Abstract
NMDA receptors (NMDARs) play a critical role in excitatory synaptic transmission, plasticity and in several forms of learning and memory. In addition, NMDAR dysfunction is believed to underlie a number of neuropsychiatric conditions. Growing evidence has demonstrated that NMDARs are tightly regulated by several G-protein-coupled receptors (GPCRs). Ligands that bind to GPCRs, such as neurotransmitters and neuromodulators, activate intracellular pathways that modulate NMDAR expression, subcellular localization and/or functional properties in a short- or a long-term manner across many synapses throughout the central nervous system. In this review article we summarize current knowledge on the molecular and cellular mechanisms underlying NMDAR modulation by GPCRs, and we discuss the implications of this modulation spanning from synaptic transmission and plasticity to circuit function and brain disease.
Keywords: Excitatory transmission, glutamate, LTP, LTD, mGluR, dopamine, GABAB, serotonin, adrenergic, adenosine, acetylcholine, metaplasticity, schizophrenia, fragile X syndrome
Introduction
In the mammalian brain, fast excitatory synaptic transmission is mediated by three glutamate receptors: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), N-methylD-aspartate receptor (NMDAR) and kainate receptors. These receptors are molecularly and functionally diverse, and can be regulated by several neuromodulatory systems (Traynelis et al., 2010), making excitatory transmission a highly complex and regulated process. NMDARs stand out because of their dual role as mediators of synaptic transmission and long-term synaptic plasticity, such as long-term potentiation and depression (LTP and LTD) of AMPAR-mediated synaptic transmission. NMDARs are also dynamically regulated and subject to activity-dependent long-term plasticity (Hunt et al., 2012;Rebola et al., 2010). In addition, NMDARs can be found in the presynaptic compartment where they regulate neurotransmitter release and presynaptic plasticity (Bouvier et al., 2018). Furthermore, NMDAR dysfunction has been linked to several neuropsychiatric and neurodegenerative diseases (Lau et al., 2007;Paoletti et al., 2013). Given their critical contribution to brain function, it is not surprising that NMDARs are tightly regulated by a number of mechanisms. A key regulatory mechanism can be found in G-protein-coupled receptors (GPCRs) (Yang et al., 2014). This review article summarizes current evidence in support of GPCRs as key regulators of NMDARs, and the consequences of this process on synaptic transmission and plasticity, circuit function and disease.
NMDARs possess three unique properties (Paoletti, et al., 2013): high calcium permeability, slow gating kinetics, and Mg2+ block of the channel pore at resting potential. NMDARs act as coincidence detectors given that both glutamate binding and membrane depolarization, which is required to relieve the Mg2+ block of current flow through the channel, must occur in order to allow current flow through the channel. NMDARs are tetramers that can be assembled as di-heteromers, with two obligatory GluN1 and two GluN2 or GluN3 subunits, or exist as tri-heteromers, with a combination of GluN1 and different splicing variants of GluN2 and/or GluN3 (Paoletti, et al., 2013). The subunit composition determines the functional properties of NMDARs (Iacobucci et al., 2018;Paoletti, et al., 2013). Each subunit possesses different intra- and extracellular- motifs that are subject to post-translational modifications that in turn can change NMDAR properties, expression levels, synaptic localization and interaction with other important intracellular components (Kotecha et al., 2003b;Sanz-Clemente et al., 2013;Traynelis, et al., 2010). In addition to their canonical function as ionotropic channels, NMDARs can signal in a metabotropic manner (Dore et al., 2016;Gray et al., 2016). In this case, glutamate binding to NMDARs induces conformational changes of the receptor that activate intracellular messengers, which can trigger synaptic plasticity independently from ion flux through the channel (Dore et al., 2015;Nabavi et al., 2013;Stein et al., 2015). Lastly, NMDAR-mediated transmission and plasticity can be modulated by the release of glutamate and co-agonists (e.g. D-serine) from astrocytes, (Harada et al., 2015;Letellier et al., 2016;Mothet et al., 2006;Navarrete et al., 2019;Panatier et al., 2006).
GPCRs belong to the large family of seven transmembrane domain receptors (7TM) which are activated by neurotransmitters, neuromodulators, peptides and sensory stimuli (Bockaert et al., 1999;Pavlos et al., 2017). GPCRs can be classified in four families, namely, Gs, Gi/o, Gq, and G12/13. The signaling pathways downstream of these GPCRs have been reviewed elsewhere (Kristiansen, 2004). Briefly, Gs stimulates adenylate cyclase which leads to the activation of the cyclic AMP (cAMP)- protein kinase A (PKA) pathway whereas Gi/o does the opposite (i.e. inhibits adenylate cyclase). Gq leads to protein kinase C (PKC) activation, inositol-3-phosphate (IP3) production and calcium release from internal stores. G12/13 activates a small Rho-GTPase through guanine nucleotide exchange factor (RhoGEF). In addition, GPCRs can impact neuronal function by regulating several membrane ion channels, such as voltage-gated calcium channels (VGCCs) and potassium channels (Dascal, 2001;McCudden et al., 2005). GPCRs also signal through Gprotein-independent intracellular effectors that rely on direct interactions between the 7TM receptor and intracellular scaffolding proteins or other molecules (Heuss et al., 2000). Thus, GPCRs are well positioned to modulate NMDARs in a number of ways, and by this means strongly regulate synaptic transmission, plasticity, and circuit function (Anwyl, 1999;Hunt, et al., 2012;Rebola, et al., 2010;Tritsch et al., 2012;Yang, et al., 2014).
NMDAR modulation by GPCRs: role of neurotransmitters and neuromodulators
Metabotropic glutamate receptors
Metabotropic glutamatergic receptors (mGluRs) are one of the best-characterized examples of GPCRs that modulate NMDARs. mGluRs are glutamate-activated GPCRs that share no sequence homology with other families of GPCRs (Conn et al., 1997). There are 8 subtypes of mGluRs (1 to 8) that can be subdivided in three groups (I, II, III). Classically, group I mGluRs (mGluR1 and 5) are Gq-coupled, while group II (mGluR2 and 3) and group III (mGluR 4,6,7,8) mGluRs are Gi/o-coupled (Nicoletti et al., 2011). Group I mGluRs have been extensively studied for their interaction with NMDARs.
