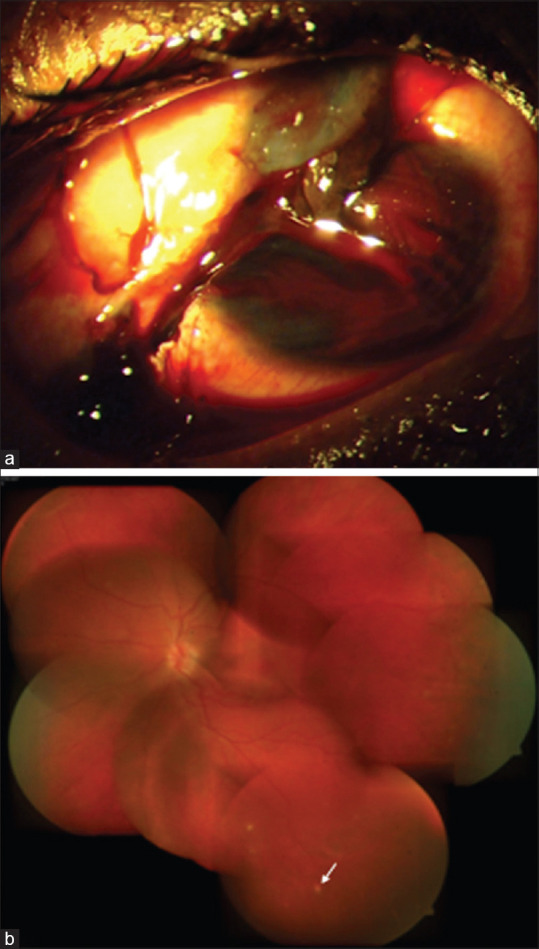
Figure 13.
Flowchart for the management of sympathetic ophthalmia
Research into the molecular pathways of sympathetic ophthalmia has opened new vistas in the management of this condition, especially targeted therapies against certain cytokines. Disruption of leukocyte recruitment by targeting matrix metalloproteinase-9, chemokine ligand 2 (CCL2), and C-X-C motif chemokine ligand 2 (CXCL12) may hold promise for future treatment of this condition.[24,62] These therapies are currently under investigation.
Local therapies
Intravitreal therapies can be helpful in the management of sympathetic ophthalmia because they help in reducing the dosage of systemic anti-inflammatory therapies and in bridging the steroid therapy while awaiting the action of systemic corticosteroids. Intravitreal triamcinolone acetonide (IVTA) has been shown to be effective in improving vitreous inflammation, visual acuity, and initial severity of the disease so that corticosteroids can be tapered even faster. Chan et al.[67] demonstrated the rapid recovery from sympathetic ophthalmia in a patient who received supplementary IVTA. Jonas described a patient who received two injections of IVTA (high dose: 25 mg) and experienced significant improvement in his visual fields without a clinically significant rise in intraocular pressure.[68] Ozdemir et al.[69] used a low dose of IVTA (4 mg; single injection) in a patient who was already on systemic corticosteroids. The patient experienced clinical remission and regained visual acuity of 20/20. Jonas et al.[70] also used multiple repeat IVTA injections in chronic cases of sympathetic ophthalmia, especially those with macular edema. The main complications of IVTA include high risk of cataract, glaucoma, and other serious side-effects such as endophthalmitis.
Intravitreal corticosteroid implant (Ozurdex ®, Allergan Inc) is a new alternative that provides a lower sustained corticosteroid dose intravitreally resulting in a substantial decrease in the risk of glaucoma and cataract. Meira et al.[71] used the dexamethasone implant in a 54-year-old monocular patient with pseudophakia with sympathetic ophthalmia, undergoing therapy with cyclosporine. Because the patient had recurrent cystoid macular edema, supplementation with a dexamethasone implant was performed. The patient was also given sustained release fluocinolone (Iluvien ®, Alimera Sciences Limited), after which the patient experienced long-term (3 year) remission and sustained control of cystoid macular edema. Therefore, intravitreal depot steroids may be helpful in managing patients of sympathetic ophthalmia with recurrent macular edema.
