Abstract
Context
Thyroid eye disease (TED) is a complex autoimmune disease process. Orbital fibroblasts represent the central orbital immune target. Involvement of the TSH receptor (TSHR) in TED is not fully understood. IGF-I receptor (IGF-IR) is overexpressed in several cell types in TED, including fibrocytes and orbital fibroblasts. IGF-IR may form a physical and functional complex with TSHR.
Objective
Review literature relevant to autoantibody generation in TED and whether these induce orbital fibroblast responses directly through TSHR, IGF-IR, or both.
Evidence
IGF-IR has traditionally been considered a typical tyrosine kinase receptor in which tyrosine residues become phosphorylated following IGF-I binding. Evidence has emerged that IGF-IR possesses kinase-independent activities and can be considered a functional receptor tyrosine kinase/G-protein-coupled receptor hybrid, using the G-protein receptor kinase/β-arrestin system. Teprotumumab, a monoclonal IGF-IR antibody, effectively reduces TED disease activity, proptosis, and diplopia. In addition, the drug attenuates in vitro actions of both IGF-I and TSH in fibrocytes and orbital fibroblasts, including induction of proinflammatory cytokines by TSH and TED IgGs.
Conclusions
Although teprotumumab has been proven effective and relatively safe in the treatment of TED, many questions remain pertaining to IGF-IR, its relationship with TSHR, and how the drug might be disrupting these receptor protein/protein interactions. Here, we propose 4 possible IGF-IR activation models that could underlie clinical responses to teprotumumab observed in patients with TED. Teprotumumab is associated with several adverse events, including hyperglycemia and hearing abnormalities. Underpinning mechanisms of these are being investigated. Patients undergoing treatment with drug must be monitored for these and managed with best medical practices.
Keywords: IGF-IR, IGF1R, IGF1, IGF-I, beta-arrestins, RTK, GPCR, biased signaling, TSHR
Clinical Background of Thyroid Eye Disease
Epidemiology of Thyroid Eye Disease
The ocular and periocular manifestations of Graves’ disease (GD) result in substantially reduced quality of life, the consequence of physical and emotional disease impact (1, 2). Orbital disease in GD, known as thyroid eye disease (TED or thyroid-associated ophthalmopathy), appears to emerge from similar/identical immunological events as those underpinning disordered thyroid gland function (3). Orbital tissue reactivity, remodeling, and the resulting clinical manifestations represent the most consequential extrathyroidal manifestations of GD. As many as 40% to 50% of patients with GD manifest clinically important TED, of which the majority is female, although older men are more likely to develop severe disease (4). GD incidence clusters in families harboring other autoimmune diseases. The temporal relationship between development of GD and TED is variable and the appearance of TED may precede, coincide with, or follow that of thyroid dysfunction. In addition, patients with autoimmune thyroiditis can also develop TED (5). A minority of patients with TED remain euthyroid throughout their lifetimes and thus never require treatment of their thyroid gland.
Potential Risk Factors Involved in TED
Risk of developing TED is substantially greater in patients exhibiting thyroid dysfunction attributable to GD than individuals in the general population. Among risk factors for TED are increasing patient age, tobacco smoking, radioactive iodine (RAI) ablative therapy, thyroid dysregulation, and high serum cholesterol levels. Prophylactic steroids may mitigate the risks associated with RAI therapy (6, 7). Identities of TED-promoting smoke constituents remain uncertain (8). Thyroid ablation with RAI has been recognized also as a risk factor for developing or worsening of TED (9). Glucocorticoid steroids may lessen the deleterious effects of RAI on TED (10).
Clinical Manifestations of TED
Inflammation and tissue remodeling occurring in TED involve the orbit and upper face. These manifestations include eyelid retraction, proptosis, diplopia, tissue congestion, and periocular discomfort/pain and tissue injection. TED typically presents as an initial, active, progressive process, followed after 1 to 4 years by the chronic/stable phase, during which these tissue abnormalities cease changing (11). The clinical manifestations of TED and how these relate to GD and thyroid autoimmunity have been reviewed in the recent past (3).
Cells Involved in Orbital Inflammation and Tissue Remodeling in TED
Recent progress in identifying which cell types participate in TED has implicated CD34 + fibrocytes, which apparently infiltrate the orbit and exhibit unique phenotypic attributes (12). Fibrocytes display relatively high constitutive levels of major histocompatibility complex II and costimulatory molecules such as CD80, CD86, and programmed death-ligand 1, all of which appear to exhibit IGF-1 receptor (IGF-IR)-dependent expression (13). CD34+ orbital fibroblasts, putative derivatives of fibrocytes, comprise a sizable cell subset in the TED orbit. They coexist with CD34- fibroblasts, which appear identical to fibroblasts uniformly present in healthy orbits. Moreover, in addition to fibrocytes and fibroblasts, T and B lymphocytes, mast cells, and monocytes may infiltrate the TED orbit and are considered critical to the development of TED by virtue of the cytokines and other inflammatory mediators they generate and their capacity to mediate antigen-specific immune responses (14, 15). Despite extensive investigation suggesting an active role of extraocular muscle cells, their involvement appears to be secondary.
TSH Receptor Pathway
Current Concepts of TSH Receptor Signaling
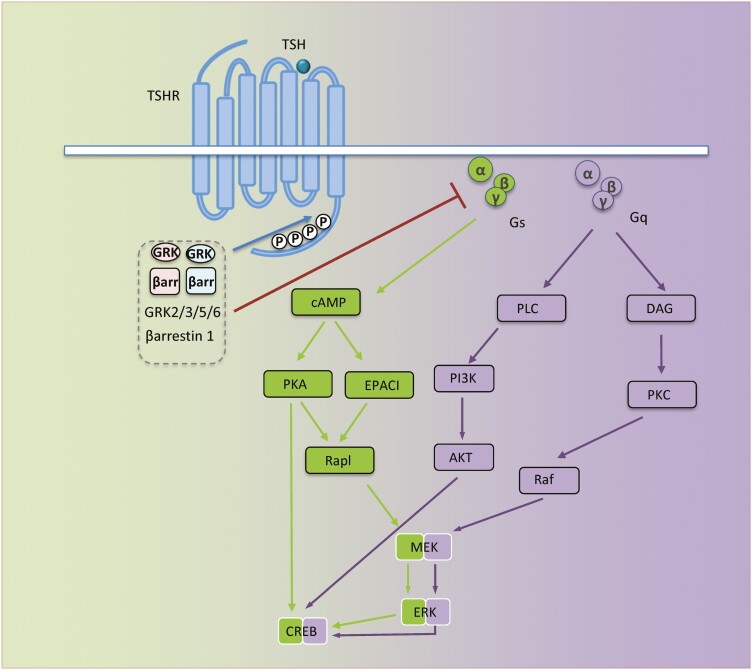
G protein-coupled receptors, also known as 7-transmembrane receptors, include multiple cell surface structures capable of initiating downstream signaling through activation of heterotrimeric G proteins. Ligand activated G-protein-coupled receptors (GPCRs) facilitate GDP-GTP exchange on the Gα subunit, resulting in heterotrimeric complex dissociation into monomeric Gα and Gβγ dimers, each component initiating discrete signaling events (16). Depending on specific Gα subunit utilization for signal activation, GPCRs are classified as Gs (stimulation of cAMP), Gi (inhibition of cAMP), Gq, or G12 (16). TSH receptor (TSHR or thyrotropin receptor) is a member of the GPCR family (17). Engagement of cell surface-displayed TSHR by TSH or agonistic subsets of anti-TSHR antibodies results in the activation of 2 major G protein classes (Gs and Gq) (18) (Fig. 1). This in turn provokes second messengers via major signaling pathways such as cAMP/protein kinase A/Ras-related protein 1/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), protein kinase C/Raf/MEK/ERK (Fig. 1) (18). Interactions of these pathways result in complex signaling crosstalk (18). Stimulated TSHR, after being sequestered by clathrin-coated pits or caveolin scaffolding proteins, can signal following internalization, a requirement for efficient protein kinase A-dependent cAMP response element binding protein phosphorylation, thus allowing induction of early-immediate response genes. An important observation made in vitro concerns significant (constitutive) stimulatory Gs activation exhibited by unliganded, wild-type TSHR. This activity results in intracellular cAMP accumulation. Intrinsic TSHR signaling is susceptible to blockade by inverse agonistic antibodies (19). Similar to other activated GPCRs, TSHR reiterates heterotrimeric G-protein recruitment/activation cycle should this interaction be perpetuated by the GPCR/G-protein interface. This process can be downregulated through a feedback mechanism known as desensitization. This process is instigated by increased local Gβγ concentrations facilitating engagement of G protein-coupled receptor kinases (GRKs) by activated receptor (20). Subsequent phosphorylation of serine/threonine residues within intracellular receptor domains creates binding sites and recruitment of specialized cytoplasmic arrestin proteins (21). This interaction prevents further G protein activation while initiating an alternative signaling pathway, commonly known as arrestin signaling (22, 23). As GRK/arrestin interplay translates conformational change into alternative downstream signals, it behaves as an “allosteric microprocessor” (22).
