Abstract
In December 2019, a new single-stranded RNA coronavirus, SARS-CoV-2, appeared in China and quickly spread around the world leading to a pandemic. Infection with SARS-CoV-2 generates symptoms ranging from asymptomatic to severe, occasionally requiring hospitalization in intensive care units, and, in more severe cases, leading to death. Scientists and researchers around the world have made a real race against time to develop various vaccines to slow down and stop the spread of the virus. In addition to conventional viral vector vaccines, new generation mRNA vaccines, BNT152b2 (Comirnaty) and mRNA-1273 (Spikevax), have been developed respectively by Pfizer/BioNTech and Moderna. These vaccines act on immune cells to induce an immune response with the production of specific antibodies against Spike protein of SARS-CoV-2, and to stimulate the differentiation of T and B memory cells. The objective of this review is to provide a detailed picture of the validity of these new vaccines and the safety of vaccination. Not only was the immunogenic effect of mRNA vaccines evaluated, but also the psychosocial impact they had on the population. The data collected show that this type of vaccine can also be an excellent candidate for future treatment and eradication of possible new pathologies with viral and non-viral etiology.
Keywords: Comirnaty, Spikevax, Immunity, SARS-CoV-2, Vaccination
1. Introduction
Pathologies with viral etiology have always represented a great danger to public health. In recent years several viral diseases have occurred such as Severe Acute Respiratory Syndrome (SARS) in 2003, H1N1 (swine fever) in 2009, and Middle East Respiratory Syndrome (MERS) in 2012 [1]. In December 2019, a newly discovered coronavirus, SARS-CoV-2, began to spread to China from the metropolis of Wuhan, giving rise to a disease known as COVID-19. In a short time, the explosion of contagions forced the World Health Organization (WHO) to proclaim the state of pandemic [2]. Several risk factors such as old age, smoking, and comorbidities may contribute to the increased possibility of infection and the severity of symptoms. Some studies show that lifestyle and eating habits can also affect the severity of symptoms [3]. For example, high alcohol consumption, increased during lockdown periods to cope with a depressive psychological state, plays a role in the worsening of the clinical picture [4]. Symptoms may change from non-existent (asymptomatic) or mild to severe, leading to bilateral interstitial pneumonia and acute respiratory distress syndrome (ARDS) [5]. Globally, 6.249.700 deaths from COVID-19 have been reported by the World Health Organization (WHO) as of May 2022 [6] disrupting social life and the economy, and also causing significant psychological damage. The spread of new strains (Alpha, Beta, Gamma, Delta, Omicron) able to increase the rate of infection and the transmissibility have further aggravated the already unstable social and economic condition, putting even more crisis the health system of the various nations [7]. The Alpha strain (B.1.1.7 lineage) was first identified in the United Kingdom in late 2020 (European Centre for Disease Prevention and Control [8]. Not longer before the Beta variant (B.1.351 lineage), also known as 20H/501Y.V2 has been first identified in South Africa [9]. The Gamma strain (P.1 lineage), also known as 20 J/501Y.V3,was first identified in Japan in December 2020 but mostly widespread in Brazil [10]; simultaneously, the Delta strain (B.1.617.2 lineage), was first identified in India becoming the most prevalent variant worldwide until the emergence of the Omicron variant (2022) [11]. The Omicron strain (B.1.1.529 lineage) was first reported in Botswana, earlier than in South Africa in November 2021 (Omicron (B.1.1.529 lineage). This variant includes BA.1, BA.2, BA.3, BA.4, BA.5 and also recombinant forms such as BA.1/BA.2 (also known as XE) [12].
In addition to licensed drug treatment already undergoing randomized clinical trials (remdesivir, dexamethasone, and bamlanivimab plus etesevimab) [13], [14], [15], many studies have shown that different nutraceuticals may play an important role both in the management of clinical symptoms induced by SARS-CoV-2 infection, showing antioxidant (Zinc, Baicalein), anti-inflammatory (Vitamin B3, B6, B12, D, Eucalyptus EO, Baicalein), immunostimulant activity as well as in boosting the immune system before and after the vaccine (Vitamin C, D) [3], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25].
However, vaccination is the most effective weapon to eradicate this virus and allow the world’s population to return to normal daily life. Over the past decade, vaccines have been responsible for improving life expectancy and the economy. Increasingly available and used vaccines have helped to eradicate completely or for the most part many infectious diseases. The management of patients suffering from viral diseases involves an enormous economic and social expenditure. Carrying out widespread vaccination can lower these costs and increase individual and public health security by aiming at the annihilation of the virus [26].
During the outbreaks of SARS and MERS, studies for the development of several vaccines were conducted. In particular, two of these vaccines, one from Sinovac Biotech ltd and one based on DNA, developed by the National Institute of Allergy and Infectious Disease (NIAID) have been processed to Stage I [27]. Almost all studies of vaccines against SARS-CoV have been abandoned, as the virus has never recurred since 2003. In contrast, nine MERS-CoV vaccines reached clinical stage I/II [28], [29]. The study of these vaccines served as an advanced starting point for the development of 63 candidate vaccines against SARS-CoV-2. Of all vaccines studied, 11 % are mRNA vaccines, of which two, mRNA 1273 Spikevax (developed by Moderna) and BNT162b Comirnaty (developed by Pfizer-BioNTech ltd), were the first to be approved for emergency use in several countries. Of the viral vector vaccines, which accounted for the remaining percentage of vaccines tested, were approved AstraZeneca (ChAdOx1, developed by Jenner Institute of Oxford University), Johnson & Johnson (JNJ-78436734, developed by Janssen Pharmaceutical companies), and Sputnik-V (Gam-COVID-vac, developed by N. F. Gamaleya Federal Research Center for Epidemiology & Microbiology). They contain recombinant non-replicating DNA within adenovirus (AdV) [30], [31], [32]. Both vaccine types aim to code for the SARS-CoV-2 Spike (S) protein to induce a direct immune response against this protein (Fig. 1 ). Other vaccines developed and used around the world include: NVX-CoV2373 (Novavax), a recombinant protein subunit vaccine composed of trimeric spike glycoproteins and a Matrix-M1 adjuvant [33]. CoronaVac (Sinovac), an inactivated COVID-19 vaccine developed in China [34]. Ad5-based COVID-19 vaccine (CanSino Biologics), based on a replication-incompetent adenovirus 5 vector that expresses the spike protein [35];Covaxin (Bharat Biotech/Indian Council of Medical Research) also called BBV152, an inactivated COVID-19 vaccine that was developed and used in India [36];WIV04 and HB02 (Sinopharm) are inactivated whole-virus vaccines based on two different SARS-CoV-2 isolates from patients in China [37]; ZyCoV-D (Zydus Cadila), the first DNA COVID-19 vaccine available and initially authorized in India [38] (Table 1 ). mRNA vaccines have many advantages, unlike conventional vaccines. First of all, mRNA vaccines provide a transcript capable of coding for a specific target antigen, whatever it is [39]. Modifying the efficacy of the vaccine is extremely simple, by modifying the mRNA sequence [40]. This type of vaccine also has intrinsic self-adjuvant abilities capable of inducing important and prolonged adaptive immune responses through tumor necrosis factor (TNF), interferon (IFN), and various cytokines secreted by the immune cell system [41]. Importantly, mRNA vaccines resolve in the cytoplasm and are transient molecules that are rapidly degraded after injection [42]. They are more biosecure than DNA-based vaccines because they do not penetrate the nucleus and thus cannot integrate with the host genome [43]. The same mRNA can be recognized by antigen presenting cells (APCs), inducing the activation of pattern recognition receptors (PRRs), such as Toll-like receptors (TLR)-3, TLR-7, TLR-8 [44]. TLRs are evolutionarily conserved receptors that play a key role in the modulation of the immune response [1], [45], [46], [47], [48], [49], [50], [51], [52], [53], [148]. Unfortunately, storage and transport of this type of vaccine require very low temperatures, but with the integration of lipid nanoparticles, mRNA vaccines are more stable and resistant to less rigid temperatures [54].
Fig. 1.
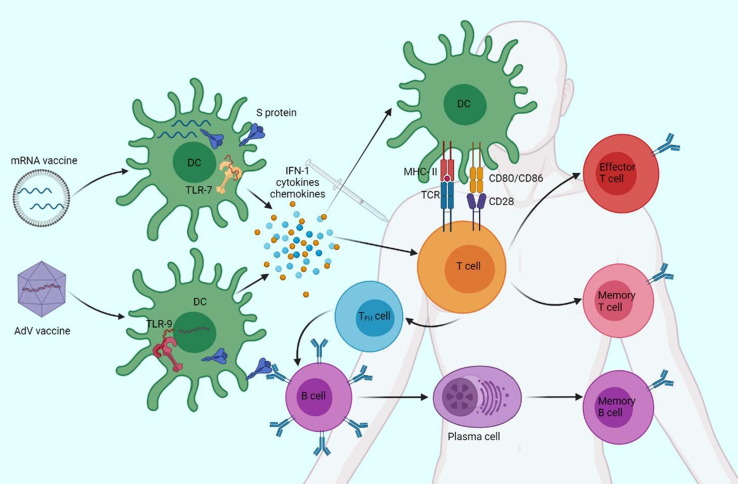
Vaccines and immune system interaction. Vaccines either penetrate the DCs at the injection site or reach the lymph nodes, producing the S protein. In addition, innate immunity cells are activated by the vaccine’s intrinsic adjuvant power, leading to rapid secretion of IFN-1 and pro-inflammatory cytokines. TLRs, in particular, TLR-7 are activated by mRNA vaccines, while TLR-9 is the predominant receptor for AdV vaccines. DCs present the S-protein to naive T cells, through the binding of T cell receptor (TCR) to MHC-II and binding of the CD80/86 and CD28 receptors, which are thus stimulated to differentiate into cytotoxic T lymphocytes and helper T lymphocytes. Follicular T-h cells (TFH) induce B cells to differentiate into plasma cells, producing anti-S antibodies. T cells and memory B cells then come into play to prevent future SARS-CoV-2 infection. Created with BioRender.com.
Table 1.
Summary chart of vaccine against COVID-19, with way of functioning.
| Type | Developer | Way of Functioning | |
|---|---|---|---|
| BNT162b2 | mRNA vaccine | Pfizer-BioNTech | The mRNA vaccine is delivered in a lipid nanoparticle to express a full-length spike protein |
| mRNA-1273 | mRNA vaccine | Moderna | The mRNA vaccine is delivered in a lipid nanoparticle to express a full-length spike protein |
| ChAdOx1 nCoV-19/AZD1222 | Replication-incompetent vector vaccine | University of Oxford, AstraZeneca, the Serum Institute of India | A replication-incompetent chimpanzee adenovirus vector that expresses the spike protein |
| Ad26.COV2.S | Replication-incompetent vector vaccine | Janssen/Johnson & Johnson | A replication-incompetent adenovirus 26 vector that encodes a stabilized spike protein |
| Gam-COVID-Vac/Sputnik V | Replication-incompetent vector vaccine | Gamaleya Institute | Two replication-incompetent adenovirus vectors that express a full-length spike glycoprotein |
| NVX-CoV2373 | Recombinant protein vaccine | Novavax | Recombinant protein subunit vaccine composed of trimeric spike glycoproteins and a potent Matrix-M1 adjuvant |
| CoronaVac | Inactivated vaccine | Sinovac | Produced by growing SARS-CoV-2 in cell culture then chemically inactivating the virus combined with an aluminum hydroxide adjuvant |
| Ad5-based COVID-19 vaccine | Replication-incompetent vector vaccine | CanSino | A replication-incompetent adenovirus 5 vector that expresses the spike protein |
| Covaxin | Inactivated vaccine | Bharat Biotech | Produced by growing SARS-CoV-2 in cell culture then chemically inactivating the virus combined with an aluminum hydroxide and a toll-like receptor agonist adjuvant |
| WIBP-CorV | Inactivated vaccine | Sinopharm | Produced by growing SARS-CoV-2 in cell culture then chemically inactivating the virus combined with an aluminum hydroxide adjuvant |
| BBIBP-CorV/HB02 | Inactivated vaccine | Covilo, Sinopharm | Produced by growing SARS-CoV-2 in cell culture then chemically inactivating the virus combined with an aluminum hydroxide adjuvant |
| ZyCoV-D | DNA vaccine | Zydus Cadila | A DNA plasmid vector pVAX1 carrying gene-expressing spikeS protein of SARS-CoV-2 and IgE signal peptide |
Our study aims to review the literature on mRNA vaccines, assessing the efficacy and safety but also the immunogenic activity and the social and psychological impact of COVID-19 vaccination, starting from the current state of the art of research in the field.
