ABSTRACT
Background:
Coronavirus disease-2019 (COVID-19) results in acute lung injury. This study examined the usefulness of serum transforming growth factor-beta 1 (TGF-β1) and connective tissue growth factor (CTGF) levels in predicting disease severity in COVID-19 patients with pulmonary involvement.
Methods:
Fifty patients with confirmed COVID-19 and pulmonary involvement between September 2020, and February 2021 (Group 1) and 45 healthy controls (Group 2) were classified into three subgroups based on clinical severity: moderate, severe, and critical pneumonia. Serum TGF-β1 and CTGF concentrations were measured on days 1 and 7 of admission in Group 1 using an enzyme-linked immunosorbent assay. These concentrations were also measured in control cases. The mean serum TGF-β1 and CTGF levels were then compared among COVID-19 patients, based on clinical severity.
Results:
Significantly higher mean serum TGF-β1 and CTGF levels were observed on both days in Group 1 than in the control group. The mean serum TGF-β1 and CTGF levels on day 7 were also significantly higher than those on day 1 in Group 1. The critical patient group had the highest serum TGF-β1 and CTGF levels on both days, and the difference between this group and the moderate and severe pneumonia groups was significant. Cutoff values of 5.36 ng/mL for TGF-β1 and 626.2 pg/mL for CTGF emerged as predictors of COVID-19 with pulmonary involvement in receiver-operating characteristic curve analysis.
Conclusions:
TGF-β1 and CTGF are potential markers that can distinguish COVID-19 patients with pulmonary involvement and indicate disease severity. These findings may be useful for initiating treatment for early-stage COVID-19.
Keywords: COVID-19, CTGF, Lung injury, SARS-COV-2, TGF-β1
INTRODUCTION
Respiratory system findings associated with coronavirus disease-2019 (COVID-19) infection derived from SARS-COV-2 virus may emerge with differing clinical severities. Advanced age, immunosuppression, cardiovascular diseases, hypertension, chronic pulmonary and renal diseases, liver diseases, malignancies, and severe obesity constitute risk factors for severe/critical COVID-19 1 .
Our knowledge of the histopathological changes in the lungs during COVID-19 infection is still limited. Changes occurring in the early stage of the disease are based on alterations in the lung tissues of patients operated on due to pulmonary tumors, and diagnosed immediately after surgery 2 , while late-stage findings rely on autopsy data 3 . Serous and/or fibrinous exudates and hyaline membranes in the alveoli, inflammatory cell infiltration, foci of fibroblastic proliferation, edema in the alveolar wall, hyaline thrombi in the vessels, and interstitial thickening accompanied by fibrosis are observed, and all signs of widespread alveolar damage are present in patients infected with SARS-CoV-2 2 , 3 .
The World Health Organization’s clinical classification considers COVID-19 under the headings of mild signs, pneumonia or severe pneumonia, acute respiratory distress syndrome (ARDS), severe sepsis, and septic shock 4 .
SARS-CoV-2 enters the upper respiratory tract through angiotensin-converting enzyme 2 (ACE2) receptors on the surface of the pulmonary epithelium. The virus infects pulmonary alveolar type II cells during their migration to the lower respiratory tract 5 , 6 .
Interstitial edema and hyaline membrane formation develop in the acute phase as a result of widespread alveolar damage in COVID-19 patients, while septal fibrosis and proliferation in fibroblasts develop in the chronic remodeling phase 7 . Whether patients will require long-term pulmonary rehabilitation after COVID-19 infection by causing chronic lung damage, is currently unclear 8 , 9 .
The excessive cytokine production seen in COVID-19 is associated lung injury development 10 , 11 . Cytokines, released as a result of macrophage , cause massive cell breakdown 12 . This is followed by a fibrotic phase involving growth factors, including connective tissue growth factor (CTGF) and transforming growth factor-beta1 (TGF-β1), in which repair mechanisms become operative 13 . TGF-β has three isoforms, TGF-β1, TGF-β2, and TGF-β3 14 . TGF-β1, in particular, leads to pulmonary fibrosis and the overproduction of extracellular matrix by stimulating the differentiation of fibroblasts into myofibroblasts 15 - 18 . CTGF is a protein whose expression is increased by TGF-β1, which is involved in fibroblast growth and extracellular matrix synthesis in the early period 19 , 20 .
This study aimed to investigate the mechanism of lung damage development in COVID-19 patients and to determine the association between serum TGF-β1 and CTGF levels and disease severity to contribute to the treatment of severe or chronic cases.
