Abstract
Aims
To develop age and sex-specific risk equations for predicting mortality following major complications of diabetes, using a large linked administrative dataset from Western Australia (WA) and to incorporate these into an existing diabetes simulation model..
Methods
The study uses linked hospital and mortality records on 13,884 patients following a major diabetes-related complication with a mean (SD) duration of 2.62 (2.25) years. Risk equations for predicting mortality were derived and integrated into the UKPDS Outcomes Model. Estimates of life expectancy and incremental QALYs gained as a result of two theoretical therapies (a reduction of HbA1c of 1%, and reduction of systolic blood pressure of 10mmHg) were determined using the original and adapted models.
Results
The two versions of the model generated differences in life expectancy following specific events; however there was little impact of using alternative mortality equations on incremental QALYs gained as a result of reducing HbA1c or systolic blood pressure, or on outcomes of life expectancy for a cohort initially free of complications.
Conclusions
Mortality following complications varies across diabetic populations and can impact on estimates of life expectancy, but appears to have less impact on incremental benefits of interventions that are commonly used in pharmoeconomic analyses.
Keywords: type 2 diabetes, mortality, complications, simulation model, administrative data
Introduction
Diabetes simulation models are increasingly being used to inform economic evaluations, particularly in the context of theraputic reimbusment decisions (The Mount Hood 4 Modeling Group; 2007). A key issue often referred to as external validation is to test their applicability in populations that were not used in their construction (American Diabetes Association Consensus Panel, 2004; Sargent, 2005), an example being the validation of the Framingham risk score in different ethnic groups (D’Agostino et al, 2001). When systematic differences arise, attempts are usually made to calibrate the model to the new setting. There is no agreement as to how calibration should be carried out, but it usually involves examination of some aggregate output of the model in the new population (Kopec et al., 2010; Weinstein et al., 2001) and if differences arise, adjustments of intercept and slope of risk model equations are made so that the model predicts appropriate levels of risk (Abbasi et al., 2012).
The difference in mortality associated with diabetes complications is a source of variation that needs to be examined when adapting diabetes simulation models across settings. Such differences may arise over time and between different settings, due to factors such as the intensity and availibility of health care interventions to treat patients’ post-event risk. A pertinent issue in health economic simulation modelling is that there are a limited number of data sources with which to construct models of diseases processes. This is particularly the case in diabetes where data from long-term studies conducted in one or two countries are often used by researchers worldwide. While there is a need to examine existing diabetes simulation models’ ability to predict mortality, this issue has received much less attention than the degree to which they are able to predict absolute event rates of major complications such as myocardial infarction (The Mount Hood 4 Modeling Group; 2007).
A plausible explanation for a lack of post-event mortality validation studies is a lack of existing data sets that follow people with diabetes after experiencing complications. A potential source of information on mortality following complications is linked administrative data that include records both of hospitalisations and deaths. Our primary objectives in this study were to use such an administrative dataset from Western Australia (WA) (Hayes et al., 2011) to estimate age and sex-specific risk equations for predicting mortality following major complications of diabetes and to incorporate these into the UKPDS Outcomes Model (Clarke et al., 2004), a well known type 2 diabetes simulation model. Whilst ‘stand alone’ models that estimate life expectancy post event have been derived from the WA dataset (Hayes et al., 2011) they cannot easily be integrated into the more complex simulation model which requires annually updated information on complication status in order to predict mortality.
A second aim of the present study is to examine the broader issue of how taking into account rates of mortality in different diabetes populations may impact on overall outcomes such as life expectancy and quality adjusted life expectancy. This involved comparing estimates of life expectancy using the orginal UKPDS Outcomes Model and a model in which post-event mortality is determined from the Western Australian population. Finally, we examine differences in estimates of life expectancy and QALYs for a representative patient under a series of hypothetical interventions that alter classical risk factors (e.g. HbA1c). This addresses whether decision analytic aspects of the model are affected by the use of Australian-specifc mortality equations.
Materials and Methods
Estimation of Australian mortality equations
The UKPDS Outcomes Model includes three equations for estimating mortality. The first equation estimates risk of death from causes unrelated to diabetes while the second two equations estimate the increased risk of death associated with major complications (Clarke et al., 2004),. In this study we replace the latter by estimating two new equations using Australian data to represent all cause mortality following any of six complications.
