SUMMARY
Medium spiny neurons (MSNs) constitute the vast majority of striatal neurons and the principal interface between dopamine reward signals and functionally diverse cortico-basal ganglia circuits. Information processing in these circuits is dependent on distinct MSN types – cell types that are traditionally defined according to their projection targets or dopamine receptor expression. Single cell transcriptional studies have revealed greater MSN heterogeneity than predicted by traditional circuit models, but the transcriptional landscape in the primate striatum remains unknown. Here, we set out to establish molecular definitions for MSN subtypes in Rhesus monkeys, and to explore the relationships between transcriptionally defined subtypes and anatomical subdivisions of the striatum. Our results suggest at least nine MSN subtypes, including dorsal striatum subtypes associated with striosome and matrix compartments, ventral striatum subtypes associated with the nucleus accumbens shell and olfactory tubercle, and an MSN-like cell type restricted to μ-opioid receptor rich islands in the ventral striatum. Although each subtype was demarcated by discontinuities in gene expression, continuous variation within subtypes defined gradients corresponding to anatomical locations and, potentially, functional specializations. These results lay the foundation for achieving cell-type-specific transgenesis in the primate striatum and provide a blueprint for investigating circuit specific information processing.
Keywords: MSNs, medium spiny neurons, striatum, primate, NHP, NUDAP, transcriptional diversity, anatomical diversity, snRNA-Seq, FISH
INTRODUCTION
The striatum serves as the major input nucleus for the basal ganglia (BG) and the principal neural interface between dopamine reward signals and cortico-basal ganglia-thalamo-cortical circuits. Information processing in the striatum is dependent on cell-type-specific circuits. In particular, Medium Spiny Neurons (MSNs), which account for the vast majority of all striatal neurons, are divided into two major cell types: D1- and D2- MSNs.1 D1-MSNs express dopamine receptor type 1 (DRD1) and form the “direct pathway” via monosynaptic projections to the basal ganglia output nuclei.2 D2-MSNs express dopamine receptor type 2 (DRD2) and form the “indirect pathway” via di-synaptic projections to the basal ganglia output nuclei.2 Activity in the direct and indirect pathways produces, broadly, opposing effects on thalamo-cortical projections. This cell-type-specific circuit model has been crucial to understanding the role of the striatum and BG in the control of movement and the mechanisms of Parkinson’s disease.3,4 However, the striatum and BG are involved in many behaviors besides the control of movement. For example, segregated neurochemical compartments in the dorsal striatum (DS) known as striosome (patch) and matrix are thought to participate in limbic and sensorimotor functions, respectively.5-7 Similarly, the ventral striatum (VS) has fundamental roles in reward processing, learning, and emotional responses.8-11 The traditional model, that involves competition between signals in the direct and indirect pathways, does not account for these broad functional roles. Rather, these broad functionalities indicate deeper cell-type and circuit heterogeneity.
Single cell technologies that classify cell types according to their overall gene expression profiles provide powerful and quantitative methods for investigating cell type heterogeneity.12 Single cell and single nucleus RNA sequencing (scRNA-Seq and snRNA-Seq, respectively) have revealed new subtypes of MSNs and striatal interneurons.13-18 Moreover, these technologies are providing novel insights into cell-type-specific mechanisms for diseases involving the striatum, including drug addiction19 and Huntington’s disease.20 Despite these advances, we know neither the extent of MSN diversity in the primate striatum, nor how that diversity corresponds to the anatomical or neurochemical divisions of the highly articulated primate brain.
The close phylogenetic relationship and the high degree of homology between human and non-human primate (NHP) brains, genes, and behaviors make NHP studies indispensable for understanding the neuronal substrates of human behavior, as well as neurological, neurodegenerative, and psychiatric diseases.21 Here, we used snRNA-Seq and Fluorescent In-Situ Hybridization (FISH) to characterize the transcriptional and anatomical diversity of MSNs and closely related neurons. The resulting cell-type-specific gene expression patterns provide insights into MSN functions and indicate potential molecular access points for cell-type-specific applications of genetically coded tools to primate brains, in scientific or translational settings.
RESULTS
Major Cell Classes in the Primate Striatum
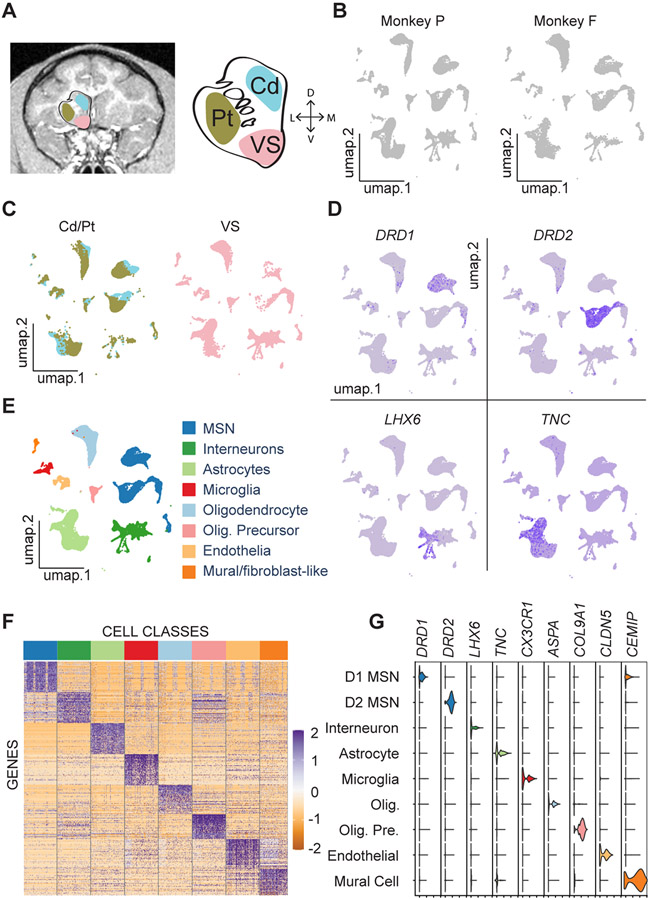
To investigate the cell-type-specific architecture of the NHP striatum, we micro-dissected the caudate nucleus (Cd), putamen (Pt), and ventral striatum (VS) from coronal sections of two monkeys (Figures 1A and S1A), performed snRNA-Seq, and clustered the nuclei profiles based on their gene expression counts (STAR Methods). Each major cluster had nuclei derived from both subjects (Figure 1B) and all regions (Figure 1C), indicating broad similarities between the subjects and the regions. Feature plots showed the expression of well-known marker genes and thus indicated the correspondence between nuclei clusters and broad cell classes including D1-MSNs, D2-MSN, striatal interneurons, and non-neuronal cell types (Figures 1D, S1C and S2). Each major cell class was signified by groups of differentially expressed genes that were specifically enriched in that cluster (Figures 1E and 1F; Data S1). The intersection of differentially expressed genes from each cluster with orthologous marker gene lists from several databases13,17,22 confirmed the identity of the clusters (p < 0.05, Benjamini-Hochberg corrected, Hypergeometric tests). Violin plots showing the expression levels of marker genes in each major class confirmed the basic validity of our experimental and analytic methods (Figure 1G). Together, these results provide a broad transcriptional catalogue of cell classes in the NHP striatum and a rich dataset for future exploration.
Figure 1. Cell Type Taxonomy in the Primate Striatum.
(A) MRI image of a Rhesus macaque coronal brain section (left) showing three striatal regions labeled with cyan (caudate nucleus), brown (putamen), and pink (ventral striatum). Schematic striatum (middle) marked by Cd (caudate nucleus), Pt (putamen), and VS (ventral striatum). The right axis shows dorsal (D), ventral (V), lateral (L), and medial (M) directions.
(B) UMAP visualizations of the samples from two subjects (P and F).
(C) UMAP visualizations of striatal nuclei colored by the three regions. The color scheme for these regions is the same as in A.
(D) Feature plots of canonical neuronal and astrocyte marker gene expression in striatal nuclei.
(E) UMAP visualization colored according to eight major classes in the NHP.
(F) Heat map of differentially expressed genes. Color bar at the top corresponds to the major classes identified in E.
(G) Violin plots of distributions of marker gene expression across nine clusters, with MSNs divided into D1- and D2-MSNs.
See also Figures S1 and S2, Table S1, and Data S1.
Transcriptional Diversity of Medium Spiny Neurons
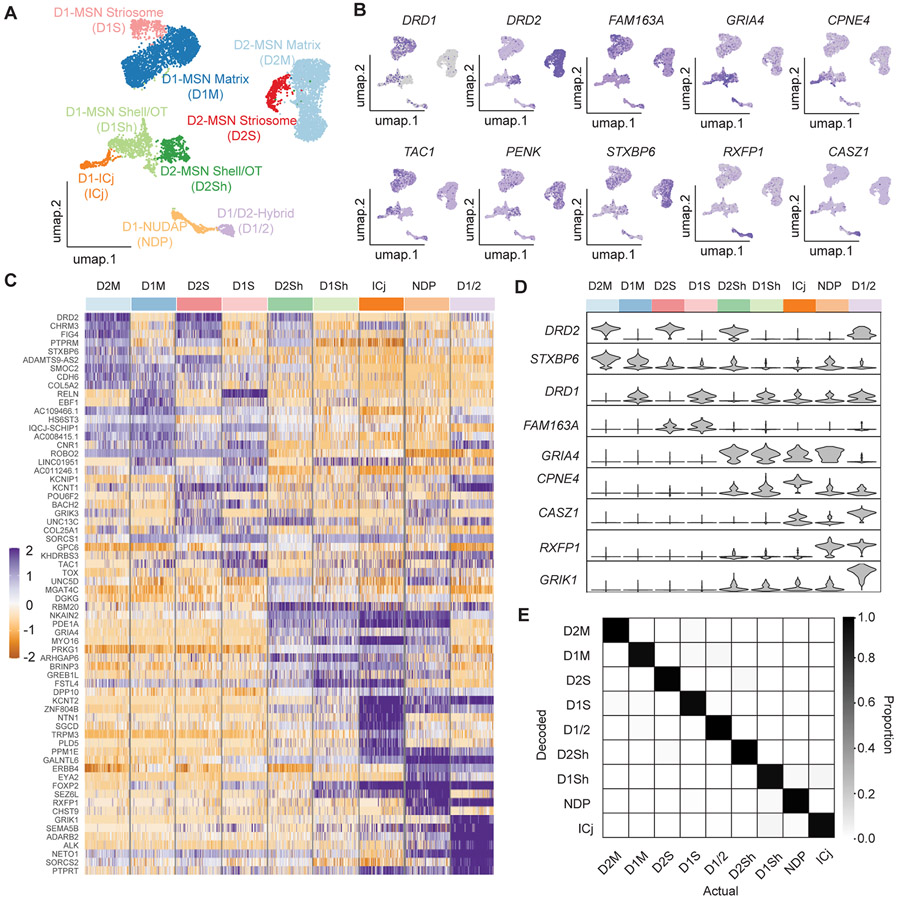
The expression of well-known MSN marker genes, including PP1R1B, BCL11B, and PDE1B,23,24 indicated which clusters contained MSNs (Figures S1E-S1G). Differential gene analysis of those MSN clusters vs. other clusters revealed several other MSN markers, including KIAA1211L, PDE2A, SLIT3, and NGEF (Figures S1H-S1K). To identify MSN subtypes, we recalculated the PCAs and performed dimensionality reduction on the isolated MSN nuclei, clustered them at a resolution that distinguished physically separated UMAP clusters, and annotated them using FISH probes against a mixture of previously described16,25,26 and novel marker genes (Data S1; Figures 2A-2C and 4-7). The clusters putatively corresponded to D1- and D2-MSNs in the matrix (D1M and D2M), D1- and D2-MSNs in striosome (D1S and D2S), D1- and D2-MSNs in the NAc shell and olfactory tubercle (OT) (D1Sh and D2Sh), and MSN-like neurons located in the interface islands (Figure 2A). One cluster was a D1/D2-hybrid (D1/2) and shared many characteristics with a novel MSN type described in rodents (D1H or eccentric-SPN).15,17 Each MSN cluster was signified by groups of differentially expressed genes that were specifically enriched in that cluster (Figures 2C and 2D; Data S1; Table S1) (Methods). We used the Single Cell Clustering Assessment Framework (SCCAF) to quantify the robustness the MSN subtype clusters.27 For all nine clusters, the model predictions were robust: the areas under the receiver operating characteristic (AUROC) was more than 0.92 and 0.94, for monkeys P and F, respectively (Figure 2E and S3D).27 Together, these results indicate that the primate striatum contains at least nine transcriptionally distinct neuron subtypes that all feature characteristics of MSNs.