mGluRs and LTP of NMDAR
mGluR-mediated modulation of NMDARs was first observed in the CA1 area of the hippocampus, where activation of mGluRs with the broad-spectrum agonist (1S,3R)-1-Aminocyclopentane-1,3-dicarboxylic acid (ACPD) triggered a transient increase in NMDAR currents (Aniksztejn et al., 1991). In addition, tetanic stimulation of synaptic inputs to dentate gyrus granule cells induced LTP of NMDAR-mediated excitatory postsynaptic currents (EPSC), and this LTP and ACPDinduced potentiation occluded each other (O’Connor et al., 1994), suggesting a common expression mechanism. NMDAR LTP induced by synaptic activity was abolished by the application of the non-selective mGluR antagonist (RS)-α-Methyl-4-carboxyphenylglycine (MCPG) or by the NMDAR competitive antagonist D-2-amino-5-phosphonovalerate (D-AP5). These results provided the first evidence that NMDAR LTP required mGluR and NMDAR coactivation by endogenous glutamate. Co-activation of mGluR5 and NMDAR is a common feature across synapses that express NMDAR LTP (Hunt, et al., 2012;Rebola, et al., 2010). In support of this notion NMDAR LTP is absent in mGluR5 knockout mice (Jia et al., 1998). In isolated hippocampal cells, the selective mGluR5 agonist (RS)-2-Chloro-5-hydroxyphenylglycine (CHPG) induced a long-lasting increase of NMDAR EPSCs in an mGluR5, IP3 receptor (IP3R), PKC and non-receptor tyrosine kinase Src (Src)-dependent manner (Kotecha et al., 2003a). The CHPGmediated potentiation required mGluR5 and NMDARs co-activation. At the hippocampal mossy fiber-to-CA3 pyramidal cell (mf-CA3) synapse, brief patterns of presynaptic activity induced mGluR5-dependent LTP of NMDAR-mediated transmission (Kwon et al., 2008;Rebola et al., 2008). This NMDAR LTP not only required mGluR5/NMDAR co-activation, but also postsynaptic calcium influx via NMDARs, G-protein activation, recruitment of PKC and Src kinase, and calcium release from the internal stores (Figure 1). Thus, PKC and Src kinase seem to be the downstream targets of mGluR5 and NMDAR co-activation. In slice cultures, the mGluR-Src pathway potentiates NMDAR-mediated transmission in a G-protein-independent manner (Benquet et al., 2002;Heuss et al., 1999). In hippocampal neurons from both cultures and acute slices, activation of PKC modulates NMDAR gating properties and enhances NMDAR delivery to the plasma membrane following phosphorylation of SNARE-proteins (Kwon, et al., 2008;Lan et al., 2001a;Lan et al., 2001b;Lau et al., 2010;Zheng et al., 1999). Intriguingly, in midbrain dopaminergic neurons, NMDAR LTP requires PKA but not PKC activation (Harnett et al., 2009). Like in the hippocampus, NMDAR LTP in these dopaminergic neurons requires mGluR5 and NMDAR co-activation, which, together with calcium rise generated by postsynaptic action potentials leads to IP3R-mediated calcium-induced calcium release. While the link between mGluR5 and PKA is unclear, recent evidence in organotypic slice cultures showed that activation of Gq-coupled receptors, including group I mGluRs, increases PKA activity (Chen et al., 2017)(Figure 1). Phosphorylation of NMDAR by PKA modifies receptor properties such as calcium permeability, channel gating and currents in both slices and heterologous system (Aman et al., 2014;Murphy et al., 2014;Skeberdis et al., 2006). While group I mGluRs-mediated PKA activation could play a role in NMDAR LTP in midbrain dopaminergic neurons, clear mechanistic evidence for this process is missing.
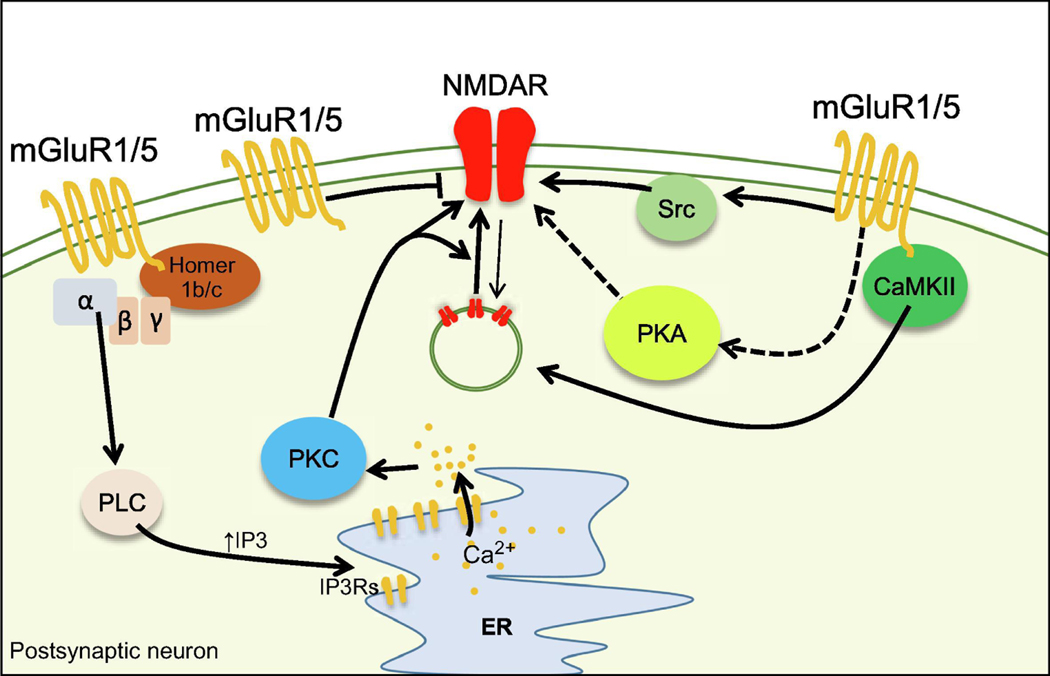
Figure 1.
Mechanisms of NMDAR modulation by mGluRs
Activation of group I mGluRs leads to calcium release from the endoplasmic reticulum (ER) and activation of PKC via the PLC/IP3 pathway. Recruitment of calcium release from the internal stores requires the interaction of group I mGluRs with Homer 1b/c. PKC can enhance NMDAR-mediated currents and/or increase NMDAR trafficking to the synapse. In addition to PKC, Gq can also modulate NMDAR via the activation of the Src kinase. Group I mGluRs could potentially enhance NMDAR function by non-canonical activation of PKA. Activation of group I mGluRs can also induce depression of NMDAR-mediated transmission. Arrowheads and bars represent positive and negative modulation of downstream targets, respectively. Dashed lines represent a pathway which is largely unknown. Thick and thin lines indicate pathways that are activated or inhibited, respectively.
mGluRs and LTD of NMDARs
mGluRs are also involved in LTD of NMDAR-mediated synaptic transmission. At the mf-CA3 synapse, mGluR5 and mGluR1 induce bidirectional plasticity –i.e. NMDAR LTP/LTD (Hunt et al., 2013). Here, coincident pre- and postsynaptic burst activity induces NMDAR LTP or LTD depending on the timing of the pre and postsynaptic burst. Interestingly, while mGluR5 are required for NMDAR LTP, mGluR1 are required for NMDAR LTD (Hunt, et al., 2013). mGluRdependent NMDAR LTD has also been reported at Schaeffer collateral to CA1 pyramidal cell (Sch-CA1) synapses (Bhouri et al., 2014;Peng et al., 2010;Yi et al., 1995), and at perforant pathway-to-granule cell synapses (Harney et al., 2006).