Figure 1.
TSH receptor (TSHR) signaling. Schematic representation of major signaling pathways downstream TSHR. When not stimulated by activated receptor, Gα (α) is bound to guanosine diphosphate (GDP) and Gβγ (βγ), forming inactive Gs or Gq containing trimer, αβγ. Ligand-activated receptor leads to GDP/GTP exchange on the α subunit, resulting in dissociation into α and βγ subunits. α and βγ protein subunits subsequently interact with second effector proteins, promoting multiple downstream pathway activation; TSHR/Gs activates adenylyl cyclase (AC), resulting in increased cAMP, which in turn regulates multiple signaling pathways, including ion channels, protein kinase A and EPAC. TSHR/Gq initiates phospholipase C (PLC)/phosphoinositide 3-kinase (PI3K) cascade activation, promoting cell proliferation and survival. Likewise, TSHR/Gq enhances diacyl glycerol (DAG) generation and protein kinase C (PKC) activation. Both Gs and Gq, eventually activate Raf/MEK/ERK, also known as mitogen-activated protein kinase pathways. βγ subunits initiate receptor-distinct signaling processes including G protein-coupled receptor kinase (GRK) recruitment. GRKs phosphorylate serine/threonine residues within the TSHR C-terminus, creating binding sites for β-arrestins (βarr). βarr recruitment prevents further G protein activation (desensitization). All signaling pathways downstream from TSHR culminate in biological effects mediated through transcription factors such as ERK and cAMP response element binding protein.
The human genome encodes 4 arrestin isoforms and 7 members of the GRK family (GRK 1-7) (24, 25). Arrestin isoforms 1 and 4, also known as visual arrestins, regulate photoreceptor signaling. Isoforms 2 and 3 (β-arrestins 1 and 2) are ubiquitously expressed and regulate most GPCRs. The GRK family is subdivided in 3 branches; GRK1/7 regulate the activity of visual arrestins, whereas GRK2/3 and GRK4/5/6 coordinate β-arrestins. GRK2/3 are cytoplasmic proteins highly dependent on specific membrane-recruitment signaling for promoting translocation of their partner receptors. Thus, GRK2/3 are recruited to membranes through a feedback mechanism involving Gβγ dimer released from activated receptors. (For a detailed review, see reference (26)). The GRK/arrestin system controls TSHR desensitization and trafficking although the processes involved remain poorly understood and experimental results are conflicting. GRK2 and GRK5 may be involved in homologous desensitization of TSHR Gs signaling in FRTL5 cells in a β-arrestin 1–dependent manner (27, 28). TSHR activation, likely the consequence of GRK-dependent phosphorylation, enables binding of either β-arrestin 1 or β-arrestin 2. These associations result in divergent signaling and altered receptor trafficking. In human embryonic kidney 293 cells, both β-arrestin isoforms internalize the receptor; however, β-arrestin 2 binds TSHR more rapidly and does not colocalize with the receptor once the protein enters endosomes (29). Unlike other GPCRs, TSHR desensitization appears ligand- and/or cell type-specific because internalization fails to alter TSH/TSHR-induced signaling in thyroid epithelial cells (30). In contrast, TSHR internalization attenuates signaling in human embryonic kidney 293 cells by β-arrestin 1 and may mediate TSHR-induced MAPK and PI3K/Akt signaling and differentiation in human osteosarcoma cells, whereas β-arrestin 2 inhibits Gs signaling through TSHR desensitization (Fig. 1) (31). GRK isoenzymes control β-arrestin recruitment, signaling, TSHR degradation, and responsiveness to various ligands, including TSH and TSHR autoantibodies.
Evidence Supporting Involvement of TSHR and TSHR Autoantibodies in TED
A single immunological abnormality may underlie Graves’ hyperthyroidism and TED, primarily involving loss of tolerance to TSHR (32). Elevated TSHR levels have been detected in orbital fibroblasts and adipocytes from patients with TED. Further, adipogenic differentiation enhances these levels. TSHR-initiated actions in orbital fibroblasts are mediated through stimulation of adenyl cyclase/cAMP and PI3K/Akt (32). These cells represent the autoimmune target in TED. Orbit-infiltrating CD8+ T cells recognize fibroblasts but not extraocular muscle extracts. The clinical observation that TSHR-directed autoantibodies (TRAbs) can be detected in most patients with TED, including those who are euthyroid, suggests that this receptor acts as the primary autoantigen in TED. In addition, TRAb levels generally correlate with TED severity and clinical activity. In aggregate, these associations suggest connectivity between TSHR and TED but fail to establish a causal relationship.
Evidence Suggesting Autoantigens/Autoantibodies Beyond TSHR/TRAb in TED
Accumulation of hyaluronan (HA) represents a cardinal feature of TED. Smith and Hoa found that GD-IgG (comprising TRAbs as well as other IgGs), but not recombinant human TSH (rhTSH), increased HA production in undifferentiated TED orbital fibroblasts (33). In contrast, Van Zeijl et al. reported that neither rhTSH nor GD-IgG increased HA production in undifferentiated fibroblasts (34). They concluded that TSHR-mediated cAMP signaling is uninvolved in GD-IgG-induced HA synthesis (34). Conversely, Kumar et al. reported enhanced HA production in undifferentiated TED fibroblasts after treatment with either bovine TSH (bTSH) or the stimulatory TRAb, M22 (35). Tsui and colleagues found that inhibiting IGF-IR or knocking-down its expression could attenuate the activation of Erk provoked by rhIGF-1, bTSH, or GD-IgG (36). This was confirmed by Kumar et al., who demonstrated that IGF-IR blockade inhibited both IGF-I and M22 provoked HA synthesis and Akt phosphorylation (35). In another study, Zhang et al. observed a small but significant stimulation of HA production by bTSH as well as by 2 monoclonal TRAbs (1 stimulatory and 1 neutral) in undifferentiated, healthy donor-derived orbital fibroblasts, but not in fibroblasts from TED patients (37). These divergent results may have resulted from use of different assays and dissimilar culture conditions. Although in vitro studies offer certain advantages, they cannot replicate conditions existing in situ in the TED orbit. Thus, based on available data, a precise pathogenetic role of TRAbs in TED is far from clarified.
Interactions Between GH-IGF and TSH-thyroid Pathways
Interaction between GH-IGF-I and TSH-thyroid pathways is required for normal somatic growth (38). For instance, triiodothyronine is necessary for normal pituitary GH secretion. In turn, IGF-I appears to inhibit triiodothyronine-induced GH gene expression through a short, negative feedback loop. Exogenous thyroxine stimulates IGF-I activity in the absence of GH in hypophysectomized or thyroidectomized animals. Circulating IGF-I levels are negatively associated with TSH in euthyroid and hyperthyroid subjects. Close physical and functional associations between the IGF-IR and TSHR have been described in thyroid epithelium and human orbital fibroblasts (36). Simultaneous activation of the 2 receptors results in synergistic up-regulation of DNA synthesis and cell proliferation compared with effects of either agonist alone (39). TSH induces insulin receptor substrate (IRS)-2 monoubiquitination in thyroid epithelial cells, thereby enhancing IGF-I signaling and mitogenic activity (40). Both IGF-I and TSH increase nuclear β-catenin content and thus promote Wnt-dependent thyroid cell proliferation (41). IGF-I can independently stimulate rat thyroid cell proliferation, suggesting that compared with TSH, it may also represent an important thyroid growth regulator.
Lung fibroblasts derived from TSHR-knockout mice exhibited reduced IGF-IR surface expression, as well as altered subcellular localization and attenuated IGF-IR-dependent signaling (42). In mice overexpressing both IGF-I and IGF-IR, reduced circulating TSH levels are required for maintaining normal thyroid function (43). In contrast, IGF-IR deficiency in thyrocytes impairs thyroid hormone secretion and completely blocks TSH-stimulated goiter formation. These findings reveal the essential role for IGF-IR signaling in the regulation of thyroid glandular function (44).
