Abstract
A systematic review and meta-analysis were conducted to estimate the prevalence of diabetic retinopathy (DR) in India’s urban and rural areas. Medline, Scopus, and ScienceDirect databases were searched for population-based studies published in English between January 1990 and April 2021, wherein the prevalence of DR among Indian residents with type 2 diabetes mellitus (DM) was reported. A random-effects model was used to estimate the overall, rural, and urban prevalence. Data from 10 eligible studies were aggregated for meta-analysis. The prevalence of DR was 17.44% (95% confidence interval [CI], 14.33–20.55) in urban and 14.00% (95% CI: 9.13–18.86) in rural population (P = 0.24). The overall DR prevalence was 16.10% (95% CI: 13.16–24.32), and the population prevalence was 1.63% [95% CI: 0.94–2.32]. Prevalence of DR in people with diabetes was lower in the age group of 40–49 years [13.57% (95% CI: 7.16–19.98)] than in the age group of 50–59 years [16.72% (95% CI: 12.80–20.64)] and the age group of 60 years and above [16.55% (95% CI: 12.09–21.00)]. Variability in studies was high: urban (I2 = 88.90%); rural (I2 = 92.14%). Pooled estimates indicate a narrow difference in DR prevalence among people with diabetes in rural and urban India. The fast urbanization and increasing diabetes prevalence in rural areas underscore the need for providing equitable eye care at the bottom of the health pyramid.
Keywords: Diabetes, retinopathy, socioeconomic status, South Asia
The worldwide prevalence of diabetic retinopathy (DR) was estimated to be 34.6% among people with diabetes mellitus (DM) in 2012.[1] A recent meta-analysis (comprising 59 studies) found a lower prevalence estimate of 22.27%, with the highest prevalence in the African and North American regions and a comparatively lower prevalence in South-East Asia.[2] In 2000, it was estimated that India would be home to nearly 80 million people living with DM by 2030.[3] However, with an estimated 77 million people with diabetes in 2019, the predicted disease burden has arrived in India nearly a decade earlier. The revised projection forecasts that 130 million people will be living in India with diabetes by 2045.[4]
The Indian ICMR-INDIAB study, a nationally representative population-based study, reported a lower prevalence of DM in rural (5.2%) than urban (11.2%) India. However, there was a distinct difference between affluent rural communities (6.4%) and lower socioeconomic rural populations (3.9%).[5] With a rapid change in the socioeconomic structure in India and the fast urbanization of rural settlements, the gap between urban and rural areas in the prevalence of DM is likely to narrow. In the last five decades, the prevalence of DM has increased in rural and urban India from 2.4% and 3.3% in 1972 to 15% and 19%, respectively, in 2015–2019;[6] it is higher than the worldwide rural (7.2%) and urban (10.8%) prevalence of DM.[4] It is, therefore, necessary to study the impact of DM on vision in this population because eye care services in rural India are scarce, and nearly 65% of people of India live in villages.[7] The recent rollout of health programs such as the National Program for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke (NPCDCS) and National Multisectoral Action Plan (NMAP) across India has a limited provision of eye care built into these systems.[8]
The rural-urban divide and the socioeconomic burden of DR in India have been studied in only some regions of India. The studies investigating the DR prevalence in the rural and urban communities of the same ethnic population have indicated a higher prevalence in urban than in rural areas, with an odds of 1.4–6 times higher in the urban population.[9,10] A wide range in DR prevalence has been reported in studies of urban and rural people alone, 10.1%–22.4% and 9.6%–32.5%, respectively.[11,12,13,14] The wide variation might have been due to differences in the study type (population/camp/hospital-based), surveyed population, and the survey period.
There is insufficient data of a nationally representative population-based study on DR burden in India and the impact of socioeconomic, dietary, and genetics in a culturally, socially, and economically diverse country. Therefore, we reviewed the published population-based studies in India and performed a meta-analysis of the aggregated data to determine the prevalence of DR among people with diabetes mellitus in rural and urban India.
Methods
Search strategy: The systematic review and meta-analysis followed the MOOSE guidelines for prevalence studies.[15] Our study question was - what is the prevalence of diabetic retinopathy among people with diabetes mellitus in rural and urban India? The literature search was conducted for articles published in the English language indexed in the Medline, Scopus, and ScienceDirect databases. The search keywords were a combination of both controlled vocabulary (MeSH- Medical Subject Heading terms) and free text words – MEDLINE (PubMed): “epidemiology”[MeSH Subheading] OR “epidemiology”[All Fields] OR “prevalence”[All Fields] OR “prevalence”[MeSH Terms]), AND “diabetes mellitus”[MeSH Terms] OR (“diabetes”[All Fields] AND “mellitus”[All Fields]) OR “diabetes mellitus”[All Fields] OR “diabetes”[All Fields] OR “diabetic”[All Fields] OR “diabetics”[All Fields] AND “diabetic retinopathy”[MeSH Terms] OR (“diabetic”[All Fields] AND “retinopathy”[All Fields]) OR “diabetic retinopathy”[All Fields]) AND (“india”[MeSH Terms] OR “india”[All Fields]. Scopus: TITLE-ABS-KEY (“diabetic retinopathy” prevalence AND india). ScienceDirect: Terms: diabetes, prevalence, India; Title, abstract, keywords: “diabetic retinopathy.” Reference lists of eligible publications and related reviews were scanned to identify relevant studies. The literature search was conducted on April 10, 2021 and was limited to studies published between January 1, 1990 and April 10, 2021. The study followed the Tenets of the Declaration of Helsinki.
Eligibility criteria: Inclusion: population-based studies, subjects residing in India, and the studies with published data on the estimated prevalence (with/without confidence intervals). From the communications on the same population sample, the highest quality (risk-of-bias assessment) study and the most recent data were included. Exclusion: studies conducted only on people with type 1 DM, or the proportion of type 1 DM participants >10% when the study included both types of DM, and studies where the number of eyes was used as the denominator for the calculation of prevalence. The study did not include conference abstracts and grey literature (articles not available via conventional publication channels, i.e., unpublished studies, governmental reports, etc.).
