Abstract
We present a comprehensive review of existing literature on surgical corneal neurotization (SCN) as a treatment modality for neurotrophic keratopathy (NK) with an interim report of seven cases where SCN was performed using the indirect approach and followed up till 18 months postoperatively to look for improvement in ocular surface, corneal sensations, and nerve regeneration by using in vivo confocal microscopy (IVCM). A literature search was performed for publications with keywords “corneal nerves,” “neurotization,” “esthesiometry,” “corneal anesthesia,” and “neurotrophic keratopathy.” All literature available till December 31, 2020 was reviewed and included to describe NK and its management options, particularly SCN. NK is associated with absent or reduced corneal sensations and is managed using a step-ladder algorithm ranging from medical management for symptomatic relief to surgical corneal neurotization. Both direct and indirect approaches of SCN have a favorable outcome with reduced surgical morbidity in the indirect approach using sural nerve graft. Post neurotization, corneal sensation recovery may take up to 3–6 months, while nerve regeneration on confocal microscopy can take as long as 6 months–1 year.
Keywords: Corneal anesthesia, corneal nerves, esthesiometry, neurotization, neurotrophic keratopathy
Corneal healing response requires the presence of normal corneal sensations.[1,2,3,4] Neurotrophic keratopathy (NK) results from impairment of corneal innervation cascade.[3] Various causes of NK include neuropathy, diabetes mellitus (DM), herpetic keratitis, trauma, chemical or electrical injury, and iatrogenic.[5,6] Neural regeneration is a remote possibility if the underlying cause is not reversible. The use of scleral contact lenses and nerve growth factors (NGF) has been described.[5,7,8,9] Surgical corneal neurotization (SCN) has shown great promise but remains to be explored further. We discuss various techniques and approaches to neurotization of the cornea in this review and describe our own experience in the form of an interim report of seven cases and their outcome post neurotization.
Method of Literature Search
The literature search was done on PubMed by using keywords “corneal nerves,” “neurotization,” “esthesiometry,” “corneal anesthesia,” and “neurotrophic keratitis.” The literature available till December 2020 was reviewed and included to explore the features of NK and its management particularly SCN. Only English-language articles were included.
Corneal Innervation
An intact neural channel is essential for the cornea to maintain its epithelial barrier and tear film function.[1,2,3,4] Cornea is the most densely innervated tissue in the human body, with over 1.5% of nerve fibers from trigeminal (TG) ganglion supplying it through 70–80 long ciliary nerves.[2] The nerve fibers terminate in free nerve endings in the corneal epithelium and lose their myelin sheath as they pass 2–3 mm deeper at the level of the anterior third of the stroma. They further interdigitate and form three plexuses at the subepithelial/subbasal, anterior stroma, and mid stromal level. The subbasal nerve plexus has thinner nerve fibers with both sensory and autonomic functions.[10] Around 1–2 mm inferonasal to the corneal apex, they form a whorl-like pattern.[11] Several neurotrophic factors such as substance P and epidermal growth factor (EGF) released from the nerve fibers help maintain a healthy epithelial barrier and promote wound healing.[12]
Neurotrophic Keratopathy
An insensate cornea with abnormal corneal nerve functions leads to the development of NK. Table 1 enumerates the various causes of NK classified as congenital and acquired ones. Congenital causes are rare and include familial corneal hypesthesia, and congenital corneal anesthesia (CCA). They are associated with recurrent red-eye in children with non-healing NK.[13] Though CCA may be an isolated disorder, it can be seen in association with mesenchymal dysgeneses such as Goldenhar’s syndrome and vertebral abnormalities, anal atresia, cardiac anomalies, trachea-esophageal fistula, and renal and limb deformities (VACTERL).[14,15] It may be seen in association with anhidrosis and congenital insensitivity to pain.[14,15] Typically, CCA is bilateral and rarely unilateral.[14,15] It may be associated with posterior fossa tumors in children.[16] Acquired causes of NK include DM, stroke, multiple sclerosis (MS), vasculitis, nutritional deficiency, leprosy, and other systemic neuropathies. Local causes include lesions affecting the neural channel starting from TG ganglion up to the corneal nerve endings such as meningioma, acoustic neuroma, post-TG neuralgia surgery, orbital/facial trauma or surgery, and orbital tumors.[17,18] Corneal neuropathies secondary to herpetic keratitis, chemical or ocular burns, and prolonged topical anesthetic use can also produce NK.[19,20] Chronic contact lens wear along with a history of prior refractive surgery are other potential risk factors.[21]
Table 1.
Causes of neurotrophic keratitis
| CONGENITAL | ACQUIRED |
| Congenital corneal hypoesthesia/anesthesia | Systemic |
| Diabetes mellitus | |
| Congenital CN palsy | Vitamin A deficiency |
| Riley–Day Syndrome | Vitamin B complex deficiency |
| Goldenhar syndrome | Leprosy |
| Mobius corneal hypesthesia | Multiple sclerosis |
| Vasculitis | |
| Other nutritional deficiency | |
| Age | |
| Local | |
| Neurological | |
| Meningioma | |
| Acoustic neuroma | |
| Infections | |
| Herpes zoster | |
| Herpes simplex | |
| Toxic topical medications | |
| Topical anesthetics | |
| Preservatives | |
| Topical beta-blockers | |
| Chemical and Thermal burns | |
| Iatrogenic | |
| Orbital or facial trauma surgery | |
| Post TG neuralgia surgery | |
| Post LASIK | |
| Chronic CL wear |
TG Trigeminal, LASIK Laser in situ Keratomileusis, CL Contact Lens
Evaluation of a case of NK involves measurement of corneal sensations/corneal nerve function or corneal esthesiometry.
Measuring corneal sensations
Cotton wisp test
The cotton wisp test is used to assess corneal sensations qualitatively. The patient looks straight with both eyes open and no topical anesthesia. A small fine-tipped sterile cotton wisp is held by the examiner standing behind the patient. It is gently applied on the patient’s cornea from a temporal aspect as the patient continues to look ahead. All four quadrants in both eyes are checked, and blink responses are compared. Normal comparable blink response is characteristic of normal corneal sensations.
Corneal aesthesiometer
Cochet–Bonnet esthesiometer (CBA) is a handheld device that can quantitatively assess corneal sensations.[22] It is more objective and useful in the diagnosis and follow-up evaluation. It consists of a fine nylon thread with adjustable length to simulate different intensities of stimuli. The longer the thread, the lighter the stimulus. The length of the thread ranges from 5 to 60 mm. The pressure increases from 11 to 200 mm/g as the length is reduced.[23] The length of the thread at which the patient responds to half of the stimuli is called corneal touch stimulus. Further, a calibration curve is used to convert this threshold value to pressure, and the reciprocal of that gives the corneal sensitivity. This method has its limitations in cost, reproducibility, and technical difficulties with the thread’s placement and alignment.
Newer techniques to measure corneal sensitivity objectively, such as a non-contact jet esthesiometer, have been developed but not popularly used.[24] Another non-contact gas esthesiometer was devised by Belmonte et al.[25] to measure mechanical, thermal, and chemical corneal sensitivities.
Stages of NK
Clinically, NK is classified into three stages as described by Mackie.[26]
Stage 1
Superficial punctate keratopathy (SPK) with corneal epithelial irregularities and corneal edema is seen. Stromal scarring and superficial corneal neovascularization may develop in the chronic phase.
Stage 2
Non-resolving persistent epithelial defect (PED) with smooth rolled up margin is seen paracentrally usually in the superior half of the cornea. It may be accompanied with stromal edema and Descemet’s membrane (DM) folds along with anterior chamber (AC) inflammation.
Stage 3
Frank corneal ulcer with associated stromal melting and thinning is seen, which may eventually lead to corneal perforation and requires urgent surgical management.
Management of NK
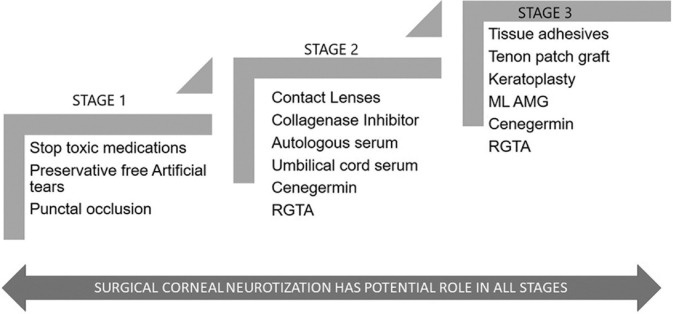
The target of therapy is to promote epithelial healing and prevent any further corneal damage. A stage-wise step-ladder pattern is followed to manage NK [Fig. 1].