Figure 2. Medium Spiny Neuron (MSN) Subtypes.
(A) UMAP projection of MSN nuclei. Each dot represents a nucleus, and the colors represent the different MSN types.
(B) Feature plots for the expression of DRD1, TAC1, DRD2, PENK showing the separation of D1- and D2-MSNs and the expression of marker genes enriched in each cluster.
(C) Heatmap showing the top ten most enriched genes in each MSN type. Colored bar at the top corresponds to the colors in A.
(D) (top) MSN type identifications colored according to A. (bottom) Violin plots showing cell type and compartment specific marker gene expression.
(E) The accuracy rate between SCCAF decoded cell type and actual cell type using the data combined from both subjects.
See also Figures S1 and S3, Table S1, and Data S1.
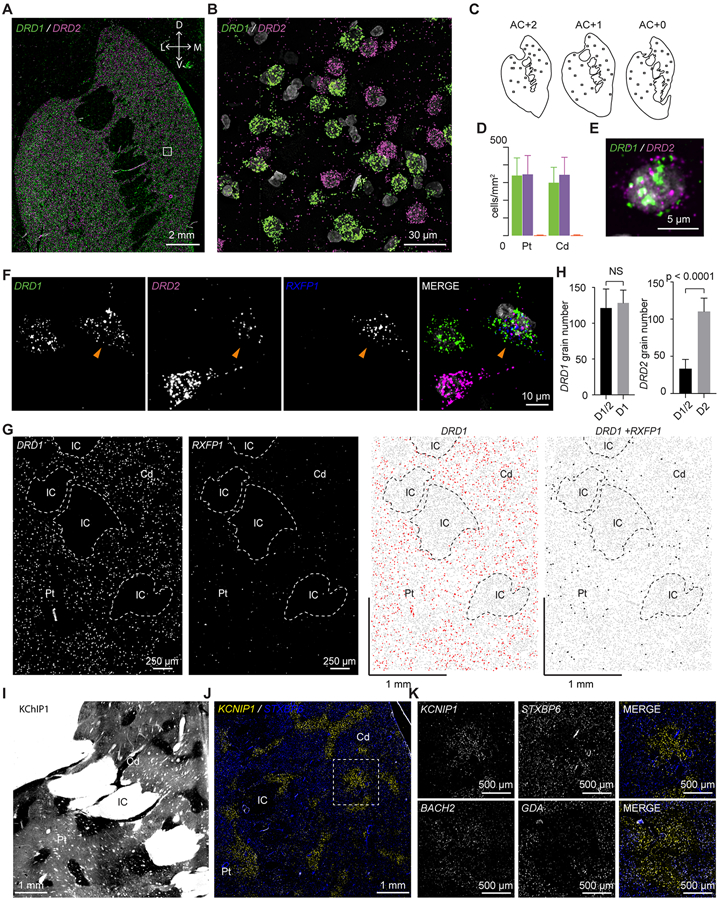
Figure 4. MSN Subtypes in the Dorsal Striatum.
(A) FISH labeling of DRD1 (green) and DRD2 (magenta). White box indicates the region shown in B. The top right axis shows dorsal (D), ventral (V), lateral (L), and medial (M) directions.
(B) High resolution image of region highlighted in A.
(C) Schematic diagrams of the three sections used for DRD1 and DRD2 quantification. The square boxes indicate the quantified regions of interest (ROIs).
(D) Quantification of cell density of neurons expressing DRD1 (green), DRD2 (magenta), or both (orange) in the caudate and putamen. Error bars are SD across ROIs.
(E) One example MSN expressing both DRD1 and DRD2.
(F) RXFP1 labels D1/D2-hybrid MSNs in the dorsal striatum. Arrowhead points to an example D1/D2-hybrid cell.
(G) Quantification of DRD1 and DRD2 grain number in D1/D2-hybrid cells and normal D1- or D2-MSNs. Unpaired t-test was used for statistical analysis and p values were indicated on the plots. Error bars represent standard deviation (SD) across 6 cells per type. NS: non-specific.
(H) FISH labeling of DRD1 and RXFP1. Left two pictures are original FISH images showing the distribution of DRD1 and RXFP1. The right two pictures are CellProfiler processed images showing DRD1 expressing (red dots) or DRD1 and RXFP1 (black dots, enlarged for display purposes) co-expressing cells. Grey dots are nuclei. Abbreviations: Cd, caudate; Pt, putamen; IC, internal capsule.
(I) Immunohistochemistry of KChIP1 showing robust striosome pattern.
(J) FISH labeling of KCNIP1 (yellow) and STXBP6 (blue) distinguishes striosome and matrix, respectively. White square indicated the region shown in K.
(K) (top) Detail of the white square in J. (bottom) As in above, for characteristic striosome and matrix markers, BACH2 and GDA. Abbreviations: Cd, caudate; Pt, putamen; IC, internal capsule.
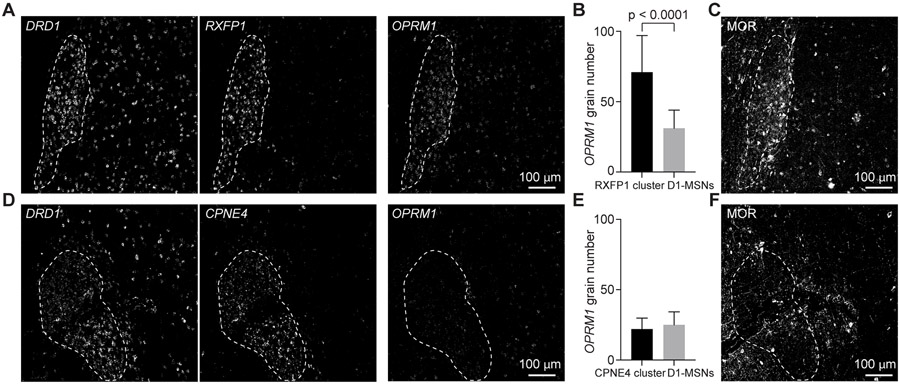
Figure 7. μ-Opioid Receptor Expression is Specifically Enriched in D1-NUDAP cells.
(A) FISH stain of DRD1 and RXFP1 as well as OPRM1 in two close sections. White dashed line delineates the boundaries of a RXFP1 cluster.
(B) Quantification of grain number of OPRM1 in RXFP1 clusters and close D1-MSNs. Unpaired t-test was used for statistical analysis. Error bars represent standard deviation (SD) across 41 cells in each group.
(C) MOR expression in an adjacent section of A.
(D) FISH stain of DRD1 and CPNE4 as well as OPRM1 in two close sections. White dashed line delineates the boundaries of a CPNE4 cluster.
(E) Quantification of grain number of OPRM1 in CPNE4 clusters and close D1-MSNs. Unpaired t-test was used for statistical analysis. Error bars represent standard deviation (SD) across 36 cells in each group.
(F) MOR expression in an adjacent section of D.
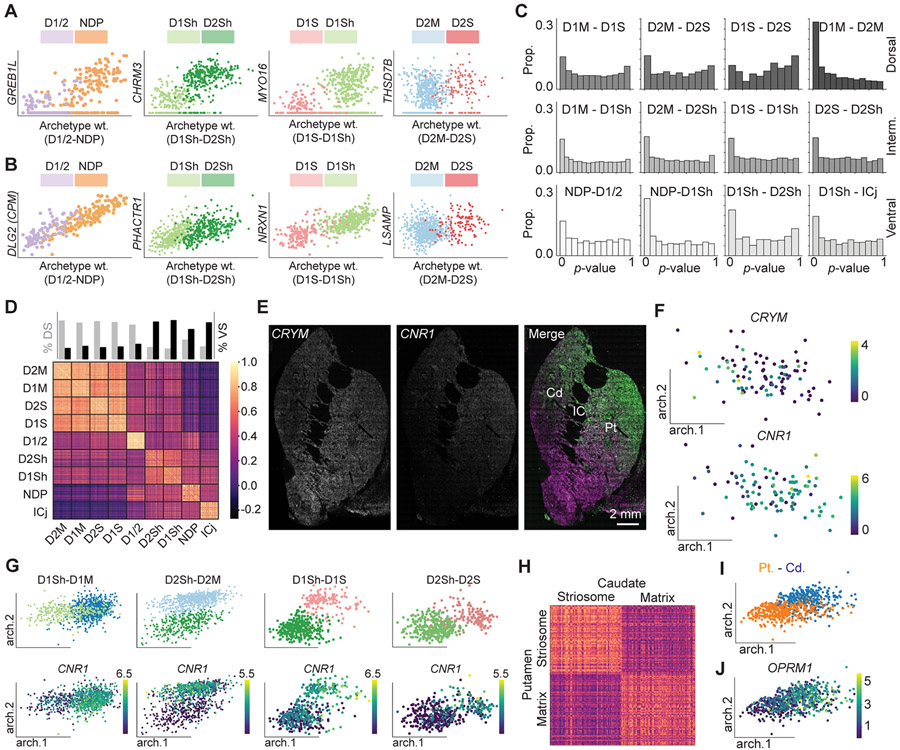
We used archetypal analysis to determine the similarity between the monkeys P and F, and the relationships between MSN subtypes.28-30 Archetypal analysis decomposes the expression matrix into gene loading vectors, or ‘archetypes,’ that correspond to cell states.28-30 We found the archetypes that defined the transition from one MSN subtype to another using the data from Monkey P. When projected onto monkey F, these archetypes maintained the same transitions between subtype clusters (Figure S3G). This result illustrates the high level of consistency between the two experimental subjects and validates the archetypes. We subsequently found all genes that were significantly correlated with the archetypes that defined the transitions between subtypes (p < 0.05, Benjamini-Hochberg corrected, Pearson’s correlation). Each subtype pair contained genes where transitions included a significant discontinuity (Figure 3A, p < 0.05, Regression discontinuity test, Methods) and other genes without significant discontinuity (Figure 3B, p > 0.05, Regression discontinuity test). The p-value distributions demonstrated the degree of discontinuity between subtype pairs (Figure 3C). For every pair, except for D1S and D2S – the subtypes for which we had the lowest sample sizes – the p-value distributions indicated more statistically significant discontinuities than predicted by chance (Figure 3C, p < 0.00001, Benjamini-Hochberg corrected, Binomial test, Methods). These results demonstrate that each identified MSN subtype is characterized by discrete gene expression patterns.
Figure 3. Archetypal Analysis of MSN Subtypes.
(A) Representative genes showing significant discontinuity between a subtype pair.
(B) Representative genes showing non-significant discontinuity between a subtype pair.
(C) The p-value distributions between each subtype pair.
(D) Heat map showing the cosine similarity between cells within and between the nine types of MSNs.
(E) FISH labeling of CRYM (magenta) and CNR1 (green) reveals a continuous gradient on the dorsal-ventral axis.
(F) CRYM (top) and CNR1 (bottom) expressions along the D1/D2-hybrid archetype axes.
(G) The archetype distribution of subtype pairs (top) that were divided between the dorsal and ventral striatum and CNR1 (bottom) distributions in these archetype axes.
(H) Heat map showing the cosine similarity between cells within and between striosome and matrix in caudate and putamen. The color scale is the same as in D.
(I) Distribution of caudate and putamen in archetype axes.
(J) OPRM1 expression in the caudate and putamen cells along the archetype axes.
