Abstract
The difference in substrate selectivity of the maltodextrin (LamB) and sucrose (ScrY) porins is attributed mainly to differences in loop L3, which is supposed to constrict the lumen of the pores. We show that even a single mutation (D201Y) in loop L3 leads to a narrowing of the substrate range of ScrY to that resembling LamB. In addition, we removed the putative N-terminal coiled-coil structure of ScrY and studied the effect of this deletion on sucrose transport.
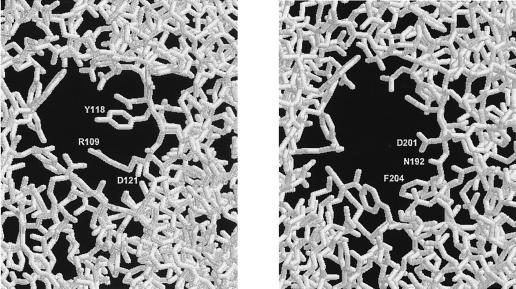
The maltodextrin (LamB) and sucrose (ScrY) porins in the outer membranes of enteric bacteria allow efficient uptake of oligosaccharides even at low substrate concentrations (for recent reviews see references 14, 24, and 28). According to X-ray studies, both functional LamB (4, 13, 16) and ScrY (7) are trimers in which each monomer forms an 18-stranded antiparallel β-barrel. The β-sheets are connected by short turns toward the periplasmic side and by longer loops facing the exterior of the cell, with the exception of loop L3. This loop is folded into the lumen of the barrel and constitutes the central part of a constriction zone within the channel, which is directly involved in substrate recognition and binding. LamB seems to be optimized for maltose and maltooligosaccharides (1, 15, 26), whereas ScrY shows a broader substrate range which also includes sucrose (19, 22, 26). From crystallographic studies (7, 27) this difference in substrate selectivity is explained mainly by the wider pore of ScrY. In LamB, residues R109 and Y118 of loop L3 seem to sterically hinder the bulky sucrose molecule to permeate through the constriction zone. In ScrY these amino acids are replaced by the shorter residues N192 and D201, respectively (Fig. 1). In addition, D121 of LamB, which is thought to be involved in substrate binding (27), is also changed in ScrY (to F204), whereas the remaining residues lining the constriction zones of both porins are highly conserved. To study the contribution of these amino acids to the substrate selectivity of these porins we replaced them in ScrY with the amino acids found in LamB at the corresponding positions (i.e., N192R, D201Y, and F204D) and determined the transport properties of these mutants. In addition, we studied the influence of the amino-terminal region of ScrY on the sucrose and maltose transport kinetics. Compared to that of LamB, the mature form of ScrY contains 71 extra amino acid residues at its N-terminal end (9, 19). Residues 4 to 45 of this extension are supposed to form a coiled-coil structure (7), the function of which is not yet known. A deletion mutant lacking amino acids 3 to 72 was described by Schülein et al. (23) as still active and showing increased similarity to LamB in regard to its channel properties. Therefore, we constructed and analyzed a mutant with a similar deletion in combination with a triple mutation in L3. Furthermore, we transferred the 5′ deleted scrY allele back into the scr operon located on a single-copy plasmid to study its effect on the sucrose metabolism under physiological conditions.
FIG. 1.
Cross section through the constriction zone of LamB (left) and ScrY (right) viewed from the periplasmic side. The three amino acid residues of loop L3 that differ in both porins are marked. Data were derived from the Protein Data Bank, Brookhaven National Laboratory, and handled by using the program RasWin Molecular Graphics.