Figure 1.
Flowchart depicting the step-ladder algorithm used in the management of neurotrophic keratopathy
All potentially toxic topical medications are stopped. The use of topical steroids and topical non-steroidal anti-inflammatory agents can impair healing and potentiate stromal lysis.[27,28] Treatable causes of NK such as exposure keratitis, limbal stem cell deficiency, lid abnormalities, and dry eye are managed synergistically. The use of preservative-free eye drops is sufficient for Stage 1 NK in most cases. Stage 2 NK is managed surgically with a tarsorrhaphy, conjunctival flap, or amniotic membrane transplantation (AMT).[29,30,31,32] Bandage contact lens (BCL) can be placed to potentiate epithelial healing. Stage 3 NK with thinning can be managed with multilayered AMT or tenons patch graft.[33,34] Chemically induced eyelid ptosis with botulinum toxin has been tried for promoting epithelial healing in Stage 2 NK.[35] Frank corneal perforation needs to be managed surgically with tissue adhesives (TA) and BCL placement in case of smaller perforations (<3 mm) and tenons patch graft or penetrating keratoplasty (PK) for large perforations.[36,37,38] The outcome of keratoplasty in NK is poor, with the possibility of corneal melt or PED in such cases.[38]
Newer pharmacological options for the promotion of corneal healing in cases of NK have been described. The use of autologous serum drops (20%–50%) has shown variable results.[39,40,41,42,43] Jeng et al.[43] demonstrated an efficacy of 50% autologous serum drops in 23 out of 25 eyes with NK with healing in a mean time of 22 days. With the presence of growth factors, cytokines, and neuro-mediators, just like natural tears, it can promote corneal healing and maintain corneal homeostasis.[39] It is contraindicated in patients with blood dyscrasia and anemia.[39,43] Major drawback is accessibility and the cumbersome nature of retrieval and storage. There are isolated reports of secondary corneal infections from contaminated serum.[44] Umbilical cord serum for epithelialization has higher promotional growth factor content.[45,46] However, the lack of ease of access limits its use in general practice.
The use of topical regenerating agent (RGTA; Cacicol20®; OTR3, Paris, France) has been described.[47,48,49,50,51] Complete healing in 73% of cases (8/11) with NK after a mean period of 8.7 weeks was seen in one study.[48] However, Arvola et al.[49] reported failure of RGTA in 67% of NK cases. RGTA contains large polymers that mimic heparan sulfates and promote corneal epithelial healing. A combination of topical substance P (SP) and insulin-like growth factor 1 (IGF-1) showed healing in 73% of cases with NK within 4 weeks in an open study.[52] Topical insulin has also proven to be effective and safe in treating refractory NK.[53] The drops are used at a concentration of 1 unit/mL and are prepared by injecting regular insulin into artificial tears with a polyethylene glycol and propylene glycol base.[53] Nerve growth factor (NGF)-containing eye drops are efficacious in moderate to severe NK.[2,5,7,8,47,54,55,56] Cenegermin is a recombinant form of human NGF and is approved for use as an ophthalmic solution 0.002% by the United States Food and drug administration (US-FDA) for managing NK. Newer medical therapy for NK has been summarized in Table 2.
Table 2.
Newer pharmacological therapy for Neurotrophic keratitis
| Recombinant Human Nerve Growth Factor (rhNGF); |
| Cenegermin (Oxervate) |
| Re Genera Ting Agent (RGTA) polymer eye drops (Cacicol20®, OTR3, Paris, France) |
| Combination of topical substance P (SP) and insulin-like growth factor 1 (IGF-1) |
| Topical Insulin |
| Coenzyme Q10 and antisense oligonucleotide that suppresses connexin 43 expression |
| Semaphorins, Neurotrophins 3 and 4 |
Surgical Corneal Neurotization
The major drawback of options discussed earlier is non-addressal of the underlying cause of corneal anesthesia with a need for life-long medications and a chance of recurrence. This challenge is addressed with SCN or minimally invasive corneal neurotization (MICN), which utilizes a healthy donor nerve graft to re-innervate the diseased cornea.[57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] Surgical neurotization is an established treatment option for brachial plexus injury.[83] The idea of SCN was first discussed in 1972 by Samii.[84] His attempt to connect major occipital nerve to proximal ophthalmic nerve with sural nerve graft had limited success.[84] In 2009, Terzis et al.[57] performed the first successful direct SCN in facial nerve palsy cases along with ipsilateral (I/L) TG nerve pathology and corneal anesthesia. The last decade saw the gradual evolution of this technique. SCN can be performed via a direct or indirect approach. Direct SCN involves the direct transfer of the donor nerve to the corneoscleral limbus to reinnervate the pathological cornea.[57,58,59,60,61,62,63,64] Usual targeted donor nerves are supratrochlear (ST) and supraorbital (SO) nerves. Indirect or minimally invasive corneal neurotization (MICN) utilizes an intermediary nerve graft connecting donor sensory nerve to the diseased cornea. Indirect SCN targets the sural nerve or greater auricular nerve.[58,65,66,67,68,69,70,71] Minimally invasive or endoscopic approach has also been utilized for direct SCN.[60,61,62]
The ideal donor nerve is rich in axons and lies close to the pathological cornea, providing easy access with minimum surgical morbidity during dissection. SO and ST are terminal branches of the frontal nerve, an extension of the ophthalmic division of the TG nerve. Domeshek et al.,[72] in a gross and histo-morphometric anatomical analysis, described SO nerve at the orbital rim to be the most robust axonal source containing greater than double the number of ST fibers at the same location, and more than three times SO or ST fibers 6 cm distally. Thus, the proximal SO nerve is the most favorable donor both for direct transfer and interposed graft. A thorough preoperative sensory nerve examination to check the status and availability of a healthy donor nerve is performed before planning SCN.
While most indications are common between two approaches, there are few exceptions, such as bilateral impairment of the TG nerve’s ophthalmic division where a direct approach is not possible. For this reason, very few studies have compared the outcome of the two approaches, and those that are available are not randomized trials.[73] To date, there is no consensus regarding the superiority of one approach, and the choice mainly depends on surgeon choice, patient preference, availability of healthy donor nerve, and site of pathology.
Direct Approach
Contralateral supraorbital and supratrochlear nerve transfer[57,74]
A bi-coronal incision is made 3 cm behind the hairline connecting one auricular helix to another. Dissection is performed until the subgaleal plane’s supraorbital rims, and the SO or ST nerve is traced. Around 6–12 cm length is needed in case of contralateral (C/L) transfer. Through an upper eyelid incision and a blephorotomy, the nerves are directly transferred to the affected eye’s superior fornix. The donor nerve branches are secured to the corneoscleral limbus of the affected eye with 10-0 monofilament nylon sutures followed by a tarsorrhaphy.
Terzis et al.[57] in 2009 performed the first successful direct SCN with open direct transfer of C/L SO and ST nerve to the pathological cornea. At a mean follow-up of 16.3 months, improvement in corneal sensations and ocular surface occurred in all six eyes while visual acuity improved significantly in half of them. The major drawback was the invasive nature with loss of sensations over the C/L forehead postoperatively along with the disfiguring large bicoronal incision and associated surgical morbidity with the formation of neuroma and subgaleal hematoma.[57] Allevi et al.[74] in 2014 described a case of a woman with unilateral TG and facial nerve palsy following surgery for vestibular schwannoma. They performed direct SCN using C/L SO and ST nerve to the diseased side followed by cornea transplantation 6 months later. The patient gained visual acuity and continued to maintain a healthy ocular surface post keratoplasty.
Ipsilateral supraorbital nerve transfer[75,76,77]
In cases where only the I/L long ciliary nerve is damaged, I/L SO nerve transfer can be performed with ease of access and minimal dissection. It was first described by Jacinto et al.[75] in 2016 in a patient with postocular surgery NK where it led to regeneration of corneal sensations 6 months later. The technique involves creating a hemi-coronal incision behind the hairline, incising the periosteum 2 cm above the superior orbital rim, and dissecting the SO nerve’s deep branches which are channeled through a supratarsal incision along the upper lid crease and then through blepharotomy into the superior conjunctival fornix and to the limbus.[76] While this approach appears more straightforward due to accessibility and ease of placement of nerves, it has very limited application with no role in conditions such as herpetic keratopathy where the TG nerve’s retrograde involvement may be seen.[78]
Ipsilateral infraorbital nerve transfer[79]
Menicacci et al.[79] in June 2016 at Congress of the Italian Society of Stem Cells and Ocular Surface described direct transfer of I/L infraorbital (IO) nerve to the anesthetic cornea in a case with I/L TG ophthalmic division damage secondary to surgery for removal of cranial nerve VIII neurinoma. Only superior fibers are transected to avoid cheek and lip paresthesia postoperatively. Recovery of corneal sensations and improvement in the ocular surface along with an increase in number and caliber of nerve fibers was noted at 1-year follow-up.