Despite our emphasis on discrete borders between subtypes, we also detected continuous gradients of gene expression. Cosine similarity between nuclei indicated that DS-derived clusters were more like other DS clusters compared to VS-derived clusters, and vice versa (Figure 3D) (p < 0.0001, Permutation tests, STAR Methods). Consistent with this and with previous studies in mouse,15,16 the crystallin mu and the cannabinoid receptor genes, CRYM and CNR1, respectively, reflect continuous gradients on the dorsal-ventral axis of the mouse striatum (Figure 3E). Archetypal analysis indicated that this gradient is reflected in the D1/D2-hybrid population (Figure 3F; Table S2), and across subtype pairs that were divided between the DS and VS (Figure 3G). This results highlights that gene expression gradients that define position on the dorsal and ventral axis are conserved between species. The primate DS is divided into the Cd and Pt by the internal capsule. Compared to the differences between the DS and VS, the Cd and Pt appeared more similar. Indeed, cosine similarities between the striosome and matrix showed that striosome nuclei in the Cd were far more similar to striosome nuclei in the Pt, as opposed to matrix nuclei in the Cd (Figure 3H, p < 0.0001, Permutation test, STAR Methods). Nevertheless, archetypal analysis identified a gradient that showed an enhanced μ-opioid receptor (OPRM1) expression in the Cd (Figures 3I and 3J; Data S2). This archetype might highlight striosome-enriched nature of the Cd.
Archetype analysis also highlighted continuous sources of variation within subtypes. Some nuclei derived from the VS clustered together with matrix nuclei (D1M and D2M). We detected archetypes that highlighted the VS nuclei fraction (Figure S3H), and the genes that were correlated with the VS weighted vector are preferentially expressed in NAc core, including ZDBF2 (r = 0.18 and 0.2, p < 10−6 and 10−7, with D1- and D2-MSNs, respectively, Pearson’s correlation) and HPCAL4 (r = 0.12, p < 10−3 with D2-MSNs, Pearson’s correlation) (Data S3).31 Another archetype of particular interest was found in D1Sh and defined by upregulation of the gene for the Neurokinin-B receptor, TAC3. Differential gene analysis of the D1Sh TAC3-archetype revealed selectively genes enriched in this archetype, including MPPED1, HPCAL1, and MEIS3. This result demonstrates within-subtype variations with potentially functional roles.
MSN Subtype and Archetype Distributions in the Dorsal Striatum
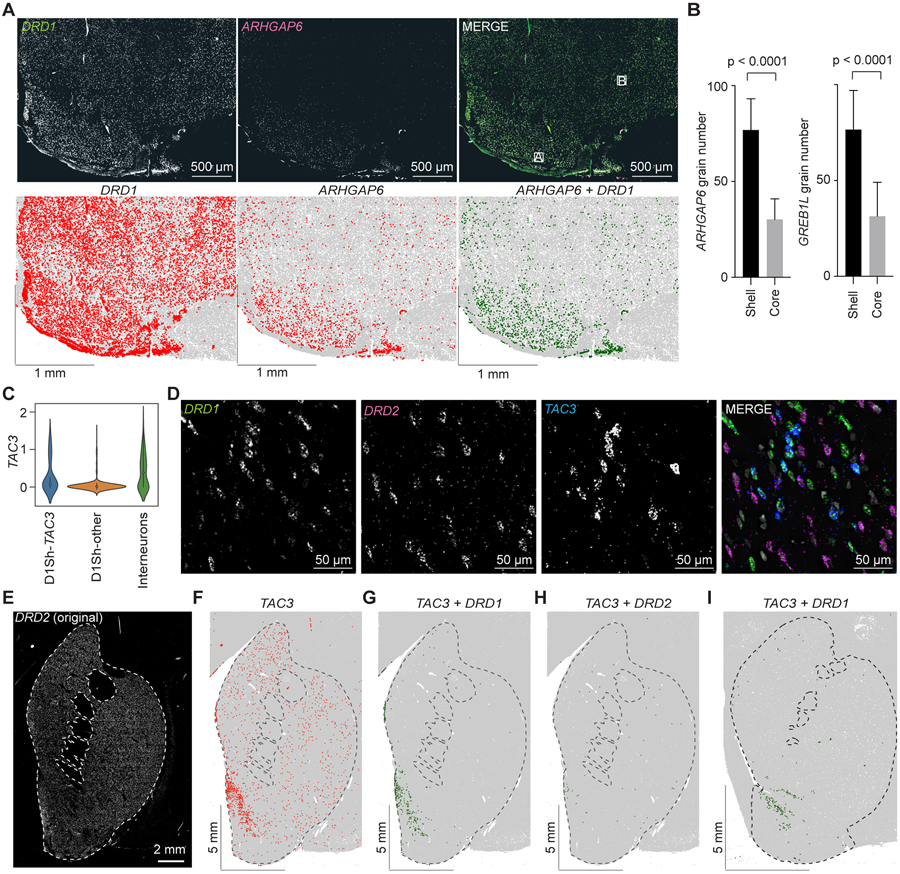
We used FISH to explore the anatomical distribution of MSN subtypes and archetypes. As expected, the majority of neurons expressed either DRD1 (325 ± 99 nuclei/mm2) or DRD2 (320 ± 99 nuclei/mm2; Figures 4A-4D). To investigate the relatively rare hybrid cell type (D1/2) that expresses both DRD1 and DRD2, we used probes against RXFP1, a highly specific marker gene for D1/2 cells (p = 2.47 x 10−135 , Wilcoxon). High resolution imaging confirmed that RXFP1 positive cells in the dorsal striatum co-labeled with DRD1 and DRD2 (Figure 4F). D1/2 cells were distributed uniformly throughout the DS (Figure 4G). Intestingly, D1/2 cells had the same amount of DRD1 expression as nearby D1-MSNs, but there was far less DRD2 expression when compared to nearby D2-MSNs (Figure 4H, p = 0.55 and p < 0.0001 respectively, unpaired t-tests). Co-clustering of the NHP data with striatum data from mouse indicates that the D1/2 shares important transcriptional characteristics with a subset of the recently described “D1H” (Figures S3A-S3C).15,17 These results confirm that a hybrid D1/2 cell type is also present in the NHP striatum.
Both D1- and D2-MSNs derived from the DS split into two clusters; one larger cluster and one smaller cluster. We reasoned that the larger D1- and D2-MSN clusters likely corresponded to the matrix (Figure 2A, dark and light blue clusters, respectively), whereas the smaller clusters likely corresponded to striosomes (Figure 2A, light and dark red clusters, respectively). The genes most enriched in the striosome MSNs (D1S and D2S) were KCNT1, KHDRBS3, FAM163A, BACH2, and KCNIP1, whereas the genes most enriched in the matrix MSNs (D1M and D2M) were EPHA4, GDA, STXBP6, and SEMA3E (Figures 2C and S4A) (p < 1.77 x 10−52 , Wilcoxon). KCNIP1 is the gene for the the potassium channel-related KChIP1, and staining for KChIP1 labels the boarders of the striosome (Figure 4I).32 We performed FISH labeling with KCNIP1 and STXBP6 – a highly specific matrix marker – and the results show a similar pattern as the KChIP1 antibody labelling (Figures 4J and 4K). Similar patterns were observed using FISH probes that labeled other identified striosome and matrix markers (Figures 4K and S4B-S4D). Thus, these results confirm that four major snRNA-Seq clusters derived mostly from the DS correspond to D1- and D2- MSNs in the striosome and matrix.
MSN Subtype and Archetype Distributions in the Ventral Striatum
Four MSN clusters were highly enriched in the nuclei from the VS (Figure 2D). Three of the clusters were DRD1 positive, whereas the fourth cluster was DRD2 positive. Differential gene analysis of the two largest clusters (Figure 2A, light and dark green) revealed selectively enriched marker genes, including GREB1L, ARHGAP6, and GRIA4 (Figure 2C). FISH labeling of the striatum with DRD1 and ARHGAP6 revealed that ARHGAP6 was enriched in a restricted portion of the VS that likely corresponds to the NAc shell and OT (Figures 5A, 5B and S5A), but was not prevalent in the core (Figure S5B). Quantification of grain number of ARHGAP6 in putative shell/OT and core regions confirmed that ARHGAP6 was more enriched in shell/OT (Figure 5B, left), and very similar pattern was observed with probes against another enriched marker gene, GREB1L (Figure 5B, right). We labeled sections with DRD1 and GREB1L probes and an adjacent section with an immunofluorescent stain for calbindin, which roughly markes the border between NAc core and shell.33 The GREB1L intensity traced the putative transition from the calbindin-poor shell to the calbinin-rich core (Figure S5C). Thus, these results indicate that NAc Shell and OT are comprised of region-specific D1- and D2-MSNs.
Figure 5. MSN Subtypes in the Ventral Striatum.
(A) (top) Double labeling with DRD1 (left) and ARHGAP6 (middle) of the NAs shell/OT shows that ARHGAP6 is selectively enriched in the shell/OT. (bottom) CellProfiler processed images for the above images. Lettered boxes indicate regions shown in Figure S5A and S5B.
(B) (left) Quantification of grain number of ARHGAP6 in shell/OT and core. Unpaired t-test was used for statistical analysis. Error bars represent standard deviation (SD) across 32 cells per each region. (right) Quantification of grain number of GREB1L in shell/OT and core. Unpaired t-test was used for statistical analysis. Error bars represent standard deviation (SD) across 29 cells per each region.
(C) Violin plot showing TAC3 levels in D1Sh-TAC3 archetype, other D1Sh cells, and TAC3 interneurons
(D) TAC3 co-localizes with DRD1 in medial shell MSNs.
(E) DRD2 FISH image showing the outline of the striatum. Dashed white line delineate the borders of striatum.
(F) CellProfiler results showing the TAC3 distribution (red dots) in the section in E. Grey dots are nuclei.
(G) CellProfiler results showing the distribution of TAC3 and DRD1 co-expressing cells (dark green dots) in the section in E.
(H) CellProfiler results showing the distribution of TAC3 and DRD2 co-expressing cells (dark green dots) in the section in E.
(I) CellProfiler results showing the distribution of TAC3 and DRD1 co-expressing cells in a second animal (dark green dots).
Archetypal analysis revealed a TAC3-positive archetype within the D1-Shell/OT subtype (Figures 5C and S5D-S5F; Table S3). In order to locate this MSN archetype and to distinguish it from TAC3-positive interneuons (Figure S2),18 we triple labelled coronal sections with probes against DRD1, DRD2, and TAC3 (Figures 5D and 5E). This labeling revealed the previously described TAC3 interneuons unifomly distributed throughout the striatum (Figure 5F); these cells did not show co-localization with DRD1 or DRD2. However, there was a cluster of TAC3-positive neurons located in the medial shell region of the NAc that co-localized with DRD1, and far fewer than that co-localized with DRD2 (Figures 5G and 5H). We observed the same DRD1- and TAC3-positive cluster in the medial shell region of a second monkey (Figure 5I). These results reveal a novel TAC3-positive MSN archetype in the primate NAc.