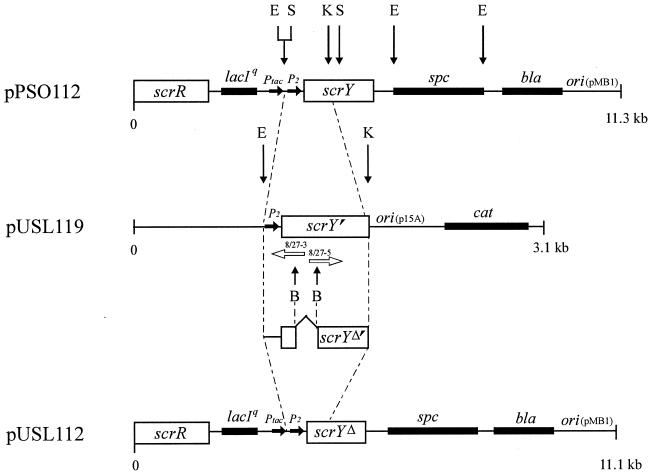
Since the unregulated high-level expression of scrY is deleterious to cells (19) we cloned in a first step an 0.8-kb EcoRI-KpnI DNA fragment of pPSO112 (19), coding only for the amino-terminal part of ScrY, into pSU19 (12), yielding pUSL119 (Fig. 2). In detail, this fragment ranges from nucleotides −71 to +745, i.e., it carries the promoter scrYp and codes for the first 229 amino acids of the ScrY precursor. Subsequently, a PCR was carried out with the divergent mutagenesis primer pair 8/27-3 and 8/27-5 (Fig. 2), leading to an amplified DNA fragment with two newly introduced BssHII restriction sites near each end. Cutting with BssHII and subsequent religation generated an in-frame deletion of 183 bp in scrY that did not cause any amino acid exchanges, as confirmed by sequencing. The deletion comprises base pairs 67 to 249 of scrY, i.e., amino acid residues 1 to 61 of the mature protein, and thus removes most of the amino-terminal extension of ScrY, including the putative coiled-coil structure. The replacement of the original 0.8-kb EcoRI-KpnI fragment of pPSO112 (ScrY+) with the deletion fragment resulted in pUSL112 (ScrYΔ61), which allows the adjustable expression of the mutant porin from the two regulated promoters scrYp and Ptac.
FIG. 2.
Schematic representation of the construction of pUSL112 carrying the 5′ deleted scrY allele under the control of the hybrid promoter Ptac (3) and the scrY promoter P2. The deletion was introduced through two newly constructed BssHII restriction sites by PCR with the primer pair 8/27-3 (5′-GGTGCTTATATCCGTGCGCGCATGGGCTGAGGCAGCGC-3′) and 8/27-5 (5′-GTGGCTCAGCGTACCGCGCGCCTTGAGAAAAAAGCCG-3′) indicated by two divergent white arrows in the figure. The BssHII restriction sites in the primer sequences are underlined. The EcoRI-KpnI fragment from pPSO112 cloned into pUSL112 is marked by dashed lines. The promoters Ptac and P2 are not drawn to scale. B, BssHII; E, EcoRI; K, KpnI; S, SphI.
To generate the amino acid exchanges D201Y, F204D, and N192R, we cloned a subfragment containing the coding region for loop L3 from both pPSO112 (ScrY+) and pUSL112 (ScrYΔ61) into pAlter-1 (Promega). The mutagenesis procedure was carried out according to the supplier’s protocol with the mutagenesis primers 5′-CGAGGAACACGACATAAGAGTCAATCCA-3′ (D201Y), 5′-CCGGCGAGGTCCACGACATCAGAGTC-3′ (F204D), and 5′-GTGAATATCGAATCTGTCGCGGTCGAAACG-3′ (N192R) and the N192R primer together with primer 5′-GGTACCGGCGAGGTCCACGACATAAGAGTCAATCCA-3′ for the triple mutation (exchanged base pairs are underlined). The mutated region of scrY was then transferred back into pPSO112 and pUSL112 to generate pPSO117 (ScrYD201Y), pPSO118 (ScrYF204D), pPSO119 (ScrYN192R), pUSL3113 (ScrYL3) and pUSL3213 (ScrYΔ61-L3) and sequenced as a control.
To check the expression levels of the different sucrose porin mutants we transferred the different scrY alleles into the LamB− mutant PS9, a spontaneous λR Mal+ mutant from wild-type Escherichia coli K-12 (19). Outer membranes were isolated by sucrose gradient centrifugation, and the proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described previously (19). Changes in the molecular weights of the deletion forms of ScrY could be observed (Mr, about 45,000 compared to 53,000 for the wild-type porin), whereas the amounts of ScrY molecules in the outer membranes remained essentially the same for the wild type and the mutants (data not shown).