METHODS
Study design and participants
Ethical approval for this study was granted by the Republic of Turkish Ministry of Health COVID-19 Scientific Research Assessment Commission and Ethical Committee at Ataturk University Medical Faculty (No:B.30.2. Ata.0.01.00/209). Fifty patients had visited the Infectious Diseases and Clinical Microbiology Department between September 2020 and February 2021 due to symptoms including cough, sore throat, fever, muscle/joint pains, nasal discharge, shortness of breath, fatigue, and sudden loss of taste or smell, and diagnosed with COVID-19 on the basis of radiological and laboratory tests and physical examination, were enrolled as Group 1. Varying levels of pulmonary involvement were observed in these patients by radiological findings. All patients were hospitalized during treatment. On the seventh day of follow-up in the moderate pneumonia group, improvement was observed in initial symptoms, and intensive care was not required. Only two members in the severe pneumonia group required admission to the intensive care unit. Mortality occurred in four patients in the critical group. Forty-five healthy individuals were enrolled in Group 2 (control group). The patient underwent provided physical examinations and laboratory test results. All members of both groups were given detailed information concerning the research, and were enrolled following the receipt of written consent forms. Individuals with cancer, high blood pressure, cardiovascular disease, diabetes mellitus, chronic renal and liver diseases, and acute or chronic inflammatory diseases were excluded.
Definitions and diagnosis
Group 1: COVID-19 was confirmed by means of a SARS-CoV-2 qPCR Detection Kit (Bio-Speedy Bioeksen) employed for the detection of the SARS-CoV-2 virus. Nucleic acid isolates were obtained from the oropharyngeal and nasopharyngeal swab specimens. The diagnosis of COVID-19 was based on the interim guidelines published by the World Health Organization 4 . Patients with COVID-19 pneumonia were then classified into three subgroups based on the clinical severity during hospital admission: moderate, severe, and critical categories requiring intensive care. Clinical evaluation relied on thoracic computed tomography and laboratory tests.
Cases exhibiting findings including high fever, sore throat, muscle-joint ache, dry cough, and findings of moderate pneumonia on thoracic tomography were classified as moderate pneumonia.
Cases with findings including high fever, sore throat, muscle-joint ache, dry cough, tachypnea (>30/min), severe respiratory distress or SpO2 levels < 90%, and bilateral diffuse pneumonia on thoracic tomography were classified as severe pneumonia.
Critical illness was defined based on criteria applicable in cases of severe respiratory distress, sepsis, septic shock, or other conditions that would necessitate mechanical ventilation or vasopressor therapy.
Group 2 comprised healthy individuals. All members of Group 2 were RT-PCR-negative upon enrolment in the study.
Blood specimens
Blood specimens were collected from all patients on the first day for routine biochemistry tests, prior to commencement of any drug therapy and on the seventh day after the start of treatment 21 , 22 . Blood specimens were allowed to clot for 30 min and then centrifuged at +4°C for 15 min. The resulting serum specimens were stored at -80°C until TGF-β and CTGF analyses.
Analyte assay techniques
TGF-β1 and CTGF values were determined using the ELISA method with a Human TGF-β1 ELISA kit (Elabscience, Human TGF-β1: cat. E-EL-H0110, Texas, USA) and a human CTGF ELISA kit (Elabscience, Human CTGF: E-EL-H0828, Texas, USA) in accordance with the manufacturer’s recommendations. The measurement ranges for TGF-β1 and CTGF were 31.25-2000 pg/mL and 62.5-4000 pg/mL, respectively. For TGF-β1, the kit exhibited an intra-assay coefficient of variation (CV) of 5.4% and an inter-assay CV of 4.8%. For CTGF, the intra-assay CV was 5.1% and the inter-assay CV was 4.9%.
Serum C-reactive protein (CRP, mg/L) and lactate dehydrogenase (LDH, U/L) levels investigated during routine tests were measured on a Beckman Coulter AU5821 device present in the biochemistry laboratory, ferritin (ng/mL) and troponin-I (ng/L) levels were measured on a Beckman Coulter DxI800 device, and procalcitonin levels (ng/mL) on a Roche Cobas 6000 device, using commercial kits. The erythrocyte sedimentation rate (ESR) was measured on a StaRRsed device using the Westergren method, and the results were presented in mm/h. White blood cell (WBC), lymphocyte, and neutrophil counts were measured using a Sysmex XN-9000 device (Japan) and expressed as cells/µL. D-dimer levels (ng/mL) were determined using a Radiometer AQT90 Flex device.