2. Structure and pathogenicity of SARS-CoV-2
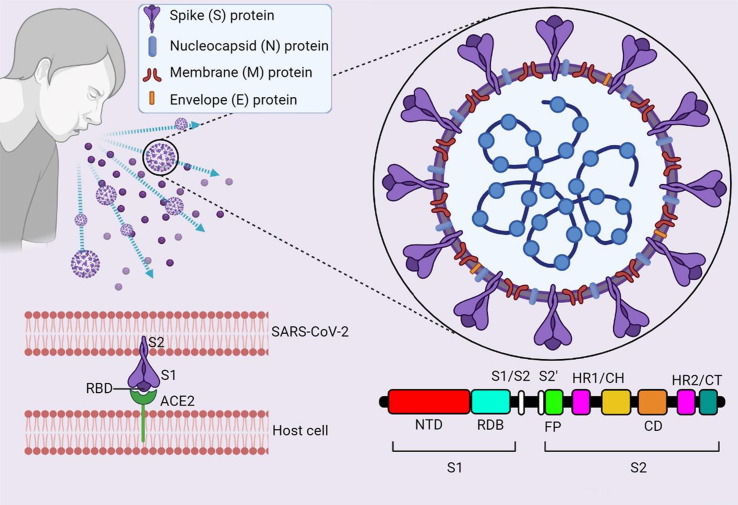
SARS-CoV-2 is a single-stranded RNA (ssRNA) virus contained in an envelope on which S glycoproteins are found, positioned like a “crown”. Interestingly, the genome of this coronavirus has 79. 6 % genetic identity with the genome of the previous SARS-CoV [55]. SARS-CoV-2 contains 4 proteins that play a key role in its replication and transmission. In fact, in addition to protein S, proteins nucleocapsid (N), membrane (M), and envelope (E) also contribute to the spread of the virus [56]. Protein S binds to the host cell’s angiotensin-converting enzyme (ACE)-2 receptors, present in the respiratory tract, heart, kidneys, intestine [57], activating Transmembrane Protease Serin (TMPRSS)-2 [58]. This protease cleaves the S protein into two subunits, S1 and S2, however, there are also other receptors sensitive to binding to the viral S protein, such as CD-147 on epithelial cells [59] and CD26 [60]. Both S1 and S2 subunits play a key role in host cell penetration: S1 presents the ACE-2 receptor-binding domain (RDB), while S2 shows the cleavage site involved in the fusion of viral membranes and host cell (Fig. 2 ) [61], [62]. Thanks to previous studies on SARS-CoV and MERS-CoV, the S protein has been fully identified, finding in S1 and S2 the epitopes for the induction of neutralizing antibodies [63], [64]. Protein N is the most present and highly conserved protein, abundant RNA-binding protein critical for viral genome packaging [65]. Its use in the field of vaccines is still controversial because many studies show that patients have had a strong antibody response against this protein, while others have had almost no response. Protein M, used to stimulate the production of antibodies, has generated a strong immune response, so much so that it is a reference antigen for the development of anti-SARS-CoV-2 vaccines [66]. Finally, protein E, which forms a cation-selective ion channel through host cell lumen release [67] has been shown to induce negligible antibody responses [68].
Fig. 2.
Structure of SARS-CoV-2 virus. ssRNA shares 79.6% of the sequence identity with SARS-CoV and encodes for the proteins S, M, N, and E. Protein S consists of two subunits: S1 and S2. S1 presents the RBD. RBD is required for binding to the ACE-2 receptor on the surface of host cells. The domain of the S2 subunit forms a trimeric structure and has the fusion peptide (FP) and two heptad repeats (HR1/Central Helics and HR2/Cytoplasmic Tale), which are necessary for the fusion of the virus and the host cell membrane. Protein N wraps the genome into a virion for efficient viral replication, protein M is involved in viral integration, protein E facilitates assembly and compacting. Created with BioRender.com.
Following entry of the virus via inhalation, lung epithelial cells recognize SARS-CoV-2 as a foreign pathogen via PRRs and initiate the innate and adaptive immune response [69]. The activation of dendritic cells (DCs), that orchestrates immune response [70], [71], [72], [147], leads to the presentation of viral antigen to CD8+ and CD4+ cells, with stimulation of naïve T cells in the lymph nodes [26]. Protein S binds to airway epithelial cells via ACE receptors [73]. This binding stimulates TLR-7 and TLR-8 to induce the production of IFN-1, which plays a key role in the management of viral infections, resulting in the production of cytokines and antiviral enzymes by other immune cells [74]. However, SARS-CoV-2 can bypass IFN [75] and this mechanism could be the basis for the development of severe clinical conditions [76]. Within 1–2 days of infection, the virus replicates locally, being detectable by polymerase chain reaction (PCR) with nasal swabs [77]. Often, patients at this stage were asymptomatic but highly infectious. For the next 3–7 days, the virus remains in the upper airway and may cause manageable mild symptoms at home [78]. In about 14 % of cases, however, the virus continues to spread, leading to modest clinical settings that require hospitalization [79]. In about 5 % of patients, the virus infects the alveolar cells, causing a serious pathological condition, increasing the extent of tissue and cell damage. This triggers a chain reaction by macrophages, monocytes, lymphocytes, and neutrophils [80] producing IFN-α, IFN-γ, interleukin (IL)-1β, IL-12, IL-6, IL-18, IL-17, IL-33, TNF-α, transforming growth factor (TGF)-β, chemokine (C—C motif) ligand (CCL)-2, CCL-3, CCL-5, chemokine (C-X-C motif) ligand (CXCL)-8, CXCL-9, CXCL-10, causing a real cytokine storm. This reaction, highly inflammatory, represents one of the main causes of the development of ARDS), bilateral pneumonia leading to death [81].
3. LNPs-mRNA complex immunogenicity
Lipid nanoparticles (LNPs) not only play a key role in the stability of mRNA, but also promote its immunogenic mechanism. LNPs are vectors of approximately 100 nm consisting of phospholipids, cholesterol, polyethylene glycol (PEG) lipids, and ionizable lipids [82]. While phospholipids and cholesterol have a stabilizing and structural function, PEGs promote prolonged circulation [83]. Ionizable lipids facilitate the release of mRNA from the endosome into the cytosol to begin translation [84]. Phospholipids and cholesterol are physiologically present in cell membranes, so it is not plausible that they could be involved in the induction of immune responses. However, ionizable lipids can elicit inflammatory responses. Several studies on the LNPs-mRNA complex have shown how the ionizable lipids of this platform can stimulate a powerful immune response [85]. Men who have been given the SARS-CoV-2 LNPs-mRNA vaccine have often experienced the classic symptoms of inflammation: pain, swelling, redness, heat at the injection site, and fever [86]. Specific studies conducted with the administration of LNPs alone or combined with non-coding RNA have demonstrated the inflammatory power of these lipids [87]. This may lead to the conclusion that most of the adverse effects of vaccination are caused by these ionizable lipid nanoparticles.
However, the activation of innate memory could help to modulate both these side effects and the inflammatory response [88]. Moreover, the presence of this complex also allows the repackaging of the mRNA into vesicles which, after expulsion, could reach even sites far from the injection site, increasing the number of cells involved in the antigenic translation and thus increasing the duration of its expression [89], [90]. Preclinical animal testing and human clinical studies by Moderna and Pfizer/BioNTech on SARS-CoV-2 to LNPs-mRNA vaccines demonstrated protection rates above 90 %. These vaccines prove to be safer than viral vector vaccines, are highly customizable, self-adjuvant, large-scale products, and easily adaptable to any emerging pathogens [91]. The success of this type of vaccine is mainly due to the improved ability of the mRNA to leave the endosomal compartment to allow translation into cytoplasmic ribosomes. The ionizable component could be protonated in most cells in the acidic environment of the endosome, leading to the release of mRNA. In contrast, the different biology of DCs hinders this release, being able to retain protein antigens for prolonged periods [92].
However, lipid vectors able to merge with the cell membrane release mRNA directly into the cytosol. In the case of mRNA vaccines with complete S protein, it is neutralized and broken down into immunogenic antigenic epitopes and presented to CD8+ cells via the major histocompatibility complex (MHC)-I [91]. DCs transfected by mRNA or immunogenic epitopes involve the MHC-II for immune cell presentation. Activated B cells differentiate into memory B cells and plasma cells (with production of specific antibodies), allowing an immune reaction after a second possible contact, leading to the neutralization of viral antigens. If the amount of antibodies is not sufficient to trigger the response, the inactive memory cells are activated to determine the secondary immune response [93]. The mechanism of mRNA translation and the subsequent presentation of the antigen to trigger the immune response is schematized in Fig. 3 .
Fig. 3.
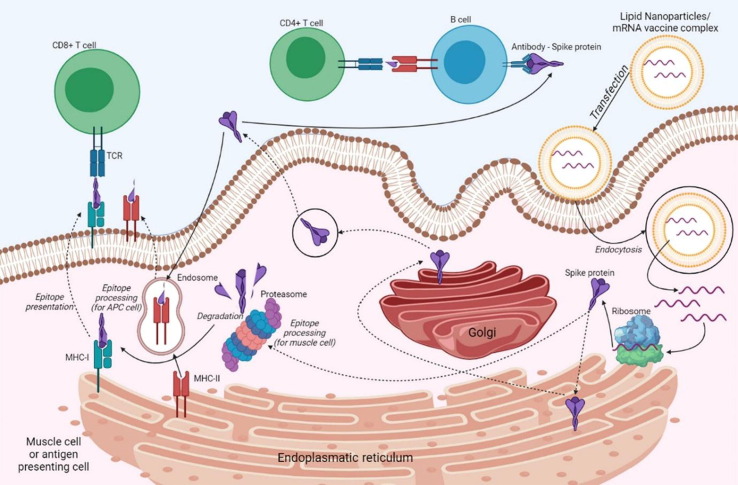
Immunogenic mechanism of mRNA vaccines. Through endocytosis, the vaccine enters a muscle cell or an antigen-presenting cell. LNP-free mRNA is translated into Spike protein at the ribosome level. The newly synthesized S protein is secreted into the extracellular space [cit] and through endocytosis enters an APC cell and is incorporated as part of the MHC-II to present the antigen to immune T and B cells. Protein antigens partially degraded by the proteasome are incorporated into MHC-I complexes and presented to immune cells. Created with BioRender.com.
