Abstract
One exercise session can elevate insulin-stimulated glucose uptake (GU) by skeletal muscle, but it is uncertain if this effect is accompanied by altered membrane cholesterol content, which is reportedly inversely related to insulin-stimulated GU. Muscles from sedentary (SED) or exercised 3hours post-exercise (3hPEX) rats were evaluated for: GU, membrane cholesterol, and phosphorylation of cholesterol regulatory proteins (pHMCGRSer872 and pABCA1Ser2054). Insulin-stimulated GU for 3hPEX exceeded SED. Membrane cholesterol, pHMCGRSer872 and pABCA1Ser2054 did not differ between groups.
Novelty
Alterations in membrane cholesterol and phosphorylation of proteins that regulate muscle cholesterol are not essential for elevated insulin-stimulated GU in skeletal muscle after acute exercise.
Keywords: glucose uptake, membrane cholesterol, exercise
Introduction
Skeletal muscle is the major site for insulin-stimulated glucose disposal (DeFronzo et al., 1981). One exercise session can increase insulin-stimulated glucose uptake (GU) in skeletal muscle for 2-48 h post-exercise (Cartee, 2015, Wojtaszewski et al., 2003, Funai et al., 2009). However, the mechanisms involved in this important exercise benefit remain incompletely understood.
An inverse relationship has been reported between membrane cholesterol content and insulin-stimulated GU in skeletal muscle. (Sanchez-Aguilera et al., 2018, Grice et al., 2019, Habegger et al., 2012b). A recent study observed that chronic exercise by insulin resistant mice normalized their elevated membrane cholesterol content concomitant with improved insulin-stimulated GU in skeletal muscle (Ambery et al., 2017). However, little is known about the acute exercise effect on muscle membrane cholesterol.
3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a rate-limiting enzyme in cholesterol synthesis, is phosphorylated and inactivated by AMPK (Clarke and Hardie, 1990, Sato et al., 1993). Increased membrane cholesterol content is associated with activation of HMGCR in myocytes (Habegger et al., 2012b). Evidence suggests that ATP-binding cassette transporter A1 (ABCA1), which plays a major role in cellular cholesterol efflux (Larrede et al., 2009, Wang and Tall, 2003), may influence glucose tolerance and insulin sensitivity (Key et al., 2017, Sanchez-Aguilera et al., 2018, de Haan et al., 2014).
Our primary goal was to investigate if increased insulin-stimulated GU post-exercise was accompanied by lower membrane cholesterol in muscle from rats. We also assessed exercise effects on the phosphorylation of key cholesterol regulatory proteins (HMGCR and ABCA1) in skeletal muscle.
Materials and Methods
Materials
Chemicals were obtained from Sigma-Aldrich (St. Louis,MO) or Fisher Scientific (Hanover Park,IL) unless otherwise noted. Pierce MemCode Reversible Protein Stain Kit (#24585), Bicinchoninic acid protein assay (#23225), Tissue Protein Extraction Reagent (TPER; #78510) and Amplex™ Red Cholesterol Assay Kit (#A12216) were obtained from Thermo Fisher Scientific (Waltham,MA). Anti-phospho Akt Ser473 (pAktSer473; #9271), anti-phospho Akt Thr308 (pAktThr308; #13038), anti-Akt (#4691), anti-phospho AS160 Thr642 (pAS160Thr642; #8881), anti-phospho AMPKα Thr172 (pAMPKαThr172; #2531), anti-AMP-activated protein kinase-α (AMPKα; #5831), anti-ryanodine receptor 1 (RyR1; #8153), anti-insulin receptor (IR; #3025), anti-α-Tubulin (#2144) and anti-rabbit IgG horseradish peroxidase conjugate (#7074) were from Cell Signaling Technology (Danvers,MA). Anti-Akt Substrate of 160 kDa (AS160; #ABS54) was from EMD Millipore (Billerica,MA). Anti-HMG-CoA Reductase (HMGCR, #BS-5068R) and anti-phospho HMG-CoA Reductase Ser872 (pHMGCRSer872, #BS-4063R) were from Bioss Antibodies (Woburn,MA). Anti-ATP Binding Cassette Subfamily A Member 1 (ABCA1, #NB400-105SS) was from Novus Biologicals (Littleton,CO). Anti-phospho ABCA1 Ser2054 was from Abcam (Cambridge,MA). 2-Deoxy-D-[3H]-glucose ([3H]-2-DG) and [14C]-mannitol were from Perkin Elmer (Boston,MA).
