Abstract
Background
Gastric cancer (GC) is a common type of gastrointestinal tumor in the world. Transfer RNA (tRNA) derived fragments (tsRNAs) implicate various cancers, but their roles in GC remain unclear. Our study aimed to investigate the potential biological functions and molecular mechanisms of tsRNAs in GC.
Methods
Differentially expressed tsRNAs were identified using high-throughput sequencing. The expression levels of tsRNAs were validated in 62 paired GC tissues and adjacent normal tissues using RT-qPCR. In vitro functional assays were used to evaluate the influences of tsRNAs on GC cells. The potential mechanisms underlying tsRNAs were explored using bioinformatics analysis,RT-qPCR, RNA immunoprecipitation assays and Western blot.
Results
We found that tiRNA-Val-CAC-001 was downregulated in GC tissues and cells, and demonstrated that tiRNA-Val-CAC-001 was a tsRNA sheared from mature tRNA-Val and mainly localized in the cytoplasm. tiRNA-Val-CAC-001 overexpression inhibited metastasis and proliferation but promoted apoptosis of GC cells; nevertheless, tiRNA-Val-CAC-001 knockdown increased metastasis and proliferation and reduced apoptosis (P<0.05). GO and KEGG analyses indicated tiRNA-Val-CAC-001 may exert its effects via Wnt/β-catenin signaling pathway by targeting LRP6. Following experiments showed that tiRNA-Val-CAC-001 could downregulated the protein levels of LRP6 and β-catenin, but up-regulated p-β-catenin, which confirmed the findings in bioinformatics analysis.
Conclusion
In conclusion, tiRNA-Val-CAC-001 works as a cancer suppressor in GC by targeting LRP6 via Wnt/β-catenin signaling pathway. tiRNA-Val-CAC-001 may serve as a therapy target and a biomarker of GC in the future.
Key Points
tiRNA-Val-CAC-001 is downregulated in gastric cancer tissues and cell lines, tiRNA-Val-CAC-001 has potential to become a novel diagnostic biomarker in gastric cancer, and tiRNA-Val-CAC-001 regulates gastric cancer cells by targeting LRP6.
Keywords: gastric cancer, tRNA-derived-fragments, LRP6, metastasis, proliferation, apoptosis
Introduction
Gastric cancer (GC) is one of the most common malignant tumors in the world, and it has the highest incidence rate in East Asia, especially in China.1 GC is an invasive disease with a poor 5-year survival rate and prone to lymph node metastasis (LNM) at the early stage of the diseases,2 which makes GC the second leading cause of cancer death in the world.3 Many factors can contribute to the oncogenesis of GC, such as Helicobacter pylori (Hp) infection, elder age, and imbalance in fruit and vegetable intake that are the risk factors of GC.4 Besides, dysregulated genes or non-coding RNAs have been shown to influence cancer occurrence, including GC.5 Therefore, these genes or non-coding RNAs can serve as biomarkers for GC. Nowadays, GC is usually diagnosed by gastroscopy and gastrointestinal angiography, however, these examinations are high cost and invasive to patients.6 Many tumor markers have been used to screen GC, including CEA, CA199 and AFP.7 However, the sensitivity of these markers is not high enough to diagnose GC at early stage. It is urgent to identify new biomarkers for the early diagnosis of GC.
Recently, a novel class of non-coding RNAs, named tRNA derived small fragments (tsRNAs), were shown to implicate in pathogenesis of disease and could serve as biomarkers.8 tsRNAs are produced from mature tRNAs or precursor tRNAs with the shearing by angiogenin (ANG) and Dicer in the cases of hypoxia or stress.9 tsRNAs mainly include two types: tRNA derived stress-induced RNAs (tiRNAs) and tRNA derived fragments (tRFs).10
The mature tRNAs are cut into two halves from the anticodon loop to obtain 3ʹ-tiRNAs and 5ʹ-tiRNAs, whose length is 31–40 nucleotides (nt).11 tRFs, 14–30 nt in length, are formed by Dicer at D-loop, T-loop and other positions of the nucleic acid ribozyme through the cleavage of the mature tRNA.12 Many researchers have paid more attentions to tsRNAs and proved that tsRNAs are involved in the occurrence and development of tumors. Our laboratory previously found that the downregulation of 5-tRF-His could inhibit proliferation, migration and invasion of breast cancer cells.13 Zhang et al reported that tRF-03357 promoted cell proliferation, migration and invasion in high-grade serous ovarian cancer.14 Studies showed that tsRNAs also exert their regulatory roles in lung cancer and GC, and even in human Alzheimer’s disease, suggesting tsRNAs implicate in various diseases.15,16 In this study, we aimed to detect the expression levels of tiRNA-Val-CAC-001 in GC and to investigate the underlying mechanisms of tiRNA-Val-CAC-001 suppressing GC progression.
Materials and Methods
Tissue Samples
Totally, 62 pairs of GC tissues and normal adjacent tissues (NATs) were collected at The Affiliated Cancer Hospital of Nanjing Medical University (Nanjing, China) from 2017 to 2020. The inclusion criteria of GC patients were as follows: (1) The diagnosis of GC was confirmed by histopathological examination; (2) The patients did not have any other tumor history; (3) Because anti-cancer therapy may affect the expression levels of tsRNAs, we only recruited GC patients who did not receive any radiotherapy, chemotherapy, biological immune agents or other treatments; (4) The patients had no history of acute or chronic gastritis or gastric polyp; (5) Complete clinical data can be extracted. The average age of the patients was 60 years. Our study was approved by the Clinical Research Ethics Committee of Nanjing Medical University, and informed consent was obtained from all the patients.
tsRNA Expression Profile
We randomly selected 6 pairs of GC tissues and matched NATs for sequencing analysis. Kangcheng Biological Science and Technology Company (Shanghai, China) was responsible for tsRNA expression profile sequencing technology service.