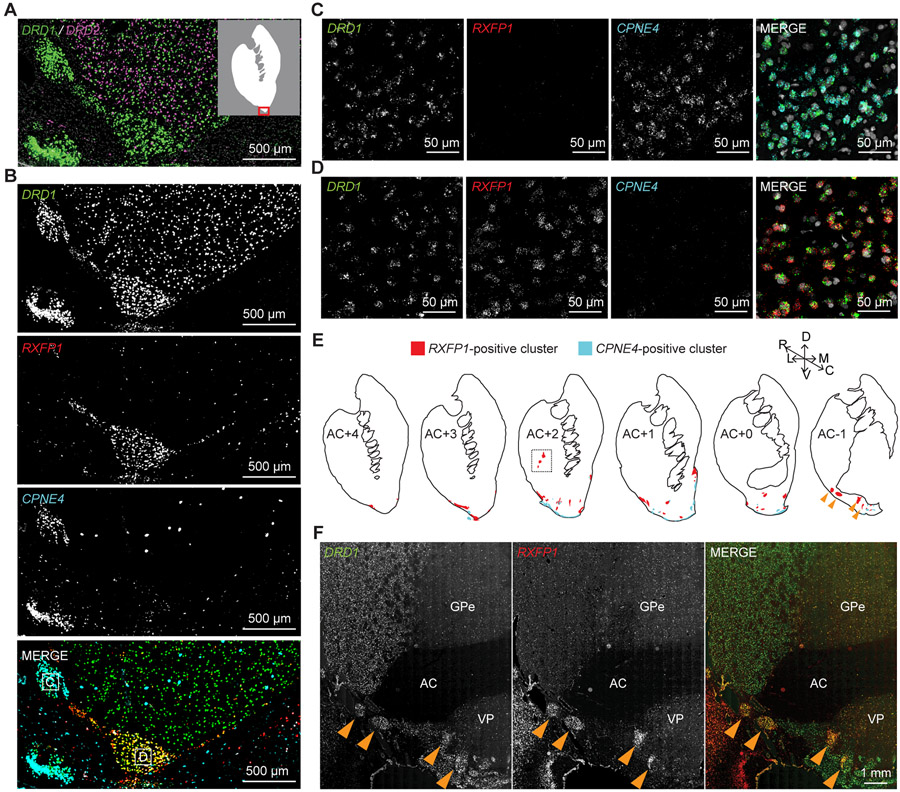
One of the most remarkable features of the DRD1 and DRD2 labelling in the VS was the presence of D1-exclusive islands that likely corresponded to “interface islands” (Figure 6A).34,35 We investigated whether the cell types within these D1-exclusive islands corresponded to the smaller DRD1 enriched VS clusters (Figure 2A, light and dark orange). We selected two respective marker genes, RXFP1 and CPNE4, for the two DRD1 enriched VS clusters (Figure S6C). We labeled one section using DRD1, RXFP1, and CPNE4 probes and revealed that RXFP1 and CPNE4 labeled distinct D1-exclusive cell islands (Figure 6B). High resolution confocal microscopy of these islands verified that CPNE4 and DRD1 co-localized in the same cells in one island (Figure 6C), whereas RXFP1 and DRD1 co-localized in the same cells of another island (Figure 6D). These results suggest that different interface islands contain different DRD1-positive cell types.
Figure 6. Cell Types in the Interface Islands.
(A) FISH stain of DRD1 (green) and DRD2 (magenta). Inset: White area indicates striatum and the red box highlights the area shown in A and B.
(B) FISH stain of DRD1, RXFP1 and CPNE4 in immediately adjacent section from A.
(C) High-resolution image of the regions indicated with the letter “C” in B.
(D) High-resolution image of the region indicated with the letter “D” in B.
(E) Distribution of RXFP1 and CPNE4 clusters across six rostral-caudal regions identified by multichannel FISH. The upper right axis shows dorsal (D), ventral (V), lateral (L), medial (M), rostral (R), and caudal (C) directions. Dashed white box denotes RXFP1 clusters in putamen, the FISH image of which is shown in Figure S5H. Yellow arrowheads point to RXFP1 clusters in caudal extent of NAc in both illustration and images shown in F.
(F) Example RXFP1 clusters in caudal extent of NAc. AC = anterior commissure, GPe = external globus pallidus, VP = ventral pallidum.
We repeated the DRD1, RXFP1, and CPNE4 FISH on regularly spaced pre- and peri-commissural coronal sections. We defined the regions of the VS by comparison of Nissl-stained sections with a high-resolution MRI and DTI Rhesus macaque brain atlas (Figures S6A and S6B)36,37 and we mapped all the nearby islands in two monkeys (Figures 6E and S5G-S5I). The CPNE4-positive islands appeared to correspond to the Islands of Calleja (ICj).38 This correspondence was verified by intense co-localization of CPNE4 and DRD1 in the cells of the major ICj, an easily identifiable landmark at the border between the NAc and the septal nuclei (Figure S6D). Previous studies have shown that cells in ICjs are granule cells.38 Likewise, the CPNE4-positive cells we examined were small and exhibited high packing density (Figures S6E and S6F). Thus, the cluster enriched with DRD1 and CPNE4 corresponded with granule cells in the ICjs. Outside of the ICjs, co-localization between DRD1 and CPNE4 was restricted to a dense cell layer at the ventral extreme of the OT, possibly corresponding to a portion of the anterior olfactory nucleus (AON) (Figures 6B and S6G). Gene enrichment analysis revealed that differentially expressed genes in these cells are implicated in neurogenesis, neurosecretion, and many other functions (Data S4).
In contrast to the CPNE4-positive ICjs, the RXFP1-positive islands had larger nuclei that were less densely packed together and did not appear different from nearby D1-MSNs (Figures S6E and S6F). Likewise, RXFP1-positive islands were not restricted to the border regions of the VS, rather, they were found throughout the NAc, putamen, and near the adjacent septal nuclei (Figure 6E, orange arrows and dashed black box, Figures S5G-S5I, S6H, S6I, and S7A). RXFP1-positive cells located in these VS islands exhibited high levels of DRD1 expression, but no detectable DRD2 expression (Figures S7A and S7B). Moreover, cells in the RXFP1-positive islands expressed high levels of the gene for the μ-opioid receptor (OPRM1) compared to regular D1- MSNs located outside of the D1-exclusive island (Figures 7A and 7B). Interestingly, expression of the κ-opioid receptor gene (OPRK1) was almost completely absent from these islands, compared to surrounding tissues (Figure S7D). Using immunohistochemistry, we confirmed that MOR was expressed in the RXFP1-positive islands (Figure 7C). Based on their distribution and the upregulation of μ-opioid receptor – upregulation that was not present in the ICjs (Figures 7D-7F) – we concluded that these RXFP1-positive interface islands corresponded with Neurochemically Unique Domains in the Accumbens and Putamen (NUDAPs).10,11 Therefore, we denoted the DRD1- and RXFP1-positive cells as D1-NUDAP neurons. Gene enrichment analysis revealed that D1-NUDAP neurons express genes that have been implicated in drug addiction and many other functions (Data S4). These data show a novel cell type that is associated with interface islands and could be critical for the hedonic aspects of reward. Altogether, these results demonstrate that the ventral striatum is characterized by the presence of discrete MSN subtypes that correspond to functionally relevant subdivisions including NAc shell, OT, and distinct types of interface islands.
DISCUSSION
The phylogenetic relationship between humans and nonhuman primates (NHPs) makes NHPs a crucial neuroscientific model. Here, single nucleus RNA-sequencing (snRNA-Seq) revealed at least nine distinct Medium Spiny Neuron (MSN) and MSN-like subtypes in the NHP striatum (Figure 2). The borders between subtype pairs were characterized by discontinuities in gene expression, though we also found continuous axes of variation (Figure 3). We identified five distinct MSN subtypes in the dorsal striatum (DS), including D1- and D2-MSN subtypes specific to the striosome and matrix compartments, as well as a hybrid cell type that contained mRNA for both D1 and D2 receptors (Figure 4). The ventral striatum (VS) contained at least four distinct subtypes, including D1- and D2-MSN subtypes located specifically in the Nucleus Accumbens (NAc) shell and Olfactory Tubercule (OT) regions (Figure 5), and two subtypes associated with the “interface islands” – dense cellular islands located within and near the ventral border of the striatum. Marker genes for one of these VS cluster subtype were highly enriched in the Islands of Calleja (ICjs). Cells from the other VS cluster subtype were restricted to Neurochemically Unique Domains in the Accumbens and Putamen (NUDAPs) (Figures 6 and 7).10,11 Within these subtypes, archetypal analysis revealed finer distinctions, including a TAC3-positive D1Sh-MSN that could represent the origin of a third pathway through cortico-basal ganglia loops (Figure 5).39 Together, these MSN subtypes and archetypes provide a blueprint for studying cell-type specific functions during sophisticated primate behaviors, and the cell-type-specific marker genes define potential molecular access points to enable the application of genetically coded tools in scientific or translational contexts.
The DS is divided into the caudate nucleus (Cd) and putamen (Pt) by the internal capsule. Spanning these structures are two neurochemical compartments, matrix and striosome, that form the ‘neostriatal mosaic.5,7 Broadly understood, matrix MSNs receive neocortical inputs from associative and sensorimotor cortex and give rise to the direct and indirect pathways.40 In contrast, striosomal MSNs, and the recently discovered, extrastriosomal ‘exo-patch’ MSNs,16,26 receive input from limbic territories, including the anterior cingulate cortex, orbitofrontal cortex, and anterior insular cortex,41 and project directly to midbrain dopamine neurons.42,43 Despite our advanced understanding of these circuit-based structures, we are only beginning to gain insights into the associated circuit-based functions. For example, striosomal MSN activations influence cognitive and emotional decision making44 and value based learning.45 A critical milestone that will enable us to accelerate functional discovery will be the development of cell-type- and compartment-specific viral vectors to enable circuit interrogation in NHPs. Here, we identified compartment-specific gene expression patterns for NHP matrix – STXBP6, GDA, and SEMA3E – and striosome – BACH2, KCNT1, KCNIP1, and KHDRBS3. Moreover, we found an extrastriosomal cell type, the D1/D2 hybrid, that expressed many of the genes associated with striosome, thus suggesting a possible homology to ‘exo-patch’ cells.26 We expect that understanding the regulatory vocabulary governing these gene expression patterns will reveal cell-type-specific enhancers that can be packaged into AAVs that grant molecular access to striosome and matrix MSNs in the NHP brain.
The VS is strongly implicated in reward processing.9 The NAc and OT complex comprises a major portion of the rostral VS, and this complex is traditionally recognized as a limbic-motor interface.46 The NAc is further divided into core and shell territories with distinct behavioral functions.47 We found several gene markers, including ARHGAP6, GREB1L, and GRIA4, that were upregulated in the VS samples (Figure 2). FISH labeling with these probes revealed that their upregulation traced the transition of the ventrally positioned, Calbindin-poor shell to the dorsally positioned, Calbindin-rich core (Figure S5C). Thus, these marker genes label D1- and D2-MSN subtypes that were specific to the NAc shell and OT. Within the D1Sh subtype, we detected an archetype that expresses the gene for Neurokinin-B (TAC3) (Figures 5C-5I). Previous tracing studies in rodents have shown that a small population Neurokinin B-positive D1-MSNs have direct projections to other basal forebrain structures, including notably the cholinergic substantia inomiata.39,48,49 Thus, this TAC3 MSN archetype could represent the genesis of an additional pathway, along with the direct and indirect pathways, for cortico-striatal signals to reach the cortex. As with the DS cell types, the regulatory code controlling these cell-type-specific VS gene expression patterns will likely hold the keys to molecular access points.
The DS striosome and the VS are both implicated in limbic functions and reward processing, and thus it is interesting to compare these subpopulations. Our data indicate that there are many transcriptional similarities between the striosome and VS cell types, but also some key differences. For example, many striosome specific markers are upregulated in D1-NUDAP cells, including KCNIP1, KCNT1, KHDRBS3, and BACH2 (Figure S7C). Even PDYN, which is a widely acknowledged D1-striosome marker gene.50 is also expressed in D1-NUDAPs. On the other hand, D1-NUDAPs also express some genes which we found to be selectively expressed in the matrix, including STXBP6, GDA, and SEMA3E. OPRM1 was upregulated in the striosome, as predicted, but the upregulation was not as dramatic as we expected. In contrast, we observed robust OPRM1 signals in the NUDAPs (Figures7A-7C). Indeed, this selective enrichment of OPRM1 in the RXFP1-positive interface islands is a key piece of evidence in favor of the NUDAP hypothesis. This selective OPRM1 enrichment suggests the intriguing possibility that NUDAPs are part of the network of “hedonic hotspots?” Hedonic hotspots are regions in the NAc and ventral pallidum that, when opioids are directly applied, produce behavioral reactions that indicate pleasure.51 As might be expected for hedonic hotspots, the κ-opioid receptor gene (OPRK1) was absent from D1-NUDAP cells (Figure S7D). These differentially expressed genes and others provide a blueprint for understanding cell-type-specific contributions of this novel cell type to reward processing and pleasure.