We used an administrative health service dataset from WA over a ten year period from 1 January 1990 to 31 December 1999, which has been described previously [8]. The dataset contains de-identified information from four separate sources: 1) insurance claims for medical and diagnostic charges; 2) information on dispensed prescriptions for pharmaceuticals for those holding Government concession and health care cards; 3) hospital records of inpatient episodes (including day-only admissions) for public and private hospitals; and 4) WA state death records. These data were confidentially linked at the individual patient level through a collaborative project undertaken by Commonwealth Health and Aging, the WA Department of Health, The University of WA, the Australian Institute of Health and Welfare, and the Health Insurance Commission. The data linkage was conducted using a protocol to ensure that the individuals privacy is protected (Boyko et al., 1996). The study was approved by the Commonwealth Department of Health and Ageing Departmental Ethics Committee.
Identification of people with diabetes was based on several criteria: use of diabetes specific medications; use of HbA1c tests; hospital admissions or discharges with diagnostic code(s) indicating diabetes (i.e. ICD9 code 250 or ICD10 codes E10-14), or diabetes listed as a cause of death on death certificates. Around 70,000 people over the age of 35 years were identified as having diabetes by fulfilling at least one of these criteria. Of those identified with diabetes, patients were further classified as having type 1 diabetes if indicated on one or more hospital records or if there was evidence of use of insulin but not of use of oral anti-diabetic agents. This is a wider definition of type 1 diabetes than used in a previous study using the same administrative dataset (Hayes et al., 2011). We supplemented the previous dataset with up to six additional months of follow-up data in which complications were coded according to ICD10 classifcations. This increased the number of focal events by around 10 percent for each type of complication. We also included foot ulcer as an additional complication as patients with this complication have been shown to have increased risk of mortality (Nelson et al., 2008).
The population used in this analysis was individuals with either type 1 or type 2 diabetes who had any one of six complications defined using the following diagnostic, procedural, or cause of death codes:
myocardial infarction (MI) -either non-fatal myocardial infarction (ICD-9 code 410), or fatal cardiac event (ICD-9 code ≥ 410 and ≤414.9, or ≥ 428 and ≤ 428.9, or sudden death (ICD-9 code ≥ 798 and ≤ 798.9; ICD10 R96); or ICD-10 I21; ICD-10 code ≥ I20 and ≤I 25) or ICD10 R96
stroke -(ICD-9 code ≥430 and ≤434.9, or 436) or fatal stroke (ICD-9 code ≥ 430 and ≤ 438.9) or any fatal cebrovascular disease ICD-10 ≥ I60 and ≤ I69.
heart failure -ICD9 Codes 428 to 428.1, or ICD-10 I50
amputation of digit or limb using hospital procedure codes ≥84.10 to ≤84.17.
ulcer ICD-9 707.1 and non-pressure chronic ulcer of skin ICD-10 L97 and L98.4
End stage renal disease ICD-9 V45.1; V56 or ICD-9 55.69, or ICD10 N18.0 and renal failure N19 and an encounter for care involving renal dialysis Z49
Statistical analysis
The outcome modelled was death following one of the six specified complications. Time to death was measured in months, the shortest unit of time available in the administrative data. Patients were censored at the end of the study period, or if there was evidence of emigration to another Australian state or country (indicated by the pattern of health care use). The date of diabetes ascertainment was determined as the earliest recorded use of diabetes-specific medications, HbA1c tests or hospital admissions involving diabetes. We classified the first complication after the individual was identified as having diabetes, as the focal event. Individuals were considered to have prior comorbidity if they had hospital records for any of the six specified complications before their date of diabetes ascertainment.
To account for the high initial mortality following complications, and to maintain consistency with the original UKPDS model, we derived a logistic regression model for survival within the same year as the event, followed by a Gompertz model for survival in subsequent years. This division allowed the mortality equations to be integrated into the structure of the UKPDS simulation model that uses annual cycles.
A multivariate logistic regression model was estimated for the probability of death in the same calendar year as the focal event. Covariates in the model included the type of focal event (MI, stroke, heart failure, amputation, renal failure, ischaemic heart disease or ulcer), age (continuous), sex, type 1 diabetes and prior comorbidities existing before the date of diabetes ascertainment. Interaction terms for the type of event with age, sex and type 1 diabetes were also investigated. Covariates were dropped through stepwise backwards elimination if their odds ratios were not significantly different from unity at the 5% level.