4. Efficacy and safety of mRNA vaccines
The mass vaccination campaign against SARS-CoV-2 is already active in most of the world. Several articles published during the pandemic described valuable studies aimed at developing an effective and safe vaccine with a reduced number of non-severe secondary effects [94]. During the first phase of the research, the vaccine is administered to humans (both young and elderly) in order to confirm its safety and immunogenic effects, as well as to determine the appropriate dose to be used. In the second stage, randomized clinical trials represent the “gold standard” for evaluating vaccine efficacy (VE). Although, limitations in terms of inclusion criteria (highly restrictive) need to be considered to evaluate its effectiveness [95]. For these and other reasons, the results of the trials may not coincide with those obtained in a mass vaccination.
4.1. BNT152b2 (“Comirnaty”; Pfizer BioNTech)
BNT152b2 is administered intramuscularly in two doses three weeks apart and has been authorized for use in various locations, including the European Union (EU) [96]. Comparable binding and neutralizing antibodies to the convalescent plasma of patients with asymptomatic or moderate SARS-CoV-2 infection were found in a randomized phase I and II study in healthy adults aged 18 to 85 years [97]. Although the response in participants aged ≥65 years old was generally lower than that of younger participants, it was comparable to the titer present in the convalescent plasma. It was also found that the neutralizing antibody titer in 12–15 years old participants was significantly higher than that of 16–25 years old participants [98]. Furthermore, several studies suggest that the neutralizing activity against variants of concern is maintained, despite the level of neutralizing antibodies in the Beta variant (B.1.351) being lower than in the Delta variant (B.1.617.2), if compared with that obtained with other circulating strains [99], [100], [101], [102].
In the phase III trial, BNT152b2 showed a capacity of 95 % (Confidence Interval (CI) 90.3–97.6) in preventing a symptomatic form of COVID-19 at or after day 7 after the second dose, calculated after the evaluation of 170 confirmed cases of COVID-19 among more than 36,000 participants ≥16 years, with a median of two months of follow-up after vaccination [103]. In the population ≥65 years old with other comorbidities, the efficacy was 95 % (CI 44.2–99.8). The efficacy of the vaccine was also high in a trial carried out in adolescents 12–15 years without evidence of a previous infection, reaching 95 % (CI 75.3–100) [98]. The data in question have been confirmed by studies carried out at national level in other states. In Israel, the effectiveness observed at ≥7 days after the second dose was 92 % for preventing a SARS-CoV-2 infection, 97 % for hospitalization from COVID-19, 97 % for symptomatic forms of COVID-19, 97 % for death from COVID-19; with high and comparable outcomes in all age groups. It was also found to be effective against the SARS-CoV-2 variants prevalent during the trial in question [104]. Some data not yet published in Israel would suggest that the increasing predominance of the Delta variant on the territory would have led to a temporary reduction of effectiveness, reaching 64 % against a symptomatic infection of SARS-CoV-2, but maintaining very high values of effectiveness against the hospitalization from COVID-19. In the United Kingdom, the estimated effectiveness against the symptomatic form of COVID-19 induced by the Delta variant was 95 % (CI 85–90) compared to 95 % (CI 92–95) for the Alpha variant [105]. A cohort study confirmed the efficacy of vaccination in protecting Delta variant against contagion [106]. The effectiveness may decrease over time. In a follow-up study, the efficacy against the symptomatic form of COVID-19 remained high for 6 months, standing at 96 % from the time of vaccination at 2 months, at 90 % between 2 and 4 months, and 84 % from 4 to 6 months. According to the data in question, only 1 case of severe infection among the 30 observed was recorded in a vaccinated subject [107].
Some adverse effects are quite frequent, especially after the administration of the second dose. Most are of moderate or mild severity and are usually limited to the first two days after vaccination [108], [109]. In post-vaccination questionnaires collected in the 16-years-or-older US population a local reaction at the injection site (pain, redness, itching, and swelling) was reported in 65 % of cases after the first and second dose; myalgia, headache, and fatigue respectively in 17 %, 25 %, and 29 % of cases after the first dose, and 37 %, 40 %, and 48 % after the second dose [108]. In addition, about one in five participants reported symptoms such as chills, joint pain, and fever. Reactions were mainly reported within the first day of vaccination, especially in the 12–15-years-old group after the second dose. Specifically, myalgia (32 %), chills (42 %), headache (65 %), and fatigue (65 %) were reported. These reactions, while quite common, occurred less frequently in subjects aged 65 years or older [98]. Anaphylaxis after vaccination has been reported with a ratio of about 5 cases per 1 million doses. In about 4 out of 5 cases, anaphylaxis occurs in subjects with a history of allergic reactions and in 9 out of 10 cases within 30 min of inoculation. Other reported cases of allergic reactions were rash, itchy throat sensation, and mild respiratory symptoms. Rare cases of Bell's palsy were recorded in phase III trials, but the percentage did not exceed the one recorded in the general population. Furthermore, post-vaccine monitoring did not identify an association between vaccination and Bell's palsy [110] (Table 2 ).
Table 2.
Summary table of mRNA vaccines safety and efficacy.
| Name of the vaccine | NT162b2 | mRNA-1273 |
|---|---|---|
| Company developer | Pfizer/BioNTech | Moderna |
| Type | mRNA | mRNA |
| Doses and suggested interval | 2 doses 3 weeks apart Booster dose 6 months following primary series for the following: Adults 65 years or older Adults 18 years or older at high risk for severe COVID-19 because of comorbidities Adults 18 years or older at occupational or institutional risk of exposure |
2 doses 4 weeks apart |
| Efficacy against symptomatic COVID-19 (in clinical trials) | 95 % (95 % CI 90–98) after a median of 2 months follow-up 91 % (95 % CI 89–93) after median of 6 months follow-up |
94 % (95 % CI 89–97) after median of 2 months follow-up |
| Effectiveness in observational studies when Delta variant prevalent | Symptomatic infection: 41 to 88 % Severe disease/ hospitalization: 86 to 95 % |
Symptomatic infection: 85 to 88 % Severe disease/ hospitalization: 89 to 96 % |
| Storage requirements | Ultracold freezer (–90 to –60 °C) then freezer (–25 to –15 °C) for up to 2 weeks cumulative time then refrigerated (2 to 8 °C) for up to 1 month | Freezer (–25 to –15 °C) then refrigerated (2 to 8 °C) for up to 30 days |
| Common side effects | Local injection site reactions Systemic symptoms (fevers, chills, fatigue, myalgias, headache) |
Local injection site reactions Systemic symptoms (fevers, chills, fatigue, myalgias, headache) |
| Rare adverse effects | Anaphylaxis (approximately 5 per million) Myocarditis/pericarditis (approximately 16 per million among 16–39-year-olds) |
Anaphylaxis (approximately 2.8 per million) Myocarditis/pericarditis (approximately 16 per million among 16–39-year-olds) |
4.2. mRNA-1273 (“Spikevax”; Moderna)
mRNA-1273 is administered intramuscularly in two doses four weeks apart and has been authorized for use in various locations, including the European Union [111]. In the phase I trial, it demonstrated the ability to produce neutralizing antibodies in values comparable to those present in convalescent plasma in a healthy population aged 18–55, and a response with CD4 cells with a T helper (Th)-1 bias, as well as an immune response in subjects 55 years old and older comparable to that of younger subjects [86], [112]. The binding and neutralizable antibodies decrease slightly after six months in all population groups, but the antibody titer maintains a high value and the neutralizing capacity persists for this time [113]. In addition, induced immunity was found in subjects between 12 and 17 years old that was comparable if not higher than that of young adults [114]. The studies carried out on the plasma of subjects vaccinated with the mRNA-1273 show that the neutralizing antibody activity is effective against the variants of concern, but the number of neutralizing antibodies against the variant Beta (B.1.351) and Delta (B.1.617.2) is lower if compared with that obtained with other circulating strains [99], [115], [116].
In a phase III study, mRNA-1273 showed an efficacy of 95 % CI 89.3–96.8 in preventing a symptomatic form of COVID-19 at ≥ 14 days after the second dose [30]. In subjects aged ≥ 65, on the other hand, the efficacy was 95 % (CI 61.4–95.5). About 5 months after follow-up, the effectiveness of the vaccine was 93.2 % in preventing a symptomatic form of COVID-19 and 98.2 % in preventing a severe form of COVID-19 [117]. The data collected in the aforementioned trial were confirmed by several observational studies on the effectiveness of the vaccine [118], [119], [120], [121]. In addition, a study carried out on hospitalized subjects (40,000) and in the context of urgent care and emergency (20,000) found that mRNA-1273 has a 91–92 % effectiveness in protecting emergency-, urgent care-, and hospitalization-related cases of COVID-19 [121].
Some adverse effects are quite common, especially after the second dose. Most are mild or moderate, do not require the interruption of daily activities or the use of painkillers, and in any case are generally limited to the first two days after vaccination [108], [109]. In post-vaccination questionnaires collected in the US population, pain, redness, itching, and swelling were reported between 74 and 82 % of the participants both after the first and second dose. Myalgia (21 %), headache (27 %), and fatigue (33 %) were also reported after the first dose, and respectively in 51 %, 53 %, and 60 % after the second dose. In addition, fever and chills (40 %) and joint pain (32 %) were reported. Reactions generally occurred within the first day after vaccination, and to a lesser extent but still frequently in subjects ≥ 65 years of age [108]. Anaphylaxis has been reported following vaccination at 2.8 events per million doses. In 86 % of cases, anaphylaxis was reported in subjects with a history of allergic reactions, and in 90 % of cases, it occurred within 30 min of administration [122]. Rare cases of Bell’s paralysis have been reported following vaccination, but the percentage did not exceed that of the general population and post-marketing monitoring did not identify an association with vaccination [110]. The report by the Advisory Committee on Immunization Practices (ACIP) on May 12, 2021 found a total of 8 cases following the administration of the mRNA-1273 dose, of which only 6 cases could be potentially incidental and none were found to have thrombocytopenia. To date, the scientific community strongly suggests that the benefits far outweigh the potential harm [123], [124] (Table 2).
4.3. Booster vaccinations
Some data from observational studies indicates that protection against SARS-CoV-2 infection could decrease over time. However, protection against hospitalization from COVID-19 as well as protection against severe forms of COVID-19 would be guaranteed [107], [125]. As a result, several countries have already started to administer booster vaccinations to individuals who have been fully vaccinated. The Centers for Disease Control and Prevention (CDC) recommends a booster dose of BNT162b2 six months after the last dose for some high-risk groups. Taking into account several studies that confirm an increased ability to produce antibodies neutralizing agents in subjects undergoing transplants with a weakening of the immune system, European Medicines Agency (EMA)'s human medicines committee (CHMP) recommends administering an extra dose of COVID-19 mRNA vaccines to subjects with severe immunosuppression at least 29 days after the second dose [126], [127], [128]. In an observational study conducted in Israel, individuals who received the booster dose at least 5 months after the previous one had an 11 times lower rate of infection than those who did not and a 20 times lower rate of severe disease. Due to the design of the study, it is unclear whether some of the differences are attributable to other factors [129]. Following the evaluation of these data, the CHMP concluded that it is possible to evaluate the administration of booster doses at least 6 months after the second dose in the population aged 18 and over.
Cases of pericarditis and myocarditis have been reported in a percentage worthy of attention following vaccination with BNT162b2 and mRNA-1273, generally within the first week after vaccine shot and after the second shot, especially in males, adolescents and/or young adults. However, given the rarity and moderate effects found, the benefits obtained from an mRNA vaccination far outweigh the slightly increased risk [130]. The CDC suggests that those who develop pericarditis or myocarditis after the first dose of an mRNA vaccine should postpone the second dose until the episode has completely resolved, in case the risk of a severe form of COVID-19 is high [131].