Cell Culture
The human GC cell lines SGC-7901, HGC-27, BGC-823, MKN-45, SNU-16, MGC-803, and AGS were obtained from National Collection of Authenticated cell cultures (Shanghai, China). All GC cells were gastric adenocarcinoma. Normal epithelium of human stomach cell GES-1 was obtained from Li Chuan Culture Collection (Shanghai, China). All human GC cell lines were incubated in RPMI 1640 medium (KeyGen BioTech, China) mixed 10% (v/v) fetal bovine serum (FBS, Gibco Invitrogen, Carlsbad, CA). GES-1 cell line was incubated in DMEM medium (KeyGen BioTech, China) with 10% (v/v) FBS. Cell incubator was at 37°C with 5% CO2. All cell lines were identified by Cell STR Identification.
Reverse‐transcriptase Quantitative Polymerase Chain Reaction (RT‐qPCR)
We used stem-loop-based RT‐qPCR to detect the expression of tsRNA. Briefly, total RNA was extracted by using TRIzol (Life Technologies, USA). Total RNA extraction was quantitatively determined by an ultraviolet spectrophotometer. The first-strand cDNA was synthesized with a specific reverse transcribed (RT) primer of a tsRNA using RT Mix Kit (ribobio, China). Primer sequences were designed using the online website Primer3 (https://primer3.ut.ee/). The primer sequences are shown in Table 1. The RT-qPCR cycling conditions were 95°C for 10 min, followed by 45 cycles of 95°C for 8 s and 58°C for 8 s. The molecular size of RT-qPCR products was detected by electrophoresis. The sequence of tiRNA-Val-CAC-001 was confirmed by cloning sequencing. RNU6B (U6) was used as normal reference for tiRNA-Val-CAC-001. GAPDH was used as a normal reference for low-density lipoprotein receptor-related protein 6 (LRP6). We used ΔΔCT method to analyze the results.
Table 1.
The Sequences of Primers Used in the Present Study
| Name | Sequence (5’-3’) |
|---|---|
| tiRNA-Val-CAC-001-F | GCTTCTGTAGTGTAGTGGTTATCA |
| tiRNA-Val-CAC-001-R | CAGTGCGTGTCGTGGAGT |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
| GAPDH-F | GCAAGAGCACAAGAGGAAGA |
| GAPDH-R | ACTGTGAGGAGGGGAGATTC |
| LRP6-F | ACGATTGTAGTTGGAGGCTTG |
| LRP6-R | ATGGCTTCTTCGCTGACATCA |
Abbreviations: F, Forward; R, Reverse.
Cell Transfection
Totally, 5 × 105 Cells were seeded into 6-well plates and when the cell density reached 70% to 80% transfected with lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s protocols. The final concentration was 100nM for single-strand mimics or negative control of mimics (mimics-NC) (ribobio, China) and 150nM for single-strand inhibitors or negative control of inhibitors (inhibitor-NC) (ribobio, China). After transfection, cells were continued to culture for 24 h for cell function assays, and 48 h for WB.
Transwell Assay
Totally, 6 × 104 cells were seeded into transwell (Corning, USA) upper chambers with 200 μL serum-free medium after transfection for 24 h. Then, we added 600 μL medium mixed in 20% (v/v) FBS to bottom chambers. In this way, the cells in the upper chambers would move to bottom chambers. Non-migration cells were carefully removed by cotton swabs after incubation for 48 h. The remaining cells were fixed with 4% paraformaldehyde for 30 mins and then stained with 0.1% crystal violet for 20 mins. Finally, we observed the results under a microscope. All the steps were the same as described above for transwell invasion assay, but the chambers were replaced with the one coated with matrigel (Corning, USA).
Cell Proliferation Assay
After transfection for 24 h, 3 × 103 cells were seeded into 96-well plates with 100μL complete medium. We added 10μL Cell Counting Kit-8 (CCK-8; Apexbio, USA) to the wells at 24 h, 48 h, and 72 h, respectively, to detect the proliferation of cells. Then, we incubated cells in the incubator for 2 h at 37°C out of light. We detected absorbance of each well at a wavelength of 450 nm using SpectraMax i3x (Molecular Devices, USA) and calculated the cell proliferation rate.
Cell Colony Formation Assay
In the cell colony formation assay, we seeded 3 × 103 cells into 6-well plates after transfection for 24 h. Then, we put the plate in an incubator for a week. Similarly, the cells were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for 20 min. Finally, we observed the number and size of colonies under a microscope to calculate the rate of cell colony formation.
Flow Cytometry for Cell Apoptosis
After transfected for 24 h, cells were collected by Trypsin (Gibco, USA) and washed twice using PBS. Annexin V-APC/7-AAD Cell Apoptosis Reagent (KeyGen BioTech, China) was used to detect cell apoptosis rate. The cells were incubated at room temperature in the dark for 15 min after adding the reagents, and then the number of apoptotic cells was measured by flow cytometry (Becton-Dickinson, USA).
Nucleus and Cytoplasm Separation Assay
Totally, 5 × 105 cells were used to perform nucleus and cytoplasm using PARISTM Kit Protein and RNA Isolation System (Invitrogen, USA) according to the manufacturer’s protocol. We detected the respective proportion of the target tiRNA in the nucleus and cytoplasm using RT-qPCR.
Fluorescence in situ Hybridization (FISH)
After placing a clean cell sliver into a 24-well plate, we seeded 1 × 104 cells in each hole and overnight. The cells were co-incubated with 4μM probe (GAGGCGAACGTGA+TAACCACTACAC+TACAGAAGC) for 24 h, and then treated with FISH Kit (GenePharma, China) according to the manufacturer’s protocol. Finally, we observed the luciferin distribution under a fluorescence microscope.
