Abstract
Background
A high proportion of clinicians experienced common anxiety, insomnia and depression during the COVID-19 pandemic. This study examined the item-level association of comorbid anxiety and insomnia symptoms among clinicians who suffered from depressive symptoms during the late stage of the COVID-19 pandemic using network analysis (NA).
Methods
Clinicians with depressive symptoms (with a Patients Health Questionnaire (PHQ-9) total score of 5 and above) were included in this study. Anxiety and insomnia symptoms were measured using the Generalized Anxiety Disorder Scale - 7-item (GAD-7) and Insomnia Severity Index (ISI), respectively. Network analysis was conducted to investigate the network structure, central symptoms, bridge symptoms, and network stability of these disturbances. Expected influence (EI) was used to measure the centrality of index.
Results
Altogether, 1729 clinicians were included in this study. The mean age was 37.1 [standard deviation (SD)=8.04 years], while the mean PHQ-9 total score was 8.42 (SD=3.33), mean GAD-7 total score was 6.45 (SD=3.13) and mean ISI total score was 8.23 (SD=5.26). Of these clinicians, the prevalence of comorbid anxiety symptoms (GAD-7≥5) was 76.8% (95% CI 74.82–78.80%), while the prevalence of comorbid insomnia symptoms (ISI≥8) was 43.8% (95% CI: 41.50–46.18%). NA revealed that nodes ISI7 (“Interference with daytime functioning”) (EI=1.18), ISI4 (“Sleep dissatisfaction”) (EI=1.08) and ISI5 (“Noticeability of sleep problem by others”) (EI=1.07) were the most central (influential) symptoms in the network model of comorbid anxiety and insomnia symptoms in clinicians. Bridge symptoms included nodes PHQ3 (“Sleep”) (bridge EI=0.55) and PHQ4 (“Fatigue”) (bridge EI=0.49). Gender did not significantly influence the network structure, but “having the experience of caring for COVID-19 patients” significantly influenced the network structure.
Conclusion
Central symptoms and key bridge symptoms identified in this NA should be targeted in the treatment and preventive measures for clinicians suffering from comorbid anxiety, insomnia and depressive symptoms during the late stage of the COVID-19 pandemic.
Keywords: depression, anxiety, sleep, network analysis, health personnel
Introduction
Depressive, anxiety and insomnia symptoms were common among clinicians during the Coronavirus disease (COVID-19) pandemic1–4 in many countries including China.5,6 For example, a meta-analysis revealed that the global prevalence of sleep disturbances was 42.47% (95% CI: 37.95–47.12%),7 anxiety symptoms were 20% (95% CI: 15–25%) and depressive symptoms were 22% (95% CI: 18–28%) among healthcare workers during the COVID-19 pandemic.8 Previous studies found that comorbid anxiety and insomnia symptoms were common in individuals with depression, which were associated with more severe outcomes compared to having depressive symptoms alone.9,10 Comorbid sleep problems may also worsen mood symptoms and treatment outcomes in patients with depression,11,12 while comorbid anxiety and depression could aggravate insomnia. Further, people at risk for insomnia were more likely to suffer from both anxiety and depression.13 However, these studies were done only at the disorder or syndrome level based on total scores of standard rating scales, and the relationships between individual anxiety, insomnia and depressive symptoms at the symptom level were rarely reported.
Network analysis (NA) is a novel approach to examine the inter-symptom relationships across different syndromes/disorders. The onset and maintenance of symptoms are determined by tracing a path through the network.14 NA has been used to examine the most central (influential) depressive and anxiety symptoms in adolescents,15,16 adults17–19 and psychiatric samples.20–22 For instance, sad mood, guilt and motor disturbances were identified as the most central symptoms in Wuhan residents during the late stage of the COVID-19 pandemic.19 NA may also be used to elucidate specific pathways by which psychiatric comorbidities occur.23 For example, one study24 explored comorbid major depressive disorder (MDD) and obsessive-compulsive disorder (OCD) and found that certain items such as “Obsessive behavior”, “Cognitions”, “Concentration problems”, “Guilt”, and “Sadness” were the bridge symptoms connecting disorders. Another NA study on comorbid sleep dysfunction and post-traumatic stress disorder (PTSD) symptoms among Filipino domestic workers found that items on “Concentration difficulties”, “Recklessness”, “Inability” and “Sleep dysfunction” were bridge symptoms.25 Additionally, NA studies on depressive and anxiety symptoms found nodes (“Guilty”), (“Sad mood”), and (“Suicide ideation”) were bridge symptoms in adolescents among China.16 To date, no NA studies have examined the relationships between comorbid anxiety and insomnia symptoms in individuals with depression.
In the past years, the relationship between comorbid anxiety and depressive has been examined using NA. For example, Kaiser et al26 found that “Sad mood” and “Inability to control worry” are the most central symptoms for comorbid depression and anxiety disorders. However, considering that the relationship between anxiety and depressive symptoms could have been moderated by sleep problems,27 sleep should be addressed in the assessment and treatment of depression and anxiety.27 A recent NA (N=226) on the association between anxiety, depression and sleep in patients with generalized anxiety disorder (GAD) found that “Anhedonia” was the most central symptom.28 However, the NA study was limited by a small sample size. A recent review on NA suggested that a large sample size should be used in future studies.29
Given that psychiatric comorbidities such as anxiety, insomnia and depressive symptoms are common among clinicians during the COVID-19 pandemic,1–4 we hypothesized that there would be close inter-relationships between certain comorbid anxiety and insomnia symptoms among clinicians. In this study, we examined comorbid of anxiety and insomnia among clinicians who suffered from depressive symptoms in China during the late stage of the pandemic using NA to understand their inter-relationships.