5. Covid vaccination psychological impact
The COVID-19 pandemic has had a strong impact on public emotion because of isolation and loneliness due in part to social distancing measures and the lockdown imposed to prevent the spread of infection, but also to fear of contracting the virus, financial difficulties, and fear of vaccine safety, causing an increase in the population of anxious and depressive states [132], [133]. Long before the COVID-19 pandemic took hold, vaccination has always been a matter of debate, thanks in particular to anti-vaccination groups on social media and the internet promoting disinformation and conspiracy theories about vaccines, often creating confusion and division [134]. These sites and social accounts play on the subject’s emotions, citing mandatory vaccination as a violation of civil rights, or hyperbolizing the dangerous side effects of vaccines [135], and the conspiracy theories mentioned by these groups are aimed at sowing mistrust of scientists, experts and government organizations [134]. This, compounded by the emotional issues of the pandemic, has caused confusion, nervousness, hesitancy, and apathy about the vaccine decision [136].
A recent study by Murphy et al. showed that among a sample of 1041 Irish adults and 2025 British adults, 35 % and 31 % respectively were hesitant/resistant to the vaccine. Despite different social backgrounds and economic, political, cultural, and geographic differences, both hesitant populations shared a similar psychological profile: they were more self-interested, more distrustful of expert figures, more impulsive, and with more religious, conspiracy, and paranoid beliefs [137]. These results coincide with those of other European nations [138], and in the United States, where 33 % of the population report resistance or hesitation to the vaccine [139], [140]. An interesting cross-sectional study carried out in Italy during the different phases of the pandemic showed that the perception of the risk of COVID-19 increased during the lockdown phase and decreased during the reopening phase, with the result that during the lockdown a higher percentage of the population was interested in vaccinating against COVID-19, even hesitant people [141]. This can be explained by the “hot–cold empathy bias”: in fact, people tend to give less importance to their emotional reactions during “cold” decisions than “hot”, thus leading to an asymmetry between their beliefs and choices [142]. This type of bias is also found in people who, before receiving the anti-COVID-19 vaccination, have already undergone tests to determine whether they are positive or negative for the disease. These subjects are more likely and less resistant to receive the vaccination than those who have not taken any kind of COVID-test [143]. Another reason why people are so hesitant about vaccination is its potential negative effect on fertility [144]. A recent study by Gonzalez et al., which measured sperm levels before and after two doses of COVID-19 mRNA vaccine, showed that there were no significant decreases, and in many cases, there were also increases in sperm parameters. This is because these vaccines contain mRNA and not the live virus, so they are unlikely to alter sperm values [145].
Reaching out to those who are still hesitant or resistant about COVID vaccination is certainly important to ensure public health, however, the distrust these individuals show about the established authority does not make the task any easier. One strategy could be to involve authoritative figures not linked to vaccine authority or competence (such as religious leaders), or to emphasize, given the lack of altruism and the internal locus of control, the personal benefits these individuals could receive with COVID vaccination, and unconventional communication channels could be used to support the vaccine campaign, such as social media [137]. Also, misinformation about the consequences of vaccination is an often widespread and difficult occurrence to control. Vaccine information campaigns could be helpful in this regard, by trying to identify which doubt afflicts a hesitant or resistant subject, and by focusing the discussion on that area [146].
6. Conclusions
The data collected demonstrate the efficacy of mRNA vaccines in stimulating immune cells to produce specific antibodies against SARS-CoV-2. Vaccination is the most effective weapon to combat and contain the COVID-19 pandemic. Therefore, it would be useful to further encourage vaccination, trying to overcome the insecurities and doubts of a minority of people who have not yet been vaccinated. These molecules are more biosecure than traditional viral vector vaccines, suggesting their possible use also in the management of diseases of broad global health interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Alessio Alesci: Conceptualization, Writing – original draft, Writing – review & editing, Data curation, Visualization. Marco Gitto: Writing – original draft. Magdalena Kotańska: Data curation, Visualization. Patrizia Lo Cascio: Data curation, Visualization. Anthea Miller: Data curation. Noemi Nicosia: Writing – original draft. Angelo Fumia: Writing – original draft, Data curation, Visualization. Simona Pergolizzi: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to all the researchers whom we cited in this review for their significant and valuable research.
References
- 1.Alesci A., Aragona M., Cicero N., Lauriano E.R. Can nutraceuticals assist treatment and improve Covid-19 symptoms? Nat. Prod. Res. 2022;36(10):2672–2691. doi: 10.1080/14786419.2021.1914032. [DOI] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alesci A., Fumia A., Lo Cascio P., Miller A., Cicero N. Immunostimulant and antidepressant effect of natural compounds in the management of Covid-19 symptoms. J. Am. Coll. Nutr. 2021:1–15. doi: 10.1080/07315724.2021.1965503. [DOI] [PubMed] [Google Scholar]
- 4.Alessio A., Pergolizzi S., Gervasi T., Aragona M., Lo Cascio P., Cicero N., Lauriano E.R. Biological effect of astaxanthin on alcohol-induced gut damage in carassius auratus used as experimental model. Nat. Prod. Res. 2021;35:5737–5743. doi: 10.1080/14786419.2020.1830396. [DOI] [PubMed] [Google Scholar]
- 5.Wang D., Hu B.o., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Coronavirus (COVID-19) Dashboard Available online: https://covid19.who.int (accessed on 9 May 2022).
- 7.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat. Rev. Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom Available online: https://lns.lu/en/the-rapid-increase-of-a-sars-cov-2-variant-with-multiple-spike-protein-mutations-observed-in-the-united-kingdom/ (accessed on 9 May 2022).
- 9.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., Mlisana K., von Gottberg A., Walaza S., Allam M., Ismail A., Mohale T., Glass A.J., Engelbrecht S., Van Zyl G., Preiser W., Petruccione F., Sigal A., Hardie D., Marais G., Hsiao N.-Y., Korsman S., Davies M.-A., Tyers L., Mudau I., York D., Maslo C., Goedhals D., Abrahams S., Laguda-Akingba O., Alisoltani-Dehkordi A., Godzik A., Wibmer C.K., Sewell B.T., Lourenço J., Alcantara L.C.J., Kosakovsky Pond S.L., Weaver S., Martin D., Lessells R.J., Bhiman J.N., Williamson C., de Oliveira T. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 10.Faria N.R., Claro I.M., Candido D., Franco L.M., Andrade P.S., Coletti T.M., Silva C.A., Sales F.C., Manuli E.R., Aguiar R.S. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. Virological. 2021;372:815–821. [Google Scholar]
- 11.Investigation of SARS-CoV-2 Variants: Technical Briefings Available online: https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings (accessed on 9 May 2022).
- 12.CDC Coronavirus Disease 2019 (COVID-19) Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html (accessed on 9 May 2022).
- 13.B. Rochwerg, A. Agarwal, L. Zeng, Y.S. Leo, J.A. Appiah, T. Agoritsas, J. Bartoszko, R. Brignardello-Petersen, B. Ergan, L. Ge, et al., Remdesivir for severe Covid-19: a clinical practice guideline. BMJ 370 (2020) m2924, doi:10.1136/bmj.m2924. [DOI] [PubMed]
- 14.Dougan M., Nirula A., Azizad M., Mocherla B., Gottlieb R.L., Chen P., Hebert C., Perry R., Boscia J., Heller B., Morris J., Crystal C., Igbinadolor A., Huhn G., Cardona J., Shawa I., Kumar P., Adams A.C., Van Naarden J., Custer K.L., Durante M., Oakley G., Schade A.E., Holzer T.R., Ebert P.J., Higgs R.E., Kallewaard N.L., Sabo J., Patel D.R., Dabora M.C., Klekotka P., Shen L., Skovronsky D.M. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl. J. Med. 2021;385(15):1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., Avezum A., Lopes R.D., Bueno F.R., Silva M.V.A.O., Baldassare F.P., Costa E.L.V., Moura R.A.B., Honorato M.O., Costa A.N., Damiani L.P., Lisboa T., Kawano-Dourado L., Zampieri F.G., Olivato G.B., Righy C., Amendola C.P., Roepke R.M.L., Freitas D.H.M., Forte D.N., Freitas F.G.R., Fernandes C.C.F., Melro L.M.G., Junior G.F.S., Morais D.C., Zung S., Machado F.R., Azevedo L.C.P. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alesci A., Miller A., Tardugno R., Pergolizzi S. Chemical analysis, biological and therapeutic activities of olea europaea L extracts. Natural Product Research. 2022;36(11):2932–2945. doi: 10.1080/14786419.2021.1922404. [DOI] [PubMed] [Google Scholar]
- 17.Alesci A., Cicero N., Salvo A., Palombieri D., Zaccone D., Dugo G., Bruno M., Vadalà R., Lauriano E.R., Pergolizzi S. Extracts deriving from olive mill waste water and their effects on the liver of the goldfish carassius auratus fed with hypercholesterolemic diet. Nat. Prod. Res. 2014;28:1343–1349. doi: 10.1080/14786419.2014.903479. [DOI] [PubMed] [Google Scholar]
