Abstract
In the last years, antiviral drugs especially used for the treatment of COVID-19 have been considered emerging contaminants because of their continuous occurrence and persistence in water/wastewater even at low concentrations. Furthermore, as compared to antiviral drugs, their metabolites and transformation products of these pharmaceuticals are more persistent in the environment. They have been found in environmental matrices all over the world, demonstrating that conventional treatment technologies are unsuccessful for removing them from water/wastewater. Several approaches for degrading/removing antiviral drugs have been studied to avoid this contamination. In this study, the present level of knowledge on the input sources, occurrence, determination methods and, especially, the degradation and removal methods of antiviral drugs are discussed in water/wastewater. Different removal methods, such as conventional treatment methods (i.e. activated sludge), advanced oxidation processes (AOPs), adsorption, membrane processes, and combined processes, were evaluated. In addition, the antiviral drugs and these metabolites, as well as the transformation products created as a result of treatment, were examined. Future perspectives for removing antiviral drugs, their metabolites, and transformation products were also considered.
Keywords: Antiviral drugs, COVID-19, Determination methods, Removal process, Virus, SARS-CoV-2
1. Introduction
Pharmaceutical compounds, such as antiviral drugs, analgesics, antibiotics, anti-inflammatory medicines, beta-blockers, lipid regulators, X-ray contrast media, antidepressants and antipyretics have become increasingly common in human and animal health care, to improve life quality and extend lifespan [1], [2]. This increase in the use of pharmaceutical compounds has emerged as a global environmental problem in recent years [3]. Therefore, the widespread use of pharmaceutical compounds for a variety of purposes across the world needs careful monitoring of their contamination of water sources [2].
Antiviral drugs are a class of pharmaceuticals that are used to treat viral infections by preventing the growth of pathogens [4]. After the approval and distribution of the first antiviral drug, idoxuridine, on the market in 1963, the alarming rate of mortality due to viral infections prompted the creation of an increasing number of antiviral drugs [5], [6]. These antiviral drugs are commonly used to treat a variety of viral infectious diseases, including influenza, human immunodeficiency virus (HIV), hepatitis and herpes [5], [7]. A novel coronavirus (COVID-19) linked to respiratory diseases in humans was discovered in China, Wuhan in December 2019 [8], [9]. COVID-19 as of March 2022 > 462,000,000 people have been infected, and >6, 000, 000 have died according to World Health Organization (WHO) [10]. In March 2020, WHO recognized the new coronavirus (COVID-19), also known as Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2), as a worldwide pandemic, based on its fatal effect [11].
These antiviral drugs are common in wastewater due to the use of personal care items and pharmaceuticals in the home, as well as in the pharmaceutical industry and hospital waste [12]. Therefore, the use of these clinically tested antiviral drugs has the potential to pose a significant threat to the water resource quality for human consumption [8]. Parts of antiviral drugs that have not been completely metabolized are extracted by patients in their urine or feces and are then mostly disposed of in the sewage system. It has been found that up to 60 % of an applied dose of antiviral drugs is excreted by patients [4], [7]. Eventually, antiviral drugs are discharged into receiving aquatic environment through the effluent release of wastewater treatment plants (WWTPs) because of inadequate removal in WWTPs [7], [13].
Continuous drug use has resulted in increased water pollution and may have negative consequences such as aquatic toxicity, development of resistance in pathogenic microbes; genotoxicity and endocrine disruption in the aquatic ecosystem [1], [14]. In addition, when an antiviral drug and the virus to be treated co-exist in the same waterbody, vulnerable organisms may acquire resistance. This might lead to the emergence of new antiviral resistance in the environment [15]. Therefore, it is essential to treat these drugs to eliminate the negative effects on the environment. Several treatment technologies including ozonation, photolysis, electrochemical advanced oxidation process, photocatalysis, adsorption, activated sludge process and membrane bioreactor have been used to remove antiviral drugs from water/wastewater up to now. Although these technologies are efficient for removing antiviral drugs from the water supply, they have their drawbacks, including large equipment costs, high energy consumption, secondary contamination and the creation of additional harmful by-products.
There is a shortage of knowledge on the combined evaluation of antiviral drugs and viruses discharged into the environment, determination methods, and their treatment in the water/wastewater using various biological and non-biological processes. Thus, it is crucial to investigate the fate and determination methods in the environment. The goal of this review was to investigate the physicochemical properties, analytical methodologies for viruses and to be treated antiviral drugs and removal methods for antiviral drugs. Furthermore, the research addresses challenges that were faced as well as prospective prospects.
2. Antiviral drugs and virus
There are many viruses and antiviral drugs used in the treatment of these viruses in the literature. These antiviral drugs, their pharmaceutical and physicochemical properties are presented in Table 1 [16], [17] .
Table 1.
Physicochemical properties of some antiviral drugs.
| Virus | Antiviral drug | Trade name | Formula | Molecular weight (MW) (g/mol) | Log Kow | pKa (acidic, basic) |
Bioavailability |
|---|---|---|---|---|---|---|---|
| HIV | Abacavir | Ziagen | C14H18N6O | 286.33 | 1.2 | 15.41, 5.8 | 1 |
| Zidovudine | Retrovir | C10H13N5O4 | 267.24 | 0.05 | 9.96, −3 | 1 | |
| Lamivudine | Epivir | C8H11N3O3S | 229.26 | −9.54 | 14.29, −0.16 | 1 | |
| Stavudine | Zerit | C10H12N2O4 | 224.21 | −0.72a | 9.95, −3 | 1 | |
| Nevirapine | Viramune | C15H12N2O4 | 266.29 | 3.89 | 14.98, 3.28 | 1 | |
| HSVs | Acyclovir | Zovirax | C8H11N5O3 | 225.20 | −1.56 | 11.98, 3.02 | 1 |
| Famciclovir | Famvir | C14H19N5O4 | 321.33 | 0.64 | 16.68, 3.65 | 1 | |
| Penciclovir | Denavir | C10H15N5O3 | 253.25 | −1.14 | 12, 2.88 | 1 | |
| Influenza | Amantadine | Gocovri | C10H17N | 151.24 | 2.44 | -, 10.71 | 1 |
| Oseltamivir | Tamiflu | C16H28N2O4 | 312.4 | 0.95 | 14.03, 9.31 | 1 | |
| Zanamivir | Relenza | C12H20N4O7 | 332.3 | −4.66 | 3.06, 11.93 | 0 | |
| SARS-CoV-2 | Favipiravir | Favipiravir | C5H4FN3O2 | 157.1 | – | 9.39, −3.7 | 1 |
| Remdesivir | Veklury | C27H35N6O8P | 602.58 | – | 10.23, 0.65 | 0 |
at 37 °C.
Antiretroviral drugs (ARVs) are medications that treat retroviral infections, especially human immunodeficiency virus type 1 (HIV-1) These drugs can significantly prolong the life of people who is HIV-positive. They consist of mainly six subdivisions such as nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), integrase inhibitors, protease inhibitors, entry & fusion inhibitors and p450-3A inhibitors [18], [19]. Also, the most commonly used ARVs for retroviral infections are abacavir, zidovudine, lamivudine, stavudine and nevirapine. These drugs can be used together to increase the curability of the HIV viruses [20].
Herpes simplex viruses (HSVs), which are doubled-stranded, enveloped DNA viruses of the Herpesviridae family, are common pathogen in humans [21], [22]. These viruses generally occur in the oral and genital areas. However, while some of them affect children by causing chickenpox, which has possible complications such as encephalitis and pneumonia, some of them are caused by neuralgia and nerve palsy in adults [23], [24]. Herpex simplex viruses consist of herpes simplex virus type 1 (HSV-1) which is the most susceptive, herpes simplex type 2 (HSV-2), varicella-zoster virus, cytomegalovirus and Epstein-Barr virus [25]. Acyclovir, one of the antiherpetics, is an antiviral drug used in the treatment of Herpex simplex viruses such as HSV-1, HSV-2 and Varicella-Zoster (VZV) [26]. Also, because of the physicochemical properties of acyclovir such as low water solubility, poor membrane permeability, and low oral bioavailability (15–30 %), its therapeutic properties may be reduced [26], [27]. Famciclovir (FCV), is a pro-antiviral drug produced to increase the bioavailability effect of penciclovir. Both penciclovir and famciclovir are antiherpetic drugs that are actively used against HSV-1, HSV-2 and VZV virüs [28], [29], [30].
Influenza is a respiratory infectious disease that ranks high as one of the deadliest diseases in the category of infectious diseases with its rapid transmission rate [31], [32]. Influenza viruses are classified as A, B and C according to their matrix proteins and nucleoproteins [33]. Influenza can be overcome as a very minor disease, in some cases, it can result in hospitalization or even death [34]. It is estimated that approximately 3 to 5 million serious infections are transmitted annually due to the influenza epidemic, resulting in 290,000 to 650,000 deaths from respiratory diseases all around the world [35]. Although vaccination is one of the effective treatments for influenza, it is less effective against special populations such as children, the elderly and people who have a weak immune system. In addition, since the production of the vaccine takes at least 6 months, antiviral drugs become a complement to vaccines [36]. Two groups of anti-influenza drugs, adamantanes (amantadine and rimantadine) and neuraminidase inhibitors (NAI) (oseltamivir and zanamivir) are used for the treatment of influenza infectious disease [33], [37].
Due to the rapid spread rate of COVID-19 that firstly appeared in China, Wuhan in December 2019, it reached the status of pandemic disease [38], [39], [40]. Although there is no specific drug for the treatment of COVID-19, the drugs favipiravir, remdesivir, hydroxychloroquine, azithromycin and chloroquine have been subjected to clinical testing [41], [42]. Favipiravir is an RNA virus polymerase inhibitor showing effective antiviral activity against several RNA viruses [43]. Therefore, favipiravir is treat COVID-19 in several countries such as Japan, Russia, Ukraine, Uzbekistan, Italy, and Turkey [44], [45]. Remdesivir, the nucleotide analog of adonesis, is a drug used in the treatment of COVID-19 for people older than 12 years in the USA [46].
3. Occurrence and determination methods in the aquatic environment
3.1. Antiviral drugs
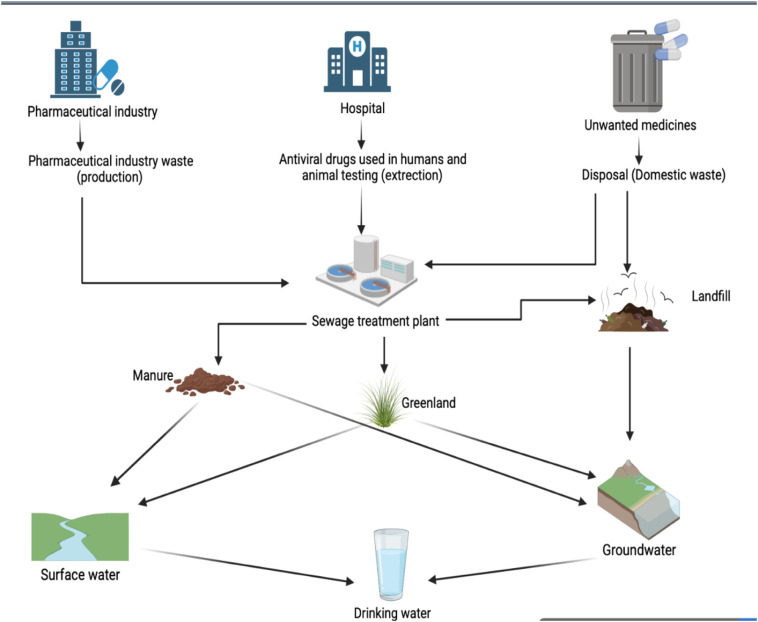
Antiviral drugs have been found in a variety of aquatic environments including raw wastewater, WWTP effluents, surface water and groundwater. Antiviral drugs reaching WWTPs are only partly eliminated, and they may find their way into the environment through hierarchical levels. Fig. 1 shows the many ways by which antiviral drugs can enter the environment from various sources and eventually reach drinking water sources. Unused antiviral drugs are thrown away in the sewage system, drains, and even in the garbage. Antiviral drugs can reach the environment through three major routes: the pharmaceutical industry's effluent, medical waste, and medicines that are out of date, unused, or undesirable are disposed of away [13] .
Fig. 1.
The fate of antiviral drugs in the environment.
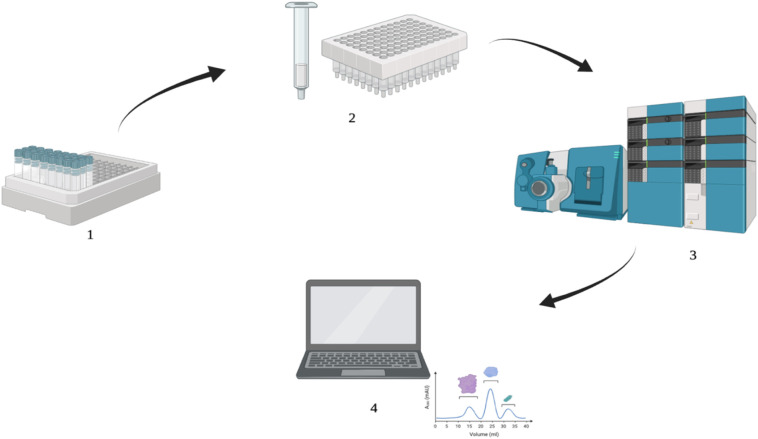
Since antiviral drugs are detected at low concentrations in environmental media, pre-concentration is necessary before analysis. Fig. 2 shows the analysis method for the detection of antiviral drugs. Analysis techniques of antiviral drugs are given in Table 2 . Solid-phase extraction (SPE) is the most often utilized isolation and enrichment procedure in the literature. The SPE procedure can be performed on-line or off-line [47]. Moreover, suspended substances (SSs) such as colloids, microorganisms, and suspended particles, from aqueous environmental samples (i.e wastewater) are removed by filtering before extraction. Otherwise, SSs can occlude both the SPE cartridges and analysis systems [48]. Several SPE cartridges have been utilized to separate antiviral drugs from aqueous matrices in the literature. In the majority of the SPE procedures mentioned in Table 2, Waters Oasis hydrophilic-lipophilic balance (Oasis HLB, USA) cartridges are employed.
