Abstract
Context
Thyroid eye disease (TED) is a sight-threatening and debilitating autoimmune condition, with limited therapies available, that often poses diagnostic and therapeutic challenges. In recent years, the treatment landscape has shifted to early intervention with targeted therapy.
Methods
A PubMed review of the literature was conducted for the period between 1979 and 2021. Search terms included thyroid eye disease, teprotumumab, targeted therapy, Graves disease, Graves ophthalmopathy, dysthyroid optic neuropathy, and related terms in different combinations. Novel biologic therapies for TED have emerged as alternatives to traditional steroid regimens in recent years. New insights into TED pathophysiology have uncovered the role of the insulin-like growth factor 1 receptor (IGF-1R) and led to the development of teprotumumab, an IGF-1R–inhibiting monoclonal antibody.
Results
Randomized clinical trials demonstrating the efficacy of teprotumumab for TED led to Food and Drug Administration approval. Teprotumumab is gradually replacing immunosuppressive agents as first-line therapy in the United States for active moderate-to-severe TED, while emerging reports also show its use in other stages of the disease. Recent data highlight risk factors for adverse events and screening protocols to maximize patient safety. Personalized therapeutic plans developed through effective partnership between endocrinologists and ophthalmologists aim to enhance the safety and outcomes of TED treatments and improve care for this complex disease.
Conclusion
TED management is shifting to an era of targeted therapy with multidisciplinary care. Teprotumumab has demonstrated superior efficacy to conventional treatments and has transformed our therapeutic and surgical algorithms. Clinical guidelines and additional studies are needed to further guide and refine therapy.
Keywords: thyroid eye disease, Graves ophthalmopathy, teprotumumab, management, targeted therapy, biologic therapy
Thyroid eye disease (TED), also known as thyroid-associated orbitopathy, Graves ophthalmopathy, or Graves orbitopathy (GO), is a complex autoimmune inflammatory condition which is frequently disfiguring and can be sight-threatening. Recent meta-analyses reported the overall prevalence of TED in Graves patients to be 30% to 40% (1, 2), although subclinical extraocular muscle enlargement is reported in nearly 70% of patients (3). Most TED patients are hyperthyroid; however, TED can occur in the setting of euthyroidism or hypothyroidism in at least 10% of cases (4). In the United States, TED affects 16 per 100 000 females and 2.9 per 100 000 males (5). Risk factors include smoking, female sex, age, radioactive iodine treatment, thyroid dysfunction, elevated levels of thyrotropin receptor antibodies, vitamin D deficiency, and potentially hypercholesterolemia (6-9). In addition, recent studies disclose that diabetes mellitus and obstructive sleep apnea are significantly associated with progression to dysthyroid optic neuropathy (DON) (10-12).
Early and accurate diagnosis of TED as well as appropriate clinical assessment of the patient are essential to determine proper management and to halt the visual and functional sequelae that can impair quality of life (QoL). Diagnosis can be challenging because of the heterogeneity in presentation. Clinical assessment involves measuring disease activity and severity. TED typically begins with an acute inflammatory (active) phase, lasting 6 to 36 months (13-15). It then transitions into a chronic stable phase where tissue remodeling ceases and proptosis, eyelid retraction, and diplopia can improve, but often persist (16). Activity of TED is assessed through the clinical activity score (CAS), which evaluates inflammatory signs and symptoms and classifies disease as active or inactive. The severity is a function of the degree of diplopia, proptosis, and soft tissue changes and their impact on the patient’s QoL. The European Group on Graves’ Orbitopathy (EUGOGO) classification system uses these variables to classify patients as having mild, moderate-to-severe, or sight-threatening disease. Mild disease refers to disease that has a minimal effect on daily life and is not treated with steroids or surgery. In moderate-to-severe patients, TED affects patients’ daily life and warrants advanced treatments. In sight-threatening TED, vision is threatened by DON or corneal breakdown (17). A recent study of newly diagnosed Graves patients found that the prevalence of concurrent mild, moderate/severe, and sight-threatening disease was 20%, 5.8%, and. 0.3%, respectively (18). While the majority of TED patients require no therapy or only supportive measures, about 25% of patients will require medical or surgical intervention, and 3% to 5% may develop sight-threatening disease (2, 19).
In the last 2 decades, considerable advances in our understanding of TED pathogenesis have resulted in a paradigm shift in management. We will review the importance of multidisciplinary care, the evolving therapeutic landscape of TED, and key considerations in the treatment algorithm.
Multidisciplinary Thyroid-Eye Clinics
Multidisciplinary care for TED was originally promoted by EUGOGO in its 2008 Consensus Statement (17). Referral pathways to joint thyroid-eye clinics for patients presenting to various health care professionals have been suggested (20). Multidisciplinary care is important for several reasons. First, the diagnosis of TED can be challenging, especially when the presentation is atypical. Collaboration between endocrinologists and ophthalmologists can rule out other ocular inflammatory processes while also establishing evidence of autoimmune thyroid disease. Second, endocrinologists and ophthalmologists have nonoverlapping expertise in the treatment of TED: control of thyroid dysfunction and localized eye treatments, respectively. Third, therapy needs to be individualized based on comorbidities and other patient characteristics that increase the risk for adverse events (AEs). A joint evaluation ensures the most customized management plan for each patient. Fourth, appropriate treatment of endocrine side effects that can develop with TED therapies is needed. Finally, multidisciplinary thyroid-eye clinics facilitate education and research.
Recommendations for Effective Comanagement Strategies
Development of TED centers requires some coordination but is feasible and highly rewarding for patients and physicians alike. The primary members of the multidisciplinary team are typically the endocrinologist and the oculoplastic or orbital specialist; however, other members may include a strabismus surgeon, neuro-ophthalmologist, radiation oncologist, orthoptist, and a specialized nurse. Psychologists can also assist, as TED causes both emotional stress and occupational impairment, affecting QoL (21, 22). Patients seen in joint thyroid-eye clinics report greater satisfaction with their care compared with those that are not, though only a minority of TED patients (25%) are seen in such clinics (23).
Effective comanagement of TED requires multiple strategies. First, the team members should develop a common approach to assessment and treatment, tailored to the availability of specific therapies. Second, the team should see patients together physically, in joint thyroid-eye clinics, or if not possible, in close temporal proximity. Third, streamlined referral pathways between endocrinology, ophthalmology, and other subspecialties involved in TED patient care are necessary to ensure prompt evaluation. Fourth, TED teams should determine management plans together and communicate frequently regarding response to therapy and AEs of treatment. Finally, collaboration in education of trainees and research in TED can add additional physician fulfillment and ultimately improve TED care.
Role of Endocrinologist and Oculoplastic Surgeon
Endocrinologists frequently have a preestablished relationship with patients, managing thyroid dysfunction. They can therefore recommend interventions to reduce the risk of developing TED, such as counseling regarding tobacco cessation, maintenance of euthyroidism, and judicious use of radioactive iodine with the addition of steroids in patients at risk (24-26). All patients with Graves disease (GD) should have a baseline eye exam with Hertel measurements. If TED signs or symptoms develop, the endocrinologist is best suited to establish an early diagnosis and triage the urgency of referral to an ophthalmologist with TED expertise. Basic assessment of optic nerve (ON) function in patients at risk is indicated, with emergent referral to ophthalmology in cases of DON suspicion. Furthermore, the endocrinologist can help weigh the various systemic TED treatments considered and perform a risk/benefit assessment based on the patient’s unique medical history. Next, the endocrinologist can help monitor for treatment complications such as hypertension and others, institute appropriate protective therapies such as bisphosphonates for bone protection, and treat changes in glycemic control. Finally, the endocrinologist can monitor patients for stability in the inactive TED phase, and refer for rehabilitative ocular/periocular surgery, when appropriate.
