Abstract
Context
Thyroid eye disease (TED), a vision-threatening and disfiguring autoimmune process, has thwarted our efforts to understand its pathogenesis and develop effective and safe treatments. Recent scientific advances have facilitated improved treatment options.
Objective
Review historically remote and recent advances in understanding TED.
Design/Setting/Participants
PubMed was scanned using search terms including thyroid-associated ophthalmopathy, thyroid eye disease, Graves’ orbitopathy, autoimmune thyroid disease, and orbital inflammation.
Main outcome measures
Strength of scientific evidence, size, scope, and controls of clinical trials/observations.
Results
Glucocorticoid steroids are widely prescribed systemic medical therapy. They can lessen inflammation-related manifestations of TED but fail to reliably reduce proptosis and diplopia, 2 major causes of morbidity. Other current therapies include mycophenolate, rituximab (anti-CD20 B cell-depleting monoclonal antibody), tocilizumab (interleukin-6 receptor antagonist), and teprotumumab (IGF-I receptor inhibitor). Several new therapeutic approaches have been proposed including targeting prostaglandin receptors, vascular endothelial growth factor, mTOR, and cholesterol pathways. Of potentially greater long-term importance are attempts to restore immune tolerance.
Conclusion
Despite their current wide use, steroids may no longer enjoy first-tier status for TED as more effective and better tolerated medical options become available. Multiple current and emerging therapies, the rationales for which are rooted in theoretical and experimental science, promise better options. These include teprotumumab, rituximab, and tocilizumab. Restoration of immune tolerance could ultimately become the most effective and safe medical management for TED.
Keywords: Graves’ disease, IL-6 receptor, orbit, ophthalmopathy, autoimmunity, IGF-I receptor, TSH receptor, teprotumumab, glucocorticoids
Thyroid eye disease (TED, also known as thyroid-associated ophthalmopathy and Graves’ orbitopathy) represents a disfiguring and potentially sight-threatening condition most commonly associated with Graves’ disease (GD) (1)(Fig. 1). In some instances, TED can accompany Hashimoto thyroiditis. It can inflict substantial physical and emotional morbidity, leading to reduced quality of life (2). Major shortfalls in the management of TED stem from health care provider unfamiliarity with the disease, leading to incorrect diagnosis and therapeutic delays. This is especially true in patients not manifesting obvious thyroid autoimmunity and those in whom subtle signs of periocular inflammation may be misinterpreted. Timely implementation of appropriate medical treatment appears to offer the best clinical outcomes.
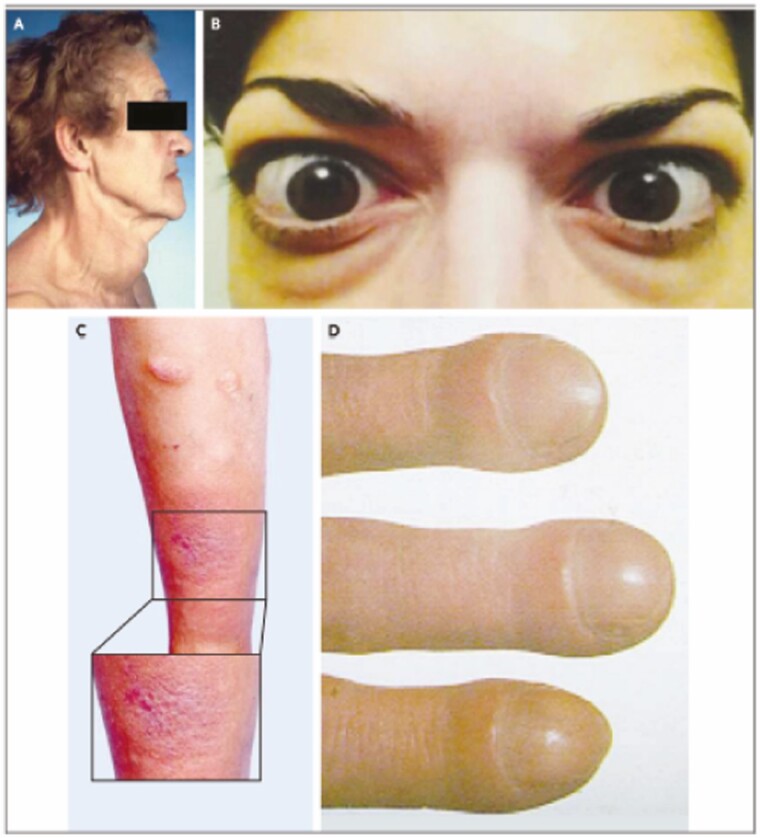
Figure 1.
Clinical manifestations of Graves’ disease. (A) Diffuse moderately enlarged goiter in a woman with hyperthyroidism from Graves’ disease. (B) Moderate to severe thyroid eye disease including bilateral proptosis, periorbital edema, scleral injection, and lid retraction in this patient. (C) Pretibial dermopathy of the plaque form affecting both legs in this patient. (D) Acropachy with fingernail clubbing. From N Engl J Med, Smith T.J. and Hegedus L. Graves’ disease. 375; 1552-1565. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission.
TED follows a characteristic disease course, described more than 70 years ago by Rundle and Wilson (3). After typically presenting with subtle signs and symptoms of activity dominated by eyelid retraction, inflammation, and tissue swelling/congestion, some patients with more severe disease develop proptosis and/or diplopia. Mild TED usually does not require systemic medical therapy and can be managed with local care, such as eyedrops, compresses, tobacco smoke avoidance, and eye protection against strong light and wind. Systemic medical therapy, most frequently glucocorticoids administered either as oral or IV preparations, is typically implemented during the active phase of moderate to severe, symptomatic disease and urgently with development of compressive optic neuropathy. Steroids lessen inflammation-related signs and symptoms of TED (4). Other currently used medical therapies including mycophenolate (MMF), rituximab, selenium, and tocilizumab, may also reduce inflammation and other components of TED activity. Importantly, none of these off-label medications reliably improves diplopia or proptosis and thus none reduces TED severity. Disease activity/progression culminates after 1 to 4 years in chronic, stable disease, when worsening and clinical activity abate. It is during the chronic phase that surgical rehabilitation has been widely viewed as the only therapeutic option remaining for residual disease. These procedures, including orbital decompression, strabismus surgery, eyelid repair, and a variety of cosmetic strategies, are typically performed sequentially (5). Any of these surgeries can reactivate TED and lead to imperfect results. Thus, better medical treatment options are very much necessary. But development of targeted and optimally effective drugs requires more complete understanding of disease pathogenesis.
This paper attempts to briefly review basic and clinical research leading directly to development of targeted therapies for TED. It should provide a roadmap followed as scientific insights have emerged from many laboratories. These developments have yielded an underlying rationale for specific TED treatments.
Deciphering the Pathogenesis of TED
Identifying Pathogenic Autoantigens
Detailed understanding of the molecular and cellular basis for TED has eluded investigators for many decades. The relationship between thyroid autoimmunity in GD and TED was explored in the past. Initial connections between thyroid and orbit were identified once shared thyroid autoantigen expression, including thyroglobulin and TSH receptor (TSHR), were detected in orbital tissues (6, 7). Although the central role for TSHR in GD is now well established, its mechanistic involvement in TED is less clear cut (Fig. 2). There is generally good correlation between TED activity/severity and detectable anti-TSHR antibodies (8), although thyroid-stimulating immunoglobulins (TSIs) are sometimes undetectable in rare patients with severe TED (9). But the importance of TSI actions within the orbit and tissues peripheral to thyroid can only be estimated, based largely on in vitro studies, in addition to inferences emerging from improved preclinical models (10).
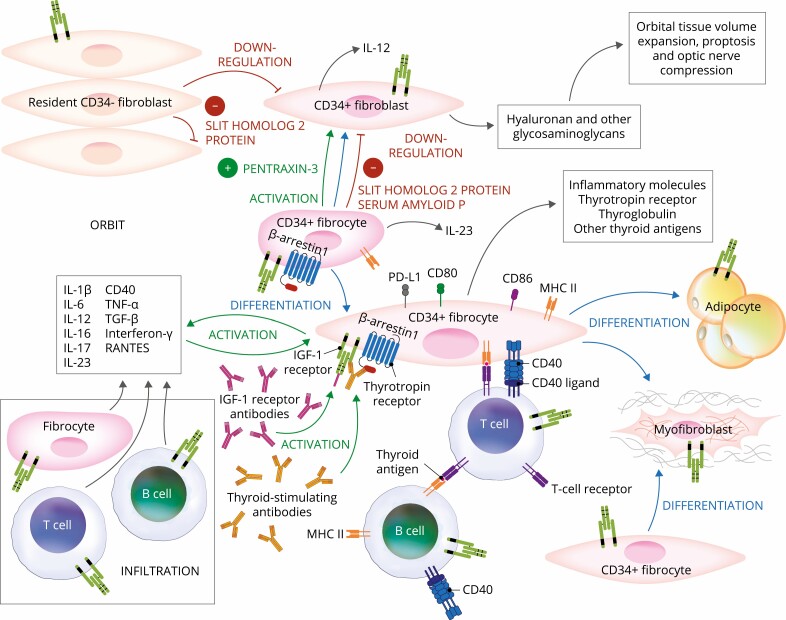
Figure 2.
Proposed theoretical model of thyroid eye disease (TED) pathogenesis. Orbital fibroblasts exhibiting robust responses to inflammatory mediators appear to represent the central effector cells. CD34+ fibroblasts derived from CD34+ CXCR4+ collagen 1 + fibrocytes, monocyte-derived progenitors traffic from bone marrow to the TED orbit. Fibrocytes express several thyroid-specific proteins, including thyrotropin receptor (TSHR), thyroglobulin, thyroperoxidase, and sodium-iodide symporter and express class II major histocompatibility complex (MHC). Fibrocytes and orbital fibroblasts undergo differentiation into myofibroblasts and adipocytes. Slit2 expressed and released by CD34- fibroblasts down regulates expression of many genes expressed by fibrocytes and CD34+ fibroblasts. Interleukins 1β, 6, 8, 10, 12, 16, and 23, tumor necrosis factor α, and Regulated on Activation, Normal T Expressed and Secreted (RANTES), CXCL-12, and CD40-CD154 are expressed in the TED orbit by various cell types and contribute to the inflammatory milieu. CD34+ and CD34- orbital fibroblasts cell-surface display insulin-like growth factor-I receptor (IGF-IR) and express 3 mammalian hyaluronan synthase isoenzymes and UDP glucose dehydrogenase and synthesize hyaluronan. This glycosaminoglycan underlies in part orbital tissue expansion in TED. Hyaluronan synthesis localizes primarily to CD34- orbital fibroblasts.