A Gompertz model, for the the hazard of death in years beyond the year of the focal event was estimated as a function of a patient’s current age, in order to allow extrapolation beyond the observed follow-up period (Nelson et al., 2008). Hence the shape function of the Gompertz depends on current age and the linear covariates were the type of focal event, prior comorbidities and time varying covariates for future events as patients are still vulnerable to further complications in years beyond the year of the focal event. Covariates were dropped through stepwise backwards elimination if they did not achieve significance at the 5% level. The proportional hazards assumption was tested using Schoenfeld residuals (Schoenfeld, 1982) in an equivalent Cox model and by examination of Cox–Snell residual plots. Where proportional hazards problems were found they were resolved by using age interacted covariates.
Simulation Modeling
The logistic and Gompertz mortality equations estimated from the Western Australian population were coded into the simulation model. Then using representative input data and clinical risk factors for patients from the Fremantle Diabetes Study (FDS) (Davis et al., 2000) and using the UKPDS Outcomes model with either the existing UK mortality equations or the WA mortality equations, we simulated post-event survival following each of five major complications of diabetes: myocardial infarction, stroke, heart failure, amputation and renal failure (foot ulcer is not presently an outcome in the current UKPDS outcomes model). The input cohort comprised patients aged 74 years with diabetes duration 13 years, equal numbers of men and women, 14% smokers, HbA1c of 7.9%, systolic blood pressure 157 mmHg, BMI 29 kg/m2 and total:HDL cholesterol 5.2, representing the summary statistics of patients in the FDS close to the time of their complication.
We then derived life expectancies and quality adjusted life expectancies following each event from the two different versions of the simulation model. Secondly we estimated outcomes for the same cohort of patients, initially free of complications. Simulations were run for a maximum of 26 years to age 100 years and remaining life expectancy and quality adjusted life expectancy were determined. In order to minimize Monte Carlo (1st order) uncertainty arising due to the probabilistic nature of simulations, we carried out 200 replications of a 1000 person dataset to derive each life expectancy. Parameter (2nd order) uncertainty was evaluated using 1000 bootstrapped coefficients of all model risk equations including the new logistic and Gompertz models, which permitted calculation of 95% confidence intervals for all life expectancy predictions. Finally we examined incremental QALYs gained as a result of two theoretical therapies: a reduction of HbA1c of 1%, and reduction of systolic blood pressure of 10mmHg. We compared these effects using both UK and Australian versions of the simulation model.
Results
Australian mortality equations
The sub-group of patients who experienced at least one of the six selected complications comprised 13,844 patients: 7,673 men and 6,211 women. Summary statistics of the frequency of different focal events and death, associated age at event, prior co-morbidities and type 1 diabetes are provided in table 1. The most common complications for patients in our dataset were MI, stroke and heart failure with approximately 88% of patients having one of these as their focal event. Amputation, renal failure and foot ulcer together comprised the remaining 12% of first events occurring during the observation period. With the exception of renal failure, women were on average 4–6 years older than men at the time of their first event (mean (SD) age women: 73.9 (11.4) years; men: 69.3 (11.4) years). The average age for renal failure was 62.6 (12.3) years for women and 63.2 (12.5) years for men. The mean (SD) duration of follow-up was 2.62 (2.25) years. Approximately half of the cohort died during this period and approximately one tenth of the cohort had prior co-morbidities at the time of diabetes ascertainment.
Table 1.