Fig. 2.
Analysis method for detection of antiviral drugs (1) samples (2) extraction (3) instrumental analysis (4) data acquisition.
Table 2.
Determination methods for antiviral drugs in water/wastewater.
| Antiviral drug | Metabolite | Matrix | Extraction | Detection method | Wavelength (nm) | Measuring range | References |
|---|---|---|---|---|---|---|---|
| Acyclovir | – | Aqueous solution | – | UV–Vis spectrophotometer | 760 | – | [66] |
| Sofosbuvir | – | Aqueous solution | – | UV–Vis spectrophotometer | 260 | – | [67] |
| Oseltamivir | Oseltamivir carboxylate | Aqueous solution | – | HPLC system tandem with a triple quadrupole mass spectrometer with electro-spray ionization (ESI) | – | – | [59] |
| Staduvine Nevirapine |
– | Wastewater treatment plant influent and effluent | – | HPLC equipped with a fluorescent detector (FLD) | – | – | [60] |
| Acyclovir | – | Aqueous solution | – | High-performance liquid chromatograph equipped with a VP-ODS column and SPD-M20A photodiode array detector | 254 | – | [70] |
| – | Oseltamivir carboxylate | Synthetic river water | SPE | LC-MS/MS | – | 2–20 ng/L (LOQ) | [49] |
| Oseltamivir | – | Aqueous solution | – | HPLC with UV detection | 230 | – | [71] |
| Acyclovir | – | Aqueous solution | – | HPLC | – | – | [61] |
| Acyclovir Lamivudine |
– | Reverse osmosis brine | – | HPLC with UV detection | 254 | – | [72] |
| Acyclovir Zidovudine Lamivudine |
Pure water Fresh water Seawater |
– | Spectrophotometer | 297, 302, 313, 334, 365 and 366 | – | [73] | |
| Oseltamivir | Oseltamivir carboxylate | River water | – | UPCL-MS/MS with electro-spray ionization (ESI) | – | – | [65] |
| Zanamivir | – | Artificial fresh water River |
– | – | – | [74] | |
| Zidovudine Lamivudine Nevirapine |
– | Municipal wastewater | SPE | LC-MS/MS | – | – | [50] |
| Favipiravir Peramivir Laninamivir Oseltamivir Amantadine Zanamivir |
Oseltamivir carboxylate | River water | SPE | LC-MS/MS | – | 0.1–0.2 ng/L (LOD) 0.2–0.7 ng/L (LOQ) |
[51] |
| Zidovudine | – | Aqueous solution | – | HPLC | – | – | [76] |
| Oseltamivir | – | Synthetic wastewater | – | HPLC with UV detection | 215 | 0.2 μM (detection limit) | [77] |
| Acyclovir | – | Aqueous solution | – | HPLC | 254 | – | [62] |
| Lamivudine | – | Aqueous solution | – | HPLC | 275 | – | [63] |
| Oseltamivir phosphate (Tamiflu) | – | Aqueous solution | – | HPLC | 215 | 0.2 μM (detection limit) | [64] |
| Abacavir | – | Aqueous solution | – | HPLC | 271 | – | [78] |
| Lamivudine | – | Aqueous solution | – | HPLC | – | – | [79] |
| Oseltamivir phosphate | Oseltamivir carboxylate | Aqueous solution | – | HPLC with UV detection | 220 | – | [80] |
| Acyclovir Penciclovir |
– | Hospital wastewater | SPE | UPLC-MS/MS | – | – | [81] |
| Acyclovir | – | Pharmaceutical wastewaters | – | HPLC/MS | – | – | [82] |
| Acyclovir Penciclovir |
– | Wastewater treatment plant influent and effluent | – | LC-MS | – | – | [52] |
| Acyclovir | – | Anaerobic sludge | – | LC-ESI-MS/MS | – | – | [53] |
| Acyclovir | – | Synthetic wastewater | SPE | UFLC- 4000 QTRAP hybrid triple quadruple-linear ion trap mass spectrometer (QqLIT-MS) |
– | – | [83] |
| Acyclovir | – | Urban and hospital wastewater | SPE | LC-MS/MS | – | 0.03–50.6 ng/L (LOD) 0.1–167.2 ng/L (LOQ) |
[54] |
| Abacavir Lamivudine Zidovudine |
– | Wastewater treatment plant influent and effluent Surface water |
– | LC-tandem-MS and HPLC | – | – | [84] |
| Oseltamivir | – | Wastewater treatment plant influent and effluent | SPE | LC/MS/MS with ESI-positive ion mode | – | 0.30 ng/L (LOD) 0.92 ng/L (LOQ) |
[55] |
| – | Oseltamivir carboxylate | Synthetic wastewater | – | LC/MS | – | – | [85] |
| Acyclovir Lamivudine | – | Municipal wastewater | SPE | LC/MS | – | – | [86] |
| Acyclovir | – | Pharmaceutical wastewater | – | HPLC/MS | – | 0.5 μg/L (detection limit) |
[87] |
| Acyclovir | – | Wastewater treatment plant effluent | – | LC/MS | – | – | [88] |
| Oseltamivir | Oseltamivir carboxylate | Hospital wastewater | SPE | HPLC-MS/MS | – | – | [89] |
| Acyclovir | – | Wastewater treatment plant secondary effluent | – | HPLC-MS | – | 25 ng/L (LOQ) | [90] |
| Acyclovir | – | Wastewater treatment plant effluent | – | HPLC-MS/MS | – | 0.025 μg/L (LOQ) | [91] |
| Acyclovir | – | Wastewater treatment plant effluent | – | LC-MS/MS | – | 0.0001 mg/L (LOQ) |
[56] |
| Abacavir Lamivudine Nevirapine Zidovudine |
– | Wastewater treatment plant influent and effluent | SPE | LC-MS/MS | – | 2–20 ng/L (LOD) 12–65 ng/L (LOQ) |
[57] |
|
Lamivudine Nevirapine Zidovudine |
– | Wastewater treatment plant influent and effluent | SPE | LC-MS/MS | – | 0.1–1.9 ng/L (LOQ and LOD) |
[58] |
Several methods such as liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], high-performance liquid chromatography (HPLC) [59], [60], [61], [62], [63], [64], ultra-performance liquid chromatography with positive electrospray ionization tandem spectrometry (UPLC-MS/MS) [65] and (ultraviolet) UV–Vis spectrophotometer [66], [67] have been used to determine antiviral drugs from aqueous samples. In most cases, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is used to quantify antiviral drugs from environmental waters and wastewaters due to high selectivity, accuracy, sensitivity and flexibility [68], [69].
3.2. Viruses
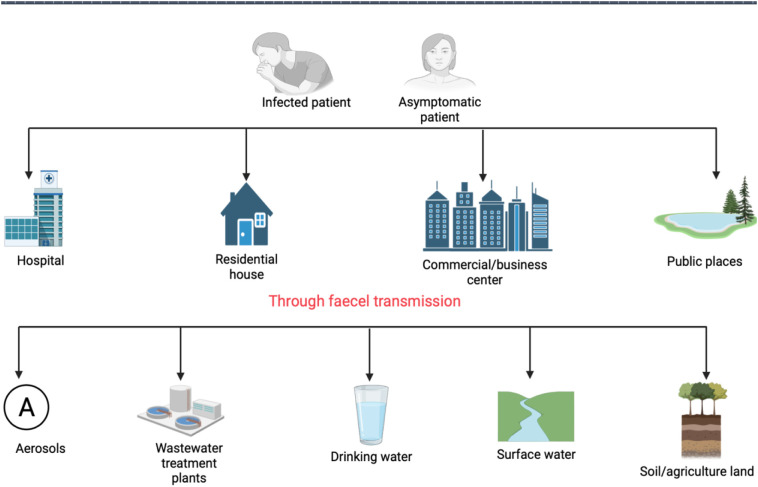
Viruses are common and persistent in raw, treated wastewater and receiving water bodies [92]. Human feces, particularly that of diseased people, is a major source of viruses in wastewater and enteric viruses are discharged in sewage systems by infected people with approximately 105 to 1012 per gram of feces [93]. The bodies that receive treated wastewater are frequently used for recreational activities, agriculture, and as a supply of raw water for the manufacture of drinking water [94]. Pathogenic viruses in wastewater are a concern because of endangering human health [95]. Fig. 3 represents the possible transport ways and the fate of the virus in the environment.
Fig. 3.
Possible transport ways and the fate of the virus in the environment.
Viral outbreaks are a global problem that has a negative impact on public health and safety. Virus diseases, particularly local influenza epidemics, have prompted researchers to focus on virus detection in wastewater and water [96], [97], [98]. COVID-19 has been spreading over the world, infecting millions of people, and causing significant loss of lives and economic damage. The present negative consequences of the COVID-19 pandemic need the development of novel detection tools for future viral outbreaks. Water-based epidemiology (WBE), which monitors viral RNA in wastewater, allows researchers to investigate COVID-19 prevalence and spread in defined populations, which is useful for guiding public health policies [99]. There are excessive data on water and wastewater-based epidemiology in the literature. Some studies are summarized in Table 3 .
Table 3.
Virus detection on water and wastewater.