The basal ganglia are highly conserved throughout vertebrate evolution,52 and a rough comparison of our results with single cell studies of the mouse striatum13-17 confirmed this pattern at the level of cell types. As with prior studies, we find a relatively even distributions of D1- and D2-MSN in the striatum. Approximately 10% of sampled MSNs were identified as striosome MSNs.17 We found that our neuronal nuclei samples contained approximately 85% MSNs and 15% interneurons; this is a higher proportion of interneurons than was recovered from single cell analysis of rodent striatum,17 but consistent with counts made in humans.53 A novel and relatively rare MSN type has been recently documented and variously described as eccentric SPNs,16,17 Pcdh8-MSNs,13 and D1H.15 Both D1/D2 hybrid and D1-NUDAP neurons shared some characteristics with this novel cell type. We formally compared our results to D1H because the sequencing depth was most similar between the studies. Co-clustering our data with the mouse data revealed that approximately half of the D1H population co-clustered with D1/D2-hybrids, and the other half co-clustered with D1-NUDAPs (Figures S3A-S3C). However, despite their similarities and their co-clustering with D1H, our data indicate that D1-NUDAP neurons and D1/D2-hybrid neurons represent distinct MSN subtypes. First, although DRD2 expression was common in D1/D2-hybrids (Figures 2D and 4F), we found no evidence of DRD2 expression in D1-NUDAPs (Figures S7A and S7B). Second, the D1-NUDAP neurons did not express other marker genes, including CASZ1 and GRIK1, that were reported in D1/D2-hybrids, D1H, and eSPNs.15-17 Third, a machine learning classifier easily distinguished between D1-NUDAP and D1/D2-hybrid neuron subtypes (Figure 2E). Finally, we only found D1/D2-hybrid MSNs in the DS, whereas D1-NUDAP cells were restricted to dense cell islands in the VS. Together, these data clearly demonstrate that in NHPs, D1-NUDAP and D1/D2-hybrid MSNs are discrete subtypes. However, the limitations involved with integrating single cell data sets,54,55 and the vast differences between the studies – differences that include species, age, single cell technology, transgenic status, MSN sampling density, and sequencing depth – preclude us from determining the role of species in determining the degree of discontinuity between cell types.
In contrast to the distinct boundaries between subtypes, we also observed continuous variation in gene expression.15,16,56 On a large scale, continuos variation in gene expression was exemplified by the dorso-ventral gradients of CRYM and CNR1 (Figure 3), but there were also axes of continuous variation within and between MSN subtypes. We examined this variation using archetypal analysis.28-30 Archetypes have biologically interpretable dimensions and concepts, in genes and cell states, respectively. Moreover, projecting archetypes learned in one biological replicate onto another biological replicate requires only a simple matrix operation. This simplicity enabled us to clearly demonstrate the similarity between the biological replicates (Figure S3G). Within most of the MSN subtypes, we observed several archetypes. For example, archetypal analysis of the matrix clusters revealed an archetype that highlighted VS derived nuclei (Figure S3H). Some of the genes correlated with this VS archetype are upregulted in the NAc core.31 Thus, this archetype analysis defined a potential NAc core signal. One challenge that remains is to determine whether archetypes indicate subtypes or ‘states,’ with the former being a stable feature found across individuals and the latter being a transitory phase that could be activity dependent. In the case of the D1Sh TAC3-archetype, the fact that we detected it in two monkeys indicates that this archetype is akin to a minor subtype, but other archetypes may indicate transitory cell states. As we gather more data about cell types – for example data on sexual dimorphisms, epigenomic features, physical circuits, and behavioral functions – we believe that the concepts captured by archetypal analysis will be crucial for organizing, describing, and modeling the vast functional heterogenity that charachterizes even simple brain structures like the striatum.
The challenge of defining “cell types” was the genesis of modern neuroscience.57 More recently, we have come to understand cell types as complex distributions of molecular processes.58,59 The dynamics of such processes rarely fit simple boundaries, but nonetheless we continue to use the idea of cell types to abstract molecular, neurophysiological, and morphological patterns that we observe in our cells of study. We foresee at least three critical reasons to avidly continue doing so in NHP. First, Old-world monkeys, like Rhesus macaques, are more similar to humans than any other research animal that allows for invasive neurophysiological experiments. Accordingly, Rhesus macaque cell types, including highly specialized neurons like Betz cells, von Economo neurons, and even striatal interneuron types, recapitulate homologous human cell types better than cells from rodents or even from marmosets.18,60,61 Second, single cell studies performed on post-mortem human tissue are subject to different ethical constraints that manifest as relatively long and highly variable postmortem intervals.62 In contrast, NHP experiments can be performed in a highly controlled and more timely fashion. Finally, NHPs have resisted the widespread application of modern genetically coded tools. Single cell technologies, including snRNA-Seq and snATAC-Seq can identify cell types and potent regulatory sequences that will break this resistance and enable the effective applications of genetically coded tools in large, wild type animals that resemble humans. Rhesus macaque behavior is readily interpretable in terms of human behavioral theory such as economic theory,63-66 learning theory,67 and even game theory.68,69 The diversity of MSN cell types presented here provides a blueprint to investigate the cell-type-specific mechanisms for such sophisticated behaviors.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for reagents and resources should be directed to and will be fulfilled by the Lead Contact, William R. Stauffer (wrs@pitt.edu).
Materials availability
FISH probes are available from ACD with the catalog numbers we list below.
Data and code availability
We deposited our snRNA-Seq data to GEO accession GSE167920. Go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE167920. Code is publicly available at https://github.com/pfenninglab/nhp_snrna_striatum_analysis and https://github.com/rewardlab/NHPstriatum. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Non-human primates (NHPs)
All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee (IACUC) (Protocol ID, 19024431). Rhesus Monkeys were single- or pair-housed with a 12h-12h light-dark cycle. Monkey F was a 12-year-old female (8.1 kg). Monkey P was a 5-year-old female (5.4kg). Monkey B was a 13-year-old male (11.78 kg). Monkey K was a 4-year-old male (6.0 kg).
METHOD DETAILS
MRI and surgery
For MRI, we anesthetized monkey F and P with ketamine and maintained general anesthesia with isoflurane. We head fixed the monkeys using a MRI-compatible stereotaxic frame and scanned (Siemens, 3T) for anatomical MRI. We generated a whole brain model for each monkey using Brainsight (Rogue Research) and 3-D printed a custom matrix for the brain with cutting guide set every 1 mm.
To maximize nuclei viability, we followed a harvesting protocol similar to the one outlined in Davenport et al.70 Briefly, animals were initially sedated with ketamine (15 mg/kg IM), and then ventilated and further anesthetized with isoflurane. The animals were transported to a surgery suite and placed in a stereotaxic frame (Kopf Instruments). We removed the calvarium and then perfused the circulatory systems with 3-4 liters of ice cold artificial cerebrospinal fluid (ACSF; 124 mM NaCl, 5 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 23 mM NaHCO3, 3 mM NaH2PO4, 10 mM glucose; pH 7.4, osmolarity 290–300 mOsm) oxygenated with 95 % O2:5 % CO2. We then opened the dura and removed the brain. We sliced on the custom brain matrix into 4 mm slabs along the rostral-caudal axis. We removed three striatal regions – the caudate nucleus, putamen, and ventral striatum – under a dissection microscope for nuclei isolation. Monkeys B and K, for FISH, were perfused with 4% paraformaldehyde (PFA, Sigma-Aldrich, Cat# P6148) in phosphate buffered saline (PBS, Fisher scientific, Cat# BP243820) supplemented with 10% sucrose (Sigma-Aldrich, Cat# S8501). The brain was post-fixed with 4% PFA and cryopreserved with a gradient of sucrose (10%, 20%, 30%) in PBS.
Nuclei isolation
We isolated nuclei isolated as previously described.71 Briefly, we homogenized tissues using a loose glass dounce homogenizer followed by a tight glass homogenizer in EZ PREP buffer (Millipore Sigma, Cat# NUC-101). We washed nuclei once with EZ PREP buffer and once with Nuclei Suspension Buffer (NSB; consisting of 1× PBS, 0.01% BSA and 0.1% RNase inhibitor (Clontech, Cat# 2313A)). We re-suspended the washed nuclei in NSB and filtered them through a 35-μm cell strainer (Corning, Cat# 352235). We counted the nuclei and diluted down to 1000 cells/μl. We loaded approximately 10,000 cells from each brain region onto a 10X chip which were then run through a 10x Genomics Chromium controller.
Single nucleus RNA-Seq
We used 10x Chromium Single Cell 3’ Reagent kits v3 Chemistry (10x Genomics, Cat# PN-1000075) for monkeys F and P. We reverse transcribed RNAs and generated libraries according to 10x Genomics protocol. Briefly, we generated Gel beads-in-emulsion (GEMs) after running through a 10x Genomics Chromium controller. We reverse transcribed mRNAs within GEMs in a Bio-Rad PCR machine (Cat# C1000). We barcoded cDNAs from individual cells with 10x Genomics Barcodes and barcoded different transcripts with unique molecular identifiers (UMIs). We purified cDNAs with Dynabeads (10x Genomics, Cat# 2000048) after breaking the emulsion with a recovery agent (10x Genomics, 220016). Then, we amplified cDNAs by PCR and purified them with SPRIselect reagent (Beckman Coulter, Cat# B23318). We analyzed the cDNA quantification and quality using Agilent Bioanalyzer 2100. We prepared libraries following fragmentation, end repair, A-tailing, adaptor ligation, and sample index PCR. We quantified the libraries by qPCR using a KAPA Library Quantification Kit (KAPA Biosystems, Cat# KK4824). We pooled together libraries from individual monkeys and loaded them onto NovaSeq S4 Flow Cell Chip. We sequenced samples from monkeys F and P to depths of 400,000 and 250,000 reads per nuclei, respectively.
FISH probes
We ordered custom FISH probes from ACD to validate MSN subtypes as follows: DRD1 (ACD #549041, a 20ZZ probe targeting 1335-2279 of NM_001206975.1), DRD2 (ACD #549031-C2, a 20ZZ probe targeting 232-1470 of XM_001085571.3), RXFP1 (ACD #801121-C2, a 20ZZ probe targeting 1508-2592 of XM_001096574.4), CPNE4 (ACD #801111-C3, a 20ZZ probe targeting of 2-943 of XM_028843706.1), KCNIP1 (ACD #889143-C3, a 20ZZ probe targeting 283-1626 of XM_015141392.2), KCNT1 (ACD #881571-C3, a 20ZZ probe targeting 964-1906 of XM_015116381.2), BACH2 (ACD #898961-C3, a 20ZZ probe targeting 927-2260 of XM_028847343.1), KHDRBS3 (ACD #881591-C3, a 20ZZ probe targeting 494-1493 of XM_028852934.1), STXBP6 (ACD #881611-C2, a 20ZZ probe targeting 2-1077 of NM_001260925.1), SEMA3E (ACD #879971-C2, a 20ZZ probe targeting 905-1889 of XM_028846126.1), GDA (ACD #881601-C2, a 20ZZ probe targeting 355-1373 of XM_015117899.2), GREB1L (ACD #898991-C3, a 20ZZ probe targeting 784-1732 of XM_015121640.2), ARHGAP6 (ACD #898981-C3, a 20ZZ probe targeting 2516-3463 of XM_001094565.3), TAC3 (ACD #520901-C3, a 17 ZZ design and targets 2- 768 bp of XM_001115535.2), and OPRK1 (ACD #518931, a 20 ZZ probe and targets 91–1392 bp of NM_001321097.1).
FISH stain and imaging
We embedded brains in optimal cutting temperature (OCT) and stored them at −80°C until cutting. We cut floating sections at 15 and 30 μm, in monkey B and K, respectively, mounted tissue on 2x3” pre-treated slides, and preserved the slides in a freezer at −80°C. We used the Advanced Cell Diagnostics (ACD) RNAscope platform and Multiplex Fluorescent Detection Reagents v2 (ACD, Cat# 323110) to perform FISH with slight modifications for monkey brain tissue. We air dried slides for 30 min after removal from −80°C freezer and baked them at 60°C for 20 min. We treated the brain sections with hydrogen peroxide (ACD, Cat# 322335) for 10 min at room temperature and then with RNAscope Target Retrieval Reagents (ACD, Cat# 322000) for 8 min at 99°C. We incubated the slides in 100% alcohol for 3 min and then dried them in 60°C for 10 min. We treated the samples with protease III (ACD, Cat# 322337) for 30 min at 40°C and incubated them with probes for 2 hr. After hybridization with AMP 1, 2 and 3, we incubated the slides with different HRP channels and fluorophores, including Opal 520 (PerkinElmer, Cat# FP1487A), Opal 570 (PerkinElmer, Cat# FP1488A) and Opal 650 (PerkinElmer, Cat# FP1496A). Lastly, we used Trueblack (Biotium, Cat# 23007) to quench Lipofuscin autofluorescence for 45 s at room temperature and counterstained every slide with DAPI before mounting with Prolong Gold Antifade Mountant (Life technologies, Cat# P36930).