Molecular mechanisms of mGluR-dependent bidirectional NMDAR plasticity
The reason why two receptors belonging to the same family can engage two opposite forms of plasticity is unclear. A possible explanation is the presence of different splicing variants of mGluR1 and mGluR5 (Conn, et al., 1997). While the two known mGluR5 isoforms possess a long C-terminal tail, some splicing variants of mGluR1, such as mGluR1b, contain a shorter intracellular C-terminal which is crucial for mGluRs coupling to intracellular effectors, such as Homer proteins (Joly et al., 1995;Niswender et al., 2010;Tu et al., 1999;Tu et al., 1998). Homer proteins comprise Homer1b/c and Homer1a. Homer 1a is a dominant negative Homer protein that binds to mGluRs and prevents their binding with Homer1b/c (Kammermeier et al., 2007). Binding of Homer1b/c to group I mGluRs is critical for their perisynaptic localization, functional interaction with NMDAR, and for the coupling to intracellular players such as IP3Rs (Lujan et al., 1996;Ohtani et al., 2014;Prezeau et al., 1996;Tu, et al., 1998;Xiao et al., 1998)(Figure 1). Since plasticity of NMDAR transmission strongly relies on postsynaptic calcium levels (Harnett, et al., 2009;Harney, et al., 2006;Hunt, et al., 2013;Kwon, et al., 2008), impaired coupling of mGluRs to IP3Rs could affect IP3R-mediated calcium rise required for NMDAR plasticity. Consistent with this hypothesis, mfCA3 synapses express the mGluR1b short-tail isoform (Hunt, et al., 2013), suggesting that mGluR1-dependent NMDAR LTD might rely on different postsynaptic calcium dynamics compared to mGluR5-dependent NMDAR LTP. At the Sch-CA1 synapse, expression of mGluR1dependent NMDAR LTD is input specific and correlates with higher levels of Homer1b/c at Schinputs compared to temporoammonic inputs, which lack NMDAR LTD, indicating that levels of Homer proteins can influence the expression of plasticity (Bhouri, et al., 2014). In the Fmr1 knockout (KO) mouse, a model of Fragile X syndrome (FXS), binding of Homer1a to mGluR5 disrupts the mGluR5/NMDAR interaction, thereby impairing NMDAR-mediated transmission, NMDAR-dependent plasticity and cognitive performance (Aloisi et al., 2017). In addition, disrupting the mGluR5/Homer1b/c interaction with a TAT-peptide, a manipulation that recapitulates the effect of Homer1a binding to mGluR5, mimicked the impairment in NMDAR function observed in Fmr1 KO mice, and the functional and behavioral deficit were rescued by selective knockdown of Homer1a (Aloisi, et al., 2017). Together, these observations highlight the role of Homer as a critical molecular hub for the interaction between group I mGluRs and NMDARs and for mGluR-dependent NMDAR plasticity.
mGluR5 can also interact with NMDARs through other intracellular mediators, one being calcium-calmodulin-dependent kinase type 2 (CaMKII) (Jin et al., 2013;Marks et al., 2018). In cultured striatal neurons, mGluR1/5 receptors anchor inactive CaMKII at the synapse. Upon NMDARmGluR1/5 co-activation and calcium influx, CaMKII becomes active and loses its affinity for the C-terminal domain of mGluR1/5 (Jin, et al., 2013). CaMKII then phosphorylates the GluN2B subunit and triggers membrane delivery of GluN2B-containing NMDARs (Jin et al., 2015). It is currently unknown if this mechanism is conserved across other central synapses and in more intact preparations. CaMKII also regulates mGluR5 surface levels and mGluR5-mediated calcium influx in heterologous systems (Marks, et al., 2018). Modulation of mGluR5-mediated calcium dynamics may have an important impact on NMDARs. In the dentate gyrus, mGluR5 mediate a form of NMDAR LTD which can be switched to LTP depending on different intracellular calcium buffer capacity (Harney, et al., 2006). This observation suggests that modulation of calcium dynamics downstream of mGluR5 activation might be important for the bidirectionality of mGluRs modulation of NMDAR –i.e. LTP vs LTD.
GABAB receptors
GABAB receptors (GABABRs) are GABA-activated Gi/o-coupled receptors that inhibit adenylate cyclase and the cAMP/PKA signaling cascade (Bettler et al., 2004). GABABRs also modulate ion channels such as VGCCs and G-protein-coupled inward-rectifying potassium (GIRK) channels (Bettler, et al., 2004) (Figure 2). GABABRs are expressed both post- and presynaptically and their activation, respectively, mediates a slow inhibitory postsynaptic potential (slow IPSP) and suppresses neurotransmitter release.
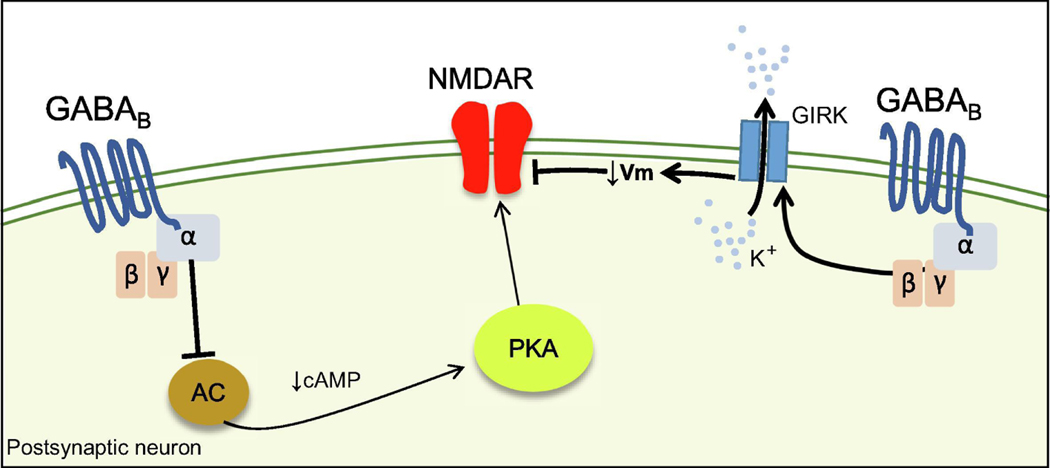
Figure 2.
Mechanisms of NMDAR modulation by GABABRs
GABABRs negatively regulate the adenylate cyclase (AC)/cAMP/PKA pathway and reduce the function of NMDAR. GABABRs can also reduce NMDAR conductance by hyperpolarizing the membrane via the activation of GIRK channels
GABABR direct modulation of NMDARs
In layer 2/3 pyramidal neurons of the prefrontal cortex (PFC), activation of postsynaptic GABABRs selectively regulates NMDAR calcium permeability but not NMDAR EPSCs (Chalifoux et al., 2010). This effect is the result of Gi/o-mediated inhibition of PKA and is independent of modulation of VGCCs and GIRK channels, consistent with the fact that phosphorylation of NMDAR by PKA at certain residues increases NMDAR calcium permeability but not NMDAR EPSCs (Higley et al., 2010;Lur et al., 2015;Skeberdis, et al., 2006). This mechanism could expand the capability of GABAergic inhibition to change the threshold for plasticity – a phenomenon called metaplasticity– by targeting NMDAR calcium permeability only. In layer 5 pyramidal neurons of the PFC, GABABRs are co-expressed with Gi/o-coupled α2 adrenergic receptors (α2ARs) (Lur, et al., 2015). Here, α2ARs activation suppresses AMPAR-EPSCs but not NMDAR-EPSCs, while GABABRs selectively inhibit NMDARs despite the common Gi/o coupling. The different effect of α2ARs and GABABRs is likely due to Regulator of G-proteins Signaling (RGS) proteins that create synaptic microdomains and prevent crosstalk between GPCRs coupled to the same pathway (Hollinger et al., 2002;Lur, et al., 2015)
GABABR indirect modulation of NMDARs
Activation of GIRK channels by GABABRs leads to membrane hyperpolarization, which strongly reduces NMDAR-mediated transmission due to enhanced Mg2+ block (Morrisett et al., 1991;Otmakhova et al., 2004). Theoretical models predict that the combined conductance of both NMDAR and GABABR-activated GIRK channels can generate robust bistability of membrane voltage and persistent firing states, a property thought to be important for working memory (Sanders et al., 2013). Current evidence suggests that GABABRs mediate a non-canonical excitatory effect by activating specific subtypes of VGCCs, or by indirect inhibition of largeconductance calcium-activated potassium channels (BK channels) (Garaycochea et al., 2016;Zhang et al., 2016). Such mechanisms could lead to membrane depolarization and facilitate NMDAR-mediated currents and potentials.