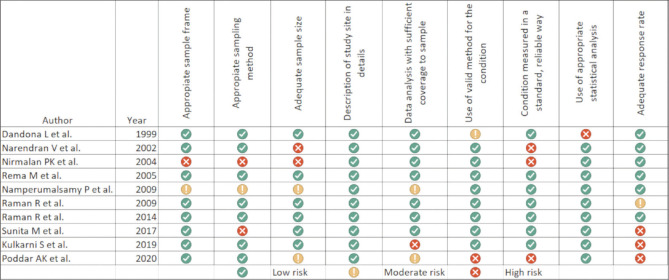
Screening, quality assessment, and data extraction: After deleting duplicate records within and between different bibliographic databases, two authors (UCB, ASB) independently reviewed the remaining titles and abstracts to identify potentially eligible articles before a full appraisal. Discrepancies between the screened abstracts were adjudicated by a third independent reviewer (JS). In the full review, a thorough quality assessment of the full-text articles was done using the Joanna Briggs Institute (JBI) standardized critical appraisal checklist for prevalence studies to ascertain the bias (JBI Critical Appraisal Checklist for Studies Reporting Prevalence Data).[16] Any disagreement between the reviewers was resolved through consensus involving a third reviewer (JS). In the meeting, a thorough discussion was carried out on methodology, the possibility of bias in design, conduct and analysis, and the extent to which this bias was addressed. Subsequently, studies were excluded that did not qualify the appraisal. In the case of multiple publications from the same survey or overlapping data, preference was given to the most recent study or the one with the most inclusive information. Discrepancies in study eligibility were resolved through discussion. Data were extracted from each article by using a standardized spreadsheet designed in advance and coded as required. All attempts were made to contact the study authors for missing/incomplete data.
Statistical analysis
Data were extracted from the selected studies and entered in Microsoft® Excel software for Mac version 16.48 and further analyzed using Stata 16 (Stata Corp. 2020. Stata Statistical Software: Release 16. College Station TX). Meta-analysis of pooled prevalence data was represented with a forest plot with effect size from each study, 95% confidence intervals, and percent weight assigned to each survey. Due to the varied methodology of the selected studies, substantial heterogeneity was expected. Thus, the random-effects method was used to calculate the pooled effect size. The estimate of heterogeneity was calculated using an inverse-variance model.[17] Publication bias was assessed through funnel plot and using the Eggers test. Heterogeneity was evaluated through Higgin’s I2 statistics expressed in percentages, tau squared statistics, and heterogeneity test statistics (Chi-square and P value). We considered Higgin’s I2 values of 25%, 50%, and 75% as low, moderate, and high heterogeneity, respectively.[18] Subgroup analysis was also conducted by rural/urban setting, age group of the study participants, study regions, epidemiological transition level (ETL) status of the study location, and survey period. Further analysis was conducted on the prevalence of sight-threatening DR (STDR) and diabetic macular edema (DME). A regression analysis of prevalence with respect to the year of study was performed.
Results
Ten articles were found to be eligible for the systematic review and meta-analysis, and the extraction process is detailed in the flow chart [Fig. 1]. The studies had recruited 98,451 individuals; 9% (n = 8,866) people had DM, and 1,327 (14.9% of people with DM and 1.3% of the cohort) of them had DR. Table 1 summarizes the study characteristics and results.
Figure 1.
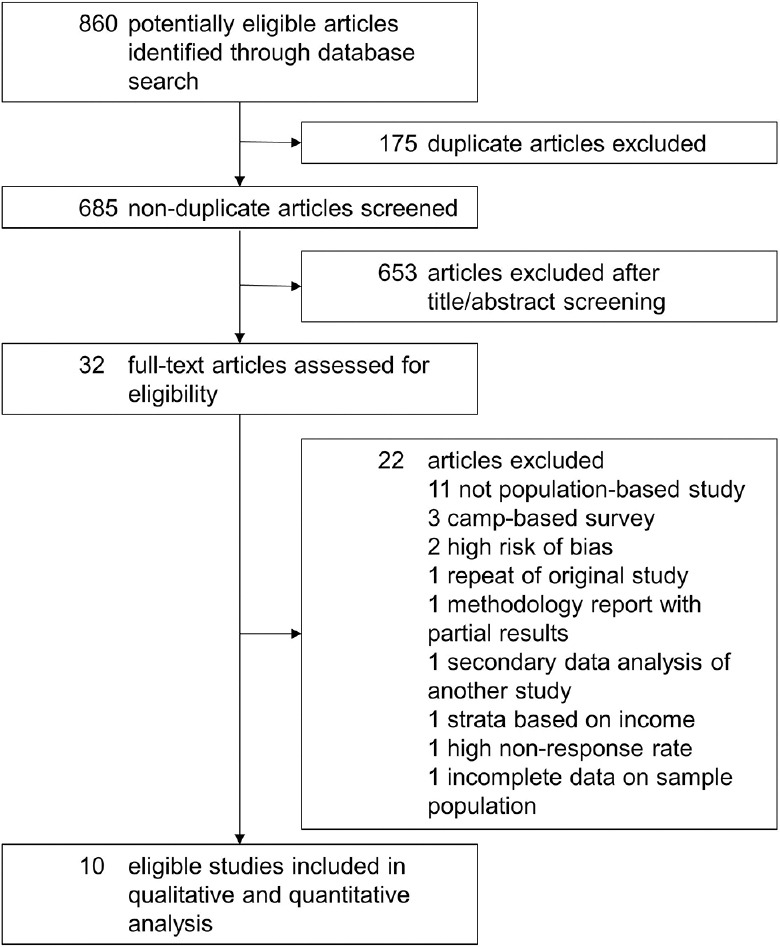
Systematic review and meta-analysis flow chart
Table 1.