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections are useful adjuncts in eyes with sympathetic ophthalmia complicated by choroidal neovascularization (CNV).[72] While there are few reports of such complications, the use of optical coherence tomography angiography can help in detecting such membranes in future, thereby expanding the spectrum of patients who require anti-VEGF therapy. There is no preference among ranibizumab, bevacizumab, or aflibercept for treating inflammatory CNV in eyes with sympathetic ophthalmia. Long-term immunosuppressive therapies in these eyes are useful adjuncts to anti-VEGF agents.[58]
Refractory sympathetic ophthalmia
Patients with sympathetic ophthalmia can develop a refractory disease, which may respond suboptimally to corticosteroid therapy. Despite immunosuppression with agents such as mycophenolate mofetil, azathioprine, or cyclosporine, these patients may continue to experience ongoing vitreous and chorioretinal inflammation. There is limited experience with biological agents in patients with sympathetic ophthalmia. Kim et al.[73] used adalimumab in the treatment of pediatric sympathetic ophthalmia (single case). Their patient was a 5-year-old girl who received penetrating trauma to the eye requiring open globe repair. Histopathology after enucleation of the traumatized eye revealed features of sympathetic ophthalmia. The patient was treated with systemic corticosteroids and adalimumab after screening for latent tuberculosis with a good outcome.
Gupta et al.[74] reported a case of pediatric sympathetic ophthalmia refractive to various immunosuppressive agents, and the patient was eventually treated with intravenous infliximab, resulting in prolonged control of inflammation on infliximab alone. Similarly, Soheilian et al.[75] reported a case of refractory sympathetic ophthalmia successfully treated with adalimumab after multiple failed treatments with immunosuppressive agents.
Mesquida et al.[76] used tocilizumab therapy for refractory macular edema because of sympathetic ophthalmia. In their series of 16 eyes (12 patients), one patient with sympathetic ophthalmia was included. Thus, refractory macular edema may be another indication for the use of biological agents in these patients.
Further research on the use of biological agents in sympathetic ophthalmia, including a study using a larger series, is warranted to understand the exact role, efficacy, and safety of these agents.
Cataract surgery in the sympathizing eye
Very few reports document the outcome of cataract surgery in the sympathizing eye.[77,78,79,80] Cataract surgery in a sympathizing eye is difficult due to (a) decreased visualization secondary to band-shaped keratopathy, corneal edema, or corneal scarring; (b) organization of inflammatory debris and formation of membranes extending the iris over the lens surface; (c) poor pupillary dilation secondary to iris atrophy, pupillary membranes, or posterior synechiae; (d) presence of chronic inflammation; (e) decision regarding intraocular lenses (IOLs); and (f) posterior segment lesions such as optic atrophy, cystoid macular edema, epiretinal membrane, and foveal atrophy. Before the era of immunosuppression, sympathetic ophthalmia was one of the absolute contraindications for intraocular lens (IOL) implantation. However, now, due to better understanding of the disease process and better experience with immunosuppressive drugs and biological agents, IOLs can be placed in selected cases. A good control of preoperative inflammation, minimization of breakdown of the blood–aqueous barrier intraoperatively, and adequate suppression of inflammation postoperatively are critical to the success of cataract surgery in such eyes. The sympathizing eye should have at least 3 months of a quiescent period before cataract surgery. It is advisable to start patients on oral steroids 3 days prior to cataract surgery and to continue it in the postoperative period with gradual tapering. Small-incision phacoemulsification, preferably a clear corneal incision, has been shown to produce less blood–aqueous barrier breakdown and fewer cellular deposits on the surface of the IOL implant. Intraoperatively, small pupils can be managed by synechiolysis, iris stretching maneuvers, or self-retaining flexible iris hooks. In cases where inflammation is well controlled with a long uveitis free interval with or without a maintenance dose of steroids, in-the-bag placement of an acrylic or heparin-surface-modified polymethylmethacrylate IOL should be done. Cataract surgery with an intravitreal dexamethasone implant (Ozurdex  , Allergan Inc) has shown a good outcome in eyes with uveitis and can be an effective alternative for postoperative systemic steroids or in eyes with coexisting CME.[81] Postoperatively, apart from aggressive treatment with corticosteroids, cycloplegics should be added to prevent formation of posterior synechiae. These eyes should be monitored for postoperative glaucoma, posterior capsular opacification, cystoid macular edema, and the epiretinal membrane. Despite all these efforts, the final visual outcome depends upon posterior segment complications. Thus, patients should be properly counselled.
, Allergan Inc) has shown a good outcome in eyes with uveitis and can be an effective alternative for postoperative systemic steroids or in eyes with coexisting CME.[81] Postoperatively, apart from aggressive treatment with corticosteroids, cycloplegics should be added to prevent formation of posterior synechiae. These eyes should be monitored for postoperative glaucoma, posterior capsular opacification, cystoid macular edema, and the epiretinal membrane. Despite all these efforts, the final visual outcome depends upon posterior segment complications. Thus, patients should be properly counselled.
Prevention
Factors that play a major role in the prevention of SO or vision loss due to SO include a) prompt and meticulous primary repair in cases with open globe injuries, b) removal of the injured eye when surgical repair is deemed impossible, and c) prompt control of inflammation.