Dopamine receptors
Dopamine (DA) receptors can be divided in two families: D1-like (which includes D1R and D5R) and D2-like (which includes D2R, D3R and D4R). D1-like receptors are mainly Gs-coupled while D2-like receptors are coupled to Gi/o (Beaulieu et al., 2011). The two receptor families are widely expressed in the brain, display different affinities for DA, and can be expressed in a cell-specific manner. At the synaptic level, D1- and D2-like receptors can be expressed on the dendritic tree but they also localize at the perisynaptic region of dendritic spines (Ladepeche et al., 2013;Smiley et al., 1994). Due to their localization on dendritic spines, DA receptors are ideally positioned to modulate NMDARs. The interaction between DA receptors and NMDARs has been investigated at several central synapses (Tritsch, et al., 2012). D1- and D2-like receptors seem to play a “pushpull” effect on NMDAR, with D1-like meditating potentiation and D2-like mediating depression of NMDAR-mediated function.
PKA-mediated modulation of NMDARs by DA receptors
PKA is a key player in the dichotomic effect of D1-like and D2-like receptors on NMDAR function. D1-like receptors are Gs-coupled and their activation increases cAMP levels and PKA activity (Tritsch, et al., 2012) (Figure 3). Several studies in both cultured neurons and acute slices from PFC, striatum and nucleus accumbens have demonstrated that activation of D1-like receptors by DA or selective agonists modulates NMDAR in a PKA-dependent manner (Dudman et al., 2003;Li et al., 2010;Snyder et al., 1998;Wang et al., 2001). Conversely, D2-like receptor activation depresses NMDAR function via PKA inhibition in cultured neurons and acute slices from PFC and hippocampus (Banks et al., 2015;Higley, et al., 2010;Kotecha et al., 2002;Wang et al., 2003). In D2-positive striatal medium-sized spiny neurons (MSNs), activation of D2Rs by an agonist reduces NMDAR-calcium permeability without affecting NMDAR EPSCs (Higley, et al., 2010). The PKA target involved in this process is still a matter of debate. In the CA1 area of the hippocampus PKA selectively regulates NMDAR calcium permeability (Skeberdis, et al., 2006), presumably by phosphorylating the GluN2B subunit at residue Ser1166 (Murphy, et al., 2014). However, in D2-positive MSNs, Ser1166 is unlikely to be the target of PKA given that GluN2Bcontaining NMDAR in these cells do not seem to contribute to D2R modulation of NMDAR-calcium permeability. In heterologous systems, phosphorylation of GluN1 and not GluN2B regulates NMDAR calcium permeability, suggesting that the GluN1 subunit might be a potential target of PKA in native brain (Aman, et al., 2014). Alternatively, NMDARs could be indirectly modulated by PKA signaling. In the nucleus accumbens, activation of PKA by D1R agonists increases the phosphorylation levels of the GluN1 subunit (Snyder, et al., 1998). This enhancement requires activation of Dopamine- and cAMP-Regulated Phosphoprotein (Mr 32 kDa) (DARPP-32), a phosphoprotein that can inhibit protein phosphatase 1 (PP1) (Figure3). GluN1 phosphorylation levels are also increased by direct blockade of PP1/2A, suggesting a tonic dephosphorylation of GluN1 subunit by PP1. The increase of GluN1 phosphorylation by a D1R agonist was prevented by co-application of D2R agonist quinpirole which dephosphorylates DARPP-32 via calcineurin A. These results indicate that PKA controls GluN1 phosphorylation levels by indirect inhibition of PP1 by DARPP-32. A similar mechanism was described in dissociated striatal MSNs, where D1R agonists increased NMDAR-mediated currents in a DARPP-32-dependent manner (Flores-Hernandez et al., 2002). The same study showed that activation of D2R strongly reduced D1Rmediated potentiation of NMDAR currents, suggesting that DARPP-32 might be a key mediator of the push-pull modulation of NMDARs by DA receptors. It is worth noting that most of these studies employed glutamate uncaging or NMDA application (iontophoresis, puff and or bath application) to test the modulation of NMDAR-mediated currents. While these approaches minimize potential presynaptic effects by potential activation of DA receptors, they do not fully mimic the dynamics of NMDAR activation during glutamate release. This stands true especially during repetitive presynaptic activity where postsynaptic depolarization, by relieving the Mg2+ block, increases NMDAR EPSCs (Hunt, et al., 2012). Activation of DA receptors can also change membrane ion channels and thus influence the membrane potential (Cepeda et al., 1998). Since NMDAR are voltage-dependent and voltage-clamp is prone to errors (Beaulieu-Laroche et al., 2018;Williams et al., 2008), changes in membrane potential could lead to misleading results.
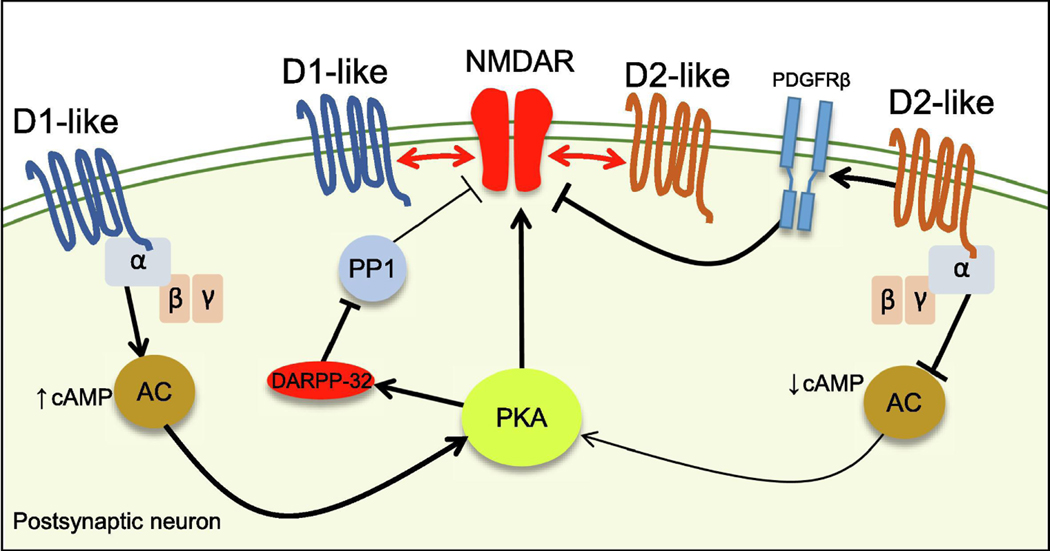
Figure 3.
Mechanisms of NMDAR modulation by DA receptors.
D1-like and D2-like-coupled receptors positively and negatively regulate the AC/cAMP/PKA pathway, respectively. PKA modulates NMDAR function directly by regulating NMDAR phosphorylation levels or indirectly by controlling other downstream targets (i.e. inhibition of PP1 via DARPP-32). Direct proteinprotein interaction between NMDARs and DA receptors receptors (red arrows) can also affect the synaptic localization of NMDAR.