To measure the effect of the mutations in ScrY on the carbohydrate uptake in intact cells, we determined the apparent Km values (Kmapps) for sucrose and maltose transport in PS9 carrying either the different ScrY plasmids or, for comparison, pTROY9 carrying lamB (3a). Maltose uptake was through the chromosomally encoded MalEFGK transport system and sucrose uptake was through the constitutively expressed sucrose-PTS (EIIScr), which was supplied by pPSO101 (scrA+) (19). Transport was assayed in whole cells as described previously (17), with 14C-labelled substrates at concentrations ranging from 6 to 156 μM for sucrose and from 3 to 40 μM for maltose. Under these conditions diffusion through the outer membrane is the limiting step in the overall uptake, unless specific porins facilitate the diffusion. Thus, in the presence of a specific porin the transport rate increases, leading to a decrease of the Kmapp for the overall transport. The following results were obtained (also summarized in Table 1). (i) Neither the N-terminal deletion nor the amino acid exchanges in ScrY significantly changed the Kmapp for the maltose transport, indicating that the general function and particularly the permeability of maltose are not substantially impaired in the mutant sucrose porins. (ii) The triple mutation in L3 of ScrY increased the Kmapp of the sucrose transport about 25-fold to 100 μM, resembling the Kmapp of 85 μM for LamB. Most of this effect can be attributed to the D201Y exchange, as tested in the single mutant (Kmapp = 90 μM). The D201Y exchange obviously constricts the eyelet in a way similar to LamB, hindering the bulky sucrose molecules from passing through the ScrYD201Y porin, as predicted from the crystal structures (7, 27) (Fig. 1). Surprisingly, only a minor effect was found for the N192R mutation (Kmapp = 10 μM), which also had been expected to lead to a smaller pore. Finally, the F204D exchange had essentially no influence on the sucrose uptake rate (Kmapp = 5 μM) during the high-level expression of the porin. (iii) The deletion of the coiled-coil structure in ScrYΔ61 did not change the Kmapp (4 μM) for the sucrose transport in comparison with that of the wild-type sucrose porin. However, in combination with the triple mutation in loop L3 this deletion lowered the Kmapp for sucrose uptake from 100 μM (ScrYL3) to 30 μM (ScrYΔ61-L3). Thus, in the absence of the coiled coil the diffusion of sucrose through the outer membrane seems to increase. At present we do not know whether this is due to an enhanced flux through the sucrose porin or whether the properties of the cell wall, including the general porins OmpF and OmpC, are influenced in some way.
TABLE 1.
Apparent Km values for sucrose and maltose uptake in strain PS9/pPSO101 (scrA+) carrying different scrY alleles or lamB+, respectivelya
| Plasmid | Glycoporin |
Kmapp (μM)
|
|
|---|---|---|---|
| Sucrose | Maltose | ||
| None | None | 250 | 400 |
| pPSO112 | ScrY | 4 | 7 |
| pTROY9 | LamB | 85 | 3 |
| pPSO117 | ScrYD201Y | 90 | 4 |
| pPSO118 | ScrYF204D | 5 | 3 |
| pPSO119 | ScrYN192R | 10 | 3 |
| pUSL3113 | ScrYL3 | 100 | 5 |
| pUSL112 | ScrYΔ61 | 4 | 4 |
| pUSL3213 | ScrYΔ61-L3 | 30 | 6 |
Strain PS9/pPSO101 lacking LamB but expressing EIIScr constitutively from pPSO101 was used as the host for the different porin-carrying plasmids. For transport experiments cells were grown in Lennox broth (11) without glucose, containing the appropriate antibiotics for maintenance of the various plasmids as well as maltose (0.2% [wt/vol]) for the induction of the chromosomally encoded MalEFGK transport system. For induction of the sucrose porins, 2 mM concentrations of IPTG (isopropyl-β-d-thiogalactopyranoside) were added 1 h before cells were harvested at approximately 4 × 108 cells per ml. Uptake was measured as described previously (17), with [14C]sucrose and [14C]maltose. Kmapp values were based on the initial uptake rates.