| Disease | Virus | Source | Concentration method | RNA detection method | Virus concentration | References |
|---|---|---|---|---|---|---|
| Herpes | Herpes Simplex virüs (HSV) | Wastewater treatment center aerosols | The Coriolis®μ (a liquid cyclone) Amicon 100 kDa tangential flow filtration devices (Millipore) |
qPCR, Illumina MiSeq | %3,4-12,1 | [98] |
| Influenza | Avian influenza virus H5N2 | River delta wetland | ND. | qRT-PCR | 0.91 EID50/ml, 0.057 TCID50/ml |
[96] |
| Influenza-A | Fecal and water samples were collected at migratory stopover sites | Filter-concentration through cation charged filters (CCF) | qRT-PCR) | 53 % stopover sites, 7 % and 4.8 % of the fecal and water samples | [97] | |
| COVID-19 | SARS-CoV-2 | WWTP | Electronegative membrane, ultrafiltration | qRT-PCR | 22 % 1.2 × 102 (copies/L) |
[100] |
| Sewage samples | Ultrafiltration | qRT-PCR | 2,6–2200 gene copies per mL | [101] | ||
| Sewage samples | PEG-NaCl precipitation | qRT-PCR | 57–303 copies per ml | [102] | ||
| Primary sewage sludge | ND | qRT-PCR | 1.7 × 103–4.6 × 105 ml−1 copies per ml | [103] | ||
| WWTP | Ultracentrifugation-based method | qRT-PCR | 3.1 to 7.1 log10 genome copies /100 mL | [104] | ||
| WWTP | Two-phase separation method, high-speed centrifugation | qRT-PCR | ND | [105] | ||
| WWTP | Ultrafiltration and an adsorption–elution method using electronegative membranes | qRT-PCR | 7.5 × 103 copies/L from the N1 assay and 3.1 × 103 and 4.3 × 103 copies/LFrom the N2 assay | [106] | ||
| Raw wastewater | PEG precipitation, centrifugation | qRT-PCR | 103 to 105 genome copies per liter (GC/L) | [107] | ||
| Municipal and hospital wastewater | Filtration 80.45-μm pore size) and centrifugal filter with a cutoff of 10 kDa | qRT-PCR | 19.73 % positive | [108] | ||
| River waters | Electronegative membrane-vortex (EMV) method | qRT-PCR | ND | [109] | ||
| River waters | Skimmed Milk Flocculation method, centrifugation | qRT-PCR | N1: 2,91E+05 to 3,19E+06 GC/L N2: 2,07E+05 to 2,22E+06 GC/L |
[110] | ||
| River waters | ND | Real-time RT-PCR and infectivity test on culture cells | Positive nucleocapsid (N) gene and the Orf1ab gene | [111] |
Medema et al. (2020) conducted the first investigation on the occurrence of SARS-CoV-2 in sewage samples in the Netherlands. Sewage water samples were obtained from WWTPs in six Dutch towns. The SARS-CoV-2 virus was found in raw wastewater at the Kaatsheuvel WWTP and the Amsterdam Schiphol Airport's Tilburg WWTP, according to the findings. Notably, the scientists stated that after the first person tested positive for SARS-CoV-2, the first water sample carrying the virus was tracked for 4 days. This was a significant and intriguing discovery in the light of the overall epidemic that is sweeping the globe [101]. Similarly, Ahmed et al. (2020) were the first to report the presence of SARS-CoV-2 in untreated wastewater (sewage) samples taken in Australia at two WWTPs and one suburban pumping station. 22.2 % of the total tested samples were positive. Furthermore, the authors estimated the number of infections using Monte Carlo simulation. If the water samples are found to be positive for SARS-CoV-2, the simulation will be carried out. As a result, the presence of SARS-CoV-2 in raw wastewater can act as an early warning signal in society for COVID-19 infections [100]. Furthermore, Sherchan et al. (2020) collected samples from two WWTPs for four months. The results showed that SARS-CoV-2 RNA was tested positive in roughly 13 % of the raw wastewater samples using RT-qPCR. However, SARS-CoV-2 RNA was not identified in the secondary-treated effluent wastewater or final effluent samples. The findings revealed that the SARS-CoV-2 was eliminated to undetectable levels during wastewater treatment operations [106]. Wu et al. (2020) verified SARS-CoV-2 positivity in Massachusetts. Using RT-qPCR, they evaluated wastewater collected from an urban treatment facility and identified SARS-CoV-2 RNA at 57–303 copies per ml of sewage. The measured viral titers were much greater than predicted based on clinically confirmed cases in Massachusetts at the time [102]. Similarly, Peccia et al. (2020) determined SARS-CoV-2 RNA quantities in primary sewage sludge from the New Haven, Connecticut, metropolitan region. SARS-CoV-2 RNA was found throughout the >10-week research. SARS-CoV-2 RNA concentrations in sludge were 0–2 days ahead of SARS-CoV-2 positive test results by specimen date of collection, 0–2 days ahead of the percentage of positive tests by specimen date of collection, 1–4 days ahead of local hospital admissions, and 6–8 days ahead of SARS-CoV-2 positive test results by reporting date. Their findings demonstrate the value of viral RNA tracking for SARS-CoV-2 infection surveillance at the community level in municipal wastewater [103]. The genome of SARS-CoV-2 was discovered in raw wastewater samples collected in three WWTPs in Italy, according to Rimoldi et al. (2020).The infectivity test, however, proved that the pathogenicity of SARS-CoV-2 coronavirus in wastewater was useless because of the lack of cytopathic impact (CPE). Viruses are frequently destroyed or rendered inactive during water treatment or purification operations [111]. Prado et al. (2021) discussed the results of sanitary sewage monitoring in Rio de Janeiro (Brazil) and its use as an additional indicator in the surveillance of COVID-19 cases, hence helping public health interventions from local authorities. Throughout 20 weeks, 12 composite raw sewage samples were obtained from two WWTPs and alternately from 17 sewer pipes (SP) from nearby neighborhoods. SARS-CoV-2 RNA was identified and quantified using RT-qPCR by the ultracentrifugation-based approach. During the peak of the pandemic, SARS-CoV-2 RNA was found in 84.3 % (188/223) of the samples, with a positive rate ranging from 42 % (5/12) in the first week of monitoring to 100 % and virus concentrations ranged from 3.1 to 7.1 log10 genome copies/100 mL during the investigation. Positive rates in WWTPs were higher than in SP, making them a useful tool for tracking trends in the evolution of the COVID-19 curve, although SP data were more efficient when public health actions were required [104]. The presence of SARS-CoV-2 in Pakistan was investigated by Sharif et al., (2021). A two-phase separation process is utilized for sample extraction and concentration. An additional high-speed centrifugation phase was performed before RNA extraction to increase viral RNA yield. SARS-CoV-2 was found in 78 wastewater samples collected from 38 different locations across Pakistan, as well as 74 wastewater samples from polio monitoring stations. 21 wastewater samples (27 %) from 13 districts tested positive for RT-qPCR. Positive SARS-CoV-2 RNA samples from locations with COVID 19 patients and a quarantine facility support the findings and future application of wastewater surveillance [105]. Recently, Monteiro et al. (2022) used the Charité assays (E Sarbecco, RdRP, and N Sarbecco) to track the dynamics of SARS-CoV-2 RNA at the five WWTPs that serve over two million people in Portugal over 32 weeks. They also studied raw wastewater from three COVID-19 hospitals. SARS-CoV-2 RNA detection was irregular in the first several weeks, with amounts ranging from 103 to 105 genome copies per liter (GC/L). The synchronicity between trends in SARS-CoV-2 RNA daily new COVID-19 cases highlights the value of WBE as a surveillance tool and in raw wastewater, especially after the epidemiological curve has been phased out and hotspots of disease re-appear in the population, which may be difficult to detect based only on contact tracing and syndromic surveillance.
Besides the studies conducted with wastewater limited research was published about tracking SARS-CoV-2 in water bodies. SARS-CoV-2 pollutes the water in a variety of ways. One method is to transfer SARS-CoV-2 infected untreated wastewater to rivers, lakes, or other bodies of water. Another option is to employ processed wastewater effluents that are infected with SARS-CoV-2 as a result of inadequate virus eradication from wastewater. The most common way in areas with poor basic sanitation is to dump raw sewage directly into bodies of water without treatment [95].
Guerrero-Latorre et al. (2020) reported the first detection of SARS-CoV-2 in a river from Quito, Ecuador. The scientists noted that the presence of SARS-CoV-2 in the river was caused by the city's direct release of wastewater into river streams without any treatment [110]. Rimoldi et al. (2020) reported a similar discovery of the SARS-CoV-2 virus in the Lambro River in Italy [111]. Furthermore, Haramoto et al. (2020) studied the presence of the SARS-CoV-2 virus in both WWTP and local river surface water in Japan. The SARS-CoV-2 RNA concentration in five secondary-treated wastewater samples (before chlorination) was quantified at 2.4 103 copies/L, according to the results of RT-qPCR analysis. In contrast, SARS-CoV-2 RNA was not found in the wastewater influent or river samples [109].
Notably, despite the presence of SARS-CoV-2 viral RNA in rivers, an infectivity assay on culture cells revealed that the coronavirus had no infectivity [112].
4. Antiviral drugs treatment technologies
4.1. Non-biological methods
4.1.1. Adsorption
Adsorption processes are used for the treatment of water/wastewater including some pharmaceuticals because of their simple design and low operation cost, low energy requirement and no production of by-products [113], [114], [115]. However, expensive adsorbents are a significant disadvantage [114], [116]. In the adsorption process, various adsorbents such as activated carbons, clays, silica particles, carbon nanotubes, minerals and hydrous metal oxides [113], [117], [118], [119]. One of the main challenges is the sustainable management of spent adsorbents [120]. To solve this problem, regeneration is a promising method that restores the adsorption capabilities of depleted adsorbents by desorbing the pollutants that have already been absorbed. Rather than replacing the adsorbents, it is commonly seen to be a less expensive and superior solution [121] . During wastewater treatment, several methods such as sedimentation, filtration, centrifugation, and magnetic separation techniques are utilized to separate and recover wasted adsorbents [120].
There are parameters such as adsorbent amount, pH, contact time and temperature that affect adsorption performance for the removal of antiviral drugs from water/wastewater. Jain et al. (2014) stated that pH, adsorbent amount and temperature considerably affected the removal of acyclovir from water using adsorption. They also reported these parameters according to their degree of impact as follows: adsorbent amount > temperature > pH [66]. In the study of Babas et al. (2020), the effect of operational parameters that consist of pH (3, 6.8 and 11), amount of adsorbent (10, 20 and 30 g/L) and initial concentration of sofosbuvir (0.05, 0.1 and 0.15 mM).
was investigated on the removal of sofosbuvir which is an antiviral drug for the treatment of Hepatit-C. They discovered that optimum operational parameters were pH 6.8, adsorbent amount 20 g/L and initial sofosbuvir concentration 0.1 mM with the highest removal efficiency of 58.5 % [67].
Wang et al. (2015) investigated the removal of oseltamivir (OE) which is an antiviral drug for the treatment of influenza and oseltamivir carboxylate (OC) which is oseltamivir's metabolite using adsorption. They concluded that when water/wastewater included initial oseltamivir and oseltamivir carboxylate concentration of 10−4 mmol/L, the removal efficiency of these compounds was above 90 % using carbon nanotubes [59]. Kebede et al. (2020) searched treatment of wastewater that included some antiretroviral drugs using an adsorption process that its adsorbent is nanofibers produced from Mondia whitei root extract. They evaluated the effect of adsorbent dose, initial drug concentration, pH, contact time and temperature on adsorption rate. They observed that most of the interaction of adsorbate- adsorbent occurred in the first 30 min. They stated that temperature and pH have a significant effect on the adsorption rate as they affect the physicochemical structure of the adsorbent and the molecule to be removed [60].
Advantages and disadvantages of removal methods for antiviral drugs are given in Table 4 .
Table 4.
Advantages and disadvantages of removal methods for antiviral drugs.
| Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Adsorption | Easy design Low operation cost Low energy requirement |
Expensive adsorbents | [114], [116] |
| Photolysis (UV-based) | Rapid reaction rate Cost-effective Easy operation |
Transformation products can be more permanent than original |
[50], [77], [122] |
| Ozonation | Generation of more biodegradable products | Generation of more harmful by-products | [75], [87] |
| Photocatalysis | Cost-effective Easy operation |
Rare applicability in the real system | [122] |
| The electrochemical advanced oxidation process | Highly effective | Expensive electrodes High energy consumption |
[122] |
| Activated sludge process | Cheaper investment cost Easy operation |
Generation of transformation products that can be harmful | [123], [124], [125], [126] |
| Membrane bioreactor | High-quality permeate Less sludge production Lower environmental impact Small footprint No need chemical High performance |
Biofouling Increase energy consumption rate |
[122], [127], [128], [129] |
4.1.2. Advanced oxidation process
4.1.2.1. Photolysis (UV-based)
Photolysis is one of the advanced oxidation processes that causes a chemical compound to decompose by exposure to artificial or natural light [130]. There are two classes of photolysis: direct and indirect. Direct photolysis is caused by UV absorption, whereas indirect photolysis occurs when an organic compound interacts with photosensitizers such as oxygen and hydroxyl or peroxy radicals [131].
Photolysis is used for the treatment of pharmaceuticals that are included in antiviral drugs in water/wastewater [61], [132], [133], [134]. The efficiency of the photolysis for the removal of antiviral drugs can be affected by the pH, initial concentration of the antiviral drug, chemical properties of the water/wastewater and light source [70], [73], [135]. Due to the pKa value of each antiviral drug, its dissolution form may change at changing pH values. In the study of Jia et al. (2019), with increasing pH from 5 to 9, the ratio of the molecular form of acyclovir was decreased and the ratio of ion form of acyclovir (ACV) increased. Because of less susceptibility of free radicals to negative form of ACV, the photodegradation rate of acyclovir was enhanced. However, the photodegradation rate of ACV was slightly affected by the initial concentration of acyclovir [70].
Blum et al. (2017) examined the treatment of active 30 pharmaceutical ingredients including oseltamivir using photolysis for 28 days in river water. Also, they evaluated the photolysis rate of these compounds in a buffer that included ammonium acetate, filtered river water and unfiltered river water oseltamivir carboxylate was removed 10 to 40 % in three different water content under UV irradiation for 28 days. The different half-life results for the target pharmaceuticals in three different water content reveal the effect of the chemical properties of water sources on the kinetics of the photolysis process [49]. Tong et al. (2011) investigated the treatment of oseltamivir phosphate (Tamiflu) using different combinations of UV-based processes that consist of only UV, UV/H2O2 and UV/H2O2/Fe2+. They reported that since the formation and interaction of hydroxyl ions increases with the addition of H2O2 and Fe2+, the kinetic rate of photolysis to which these chemicals are added increases [71].
4.1.2.2. Ozonation
The breakdown of ozone, which is often used for water/wastewater treatment as a disinfectant and oxidant, leads to the formation of hydroxyl radicals during the ozonation process [136], [137]. Pharmaceuticals are removed using an ozonation method with a high oxidation potential (Eo = 2.07 V) that targets the oxidation of double bonds, amine groups or aromatic structures in their structures [138], [139]. However, more harmful by-products than the original may be generated in water/wastewater treated with ozone treatment, and this toxicity may remain in water/wastewater to some extent [123]. Furthermore, since ozonation generates more biodegradable compounds, it can be used with biological treatment for the elimination of drugs [139], [140]. Prasse et al. (2012) studied the removal of acyclovir and carboxy-acyclovir which is acyclovir's biotransformation product in drinking and surface water using the ozonation process. Results showed that the degradation of acyclovir and carboxy-acyclovir were significantly affected by the pH change, especially due to the effect of pH on amine parts in acyclovir and carboxy-acyclovir structures. COFA which is a by-product of ozonation did not oxidize even with increasing ozone dose while COFA found in wastewater treatment plant (WWTP) effluent decreased with rising ozone dose. They stated that there could be two reasons: (I) due to other reagents formed as a result of ozonation in wastewater and (II) increase of OH radicals with the acceleration of ozonation due to organic matter in wastewater. To test the biodegradability of COFA, COFA was added to the WWTP effluent and treated using biological treatment. There was no degradability of COFA in the first 48 h, but 40 % degraded after 14 days [75]. Therefore, intermediate products that may occur during the ozonation removal of antiviral drugs and their effects on the environment should be analyzed in-depth [76]. Table 5 shows non-biological technologies for the treatment of antiviral drugs.
Table 5.