Improved management strategies and surgical techniques while dealing with open globe injury have led to a decrease in the incidence of SO, which was reported to be around 0.2%–2% in the twentieth century to less than 0.5% in the last three decades.[1,62,82] A delay in primary repair has been shown to increase the risk of SO, thereby indicating the importance of prompt intervention to facilitate its prevention.[27,83,84]
The role of enucleation and evisceration in the prevention of sympathetic ophthalmia has been controversial, particularly when the injured eye has good visual potential, as it could be the eye with better vision if sympathetic ophthalmia develops eventually.[85] However, eyes that are deemed non-operable and have no visual potential can be subjected to either enucleation or evisceration following trauma.[85,86] Between enucleation and evisceration, the choice of procedure is based on the surgeon’s decision. Although evisceration has a cosmetic advantage over enucleation, the theoretical risk of SO is always there. Moreover, the exact incidence of SO following evisceration cannot be estimated as SO is a rare disease and evisceration is also a procedure that is not performed very commonly.[82,87,88] Levine et al.[89] reported no cases of SO following evisceration in 51 eyes that completed follow-up examination. They also carried out a survey within the American Society of Ophthalmic Plastic and Reconstructive Surgery, which included 841 eviscerations, where 5 cases of SO were reported anecdotally without any clinical or histological evidence. They concluded that evisceration is an effective and safe procedure with a low risk of SO.
Savar et al.[86] showed that the rate of SO following enucleation in eyes with open globe injury is very low and ranged between 0.3 and 0.9%. Enucleation or evisceration, if needed, is usually recommended within 2 weeks of trauma or as early as possible because SO has been reported as early as 5 days from injury.[62]
Sympathetic ophthalmia is a rare disease, and with the judicious use of steroids and immunosuppressive agents, it is no longer considered to be a disease resulting in vision loss. This obviates the role of destructive surgeries such as enucleation or evisceration in the prevention of SO. High-dose corticosteroids are the mainstay for the treatment of SO, but there is no data to support the role it plays in the prevention of the disease. The aqueous has an innate property to prevent the activation of the autoimmune response due to the inhibitory activity on immune cells which is attributed to transforming growth factor-β.[90] This property of the aqueous is subdued because of the inflammation caused by trauma, resulting in the activation of a cascade of events leading to SO.[90,91] Inflammation controlled with corticosteroids during this period could play a pivotal role in preventing the development of SO. However, the evidence so far does not support its benefit in the prevention of SO.
Prognosis
Better health care facilities, early intervention, superior wound repair techniques, and increased awareness have improved the prognosis of sympathetic ophthalmia. Prompt and aggressive treatment with corticosteroids along with long-term immunosuppressive therapy has significantly improved visual outcomes in these eyes. With aggressive treatment, approximately 70% of patients have improvement in visual acuity in the sympathizing eye, of which 58.2% achieve visual acuity of 20/40 or better.[38] However, the relapsing nature of the disease and complications arising from chronic panuveitis remain the biggest challenges.
Conclusion