Characteristics of included studies
| Author, Publication Year | Study period (years) & location | Population (C, D, V, S; Age criteria [years]; Region) | Sampling method, sample size (n=screened for DM; assessed for DR) | Diabetes Mellitus | Diabetic Retinopathy | Prevalence of DR (Percentage [95% CI]) | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Diagnostic criteria | Inclusion criteria | Classification | Screening Methodology | Urban | Rural | ||||
| Dandona et al. (1999)[12] | 1996-1997 Urban |
C: Hyderabad; S: Telangana; Age: ≥30; South India | Cluster (stratified random) sampling (1399; 124) | Self-reported | Known DM | Modified ETDRS-Klein et al.[19] | Clinical (FP for documentation) | 22.4% [15.2-29.9] | - |
| Narendran et al. (2002)[20] | 2001 Rural & Urban* |
D: Palakkad; S: Kerala; Age: ≥50; South India | Cluster sampling (5212; 260) | Self-reported | Known DM | Modified Klein et al.’s criteria[19] | Clinical | - | 26.8% [19.2-34.4] |
| Nirmalan et al. (2004)[21] | 1995-1997 Rural |
D: Madurai, Tirunelveli; V: Chidambaranar; S: Tamil Nadu; Age: ≥40; South India | Stratified, multistage cluster sampling (5150; 228) | NR | Known, + PPBS ≥180 mg/dl | Klein et al.[19] | Clinical | - | 10.5% [6.5-14.5] |
| Rema et al. (2005)[22] | NR Urban |
C: Chennai; S: Tamil Nadu; Age: ≥20; South India | Systematic random (26001; 1715) | WHO, 1999 | OGTT | ETDRS | FP (4 fields) | 17.6% [15.8-19.5] | - |
| Raman et al. (2009)[23] | 2003-2006 Urban |
C: Chennai; S: Tamil Nadu; Age: ≥40; South India | Multistage random sampling (5999; 1414) | ADA | Known + FBS ≥110 mg/dL (venous) | Klein et al.[19] | FP (4 fields) | 18.0% [16.0-20.1] | - |
| Namperumalsamy et al. (2009)[9] | 2005-2006 Rural & Urban† |
D: Theni; S: Tamil Nadu; Age: ≥30; South India | Cluster sampling (28039; 2436) | WHO, 1999 | Known + FBS ≥126 mg/dL (capillary) | Klein et al.[19] | Clinical | 13.4% [11.2-16.0] | 10.2% [7.9-13.1] |
| Raman et al. (2014)[24] | 2007-2010 Rural |
D: Thiruvallur Kanchipuram; S: Tamil Nadu; Age: ≥40; South India |
Multistage cluster sampling (13079; 1190) | NR | Known + OGTT | Klein et al.[19] | FP (4-7 field) | - | 10.3% [8.5-11.9] |
| Sunita et al. (2017)[25] | 2011-2014 Urban |
C: Mumbai; S: Maharashtra; Age: ≥40; West India | Single stage random sampling (6569; 592) | ADA | FBS ≥126 (capillary) | Klein et al.[19] | Clinical+FP (7 field) | 15.3% [8.8-21.8] | - |
| Kulkarni et al. (2019)[26] | 2017 Urban |
C: Pune; S: Maharashtra; Age: ≥50; West India | RAAB + DR (cluster sampling, probability proportionate to size) (3527; 706) | NR | Known + RBS ≥200 | Scottish[27] | Clinical | 14.3% [11.7-16.9] | - |
| Poddar et al. (2020)[28] | 2016 Rural |
D: Siwan; S: Bihar; Age: ≥50; East India | RAAB + DR (stratified random cluster sampling) (3476; 201) | NR | Known + RBS ≥200 | Scottish[27] | Clinical | - | 14.9% [10.3-19.5] |
ADA=American Diabetes Association, C=City, D=District, DM=Diabetes Mellitus, DR=Diabetic Retinopathy, ETDRS=Early Treatment of Diabetic Retinopathy Study, FBS=Fasting Blood Sugar, FP=Fundus Photography, NR=Not reported, OGTT=Oral glucose tolerance test, PPBS=Post Prandial Blood Sugar, RAAB=Rapid Assessment of Avoidable Blindness, RBS=Random Blood Sugar S=State, V=Village, WHO=World Health Organization. *22 rural & 3 urban clusters, †55% rural participants
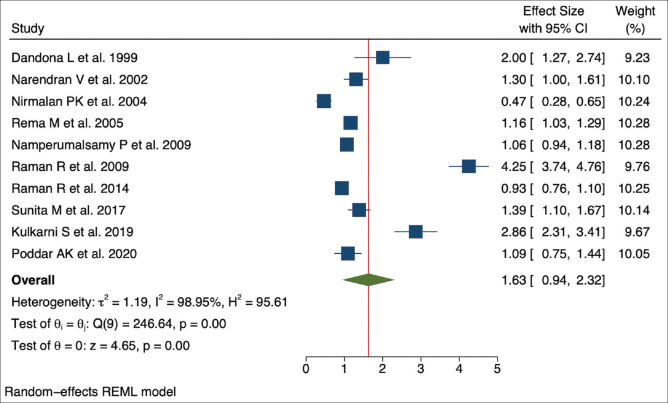
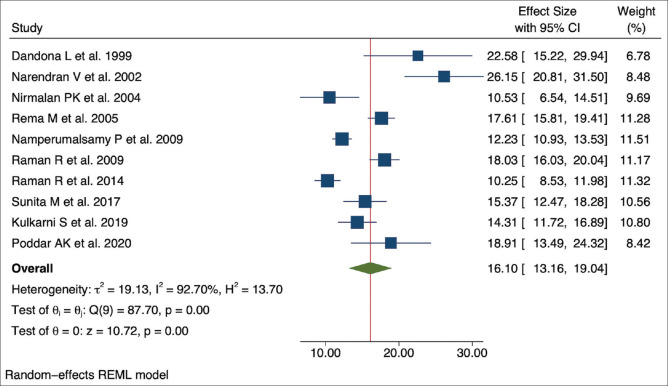
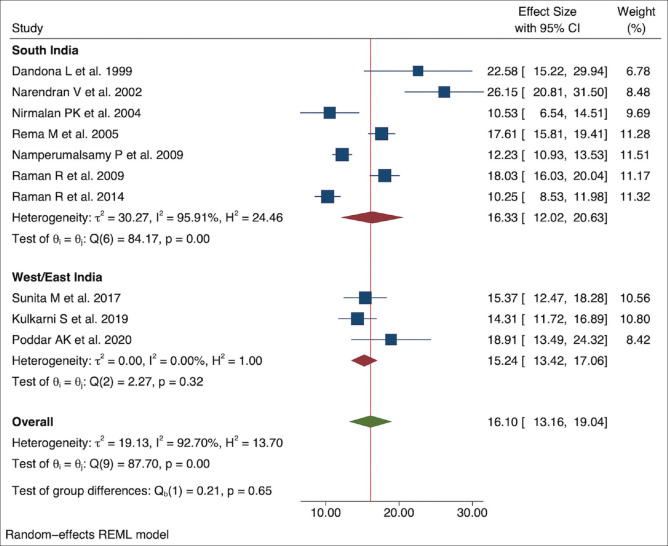
The selected studies for the meta-analysis had methodological variations. We used the random-effects model using effect size and standard error of the effect size (effect size is the prevalence of DR among the people with DM). The meta-analysis suggested high heterogeneity or variability between the studies (tau squared = 19.13, Higgins I2 = 92.70% with heterogeneity Chi-squared = 87.70, degree of freedom = 9, and P < 0.001). The overall DR prevalence estimate in the population was 1.63% [95% Confidence Interval [CI]: 0.94–2.32]. The DR prevalence in the individual studies within the diabetic group ranged between 10.25% and 26.15%, with a pooled prevalence of 16.10% (95% CI: 13.16–19.04). High heterogeneity resulted in approximately equal weightage given to each study (range: 6.78–11.51) by the random-effects model. Figs. 2 and 3 present the forest plot showing the pooled effect size from the meta-analysis.