RNA Immunoprecipitation (RIP) Assays
Totally, 1 × 107 cells were used to follow experimental according to the Magna RIPTM Kit (Millipore, USA) manufacturer’s protocol. Briefly, AGO2 protein antibody (proteintech, China) and negative control IgG antibody were firstly co-incubated with magnetic beads for 30 mins to form the binder at room temperature. We treated the cells with a lysis buffer and used a bead-antibody complex previously formed to bind the protein-RNA complex within the cells. The obtained RNA was detected using RT-qPCR and compared its expression level with Input group and IgG group.
Western Blot
RIPA Lysis Buffer (Servicebio, China) was used to extract the total protein. Then, the protein and SDS-Loading Buffer mixture were boiled together. Proteins were electrophoresed on SDS-PAGE gels (Genscirpt, China) and transferred onto polyvinylidene fluoride membranes. Next, the primary antibody (LRP6, Abcam USA; β-catenin, β-actin, CST USA; p-β-catenin, Affinity USA) was incubated overnight at 4°C and the fluorescent secondary antibody (ROCKLAND, USA) was incubated at room temperature and out of light for 2 h. Finally, we put the membranes under Bio-Imaging Systems to detect the grayscale value of the bandings.
Data Analysis
We normalized the raw read count of the sequencing output and calculated differentially expressed genes by using DESeq2 (Love et al 2014) (R package version 1.18.1) in the R software (version 4.0.1). DEGs were screened with the following criteria: fold-change >2 and P value <0.05. MINTbasev2.0 (https://cm.jefferson.edu/MINTbase/) and UCSC Genome Browser database (https://xena.ucsc.edu/) were used to identify the source tRNA of tiRNA-Val-CAC-001. We performed gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses using DAVID (https://david.ncifcrf.gov/tools.jsp).
The experimental data were analyzed using GraphPad Prism and IBM SPSS Statistics 26. When the data are normally distributed, the differences between two groups were assessed using the student’s-t test. The correlation between tiRNA-Val-CAC-001 expression level and clinicopathological factors of GC patients was evaluated with Pearson’s chi-square test. The sensitivity, specificity and area under curve (AUC) were calculated using receiver operating characteristic (ROC) curve. The assay results were from three independent experiments. The results were considered statistically significant when P<0.05.
Results
tiRNA-Val-CAC-001 is Downregulated in GC Cell Lines and Tissues
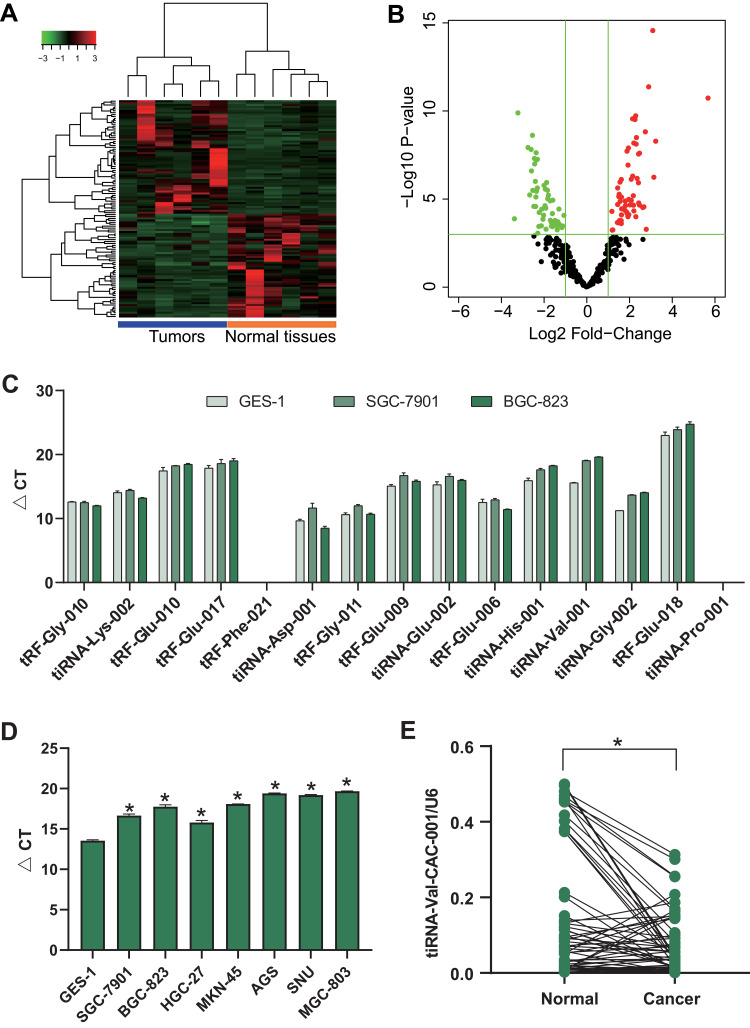
We obtained 59 up-regulated and 54 down-regulated tsRNAs in GC tissues compared to NATs (Figure 1A and B). First, we validated the expression levels of top 15 differentially expressed tsRNAs in GES-1, SGC-7901 and BGC-823 using RT-qPCR and obtained tiRNA-Val-CAC-001 which was significantly downregulated in SGC-7901 and BGC-823 (fold change = 7.20, P = 0.0003) (Figure 1C). Then, we validated the expression levels of tiRNA-Val-CAC-001 in other GC cell lines, and the results showed that tiRNA-Val-CAC-001 had a lower expression level in GC cell lines than that in GES-1 (Figure 1D). The RT-qPCR result was inconsistent with sequencing analysis result. After detecting tissues, we found that tiRNA-Val-CAC-001 was significantly downregulated in 42 GC tissues compared to the corresponding adjacent normal tissues (Figure 1E).