Animals
Animal care procedures were approved by the University of Michigan Committee on Use and Care of Animals and performed based on the guidelines from the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA. Male Wistar rats (12-15 weeks-old) were housed on a 12:12 hour light:dark cycle (lights out at 1700) with access to water and chow.
Exercise
Food was removed from rats at ~17:00 the night before the experiment. The following morning (~08:30), rats were randomly assigned to sedentary or exercise (swimming in a barrel filled with water,35°C, for 4×30min bouts, with 5min rest between bouts) groups.
Muscle Incubation for Glucose Uptake
Rats were anesthetized (intraperitoneal ketamine/xylazine injection) at 3h post-exercise (3hPEX) along with sedentary rats. Both epitrochlearis muscles were dissected out and placed in vials that were gassed (95% O2, 5% CO2) in a temperature-controlled water bath (35°C). During step 1 (30minutes), muscles were incubated with Krebs Henseleit (KHB) supplemented with 0.1% bovine serum albumin (BSA), 2mM sodium pyruvate, 6mM mannitol ±insulin (0.6nM). During step 2 (20minutes), muscles were incubated with KHB/BSA, the same insulin concentration as step 1, 1mM 2-DG (2.25 mCi/mmol3H–2-DG), and 9 mM mannitol (0.022 mCi/mmol 14C-mannitol). After step 2, muscles were blotted, freeze-clamped, and stored (−80°C) until analyzed.
Muscle Lysate Preparation
Frozen muscles were weighed and homogenized as previously described (Arias et al., 2007).
2-Deoxy-D-glucose (2-DG) Uptake
2-DG uptake was calculated as previously described (Cartee and Bohn, 1995).
Membrane fractionation and cholesterol content
Membrane-enriched and cytosol-depleted fractions were obtained by differential centrifugation as previously described (Grice et al., 2019). Membrane enrichment was assessed by immunoblotting with antibodies against membrane marker proteins (insulin receptor, IR; Na+/K+-ATPase; ryanodine receptor 1, RYR1) and a cytosolic marker protein (α-tubulin). Cholesterol in the membrane-enriched fraction was determined using the Amplex Red Cholesterol Assay Kit (Thermo Scientific; #A12216) as previously described (Grice et al., 2019).
Immunoblotting
Immunblotting was performed as previously described (Arias et al., 2007). Samples from SED and 3hPEX groups were loaded in alternating lanes of the gels.
Statistical Analysis
Student’s t-test was used for comparison between two groups. Two-way analysis of variance (ANOVA) was used to identify main effects of insulin (basal or insulin) and exercise (SED or 3hPEX). Post-hoc analysis used the Tukey test.
Results
2-Deoxy-D-glucose Uptake
There were significant main effects of insulin (insulin>basal, P<0.01) and exercise (3hPEX>SED; P<0.01, Figure 1) for GU. Post-hoc analysis indicated insulin-stimulated muscles had greater GU than paired muscles incubated without insulin for both SED and 3hPEX groups (P<0.05). GU by insulin-stimulated muscles from the 3hPEX group exceeded SED-controls (P<0.01).
Figure 1.