The ophthalmologist, typically an oculoplastic or orbital surgeon, is critical in the accurate diagnosis and management of TED. An initial comprehensive ophthalmologic evaluation is needed to detect and treat ocular comorbidities, stage TED, identify sight-threatening disease, and establish a baseline exam against which response to therapy can be measured. During the active phase, the oculoplastic surgeon may recommend treatment based on disease severity, provide local treatments such as ophthalmic ointments and drops, and monitor ophthalmic disease manifestations. During the inactive phase, the ophthalmologist may recommend surgical or nonsurgical treatment options.
Medical Management
In recent years, a better understanding of the underlying disease mechanisms has changed the therapeutic landscape of TED. We will briefly review traditional therapies, and then focus on the role of teprotumumab, the only Food and Drug Administration (FDA)-approved therapeutic option for TED.
Traditional Treatments
Management of TED depends on the activity and severity of the disease.
All patients with GD should be counseled on strict avoidance of tobacco and monitored closely for thyroid dysfunction, with prompt correction, if it develops.
Active mild thyroid-eye disease
General supportive measures include ophthalmic lubricants, oral selenium 100 mcg twice daily for 6 months, and close observation. Selenium, given orally as sodium selenite 100 mcg twice daily for 6 months, has been shown to reduce inflammatory symptoms, improve QoL, and decrease risk of TED progression in a large European randomized controlled trial (RCT) (27).
Active moderate-to-severe thyroid-eye disease
Traditionally, these patients have been treated with steroids, which continue to be the first-line treatment recommendation in the 2021 EUGOGO guidelines, in combination with mycophenolate, or alone, in higher doses (28). After RCTs proved the superior efficacy (about 80% vs 50%-60%) and safety of intravenous (IV) steroids compared with oral steroids (29, 30) in TED patients, a 12-week course of IV steroids became the most common initial treatment (IV methylprednisolone [IVMP] 500 mg weekly × 6 followed by 250 mg IV weekly × 6). In a meta-analysis of all RCTs involving IV steroids, mainly as compared to oral steroids, they were shown to result in TED inactivation in 59% of cases, improve diplopia in one-third of patients, and have a minor effect on proptosis (mean: 1.14 mm) (31). Importantly, these RCTs were not randomized to placebo (32). Higher doses of IV steroids may be more effective but have side effects more commonly (33). Orbital radiation (ORT) has also been used in the treatment of TED, even though efficacy data from RCTs are mixed, with a recent meta-analysis showing its main effect is ameliorating diplopia (odds ratio, 4.88) with no improvement in proptosis (34). Patients most likely to benefit are those with early, active, moderate-severe, or rapidly progressive disease, especially associated with evolving motility deficits (35). ORT in combination with oral steroids has been shown in some RCTs to be superior to monotherapy with either modality in improving the ophthalmopathy index (36, 37), though a more recent RCT did not find the combination more beneficial (38). The combination of ORT and IV steroids has not been studied in RCTs. However, efficacy has been shown in retrospective studies and the 2021 EUGOGO guidelines have recommended this as one of the second-line treatment options (28). Another combination of treatments that has been studied in a recent RCT is IV steroids plus mycophenolate. While combination therapy was not shown to be superior to IV steroid monotherapy with respect to the primary end point of response at 12 weeks or relapse at 24 or 36 weeks, post hoc analysis at 36 months showed improvement in a composite index with combination therapy over IV steroid monotherapy (67% vs 46%) (39), while being similarly tolerated. There was no significant effect on diplopia or proptosis. The aforementioned benefit seen in inflammatory parameters with the combination therapy prompted the EUGOGO guidelines to recommend it as one of the 2 first-line options in the management of TED (28). Limitations of the recent EUGOGO treatment guidelines is that most recommendations are supported by moderate-quality evidence. In addition, the fact that teprotumumab does not have regulatory approval in the European Union, where the guidelines originated, has limited its role in the suggested therapeutic algorithms.
Sight-threatening thyroid-eye disease (dysthyroid optic neuropathy)
Traditional therapy for DON is largely based off a small RCT of 15 patients randomly assigned to a coronal 3-wall orbital decompression vs high-dose steroids (1 g IVMP daily × 3), repeated after 1 week, then followed by an oral prednisone taper for 4 months. The cumulative dose of methylprednisolone was approximately 8 g. The study determined that 5 of the 6 patients in the decompression group did not respond to surgery, 3 because of insufficient improvement of visual acuity and 2 because of severe chemosis. In the group randomly assigned to high-dose steroids, 4 of 9 patients did not respond to therapy because of insufficient improvement in visual acuity. There was no statistically significant difference in the number of responders between the 2 groups (P = .13) (39). Based on these results and a small retrospective study that showed that 40% of patients had improvement in visual function after high-dose IVMP (40), EUGOGO recommended high-dose steroids as the first-line therapy for DON (27). When the response is absent or poor, urgent orbital decompression surgery is necessary (27). Limitations to the RCT include the small sample size and the invasive and outdated surgical approach to which high-dose steroids were compared. Alternatively, a retrospective review of 104 patients with DON demonstrated that ORT with oral steroids avoided orbital decompression in the majority of patients during the acute active phase, though 13% required orbital decompression during the chronic phase of disease (41). Importantly, patients with a poor steroid response are unlikely to benefit from ORT treatment (35). Larger RCTs comparing IV steroids alone to current minimally invasive surgical decompression techniques are in order. Prospective studies are needed to determine the efficacy of ORT with oral steroids as well as newer targeted therapies such as teprotumumab for the treatment of DON.
Chronic Thyroid-Eye Disease
Conventional medical therapies have no role in chronic TED. The standard teaching is to monitor patients until they demonstrate stable inactive disease for 6 months, at which point surgical intervention is typically recommended for visually significant proptosis, diplopia, eyelid retraction, or eyelid asymmetries.
Early Biologics
After 2010, monoclonal antibody therapies were popularized for TED. Rituximab, an anti-CD20 monoclonal antibody that depletes B cells, had mixed results in 2 small RCTs. An Italian RCT demonstrated efficacy in reducing CAS and decreasing disease reactivation when compared to IV steroids in patients treated early (4.5 months) (42); however, the US RCT (43) demonstrated no efficacy when compared against placebo in patients with an 11.2 month average history of TED. Given its side effect profile and some risk for DON, rituximab is considered a second- or third-line option. An RCT of steroid-refractory TED patients treated with tocilizumab, an anti-interleukin-6 receptor antagonist, as 4 monthly infusions, reduced CAS significantly more than placebo at 16 weeks with no sustained benefit at 40 weeks (44). The effect on proptosis was minimal (1.5 mm), whereas there was no effect on diplopia or QoL. Tocilizumab is also considered a second- or third-line option for TED.
Teprotumumab
Recent advances in our understanding of TED pathophysiology have uncovered the role of the insulin-like growth factor 1 receptor (IGF-1R). The IGF-1R forms a physical and signaling complex with the thyrotropin receptor, which is believed to be a key factor in TED pathogenesis (45). Patients with GD overexpress the IGF-1R on their orbital fibroblasts (OFs), B cells, and T cells (46). Once activated, orbital fibroblasts function as key effector cells in the TED orbit, producing cytokines, including interleukin-6, in addition to hyaluronan and extracellular matrix molecules. The expression of such molecules leads to clinical signs of inflammation and soft-tissue expansion (fat and muscle) in the orbit (47). The newfound understanding of the importance of the IGF-1R in TED pathogenesis led to the use of teprotumumab, a monoclonal antibody that blocks the IGF-1R signaling, as a therapeutic agent.
In phase 2 and 3 double-masked, randomized, placebo-controlled studies, 77% of the active moderate-to-severe TED patients treated with teprotumumab demonstrated an improvement in proptosis greater than or equal to 2 mm compared to 15% in the placebo group at 24 weeks (32, 48). In a pooled analysis of patients from the phase 2 and 3 trials, there was a mean decrease of 3.14 mm in proptosis from baseline (similar to what can be achieved with 2-wall orbital decompression) (49). Further, in the pooled phase 2 and 3 data, diplopia improved by 1 or more grades (Gorman diplopia score) in 70% of treated patients, compared to 31% in the placebo group, with 53% having complete resolution of diplopia at 24 weeks (32, 48). At 24 weeks, 62% had disease inactivation (CAS of 0/1) compared with 22% for placebo. An improvement in overall response, defined as a reduction of 2 or more in the CAS plus a reduction in proptosis greater than or equal to 2 mm, was seen in 74% of treated individuals compared to 14% with placebo at 24 weeks.