PKA-independent modulation of NMDARs by DA receptors
DA receptors can also modulate NMDARs in a PKA-independent manner. In dissociated PFC neurons, D1R agonists increase NMDAR currents via PKC (Chen et al., 2004). The link between D1Rs and PKC is unclear but does not require PLC activation, suggesting that D1R are not acting via Gq -PLC pathway (Chen, et al., 2004). In cultured CA1 pyramidal neurons, activation of D4Rs triggers depression of NMDAR currents, and this effect is mediated by D4R-mediated transactivation of platelet derived growth factor receptor β (PDGFR) (Figure 3), which leads to calcium/calmodulin-dependent desensitization of NMDARs (Kotecha, et al., 2002). In the PFC, activation of D2/3Rs (but not D4Rs) triggers a PDGFR-dependent depression of NMDAR currents (Beazely et al., 2006). These studies suggest that D1- and D2-like receptors can modulate NMDAR currents in a PKA-independent manner, but do not eliminate the possibility that PKA could also modulate NMDAR calcium permeability.
Modulation of NMDARs via direct DA receptor/NMDAR interaction
In addition to modulating NMDARs via intracellular pathways, both D1- and D2-like receptors can modulate NMDARs via direct protein-protein interaction (Cepeda et al., 2006;Tritsch, et al., 2012) (Figure 3). In hippocampal lysates the C-terminal domain of D1R interacts with the GluN1 and GluN2 subunits (Lee et al., 2002). The interaction with the GluN1 subunit seems to be required for D1R-dependent activation of PI3 kinase, while the D1R-GluN2 interaction determines D1Rdependent reduction of NMDAR surface levels. Super-resolution microscopy demonstrated that D1R-NMDAR interaction occurs at perisynaptic areas of excitatory spines of hippocampal neuronal cultures, and that D1R activation decreased the size of D1R and NMDAR clusters, which increased the motility and synaptic localization of NMDARs, thereby facilitating the induction of NMDAR-dependent plasticity in acute hippocampal slices (Ladepeche, et al., 2013). In contrast, acute cocaine administration, which increases synaptic levels of dopamine, led to D2R activation and the formation of D2R/GluN2B complexes that resulted in less phosphorylation of GluN2B by CaMKII (Liu et al., 2006b). This mechanism seems to play a significant role in the behavioral changes induced by cocaine (Liu, et al., 2006b). The D2R/GluN2B complex is also required for the inhibitory effect of D2R-agonists on NMDAR-currents (Liu, et al., 2006b), suggesting that the D2R-NMDAR direct interaction might play a role in the physiological modulation of NMDAR by DA.
Serotonin Receptors
The large family of serotonin (5HT) receptors comprises seven groups (5HT1 to 5HT7) and a few subtypes within some groups (Hoyer et al., 2002). Except for the 5HT3 receptor, which is an ionotropic receptor, all other 5HT receptors are GPCRs. Each group couples to different G proteins: 5HT1 and 5HT5 are coupled to Gi/o; 5HT2 are coupled to Gq; 5HT4, 5HT6 and 5HT7 are coupled to Gs. The presence of multiple splice variants, RNA editing, and other posttranslational modifications add several layers of complexity to GPCR-coupled 5HT receptor signaling. 5HT receptors can be expressed in neuronal subcellular compartments including the soma, dendritic spines and axon terminals (Cornea-Hebert et al., 1999;Peddie et al., 2008;Riad et al., 2000), suggesting that their activation can regulate neuronal excitability, as well as synaptic transmission and plasticity at different levels.
Modulation of NMDAR-mediated transmission and plasticity by 5HT receptors
Early studies showed that 5HT potentiated NMDAR-mediated responses in the neocortex (Nedergaard et al., 1986;Reynolds et al., 1988) a phenomenon presumably mediated by 5HT2 receptors (Rahman et al., 1993). The first investigations of the role of 5HT on NMDAR-dependent LTP yielded conflicting results. 5HT depletion abolished LTP at PP-GC synapse in the dentate gyrus (Bliss et al., 1983), and others showed a concentration-dependent inhibition of LTP by exogenous 5HT at Sch-CA1 synapse, in part presumably by modulating NMDARs (Staubli et al., 1994). These results highlight that 5HT may have synapse-specific effects that might arise from the diversity of 5HT receptors or from different synapse-specific properties. In the visual cortex, inhibition of NMDAR-dependent LTP relied on inhibition of NMDAR responses via activation of 5HT1A, 5HT2 and 5HT7 receptors (Edagawa et al., 1998, 1999). Another study showed that 5HT application, by co-activation of 5HT1 and 5HT2A/C, abolished NMDAR-dependent LTP in the visual cortex of young rats (Kim et al., 2006). This LTP was absent in older animals, which could result from an age-dependent increase in 5HT levels as depletion of endogenous 5HT restored LTP. Thus, the effect of 5HT on NMDAR-dependent plasticity seems to be area-, synapse- and age-specific.
Molecular mechanisms of NMDARs modulation by 5HT1 and 5HT2 receptors
5HT modulates NMDAR function through different molecular pathways. In the PFC, 5HT1A receptor activation decreases NMDAR currents and membrane levels of the GluN2B subunit (Yuen et al., 2005). 5HT1A activation triggers destabilization of the complex formed by microtubule associated protein 2 (MAP2) and the molecular motor Kif17, which leads to a reduction of NMDAR trafficking to the membrane. Consistent with the Gi/o coupling of 5HT1A receptors, this effect results from a decrease in PKA activity, which reduces phosphorylation of PKA targets such as CaMKII and extracellular signal-regulated kinase (ERK) that are known to phosphorylate MAP2 on the microtubule binding domain (Figure 4). Blockade and overexpression of RGS4 respectively enhanced and attenuated the modulation of NMDAR by 5HT1A, suggesting that 5HT1A-mediated NMDAR inhibition is negatively regulated by RGS4 (Figure 4). The inhibitory effect of 5HT1A receptors in the PFC is compensated by 5HT2A/C receptors (Yuen et al., 2008). 5HT2A/C counteract the inhibitory effect of 5HT1A by increasing ERK activity in a β-arrestin-dependent manner (Figure 4). A similar G-protein-independent modality for 5HT2C receptors was also found in motoneurons of the frog spinal cord, where 5HT2C activation induced a Src-dependent increase in NMDAR currents (Bigford et al., 2012)(Figure 4). In the PFC, 5HT1A and 5HT2A/C receptors agonists used at much lower concentration than other studies (Yuen, et al., 2008;Yuen, et al., 2005) regulated NMDAR-induced depolarization and firing in a bidirectional manner (Zhong et al., 2008). At lower doses, 5HT1A and 5HT2A/C receptors agonists alone did not change NMDAR currents or membrane properties, but modulated NMDAR-induced depolarization only when co-applied with NMDA. Moreover, low doses of 5HT1A and 5HT2A/C agonists alone did not change ERK activity as reported with higher concentrations of the same antagonists, suggesting a synergistic effect of 5HT receptor/NMDAR co-activation (Yuen, et al., 2008;Yuen, et al., 2005). Whether this dosedependent 5HT receptor-activation might somehow mimic physiological (or pathological) changes in 5HT levels is unclear. Of note, both 5HT receptors and NMDARs can be expressed presynaptically (Arvanov et al., 1999;Barre et al., 2016;Bouvier, et al., 2018) (Figure 4). 5HT2A are expressed at presynaptic terminals of thalamocortical inputs to PFC where they enhance neurotransmitter release via modulation of presynaptic NMDAR in a PLC and PKC-dependent manner (Barre, et al., 2016).