Statistical analysis
Data were recorded and analyzed using SPSS software for Windows (version 20.0; SPSS Inc., Chicago, IL, USA). Categorical variables were presented as numbers and percentages. Numerical variables are presented as mean ± standard deviation. Visual (histogram) and analytical methods (Kolmogorov-Smirnov or Shapiro-Wilk tests) were used to evaluate the normality of the distribution of variables. The chi-square test was applied to compare categorical variables, and the t-test or one-way analysis of variance, as appropriate, was used to compare continuous variables. The significance of differences between groups was assessed using the post-hoc Tukey test. Pearson’s correlation coefficient was used for linear correlation analysis. The receiver-operating characteristic curve (ROC) method, which indicates the predictive power of a specific method, was employed to determine TGF-β1 and the sensitivity, specificity, area under the curve (AUC), and cutoff values. Statistical significance was set at P < 0.05.
RESULTS
Group 1 comprised 24 (48%) women and 26 (52%) men, while the control group comprised 21 (46.7%) women and 24 (53.3%) men. The mean ages were 47.02 ± 15.22 and 51.69 ± 16.17 years in Group 1 and the control group, respectively. No significant age or sex differences were observed between the groups (P = 0.89 and P = 0.16, respectively). When COVID-19 patients were classified in terms of clinical characteristics, moderate pneumonia was present in 20 (40%), severe pneumonia in 15 (30%), and critical pneumonia in 15 (30%). The patients' symptoms on hospital admission and clinical outcomes are shown in Table 1. Radiological examination of the patients revealed a ground glass appearance in the pulmonary parenchyma, ground glass together with consolidation, septal thickening, fibrosis, air bronchograms, reverse halo sign, nodule, and pleural effusion (Supplementary Table 1).
TABLE 1: Patients' symptoms at hospital admission and clinical outcomes.
| General data | Overall (n = 50) |
|---|---|
| Onset-inpatient interval (n) | 50 (100%) |
| Signs and symptoms on admission | |
| High fever | 50 (100%) |
| Dry cough | 50 (100%) |
| Expectoration | 22 (44%) |
| Dyspnea | 32 (64%) |
| Muscle ache | 27 (54%) |
| Headache | 15 (30%) |
| Sore throat | 18 (36%) |
| Chest tightness | 15 (30%) |
| Nausea and vomiting | 5 (10%) |
| Treatment | n = 50 |
| Oxygen therapy | 30 (60%) |
| Intensive care unit | 15 (30%) |
| Antibiotic treatment | 30 (60%) |
| Antiviral treatment | 31 (62%) |
| Glucocorticoids | 28 (56%) |
| Clinical outcome | |
| Length of hospitalization (days) | |
| Median (min-max) | 9 (4-30) |
| Died | 4 (8%) |
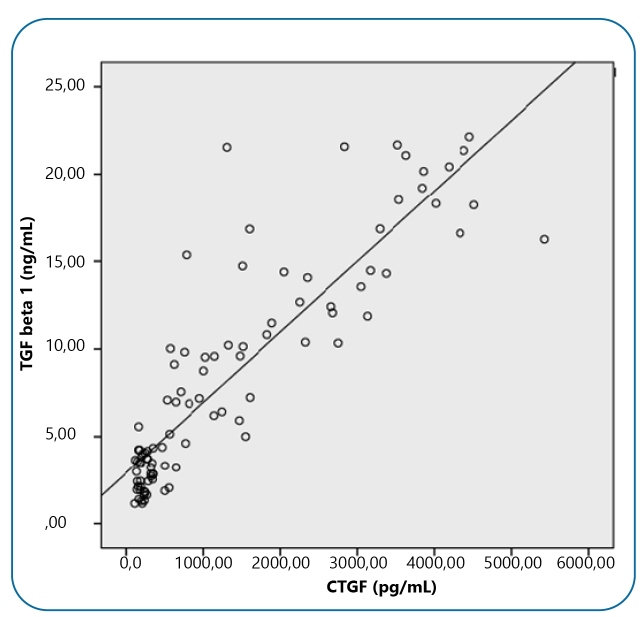
TGF-β1 and CTGF levels and routine laboratory parameters of the groups are shown in Table 2. Routine test results on day 1, such as LDH, D-dimer, troponin-I, ESR, CRP, and ferritin levels, were significantly higher in the patient group than in the control group (P < 0.05), while WBC, lymphocyte, and neutrophil counts were lower than those in the control group (P < 0.05). Routine laboratory test results on day 7 after medical treatment were similar to those of the control group (P > 0.05). However, the serum TGF-β1 and CTGF levels on day 1 and 7 were significantly higher than those in the control group (P < 0.05). Moreover, serum TGF-β1 and CTGF levels on day 7 were significantly higher than those on day 1 (P < 0.001). A strong, significant, positive correlation was observed between serum TGF-β1 and CTGF levels (r=0.884, P < 0.001) (Supplementary Figure 1).