IGF-I receptor (IGF-IR) has been implicated in TED development, representing a second autoantigen (11). IGF-IR is overexpressed by orbital fibroblasts and B and T cells in GD (12-14). TSHR and IGF-IR form a physical and functional signaling complex (12). Importantly, pathogenic signaling initiated at either receptor is dependent on IGF-IR activity, suggesting a mechanism involving receptor:receptor cross-talk and perhaps TSHR transactivation of IGF-IR (12). In addition, anti-IGF-IR autoantibodies have been detected by some investigators in patients with GD (11, 15-17). In contrast, other laboratory groups have failed to experimentally connect these autoantibodies to GD or TED pathogenesis (18, 19). The central involvement of IGF-IR in mediating GD autoantibody-provoked signaling provided the core rationale for targeting IGF-IR therapeutically. It remains uncertain whether functional, activating autoantibodies directed at epitopes on IGF-IR play an important role in disease development. That issue has generated substantial controversy that remains unresolved. The most likely explanation for disparate findings emerging from various laboratories concerning IGF-IR activating autoantibodies is wide variation in experimental design, culture conditions, and target cell-types used (12, 17, 18, 20-22). Although the precise mechanisms through which TED pathogenesis involves TSHR and IGF-IR remain to be clarified, important evidence clearly implicates both pathways. Potential mechanisms through which TSHR:IGF-IR interactions occur and their impact on TED are detailed in another paper appearing in this issue (23). Importantly, targeting IGF-IR has yielded an effective therapeutic strategy.
Proposed Mechanisms Underlying Disease in the TED Orbit
Importance of circulating and orbit-infiltrating B cells, T cells, and mast cells in the pathogenesis of TED has been investigated for many years by several laboratory groups (24-26). By virtue of their cytokine expression repertoires and interactions with the residential fibroblast population within the orbit, lymphocytes are viewed as critical to the initiation and perpetuation of inflammation and tissue remodeling occurring in TED. Mast cells are also implicated in disease pathogenesis (27). HMC-1 mast cells activate orbital fibroblast production of hyaluronan and PGE2 (28).
Less well-studied in the context of TED have been monocytes and their putative derivatives, cluster of differentiation 34+ (CD34+) fibrocytes (29). The fibroblast population inhabiting the TED orbit comprises distinct subsets which can be segregated based on either CD90 (Thy-1) (30, 31) or the myeloid cell marker, CD34 (32). Thy-1+ orbital fibroblasts differentiate into myofibroblasts when activated by TGF-β and the activation of the Smad signaling pathway (33), whereas Thy-1- fibroblasts differentiate into mature adipocytes when exposed to peroxisome proliferator-activated receptor γ agonists (34). CD34+ orbital fibroblasts (CD34+ OF) are putative derivatives of circulating, bone marrow-derived CD34+ fibrocytes, and cells involved in wound healing, scar formation, and fibrosis. Fibrocytes cultivated from the blood exhibit a CD34+ CXCR4 + TSHRhigh Collagen 1+ phenotype. They possess substantial antigen presenting and T-cell costimulatory capacity (35, 36). CD34+ OF are uniquely identified in the TED orbit and express multiple thyroid autoantigens (37, 38). These cells coexist with CD34- OF. The interplay between CD34+ OF and CD34- OF subsets results in modulation of the CD34+ OF gene expression repertoire, mediated through the Slit2/roundabout 1 pathway (39, 40). Slit2, a TSH-inducible glycoprotein, is expressed and released by CD34- OF and downregulates several immune-related proteins in CD34+ OF. These include thyroid autoantigens such as thyroglobulin, TSHR, thyroperoxidase, and sodium iodide symporter. Further, fibrocytes and, to a lesser degree, CD34+ OF, display CD80, CD86, PD-L1, and major histocompatibility complex II (MHC II) proteins, the levels of which are also downregulated by Slit2 (36). Fibrocytes and CD34+ OF express and release several important cytokines (40). These include IL-1β, IL-6, IL-8, IL-12, and IL-23. Levels of specific cytokines in CD34+ OF are regulated by Slit2 (41).
Debate continues as to whether immune responses occurring in GD and TED are similar or identical in thyroid and orbit, respectively. Recent evidence suggests that peripheral Th1, Th2, and Th17 biased responses can dominate GD, perhaps at different disease stages. Further, autoimmune processes occurring in rodent models of GD may diverge from those in human beings. Th17 cells have been identified as playing potentially important roles in orbital tissue activation (42). IL-17A can induce Regulated upon Activation, Normal T Cell Expressed and Secreted (RANTES) expression in GD-OF (43) and may promote both inflammation and fibrosis in TED (44). Both autoreactive immunoglobulins and T cells appear to promote inflammation and remodeling of TED orbital tissues (1, 45). CD4+ and CD8+ T cells infiltrate the orbit as do B cells, CD34+ fibrocytes, and mast cells (27, 32, 44, 46, 47). These infiltrating cells generate several cytokines that act on residential fibroblasts to induce TED-relevant cytokines. GD-OF exhibit particularly robust responses to molecular cues within the diseased orbit (48, 49). They generate prostanoids, including PGE2, when engaged with IL-1β or CD154. These responses suggest that prostaglandin endoperoxide H synthase-2, the inflammatory cyclooxygenase, might be therapeutically targeted in TED (50, 51). A number of proinflammatory cytokines and their receptors remain relatively unexplored as clinical targets for TED, including IL-1β, IL-1 receptor, IL-1 receptor antagonist, IL-8, IL-12, IL-16, IL-17A, and IL-23, RANTES, and interferon γ (40, 43, 52-55). Each appears potentially relevant to TED because all induce target genes in GD-OF at both transcriptional and posttranscriptional levels (56, 57).
Because many of these cytokines comprise regulatory pathways mediating numerous types of immune responses, therapeutically altering their activities could result in off-target consequences. Thus, one could anticipate substantial barriers in bringing novel or repurposed cytokine-targeting medications for TED to the clinic.
Oxidative stress plays important roles in autoimmune disease (58). TED represents a process in which oxidative stress is likely to be involved (59) and therefore might be targeted therapeutically. Caveolin-1 is downregulated in orbital adipocytes, whereas deiodinase 3 and hypoxia-inducible factor-1α are upregulated in TED-derived orbital adipose tissue (60). Hypoxia-inducible factor-1α activation has been detected in cigarette smokers developing TED (61). Thiol-disulfide homeostasis may be disrupted in active TED (62). The increased oxidative stress in TED has led some investigators to propose therapeutic antioxidants for the disease (63). It is possible that the benefit of dietary selenium supplementation in deficient patients with TED results from the antioxidant properties of this element (64).
Among the emerging factors determining immune function and underlying autoimmune diseases are the microbiological inhabitants of the human gut, oral mucosa, and other tissue niches (65-68). Attention has been paid to viral constituents of the gut (69). In aggregate, findings to date suggest that probiotic therapies might benefit patients with autoimmune phenotypes (70). The potential relationship between thyroid autoimmunity, TED, and microbiota has prompted several studies yielding interesting relationships (71). In animal models of TED, gut microbiota may in part determine immune responses associated with disease development (72). Changes in gut microbiota have been identified in patients with severe, active TED (73). Propionic acid production by gut bacteria has been linked to alterations in Th17/Treg balance in GD (74). Altering gut flora may alter the resulting disease phenotype in animal models of GD (75). Whether the impact of gut flora modification in animal models of GD will translate into treating patients with TED has yet to be demonstrated.
Vascular endothelial growth factor (VEGF) is elevated in patients with TED (76). Levels of VEGF receptor 2 mRNA and VEGF-A, VEGF-C, and VEGF-D are increased in orbital tissues from patients (77). Tear VEGF levels are elevated in patients with active TED and are reduced in response to glucocorticoids (78).
Current Therapeutic Landscape for TED
Medical treatments historically available for TED have generally failed to meet all needs of many patients, especially those with moderate to severe, debilitating, and vision-threatening disease (Fig. 3). Most cases of mild TED can be effectively managed with conservative, local measures and do not require systemic medications. For moderate to severe active TED, frequently used drugs exhibit broad anti-inflammatory and immune-suppressing properties, thus limiting both their dosages and duration of exposure. The concerning properties and limitations of these drugs have driven attempts to identify new options for targeted therapy through better understanding of TED pathogenesis. The array of off-label medications currently in use represents in large part several “old standbys.” Many of these lack adequately powered, placebo-controlled studies validating their effectiveness. Newly introduced and repurposed drugs and others “waiting in the wings” promise a dramatically changed treatment paradigm for TED in the immediate future.
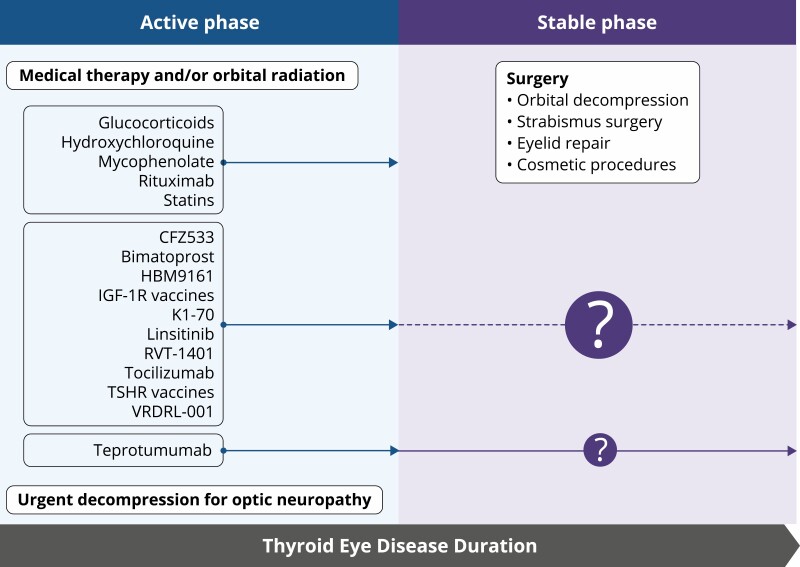
Figure 3.