Sympathetic Ophthalmia is a serious potentially sight-threatening bilateral panuveitis that follows penetrating injury or an intraocular surgery. The exact pathophysiology is still unknown but believed to be related to an autoimmune response to retinal or uveal antigens or both. The disease responds rapidly to systemic corticosteroids but recurrence is common. Hence, concomitant use of immunosuppressive drugs is warranted to prevent recurrence while few recalcitrant cases might also require use of biological agents.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Albert D, Diaz-Rohena R. A historical review of sympathetic ophthalmia and its epidemiology. Surv Ophthalmol. 1989;34:1–14. doi: 10.1016/0039-6257(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie W. 3rd edition. London: Longmans; 1840. A Practical Treatise on the Diseases of the Eye; pp. 523–34. [Google Scholar]
- 3.Dalen A. Zur Kenntnis der sogenannten Choroiditis sympathetica. Mitt Augen Klin Carolin Med. Chirurg Inst Stockholm. 1904;6:1. [Google Scholar]
- 4.Fuchs E. On sympathetic inflammation (initially remarks on serous iritis) Graefes Arch Clinical Exp Ophthalmology. 1905;61:365–456. [Google Scholar]
- 5.Elschnig A. Studies on sympathetic ophthalmia, II:The antigenic effects of eye pigments. Graefes Arch Clin Exp Ophthalmol. 1910;76:509–46. [Google Scholar]
- 6.Rao NA, Xu S, Font RL. Sympathetic ophthalmia. An immunohistochemical study of epithelioid cells and giant cells. Ophthalmology. 1985;92:1660–2. doi: 10.1016/s0161-6420(85)34087-3. [DOI] [PubMed] [Google Scholar]
- 7.Prichard A. Surgical cases admitted under Augustin Prichard, Esq, Surgeon to the Infirmary. Prov Med Surg J. 1851;15:66–7. [Google Scholar]
- 8.Critchett G. Ueber sympathische ophthalmie. Klin Monatsbl Augenheikd 1 (Heidelberg Supplement) 1863;440 [Google Scholar]
- 9.Marak GE. Recent advances in sympathetic ophthalmia. Surv Ophthalmol. 1979;23:141–56. doi: 10.1016/0039-6257(79)90018-3. [DOI] [PubMed] [Google Scholar]
- 10.Kilmartin DJ, Dick AD, Forrester JV. Prospective surveillance of sympathetic ophthalmia in the UK and Republic of Ireland. Br J Ophthalmol. 2000;84:259–63. doi: 10.1136/bjo.84.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubin JR, Albert DM, Weinstein M. Sixty-five years of sympathetic ophthalmia:A clinicopathologic review of 105 cases (1913–1978) Ophthalmology. 1980;87:109–21. doi: 10.1016/s0161-6420(80)35270-6. [DOI] [PubMed] [Google Scholar]
- 12.Dutta Majumder P, Anthony E, George AE, Ganesh SK, Biswas J. Postsurgical sympathetic ophthalmia:Retrospective analysis of a rare entity. Int Ophthalmol. 2018;38:2487–93. doi: 10.1007/s10792-017-0759-0. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Gupta A, Dogra MR. Posterior sympathetic ophthalmia:A single centre long-term study of 40 patients from North India. Eye (Lond) 2008;22:1459–64. doi: 10.1038/sj.eye.6702927. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi M, Agarwal K, Reddy Pappuru RR, Dedhia C, Agarwal H, Nayak S, et al. Sympathetic ophthalmia after vitreoretinal surgeries:Incidence, clinical presentations and outcomes of a rare disease. Semin Ophthalmol. 2019;34:157–62. doi: 10.1080/08820538.2019.1610464. [DOI] [PubMed] [Google Scholar]
- 15.Harrison TJ. Sympathetic ophthalmia after cyclocryotherapy of neovascular glaucoma without ocular penetration. Ophthalmic Surg. 1993;24:44–6. [PubMed] [Google Scholar]
- 16.Biswas J, Fogla R. Sympathetic ophthalmia following cyclocryotherapy with histopathologic correlation. Ophthalmic Surg Lasers. 1996;27:1035–8. [PubMed] [Google Scholar]
- 17.Wang Y, Chan CC. Gender differences in Vogt-Koyanagi-Harada disease and sympathetic ophthalmia. J Ophthalmol. 2014;2014:157803. doi: 10.1155/2014/157803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollack AL, McDonald HR, Ai E, Green WR, Halpern LS, Jampol LM, et al. Sympathetic ophthalmia associated with pars plana vitrectomy without antecedent penetrating trauma. Retina. 2001;21:146–54. doi: 10.1097/00006982-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Gass JD. Sympathetic ophthalmia following vitrectomy. Am J Ophthalmol. 1982;93:552–8. doi: 10.1016/s0002-9394(14)77368-4. [DOI] [PubMed] [Google Scholar]
- 20.Kilmartin DJ, Dick AD, Forrester JV. Commentary:Sympathetic opthalmia risk following vitrectomy:Should we counsel patients? Br J Ophthalmol. 2000;84:448–9. doi: 10.1136/bjo.84.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheppard JD. Sympathetic ophthalmia. Semin Ophthalmol. 1994;9:177–84. doi: 10.3109/08820539409060013. doi:10.3109/08820539409060013. Erratum in:Semin Ophthalmol. 1995. 10. 87. [DOI] [PubMed] [Google Scholar]