Endoscopic ipsilateral or contralateral supraorbital nerve transfer[62]
It minimizes surgical trauma and decreases incision size and postoperative complication after direct nerve transfer. Both I/L and C/L transfer can be performed endoscopically. I/L endoscopic SO transfer was first performed by Leyngold et al.[62] in cadaveric eyes. The same authors performed endoscopic C/L SO transfer by using the combined transpalpebral and endoscopic forehead approach to dissect the nerve via C/L upper eyelid skin-crease incision to expose the superior orbital rim.[62] SO nerve is then dissected in the subgaleal plane. A vertical incision is then made 5 mm behind the hairline. Dissection is carried out in the subperiosteal plane up to the isolated SO nerve. The periosteum is opened to complete the dissection endoscopically. Isolated nerve branches are tunneled to the I/L upper eyelid incision in the subgaleal plane and directly transferred to the superior fornix through a blepharotomy and then to the limbus. The major advantage is reduced surgical trauma and incision size.
Indirect Approach
The indirect approach can be used in the case of bilateral corneal anesthesia without extensive local dissection. Intermediary nerve graft connects donor sensory nerve to the diseased cornea. Coaptation of intermediary nerve to donor sensory nerve may be end-to-end or end-to-side. After coaptation, the extent of axons’ growth into the cornea depends on the distance from the affected eye, time since surgery, and patient’s age.
Sural nerve graft to ipsilateral or contralateral supraorbital and supratrochlear nerve[58,69,76]
The sural nerve innervates the calf and foot and is the most easily accessible sensory nerve for grafting. Dissection of 10–15-cm length of sural nerve graft is followed by the creation of subbrow incision to dissect the C/L or I/L SO or ST nerves. Fibrin glue and 10-0 monofilament nylon sutures are used to perform end-to-end or end-to-side coaptation of the graft with the donor nerves. End-to-end coaptation is preferred for most cases.[80] C/L cases would need additional tunneling across the nasal bridge. Like in the direct approach, nerve branches are further tunneled through the upper lid incision and blepharotomy into the superior conjunctival fornix and onwards to the limbus. This is the most employed technique of SCN to date.[81]
Elbaz et al.[58] in 2014 first used sural nerve graft to connect C/L STN to pathological cornea in five eyes with unilateral TG and facial nerve palsy secondary to vestibular schwannoma. Significant improvement in corneal sensations was reported in all cases 6 months post-operatively.[58]
Catapano et al.[66] in 2019 described indirect SCN with sural nerve graft in 19 young eyes with long-standing NK. Significant recovery of corneal sensations with improved ocular surface and stable BCVA was noted in all cases. Among these, four eyes underwent keratoplasty 24–33 months after SCN [two deep anterior lamellar keratoplasty (DALK), one penetrating keratoplasty (PK), and one PK with cataract surgery]. Complete re-epithelialization occurred in these eyes by 4–8 weeks after keratoplasty.[66]
Greater auricular nerve graft to ipsilateral supratrochlear nerve[65]
The greater auricular nerve (GAN) is a sensory nerve and arises from the cervical nerve plexus. Limited length of the nerve limits its use to I/L transfer only. Benkhatar et al.[65] performed end-to-end anastomosis of the GAN graft to I/L ST nerve in a case of unilateral NK.
Sural nerve graft to ipsilateral greater auricular nerve[70]
Jowett et al.[70] performed endoscopic dissection of GAN through infra-auricular incision followed by coaptation of sural nerve graft to GAN. Coapted nerves are then directed to the pathological eye.
Acellular nerve allograft for minimally invasive corneal neurotization[82]
Most early approaches were associated with disfiguring large scars, alopecia, and donor site morbidity. To overcome these limitations, Leyngold et al.[82] in 2019 described minimally invasive surgical technique by using acellular nerve allograft for SCN in patients with NK. After dissecting the donor nerve locally, a 70 mm × 1–3 mm processed acellular nerve allograft (Avance Nerve Graft) is coapted to it. A nerve connector or AMT is used to protect end-to-end coaptation. No such material is needed for end-to-side coaptation. Coapted bundle is then directed to the affected eye. This is minimally invasive with reduced donor site morbidity.
Outcome Measures
Fogagnolo et al.[73] in 2020 compared safety and efficacy of the direct and indirect approaches in a non-randomized multicentric interventional study. While comparing the outcomes of 16 eyes that underwent Direct SCN versus 10 eyes in which indirect SCN was performed, they found that NK healed in all patients irrespective of the technique after a mean period of 3.9 months. They further went on to conclude that direct and indirect SCN showed comparable outcomes one year postoperatively.[73]
Indirect SCN offers the flexibility of choice of donor nerve graft and avoids large coronal incision, thereby reducing surgical morbidity. With indirect SCN, axon-rich proximal donor sensory nerve can also be used for corneal neurotization.[58] Indirect SCN may be associated with neurinoma formation at the neurorrhaphy.[75]
Outcome measures used include the recovery of corneal sensations, corneal nerve recovery as seen on in vivo confocal microscopy (IVCM), and histopathology. Table 3 summarizes the outcome as reported in the reviewed literature.
Table 3.
Review of the literature on surgical corneal neurotization
| Author | Year | Number of eyes | Approach | Donor nerve | Follow-up | Recovery of Corneal sensations | Histo pathology | Confocal microscopy | Outcome | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Terzis et al.[57] | 2009 | 6 Unilateral facial and TG palsy secondary to intracranial pathology |
Open Direct | C/L SO ST | 16.3 months | Improved CBA |
NA | NA | VA improved in 3, Ocular surface health improved in all | Accessible donor nerve, Avoids neurorrhaphy | Large bicoronal incision |
| Allevi et al.[74] | 2014 | 1 Unilateral TG and facial palsy secondary to vestibular schwannoma |
Open direct | C/L SO ST | 6 months | Improved CBA |
NA | NA | PKP done 6 months after SCN | Accessible donor nerve, Avoids neurorrhaphy | Large bicoronal incision |
| Elbaz et al.[58] | 2014 | 5 Intracranial pathologies posterior fossa tumor, basal skull fracture |
Indirect | Sural nerve end to end with C/L ST | 6 months | Improved CBA |
NA | NA | -- | Reduced surgical morbidity | Stump neuronima at Neurorrhaphy site |
| Jacinto et al.[75] | 2016 | 1 Local injury to the long ciliary nerve |
Open Direct | I/L SO | 8 months | Improved CW |
NA | NA | Significantly improved VA and ocular surface, maintained at 2 years follow-up | Accessible donor nerve, Avoids neurorrhaphy Smaller hemicoronal scalp incision |
Limited application only in cases where I/L frontal nerve is intact |
| Menicacci et al.[79] | 2016 | 1 I/L TG nerve pathology |
Direct | I/L IO | 12 months | Improved | NA | Increase in number and caliber of nerve fibres | NA | - | Limited application |
| Leyngold et al.[62] | 2018-2020 | 5 | Endoscopic Direct | C/L SO | 2-4 months | Improved CBA |
NA | NA | Minimally invasive | Cost, technically challenging |
|
| Catapeno et al.[66] | 2018 | 19 | Indirect | Sural nerve end to end with C/L ST Nerve |
24 months | Improved CBA |
Character dot and linear axon profile seen | NA | 2 eyes post SCN DALK, 1 eye post SCN PK, 1 eye post SCN PK Triple, Re-epithelialization by 4-8 weeks post keratoplasty |
Reduced surgical morbidity | One PK case developed endothelial rejection with keratitis and PED at 12 months follow-up |
| Benkhatar et al.[65] | 2018 | 1 | Indirect | GAN to I/L ST end to end | 12 months | Improved CBA | NA | Regrowth of nerve fibres | -- | Reduced surgical morbidity | Crowding of single surgical field |
| Jowett et al.[70] | 2019 | 2 | Indirect | Sural Nerve to I/L GAN end to end | 9 months | Improved CBA |
NA | Regrowth of nerve fibres | Improved visual acuity and pachymetry | - | - |
| Leyngold et al.[82] | 2020 | 7 multicentric | Indirect | Acellular nerve graft | 6 months | All improved peripheral corneal sensations 5 improved central corneal sensations | NA | Increased nerve density | Improved corneal health | Reduced donor site morbidity | Cost and access |
| Fogagnolo et al.[73] | 2020 | 26 | 16 Direct SCN 10 Indirect SCN |
Direct (C/L SO and ST) Indirect (Sural nerve graft) |
12 months | Improved in 80% cases of DCN and 83.3% of ICN group, no significant difference | NA | Comparable regrowth of nerve fibres but not up to normal level | - | - | Not randomised (varying Indications) Small sample size |