- 18.Alesci A., Salvo A., Lauriano E.R., Gervasi T., Palombieri D., Bruno M., Pergolizzi S., Cicero N. Production and extraction of astaxanthin from phaffia rhodozyma and its biological effect on alcohol-induced renal hypoxia in carassius auratus. Nat. Prod. Res. 2015;29:1122–1126. doi: 10.1080/14786419.2014.979417. [DOI] [PubMed] [Google Scholar]
- 19.A. Di Sotto, A. Vitalone, S. Di Giacomo, Plant-derived nutraceuticals and immune system modulation: an evidence-based overview, Vaccines (Basel) 8 (2020) doi: 10.3390/vaccines8030468. [DOI] [PMC free article] [PubMed]
- 20.Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C., Apostolopoulos V., Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mrityunjaya M., Pavithra V., Neelam R., Janhavi P., Halami P.M., Ravindra P.V. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.570122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fumia A., Cicero N., Gitto M., Nicosia N., Alesci A. Role of nutraceuticals on neurodegenerative diseases: neuroprotective and immunomodulant activity. Nat. Prod. Res. 2021:1–18. doi: 10.1080/14786419.2021.2020265. [DOI] [PubMed] [Google Scholar]
- 23.Alesci A., Nicosia N., Fumia A., Giorgianni F., Santini A., Cicero N. Resveratrol and immune cells: a link to improve human health. Molecules. 2022;27:424. doi: 10.3390/molecules27020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alesci A., Lauriano E.R., Fumia A., Irrera N., Mastrantonio E., Vaccaro M., Gangemi S., Santini A., Cicero N., Pergolizzi S. Relationship between immune cells, depression, stress, and psoriasis: could the use of natural products be helpful? Molecules. 1953;2022:27. doi: 10.3390/molecules27061953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alesci A., Pergolizzi S., Fumia A., Miller A., Cernigliaro C., Zaccone M., Salamone V., Mastrantonio E., Gangemi S., Pioggia G., Cicero N. Immune system and psychological state of pregnant women during COVID-19 pandemic: are micronutrients able to support pregnancy? Nutrients. 2022;14(12):2534. doi: 10.3390/nu14122534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin J.E., Louder M.K., Holman L.A., Gordon I.J., Enama M.E., Larkin B.D., Andrews C.A., Vogel L., Koup R.A., Roederer M., Bailer R.T., Gomez P.L., Nason M., Mascola J.R., Nabel G.J., Graham B.S. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26(50):6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folegatti P.M., Bittaye M., Flaxman A., Lopez F.R., Bellamy D., Kupke A., Mair C., Makinson R., Sheridan J., Rohde C., Halwe S., Jeong Y., Park Y.-S., Kim J.-O., Song M., Boyd A., Tran N., Silman D., Poulton I., Datoo M., Marshall J., Themistocleous Y., Lawrie A., Roberts R., Berrie E., Becker S., Lambe T., Hill A., Ewer K., Gilbert S. Safety and immunogenicity of a candidate middle east respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020;20(7):816–826. doi: 10.1016/S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koch T., Dahlke C., Fathi A., Kupke A., Krähling V., Okba N.M.A., Halwe S., Rohde C., Eickmann M., Volz A., Hesterkamp T., Jambrecina A., Borregaard S., Ly M.L., Zinser M.E., Bartels E., Poetsch J.S.H., Neumann R., Fux R., Schmiedel S., Lohse A.W., Haagmans B.L., Sutter G., Becker S., Addo M.M. Safety and immunogenicity of a modified vaccinia virus ankara vector vaccine candidate for middle east respiratory syndrome: an open-label, Phase 1 trial. Lancet Infect Dis. 2020;20(7):827–838. doi: 10.1016/S1473-3099(20)30248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the MRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aban M., Abayomi F., Abeyskera K., Aboagye J., Adam M., Adams K., Adamson J., Adelaja Y.A., Adewetan G., Adlou S., Ahmed K., Akhalwaya Y., Akhalwaya S., Alcock A., Ali A., Allen E.R., Allen L., Almeida T.C.D.S.C., Alves M.P.S., Amorim F., Andritsou F., Anslow R., Appleby M., Arbe-Barnes E.H., Ariaans M.P., Arns B., Arruda L., Azi P., Azi L., Babbage G., Bailey C., Baker K.F., Baker M., Baker N., Baker P., Baldwin L., Baleanu I., Bandeira D., Bara A., Barbosa M.A.S., Barker D., Barlow G.D., Barnes E., Barr A.S., Barrett J.R., Barrett J., Bates L., Batten A., Beadon K., Beales E., Beckley R., Belij-Rammerstorfer S., Bell J., Bellamy D., Bellei N., Belton S., Berg A., Bermejo L., Berrie E., Berry L., Berzenyi D., Beveridge A., Bewley K.R., Bexhell H., Bhikha S., Bhorat A.E., Bhorat Z.E., Bijker E., Birch G., Birch S., Bird A., Bird O., Bisnauthsing K., Bittaye M., Blackstone K., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bodalia P., Boettger B.C., Bolam E., Boland E., Bormans D., Borthwick N., Bowring F., Boyd A., Bradley P., Brenner T., Brown P., Brown C., Brown-O'Sullivan C., Bruce S., Brunt E., Buchan R., Budd W., Bulbulia Y.A., Bull M., Burbage J., Burhan H., Burn A., Buttigieg K.R., Byard N., Cabera Puig I., Calderon G., Calvert A., Camara S., Cao M., Cappuccini F., Cardoso J.R., Carr M., Carroll M.W., Carson-Stevens A., Carvalho Y.d.M., Carvalho J.A.M., Casey H.R., Cashen P., Castro T., Castro L.C., Cathie K., Cavey A., Cerbino-Neto J., Chadwick J., Chapman D., Charlton S., Chelysheva I., Chester O., Chita S., Cho J.-S., Cifuentes L., Clark E., Clark M., Clarke A., Clutterbuck E.A., Collins S.L.K., Conlon C.P., Connarty S., Coombes N., Cooper C., Cooper R., Cornelissen L., Corrah T., Cosgrove C., Cox T., Crocker W.E.M., Crosbie S., Cullen L., Cullen D., Cunha D.R.M.F., Cunningham C., Cuthbertson F.C., Da Guarda S.N.F., da Silva L.P., Damratoski B.E., Danos Z., Dantas M.T.D.C., Darroch P., Datoo M.S., Datta C., Davids M., Davies S.L., Davies H., Davis E., Davis J., Davis J., De Nobrega M.M.D., De Oliveira Kalid L.M., Dearlove D., Demissie T., Desai A., Di Marco S., Di Maso C., Dinelli M.I.S., Dinesh T., Docksey C., Dold C., Dong T., Donnellan F.R., Dos Santos T., dos Santos T.G., Dos Santos E.P., Douglas N., Downing C., Drake J., Drake-Brockman R., Driver K., Drury R., Dunachie S.J., Durham B.S., Dutra L., Easom N.J.W., van Eck S., Edwards M., Edwards N.J., El Muhanna O.M., Elias S.C., Elmore M., English M., Esmail A., Essack Y.M., Farmer E., Farooq M., Farrar M., Farrugia L., Faulkner B., Fedosyuk S., Felle S., Feng S., Ferreira Da Silva C., Field S., Fisher R., Flaxman A., Fletcher J., Fofie H., Fok H., Ford K.J., Fowler J., Fraiman P.H.A., Francis E., Franco M.M., Frater J., Freire M.S.M., Fry S.H., Fudge S., Furze J., Fuskova M., Galian-Rubio P., Galiza E., Garlant H., Gavrila M., Geddes A., Gibbons K.A., Gilbride C., Gill H., Glynn S., Godwin K., Gokani K., Goldoni U.C., Goncalves M., Gonzalez I.G.S., Goodwin J., Goondiwala A., Gordon-Quayle K., Gorini G., Grab J., Gracie L., Greenland M., Greenwood N., Greffrath J., Groenewald M.M., Grossi L., Gupta G., Hackett M., Hallis B., Hamaluba M., Hamilton E., Hamlyn J., Hammersley D., Hanrath A.T., Hanumunthadu B., Harris S.A., Harris C., Harris T., Harrison T.D., Harrison D., Hart T.C., Hartnell B., Hassan S., Haughney J., Hawkins S., Hay J., Head I., Henry J., Hermosin Herrera M., Hettle D.B., Hill J., Hodges G., Horne E., Hou M.M., Houlihan C., Howe E., Howell N., Humphreys J., Humphries H.E., Hurley K., Huson C., Hyder-Wright A., Hyams C., Ikram S., Ishwarbhai A., Ivan M., Iveson P., Iyer V., Jackson F., De Jager J., Jaumdally S., Jeffers H., Jesudason N., Jones B., Jones K., Jones E., Jones C., Jorge M.R., Jose A., Joshi A., Júnior E.A.M.S., Kadziola J., Kailath R., Kana F., Karampatsas K., Kasanyinga M., Keen J., Kelly E.J., Kelly D.M., Kelly D., Kelly S., Kerr D., Kfouri R.d.Á., Khan L., Khozoee B., Kidd S., Killen A., Kinch J., Kinch P., King L.D.W., King T.B., Kingham L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Lang M., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lazarus E.M., Leach A., Lees E.A., Lemm N.-M., Lessa A., Leung S., Li Y., Lias A.M., Liatsikos K., Linder A., Lipworth S., Liu S., Liu X., Lloyd A., Lloyd S., Loew L., Lopez Ramon R., Lora L., Lowthorpe V., Luz K., MacDonald J.C., MacGregor G., Madhavan M., Mainwaring D.O., Makambwa E., Makinson R., Malahleha M., Malamatsho R., Mallett G., Mansatta K., Maoko T., Mapetla K., Marchevsky N.G., Marinou S., Marlow E., Marques G.N., Marriott P., Marshall R.P., Marshall J.L., Martins F.J., Masenya M., Masilela M., Masters S.K., Mathew M., Matlebjane H., Matshidiso K., Mazur O., Mazzella A., McCaughan H., McEwan J., McGlashan J., McInroy L., McIntyre Z., McLenaghan D., McRobert N., McSwiggan S., Megson C., Mehdipour S., Meijs W., Mendonça R.N.