We scanned labeled sections using a Hamamatsu NanoZoomer S360 or a Nikon Eclipse Ti2 under 20x objective. For high resolution images, we used a Nikon Eclipse Ti2 or an Olympus IX83 under 40x or 63x objective or 40x with additional 2x built in objective (equal to 80x). We took multi-layer images and did deconvolution using Nikon's NIS-Elements deconvolution software and used maximum intensity projections to create single images. We used NDP software to convert the NanoZoomer original files to tiff format and ImageJ and Adobe Photoshop to adjust brightness and overlay images.
Immunohistochemistry (fluorescent)
We sought to verify previously labeled sections containing FISH probes for possible shell markers using adjacent tissue sections and immunohistochemistry to label for Calbindin. The sections were rinsed in Phosphate Tris (PT, pH 7.2-7.4) buffer and blocked in 10% normal donkey serum (Jackson, Cat# 017-000-121) solution for one hour. The sections were then incubated with primary antibody solution (Swant, Calbindin D-28K, 300, 1: 7,000) overnight at 4°C. The sections were then rinsed in PT buffer and incubated in secondary solution (Alexa Fluor 568, Donkey anti-mouse, 1:300) at room temperature for two hours. They were then rinsed in PT buffer and counterstained with Hoechst (Invitrogen, Cat# H21486, 1: 10,000) for 10 minutes. Lastly, the sections were rinsed in PT buffer and mounted with Prolong Gold Antifade (ThermoFisher, Cat# P36930). To verify μ-opioid receptor proteins were enriched in NUDAPs as well, we did immunostaining with MOR antibodies on an adjacent section performed with OPRM1 FISH labeling (Abcam, ab-10275, 1:500) using similar approach except that PBS buffer instead of PT buffer was used. To verify DRD2 and CPNE4 labels cholinergic neurons, after FISH labeling with the two probes, we performed the following immunostaining with ChAT antibodies (ProSci, Cat# 45-037, 1:1000) using similar approach except that TBS buffer instead of PT buffer was used.
Immunohistochemistry (DAB)
Free-floating sections were selected based on previous FISH labeling to provide verification of common protein marker identification. Sections selected for KChIP1 were rinsed in Phosphate Tris (PT, pH 7.2-7.4) buffer and Phosphate-Buffered Saline with 0.2% Triton (PBST, pH 7.2-7.4) respectively. They were then moved into a 0.5% H2O2 buffer for 10 minutes followed by a series of rinses before incubating in a 10% Normal Horse Serum (NHS, Vector, S-2000) solution for one hour. The tissue was transferred to primary antibody solution (NeuroMab, Anti-KChIP1, clone K55/7, 1:200) for overnight incubation at 4°C. The next day, sections were again serially rinsed in respective buffers and incubated in secondary antibody (Vector, Vectastain ABC, Peroxidase Kit, PK-4002, 1:200) for 30 minutes. Sections were again serially rinsed and placed into ABC (Vector, Vectastain ABC, Peroxidase Kit, PK-4002) buffer for one hour incubation before being rinsed. Tissue was placed in DAB substrate solution (3,3’-diaminobenzidine, Vector, SK-4100) until reacted. Following a final serial rinse, sections were mounted for imaging and analysis.
QUANTIFICATION AND STATISTICAL ANALYSIS
Custom annotation file
We downloaded the macaque rheMac10 genome72 (https://hgdownload.soe.ucsc.edu/goldenPath/rheMac10/bigZips/rheMac10.fa.gz) and human NCBI RefSeq transcriptome annotation gtf file from the UCSC genome browser (https://hgdownload.soe.ucsc.edu/goldenPath/hg38/bigZips/genes/hg38.ncbiRefSeq.gtf.gz). We used the UCSC liftOver tool with the hg38toRheMac10 chain file (https://hgdownload.soe.ucsc.edu/goldenPath/hg38/liftOver/hg38ToRheMac10.over.chain.gz) to overlay the human transcriptome gtf file onto the rheMac10 genome, with a minimal match threshold of 0.85. The human “liftOver” annotations were used to extend and supplement the macaque annotations, leading to greater numbers of genes called (Figure S1B).
Single nucleus RNA sequencing analysis
We converted original bcl format of sequences to fastq files using cellranger mkfastq. Alignment of the reads by cellranger count to the rheMac10 genome using the custom rheMac10 gtf yielded 61,609 and 23,690 nuclei for monkey P and F, respectively. We used a Seurat v3 pipeline to perform an integrated analysis of monkey F and P (85,299 nuclei in total). First, we removed ambient RNA in monkey P using SoupX.73 We then deleted ribosomal genes for both monkeys, and removed doublets using DoubletDetection,74 which lowered the number of nuclei to 80,902. Neuronal cells express higher numbers of genes compared to non-neuronal cells62,75,76 and therefore we used two different thresholds to remove low quality neuronal and non-neuronal nuclei. We chose these thresholds as the minimal level that produced clear cluster separation (Figure 1E). A total of 31,258 genes were identified, with an average of 3,200 per nucleus. We performed standard log-normalization and a variance stabilizing transformation prior to finding anchors and identified variable features individually for each monkeys’ data set using Seurat’s FindVariableFeatures function with number of features set to 7000. Next, we identified anchors using the FindIntegrationAnchors function with default parameters and passed these anchors to the IntegrateData function. This returned a Seurat object with an integrated expression matrix for all nuclei. We scaled the integrated data with the ScaleData function, ran PCA using the RunPCA function, and visualized the results with UMAP. We used Louvain clustering and chose a resolution that reflected the major cell classes of striatum including D1- and D2- MSNs, interneurons and astrocytes. We calculated the differentially expressed genes for each cell class with the FindMarkers function (Data S1). We annotated the clusters based on feature plots of well-known marker genes13,17,22 and verified the identities of cell clusters by using the hypergeometric test to compare differentially expressed genes for each cluster to markers from single cell rodent studies.77 Given the robust conservation of major cell types, the rodent markers were sufficient for annotation.77 We converted rodent genes to rhesus macaque genes by BioMart Ensemble, keeping one-to-one orthologs only.78,79
For MSN analysis, we isolated the clusters that were enriched with well-known marker MSN genes including PP1R1B, BCL11B, and PDE1B. In order to balance the number of nuclei per animal, we randomly sampled MSN and MSN-like nuclei from monkey P to levels of monkey F (7,387 nuclei). We then re-calculated principal components (PCs) and performed UMAP dimensionality reduction on the first 15 PCs. We used Louvain clustering and chose a resolution that separated clusters that were distinct in UMAP space. We calculated the differentially expressed genes for each MSN subtype with the FindMarkers function (Data S1). To determine whether the clustering of MSNs was exhaustive, we further isolated ‘D1-’ and ‘D2-MSNs’. D1-MSNs including D1-striosome, D1-matrix and D1-shell/OT, whereas D2-MSNs included D2-striosome, D2-matrix and D2-shell/OT. We re-calculated PCs and performed UMAP dimensionality reduction based on the top 15 PCs for both subclusters. This analysis recovered the same, physically distinct clusters observed in the integrated analysis. To analyze the interneuron populations, we isolated clusters based on interneuron markers.18 Similarly, we re-calculated PCs and performed UMAP dimensionality reduction on the first 30 PCs. We used Louvain clustering and annotated the resulting clusters based on known interneuron markers. To explore the functional roles of these cell types, gene enrichment analysis was run using gprofiler80 with the following GO databases: Biological Process, Molecular Pathway, KEGG, and Human Phenotype ontology (Data S4). We calculated the cosine similarity for nuclei within and between clusters based on PCA space. We used a permutation test on the cosine similarity between pairs of clusters. We randomly shuffled the nuclei to mask the nuclei identity and recalculated cosine similarity. We repeated these 10,001 times and used within group and between group variance ratio to determine a P value. To compare the cell types between monkey and mouse MSNs, we first generated an orthologous gene list between mouse and rhesus macaque from BioMart Ensemble with one-to-one orthologous genes78,79 used for downstream analysis. We integrated our MSNs and MSN-like nuclei from the two monkeys with MSN types in Stanley et. al.15 based on the homologous genes using the Seurat integration method as above for the two animals.
Archetypal analysis
We used archetypal analysis to characterize the gradients within and between subtypes. We used partition-based graph abstraction (PAGA) to find those pairs that warranted a gradient-based analysis. PAGA uses the formalism of graph theory and community detection to define a statistic quantifying the presence of connectivity between two clusters.81 To calculate the PAGA graph we ran scanpy.tl.paga on our Seurat integrated data (Figure S3F). We defined subtype pairs as connected if the PAGA edge weight was greater than 0.02. For each pair of connected subtypes – or within a single subtype – we used the Dirichlet Simplex Nest (DSN) implementation of archetype analysis (https://arxiv.org/abs/1905.11009) to define gradients of gene expression. Because we used the raw gene counts, we ran the DSN algorithm in the Poisson configuration.82 Across several runs of the DSN algorithm, we located the archetype most correlated with the subtypes’ annotations and designated those archetypes as the transition axes between the subtype pairs. To determine if the transitions were discrete, we used regression discontinuity design (RDD).83 The null hypothesis of this test is that the gene expression is, solely, a linear function of the transition archetype weights, and adding a threshold does not add additional information. Therefore, we computed p-value distributions across all correlated genes for each connected pair. We then tested whether the number of genes that had significant discontinuities (p < 0 .05, RDD) was greater than expected by chance using a binomial test. We performed the binomial test with two different null distributions, a uniform null distribution, and a simulated null distribution where we randomly shuffled the cell labels and performed the RDD 100 times per gene. Regardless of which null distribution we used, the binomial tests indicated more statistically significant discontinuities than predicted by chance for every subtype pair except for D1S and D2S (the subtypes for which we had the lowest sample sizes) (p < 0.00001 for uniform null, and p < 10−12 for simulated null). We corrected the binomial test p-values using the Benjamini-Hochberg procedure. To verify biological reproducibility, and because defining an archetype and computing discontinuities from the same data can lead to inflated p-value distributions,84 we calculated the archetypes using data from monkey P and computed the discontinuities on data from monkey F. To project the archetypes learned from monkey P onto monkey F, we multiplied the normalized expression matrices of monkey F by the pseudoinverse of the archetype vectors. Using the DSN algorithm we found several archetypes of particular interest including an archetype representing CNR1 signal in hybrid cells, caudate signal in D1- and D2- MSNs, core signal in D1- and D2- MSNs and TAC3 signal in shell D1- MSNs. To provide molecular markers for these archetypes we calculated each gene's Pearson correlation with each archetype of interest (Data S2 and S3; Tables S2 and S3).
Assessing clustering robustness
We used Single Cell Clustering Assessment Framework (SCCAF)27 to test the robustness of our MSN subtype classification. The concept behind such 'self projection' tests is that the gene expression patterns in a subsample of the cells should be sufficient to classify the remaining cells in the labeled clusters with a high level of accuracy.85 SCCAF splits the expression data into a training and test set, and then fits a classifier on the training set on each cluster provided (MSN subtypes in our case). We used the default parameters of a 50% train/test split and a logistic regression classifier. We also used SCCAF to test whether our snRNA-seq sampling was sufficient for accurately detecting the MSN subtype heterogeneity. Because the number of cells of each MSN subtype could vary during the down sampling, we opted to use SCCAF classifier's macro-f1 score for evaluation. The f1 score is the geometric mean between the precision and recall. To compute our macro-f1 score, we computed the average across f1 scores for each MSN subtype. Using the scanpy.pp.subsample method, we repeatedly subsampled fractions of our cells from both monkeys and calculated the macro-f1 across each trial.