| Current study | 2021 | 7 | Indirect | Sural nerve graft to C/L SO nerve | 3-18 months | Improved in all cases (Cotton wisp method) | NA | Comparable regrowth of nerve fibers but not up to normal level | VA improved in 6 cases; Ocular surface health improved in all | Accessible donor nerve | Donor site keloid formation in one case |
TG Trigeminal, C/L contralateral, I/L Ipsilateral, SO Supraorbital, ST Supratrochlear, GAN Greater Auricular Nerve, CBA Cochet Bonnet aesthesiometer, VA Visual Acuity, PKP Penetrating keratoplasty, CW Cotton wisp, DALK Deep anterior lamellar keratoplasty, SCN Surgical corneal neurotization, DCN Direct corneal neurotization, ICN Indirect Corneal neurotization, NA Not applicable
Recovery of corneal sensations
CBA is the most employed objective tool to assess corneal sensations perioperatively.[57,58,69] Some authors used the cotton wisp method.[61,62,74] Park et al.[81] in 2020 published a systematic review of the clinical outcomes of corneal neurotization where they included all published articles and meeting abstracts between December 2008 and February 2019. They identified 54 eyes that underwent SCN. Baseline corneal sensitivity of 2.18 ± 5.37 mm was reported with CBA. Post SCN, it significantly improved to 40.10 ± 18.95 mm with a mean filament length change of 38.00 mm. Return of corneal sensitivity is more complete in younger and pediatric age groups. All included studies in this review showed a significant return of corneal sensations in all cases at an average of 11.84 ± 13.8 months.[81]
Fogagnolo et al.[73] compared the outcomes of direct and indirect SCN in terms of recovery of corneal sensitivity and reported comparable results with 80% of the direct SCN group and 83.3% of the indirect SCN group showing corneal sensation recovery at 1 year. They stated that direct SCN might guarantee earlier corneal sensitivity recovery than indirect SCN, which is an expected outcome owing to the distance of the target site from the neurorrhaphy.
In vivo confocal microscopy
IVCM is non-invasive and provides high-resolution images of the subbasal nerve plexus. Only five studies to date have described the outcome of SCN in terms of reinnervation as assessed with IVCM. Fung et al.[67] were the first to describe its use for this purpose. They performed two cases of SCN, with indirect sural nerve graft transfer to SO nerve approach in one and IO nerve in the other. They documented the growth of corneal nerves on IVCM 6 months later. Following this, Ting et al.[63] published two cases showing corneal nerve regeneration postoperatively by using IVCM. Two other studies used nerve regeneration on IVCM as an outcome measure, and all four cases revealed corneal nerve regeneration 6 months post-SCN.[75,70] Fogagnolo et al.[73] while evaluating the outcome of 26 eyes (16 Direct and 10 indirect SCN) noted that in four eyes, corneal subbasal nerve plexus (SNP) was detected pre-operatively as well, while in the remaining 22, it developed as early as 3 months postoperatively. In all 26 cases, it reached near normal status at the 1-year post-operative visit. They reported a comparable outcome with both approaches.
Histopathology
Ting et al.[63] performed SCN for severe unilateral NK secondary to cerebellopontine angle meningioma. In the postoperative period, corneal sensations returned centrally but remained absent infero-temporally. However, at 2 years post-SCN, the authors reported a complete loss of sensation again followed by recurrence of NK and nil vision in the eye, which then underwent an evisceration. Interestingly, on histopathological assessment of eviscerated corneoscleral disc, after fixing it in standard 10% buffered formalin for 24 h, the transplanted nerve was seen with corneal stroma. Hematoxylin and eosin (H&E) stain and special immunohistochemical stains for neurofilaments confirmed the presence of a viable grafted nerve near the limbus. Fogagnolo et al.[73] also performed a histopathological examination of a cornea where optical keratoplasty was performed 18 months post-SCN, and the presence of the transplanted nerve in the corneal button was confirmed.
Re-innervation Physiology
Regeneration of nerves post neurotization is due to neurotrophic factors rather than direct nerve sprouting.[63,85] Release of these factors is secondary to Wallerian degeneration as demonstrated in a rat model study on lower limb nerves where an intact donor nerve graft laid parallel to the denervated peripheral nerve led to gradual nerve regeneration.[85] New nerves may have abnormal branching and accessory thin nerve fibers but are functionally capable of corneal recovery and healthy blink response.[80]
Our Experience
We present an interim report of seven cases where SCN was performed for its various indications. Table 4 summarizes the patient characteristics and outcomes. Seven cases with severe unilateral NK were included. Written informed consent was obtained. Preoperative evaluation included detailed slit-lamp biomicroscopy examination, BCVA, and dilated fundus examination where possible. Central corneal sensation was measured using the cotton wisp method in both eyes at baseline as well as at each follow-up. IVCM (Nidek Confoscan 4; Nidek Technologies, Padova, Italy) was performed at baseline and at follow-up. At each follow-up, the improvement in the ocular surface was assessed based on symptomatic improvement and clinical examination including blink rate, improvement in corneal luster, tear dynamics, Schirmer’s values, surface staining, and corneal sensations.
Table 4.
Demography, clinical characteristics, and interim report of patients who underwent indirect surgical corneal neurotization
| Patient number | Age at surgery/Sex | Laterality | Etiology | Mackie stage | BCVA Pre operative | Corneal findings | Facial Palsy (Y/N) | Pre operative Corneal sensation (cotton wisp) | Surgery | Last follow-up (months) | Post-operative BCVA at last follow-up | Corneal Sensations at last follow-up | Regrowth Of nerve fibers IVCM at last follow-up (Y/N) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5y/M | RE | NK | 2 | 20/400 | Corneal scar | N | Absent | Indirect SCN | 18 months | 20/320 | Improved, Lesser than normal eye | Y |
| 2 | 7y/F | LE | NK post neurological illness | 2 | 20/125 | Corneal scar | N | Absent | Indirect SCN | 18 months | 20/50 | Improved | NA |
| 3 | 1.5y/M | RE | NK Post traumatic | 2 | 20/320 | Exposure keratopathy | Y | Absent | Indirect SCN | 3 months | 20/250 | Improved | NA |
| 4 | 9m/F | LE | NK post herpetic | 2 | CFCF | Neurotrophic keratopathy | N | Absent | Indirect SCN | 18 months | CF 1m | Improved | NA |
| 5 | 28y/F | RE | NK post herpetic | 2 | 20/50 | Neurotrophic keratopathy | N | Absent | Indirect SCN | 18 months | 20/80 | Improved | Y |
| 6 | 20y/F | RE | NK post herpetic | 2 | 20/50 | Neurotrophic keratopathy | N | Absent | Indirect SCN | 18 months | 20/25 | Improved | Y |
| 7 | 38y/F | RE | NK post herpetic | 2 | CFCF | Punched out corneal ulcer with TA BCL | N | Absent | Indirect SCN | 13 months | CF 1 m | Improved | NA |
RE Right eye, LE Left eye, BCVA Best corrected visual acuity, NK Neurotrophic keratopathy, SCN Surgical corneal neurotization, CFCF Counting fingers close to face, CF 1 m Counting fingers at 1 m, IVCM In vivo confocal microscopy, TA BCL Tissue adhesive bandage contact lens
Surgical Technique
After a thorough preoperative assessment verifying sensory function in the donor nerve, the patient is placed in a supine position and the left leg is prepared for sural nerve graft harvest. Brow incision is made on the C/L side and the SO nerve is exposed. Distance between the dissected SO nerve and the opposite corneal limbus is measured to estimate the length of nerve graft required. An additional 2 cm is added as a safety margin. A subcutaneous tunnel is made to the opposite upper eyelid. The sural nerve is identified in the left leg via an incision posterior to the lateral malleolus. The appropriate length of the nerve is harvested based on the previous measurement. Sural nerve graft is then transferred for coaptation. One end of the nerve graft is coapted to the dissected supraorbital nerve (end-to-end with 10-0 monofilament nylon suture). The other end of the nerve graft is delivered to the recipient corneal limbus through a blepharotomy incision. Nerve fascicles are exposed after incising the epineurium. Fibers are tunneled across the limbus with free ends toward the cornea. After conjunctivotomy and tenotomy, fibers are delivered to the subtenon space where they are fixed using 10-0 monofilament nylon suture.