It is conceivable that the amino-terminal extension of ScrY becomes more important under limiting conditions, e.g., during prolonged growth at low substrate concentrations or when the truncated porin is not overexpressed from a multicopy plasmid. We thus reintroduced the 5′ deleted scrY allele into an otherwise complete scr regulon which was then transposed onto a single-copy F′ plasmid. In particular, we started with plasmid pKJL710 (gift from K. Jahreis, Osnabrück, Germany), which contains the scr regulon of pUR400 (18) cloned between two inverted repeats of the transposon Tn1721 (21). The 1.0-kb SphI fragment of that plasmid carrying the N-terminal part of wild-type scrY was exchanged with the corresponding truncated 0.8-kb SphI fragment of pUSL112 encoding ScrYΔ61. Both the mutated and the wild-type scr regulons were then allowed to transpose onto the F′8 (gal+) plasmid (6). The transposase of Tn1721 was expressed from pPSO110, a derivative of pACYC184 (2) that carries the gene for the transposase (tnpA), under the control of the hybrid promoter Ptac (3).
The F′ plasmids containing either the wild-type (F′scr1) or the mutated (F′scr3) scr regulon were transferred by conjugation into E. coli K-12 strain S136 (20), and the presence of the different scrY alleles was confirmed by PCR. To test the influence of the N-terminal deletion in ScrYΔ61 on the porin function we measured the sucrose transport of induced and uninduced cells of both S136/F′scr1 and S136/F′scr3. As an internal control for appropriate induction levels of the scr regulons we also assayed the invertase activities of both strains (Table 2). No significant differences in sucrose transport and invertase activities were found between the two strains under these conditions, indicating again that the mutation in scrY has no or only a little effect on the function of the sucrose porin.
TABLE 2.
Sucrose transport and invertase activity in strain S136 carrying either the wild-type (F′scr1) or the mutated (F′scr3) scr regulon on single-copy F8 plasmids
| Plasmid | Glycoporin | Inductiona | Sucrose transportb | Invertase activityb |
|---|---|---|---|---|
| F′scr1 | ScrY+ | Noninduced | 28 | 6 |
| Induced | 522 | 71 | ||
| F′scr3 | ScrYΔ61 | Noninduced | 25 | 4 |
| Induced | 577 | 69 |
Cells were grown in minimal medium (25) with either glycerol (noninduced) or sucrose (induced for the scr genes) as the carbon source (0.2% [wt/vol]).
Sucrose uptake and invertase activity were tested as described previously (17). Transport activities for sucrose (0.8 μM) are given in picomoles per minute per milligram of protein. Invertase activities are expressed in nanomoles per minute per milligram of protein.
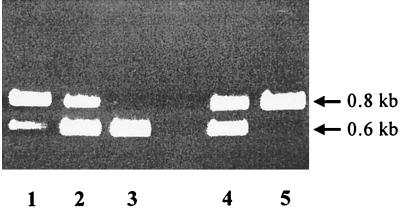
Growth competition experiments can reveal even minor changes in the fitness of bacteria exposed to selective environmental conditions. For example, low substrate concentrations strongly select for the optimization of transport activities (references in references 5, 8, and 10), and cells with even slightly improved porins should be enriched in such media. Therefore, we mixed equal amounts of strains S136/F′scr1 and S136/F′scr3 and incubated this mixture in 10 ml of a minimal medium containing either sucrose or glucose as the sole carbon source (5 μM). The cultures were inoculated with a titer of 103 cells per ml, grown for approximately 10 generations to stationary phase, diluted in fresh media to 103 cells per ml, and grown once more for a further 10 generations. We subsequently estimated the cell number ratios of both strains by PCR amplifying a DNA fragment that differed in length for both strains due to the deletion in scrY. As a result of four independent experiments a significant shift in the cell ratios towards the strain with the wild-type ScrY (S136/F′scr1) was observed in the sucrose medium, while the ratio remained constant in the glucose control medium (Fig. 3). Thus, the presence of the coiled-coil structure in ScrY is apparently advantageous for cells growing at low sucrose concentrations. This at first sight contradicts the enhanced sucrose uptake found in the deletion mutant ScrYΔ61-L3, compared to ScrYL3 (Table 1). However, considering the different synthesis rate of the sucrose porin in both experiments (single-copy versus multicopy vectors) one can speculate that on the one hand, the coiled-coil structure reduces the flux through a porin for sterical reasons and, on the other hand, it stabilizes the active trimer structure which becomes relevant especially when only a few porins are present in the outer membrane. Further studies are needed to elucidate the putative function of the coiled-coil structure in the facilitated diffusion of sucrose through ScrY.