Figure 2.
Forest plot showing the pooled effect size of the overall DR prevalence in the population
Figure 3.
Forest plot showing the pooled effect size of the DR prevalence within the diabetic group in the included studies
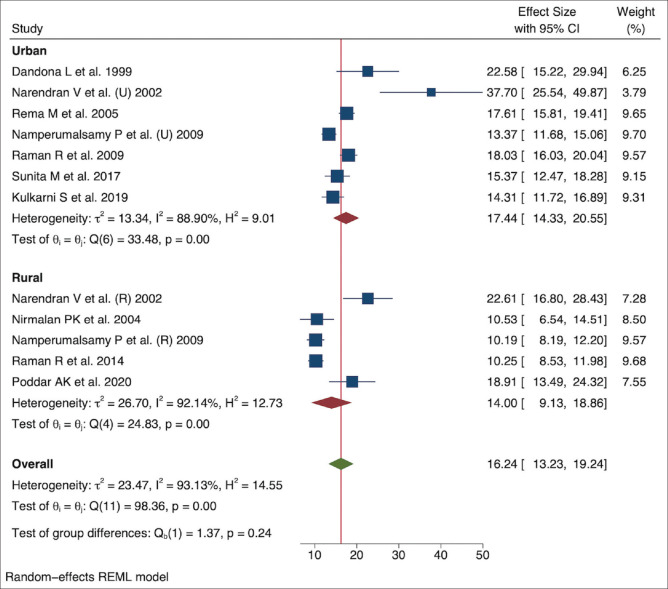
Subgroup analyses were performed for the study settings (urban and rural), age groups (40–49 years, 50–59 years, and ≥60 years), study regions (south India and west/east India), epidemiological transition level (ETL) status of the Indian state in which the study was conducted, survey period (in blocks of 10 years), STDR, and DME. With respect to the setting, the pooled prevalence of DR in urban India was higher, that is, 17.44% (95% CI: 14.33–20.55), than in rural India, that is, 14.00% (95% CI: 9.13–18.86), but the difference was not statistically significant (P = 0.24). Variations in studies in both urban (I2 = 88.90%) and rural (I2 = 92.14%) areas were high [Fig. 4].
Figure 4.
Pooled prevalence of DR in urban and rural India
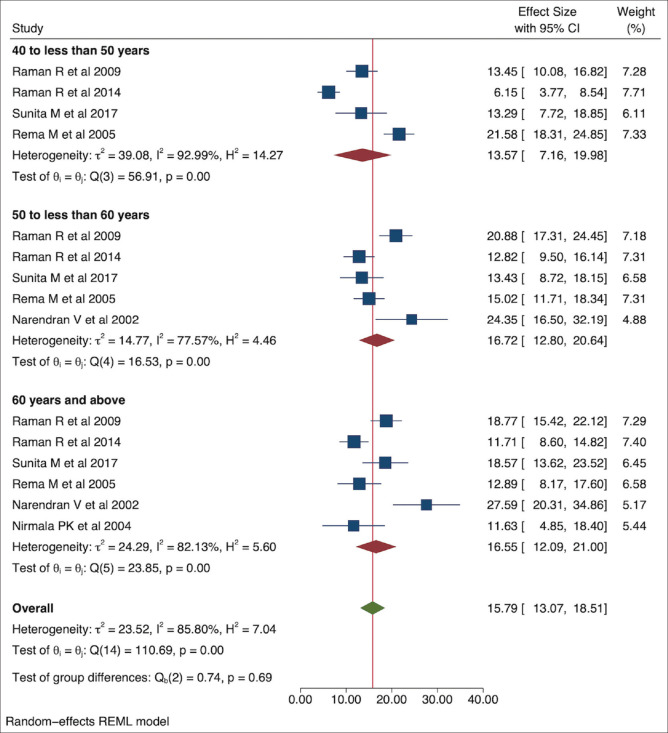
The age categories were different in the studies included in the current meta-analysis. Pooled prevalence of DR in people with DM between age 40 and 49 years was lower [13.57% (95% CI: 7.16–19.98)] than in the age group of 50–59 years [16.72% (95% CI: 12.80–20.64)] and ≥60 years [16.55% (95% CI: 12.09–21.00)] [Fig. 5].
Figure 5.
Pooled prevalence of DR in people with diabetes in various age groups
Seven of ten studies were conducted in south India (Kerala, Tamil Nadu, and Telangana), two studies were conducted in western India (Maharashtra), and one study was conducted in eastern India (Bihar). Pooled prevalence of DR in people with DM was higher in south India [16.33% (95% CI: 12.02–20.63)] with high variability (I2 = 95.91%) compared to west/east India [15.24% (95% CI: 13.42–17.06)] [Fig. 6]. Of the Indian states included in the current meta-analysis, one was a low ETL state (Bihar), two were higher middle ETL states (Maharashtra and Telangana), and two were high ETL states (Kerala and Tamil Nadu).[29] Though the low ETL state (Bihar) had a higher prevalence [18.91% (95% CI: 13.49–24.32)] as compared to the middle [15.28% (95% CI: 13.41–17.15)] and high [15.56% (95% CI: 11.02–20.09)] ETL states, the difference in DR prevalence between the subgroups was not statistically significant. Subgroup analysis of studies conducted in the past three decades showed higher DR prevalence in 1990–2000 [22.58% (95% CI: 15.22–29.94)] than the blocks of 2001–2010 [16.68% (95% CI: 11.72–21.64)] and 2011–2020 [14.18% (95% CI: 10.85–17.50)]. A regression analysis of prevalence with respect to the year of study showed a negative relationship [Fig. 7]. Details of subgroup analysis are given in Table 2. STDR was reported in four studies with an overall prevalence estimate of 4.52% [95% CI: 2.93–6.11] among people with DM. DME was calculated from five studies, with the overall prevalence estimate, among people with DM, of 2.10% [95% CI: 1.54–2.65].