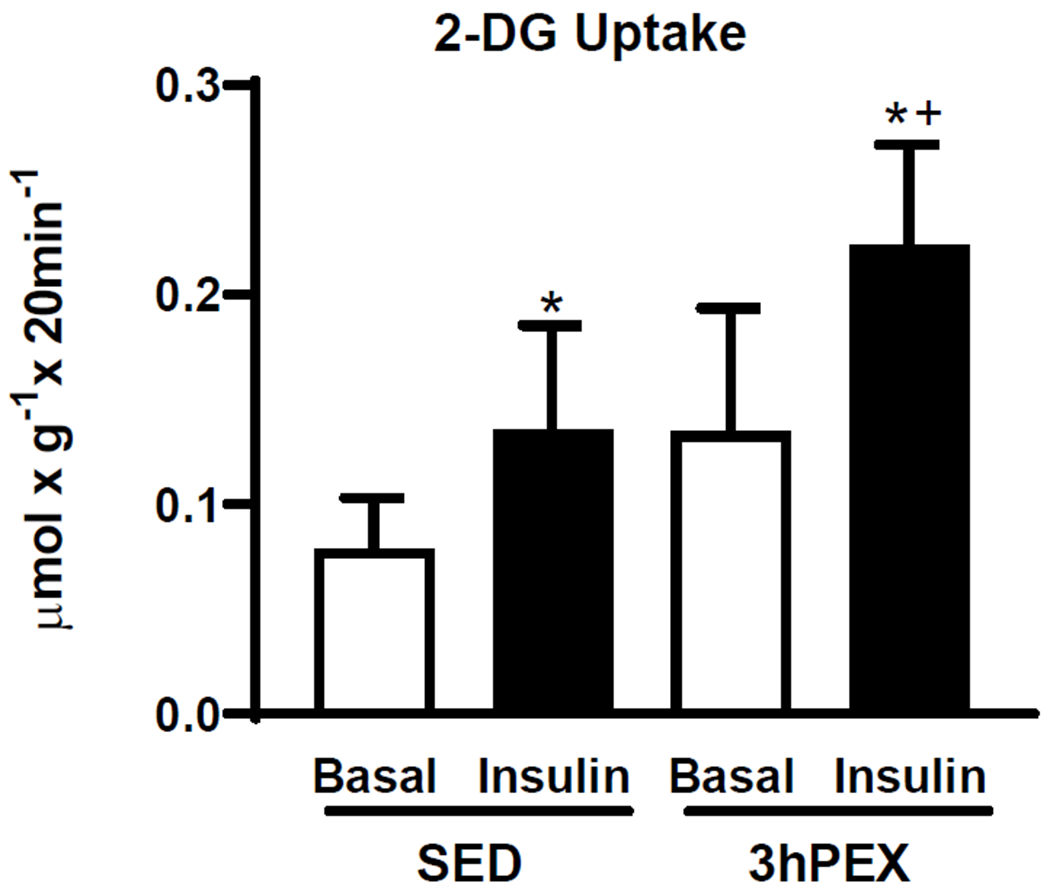
2-DG uptake in paired muscles incubated±insulin (100μU/ml). *P<0.05 Insulin versus Basal in both SED and 3hPEX; †P<0.01, 3hPEX versus SED with insulin. Data were analyzed by two-way ANOVA, values are means±SD; n=5-6 per group.
Membrane Cholesterol Content
The membrane fraction was enriched with membrane markers (IR, Na+/K+-ATPase, and RYR1) and depleted for the cytosolic marker (α-tubulin, Figure 2A). Membrane cholesterol was unaltered after exercise (Figure 2B).
Figure 2.
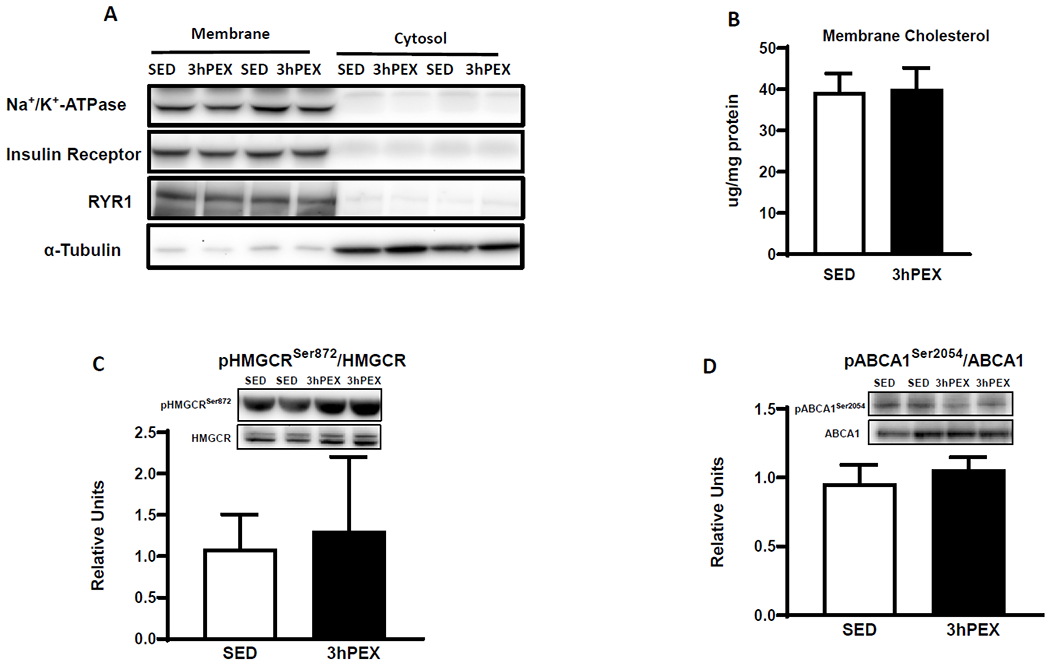
A: Blots of membrane and cytosolic marker proteins, B: Membrane cholesterol content, n=9 per group. C: pHMGCRSer872/HMGCR and D: pABCA1Ser2054/ABCA1, in muscles, n=5-6 per group. Data were analyzed by Student’s t-test, values are means±SD.