Characteristics of patients at the time of their focal event
| Men (n=7673) | Mean (sd) Age at event |
Women (n=6211) | Mean (sd) Age at event |
All (n=13884) | |
|---|---|---|---|---|---|
| Number events | |||||
| Focal event type n (%) | |||||
| Myocardial infarction | 2624 | 68.0 (11.7) | 1844 | 74.1 (11.4) | 4468 (32.2) |
| Stroke | 2174 | 71.3 (10.5) | 1764 | 75.4 (11.1) | 3938 (28.4) |
| Heart failure | 1903 | 72.4 (10.6) | 1924 | 75.9 (10.5) | 3827 (27.6) |
| Ulcer | 424 | 68.0 (12.6) | 396 | 72.3 (12.9) | 820 (5.9) |
| Amputation | 505 | 68.7(11.3) | 274 | 72.4 (12.2) | 779 (5.6) |
| Renal failure | 222 | 63.2 (12.5) | 158 | 62.6 (12.3) | 380 (2.7) |
| Total Deaths (1991–1999) | 3784 (49.3) | 74.9 (10.3) | 3528 (56.8) | 78.5 (10.3) | 7312 (52.7) |
| Death in year of focal event | 1962 (25.6) | 75.2 (10.2) | 1912 (30.8) | 79.0 (10.1) | 3874 (27.9) |
| Deaths in subsequent years | 1822 (23.7) | 74.7 (10.5) | 1616 (26.0) | 78.0 (10.4) | 3438 (24.8) |
| Prior comorbidities | 283 (3.7) | 265 (4.3) | 548 (3.9) | ||
| Type 1 diabetes | 1097 (14.3) | 772 (12.4) | 1869 (13.5) | ||
Statistical analysis
Parameters and estimated odds ratios from the logistic regression model for death within the same year as the focal complication are presented in table 2. All events apart from heart failure had lower initial mortality than MI which was the referent in the model. The risk of mortality increased by about 6% for every year of age (p<0.001) for MI, amputation and foot ulcer but the interactions terms indicated differences in age-dependency for stroke and heart failure. Death in the year of renal failure was much more strongly age-dependent, increasing by about 11% for every year of age. The probability of dying was between 2 and 2.5 times higher for patients who had co-morbidities (prior stroke (p=0.004), heart failure (p<0.001), or amputation (p=0.03)).
Table 2.
Logistic Model for death within same calendar year as the focal event
| Coefficient (se) β0 |
Odds ratio (95% CI) | p | |
|---|---|---|---|
| Constant | −5.019 (0.189) | ||
| β | |||
| Male | −0.195 (0.049) | 0.82 (0.75–0.91) | <0.001 |
| Age at time of event (years) | 0.0629 (0.003) | 1.065 (1.060–1.070) | <0.001 |
| Focus event | |||
| MI (base case) | 0 | 1 | |
| Stroke | −1.659 (0.357) | 0.190 (0.094–0.383) | <0.001 |
| Heart failure | 0 | 1 | |
| Amputation | −1.435 (0.179) | 0.238 (0.168–0.338) | <0.001 |
| Renal failure | −3.711 (0.986) | 0.024 (0.004 – 0.169) | <0.001 |
| Ulcer | −1.538 (0.129) | 0.215 (0.167–0.276) | <0.001 |
| Age and event interactions | |||
| Stroke*age | 0.017 (0.005) | 1.017 (1.008–1.027) | <0.001 |
| Heart failure*age | −0.016 (0.001) | 0.984 (0.982–0.986) | <0.001 |
| Renal *age | 0.051 (0.014) | 1.052 (1.024–1.081) | <0.001 |
| Sex and event interactions | |||
| Heart failure * male | 0.519 (0.088) | 1.681 (1.413–1.998) | <0.001 |
| Amputation * male | 0.563 (0.218) | 1.756 (1.145–2.693) | 0.01 |
| Type 1 event interactions | |||
| Heart failure * type 1 | 0.477 (0.103) | 1.612 (1.316–1.974) | <0.001 |
| Ulcer * type 1 | 0.856 (0.242) | 2.354 (1.464–3.784) | <0.001 |
| History of events | |||
| Prior Stroke | 0.638 (0.224) | 1.893 (1.220–2.937) | 0.004 |
| Prior Heart failure | 0.972 (0.144) | 2.643 (1.991–3.508) | <0.001 |
| Prior amputation | 0.638 (0.295) | 1.893 (1.063–3.372) | 0.03 |
The Gompertz model for mortality in years beyond the year of the complication is shown in table 3. Similarly to the logistic model, every year increase in age resulted in an approximate 6% increase in risk of death. Compared with MI as the referent, all other complications examined conferred higher long term mortality risk (all hazard ratios >1). For example, someone with renal failure and who survives till the end of that year is three times more likely to die in the following year than someone who survived a MI.
Table 3.