Figure 1.
tiRNA-Val-CAC-001 expression level in gastric cancer (GC) tissues and GC cells. (A) Heatmap of differentially expressed tsRNA in 6 pairs of GC tissues and normal adjacent tissues (NATs). (B) Volcano plot of differentially expressed tsRNA (P<0.05, fold change>2). (C) The expression level of top 15 significantly differentially expressed tsRNA. (D) Expression levels of tiRNA-Val-CAC-001 in GES-1 and GC cell lines were detected by RT-qPCR (*P<0.05). (E) Expression levels of tiRNA-Val-CAC-001 in GC tissues and NATs (*P<0.05).
tiRNA-Val-CAC-001 is a Derivative from tRNA152-Val
tiRNA-Val-CAC-001 is a tiRNA 34nt in length (GCTTCTGTAGTGTAGTGGTTATCACGTTCGCCTC), derived from 5’-half of tRNA152-Val and localized on chr6 (Figure 2A and B). The results of agarose gel electrophoresis showed that a single, correct band was obtained for tiRNA-Val-CAC-001, and cloning sequencing confirmed the sequence of tiRNA-Val-CAC-001 (Figure 2C). We also obtain the complete base structure of tRNA152-Val, indicating tiRNA-Val-CAC-001ʹs cleavage site was located at the anticodon loop of tRNA152-Val (Figure 2D).
Figure 2.
tiRNA-Val-CAC-001 is derived from tRNAVal-152. (A) tiRNA-Val-CAC-001 is located on chr6 and derived from tRNA-Val (anticodon CAC) which is 73nt in UCSC Genome Browser database. (B) tiRNA-Val-CAC-001 is 5’-half fragment of tRNA-Val-CAC-2-1 with a MINTbase unique ID of tRF-34-Q99P9P9NH57S15. (C) Electrophoresis and sequencing confirmed the existence of tiRNA-Val-CAC-001. (D) The complete structure of tRNA-Val-CAC and the location of tiRNA-Val-CAC-001.
tiRNA-Val-CAC-001 Mainly Localized in the Cytoplasm
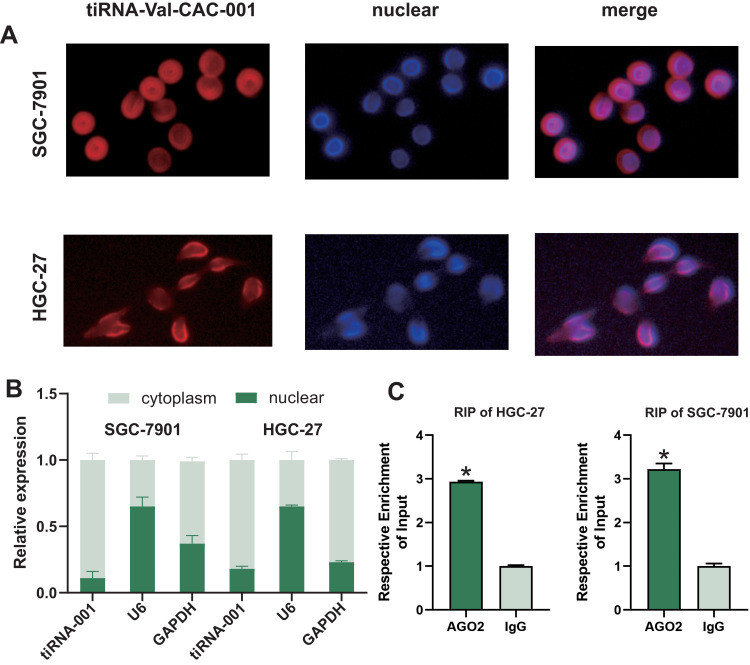
FISH assay was performed to determine the subcellular localization of the tiRNA-Val-CAC-001. The results showed that the tiRNA-Val-CAC-001 is mainly localized in the cytoplasm (Figure 3A). We also detected the proportion of tiRNA-Val-CAC-001 distribution by nucleus-cytoplasmic separation assay, which further confirmed the findings in FISH assay (Figure 3B). RIP assay showed that the tiRNA-Val-CAC-001 could bind to AGO2 protein (Figure 3C), suggesting tiRNA-Val-CAC-001 may exert its functions by combining 3’-untranslated region (3’-UTR) of target genes.
Figure 3.
tiRNA-Val-CAC-001ʹs subcellular localization. (A) The subcellular location of tiRNA-Val-CAC-001 was detected using FISH. (B) tiRNA-Val-CAC-001 is mainly distributed in cytoplasm. tiRNA-001: tiRNA-Val-CAC-001 (C) tiRNA-Val-CAC-001 could bind with AGO2 protein (*P<0.05).
tiRNA-Val-CAC-001 Inhibits Metastasis, Proliferation, and Promotes Apoptosis of GC Cells
We used an in vitro cell function assay to detect the effect of tiRNA-Val-CAC-001 on GC cells. We transfected SGC-7901 and HGC-27 with artificially synthesized mimics and/or mimics-NC. Transwell assays showed that overexpression of tiRNA-Val-CAC-001 inhibited the migration and invasion of GC cells (Figure 4A and B); colony assays suggested that overexpressed tiRNA-Val-CAC-001 reduced the number of colony formation (Figure 4C); apoptosis assays showed that the overexpression of tiRNA-Val-CAC-001 promoted the apoptosis rate of GC cells slightly (Figure 4D); and CCK-8 assays showed that the overexpression of tiRNA-Val-CAC-001 inhibited proliferation of GC cells (Figure 4E); To further validate the function of tiRNA-Val-CAC-001, we knocked its expression in GES-1 and the results showed that down-regulated tiRNA-Val-CAC-001 was associated with increased migration, invasion, and proliferation, as well as decreased apoptosis (Figure 5A–E).
Figure 4.