- 22.Rathinam SR, Rao NA. Sympathetic ophthalmia following postoperative bacterial endophthalmitis:A clinicopathologic study. Am J Ophthalmol. 2006;141:498–507. doi: 10.1016/j.ajo.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Jakobiec FA, Marboe CC, Knowles DM, 2nd, Iwamoto T, Harrison W, Chang S, et al. Human sympathetic ophthalmia. An analysis of the inflammatory infiltrate by hybridoma-monoclonal antibodies, immuno- chemistry, and correlative electron microscopy. Ophthalmology. 1983;90:76–95. doi: 10.1016/s0161-6420(83)34602-9. [DOI] [PubMed] [Google Scholar]
- 24.Abu El-Asrar AM, Struyf S, Van den Broeck C, Van Damme J, Opdenakker G, Geboes K, et al. Expression of chemokines and gelatinase B in sympathetic ophthalmia. Eye (Lond) 2007;21:649–57. doi: 10.1038/sj.eye.6702342. [DOI] [PubMed] [Google Scholar]
- 25.Reynard M, Riffenburgh RS, Minckler DS. Morphological variation of Dalen-Fuchs nodules in sympathetic. Br J Ophthalmol. 1985;69:197–201. doi: 10.1136/bjo.69.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis JL, Mittal KK, Freidlin V, Mellow SR, Optican DC, Palestine AG, et al. HLA associations and ancestry in Vogt-Koyanagi-Harada disease and sympathetic ophthalmia. Ophthalmology. 1990;97:1137–42. doi: 10.1016/s0161-6420(90)32446-6. [DOI] [PubMed] [Google Scholar]
- 27.Arevalo JF, Garcia RA, Al-Dhibi HA, Sanchez JG, Suarez-Tata L. Update on sympathetic ophthalmia. Middle East Afr J Ophthalmol. 2012;19:13–21. doi: 10.4103/0974-9233.92111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao NA, Robin J, Hartmann D, Sweeney JA, Marak GE., Jr The role of the penetrating wound in the development of sympathetic ophthalmia experimental observations. Arch Ophthalmol. 1983;101:102–4. doi: 10.1001/archopht.1983.01040010104019. [DOI] [PubMed] [Google Scholar]
- 29.Rao NA, Wong VG. Aetiology of sympathetic ophthalmitis. Trans Ophthalmol Soc U K. 1981;101:357–60. [PubMed] [Google Scholar]
- 30.Nussenblatt RB. Sympathetic ophthalmia. In: Nussenblatt RB, Whitcup SM, editors. Uveitis:Fundamental and Clinical Practice. 3rd ed. St Louis, Missouri: Mosby; 2004. pp. 311–23. [Google Scholar]
- 31.Chan CC, Roberge RG, Whitcup SM, Nussenblatt RB. Thirty-two cases of sympathetic ophthalmia. Arch Ophthalmol. 1995;113:597–601. doi: 10.1001/archopht.1995.01100050065032. [DOI] [PubMed] [Google Scholar]
- 32.Goto H, Rao NA. Sympathetic ophthalmia and Vogt-Koyanagi-Harada syndrome. Int Ophthalmol Clin. 1990;30:279–85. doi: 10.1097/00004397-199030040-00014. [DOI] [PubMed] [Google Scholar]
- 33.Makley TA, Jr, Azar A. Sympathetic ophthalmia. A long-term follow-up. Arch Ophthalmol. 1978;96:257–62. doi: 10.1001/archopht.1978.03910050125004. [DOI] [PubMed] [Google Scholar]
- 34.Croxatto JO, Rao NA, McLean IW, Marak GE. Atypical histopathologic features in sympathetic ophthalmia. A study of a hundred cases. Int Ophthalmol. 1982;4:129–35. doi: 10.1007/BF00161902. [DOI] [PubMed] [Google Scholar]
- 35.Ganesh SK, Narayana KM, Biswas J. Peripapillary choroidal atrophy in sympathetic ophthalmia and management with triple-agent immunosuppression. Ocul Immunol Inflamm. 2003;11:61–5. doi: 10.1076/ocii.11.1.61.15581. [DOI] [PubMed] [Google Scholar]
- 36.Damico FM, Kiss S, Young LH. Sympathetic ophthalmia. Semin Ophthalmol. 2005;20:191–7. doi: 10.1080/08820530500232100. [DOI] [PubMed] [Google Scholar]
- 37.Vote BJ, Hall A, Cairns J, Buttery R. Changing trends in sympathetic ophthalmia. Clin Exp Ophthalmol. 2004;32:542–5. doi: 10.1111/j.1442-9071.2004.00876.x. [DOI] [PubMed] [Google Scholar]
- 38.Castiblanco CP, Adelman RA. Sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2009;247:289–302. doi: 10.1007/s00417-008-0939-8. [DOI] [PubMed] [Google Scholar]
- 39.Rao NA, Marak GE. Sympathetic ophthalmia simulating Vogt-Koyanagi-Harada's disease:A clinico-pathologic study of four cases. Jpn J Ophthalmol. 1983;27:506–11. [PubMed] [Google Scholar]