Gompertz equation for hazard of death in years beyond the focal event year.
| Coefficient (se) | HR (95% CI) | p | |
|---|---|---|---|
| Baseline hazard | |||
| gamma | 0.056 (0.002) | <0.001 | |
| Constant, β0 | −7.068 (0.186) | <0.001 | |
| β coefficients | β | HR (95% CI) | |
| Male | 0.115 (0.035) | 1.12 (1.05–1.20) | 0.001 |
| Type 1 diabetes | 0.267 (0.047) | 1.31 (1.19–1.43) | <0.001 |
| Focus event | |||
| MI (referrant) | 0 | 1 | |
| Stroke | 0.286 (0.044) | 1.33 (1.22–1.45) | <0.001 |
| Heart failure if age under 60 years | 1.62 (0.104) | 5.05 (4.13–6.19) | <0.001 |
| Heart failure at 60–69 years | 1.28 (0.071) | 3.62 (3.15–4.16) | <0.001 |
| Heart failure at 70–79 years | 0.966 (0.053) | 2.63 (2.37–2.92) | <0.001 |
| Heart failure if age >80 years | 0.721 (0.055) | 2.06 (1.85–2.29) | <0.001 |
| Amputation | 0.518 (0.057) | 1.68 (1.50–1.88) | <0.001 |
| Renal failure | 1.043 (0.086) | 2.84 (2.40–3.36) | <0.001 |
| Ulcer | 0.289 (0.058) | 1.34 (1.19–1.50) | <0.001 |
| Future events | |||
| MI and aged under 70 | 2.083 (0.082) | 8.03 (6.84–9.42) | <0.001 |
| MI and aged over 70 | 1.788 (0.048) | 5.98 (5.44–6.57) | <0.001 |
| Stroke | 1.300 (0.054) | 3.67 (3.30–4.08) | <0.001 |
| Heart failure | 0.566 (0.082) | 1.76 (1.50–2.07) | <0.001 |
| Renal failure | 0.865 (0.145) | 2.37 (1.77–3.18) | <0.001 |
| Amputation | 0.556 (0.117) | 1.74 (1.38–2.19) | <0.001 |
| Ulcer | 0.299 (0.151) | 1.35 (1.00–1.81) | 0.047 |
Simulation modeling
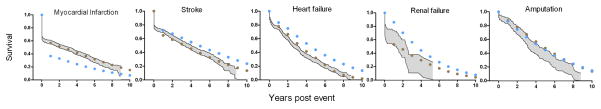
Figure 1 shows the observed Kaplan Meier survival of men and women 70–79 years, following each of 5 complications, compared with the simulated survival from the original UKPDS model and the Australian version of the model. Simulations using WA equations were closer to the observed survival in all cases, with simulated survival falling within the 95% confidence interval of the data. Simulations using UK mortality equations generally fell outside of the 95% confidence interval with survival post MI being under predicted and survival following stroke, heart failure and renal failure being over predicted.
Figure 1.
Comparison of survival following complications for 70–79 age group, with simulated survival from two different models.
Shaded area= Kaplan Meier 95% confidence interval of observed WA survival; blue circles = simulation using UK death equations; brown circles = simulation using WA death equations.
Simulated life expectancies and quality adjusted life expectancies following events derived from both versions of the simulation model are shown in table 4. Point estimates of life expectancy using the Australian version of the model were shorter following heart failure, stroke and renal failure, longer following MI. Life expectancies following amputation were similar regardless of whether UK or WA death equations were used. However, 95% confidence intervals were overlapped for UK and Australian predictions following most events except MI and heart failure. The Australian version of the model predicted shorter life expectancy following heart failure (approximately 1.4 years or 1 QALY) than the original UKPDS model and longer life expectancy following MI of about 1.2 years or 0.9 QALYs. Also notable is that 95% confidence intervals of life expectancies (which take into account parameter uncertainty) derived from the Australian version of the simulation model were much narrower than from the UK version. This relates directly to the greater parameter uncertainty of the coefficients in the UK mortality equations.
Table 4.