TABLE 2: Study groups’ TGF-β1, CTGF, and other laboratory parameter results .
| Group 1 (n=50) | Group 2 (n=45) | P | |
|---|---|---|---|
| TGF-β1 | |||
| 1st day (ng/mL) | 3.86±1.86 | 2.95±1.14 | 0.005a |
| 7th day (ng/mL) | 13.14±5.10 | <0.001b | |
| CTGF | |||
| 1st day (pg/mL) | 762.14±465.10 | 276.96±146.46 | <0.001 |
| 7th day (pg/mL) | 2291.27±1323.42 | ||
| WBC | |||
| 1st day (cells/µL) | 4939.80±1204.61 | 6892.19±1638.15 | <0.001a |
| 7th day(cells/µL) | 6556.24±1535.87 | 0.305b | |
| Neutrophil | |||
| 1st day (cells/µL) | 2846.20±959.09 | 3953.56±995.86 | <0.001a |
| 7th day (cells/µL) | 3702.5±1212.64 | 0.276b | |
| Lymphocyte | |||
| 1st day (cells/µL) | 1152±413.03 | 2000.56±654.63 | <0.001a |
| 7th day (cells/µL) | 2319±2270.656 | 0.366b | |
| LDH | |||
| 1st day (U/L) | 427.24±213.79 | 192.84±39.62 | <0.001a |
| 7th day (U/L) | 199.62±32.50 | 0.363b | |
| D-DIMER | |||
| 1st day (ng/mL) | 1310.74±935.06 | 290.31±102.84 | <0.001a |
| 7th day (ng/mL) | 324.1±128.77 | 0.164b | |
| Troponin-I | |||
| 1st day (ng/L) | 5.79±7,81 | 2.5±1.33 | 0.006a |
| 7th day (ng/L) | 2.38±1.44 | 0.669b | |
| ESR | |||
| 1st day (mm/h) | 44.8±27.35 | 9.24±3.63 | <0.001a |
| 7th day (mm/h) | 10.52±8.84 | 0.37b | |
| CRP | |||
| 1st day (mm/h) | 75.89±58.23 | 3.63±1.42 | <0.001a |
| 7th day (mm/h) | 4.37±2.27 | 0.062b | |
| Ferritin | |||
| 1st day (ng/mL) | 453.12±312.72 | 208.49±66.48 | <0.001a |
| 7th day (ng/mL) | 183.23±74.47 | 0.086b | |
| Procalcitonin | |||
| 1st day (ng/mL) | 0.13±0.43 | 0.05±0.08 | 0.194a |
| 7th day (ng/mL) | 0.03±0.02 | 0.189b |
Values are presented as the mean ± standard deviation. a: Group 1-1st day and Group 2, b: Group 1-7th day and Group 2. TGF-β1: transforming growth factor-beta 1; CTGF: connective tissue growth factor; WBC: white blood cell; LDH: lactate dehydrogenase; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein.
TGF-β1 and CTGF levels of the patients in Group 1 were compared in terms of clinical severity (Figure 1). Serum TGF-β1 and CTGF levels correlated with clinical severity levels. The critical patient group had the highest serum TGF-β1 and CTGF levels on day 1 and 7. Two-way comparisons of all groups established on the basis of clinical severity revealed significant variation between CTGF levels on day 1 and 7. However, while there was no statistically significant difference between day 1 serum TGF-β1 levels in the two-way comparisons, a significant variation was observed on day 7 (Table 3).
FIGURE 1: Box-plot presentation of serum TGF-β1 and CTGF levels on day 7 of admission in Group 1 divided by severity of COVID-19 disease. *:p<0.001 (analysis of variance) two-way comparison of subgroups.