Immunoblotting
HMGCR and ABCA1 Phosphorylation
pHMGCRSer872/HMGCR and pABCA1Ser2054/ABCA1 values did not differ between 3hPEX and SED groups (Figures 2C and 2D).
Akt and AS160 Phosphorylation
There was a significant main effect of insulin (insulin>basal, P<0.001, Figures 3A and 3B) for pAkt on Thr308 and Ser473. Post-hoc analysis indicated insulin-treated muscles exceeded paired muscles incubated without insulin for SED and 3hPEX groups (P<0.01 for pAktThr308/Akt; P<0.001 for pAktSer473/Akt).
Figure 3.
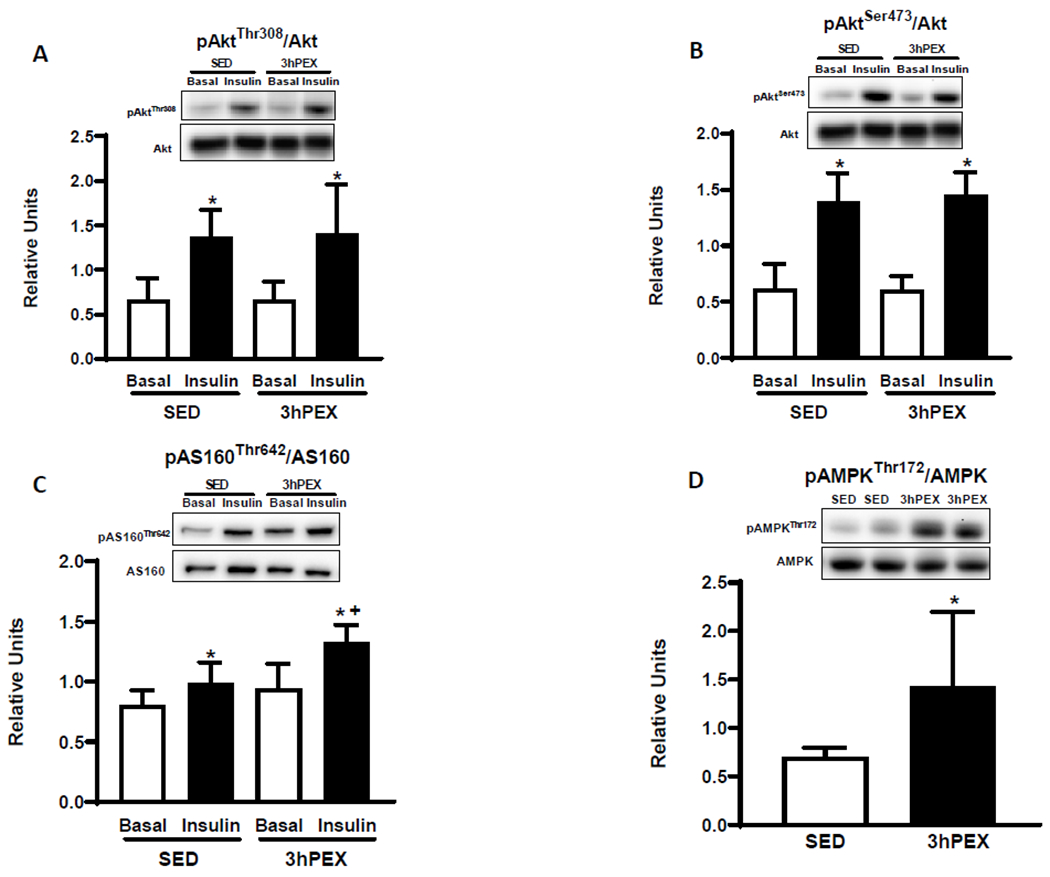
A: pAktThr308/Akt, B: pAktSer473/Akt, C: pAS160Thr642/AS160 in paired muscles incubated±insulin (100μU/ml). *Insulin versus Basal in both the SED and the 3hPEX groups (P<0.01 for pAktThr308/Akt, P<0.001 for pAktSer473/Akt, P<0.05 for pAS160Thr642/AS160). †P<0.01, 3hPEX versus SED with insulin. Data were analyzed by two-way ANOVA, Values are means ±SD; n=5-6 per group. D: pAMPKThr172/AMPK, in muscles. *P<0.05; 3hPEX versus SED. Data were analyzed by Student’s t-test, values are means±SD; n=5-6 per group.