Non-biological technologies for the treatment of antiviral drugs.
| Antiviral Drugs | Matrix | Concentration | Treatment Technology | Process Conditions | Removal or Q(mg/g) | References |
|---|---|---|---|---|---|---|
| Acyclovir | Distilled water | 400 mg/L | Adsorption | Temperature: 39 °C, pH:8: powdered adsorbent activated carbon, adsorbent dose: 2 g/L | 90.3 % | [66] |
| Sofosbuvir | Distilled water | 0.1 mM | Adsorption | pH: 6.8, adsorbent: e- perlite adsorbent dose: 20 g/L |
58.5 % | [67] |
| Didanosine, Nevirapine, Ritonavir, Efavirenz, Stavudine | Wastewater treatment plant influent and effluent Distilled water |
0.5–1.25 mg/L | Adsorption | Contact time:15 min to 120 min, temperature: 15 to 60 °C,pH: 3 to 12 | 64.9 mg/g-200.5 mg/g | [60] |
| Acyclovir | Distilled water | 100 mg/L | Adsorption | Temperature: 45 °C, pH:11, adsorbent: powdered activated charcoal, adsorbent dose: 4 g/L, equilibrium contact time: 75 min. | 98 % | [141] |
| Zidovudine (ZDV) Lamivudine (3TC) Nevirapine (NVP) |
Wastewater treatment plant effluent | 20 μM | Photolysis (UV) | pH:7.7–8.1, electrical energy dose: 6.67 kWh/103 L H2O2 dose:20.4 mg/L, Cl2 dose:42.6 mg/L | ZDV: >90 % 3TC:~50 % NVP:<20 % |
[50] |
| UV/H2O2 | ZDV: >90 % 3TC:~75 % NVP:>55 % |
|||||
| UV/Cl2 | ZDV:~90 % 3TC:~80 % NVP:~20 % |
|||||
| Acyclovir | Open-water treatment wetland |
302 ± 58 ng/L | Photolysis (solar energy) |
Depth: 25–30 cm, pH: 7.7–9.0 NO3−:3.5–7.5 mg/L, DOC: 3.7–5.6 mg/L C, DIC:45–54 mg/L C |
70 % | [142] |
| Acyclovir (ACV) Lamivudine (LMVD) |
RO brine A and B from municipal wastewater reuse facilities | 5 μM | UV/H2O2 | Incident UV fluence: 1000 mJ/cm2 H2O2: 5 mM, K2S2O8: 5 mM |
ACV: ~35 % (RO Brine A) ACV: ~45 % (RO Brine B) LMVD: ~80 % (RO Brine A) LMVD:~95 % (RO Brine B) |
[72] |
| UV/S2O82− | ACV: ~30 % (RO Brine A) ACV:~45 % (RO Brine B) LMVD: 100 % (RO Brine A) LMVD:~100 % (RO Brine B) |
|||||
| Oseltamivir acid (the active metabolite of Tamiflu®) | Secondary effluent in pilot-scale WWTP | 1 μM | Ozonation (O3) | >0.3 g O3 g−1 DOC 0.5 g O3 g−1 DOC |
>50 % < detection limit |
[77] |
| Tamiflu (oseltamivir phosphate) | Ultrapure water | 21 μM | Photocatalysis | P25 (one of the powdered TiO2) concentration: 20 and 100 mg/ L UV-A irradiation time: 80 min pH: 5.8 ± 0.1 |
>95 % | [64] |
| Abacavir | Deionized water | – | Electrochemical Degradation | Anode:Ti/SnO2-Sb Time: in 10 min Current density: 0.2 mA/cm2 |
>97 % | [78] |
| Lamivudine | Deionized water | 2.5 mg/L | Electrochemical Degradation | Current density: 10 mA/cm2 | 98.3 % | [79] |
| Initial pH:6.7 |
4.1.2.3. Photocatalysis
Photocatalysis, which is the interaction of a catalyst with a substrate or photographic result to accelerate a photochemical process, is used to remove pharmaceutical compounds such as antiviral drugs and antibiotics [62], [143], [144], [145]. Although various photocatalysts such as ZnO, Fe2O3, SnO2, ZnS, WO3, CeO2, CdS and TiO2 were used in this process, TiO2 especially is a promising photocatalyst due to its inexpensive, non-toxic and chemical stability [146], [147], [148]. Several parameters affect the removal efficiency of photocatalysis such as pH, irradiation type and concentration, temperature, catalyst concentration and type, and initial pollutant concentration [149], [150], [151], [152], [153]. An et al. (2011) evaluated the effects of pH, amount of TiO2 and initial concentration of lamivudine on the photocatalysis process by using TiO2 under UV for the removal efficiency of lamivudine. While the pH (7.0) and lamivudine concentration (100 μM) were constant, the removal efficiency enhanced to some extent (TiO2 concentration of 1.0 g/L) with increasing TiO2 concentration (0.5–3.0 g/L) and then gradually decreased. The reason for this decrease can be explained as the decrease in UV activity and less stimulation of TiO2 particles with the increase of TiO2 concentration from 1 g/L to 3 g/L. To investigate of pH effect, experiments were carried out from pH 3 to 11. The results showed that the removal efficiency of 98 % between pH 3–9 did not change much, depending on the surface load of the TiO2 particle (change point is 6.3) and the pKa value (4.4) of lamivudine. However, with increasing pH 11, the degradation efficiency decreased by almost 85 %. When the initial lamivudine concentration was increased from 50 to 200 μM, the removal efficiency of lamivudine was decreased due to the reduction of the TiO2 photon absorption rate by taking more photons into the drug [63].
Reactive species called radical scavengers affect the efficiency of photocatalysis reactions. Wang et al. (2015) examined the effect of radical scavengers such as KI, ISO, and NaF on the removal of oseltamivir phosphate (OP) using photocatalytic degradation. Removal of OP was decreased with increasing potassium iodine (KI) while degradation of OP was enhanced with increasing NaF. The use of ISO did not give considerable effect on the removal of OP until CISO /COP = 2, while degradation of OP was improved at CISO /COP > 5. Also, the removal efficiency of OP was >95 % with 20 and 100 mg/L P25 which is one of the powdered TiO2 after 80 min of irradiation of UV-A [64].
4.1.2.4. The electrochemical advanced oxidation process
Electrochemical advanced oxidation processes (EAOPs) are alternative technologies for the removal of pharmaceutical compounds such as antiviral drugs [80], [154], [155]. Electrical current instead of chemicals was used for the production of OH radicals in the electrochemical advanced oxidation process [156]. EAOPs can be applied as two mechanisms for removing target pollutants: (I) direct oxidation is occurred at the surface of an anode or physically and chemically sorbed OH radicals (II) indirect oxidation is defined as the electrochemical production of compounds such as ozone (O3), active bromine or S2O8 2−, hydrogen peroxide (H2O2), active chlorine [157].
The removal of antiviral drugs by electrochemical degradation is affected by parameters such as pH, current density, initial concentration of antiviral drug and various inorganic ions [78], [79]. The degradation of abacavir using electrochemical oxidation was examined by Zhou et al. (2019). They evaluated the effects of current density, pH and some ions on process efficiency for the removal of abacavir. As the electric current density was increased, antiviral drug removal was increased because of the increase in OH radical formation. After the current density was >0.2 mA/cm2 of current, almost all of the abacavir was removed. Abacavir removal can be affected by pH because of its pKa and chemical structure containing an amide group, and this effect may not be significant in some pH ranges. Also, among inorganic ions such as NO3 −, HCO3 − and Cl−, HCO3 − was the most inhibitive ion because of its preventing feature of OH radicals formation [78]. Wang et al. (2019) conducted electrochemical oxidation experiments with different parameters such as pH, current density, initial antiviral drug concentration, and ions to remove lamivudine. They stated that degradation of lamivudine was improved with increasing electrical current density while removal of lamivudine was decreased with increasing initial lamivudine concentration because of the production of OH radicals on the anode surface. pH change did not considerably affect lamivudine removal. Since CO3 .- can occur in the presence of HCO3 − and lamivudine removal was increased because of oxidizing the lamivudine by CO3 .-. However, NO3 − was an inhibiter for the degradation of lamivudine due to the formation of NH3 [79].
4.2. Biological methods
4.2.1. Activated sludge processes
Activated sludge processes consist of many physical, chemical and biological processes containing oxidation, sorption, volatilization and mainly biodegradation and can be used for the removal of pharmaceuticals including antiviral drugs [52], [158]. Compared to advanced processes, the activated sludge process has cheaper investment cost than most advanced processes and can be easily operated. Pharmaceutical compounds can be transformed into a toxic form as a result of removal using other treatment processes such as chlorination and ozonation while organic and inorganic materials are oxidized and turned into gases and sludge in the activated sludge process [123], [124], [125], [126].
The degradation of antiviral drugs by the activated sludge process can be explained by the oxidation of the hydroxyl-moiety to the carboxyl-moiety [84]. Xu et al. (2017) examined the biological degradation of acyclovir using an activated sludge process under different ammonium conditions. Results showed that acyclovir biodegraded to carboxyacyclovir even with different initial concentrations and different ammonium conditions. Also, the removal of acyclovir was enhanced with an increasing ammonium oxidation rate [83].
There are several parameters such as pH, the amount of dissolved oxygen, hydraulic retention time (HRT), organic loading rate (OLR), solids residence time (SRT), temperature and microbial community that affects on removal performance of the activated sludge process [159], [160], [161], [162], [163], [164]. Matsua et al. investigated the biodegradation rate of pharmaceuticals including oseltamivir in wastewater treatment plant using activated sludge process. They stated that oseltamivir was removed at <50 %. Four different wastewater that have different SRT and temperature values were used in their study. The removal efficiency of oseltamivir and other pharmaceuticals was increased when the temperature was high and SRT was long [55]. Treatment of three pharmaceutical wastewaters that have different acyclovir concentrations and other characteristics such as TOC, and COD using aerobic biological process were examined by Mascolo et al. (2010). They found that almost all acyclovir was biologically removed. But acyclovir in wastewater that has the highest TOC and acyclovir concentration was treated slower than the other two wastewater because of the degradation time of the TOC [82]. The removal efficiency of pharmaceutical compounds including antiviral drugs by activated sludge is related to LogKow values which are measures of hydrophobicity. Muriuki et al. (2020) stated that since nevirapine and lamivudine have high hydrophobicity properties, these antiviral drugs can be easily adsorbed to solid. This adsorption capacity was also affected by sludge age. The adsorption capacity decreased with increasing sludge aging [58]. Also, Azuma et al. (2018) stated that the degradation of antiviral drugs in activated sludge is associated with log Kd value which is the solid-water partition coefficient [81].
Biological technologies for the treatment of antiviral drugs are presented in Table 6 .
Table 6.
Biological technologies for the treatment of antiviral drugs.
| Antiviral drugs | Matrix | Concentration | Treatment technology | Process conditions | Removal | References |
|---|---|---|---|---|---|---|
| Oseltamivir | Municipal sewage treatment plant influent | 5–100 ng/L | Activated sludge process | Effluent quantity:~49 m3, Temperature: 27 °C SRT: ~14 day |
<50 % | [55] |
| Acyclovir | Three pharmaceutical wastewater | 240 mg/L | Activated sludge process | TOC: 9900 mg/L COD: 26700 mg/L pH:6.7 Time: 28 day Temperature: 20–25 °C |
>90 % | [82] |
| 170 mg/L | TOC: 20200 mg/L COD: 54800 mg/L pH:6.4 Time: 28 day Temperature: 20–25 °C |
>90 % | ||||
| 2580 mg/L | TOC: 29250 mg/L COD: 81550 mg/L pH:6.3 Time: 28 day Temperature: 20–25 °C |
>90 % | ||||
| Abacavir | Municipal wastewater | ~30 ng/L | Aerobic treatment system | HRT: 9.9–11.4 h SRT: 24.2–27.7 days Flow rate: ~27,000 m3/gün |
~80 % | [86] |
| Acyclovir | 600 ng/L | ~65 % | ||||
| Emtricitabine | 15–20 ng/L | <10 % | ||||
| Lamivudine | 90–100 ng/L | ~70–75 % | ||||
| Acyclovir | Hospital wastewater | – | Activated sludge treatment | Dissolved oxygen concentration: 8 mg/L Temperature: 20 °C and dark Reactor volume:2 L |
~70 % | [81] |
| Famciclovir | 100 % | |||||
| Penciclovir | ~90 % | |||||
| Valaciclovir | 100 % | |||||
| Acyclovir | Milli-Q water | 15 mg/L | Activated sludge treatment with the addition of nitrifying culture | HRT: 24 h SRT: 15 day Dissolved oxygen concentration: 2.5–3 mg/L pH: 7.5–8 Initial ammonium: 50 mg/L |
65.1 % | [83] |
| 15 μg/L | 88.2 % | |||||
| Acyclovir | Urban and hospital wastewater | – | Aerobic biological treatment | Secondary treatment with disinfection | ~80 % | [54] |
| Acyclovir | Pharmaceutical wastewater | 154 mg/L | Membrane bioreactor | Feed flow rate: 1.6 L/day Recirculation flow rate: 4.8 L/day Membrane module: hollow fiber membrane Membrane surface area: 0.047 m2 |
~98 % | [87] |
| Acyclovir | Wastewater treatment effluent | – | Membrane bioreactor | MBR system volume: 250 L HRT: 10 h |
60–90 % | [88] |
| Abacavir | Municipal wastewater | ~30 ng/L | Staged anaerobic fluidized membrane bioreactor | Flow rate: 5.5 m3/day Two anaerobic fluidized bed reactor HRT: 6.8 h SRT: 36 days |
~80 % | [86] |
| Acyclovir | 600 ng/L | >95 % | ||||
| Emtricitabine | 15–20 ng/L | ~50 % | ||||
| Lamivudine | 90–100 ng/L | >90 % |
4.2.2. Membrane bioreactor
Membrane bioreactor (MBR), which is a modification of the activated sludge process, is used for the treatment of pharmaceuticals including antiviral drugs and consists of a biological reactor and a membrane module. Membrane filters the particulate from waste in the reactor and ensures the purification of the wastewater [89], [165], [166], [167]. The membrane bioreactor is operated under two major configurations: submerged MBR and external MBR [168]. Compared to the conventional activated sludge process, the membrane bioreactor has several advantages such as high-quality permeate, less sludge production, operation at higher mixed liquor suspended solids (MLSS) concentrations, lower environmental impact and small footprint [127], [128], [129].