Case 1
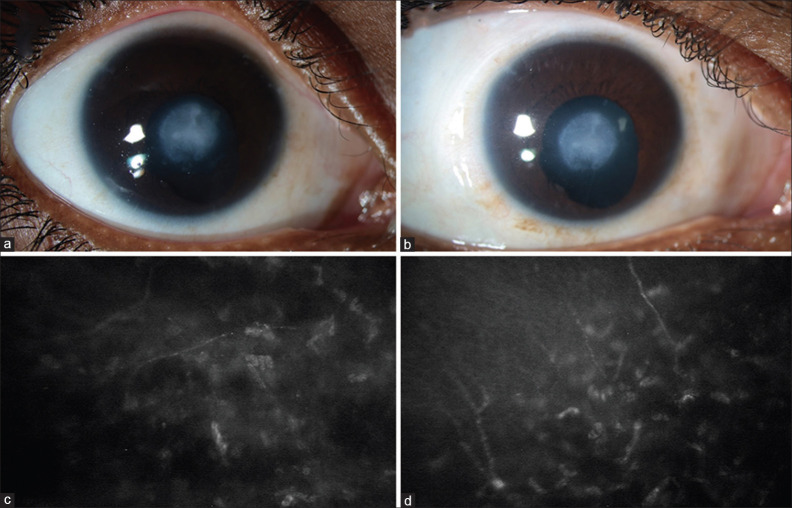
A 5-year-old boy presented with a history of pain and redness in the right eye (RE) following injury with stone 15 days prior. The child was fixing and following light with the RE at presentation. Central corneal ulcer with rolled edges and stromal edema was noted. Clinical diagnosis of NK in the RE was made and managed medically. Best-corrected visual acuity (BCVA) improved to 20/400 (1.3 logMAR). Ten years post-trauma, absent corneal sensations persisted even though epithelial defect healed with corneal scarring [Fig. 2a]. SCN was performed for RE at this time. At the 1-week postoperative visit, BCVA in the RE was maintained at 20/400 (1.3 logMAR), and corneal sensations were still absent. The patient reported absent sensations on a small part of the C/L forehead in the ST nerve distribution. At 1 month follow-up, corneal sensations were still lacking, but BCVA improved to 20/320 (1.2 logMAR). The ocular surface was well maintained with no incidence of epithelial breakdown or persistent epithelial defects, or NK. BCVA was maintained till the last follow-up at 18 months with a healthy ocular surface and improved corneal sensations [Fig. 2b]. IVCM also demonstrated regrowth of nerve fibers at last follow-up [Fig. 2c and d].
Figure 2.
Clinical photograph of the eye of a patient with post-traumatic neurotrophic keratopathy preoperatively (a) and at 18 months (b) after indirect surgical corneal neurotization and in vivo confocal microscopy images of the same eye preoperatively (c) and 18 months (d) after neurotization
Case 2
A 7-year-old girl presented with a history of unknown neurological illness, following which she was in a comatose state for 3 months. She presented with pain and redness in the left eye (LE) for 8 days. Clinically, she was diagnosed with NK in the LE, managed medically, and BCVA gradually improved from counting fingers 1 m (CF1m) to 20/125 (0.79 logMAR) with medical management. She was noted to have bilateral optic atrophy owing to the underlying neurological pathology. One year later, as absent corneal sensations persisted, SCN was performed for the LE. At 1 week, BCVA was noted to be 20/200 (1 logMAR), which improved further to 20/50 with a healthier ocular surface at 1 month. Ocular surface stabilized further by 18 months with improved corneal sensations.
Case 3
A 1.5-year-old boy was brought by the parents with a history of injury to the right side of the head 10 days prior, following which he developed pain and redness in the RE. The child was noted to have right-sided facial nerve palsy with 2-mm lagophthalmos RE. He was diagnosed with exposure keratopathy with microbial keratitis in the RE after clinical examination and was managed medically. The infiltrates had completely resolved with persisting corneal scar and absent corneal sensations in all quadrants on follow-up. Nine years after the initial trauma, SCN was performed for the RE. Postoperatively at 1 week, BCVA was maintained at 20/320 (1.2 logMAR), and absent corneal sensations persisted. At 1 month, corneal surface further improved with tarsorrhaphy in place and BCVA improved to 20/250. Corneal sensations recovered partially at 3 months follow-up while BCVA was maintained at 20/250.
Case 4
A 9-month-old baby girl was brought with a history of pain, photophobia, and redness in the LE for 1 week. There was no history of predisposing trauma or any neurological illness or surgery. On clinical examination, corneal infiltrates with microbial keratitis were noted, and the condition was medically managed on the lines of herpetic NK. Subsequently, 7.5 years after the initial presentation, SCN was performed for absent corneal sensations in the LE. BCVA in LE prior to surgery was only counting fingers close to the face (CFCF) due to dense corneal scar and amblyopia. Ocular surface improved considerably at 1-month postoperative visit with BCVA of 20/1200 and improved corneal sensations. At 18 months postoperative follow-up, her BCVA improved to CF 1 m and ocular surface as well as corneal sensations improved further.
Case 5
A 28-year-old lady presented with a history of pain, redness, and whitish opacification in RE for the last 1 month. Clinically, a diagnosis of RE Herpes zoster ophthalmicus (HZO) was made, and the condition was managed medically. Corneal sensations in the affected eye were noted to be significantly reduced. One year later, SCN was performed for RE. Baseline BCVA before surgery was 20/50, which improved to 20/30 at 1 month follow-up with a significant improvement in the ocular surface. Postoperative 18 months follow-up revealed further improvement in ocular surface and corneal sensations while BCVA was 20/80 at this time due to corneal scar. IVCM revealed regenerated corneal nerve fibers at 18 months.
Case 6
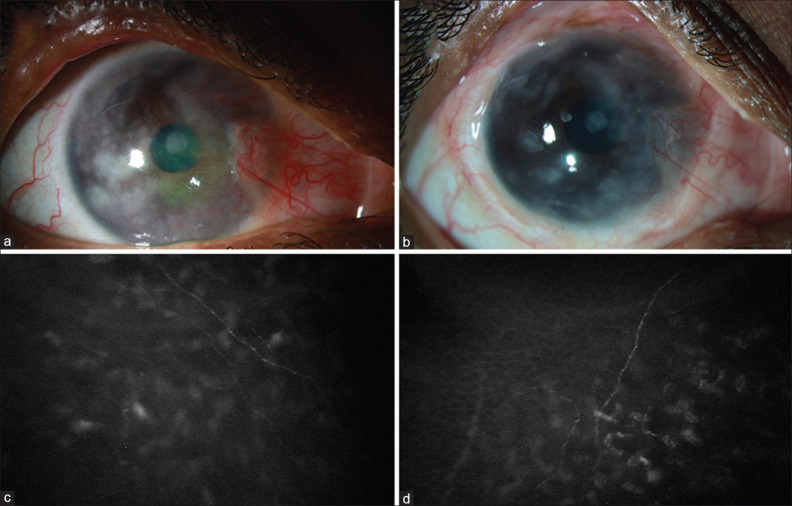
A 20-year-old lady presented to our clinic with pain and redness in RE for the last 3 months following the occurrence of lesions on the right side of the face. On clinical examination, a diagnosis of RE HZO was made, and medical management was instituted. One year after primary pathology, SCN was performed for RE. BCVA in RE improved from 20/50 to 20/30, and corneal sensations also recovered partially at 3 months follow-up. By 18 months, BCVA improved further to 20/25 with significantly improved ocular surface and corneal sensations. IVCM also revealed regrowth of corneal nerve fibers [Fig. 3].
Figure 3.