For pAS160Thr642/AS160, there were significant main effects of insulin (insulin>basal, P< 0.001) and exercise (3hPEX>SED; P<0.01, Figure 3C). Post-hoc analysis indicated insulin-treated muscles had greater pAS160Thr642/AS160 than paired muscles incubated without insulin for both SED and 3hPEX groups (P<0.05). pAS160Thr642/AS160 was significantly greater (P<0.01) for 3hPEX compared with SED controls with insulin.
AMPKα Phosphorylation
The pAMPKαThr172/AMPKα values were significantly greater (P<0.05, Figure 3D) for the 3hPEX group versus SED controls.
Discussion
Previous studies reported an inverse relationship between membrane cholesterol and insulin-stimulated GU in muscle (Llanos et al., 2015, Habegger et al., 2012b). Insulin-stimulated GU was reportedly decreased in muscles from insulin-resistant mice by a mechanism related to membrane cholesterol content (Ambery et al., 2017, Grice et al., 2019). Impaired insulin-stimulated GU was found to be associated with increased membrane cholesterol content in rat L6 myotubes (Habegger et al., 2012a, Habegger et al., 2012b). Normalization of excess membrane cholesterol improved insulin-stimulated GU in myocytes and myofibers (Habegger et al., 2012b, Sanchez-Aguilera et al., 2018, Llanos et al., 2015). A recent study reported that 2 weeks of exercise training improved insulin-stimulated GU concomitant with reduced membrane cholesterol content in muscle from high-fat-fed, insulin-resistant mice (Ambery et al., 2017). These studies suggest that membrane cholesterol can regulate insulin-stimulated GU. However, earlier studies have not probed the effect of acute exercise on muscle membrane cholesterol. Consistent with earlier research (Arias et al., 2007, Wang et al., 2018, Schweitzer et al., 2012), greater insulin-stimulated GU was observed in the current study after one exercise session. The major novel finding was that this exercise-benefit on insulin-stimulated GU was not attributable to reduced membrane cholesterol.
HMGCR and ABCA1 are key cholesterol regulatory proteins. Earlier research indicated activation of HMGCR regulates membrane cholesterol in myocytes (Habegger et al., 2012b). Cholesterol was elevated and insulin-stimulated GU was reduced in the adipose tissue of ABCA1-deficient mice (de Haan et al., 2014). Insulin-resistant humans versus healthy humans were characterized by increased plasma cholesterol and downregulation of the ABC transporter pathway in muscle (Tonks et al., 2016). One study reported evidence that the expression of ABCA1 contributes to lowering cholesterol accumulation and improved GU of myofibers in the insulin-resistance condition (Sanchez-Aguilera et al., 2018). In this context, it is notable that the absence of an exercise effect on membrane cholesterol content in muscles in the current study was accompanied by unaltered phosphorylation of HMGCR or ABCA1. The current results for acute exercise in healthy rats do not eliminate the possibility that improved insulin sensitivity after acute or chronic exercise by insulin resistant animals or humans may involve mechanisms related to membrane cholesterol.
AMPK activation has been linked to enhanced insulin sensitivity (Fisher et al., 2002, Kjobsted et al., 2016), and AMPK can phosphorylate and thereby inhibit HMGCR (Sato et al., 1993, Clarke and Hardie, 1990). A previous study provided evidence that activating AMPK using 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside enhanced insulin sensitivity via lowering membrane cholesterol in rat L6 myotubes (Habegger et al., 2012a). In the current study, the effect of exercise pAMPKThr172 remained detectable at 3hPEX. However, pHMGCRSer872 and membrane cholesterol content did not differ between the 3hPEX and SED-controls, suggesting that the putative relationship between AMPK and insulin sensitivity post-exercise depends on other mechanisms.