Overexpressed tiRNA-Val-CAC-001 inhibited gastric cancer (GC) cells metastasis, proliferation and apoptosis. (A) Transwell invasion assay indicated overexpressed tiRNA-Val-CAC-001 inhibited invasion of GC cells (*P<0.05). (B) Transwell migration assay indicated overexpressed tiRNA-Val-CAC-001 inhibited migration of GC cells (*P<0.05). (C) Colony formation assay indicated overexpressed tiRNA-Val-CAC-001 inhibited proliferation of GC cells (*P<0.05). (D) Overexpressed tiRNA-Val-CAC-001 promoted apoptosis of GC cells. (E) CCK-8 assay was used to examine cell proliferation in GC cell lines transfected with tiRNA-Val-CAC-001 mimics or mimics-NC (*P<0.05).
Abbreviations: mimics, tiRNA-Val-CAC-001 mimics; mimics-NC, negative control of mimics.
Figure 5.
Downregulated tiRNA-Val-CAC-001 promoted GES-1 cell metastasis, proliferation and apoptosis. (A) Transwell invasion assay indicated downregulatedtiRNA-Val-CAC-001 promoted invasion in GES-1 cell (*P<0.05). (B) Transwell migration assay indicated downregulated tiRNA-Val-CAC-001 promoted migration inGES-1 cell (*P<0.05). (C) Colony formation assay indicated downregulated tiRNA-Val-CAC-001 promoted proliferation of GES-1 cell (*P<0.05). (D) DownregulatedtiRNA-Val-CAC-001 inhibited apoptosis of GES-1 cell. (E) CCK-8 assay was used to examine cell proliferation in GES-1 cell transfected with tiRNA-Val-CAC-001 inhibitor or inhibitor-NC (*P<0.05).
Abbreviations: inhibitor, tiRNA-Val-CAC-001 inhibitor; inhibitor-NC, negative control of inhibitor.
tiRNA-Val-CAC-001 Regulated GC Malignancy by Targeting LRP6 via Wnt Signaling Pathway
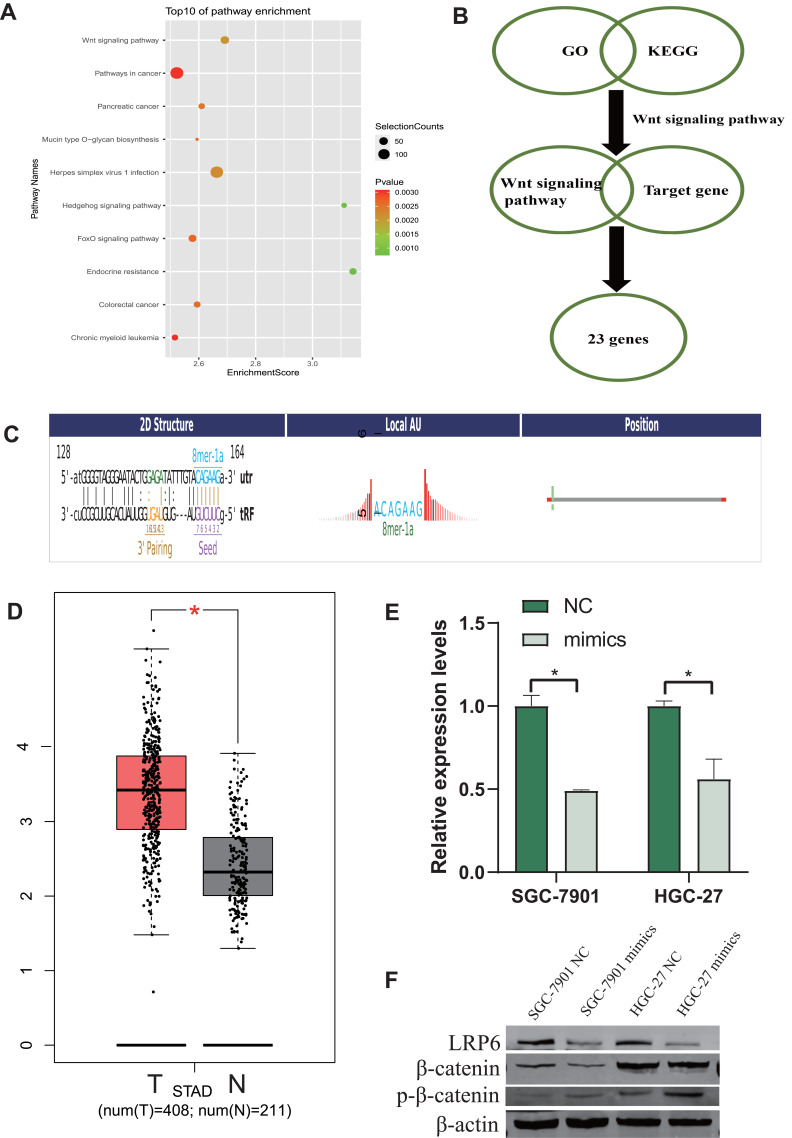
We used GO and KEGG analyses to explore the molecular mechanism and predict target genes of tiRNA-Val-CAC-001 (Figures 6 and 7). The target genes were enriched into 3 categories in GO analysis, including biological process (BP), cellular component (CC), and molecular function (MF) (Figure 6A). The enriched GO terms in BP category included nucleic acid metabolic process, cellular metabolic process, regulation of RNA process and transcription (Figure 6B). KEGG suggested that the top 10 significant (P<0.05) pathways, including Wnt signaling pathway, Pathway in cancer, Hedgehog signaling pathway and FOXO signaling pathway (Figure 7A). The detailed information of Wnt signaling pathway is shown in Figure 6C and D. From the intersection results of GO and KEGG enriched analyses, we obtained the Wnt signaling pathway. Wnt signaling pathway is involved in a variety of pathological processes including cancers.17 It has been demonstrated involving various diseases, such as breast cancer, colorectal cancer and non-small cell lung cancer.18,19 On the basis of tiRNA-Val-CAC-001 seed region sequences, we obtained some genes that may be targeted by tiRNA-Val-CAC-001 directly using TargetScan (http://www.targetscan.org/) and miRDB (http://mirdb.org/custom.html). We obtained 23 genes from the intersection results of Wnt signaling pathway and target genes list (Figure 7B). Figure 7C shows tiRNA-Val-CAC-001 seed region sequence and LRP6 3’-UTR binding site. We obtained the expression of LRP6 in GC tissues from TCGA database (Figure 7D). We detected the expression levels of 23 candidate genes and found that LRP6 was significantly decreased in SGC-7901 and HGC-27 transfected with tiRNA-Val-CAC-001 mimics by RT-qPCR (Figure 7E). In addition, WB results also suggested that LRP6 protein expression level was decreased in tiRNA-Val-CAC-001 overexpression group compared with control group, β-catenin expression was also decreased, but p-β-catenin expression level was increased (Figure 7F). Based on the above results,tiRNA-Val-CAC-001 could regulate GC malignancy by targeting LRP6 via Wnt signaling pathway.