Figure 6.
Pooled prevalence of DR in people with diabetes in different regions of India
Figure 7.
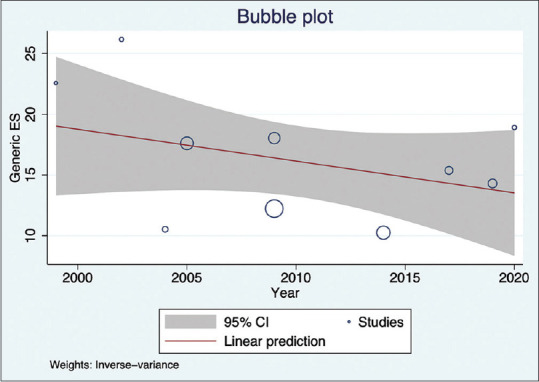
DR prevalence trend through time according to the publication year of the studies
Table 2.
Subgroup analysis for comparison of prevalence of diabetic retinopathy along with statistics related to heterogeneity
| Subgroup analysis | Prevalence (95% Confidence Interval, CI) | I 2 | Tau squared value | Heterogeneity test | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Chi value | DF* | P | ||||
| Region | ||||||
| Urban | 17.44 (14.33-20.55) | 88.90 | 13.34 | 33.48 | 6 | <0.001 |
| Rural | 14.00 (9.13-18.86) | 92.14 | 26.70 | 24.83 | 4 | <0.001 |
| Overall | 16.24 (13.23-19.24) | 93.13 | 23.47 | 98.36 | 11 | <0.001 |
| Age group | ||||||
| 40-49 years | 13.57 (7.16-19.98) | 92.99 | 39.08 | 56.91 | 3 | <0.001 |
| 50-59 years | 16.72 (12.80-20.64) | 77.57 | 14.77 | 16.53 | 4 | <0.001 |
| ≥60 years | 16.55 (13.07-18.51) | 82.13 | 24.29 | 23.85 | 5 | <0.001 |
| Overall | 15.79 (13.07-18.51) | 85.80 | 23.52 | 110.6 | 14 | <0.001 |
| Location | ||||||
| South India | 16.33 (12.02-20.63) | 95.92 | 30.27 | 84.17 | 6 | <0.001 |
| West/East India | 15.24 (13.42-17.06) | 0 | 0 | 2.27 | 2 | 0.32 |
| Overall | 16.10 (13.16-19.04) | 92.70 | 19.13 | 87.70 | 9 | <0.001 |
| Epidemiological Transition Level | ||||||
| Low | 18.91 (13.49-24.32) | - | - | - | - | -# |
| Higher middle | 15.28 (13.41-17.15) | 0 | 0 | 4.33 | 2 | 0.11 |
| Higher | 15.56 (11.02-20.09) | 96.46 | 29.88 | 79.02 | 5 | <0.001 |
| Survey Period | ||||||
| 1990-2000 | 22.58 (15.22-29.94) | - | - | - | - | -# |
| 2001-2010 | 16.68 (11.72-21.64) | 95.93 | 29.42 | 55.81 | 4 | <0.001 |
| 2011-2020 | 14.18 (10.85-17.50) | 82.13 | 8.91 | 17.51 | 3 | <0.001 |
| Sight threatening diabetic retinopathy | 4·52 (2·93-6·11) | 72·32 | 1·75 | 9·13 | 3 | 0·03 |
| Diabetic macular edema | 2·10 (1·54-2·65) | 44·68 | 0·17 | 8·32 | 4 | 0·08 |
*DF=degree of freedom, #=less than three studies
We also explored the effect sample size in each study, impacting the effect size through meta-regression analysis. The analysis suggested a minor negative correlation of effect size with increasing sample size [Fig. 8]. Assessment of publication bias is less reliable in the presence of high variability/heterogeneity between the studies. Nevertheless, we assessed bias and minor study effects by observing the funnel plot and Egger’s test for small-study effects. The Egger’s test result of effect estimates against its standard error suggested a publication bias (P = 0.023; beta = 3.25, and z value = 2.27). The funnel plot confirmed this observation [Fig. 9].
Figure 8.
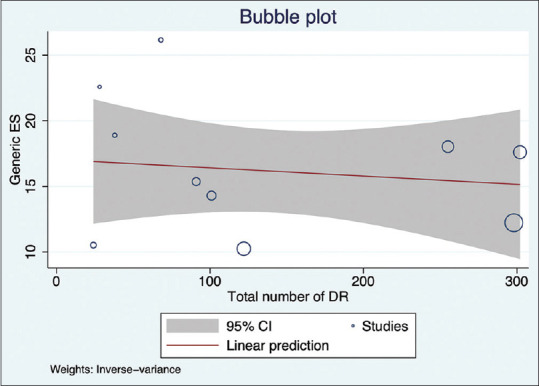
Meta-regression analysis shows the correlation of effect size with increasing sample size in the studies
Figure 9.
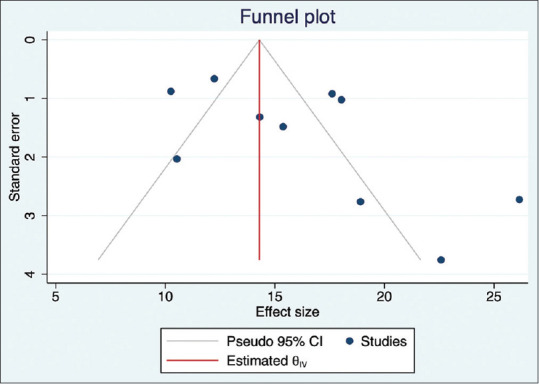
Funnel plot showing publication bias
Discussion
Our meta-analysis findings of a lower DR prevalence in rural India (14.0% rural vs. 17.4% urban) concurs with India’s published rural-urban diabetes trend, but the difference was not statistically significant.[5] The older adults had a higher prevalence, the same as reported in the previous studies, and reflect the duration of diabetes.[10,30] Comparison of the prevalence of DR in rural and urban India has not been performed earlier, although the SMART India multicenter study is underway;[31] the current one is the first meta-analysis. Incidentally, most studies that met the inclusion criteria of the present meta-analysis were from south India, limiting the extrapolation of the study results to the entire country. The pooled prevalence in the south Indian studies showed a high degree of variability and a higher prevalence of DR than the rest of the country. The prevalence of DME in this meta-analysis (2.1%) is apparently lower than an estimate from an urban South-Indian population survey (the prevalence of center-involving DME was 3.03% as against 10.8% for non-center-involving DME).[32] The studies that reported DME in this meta-analysis did not have a uniform reporting method. Optical coherence tomography use in the diagnosis of DME was limited.