Á., Mentzer A.J., Mirtorabi N., Mitton C., Mnyakeni S., Moghaddas F., Molapo K., Moloi M., Moore M., Moraes-Pinto M.I., Moran M., Morey E., Morgans R., Morris S., Morris S., Morris H.C., Morselli F., Morshead G., Morter R., Mottal L., Moultrie A., Moya N., Mpelembue M., Msomi S., Mugodi Y., Mukhopadhyay E., Muller J., Munro A., Munro C., Murphy S., Mweu P., Myasaki C.H., Naik G., Naker K., Nastouli E., Nazir A., Ndlovu B., Neffa F., Njenga C., Noal H., Noé A., Novaes G., Nugent F.L., Nunes G., O'Brien K., O'Connor D., Odam M., Oelofse S., Oguti B., Olchawski V., Oldfield N.J., Oliveira M.G., Oliveira C., Oosthuizen A., O'Reilly P., Osborne P., Owen D.R.J., Owen L., Owens D., Owino N., Pacurar M., Paiva B.V.B., Palhares E.M.F., Palmer S., Parkinson S., Parracho H.M.R.T., Parsons K., Patel D., Patel B., Patel F., Patel K., Patrick-Smith M., Payne R.O., Peng Y., Penn E.J., Pennington A., Peralta Alvarez M.P., Perring J., Perry N., Perumal R., Petkar S., Philip T., Phillips D.J., Phillips J., Phohu M.K., Pickup L., Pieterse S., Piper J.o., Pipini D., Plank M., Du Plessis J., Pollard S., Pooley J., Pooran A., Poulton I., Powers C., Presa F.B., Price D.A., Price V., Primeira M., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Quaid S., Rabara R., Radford A., Radia K., Rajapaska D., Rajeswaran T., Ramos A.S.F., Ramos Lopez F., Rampling T., Rand J., Ratcliffe H., Rawlinson T., Rea D., Rees B., Reiné J., Resuello-Dauti M., Reyes Pabon E., Ribiero C.M., Ricamara M., Richter A., Ritchie N., Ritchie A.J., Robbins A.J., Roberts H., Robinson R.E., Robinson H., Rocchetti T.T., Rocha B.P., Roche S., Rollier C., Rose L., Ross Russell A.L., Rossouw L., Royal S., Rudiansyah I., Ruiz S., Saich S., Sala C., Sale J., Salman A.M., Salvador N., Salvador S., Sampaio M., Samson A.D., Sanchez-Gonzalez A., Sanders H., Sanders K., Santos E., Santos Guerra M.F.S., Satti I., Saunders J.E., Saunders C., Sayed A., Schim van der Loeff I., Schmid A.B., Schofield E., Screaton G., Seddiqi S., Segireddy R.R., Senger R., Serrano S., Shah R., Shaik I., Sharpe H.E., Sharrocks K., Shaw R., Shea A., Shepherd A., Shepherd J.G., Shiham F., Sidhom E., Silk S.E., da Silva Moraes A.C., Silva-Junior G., Silva-Reyes L., Silveira A.D., Silveira M.B.V., Sinha J., Skelly D.T., Smith D.C., Smith N., Smith H.E., Smith D.J., Smith C.C., Soares A., Soares T., Solórzano C., Sorio G.L., Sorley K., Sosa-Rodriguez T., Souza C.M.C.D.L., Souza B.S.D.F., Souza A.R., Spencer A.J., Spina F., Spoors L., Stafford L., Stamford I., Starinskij I., Stein R., Steven J., Stockdale L., Stockwell L.V., Strickland L.H., Stuart A.C., Sturdy A., Sutton N., Szigeti A., Tahiri-Alaoui A., Tanner R., Taoushanis C., Tarr A.W., Taylor K., Taylor U., Taylor I.J., Taylor J., te Water Naude R., Themistocleous Y., Themistocleous A., Thomas M., Thomas K., Thomas T.M., Thombrayil A., Thompson F., Thompson A., Thompson K., Thompson A., Thomson J., Thornton-Jones V., Tighe P.J., Tinoco L.A., Tiongson G., Tladinyane B., Tomasicchio M., Tomic A., Tonks S., Towner J., Tran N., Tree J., Trillana G., Trinham C., Trivett R., Truby A., Tsheko B.L., Turabi A., Turner R., Turner C., Ulaszewska M., Underwood B.R., Varughese R., Verbart D., Verheul M., Vichos I., Vieira T., Waddington C.S., Walker L., Wallis E., Wand M., Warbick D., Wardell T., Warimwe G., Warren S.C., Watkins B., Watson E., Webb S., Webb-Bridges A., Webster A., Welch J., Wells J., West A., White C., White R., Williams P., Williams R.L., Winslow R., Woodyer M., Worth A.T., Wright D., Wroblewska M., Yao A., Zimmer R., Zizi D., Zuidewind P. Safety and efficacy of the ChAdOx1 NCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V., Shcherbinin D.N., Semikhin A.S., Simakova Y.V., Tokarskaya E.A., Egorova D.A., Shmarov M.M., Nikitenko N.A., Gushchin V.A., Smolyarchuk E.A., Zyryanov S.K., Borisevich S.V., Naroditsky B.S., Gintsburg A.L. Safety and efficacy of an RAd26 and RAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunkle L.M., Kotloff K.L., Gay C.L., Áñez G., Adelglass J.M., Barrat Hernández A.Q., Harper W.L., Duncanson D.M., McArthur M.A., Florescu D.F., McClelland R.S., Garcia-Fragoso V., Riesenberg R.A., Musante D.B., Fried D.L., Safirstein B.E., McKenzie M., Jeanfreau R.J., Kingsley J.K., Henderson J.A., Lane D.C., Ruíz-Palacios G.M., Corey L., Neuzil K.M., Coombs R.W., Greninger A.L., Hutter J., Ake J.A., Smith K., Woo W., Cho I., Glenn G.M., Dubovsky F. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N. Engl. J. Med. 2022;386(6):531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., Köse Ş., Erdinç F.Ş., Akalın E.H., Tabak Ö.F., Pullukçu H., Batum Ö., Şimşek Yavuz S., Turhan Ö., Yıldırmak M.T., Köksal İ., Taşova Y., Korten V., Yılmaz G., Çelen M.K., Altın S., Çelik İ., Bayındır Y., Karaoğlan İ., Yılmaz A., Özkul A., Gür H., Unal S., Kayaaslan B., Hasanoğlu İ., Dalkıran A., Aydos Ö., Çınar G., Akdemir-Kalkan İ., İnkaya A.Ç., Aydin M., Çakir H., Yıldız J., Kocabıyık Ö., Arslan S., Nallı B., Demir Ö., Singil S., Ataman-Hatipoğlu Ç., Tuncer-Ertem G., Kınıklı S., Önal U., Mete B., Dalgan G., Taşbakan M., Yamazhan T., Kömürcüoğlu B., Yalnız E., Benli A., Keskin-Sarıtaş Ç., Ertosun M.G., Özkan Ö., Emre S., Arıca S., Kuşçu F., Candevir A., Ertürk-Şengel B., Ayvaz F., Aksoy F., Mermutluoğlu Ç., Demir Y., Günlüoğlu G., Tural-Önür S., Kılıç-Toker A., Eren E., Otlu B., Mete A.Ö., Koçak K., Ateş H., Koca-Kalkan İ., Aksu K. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. The Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halperin S.A., Ye L., MacKinnon-Cameron D., Smith B., Cahn P.E., Ruiz-Palacios G.M., Ikram A., Lanas F., Lourdes Guerrero M., Muñoz Navarro S.R., Sued O., Lioznov D.A., Dzutseva V., Parveen G., Zhu F., Leppan L., Langley J.M., Barreto L., Gou J., Zhu T., Mao H., Gagnon L., Tran S.-P., Khan S.T., Becerra Aquino A.G., Saldaña Montemayor E.E., Rivera Martínez N.E., Bohórquez López V.C., Simón Campos J.A., Pineda Cárdenas F.d.J., Chen W., Hou L., Zhang Z., Corral G., López E., Teijeiro R., Alzogaray M.F., Zaidman C., Lopardo G., Goecke B., Feijooó Seoane R.M., Mahmood S.F., Khan E.A., Akram J., Abbas S., Salahuddin N., Rozhkova E., Zubkova T. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. The Lancet. 2022;399(10321):237–248. doi: 10.1016/S0140-6736(21)02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ella R., Reddy S., Blackwelder W., Potdar V., Yadav P., Sarangi V., Aileni V.K., Kanungo S., Rai S., Reddy P., Verma S., Singh C., Redkar S., Mohapatra S., Pandey A., Ranganadin P., Gumashta R., Multani M., Mohammad S., Bhatt P., Kumari L., Sapkal G., Gupta N., Abraham P., Panda S., Prasad S., Bhargava B., Ella K., Vadrevu K.M., Aggarwal P., Aglawe V., Ali A., Anand N., Awad N., Bafna V., Balasubramaniyam G., Bandkar A., Basha P., Bharge V., Bhate A., Bhate S., Bhavani V., Bhosale R., Chalapathy D.V., Chaubal C., Chaudhary D., Chavan A., Desai P., Dhodi D., Dutta S., Garg R., Garg K., George M., Goyal P., Guleria R., Gupta S., Jain M., Jain M.K., Jindal S., Kalra M., Kant S., Khosla P., Kulkarni P., Kumar P., Kumar Y., Majumdar A., Meshram P., Mishra V., Mohanty S., Nair J., Pandey S., Panigrahi S.K., Patil B., Patil V., Rahate P., Raj V., Ramanand S., Rami K., Ramraj B., Rane S., Rao E.V., Rao N., Raphael R., Reddy G., Redkar V., Redkar S., Sachdeva A., Saha J., Sahoo J., Sampath P., Savith A., Shah M., Shanmugam L., Sharma R., Sharma P., Sharma D., Singh A., Singh J., Singh P., Sivaprakasam S., Subramaniam S., Sudheer D., Tandon S., Tariq M., Tripathi V., Vable M., Verma R., Waghmare S. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. The Lancet. 2021;398(10317):2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., Al Nusair M., Hassany M., Jawad J.S., Abdalla J., Hussein S.E., Al Mazrouei S.K., Al Karam M., Li X., Yang X., Wang W., Lai B., Chen W., Huang S., Wang Q., Yang T., Liu Y., Ma R., Hussain Z.M., Khan T., Saifuddin Fasihuddin M., You W., Xie Z., Zhao Y., Jiang Z., Zhao G., Zhang Y., Mahmoud S., ElTantawy I., Xiao P., Koshy A., Zaher W.A., Wang H., Duan K., Pan A.n., Yang X. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khobragade A., Bhate S., Ramaiah V., Deshpande S., Giri K., Phophle H., Supe P., Godara I., Revanna R., Nagarkar R., Sanmukhani J., Dey A., Rajanathan T.M.C., Kansagra K., Koradia P. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. The Lancet. 2022;399(10332):1313–1321. doi: 10.1016/S0140-6736(22)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson N.A.C., Kester K.E., Casimiro D., Gurunathan S., DeRosa F. The promise of MRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardi N., Hogan M.J., Porter F.W., Weissman D. MRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linares-Fernandez S., Lacroix C., Exposito J.Y., Verrier B. Tailoring MRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Iavarone C., O’hagan D.T., Yu D., Delahaye N.F., Ulmer J.B. Mechanism of action of MRNA-based vaccines. Expert Rev Vaccines. 2017;16(9):871–881. doi: 10.1080/14760584.2017.1355245. [DOI] [PubMed] [Google Scholar]
- 43.Sahin U., Kariko K., Tureci O. MRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 44.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic MRNA delivery. Mol Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alesci A., Pergolizzi S., Lo Cascio P., Fumia A., Lauriano E.R. Neuronal regeneration: vertebrates comparative overview and new perspectives for neurodegenerative diseases. Acta Zoologica. 2022;103(2):129–140. [Google Scholar]
- 46.Lauriano E.R., Silvestri G., Kuciel M., Żuwala K., Zaccone D., Palombieri D., Alesci A., Pergolizzi S. Immunohistochemical localization of toll-like receptor 2 in skin langerhans’ cells of striped dolphin (Stenella Coeruleoalba) Tissue Cell. 2014;46:113–121. doi: 10.1016/j.tice.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Lauriano E.R., Pergolizzi S., Capillo G., Kuciel M., Alesci A., Faggio C. Immunohistochemical characterization of toll-like receptor 2 in gut epithelial cells and macrophages of goldfish carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016;59:250–255. doi: 10.1016/j.fsi.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Lauriano E.R., Aragona M., Alesci A., Lo Cascio P., Pergolizzi S. Toll-like receptor 2 and α-smooth muscle actin expressed in the tunica of a urochordate, Styela Plicata. Tissue Cell. 2021;71 doi: 10.1016/j.tice.2021.101584. [DOI] [PubMed] [Google Scholar]
- 49.A. Alesci, S. Pergolizzi, P. Lo Cascio, G. Capillo, E.R. Lauriano, Localization of vasoactive intestinal peptide and toll‐like receptor 2 immunoreactive cells in endostyle of urochordate Styela plicata (Lesueur, 1823). Microscopy Res & Technique (2022), jemt.24119, doi:10.1002/jemt.24119. [DOI] [PMC free article] [PubMed]
- 50.A. Alesci, S. Pergolizzi, G. Capillo, P.L. Cascio, E.R. Lauriano, Rodlet cells in kidney of goldfish (Carassius Auratus, Linnaeus 1758): A light and confocal microscopy study, Acta Histochemica 124 (2022) 151876, doi: 10.1016/j.acthis.2022.151876. [DOI] [PubMed]
- 51.A. Alesci, S. Pergolizzi, A. Fumia, C. Calabrò, P. Lo Cascio, E.R. Lauriano, Mast cells in Goldfish (Carassius Auratus) gut: immunohistochemical characterization. Acta Zoologica 2022, azo.12417, doi:10.1111/azo.12417.