IGF and its Related Molecules
IGF system comprises 3 ligands (insulin, IGF-I, and IGF-II), 3 cell-surface receptors (insulin receptor [IR], IGF-IR, and IGF-IIR) and 6 IGF binding proteins (IGFBPs) that modulate ligand-receptor interactions (45). Noncanonical family members include the antimicrobial peptide, LL-37, which functions as an IGF-IR ligand (46), the orphan insulin-receptor-related receptor (47), and the insulin receptor-IGF-IR hybrid receptor (48, 49). IGF-I and IGF-II exert both systemic and local (autocrine/paracrine) effects. IGFs are expressed by virtually all mammalian cells and are secreted unmodified following synthesis through a constitutive secretory pathway. Most circulating IGF-I (about 99%) is bound to IGFBPs, whereas a small component (0.5%-1%) circulates in a free (unbound) form. IGFs exhibit higher affinity for IGFBPs than IGF-IR. Consequently, most IGF molecules in the mammalian body are inaccessible to IGF-IR binding. IGF-I and IGF-II act in large part through IGF-IR at concentrations of 1 to 2 nM (50). Extracellular IGF concentrations are considerably lower than those in the circulation (50). IGF secretion rates modestly correlate with tissue activity. IGFBPs can associate with cell membranes, and in so doing increase local IGF concentrations proximate to IGF-IR. They may prevent IGF-IR down-regulation and thereby increase IGF activity. Each IGFBP appears susceptible to specific IGFBP proteases, resulting in narrow, well-controlled proteolysis and lower binding affinity. This can shift complex equilibria, making IGFs more available for IGF-IR binding. IGFBP protease activity is regulated in turn by protease inhibitors, the balance of which can be altered by disease.
Current Concepts of IGF-IR Signaling
Canonical Signaling
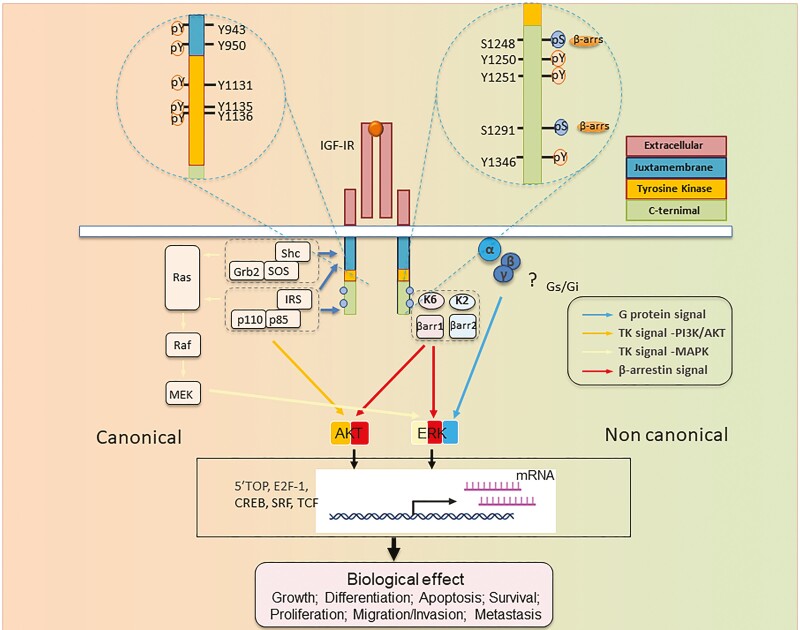
IGF-IR is dimeric in structure, with each protomer comprising an extracellular alpha-domain and a transmembrane beta-subunit with a cytoplasmic kinase-domain. Within the classical paradigm, signal transduction initiated at IGF-IR is triggered by ligand binding to the receptor ectodomain, conformational intracellular beta-subunit rearrangements, resulting in autophosphorylation (in Trans) of specific tyrosine residues within activation domains (Fig. 2) (51, 52).
Figure 2.
Structure-function relationship of IGF-I receptor (IGF-IR) informing RTK/GPCR dualism. IGF-IR is annotated with numbered aa residues. Known key residues/sites of posttranslational modifications (PTMs) as determinant substrates/adaptor protein binding within β-subunit, thus controlling both canonical RTK signaling (left) and noncanonical GPCR-like signaling. IGF-IR kinase-dependent signaling pathways: IGF-I (or IGF-II) binding to IGF-IR promotes intrinsic tyrosine kinase activity and autophosphorylation. Activated receptor can recruit and phosphorylate substrates such as IRS and Shc. Phosphorylated tyrosine 950 within Asn-Pro-X-Tyr juxta membrane motif is essential for insulin receptor substrate-1 (IRS1)/IRS-2 and SHC-transforming protein (Shc) recruitment. Tyrosine phosphorylation of IRS and Shc proteins leads to as Grb2 and PI 3-kinase (p110/p85) binding. These protein associations induce downstream signaling activation, primarily through the rat sarcoma virus/Raf/MAPK and PI3K/Akt pathways, which in turn activate transcription factors coordinating downstream IGF biological effects. In addition to kinase signaling, agonist-induced IGF-IR stimulation results in noncanonical GPCR signaling through heterotrimeric G proteins (α, β, γ) (? = not yet fully understood pathways), followed by rapid IGF-IR phosphorylation by G protein-coupled receptor kinases (GRKs, K2, K6) at serine residues within the C-terminus. Serine-phosphorylated receptors present high-affinity binding sites for multifunctional adaptor protein β-arrestin 1/2 (βarr1/2). βarr recruitment: βarr acquires an active conformation following IGF-IR binding with MAPK pathway scaffold components. These events result in second wave IGF-IR kinase-dependent MAPK/ERK signaling activation. Abbreviations: Akt, protein kinase B; ERK, extracellular-signal-regulated kinase; IGF-IR, IGF receptor; P = major phosphorylation sites; IRS, insulin receptor substrate; MEK, mitogen-activated protein kinase/Erk kinase; Shc, Src homology and collagen domain protein.
Because high resolution of full-length IGF-IR has yet to be accomplished, the complete mechanism through which ligand-receptor interactions facilitate receptor signaling remains poorly understood. This includes a granular understanding of how responses are mediated through conformational changes conveyed from extracellular alpha-domains to intracellular domains and consequent tyrosine phosphorylation.
Following kinase activation and structural rearrangements, several tyrosine residues are phosphorylated, creating docking sites for downstream signaling proteins (53). For each IGF-IR monomer, 8 tyrosine phosphorylation sites have been identified: Y943, Y950, Y1131, Y1135, Y1136, 1250, 1251, and Y1346 (Fig. 2). Because multiple partners compete for each binding site, pathway specificity may be orchestrated through relative signaling protein expression levels (53).
Once bound to phosphorylated tyrosine, signal transduction molecules direct activated receptor to multiple, specific downstream pathways, including MAPK and PI3K (54, 55) (Fig. 2). Bulky docking proteins bound to the juxta membrane Asn-Pro-X-Tyr 950 motif undergo C-terminal region tyrosine-phosphorylation (52, 56). These are recognized by the signaling molecule Grb2 through its SH2 domain (57). In turn, Grb2 complexes with SOS, resulting in Ras activation through GDP/GTP exchange. This in turn generates phosphorylation-dependent, domino activation of MAPK components, including RAF, MEK, and ERK. The ultimate effectors, namely, phosphorylation-activated ERKs, undergo nuclear translocation, where they modify gene expression involved in cell growth and proliferation (58).
The second key IGF-IR signaling pathway, initiated by p85 regulatory subunit of PI3K recruitment to phosphorylated receptors via IRS (Fig. 1), generates phosphatidylinositol 3,4,5-trisphosphate (56). This allows phosphoinositide-dependent kinase-1 to phosphorylate and activate Akt (59). Through its phosphorylation of several substrates, including mammalian target of rapamycin, glycogen synthase kinase 3β, FOXO, and Bcl-2, Akt regulates protein synthesis, glucose metabolism, and cell survival (60).
Noncanonical Signaling: IGF-IR Uses GPCR Components
IGF-IR Uses G Proteins
Although G protein signaling has received much attention and is well-established downstream from GPCRs, it has become increasingly evident that heterotrimeric G proteins also mediate signaling from several receptor tyrosine kinases (RTKs) (61, 62). Among the first RTKs described to engage G proteins were IR and IGF-IR, the downstream MAPK signals sensitive to pertussis toxin, a prototypical Gi inhibitor (63). IGF-IR constitutively binds Gi and Gβ (64) and its signaling is dependent on Gβγ (24) (Fig. 2).