Simulated remaining life expectancy and quality adjusted life expectancy following one of 5 complications and with no complications.
| Life expectancy years (95% CI) | Quality adjusted life expectancy years (95% CI) | |||
|---|---|---|---|---|
| UK version | WA version | UK version | WA version | |
| Post MI | 2.74 (2.07–3.42) | 4.33 (3.85–4.72) | 1.97 (1.49–2.46) | 3.10 (2.76–3.37) |
| Post stroke | 5.73 (4.73–6.88) | 4.39 (4.01–4.78) | 3.57 (2.95–4.28) | 2.74 (2.51–2.98) |
| Post CHF | 4.69 (3.88–5.65) | 3.32 (3.14–3.50) | 3.12 (2.58–3.75) | 2.21 (2.09–2.34) |
| Post amputation | 5.10 (3.79–6.72) | 4.77 (4.33–5.20) | 2.58 (1.92–3.4) | 2.41 (2.19–2.63) |
| Post renal failure | 4.17 (2.82–5.57) | 2.82 (2.43–3.19) | 2.18 (1.48–2.91) | 1.48 (1.27–1.67) |
| No complications | 8.17 (7.08–9.38) | 8.17 (7.07–9.31) | 6.19 (5.37–7.07) | 6.20 (5.37–7.05) |
Whilst differences in life expectancy of up to 1.5 years were simulated from the two versions of the model following specific events, overall life expectancy for this FDS cohort assumed free of complications at baseline was similar from both simulation models (table 4).
The incremental quality adjusted life years gained under scenarios of changes in risk factors from both original and Australian specific versions of the UKPDS Outcomes model are shown in table 5. Point estimates for incremental QALYs gained as a result of reducing systolic blood pressure of the patient cohort from 157 to 147 mmHg and as a result of reducing HbA1c by 1% were slightly greater for the UK model, but when 2nd order uncertainty was incorporated, 95% confidence limits of all incremental QALY estimates were overlapped.
Table 5.
Incremental QALYs gained from different theoretical therapies using original and Australian versions of the model.
| Theoretical therapy | UK version QALYs | Incremental QALYs | Australian version QALYs | Incremental QALYs |
|---|---|---|---|---|
| None –base model | 6.19 | 6.20 | ||
| HbA1c reduced by 1% | 6.34 | 0.15 (0.04–0.26) | 6.32 | 0.12 (0.02–0.24) |
| SBP reduced by 10 mmHg | 6.33 | 0.14 (0.03–0.26) | 6.32 | 0.12 (0.02–0.27) |
Footnote: 95% confidence limits represent 2nd order uncertainty
Discussion
In this study, we have derived mortality risk equations to estimate survival following major complications of diabetes based on a large linked administrative healthcare data set from Australia. Not only did this enable us to adapt the UKPDS outcomes model to another setting (Australia), it also allowed us to examine the impact of using different mortality equations on outcomes such as QALYs and life expectancy that are typically used by health economists when modeling chronic diseases. This is a form of model uncertainly which is often difficult to examine when models are developed from a single population, or synthesized from a limited number of published sources.
Simulations using the Australian and UK models resulted in different predictions of post event survival and life expectancy, particularly following MI and heart failure. These suggest substantial differences in mortality risk between the two populations. The higher survival following MI in the Australian population may be due to differences across time as the original UKPDS equations were based on data from 1977–1997, whilst the Australian equations were based on data from 1990 until 1999. This is consistent with the declining trend in MI case fatality observed in other studies (Smolina et al., 2010) and, in this particular case, may largely reflect the fact that the UKPDS was conducted before statin therapy was available as a risk modifying strategy. Indeed, <2% of UKPDS patients received lipid-modifying therapy during the study. The mortality discrepancy may also represent differences across settings which may be related to health system performance, or other factors such as socio-economic differences. The poorer survival after heart failure predicted by the Australian–specific model is a little harder to explain, but could be due to non-proportional effects using the models from the UKPDS which had limited data on older patients. These differences may also reflect the better initial clinical state and management of patients who are part of a clinical trial over that of community dwelling patients with type 2 diabetes.
Whilst the original and the Australian version of the outcomes model generated differences in point estimates of life expectancy following MI, stroke, heart failure and renal failure, there were only small differences in aggregate outcomes of average life expectancy for a cohort initially free of complications. To some extent this is due to the fact that the over predictions of mortality from the UKPSD model following some events are cancelled out by under predictions of mortality following other events. Additionally, only a proportion of this cohort would experience complications over their lifetime (e.g. in 25 years of simulation around 25% of the cohort are predicted to have an MI and only 14% a stroke) and so the effect of mortality differences are diluted.