- 52.Alesci A., Fumia A., Miller A., Calabrò C., Santini A., Cicero N., Lo Cascio P. Spirulina promotes macrophages aggregation in Zebrafish (Danio Rerio) liver. Nat. Prod. Res. 2022:1–7. doi: 10.1080/14786419.2022.2089883. [DOI] [PubMed] [Google Scholar]
- 53.A. Alesci, N. Cicero, A. Fumia, C. Petrarca, R. Mangifesta, V. Nava, P. Lo Cascio, S. Gangemi, M. Di Gioacchino, E.R. Lauriano, Histological and chemical analysis of heavy metals in kidney and gills of boops boops: melanomacrophages centers and rodlet cells as environmental biomarkers. Toxics 10 (2022) 218, doi: 10.3390/toxics10050218. [DOI] [PMC free article] [PubMed]
- 54.Hodgson J. The pandemic pipeline. Nat Biotechnol. 2020;38(5):523–532. doi: 10.1038/d41587-020-00005-z. [DOI] [PubMed] [Google Scholar]
- 55.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X.i., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.-E., Barbry P., Leslie A., Kiem H.-P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., Banovich N., Barbry P., Brazma A., Desai T., Duong T.E., Eickelberg O., Falk C., Farzan M., Glass I., Haniffa M., Horvath P., Hung D., Kaminski N., Krasnow M., Kropski J.A., Kuhnemund M., Lafyatis R., Lee H., Leroy S., Linnarson S., Lundeberg J., Meyer K., Misharin A., Nawijn M., Nikolic M.Z., Ordovas-Montanes J., Pe’er D., Powell J., Quake S., Rajagopal J., Tata P.R., Rawlins E.L., Regev A., Reyfman P.A., Rojas M., Rosen O., Saeb-Parsy K., Samakovlis C., Schiller H., Schultze J.L., Seibold M.A., Shalek A.K., Shepherd D., Spence J., Spira A., Sun X., Teichmann S., Theis F., Tsankov A., van den Berge M., von Papen M., Whitsett J., Xavier R., Xu Y., Zaragosi L.-E., Zhang K. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang K.e., Chen W., Zhang Z., Deng Y., Lian J.-Q., Du P., Wei D., Zhang Y., Sun X.-X., Gong L.i., Yang X.u., He L., Zhang L., Yang Z., Geng J.-J., Chen R., Zhang H., Wang B., Zhu Y.-M., Nan G., Jiang J.-L., Li L., Wu J., Lin P., Huang W., Xie L., Zheng Z.-H., Zhang K., Miao J.-L., Cui H.-Y., Huang M., Zhang J., Fu L., Yang X.-M., Zhao Z., Sun S., Gu H., Wang Z., Wang C.-F., Lu Y., Liu Y.-Y., Wang Q.-Y., Bian H., Zhu P., Chen Z.-N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1) doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J., Yang Y.L., Jeong Y., Jang Y.S. Middle east respiratory syndrome-coronavirus infection into established HDPP4-transgenic mice accelerates lung damage via activation of the pro-inflammatory response and pulmonary fibrosis. J. Microbiol. Biotechnol. 2020;30:427–438. doi: 10.4014/jmb.1910.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;14:7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Xiao T., Cai Y., Chen B. Structure of SARS-CoV-2 spike protein. Curr. Opin. Virol. 2021;50:173–182. doi: 10.1016/j.coviro.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tong P.B., Lin L.Y., Tran T.H. Coronaviruses pandemics: can neutralizing antibodies help? Life Sci. 2020;255 doi: 10.1016/j.lfs.2020.117836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cubuk J., Alston J.J., Incicco J.J., Singh S., Stuchell-Brereton M.D., Ward M.D., Zimmerman M.I., Vithani N., Griffith D., Wagoner J.A., Bowman G.R., Hall K.B., Soranno A., Holehouse A.S. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-21953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J., Sun Y., Qi J., Chu F., Wu H., Gao F., Li T., Yan J., Gao G.F. The membrane protein of severe acute respiratory syndrome coronavirus acts as a dominant immunogen revealed by a clustering region of novel functionally and structurally defined cytotoxic T-lymphocyte epitopes. J Infect Dis. 2010;202:1171–1180. doi: 10.1086/656315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandala V.S., McKay M.J., Shcherbakov A.A., Dregni A.J., Kolocouris A., Hong M. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 2020;27:1202–1208. doi: 10.1038/s41594-020-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li K., Hao Z., Zhao X., Du J., Zhou Y. SARS-CoV-2 infection-induced immune responses: friends or foes? Scand J Immunol. 2020;92 doi: 10.1111/sji.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.A. Alesci, E.R. Lauriano, M. Aragona, G. Capillo, S. Pergolizzi, Marking vertebrates langerhans cells, from fish to mammals, Acta Histochemica 122 (2020) 151622, doi:10.1016/j.acthis.2020.151622. [DOI] [PMC free article] [PubMed]
- 71.S. Pergolizzi, G. Rizzo, A. Favaloro, A. Alesci, S. Pallio, G. Melita, G. Cutroneo, E.R. Lauriano, Expression of VAChT and 5-HT in ulcerative colitis dendritic cells, Acta histochemica 13 (2021) 151715, doi:10.1016/j.acthis.2021.151715. [DOI] [PubMed]
- 72.Pergolizzi S., Alesci A., Centofanti A., Aragona M., Pallio S., Magaudda L., Cutroneo G., Lauriano E.R. Role of Serotonin in the maintenance of inflammatory state in Crohn’s disease. Biomedicines. 2022;10:765. doi: 10.3390/biomedicines10040765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sungnak W., Huang N.i., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Y.X. Lim, Y.L. Ng, J.P. Tam, D.X. Liu, Human coronaviruses: a review of virus-host interactions, Diseases 4 (2016) doi:10.3390/diseases4030026. [DOI] [PMC free article] [PubMed]
- 75.Oudshoorn D., Rijs K., Limpens R.W.A.L., Groen K., Koster A.J., Snijder E.J., Kikkert M., Bárcena M., Denison M.R. Expression and cleavage of middle east respiratory syndrome coronavirus Nsp3-4 polyprotein induce the formation of double-membrane vesicles that mimic those associated with coronaviral RNA replication. mBio. 2017;8(6) doi: 10.1128/mBio.01658-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hidden Immune Weakness Found in 14% of Gravely Ill COVID-19 Patients Available online: https://www.sciencemag.org/news/2020/09/hidden-immune-weakness-found-14-gravely-ill-covid-19patients/2020.
- 77.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 79.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 80.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C., Zhang M., Tan J., Xu Y., Song R., Song M., Wang L., Zhang W., Han B., Yang L.i., Wang X., Zhou G., Zhang T., Li B., Wang Y., Chen Z., Wang X. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1) doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L.i., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for MRNA delivery. Nat Rev Mater. 2021;6(12):1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samaridou E., Heyes J., Lutwyche P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv Drug Deliv Rev. 2020;154–155:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Meng C., Chen Z., Li G., Welte T., Shen H. Nanoplatforms for mRNA therapeutics. Adv. Therap. 2021;4(1):2000099. [Google Scholar]
- 85.Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., Moody M.A., Verkerke H.P., Myles A., Willis E., LaBranche C.C., Montefiori D.C., Lobby J.L., Saunders K.O., Liao H.-X., Korber B.T., Sutherland L.L., Scearce R.M., Hraber P.T., Tombácz I., Muramatsu H., Ni H., Balikov D.A., Li C., Mui B.L., Tam Y.K., Krammer F., Karikó K., Polacino P., Eisenlohr L.C., Madden T.D., Hope M.J., Lewis M.G., Lee K.K., Hu S.-L., Hensley S.E., Cancro M.P., Haynes B.F., Weissman D. Nucleoside-modified MRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215(6):1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O’Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An MRNA vaccine against SARS-CoV-2 - preliminary report. N Engl. J. Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.S. Ndeupen, Z. Qin, S. Jacobsen, H. Estanbouli, A. Bouteau, B.Z. Igyarto, The MRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. bioRxiv 2021, doi:10.1101/2021.03.04.430128. [DOI] [PMC free article] [PubMed]
- 88.Netea M.G., Quintin J., van der Meer J.W. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Maugeri M., Nawaz M., Papadimitriou A., Angerfors A., Camponeschi A., Na M., Hölttä M., Skantze P., Johansson S., Sundqvist M., Lindquist J., Kjellman T., Mårtensson I.-L., Jin T., Sunnerhagen P., Östman S., Lindfors L., Valadi H. Linkage between endosomal escape of LNP-MRNA and loading into EVs for transport to other cells. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-12275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gebre M.S., Brito L.A., Tostanoski L.H., Edwards D.K., Carfi A., Barouch D.H. Novel approaches for vaccine development. Cell. 2021;184:1589–1603. doi: 10.1016/j.cell.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Igyarto B.Z., Jacobsen S., Ndeupen S. Future considerations for the MRNA-lipid nanoparticle vaccine platform. Curr Opin Virol. 2021;48:65–72. doi: 10.1016/j.coviro.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouteau A., Kervevan J., Su Q., Zurawski S.M., Contreras V., Dereuddre-Bosquet N., Le Grand R., Zurawski G., Cardinaud S., Levy Y., et al. DC subsets regulate humoral immune responses by supporting the differentiation of distinct Tfh cells. Front Immunol. 2019;10:1134. doi: 10.3389/fimmu.2019.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park J.W., Lagniton P.N.P., Liu Y., Xu R.H. MRNA vaccines for COVID-19: what, why and how. Int J Biol Sci. 2021;17:1446–1460. doi: 10.7150/ijbs.59233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Cheng L., Lian R., Song Z., Tian J. COVID-19 vaccine research focusses on safety, efficacy, immunoinformatics, and vaccine production and delivery: a bibliometric analysis based on VOSviewer. Biosci Trends. 2021;15:64–73. doi: 10.5582/bst.2021.01061. [DOI] [PubMed] [Google Scholar]
- 95.Sharma O., Sultan A.A., Ding H., Triggle C.R. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pinho, A.C. Ema Recommends First COVID-19 Vaccine for Authorisation in the EU Available online: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu.
- 97.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Şahin U., Gruber W.C. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., Perez J.L., Walter E.B., Senders S., Bailey R., Swanson K.A., Ma H., Xu X., Koury K., Kalina W.V., Cooper D., Jennings T., Brandon D.M., Thomas S.J., Türeci Ö., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Edara V.-V., Pinsky B.A., Suthar M.S., Lai L., Davis-Gardner M.E., Floyd K., Flowers M.W., Wrammert J., Hussaini L., Ciric C.R., Bechnak S., Stephens K., Graham B.S., Bayat Mokhtari E., Mudvari P., Boritz E., Creanga A., Pegu A., Derrien-Colemyn A., Henry A.R., Gagne M., Douek D.C., Sahoo M.K., Sibai M., Solis D., Webby R.J., Jeevan T., Fabrizio T.P. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385(7):664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., Prot M., Gallais F., Gantner P., Velay A., Le Guen J., Kassis-Chikhani N., Edriss D., Belec L., Seve A., Courtellemont L., Péré H., Hocqueloux L., Fafi-Kremer S., Prazuck T., Mouquet H., Bruel T., Simon-Lorière E., Rey F.A., Schwartz O. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 101.Tada T., Dcosta B.M., Samanovic M.I., Herati R.S., Cornelius A., Zhou H., Vaill A., Kazmierski W., Mulligan M.J., Landau N.R., Goff S.P. Convalescent-phase sera and vaccine-elicited antibodies largely maintain neutralizing titer against global SARS-CoV-2 variant spikes. mBio. 2021;12(3) doi: 10.1128/mBio.00696-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wall E.C., Wu M., Harvey R., Kelly G., Warchal S., Sawyer C., Daniels R., Hobson P., Hatipoglu E., Ngai Y., Hussain S., Nicod J., Goldstone R., Ambrose K., Hindmarsh S., Beale R., Riddell A., Gamblin S., Howell M., Kassiotis G., Libri V., Williams B., Swanton C., Gandhi S., Bauer D.LV. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 MRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., Southern J.o., Swerdlow D.L., Jodar L., Levy Y., Alroy-Preis S. Impact and effectiveness of MRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K.E., Hopkins S., Chand M., Ramsay M. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singanayagam A., Hakki S., Dunning J., Madon K.J., Crone M.A., Koycheva A., Derqui-Fernandez N., Barnett J.L., Whitfield M.G., Varro R., Charlett A., Kundu R., Fenn J., Cutajar J., Quinn V., Conibear E., Barclay W., Freemont P.S., Taylor G.P., Ahmad S., Zambon M., Ferguson N.M., Lalvani A., Badhan A., Dustan S., Tejpal C., Ketkar A.V., Narean J.S., Hammett S., McDermott E., Pillay T., Houston H., Luca C., Samuel J., Bremang S., Evetts S., Poh J., Anderson C., Jackson D., Miah S., Ellis J., Lackenby A. Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet. Infect. Dis. 2022;22(2):183–195. doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., Bailey R., Swanson K.A., Xu X., Roychoudhury S., Koury K., Bouguermouh S., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Yang Q.i., Liberator P., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Gruber W.C., Jansen K.U. Safety and efficacy of the BNT162b2 MRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chapin-Bardales J., Gee J., Myers T. Reactogenicity following receipt of MRNA-based COVID-19 vaccines. JAMA. 2021;325:2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 109.Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T., Nair N., Martin S., Clark T., Markowitz L., Lindsey N., Zhang B., Licata C., Jazwa A., Sotir M., Shimabukuro T. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimabukuro T. COVID-19 vaccine safety update, advisory committee on immunization practices (ACIP) Meeting. 2021 [Google Scholar]
- 111.D. Glanville, EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU. Available online: (accessed on 20 March 2021), 2021.