IGF-IR Uses GRK/β-arrestin System
All GRK family members are serine/threonine kinases facilitating receptor-arrestin associations. Initially discovered for its role in controlling GPCR signaling, internalization, and trafficking, the GRK/arrestin system is now considered a key regulator of RTK expression and function (23, 52, 62, 65).
Among RTKs, IGF-IR is fully characterized regarding its GRK/β-arrestin engagement (23, 52, 62). Individual GRKs and β-arrestin isoforms can exhibit similar or opposing functions with other family members. GRK 5/6 inhibition abolishes IGF-I-mediated signaling and receptor degradation whereas GRK 2/3 inhibition increases ERK activity while enhancing receptor degradation (66, 67). These effects are mediated via serine phosphorylation and β-arrestin recruitment to IGF-IR (67, 68). Both β-arrestin isoforms coimmunoprecipitate with IGF-IR and control receptor recycling. Ligand occupancy increases IGF-IR affinity for β-arrestin 1, leading to stable complexes that internalize and sustain MAPK/ERK signaling even when localized within endosomes (68-70) (Fig. 2). In contrast, β-arrestin 2 has greater affinity for unligated receptor; this interaction is transient and can trigger receptor ubiquitination and degradation, but fails to activate signaling (68-71) (Fig. 2). In addition, β-arrestin 2 protects the receptor against ligand-dependent degradation, and attenuates β-arrestin 1 signaling (72, 73). Based on receptor/β-arrestin interaction stability, functional GRK2/β-arrestin 2 and GRK6/β-arrestin 1 partnerships have been proposed. GRK2/β-arrestin 2 pairing occurs at low ligand concentrations, has transient signaling effects maintaining receptor homeostasis, and prevents GRK6/β-arrestin 1 complex formation. GRK6/β-arrestin 1 represents a very powerful activator of IGF-IR-mediated β-arrestin 1 signaling and promotes cell proliferation and survival (70-72, 74). This type of signaling is triggered by IGF-IR, not only under conditions of abundant natural ligand availability (66), but also during oncogenic IGF-IR hyperactivation (eg, by Mdm2). It can occur with noncanonical ligands, such as IGF-IR-targeting therapeutic antibodies and LL-37 (59, 65, 67, 70-72, 74, 75). GRK6/β-arrestin 1 signaling downstream IGF-IR not only activates robust MAPK/AKT survival pathways but also inhibits tumor suppressor p53 signaling; however, this occurs at the cost of substantial IGF-IR internalization and degradation (Fig. 2) (59, 65, 67, 70-72, 74)
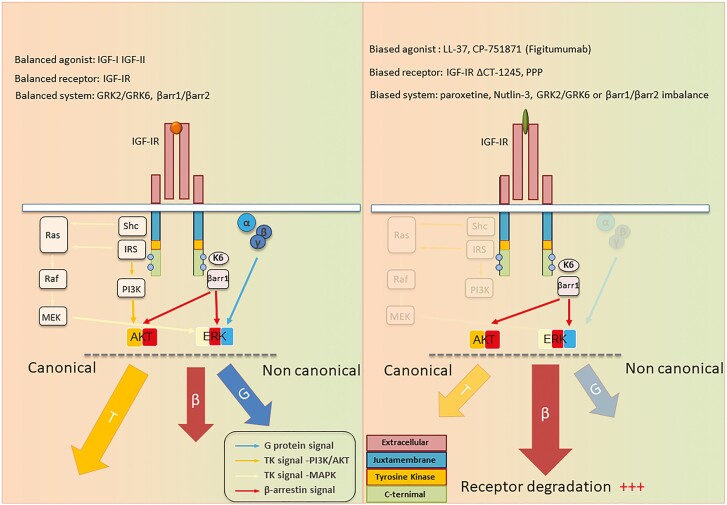
Recognition of multiple signaling pathways downstream IGF-IR, including those associated with G proteins and GRK/β-arrestins, provides a plausible mechanistic explanation for the apparent paradox underlying intrinsic receptor kinase-disengaged signaling. In this scenario, kinase-independent receptor downregulation by the anti-IGF-IR targeting antibody, CP (67) or kinase-independent signaling activation (68), becomes integral to functional selectivity (biased signaling [BS]) of IGF-IR (52, 59, 65, 67, 70-72, 74) (Fig. 3).
Figure 3.
Balanced vs biased IGF-IR signaling. IGF-I (balanced agonist) binding to IGF-IR within an unbiased system (GRKs and β-arrestin [βarr] balance) exhibits equivalent potencies for all signaling pathways, tyrosine kinase (T), βarr signaling (β), and G-protein signaling (G). Cell surface IGF-IR levels are preserved by GRK2/βarr2 (receptor recycling) and balanced by GRK6/βarr 1 (receptor degradation). Functional selectivity (biased signaling toward specific pathways) could be achieved by biased ligands (eg, anti-IGF-IR antibodies) or biased receptors (eg, TK inhibitors). System bias may be achieved through differential expression/inhibition of signaling effectors or cofactors, such as GRK and/or βarr isoforms. Demonstrated here is a model based on βarr biased signaling activation by anti-IGF-IR antibodies (eg, figitumumab), resulting in IGF-IR downregulation with cancer-protective βarr-ERK biased signaling activation.
IGF-IR Biased Signaling
BS (functional selectivity, allosteric modulation) is defined as the capacity of a receptor/ligand complex to preferentially activate a particular downstream signaling pathway accessible to that receptor while not initiating signaling through others. Because of receptor/ligand pairing, BS could be initiated by biased ligands (agonists that preferentially activate certain signaling pathways), biased receptors (specific modifications of the receptor/transducer interface), or system bias (relative expression levels of signal transducers) (Fig. 3).
Studies identifying GRK/β-arrestin as a signaling microprocessor complex downstream from IGF-IR reveals a remarkable characteristic for this prototypical RTK. Namely, β-arrestin not only triggers IGF-IR internalization, but also initiates its own second signaling wave mediated through MAPK/ERK. This β-arrestin-mediated ERK signaling, similar to the β-arrestin role in GPCR signaling, is independent of intrinsic IGF-IR kinase signal activation. Importantly, it may occur when the tyrosine kinase domain is inhibited (76) or following receptor interactions with kinase-inhibitory ligands such as anti-IGF-IR antibodies (67). Just as G-protein signaling may be unbalanced, so too might downstream IGF-IR signaling be unbalanced and kinase-, β arrestin-, or G protein-biased (Fig. 3). Kinase-BS was demonstrated with biased receptors (mutant IGF-IRs). These proteins are incapable of binding β-arrestins (77). In contrast, β arrestin-BS can occur with biased ligands (eg, LL-37, antibodies targeting IGF-IR), biased receptors or as a consequence of system bias.
IGF-IR Bioactivities
The IGF System and Immune Function
IGF-I and its extensive pathways regulate immune function (reviewed in Smith and Janssen (13, 78)). Professional immune cells express IGF-I, IGF-IR, and IGFBPs. Further, IGF-I may stimulate their proliferation and modulate humoral and cellular immune functions. These regulatory effects include B-cell immunoglobulin secretion, thymulin expression/release from thymic epithelial cells, natural killer cell activity, and neutrophil/macrophage oxidative burst and killing capacity (79). IGF-I can expand peripheral circulating and lymphoid organ residing T- and B-cell populations, increasing their biological impact. It enhances antibody responses (80), and IL-10 and IL-4 generation. IL-10 typically exhibits anti-inflammatory actions; thus, IGF-I might inhibit Th1-mediated responses through production of the cytokine in T cells (81). Myeloid cells, including monocytes and macrophages, generate higher IGF-I levels than do peripheral lymphocytes (80). Bone marrow stromal cells and thymic epithelial cells also generate IGF-I (80). Autocrine/paracrine-stimulated IGF-I release occurs immediately following T-cell activation, causing IGF-IR down-regulation (82), coincident with receptor activation. IGF-I in culture medium increases IGF-IR internalization and phosphorylation, resulting in MAPK phosphorylation. Consequently, IL-2 and CD25 synthesis is upregulated (82). TNF-α, colony-stimulating factors, and prostaglandin E2 all induce IGF-I production in macrophages through separate pathways (80), whereas interferon-γ attenuates its expression (80).