External validation of models is uncommon (Altman at al., 2009) and there has been discussion as to whether it is required before a model is used for decision analysis (Kopec et al., 2010; Weinstein et al., 2001). We are aware of only one previous validation of a diabetes simulation model that specific focuses on mortality as an outcome (Song et al., 2011). Here we have been able to examine external validity at two levels, for specific patient subgroups that have experienced a particular complication, and at the aggregate level of a patient cohort. The predictive validity of the original UKPDS Outcomes model was relatively poor in examining post–event survival among these specific patient subgroups in Australia. We also examined whether decision analytic validity of the model might change depending on the source of the mortality risk equations. Incremental QALYs predicted from a reduction in systolic blood pressure of 10 mmHg or a reduction in HbA1c of 1%, were similar irrespective of the source of mortality risk equations.
The question of whether the original outcomes model is valid in the Australian setting depends on how it will be used. Applications of the UKPDS Outcomes model to date include cost effectiveness analysis (Clarke et al., 2006, O’Reilly et al., 2007), prediction of life expectancies (Leal et al., 2009) and prediction of the incidence of cardiovascular complications (Reynoso-Noverón, 2011). On the basis of the results in this study, we would conclude that the calibration by direct estimation of mortality risk appropriate to Australia was necessary to have confidence in predictions of life expectancy following specific events, but that incremental outcomes of simulations such as used in economic evaluation were robust to the data source of the equations for death following events.
There are some limitations to our research. There were no records of clinical risk factors in the administrative dataset, hence the Australian mortality equations do not discriminate between people of high or low clinical risk. Additionally, there could be incorrect assignment of type 1 and type 2 status, but the overall proportion of type 1 patients in the administrative dataset (13.5%) was similar to that reported in national statistics (AIHW 2011).. Finally, the Australian mortality equations were based on 10 years of data prior to 2000 so would not reflect any more recent improvements in survival following complications that may have occurred in the last decade.
The uncertainty surrounding the estimates of life expectancy was much greater using the UK than the Australian version of the model, which is consistent with the larger number of events used to derive mortality equations. For example there were a total of 13884 events in the Australian data whereas the original UKPDS mortality equations were based on only 717 patients with events. Record linked data are increasingly being used in epidemiological studies (Hayes et al., 2011; Smolina et al., 2012) and their large size makes them particularly useful for estimating mortality equations. When collected in countries such as Australia which has a system of universal health care, such data also have the advantage that they cover the entire population. The use of these data facilitates the estimation of mortality risks based on a large number of patients and avoids the selection issues that are often a source of criticism of comparable data from clinical studies. Furthermore administrative data sets tend to capture more events than clinicial trials. These advantages suggest they are likely to become increasingly used in health economic simulation modelling.
Acknowledgments
Grant support: AJH was funded though NHMRC grants 571372 and 512463. PMC was supported by an Australian NHMRC Career Development Award 571122 and NIH Grant 5R01DK090435-02. TD was funded through a NHMRC Practitioner Fellowship.
We are grateful to Dr. John Bass and Richard Solon who were instrumental in performing the data linkage, also to the Department of Health and Aging, the WA Department of Health, The University of WA, the Australian Institute of Health and Welfare, and the Health Insurance Commission, all of whom were signatories to this unique collaborative project between the Commonwealth and the State of WA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbasi A, Corpeleijn E, Peelen LM, Gannsevoort RT, de Jong PE, Gans ROB, et al. External validation of the KORA S4/F4 prediction models for the risk of developing type 2 diabetes in older adults: the PREVEND study. European Journal of Epidemiology. 2012;27:47–52. doi: 10.1007/s10654-011-9648-4. [DOI] [PubMed] [Google Scholar]