TABLE 3: Changes in serum TGF-β1 and CTGF levels among disease severity subgroups.
| Moderate pneumonia (n=20) | Severe pneumonia (n=15) | Critical level (n=15) | P | |
|---|---|---|---|---|
| TGF-β1 | ||||
| 1st day (ng/mL) | 3.59±1,67 | 3.78±1.76 | 4.31±2.20 | >0.05* |
| 7th day (ng/mL) | 8.51±2.54 | 12.86±1.67 | 9.59±2.03 | <0.001* |
| CTGF | ||||
| 1st day (pg/mL) | 446.13±250.30 | 750.88±449.03 | 1194.74±356.59 | <0.05* |
| 7th day (pg/mL) | 1080.15±373.95 | 2385.23±704.44 | 3812.13±927.88 | <0.001* |
Values expressed as mean±standard deviation. *Two-way comparison of subgroups.
Serum TGF-β1 levels were moderately, positively, and significantly correlated with D-dimer, ESR, ferritin, CRP, and LDH levels (r = 0.692, r = 0.724, r = 0.545, r = 0.808, and r = 0.663, respectively; P< 0.001 for all). In addition, CTGF levels were positively and moderately correlated with D-dimer, ESR, ferritin, C-reactive protein, and lactate dehydrogenase levels (r = 0.671, r = 0.645, r = 0.576, r = 0.800, and r = 0.694, respectively; P < 0.001 for all). Serum TGF-β1 and CTGF levels were strongly correlated with CRP levels.
The ROC curve method was used to establish the diagnostic sensitivity and specificity of COVID-19 patients’ serum TGF-β1 and CTGF levels. At cutoff values of 345.51 pg/mL for day 1 serum CTGF and 626.2 pg/mL for day 7 serum CTGF (AUC = 0.858, P< 0.001, 95% confidence interval [CI] = 0.78 - 0.94 and AUC = 0.984, P < 0.001, 95% confidence interval [CI] =0.85 - 0.99 respectively), sensitivity was 84% and 94%, and specificity was 82% and 91%, respectively, for distinguishing patients with diagnosed with COVID-19 with lung injury from those without lung injury (Figure 2).
FIGURE2: Determination of the diagnostic sensitivity and specificity of serum TGF-β1 and CTGF levels on days 1 and 7 of admission in patients diagnosed with COVID-19 using ROC curve analysis. ROC: receiver-operating characteristic curve.

At cutoff levels of 3.39 ng/mL for serum TGF-β1 on day 1 and 5.36 ng/mL for serum TGF-β1 on day 7, (AUC = 0.638, P = 0.021, 95% confidence interval [CI] = 0.53 -0.75 and AUC = 0.976, P < 0.001, 95% confidence interval [CI] = 0.82 - 0.97 respectively), sensitivity was 64% and 96%, and specificity was 51% and 89%, respectively, for discriminating patients diagnosed with COVID-19 developing lung injury from those without lung injury (Figure 2).
DISCUSSION
The principal findings of this prospective study may be summarized as follows. First, COVID-19 patients with lung injury showed higher TGF-β1 and CTGF levels than the control group. Second, routine laboratory test values began to fall on the seventh day, after medical treatment. However, serum TGF-β1 and CTGF levels continued to increase. They may be a useful guide for clinicians in evaluating lung damage. Third, TGF-β1 and CTGF levels on day 7 in particular exhibited high sensitivity and specificity for distinguishing COVID-19 patients with lung damage from those without such damage. Finally, both TGF-β1 and CTGF levels significantly correlated with illness severity in COVID-19 patients. The study outcomes emphasize the role of TGF-β1 and CTGF in lung injury in COVID-19 patients.
TGF-β is synthesized as an inactive large precursor protein called the latency-associated peptide (LAP). LAP, a latent TGF-β is unable to bind to its receptor and is known as the inactive form of TGF-β. The intracellular protease cleaves LAP-1 from the rest of the protein to yield the biologically active TGF- β 23 . TGF-β is a multifunctional cytokine known to exhibit profibrogenic, anti-inflammatory, and immunosuppressive activities that increase during and after both sepsis and COVID-19, with the probability of halting the hyperinflammatory response 24 . TGF-β is also a powerful immunosuppressive factor that significantly inhibits immune functions and delays recovery by reducing cell recruitment and downregulating cytokine production 25 , 26 .