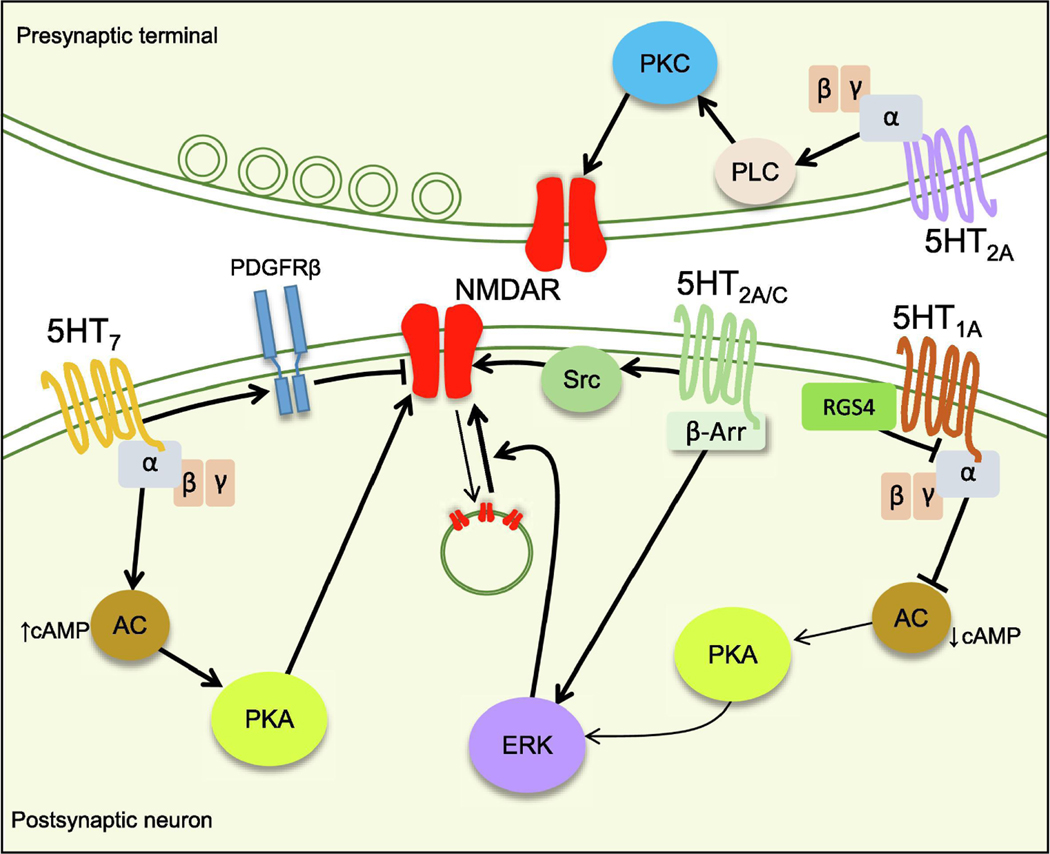
Figure 4.
Mechanisms of NMDAR modulation by 5HT receptors.
5HT7 and 5HT1A receptors regulate PKA activity and have a positive and negative effect on NMDAR function respectively. 5HT1A receptors inhibit ERK phosphorylation by PKA and reduce trafficking of NMDAR to the membrane. RGS4 negatively regulates the effect 5HT1A receptors effect on PKA activity. 5HT7 can also negatively regulate NMDAR by inducing transactivation of PDGFRβ which has an inhibitory effect on NMDAR-mediated transmission. 5HT2A/C receptors enhance NMDAR function by activation of ERK via the β-arrestin pathway or via activation of Src kinase. At the presynaptic level, 5HT2A increase NMDAR function via the PLC/PKC pathway.
Modulation of NMDARs by 5HT7 receptors
Activation of 5HT7 receptors seems to have a differential effect on NMDAR depending on the time of exposure to the agonist. A first report in isolated hippocampal neurons demonstrated that acute application of 5HT7 agonist induces an increase in NMDAR currents and phosphorylation of GluN1 and GluN2B subunit via PKA activation (Vasefi et al., 2013b) (Figure 4). Instead, a longer exposure to the 5HT7 agonist induced a PDGFR-dependent decrease in NMDAR surface which endows 5HT7 receptor with a neuroprotective effect against NMDA-induced excitotoxicity (Vasefi et al., 2013a;Vasefi et al., 2012) (Figure 4). 5HT7 receptor activation also affects NMDAR-dependent plasticity in the medial vestibular neurons where it blocks NMDAR-dependent LTD in a PKA-dependent manner (Li et al., 2017).
Muscarinic Acetylcholine Receptors
Muscarinic acetylcholine receptors include 5 subtypes of receptors (M1–5) with broad distribution in the CNS. M1, M3 and M5 receptors are Gq -coupled while M2 and M5 receptors are Gi/o-coupled (Thiele, 2013). Muscarinic receptors can be expressed both pre- and postsynaptically. Early evidence suggested that blockade of muscarinic receptors impaired memory performance (Berger et al., 1969;Drachman et al., 1974). In addition, blockade of muscarinic receptors during induction blocked LTP (Hirotsu et al., 1989), suggesting these receptors could facilitate LTP induction presumably by targeting NMDARs.
Bidirectional modulation of NMDARs by M1 receptors
In the hippocampus, both acetylcholine and muscarinic agonists transiently increased NMDAR-mediated transmission in a calcium- and IP3R-dependent manner (Harvey et al., 1993;Markram et al., 1990, 1992). This effect was mediated by M1 receptors, which colocalize with NMDARs at excitatory synapses in hippocampal pyramidal neurons (Marino et al., 1998). The mechanisms underlying M1-dependent potentiation of NMDAR are diverse. In CA1 pyramidal neurons, M1dependent potentiation requires postsynaptic calcium rise and activation of IP3Rs, but not PKC (Harvey, et al., 1993;Markram, et al., 1992). Other studies reported that M1 activation with acetylcholine was sufficient to trigger LTP of NMDAR-mediated synaptic transmission, and this potentiation relied on CaMKII, PKC and Src activation and was independent of SNAREdependent exocytosis (Fernandez de Sevilla et al., 2010) (Figure 5). As in the hippocampus, activation of M1 receptors by exogenous agonists and endogenous acetylcholine triggered a PKC-dependent increase of NMDAR-evoked depolarizations in striatal neurons (Calabresi et al., 1998). While most studies have reported M1-dependent potentiation of NMDAR-mediated transmission, there is also evidence for an M1-dependent form of NMDAR LTD at Sch-CA1 synapses that is induced by agonists or endogenous acetylcholine (Jo et al., 2010). This form of LTD requires calcium rise, ryanodine receptor activation and the calcium sensor hippocalcin, and is expressed via dynamin-dependent internalization of NMDARs (Figure 5). Thus, activation of M1 receptors can induce potentiation or depression of NMDAR-mediated synaptic transmission. A clear explanation has not emerged but these opposite effects could be due to a different developmental stage – i.e. 14–16 day-old rats (Fernandez de Sevilla, et al., 2010) vs 4–5 week-old rats (Jo, et al., 2010) –, or the duration of M1 activation, where brief activation (< 1s) leads to potentiation (Fernandez de Sevilla, et al., 2010), whereas longer M1 activation (~10 min) leads to depression (Jo, et al., 2010).