FISH image quantification
We quantified expression using high resolution images collected using a Nikon Eclipse Ti2 (40x objective with or without additional 2x built in objective) or an Olympus IX83 under 40x or 63x magnification. For comparison between different regions, we scanned images using the same settings. To quantify cells expressing DRD1 and DRD2 in the caudate and putamen (Figure 4D), we chose ten representative areas from each striatal region (Figure 4C). We adjusted the threshold of the nuclei images and converted them to black and white using the “Make Binary” function in ImageJ.86 We then filled the holes using the “Fill Holes” function and separated overlapping nuclei with the “Watershed” function. We identified the number and regions for nuclei using the “Analyze Particles” function and added the nuclear contours to “ROI Manager”. We then opened the DRD1 and DRD2 images and identified the DRD1 and DRD2 grains using the “Find Maxima” function in ImageJ and then obtained binary images with a single pixel for each local maxima by choosing output type of “Single points”. We measured the integrated intensity of DRD1 and DRD2 above each nucleus using the “Measure” function within the “ROI Manager”. The number of grains in each nucleus is equal to the integrated intensity divided by 255. We considered cells containing three or more grains above the nucleus as positive for that gene. We chose this threshold because it provides clear separation from the background. We quantified the total number of DRD1-positive and DRD2-positive cells for each striatal region of interest (ROI) in each section and counted positive cells on three rostro-caudal sections. We calculated cell density as the number of positive cells divided by total number of nuclei in the area. We used a similar method to quantify the cell density in CPNE4 and RXFP1 clusters (Figure S6E) except that we drew ROIs for the whole CPNE4 and RXFP1 clusters after separating overlapping nuclei with the “Watershed” function. As control, we drew ROIs of roughly similar size to the RXFP1 or CPNE4 clusters in nearby regions and quantified the cells expressing DRD1.
To quantify DRD1 and DRD2 grains in D1/D2-hybrid cells, D1- and D2- MSNs (Figure 4H), we scanned high resolution images for RXFP1-positive cells in triple stained DRD1, DRD2, and RXFP1 sections. The majority of RXFP1-positive cells expressed both DRD1 and DRD2 in dorsal striatum. We quantified the number of grains for DRD1 and DRD2 in these D1/D2-hybrid cells as well as adjacent normal D1 and D2 MSNs in ImageJ using similar methods as above except that we quantified the total grains in the cells instead of nuclei. To quantify DRD1 and DRD2 grains in D1/D2-hybrid cells, we first draw ROIs for RXFP1 expressing cells and added the ROIs to the “ROI Manager” based on the RXFP1 signal. We then opened the DRD1 and DRD2 images and identified the DRD1 and DRD2 grains using the “Find Maxima” function in ImageJ and then output binary images with a single pixel for each local maxima by choosing an output type of “Single points”. We measured the integrated intensity and calculated the number of grains for each ROI using the “Measure” function within the “ROI Manager”. To quantify DRD1 and DRD2 grains in D1- and D2- MSNs, we used the same method except that we drew ROIs for D1- and D2- MSNs. We confirmed the identity of D1- or D2- MSN instead of a D1/D2-hybrid cell by quantifying the DRD2 or DRD1 and RXFP1 grain number less than three in D1- or D2- MSNs. We used a similar method to quantify ARHGAP6 and GREB1L grains in the core and shell except that we first adjusted the threshold of the images to overexpose the ARHGAP6 and GREB1L signals to guide the ROI drawing. To quantify OPRM1 grain numbers in RXFP1 and CPNE4 islands and nearby D1-MSNs, we labeled DRD1 and OPRM1 on one section and DRD1, RXFP1 and CPNE4 on an adjacent section because of overlapping channels in the OPRM1 and RXFP1 probes. The locations of RXFP1 and CPNE4 islands in the OPRM1 and DRD1 double stained sections were determined by adjacent section labeled with RXFP1 and CPNE4. The quantification of OPRM1 grains numbers was similar to ARHGAP6 or GREB1L quantification except that we used overexposed DRD1 to draw ROIs for each cell.
To quantify nuclei size for RXFP1 and CPNE4 islands (Figure S6F), we first scanned high resolution images for DRD1, RXFP1, and CPNE4 triple labeled sections. We performed automatic quantification of the areas of nuclei using ImageJ: 1) adjust the threshold and convert images to black and white using the “Make Binary” function; 2) fill the holes using the “Fill Holes” function; 3) separate overlapping nuclei with the “Watershed” function; 4) then draw the ROI and automatically detect the areas of nuclei using the “Analyze Particles” function. To produce a more accurate quantification of the nuclei size, we chose to randomly sample dozens of cells expressing DRD1 in each island per section due to high packing density of the nuclei in ICjs (Figure S6E). We calculated the area of nuclei by the similar method except that we drew an area of interest around individual nucleus. Three sections in total were quantified and the area for each nucleus was normalized to the control D1-MSNs in each section.
D1 islands mapping
In order to map the distribution of D1-RXFP1 and D1- CPNE4 islands, we triple labeled six sections spaced at 750 μm intervals with probes against DRD1, RXFP1, and CPNE4 in monkey B. We scanned the whole sections and ran a custom CellProfiler pipeline87 to determine the spatial distribution of nuclei and signal intensity of individual channel for each nucleus. We marked regions as D1-RXFP1 (D1-CPNE4) that had a majority of cells double-labeled with DRD1 and RXFP1 (CPNE4). To confirm that these islands were D1 exclusive, we double-labeled sections with DRD1 and DRD2 adjacent to the above six sections. We confirmed that these islands are exclusively the D1 clusters from the similar CellProfiler analysis. We did the similar process for eight sections spaced at 600 μm intervals for monkey K.
Supplementary Material
Table S2. CNR1 archetype signal in hybrid cells, related to STAR Methods and Figure 3.
Table S3. TAC3 archetype signal in shell D1-MSNs, related to STAR Methods Figure 5.
Data S1. Markers in major cell classes and MSN subtypes, related to STAR Methods and Figure 1 and 2.
Data S2. Caudate signal in D1- and D2- MSNs, related to STAR Methods and Figure 3.
Data S4. Gene enrichment analysis for each MSN subtype, related to STAR Methods and Figures 4-7.
Data S3. Core signal in D1- and D2- MSNs, related to STAR Methods and Figure 5.
ACKNOWLEDGMENTS
We thank Jacquelyn Breter for taking excellent care of animals. We also thank Jaimi Nagashima and Mitsutoshi Hanada for their assistance with large image scanning. This works was supported by NIH grants UG3MH120094 (WRS) and DP2MH113095 (WRS).
INCLUSION AND DIVERSITY
We worked to ensure sex balance in the selection of non-human subjects. One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list. The author list of this paper includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.DiFiglia M, Pasik P, and Pasik T (1976). A Golgi study of neuronal types in the neostriatum of monkeys. Brain Res 114, 245–256. [DOI] [PubMed] [Google Scholar]
- 2.Parent A, and Hazrati L-N (1995). Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Reviews 20, 91–127. [DOI] [PubMed] [Google Scholar]
- 3.Albin RL, Young AB, and Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci 12, 366–375. [DOI] [PubMed] [Google Scholar]
- 4.DeLong MR, and Wichmann T (2015). Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA Neurol 72, 1354–1360. [DOI] [PubMed] [Google Scholar]
- 5.Graybiel AM, and Ragsdale CW Jr. (1978). Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A 75, 5723–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong S, Amemori S, Chung E, Gibson DJ, Amemori KI, and Graybiel AM (2019). Predominant Striatal Input to the Lateral Habenula in Macaques Comes from Striosomes. Curr Biol 29, 51–61 e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerfen CR (1984). The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311, 461–464. [DOI] [PubMed] [Google Scholar]
- 8.Haber SN, and McFarland NR (1999). The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci 877, 33–48. [DOI] [PubMed] [Google Scholar]
- 9.Heimer L, and Wilson R (1975). The subcortical projections of allocortex: similarities in the neuronal associations of the hippocampus, the piriform cortex and the neocortex. (Raven), pp. 173–193. [Google Scholar]
- 10.Voorn P, Brady LS, Berendse HW, and Richfield EK (1996). Densitometrical analysis of opioid receptor ligand binding in the human striatum--I. Distribution of mu opioid receptor defines shell and core of the ventral striatum. Neuroscience 75, 777–792. [DOI] [PubMed] [Google Scholar]
- 11.Daunais JB, Letchworth SR, Sim-Selley LJ, Smith HR, Childers SR, and Porrino LJ (2001). Functional and anatomical localization of mu opioid receptors in the striatum, amygdala, and extended amygdala of the nonhuman primate. J Comp Neurol 433, 471–485. [DOI] [PubMed] [Google Scholar]
- 12.Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, et al. (2009). mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods 6, 377–382. [DOI] [PubMed] [Google Scholar]
- 13.Gokce O, Stanley GM, Treutlein B, Neff NF, Camp JG, Malenka RC, Rothwell PE, Fuccillo MV, Sudhof TC, and Quake SR (2016). Cellular Taxonomy of the Mouse Striatum as Revealed by Single-Cell RNA-Seq. Cell Rep 16, 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz-Manchado AB, Bengtsson Gonzales C, Zeisel A, Munguba H, Bekkouche B, Skene NG, Lonnerberg P, Ryge J, Harris KD, Linnarsson S, and Hjerling-Leffler J (2018). Diversity of Interneurons in the Dorsal Striatum Revealed by Single-Cell RNA Sequencing and PatchSeq. Cell Rep 24, 2179–2190 e2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley G, Gokce O, Malenka RC, Sudhof TC, and Quake SR (2020). Continuous and Discrete Neuron Types of the Adult Murine Striatum. Neuron 105, 688–699 e688. 10.1016/j.neuron.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Martin A, Calvigioni D, Tzortzi O, Fuzik J, Warnberg E, and Meletis K (2019). A Spatiomolecular Map of the Striatum. Cell Rep 29, 4320–4333 e4325. [DOI] [PubMed] [Google Scholar]
- 17.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, et al. (2018). Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 174, 1015–1030 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krienen FM, Goldman M, Zhang Q, del Rosario RCH, Florio M, Machold R, Saunders A, Levandowski K, Zaniewski H, Schuman B, et al. (2020). Innovations present in the primate interneuron repertoire. Nature 586, 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savell KE, Tuscher JJ, Zipperly ME, Duke CG, Phillips RA 3rd, Bauman AJ, Thukral S, Sultan FA, Goska NA, Ianov L, and Day JJ (2020). A dopamine-induced gene expression signature regulates neuronal function and cocaine response. Sci Adv 6, eaba4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H, Fenster RJ, Pineda SS, Gibbs WS, Mohammadi S, Davila-Velderrain J, Garcia FJ, Therrien M, Novis HS, Gao F, et al. (2020). Cell Type-Specific Transcriptomics Reveals that Mutant Huntingtin Leads to Mitochondrial RNA Release and Neuronal Innate Immune Activation. Neuron 107, 891–908 e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izpisua Belmonte JC, Callaway EM, Caddick SJ, Churchland P, Feng G, Homanics GE, Lee KF, Leopold DA, Miller CT, Mitchell JF, et al. (2015). Brains, genes, and primates. Neuron 86, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, and Macklis JD (2008). Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci 28, 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Z, Adamowicz WO, Eldred WD, Jakowski AB, Kleiman RJ, Morton DG, Stephenson DT, Strick CA, Williams RD, and Menniti FS (2006). Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience 139, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crittenden JR, and Graybiel AM (2011). Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat 5, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JB, Klug JR, Ross DL, Howard CD, Hollon NG, Ko VI, Hoffman H, Callaway EM, Gerfen CR, and Jin X (2016). Genetic-Based Dissection Unveils the Inputs and Outputs of Striatal Patch and Matrix Compartments. Neuron 91, 1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao Z, Moreno P, Huang N, Papatheodorou I, Brazma A, and Teichmann SA (2020). Putative cell type discovery from single-cell gene expression data. Nat Methods 17, 621–628. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi S, Davila-Velderrain J, and Kellis M (2020). A multiresolution framework to characterize single-cell state landscapes. Nature Communications 11, 5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadi S, Ravindra V, Gleich DF, and Grama A (2018). A geometric approach to characterize the functional identity of single cells. Nature Communications 9, 1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei SC, Sharma R, Anang N-AAS, Levine JH, Zhao Y, Mancuso JJ, Setty M, Sharma P, Wang J, Pe’er D, and Allison JP (2019). Negative Co-stimulation Constrains T Cell Differentiation by Imposing Boundaries on Possible Cell States. Immunity 50, 1084–1098.e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puighermanal E, Castell L, Esteve-Codina A, Melser S, Kaganovsky K, Zussy C, Boubaker-Vitre J, Gut M, Rialle S, Kellendonk C, et al. (2020). Functional and molecular heterogeneity of D2R neurons along dorsal ventral axis in the striatum. Nature Communications 11, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikula S, Parrish SK, Trimmer JS, and Jones EG (2009). Complete 3D visualization of primate striosomes by KChIP1 immunostaining. J Comp Neurol 514, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meredith GE, Pattiselanno A, Groenewegen HJ, and Haber SN (1996). Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. J Comp Neurol 365, 628–639. [DOI] [PubMed] [Google Scholar]