The reduction of proptosis by teprotumumab relates to the reversal of soft-tissue expansion in the orbit (50). Subsequent to the phase 2 and 3 trials, in the United States, the FDA approved teprotumumab as the first and only medical therapy for TED.
Teprotumumab May Benefit Thyroid-Eye Disease in Active and Chronic Disease
Clinical trials studying the use of teprotumumab in TED included only patients with active, moderate-to-severe disease; however, FDA approval is for TED, without specific reference to disease stage or grade. Since approval, data on its effect in the inactive stage as well as mild and sight-threatening disease are accumulating. Importantly, without RCTs in these populations, the clinical implications of these reports are challenging to interpret. Table 1 includes a list of reports, cited in this paper, that describe the efficacy of teprotumumab for the treatment of TED in all stages and grades of disease.
Table 1.
Reports of teprotumumab efficacy for thyroid eye disease in all phases of disease
| Author | Year | Design | Intervention | Patient type | Sample size | Follow-up, wk |
|---|---|---|---|---|---|---|
| Smith (48) | 2017 | RCT | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | Active moderate-to-severe TED | 88 | 24 |
| Douglas (32) | 2020 | RCT | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | Active moderate-to-severe TED | 83 | 24 |
| Ozzello (51) | 2020 | Case report | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | DON | 1 | 6 |
| Sears (52) | 2020 | Case report | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wks | DON | 1** | 6 |
| Sears (53) | 2020 | Case series | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | DON | 10 | 15 |
| Jain (50) | 2020 | Retrospective study | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | Active moderate-to-severe TED | 6* | 24 |
| Slentz (54) | 2021 | Case report | Teprotumumab (10mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | DON | 1 | 25 |
| Chiou (55) | 2021 | Case series | Teprotumumab (10 mg/kg IV × 1 dose, 20 mg/kg × 7 doses every 3 wk) | DON | 2 | 6 |
| Diniz (56) | 2021 | Case series | Teprotumumab (10 mg/kg IV × 1 dose, 20 mg/kg × 7 doses every 3 wk) | TED and DON | 21 | 30 |
| Lopez (57) | 2021 | Case report | Teprotumumab (10 mg/kg IV × 1 dose, 20 mg/kg × 7 doses every 3 wk) | DON | 1 | 6 |
| Hwang (58) | 2021 | Case report | Teprotumumab (10 mg/kg IV × 1 dose, 20 mg/kg × 7 doses every 3 wk) | DON | 1 | 24 |
| Ugradar (59) | 2021 | Retrospective study | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | Noninflammatory TED | 4 | 24 |
| Ugradar (60) | 2021 | Retrospective study | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | Chronic stable TED (> 2 y) | 31 | 30 |
| Ugradar (61) | 2021 | Retrospective study | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | TED | 11 | 24 |
| Kahaly (49) | 2021 | Retrospective study | Teprotumumab (10 mg/kg IV × 1 infusion, 20 mg/kg × 7 doses every 3 wk) | Active moderate-to-severe TED | 171*** | 72 |
Abbreviations: DON, dysthyroid optic neuropathy; IV, intravenous; RCT, randomized controlled trial; TED, thyroid eye disease.
Mild active thyroid-eye disease
According to the EUGOGO severity classification guidelines, patients with mild active TED experience only a minor effect on daily life that is insufficient to justify immunomodulation or surgical treatment. Therefore, to date, there are no studies exclusively evaluating teprotumumab for mild TED. A report of 21 patients with various stages and grades of TED, including 2 patients with active, mild TED, found improvement in proptosis, CAS, and ductions after teprotumumab, with no significant differences in the response to therapy between different stages and grades of disease (56). This observational report suggests that even mild TED may benefit from teprotumumab. However, this study may not be sufficiently powered to detect differences in the response to therapy between the mild and the moderate/severe TED groups. Furthermore, the effect on daily life and ocular function should be measured against the cost of the drug, lack of rigorous data in this population, and side effect profile. It is still unknown if the treatment of mild active disease significantly decreases progression to more moderate or severe disease.
Sight-threatening thyroid-eye disease/dysthyroid optic neuropathy
Since teprotumumab’s approval, several case reports and uncontrolled studies have suggested improvement of DON in teprotumumab-treated patients who were refractory to steroids or radiation and poor surgical candidates (52-55, 57, 58). Sears et al (52) first reported a patient with unilateral DON, refractory to ORT and a poor surgical and steroid candidate, who experienced improvement in best-corrected visual acuity (BCVA), color vision, visual fields, proptosis (by 4 mm), and reduction of extraocular muscle size on magnetic resonance imaging after 2 infusions of teprotumumab. To date, 19 published cases of refractory DON (to steroids, ORT, and/or surgical decompression) have been managed with teprotumumab (51-55, 57, 58). Of these, 79% (15) experienced an improvement in BCVA or pupil function after a mean follow-up of 16 weeks, with mean improvement in CAS and proptosis of 4.3 and 4 mm, respectively. Four patients did not experience an improvement in DON (51, 53); all had long-standing DON with hand motion BCVA and likely irreversible ON atrophy. These reports are limited by short follow-up, variability in data collection, and possibly selection bias. While the quality of evidence from these studies is not very strong, they support the need for further investigation into teprotumumab’s efficacy in the treatment of DON, particularly in cases refractory to other therapies, and before irreversible ON damage. Additional studies are needed to determine whether teprotumumab will prove as effective as steroids, ORT, and orbital decompression for DON.
Chronic Thyroid-Eye Disease
The IGF-1R is overexpressed in the orbital fibroblasts of patients with chronic TED (59). In addition, preclinical studies demonstrate that the IGF-1R, at least in part, controls the metabolic turnover of macromolecules in the extracellular matrix, including hyaluronan production, by TED fibroblasts (62). Therefore, it is understandable that downregulating this pathway can result in deturgescence of orbital tissues to reduce soft-tissue volume, resultant proptosis, and extraocular muscle enlargement, regardless of disease stage. Several retrospective studies have demonstrated improvement in proptosis in patients with chronic TED treated with teprotumumab (51, 56, 60). A retrospective uncontrolled study of 31 patients with chronic TED (average 7 years after diagnosis), with a CAS less than or equal to 3, demonstrated significant reductions in proptosis, inflammation, diplopia, strabismus, and orbital soft-tissue volume after teprotumumab (60). Ninety percent of study orbits had a clinically significant improvement (≥ 2 mm) in proptosis, 67% of patients experienced a clinically significant improvement in diplopia, while 47% had complete resolution of diplopia following therapy. Similarly, a study of 21 TED patients of different grades and stages, including 11 patients with chronic TED, found significant improvement in CAS, proptosis, and ductions, with no differences based on disease stage (56). These studies support the notion that chronic TED may not be as inactive as previously thought. Limitations of these reports include their retrospective uncontrolled nature, small sample size, and short follow-up time; therefore, placebo-controlled RCTs are warranted to evaluate the use of teprotumumab for chronic TED.
Special Circumstances
Asymmetric disease
In a study of 269 patients with TED, 31% of patients had asymmetric (2.5-mm difference between both eyes) proptosis (63). This was associated with a higher disease burden and substantial QoL deterioration. In a recent pooled analysis of the phase 2 and 3 trials for teprotumumab, 10 of 84 patients in the active treatment arm and 12 of 87 patients in the placebo group were found to have asymmetric proptosis (difference of ≥ 3 mm between eyes) (64). In the treatment group, there was a greater mean (SD) reduction from baseline in proptosis in the study eye (4.7 mm [1.6 mm]) than the fellow eye (1.9 mm [1.5 mm]); P < .01 for both). Seventy percent of patients with asymmetric disease in the treated group no longer demonstrated asymmetry at week 24. In contrast, 58% of patients in the placebo group still had asymmetric proptosis at 24 weeks. The mean (SD) change from baseline in this group was 0.1 mm (1.5 mm) for the study eye and 0.6 mm (1.4 mm) for the fellow eye (P = .3). These results point to the specificity of teprotumumab in targeting tissue affected by TED as opposed to considering any nonspecific effects.