Implication of IGF-IR and Anti-IGF-IR Autoantibodies in TED
Close association between the thyroid gland and IGF-I was identified several decades ago when Ingbar and colleagues described enhancement of TSH action by IGF-I and insulin (83). An additional twist was added by Weightman et al., who reported the IGF-I displacing ability of GD-IgG from fibroblast surface binding sites (84). Pritchard et al. ultimately established the binding site identity as IGF-IR by using the specific IGF-I analogue, Des 1-3 (85). Subsequently, Pritchard et al. found that GD-IgGs can induce chemokines in TED orbital fibroblasts (85, 86). Their studies further demonstrated that GD-IgG could mimic the actions of IGF-I , whereas rhTSH failed to do so (85). Those binding sites had an apparent dissociation constant of 0.5 nM, which is consistent with earlier studies of IGF-IR binding characteristics (85). In contrast, IgG from healthy controls failed to compete with IGF-I binding to these cells. This same group then reported that IGF-IR and TSHR form a physical and functional signaling complex and that inhibiting IGF-IR attenuates signaling initiated from both receptors (36). Thus, several clues suggest IGF-IR involvement in GD and TED, providing the rationale for considering this pathway as a potential therapeutic target. The existence of autoantibodies directed at IGF-IR, and especially those with IGF-IR-activating properties, as distinct from TSHR antibodies, has led to contentious debate (87, 88). Some studies have demonstrated IGF-IR antibodies, whereas others have failed to detect them (89-92). Use of substantially different assays for detecting IGF-IR autoantibodies and lack of experimental standardization may underlie these divergent findings. In addition, these studies examined only effects of GD-IgGs on intrinsic IGF-IR tyrosine phosphorylation. As discussed previously, alternative signaling pathways downstream of IGF-IR use G proteins and GRK/β-arrestins in a kinase-independent manner. Further, IGF-IR antibodies have been detected in a mouse model of GD manifesting some features of TED (93). That model involved the immunization of mice with TSHR A subunit encoding plasmids. At the heart of the debate over IGF-IR autoantibodies is whether all GD-IgG activities can correctly be attributed to those targeting TSHR. Speaking against that possibility, Pritchard et al. demonstrated identical IGF-IR signal-initiating activities in sera and IgGs from patients with rheumatoid arthritis but not manifesting GD or TED (94). These findings in aggregate in 2010 provided a plausible predicate for empirically determining whether inhibiting IGF-IR represented a potential therapy for TED. Two placebo-controlled, double-masked trials of teprotumumab, an IGF-IR-blocking monoclonal antibody, were conducted in multiple centers across North America and Europe (95, 96). Both studies demonstrated that the drug could effectively reduce both activity and severity of TED. Importantly, 2 impactful manifestations of TED, proptosis and diplopia, which are not reliably improved with any other medical therapies, responded dramatically to teprotumumab. The magnitude of response of proptosis was equivalent to the best surgical results thus far reported. In our view, these remarkable therapeutic responses constitute the most unambiguous evidence supporting the important role of IGF-IR in the pathogenesis of TED.
What Is the Basis for Teprotumumab Effectiveness in TED?
IGF-I signaling pathways promote and sustain the malignant cellular phenotype. Thus, potential for therapeutically targeting IGF-IR in cancer has been extensively investigated (51, 97, 98). To that end, multiple IGF-IR inhibitors (eg, IGF-IR blocking antibodies and kinase inhibitors) were developed for clinical testing against a variety of neoplasms. To date, none of these drug candidates demonstrated convincing effectiveness in multiple clinical trials. As a consequence, the potential therapeutic value of IGF-IR inhibition for cancer therapy remains widely debated. On the other hand, the clinical success of IGF-IR inhibition in TED by teprotumumab both in formal, placebo-controlled clinical trials and in widespread use in North America has reinvigorated interest in therapeutic targeting IGF-IR.
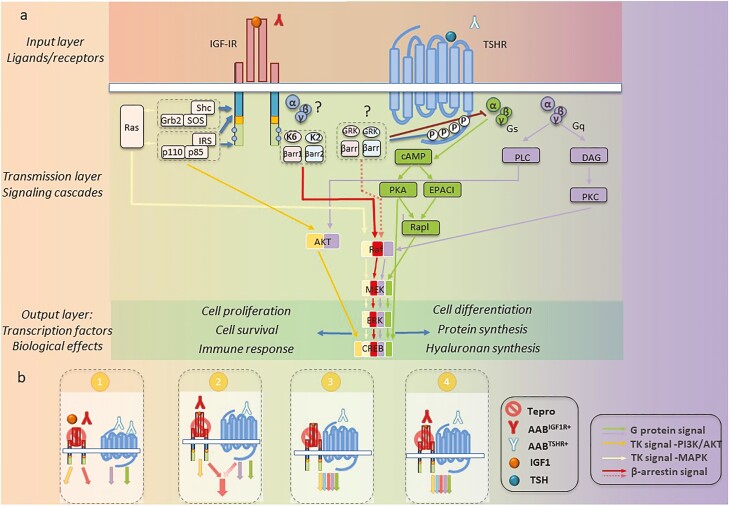
Although teprotumumab has proven effective and relatively safe in the treatment of TED, long-term observations, beyond those thus far reported (99) are currently unavailable. Many questions pertaining to IGF-IR, its relationship with TSHR, and how the drug might be disrupting this protein/protein interaction remain to be answered (Fig. 4).
Figure 4.
Modeling proposed for teprotumumab mechanism of action in TED. (A) IGF-IR/TSHR system can be dissected into 3 distinct layers. The input layer comprises ligands (canonical or autoantibodies) and surface receptors. Following stimulation, signal transmission within second layer is controlled by a variety of messenger proteins. Confluence of signaling cascade components results in transcription factor activation within output layer. These factors control site-specific transcription and generate distinct biological effects. (B) Potential alternative signaling in TED, based on different mechanisms of IGF-IR activation. (1) Independent model: TSHR and IGF-IR function as separate entities. IGF-IR and TSHR activated as indicated with independent downstream signaling. Teprotumumab effects result entirely from IGF-IR inhibition. (2) Codependent IGF-IR signaling: IGF-IR activated by AABIGF-IR ligation. Biological effects are codependent on crosstalk between IGF-IR and TSHR downstream signaling pathways. Teprotumumab effects result from both IGF-IR and codependent TSHR inhibition. (3) Codependent TSHR signaling: AABTSHR activates TSHR, which in turn transactivates IGF-IR. Downstream IGF-IR/TSHR signaling pathways overlap extensively and are simultaneously triggered by activation of both receptor proteins. In this scenario, teprotumumab-engaged IGF-IR fails to become transactivated, whereas TSHR signaling remains unaffected. (4) Codependent IGF-IR/TSHR signaling: ABBTSHR engages TSHR, whereas IGF-IR becomes ligated with ABBIGF-IR. Both receptors are activated, generating protein crosstalk at both first and second layers. Teprotumumab would affect both IGF-IR activation as well as the receptor/signaling crosstalk.
Both receptor pathways need to be dissected at three distinct mechanistic layers. The input layer, represents each receptor protein and its respective ligands, including canonical (eg, IGF-I, TSH) and noncanonical (eg, autoantibodies directed against TSHR [AABTSHR] and IGF-IR [AABIGF-IR]). For both TSHR and IGF-IR, these antibodies can be stimulatory (+), neutral, and inhibitory (-). Within the second mechanistic layer, G proteins and GRK/β-arrestin signaling cascades are triggered by TSHR, IGF-IR, or both, with the latter additionally activating the tyrosine kinase-dependent pathways. Various downstream signaling pathways ultimately activate specific transcription factors, generating a narrowly regulated biological effect within the response layer. Fundamental to this paradigm is acceptance that TED pathogenesis involves stimulatory AABTSHR+ representing the dominant pathogenic TSHR ligand. In contrast, the mechanism through which IGF-IR activation occurs remains debated. Canonical ligands, stimulatory AABIGF-IR and TSHR-dependent transactivation remain viable mechanistic possibilities, based on the known mechanisms of IGF-IR activation. Several models involved in IGF-IR activation deserve consideration:
(1) An independent model involves TSHR and IGF-IR functioning as separate entities. In this scenario, TED results from canonical IGF-IR activation by systemic or locally generated IGF-I or IGF-II. Within this model, stimulatory AABIGF-IR could be proposed as potential IGF-IR signaling activators.