FIG. 3.
Agarose gel electrophoresis of PCR products obtained from mixed cultures of strains S136/F′scr1 and S136/F′scr3. PCR products of 0.8 kb were expected with plasmid F′scr1 as a template carrying the wild-type scrY allele, and products of 0.6 kb were expected with plasmid F′scr3 containing the deleted form of scrY. Lanes 1 through 3, samples from a reconstruction experiment with premixed cultures of S136/F′scr1 and S136/F′scr3 without competitive growth. Strains were mixed to the following ratios of S136/F′scr1 to S136/F′scr3: 10:1 (lane 1), 1:1 (lane 2), and 1:10 (lane 3). Lanes 4 and 5, mixed cultures grown for about 20 generations in minimal media containing either glucose (lane 4) or sucrose (lane 5) as the sole carbon source (5 μM concentrations of each). The lengths of the fragments are indicated at the right.
Acknowledgments
We thank Knut Jahreis for providing plasmid pJKL710 and Bernadette Wulfern for preparing the photographs.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB171) and the Verband der Chemischen Industrie.
REFERENCES
- 1.Benz R, Schmid A, Vos-Scheperkeuter G H. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J Membr Biol. 1987;100:21–29. doi: 10.1007/BF02209137. [DOI] [PubMed] [Google Scholar]
- 2.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Boer H W, Comstock L J, Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci USA. 1983;80:21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.de Vries G E, Raymond C R, Ludwig R A. Extension of bacteriophage λ host range: selection, cloning, and characterization of a constitutive λ receptor gene. Proc Natl Acad Sci USA. 1984;81:6080–6084. doi: 10.1073/pnas.81.19.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutzler R, Wang Y-F, Rizkallah P J, Rosenbusch J P, Schirmer T. Crystal structures of various maltooligosaccharides bound to maltoporin reveal a specific sugar translocation pathway. Structure. 1996;4:127–134. doi: 10.1016/s0969-2126(96)00016-0. [DOI] [PubMed] [Google Scholar]
- 5.Ferenci T. Adaptation to life at micromolar nutrient levels: the regulation of Escherichia coli glucose transport by endoinduction and cAMP. FEMS Microbiol Rev. 1996;532:1–17. doi: 10.1111/j.1574-6976.1996.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 6.Fiethen L, Starlinger P. Mutations in the galactose operator. Mol Gen Genet. 1970;108:322–330. doi: 10.1007/BF00267769. [DOI] [PubMed] [Google Scholar]
- 7.Forst D, Welte W, Wacker T, Diederichs K. Structure of the sucrose-specific porin ScrY from Salmonella typhimurium and its complex with sucrose. Nat Struct Biol. 1998;5:37–46. doi: 10.1038/nsb0198-37. [DOI] [PubMed] [Google Scholar]
- 8.Harder W, Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–24. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- 9.Hardesty C, Ferran C, DiRienzo J M. Plasmid-mediated sucrose metabolism in Escherichia coli: characterization of scrY, the structural gene for a phosphoenolpyruvate-dependent sucrose phosphotransferase system outer membrane porin. J Bacteriol. 1991;173:449–456. doi: 10.1128/jb.173.2.449-456.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lengeler J W. Carbohydrate transport in bacteria under environmental conditions, a black box? Antonie Leeuwenhoek. 1993;63:275–288. doi: 10.1007/BF00871223. [DOI] [PubMed] [Google Scholar]
- 11.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 12.Martinez E, Bartolomé B, de la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacZα reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988;68:159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- 13.Meyer J E W, Hofnung M, Schulz G E. Structure of maltoporin from Salmonella typhimurium ligated with a nitrophenyl-maltotrioside. J Mol Biol. 1997;266:761–775. doi: 10.1006/jmbi.1996.0823. [DOI] [PubMed] [Google Scholar]