- 40.Lim WK, Chee SP, Sng I, Nussenblatt RB, Chan CC. Immunopathology of progressive subretinal fibrosis:Variant of sympathetic ophthalmia. Am J Ophthalmol. 2004;138:475–7. doi: 10.1016/j.ajo.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Goudot M, Groh M, Salah S, Monnet D, Blanche P, Brézin AP. Lymphocytic meningitis in patients with sympathetic ophthalmia. Ocul Immunol Inflamm. 2017;25:196–201. doi: 10.1080/09273948.2017.1291841. [DOI] [PubMed] [Google Scholar]
- 42.Tandon R, Vanathi M, Verma L, Bharadwaj A. Central serous retinopathy masquerading as sympathetic ophthalmia. Eye. 2003;17:666–7. doi: 10.1038/sj.eye.6700423. [DOI] [PubMed] [Google Scholar]
- 43.Chu DS, Foster CS. Sympathetic ophthalmia. Int Ophthalmol Clin. 2002;42:179–85. doi: 10.1097/00004397-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Wang RC, Zamir E, Dugel PU, Sipperley JO, Thirkill CE, Shabatian B, et al. Progressive subretinal fibrosis and blindness associated with multifocal granulomatous chorioretinitis:A variant of sympathetic ophthalmia. Ophthalmology. 2002;109:1527–31. doi: 10.1016/s0161-6420(02)01108-9. [DOI] [PubMed] [Google Scholar]
- 45.Castiblanco C, Adelman RA. Imaging for sympathetic ophthalmia:Impact on the diagnosis and management. Int Ophthalmol Clin. 2012;52:173–81. doi: 10.1097/IIO.0b013e318265d5c7. [DOI] [PubMed] [Google Scholar]
- 46.Mahajan S, Invernizzi A, Agrawal R, Biswas J, Rao NA, Gupta V. Multimodal imaging in sympathetic ophthalmia. Ocul Immunol Inflamm. 2017;25:152–9. doi: 10.1080/09273948.2016.1255339. [DOI] [PubMed] [Google Scholar]
- 47.Burkholder BM, Dunn JP. Multiple serous retinal detachments seen on wide-field imaging in a patient with sympathetic ophthalmia. JAMA Ophthalmol. 2014;132:1220. doi: 10.1001/jamaophthalmol.2014.52. [DOI] [PubMed] [Google Scholar]
- 48.Lewis ML, Gass DM, Spencer WH. Sympathetic uveitis after trauma and vitrectomy. Arch Ophthalmol Chic Ill 1960. 1978;96:263–7. doi: 10.1001/archopht.1978.03910050131005. [DOI] [PubMed] [Google Scholar]
- 49.Sharp DC, Bell RA, Patterson E, Pinkerton RM. Sympathetic ophthalmia. Histopathologic and fluorescein angiographic correlation. Arch Ophthalmol Chic Ill 1960. 1984;102:232–5. doi: 10.1001/archopht.1984.01040030182022. [DOI] [PubMed] [Google Scholar]
- 50.Saatci AO, Paşa E, Söylev MF, Koçak N, Durak I, Kaynak S. Sympathetic ophthalmia and indocyanine green angiography. Arch Ophthalmol Chic Ill 1960. 2004;122:1568–9. doi: 10.1001/archopht.122.10.1568. [DOI] [PubMed] [Google Scholar]
- 51.Bernasconi O, Auer C, Zografos L, Herbort CP. Indocyanine green angiographic findings in sympathetic ophthalmia. Graefes Arch Klin Exp Ophthalmol. 1998;236:635–8. doi: 10.1007/s004170050134. [DOI] [PubMed] [Google Scholar]
- 52.Fleischman D, Say EAT, Wright JD, Landers MB. Multimodality diagnostic imaging in a case of sympathetic ophthalmia. Ocul Immunol Inflamm. 2012;20:300–2. doi: 10.3109/09273948.2012.682637. [DOI] [PubMed] [Google Scholar]
- 53.Gupta V, Gupta A, Dogra MR, Singh I. Reversible retinal changes in the acute stage of sympathetic ophthalmia seen on spectral domain optical coherence tomography. Int Ophthalmol. 2011;31:105–10. doi: 10.1007/s10792-011-9432-1. [DOI] [PubMed] [Google Scholar]
- 54.Behdad B, Rahmani S, Montahaei T, Soheilian R, Soheilian M. Enhanced depth imaging OCT (EDI-OCT) findings in acute phase of sympathetic ophthalmia. Int Ophthalmol. 2015;35:433–9. doi: 10.1007/s10792-015-0058-6. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal A, Freund KB, Kumar A, Aggarwal K, Sharma D, Katoch D, et al. Bacillary layer detachment in acute Vogt-Koyanagi-Harada disease:A novel swept-source optical coherence tomography analysis. Retina Phila Pa. 2021;41:774–83. doi: 10.1097/IAE.0000000000002914. [DOI] [PubMed] [Google Scholar]
- 56.Agrawal R, Jain M, Khan R, Jaisankar D, Xin W, Ding J, et al. Choroidal structural changes in sympathetic ophthalmia on swept-source optical coherence tomography. Ocul Immunol Inflamm. 2021;29:537–42. doi: 10.1080/09273948.2019.1685110. [DOI] [PubMed] [Google Scholar]