Use of current therapies and those under development in active and stable, moderate to severe TED. Medical treatment for mild active TED is typically limited to local, nonsurgical measures. In contrast, management of moderate to severe, active TED often involves systemic medications. Some of these drugs can reduce disease activity (signs and symptoms related to inflammation) but most appear relatively ineffective in stable TED. Preliminary reports suggest that teprotumumab may also be effective during stable disease, but clinical trials will be necessary to establish its effectiveness in nonprogressive TED. Relative size of question marks reflects the relative uncertainty of specific treatment effectiveness in stable TED. Surgery is typically reserved for TED reaching the stable phase. The exception is the development of vision loss from compressive optic neuropathy or severe anterior eye surface deterioration. Rituximab (anti-CD20 displayed on B cells), tocilizumab (IL-6 receptor inhibitor), teprotumumab, VRDN-001, linsitinib (IGF-IR inhibitors), K1-70 (TSHR inhibitor), RVT-1401 and HBM9161 (FcRn antagonists), CFZ533 (anti-CD40 antagonist), and TSHR and IGF-IR vaccines (for potentially restoring immune tolerance).
Steroids Remain the Most Frequently Used Systemic Medications for TED
Because inflammation is frequently a dominant presenting feature of TED, enthusiasm for steroid use, beginning decades ago, made good sense (79). Active, moderate to severe, progressive TED is frequently treated with relatively high-dosage, IV glucocorticoids (80, 81). Steroids exemplify the broadly targeted therapies currently in wide use for TED, given the extensive clinical experience with them, both in formal studies and in real-world patient care (82). In addition, they enjoy relatively low cost and wide availability. Like other nonspecific anti-inflammatory agents, steroids are used off-label for TED. Glucocorticoids act through both nuclear and plasma membrane receptors (Fig. 4). They induce or attenuate expression of several genes, including those encoding proteins involved in proinflammatory pathways in T and B cells, GD-OF, and CD34+ fibrocytes. Many of these glucocorticoid steroid effects are mediated through their regulation of transcription factor, nuclear factor-κB (83-85). The multiple consequential genes lying downstream from glucocorticoid receptor/nuclear factor-κB interactions are likely to underly, in large part, the common side effects associated with steroid use in TED (86, 87). These off-target consequences severely limit their dosages and treatment duration (1, 88-90).
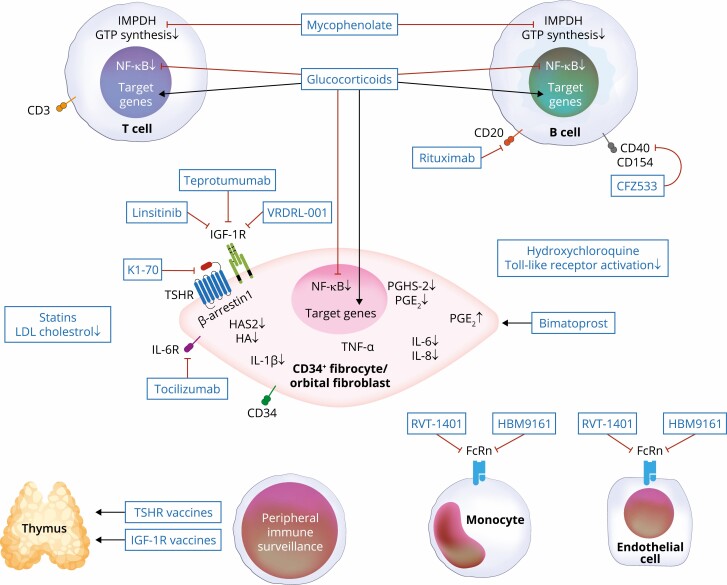
Figure 4.
Putative molecular targets for current medical therapies and those under development. Glucocorticoid steroids target many cell types, where they induce several target genes while inhibiting the expression of others. Many of their anti-inflammatory actions are mediated through nuclear factor-κB. Other agents are more target-specific, such as mycophenolate (targets inosine monophosphate dehydrogenase [IMPDH] and GTP synthesis in lymphocytes), rituximab (anti-CD20 displayed on B cells), tocilizumab (IL-6 receptor inhibitor), teprotumumab, VRDN-001, linsitinib (IGF-IR inhibitors), K1-70 (TSHR inhibitor), RVT-1401, HBM9161 (FcRn antagonists), CFZ533 (anti-CD40 antagonist), and TSHR and IGF-IR vaccines. It is possible that additional, as-yet unidentified targets may play important roles in mediating clinical responses.
Pulse IV administration of steroids is preferred over oral dosing based on studies showing greater efficacy and a reduced side effect profile (91, 92). Importantly, steroid popularity, regardless of administration route, rests largely on common interpretations of results from studies/trials not involving placebo controls where clinical activity scores (CAS) improve. Unfortunately, little evidence exists demonstrating the reliable effectiveness of steroids in TED beyond reducing the consequences of inflammation, including pain and ocular discomfort. Many facets of clinical activity typically improve as a consequence of natural disease course, as is pictorialized by the Rundle curve (3). Only a single, placebo-controlled clinical study of steroids has been reported. That report by van Geest et al (93) included 15 patients with moderate-severe TED. The authors concluded steroid superiority over placebo based on responses observed in 6 patients treated with methylprednisolone (6 g total) over 3 months compared with 9 patients receiving placebo. Further, their conclusions were based on outcomes heavily weighted on inflammation and its consequences over a 48-week observation period. Improvement in other parameters, such as proptosis and diplopia, which represent important sources of morbidity and diminished patient quality of life (QoL), were not essential for achieving response in this study. Many more recent studies of steroids have focused on establishing the most effective and safest dosage and route of administration. These have uniformly not included placebo controls (92).
Widespread availability of glucocorticoids, prescriber familiarity with their use in TED, and limited access to newly developed biologicals perpetuate calls for their first-line status in Europe. The recently published European Group on Graves’ Orbitopathy (EUGOGO) guidelines for medical treatment of TED list high-dosage steroids in combination with MMF as “first-line therapy” (94). Recommendation for this 2-drug approach appears to rest on a single study at 4 different institutions where the medications were combined (95). The relative strength of evidence supporting this combined therapy for TED is discussed in the following section. Another aspect of European preference for steroids stems from the unavailability of 1 newly available therapy. Teprotumumab has not yet been approved for clinical use outside of the United States. Many authors agree that steroids may be very useful in patients acutely experiencing vision threatening compressive optic neuropathy.
Steroid therapy in TED is frequently associated with development or worsening carbohydrate intolerance/diabetes mellitus, hypertension, Cushing syndrome, increased infections, thrush, psychiatric events, and accelerated bone loss (88, 96). These side effects are similar to those widely recognized in patients undergoing systemic, supraphysiological steroid therapy for other medical indications. In addition, high-dose IV pulse steroids can result in severe liver toxicity, although this is rare (89, 90). From aggregate evidence, one can conclude that high dosages of steroids, administered either orally or IV, are associated with increased incidence of side effects (4, 80).
In summary, many steroid-treated patients with TED experience reduced CAS by diminishing inflammatory-related signs and symptoms of active, progressive TED. Benefit of steroids is limited by the lack of consistent response regarding proptosis and diplopia, both of which greatly impact the morbidity of TED. Importantly, steroid therapy in TED does not appear to improve disease outcomes or alter its clinical course. Thus, many steroid-treated patients with relatively severe TED must undergo subsequent surgical procedures, typically performed once the active phase has run its course. Further, exposure to therapeutic dosages of glucocorticoids can be associated with potentially serious side effects. Recent progress in identifying more effective and safer therapeutic options has stirred controversy as to the likely future role of systemic steroids in TED (80).
Mycophenolate
Antimetabolites such as MMF have been used for some time to immunosuppress renal transplant patients and thus reduce organ rejection (97). MMF is the pro-drug of mycophenolic acid that inhibits type II inosine monophosphate dehydrogenase, the rate limiting-enzyme in de novo GTP nucleotide synthesis. This enzyme is expressed widely in activated B and T lymphocytes (98-100). MMF inhibits PI3K/AKT/mTOR and thus attenuates the actions of IL-2 and IL-1β (101). Thus, its cellular targets, by and large, are indiscriminate, leading to a wide scope of immunologic consequences.
MMF was repurposed for use in TED, a practice with thinly documented benefit as the rationale derives from limited studies. A randomized clinical trial comparing MMF and steroids in 174 patients with moderate to severe TED was conducted in China (96). The primary outcome was “overall response,” consisting of at least 3 of the following elements: improvement in CAS ≥ 2 points/disease inactivation (CAS ≤ 3), soft-tissue improvement by 1 grade of eyelid swelling, eyelid erythema, conjunctival redness/edema, proptosis improvement ≥ 2 mm, eye movement improvement, diplopia improvement, and increased visual acuity ≥ 2/10, adjudicated at weeks 12 and 24. Analysis of week 24 data indicates a higher overall response rate for MMF compared with steroids. CAS, diplopia, and proptosis (2-3 mm reduction) responses were greater in those receiving MMF. The same study found more modest but sizable improvement in these parameters following glucocorticoid therapy. Another, randomized, single masked study compared methylprednisolone (cumulative 4.5 g) alone over 6 weeks versus in combination with MMF (cumulative 120 g) for 24 weeks (EUDRACT number 2008-002123-93) in 164 patients at 4 European centers (95). The primary outcome was response rate at 12 and 24 weeks, including CAS reduction of > 2 parameters (eyelid swelling, proptosis, lid width, diplopia grade) in the absence of worsening of any parameter, and sustained response at week 36. Addition of MMF to steroid offered no additional benefit to glucocorticoids alone in the intention-to-treat population at weeks 12 and 24 and week 46 relapse rates. Post hoc analysis of week 24 data suggested that MMF combined with methylprednisolone increased response rate compared with steroids alone. Safety evaluation of MMF in TED, whether as a single agent or combined with steroids, was also limited in terms of observational duration and numbers of patients followed. Importantly, primary responses in the European study did not require meeting disease severity endpoints. Because neither study was placebo controlled, the potential contributions of either medication, administered as a single agent or in combination, to naturally occurring clinical improvement cannot be determined unambiguously. The Chinese study revealed improved proptosis and diplopia, responses not seen in reports from other clinical studies. Smith (102) commented on what he considered to be important weaknesses in the existing evidence supporting the 2-drug therapy proposed by EUGOGO (94). Those comments stressed the importance of evenhandedness in evaluating newly introduced medications for TED, a goal he felt was not achieved by the guideline authors. Those authors have in turn responded, noting that teprotumumab availability is currently limited to North America. Importantly, they left unaddressed the limited evidence for efficacy and lack of adequate long-term follow-up specifically regarding combined steroid and MMF therapy (103). An analysis of safety data from the published trials with MMF suggests that the drug, alone or in combination with steroids, may be well-tolerated (104).