Clinical photograph of the eye of a patient with post-herpetic neurotrophic keratopathy preoperatively (a) and at 18 months (b) after indirect surgical corneal neurotization and in vivo confocal microscopy images of the same eye preoperatively (c) and 18 months (d) after neurotization
Case 7
A 38-year-old lady presented to our clinic with recurrent episodes of pain, redness in the RE for the past 13 years. She had Varicella infection during childhood along with ocular involvement in the RE. After clinical examination, a diagnosis of NK with significant thinning was made for which she underwent TA and BCL placement. Local examination revealed absent sensations along the supratrochlear and supraorbital nerve distribution with intact infra-trochlear nerve sensation. BCVA in RE was CFCF. After 11 months, she underwent SCN in the RE. Postoperatively at 13 months follow-up, her ocular surface had stabilized, and the density of corneal scarring reduced with signs of reinnervation along the supra-trochlear nerve distribution. Her vision improved to CF at 1 meter. The patient emphasized that she can now appreciate the instillation of eyedrops in the RE.
Discussion
SCN is an evolving and promising technique with a favorable outcome irrespective of approach. It can offer a permanent cure to NK. In this interim report of the seven cases where we performed indirect SCN, the mean age of patients at clinical presentation of NK was 14.32 ± 13.46 years (range: 9 months–38 years). Of these, two were males and five were females. The mean age of these patients when SCN was performed was 14.87 ± 8.07 years. The cause of NK in four patients was post-herpetic, post-traumatic in two cases, and it developed post neurological illness in one case. The mean follow-up was 15.14 ± 5.24 months (range: 3–18 months). All cases were noted to have absent corneal sensations at baseline by using the cotton wisp method. Visual outcome post SCN was favorable, while corneal sensations were also seen to be gradually recovering in all cases. In two cases, patients reported reduced tactile sensations on the C/L forehead. Corneal surface improved significantly post SCN in all cases. The last follow-up ranged from 3–18 months with five cases followed up till 18 months postoperatively, while one case was followed up till 3 months and another till 13 months. The outcome at further follow-up remains to be studied. Most of these cases shall be planned for future keratoplasty around 24 months postoperatively once the ocular surface has further stabilized.
Our results are comparable with previous studies in terms of recovery of corneal sensations. Most studies discussed above report an improvement in the sensations only after 3–6 months of SCN. As nerve growth usually begins a month after coaptation and progresses at a rate of 1 mm/day in a healthy individual, we can expect nerve growth to reach the cornea only after 3 months, following which the fascicle free nerve endings go on to innervate the cornea, which may take a few additional weeks. This is to say that in the best-case scenario, we expect corneal sensations to appear only 5–6 months after the procedure, and regrowth of corneal nerve fibers on IVCM may take as long as 6 months–1 year. We performed IVCM in three of our cases, which revealed a favorable outcome with newly sprouted nerve fibers seen in all at 18 months follow-up. These nerve fibers are expectedly less in number as compared to a normal eye. Thus, the course of improvement in our cases needs to be ascertained with further follow-up.
Future Directions
Surgical corneal neurotization in cases of NK can potentially create a healthy bed for any future corneal transplants. It remains to be seen how postoperative adjuvant therapy in the form of nerve growth factor can potentiate the outcome after a successful SCN. The long-term outcome of SCN and the shelf life of its efficacy need to be ascertained with planned long-term prospective studies.
Conclusion
Neurotrophic keratopathy is a sight-threatening corneal pathology associated with absent or reduced corneal sensations. Management follows a step-ladder algorithm. Medical management corrects the symptomatology and not the underlying cause. Surgical corneal neurotization may correct the underlying pathology. SCN can be performed using the direct or indirect approach with favorable and comparable outcomes. Reduced surgical morbidity in the indirect approach using sural nerve graft makes it the most employed technique. Post neurotization, recovery usually takes at least 3–6 months to recover corneal sensations, while nerve regeneration on confocal microscopy can take as long as 6 months–1 year.
Author contributions
Conception, design, and manuscript preparation: Rathi, Bothra, Yellinedi, Ramappa.
Manuscript correction, data interpretation, and critical review: Rathi, Ramappa, Bothra, Priyadarshini, Fernandes, Murthy, Yellinedi, Fernandes.
Data collection: Achanta, Rathi, Ramappa.
Obtained funding: Hyderabad eye institute, Hyderabad, India.
Overall responsibility: Rathi, Ramappa.
Financial support and sponsorship
Hyderabad Eye Research Foundation and Hyderabad Eye Institute, Hyderabad, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Eguchi H, Hiura A, Nakagawa H, Kusaka S, Shimomura Y. Corneal nerve fiber structure, its role in corneal function, and its changes in corneal diseases. Biomed Res Int. 2017;2017:3242649. doi: 10.1155/2017/3242649. doi:10.1155/2017/3242649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dua HS, Said DG, Messmer EM, Rolando M, Benitez-Del-Castillo JM, Hossain PN, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–31. doi: 10.1016/j.preteyeres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Versura P, Giannaccare G, Pellegrini M, Sebastiani S, Campos EC. Neurotrophic keratitis:Current challenges and future prospects. Eye Brain. 2018;10:37–45. doi: 10.2147/EB.S117261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–9. doi: 10.2147/OPTH.S45921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107:1347–51. doi: 10.1016/s0161-6420(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 6.Hsu HY, Modi D. Etiologies, quantitative hypoesthesia, and clinical outcomes of neurotrophic keratopathy. Eye Contact Lens. 2015;41:314–7. doi: 10.1097/ICL.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 7.Sacchetti M, Bruscolini A, Lambiase A. Cenegermin for the treatment of neurotrophic keratitis. Drugs Today (Barc) 2017;53:585–95. doi: 10.1358/dot.2017.53.11.2722395. [DOI] [PubMed] [Google Scholar]
- 8.Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. 2006;25:352–5. doi: 10.1097/01.ico.0000176609.42838.df. [DOI] [PubMed] [Google Scholar]
- 9.Grey F, Carley F, Biswas S, Tromans C. Scleral contact lens management of bilateral exposure and neurotrophic keratopathy. Cont Lens Anterior Eye. 2012;35:288–91. doi: 10.1016/j.clae.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–25. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–85. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura M, Nishida T, Ofuji K, Reid TW, Mannis MJ, Murphy CJ. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp Eye Res. 1997;65:321–9. doi: 10.1006/exer.1997.0345. [DOI] [PubMed] [Google Scholar]
- 13.Clarke MP, Sullivan TJ, Kobayashi J, Rootman DS, Cherry PM. Familial congenital corneal anaesthesia. Aust N Z J Ophthalmol. 1992;20:207–10. doi: 10.1111/j.1442-9071.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 14.Cruysberg JR, Draaijer RW, Pinckers A, Brunner HG. Congenital corneal anesthesia in children with the VACTERL association. Am J Ophthalmol. 1998;125:96–8. doi: 10.1016/s0002-9394(99)80241-4. [DOI] [PubMed] [Google Scholar]
- 15.Villanueva O, Atkinson DS, Lambert SR. Trigeminal nerve hypoplasia and aplasia in children with Goldenhar syndrome and corneal hypoesthesia. J AAPOS. 2005;9:202–4. doi: 10.1016/j.jaapos.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Lambley RG, Pereyra-Muñoz N, Parulekar M, Mireskandari K, Ali A. Structural and functional outcomes of anaesthetic cornea in children. Br J Ophthalmol. 2015;99:418–24. doi: 10.1136/bjophthalmol-2014-305719. [DOI] [PubMed] [Google Scholar]