Figure 6.
tiRNA-Val-CAC-001 regulated GC malignancy by targeting LRP6 via Wnt signaling pathway. (A) Bar graph of the GO analysis of tiRNA-Val-CAC-001 target genes. (B) Pie chart of biological process. (C) Wnt signaling pathway in GO analysis. (D) Wnt signaling pathway in KEGG analysis.
Figure 7.
tiRNA-Val-CAC-001 regulated GC malignancy by targeting LRP6 via Wnt signaling pathway. (A) Bubble chart of the KEGG enrichment analyses of tiRNA-Val-CAC-001 target genes showed that the top 10 significant pathways in gastric cancer tissues. (B) The downstream target genes of tiRNA-Val-CAC-001 were analyzed by GO and KEGG enrichment analyses. (C) The seed region sequence of tiRNA-Val-CAC-001 and 3’-UTR of LRP6 binding site. (D) The mRNA expression levels of LRP6 were detected in gastric cancer cells transfected with tiRNA-Val-CAC-001 mimics or negative control (NC) of mimics (*P<0.05). (E) LRP6 expression in GC tumor in TCGA (*P<0.05). (F) Protein expression level was confirmed in transfected gastric cancer cells.
Diagnostic Efficacy of tiRNA-Val-CAC-001
ROC curve analysis showed the AUC was 0.748 for tiRNA-Val-CAC-001 in detecting GC (Supplementary Figure 1). At the same time, the clinicopathological parameter analysis showed that the expression level of tiRNA-Val-CAC-001 was significantly correlated with the gender and TNM stage of GC patients (Table 2).
Table 2.
The Correlations Between the Expression Level of tiRNA-Val-CAC-001 and the Clinicopathological Features in Gastric Cancer Tissues (n = 62)
| Characteristic | Number | tiRNA-Val-CAC-001 | P-value | |
|---|---|---|---|---|
| ≤0.67 | >0.67 | |||
| Number | 62 | 31 | 31 | |
| Age (years) | 0.788 | |||
| ≤ 60 | 21 | 11 | 10 | |
| >60 | 41 | 20 | 21 | |
| Gender | 0.022* | |||
| Male | 50 | 21 | 29 | |
| Female | 12 | 10 | 2 | |
| LN metastasis | 0.473 | |||
| Yes | 53 | 25 | 28 | |
| No | 9 | 6 | 3 | |
| Distant metastasis | 0.64 | |||
| M1 | 5 | 3 | 2 | |
| M0 | 57 | 28 | 29 | |
| TNM stage | 0.008* | |||
| I—II | 46 | 28 | 18 | |
| III—IV | 16 | 3 | 13 | |
| Local invasion | 0.688 | |||
| T1—T2 | 7 | 4 | 3 | |
| T3—T4 | 55 | 27 | 28 | |
Note: *P<0.05.
Abbreviations: LN, lymph node; TNM, tumor node metastasis.
Discussion
tsRNAs were first discovered in 1977.20 They were considered random products of tRNAs degradation and attracted little attention at that moment.21 Until recently, studies found that tsRNAs play crucial roles in diseases. Some studies showed that tsRNAs are closely associated with metastasis, proliferation, cell cycle, apoptosis, and drug-resistance.22,23 For example, Hu et al reported that tsRNA-5001a promotes the proliferation of lung adenocarcinoma cells and increases the risk of postoperative recurrences in lung adenocarcinoma patients.24 Zhou et al showed that tRF5-Glu contribute to the complex tumor heterogeneity of ovarian cancer cells and may provide new targets for therapeutic intervention.25 Additionally, tsRNAs have certainly diagnostic efficiency for tumors. Wang et al identified six tsRNAs derived from the 5’ ends of tRNAs as novel diagnostic biomarkers for early breast cancer.26 In general, tsRNAs could participate in tumor progression and had the potential to become biomarkers for cancer.
In the present study, we explored the roles of tsRNAs in GC and identified that tiRNA-Val-CAC-001 was a significantly downregulated tsRNA in both GC cells and tissues. Further analyses showed that knockdown of tiRNA-Val-CAC-001 could not only promote proliferation and metastasis but also suppress apoptosis of GC cells. The effects of tiRNA-Val-CAC-001 on GC cells were further confirmed in cells transfected with tiRNA-Val-CAC-001 mimics, suggesting tiRNA-Val-CAC-001 may be a GC suppressor. Studies have explored tsRNAs in GC. Tong et al showed tRF-3017A promoted metastasis of GC.27 Dong et al found that tRF-24-V29K9UV3IU may hinder GC tumor progression by inhibiting cell proliferation, migration, and invasion while promoting cell apoptosis.28 Meanwhile, Zhang et al reported that tRF-3019a modulated GC cell progression by targeting FBXO47.16 Taken together, these results indicated that tsRNAs are implicated in GC pathogenesis.