- 57.Brar M, Sharma M, Grewal SPS, Grewal DS. Treatment response in sympathetic ophthalmia as assessed by widefield OCT angiography. Ophthalmic Surg Lasers Imaging Retina. 2018;49:726–30. doi: 10.3928/23258160-20180831-13. [DOI] [PubMed] [Google Scholar]
- 58.Agarwal A, Invernizzi A, Singh RB, Foulsham W, Aggarwal K, Handa S, et al. An update on inflammatory choroidal neovascularization:Epidemiology, multimodal imaging, and management. J Ophthalmic Inflamm Infect. 2018;8:13. doi: 10.1186/s12348-018-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karti O, Saatci AO. Optical coherence tomography angiography in eyes with non-infectious posterior uveitis;Some practical aspects. Med Hypothesis Discov Innov Ophthalmol. 2019;8:312–22. [PMC free article] [PubMed] [Google Scholar]
- 60.Pichi F, Aggarwal K, Neri P, Salvetti P, Lembo A, Nucci P, et al. Choroidal biomarkers. Indian J Ophthalmol. 2018;66:1716–26. doi: 10.4103/ijo.IJO_893_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hebestreit H, Huppertz HI, Sold JE, Dämmrich J. Steroid-pulse therapy may suppress inflammation in severe sympathetic ophthalmia. J Pediatr Ophthalmol Strabismus. 1997;34:124–6. doi: 10.3928/0191-3913-19970301-16. [DOI] [PubMed] [Google Scholar]
- 62.Chu XK, Chan CC. Sympathetic ophthalmia:To the twenty-first century and beyond. J Ophthalmic Inflamm Infect. 2013;3:49. doi: 10.1186/1869-5760-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J, Li Y, Xie R, Li X, Zhang X. Sympathetic ophthalmia:Report of a case series and comprehensive review of the literature. Eur J Ophthalmol. 2021;31:3099–109. doi: 10.1177/1120672120977359. [DOI] [PubMed] [Google Scholar]
- 64.Dutta Majumder P, Mistry S, Sridharan S, George AE, Rao V, Ganesh SK, et al. Pediatric sympathetic ophthalmia:20 years of data from a tertiary eye center in India. J Pediatr Ophthalmol Strabismus. 2020;57:154–8. doi: 10.3928/01913913-20200219-01. [DOI] [PubMed] [Google Scholar]
- 65.Tan XL, Seen S, Dutta Majumder P, Ganesh SK, Agarwal M, Soni A, et al. Analysis of 130 cases of sympathetic ophthalmia-A retrospective multicenter case series. Ocul Immunol Inflamm. 2019;27:1259–66. doi: 10.1080/09273948.2018.1517894. [DOI] [PubMed] [Google Scholar]
- 66.Mérida S, Palacios E, Navea A, Bosch-Morell F. New immunosuppressive therapies in uveitis treatment. Int J Mol Sci. 2015;16:1–95. doi: 10.3390/ijms160818778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan RV, Seiff BD, Lincoff HA, Coleman DJ. Rapid recovery of sympathetic ophthalmia with treatment augmented by intravitreal steroids. Retina. 2006;26:243–7. doi: 10.1097/00006982-200602000-00029. [DOI] [PubMed] [Google Scholar]
- 68.Jonas JB. Intravitreal triamcinolone acetonide for treatment of sympathetic ophthalmia. Am J Ophthalmol. 2004;137:367–8. doi: 10.1016/S0002-9394(03)00899-7. [DOI] [PubMed] [Google Scholar]
- 69.Ozdemir H, Karacorlu M, Karacorlu S. Intravitreal triamcinolone acetonide in sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2005;243:734–6. doi: 10.1007/s00417-004-1064-y. [DOI] [PubMed] [Google Scholar]
- 70.Jonas JB, Spandau UH. Repeated intravitreal triamcinolone acetonide for chronic sympathetic ophthalmia. Acta Ophthalmol Scand. 2006;84:436. doi: 10.1111/j.1600-0420.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 71.Meira J, Madeira C, Falcão-Reis F, Figueira L. sustained control from recurring non-infectious uveitic macular edema with 0.19 mg fluocinolone acetonide intravitreal implant-A case report. Ophthalmol Ther. 2019;8:635–41. doi: 10.1007/s40123-019-00209-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saatçi AO, Ayhan Z, İpek ŞC, Söylev Bajin M. Intravitreal aflibercept as an adjunct to systemic therapy in a case of choroidal neovascular membrane associated with sympathetic ophthalmia. Turk J Ophthalmol. 2018;48:209–11. doi: 10.4274/tjo.09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JB, Jeroudi A, Angeles-Han ST, Grossniklaus HE, Yeh S. Adalimumab for pediatric sympathetic ophthalmia. JAMA Ophthalmol. 2014;132:1022–4. doi: 10.1001/jamaophthalmol.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta SR, Phan IT, Suhler EB. Successful treatment of refractory sympathetic ophthalmia in a child with infliximab. Arch Ophthalmol. 2011;129:250–2. doi: 10.1001/archophthalmol.2010.358. [DOI] [PubMed] [Google Scholar]