Substantial evidence links Akt substrate of 160 kDa (AS160) to insulin-stimulated GU (Sano et al., 2003, Chen et al., 2011, Cartee, 2015). In the current study, greater AS160 phosphorylation on Thr642 was accompanied by improved insulin-stimulated GU, consistent with earlier research (Schweitzer et al., 2012, Wang et al., 2018, Castorena et al., 2014). These results indicate that altered membrane cholesterol content was unnecessary for elevated AS160 phosphorylation post-exercise.
The enhanced insulin-stimulated GU in this study was reminiscent of results after treadmill exercise by rodents, or after treadmill or cycling exercise by humans (Cartee, 2015). In rats performing the exercise protocol used in this study, we previously measured insulin-stimulated GU by the extensor digiti quinti proprius (EDQP), a forelimb muscle with a fiber-type composition similar to the epitrochlearis (Wang et al., 2018). Swim-exercise did not alter insulin-stimulated GU in the EDQP, arguing against the possibility that the exercise-effect in the epitrochlearis was a nonspecific stress response.
The current study revealed that acute exercise-induced reduction in membrane cholesterol content is not essential for elevated insulin-stimulated GU in muscle from healthy rats. Furthermore, the lack of decreased membrane cholesterol content post-exercise was consistent with the observation of unaltered phosphorylation of HMGCR Ser872 and ABCA1 Ser2054, proteins with important roles in regulating cholesterol.
Acknowledgement
This research was supported by grants from the National Institutes of Health (DK71771). We thank Dominic Thorley and Gengfu Dong for research assistance.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- AMBERY AG, TACKETT L, PENQUE BA, BROZINICK JT & ELMENDORF JS 2017. Exercise training prevents skeletal muscle plasma membrane cholesterol accumulation, cortical actin filament loss, and insulin resistance in C57BL/6J mice fed a western-style high-fat diet. Physiological Reports, 5. doi: ARTN e1336310.14814/phy2.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARIAS EB, KIM J, FUNAI K & CARTEE GD 2007. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism, 292, E1191–E1200. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- CARTEE GD 2015. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab, 309, E949–59. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTEE GD & BOHN EE 1995. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol, 268, E902–9. [DOI] [PubMed] [Google Scholar]
- CASTORENA CM, ARIAS EB, SHARMA N & CARTEE GD 2014. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes, 63, 2297–308. doi: 10.2337/db13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN S, WASSERMAN DH, MACKINTOSH C & SAKAMOTO K 2011. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab, 13, 68–79. doi: S1550-4131(10)00447-X [pii] 10.1016/j.cmet.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE PR & HARDIE DG 1990. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. Embo j, 9, 2439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE HAAN W, BHATTACHARJEE A, RUDDLE P, KANG MH & HAYDEN MR 2014. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. Journal of Lipid Research, 55, 516–523. doi: 10.1194/jlr.M045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEFRONZO RA, JACOT E, JEQUIER E, MAEDER E, WAHREN J & FELBER JP 1981. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes, 30, 1000–7. [DOI] [PubMed] [Google Scholar]
- FISHER JS, GAO J, HAN DH, HOLLOSZY JO & NOLTE LA 2002. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab, 282, E18–23. [DOI] [PubMed] [Google Scholar]