Treatment of pharmaceutical wastewater containing acyclovir using membrane bioreactor was investigated by Mascolo et al. (2010). Results showed that the removal efficiency of acyclovir using MBR was approximately 98 %. Some by-products formed as negative ions were found after MBR treatment. These by-products were reduced by 90 % with the MBR treatment method [87]. Arriaga et al. carried out a study about the treatment of organic micropollutants such as acyclovir in the effluent of wastewater treatment plant using MBR. They operated MBR in two stages. Stage 1 operated with continuous feeding that included some pharmaceuticals while stage 2 operated without the addition of pharmaceuticals. Acyclovir removal was approximately 60 % for stage 1 and around 90 % for stage 2 [88]. Performance of two-staged anaerobic fluidized membrane bioreactor (SAF-MBR) with granular activated carbon was compared to activated sludge for treatment of pharmaceutical compounds including some antiviral drugs and their by-products after disinfection by McCurry et al. (2014). They stated that similar to aerobic processes, the anaerobic system occurs as sorption due to hydrophobicity in the removal of pharmaceuticals containing antiviral drugs. It was reported that except for emtricitabine whose removal rate was almost 50 %, acyclovir, abacavir and lamivudine were removed at >80 % using SAF-MBR [86].
4.3. Combined processes
The removal of antiviral drugs in water/wastewater has been investigated by combining processes. Knopp et al. (2016) investigated the removal of micropollutants containing antiviral drugs using biological treatment combined with ozonation. Then, experiments were carried out with two biological filters or granular activated carbon filters. They stated that acyclovir was removed 94 % with only biological treatment and the concentration of carboxy-acyclovir that is transformation product of acyclovir was increased. Acyclovir and carboxy-acyclovir were removed 100 % and carboxy-acyclovir converted into N-(4-carbamoyl-2-imino-5-oxo imidazolidin)-formamido-N-methoxyacetetic acid (COFA) which is more toxic than acyclovir during ozonation. They reported that both GAC filters and biological filters failed to reduce COFA [91]. Mascolo et al. (2010) examined the treatment of pharmaceutical wastewater that contained acyclovir using a membrane bioreactor coupled with ozonation. Results showed that removal of acyclovir was approximately 100 % using membrane bioreactor-ozonation. Removal of the by-product formed during ozonation has been effectively achieved once it has been re-entered into the MBR system. If this treatment configuration (ozonation after MBR) is used, similar by-product elimination can only be achieved when the ozonation is run for >60 min, resulting in a high operational cost [87]. Schlüter-Vorberg et al. (2015) investigated the degradation of acyclovir by biological treatment integrated ozonation. Also, they evaluated the toxicity of acyclovir and its transformation products for the environment. Acyclovir was completely converted to carboxy-acyclovir which is the only transformation product from acyclovir during biological treatment. During ozonation, carboxy-acyclovir converted to COFA and unidentified transformation products. According to ecotoxicological tests, while the reported toxicity of C-ACV and COFA does not imply an intolerable environmental risk, the findings highlight the need to research the toxicity of TPs in general, especially if they are generated from parent pharmaceuticals such as ACV that have no aquatic toxicity [56].
5. Future perspectives
In recent years, the occurrence and fate of antiviral drugs in the environment have attracted the attention of scientists, particularly in light of the COVID-19 pandemic. These drugs accumulate in the environment since they are persistent and resistant to biodegradation. Even at low concentrations, they can have negative effects on the aquatic environment. Therefore, various removal processes have been investigated to address environmental pollution issues.
Several antiviral drugs enter the environment and can eventually reach some even drinking water supplies [13]. Therefore, effective antiviral drug treatment in WWTPs is critical. The removal of these pharmaceuticals by most current treatments used in WWTPs (such as coagulation, flocculation, sedimentation, and filtration) can be ineffective [169]. Also, there is a great lack of the removal of antiviral drugs from WWTPs in the literature. Future studies should be performed on the occurrence, removal and mass loads of antiviral drugs in WWTPs.
Advanced oxidation processes (AOPs) have emerged as a viable option because of the resistant nature of effluents containing antiviral drugs. Ozonation and photodegradation that have been mostly used in the literature are successful in removing antiviral drugs in several studies [49], [70], [71], [75], [76]. However, these treatment methods can cause the production of more permanent products than the original antiviral drug [65]. Therefore, novel treatment methods for removing antiviral drugs from water/wastewater should be investigated. Adsorption, membrane processes and electrolysis can also be utilized to remove antiviral drugs, their metabolites and transformation products from water/wastewater.
Adsorption can be used as an alternative removal technique for the treatment of antiviral drugs, although it is not commonly employed to remove antiviral drugs. This approach was found to be quite successful (antiviral drug removal efficiency of 58.5–90) [59], [67]. In addition, the adsorbent materials can be fabricated from agricultural residues and offer an economical alternative because of their low cost [170]. Therefore, there is a need for more studies on the treatment of antiviral drugs from water/wastewater using an adsorption process with different low-cost adsorbents.
Membrane processes, such as reverse osmosis, nanofiltration and membrane bioreactors, have attracted a lot of attention in the pharmaceutical industry [171], [172], [173], [174]. Antiviral drugs can be removed and recovered without any chemical modifications by the use of appropriate membranes. The generated water was quite clean and could be reused without additional treatment in a circular economy approach.
Although combined techniques are not widely used, they are one of the most effective strategies for removing antiviral drugs from water/wastewater and significantly reducing the toxicity of treated water/wastewater. The most common combination approach is an AOPs followed by biological treatment, membrane, or even an adsorption process [56], [87], [91]. Because of their complexity, high operation costs, and in most cases, inability to operate in a continuous mode, these approaches are rarely used [130].
A wide range of antiviral drugs have been utilized to treat COVID-19 patients. Favipiravir is one of the antiviral drugs used to treat COVID-19. In the literature, there are still few investigations on the presence, removal, fate, and ecotoxicological impacts of favipiravir and other antiviral drugs used to treat COVID-19 [175], [176] . Because of the potential negative impacts of these antiviral drugs on the environment, both their transportation and their environmental impact must be thoroughly examined.
6. Conclusions
In recent years, the presence and fate of antiviral drugs in environmental matrices have gotten a lot of interest from the scientific community. These pharmaceuticals are chemically stable and resistant to biodegradation, accumulating in the environment. Even at low concentration levels, they can negatively affect aquatic and terrestrial ecosystems. Therefore, it is of great importance to investigate various degradation/removal methods of these drugs to address environmental pollution concerns. Robust and sensitive analytical approaches are necessary to investigate the risks posed by antiviral drugs in terms of consumption and persistence in the environment. Given the restricted methods for detecting antiviral drugs in an aqueous solution, more accurate detection approaches are required. The majority of the research is focused on the removal of oseltamivir. In recent years, the use of antiviral drugs has increased, especially with COVID-19, and this causes an increase in the concentration of these drugs in the environment. Thus, more study is needed to effectively remove additional antiviral drugs from water/wastewater. However, data on the metabolites of antiviral drugs, their removal products, measurement and fate are largely lacking in knowledge. The gap in this area needs to be filled.
Abbreviations
- 3TC
Lamivudine
- ACV
Acyclovir
- AOPs
Advanced oxidation processes
- ARVs
Antiretroviral drugs
- CCF
Cation-charged filters
- COD
Chemical oxygen demand
- COFA
N-(4-carbamoyl-2-imino-5-oxo imidazolidin)-formamido-N-methoxyacetetic acid
- COVID-19
Novel coronavirus
- EAOPs
Electrochemical advanced oxidation process
- EID50
Egg infectious doses
- EMV
Electronegative membrane-vortex
- FCV
Famciclovir
- GC
Genome copies
- H2O2
Hydrogen peroxide
- HIV-1
Human immunodeficiency virus type 1
- HIV
Human immunodeficiency virus
- HPLC
High-performance liquid chromatography
- HRT
Hydraulic retention time
- HSV-1
Herpes simplex virüs type 1
- HSV-2
Herpes simplex type 2
- HSVs
Herpes simplex viruses
- KI
Potassium iodine
- LC-MS/MS
Liquid chromatography coupled with tandem mass spectrometry
- LMV
Lamivudine
- MBR
Membrane bioreactor
- MLSS
Mixed liquor suspended solids
- MW
Molecular weight
- NAI
Neuraminidase inhibitors
- ND
Not detected
- NNRTIs
Non-nucleoside reverse transcriptase inhibitors
- NRTs
Nucleoside/nucleotide reverse transcriptase inhibitors
- NVP
Nevirapine
- O3
Ozone
- OASIS HLB
Oasis hydrophilic-lipophilic balance
- OC
Oseltamivir carboxylate
- OE
Oseltamivir
- OLR
Organic loading rate
- OP
Oseltamivir phosphate
- qPCR
Quantitative polymerase chain reaction
- qRT-PCR
Quantitative reverse transcription-polymerase chain reaction
- S2O82−
Active bromine
- SAF-MBR
Staged anaerobic fluidized membrane bioreactor
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus
- SPE
Solid-phase extraction
- SRT
Solids residence time
- SSs
Suspended substances
- TCID50
Tissue culture infectious dose
- TOC
Total organic carbon
- UPLC-MS/MS
Ultra-performance liquid chromatography with positive electrospray ionization tandem spectrometry
- UV
Ultraviolet
- VZV
Varicella-Zoster
- WBE
Wastewater-based epidemiology
- WWTPs
Wastewater treatment plants
- ZDV
Zidovudine
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The authors do not have permission to share data.
References
- 1.Couto C.F., Lange L.C., Amaral M.C.S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—a review. J. Water Process Eng. 2019;32:August. [Google Scholar]
- 2.Bilal M., Rizwan K., Adeel M., Iqbal H.M.N. Hydrogen-based catalyst-assisted advanced oxidation processes to mitigate emerging pharmaceutical contaminants. Int. J. Hydrogen Energy. 2021;(xxxx) [Google Scholar]
- 3.Krasucka P., Rombel A., Yang X.J., Rakowska M., Xing B., Oleszczuk P. Adsorption and desorption of antiviral drugs (ritonavir and lopinavir) on sewage sludges as a potential environmental risk. J. Hazard. Mater. 2022;425(November 2021):127901. doi: 10.1016/j.jhazmat.2021.127901. [DOI] [PubMed] [Google Scholar]
- 4.Yao L., Dou W.Y., Ma Y.F., Liu Y.S. Development and validation of sensitive methods for simultaneous determination of 9 antiviral drugs in different various environmental matrices by UPLC-MS/MS. Chemosphere. 2021;282(May) doi: 10.1016/j.chemosphere.2021.131047. [DOI] [PubMed] [Google Scholar]
- 5.Nannou C., Ofrydopoulou A., Evgenidou E., Heath D., Heath E., Lambropoulou D. Antiviral drugs in aquatic environment and wastewater treatment plants: a review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020;699 doi: 10.1016/j.scitotenv.2019.134322. [DOI] [PubMed] [Google Scholar]
- 6.Erik De Clercq G.L. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29 doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao L., et al. Occurrence, removal and mass loads of antiviral drugs in seven wastewater treatment plants with various treatment processes. Water Res. 2021;207(August) doi: 10.1016/j.watres.2021.117803. [DOI] [PubMed] [Google Scholar]
- 8.Nippes R.P., Macruz P.D., da Silva G.N., Neves Olsen Scaliante M.H. A critical review on environmental presence of pharmaceutical drugs tested for the COVID-19 treatment. Process Saf. Environ. Prot. 2021;152:568–582. doi: 10.1016/j.psep.2021.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020;8(4) doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . 2022. Coronavirus (COVID-19) Dashboard. [Google Scholar]
- 11.Kumari M., Kumar A. Can pharmaceutical drugs used to treat COVID-19 infection leads to human health risk? A hypothetical study to identify potential risk. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva M., Baltrus J.P., Williams C., Knopf A., Zhang L., Baltrusaitis J. General heterogeneous photo-Fenton-like degradation of emerging pharmaceutical contaminants in wastewater using Cu-doped MgO nanoparticles. Appl. Catal. A. 2022;630(December 2021):1–13. [Google Scholar]
- 13.Jain S., Kumar P., Vyas R.K., Pandit P., Dalai A.K. Occurrence and removal of antiviral drugs in environment: a review. Water. Air. Soil Pollut. 2013;224(2) [Google Scholar]
- 14.Kumari M., Kumar A. Environmental and human health risk assessment of mixture of COVID-19 treating pharmaceutical drugs in environmental waters. Sci. Total Environ. 2022;812 doi: 10.1016/j.scitotenv.2021.152485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nannou C., Ofrydopoulou A., Evgenidou E., Heath D., Heath E., Lambropoulou D. Analytical strategies for the determination of antiviral drugs in the aquatic environment. Trends Environ. Anal. Chem. 2019;24 [Google Scholar]
- 16.National Center for Biological Information , “No Title,” 2021.