Facial Changes
The concept of thyroid orbitopathy has been recently expanded to include other regions of the face (61). In a prospective study, 23 patients scheduled to receive teprotumumab for TED were assessed using stereophotogrammetric imaging (3-dimensional facial volume analysis). Following teprotumumab therapy, the mean (SD) decrease in full facial volume was 8.9 mL (8.7 mL). More specifically, when the face was divided anatomically into regions, there was a 1.62-mL (3.16-mL) decrease in the midface, and 2.67 mL (4.6 mL) in the lower face. While this study has yet to be validated, it raises the concept of thyroid facial disease, as opposed to an orbitopathy, and further expands the potential beneficial role of teprotumumab in TED.
Adverse Event Assessment and Management
In the phase 2 and 3 clinical trials, teprotumumab was overall well tolerated, although mild to moderate side effects were common in treated and placebo groups. The combined results of 84 teprotumumab-treated patients showed that 80% of patients experienced an AE during therapy compared with 71% in the placebo group (32, 48). The majority of AEs (94%) were mild to moderate (grade 1 or 2), with 4% of patients experiencing a serious AE related or possibly related to teprotumumab. The most common AEs (each occurring in ≥ 10%) were muscle spasms, nausea, alopecia, diarrhea, fatigue, hyperglycemia, and hearing impairment. Most AEs are likely related to downstream effects of IGF-1R inhibition. Since FDA approval of teprotumumab, cases of new-onset ulcerative colitis and encephalopathy have been reported (65-67). Dose adjustment or discontinuation of therapy should be considered in any patient with severe AEs.
Management of AEs during therapy is recommended. Patients are encouraged to increase hydration and nutritional supplementation during therapy. For symptomatic muscle cramps, massage, Epsom salt baths, or dietary supplementation with magnesium, calcium, and potassium may be beneficial. Nausea can be managed with antiemetics. Diarrhea is managed with increased hydration, dietary changes, or over-the-counter antidiarrheal medications. For severe gastrointestinal symptoms, a gastroenterology consult is prudent. Exacerbation of IBD and hearing loss are concerning AEs that require co-management with gastroenterology and otolaryngology.
Inflammatory bowel disease
In the gastrointestinal tract, IGF-1 likely has a key role in promoting growth of the mucosa, preventing apoptosis, supporting the mucosa’s barrier function, and reducing inflammation (68-73). In the phase 2 study for TED, 2 patients with preexisting colitis showed serious exacerbations on teprotumumab. Therefore, patients with inflammatory bowel disease (IBD) were subsequently excluded from the phase 3 study and preexisting IBD is currently considered a relative contraindication to therapy. A case report demonstrated new-onset IBD in a patient with a strong family history of IBD (65), suggesting that patients with a strong family history of IBD should be educated regarding the potential risks of therapy. Should IBD exacerbation, severe persistent diarrhea, or bloody stools occur, urgent evaluation by gastroenterology is warranted and discontinuation of therapy should be considered pending evaluation.
Hearing loss
Hearing loss is a concerning AE that warrants further study. It is known that IGF-1 plays a critical physiologic role in inner ear development (74), with neurotrophic effects on cochlear hair cells (75-77) and synapses (78). In longitudinal aging studies, lower IGF-1 levels were associated with an increased risk of developing age-related hearing loss (77). Clinical trials have used gelatin hydrogels impregnated with topical IGF-1 in the middle ear to treat sudden sensorineural hearing loss (SNHL) (79, 80). These data suggest a mechanism through which IGF-1R inhibition could result in auditory side effects. The RCTs found that 10% of patients experienced hearing symptoms, which were unsolicited, that resolved in most patients after cessation of teprotumumab; however, baseline and posttreatment audiology testing were not reported. In a prospective observational study of 27 patients treated with teprotumumab, 81.5% (22) complained of new subjective otologic symptoms after a mean of 3.8 infusions. The majority of patients with tinnitus, ear plugging, and autophony experienced symptom resolution at 39.2-week average follow-up. However, only 45.5% of patients with hearing loss symptoms recovered fully. In this study, 5 patients, with baseline and posttreatment audiograms, developed teprotumumab-related SNHL and 1 patient developed a patulous eustachian tube. A prior history of hearing loss was identified as a risk factor for teprotumumab-related hearing loss (81). This led the authors to recommend a baseline audiogram and patulous eustachian testing on all patients with repeat testing should new symptoms develop or persist. Patients with baseline audiogram changes should repeat audiogram testing during and after therapy even if asymptomatic (82). Patients with abnormal audiologic testing should be referred to an otolaryngologist. If SNHL is attributed to therapy, the risk of permanent or worsening hearing loss should be discussed with the patient and weighed against the risk of worsening visual function from TED. Depending on the level of hearing loss, therapy cessation should be considered.
Hyperglycemia
Owing to the existence of hybrid heterodimeric IGF-1R/insulin receptor complexes, some insulin resistance is expected with IGF-1R inhibition. In phase 2/3 teprotumumab trials, patients with baseline glycated hemoglobin A1C (HbA1C) greater than or equal to 9% were excluded, while hyperglycemia developed in 8 of 84 patients treated with teprotumumab (10%), the majority of whom had preexisting diabetes or impaired glucose tolerance (32, 48). The manufacturer recommends monitoring glucose levels in all patients and treating hyperglycemia, but formal guidelines do not yet exist. Recommendations for screening and management of hyperglycemia developing with targeted oncologic treatments affecting cell proliferation pathways, including with IGF-1R inhibition, support a goal fasting blood glucose (BG) of less than 160 mg/dL and/or HbA1C of 8% or less (83). Glucose monitoring is recommended at baseline and every visit, with more frequent home glucose monitoring in diabetic patients or those at high risk for diabetes (83). Lee and colleagues (84) recently published recommendations for different monitoring strategies in teprotumumab-treated patients with known diabetes, prediabetes, and normal glucose tolerance. They recommend ideally a pretreatment HbA1C of 7% or less and daily fasting BG in diabetic patients with weekly communication with the endocrinologist during the first few months of treatment, and adjustments if fasting BG is greater than or equal to 150 mg/dL or postprandial BG is greater than or equal to 200 mg/dL. Weekly fasting BG throughout treatment in prediabetics and weekly fasting BG the first few months of treatment in nondiabetic patients is suggested, with consideration of antidiabetic therapy adjustment or initiation if fasting BG is greater than or equal to 150 mg/dL.
Considerations Before Initiating Treatment
The risks and benefits of teprotumumab should be discussed with each patient. The patient’s medical and surgical history should be reviewed, assessing for a history of thyroid dysfunction, diabetes, IBD, hearing loss, current medications, and previous TED therapies and procedures. In addition, a risk factor assessment for potential AEs and contraindications should be completed. Baseline laboratory testing includes thyroid function tests, fasting BG, HbA1C, and a pregnancy test (premenopausal female patients only). Thyroid function should be corrected before or concomitantly with teprotumumab therapy for optimal patient outcomes, while glycemic and pregnancy assessments determine eligibility for therapy.
IGF-1 is critical in fetal skeletal development; therefore, appropriate contraception is strictly recommended before initiation, during treatment, and, given the long half-life of the drug (20 days), for 6 months following completion of therapy in women of reproductive age. Pregnancy, lactation, uncontrolled diabetes, and active IBD are contraindications. Of note, patients with uncontrolled diabetes (defined as HbA1C ≥ 9) or a diagnosis of IBD were excluded from the phase 3 trials. Patients with controlled diabetes, stable IBD, or at risk for IBD should be counseled on the risks of therapy, which should be weighed against the potential benefits. These patients should be monitored with blood sugar checks and AE assessment before each infusion. In addition, given a 10% prevalence of hearing dysfunction reported in the trials, and a recent observational study noting a high rate of hearing dysfunction symptoms, baseline audiologic evaluation (audiogram plus patulous eustachian testing) should be offered to each patient. After a patient agrees to proceed with teprotumumab therapy, insurance authorization is obtained. Time for approval varies, as different insurance carriers use separate criteria for approval.