- 17.Semeraro F, Forbice E, Romano V, Angi M, Romano MR, Filippelli ME, et al. Neurotrophic keratitis. Ophthalmologica. 2014;231:191–7. doi: 10.1159/000354380. [DOI] [PubMed] [Google Scholar]
- 18.Lambiase A, Sacchetti M, Mastropasqua A, Bonini S. Corneal changes in neurosurgically induced neurotrophic keratitis. JAMA Ophthalmol. 2013;131:1547–53. doi: 10.1001/jamaophthalmol.2013.5064. [DOI] [PubMed] [Google Scholar]
- 19.Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, et al. Corneal sensation and sub-basal nerve alterations in patients with herpes simplex keratitis:An in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–36. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabbarvand M, Hashemian H, Khodaparast M, Rafatnejad A, Beheshtnejad A, Salami A. Do unilateral herpetic stromal keratitis and neurotrophic ulcers cause bilateral dry eye? Cornea. 2015;34:768–72. doi: 10.1097/ICO.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 21.Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WMl. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002;43:3660–4. [PubMed] [Google Scholar]
- 22.Chao C, Stapleton F, Badarudin E, Golebiowski B. Ocular surface sensitivity repeatability with Cochet-Bonnet esthesiometer. Optom Vis Sci. 2015;92:183–9. doi: 10.1097/OPX.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 23.Lum E, Murphy PJ. Effects of ambient humidity on the Cochet-Bonnet aesthesiometer. Eye (Lond) 2018;32:1644–51. doi: 10.1038/s41433-018-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vega JA, Simpson TL, Fonn D. A noncontact pneumatic esthesiometer for measurement of ocular sensitivity:A preliminary report. Cornea. 1999;18:675–81. doi: 10.1097/00003226-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999;40:513–9. [PubMed] [Google Scholar]
- 26.Mackie IA. Neuroparalytic keratitis. In: Fraunfelder F, Roy FH, Meyer SM, editors. Current Ocular Therapy. Philadelphia, PA, USA: WB Saunders; 1995. [Google Scholar]
- 27.Petroutsos G, Guimaraes R, Giraud JP, Pouliquen Y. Corticosteroids and corneal epithelial wound healing. Br J Ophthalmol. 1982;66:705–8. doi: 10.1136/bjo.66.11.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hersh PS, Rice BA, Baer JC, Wells PA, Lynch SE, McGuigan LJ. Topical nonsteroidal agents and corneal wound healing. Arch Ophthalmol. 1990;108:577–83. doi: 10.1001/archopht.1990.01070060125062. [DOI] [PubMed] [Google Scholar]
- 29.Cosar CB, Cohen EJ, Rapuano CJ, Maus M, Penne RP, Flanagan JC, et al. Tarsorrhaphy clinical experience from a cornea practice. Cornea. 2001;20:789–91. doi: 10.1097/00003226-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Gundersen T. Conjunctival flaps in the treatment of corneal disease with reference to a new technique of application. AMA Arch Ophthalmol. 1958;60:880–8. doi: 10.1001/archopht.1958.00940080900008. [DOI] [PubMed] [Google Scholar]
- 31.Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84:826–33. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB, et al. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers:A randomized, controlled clinical trial. Cornea. 2005;24:654–60. doi: 10.1097/01.ico.0000153102.19776.80. [DOI] [PubMed] [Google Scholar]
- 33.Prabhasawat P, Tesavibul N, Komolsuradej W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br J Ophthalmol. 2001;85:1455–63. doi: 10.1136/bjo.85.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma N, Singhal D, Maharana PK, Vajpayee RB. Tuck-in tenon patch graft in corneal perforation. Cornea. 2019;38:951–4. doi: 10.1097/ICO.0000000000001955. [DOI] [PubMed] [Google Scholar]
- 35.Kirkness CM, Adams GG, Dilly PN, Lee JP. Botulinum toxin A-induced protective ptosis in corneal disease. Ophthalmology. 1988;95:473–80. doi: 10.1016/s0161-6420(88)33163-5. [DOI] [PubMed] [Google Scholar]
- 36.Fogle JA, Kenyon KR, Foster CS. Tissue adhesive arrests stromal melting in the human cornea. Am J Ophthalmol. 1980;89:795–802. doi: 10.1016/0002-9394(80)90168-3. [DOI] [PubMed] [Google Scholar]
- 37.Jhanji V, Young AL, Mehta JS, Sharma N, Agarwal T, Vajpayee RB. Management of corneal perforation. Surv Ophthalmol. 2011;56:522–38. doi: 10.1016/j.survophthal.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Margo JA, Jeng BH. Corneal transplantation in the setting of neurotrophic keratopathy—risks and considerations. Curr Ophthalmol Rep. 2017;5:14–22. [Google Scholar]
- 39.Bernabei F, Roda M, Buzzi M, Pellegrini M, Giannaccare G, Versura P. Blood-based treatments for severe dry eye disease:The need of a consensus. J Clin Med. 2019;8:1478. doi: 10.3390/jcm8091478. doi:10.3390/jcm8091478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saad S, Abdelmassih Y, Saad R, Guindolet D, Khoury SE, Doan S, et al. Neurotrophic keratitis:Frequency, etiologies, clinical management and outcomes. Ocul Surf. 2020;18:231–6. doi: 10.1016/j.jtos.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Mixon W, Angelle PP, Chang RI. Autologous eye drops for the treatment of dry eye and neurotrophic keratitis. Int J Pharm Compd. 2009;11:506–15. [PubMed] [Google Scholar]
- 42.Turkoglu E, Celik E, Alagoz G. A comparison of the efficacy of autologous serum eye drops with amniotic membrane transplantation in neurotrophic keratitis. Semin Ophthalmol. 2014;29:119–26. doi: 10.3109/08820538.2013.768678. [DOI] [PubMed] [Google Scholar]
- 43.Jeng BH, Dupps WJ., Jr Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28:1104–8. doi: 10.1097/ICO.0b013e3181a2a7f6. [DOI] [PubMed] [Google Scholar]
- 44.Sanz-Marco E, Lopez-Prats MJ, Garcia-Delpech S, Udaondo P, Diaz-Llopis M. Fulminant bilateral Haemophilus influenzae keratitis in a patient with hypovitaminosis A treated with contaminated autologous serum. Clin Ophthalmol. 2011;5:71–3. doi: 10.2147/OPTH.S15847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon KC, You IC, Im SK, Jeong TS, Park YG, Choi J. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114:1637–42. doi: 10.1016/j.ophtha.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Erdem E, Yagmur M, Harbiyeli I, Taylan-Sekeroglu H, Ersoz R. Umbilical cord blood serum therapy for the management of persistent corneal epithelial defects. Int J Ophthalmol. 2014;7:807–10. doi: 10.3980/j.issn.2222-3959.2014.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bremond-Gignac D, Daruich A, Robert MP, Chiambaretta F. Recent innovations with drugs in clinical trials for neurotrophic keratitis and refractory corneal ulcers. Expert Opin Investig Drugs. 2019;28:1013–20. doi: 10.1080/13543784.2019.1677605. [DOI] [PubMed] [Google Scholar]
- 48.Aifa A, Gueudry J, Portmann A, Delcampe A, Muraine M. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Invest Ophthalmol Vis Sci. 2012;53:8181–5. doi: 10.1167/iovs.12-10476. [DOI] [PubMed] [Google Scholar]
- 49.Arvola RP, Robciuc A, Holopainen JM. Matrix regeneration therapy:A case series of corneal neurotrophic ulcers. Cornea. 2016;35:451–5. doi: 10.1097/ICO.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 50.Salazar-Quiñones L, Molero-Senosiáin M, Aguilar-Munoa S, Gegúndez-Fernández JA, Díaz-Valle D, Muñoz-Hernández AM, et al. Management of corneal neurotrophic ulcers with Cacicol®-RGTA (ReGeneraTing Agent):A case series. Arch Soc Esp Oftalmol. 2020;95:421–8. doi: 10.1016/j.oftal.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Guerra M, Marques S, Gil JQ, Campos J, Ramos P, Rosa AM, et al. Neurotrophic keratopathy:Therapeutic approach using a novel matrix regenerating agent. J Ocul Pharmacol Ther. 2017;33:662–9. doi: 10.1089/jop.2017.0010. [DOI] [PubMed] [Google Scholar]