The high variation in the pooled prevalence in urban and rural areas may partly reflect rapid urbanization and uneven development in different parts of India in the last two decades when these studies were carried out and partly reflect the high prevalence of DM in south India.[5,33] The critical urban-rural wage gap from 51% in 1983 to 27% in 2010 and the increase in urban population from 28.5% in 2001 to 34% in 2019 testify the changing lifestyle and urban migration from rural agriculture-based jobs.[6,34]
Three global meta-analyses have attempted to address the question of the prevalence of DR among people with diabetes. The META-EYE study included participant-level data from three Indian studies: Andhra Pradesh Eye Disease Study (APEDS), Chennai Urban Rural Epidemiology Study (CURES), and Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS); the meta-analysis by Ruta et al. had included two studies by Rema et al., and Namperumalsamy et al.[1,35] The recent study by Teo et al.[2] included 11 studies from India and reported an estimate of DR prevalence [16.99% (95% CI: 14.13–20.28)] in the South-East Asian region similar to our study analysis. Teo et al.[2] reported a lower prevalence of DR in people of Asian ethnicity. However, South Asia has a high population density. The absolute number of individuals with DM is on the rise; therefore, it deserves a further analysis of the regional prevalence and variation. Two meta-analyses from China reported DR prevalence of 18.45% and 23%, and these studies reported a higher rural than urban prevalence.[36,37]
India is a developing country with varying levels of socioeconomic development and cultural disparities. Asaria et al.[38] explored the inequalities in health across social groups and various diseases in India. Higher life expectancy at birth has been reported in urban households than rural ones, irrespective of the wealth status. This inequality is further exemplified in the distribution of life expectancy by gender—men in urban areas had the widest distribution, and women had the narrowest. The impact of the duration of DM on the incidence of DR is known. A lower life expectancy in rural populations may contribute to the lower DR prevalence in rural India.[13] The impact of life expectancy on microvascular complications in the diabetic population in rural and urban areas merits further investigation.
There is a clear socioeconomic status (SES) and ETL divide in India.[29] Socioeconomic status plays a vital role in the prevalence and care of diabetes worldwide.[39,40,41,42,43] The Indian data on the prevalence in various socioeconomic strata report equivocal results, and the study settings are primarily urban.[10,12,23,44] A rising prevalence of DM in individuals with higher SES in the rural community may put a large population of rural India at risk of vision loss due to DR.[5] Variation in DM and DR prevalence between different regions and states of India reported by various studies agreed with our subgroup analyses. However, there was high heterogeneity in south Indian studies;[33,45] a majority of population-based DR surveys in south India or the higher ETL of the southern states may be ascribed to the variability.
The negative attributes of changing dietary habits coupled with physical inactivity have increased obesity and diabetes in rural and semi-urban areas.[46] The increasing risk of DM has also been recorded in individuals who had a history of childhood malnutrition and rural-to-urban migrants.[47,48] There are considerable differences in India’s dietary patterns of rural and urban and rich and poor households.[49] In rural and urban areas, the affluent families consume >3000 kcal/day, that is, 20% more than the reference diet. Their calorie intake per person/day is almost twice as high as their poorest counterparts, who consume only 1645 kcal/person/day.[50] Social welfare programs for rural India favoring heavy subsidies on rice, sugar, and palm oil through public distribution systems lead to increased consumption of low nutrient calorie-rich food. Relative low price and high accessibility of energy-dense but low-nutrient food decrease the consumption of whole grains, fruits, and vegetables.[51] High total caloric intake has been linked with a higher risk of DR.[52]
The rising rural prevalence of DR can be attributed to the limited access to health care and poor health-seeking behavior resultant of low education levels prevalent in rural India. Low education may directly impair an individual’s ability to obtain adequate care and reduce life opportunities that may hold them from meeting the health care expense in general and force them to live in neighborhoods with worse access to healthcare facilities.[53] A population with no history of schooling in a rural setting was found to have the highest risk of DR in a south Indian study.[14] Low awareness about the importance of seeking timely care and the limited access to healthcare in rural settings may contribute to the higher prevalence. Association between low education level in the lower socioeconomic group and the development of DR have been observed in studies by various research groups in different geographic locations.[54,55,56]
These conclusions on the DR prevalence in rural and urban India in this meta-analysis drawn from studies in India conducted during the past two decades indicate no statistically significant trend in DR prevalence over time, though a regression analysis hinted at a negative correlation. The results are in agreement with the world diabetic retinopathy trend.[2] The other strength of the study was the large effect sample allowing subgroup meta-analysis.
The included studies had some limitations: they were restricted to only a few regions of India and used varying sampling methods and diagnostic criteria for DM and DR [Table 1]; not all studies provided prevalence data on STDR, proliferative and nonproliferative DR. This meta-analysis study included only peer-reviewed articles and did not include governmental reports. In addition, the urban and rural criterion was not objectively defined; instead, it was self-declared by each study. With rapid urbanization and uneven development in different parts of India, the definitions of urban and rural regions must have changed during the past decades, contributing further to the study heterogeneity. The publication bias for reporting the DR prevalence in urban and rural India as brought out by this study suggests the nonreporting of negative studies or selective outcome reporting in the published studies. The cumulative risk of bias assessment is presented in Fig. 10.
Figure 10.
Risk of bias assessment in the included studies
The study, however, shows a narrowing gap in DR prevalence between urban and rural India. This should alert the policymakers as the absolute number of the affected individuals in the rural population will be significant because of the larger rural Indian population. It calls for strategic planning and integration of eye care into the national health programs focusing on noncommunicable diseases.[57] Strengthening the teleophthalmology platforms by capitalizing on expanding mobile internet penetration to rural India is feasible. A nationally representative study to determine the prevalence of DR and DME across states and several sociocultural and economic factors is a long-felt need.
Conclusion
The population-based studies in India have not yielded consistently convergent prevalence estimates on diabetic retinopathy. Wide variation in the rural-urban prevalence has been reported. Pooled estimates in this meta analysis study show a lower prevalence of DR in rural India, gradually inching up to the urban prevalence, the difference statistically insignificant. This underscores the need for improving eye care at the primary and secondary levels in India. Inclusion of comprehensive eye care with screening for diabetic retinopathy in the national programs in India could be the first logical step in the care of this emerging disease.