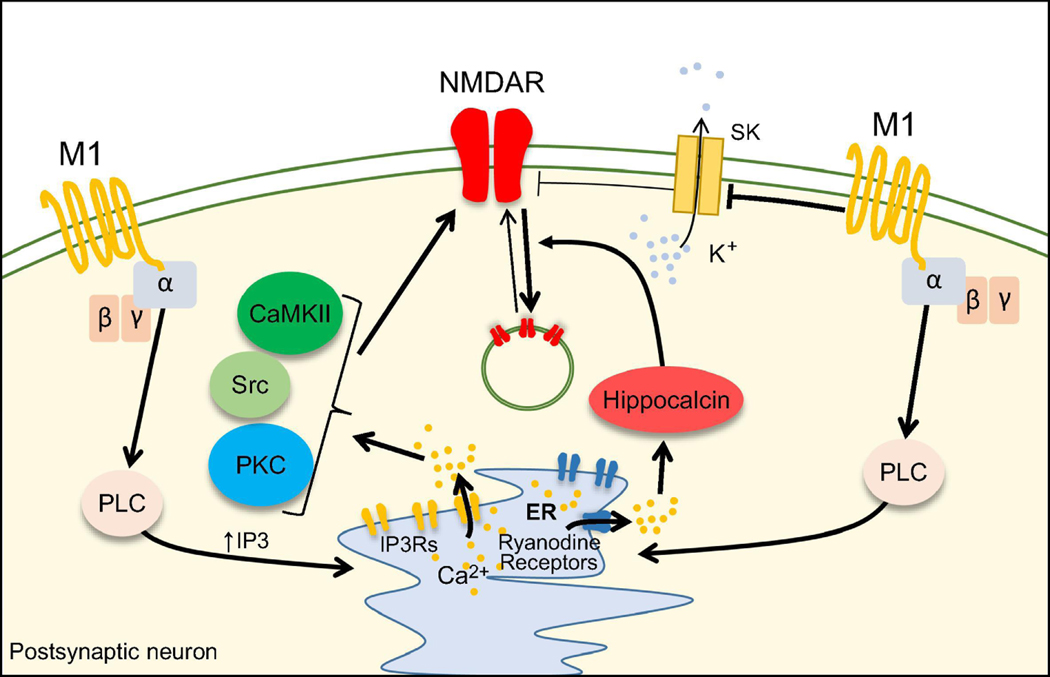
Figure 5.
Mechanisms of NMDAR modulation by muscarinic acetylcholine receptors.
Activation of M1 receptors leads to calcium release from the ER and recruitment of PKC, CaMKII and Src which in turn enhance NMDAR-mediated transmission. M1 receptors reduce membrane hyperpolarization by blocking SK-channel thus disinhibiting NMDAR function. M1 receptors can induce depression of NMDAR-mediated transmission via a pathway that involves hippocalcin-mediated dynamin-dependent internalization of NMDAR.
Indirect modulation of NMDARs by M1 receptor and SK channels
Activation of M1 receptors can indirectly affect NMDARs via inhibition of small conductance calcium-activated potassium (SK) channels (Giessel et al., 2010;Tigaret et al., 2018). Upon intracellular calcium rise, SK channels open and hyperpolarize the membrane. In dendritic spines of CA1 pyramidal neurons, activation of M1 receptors decreases the sensitivity of SK channels for calcium (Giessel, et al., 2010) (Figure 5), which boosts both NMDAR- and AMPAR-mediated depolarizations. This M1-mediated modulation of SK channels is likely implicated in the regulation of postsynaptic calcium dynamics during the induction of LTP at Sch-CA1 synapse (Faber et al., 2005;Ngo-Anh et al., 2005;Tigaret, et al., 2018).
Adrenergic receptors
The major source of norepinephrine (NE) in the brain is the locus coeruleus from which thousands of NE-producing neurons send extensive projections throughout the brain (Berridge et al., 2003). NE is released in the extracellular space and by volume transmission targets α(1–2) and β(1–3) adrenergic receptors (ARs). α1ARs and α2ARs couple to Gq and Gi/o proteins, respectively, while βARs are Gs-coupled (Berridge, et al., 2003). At central synapses ARs can be expressed pre- and postsynaptically where they modulate synaptic transmission and neuronal properties.
Modulation of NMDARs by βAR
Given its implication in behavior (Bouret et al., 2005) and in learning and memory (Tully et al., 2010), several groups have tested the role of NE in NMDAR-dependent LTP in the hippocampus. In the dentate gyrus, manipulations that depleted NE content, or blocked βARs impaired LTP (Bliss, et al., 1983;Stanton et al., 1985), while NE or a βARs agonist was sufficient to trigger long-lasting potentiation of EPSPs and population spikes both in the dentate gyrus (Lacaille et al., 1985;Neuman et al., 1983) and in the amygdala (Gean et al., 1992). Moreover, activation of βARs facilitates the induction of AMPAR LTP at Sch-CA1 in a NMDAR-dependent manner (Thomas et al., 1996), consistent with an effect of βARs activation on NMDAR.
PKA-dependent modulation of NMDARs by βARs
In autaptic CA1 neurons, NMDARs are continuously phosphorylated by PKA under basal conditions, and a brief burst of synaptic activity induced calcineurin-dependent dephosphorylation of NMDARs that reduced NMDAR currents (Raman et al., 1996) (Figure 6). Activation of βARs prevented calcineurin-induced desensitization of NMDARs by increasing the activity of PKA. The putative aminoacidic residue responsible for βARs-mediated phosphorylation of NMDAR by PKA was found nearly 20 years after. A serine-to-alanine mutation on Ser1166 of the GluN2B prevented βAR-mediated increase in NMDAR EPSCs, dendritic spine calcium rise and phosphorylation of GluN2B subunit by PKA (Murphy, et al., 2014). Remarkably, PKA phosphorylation can also modulate NMDAR calcium permeability without significantly changing NMDAR-mediated EPSCs (Chalifoux, et al., 2010;Higley, et al., 2010;Lur, et al., 2015;Skeberdis, et al., 2006). Since PKA has several targets on the NMDAR (Aman, et al., 2014), different neuromodulators might induce NMDAR phosphorylation by PKA at specific residues/subunits with diverse consequences for NMDAR function.
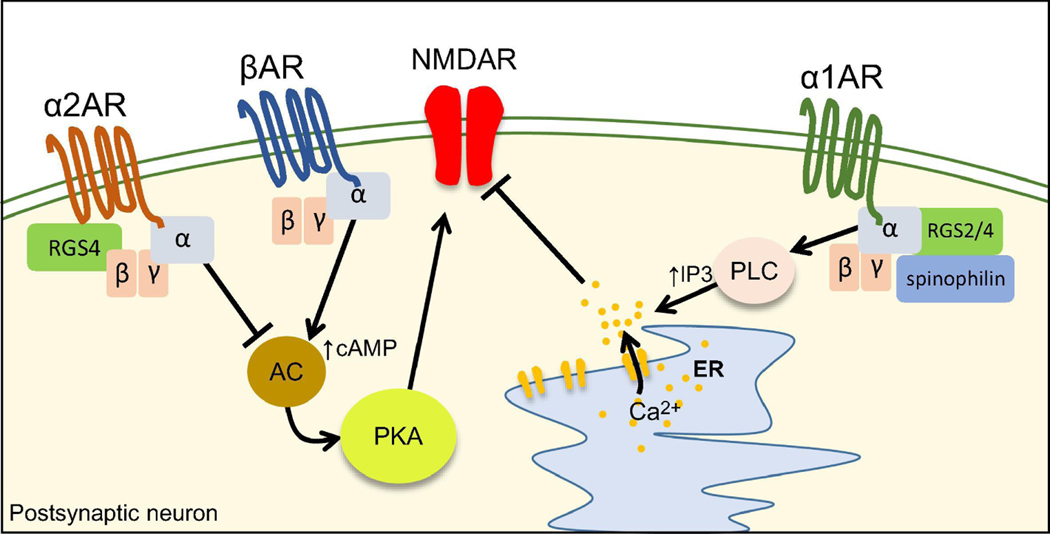
Figure 6.