- 34.Prensa L, Richard S, and Parent A (2003). Chemical anatomy of the human ventral striatum and adjacent basal forebrain structures. J Comp Neurol 460, 345–367. [DOI] [PubMed] [Google Scholar]
- 35.Heimer L (2000). Basal forebrain in the context of schizophrenia. Brain Res Brain Res Rev 31, 205–235. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese E, Badea A, Coe CL, Lubach GR, Shi Y, Styner MA, and Johnson GA (2015). A diffusion tensor MRI atlas of the postmortem rhesus macaque brain. Neuroimage 117, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakker R, Tiesinga P, and Kotter R (2015). The Scalable Brain Atlas: Instant Web-Based Access to Public Brain Atlases and Related Content. Neuroinformatics 13, 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer G, Gonzalez-Hernandez T, Carrillo-Padilla F, and Ferres-Torres R (1989). Aggregations of granule cells in the basal forebrain (islands of Calleja): Golgi and cytoarchitectonic study in different mammals, including man. J Comp Neurol 284, 405–428. [DOI] [PubMed] [Google Scholar]
- 39.Furuta T, and Kaneko T (2006). Third pathway in the cortico-basal ganglia loop: Neurokinin B-producing striatal neurons modulate cortical activity via striato-innominato-cortical projection. Neuroscience Research 54, 1–10. [DOI] [PubMed] [Google Scholar]
- 40.Huerta-Ocampo I, Mena-Segovia J, and Bolam JP (2014). Convergence of cortical and thalamic input to direct and indirect pathway medium spiny neurons in the striatum. Brain Struct Funct 219, 1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eblen F, and Graybiel AM (1995). Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci 15, 5999–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, and Kaneko T (2011). Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. European Journal of Neuroscience 33, 668–677. [DOI] [PubMed] [Google Scholar]
- 43.Crittenden JR, Tillberg PW, Riad MH, Shima Y, Gerfen CR, Curry J, Housman DE, Nelson SB, Boyden ES, and Graybiel AM (2016). Striosome–dendron bouquets highlight a unique striatonigral circuit targeting dopamine-containing neurons. Proceedings of the National Academy of Sciences 113, 11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman A, Homma D, Gibb Leif G., Amemori K.-i., Rubin Samuel J., Hood Adam S., Riad Michael H., and Graybiel Ann M. (2015). A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict. Cell 161, 1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman A, Hueske E, Drammis SM, Toro Arana SE, Nelson ED, Carter CW, Delcasso S, Rodriguez RX, Lutwak H, DiMarco KS, et al. (2020). Striosomes Mediate Value-Based Learning Vulnerable in Age and a Huntington’s Disease Model. Cell 183, 918–934.e949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mogenson GJ, Jones DL, and Yim CY (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14, 69–97. [DOI] [PubMed] [Google Scholar]
- 47.Floresco SB (2015). The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66, 25–52. [DOI] [PubMed] [Google Scholar]
- 48.Furuta T, Mori T, Lee T, and Kaneko T (2000). Third group of neostriatofugal neurons: Neurokinin B-producing neurons that send axons predominantly to the substantia innominata. Journal of Comparative Neurology 426, 279–296. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Furuta T, and Kaneko T (2004). Neurokinin B-producing projection neurons in the lateral stripe of the striatum and cell clusters of the accumbens nucleus in the rat. Journal of Comparative Neurology 480, 143–161. [DOI] [PubMed] [Google Scholar]
- 50.Xiao X, Deng H, Furlan A, Yang T, Zhang X, Hwang GR, Tucciarone J, Wu P, He M, Palaniswamy R, et al. (2020). A Genetically Defined Compartmentalized Striatal Direct Pathway for Negative Reinforcement. Cell 183, 211–227 e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berridge KC, and Kringelbach ML (2015). Pleasure systems in the brain. Neuron 86, 646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grillner S, Robertson B, and Stephenson-Jones M (2013). The evolutionary origin of the vertebrate basal ganglia and its role in action selection. The Journal of Physiology 591, 5425–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lecumberri A, Lopez-Janeiro A, Corral-Domenge C, and Bernacer J (2018). Neuronal density and proportion of interneurons in the associative, sensorimotor and limbic human striatum. Brain Struct Funct 223, 1615–1625. [DOI] [PubMed] [Google Scholar]
- 54.Tran HTN, Ang KS, Chevrier M, Zhang X, Lee NYS, Goh M, and Chen J (2020). A benchmark of batch-effect correction methods for single-cell RNA sequencing data. Genome Biology 21, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mereu E, Lafzi A, Moutinho C, Ziegenhain C, McCarthy DJ, Álvarez-Varela A, Batlle E, Sagar, Grün D, Lau JK, et al. (2020). Benchmarking single-cell RNA-sequencing protocols for cell atlas projects. Nature Biotechnology 38, 747–755. [DOI] [PubMed] [Google Scholar]
- 56.O'Leary TP, Sullivan KE, Wang L, Clements J, Lemire AL, and Cembrowski MS (2020). Extensive and spatially variable within-cell-type heterogeneity across the basolateral amygdala. eLife 9, e59003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramón y Cajal S (1909). Histologie du système nerveux de l'homme & des vertébrés (Maloine). [Google Scholar]
- 58.Stein-O’Brien GL, Clark BS, Sherman T, Zibetti C, Hu Q, Sealfon R, Liu S, Qian J, Colantuoni C, Blackshaw S, et al. (2019). Decomposing Cell Identity for Transfer Learning across Cellular Measurements, Platforms, Tissues, and Species. Cell Systems 8, 395–411.e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Bella DJ, Habibi E, Stickels RR, Scalia G, Brown J, Yadollahpour P, Yang SM, Abbate C, Biancalani T, Macosko EZ, et al. (2021). Molecular logic of cellular diversification in the mouse cerebral cortex. Nature 595, 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakken TE, Jorstad NL, Hu Q, Lake BB, Tian W, Kalmbach BE, Crow M, Hodge RD, Krienen FM, Sorensen SA, et al. (2020). Evolution of cellular diversity in primary motor cortex of human, marmoset monkey, and mouse. bioRxiv, 2020.2003.2031.016972. [Google Scholar]
- 61.Evrard HC, Forro T, and Logothetis NK (2012). Von Economo neurons in the anterior insula of the macaque monkey. Neuron 74, 482–489. [DOI] [PubMed] [Google Scholar]
- 62.Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Johansen N, Penn O, et al. (2019). Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genest W, Stauffer WR, and Schultz W (2016). Utility functions predict variance and skewness risk preferences in monkeys. Proc Natl Acad Sci U S A 113, 8402–8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lak A, Stauffer WR, and Schultz W (2014). Dopamine prediction error responses integrate subjective value from different reward dimensions. Proc Natl Acad Sci U S A 111, 2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stauffer WR, Lak A, Bossaerts P, and Schultz W (2015). Economic choices reveal probability distortion in macaque monkeys. J Neurosci 35, 3146–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stauffer WR, Lak A, and Schultz W (2014). Dopamine reward prediction error responses reflect marginal utility. Curr Biol 24, 2491–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waelti P, Dickinson A, and Schultz W (2001). Dopamine responses comply with basic assumptions of formal learning theory. Nature 412, 43–48. [DOI] [PubMed] [Google Scholar]
- 68.Barraclough DJ, Conroy ML, and Lee D (2004). Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci 7, 404–410. [DOI] [PubMed] [Google Scholar]
- 69.Haroush K, and Williams ZM (2015). Neuronal prediction of opponent's behavior during cooperative social interchange in primates. Cell 160, 1233–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davenport AT, Grant KA, Szeliga KT, Friedman DP, and Daunais JB (2014). Standardized method for the harvest of nonhuman primate tissue optimized for multiple modes of analyses. Cell Tissue Bank 15, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Habib N, Avraham-Davidi I, Basu A, Burks T, Shekhar K, Hofree M, Choudhury SR, Aguet F, Gelfand E, Ardlie K, et al. (2017). Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat Methods 14, 955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warren WC, Harris RA, Haukness M, Fiddes IT, Murali SC, Fernandes J, Dishuck PC, Storer JM, Raveendran M, Hillier LW, et al. (2020). Sequence diversity analyses of an improved rhesus macaque genome enhance its biomedical utility. Science 370, eabc6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young MD, and Behjati S (2020). SoupX removes ambient RNA contamination from droplet based single-cell RNA sequencing data. bioRxiv, 303727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McGinnis CS, Murrow LM, and Gartner ZJ (2019). DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst 8, 329–337 e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tasic B, Yao Z, Graybuck LT, Smith KA, Nguyen TN, Bertagnolli D, Goldy J, Garren E, Economo MN, Viswanathan S, et al. (2018). Shared and distinct transcriptomic cell types across neocortical areas. Nature 563, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, et al. (2018). Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014 e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grillner S, and Robertson B (2016). The Basal Ganglia Over 500 Million Years. Curr Biol 26, R1088–R1100. [DOI] [PubMed] [Google Scholar]
- 78.Khrameeva E, Kurochkin I, Han D, Guijarro P, Kanton S, Santel M, Qian Z, Rong S, Mazin P, Sabirov M, et al. (2020). Single-cell-resolution transcriptome map of human, chimpanzee, bonobo, and macaque brains. Genome Res 30, 776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yin S, Lu K, Tan T, Tang J, Wei J, Liu X, Hu X, Wan H, Huang W, Fan Y, et al. (2020). Transcriptomic and open chromatin atlas of high-resolution anatomical regions in the rhesus macaque brain. Nat Commun 11, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, and Vilo J (2019). g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47, W191–W198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolf FA, Hamey FK, Plass M, Solana J, Dahlin JS, Gottgens B, Rajewsky N, Simon L, and Theis FJ (2019). PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol 20, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Townes FW, Hicks SC, Aryee MJ, and Irizarry RA (2019). Feature selection and dimension reduction for single-cell RNA-Seq based on a multinomial model. Genome Biol 20, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bor J, Moscoe E, Mutevedzi P, Newell ML, and Barnighausen T (2014). Regression discontinuity designs in epidemiology: causal inference without randomized trials. Epidemiology 25, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang JM, Kamath GM, and Tse DN (2019). Valid Post-clustering Differential Analysis for Single-Cell RNA-Seq. Cell Syst 9, 383–392 e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, Saadatpour A, Zhou Z, Chen H, Ye F, et al. (2018). Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 173, 1307. [DOI] [PubMed] [Google Scholar]
- 86.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, et al. (2006). CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7, R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. CNR1 archetype signal in hybrid cells, related to STAR Methods and Figure 3.
Table S3. TAC3 archetype signal in shell D1-MSNs, related to STAR Methods Figure 5.
Data S1. Markers in major cell classes and MSN subtypes, related to STAR Methods and Figure 1 and 2.
Data S2. Caudate signal in D1- and D2- MSNs, related to STAR Methods and Figure 3.
Data S4. Gene enrichment analysis for each MSN subtype, related to STAR Methods and Figures 4-7.
Data S3. Core signal in D1- and D2- MSNs, related to STAR Methods and Figure 5.
Data Availability Statement
We deposited our snRNA-Seq data to GEO accession GSE167920. Go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE167920. Code is publicly available at https://github.com/pfenninglab/nhp_snrna_striatum_analysis and https://github.com/rewardlab/NHPstriatum. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.