Since previous studies demonstrated tsRNAs could exert biological mechanisms in a manner similar to miRNAs by binding to the 3’-UTR of target genes,29 we predicted target genes of tiRNA-Val-CAC-001 and explored its roles using bioinformatics method. We analyzed the target genes of tiRNA-Val-CAC-001 with GO and KEGG enrichment analysis. GO analysis results consisted with biological process, cellular component and molecular function. Biological process mainly includes DNA-templated, RNA biosynthetic, and nucleic acid metabolic process. KEGG pathway results mainly involved in Wnt signaling pathway, FOXO signaling pathway and pathways in cancer. Wnt signaling pathway and FOXO signaling pathway have been confirmed to be involved in signaling transmission in tumors among the pathways enriched by the predicted genes.17,30 We obtained candidate genes from the intersection results of pathways and target genes of tiRNA-Val-CAC-001. In this present study, we found that tiRNA-Val-CAC-001 is mainly localized in the cytoplasm. Previous studies have demonstrated that tsRNAs are similar to miRNAs when tsRNAs are localized in the cytoplasm.31 Bioinformatics analysis indicated that LRP6 was a potential target gene for tiRNA-Val-CAC-001. Following experiments confirmed that tiRNA-Val-CAC-001 could complex with AGO2 to silence LRP6 mRNA, and tiRNA-Val-CAC-001 could down-regulate the levels of LRP6 mRNA and protein, indicating tiRNA-Val-CAC-001 may target LRP6 in a similar manner to miRNAs. LRP6 is one of the low-density lipoprotein (LDL) receptor-related protein families.32 Previous studies have demonstrated that LDL oxidation can upregulate the levels of inflammatory factors, such as TNF-α and IL-6, decrease trophoblast invasion, and promote apoptosis.32 In addition, other studies have also suggested LRP6 plays an important role in promoting metastasis of various tumors, including GC.33 In this study, we found that the expression levels of LRP6 in GC tissues are lower than those in normal adjacent tissues in TCGA. LRP6 is also a key membrane protein receptor in Wnt/β-catenin signaling pathway.34 Wnt/β-catenin signaling pathway is of physiological importance to the organism.35 It could maintain cancer stem cells and putative stem cell markers, such as LGr5/GPr49, CD44, CD24.17 Importantly, Wnt/β-catenin signaling pathway is also a crucial pathway in cancer progression.17 Our previous study demonstrated 5ʹtiRNA-Val suppresses Wnt/β-catenin signaling pathway by targeting FZD3 in breast cancer.18 Wnt/β-catenin signaling pathway substantially impacts non–small cell lung cancer tumorigenesis, prognosis, and drug-resistance.19 Taken together, we speculated that tiRNA-Val-CAC-001 inhibits GC progression by targeting LRP6 via Wnt/β-catenin signaling pathway.
However, there are a few limitations in the present study. First, we only detected the expression level of tiRNA-Val-CAC-001 in 62 paired tissues. The sample size is relatively small. Secondly, we only explored one potential gene of tiRNA-Val-CAC-001. tiRNA-Val-CAC-001 may exert its effects by binding to other genes or regulating other pathways. Further studies are needed to confirm the findings in the presented study, as well as uncover other mechanisms underlying tiRNA-Val-CAC-001.
Conclusion
In conclusion, we identified tiRNA-Val-CAC-001 as a downregulated tsRNA in GC cells and tissues. tiRNA-Val-CAC-001 inhibits malignancy of GC cells and works as a cancer suppressor in GC by targeting LRP6 via Wnt/β-catenin signaling pathway. tiRNA-Val-CAC-001 may serve as a therapy target and biomarker of GC in the future.
Funding Statement
This study was supported by the National Natural Science Foundation of China (NO. 81871718).
Compliance with Ethical Standards
This study was approved by the Clinical Research Ethics Committee of Nanjing Medical University and the ethical permit number is (2018) 471. Written informed consent of all the patients was obtained for research purposes. All procedures performed in studies involving human participants were in committee with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Disclosure
All authors declare that they have no potential conflicts of interest.