Methods
Study Design and Settings
This was a cross-sectional study on psychiatric comorbidities in clinicians during the late stage of the COVID-19 pandemic conducted from October 13 and 22, 2020 in public hospitals at Beijing, China. Due to the COVID-19 pandemic, face-to-face assessments could not be adopted. Instead, following other studies,30,31 the WeChat-based “QuestionnaireStar” program was used in this study. WeChat is a widely used social communication application, with more than 1.2 billion active users in China. All clinicians in Beijing needed to regularly report personal health status using WeChat during the pandemic; therefore, all participants were presumed to be WeChat users.
Consecutive sampling was used. All clinicians working in public hospitals in Beijing during the late stage of the pandemic were invited by the Beijing Health Authority to participate in this study on a voluntary and anonymous basis. A Quick Response code (QR Code) linked to the introduction of this study and the access to the questionnaire was designed and adopted. After providing electronic written informed consent, participants could access the data collection form and questionnaire by scanning the QR code using their WeChat. To be eligible, participants needed to meet the following criteria: 1) aged 18 years or older; 2) had a Patients Health Questionnaire (PHQ-9) total score of 5 and above, indicating depressive symptoms;32 3) was able to read and understand Chinese. The study protocol was centrally approved by the ethics committee of the Beijing Anding Hospital, Capital Medical University according to the declaration of Helsinki.33
Data Collection and Measures
Severity of depressive symptoms was assessed using the validated Chinese version of the self-report PHQ-9,34,35 with each item scored from “0” (not at all) to “3” (nearly every day) and total score ranged from 0 to 27. A PHQ-9 total score of “5–9” was considered “having mild depressive symptoms”,32 “10–14” was considered “having moderate depressive symptoms”, “15–19” was considered “having moderately-severe depressive symptoms” and “20–27” was considered “having severe depressive symptoms” The internal consistency reliability and criterion validity of the Chinese version of the PHQ-9 was 0.86 and 0.86, respectively.34,35 Severity of anxiety was measured using the validated Chinese version of the self-report Generalized Anxiety Disorder Scale 7-item (GAD-7),36,37 which comprises 7 items with each scoring from “0” (not at all) to 3 (nearly every day) and total score ranged from 0 to 21. Those having a total GAD score ≥5 were considered “having anxiety symptoms”.36 The internal consistency reliability and criterion validity of the Chinese version of the GAD-7 was 0.90 and 0.86, respectively.36,37 The validated Chinese version of the self-report Insomnia Severity Index (ISI)38,39 was used to assess severity of insomnia. The ISI consists of seven items scoring from “0” (none) to “4” (very severe) with the total score ranged from 0 to 28.40 Those with an ISI total score ≥8 were considered “having insomnia symptoms”.40 The internal consistency reliability and criterion validity of the Chinese version of the ISI was 0.80 and 0.89, respectively.38,39 Quality of life (QOL) was measured with the first two 2 items on global QOL of the self-report World Health Organization Quality of Life Scale Brief version (WHOQOL-BREF),41,42 with the total score ranged from 2 to 10 and a higher score reflecting a higher QOL. The internal consistency reliability and construct validity of the Chinese version of the WHOQOL-BREF was 0.89 and 0.86, respectively.41,42
Statistical Analyses
Network Structure
The network of comorbid anxiety, insomnia and depression was computed using the R software.43 We computed the polychoric correlations between all the PHQ-9, GAD-7 and ISI items to investigate the edges of the network, and estimated the Graphical Gaussian Model (GGM), a popular network model, with the graphic least absolute shrinkage and selection operator (LASSO) and Extended Bayesian Information Criterion (EBIC) model using the R package “qgraph”.44 GGM is a pairwise Markov random field (PMRF) model used for interval or ordinal data. The edges in the GGM could be interpreted as partial correlation coefficients. The network was visualized using the “qgraph” package, where thicker edges represented stronger relationships between nodes. We also estimated the centrality index Expected Influence (EI) of nodes to determine which symptoms are more central (influential) in the network.20 EI refers to the summed weight of edges shared with the remaining nodes in the network.45 To further assess the accuracy of the index, we used the bootstrap method in the “bootnet” package to investigate the stability of the central index – EI using the correlation stability coefficient (CS-coefficient). We set the cut-off for CS-coefficient at 0.25 for all the indices, because evidence showed that when centralities do not differ from one another, CS-coefficient is mostly below 0.25.44
In addition, we estimated the bridge EI centrality index using the bridge function of the R package “networktools”.46,47 Based on previous findings,26,48 bridge EI was the best index in identifying nodes that, if deactivated, could prevent activation spreading from one disorder to another one. Therefore, the network that showed the nodes with the highest bridge EI was selected in this study. Further, the predictability of each node was estimated using the package “mgm”. Predictability was defined as the variance in a node that could be explained by all other nodes in the network.49
Network Comparison
Following previous studies,6,50 the differences of network characteristics between male and female participants, and between those with and without the experience of caring for COVID-19 patients were compared, using the R “NetworkComparisonTest” package (Version 2.2.1)51 with 1000 permutations. Differences in network structure (eg, distributions of edge weights), global strength (eg, total absolute connectivity among the symptoms), and each specific edge between subsamples (ie, females vs males; those with experiences of caring for COVID-19 vs without experiences caring for COVID-19) were also examined.