(2) Codependent IGF-IR signaling model in which IGF-IR activation results from stimulatory AABIGF-IR ligation and resulting biological effects are also dependent on crosstalk at the second layer of the signaling pathways downstream from IGF-IR and TSHR.
(3) Codependent TSHR signaling model in which unligated IGF-IR becomes transactivated by TSHR. This scenario excludes the possibility of IGF-IR activation by AABIGF-IR and localizes IGF-IR/TSHR codependency to the first layer where: AABTSHR activates TSHR, which in turn transactivates IGF-IR. In this scenario, partial IGF-IR activation could result in substantially overlapping downstream signaling pathways triggered by the IGF-IR/TSHR complex. In this model, teprotumumab-engaged IGF-IR cannot be transactivated.
(4) Codependent IGF-IR/TSHR signaling model in which both ligated IGF-IR and TSHR interact at the first and second layer. In this scenario, TSHR is engaged with ABBTSHR, whereas IGF-IR becomes ligated with ABBIGF-IR. In this scenario, autoantibody engagement of each receptor results in distinct signaling properties, each providing a necessary component(s) of cellular response (not provided by the other receptor pathway).
These 4 theoretical models appear congruent with the clinical responses to teprotumumab; however, only scenarios embracing the role of AABIGF-IR can fully explain the previous observations that (1) GD-IgG competes with IGF-I binding to IGF-IR and (2) IgG from patients with rheumatoid arthritis initiate IGF-IR signaling (94). The IGF-IR codependent models (ie, scenarios 2 and 4) appear fully supported by experimental data demonstrating impaired TSHR signaling followed IGF-IR inhibition. These IGF-IR codependent models could explain why TED responds to teprotumumab in conditions of TSHR/IGF-IR codependency. Within these proposed mechanisms, an AABIGF-IR/IGF-1R interaction is anticipated to induce conformational changes that could be selectively prevented by therapeutic IGF-IR-targeting antibodies. As such, the therapeutic responses to teprotumumab might prove antibody and/or patient specific.
Concluding Remarks
Expanding insights into TED pathogenesis have yielded unprecedented opportunities for better treating the disease. Our current understanding of the central role played by CD34+ fibrocytes suggests that these cells and their putative derivatives, CD34+ orbital fibroblasts, can be therapeutically targeted. Fibrocytes display functional TSHR/IGF-IR signaling complexes, through which these cells can be activated to express disease-consequential genes. The specific pathogenic roles played by autoantibodies against TSHR and IGF-IR remain to be disentangled as do the physical protein-protein interactions between the 2 receptors. The GRK/arrestin system downstream from TSHR also remains incompletely understood. Consequently, the nature of TSHR/IGF-IR crosstalk must be elucidated. The distinction between canonical, tyrosine kinase-dependent, and noncanonical, tyrosine kinase-independent signaling represents an evolving concept, the dimensions of which may have proximate relevance to TED. Although the mechanisms underpinning its actions remain to be unambiguously resolved, the monoclonal IGF-IR inhibitor teprotumumab improves the activity and severity of TED, in large part by reducing inflammation, proptosis, and diplopia. Teprotumumab use is associated with several adverse event, including hyperglycemia and hearing abnormalities; thus, best medical practices must be followed in patients undergoing treatment with this drug. The advent of targeted therapies for TED represents the initial migration toward developing strategies for retolerizing the immune system to relevant autoantigens, including TSHR and IGF-IR.
Acknowledgments
The authors thank Dana Barnhart for excellent help in preparing the manuscript.
Glossary
Abbreviations
- AABIGF-IR
antibodies directed against IGF-IR
- AABTSHR
antibodies directed against TSHR
- Akt
protein kinase B
- bTSH
bovine thyroid-stimulating hormone
- BS
biased signaling
- ERK
extracellular signal-regulated kinase
- GD
Graves’ disease
- GPCR
G-protein-coupled receptor
- GRK
G-protein receptor kinase
- HA
hyaluronan
- IGF-I
IGF-I
- IGF-II
IGF-II
- IGFBP
IGF binding proteins
- IGF-IR
IGF-I receptor
- IR
insulin receptor
- IRS-1/2
insulin receptor substrate 1/2
- MEK
mitogen-activated protein kinase kinase
- PI3K
phosphatidylinositol 3-kinase
- RAI
radioactive iodine
- rhTSH
recombinant human thyroid-stimulating hormone
- RTK
receptor tyrosine kinases
- SHC
SHC-transforming protein
- TED
thyroid eye disease
- TRAb
TSH receptor autoantibody
- TSHR
TSH receptor
Contributor Information
Leonard Girnita, Department of Oncology and Pathology, BioClinicum, Karolinska Institutet and Karolinska University Hospital, 17164 Stockholm, Sweden.
Terry J Smith, Kellogg Eye Center, Department of Ophthalmology and Visual Sciences, University of Michigan Medical School, Ann Arbor, MI 48105, USA; Division of Metabolism, Endocrinology, and Diabetes, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI 48105, USA.
Joseph A M J L Janssen, Erasmus Medical Center, Department of Internal Medicine, Division of Endocrinology, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
Funding
Research support was received from the Swedish Research Council #2021-01247, Swedish Cancer Society grants CAN 2017/1103 and 20 0989 PjF, the Swedish Childhood Cancer Foundation, grant PR2020-0123 Crown Princess Margareta’s Foundation for the Visually Impaired, Welander Finsen Foundation, King Gustaf V Jubilee Foundation, Stockholm Cancer Society, Stockholm County and Karolinska Institute, National Institutes of Health grant EY008976, and National Eye Institute core grant EY007003. Schematics were created in part with BioRender.com.
Disclosures
T.J.S. has been issued patents covering his inventions concerning the use of IGF-IR inhibitors as therapy in Graves’ disease and other autoimmune diseases. These patents are held by UCLA School of Medicine and Los Angeles Biomedical Research/Lundquist Institute. He is a paid consultant for Horizon Therapeutics and Veridian. L.G. and J.A.M.J.L.J. have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Watt T, Cramon P, Hegedüs L, et al. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J Clin Endocrinol Metab. 2014;99(10):3708-3717. [DOI] [PubMed] [Google Scholar]
- 2. Bartalena L, Kahaly GJ, Baldeschi L, et al. ; EUGOGO † . The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43-G67. [DOI] [PubMed] [Google Scholar]
- 3. Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552-1565. [DOI] [PubMed] [Google Scholar]
- 4. Chin YH, Ng CH, Lee MH, et al. Prevalence of thyroid eye disease in Graves’ disease: a meta-analysis and systematic review. Clin Endocrinol (Oxf). 2020;93(4):363-374. [DOI] [PubMed] [Google Scholar]
- 5. Leo M, Menconi F, Rocchi R, et al. Role of the underlying thyroid disease on the phenotype of Graves’ orbitopathy in a tertiary referral center. Thyroid. 2015;25(3):347-351. [DOI] [PubMed] [Google Scholar]
- 6. Bartalena L, Martino E, Marcocci C, et al. More on smoking habits and Graves’ ophthalmopathy. J Endocrinol Invest. 1989;12(10):733-737. [DOI] [PubMed] [Google Scholar]
- 7. Cao J, Su Y, Chen Z, Ma C, Xiong W. The risk factors for Graves’ ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2021. doi: 10.1007/s00417-021-05456-x. [DOI] [PubMed] [Google Scholar]
- 8. Mack WP, Stasior GO, Cao HJ, Stasior OG, Smith TJ. The effect of cigarette smoke constituents on the expression of HLA-DR in orbital fibroblasts derived from patients with Graves ophthalmopathy. Ophthalmic Plast Reconstr Surg. 1999;15(4):260-271. [DOI] [PubMed] [Google Scholar]
- 9. Kung AW, Yau CC, Cheng A. The incidence of ophthalmopathy after radioiodine therapy for Graves’ disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994;79(2):542-546. [DOI] [PubMed] [Google Scholar]
- 10. Lai A, Sassi L, Compri E, et al. Lower dose prednisone prevents radioiodine-associated exacerbation of initially mild or absent graves’ orbitopathy: a retrospective cohort study. J Clin Endocrinol Metab. 2010;95(3):1333-1337. [DOI] [PubMed] [Google Scholar]
- 11. Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5(3-4):177-194. [PubMed] [Google Scholar]