SARS-CoV-2, the agent involved in the transmission of COVID-19, causes pulmonary parenchymal damage through ACE2 27 . Damage occurring in the lung tissue leads to the overexpression and release of cytokines and a series of growth factors, such as TGF-β and CTGF 28 . Studies have shown an association between COVID-19 and ARDS, which causes widespread lung damage. Pulmonary fibrosis can develop in long-term ARDS patients 29 . Pulmonary fibrosis characteristically exhibits the overproduction and accumulation of extracellular matrix proteins, including fibronectin. COVID-19 patients with pulmonary involvement have a high risk of pulmonary fibrosis 30 . Although SARS-CoV-2 infection significantly affects lung tissue, serum TGF-β1 and CTGF levels in Covid-19 patients on day 1 and 7, have not been previously reported. The multifunctional roles of TGF-β and CTGF in lung injury and fibrosis are well known 13 , 15 - 17 .
In their study of transgenic mice, Ponticos et al. demonstrated the profibrotic effect of CTGF in pulmonary fibrosis and reported that CTGF may represent a target molecule in the treatment of diseases that progress with fibrosis 31 .
Cui et al. showed that inhibition of the NF-kB/TGF-beta/Smad2/3 pathway in mice exhibited a protective effect against pulmonary fibrosis 32 .
Increased synthesis of TGF-β and its correlation with disease severity have also been reported in chronic lung diseases, such as idiopathic pulmonary fibrosis 33 .
Xu et al. 34 investigated the mechanism of pulmonary fibrosis in patients with COVID-19 pneumonia. The authors reported that SARS-COV-2 virus binds to the ACE-2 receptor, and that this activation activates genes associated with fibrosis. They also suggested that expression of proteins such as TGF-β and CTGF at the genetic level increases as a result of that activation, and that the various stages of pulmonary fibrosis are stimulated. In the present study, TGF-β1 and CTGF levels on day 1 and 7 were higher in COVID-19 patients than in the control group (P < 0.05). The levels of ESR, CRP, LDH, troponin-I, and D-dimer used in the diagnosis and follow-up of treatment in COVID-19 patients were higher than those in the control group on day 1 (P < 0.05), while WBC, neutrophil, and lymphocyte values were low (P < 0.05). Laboratory tests on day 7 of treatment approached the reference range in the healthy control group (P > 0.05). However, TGF-β1 and CTGF levels continued to increase (P < 0.05). This suggests that the tests used are insufficient for follow-up of the damage caused by the virus in the lungs. However, TGF-β1 and CTGF levels correlated with disease severity.
Radiography and computed tomography are of indisputable importance in the diagnosis and follow-up of lung damage in COVID-19 patients. However, notwithstanding their considerable benefits to both diagnosis and disease management, the most important disadvantage of the widespread use of imaging methods, is their potential contribution to radiation exposure and the spread of disease. Disease transmission in radiology units is primarily caused by surface contamination following droplet spread 35 . Radiological imaging revealed various lesions in the lung parenchyma of patients included in the present study. Day 7 serum TGF-β1 and CTGF levels exhibited high sensitivity and specificity for differentiating lung damage in COVID-19 patients (sensitivity, 96%; specificity, 89% for TGF-β1; sensitivity, 94%; and specificity, 91% for CTGF). There is an urgent need for new assistant laboratory markers capable of evaluating lung damage in COVID-19 patients, which can be easily analyzed and interpreted with high sensitivity and specificity, and that can be measured in blood. TGF-β1 and CTGF are two important potential candidates for use in blood tests and imaging methods.
In conclusion, the findings of this study suggest that serum TGF-β1 and CTGF levels may be used to estimate clinical severity in patients with COVID-19 and pulmonary involvement. Further studies investigating the power of serum TGF-β1 and CTGF levels in predicting the development of persistent lung disease in patients with COVID-19 are required. Moreover, the efficiency of various anti-TGF-β1 therapies in attenuating post-COVID lung sequelae should be investigated.
SUPPLEMENTARY MATERIAL.
SUPPLEMENTARY TABLE 1: Frequencies and morphological characteristics of patient lesions on computed tomography.
| Tomography Findings | Patient | |
|---|---|---|
| Number | % | |
| Ground glass | 34 | 68 |
| Consolidation | 23 | 46 |
| Ground glass+consolidation | 21 | 42 |
| Nodule | 8 | 16 |
| Septal thickening | 10 | 20 |
| Fibrosis | 11 | 22 |
| Air bronchogram | 7 | 15 |
| Reverse halo | 3 | 6 |
| Pleural effusion | 5 | 10 |
SUPPLEMENTARY FIGURE 1: Correlation chart between serum TGF-β1 and CTGF levels on days 1 and 7 of admission in Group 1.