Results
Study Sample
Altogether 1729 clinicians, who fulfilled the study entry criteria and completed the assessment, were included in this study. The prevalence of mild depressive symptoms was 80.05%, moderate depressive symptoms was 14.5%, moderately severe depressive symptoms was 4.51% and severe depressive symptoms was 1.39%. The mean age was 37.1 [standard deviation (SD)=8.04 years], mean PHQ-9 score was 8.42 (SD=3.33), mean GAD-7 score was 6.45 (SD=3.13) and mean ISI score was 8.23 (SD=5.26). Of clinicians with depressive symptoms, the prevalence of comorbid anxiety symptoms was 76.8% (95% CI 74.82–78.80%) (GAD-7≥5), while prevalence of comorbid insomnia symptoms was 43.8% (95% CI: 41.50–46.18%) (ISI≥8) (Table 1).
Table 1.
Demographic and Clinical Characteristics of Participants (n=1729)
| Variables | |
|---|---|
| N (%) | |
| Male gender | 371 (21.46) |
| Occupation type | |
| Doctor | 428 (24.75) |
| Nurse | 907 (52.46) |
| Medical technician | 280 (16.19) |
| Administration staff | 114 (6.60) |
| Experience of caring for COVID-19 patients | 987 (57.09) |
| Mean (SD) | |
| Age (year) | 37.1 (8.04) |
| PHQ-9 total score | 8.42 (3.33) |
| GAD-7 total score | 6.45 (3.13) |
| ISI total score | 8.23 (5.26) |
| WHOQOL-BREF | 6.17 (1.45) |
Abbreviations: SD, standard deviation; PHQ-9, 9-items Patients Health Questionnaire; GAD-7, Generalized Anxiety Disorder Scale 7-item; ISI, Insomnia Severity Index; WHOQOL-BREF, World Health Organization Quality of Life Scale Brief version.
Network Structure
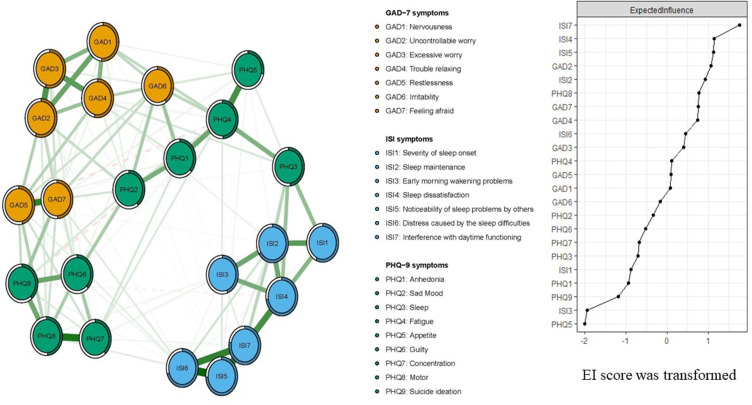
Figure 1 presents the network structure of comorbid anxiety and insomnia symptoms in clinicians with depressive symptoms. The predictability of symptoms is shown as ring-shaped pie charts. The mean predictability was 0.51, indicating that on average 51% of each node’s variance could be accounted for by other nodes. The model showed that the edge between GAD7 (“Feeling afraid”) and GAD5 (“Restlessness”) was the strongest positive edge in the anxiety community, followed by the edge between GAD2 (“Uncontrollable worry”) and GAD3 (“Excessive worry”), and the edge between GAD3 (“Excessive worry”) and GAD4 (“Trouble relaxing”). In the depression community, the edge between PHQ7 (“Concentration”) and PHQ9 (“Suicide ideation”) was the strongest connection, followed by the edge between nodes PHQ4 (“Fatigue”) and PHQ5 (“Appetite”) and the edge between nodes PHQ6 (“Guilty”) and PHQ9 (“Suicide ideation”). In the insomnia community, the edge between ISI5 (“Noticeability of sleep problem by others”) and ISI6 (“Distress caused by the sleep difficulties”) was the strongest connection, followed by the edge between ISI5 (“Noticeability of sleep problem by others”) and ISI7 (“Interference with daytime functioning”) and the edge between ISI6 (“Distress caused by the sleep difficulties”) and ISI7 (“Interference with daytime functioning”).
Figure 1.
Network structure of depression, anxiety and insomnia in clinicians during the late stage of the COVID-19 pandemic.