Untargeted Therapies
Hypercholesterolemia has been associated with a seemingly vast array of human diseases, including those of established or putative autoimmune nature (105, 106). An approach to potentially enhance the effectiveness of steroids in TED was examined in a study in which statins were administered as therapy in 88 patients with TED and low-density lipoprotein cholesterol levels between 2.97 and 4.88 mmol/L in an open label, phase 2 trial conducted at a single institution (STAGO) (107). Patients were randomized to receive either methylprednisolone 500 mg/wk for 6 weeks followed by 250 mg/wk for an additional 6 weeks alone or in combination with oral atorvastatin (20 mg/d) for 24 weeks. Primary response was achieving 2 of the following: (1) exophthalmos reduction ≥ 2 mm or more; (2) clinical activity score reduction ≥ 2 points; (3) eyelid aperture reduction ≥ 2 mm; (4) disappearance/improvement of diplopia; and (5) improved visual acuity ≥ 0.2 decimals. The investigators interpreted their study results as demonstrating that the addition of atorvastatin to high-dose steroids improves the clinical outcomes of patients with TED and hypercholesterolemia. They further state an intention to conduct a phase 3 trial where patients with TED are included, regardless of whether they exhibited elevated serum cholesterol levels. Concern arises for administering 2 medications with potentially severe side effects, especially to patients without medically indicated treatments for lipid abnormalities. Such an approach could limit enthusiasm for participation in such a study. The potential for statins to cause autoimmune diseases (108, 109) makes them a particularly dubious choice as therapy for TED. Several experimental studies suggest that lowering serum cholesterol levels, in and of themselves, may be ineffective in altering the development or activity/severity of autoimmune diseases such as TED (110). In any event, calls for caution in the use of statins in individuals with autoimmune diseases have been voiced (111).
Hydroxychloroquine has been used to treat cultured GD-OF and OFs from healthy donors. The drug inhibited cell proliferation, adipogenesis, hyaluronan accumulation, and autophagy while enhancing apoptosis in GD-OF (112). Whether these observations suggest therapeutic utility in TED is uncertain.
Steroid-sparing drugs such as methotrexate were examined for their potential to reduce needed dosage of IV methylprednisolone in 24 moderate-to-severe TED patients with vision, inflammation, strabismus and appearance (VISA) 5.5/10 in a retrospective study (113). All patients received IV methylprednisolone combined with a steroid-sparing drug. The authors reported reduction in the methylprednisolone dosage (mean, 2.72 g) compared to the EUGOGO recommended 4.5 g in 22 patients.
Birth of Biological Therapies for TED
Rituximab
The relatively poor effectiveness of steroids and other nonspecific medications for TED in reliably reducing proptosis and diplopia has resulted in a search for better, more specific therapies with fewer side effects. Among these, rituximab, an anti-CD20 monoclonal antibody targeting a subset of B cells, was among the first examined, initially in case reports/series (114, 115), and subsequently in 2 small, single institution trials (116, 117). The study from Milan examined 32 patients randomized to receive either methylprednisolone (7.5 g) or rituximab (2 g or 500 mg) (116). The primary endpoint was improvement in CAS, whereas the secondary responses included changes in proptosis, diplopia, eye muscle motility, lid fissure, and QoL score at week 24 following treatment initiation. Rituximab was found more effective than steroids in reducing CAS (116). The other trial, conducted at the Mayo Clinic, Rochester, MN, compared rituximab (2 g) with placebo in 25 patients and failed to detect differences in inflammation responses between the 2 treatment arms at 24 weeks (117). Neither study revealed a proptosis response. Differences in patient age, disease duration, and TSH receptor antibody test levels were postulated as potential explanations for the divergent results from the 2 trials.
In aggregate, the existing evidence fails to unambiguously support rituximab as superior to placebo or glucocorticoids in the treatment of active TED. Further, anti-CD20 B-cell depletion does not appear to reliably improve either proptosis or diplopia, 2 major manifestations negatively affecting QoL. Given the absence of a placebo comparator in the Italian study, it is not possible to determine whether either steroids or rituximab offers clinical benefit beyond that attributable to the natural course of TED (3). Thus, further consideration of rituximab as therapy for TED will require additional clinical studies involving larger patient cohorts and appropriate controls.
Teprotumumab
Rationale for therapeutically targeting IGF-IR in TED was born out of empirical observations suggesting that this tyrosine kinase receptor was involved in the pathogenesis of GD and its connective tissue manifestations (118). The therapeutic landscape for TED changed following 2 clinical trials for teprotumumab (119, 120) and its subsequent approval by the US Food and Drug Administration in January 2020 (121). For the first time, a registered medical therapy for TED has become available for wide clinical use. Teprotumumab, a fully human IGF-IR inhibitor, was developed and evaluated initially for treatment of several types of cancer but clinical responses to it and other similar molecules proved inadequate to sustain those programs (122). Thus, the drug became available for repurposing.
Eighty-eight patients with active, moderate to severe TED were enrolled in an initial phase 2 trial, whereas a phase 3 trial enrolled 83 patients in North America and Europe. Both studies were double masked and multicentered. Patients in both trials were within 9 months of initially developing TED, were clinically active (clinical activity scores ≥ 4 on a 7-point scale), and were clinically euthyroid at baseline. None had undergone orbital radiotherapy or remedial surgery for TED. Enrolled patients must have received less than 1 g of prednisolone or equivalent previously and must have undergone a 6-week steroid washout period. Patients were randomized to either placebo or teprotumumab treatment (1:1). All participants were administered a total of 8 infusions, each delivered at 3 weekly intervals over the 24-week treatment phase. If the initial infusion of 10 mg/kg per body weight teprotumumab was uneventful, subsequent doses of 20 mg/kg per body weight were administered for the remaining infusions. Responses to therapy were adjudicated at week 24. For the phase 2 study, the primary endpoint was an aggregate of ≥ 2-point improvement in CAS using a 7-point scale AND ≥ 2 mm proptosis reduction in the more severely affected (study) eye. The phase 3 trial had an endpoint of reduction from baseline in proptosis ≥ 2 mm in the study eye.
A total of 29/42 patients in the intention-to-treat cohort receiving teprotumumab met the primary response at 24 weeks in phase 2. In contrast, 9/45 patients treated with placebo responded (P < 0.001). The primary outcome in phase 3 was achieved by more patients receiving active drug (teprotumumab, 83% vs placebo controls, 10%, P < 0.001). When intention-to-treat data from the 2 trials were pooled (including 84 patients receiving teprotumumab and 87 treated with placebo), more teprotumumab-treated patients (65/84, 77%) achieved a reduction of ≥ 2 mm in proptosis at week 24 versus placebo (13/87, 15%); stratified treatment difference 63%; 95% CI, 51-75 (P < 0.0001) (123). Responses tended to be durable (67% and 69% of proptosis and diplopia responders, respectively maintained 51 weeks after the final dose of teprotumumab).
The safety profile of teprotumumab was encouraging (119, 120, 123). A total of 63/67 (94%) of teprotumumab and 59/60 (98%) of placebo-treated patients experienced mild to moderate (grade 1 or 2) adverse events (AEs), whereas 3 (4%) receiving teprotumumab had serious AEs related or possibly related to the drug. These included diarrhea, infusion reaction, and confusion (potentially Hashimoto encephalopathy), the last of these leading to study discontinuation. Muscle cramping (18%; 95% CI, 7.3-28.7), hearing loss or abnormalities (10%), and hyperglycemia (8%, 1.7-15.0, grade 2-3) were the most commonly reported AEs. Glycemic excursions were most commonly detected in patients with baseline diabetes mellitus or carbohydrate intolerance. In general, glycemic control was restored with medication adjustment. Those dosage requirements reverted to baseline requirements following the treatment phase. Hearing abnormalities have also been identified in clinical use, likely reflecting the importance of the IGF-I pathway and ear development and function (124-126). Sensorineural hearing loss, tinnitus, patulous Eustachian tube, hypoacusis, hyperacusis, and autophony have been reported (126-129). Although some of the cases of hearing abnormalities have proven transient or improved, others have continued to the present time. Issues concerning impaired cognition have also arisen in a single elderly patient (130). The important role of IGF-I and its pathway in central nervous system development and function provide plausibility for an association between IGF-IR inhibition and cognitive changes/decline (131, 132). Clearly the AEs associated with teprotumumab require continued pharmacovigilance and potentially additional studies.
Details concerning the mechanisms of action for teprotumumab remain incomplete. The drug may act on tissues peripheral to the orbit (36). Circulating fibrocytes from teprotumumab-treated patients exhibited a dramatic reduction of CD80, CD86, PD-L1, and MHC class II surface display. Further, teprotumumab treatment was associated with marked reduction of IL-17A- and interferon γ-expressing T cells, actions mediated through gene transcription and mRNA stability. Those observations suggest that teprotumumab clinical responses involve its effects on T-cell activation, mediated though antigen-presenting cells such as fibrocytes. Teprotumumab actions within the orbital tissues also remain plausible. Impact of IGF-IR disruption has been shown in cultured GD-OF. A very recent report has implicated cell-extrinsic pathways in GD-OF mediating apoptosis because of IGF-IR inhibition (133). Whether either/both intraorbital and/or peripheral mechanisms are involved in clinical responses to teprotumumab will require further studies. Additional anti-IGF-IR inhibitory molecules are being evaluated for their efficacy in TED, including VRDN 001, which is undergoing evaluation in a phase 1 trial (NCT05176639). A phase 2b trial for linsitinib, a tyrosine kinase inhibitor of IGF-IR and the insulin receptor, is being investigated for its benefit in TED (NCT05276063).