- 112.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O’Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H. Safety and immunogenicity of SARS-CoV-2 MRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Doria-Rose N., Suthar M.S., Makowski M., O’Connell S., McDermott A.B., Flach B., Ledgerwood J.E., Mascola J.R., Graham B.S., Lin B.C., O’Dell S., Schmidt S.D., Widge A.T., Edara V.-V., Anderson E.J., Lai L., Floyd K., Rouphael N.G., Zarnitsyna V., Roberts P.C., Makhene M., Buchanan W., Luke C.J., Beigel J.H., Jackson L.A., Neuzil K.M., Bennett H., Leav B., Albert J., Kunwar P. Antibody persistence through 6 months after the second dose of MRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ali K., Berman G., Zhou H., Deng W., Faughnan V., Coronado-Voges M., Ding B., Dooley J., Girard B., Hillebrand W., Pajon R., Miller J.M., Leav B., McPhee R. Evaluation of MRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl. J. Med. 2021;385(24):2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen X., Tang H., Pajon R., Smith G., Glenn G.M., Shi W., Korber B., Montefiori D.C. Neutralization of SARS-CoV-2 variants B.1.429 and B.1.351. N. Engl. J. Med. 2021;384:2352–2354. doi: 10.1056/NEJMc2103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Stewart-Jones G.B.E., Colpitts T., Bennett H., Boyoglu-Barnum S., Shi W., Moliva J.I., Sullivan N.J., Graham B.S., Carfi A., Corbett K.S., Seder R.A., Edwards D.K. Serum neutralizing activity elicited by MRNA-1273 vaccine. N Engl. J. Med. 2021;384(15):1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.El Sahly H.M., Baden L.R., Essink B., Doblecki-Lewis S., Martin J.M., Anderson E.J., Campbell T.B., Clark J., Jackson L.A., Fichtenbaum C.J., Zervos M., Rankin B., Eder F., Feldman G., Kennelly C., Han-Conrad L., Levin M., Neuzil K.M., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Polakowski L., Mascola J.R., Ledgerwood J.E., Graham B.S., August A., Clouting H., Deng W., Han S., Leav B., Manzo D., Pajon R., Schödel F., Tomassini J.E., Zhou H., Miller J. Efficacy of the MRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl. J. Med. 2021;385(19):1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Butt A.A., Omer S.B., Yan P., Shaikh O.S., Mayr F.B. SARS-CoV-2 vaccine effectiveness in a high-risk national population in a real-world setting. Ann. Int. Med. 2021;174:1404–1408. doi: 10.7326/M21-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tenforde M.W., Olson S.M., Self W.H., Talbot H.K., Lindsell C.J., Steingrub J.S., Shapiro N.I., Ginde A.A., Douin D.J., Prekker M.E., et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged >/=65 Years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A.L., Lutrick K., Groom H.C., Dunnigan K., Odean M.J., Hegmann K., Stefanski E., Edwards L.J., Schaefer-Solle N., Grant L., Ellingson K., Kuntz J.L., Zunie T., Thiese M.S., Ivacic L., Wesley M.G., Mayo Lamberte J., Sun X., Smith M.E., Phillips A.L., Groover K.D., Yoo Y.M., Gerald J., Brown R.T., Herring M.K., Joseph G., Beitel S., Morrill T.C., Mak J., Rivers P., Poe B.P., Lynch B., Zhou Y., Zhang J., Kelleher A., Li Y., Dickerson M., Hanson E., Guenther K., Tong S., Bateman A., Reisdorf E., Barnes J., Azziz-Baumgartner E., Hunt D.R., Arvay M.L., Kutty P., Fry A.M., Gaglani M. Prevention and attenuation of Covid-19 with the BNT162b2 and MRNA-1273 vaccines. N Engl. J. Med. 2021;385(4):320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thompson M.G., Stenehjem E., Grannis S., Ball S.W., Naleway A.L., Ong T.C., DeSilva M.B., Natarajan K., Bozio C.H., Lewis N., Dascomb K., Dixon B.E., Birch R.J., Irving S.A., Rao S., Kharbanda E., Han J., Reynolds S., Goddard K., Grisel N., Fadel W.F., Levy M.E., Ferdinands J., Fireman B., Arndorfer J., Valvi N.R., Rowley E.A., Patel P., Zerbo O., Griggs E.P., Porter R.M., Demarco M., Blanton L., Steffens A., Zhuang Y., Olson N., Barron M., Shifflett P., Schrag S.J., Verani J.R., Fry A., Gaglani M., Azziz-Baumgartner E., Klein N.P. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385(15):1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14–23, 2020. Am J Transplant. 2021;21:1332–1337. doi: 10.1111/ajt.16516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hunter P.R. Thrombosis after Covid-19 vaccination. BMJ. 2021;373 doi: 10.1136/bmj.n958. [DOI] [PubMed] [Google Scholar]
- 124.Shimabukuro T.T.U. Thrombosis with thrombocytopenia syndrome (TTS) Following COVID-19 Vaccination. 2021 [Google Scholar]
- 125.Nanduri S., Pilishvili T., Derado G., Soe M.M., Dollard P., Wu H., Li Q., Bagchi S., Dubendris H., Link-Gelles R., Jernigan J.A., Budnitz D., Bell J., Benin A., Shang N., Edwards J.R., Verani J.R., Schrag S.J. Effectiveness of Pfizer-BioNTech and moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant — national healthcare safety network, March 1–August 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70(34):1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hall V.G., Ferreira V.H., Ku T., Ierullo M., Majchrzak-Kita B., Chaparro C., Selzner N., Schiff J., McDonald M., Tomlinson G., Kulasingam V., Kumar D., Humar A. Randomized trial of a third dose of MRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.G. Hrabovszki, Comirnaty and Spikevax: EMA Recommendations on Extra Doses and Boosters, 2021.
- 128.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an MRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., Huppert A. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gargano J.W., Wallace M., Hadler S.C., Langley G., Su J.R., Oster M.E., Broder K.R., Gee J., Weintraub E., Shimabukuro T., Scobie H.M., Moulia D., Markowitz L.E., Wharton M., McNally V.V., Romero J.R., Talbot H.K., Lee G.M., Daley M.F., Oliver S.E. Use of MRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: Update from the advisory committee on immunization practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Interim Clinical Considerations for Use of COVID-19 Vaccines. 2021.
- 132.World Health, O. Coronavirus Disease (COVID-19) Advice for the Public: Myth Busters. URL: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters [accessed 2020-05-25] 2021.
- 133.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Broniatowski D.A., Jamison A.M., Qi S., AlKulaib L., Chen T., Benton A., Quinn S.C., Dredze M. Weaponized health communication: twitter bots and Russian trolls amplify the vaccine debate. Am J Public Health. 2018;108:1378–1384. doi: 10.2105/AJPH.2018.304567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bean S.J. Emerging and continuing trends in vaccine opposition website content. Vaccine. 2011;29:1874–1880. doi: 10.1016/j.vaccine.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 136.Chou W.S., Budenz A. Considering emotion in COVID-19 vaccine communication: addressing vaccine hesitancy and fostering vaccine confidence. Health Commun. 2020;35:1718–1722. doi: 10.1080/10410236.2020.1838096. [DOI] [PubMed] [Google Scholar]
- 137.Murphy J., Vallières F., Bentall R.P., Shevlin M., McBride O., Hartman T.K., McKay R., Bennett K., Mason L., Gibson-Miller J., Levita L., Martinez A.P., Stocks T.V.A., Karatzias T., Hyland P. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-020-20226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neumann-Böhme, S., Varghese, N.E., Sabat, I., Barros, P.P., Brouwer, W., van Exel, J., Schreyögg, J., Stargardt, T., 2020. Once We Have It, Will We Use It? A European Survey on Willingness to Be Vaccinated against COVID-19. doi:10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed]
- 139.Malik A.A., McFadden S.M., Elharake J., Omer S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Loomba S., de Figueiredo A., Piatek S.J., de Graaf K., Larson H.J. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Hum. Behav. 2021;5(3):337–348. doi: 10.1038/s41562-021-01056-1. [DOI] [PubMed] [Google Scholar]
- 141.Caserotti M., Girardi P., Rubaltelli E., Tasso A., Lotto L., Gavaruzzi T. Associations of COVID-19 risk perception with vaccine hesitancy over time for Italian residents. Soc. Sci. Med. 2021;272 doi: 10.1016/j.socscimed.2021.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Loewenstein G. Hot-cold empathy gaps and medical decision making. Health Psychol. 2005;24:S49–S56. doi: 10.1037/0278-6133.24.4.S49. [DOI] [PubMed] [Google Scholar]
- 143.L. Salerno, L. Craxi, E. Amodio, G. Lo Coco, Factors affecting hesitancy to MRNA and viral vector COVID-19 vaccines among college students in Italy. Vaccines (Basel) 9 (2021) doi: 10.3390/vaccines9080927. [DOI] [PMC free article] [PubMed]
- 144.Berry S.D., Johnson K.S., Myles L., Herndon L., Montoya A., Fashaw S., Gifford D. Lessons learned from frontline skilled nursing facility staff regarding COVID-19 vaccine hesitancy. J. Am. Geriatr. Soc. 2021;69:1140–1146. doi: 10.1111/jgs.17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gonzalez D.C., Nassau D.E., Khodamoradi K., Ibrahim E., Blachman-Braun R., Ory J., Ramasamy R. Sperm parameters before and after COVID-19 MRNA vaccination. JAMA. 2021;326:273–274. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rief W. Fear of adverse effects and COVID-19 vaccine hesitancy: recommendations of the treatment expectation expert group. JAMA Health Forum. 2021;2(4):e210804. doi: 10.1001/jamahealthforum.2021.0804. [DOI] [PubMed] [Google Scholar]
- 147.Alesci Alessio, Capillo Gioele, Fumia Angelo, Emmanuele Messina, Albano Marco, Aragona Marialuisa, Lo Cascio Patrizia, Spanò Nunziacarla, Pergolizzi Simona, Lauriano Eugenia Rita. Confocal characterization of intestinal dendritic cells from myxines to teleosts. Biology. 2022;11(7):1045. doi: 10.3390/biology11071045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lauriano Eugenia Rita, Zuwala Krystyna, Kuciel Michal, Budzik Karolina A., Capillo Gioele, Alesci Alessio, Pergolizzi Simona, Dugo Giacomo, Zaccone Giacomo. Confocal immunihistochemistry of the Dermal glands and evolutionary considerations in the Caecilian, Typhlonectes natans (Amphibia: Gymnophiona) Acta Zoologica. 2014;97(2):154–164. doi: 10.1111/azo.12112. [DOI] [Google Scholar]