- Altman DG, Vergouwe Y, Royston P, Moions KGM. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Consensus Panel. Guidelines for Computer Modeling of Diabetes and its Complications. Diabetes Care. 2004;27:2262–2265. doi: 10.2337/diacare.27.9.2262. [DOI] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare. Diabetes series no. 17. Cat. no. CVD 56. Canberra: AIHW; 2011. Diabetes prevalence in Australia: detailed estimates for 2007–08. Viewed 3 December 2012 < http://www.aihw.gov.au/publication-detail/?id=10737419311>. [Google Scholar]
- D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabetic Medicine. 1996;13:967–72. doi: 10.1002/(SICI)1096-9136(199611)13:11<967::AID-DIA266>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Clarke PM, Gray AM, Briggs A, Farmer A, Fenn P, Stevens R, et al. A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 68) Outcomes Model. Diabetologia. 2004;47:1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- Clarke PM, Gray AM, Briggs A, Stevens RJ, Matthews DR, Holman RR. Cost–utility analyses of intensive blood glucose and tight blood pressure control in Type 2 diabetes (UKPDS 72) Diabetologia. 2005;48:868–877. doi: 10.1007/s00125-005-1717-3. [DOI] [PubMed] [Google Scholar]
- Davis TM, Zimmet P, Davis WA, Bruce DG, Fida S, Mackay IR. Autoantibodies to glutamic acid decarboxylase in diabetic patients from a multi-ethnic urban Australian community: The Fremantle Diabetes Study. Diabetic Medicine. 2000;2000:17, 667–674. doi: 10.1046/j.1464-5491.2000.00359.x. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Leal J, Kelman C, Clarke PM. Risk equations to predict life expectancy of people with type 2 diabetes mellitus following major complications: a study from Western Australia. Diabetic Medicine. 2011;28:428–435. doi: 10.1111/j.1464-5491.2010.03189.x. [DOI] [PubMed] [Google Scholar]
- Kelman CW, Bass AJ, Holman CD. Research use of linked health data--a best practice protocol. Australian & N Z Journal of Public Health. 2002;26(3):251–5. doi: 10.1111/j.1467-842x.2002.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Kopec JA, Finès P, Manuel DG, et al. Validation of population-based disease simulation models: a review of concepts and methods. BMC Public Health. 2010;10:710. doi: 10.1186/1471-2458-10-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal J, Gray AM, Clarke PM. Development of life-expectancy tables for people with type 2 diabetes. European Heart Journal. 2009;30:834–839. doi: 10.1093/eurheartj/ehn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Mount Hood 4 Modeling Group. Computer Modeling of Diabetes and its Complications A report on the Fourth Mount Hood Challenge Meeting. Diabetes Care. 2007;30:1638–1646. doi: 10.2337/dc07-9919. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sun JL, Tsiatis AA, Mark DB. Empirical estimation of life expectancy from large clinical trials: Use of left-truncated, right-censored survival analysis methodology. Statistics in Medicine. 2008;27:5525–5555. doi: 10.1002/sim.3355. [DOI] [PubMed] [Google Scholar]
- O’Reilly D, Hopkins R, Blackhouse G, Clarke P, Hux J, Tarride JE, et al. Long-term cost–utility analysis of a multidisciplinary primary care diabetes management program in Ontario. Canadian Journal of Diabetes. 2007;31:205–214. [Google Scholar]
- Reynoso-Noverón N, Mehta R, Almeda-Valdes P, Rojas-Martinez R, Villalpando S, Hernández-Ávila M, et al. Estimated incidence of cardiovascular complications related to type 2 diabetes in Mexico using the UKPDS outcome model and a population-based survey. Cardiovascular Diabetology. 2011;10:1–9. doi: 10.1186/1475-2840-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent RG. Verification and validation of simulation models. In: Kuhl ME, Steiger NM, Armstrong FB, Joines JA, editors. Proceedings of the 2005 Winter Simulation Conference. 2005. pp. 130–143. [Google Scholar]
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Incidence and 30-day case fatality for acute myocardial infarction in England in 2010: national-linked database study. European Journal of Public Health. 2012 Jan 11; doi: 10.1093/eurpub/ckr196. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Alexander CM, Mavros P, Lopez VA, Malik S, Phatak HM, et al. Use of the UKPDS Outcomes Model to predict all-cause mortality in U.S. adults with type 2 diabetes mellitus: comparison of predicted versus observed mortality. Diabetes Research and Clinical Practise. 2011;91(1):121–6. doi: 10.1016/j.diabres.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Weinstein MC, Toy LE, Sandberg AE, Neumann PJ, Evans JS, Kuntz KM, et al. Modeling for Health Care and Other Policy Decisions: Uses, Roles, and Validity. Value in Health. 2001;4:348–361. doi: 10.1046/j.1524-4733.2001.45061.x. [DOI] [PubMed] [Google Scholar]