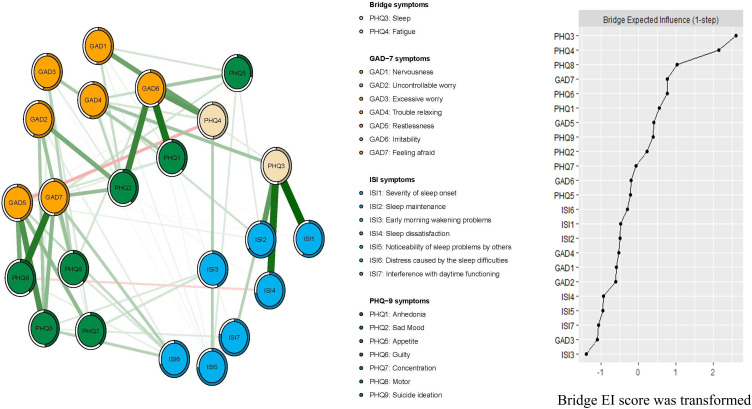
In terms of EI, ISI7 (“Interference with daytime functioning”) (EI=1.18) had the highest EI centrality, followed by ISI4 (“Sleep dissatisfaction”) (EI=1.08) and ISI5 (“Noticeability of sleep problem by others”) (EI=1.07) in the whole network (Figure 1), indicating that these three symptoms were important and central for understanding comorbid depression, anxiety and insomnia among clinicians. In contrast, several other symptoms were marginal, such as PHQ9 (“Suicide ideation”) (EI=0.70), ISI3 (“Early morning wakening problems”) (EI=0.57) and PHQ5 (“Appetite”) (EI=0.56) (Figure 1). In terms of bridge EI, PHQ3 (“Sleep”) (bridge EI=0.55) in the depression community was the most key bridge symptom linking depression, anxiety and insomnia communities, followed by PHQ4 (“Fatigue”) (bridge EI=0.49) (Figure 2). In the depression-anxiety -insomnia network, the connection between PHQ3 (“Sleep”) and ISI3 (“Early morning wakening problems”) (average edge weight=0.16) was the strongest edge, followed by the connection between PHQ3 (“Sleep”) and ISI4 (“ Sleep dissatisfaction”) (average edge weight=0.15), and the connection between PHQ9 (“Suicide ideation”) and GAD7 (“Feeling afraid”) (average edge weight=0.14) (Figure 2, Supplementary Tables S1 and S2).
Figure 2.
Network structure of depression, anxiety and insomnia in clinicians only showing bridge connection during the late stage of the COVID-19 pandemic.
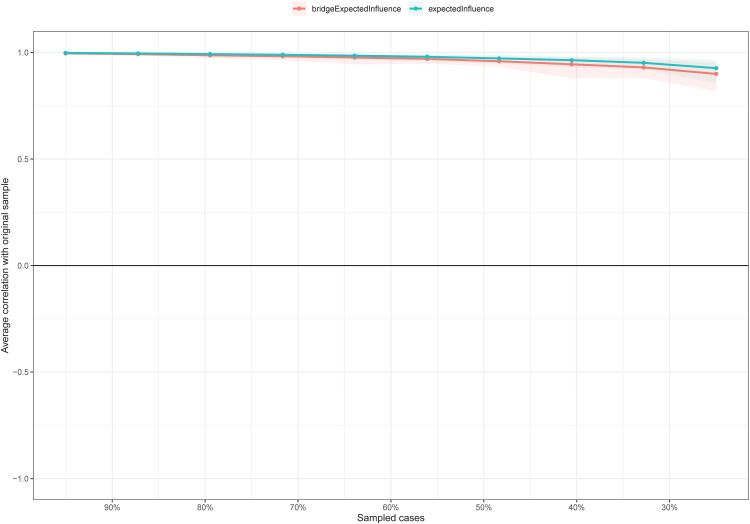
For stability of the network, the centrality of EI had an excellent level of stability (ie, CS-coefficient = 0.75), meaning 75% of the sample could be dropped and the structure of the network would not significantly change (Figure 3). The bootstrap difference test showed that most comparisons between expected influence were statistically significant.
Figure 3.
The stability of centrality and bridge centrality indices using case-dropping bootstrap.
Flow Network of Quality of Life
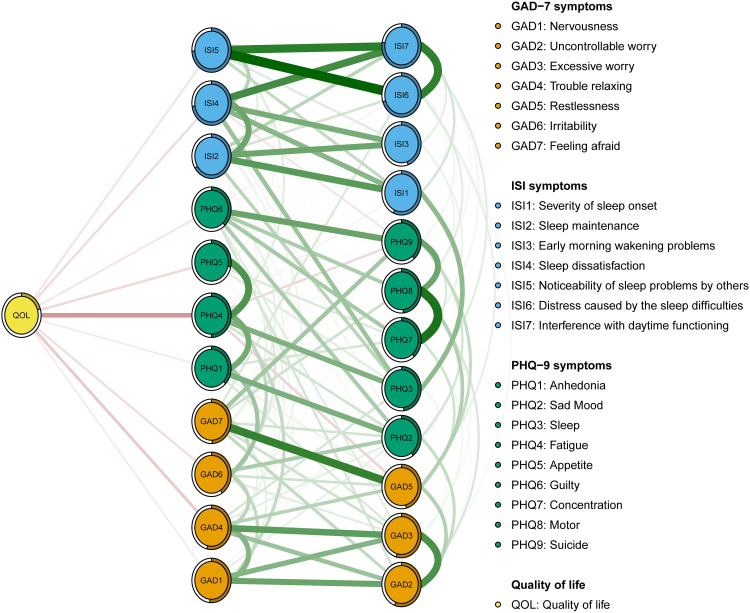
We found that PHQ4 (“Fatigue”) had the strongest negative association with QOL (average edge weight=−0.15), followed by the GAD4 (“Trouble relaxing”) (average edge weight=−0.08) and PHQ5 (“Appetite”) (average edge weight=−0.08) (Figure 4).
Figure 4.
Flow network of quality of life.
The confounding effects of age, gender, and experience of caring for COVID-19 patients on the network model of comorbid depression, anxiety and insomnia
Previous studies1,52,53 found that gender, age and the experience of caring for COVID-19 patients were significantly associated with depression, anxiety and insomnia. Therefore, we examined their potential confounding influence on the network model. When comorbid depression-anxiety-insomnia network model and structure indexes were re-estimated after controlling for age, gender and the experience of caring for COVID-19 patients, and compared with the original network model, there was significant structure change in the expected influence (rs=0.03 [−0.43;0.38]) (Supplementary Figure S1).