Footnotes
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial Support: Supported by the Ataturk University “Individual Research Project” under project code no. TSA-2020-8564.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Ethical consent: Ethical approval for the study was granted by the Ethical Committee of the Ataturk University Medical Faculty (No:B.30.2. ATA.0.01.00/209).
REFERENCES
- 1.Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76(2):428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 2.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. [2021 Sep 20]. Available from: https://www.who.int/publications/i/item/10665-332299 .
- 5.Mossel EC, Wang J, Jeffers S, Edeen KE, Wang S, Cosgrove GP, et al. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372(1):127–135. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinheimer VK, Becher A, Tönnies M, Holland G, Knepper J, Bauer TT, et al. Influenza A viruses target type II pneumocytes in the human lung. J Infect Dis. 2012;206(11):1685–1694. doi: 10.1093/infdis/jis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaz de Paula CB, de Azevedo MLV, Nagashima S, Martins APC, Malaquias MAS, Miggiolaro A, et al. IL-4/IL-13 remodeling pathway of COVID-19 lung injury. Sci Rep. 2020;10(1):18689–18689. doi: 10.1038/s41598-020-75659-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4–4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazzeri M, Lanza A, Bellini R, Bellofiore A, Cecchetto S, Colombo A, et al. Respiratory physiotherapy in patients with COVID-19 infection in acute setting: a Position Paper of the Italian Association of Respiratory Physiotherapists (ARIR) Monaldi Arch Chest Dis. 2020;90(1) doi: 10.4081/monaldi.2020.1285. [DOI] [PubMed] [Google Scholar]
- 10.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607–2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422–422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 14.Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des. 2003;9(1):39–49. doi: 10.2174/1381612033392341. [DOI] [PubMed] [Google Scholar]
- 15.Wei P, Xie Y, Abel PW, Huang Y, Ma Q, Li L, et al. Transforming growth factor (TGF)-β1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis. Cell Death Dis. 2019;10(9):670–670. doi: 10.1038/s41419-019-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9(3):111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 18.Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, et al. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol. 2008;40(3):484–495. doi: 10.1016/j.biocel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21(6):473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 20.Moussad EE, Brigstock DR. Connective tissue growth factor: what's in a name? Mol Genet Metab. 2000;71(1-2):276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- 21.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34:101663–101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boivin GP, O'Toole BA, Orsmby IE, Diebold RJ, Eis MJ, Doetschman T, et al. Onset and progression of pathological lesions in transforming growth factor-beta 1-deficient mice. Am J Pathol. 1995;146(1):276–288. [PMC free article] [PubMed] [Google Scholar]
- 23.Coutts A, Chen G, Stephens N, Hirst S, Douglas D, Eichholtz T, et al. Release of biologically active TGF-beta from airway smooth muscle cells induces autocrine synthesis of collagen. Am J Physiol Lung Cell Mol Physiol. 2001;280(5):L999-1008. doi: 10.1152/ajplung.2001.280.5.L999. [DOI] [PubMed] [Google Scholar]
- 24.Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022–1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng J, Chen W, Zhu HJ. The immune suppressive function of transforming growth factor-β (TGF-β) in human diseases. Growth Factors. 2015;33(2):92–101. doi: 10.3109/08977194.2015.1010645. [DOI] [PubMed] [Google Scholar]
- 26.Didierlaurent A, Goulding J, Hussell T. The impact of successive infections on the lung microenvironment. Immunology. 2007;122(4):457–465. doi: 10.1111/j.1365-2567.2007.02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med. 2010;4(6):759–771. doi: 10.1586/ers.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J Med Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60(7):2142–2155. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 32.Cui Y, Xin H, Tao Y, Mei L, Wang Z. Arenaria kansuensis attenuates pulmonary fibrosis in mice via the activation of Nrf2 pathway and the inhibition of NF-kB/TGF-beta1/Smad2/3 pathway. Phytother Res. 2021;35(2):974–986. doi: 10.1002/ptr.6857. [DOI] [PubMed] [Google Scholar]
- 33.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125(2):754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Xu X, Jiang L, Dua K, Hansbro PM, Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res. 2020;21(1):182–182. doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296(2):E32-e40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]