Monitoring during treatment
An ophthalmologist and/or endocrinologist should monitor patients every 6 to 12 weeks during therapy, depending on the severity of the disease. Patients with DON are seen more frequently at the discretion of the physician. BG and pregnancy tests (when appropriate) should be monitored before each infusion. Patients tolerating the medication well with a good clinical response should continue the full course of 8 infusions.
Posttreatment Monitoring
Each patient’s response to therapy, as well as the status of any AEs, should be recorded after therapy cessation. Indicators of a good clinical response include a reduction in proptosis of 2 mm or more, CAS improvement of 2 or more, and diplopia reduction of 1 or more points on the Gorman diplopia score, from baseline to week 24. Currently there is no consensus on posttreatment monitoring or when to initiate surgery or additional therapies. Until more is known, providers should consider monitoring patients for a durable response to therapy and AE resolution for at least 12 months. Signs concerning for relapse include an increase in proptosis of 2 mm or more or CAS of 2 or more points compared to week 24, or an overall CAS of 4 or more following the week 24 visit. If relapse is suspected, patients should be counseled on additional medical treatment options including steroids with or without mycophenolate, ORT, a second course of teprotumumab, or other second-line TED therapies. Once the clinical exam remains stable for 3 to 6 months following therapy, then surgical rehabilitation of remnant proptosis, strabismus, or eyelid asymmetries can be performed if needed.
Long-term results
The long-term efficacy results for teprotumumab are promising. Seventy-two week data from pooled analyses of the phase 2 and 3 clinical trials demonstrated, in patients with available long-term follow-up data, maintenance of proptosis response in 38 (67%) of 57 initial responders and maintenance of diplopia response in 33 (69%) of 48 initial responders. Additionally, QoL scores improved by 19 points at week 24 after therapy and improvement remained at 21.2 points at 72-week follow-up (49). The approximate 30% relapse rate in teprotumumab responders is comparable to that seen in the large IV steroid trial, in which 31% (19/61) of week 12 responders showed disease progression by week 24 (33). However, it is important to note that in the Bartalena trial, response and relapse were defined as changes in overall ophthalmic improvement (in large part determined by soft-tissue changes) and not as changes in proptosis or subjective diplopia, which in fact did not change with treatment. This contrasts with the teprotumumab trials, in which proptosis and subjective diplopia were 2 of the main metrics quantifying response and relapse.
Unanswered Questions and Barriers to Use
Unanswered questions remain regarding the use of teprotumumab. Different doses, different durations of treatment, and different spacing intervals between doses need to be studied. Predictors of response and durability need to be identified, as about one-third of responders will lose the diplopia and proptosis benefit of the drug during the year following discontinuation. The effect of teprotumumab on mild active TED, DON, and inactive disease needs to be evaluated in RCTs. Teprotumumab should be compared in RCTs with IV steroids and further studied in steroid-resistant patient populations. In addition, the cost of the drug should be considered. Treatment costs vary depending on geographic location and insurance carrier. However, for a 70-kg patient on Medicare, outpatient centers are currently reimbursed approximately $351 574 for the full treatment regimen of teprotumumab compared to approximately $490 for a 12-week course of IVMP (500 mg weekly × 6 followed by 250 mg IV weekly × 6). Further, teprotumumab is currently available only in the United States, where access to this medication can be quite restricted by different insurance plans, with nonuniform criteria used for coverage. Cost reduction and greater access to the drug internationally are needed if this is to become the standard first-line therapy for TED worldwide. It is still unclear if in the long run teprotumumab will decrease the need for rehabilitative surgery for constant or inconstant diplopia or significant proptosis. Finally, drug-related AEs need to be more carefully studied.
How the Role of Steroids Is Changing in the Era of Biologics
The role of IV steroids in TED management is evolving, with the introduction of new biologics, especially teprotumumab. Teprotumumab has shown impressive efficacy in proptosis and diplopia, parameters previously considered treatable only through surgery. Even though clinical trials directly comparing it to steroids are not available, the same composite ophthalmic end point at 24 weeks was reached by 90% of teprotumumab-treated patients compared with 51% of patients treated with IV steroids and 71% of patients treated with IV steroids plus mycophenolate (39, 49). Still, steroids are inexpensive, widely available, have been studied for years, have known side effects, and do improve inflammatory signs/symptoms and QoL. EUGOGO’s recently published revised TED guidelines still promote IV steroids as the first-line treatment for moderate-to-severe active TED, either as the standard 12-week protocol in combination with mycophenolate, or as a single-agent, high-dose, 12-week regimen (28). This is an understandable recommendation currently in Europe and other geographical areas, where teprotumumab is unavailable, recognizing that data for the benefit of combination with mycophenolate over steroid monotherapy are quite limited and the incremental effect of mycophenolate appears to be marginal. In the United States, however, a recent provider survey investigating practice patterns has shown that teprotumumab is replacing IV steroids as the first-line treatment in patients with moderate-to-severe active TED (85). Even though formal clinical guidelines for the United States are not yet available, the rising popularity of teprotumumab likely reflects both its efficacy and its status as the first drug approved by the FDA for TED.
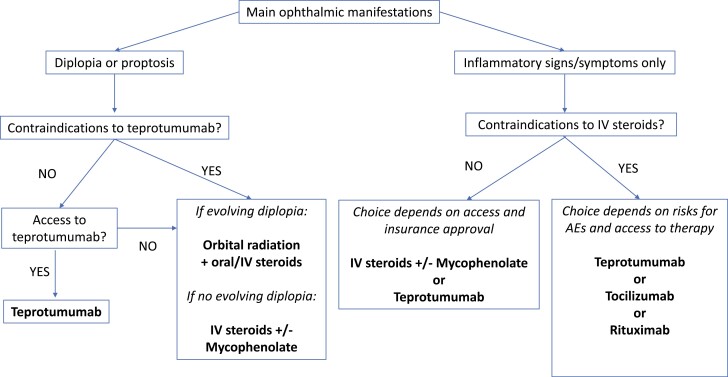
We believe that the time has come to redefine the role of steroids in TED, as one of many options to consider, rather than the default first-line option. An RCT comparing teprotumumab with IV steroids with respect both to long-term efficacy, side effects, ability to reduce need for rehabilitative surgery, and cost-effectiveness is warranted. In the meantime, treatment decisions need to be individualized and consideration paid to each patient’s main eye symptoms, risk for different AEs, as well as access to various treatments. An algorithm for TED treatment using these principles is suggested in Fig. 1.
Figure 1.
Treatment algorithm for active moderate-to-severe thyroid eye disease.
Surgical Management
The Evolving Surgical Approach to Thyroid Eye Disease
TED’s widespread chronic sequelae range from simple irritation of the eyes to disabling diplopia or loss of vision. Until recently, moderate-to-severe chronic TED has been considered a surgical disease, with no role for medical therapies. Surgery is carried out in the chronic “inactive” phase of TED, except in cases of sight-threatening disease, as there is a risk of worsening orbital inflammation if surgery is performed in the active phase. It is common practice to ensure stable disease is present for at least 6 months before surgery. This practice is prudent even for patients previously treated with teprotumumab, as long-term studies showed that approximately one-third of patients will lose proptosis and diplopia benefit at 51 weeks’ follow-up after treatment. Surgical management of chronic TED is customized to individual patient needs and their unique presentation. The sequence of surgery considers 4 components of TED: 1) proptosis, 2) restrictive strabismus, 3) eyelid abnormality (retraction), and 4) cosmetic concerns (fat bags, rhytids, facial asymmetries) (86).