Financial support and sponsorship
Hyderabad Eye Research Foundation (HERF), Hyderabad, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teo ZL, Tham Y-C, Yan Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045:Systematic review and meta-analysis. Ophthalmology. 2021;128:1580–91. doi: 10.1016/j.ophtha.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes:Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045:Results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. (9th edition) 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 5.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India:Results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 6.Ranasinghe P, Jayawardena R, Gamage N, Sivanandam N, Misra A. Prevalence and trends of the diabetes epidemic in urban and rural India:A pooled systematic review and meta-analysis of 1.7 million adults. Ann Epidemiol. 2021;58:128–48. doi: 10.1016/j.annepidem.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 7.India population 2020-StatisticsTimes.com. [Last accessed on 2021 May 15]. Available from:https://statisticstimes.com/demographics/country/india-population.php .
- 8.Home:National Health Mission. [Last accessed on 2021 May 13]. Available from:https://nhm.gov.in/
- 9.Namperumalsamy P, Kim R, Vignesh TP, Nithya N, Royes J, Gijo T, et al. Prevalence and risk factors for diabetic retinopathy:A population-based assessment from Theni District, south India. Br J Ophthalmol. 2009;93:429–34. doi: 10.1136/bjo.2008.147934. [DOI] [PubMed] [Google Scholar]
- 10.Krishnaiah S, Das T, Nirmalan PK, Shamanna BR, Nutheti R, Rao GN, et al. Risk factors for diabetic retinopathy:Findings from the Andhra Pradesh eye disease study. Clin Ophthalmol. 2007;1:475–82. [PMC free article] [PubMed] [Google Scholar]
- 11.Vyas U, Khandekar R, Trivedi N, Desai T, Danayak P. Magnitude and determinants of ocular morbidities among persons with diabetes in a project in Ahmedabad, India. Diabetes Technol Ther. 2009;11:601–7. doi: 10.1089/dia.2009.0033. [DOI] [PubMed] [Google Scholar]
- 12.Dandona L, Dandona R, Naduvilath T, McCarty C, Rao G. Population-based assessment of diabetic retinopathy in an urban population in southern India. Br J Ophthalmol. 1999;83:937–40. doi: 10.1136/bjo.83.8.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonas JB, Nangia V, Khare A, Matin A, Bhojwani K, Kulkarni M, et al. Prevalence and associated factors of diabetic retinopathy in rural central India. Diabetes Care. 2013;36:e69. doi: 10.2337/dc12-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadarajan B, Saya GK, Krishna RB, Lakshminarayanan S. Prevalence of diabetic retinopathy and its associated factors in a rural area of Villupuram District of Tamil Nadu, India. J Clin Diagn Res. 2017;11:LC23–6. doi: 10.7860/JCDR/2017/20946.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology:A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–53. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Klein BE, Magli YL, Brothers RJ, Meuer SM, Moss SE, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93:1183–7. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 20.Narendran V, John RK, Raghuram A, Ravindran RD, Nirmalan PK, Thulasiraj RD. Diabetic retinopathy among self reported diabetics in southern India:A population based assessment. Br J Ophthalmol. 2002;86:1014. doi: 10.1136/bjo.86.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nirmalan PK, Katz J, Robin AL, Tielsch JM, Namperumalsamy P, Kim R, et al. Prevalence of vitreoretinal disorders in a rural population of southern India:The Aravind comprehensive eye study. Arch Ophthalmol. 2004;122:581–6. doi: 10.1001/archopht.122.4.581. [DOI] [PubMed] [Google Scholar]
- 22.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India:The Chennai urban rural epidemiology study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 23.Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India:Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study report 2. Ophthalmology. 2009;116:311–8. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara nethralaya diabetic retinopathy epidemiology and molecular genetic study III (SN-DREAMS III), report no 2. BMJ Open Diab Res Care. 2014;2:e000005. doi: 10.1136/bmjdrc-2013-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunita M, Singh AK, Rogye A, Sonawane M, Gaonkar R, Srinivasan R, et al. Prevalence of diabetic retinopathy in urban slums:The Aditya Jyot diabetic retinopathy in urban Mumbai slums study-report 2. Ophthalmic Epidemiol. 2017;24:303–10. doi: 10.1080/09286586.2017.1290258. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni S, Kondalkar S, Mactaggart I, Shamanna BR, Lodhi A, Mendke R, et al. Estimating the magnitude of diabetes mellitus and diabetic retinopathy in an older age urban population in Pune, western India. BMJ Open Ophthalmol. 2019;4:e000201. doi: 10.1136/bmjophth-2018-000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scottish Diabetes Group. Diabetic Retinopathy Screening Implementation Group, Scotland, Scottish Executive, Diabetic Retinopathy Screening Services in Scotland:Recommendations for Implementation:A Report. Scottish Executive. 2003:61–2. [Google Scholar]
- 28.Poddar AK, Khan TA, Sweta K, Tiwary MK, Borah RR, Ali R, et al. Prevalence and causes of avoidable blindness and visual impairment, including the prevalence of diabetic retinopathy in Siwan district of Bihar, India:A population-based survey. Indian J Ophthalmol. 2020;68:375–80. doi: 10.4103/ijo.IJO_1709_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.India State-Level Disease Burden Initiative Collaborators. Nations within a nation:Variations in epidemiological transition across the states of India, 1990-2016 in the Global Burden of Disease Study. Lancet. 2017;390:2437–60. doi: 10.1016/S0140-6736(17)32804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy in India:The All India ophthalmological society diabetic retinopathy eye screening study 2014. Indian J Ophthalmol. 2016;64:38–44. doi: 10.4103/0301-4738.178144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivaprasad S, Raman R, Rajalakshmi R, Mohan V, Deepa M, Das T, et al. Protocol on a multicentre statistical and economic modelling study of risk-based stratified and personalised screening for diabetes and its complications in India (SMART India) BMJ Open. 2020;10:e039657. doi: 10.1136/bmjopen-2020-039657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pradhana D, Priya MNS, Surya J, Bhende M, Laxmi G, Sharma T, et al. Optical coherence tomography-based prevalence of diabetic macular Edema and its associated risk factors in urban South India:A population-based study. Ophthalmic Epidemiol. 2021:1–7. doi: 10.1080/09286586.2021.1907846. doi:10.1080/09286586.2021.1907846. [DOI] [PubMed] [Google Scholar]
- 33.India state-level disease burden initiative diabetes collaborators. The increasing burden of diabetes and variations among the states of India:The global burden of disease study 1990-2016. Lancet Glob Health. 2018;6:e1352–62. doi: 10.1016/S2214-109X(18)30387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Development Indicators |DataBank. [Last accessed on 2021 May 15]. Available from:https://databank.worldbank.org/reports.aspx?source=2&country=IND .