- FUNAI K, SCHWEITZER GG, SHARMA N, KANZAKI M & CARTEE GD 2009. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab, 297, E242–51. doi: 00194.2009 [pii] 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRICE BA, BARTON KJ, COVERT JD, KREILACH AM, TACKETT L, BROZINICK JT & ELMENDORF JS 2019. Excess membrane cholesterol is an early contributing reversible aspect of skeletal muscle insulin resistance in C57BL/6NJ mice fed a Western-style high-fat diet. American Journal of Physiology-Endocrinology and Metabolism, 317, E362–E373. doi: 10.1152/ajpendo.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEGGER KM, HOFFMAN NJ, RIDENOUR CM, BROZINICK JT & ELMENDORF JS 2012a. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology, 153, 2130–41. doi: 10.1210/en.2011-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEGGER KM, PENQUE BA, SEALLS W, TACKETT L, BELL LN, BLUE EK, GALLAGHER PJ, STUREK M, ALLOOSH MA, STEINBERG HO, CONSIDINE RV & ELMENDORF JS 2012b. Fat-induced membrane cholesterol accrual provokes cortical filamentous actin destabilisation and glucose transport dysfunction in skeletal muscle. Diabetologia, 55, 457–67. doi: 10.1007/S00125-011-2334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEY CC, LIU M, KURTZ CL, CHUNG S, BOUDYGUINA E, DINH TA, BASHORE A, PHELAN PE, FREEDMAN BI, OSBORNE TF, ZHU X, MA L, SETHUPATHY P, BIDDINGER SB & PARKS JS 2017. Hepatocyte ABCA1 Deletion Impairs Liver Insulin Signaling and Lipogenesis. Cell Rep, 19, 2116–2129. doi: 10.1016/j.celrep.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJOBSTED R, WOJTASZEWSKI JF & TREEBAK JT 2016. Role of AMP-Activated Protein Kinase for Regulating Post-exercise Insulin Sensitivity. Exs, 107, 81–126. doi: 10.1007/978-3-319-43589-3_5. [DOI] [PubMed] [Google Scholar]
- LARREDE S, QUINN CM, JESSUP W, FRISDAL E, OLIVIER M, HSIEH V, KIM MJ, VAN ECK M, COUVERT P, CARRIE A, GIRAL P, CHAPMAN MJ, GUERIN M & LE GOFF W 2009. Stimulation of Cholesterol Efflux by LXR Agonists in Cholesterol-Loaded Human Macrophages Is ABCA1-Dependent but ABCG1-Independent. Arteriosclerosis Thrombosis and Vascular Biology, 29, 1930–U538. doi: 10.1161/Atvbaha.109.194548. [DOI] [PubMed] [Google Scholar]
- LLANOS P, CONTRERAS-FERRAT A, GEORGIEV T, OSORIO-FUENTEALBA C, ESPINOSA A, HIDALGO J, HIDALGO C & JAIMOVICH E 2015. The cholesterol-lowering agent methyl-β-cyclodextrin promotes glucose uptake via GLUT4 in adult muscle fibers and reduces insulin resistance in obese mice. Am J Physiol Endocrinol Metab, 308, E294–305. doi: 10.1152/ajpendo.00189.2014. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-AGUILERA P, DIAZ-VEGAS A, CAMPO C, QUINTEROS-WALTEMATH O, CERDA-KOHLER H, BARRIENTOS G, CONTRERAS-FERRAT A & LLANOS P 2018. Role of ABCA1 on membrane cholesterol content, insulin-dependent Akt phosphorylation and glucose uptake in adult skeletal muscle fibers from mice. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids, 1863, 1469–1477. doi: 10.1016/j.bbalip.2018.09.005. [DOI] [PubMed] [Google Scholar]
- SANO H, KANE S, SANO E, MIINEA CP, ASARA JM, LANE WS, GARNER CW & LIENHARD GE 2003. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem, 278, 14599–602. [DOI] [PubMed] [Google Scholar]
- SATO R, GOLDSTEIN JL & BROWN MS 1993. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc Natl Acad Sci U S A, 90, 9261–5. doi: 10.1073/pnas.90.20.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWEITZER GG, ARIAS EB & CARTEE GD 2012. Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation. J Appl Physiol (1985), 113, 1852–61. doi: 10.1152/japplphysiol.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONKS KT, COSTER AC, CHRISTOPHER MJ, CHAUDHURI R, XU A, GAGNON- BARTSCH J, CHISHOLM DJ, JAMES DE, MEIKLE PJ & GREENFIELD JR 2016. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity, 24, 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, ARIAS EB, PATAKY MW, GOODYEAR LJ & CARTEE GD 2018. Postexercise improvement in glucose uptake occurs concomitant with greater γ3-AMPK activation and AS160 phosphorylation in rat skeletal muscle. Am J Physiol Endocrinol Metab, 315, E859–e871. doi: 10.1152/ajpendo.00020.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG N & TALL AR 2003. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol, 23, 1178–84. doi: 10.1161/01.ATV.0000075912.83860.26. [DOI] [PubMed] [Google Scholar]
- WOJTASZEWSKI JF, JØRGENSEN SB, FRØSIG C, MACDONALD C, BIRK JB & RICHTER EA 2003. Insulin signalling: effects of prior exercise. Acta Physiol Scand, 178, 321–8. doi: 10.1046/j.1365-201X.2003.01151.x. [DOI] [PubMed] [Google Scholar]