Recent work, however, has challenged the concept that surgical rehabilitation is the only option for chronic TED patients. Given the importance of the IGF-1R pathway in causing the soft-tissue expansion leading to chronic TED and the fact that IGF-1R continues to be overexpressed in orbital tissues in the chronic phase, treating chronic TED with IGF-1R inhibition is a logical step forward (59). A recent case series of chronic (> 3 years) TED patients treated with teprotumumab confirmed a significant improvement in inflammatory signs, proptosis, and diplopia (60). Teprotumumab thus provides a new potential opportunity for treatment of chronic TED patients. Still, more studies are needed to evaluate the long-term durability of the teprotumumab response and the effect on surgical management.
Algorithm/Guidelines for Treating Thyroid Eye Disease
Recently teprotumumab has emerged as an effective therapy for active moderate/severe TED, with benefit demonstrated in 2 RCTs (32, 48). We therefore believe it has an important role to play as one of the first-line therapies in patients with this phase and stage of disease. On the other hand, even though the data presented in this review clearly demonstrate the potential efficacy of teprotumumab for other disease phases and stages, RCTs and long-term data are lacking to advocate for major changes to current practice recommendations for mild, sight-threatening, or chronic moderate-to-severe TED as outlined by the recent EUGOGO guidelines (28). For patients with moderate-to-severe TED, we believe that therapeutic algorithms should be flexible and individualized, and take into consideration 3 main factors: 1) efficacy for the particular disease manifestations in each individual patient, 2) patient characteristics that affect risk of side effects, and 3) access to various treatments.
Teprotumumab is unique among all the medical therapies in showing dramatic changes in proptosis and diplopia. Therefore, for patients with active moderate-to-severe TED and significant proptosis or diplopia as their main disease feature, the authors recommend teprotumumab as the first-line option, where available (see Fig. 1). For patients with recent onset and evolving diplopia as the main finding, ORT with concomitant steroids (oral or IV) may also be considered. For patients with symptoms caused predominantly by soft-tissue inflammation, teprotumumab or IV steroids should be considered. If there are contraindications or side effects, second-line options include tocilizumab or rituximab, recognizing that uncertainties remain regarding their effectiveness.
The individual patient’s underlying comorbidities, age, plans for conception, and other characteristics that influence risk of AEs need consideration. Uncontrolled diabetes is a relative contraindication both for IV steroids and teprotumumab. Osteoporosis or the presence of multiple cardiac risk factors also makes steroids less desirable. Other contraindications to steroids include considerable liver dysfunction, severe hypertension, and severe steroid-responsive glaucoma (31). Steroids, tocilizumab, and rituximab should be used with caution in immunocompromised patients. With teprotumumab, women of reproductive age must be willing to use strict contraception for 1 year (including 6 months after stopping therapy). Caution should also be used in patients with IBD and in patients older than 80 years. Patients younger than 18 years are not candidates for teprotumumab, while patients younger than 35 are traditionally not offered ORT. Severe hypertension and diabetic retinopathy are contraindications to ORT (17, 87).
The final factor to consider is access to the various therapies. This may be limited by drug availability, cost, or lack of expertise. Teprotumumab is not available in most of the world, and access to ORT is also restricted in many countries. Biologic agents are substantially more costly than IV steroids or ORT, with teprotumumab being the most expensive. Finally, expertise in administering certain therapies is limited depending on the geography, experience, and training of clinicians, and the proximity to academic centers.
Until long-term data from RCTs comparing TED treatments for active moderate-to-severe disease are available, all the aforementioned factors should shape the ideal choice of therapy for each individual patient. In the United States, teprotumumab is the only FDA-approved treatment option for TED. It has demonstrated efficacy in not only improving composite ophthalmic end points, like other medical therapies, but also in causing clinically meaningful changes in proptosis and diplopia in patients with active moderate-to-severe TED. Clinical guidelines and additional studies, including RCTs comparing teprotumumab with other therapies, are needed to further guide and refine therapy.
Conclusions
Developments in understanding the pathophysiology of TED have led to a paradigm shift in TED management. Teprotumumab, the only FDA-approved therapy for TED, has changed the therapeutic landscape for TED. As management algorithms evolve, treatment is directed at early intervention for active TED with targeted therapies that not only halt the inflammatory signs of disease, but also modify functional sequelae such as proptosis and diplopia. Questions regarding the cost of new therapies, access, AEs, and the effect on rehabilitative surgery rates still remain. Before the true place of teprotumumab is clear, better assessment of its risks, as well as larger and longer-term studies, are needed. As we move into an era of early and targeted therapy of TED, management should be multidisciplinary with ophthalmologists and endocrinologists at the center of care.
Glossary
Abbreviations
- AE
adverse event
- BCVA
best-corrected visual acuity
- BG
blood glucose
- CAS
clinical activity score
- DON
dysthyroid optic neuropathy
- EUGOGO
European Group on Graves’ Orbitopathy
- FDA
Food and Drug Administration
- GD
Graves disease
- GO
Graves orbitopathy
- HbA1C
hemoglobin A1C
- IBD
Inflammatory bowel disease
- IGF-1R
insulin-like growth factor 1 receptor
- IV
intravenous
- IVMP
intravenous methylprednisolone
- ON
optic nerve
- ORT
orbital radiation
- QoL
quality of life
- RCT
randomized controlled trial
- SNHL
sensorineural hearing loss
- TED
thyroid eye disease.
Contributor Information
Andrea Lora Kossler, Department of Ophthalmology, Stanford University School of Medicine, Palo Alto, California 94303, USA.
Raymond Douglas, Cedars–Sinai Medical Center, Los Angeles, California 90048, USA.
Chrysoula Dosiou, Division of Endocrinology, Stanford University School of Medicine, Palo Alto, California 94305, USA.
Financial Support
This work was supported by an unrestricted grant from Research to Prevent Blindness and the National Institutes of Health (grant No. P30 026877).