- 52.Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI, Nishida T, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92:896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- 53.Wang AL, Weinlander E, Metcalf BM, Barney NP, Gamm DM, Nehls SM, et al. Use of topical insulin to treat refractory neurotrophic corneal ulcers. Cornea. 2017;36:1426–8. doi: 10.1097/ICO.0000000000001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pflugfelder SC, Massaro-Giordano M, Perez VL, Hamrah P, Deng SX, Espandar L, et al. Topical recombinant human nerve growth factor (Cenegermin) for neurotrophic keratopathy:A multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127:14–26. doi: 10.1016/j.ophtha.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Sheha H, Tighe S, Hashem O, Hayashida Y. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol. 2019;13:1973–80. doi: 10.2147/OPTH.S185184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanco-Mezquita T, Martinez-Garcia C, Proença R, Zieske JD, Bonini S, Lambiase A, et al. Nerve growth factor promotes corneal epithelial migration by enhancing expression of matrix metalloprotease-9. Invest Ophthalmol Vis Sci. 2013;54:3880–90. doi: 10.1167/iovs.12-10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terzis JK, Dryer MM, Bodner BI. Corneal neurotization:A novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009;123:112–20. doi: 10.1097/PRS.0b013e3181904d3a. [DOI] [PubMed] [Google Scholar]
- 58.Elbaz U, Bains R, Zuker RM, Borschel GH, Ali A. Restoration of corneal sensation with regional nerve transfers and nerve grafts:A new approach to a difficult? problem. JAMA Ophthalmol. 2014;132:1289–95. doi: 10.1001/jamaophthalmol.2014.2316. [DOI] [PubMed] [Google Scholar]
- 59.Giannaccare G, Bolognesi F, Biglioli F, Marchetti C, Mariani S, Weiss JS, et al. In vivo and ex vivo comprehensive evaluation of corneal reinnervation in eyes neurotized with contralateral supratrochlear and supraorbital nerves. Cornea. 2020;39:210–4. doi: 10.1097/ICO.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 60.Wisely CE, Rafailov L, Cypen S, Proia AD, Boehlke CS, Leyngold IM, et al. Clinical and morphological outcomes of minimally invasive direct corneal neurotization. Ophthalmic Plast Reconstr Surg. 2020;36:451–7. doi: 10.1097/IOP.0000000000001586. [DOI] [PubMed] [Google Scholar]
- 61.Leyngold I, Weller C, Leyngold M, Espana E, Black KD, Hall KL, et al. Endoscopic corneal neurotization:Cadaver feasibility study. OPRS. 2018;34:213–6. doi: 10.1097/IOP.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 62.Leyngold I, Weller C, Leyngold M, Tabor M. Endoscopic corneal neurotization:Technique and initial experience. OPRS. 2018;34:82–5. doi: 10.1097/IOP.0000000000001023. [DOI] [PubMed] [Google Scholar]
- 63.Ting DSJ, Figueiredo GS, Henein C, Barnes E, Ahmed O, Mudhar HS, et al. Corneal neurotization for neurotrophic keratopathy:Clinical outcomes and in vivo confocal microscopy and histopathological findings. Cornea. 2018;37:641–6. doi: 10.1097/ICO.0000000000001522. [DOI] [PubMed] [Google Scholar]
- 64.Lin CH, Lai LJ. Herpetic corneal keratopathy management using ipsilateral supratrochlear nerve transfer for corneal neurotization. Ann Plast Surg. 2019;83:553–7. doi: 10.1097/SAP.0000000000002120. [DOI] [PubMed] [Google Scholar]
- 65.Benkhatar H, Levy O, Goemaere I, Borderie V, Laroche L, Bouheraoua N. Corneal neurotization with a great auricular nerve graft:Effective reinnervation demonstrated by in vivo confocal microscopy. Cornea. 2018;37:647–50. doi: 10.1097/ICO.0000000000001549. [DOI] [PubMed] [Google Scholar]
- 66.Catapano J, Fung SSM, Halliday W, et al. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation:Long-term clinical outcomes and evidence of corneal reinnervation. Br J Ophthalmol. 2019;103:1724–31. doi: 10.1136/bjophthalmol-2018-313042. [DOI] [PubMed] [Google Scholar]
- 67.Fung SSM, Catapano J, Elbaz U, Jobst C, Cheyne D, Ho ES, et al. In vivo confocal microscopy reveals corneal reinnervation after treatment of neurotrophic keratopathy with corneal neurotization. Cornea. 2018;37:109–12. doi: 10.1097/ICO.0000000000001315. [DOI] [PubMed] [Google Scholar]
- 68.Bourcier T, Henrat C, Heitz A, Kremer SF, Labetoulle M, Liverneaux P, et al. Lateral antebrachial cutaneous nerve as autologous graft for mini-invasive corneal neurotization (MICORNE) Cornea. 2019;38:1029–32. doi: 10.1097/ICO.0000000000002004. [DOI] [PubMed] [Google Scholar]
- 69.Bains RD, Elbaz U, Zuker RM, Ali A, Borschel GH. Corneal neurotization from the supratrochlear nerve with sural nerve grafts:A minimally invasive approach. Plast Reconstr Surg. 2015;135:397–400. doi: 10.1097/PRS.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 70.Jowett N, Pineda li R. Corneal neurotisation by great auricular nerve transfer and scleral-corneal tunnel incisions for neurotrophic keratopathy. Br J Ophthalmol. 2019;103:1235–8. doi: 10.1136/bjophthalmol-2018-312563. [DOI] [PubMed] [Google Scholar]
- 71.Weis E, Rubinov A, Al-Ghoul AR, Yau FM. Sural nerve graft for neurotrophic keratitis:Early results. Can J Ophthalmol. 2018;53:24–9. doi: 10.1016/j.jcjo.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 72.Domeshek LF, Hunter DA, Santosa K, Couch SM, Ali A, Borschel GH, et al. Anatomic characteristics of supraorbital and supratrochlear nerves relevant to their use in corneal neurotization. Eye (Lond) 2019;33:398–403. doi: 10.1038/s41433-018-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fogagnolo P, Giannaccare G, Bolognesi F, Digiuni M, Tranchina L, Rossetti L, et al. Direct versus indirect corneal neurotization for the treatment of neurotrophic keratopathy:A multicenter prospective comparative study. Am J Ophthalmol. 2020;220:203–14. doi: 10.1016/j.ajo.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Allevi F., Fogagnolo P., Rossetti L, Biglioli F. Eyelid reanimation, neurotisation, and transplantation of the cornea in a patient with facial palsy. BMJ Case Rep. 2014;2014:bcr2014205372. doi: 10.1136/bcr-2014-205372. doi:10.1136/bcr-2014-205372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacinto F, Espana E, Padilla M, Ahmad A, Leyngold I. Ipsilateral supraorbital nerve transfer in a case of recalcitrant neurotrophic keratopathy with an intact ipsilateral frontal nerve:A novel surgical technique. Am J Ophthalmol Case Rep. 2016;4:14–7. doi: 10.1016/j.ajoc.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koaik M, Baig K. Corneal neurotization. Curr Opin Ophthalmol. 2019;30:292–8. doi: 10.1097/ICU.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 77.Kikuta S, Yalcin B, Iwanaga J, Watanabe K, Kusukawa J, Tubbs RS, et al. The supraorbital and supratrochlear nerves for ipsilateral corneal neurotization:Anatomical study. Anat Cell Biol. 2020;53:2–7. doi: 10.5115/acb.19.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cruzat A, Hamrah P, Cavalcanti BM, Zheng L, Colby K, Pavan-Langston D, et al. Corneal reinnervation and sensation recovery in patients with herpes zoster ophthalmicus:An in vivo and ex vivo study of corneal nerves. Cornea. 2016;35:619–25. doi: 10.1097/ICO.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menicacci C, Gennaro P, Menicacci FI, Bagaglia SA, Menicacci F. Sicsso Congress. Caserta: 2016. Jun 23-25, New “four hands“corneal neurotization technique in the treatment of iatrogenic paralysis of the facial nerve with involvement of the ophthalmic nerve:A case report. [Google Scholar]
- 80.Malhotra R, Elalfy MS, Kannan R, Nduka C, Hamada S. Update on corneal neurotisation. Br J Ophthalmol. 2019;103:26–35. doi: 10.1136/bjophthalmol-2018-312104. [DOI] [PubMed] [Google Scholar]
- 81.Park JK, Charlson ES, Leyngold I, Kossler AL. Corneal neurotization:A review of pathophysiology and outcomes. Ophthalmic Plast Reconstr Surg. 2020;36:431–7. doi: 10.1097/IOP.0000000000001583. [DOI] [PubMed] [Google Scholar]
- 82.Leyngold IM, Yen MT, Tian J, Leyngold MM, Vora GK, Weller C, et al. Minimally invasive corneal neurotization with acellular nerve allograft:Surgical technique and clinical outcomes. Ophthalmic Plast Reconstr Surg. 2019;35:133–40. doi: 10.1097/IOP.0000000000001181. [DOI] [PubMed] [Google Scholar]
- 83.Davidge KM, Clarke HM, Borschel GH. Nerve transfers in birth related brachial plexus injuries. Hand Clinics. 2016;32:175–90. doi: 10.1016/j.hcl.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Samii M. Autologe nerven-transplantation im trigeminusbereich. Med Mitt. 1972;46:189–94. [Google Scholar]
- 85.Gordon T, Hendry M, Lafontaine CA, Cartar H, Zhang JJ, Borschel GH. Nerve cross-bridging to enhance nerve regeneration in a rat model of delayed nerve repair. PLoS One. 2015;10:e0127397. doi: 10.1371/journal.pone.0127397. [DOI] [PMC free article] [PubMed] [Google Scholar]