Network Comparison Tests for Gender and the Experience of Caring for COVID-19 Patients
The comparison of network models between female and male clinicians did not show significant differences in network global strengths (10.98 in 10.31 female and male participants, respectively; S= 0.67, p=0.131), and edge weights (M=0.13, p=0.8; Supplementary Figures S2 and S3). The comparison of network models between those with and without the experience of caring for COVID-19 patients revealed significant differences in network global strength (the network strength was 11.32 in clinicians with experience of caring for COVID-19 patients and 10.63 in those without such experience, respectively; S: 0.67, p=0.022). There were no significant differences in edge weight (M=0.11, p=0.793; Supplementary Figures S4 and S5).
Discussion
To the best of our knowledge, this was the first study using network analysis that explored comorbid anxiety and insomnia among clinicians with depressive symptoms during the late stage of the COVID-19 pandemic. Overall, ISI7 (“Interference with daytime functioning”), ISI4 (“Sleep dissatisfaction”) and ISI5 (“Noticeability of sleep problem by others”) were the most influential symptoms in the whole network, while bridge symptoms included PHQ3 (“Sleep”) and PHQ4 (“Fatigue”).
This NA revealed that “Interference with daytime functioning” (eg, fatigue/malaise, daytime sleepiness, mood disturbance/irritability, motivation/energy initiative reduction and attention/concentration/memory impairment) was one of the core symptoms in the whole model. A previous study found that development of depression was usually associated with stable, self-reinforcing and maladaptive negative schemata, dysfunctional attitudes, attributional styles, and negative expectations. These factors could contribute to the emergence of typical depressive thinking processes such as negative automatic thoughts and impairment in daytime function.54 Neurobehavioral studies found that impairment in daytime function may be partly due to altered functioning of the pre-frontal cortex, including reduced orbitofrontal volume, hypometabolism in the prefrontal cortex during wake, and hypoactivation in the prefrontal (and medial) areas.55,56 These findings could largely account for the important role of “Interference with daytime functioning” on the whole model. Considering that clinicians needed to concentrate on their daily clinical work, treatments targeting impaired daytime function among clinicians with comorbid anxiety, insomnia and depression would be important.
“Sleep dissatification”, that involve sleep initiation, sleep maintenance, sleep quantity, and refreshment upon awakening,57 was also the core symptom in this NA network. Poor sleep quality and insufficient sleep were common among clinicians during the COVID-19 pandemics,1 and might result from high clinical pressure, irregular work schedule, frequent work shifts and fear of pandemic, which could lead to sleep dissatisfaction.1,58 In addition, “Noticeability of sleep problems by others” was another central symptom. In persons with depression, the latency to fall asleep was often prolonged; frequent awakenings at night often led to fragmented sleep and low sleep efficiency.59 The negative impacts of sleep problems (such as physical and mental health problems, and impaired cognitive and occupational functioning60) were likely to be observed by others.
The node “Sleep” was the most important bridge symptom in network model, which is consistent with the previous findings that sleep disturbances are bidirectionally related to anxiety and depression in adolescents, adults and the elderly.10 “Fatigue” was another bridge symptom in this NA model, which supports previous findings.61 The association of fatigue with anxiety, insomnia and depression might be due to the fact that fatigue was associated with dysregulation among the diffuse cortical projections of norepinephrine (NE), dopamine, acetylcholine, and histamine systems,62 whereas perception of fatigue could be also modulated by noradrenergic descending fibers.63 QOL is a multidimensional concept that measures one’s overall wellbeing, involving physical and mental health aspects. Previous studies found that the QOL was negatively associated with “Fatigue” and “Trouble relaxing” in patients with depression,28,64 which was confirmed in this study. We found that edges between QOL and “Fatigue”, and between QOL and “Trouble relaxing” were the two strongest connections in clinicians.
Considering that anxiety, depressive and insomnia symptoms are common among clinicians during the COVID-19 pandemic,65,66 appropriate strategies, such as emotional and mental health support (eg, psychological counseling hotlines or tele-health), are important to minimize the risk of these problems. Timely screening of comorbid psychiatric symptoms, such as depressive, anxiety and insomnia symptoms, could be performed using certain electronic applications, such as WeChat-based standard measures on psychiatric symptoms, for clinicians during the pandemic.67–70 Cognitive behavioral therapy of insomnia (CBT-i)71 could be used as the first-line treatment for insomnia symptoms.71 In addition, other preventive interventions72–75 could reduce the risk of psychiatric comorbidities of health professionals during the pandemic such as provision of financial and material supports, education on stress control, and online counseling services (eg, 24-h hotlines). Importantly, the identified central and bridge symptoms in the current network model could be targeted in the treatment and preventive strategies for clinicians in need during the COVID-19 pandemic.
The strengths of this study included the large sample size and the use of network analysis to examine the comorbid anxiety and insomnia symptoms among clinicians with depressive symptoms. Several limitations should also be noted. This was a cross-sectional study using undirected network analysis; hence, the causality and dynamic changes between individual symptoms could not be inferred. However, the “snapshot” of the depression-anxiety-insomnia network provided a foundation for more costly, time-consuming longitudinal studies in the future. Additionally, this study focused on clinicians with depressive symptoms, therefore the findings could not be generalized to clinicians without depressive symptoms. Finally, for logistical reasons and the risk of contagion during the pandemic, only self-reported measures on anxiety, insomnia and depressive symptoms were used in this study, which might cause recall bias.