Emerging Medical Therapies for TED
Tocilizumab
Among the more plausible candidates within the therapeutic pipeline for TED is tocilizumab, an IL-6 receptor (IL-6R) antagonist (134). The plasma membrane IL-6R comprises 2 transmembrane protein subunits, IL-6Rα (gp80) and IL-6Rβ (gp130). The receptor interfaces with Janus kinase and STAT3, which are integral to its downstream signaling and target gene activation. IL-6 induces several important genes, including immediate-early genes and those encoding critically important transcription factors (135, 136). IL-6 has been implicated in several autoimmune diseases, including GD and TED (137, 138). Underlying this involvement are its diverse actions in determining the characteristics of immune responses and T-cell polarization. Tocilizumab has been used clinically for treating rheumatoid arthritis and systemic sclerosis (139, 140). The drug was first examined in TED when Pérez-Moreiras et al (141) performed an uncontrolled, single institution trial of 18 patients with active disease, the majority of whom (15/18) experienced substantial reduction in proptosis (mean, 3.92 mm), whereas 13/18 patients exhibited diplopia responses. This was followed by a placebo-controlled, randomized study including 32 patients with moderate to severe, steroid-resistant TED (142). Proportion of patients with ≥ 2-point change in CAS from baseline at week 16 was the primary response. A total of 93.3% of patients receiving active drug responded, compared with 58.8% on placebo. Mean proptosis reduction was 1.5 mm in tocilizumab-treated patients compared with 0 mm in placebo. An open-label, unmasked, and uncontrolled observational study of 48 steroid or selenium treatment-resistant patients was conducted at several centers in Spain (143). Improvement in disease activity, intraocular pressure, and best-corrected visual acuity was reported. In another study, 21 patients were treated with either tocilizumab (7/21) or rituximab (14/21) (144). The primary endpoint, CAS reduction ≥ 2 points, was achieved in 7/7 patients receiving tocilizumab and in 9/14 rituximab-treated patients. As with many clinical studies in TED patients, small numbers, lack of standardization and placebo controls, and heavy reliance on CAS-related response parameters reduce the strength of argument for tocilizumab as effective in this disease. Future randomized, double masked trials of tocilizumab seem warranted, given the preliminary, encouraging results. These should include placebo controls and adequate statistical power.
K1-70, A Monoclonal TSHR Blocking Antibody
Plausibility for therapeutically targeting TSHR derives in large part from finding the receptor expressed in orbital tissues (145). Therapeutic interruption of TSHR activity has long been viewed as the most logical and obvious approach to medically managing GD and TED. In fact, many investigators have advocated that TSHR and the stimulatory autoantibodies directed against it represent the only therapeutic targets against which TED severity and activity might be mitigated. But the biology of human disease rarely proves that simple, given the elaborate interplay occurring between genes, cells, and signaling pathways. To determine the therapeutic potential of TSHR inhibition in GD, both monoclonal antibodies and small molecules targeting that receptor protein have been under development for many years (146, 147). One such blocking antibody, K1-70, was found to inhibit cAMP generation in vitro (147) and reduce circulating total and free T4 levels in vivo in rats (148). Those studies were followed with pharmacokinetic investigation in rats and cynomolgus monkeys (149). Eighteen patients with GD were enrolled in a phase 1 interventional trial of K1-70 in GD (NCT02904330), which was very recently reported (150). Findings from that study included improvements in thyroid function tests and manifestations of TED. As many as 5 patients experienced clinically meaningful reductions. Earlier, Ryder and colleagues (151) reported metastatic follicular thyroid cancer and TED presenting in a single patient treated with lenvatinib, radioiodine ablation, and K1-70 (made available through US Food and Drug Administration expanded access). Within 22 days of her initial dose of K1-70 (administered intramuscularly), the patient experienced decreased proptosis (2-mm bilateral reduction from baseline) and improved clinical activity score from 6/7 to 0-1/7 while continuing to smoke cigarettes. At this point, the patient underwent ophthalmic surgery for the correction of diplopia. The patient continued receiving K1-70 until, after 16 doses, each at 3-week intervals, the drug was withheld for radionuclide scanning. Her TED then worsened but responded to glucocorticoids. Over the course of her K1-70 treatment, serum TSAb levels decreased but increased after the therapy pause. This single case, although complicated by comorbidities, and the positive results of the phase 1 study, could reflect potential benefit of TSHR inhibition in TED. These results will require later stage, controlled trials before any conclusions can be drawn as to the role of TSHR inhibition in TED therapy.
Targeting CD40/CD154 Bridge
Another potential therapeutic approach for TED involves interrupting the CD40/CD154 pathway. This cascade represents an important conduit through which molecular information is conveyed between T and B cells. Moreover, it has been therapeutically targeted in other autoimmune diseases. Thyroid epithelial cells in GD express elevated CD40 levels (152), and this receptor mediates the induction in orbital fibroblasts by CD154 of IL-6, IL-8, prostaglandin endoperoxide H synthase 2, and hyaluronan (51). A recent clinical study demonstrated the response to CFZ533, an anti-CD40 monoclonal antibody, in hyperthyroid patients with GD (153). Seven of 15 patients achieved a biochemical euthyroid state with the drug. Follow-up studies will be necessary.
Neonatal Fragment Crystallizable Receptor
The neonatal fragment crystallizable receptor (FcRn) plays a determinative role in regulating IgG and albumin clearance from the circulation and degradation through lysosomal pathways (154). Targeting FcRn with monoclonal antibodies can disrupt FcRn-IgG interactions, thereby shortening the circulating half-life of pathogenic IgG autoantibodies (155). This approach has been applied with apparent success to myasthenia gravis (156). Two such therapeutic antibodies are being developed to target FcRn in patients with GD and TED, including RVT-1401 (Immunovant) and HBM9161 (Batoclimab, Harbour Biomed). The trial of RTV-1401 has been suspended over concerns of drug-induced hypercholesterolemia.
Bimatoprost
EP2-specific agonists activate cAMP-mediated responses in GD-OF (157). More recent studies have demonstrated that bimatoprost inhibits adipogenesis in these cells (158). PGF2α and EP2 agonists are active in 3-dimensional OF organoids, where they downregulate fibronectin, collagen 1, and collagen 6 gene expression while upregulating IL-6 (159). A small phase 1 clinical trial is being conducted for TED with proptosis reduction being the primary response (NCT03708627).
Immune Tolerization
Restoring immune tolerance has been attempted in a number of autoimmune diseases, including type 1 diabetes mellitus (160), systemic lupus erythematosus (161), and neuromyelitis optica (162). This strategy offers the potentially enormous advantage of leaving host defense completely intact while, in some instances, curing the underlying disease. One retolerizing approach currently under study uses noninflammatory mRNA vaccines (163). Another involves intradermal injection of antigen-processing independent epitopes (termed apitopes) (164). ATX-GD-59, which contains a combination of 2 TSHR peptides, was evaluated in a phase 1 study of 12 hyperthyroid patients (165). Seven of 10 patients receiving all 10 doses at week 18 of the study exhibited improvement or normalization of thyroid function, whereas 3 subjects developed worsening thyrotoxicosis. Antigen-specific immune retolerization as a treatment in TED seems possible: at least 2 autoantigens with likely important involvement in disease pathogenesis, TSHR and IGF-IR, have been identified, and their roles in disease development partially characterized. These advancements should “set the table” for successfully reestablishing immune tolerance.
Concluding Remarks Concerning Therapies for TED
Traditional medical management of TED, including the use of anti-inflammatory drugs such as glucocorticoid steroids, has served an important role in reducing some aspects of disease burden. However, these agents have failed to consistently improve several important disease manifestations, including proptosis and diplopia. These disease-related signs and the resulting symptoms can substantially reduce QoL, including the inability to engage in normal daily activities. Historical limitations in treatment have frequently resulted in loss of personal and financial productivity and diminished self-image. Steroids can offer temporary relief of signs and symptoms related to inflammation and congestion in TED but are not disease modifying. Further, they are frequently associated with serious side effects. Absence of placebo-controlled trials of steroids, in my view, substantially weakens the case promoting their continued use in TED, either as a single agent or in combination with MMF. That drug combination is currently advocated as “first-line” therapy in the recently published 2021 EUGOGO guidelines (94, 95). Lack of availability and the unfamiliarity of newly developed or repurposed therapies for any disease can slow drug acceptance and uptake. Attitudes toward previously unavailable treatments vary among patients and their health care providers, as has been the case with drugs being introduced for TED.
Despite the reticence expressed by some, novel therapies, including those repurposed from other diseases or developed de novo, have ushered in a new era of TED treatment. Among these are medications specifically targeting pathogenic mechanisms identified from studies conducted in the research laboratory. Teprotumumab, a monoclonal anti-IGF-IR inhibitor, was recently US Food and Drug Administration-approved for TED. It is effective in reducing inflammation, diplopia, and proptosis, thereby improving QoL. Continued vigilance remains critical when assessing the effectiveness, durability, and safety of all newly introduced drugs. Restoration of immune tolerance to TSHR, IGF-IR, and potentially other important autoantigens involved in TED pathogenesis remains the ultimate goal of many researchers. Its attainment could offer disease-free life without ongoing treatment.
Acknowledgments
The author is grateful to Dana Barnhart for expert help in manuscript preparation and Linda Polonsky for terrific editorial oversight.
Glossary
Abbreviations
- AE
adverse event
- CAS
clinical activity score
- CD
cluster of differentiation
- EUGOGO
European Group on Graves’ Orbitopathy
- FcRn
neonatal fragment crystallizable receptor
- GD
Graves’ disease
- IGF-IR
IGR-I receptor
- MHC
major histocompatibility complex
- MMF
mycophenolate mofetil
- OF
orbital fibroblasts
- QoL
quality of life
- RANTES
Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted
- TED
thyroid eye disease
- TSHR
thyrotropin receptor
- VEGF
vascular endothelial growth factor
Financial Support
This work was supported in part by National Institutes of Health grants R01 EY008976, National Institutes of Health Autoimmune Center of Excellence grant AR088974, National Eye Institute Core grant EY007003, and Research to Prevent Blindness.