Disclosures
A.K. is a consultant for Horizon and has served on Immunovant advisory boards; C.D. has served on Horizon advisory boards and as informal consultant (no compensation) for Vasaragen; and R.D. is a consultant for Horizon.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Chin YH, Ng CH, Lee MH, et al. . Prevalence of thyroid eye disease in Graves’ disease: a meta-analysis and systematic review. Clin Endocrinol (Oxf). 2020;93(4):363-374. [DOI] [PubMed] [Google Scholar]
- 2. Ippolito S, Cusini C, Lasalvia P, et al. . Change in newly diagnosed Graves’ disease phenotype between the twentieth and the twenty-first centuries: meta-analysis and meta-regression. J Endocrinol Invest. 2021;44(8):1707-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enzmann DR, Donaldson SS, Kriss JP. Appearance of Graves’ disease on orbital computed tomography. J Comput Assist Tomogr. 1979;3(6):815-819. [PubMed] [Google Scholar]
- 4. Eckstein AK, Lösch C, Glowacka D, et al. . Euthyroid and primarily hypothyroid patients develop milder and significantly more asymmetrical Graves ophthalmopathy. Br J Ophthalmol. 2009;93(8):1052-1056. [DOI] [PubMed] [Google Scholar]
- 5. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy: impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol (Oxf). 1996;45(4):477-481. [DOI] [PubMed] [Google Scholar]
- 7. McAlinden C. An overview of thyroid eye disease. Eye Vis (Lond). 2014;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heisel CJ, Riddering AL, Andrews CA, Kahana A. Serum vitamin D deficiency is an independent risk factor for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2020;36(1):17-20. [DOI] [PubMed] [Google Scholar]
- 9. Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML. Epidemiology, natural history, risk factors, and prevention of Graves’ orbitopathy. Front Endocrinol (Lausanne). 2020;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habib LA, Godfrey KJ, Mathews P, De Rojas J, Kazim M. Association of risk of obstructive sleep apnea with thyroid eye disease: compressive optic neuropathy. Ophthalmic Plast Reconstr Surg. 2019;35(3):232-234. [DOI] [PubMed] [Google Scholar]
- 11. Godfrey KJ, Schmuter G, Hu B, et al. . Prospective correlation of risk of obstructive sleep apnea with severe clinical features of thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2021;37(3S):S58-S61. [DOI] [PubMed] [Google Scholar]
- 12. Rath S, Pattnaik M, Tripathy D, Mohapatra S, Panigrahy B, Ali MH. Sight-threatening thyroid eye disease: role of diabetes mellitus and interaction with other risk factors. Ophthalmic Plast Reconstr Surg. 2021;37(4):352-360. [DOI] [PubMed] [Google Scholar]
- 13. Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5(3-4):177-194. [PubMed] [Google Scholar]
- 14. Terwee CB, Prummel MF, Gerding MN, Kahaly GJ, Dekker FW, Wiersinga WM. Measuring disease activity to predict therapeutic outcome in Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2005;62(2):145-155. [DOI] [PubMed] [Google Scholar]
- 15. Bothun ED, Scheurer RA, Harrison AR, Lee MS. Update on thyroid eye disease and management. Clin Ophthalmol. 2009;3:543-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perros P, Crombie AL, Kendall-Taylor P. Natural history of thyroid associated ophthalmopathy. Clin Endocrinol (Oxf). 1995;42(1):45-50. [DOI] [PubMed] [Google Scholar]
- 17. Bartalena L, Baldeschi L, Dickinson A, et al. . European Group on Graves' Orbitopathy (EUGOGO). Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158(3):273-285. [DOI] [PubMed] [Google Scholar]
- 18. Tanda ML, Piantanida E, Liparulo L, et al. . Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed raves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98(4):1443-1449. [DOI] [PubMed] [Google Scholar]
- 19. Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21(2):168-199. [DOI] [PubMed] [Google Scholar]
- 20. Perros P, Dayan CM, Dickinson AJ, et al. . Management of patients with Graves’ orbitopathy: initial assessment, management outside specialised centres and referral pathways. Clin Med. 2015;15(2):173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiersinga WM. Quality of life in Graves’ ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):359-370. [DOI] [PubMed] [Google Scholar]
- 22. Ponto KA, Pitz S, Pfeiffer N, Hommel G, Weber MM, Kahaly GJ. Quality of life and occupational disability in endocrine orbitopathy. Dtsch Arztebl Int. 2009;106(17):283-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Estcourt S, Hickey J, Perros P, Dayan C, Vaidya B. The patient experience of services for thyroid eye disease in the United Kingdom: results of a nationwide survey. Eur J Endocrinol. 2009;161(3):483-487. [DOI] [PubMed] [Google Scholar]
- 24. Bartalena L, Marcocci C, Bogazzi F, Panicucci M, Lepri A, Pinchera A. Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med. 1989;321(20):1349-1352. [DOI] [PubMed] [Google Scholar]
- 25. Shiber S, Stiebel-Kalish H, Shimon I, Grossman A, Robenshtok E. Glucocorticoid regimens for prevention of Graves’ ophthalmopathy progression following radioiodine treatment: systematic review and meta-analysis. Thyroid. 2014;24(10):1515-1523. [DOI] [PubMed] [Google Scholar]
- 26. Lai A, Sassi L, Compri E, et al. . Lower dose prednisone prevents radioiodine-associated exacerbation of initially mild or absent Graves’ orbitopathy: a retrospective cohort study. J Clin Endocrinol Metab. 2010;95(3):1333-1337. [DOI] [PubMed] [Google Scholar]
- 27. Marcocci C, Kahaly GJ, Krassas GE, et al. . European Group on Graves’ Orbitopathy. Selenium and the course of mild Graves’ orbitopathy. N Engl J Med. 2011;364(20):1920-1931. [DOI] [PubMed] [Google Scholar]
- 28. Bartalena L, Kahaly GJ, Baldeschi L, et al. . EUGOGO. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43-G67. [DOI] [PubMed] [Google Scholar]
- 29. Marcocci C, Bartalena L, Tanda ML, et al. . Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86(8):3562-3567. [DOI] [PubMed] [Google Scholar]
- 30. Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90(9):5234-5240. [DOI] [PubMed] [Google Scholar]
- 31. Zang S, Ponto KA, Kahaly GJ. Clinical review: intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96(2):320-332. [DOI] [PubMed] [Google Scholar]
- 32. Douglas RS, Kahaly GJ, Patel A, et al. . Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-352. [DOI] [PubMed] [Google Scholar]
- 33. Bartalena L, Krassas G, Wiersinga W, et al. . European Group on Graves’ Orbitopathy. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(12):4454-4463. [DOI] [PubMed] [Google Scholar]
- 34. Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L. Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94(8):2708-2716. [DOI] [PubMed] [Google Scholar]
- 35. Godfrey KJ, Kazim M. Radiotherapy for active thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34(4S Suppl 1):S98-S104. [DOI] [PubMed] [Google Scholar]
- 36. Bartalena L, Marcocci C, Chiovato L, et al. . Orbital cobalt irradiation combined with systemic corticosteroids for Graves’ ophthalmopathy: comparison with systemic corticosteroids alone. J Clin Endocrinolo Metab. 1983;56(6):1139-1144. [DOI] [PubMed] [Google Scholar]
- 37. Marcocci C, Bartalena L, Bogazzi F, Bruno-Bossio G, Lepri A, Pinchera A. Orbital radiotherapy combined with high dose systemic glucocorticoids for Graves’ ophthalmopathy is more effective than radiotherapy alone: results of a prospective randomized study. J Endocrinol Invest. 1991;14(10):853-860. [DOI] [PubMed] [Google Scholar]
- 38. Rajendram R, Taylor PN, Wilson VJ, et al. . Combined immunosuppression and radiotherapy in thyroid eye disease (CIRTED): a multicentre, 2 × 2 factorial, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(4):299-309. [DOI] [PubMed] [Google Scholar]
- 39. Kahaly GJ, Riedl M, König J, et al. . European Group on Graves' Orbitopathy (EUGOGO). Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol. 2018;6(4):287-298. [DOI] [PubMed] [Google Scholar]
- 40. Currò N, Covelli D, Vannucchi G, et al. . Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid. 2014;24(5):897-905. [DOI] [PubMed] [Google Scholar]
- 41. Gold KG, Scofield S, Isaacson SR, Stewart MW, Kazim M. Orbital radiotherapy combined with corticosteroid treatment for thyroid eye disease-compressive optic neuropathy. Ophthalmic Plast Reconstr Surg. 2018;34(2):172-177. [DOI] [PubMed] [Google Scholar]
- 42. Salvi M, Vannucchi G, Currò N, et al. . Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100(2):422-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015;100(2):432-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, et al. . Tocilizumab in Graves Orbitopathy Study Group. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant Graves orbitopathy: a randomized clinical trial. Am J Ophthalmol. 2018;195:181-190. [DOI] [PubMed] [Google Scholar]