- 17.Drugbank Online , “No Title,” 2021. .
- 18.Friedman W.H. Antiretroviral drug access and behavior change. J. Dev. Econ. 2018;135(August):392–411. [Google Scholar]
- 19.Ncube S., Madikizela L.M., Chimuka L., Nindi M.M. Environmental fate and ecotoxicological effects of antiretrovirals: a current global status and future perspectives. Water Res. 2018;145:231–247. doi: 10.1016/j.watres.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Russo D., et al. Removal of antiretroviral drugs stavudine and zidovudine in water under UV254 and UV254/H2O2 processes: quantum yields, kinetics and ecotoxicology assessment. J. Hazard. Mater. 2018;349(January):195–204. doi: 10.1016/j.jhazmat.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Allahverdiyev A.M., et al. Fight. Multidrug Resist. With Herb. Extr. Essent. Oils Their Components. 2013. Development of new antiherpetic drugs based on plant compounds; pp. 245–259. [Google Scholar]
- 22.Faccin-Galhardi L.C., et al. Assessment of antiherpetic activity of nonsulfated and sulfated polysaccharides from Azadirachta indica. Int. J. Biol. Macromol. 2019;137:54–61. doi: 10.1016/j.ijbiomac.2019.06.129. [DOI] [PubMed] [Google Scholar]
- 23.Nováková L., Pavlík J., Chrenková L., Martinec O., Červený L. Current antiviral drugs and their analysis in biological materials – part II: antivirals against hepatitis and HIV viruses. J. Pharm. Biomed. Anal. 2018;147:378–399. doi: 10.1016/j.jpba.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Greeley Z.W., Giannasca N.J., Porter M.J., Margulies B.J. Acyclovir, cidofovir, and amenamevir have additive antiviral effects on herpes simplex virus TYPE 1. Antivir. Res. 2019;176(October):2020. doi: 10.1016/j.antiviral.2020.104754. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien J.J., Campoli-Richards D.M. Acyclovir: an updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989;37(3):233–309. doi: 10.2165/00003495-198937030-00002. [DOI] [PubMed] [Google Scholar]
- 26.Celebioglu A., Uyar T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast- dissolving antiviral drug delivery. Mater. Sci. Eng. C. 2020;(September) doi: 10.1016/j.msec.2020.111514. [DOI] [PubMed] [Google Scholar]
- 27.Saifi Z., Mir S.R., Amin S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: a complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Technol. 2020;(February) [Google Scholar]
- 28.Litster A.L., Lohr B.R., Bukowy R.A., Thomasy S.M., Maggs D.J. Clinical and antiviral effect of a single oral dose of famciclovir administered to cats at intake to a shelter. Vet. J. 2015;203(2):199–204. doi: 10.1016/j.tvjl.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Rezk M.S., El Nashar R.M. Dissolution testing and potentiometric determination of famciclovir in pure, dosage forms and biological fluids. Bioelectrochemistry. 2013;89:26–33. doi: 10.1016/j.bioelechem.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Rizwana B F., Prasana J.C., Muthu S., Abraham C.S. Wavefunction analysis, charge transfer and molecular docking studies on famciclovir and entecavir: potential anti-viral drugs. Chem. Data Collect. 2020;26:1–13. [Google Scholar]
- 31.Suttapanit K., Boriboon J., Sanguanwit P. Risk factors for non-invasive ventilation failure in influenza infection with acute respiratory failure in emergency department. Am. J. Emerg. Med. 2020;(xxxx) doi: 10.1016/j.ajem.2020.08.094. [DOI] [PubMed] [Google Scholar]
- 32.Chan Y., et al. Advanced drug delivery systems can assist in managing influenza virus infection: a hypothesis. Med. Hypotheses. 2020;144(September) doi: 10.1016/j.mehy.2020.110298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu P.H., et al. Acylguanidine derivatives of zanamivir and oseltamivir: potential orally available prodrugs against influenza viruses. Eur. J. Med. Chem. 2018;154:314–323. doi: 10.1016/j.ejmech.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 34.2021. Y. Sharma, C. Horwood, and P. Hakendorf. ur na of. [Google Scholar]
- 35.Chughtai A.A., Tan T.C., Hitchen E.M., Kunasekaran M., MacIntyre C.R. Association of influenza infection and vaccination with cardiac biomarkers and left ventricular ejection fraction in patients with acute myocardial infarction. IJC Heart Vasc. 2020;31 doi: 10.1016/j.ijcha.2020.100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Y., et al. An M2–V27A channel blocker demonstrates potent in vitro and in vivo antiviral activities against amantadine-sensitive and -resistant influenza A viruses. Antivir. Res. 2017;140:45–54. doi: 10.1016/j.antiviral.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kode S.S., Pawar S.D., Tare D.S., Keng S.S., Mullick J. Amantadine resistance markers among low pathogenic avian influenza H9N2 viruses isolated from poultry in India, during 2009–2017. Microb. Pathog. 2019;137(October) doi: 10.1016/j.micpath.2019.103779. [DOI] [PubMed] [Google Scholar]
- 38.Naveja J.J., et al. Union is strength: antiviral and anti-inflammatory drugs for COVID-19. Drug Discov. Today. 2020;00(00) doi: 10.1016/j.drudis.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S.W., et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antivir. Res. 2020;184(August):1–10. doi: 10.1016/j.antiviral.2020.104955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization, “No Title,” 2021. .
- 41.Frediansyah A., Tiwari R., Sharun K., Dhama K., Harapan H. Antivirals for COVID-19: a critical review. Clin. Epidemiol. Glob. Health. 2021;9(July):90–98. doi: 10.1016/j.cegh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acquavia M.A., et al. Detection and quantification of COVID-19 antiviral drugs in biological fluids and tissues. Talanta. 2021;224(August2020) doi: 10.1016/j.talanta.2020.121862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madelain V., et al. Ribavirin does not potentiate favipiravir antiviral activity against Ebola virus in non-human primates. Antivir. Res. 2020;177(December2019) doi: 10.1016/j.antiviral.2020.104758. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020;76(4):370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuboi H., Kasamatsu Y., Matsubara S., Sasao A., Kunimitsu K., Munakata N., Ito T., Tsuchido Y., Yamawaki M., Fujita N. Two cases of novel coronavirus infection (COVID-19) with transient viral elevation using semi-quantitative real-time reverse transcription PCR and symptom relapse after completion of 10 days of favipiravir treatment. J. Infect. Chemother. 2021;27:1072–1075. doi: 10.1016/j.jiac.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy Vegivinti C.T., et al. Remdesivir therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Ann. Med. Surg. 2021;62(December 2020):43–48. doi: 10.1016/j.amsu.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Júnior C.A.Marasco, Sartore D.M., Lamarca R.S., da Silva B.F., Santos-Neto Á.J., lima Gomes P.C.F.d. On-line solid-phase extraction of pharmaceutical compounds from wastewater treatment plant samples using restricted access media in column-switching liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021;1180(August) doi: 10.1016/j.jchromb.2021.122896. [DOI] [PubMed] [Google Scholar]
- 48.da Cunha C.C.R.F., et al. Low-temperature partitioning extraction followed by liquid chromatography tandem mass spectrometry determination of multiclass antibiotics in solid and soluble wastewater fractions. J. Chromatogr. A. 2021;1650 doi: 10.1016/j.chroma.2021.462256. [DOI] [PubMed] [Google Scholar]
- 49.Blum K.M., et al. Removal of 30 active pharmaceutical ingredients in surface water under long-term artificial UV irradiation. Chemosphere. 2017;176:175–182. doi: 10.1016/j.chemosphere.2017.02.063. [DOI] [PubMed] [Google Scholar]
- 50.Ngumba E., Gachanja A., Tuhkanen T. Removal of selected antibiotics and antiretroviral drugs during post-treatment of municipal wastewater with UV, UV/chlorine and UV/hydrogen peroxide. Water Environ. J. 2020;34(4):692–703. [Google Scholar]
- 51.Azuma T., et al. Fate of new three anti-influenza drugs and one prodrug in the water environment. Chemosphere. 2017;169:550–557. doi: 10.1016/j.chemosphere.2016.11.102. [DOI] [PubMed] [Google Scholar]
- 52.Prasse C., Wagner M., Schulz R., Ternes T.A. Biotransformation of the antiviral drugs acyclovir and penciclovir in activated sludge treatment. Environ. Sci. Technol. 2011;45(7):2761–2769. doi: 10.1021/es103732y. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Gil L., et al. Biotransformation of organic micropollutants by anaerobic sludge enzymes. Water Res. 2019;152:202–214. doi: 10.1016/j.watres.2018.12.064. [DOI] [PubMed] [Google Scholar]
- 54.Papageorgiou M., Zioris I., Danis T., Bikiaris D., Lambropoulou D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci. Total Environ. 2019;694 doi: 10.1016/j.scitotenv.2019.07.371. [DOI] [PubMed] [Google Scholar]
- 55.Matsuo H., Sakamoto H., Arizono K., Shinohara R. Behavior of pharmaceuticals in waste water treatment plant in Japan. Bull. Environ. Contam. Toxicol. 2011;87(1):31–35. doi: 10.1007/s00128-011-0299-7. [DOI] [PubMed] [Google Scholar]
- 56.Schlüter-Vorberg L., Prasse C., Ternes T.A., Mückter H., Coors A. Toxification by transformation in conventional and advanced wastewater treatment: the antiviral drug acyclovir. Environ. Sci. Technol. Lett. 2015;2(12):342–346. [Google Scholar]
- 57.Abafe O.A., et al. LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere. 2018;200:660–670. doi: 10.1016/j.chemosphere.2018.02.105. [DOI] [PubMed] [Google Scholar]
- 58.Muriuki C., et al. Mass loading, distribution, and removal of antibiotics and antiretroviral drugs in selected wastewater treatment plants in Kenya. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140655. [DOI] [PubMed] [Google Scholar]
- 59.Wang W.L., et al. Adsorption removal of antiviral drug oseltamivir and its metabolite oseltamivir carboxylate by carbon nanotubes: effects of carbon nanotube properties and media. J. Environ. Manag. 2015;162:326–333. doi: 10.1016/j.jenvman.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 60.Kebede T.G., Seroto M.B., Chokwe R.C., Dube S., Nindi M.M. Adsorption of antiretroviral (ARVs) and related drugs from environmental wastewaters using nanofibers. J. Environ. Chem. Eng. 2020;8(5) [Google Scholar]
- 61.Russo D., et al. Photodegradation and ecotoxicology of acyclovir in water under UV254 and UV254/H2O2 processes. Water Res. 2017;122:591–602. doi: 10.1016/j.watres.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 62.An T., An J., Gao Y., Li G., Fang H., Song W. Photocatalytic degradation and mineralization mechanism and toxicity assessment of antivirus drug acyclovir: experimental and theoretical studies. Appl. Catal. B Environ. 2015;164:279–287. [Google Scholar]
- 63.An T., An J., Yang H., Li G., Feng H., Nie X. Photocatalytic degradation kinetics and mechanism of antivirus drug-lamivudine in TiO 2 dispersion. J. Hazard. Mater. 2011;197:229–236. doi: 10.1016/j.jhazmat.2011.09.077. [DOI] [PubMed] [Google Scholar]
- 64.Wang W., Wu Q., Wang Z., Hu H., Negishi N. Photocatalytic degradation of the antiviral drug Tamiflu by UV-A/TiO 2: kinetics and mechanisms. Chemosphere. 2015;131:41–47. doi: 10.1016/j.chemosphere.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 65.Gonçalves C., Pérez S., Osorio V., Petrovic M., Alpendurada M.F., Barceló D. Photofate of oseltamivir (Tamiflu) and oseltamivir carboxylate under natural and simulated solar irradiation: kinetics, identification of the transformation products, and environmental occurrence. Environ. Sci. Technol. 2011;45(10):4307–4314. doi: 10.1021/es1032629. [DOI] [PubMed] [Google Scholar]
- 66.Jain S., Kumar P., Vyas R.K., Pandit P., Dalai A.K. Adsorption optimization of acyclovir on prepared activated carbon. Can. J. Chem. Eng. 2014;92(9):1627–1635. [Google Scholar]
- 67.Babas H., Kaichouh G., Khachani M., Karbane M.E., Chakir A., Guenbour A., Bellaouchou A., Warad I., Zarrouk A. Equilibrium and kinetic studies for removal of antiviral sofosbuvir from aqueous solution by adsorption on expanded perlite: experimental, modelling and optimization. Surf. Interfaces. 2021;23 doi: 10.1016/j.surfin.2021.100962. [DOI] [Google Scholar]
- 68.Zhang Y., et al. Efficient multiresidue determination method for 168 pharmaceuticals and metabolites: optimization and application to raw wastewater, wastewater effluent, and surface water in Beijing, China. Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114113. [DOI] [PubMed] [Google Scholar]
- 69.Martínez-Piernas A.B., Plaza-Bolaños P., Gilabert A., Agüera A. Application of a fast and sensitive method for the determination of contaminants of emerging concern in wastewater using a quick, easy, cheap, effective, rugged and safe-based extraction and liquid chromatography coupled to mass spectrometry. J. Chromatogr. A. 2021;1653 doi: 10.1016/j.chroma.2021.462396. [DOI] [PubMed] [Google Scholar]