Disclosures
T.J.S. was issued patents covering treatment of TED and other autoimmune diseases with inhibitors of the insulin-like growth factor I receptor. These are held by UCLA/Lundquist Institute. He is a paid consultant for Horizon Therapeutics and Veridian
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Smith TJ, Hegedus L. Graves’ disease. N Engl J Med. 2016;375(16):1552-1565. [DOI] [PubMed] [Google Scholar]
- 2. Gerding MN, Terwee CB, Dekker FW, Koornneef L, Prummel MF, Wiersinga WM. Quality of life in patients with Graves’ ophthalmopathy is markedly decreased: measurement by the medical outcomes study instrument. Thyroid. 1997;7(6):885-889. [DOI] [PubMed] [Google Scholar]
- 3. Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5(3-4):177-194. [PubMed] [Google Scholar]
- 4. Bartalena L, Krassas GE, Wiersinga W, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(12):4454-4463. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg RA. Advances in surgical rehabilitation in thyroid eye disease. Thyroid. 2008;18(9):989-995. [DOI] [PubMed] [Google Scholar]
- 6. Konishi J, Herman MM, Kriss JP. Binding of thyroglobulin and thyroglobulin-antithyroglobulin immune complex to extraocular muscle membrane. Endocrinology. 1974;95(2):434-446. [DOI] [PubMed] [Google Scholar]
- 7. Davies TF, Teng CS, McLachlan SM, Smith BR, Hall R. Thyrotropin receptors in adipose tissue, retro-orbital tissue and lymphocytes. Mol Cell Endocrinol. 1978;9(3):303-310. [DOI] [PubMed] [Google Scholar]
- 8. Jang SY, Shin DY, Lee EJ, Lee SY, Yoon JS. Relevance of TSH-receptor antibody levels in predicting disease course in Graves’ orbitopathy: comparison of the third-generation TBII assay and Mc4-TSI bioassay. Eye (Lond). 2013;27(8):964-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tabasum A, Khan I, Taylor P, Das G, Okosieme OE. Thyroid antibody-negative euthyroid Graves’ ophthalmopathy. Endocrinol Diabetes Metab Case Rep. 2016;2016:160008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moshkelgosha S, So PW, Deasy N, Diaz-Cano S, Banga JP. Cutting edge: retrobulbar inflammation, adipogenesis, and acute orbital congestion in a preclinical female mouse model of Graves’ orbitopathy induced by thyrotropin receptor plasmid-in vivo electroporation. Endocrinology. 2013;154(9):3008-3015. [DOI] [PubMed] [Google Scholar]
- 11. Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348-6354. [DOI] [PubMed] [Google Scholar]
- 12. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douglas RS, Naik V, Hwang CJ, et al. B cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol. 2008;181(8):5768-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol. 2007;178(5):3281-3287. [DOI] [PubMed] [Google Scholar]
- 15. Pritchard J, Tsui S, Horst N, Cruikshank WW, Smith TJ. Synovial fibroblasts from patients with rheumatoid arthritis, like fibroblasts from Graves’ disease, express high levels of IL-16 when treated with Igs against insulin-like growth factor-1 receptor. J Immunol. 2004;173(5):3564-3569. [DOI] [PubMed] [Google Scholar]
- 16. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89(10):5076-5080. [DOI] [PubMed] [Google Scholar]
- 17. Varewijck AJ, Boelen A, Lamberts SW, et al. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2013;98(2):769-776. [DOI] [PubMed] [Google Scholar]
- 18. Minich WB, Dehina N, Welsink T, et al. Autoantibodies to the IGF1 receptor in Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98(2):752-760. [DOI] [PubMed] [Google Scholar]
- 19. Marcus-Samuels B, Krieger CC, Boutin A, Kahaly GJ, Neumann S, Gershengorn MC. Evidence that Graves’ ophthalmopathy immunoglobulins do not directly activate IGF-1 receptors. Thyroid. 2018;28(5):650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168(2):942-950. [DOI] [PubMed] [Google Scholar]
- 21. Manzella L, Massimino M, Stella S, et al. Activation of the IGF axis in thyroid cancer: implications for tumorigenesis and treatment. Int J Mol Sci. 2019;20(13):3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krieger CC, Place RF, Bevilacqua C, et al. TSH/IGF-1 receptor cross talk in Graves’ ophthalmopathy pathogenesis. J Clin Endocrinol Metab. 2016;101(6):2340-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girnita L, Smith T, Janssen J. IGF-I receptor, TSH receptor and thyroid eye disease. J Endocrinol Metab. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Felberg NT, Sergott RC, Savino PJ, Blizzard JJ, Schatz NJ, Amsel J. Lymphocyte subpopulations in Graves’ ophthalmopathy. Arch Ophthalmol. 1985;103(5):656-659. [DOI] [PubMed] [Google Scholar]
- 25. de Carli M, D’Elios MM, Mariotti S, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1993;77(5):1120-1124. [DOI] [PubMed] [Google Scholar]
- 26. Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderl H, Wick G. Retrobulbar T cells from patients with Graves’ ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93(6):2738-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Steensel L, Paridaens D, van Meurs M, et al. Orbit-infiltrating mast cells, monocytes, and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2012;97(3):E400-E408. [DOI] [PubMed] [Google Scholar]
- 28. Smith TJ, Parikh SJ. HMC-1 mast cells activate human orbital fibroblasts in coculture: evidence for up-regulation of prostaglandin E2 and hyaluronan synthesis. Endocrinology. 1999;140(8):3518-3525. [DOI] [PubMed] [Google Scholar]
- 29. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71-81. [PMC free article] [PubMed] [Google Scholar]
- 30. Smith TJ, Sempowski GD, Wang HS, Del Vecchio PJ, Lippe SD, Phipps RP. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995;80(9):2620-2625. [DOI] [PubMed] [Google Scholar]
- 31. Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87(1):385-392. [DOI] [PubMed] [Google Scholar]
- 32. Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95(1):430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163(4):1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(31):22910-22920. [DOI] [PubMed] [Google Scholar]
- 35. Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94(12):6307-6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernando R, Caldera O, Smith TJ. Therapeutic IGF-I receptor inhibition alters fibrocyte immune phenotype in thyroid-associated ophthalmopathy. Proc Natl Acad Sci USA. 2021;118(52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109(19):7427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, Smith TJ. Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab. 2014;99(7):E1236-E1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fernando R, Grisolia ABD, Lu Y, Atkins S, Smith TJ. Slit2 modulates the inflammatory phenotype of orbit-infiltrating fibrocytes in Graves’ disease. J Immunol. 2018;200(12):3942-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernando R, Smith TJ. Slit2 regulates hyaluronan & cytokine synthesis in fibrocytes: potential relevance to thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2021;106(1):e20-e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernando R, Atkins SJ, Smith TJ. Slit2 may underlie divergent induction by thyrotropin of IL-23 and IL-12 in human fibrocytes. J Immunol. 2020;204(7):1724-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang S, Huang Y, Wang N, et al. Insights into local orbital immunity: evidence for the involvement of the Th17 cell pathway in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2019;104(5):1697-1711. [DOI] [PubMed] [Google Scholar]
- 43. Fang S, Huang Y, Zhong S, et al. IL-17A promotes RANTES expression, but not IL-16, in orbital fibroblasts via CD40-CD40L combination in thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2016;57(14):6123-6133. [DOI] [PubMed] [Google Scholar]
- 44. Fang S, Huang Y, Wang S, et al. IL-17A exacerbates fibrosis by promoting the proinflammatory and profibrotic function of orbital fibroblasts in TAO. J Clin Endocrinol Metab. 2016;101(8):2955-2965. [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1735-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weetman AP, Cohen S, Gatter KC, Fells P, Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves’ ophthalmopathy. Clin Exp Immunol. 1989;75(2):222-227. [PMC free article] [PubMed] [Google Scholar]
- 47. Jaume JC, Portolano S, Prummel MF, McLachlan SM, Rapoport B. Molecular cloning and characterization of genes for antibodies generated by orbital tissue-infiltrating B-cells in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1994;78(2):348-352. [DOI] [PubMed] [Google Scholar]
- 48. Smith TJ. Unique properties of orbital connective tissue underlie its involvement in Graves’ disease. Minerva Endocrinol. 2003;28(3):213-222. [PubMed] [Google Scholar]
- 49. Chen B, Tsui S, Smith TJ. IL-1 beta induces IL-6 expression in human orbital fibroblasts: identification of an anatomic-site specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J Immunol. 2005;175(2):1310-1319. [DOI] [PubMed] [Google Scholar]
- 50. Wang HS, Cao HJ, Winn VD, et al. Leukoregulin induction of prostaglandin-endoperoxide H synthase-2 in human orbital fibroblasts. An in vitro model for connective tissue inflammation. J Biol Chem. 1996;271(37):22718-22728. [PubMed] [Google Scholar]
- 51. Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273(45):29615-29625. [DOI] [PubMed] [Google Scholar]
- 52. Han R, Chen B, Smith TJ. Jak2 dampens the induction by IL-1beta of prostaglandin endoperoxide H synthase 2 expression in human orbital fibroblasts: evidence for divergent influence on the prostaglandin E2 biosynthetic pathway. J Immunol. 2007;179(10):7147-7156. [DOI] [PubMed] [Google Scholar]
- 53. Hwang CJ, Afifiyan N, Sand D, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009;50(5):2262-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991;72(5):1169-1171. [DOI] [PubMed] [Google Scholar]
- 55. Sciaky D, Brazer W, Center DM, Cruikshank WW, Smith TJ. Cultured human fibroblasts express constitutive IL-16 mRNA: cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. J Immunol. 2000;164(7):3806-3814. [DOI] [PubMed] [Google Scholar]
- 56. Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem. 2011;286(27):24487-24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Neag EJ, Smith TJ. 2021 update on thyroid-associated ophthalmopathy. J Endocrinol Invest. 2021;45(2):235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aruoma OI, Kaur H, Halliwell B. Oxygen free radicals and human diseases. J R Soc Health. 1991;111(5):172-177. [DOI] [PubMed] [Google Scholar]