In conclusion, central symptoms (eg, “Interference with daytime functioning”, “Sleep dissatisfaction”, “Noticeability of sleep problem by others”) and key bridge symptoms (eg, “Sleep” and “Fatigue”) identified in this NA study could be important targets for interventions and preventive measures for comorbid anxiety and insomnia symptoms among clinicians with depressive symptoms during the pandemic. Further research should focus on developing appropriate interventions (eg, shorter shift duties and online clinician counselling platforms) that focus on the central and bridge symptoms among clinicians in need.
Acknowledgments
We thank the trade union of Beijing Municipal Administration of Hospitals and all staff who supplied this study. Meanwhile, we would like to thank Mr. Changshun Xu, deputy director of Beijing Hospital Authority, Mr. Cunliang Wang, vice chairman of the trade union of Beijing Municipal Administration of Hospitals, and Mr. Yan Li, the trade union of Beijing Municipal Administration of Hospitals for their work to this study.
Funding Statement
This study was supported by the Beijing Municipal Administration of Hospitals Incubating Program (PX2018063).
Data Sharing Statement
Stringent restrictions apply in making the research dataset and the R codes of the clinical studies publicly available according to the regulation of the ethics committee of the Beijing Anding Hospital, Capital Medical University. Readers and all interested researchers may contact Dr. YT Xiang (Email address: xyutly@gmail.com) to apply for exemptions from the Beijing Anding Hospital, Capital Medical University if appropriate.
Author Contributions
All authors made a significant contribution to the work reported: whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; and gave final approval of the version to be published; All authors agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Silva ML, Rocha RSB, Buheji M, et al. A systematic review of the prevalence of anxiety symptoms during coronavirus epidemics. J Health Psychol. 2021;26(1):115–125. doi: 10.1177/1359105320951620 [DOI] [PubMed] [Google Scholar]
- 3.Al Maqbali M, Al Sinani M, Al-Lenjawi B. Prevalence of stress, depression, anxiety and sleep disturbance among nurses during the COVID-19 pandemic: a systematic review and meta-analysis. J Psychosom Res. 2021;141:110343. doi: 10.1016/j.jpsychores.2020.110343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahrami H, BaHammam AS, Bragazzi NL, et al. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(2):299–313. doi: 10.5664/jcsm.8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Yang L, Liu S, et al. Survey of insomnia and related social psychological factors among medical staff involved in the 2019 novel coronavirus disease outbreak. Front Psychiatry. 2020;11:306. doi: 10.3389/fpsyt.2020.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi: 10.1001/jamanetworkopen.2020.3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahrami HA, Alhaj OA, Humood AM, et al. Sleep disturbances during the COVID-19 pandemic: a systematic review, meta-analysis, and meta-regression. Sleep Med Rev. 2022;62:101591. doi: 10.1016/j.smrv.2022.101591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang SX, Chen RZ, Xu W, et al. A systematic review and meta-analysis of symptoms of anxiety, depression, and insomnia in Spain in the COVID-19 crisis. Int J Environ Res Public Health. 2022;19(2):1018. doi: 10.3390/ijerph19021018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700–708. doi: 10.1016/j.jpsychires.2006.07.008 [DOI] [PubMed] [Google Scholar]
- 10.Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy M, Peterson MJ. Sleep disturbances in depression. Sleep Med Clin. 2015;10(1):17. doi: 10.1016/j.jsmc.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alimoradi Z, Broström A, Tsang HWH, et al. Sleep problems during COVID-19 pandemic and its’ association to psychological distress: a systematic review and meta-analysis. EClinicalMedicine. 2021;36:100916. doi: 10.1016/j.eclinm.2021.100916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baglioni C, Spiegelhalder K, Lombardo C, et al. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–238. doi: 10.1016/j.smrv.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 14.McNally RJ, Robinaugh DJ, Wu GWY, et al. Mental disorders as causal systems: a network approach to posttraumatic stress disorder. Clin Psychol Sci. 2015;3(6):836–849. doi: 10.1177/2167702614553230 [DOI] [Google Scholar]
- 15.Osborn TL, Campbell S, Ndetei D, Weisz J. Network analysis reveals central symptoms of adolescent depression and anxiety in sub-Saharan Africa. 2020.