- 75.Soheilian M, Jabbarpourbonyadi M, Soheilian R, Peyman GA. Bilateral uveitis after phakic intraocular lens implantation and management with adalimumab. J Cataract Refract Surg. 2012;38:1094–6. doi: 10.1016/j.jcrs.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 76.Mesquida M, Molins B, Llorenç V, Hernández MV, Espinosa G, Sainz de la Maza M, et al. Twenty-four month follow up of tocilizumab therapy for refractory uveitis-related macular edema. Retina. 2018;38:1361–70. doi: 10.1097/IAE.0000000000001690. [DOI] [PubMed] [Google Scholar]
- 77.Henkind P, Wise G. Cataract extraction in a sympathizing eye. Am J Ophthalmol. 1974;77:112–4. doi: 10.1016/0002-9394(74)90616-3. [DOI] [PubMed] [Google Scholar]
- 78.Angelis D, Gupta N, Howcroft M, Leznoff A. Cataract extraction by phacoemulsification in a sympathizing eye. Can J Ophthalmol. 2000;35:26–8. doi: 10.1016/s0008-4182(00)80106-4. [DOI] [PubMed] [Google Scholar]
- 79.Raynard M, Minckler D. Cataract extraction in the sympathising eye. Arch Ophthalmol. 1983;101:1701–3. doi: 10.1001/archopht.1983.01040020703006. [DOI] [PubMed] [Google Scholar]
- 80.Mehta S, Linton MM, Kempen JH. Outcomes of cataract surgery in patients with uveitis:A systematic review and meta-analysis. Am J Ophthalmol. 2014;158:676–92.e7. doi: 10.1016/j.ajo.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 81.Gupta A, Ram J, Gupta A, Gupta V. Intraoperative dexamethasone implant in uveitis patients with cataract undergoing phacoemulsification. Ocul Immunol Inflamm. 2013;21:462–7. doi: 10.3109/09273948.2013.822087. [DOI] [PubMed] [Google Scholar]
- 82.Bilyk JR. Enucleation, evisceration, and sympathetic ophthalmia. Curr Opin Ophthalmol. 2000;11:372–86. doi: 10.1097/00055735-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 83.Sen HN, Nussenblatt RB. Sympathetic ophthalmia:What have we learned? Am J Ophthalmol. 2009;148:632–3. doi: 10.1016/j.ajo.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Page RD, Gupta SK, Jenkins TL, Karcioglu ZA. Risk factors for poor outcomes in patients with open-globe injuries. Clin Ophthalmol. 2016;10:1461–6. doi: 10.2147/OPTH.S108901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gürdal C, Erdener U, Irkeç M, Orhan M. Incidence of sympathetic ophthalmia after penetrating eye injury and choice of treatment. Ocul Immunol Inflamm. 2002;10:223–7. doi: 10.1076/ocii.10.3.223.15600. [DOI] [PubMed] [Google Scholar]
- 86.Savar A, Andreoli MT, Kloek CE, Andreoli CM. Enucleation for open globe injury. Am J Ophthalmol. 2009;147:595–600.e1. doi: 10.1016/j.ajo.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 87.du Toit N, Motala MI, Richards J, Murray AD, Maitra S. The risk of sympathetic ophthalmia following evisceration for penetrating eye injuries at Groote Schuur Hospital. Br J Ophthalmol. 2008;92:61–3. doi: 10.1136/bjo.2007.120600. [DOI] [PubMed] [Google Scholar]
- 88.Zheng C, Wu AY. Enucleation versus evisceration in ocular trauma:A retrospective review and study of current literature. Orbit. 2013;32:356–61. doi: 10.3109/01676830.2013.764452. [DOI] [PubMed] [Google Scholar]
- 89.Levine MR, Pou CR, Lash RH. The 1998 Wendell Hughes lecture. Evisceration:Is sympathetic ophthalmia a concern in the new millennium? Ophthalmic Plast Reconstr Surg. 1999;15:4–8. doi: 10.1097/00002341-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Ramadan A, Nussenblatt RB. Visual prognosis and sympathetic ophthalmia. Curr Opin Ophthalmol. 1996;7:39–45. doi: 10.1097/00055735-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Punnonen E. Pathological findings in eyes enucleated because of perforating injury. Acta Ophthalmol (Copenh) 1990;68:265–9. doi: 10.1111/j.1755-3768.1990.tb01920.x. [DOI] [PubMed] [Google Scholar]