- 12. Smith TJ. Potential roles of CD34+ fibrocytes masquerading as orbital fibroblasts in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2019;104(2):581-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernando R, Caldera O, Smith TJ. Therapeutic IGF-I receptor inhibition alters fibrocyte immune phenotype in thyroid-associated ophthalmopathy. Proc Natl Acad Sci USA. 2021;118(52):e2114244118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1735-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Carli M, D’Elios MM, Mariotti S, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1993;77(5):1120-1124. [DOI] [PubMed] [Google Scholar]
- 16. Syrovatkina V, Alegre KO, Dey R, Huang XY. Regulation, signaling, and physiological functions of G-proteins. J Mol Biol. 2016;428(19):3850-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63(6):1256-1272. [DOI] [PubMed] [Google Scholar]
- 18. Morshed SA, Latif R, Davies TF. Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology. 2009;150(1):519-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleinau G, Biebermann H. Constitutive activities in the thyrotropin receptor: regulation and significance. Adv Pharmacol. 2014;70:81-119. [DOI] [PubMed] [Google Scholar]
- 20. Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17(4):159-165. [DOI] [PubMed] [Google Scholar]
- 21. Nobles KN, Xiao K, Ahn S, et al. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4(185):ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith JS, Lefkowitz RJ, Rajagopal S. Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov. 2018;17(4):243-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crudden C, Shibano T, Song D, Suleymanova N, Girnita A, Girnita L. Blurring boundaries: receptor tyrosine kinases as functional G protein-coupled receptors. Int Rev Cell Mol Biol. 2018;339:1-40. [DOI] [PubMed] [Google Scholar]
- 24. Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Touhara K, Lefkowitz RJ. G beta gamma subunits mediate mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J Biol Chem. 1995;270(28):16495-16498. [DOI] [PubMed] [Google Scholar]
- 25. Penela P. Chapter three - ubiquitination and protein turnover of G-protein-coupled receptor kinases in GPCR signaling and cellular regulation. Prog Mol Biol Transl Sci. 2016;141:85-140. [DOI] [PubMed] [Google Scholar]
- 26. Gurevich VV, Gurevich EV. GPCR signaling regulation: the role of GRKs and arrestins. Front Pharmacol. 2019;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagayama Y, Tanaka K, Namba H, Yamashita S, Niwa M. Expression and regulation of G protein-coupled receptor kinase 5 and beta-arrestin-1 in rat thyroid FRTL5 cells. Thyroid. 1996;6(6):627-631. [DOI] [PubMed] [Google Scholar]
- 28. Iacovelli L, Franchetti R, Masini M, De Blasi A. GRK2 and beta-arrestin 1 as negative regulators of thyrotropin receptor-stimulated response. Mol Endocrinol. 1996;10(9):1138-1146. [DOI] [PubMed] [Google Scholar]
- 29. Frenzel R, Voigt C, Paschke R. The human thyrotropin receptor is predominantly internalized by beta-arrestin 2. Endocrinology. 2006;147(6):3114-3122. [DOI] [PubMed] [Google Scholar]
- 30. Calebiro D, Nikolaev VO, Gagliani MC, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7(8):e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boutin A, Eliseeva E, Gershengorn MC, Neumann S. β-Arrestin-1 mediates thyrotropin-enhanced osteoblast differentiation. FASEB J. 2014;28(8):3446-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iyer S, Bahn R. Immunopathogenesis of Graves’ ophthalmopathy: the role of the TSH receptor. Best Pract Res Clin Endocrinol Metab. 2012;26(3):281-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89(10):5076-5080. [DOI] [PubMed] [Google Scholar]
- 34. van Zeijl CJ, Fliers E, van Koppen CJ, et al. Effects of thyrotropin and thyrotropin-receptor-stimulating Graves’ disease immunoglobulin G on cyclic adenosine monophosphate and hyaluronan production in nondifferentiated orbital fibroblasts of Graves’ ophthalmopathy patients. Thyroid. 2010;20(5):535-544. [DOI] [PubMed] [Google Scholar]
- 35. Kumar S, Iyer S, Bauer H, Coenen M, Bahn RS. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in Graves’ orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. J Clin Endocrinol Metab. 2012;97(5):1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang L, Bowen T, Grennan-Jones F, et al. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem. 2009;284(39):26447-26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodriguez-Arnao J, Miell JP, Ross RJ. Influence of thyroid hormones on the GH-IGF-I axis. Trends Endocrinol Metab. 1993;4(5):169-173. [DOI] [PubMed] [Google Scholar]
- 39. Brenner-Gati L, Berg KA, Gershengorn MC. Thyroid-stimulating hormone and insulin-like growth factor-1 synergize to elevate 1,2-diacylglycerol in rat thyroid cells. Stimulation of DNA synthesis via interaction between lipid and adenylyl cyclase signal transduction systems. J Clin Invest. 1988;82(3):1144-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fukushima T, Yoshihara H, Furuta H, et al. Nedd4-induced monoubiquitination of IRS-2 enhances IGF signalling and mitogenic activity. Nat Commun. 2015;6:6780. [DOI] [PubMed] [Google Scholar]
- 41. Sastre-Perona A, Santisteban P. Wnt-independent role of β-catenin in thyroid cell proliferation and differentiation. Mol Endocrinol. 2014;28(5):681-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atkins SJ, Lentz SI, Fernando R, Smith TJ. Disrupted TSH receptor expression in female mouse lung fibroblasts alters subcellular IGF-1 receptor distribution. Endocrinology. 2015;156(12):4731-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clément S, Refetoff S, Robaye B, Dumont JE, Schurmans S. Low TSH requirement and goiter in transgenic mice overexpressing IGF-I and IGF-Ir receptor in the thyroid gland. Endocrinology. 2001;142(12):5131-5139. [DOI] [PubMed] [Google Scholar]
- 44. Ock S, Ahn J, Lee SH, et al. IGF-1 receptor deficiency in thyrocytes impairs thyroid hormone secretion and completely inhibits TSH-stimulated goiter. FASEB J. 2013;27(12):4899-4908. [DOI] [PubMed] [Google Scholar]
- 45. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3-34. [DOI] [PubMed] [Google Scholar]
- 46. Girnita A, Zheng H, Grönberg A, Girnita L, Ståhle M. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene. 2012;31(3):352-365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Petrenko AG, Zozulya SA, Deyev IE, Eladari D. Insulin receptor-related receptor as an extracellular pH sensor involved in the regulation of acid-base balance. Biochim Biophys Acta. 2013;1834(10):2170-2175. [DOI] [PubMed] [Google Scholar]
- 48. Bailyes EM, Navé BT, Soos MA, Orr SR, Hayward AC, Siddle K. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J. 1997;327 (Pt 1):209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586-623. [DOI] [PubMed] [Google Scholar]
- 50. Holly J, Perks C. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 2006;83(3-4):154-160. [DOI] [PubMed] [Google Scholar]
- 51. Crudden C, Girnita A, Girnita L. Targeting the IGF-1R: the tale of the tortoise and the hare. Front Endocrinol (Lausanne). 2015;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Girnita L, Worrall C, Takahashi S, Seregard S, Girnita A. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci. 2014;71(13):2403-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chattopadhyay A, Vecchi M, Ji Q, Mernaugh R, Carpenter G. The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J Biol Chem. 1999;274(37):26091-26097. [DOI] [PubMed] [Google Scholar]
- 54. Craparo A, O’Neill TJ, Gustafson TA. Non-SH2 domains within insulin receptor substrate-1 and SHC mediate their phosphotyrosine-dependent interaction with the NPEY motif of the insulin-like growth factor I receptor. J Biol Chem. 1995;270(26):15639-15643. [DOI] [PubMed] [Google Scholar]
- 55. Fukushima T, Nakamura Y, Yamanaka D, et al. Phosphatidylinositol 3-kinase (PI3K) activity bound to insulin-like growth factor-I (IGF-I) receptor, which is continuously sustained by IGF-I stimulation, is required for IGF-I-induced cell proliferation. J Biol Chem. 2012;287(35):29713-29721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yoshihara H, Fukushima T, Hakuno F, et al. Insulin/insulin-like growth factor (IGF) stimulation abrogates an association between a deubiquitinating enzyme USP7 and insulin receptor substrates (IRSs) followed by proteasomal degradation of IRSs. Biochem Biophys Res Commun. 2012;423(1):122-127. [DOI] [PubMed] [Google Scholar]