- 16.Cai H, Bai W, Liu H, et al. Network analysis of depressive and anxiety symptoms in adolescents during the later stage of the COVID-19 pandemic. Transl Psychiatry. 2022;12(1):1–8. doi: 10.1038/s41398-022-01838-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zavlis O, Butter S, Bennett K, et al. How does the COVID-19 pandemic impact on population mental health? A network analysis of COVID influences on depression, anxiety and traumatic stress in the UK population. Psychol Med. 2021;1–9. doi: 10.1017/S0033291721000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren L, Wang Y, Wu L, et al. Network structure of depression and anxiety symptoms in Chinese female nursing students. BMC Psychiatry. 2021;21(1):1–12. doi: 10.1186/s12888-021-03276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao N, Li W, Zhang S-F, et al. Network analysis of depressive symptoms among residents of Wuhan in the later stage of the CoViD-19 pandemic. Front Psychiatry. 2021;12. doi: 10.3389/fpsyt.2021.735973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beard C, Millner AJ, Forgeard MJC, et al. Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychol Med. 2016;46(16):3359–3369. doi: 10.1017/S0033291716002300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried EI, Epskamp S, Nesse RM, et al. What are’good’depression symptoms? Comparing the centrality of DSM and non-DSM symptoms of depression in a network analysis. J Affect Disord. 2016;189:314–320. doi: 10.1016/j.jad.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 22.Park S-C, Kim D. The centrality of depression and anxiety symptoms in major depressive disorder determined using a network analysis. J Affect Disord. 2020;271:19–26. doi: 10.1016/j.jad.2020.03.078 [DOI] [PubMed] [Google Scholar]
- 23.Price M, Legrand AC, Brier ZMF, et al. The symptoms at the center: examining the comorbidity of posttraumatic stress disorder, generalized anxiety disorder, and depression with network analysis. J Psychiatr Res. 2019;109:52–58. doi: 10.1016/j.jpsychires.2018.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones PJ, Mair P, Riemann BC, et al. A network perspective on comorbid depression in adolescents with obsessive-compulsive disorder. J Anxiety Disord. 2018;53:1–8. doi: 10.1016/j.janxdis.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Sit HF, Garabiles MR, et al. A network analysis investigation of the comorbidity between sleep dysfunction and PTSD symptomatology among Filipino domestic workers in Macao (SAR) China. J Psychiatr Res. 2021;140:337–345. doi: 10.1016/j.jpsychires.2021.05.040 [DOI] [PubMed] [Google Scholar]
- 26.Kaiser T, Herzog P, Voderholzer U, et al. Unraveling the comorbidity of depression and anxiety in a large inpatient sample: network analysis to examine bridge symptoms. Depress Anxiety. 2021;38(3):307–317. doi: 10.1002/da.23136 [DOI] [PubMed] [Google Scholar]
- 27.Spoormaker VI, van den Bout J. Depression and anxiety complaints; relations with sleep disturbances. Eur Psychiatry. 2005;20(3):243–245. doi: 10.1016/j.eurpsy.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 28.Barthel AL, Pinaire MA, Curtiss JE, et al. Anhedonia is central for the association between quality of life, metacognition, sleep, and affective symptoms in generalized anxiety disorder: a complex network analysis. J Affect Disord. 2020;277:1013–1021. doi: 10.1016/j.jad.2020.08.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinaugh DJ, Hoekstra RHA, Toner ER, et al. The network approach to psychopathology: a review of the literature 2008–2018 and an agenda for future research. Psychol Med. 2020;50(3):353–366. doi: 10.1017/S0033291719003404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D, Yang D, Li Y, et al. Psychological impact of 2019 novel coronavirus (2019-nCoV) outbreak in health workers in China. Epidemiol Infect. 2020;148:e96. doi: 10.1017/S0950268820001090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W, Zhang Y, Wang P, et al. Psychological stress of medical staffs during outbreak of COVID-19 and adjustment strategy. J Med Virol. 2020;92(10):1962–1970. doi: 10.1002/jmv.25914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JBW, et al. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 33.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81(3):14–18. [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang YL, Liang W, Chen Z-M, et al. Validity and reliability of Patient Health Questionnaire-9 and Patient Health Questionnaire-2 to screen for depression among college students in China. Asia Pac Psychiatry. 2013;5(4):268–275. doi: 10.1111/appy.12103 [DOI] [PubMed] [Google Scholar]
- 36.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 37.He XY, Li CB, Qian J, Cui HS, Wu WY. Reliability and validity of a generalized anxiety disorder scale in general hospital outpatients (in Chinese). Shanghai Archiv Psychiatry. 2010;22(4):200–203. [Google Scholar]
- 38.Bastien C. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 39.Bai JD, Chen LX, Li L, Wang CX. Reliability and validity of Insomnia Severity Index in clinical insomnia patients(in Chinese). Chin J Pract Nurs. 2018;34(28):2182–2186. [Google Scholar]
- 40.Smith MT, Wegener ST. Measures of sleep: the Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Rheum. 2003;49(S5):S184–S196. doi: 10.1002/art.11409 [DOI] [Google Scholar]
- 41.Skevington SM, Lotfy M, O’Connell KA, et al. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13(2):299–310. doi: 10.1023/B:QURE.0000018486.91360.00 [DOI] [PubMed] [Google Scholar]
- 42.Cheung YB, Yeo KK, Chong KJ, et al. Measurement equivalence of the English, Chinese and Malay versions of the World Health Organization quality of life (WHOQOL-BREF) questionnaires. Health Qual Life Outcomes. 2019;17(1):67. doi: 10.1186/s12955-019-1130-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team. R: a language and environment for statistical computing; 2020. Available from: https://www.R-project.org/. Accessed July 20, 2022.
- 44.Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods. 2018;50(1):195–212. doi: 10.3758/s13428-017-0862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinaugh DJ, Millner AJ, McNally RJ. Identifying highly influential nodes in the complicated grief network. J Abnorm Psychol. 2016;125(6):747. doi: 10.1037/abn0000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cramer AOJ, Waldorp LJ, van der Maas HLJ, et al. Complex realities require complex theories: refining and extending the network approach to mental disorders. Behav Brain Sci. 2010;33(2–3):178–193. doi: 10.1017/S0140525X10000920 [DOI] [Google Scholar]
- 47.Payton J. Tools for identifying important nodes in networks; 2020. Available from: https://cran.r-project.org/web/packages/networktools/index.html. Accessed July 20, 2022.