- 70.Jia T.C., et al. Photodegradation mechanisms of acyclovir in water and the toxicity of photoproducts. J. Radioanal. Nucl. Chem. 2019;320(3):823–830. [Google Scholar]
- 71.Tong A.Y.C., et al. UV-induced photodegradation of oseltamivir (Tamiflu) in water. Environ. Chem. 2011;8(2):182–189. [Google Scholar]
- 72.Yang Y., Pignatello J.J., Ma J., Mitch W.A. Effect of matrix components on UV/H2O2 and UV/S2O82- advanced oxidation processes for trace organic degradation in reverse osmosis brines from municipal wastewater reuse facilities. Water Res. 2016;89:192–200. doi: 10.1016/j.watres.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 73.Zhou C., Chen J., Xie Q., Wei X., nan Zhang Y., Fu Z. Photolysis of three antiviral drugs acyclovir, zidovudine and lamivudine in surface freshwater and seawater. Chemosphere. 2015;138:792–797. doi: 10.1016/j.chemosphere.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 74.Zonja B., et al. Evaluation of the phototransformation of the antiviral zanamivir in surface waters through identification of transformation products. J. Hazard. Mater. 2014;265:296–304. doi: 10.1016/j.jhazmat.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Prasse C., Wagner M., Schulz R., Ternes T.A. Oxidation of the antiviral drug acyclovir and its biodegradation product carboxy-acyclovir with ozone: kinetics and identification of oxidation products. Environ. Sci. Technol. 2012;46:2169–2178. doi: 10.1021/es203712z. [DOI] [PubMed] [Google Scholar]
- 76.Funke J., Prasse C., Dietrich C., Ternes T.A. Ozonation products of zidovudine and thymidine in oxidative water treatment. Water Res. X. 2021;11 doi: 10.1016/j.wroa.2021.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mestankova H., Schirmer K., Escher B.I., Von Gunten U., Canonica S. Removal of the antiviral agent oseltamivir and its biological activity by oxidative processes. Environ. Pollut. 2012;161:30–35. doi: 10.1016/j.envpol.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 78.Zhou C., Wang Y., Chen J., Xu L., Huang H., Niu J. High-efficiency electrochemical degradation of antiviral drug abacavir using a penetration flux porous Ti/SnO2–Sb anode. Chemosphere. 2019;225:304–310. doi: 10.1016/j.chemosphere.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y., Zhou C., Chen J., Fu Z., Niu J. Vol. 848. 2019. Bicarbonate enhancing electrochemical degradation of antiviral drug lamivudine in aqueous solution. [Google Scholar]
- 80.Kobayashi T., et al. Application of electrolysis for inactivation of an antiviral drug that is one of possible selection pressure to drug-resistant influenza viruses. J. Virol. Methods. 2013;194(1–2):154–160. doi: 10.1016/j.jviromet.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Azuma T., et al. Performance and efficiency of removal of pharmaceutical compounds from hospital wastewater by lab-scale biological treatment system. Environ. Sci. Pollut. Res. 2018;25(15):14647–14655. doi: 10.1007/s11356-018-1688-9. [DOI] [PubMed] [Google Scholar]
- 82.Mascolo G., et al. Biodegradability of pharmaceutical industrial wastewater and formation of recalcitrant organic compounds during aerobic biological treatment. Bioresour. Technol. 2010;101(8):2585–2591. doi: 10.1016/j.biortech.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 83.Xu Y., Yuan Z., Ni B.J. Biotransformation of acyclovir by an enriched nitrifying culture. Chemosphere. 2017;170:25–32. doi: 10.1016/j.chemosphere.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Funke J., Prasse C., Ternes T.A. Identification of transformation products of antiviral drugs formed during biological wastewater treatment and their occurrence in the urban water cycle. Vol. 98. 2016. pp. 75–83. [DOI] [PubMed] [Google Scholar]
- 85.Slater F.R., Singer A.C., Turner S., Barr J.J., Bond P.L. Pandemic pharmaceutical dosing effects on wastewater treatment: no adaptation of activated sludge bacteria to degrade the antiviral drug Oseltamivir (Tamiflu®) and loss of nutrient removal performance. FEMS Microbiol. Lett. 2011;315(1):17–22. doi: 10.1111/j.1574-6968.2010.02163.x. [DOI] [PubMed] [Google Scholar]
- 86.McCurry D.L., Bear S.E., Bae J., Sedlak D.L., McCarty P.L., Mitch W.A. Superior removal of disinfection byproduct precursors and pharmaceuticals from wastewater in a staged anaerobic fluidized membrane bioreactor compared to activated sludge. Environ. Sci. Technol. Lett. 2014;1(11):459–464. [Google Scholar]
- 87.Mascolo G., et al. Effective organics degradation from pharmaceutical wastewater by an integrated process including membrane bioreactor and ozonation. Chemosphere. 2010;78(9):1100–1109. doi: 10.1016/j.chemosphere.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 88.Arriaga S., et al. Evaluation of a membrane bioreactor system as post-treatment in waste water treatment for better removal of micropollutants. Water Res. 2016;107:37–46. doi: 10.1016/j.watres.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 89.Kovalova L., Siegrist H., Singer H., Wittmer A., McArdell C.S. Hospital wastewater treatment by membrane bioreactor: performance and efficiency for organic micropollutant elimination. Environ. Sci. Technol. 2012;46(3):1536–1545. doi: 10.1021/es203495d. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y., Zhu H., Szewzyk U., Lübbecke S., Uwe Geissen S. Removal of emerging organic contaminants with a pilot-scale biofilter packed with natural manganese oxides. Chem. Eng. J. 2017;317:454–460. [Google Scholar]
- 91.Knopp G., Prasse C., Ternes T.A., Cornel P. Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters. Water Res. 2016;100:580–592. doi: 10.1016/j.watres.2016.04.069. [DOI] [PubMed] [Google Scholar]
- 92.Tm F., Jm F., Jh L., Ial D.S., Pa W., Mp M. Detection of norovirus epidemic genotypes in raw sewage using next generation sequencing. Environ. Int. 2019;123 doi: 10.1016/j.envint.2018.11.054. [DOI] [PubMed] [Google Scholar]
- 93.Gerba C.P., Betancourt W.Q., Kitajima M. How much reduction of virus is needed for recycled water: a continuous changing need for assessment? Water Res. Jan. 2017;108:25–31. doi: 10.1016/j.watres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dias E., Ebdon J., Taylor H. Estimating the concentration of viral pathogens and indicator organisms in the final effluent of wastewater treatment processes using stochastic modelling. Microb. Risk Anal. Apr. 2019;11:47–56. [Google Scholar]
- 95.Corpuz M.V.A., et al. Viruses in wastewater: occurrence, abundance and detection methods. Sci. Total Environ. Nov. 2020;745 doi: 10.1016/j.scitotenv.2020.140910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dovas C.I., Papanastassopoulou M., Georgiadis M.P., Chatzinasiou E., Maliogka V.I., Georgiades G.K. Detection and quantification of infectious avian influenza A (H5N1) virus in environmental water by using real-time reverse transcription-PCR. Appl. Environ. Microbiol. 2010;76(7):2165–2174. doi: 10.1128/AEM.01929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lickfett T.M., Clark E., Gehring T.M., Alm E.W. Detection of Influenza A viruses at migratory bird stopover sites in Michigan, USA. Infect. Ecol. Epidemiol. 2018;8:1. doi: 10.1080/20008686.2018.1474709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brisebois E., et al. Human viral pathogens are pervasive in wastewater treatment center aerosols. J. Environ. Sci. (China) 2018;67:45–53. doi: 10.1016/j.jes.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu F., et al. SARS-CoV-2 RNA concentrations in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmed W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. Aug. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. Jul. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- 102.Wu F., et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. Aug. 2020;5(4) doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peccia J., et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. Oct. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prado T., et al. Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Res. 2021;191 doi: 10.1016/j.watres.2021.116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharif S., et al. Detection of SARs-CoV-2 in wastewater using the existing environmental surveillance network: a potential supplementary system for monitoring COVID-19 transmission. PLoS One. Jun. 2021;16(6) doi: 10.1371/journal.pone.0249568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sherchan S.P., et al. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. Nov. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monteiro S., et al. A wastewater-based epidemiology tool for COVID-19 surveillance in Portugal. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karami C., Dargahi A., Vosoughi M., Normohammadi A., Jeddi F., Asghariazar V., Mokhtari A., Sedigh A., Zandian H., Alighadri M. SARS-CoV-2 in municipal wastewater treatment plant, collection network, and hospital wastewater. Environ. Sci. Pollut. Res. 2021:1–9. doi: 10.1007/s11356-021-15374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. Oct. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. Nov. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rimoldi S.G., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. Nov. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rimoldi S.G., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shin J., et al. Competitive adsorption of pharmaceuticals in lake water and wastewater effluent by pristine and NaOH-activated biochars from spent coffee wastes: contribution of hydrophobic and π-π interactions. Environ. Pollut. 2021;270 doi: 10.1016/j.envpol.2020.116244. [DOI] [PubMed] [Google Scholar]
- 114.Moreno-Pérez J., Pauletto P.S., Cunha A.M., Bonilla-Petriciolet Á., Salau N.P.G., Dotto G.L. Three-dimensional mass transport modeling of pharmaceuticals adsorption inside ZnAl/biochar composite. Colloids Surf.A Physicochem. Eng. Asp. 2021;614(January) [Google Scholar]
- 115.Kim S., et al. Enhanced adsorption performance for selected pharmaceutical compounds by sonicated Ti3C2TX MXene. Chem. Eng. J. 2021;406(June) [Google Scholar]
- 116.Calisto V., Jaria G., Silva C.P., Ferreira C.I.A., Otero M., Esteves V.I. Single and multi-component adsorption of psychiatric pharmaceuticals onto alternative and commercial carbons. J. Environ. Manag. 2017;192:15–24. doi: 10.1016/j.jenvman.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 117.Kadirvelu K., Thamaraiselvi K., Namasivayam C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001;76(1):63–65. doi: 10.1016/s0960-8524(00)00072-9. [DOI] [PubMed] [Google Scholar]
- 118.Pan B., Xing B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008;42(24):9005–9013. doi: 10.1021/es801777n. [DOI] [PubMed] [Google Scholar]
- 119.Vimonses V., Lei S., Jin B., Chow C.W.K., Saint C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009;148(2–3):354–364. [Google Scholar]
- 120.Baskar A.V., et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: a review. Sci. Total Environ. 2022;822 doi: 10.1016/j.scitotenv.2022.153555. [DOI] [PubMed] [Google Scholar]
- 121.Jain A., Kumari S., Agarwal S., Khan S. Water purification via novel nano-adsorbents and their regeneration strategies. Process Saf. Environ. Prot. 2021;152:441–454. [Google Scholar]
- 122.Zhou Y., et al. Synthetic organic antibiotics residues as emerging contaminants waste-to-resources processing for a circular economy in China: challenges and perspective. Environ. Res. 2022;211(March) doi: 10.1016/j.envres.2022.113075. [DOI] [PubMed] [Google Scholar]
- 123.Tang K., et al. Removal of pharmaceuticals, toxicity and natural fluorescence through the ozonation of biologically-treated hospital wastewater, with further polishing via a suspended biofilm. Chem. Eng. J. 2019;359(August):321–330. [Google Scholar]
- 124.Mareai B.M., Fayed M., Aly S.A., Elbarki W.I. Performance comparison of phenol removal in pharmaceutical wastewater by activated sludge and extended aeration augmented with activated carbon. Alex.Eng. J. 2020;59(6):5187–5196. [Google Scholar]
- 125.Yan X., et al. Effects of exogenous N-acyl-homoserine lactones on nutrient removal, sludge properties and microbial community structures during activated sludge process. Chemosphere. 2020;255 doi: 10.1016/j.chemosphere.2020.126945. [DOI] [PubMed] [Google Scholar]
- 126.Shao Y., et al. Sludge characteristics, system performance and microbial kinetics of ultra-short-SRT activated sludge processes. Environ. Int. 2020;143(July) doi: 10.1016/j.envint.2020.105973. [DOI] [PubMed] [Google Scholar]
- 127.Judd S. The status of membrane bioreactor technology. Trends Biotechnol. 2008;26(2):109–116. doi: 10.1016/j.tibtech.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 128.González-Hernández Y., Jáuregui-Haza U.J. Improved integrated dynamic model for the simulation of submerged membrane bioreactors for urban and hospital wastewater treatment. J. Membr. Sci. 2020;624(September):2021. [Google Scholar]