- 35.Ruta LM, Magliano DJ, LeMesurier R, Taylor HR, Zimmet PZ, Shaw JE. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med. 2013;30:387–98. doi: 10.1111/dme.12119. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Wu X, Liu L, Geng J, Yuan Z, Shan Z, et al. Prevalence of diabetic retinopathy in Mainland China:A meta-analysis. PLoS One. 2012;7:e45264. doi: 10.1371/journal.pone.0045264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song P, Yu J, Chan KY, Theodoratou E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China:A systematic review and meta-analysis. J Glob Health. 2018;8:010803. doi: 10.7189/jogh.08.010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asaria M, Mazumdar S, Chowdhury S, Mazumdar P, Mukhopadhyay A, Gupta I. Socioeconomic inequality in life expectancy in India. BMJ Glob Health. 2019;4:e001445. doi: 10.1136/bmjgh-2019-001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suwannaphant K, Laohasiriwong W, Puttanapong N, Saengsuwan J, Phajan T. Association between socioeconomic status and diabetes mellitus:The national socioeconomics survey, 2010 and 2012. J Clin Diagn Res. 2017;11:LC18–22. doi: 10.7860/JCDR/2017/28221.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assari S, Moghani Lankarani M, Piette J, Aikens J. Socioeconomic status and glycemic control in type 2 diabetes;Race by gender differences. Healthcare (Basel) 2017;5:83. doi: 10.3390/healthcare5040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly V, Unwin N, Sherriff P, Bilous R, Kelly W. Diabetes prevalence and socioeconomic status:A population based study showing increased prevalence of type 2 diabetes mellitus in deprived areas. J Epidemiol Community Health. 2000;54:173–7. doi: 10.1136/jech.54.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim NH, Kim TJ, Kim NH, Choi KM, Baik SH, Choi DS, et al. Relative and combined effects of socioeconomic status and diabetes on mortality:A nationwide cohort study. Medicine. 2016;95:e4403. doi: 10.1097/MD.0000000000004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee TC, Glynn RJ, Peña JM, Paynter NP, Conen D, Ridker PM, et al. Socioeconomic status and incident type 2 diabetes mellitus:Data from the women's health study. PLoS One. 2011;6:e27670. doi: 10.1371/journal.pone.0027670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramachandran A, Snehalatha C, Vijay V, King H. Impact of poverty on the prevalence of diabetes and its complications in urban southern India. Diabet Med. 2002;19:130–5. doi: 10.1046/j.1464-5491.2002.00656.x. [DOI] [PubMed] [Google Scholar]
- 45.Das T, Murthy GVS, Pant HB, Gilbert C, Rajalakshmi R, Behera UC. Regional variation in diabetic retinopathy and associated factors in spectrum of eye disease in diabetes study in India- Report #5. Indian J Ophthalmol. 2021;69:3095–101. doi: 10.4103/ijo.IJO_3620_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Misra R, Misra A, Kamalamma N, Vikram NK, Gupta S, Sharma S, et al. Difference in prevalence of diabetes, obesity, metabolic syndrome and associated cardiovascular risk factors in a rural area of Tamil Nadu and an urban area of Delhi. Int J Diabetes Dev Ctries. 2011;31:82–90. [Google Scholar]
- 47.Misra A, Ganda OP. Migration and its impact on adiposity and type 2 diabetes. Nutrition. 2007;23:696–708. doi: 10.1016/j.nut.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Misra A, Sharma R, Pandey RM, Khanna N. Adverse profile of dietary nutrients, anthropometry and lipids in urban slum dwellers of northern India. Eur J Clin Nutr. 2001;55:727–34. doi: 10.1038/sj.ejcn.1601214. [DOI] [PubMed] [Google Scholar]
- 49.Green R, Milner J, Joy EJM, Agrawal S, Dangour AD. Dietary patterns in India:A systematic review. Br J Nutr. 2016;116:142–8. doi: 10.1017/S0007114516001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma M, Kishore A, Roy D, Joshi K. A comparison of the Indian diet with the EAT-Lancet reference diet. BMC Public Health. 2020;20:812. doi: 10.1186/s12889-020-08951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popkin BM, Adair LS, Ng SW. Now and then:The global nutrition transition:The pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong MYZ, Man REK, Fenwick EK, Gupta P, Li LJ, van Dam RM, et al. Dietary intake and diabetic retinopathy:A systematic review. PLoS One. 2018;13:e01–2. doi: 10.1371/journal.pone.0186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross C, Mirowsky J. Why education is the key to socioeconomic differentials in health. In: Bird CE, Conrad P, Fremont AM, Timmermans S, editors. Handbook of Medical Sociology. 6th ed. Vanderbilt University Press; 2010. pp. 33–51. [Google Scholar]
- 54.Tao X, Li J, Zhu X, Zhao B, Sun J, Ji L, et al. Association between socioeconomic status and metabolic control and diabetes complications:A cross-sectional nationwide study in Chinese adults with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:61. doi: 10.1186/s12933-016-0376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachmann MO, Eachus J, Hopper CD, Davey Smith G, Propper C, Pearson NJ, et al. Socioeconomic inequalities in diabetes complications, control, attitudes and health service use:A cross-sectional study. Diabet Med. 2003;20:921–9. doi: 10.1046/j.1464-5491.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Ramos P, Jimenez-Carmona S, Alemany-Marquez P, Cordoba-Doña JA, Aguilar-Diosdado M. Socioeconomic deprivation and development of diabetic retinopathy in patients with type 1 diabetes mellitus. BMJ Open Diabetes Res Care. 2020;8:e001387. doi: 10.1136/bmjdrc-2020-001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das T, Murthy GS. Commentary:A health policy change would benefit a protocol-based screening for diabetic retinopathy in India. Indian J Ophthalmol. 2021;69:689–90. doi: 10.4103/ijo.IJO_2363_20. [DOI] [PMC free article] [PubMed] [Google Scholar]