- 59. Bednarek J, Wysocki H, Sowiński J. Peripheral parameters of oxidative stress in patients with infiltrative Graves’ ophthalmopathy treated with corticosteroids. Immunol Lett. 2004;93(2-3):227-232. [DOI] [PubMed] [Google Scholar]
- 60. Van Regemorter E, Joris V, Van Regemorter V, et al. Downregulation of caveolin-1 and upregulation of deiodinase 3, associated with hypoxia-inducible factor-1α increase, are involved in the oxidative stress of Graves’ orbital adipocytes. Thyroid. 2021;31(4):627-637. [DOI] [PubMed] [Google Scholar]
- 61. Görtz GE, Horstmann M, Aniol B, et al. Hypoxia-dependent HIF-1 activation impacts on tissue remodeling in Graves’ ophthalmopathy-implications for smoking. J Clin Endocrinol Metab. 2016;101(12):4834-4842. [DOI] [PubMed] [Google Scholar]
- 62. Yuksel N, Tanriverdi B, Ipteç B, Erel O. Thiol-disulfide homeostasis as an oxidative stress marker in patients with Graves’ ophthalmopathy. Orbit. 2019;38(5):370-375. [DOI] [PubMed] [Google Scholar]
- 63. Rotondo Dottore G, Ionni I, Menconi F, et al. Action of three bioavailable antioxidants in orbital fibroblasts from patients with Graves’ orbitopathy (GO): a new frontier for GO treatment? J Endocrinol Invest. 2018;41(2):193-201. [DOI] [PubMed] [Google Scholar]
- 64. Marcocci C, Kahaly GJ, Krassas GE, et al. Selenium and the course of mild Graves’ orbitopathy. N Engl J Med. 2011;364(20): 1920-1931. [DOI] [PubMed] [Google Scholar]
- 65. Wang Q, Zhang SX, Chang MJ, et al. Characteristics of the gut microbiome and its relationship with peripheral CD4(+) T cell subpopulations and cytokines in rheumatoid arthritis. Front Microbiol. 2022;13:799602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu Q, Ni JJ, Han BX, et al. Causal relationship between gut microbiota and autoimmune diseases: a two-sample mendelian randomization study. Front Immunol. 2021;12:746998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maglione A, Zuccalà M, Tosi M, Clerico M, Rolla S. Host genetics and gut microbiome: perspectives for multiple sclerosis. Genes. 2021;12(8):1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang W, Cong Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol Immunol. 2021;18(4):866-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tomofuji Y, Kishikawa T, Maeda Y, et al. Whole gut virome analysis of 476 Japanese revealed a link between phage and autoimmune disease. Ann Rheum Dis. 2022;81(2):278-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bungau SG, Behl T, Singh A, et al. Targeting probiotics in rheumatoid arthritis. Nutrients. 2021;13(10):3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Covelli D, Ludgate M. The thyroid, the eyes and the gut: a possible connection. J Endocrinol Invest. 2017;40(6):567-576. [DOI] [PubMed] [Google Scholar]
- 72. Masetti G, Moshkelgosha S, Köhling HL, et al. Gut microbiota in experimental murine model of Graves’ orbitopathy established in different environments may modulate clinical presentation of disease. Microbiome. 2018;6(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shi TT, Xin Z, Hua L, et al. Alterations in the intestinal microbiota of patients with severe and active Graves’ orbitopathy: a cross-sectional study. J Endocrinol Invest. 2019;42(8):967-978. [DOI] [PubMed] [Google Scholar]
- 74. Su X, Yin X, Liu Y, et al. Gut dysbiosis contributes to the imbalance of Treg and Th17 cells in Graves’ disease patients by propionic acid. J Clin Endocrinol Metab. 2020;105(11):dgaa511. [DOI] [PubMed] [Google Scholar]
- 75. Moshkelgosha S, Verhasselt HL, Masetti G, et al. Modulating gut microbiota in a mouse model of Graves’ orbitopathy and its impact on induced disease. Microbiome. 2021;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ye X, Liu J, Wang Y, Bin L, Wang J. Increased serum VEGF and b-FGF in Graves’ ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wong LL, Lee NG, Amarnani D, et al. Orbital angiogenesis and lymphangiogenesis in thyroid eye disease: an analysis of vascular growth factors with clinical correlation. Ophthalmology. 2016;123(9):2028-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xu N, Cui Y, Fu D, Sun F. Tear inflammatory cytokines and ocular surface changes in patients with active thyroid eye disease treated with high-dose intravenous glucocorticoids. J Endocrinol Invest. 2020;43(7):901-910. [DOI] [PubMed] [Google Scholar]
- 79. Evans WH. Prednisone therapy in ophthalmic Graves’ disease. Trans Ophthalmol Soc U K. 1961;81:657-662. [PubMed] [Google Scholar]
- 80. Smith TJ, Bartalena L. Will biological agents supplant systemic glucocorticoids as the first-line treatment for thyroid-associated ophthalmopathy? Eur J Endocrinol. 2019;181(5):D27-D43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bartalena L, Krassas GE, Wiersinga W, et al. ; European Group on Graves, O. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(12):4454-4463 [DOI] [PubMed] [Google Scholar]
- 82. Perros P, Krassas GE. Graves orbitopathy: a perspective. Nat Rev Endocrinol. 2009;5(6):312-318. [DOI] [PubMed] [Google Scholar]
- 83. Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91(2):752-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. van de Stolpe A, Caldenhoven E, Stade BG, et al. 12-O-tetradecanoylphorbol-13-acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J Biol Chem. 1994;269(8):6185-6192. [PubMed] [Google Scholar]
- 85. Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1beta in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002;277(19):16355-16364. [DOI] [PubMed] [Google Scholar]
- 86. Marino M, Morabito E, Altea MA, et al. Autoimmune hepatitis during intravenous glucocorticoid pulse therapy for Graves’ ophthalmopathy treated successfully with glucocorticoids themselves. J Endocrinol Invest. 2005;28(3): 280-284. [DOI] [PubMed] [Google Scholar]
- 87. Marcocci C, Watt T, Altea MA, et al. Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol. 2012;166(2):247-253. [DOI] [PubMed] [Google Scholar]
- 88. Hardy RS, Zhou H, Seibel MJ, Cooper MS. Glucocorticoids and bone: consequences of endogenous and exogenous excess and replacement therapy. Endocr Rev. 2018;39(5):519-548. [DOI] [PubMed] [Google Scholar]
- 89. Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid. 2007;17(4):357-362. [DOI] [PubMed] [Google Scholar]
- 90. Moleti M, Giuffrida G, Sturniolo G, et al. Acute liver damage following intravenous glucocorticoid treatment for Graves’ ophthalmopathy. Endocrine. 2016;54(1):259-268. [DOI] [PubMed] [Google Scholar]
- 91. Kauppinen-Mäkelin R, Karma A, Leinonen E, et al. High dose intravenous methylprednisolone pulse therapy versus oral prednisone for thyroid-associated ophthalmopathy. Acta Ophthalmol Scand. 2002;80(3):316-321. [DOI] [PubMed] [Google Scholar]
- 92. Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90(9): 5234-5240. [DOI] [PubMed] [Google Scholar]
- 93. van Geest RJ, Sasim IV, Koppeschaar HP, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol. 2008;158(2):229-237. [DOI] [PubMed] [Google Scholar]
- 94. Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ Orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43-G67. [DOI] [PubMed] [Google Scholar]
- 95. Kahaly GJ, Riedl M, Konig J, et al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol. 2018;6(4):287-298. [DOI] [PubMed] [Google Scholar]
- 96. Ye X, Bo X, Hu X, et al. Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves’ orbitopathy. Clin Endocrinol. 2017;86(2):247-255. [DOI] [PubMed] [Google Scholar]
- 97. Meier-Kriesche HU, Morris JA, Chu AH, et al. Mycophenolate mofetil vs azathioprine in a large population of elderly renal transplant patients. Nephrol Dial Transplant. 2004;19(11):2864-2869. [DOI] [PubMed] [Google Scholar]
- 98. Allison AC. Mechanisms of action of mycophenolate mofetil in preventing chronic rejection. Transplant Proc. 2002;34(7):2863-2866. [DOI] [PubMed] [Google Scholar]
- 99. Fujiyama N, Miura M, Kato S, Sone T, Isobe M, Satoh S. Involvement of carboxylesterase 1 and 2 in the hydrolysis of mycophenolate mofetil. Drug Metab Dispos. 2010;38(12):2210-2217. [DOI] [PubMed] [Google Scholar]
- 100. Carr SF, Papp E, Wu JC, Natsumeda Y. Characterization of human type I and type II IMP dehydrogenases. J Biol Chem. 1993;268(36):27286-27290. [PubMed] [Google Scholar]
- 101. Mazumder AG, Patial V, Singh D. Mycophenolate mofetil contributes to downregulation of the hippocampal interleukin type 2 and 1β mediated PI3K/AKT/mTOR pathway hyperactivation and attenuates neurobehavioral comorbidities in a rat model of temporal lobe epilepsy. Brain Behav Immun. 2019;75:84-93. [DOI] [PubMed] [Google Scholar]
- 102. Smith TJ. Comment on the 2021 EUGOGO clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(6):L13-L14. [DOI] [PubMed] [Google Scholar]
- 103. Kahaly GJ, Bartalena L. Reply to: comment on EUGOGO clinical practice guidelines. Eur J Endocrinol. 2021;185(6)L15-L16. [DOI] [PubMed] [Google Scholar]
- 104. Lee ACH, Riedl M, Frommer L, Diana T, Kahaly GJ. Systemic safety analysis of mycophenolate in Graves’ orbitopathy. J Endocrinol Invest. 2020;43(6):767-777. [DOI] [PubMed] [Google Scholar]
- 105. Prieto-Potín I, Roman-Blas JA, Martínez-Calatrava MJ, Gómez R, Largo R, Herrero-Beaumont G. Hypercholesterolemia boosts joint destruction in chronic arthritis. An experimental model aggravated by foam macrophage infiltration. Arthritis Res Ther. 2013;15(4):R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mahmoudi M, Aslani S, Fadaei R, Jamshidi AR. New insights to the mechanisms underlying atherosclerosis in rheumatoid arthritis. Int J Rheum Dis. 2017;20(3):287-297. [DOI] [PubMed] [Google Scholar]
- 107. Lanzolla G, Sabini E, Leo M, et al. Statins for Graves’ orbitopathy (STAGO): a phase 2, open-label, adaptive, single centre, randomised clinical trial. Lancet Diabetes Endocrinol. 2021;9(11):733-742. [DOI] [PubMed] [Google Scholar]
- 108. Kawasaki E, Fukuyama T, Kuriyama E, et al. Statin-induced autoimmune hepatitis in patients with type 1 diabetes: A report of two cases and literature review. J Diabetes Investig. 2020;11(6):1673-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Vigne S, Duc D, Peter B, et al. Lowering blood cholesterol does not affect neuroinflammation in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2022;19(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ciurleo R, Bramanti P, Marino S. Role of statins in the treatment of multiple sclerosis. Pharmacol Res. 2014;87:133-143. [DOI] [PubMed] [Google Scholar]