- 129.Jang Y., Kim H.-S., Ham S.-Y., Park J.-H., Park H.-D. Investigation of critical sludge characteristics for membrane fouling in a submerged membrane bioreactor: role of soluble microbial products and extracted extracellular polymeric substances. Chemosphere. 2021;271 doi: 10.1016/j.chemosphere.2021.129879. [DOI] [PubMed] [Google Scholar]
- 130.Homem V., Santos L. Degradation and removal methods of antibiotics from aqueous matrices - a review. J. Environ. Manag. 2011;92(10):2304–2347. doi: 10.1016/j.jenvman.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 131.Giokas D.L., Vlessidis A.G. Application of a novel chemometric approach to the determination of aqueous photolysis rates of organic compounds in natural waters. Talanta. 2007;71(1):288–295. doi: 10.1016/j.talanta.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 132.Pereira V.J., Weinberg H.S., Linden K.G., Singer P.C. UV degradation kinetics and modeling of pharmaceutical compounds in laboratory grade and surface water via direct and indirect photolysis at 254 nm. Environ. Sci. Technol. 2007;41(5):1682–1688. doi: 10.1021/es061491b. [DOI] [PubMed] [Google Scholar]
- 133.Yamamoto H., et al. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: laboratory photolysis, biodegradation, and sorption experiments. Water Res. 2009;43(2):351–362. doi: 10.1016/j.watres.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 134.Gonc̀alves C., Pérez S., Osorio V., Petrovic M., Alpendurada M.F., Barceló D. Photofate of oseltamivir (Tamiflu) and oseltamivir carboxylate under natural and simulated solar irradiation: kinetics, identification of the transformation products, and environmental occurrence. Environ. Sci. Technol. 2011;45(10):4307–4314. doi: 10.1021/es1032629. [DOI] [PubMed] [Google Scholar]
- 135.Zonja B., Gonc C. Evaluation of the phototransformation of the antiviral zanamivir in surface waters through identification of transformation products. Vol. 265. 2014. pp. 296–304. [DOI] [PubMed] [Google Scholar]
- 136.Sgroi M., Anumol T., Vagliasindi F.G.A., Snyder S.A., Roccaro P. Comparison of the new Cl2/O3/UV process with different ozone- and UV-based AOPs for wastewater treatment at pilot scale: removal of pharmaceuticals and changes in fluorescing organic matter. Sci. Total Environ. 2020;(xxxx) doi: 10.1016/j.scitotenv.2020.142720. [DOI] [PubMed] [Google Scholar]
- 137.Pelalak R., Alizadeh R., Ghareshabani E. Enhanced heterogeneous catalytic ozonation of pharmaceutical pollutants using a novel nanostructure of iron-based mineral prepared via plasma technology: a comparative study. J. Hazard. Mater. 2020;392(February) doi: 10.1016/j.jhazmat.2020.122269. [DOI] [PubMed] [Google Scholar]
- 138.Li X., Wang Y., Wang B., Huang J., Deng S., Yu G. Combination of ozonation and electrolysis process to enhance elimination of thirty structurally diverse pharmaceuticals in aqueous solution. J. Hazard. Mater. 2019;368(February 2018):281–291. doi: 10.1016/j.jhazmat.2019.01.062. [DOI] [PubMed] [Google Scholar]
- 139.de Wilt A., et al. Enhanced pharmaceutical removal from water in a three step bio-ozone-bio process. Water Res. 2018;138:97–105. doi: 10.1016/j.watres.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 140.Malik S.N., Khan S.M., Ghosh P.C., Vaidya A.N., Kanade G., Mudliar S.N. Treatment of pharmaceutical industrial wastewater by nano-catalyzed ozonation in a semi-batch reactor for improved biodegradability. Sci. Total Environ. 2019;678:114–122. doi: 10.1016/j.scitotenv.2019.04.097. [DOI] [PubMed] [Google Scholar]
- 141.Jain S., Vyas R.K., Pandit P., Dalai A.K. Adsorption of antiviral drug, acyclovir from aqueous solution on powdered activated charcoal: kinetics, equilibrium, and thermodynamic studies. Desalin. Water Treat. 2014;52(25–27):4953–4968. [Google Scholar]
- 142.Bear S.E., Nguyen M.T., Jasper J.T., Nygren S., Nelson K.L., Sedlak D.L. Removal of nutrients, trace organic contaminants, and bacterial indicator organisms in a demonstration-scale unit process open-water treatment wetland. Ecol. Eng. 2017;109(July):76–83. [Google Scholar]
- 143.Omatoyo M.A.T., Dalrymple K., Yeh Daniel H. Review- Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatalysis. J. Chem. Technol. Biotechnol. 2007;82:121–134. [Google Scholar]
- 144.Ramasundaram S., Yoo H.N., Song K.G., Lee J., Choi K.J., Hong S.W. Titanium dioxide nanofibers integrated stainless steel filter for photocatalytic degradation of pharmaceutical compounds. J. Hazard. Mater. 2013;258–259:124–132. doi: 10.1016/j.jhazmat.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 145.Elmolla E.S., Chaudhuri M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination. 2010;252(1–3):46–52. doi: 10.1016/j.jhazmat.2009.08.104. [DOI] [PubMed] [Google Scholar]
- 146.Kanakaraju D., Glass B.D., Oelgemöller M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014;12(1):27–47. [Google Scholar]
- 147.Orona-Návar C., et al. Removal of pharmaceutically active compounds (PhACs) and bacteria inactivation from urban wastewater effluents by UVA-LED photocatalysis with Gd3+ doped BiVO4. J. Environ. Chem. Eng. 2020;8:6. [Google Scholar]
- 148.El Mouchtari E.M., et al. TiO2 and activated carbon of Argania Spinosa tree nutshells composites for the adsorption photocatalysis removal of pharmaceuticals from aqueous solution. J. Photochem. Photobiol. A Chem. 2020;388(July) [Google Scholar]
- 149.Wang K.H., Hsieh Y.H., Wu C.H., Chang C.Y. The pH and anion effects on the heterogeneous photocatalytic degradation of o-methylbenzoic acid in TiO2 aqueous suspension. Chemosphere. 2000;40(4):389–394. doi: 10.1016/s0045-6535(99)00252-0. [DOI] [PubMed] [Google Scholar]
- 150.De la Cruz N., Dantas R.F., Giménez J., Esplugas S. Photolysis and TiO2 photocatalysis of the pharmaceutical propranolol: solar and artificial light. Appl. Catal. B Environ. 2013;130–131:249–256. [Google Scholar]
- 151.Benabbou A.K., Derriche Z., Felix C., Lejeune P., Guillard C. Photocatalytic inactivation of Escherischia coli. Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B Environ. 2007;76(3–4):257–263. [Google Scholar]
- 152.Yu J., Yu H., Cheng B., Trapalis C. Effects of calcination temperature on the microstructures and photocatalytic activity of titanate nanotubes. J. Mol. Catal. A Chem. 2006;249(1–2):135–142. [Google Scholar]
- 153.Daneshvar N., Salari D., Khataee A.R. Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J. Photochem. Photobiol. A Chem. 2003;157(1):111–116. [Google Scholar]
- 154.Lan Y., Coetsier C., Causserand C., Groenen Serrano K. An experimental and modelling study of the electrochemical oxidation of pharmaceuticals using a boron-doped diamond anode. Chem. Eng. J. 2018;333(June):486–494. [Google Scholar]
- 155.Ashrafi A.M., et al. Determination and detailed mechanism study of antiviral drugfosamprenavir using carbon paste electrode in the presenceof Triton X-100. Electrochim. Acta. 2013;109:381–388. [Google Scholar]
- 156.Bocos E., Alfaya E., Iglesias O., Pazos M., Ángeles Sanromán M. Application of a new sandwich of granular activated and fiber carbon as cathode in the electrochemical advanced oxidation treatment of pharmaceutical effluents. Sep. Purif. Technol. 2015;151:243–250. [Google Scholar]
- 157.García-Espinoza J.D., Nacheva P.M. Degradation of pharmaceutical compounds in water by oxygenated electrochemical oxidation: parametric optimization, kinetic studies and toxicity assessment. Sci. Total Environ. 2019;691:417–429. doi: 10.1016/j.scitotenv.2019.07.118. [DOI] [PubMed] [Google Scholar]
- 158.Wei Z., et al. Electrophilicity index as a critical indicator for the biodegradation of the pharmaceuticals in aerobic activated sludge processes. Water Res. 2019;160:10–17. doi: 10.1016/j.watres.2019.05.057. [DOI] [PubMed] [Google Scholar]
- 159.Hu B., Quan J., Huang K., Zhao J., Xing G. Effects of C/N ratio and dissolved oxygen on aerobic denitrification process: a mathematical modeling study. Chemosphere. 2021;272 doi: 10.1016/j.chemosphere.2020.129521. [DOI] [PubMed] [Google Scholar]
- 160.Izadi P., Izadi P., Eldyasti A. Understanding microbial shift of enhanced biological phosphorus removal process (EBPR) under different dissolved oxygen (DO) concentrations and hydraulic retention time (HRTs) Biochem. Eng. J. 2020;166(September):2021. [Google Scholar]
- 161.Kumar K., Singh G.K., Dastidar M.G., Sreekrishnan T.R. Effect of mixed liquor volatile suspended solids (MLVSS) and hydraulic retention time (HRT) on the performance of activated sludge process during the biotreatment of real textile wastewater. Water Resour. Ind. 2014;5:1–8. [Google Scholar]
- 162.Dionisi D., Rasheed A.A. Maximisation of the organic load rate and minimisation of oxygen consumption in aerobic biological wastewater treatment processes by manipulation of the hydraulic and solids residence time. J. Water Process Eng. 2018;(October 2017) [Google Scholar]
- 163.Pantea L.M., Timothy J.E.A., Lapara M., Nakatsu Cindy H. Aerobic biological treatment of a pharmaceutical wastewater: effect of temperature on cod removal and bacterial community development. Water Res. 2001;35(18):4417–4425. doi: 10.1016/s0043-1354(01)00178-6. [DOI] [PubMed] [Google Scholar]
- 164.Hashimoto K., Matsuda M., Inoue D., Ike M. Bacterial community dynamics in a full-scale municipal wastewater treatment plant employing conventional activated sludge process. J. Biosci. Bioeng. 2014;118(1):64–71. doi: 10.1016/j.jbiosc.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 165.Wisniewski C., Grasmick A. Floc size distribution in a membrane bioreactor and consequences for membrane fouling. Colloids Surf.A Physicochem. Eng. Asp. 1998;138(2–3):403–411. [Google Scholar]
- 166.De Luca G., Sacchetti R., Leoni E., Zanetti F. Removal of indicator bacteriophages from municipal wastewater by a full-scale membrane bioreactor and a conventional activated sludge process: implications to water reuse. Bioresour. Technol. 2013;129:526–531. doi: 10.1016/j.biortech.2012.11.113. [DOI] [PubMed] [Google Scholar]
- 167.Radjenovic J., Petrovic M., Barceló D. Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal. Bioanal. Chem. 2007;387(4):1365–1377. doi: 10.1007/s00216-006-0883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Melin T., et al. Membrane bioreactor technology for wastewater treatment and reuse. Desalination. 2006;187(1–3):271–282. [Google Scholar]
- 169.Ghosh G.C., Nakada N., Yamashita N., Tanaka H. Occurrence and fate of oseltamivir carboxylate (Tamiflu) and amantadine in sewage treatment plants. Chemosphere. 2010;81(1):13–17. doi: 10.1016/j.chemosphere.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 170.Santos D.H.S., et al. Regeneration of activated carbon adsorbent by anodic and cathodic electrochemical process. Process Saf. Environ. Prot. 2022;159:1150–1163. [Google Scholar]
- 171.Lin Y.L., Chiou J.H., Lee C.H. Effect of silica fouling on the removal of pharmaceuticals and personal care products by nanofiltration and reverse osmosis membranes. J. Hazard. Mater. 2014;277:102–109. doi: 10.1016/j.jhazmat.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 172.Prasertkulsak S., Chiemchaisri C., Chiemchaisri W., Yamamoto K. Removals of pharmaceutical compounds at different sludge particle size fractions in membrane bioreactors operated under different solid retention times. J. Hazard. Mater. 2019;368(January):124–132. doi: 10.1016/j.jhazmat.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 173.Dolar D., Vuković A., Ašperger D., Košutić K. Effect of water matrices on removal of veterinary pharmaceuticals by nanofiltration and reverse osmosis membranes. J. Environ. Sci. 2011;23(8):1299–1307. doi: 10.1016/s1001-0742(10)60545-1. [DOI] [PubMed] [Google Scholar]
- 174.Couto C.F., et al. Assessing potential of nanofiltration, reverse osmosis and membrane distillation drinking water treatment for pharmaceutically active compounds (PhACs) removal. J. Water Process Eng. 2020;33(October 2019):101029. [Google Scholar]
- 175.Allahverdiyeva S., Yunusoğlu O., Yardım Y., Şentürk Z. First electrochemical evaluation of favipiravir used as an antiviral option in the treatment of COVID-19: a study of its enhanced voltammetric determination in cationic surfactant media using a boron-doped diamond electrode. Anal. Chim. Acta. 2021;1159 doi: 10.1016/j.aca.2021.338418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kiyanmehr K., Moussavi G., Mohammadi S., Naddafi K. Chemosphere the efficacy of the VUV/O 3 process run in a continuous-flow fluidized bed reactor for simultaneous elimination of favipiravir and bacteria in aqueous matrices. Chemosphere. 2022;304(April) doi: 10.1016/j.chemosphere.2022.135307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.