- 45. Smith TJ, Janssen JAMJL. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocr Rev. 2019;40(1):236-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol. 2007;178(5):3281-3287. [DOI] [PubMed] [Google Scholar]
- 47. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89(10):5076-5080. [DOI] [PubMed] [Google Scholar]
- 48. Smith TJ, Kahaly GJ, Ezra DG, et al. . Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021;9(6):360-372. [DOI] [PubMed] [Google Scholar]
- 50. Jain AP, Gellada N, Ugradar S, Kumar A, Kahaly G, Douglas R. Teprotumumab reduces extraocular muscle and orbital fat volume in thyroid eye disease. Br J Ophthalmol. 2022;106(2):165-171. [DOI] [PubMed] [Google Scholar]
- 51. Ozzello DJ, Kikkawa DO, Korn BS. Early experience with teprotumumab for chronic thyroid eye disease. Am J Ophthalmol Case Rep. 2020;19:100744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sears CM, Azad AD, Dosiou C, Kossler AL. Teprotumumab for dysthyroid optic neuropathy: early response to therapy. Ophthalmic Plast Reconstr Surg. 2021;37(3S):S157-S160. [DOI] [PubMed] [Google Scholar]
- 53. Sears CM, Wang Y, Bailey LA, et al. . Early efficacy of teprotumumab for the treatment of dysthyroid optic neuropathy: a multicenter study. Am J Ophthalmol Case Rep. 2021;23:101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Slentz DH, Smith TJ, Kim DS, Joseph SS. Teprotumumab for optic neuropathy in thyroid eye disease. JAMA Ophthalmol. 2021;139(2):244-247. [DOI] [PubMed] [Google Scholar]
- 55. Chiou CA, Reshef ER, Freitag SK. Teprotumumab for the treatment of mild compressive optic neuropathy in thyroid eye disease: a report of two cases. Am J Ophthalmol Case Rep. 2021;22:101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Diniz SB, Cohen LM, Roelofs KA, Rootman DB. Early experience with the clinical use of teprotumumab in a heterogenous thyroid eye disease population. Ophthalmic Plast Reconstr Surg. 2021;37(6):583-591. [DOI] [PubMed] [Google Scholar]
- 57. Lopez MJ, Herring JL, Thomas C, Bertram BA, Thomas DA. Visual recovery of dysthyroid optic neuropathy with teprotumumab. J Neuroophthalmol. Published online June 25, 2021. doi: 10.1097/wno.0000000000001298 [DOI] [PubMed] [Google Scholar]
- 58. Hwang CJ, Nichols EE, Chon BH, Perry JD. Bilateral dysthyroid compressive optic neuropathy responsive to teprotumumab. Eur J Ophthalmol. Published online February 1, 2021. doi: 10.1177/1120672121991042 [DOI] [PubMed] [Google Scholar]
- 59. Ugradar S, Shi L, Wang Y, Mester T, Yang H, Douglas RS. Teprotumumab for non-inflammatory thyroid eye disease (TED): evidence for increased IGF-1R expression. Eye (Lond). 2021;35(9):2607-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ugradar S, Kang J, Kossler AL, et al. . Teprotumumab for the treatment of chronic thyroid eye disease. Eye (Lond). Published online July 9, 2021. doi: 10.1038/s41433-021-01593-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ugradar S, Braun J, Wang Y, Zimmerman E, Douglas RS. Facial and eyelid changes in thyroid eye disease are reversed by teprotumumab. Plast Reconstr Surg Glob Open. 2021;9(9):e3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Naik VM, Naik MN, Goldberg RA, Smith TJ, Douglas RS. Immunopathogenesis of thyroid eye disease: emerging paradigms. Surv Ophthalmol. 2010;55(3):215-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Perros P, Žarković M, Panagiotou GC, et al. . European Group on Graves’ Orbitopathy. Asymmetry indicates more severe and active disease in Graves’ orbitopathy: results from a prospective cross-sectional multicentre study. J Endocrinol Invest. 2020;43(12):1717-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ugradar S, Wang Y, Mester T, Kahaly GJ, Douglas R. Improvement of asymmetric thyroid eye disease with teprotumumab. Br J Ophthalmol. Published online February 12, 2021. doi: 10.1136/bjophthalmol-2020-318314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ashraf DC, Jankovic I, El-Nachef N, Winn BJ, Kim GE, Kersten RC. New-onset of inflammatory bowel disease in a patient treated with teprotumumab for thyroid associated ophthalmopathy. Ophthalmic Plast Reconstr Surg. 2021;37(5):e160-e164. [DOI] [PubMed] [Google Scholar]
- 66. Safo MB, Silkiss RZ. A case of ulcerative colitis associated with teprotumumab treatment for thyroid eye disease. Am J Ophthalmol Case Rep. 2021;22:101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hoang TD, Nguyen NT, Chou E, Shakir MK. Rapidly progressive cognitive decline associated with teprotumumab in thyroid eye disease. BMJ Case Rep. 2021;14(5):e242153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dubé PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology. 2006;131(2):589-605. [DOI] [PubMed] [Google Scholar]
- 69. Dong CX, Zhao W, Solomon C, et al. . The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology. 2014;155(2):370-379. [DOI] [PubMed] [Google Scholar]
- 70. Baregamian N, Song J, Jeschke MG, Evers BM, Chung DH. IGF-1 protects intestinal epithelial cells from oxidative stress-induced apoptosis. J Surg Res. 2006;136(1):31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bortvedt SF, Lund PK. Insulin-like growth factor 1: common mediator of multiple enterotrophic hormones and growth factors. Curr Opin Gastroenterol. 2012;28(2):89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lorenzo-Zúñiga V, Rodríguez-Ortigosa CM, Bartolí R, et al. . Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut. 2006;55(9):1306-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Huang KF, Chung DH, Herndon DN. Insulinlike growth factor 1 (IGF-1) reduces gut atrophy and bacterial translocation after severe burn injury. Arch Surg. 1993;128(1):47-53; discussion 53-54. [DOI] [PubMed] [Google Scholar]
- 74. Attias J, Zarchi O, Nageris BI, Laron Z. Cochlear hearing loss in patients with Laron syndrome. Eur Arch Otorhinolaryngol. 2012;269(2):461-466. [DOI] [PubMed] [Google Scholar]
- 75. Ester WA, van Duyvenvoorde HA, de Wit CC, et al. . Two short children born small for gestational age with insulin-like growth factor 1 receptor haploinsufficiency illustrate the heterogeneity of its phenotype. J Clin Endocrinol Metab. 2009;94(12):4717-4727. [DOI] [PubMed] [Google Scholar]
- 76. Jezela-Stanek A, Kucharczyk M, Pelc M, Chrzanowska KH, Krajewska-Walasek M. Minimal clinical findings in a patient with 15qter microdeletion syndrome: delineation of the associated phenotype. Am J Med Genet A. 2012;158(4):922-926. [DOI] [PubMed] [Google Scholar]
- 77. Lassale C, Batty GD, Steptoe A, Zaninotto P. Insulin-like growth factor 1 in relation to future hearing impairment: findings from the English Longitudinal Study of Ageing. Sci Rep. 2017;7(1):4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Walenkamp MJE, Robers JML, Wit JM, et al. . Phenotypic features and response to GH treatment of patients with a molecular defect of the IGF-1 receptor. J Clin Endocrinol Metab. 2019;104(8):3157-3171. [DOI] [PubMed] [Google Scholar]
- 79. Nakagawa T, Sakamoto T, Hiraumi H, et al. . Topical insulin-like growth factor 1 treatment using gelatin hydrogels for glucocorticoid-resistant sudden sensorineural hearing loss: a prospective clinical trial. BMC Med. 2010;8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nakagawa T, Kumakawa K, Usami SI, et al. . A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 2014;12:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sears CM, Azad AD, Amarikwa L, et al. . Hearing dysfunction after treatment with teprotumumab for thyroid eye disease. Am J Ophthalmol. Published online February 25, 2022. doi:10.1016/j.ajo.2022.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Douglas R, Cockerham K, Hansen MR, Kossler A, Michaelides E, Shriver EM.. Insulin-like Growth Factor 1-Receptor Inhibitors in Thyroid Eye Disease: Developing a Consensus to Address and Manage Hearing-related Impairment. Vindico; 2022. [Google Scholar]
- 83. Goldman JW, Mendenhall MA, Rettinger SR. Hyperglycemia associated with targeted oncologic treatment: mechanisms and management. Oncologist. 2016;21(11):1326-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee HBH, Mariash CN, Yoon MK, et al. . Teprotumumab and hyperglycemia guidelines to monitor for hyperglycemia in teprotumumab. Ophthalmic Plast Reconstr Surg. 2021;37(4):393. [DOI] [PubMed] [Google Scholar]
- 85. Brito J. Management of thyroid eye disease: ATA/ETA member survey. Presented at: American Thyroid Association 2021 Annual Meeting; New Rochelle, NY: Mary Ann Liebert Inc.. 30 September 2021 [online]. [Google Scholar]
- 86. Dosiou C, Kossler AL. Thyroid eye disease: navigating the new treatment landscape. J Endocr Soc. 2021;5(5):bvab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tanda ML, Bartalena L. Efficacy and safety of orbital radiotherapy for Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(11):3857-3865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.