References
- 1.Zang Y, Wang T, Pan J, Gao F. miR-215 promotes cell migration and invasion of gastric cancer cell lines by targeting FOXO1. Neoplasma. 2017;64:579–587. doi: 10.4149/neo_2017_412 [DOI] [PubMed] [Google Scholar]
- 2.Mihmanli M, Ilhan E, Idiz UO, Alemdar A, Demir U. Recent developments and innovations in gastric cancer. World J Gastroenterol. 2016;22:4307–4320. doi: 10.3748/wjg.v22.i17.4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin VY, Chu K-M. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World j Gastroenterol. 2014;20:10432–10439. doi: 10.3748/wjg.v20.i30.10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- 5.Li P-F, Chen S-C, Xia T, et al. Non-coding RNAs and gastric cancer. World j Gastroenterol. 2014;20:5411–5419. doi: 10.3748/wjg.v20.i18.5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842–13862. doi: 10.3748/wjg.v20.i38.13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng F, Tian Y, Xu G, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. doi: 10.1186/s12885-017-3738-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C, Liang T, Zhang F, Liu J, Fu Y. tRNA-derived fragments as novel potential biomarkers for relapsed/refractory multiple myeloma. BMC Bioinform. 2021;22:238. doi: 10.1186/s12859-021-04167-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y, Yu X, Zhu L, Li T, Yan Z, Guo J. Transfer RNA-derived fragments and tRNA halves: biogenesis, biological functions and their roles in diseases. J Mol Med. 2018;96:1167–1176. doi: 10.1007/s00109-018-1693-y [DOI] [PubMed] [Google Scholar]
- 10.Pekarsky Y, Balatti V, Palamarchuk A, et al. Dysregulation of a family of short noncoding RNAs, tsRNAs, in human cancer. Proc Natl Acad Sci U S A. 2016;113:5071–5076. doi: 10.1073/pnas.1604266113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang P, Yan F. tiRNAs & tRFs biogenesis and regulation of diseases: a review. Curr Med Chem. 2019;26:5849–5861. doi: 10.2174/0929867326666190124123831 [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42. doi: 10.1083/jcb.200811106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang X, Jiang P, Chen H, Tang L. 5-tRF-His, tRNA-derived fragments, regulate CKAP2 to inhibit the proliferation of breast cancer. J Clin Med. 2020;2020:9. [Google Scholar]
- 14.Zhang M, Li F, Wang J, et al. tRNA-derived fragment tRF-03357 promotes cell proliferation, migration and invasion in high-grade serous ovarian cancer. Onco Targets Ther. 2019;12:6371–6383. doi: 10.2147/OTT.S206861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao Y, Sun Q, Liu X, Wang P, Wu R, Ma Z. tRF-Leu-CAG promotes cell proliferation and cell cycle in non-small cell lung cancer. Chem Biol Drug Des. 2017;90:730–738. doi: 10.1111/cbdd.12994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F, Shi J, Wu Z, et al. A 3’-tRNA-derived fragment enhances cell proliferation, migration and invasion in gastric cancer by targeting FBXO47. Arch Biochem Biophys. 2020;690:108467. doi: 10.1016/j.abb.2020.108467 [DOI] [PubMed] [Google Scholar]
- 17.Nusse R, Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 18.Mo D, Jiang P, Yang Y, et al. A tRNA fragment, 5’-tiRNA, suppresses the Wnt/β-catenin signaling pathway by targeting FZD3 in breast cancer. Cancer Lett. 2019;457:60–73. doi: 10.1016/j.canlet.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 19.Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:djt356. doi: 10.1093/jnci/djt356 [DOI] [PubMed] [Google Scholar]
- 20.Borek E, Baliga BS, Gehrke CW, et al. High turnover rate of transfer RNA in tumor tissue. Cancer Res. 1977;37:3362–3366. [PubMed] [Google Scholar]
- 21.Balatti V, Nigita G, Veneziano D, et al. tsRNA signatures in cancer. Proc Natl Acad Sci U S A. 2017;114:8071–8076. doi: 10.1073/pnas.1706908114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, Zhou B, Wang J, et al. tRNA-derived fragment tRF-Glu-TTC-027 regulates the progression of gastric carcinoma via MAPK signaling pathway. Front Oncol. 2021;11:733763. doi: 10.3389/fonc.2021.733763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Xu Z, Sheng J. tRNA-derived small RNA: a novel regulatory small non-coding RNA. Genes. 2018;9:246. doi: 10.3390/genes9050246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu F, Niu Y, Mao X, et al. tsRNA-5001a promotes proliferation of lung adenocarcinoma cells and is associated with postoperative recurrence in lung adenocarcinoma patients. Transl Lung Cancer Res. 2021;10:3957–3972. doi: 10.21037/tlcr-21-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou K, Diebel KW, Holy J, et al. A tRNA fragment, tRF5-Glu, regulates BCAR3 expression and proliferation in ovarian cancer cells. Oncotarget. 2017;8:95377–95391. doi: 10.18632/oncotarget.20709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Ma G, Li M, et al. Plasma tRNA fragments derived from 5’ ends as novel diagnostic biomarkers for early-stage breast cancer. Mol Ther Nucleic Acids. 2020;21:954–964. doi: 10.1016/j.omtn.2020.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong L, Zhang W, Qu B, et al. The tRNA-derived fragment-3017A promotes metastasis by inhibiting NELL2 in human gastric cancer. Front Oncol. 2020;10:570916. doi: 10.3389/fonc.2020.570916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong X, Fan X, He X, et al. Comprehensively identifying the key tRNA-derived fragments and investigating their function in gastric cancer processes. Onco Targets Ther. 2020;13:10931–10943. doi: 10.2147/OTT.S266130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L, Li Z, Yu X, et al. The tRNA-derived fragment 5026a inhibits the proliferation of gastric cancer cells by regulating the PTEN/PI3K/AKT signaling pathway. Stem Cell Res Ther. 2021;12:418. doi: 10.1186/s13287-021-02497-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci. 2017;13:815–827. doi: 10.7150/ijbs.20052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mo D, He F, Zheng J, Chen H, Tang L, Yan F. tRNA-derived fragment tRF-17-79MP9PP attenuates cell invasion and migration via THBS1/TGF-β1/Smad3 axis in breast cancer. Front Oncol. 2021;11:656078. doi: 10.3389/fonc.2021.656078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Li H, Li M, Zhang W, Yang Z, Zhang S. LRP6 is involved in the proliferation, migration and invasion of trophoblast cells via miR‑346. Int J Mol Med. 2020;46:211–223. doi: 10.3892/ijmm.2020.4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Lu B, Dai C, et al. Caveolin-1 promotes chemoresistance of gastric cancer cells to cisplatin by activating WNT/β-Catenin pathway. Front Oncol. 2020;10:46. doi: 10.3389/fonc.2020.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu W, Lin C, Li Y. Rottlerin induces Wnt co-receptor LRP6 degradation and suppresses both Wnt/β-catenin and mTORC1 signaling in prostate and breast cancer cells. Cell Signal. 2014;26:1303–1309. doi: 10.1016/j.cellsig.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–481. doi: 10.1016/j.biopha.2018.11.082 [DOI] [PubMed] [Google Scholar]