- 57. Skolnik EY, Lee CH, Batzer A, et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993;12(5):1929-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9-18. [DOI] [PubMed] [Google Scholar]
- 59. Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15(23):6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 60. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Delcourt N, Bockaert J, Marin P. GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol Sci. 2007;28(12):602-607. [DOI] [PubMed] [Google Scholar]
- 62. Crudden C, Ilic M, Suleymanova N, Worrall C, Girnita A, Girnita L. The dichotomy of the Insulin-like growth factor 1 receptor: RTK and GPCR: friend or foe for cancer treatment? Growth Horm IGF Res. 2015;25(1):2-12. [DOI] [PubMed] [Google Scholar]
- 63. Luttrell LM, Hewlett EL, Romero G, Rogol AD. Pertussis toxin treatment attenuates some effects of insulin in BC3H-1 murine myocytes. J Biol Chem. 1988;263(13):6134-6141. [PubMed] [Google Scholar]
- 64. Hallak H, Seiler AE, Green JS, Ross BN, Rubin R. Association of heterotrimeric G(i) with the insulin-like growth factor-I receptor. Release of G(betagamma) subunits upon receptor activation. J Biol Chem. 2000;275(4):2255-2258. [DOI] [PubMed] [Google Scholar]
- 65. Crudden C, Song D, Cismas S, et al. Below the surface: IGF-1R therapeutic targeting and its endocytic journey. Cells. 2019;8(10): 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng H, Worrall C, Shen H, et al. Selective recruitment of G protein-coupled receptor kinases (GRKs) controls signaling of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA. 2012;109(18):7055-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng H, Shen H, Oprea I, et al. β-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing’s sarcoma. Proc Natl Acad Sci USA. 2012;109(50):20620-20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suleymanova N, Crudden C, Worrall C, Dricu A, Girnita A, Girnita L. Enhanced response of melanoma cells to MEK inhibitors following unbiased IGF-1R down-regulation. Oncotarget. 2017;8(47):82256-82267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Girnita L, Shenoy SK, Sehat B, et al. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem. 2007;282(15):11329-11338. [DOI] [PubMed] [Google Scholar]
- 70. Crudden C, Shibano T, Song D, et al. Inhibition of G protein-coupled receptor Kinase 2 promotes unbiased downregulation of IGF1 receptor and restrains malignant cell growth. Cancer Res. 2021;81(2):501-514. [DOI] [PubMed] [Google Scholar]
- 71. Song D, Cismas S, Crudden C, et al. IGF-1R is a molecular determinant for response to p53 reactivation therapy in conjunctival melanoma. Oncogene. 2022;41(4):600-611. [DOI] [PubMed] [Google Scholar]
- 72. Worrall C, Suleymanova N, Crudden C, et al. Unbalancing p53/Mdm2/IGF-1R axis by Mdm2 activation restrains the IGF-1-dependent invasive phenotype of skin melanoma. Oncogene. 2017;36(23):3274-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Girnita L, Takahashi SI, Crudden C, et al. Chapter seven - when phosphorylation encounters ubiquitination: a balanced perspective on IGF-1R signaling. Prog Mol Biol Transl Sci. 2016;141:277-311. [DOI] [PubMed] [Google Scholar]
- 74. Suleymanova N, Crudden C, Shibano T, et al. Functional antagonism of β-arrestin isoforms balance IGF-1R expression and signalling with distinct cancer-related biological outcomes. Oncogene. 2017;36(41):5734-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Economou MA, Wu J, Vasilcanu D, et al. Inhibition of VEGF secretion and experimental choroidal neovascularization by picropodophyllin (PPP), an inhibitor of the insulin-like growth factor-1 receptor. Invest Ophthalmol Vis Sci. 2008;49(6):2620-2626. [DOI] [PubMed] [Google Scholar]
- 76. Vasilcanu R, Vasilcanu D, Sehat B, et al. Insulin-like growth factor type-I receptor-dependent phosphorylation of extracellular signal-regulated kinase 1/2 but not Akt (protein kinase B) can be induced by picropodophyllin. Mol Pharmacol. 2008;73(3):930-939. [DOI] [PubMed] [Google Scholar]
- 77. Chen B, Dragomir MP, Fabris L, et al. The long noncoding RNA CCAT2 induces chromosomal instability through BOP1-AURKB signaling. Gastroenterology. 2020;159(6):2146-2162.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smith TJ, Janssen JAMJL. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocr Rev. 2019;40(1):236-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Auernhammer CJ, Strasburger CJ. Effects of growth hormone and insulin-like growth factor I on the immune system. Eur J Endocrinol. 1995;133(6):635-645. [DOI] [PubMed] [Google Scholar]
- 80. Clark R. The somatogenic hormones and insulin-like growth factor-1: stimulators of lymphopoiesis and immune function. Endocr Rev. 1997;18(2):157-179. [DOI] [PubMed] [Google Scholar]
- 81. Kooijman R, Coppens A. Insulin-like growth factor-I stimulates IL-10 production in human T cells. J Leukoc Biol. 2004;76(4):862-867. [DOI] [PubMed] [Google Scholar]
- 82. Segretin ME, Galeano A, Roldán A, Schillaci R. Insulin-like growth factor-1 receptor regulation in activated human T lymphocytes. Horm Res. 2003;59(6):276-280. [DOI] [PubMed] [Google Scholar]
- 83. Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves’-IgG. Endocrinology. 1986;119(2):940-942. [DOI] [PubMed] [Google Scholar]
- 84. Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993;16(4):251-257. [DOI] [PubMed] [Google Scholar]
- 85. Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348-6354. [DOI] [PubMed] [Google Scholar]
- 86. Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168(2):942-950. [DOI] [PubMed] [Google Scholar]
- 87. Neumann S, Gershengorn MC. Rebuttal to Smith and Janssen (Thyroid 2017;27:746-747. DOI: 10.1089/thy.2017.0281). Thyroid. 2017;27(11):1459-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smith TJ, Janssen JAMJL. Response to Krieger et al. re: “TSHR/IGF-1R cross-talk, not IGF-1R stimulating antibodies, mediates Graves’ ophthalmopathy pathogenesis” (Thyroid 2017;27:746-747). Thyroid. 2017;27(11):1458-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Minich WB, Dehina N, Welsink T, et al. Autoantibodies to the IGF1 receptor in Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98(2):752-760. [DOI] [PubMed] [Google Scholar]
- 90. Varewijck AJ, Boelen A, Lamberts SW, Fliers E, Hofland LJ, Wiersinga WM, Janssen JA. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2013;98(2):769-776. [DOI] [PubMed] [Google Scholar]
- 91. Lanzolla G, Ricci D, Nicolì F, et al. Putative protective role of autoantibodies against the insulin-like growth factor-1 receptor in Graves’ disease: results of a pilot study. J Endocrinol Invest. 2020;43(12):1759-1768. [DOI] [PubMed] [Google Scholar]
- 92. Marcus-Samuels B, Krieger CC, Boutin A, Kahaly GJ, Neumann S, Gershengorn MC. Evidence that Graves’ ophthalmopathy immunoglobulins do not directly activate IGF-1 receptors. Thyroid. 2018;28(5):650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moshkelgosha S, So PW, Deasy N, Diaz-Cano S, Banga JP. Cutting edge: retrobulbar inflammation, adipogenesis, and acute orbital congestion in a preclinical female mouse model of Graves’ orbitopathy induced by thyrotropin receptor plasmid-in vivo electroporation. Endocrinology. 2013;154(9):3008-3015. [DOI] [PubMed] [Google Scholar]
- 94. Pritchard J, Tsui S, Horst N, Cruikshank WW, Smith TJ. Synovial fibroblasts from patients with rheumatoid arthritis, like fibroblasts from Graves’ disease, express high levels of IL-16 when treated with Igs against insulin-like growth factor-1 receptor. J Immunol. 2004;173(5):3564-3569. [DOI] [PubMed] [Google Scholar]
- 95. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-352. [DOI] [PubMed] [Google Scholar]
- 96. Douglas RS, Kahaly GJ. Teprotumumab for active thyroid eye disease. Reply. N Engl J Med. 2020;382(20):1959-1960. [DOI] [PubMed] [Google Scholar]
- 97. Chen B, Li J, Chi D, et al. Non-coding RNAs in IGF-1R signaling regulation: the underlying pathophysiological link between diabetes and cancer. Cells. 2019;8(12): 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Osher E, Macaulay VM. Therapeutic targeting of the IGF axis. Cells. 2019;8(8): 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X study. Ophthalmology. 2021:S0161-6420(21)00818-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.