- 14.Nikaido H. Porins and specific diffusion channels in bacterial outer membranes. J Biol Chem. 1994;269:3905–3908. [PubMed] [Google Scholar]
- 15.Saurin W, Francoz E, Martineau P, Charbit A, Dassa E, Duplay P, Gilson E, Molla A, Ronco G, Szmelcman S, Hofnung M. Periplasmic binding protein dependent transport systems for maltose and maltodextrins: some recent studies. FEMS Microbiol Rev. 1989;63:53–60. doi: 10.1111/j.1574-6968.1989.tb14100.x. [DOI] [PubMed] [Google Scholar]
- 16.Schirmer T, Keller T A, Wang Y-F, Rosenbusch J P. Structural basis for sugar translocation through maltoporin channels at 3.2 Å resolution. Science. 1995;267:512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- 17.Schmid K, Schupfner M, Schmitt R. Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol. 1982;151:68–76. doi: 10.1128/jb.151.1.68-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid K, Ebner R, Altenbuchner J, Schmitt R, Lengeler J W. Plasmid-mediated sucrose metabolism in Escherichia coli K-12: mapping of the scr genes of pUR400. Mol Microbiol. 1988;2:1–8. doi: 10.1111/j.1365-2958.1988.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmid K, Ebner R, Jahreis K, Lengeler J W, Titgemeyer F. A sugar-specific porin, ScrY, is involved in sucrose uptake in enteric bacteria. Mol Microbiol. 1991;5:941–950. doi: 10.1111/j.1365-2958.1991.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt R. Analysis of melibiose mutants deficient in α-galactosidase and thiomethylgalactoside permease II in Escherichia coli K-12. J Bacteriol. 1968;96:462–471. doi: 10.1128/jb.96.2.462-471.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt R, Altenbuchner J, Wiebauer K, Arnold W, Pühler A, Schöffl F. Basis of transposition and gene amplification by Tn1721 and related tetracycline resistance transposons. Cold Spring Harbor Symp Quant Biol. 1981;45:59–65. doi: 10.1101/sqb.1981.045.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Schülein K, Schmid K, Benz R. The sugar-specific outer membrane channel ScrY contains functional characteristics of general diffusion pores and substrate-specific porins. Mol Microbiol. 1991;5:2233–2241. doi: 10.1111/j.1365-2958.1991.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 23.Schülein K, Andersen C, Benz R. The deletion of 70 amino acids near the N-terminal end of the sucrose-specific porin ScrY causes its functional similarity to LamB in vivo and in vitro. Mol Microbiol. 1995;17:757–767. doi: 10.1111/j.1365-2958.1995.mmi_17040757.x. [DOI] [PubMed] [Google Scholar]
- 24.Schulz G E. Porins: general to specific, native to engineered passive pores. Curr Opin Struct Biol. 1996;6:485–490. doi: 10.1016/s0959-440x(96)80113-8. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka S, Lerner S A, Lin E C C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967;93:642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulmke C, Lengeler J W, Schmid K. Identification of a new porin, RafY, encoded by raffinose plasmid pRSD2 of Escherichia coli. J Bacteriol. 1997;179:5783–5788. doi: 10.1128/jb.179.18.5783-5788.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y-F, Dutzler R, Rizkallah P J, Rosenbusch J P, Schirmer T. Channel specificity: structural basis for sugar discrimination and differential flux rates in maltoporin. J Mol Biol. 1997;272:56–63. doi: 10.1006/jmbi.1997.1224. [DOI] [PubMed] [Google Scholar]
- 28.Welte W, Nestel U, Wacker T, Diederichs K. Structure and function of the porin channel. Kidney Int. 1995;48:930–940. doi: 10.1038/ki.1995.374. [DOI] [PubMed] [Google Scholar]