- 112. Guo Y, Li H, Chen X, et al. Novel roles of chloroquine and hydroxychloroquine in Graves’ orbitopathy therapy by targeting orbital fibroblasts. J Clin Endocrinol Metab. 2020;105(6):1906-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sipkova Z, Insull EA, David J, Turner HE, Keren S, Norris JH. Early use of steroid-sparing agents in the inactivation of moderate-to-severe active thyroid eye disease: a step-down approach. Clin Endocrinol. 2018;89(6):834-839. [DOI] [PubMed] [Google Scholar]
- 114. El Fassi D, Nielsen CH, Hasselbalch HC, Hegedus L. Treatment-resistant severe, active Graves’ ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid. 2006;16(7):709-710. [DOI] [PubMed] [Google Scholar]
- 115. Khanna D, Chong KK, Afifiyan NF, et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology. 2010;117(1):133-139.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Salvi M, Vannucchi G, Curro N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100(2):422-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015;100(2):432-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993;16(4):251-257. [DOI] [PubMed] [Google Scholar]
- 119. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-352. [DOI] [PubMed] [Google Scholar]
- 121. Markham A. Teprotumumab: first approval. Drugs. 2020;80(5):509-512. [DOI] [PubMed] [Google Scholar]
- 122. Mahadevan D, Sutton GR, Arteta-Bulos R, et al. Phase 1b study of safety, tolerability and efficacy of R1507, a monoclonal antibody to IGF-1R in combination with multiple standard oncology regimens in patients with advanced solid malignancies. Cancer Chemother Pharmacol. 2014;73(3):467-473. [DOI] [PubMed] [Google Scholar]
- 123. Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021;9(6):360-372. [DOI] [PubMed] [Google Scholar]
- 124. Murillo-Cuesta S, Rodríguez-de la Rosa L, Cediel R, Lassaletta L, Varela-Nieto I. The role of insulin-like growth factor-I in the physiopathology of hearing. Front Mol Neurosci. 2011; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Camarero G, Avendano C, Fernandez-Moreno C, et al. Delayed inner ear maturation and neuronal loss in postnatal Igf-1-deficient mice. J Neurosci. 2001;21(19):7630-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hayashi Y, Yamamoto N, Nakagawa T, Ito J. Insulin-like growth factor 1 inhibits hair cell apoptosis and promotes the cell cycle of supporting cells by activating different downstream cascades after pharmacological hair cell injury in neonatal mice. Mol Cell Neurosci. 2013;56(Sept):29-38. [DOI] [PubMed] [Google Scholar]
- 127. Reed DS, Kostosky N, Davies BW, Epstein A, Durairaj VD. Rifle blast exacerbating hearing loss in a patient treated with teprotumumab for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2021;38(2):e41-e43. [DOI] [PubMed] [Google Scholar]
- 128. Yu CY, Correa T, Simmons BA, Hansen MR, Shriver EM. Audiology findings in patients with teprotumumab associated otologic symptoms. Am J Ophthalmol Case Rep. 2021;24(Sept 16): 101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chern A, Dagi Glass LR, Gudis DA. Thyroid eye disease, teprotumumab, and hearing loss: an evolving role for otolaryngologists. Otolaryngol Head Neck Surg. 2021;165(6):1945998211004240. [DOI] [PubMed] [Google Scholar]
- 130. Hoang TD, Nguyen NT, Chou E, Shakir MK. Rapidly progressive cognitive decline associated with teprotumumab in thyroid eye disease. BMJ Case Rep. 2021;14(5):e242153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lauterio TJ. The effects of IGF-I and IGF-II on cell growth and differentiation in the central nervous system. Adv Exp Med Biol. 1992;321:31-36. [DOI] [PubMed] [Google Scholar]
- 132. D’Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol. 1996;13(3):227-255. [DOI] [PubMed] [Google Scholar]
- 133. Morshed SA, Ma R, Latif R, Davies TF. Mechanisms in Graves eye disease: apoptosis as the end point of igf-1 receptor inhibition. Thyroid. 2021;32(4):429-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5(12):1731-1740. [DOI] [PubMed] [Google Scholar]
- 135. Lord KA, Abdollahi A, Thomas SM, et al. Leukemia inhibitory factor and interleukin-6 trigger the same immediate early response, including tyrosine phosphorylation, upon induction of myeloid leukemia differentiation. Mol Cell Biol. 1991;11(9):4371-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yuan J, Wegenka UM, Lutticken C, et al. The signalling pathways of interleukin-6 and g interferon converge by the activation of different transcription factors which bind to common responsive DNA elements. Mol Cell Biol. 1994;14(5):1657-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Grubeck-Loebenstein B, Buchan G, Chantry D, Turner M, Londei M, Feldmann M. Intrathyroidal cytokine production in thyroid disease. J Autoimmun. 1989;2(Suppl):171-176. [DOI] [PubMed] [Google Scholar]
- 138. Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2000;85(3):1194-1199. [DOI] [PubMed] [Google Scholar]
- 139. Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol. 2008;(181):151-160. [DOI] [PubMed] [Google Scholar]
- 140. Khanna D, Lin CJF, Furst DE, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020;8(10):963-974. [DOI] [PubMed] [Google Scholar]
- 141. Perez-Moreiras JV, Alvarez-Lopez A, Gomez EC. Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthal Plast Reconstr Surg. 2014;30(2):162-167. [DOI] [PubMed] [Google Scholar]
- 142. Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant Graves orbitopathy: a randomized clinical trial. Am J Ophthalmol. 2018;195(Nov):181-190. [DOI] [PubMed] [Google Scholar]
- 143. Sánchez-Bilbao L, Martínez-López D, Revenga M, et al. Anti-IL-6 receptor tocilizumab in refractory Graves’ orbitopathy: national multicenter observational study of 48 patients. J Clin Med. 2020;9(9):2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bennedjaï A, Bouheraoua N, Gatfossé M, et al. Tocilizumab versus rituximab in patients with moderate to severe steroid-resistant Graves’ orbitopathy. Ocul Immunol Inflamm. 2020;30(2):500-505. [DOI] [PubMed] [Google Scholar]
- 145. Feliciello A, Porcellini A, Ciullo I, Bonavolonta G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet (London, England). 1993;342(8867):337-338. [DOI] [PubMed] [Google Scholar]
- 146. Neumann S, Kleinau G, Costanzi S, et al. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149(12):5945-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Evans M, Sanders J, Tagami T, et al. Monoclonal autoantibodies to the TSH receptor, one with stimulating activity and one with blocking activity, obtained from the same blood sample. Clin Endocrinol. 2010;73(3):404-412. [DOI] [PubMed] [Google Scholar]
- 148. Furmaniak J, Sanders J, Young S, Kahelis K. Autoimmune highlight. 2011;3(1):19-25. [Google Scholar]
- 149. Furmaniak J, Sanders J, Clark J, et al. Preclinical studies on the toxicology, pharmacokinetics and safety of K1-70(TM) a human monoclonal autoantibody to the TSH receptor with TSH antagonist activity. Auto Immun Highlights. 2019;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Furmaniak J, Sanders J, Sanders P, Li Y, Rees Smith B. TSH receptor specific monoclonal autoantibody K1-70(TM) targeting of the TSH receptor in subjects with Graves’ disease and Graves’ orbitopathy-results from a phase I clinical trial. Clin Endocrinol. 2022;96(6):878-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ryder M, Wentworth M, Algeciras-Schimnich A, et al. Blocking the thyrotropin receptor with K1-70 in a patient with follicular thyroid cancer, Graves’ disease, and Graves’ ophthalmopathy. Thyroid. 2021;31(10):1597-1602. [DOI] [PubMed] [Google Scholar]
- 152. Faure GC, Bensoussan-Lejzerowicz D, Bene MC, Aubert V, Leclere J. Coexpression of CD40 and class II antigen HLA-DR in Graves’ disease thyroid epithelial cells. Clin Immunol Immunopathol. 1997;84(2):212-215. [DOI] [PubMed] [Google Scholar]
- 153. Kahaly GJ, Stan MN, Frommer L, et al. A novel anti-CD40 monoclonal antibody, iscalimab, for control of Graves hyperthyroidism-a proof-of-concept trial. J Clin Endocrinol Metab. 2020;105(3):dgz013. [DOI] [PubMed] [Google Scholar]
- 154. Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172(4):2021-2029. [DOI] [PubMed] [Google Scholar]
- 155. Liu L, Garcia AM, Santoro H, et al. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol. 2007;178(8):5390-5398. [DOI] [PubMed] [Google Scholar]
- 156. Wolfe GI, Ward ES, de Haard H, et al. IgG regulation through FcRn blocking: a novel mechanism for the treatment of myasthenia gravis. J Neurol Sci. 2021;430(Nov 15):118074. [DOI] [PubMed] [Google Scholar]
- 157. Wang HS, Keese CR, Giaever I, Smith TJ. Prostaglandin E2 alters human orbital fibroblast shape through a mechanism involving the generation of cyclic adenosine monophosphate. J Clin Endocrinol Metab. 1995;80(12):3553-3560. [DOI] [PubMed] [Google Scholar]
- 158. Choi CJ, Tao W, Doddapaneni R, et al. The effect of prostaglandin analogue bimatoprost on thyroid-associated orbitopathy. Invest Ophthalmol Vis Sci. 2018;59(15):5912-5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ichioka H, Ida Y, Watanabe M, Ohguro H, Hikage F. Prostaglandin F2α and EP2 agonists, and a ROCK inhibitor modulate the formation of 3D organoids of Grave’s orbitopathy related human orbital fibroblasts. Exp Eye Res. 2021;205(April):108489. [DOI] [PubMed] [Google Scholar]
- 160. Roep BO, Solvason N, Gottlieb PA, et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci Transl Med. 2013;5:191ra-19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Robinson S, Thomas R. Potential for antigen-specific tolerizing immunotherapy in systematic lupus erythematosus. Front Immunol. 2021;12:654701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Steinman L, Bar-Or A, Behne JM, et al. Restoring immune tolerance in neuromyelitis optica: part I. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Krienke C, Kolb L, Diken E, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371(6525):145-153. [DOI] [PubMed] [Google Scholar]
- 164. Jansson L, Vrolix K, Jahraus A, Martin KF, Wraith DC. Immunotherapy with apitopes blocks the immune response to TSH receptor in HLA-DR transgenic mice. Endocrinology. 2018;159(9):3446-3457. [DOI] [PubMed] [Google Scholar]
- 165. Pearce SHS, Dayan C, Wraith DC, et al. Antigen-specific immunotherapy with thyrotropin receptor peptides in Graves’ hyperthyroidism: a phase I study. Thyroid. 2019;29(7):1003-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.