- 48.Jones PJ, Ma R, McNally RJ. Bridge centrality: a network approach to understanding comorbidity. Multivariate Behav Res. 2019;2019:1–15. [DOI] [PubMed] [Google Scholar]
- 49.Haslbeck JMB, Waldorp LJ. mgm: estimating time-varying mixed graphical models in high-dimensional data. J Stat Softw. 2020;1(8). doi: 10.18637/jss.v093.i08 [DOI] [Google Scholar]
- 50.Zhang WR, Wang K, Yin L, et al. Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychother Psychosom. 2020;89(4):242–250. doi: 10.1159/000507639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Borkulo CD, van Bork R, Boschloo L, et al. Comparing network structures on three aspects: a permutation test. 2017. [DOI] [PubMed]
- 52.Cai Q, Feng H, Huang J, et al. The mental health of frontline and non-frontline medical workers during the coronavirus disease 2019 (COVID-19) outbreak in China: a case-control study. J Affect Disord. 2020;275:210–215. doi: 10.1016/j.jad.2020.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chew NWS, Lee GKH, Tan BYQ, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–565. doi: 10.1016/j.bbi.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165(8):969–977. doi: 10.1176/appi.ajp.2008.08050721 [DOI] [PubMed] [Google Scholar]
- 55.Shekleton JA, Flynn-Evans EE, Miller B, et al. Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. Sleep. 2014;37(1):107–116. doi: 10.5665/sleep.3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altena E, Vrenken H, Van Der Werf YD, et al. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67(2):182–185. doi: 10.1016/j.biopsych.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 57.Kline C. Sleep quality. Encyclopedia Behav Med. 2013;2013:1811–1813. [Google Scholar]
- 58.Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020;288:112954. doi: 10.1016/j.psychres.2020.112954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterson MJ, Benca RM. Sleep in mood disorders. Sleep Med Clin. 2008;3(2):231–249. doi: 10.1016/j.jsmc.2008.01.009 [DOI] [Google Scholar]
- 60.Bos SC, Macedo AF. Literature review on insomnia (2010–2016). Biol Rhythm Res. 2019;50(1):94–163. doi: 10.1080/09291016.2017.1413766 [DOI] [Google Scholar]
- 61.Baldwin DS, Papakostas GI. Symptoms of fatigue and sleepiness in major depressive disorder. J Clin Psychiatry. 2006;67:6. [PubMed] [Google Scholar]
- 62.Stahl SM, Zhang L, Damatarca C, Grady M. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64:6–17. [PubMed] [Google Scholar]
- 63.Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93–105. doi: 10.1017/S1461145704004729 [DOI] [PubMed] [Google Scholar]
- 64.Ferguson M, Dennehy EB, Marangell LB, et al. Impact of fatigue on outcome of selective serotonin reuptake inhibitor treatment: secondary analysis of STAR* D. Curr Med Res Opin. 2014;30(10):2109–2118. doi: 10.1185/03007995.2014.936553 [DOI] [PubMed] [Google Scholar]
- 65.Sahebi A, Abdi K, Moayedi S, et al. The prevalence of insomnia among health care workers amid the COVID-19 pandemic: an umbrella review of meta-analyses. J Psychosom Res. 2021;149:110597. doi: 10.1016/j.jpsychores.2021.110597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sahebi A, Nejati-Zarnaqi B, Moayedi S, et al. The prevalence of anxiety and depression among healthcare workers during the COVID-19 pandemic: an umbrella review of meta-analyses. Prog Neuropsychopharmacol Biol Psychiatry. 2021;107:110247. doi: 10.1016/j.pnpbp.2021.110247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Folkman S, Greer S. Promoting psychological well‐being in the face of serious illness: when theory, research and practice inform each other. Soc Behav Dimensions Cancer. 2000;9(1):11–19. [DOI] [PubMed] [Google Scholar]
- 68.Xiang Y-T, Yang Y, Li W, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7(3):228–229. doi: 10.1016/S2215-0366(20)30046-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jimenez AL, Della CD, Arcenas AMA, et al. Examining the competency of Filipino mental health professionals in psychodynamic psychotherapy. Asia-Pacific Psychiatry. 2021;13(1):e12441. doi: 10.1111/appy.12441 [DOI] [PubMed] [Google Scholar]
- 70.Orsolini L, Jatchavala C, Noor IM, et al. Training and education in digital psychiatry: a perspective from Asia-Pacific region. Asia-Pacific Psychiatry. 2021;13(4):e12501. doi: 10.1111/appy.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594 [DOI] [PubMed] [Google Scholar]
- 72.Rawtaer I, Mahendran R, Chan HY, et al. A nonpharmacological approach to improve sleep quality in older adults. Asia-Pacific Psychiatry. 2018;10(2):e12301. doi: 10.1111/appy.12301 [DOI] [PubMed] [Google Scholar]
- 73.Caruso CC, Arbour MW, Berger AM, et al. Research priorities to reduce risks from work hours and fatigue in the healthcare and social assistance sector. Am J Ind Med. 2022. doi: 10.1002/ajim.23363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen W-C, Chen S-J, Zhong B-L. Sense of alienation and its associations with depressive symptoms and poor sleep quality in older adults who experienced the lockdown in Wuhan, China, during the COVID-19 pandemic. J Geriatr Psychiatry Neurol. 2022;35:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ismail M, Lee KY, Sutrisno Tanjung A, et al. The prevalence of psychological distress and its association with coping strategies among medical interns in Malaysia: a national-level cross-sectional study. Asia-Pacific Psychiatry. 2021;13(2):e12417. doi: 10.1111/appy.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]