Mechanisms of NMDAR modulation by adrenergic receptors.
α2ARs inhibit NMDAR function via negative-regulation of the AC/cAMP/PKA pathway. α2AR inhibitory effect on NMDAR function relies on the interaction with RGS4. βARs enhance NMDAR function by positively modulating the cAMP/PKA pathway. Activation of α1ARs triggers a PLC/IP3/calcium-dependent depression of NMDAR transmission. The inhibitory effect of α1ARs relies on the interaction with RGS2/4 and the scaffold protein spinophilin.
Modulation of NMDARs by αARs
In PFC slices and dissociated neurons, activation of α1ARs and α2ARs by agonists triggers a transient and reversible reduction in NMDAR-mediated transmission (Liu et al., 2006a). Activation of α1Rs triggers a PLC/IP3/calcium-dependent reduction of NMDAR EPSCs (Figure 6). α1ARs modulation of NMDAR required both RGS2/4 and spinophilin, a scaffold protein that binds GPCRs and RGS allowing their interaction(Liu, et al., 2006a). On the other hand, α2ARs activation induced microtubule destabilization by inhibiting the cAMP/PKA/ERK pathway. The effect of α2ARs also required the activation of RGS4 but not RGS2, suggesting that different RGS can shape the net effect of neuromodulators (Liu, et al., 2006a) (Figure 6).
Adenosine receptors
Adenosine is a purinergic neuromodulator widely released throughout the brain. Unlike classic neurotransmitters, adenosine is not stored in vesicles, but it is either a product of extracellular ATP hydrolysis or is released via specific membrane transporters (Chen et al., 2014). Adenosine binds to four metabotropic receptors: A1, A2A, A2B and A3. Adenosine has been shown to modulate NMDARs mainly through Gs–coupled A2A receptors (A2ARs) (Figure 7).
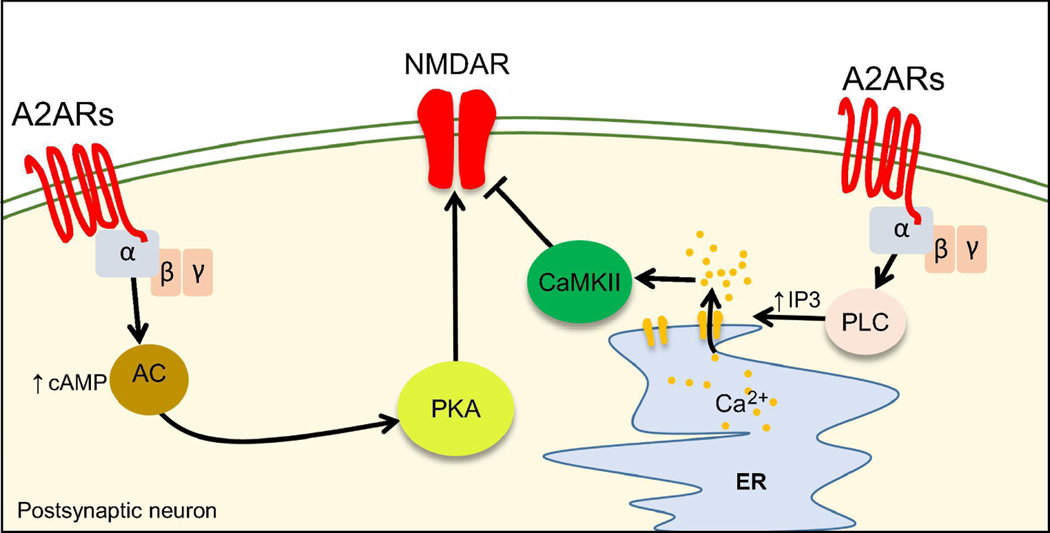
Figure 7.
Mechanisms of NMDAR modulation by A2ARs.
Activation of A2ARs can both inhibit or enhance NMDAR function. A2ARs-mediate inhibition of NMDAR relies on the activation of the PLC/IP3 pathway and on the activation of CaMKII. Positive modulation of NMDAR by A2ARs occurs through the activation of the AC/cAMP/PKA pathway.
Inhibition of NMDARs by A2A receptors
Early studies using acute striatal slices have shown that activation of A2ARs in MSNs reduced NMDA-evoked currents in a PLC/IP3/calcium/CaMKII-dependent manner (Norenberg et al., 1998;Norenberg et al., 1997;Wirkner et al., 2000) (Figure 7). Intriguingly, despite the Gs coupling, the inhibitory effect of A2ARs did not rely on the cAMP/PKA pathway, suggesting these receptors in MSNs might signal in a non-canonical manner. It should be highlighted that these studies did not target a specific subset of striatal MSN (i.e. D1-positive or D2-positive MSNs). A subset of MSNs did not display a decrease of NMDA-evoked currents in response to A2AR activation (Wirkner, et al., 2000) suggesting that A2ARs might have a cell-type specific effect (i.e. D1positive vs D2-positive MSNs).
Potentiation of NMDARs by A2ARs
More recently, it was found that activation of A2ARs in D2R-positive MSNs enhanced NMDAR-EPSCs and calcium signals in dendritic spines (Higley, et al., 2010). In the hippocampus, A2AR activation in CA1 pyramidal neurons enhanced NMDAR currents in a transient manner, while at the mf-CA3 synapse activation of A2ARs is necessary and sufficient to trigger LTP of NMDARmediated synaptic transmission (Rebola, et al., 2008). A possible explanation for these diverse effects is the colocalization and interaction of A2ARs with other GPCRs (Lohse, 2010;Prezeau et al., 2010). A2ARs colocalize and display structural/functional interactions with both mGluR5 (Ferre et al., 2002;Nishi et al., 2003;Tebano et al., 2005) and D2Rs (Fuxe et al., 2005;Hettinger et al., 2001;Torvinen et al., 2004).
NMDAR modulation by GPCRs: implications for circuit function and disease
Modulation of NMDARs by GPCRs in synaptic plasticity and circuit function
NMDARs are a critical trigger of most forms LTP and LTD of AMPAR-mediated transmission (Luscher et al., 2012). NMDAR-mediated calcium influx during the induction of plasticity is a key step in the induction of LTP and LTD. By changing the gain of NMDAR-mediated transmission, GPCRs can either decrease or increase the threshold for NMDAR-mediated forms of plasticity in a process known as metaplasticity (Abraham et al., 1996).
NMDARs also play a major role in temporal and spatial integration of excitatory inputs (Branco et al., 2010b;Hunt, et al., 2012). Activation of spatially clustered NMDARs allow supralinear summation of excitatory inputs and the generation of regenerative “all-or-none” potentials called NMDA-spikes/plateau potentials (Branco et al., 2010a;Schiller et al., 2001). NMDA spikes are important computational units that allow for the communication between remote dendritic inputs and the soma (Larkum et al., 2009) and for the encoding and decoding of incoming synaptic activity (Polsky et al., 2009). These functions of NMDA spikes may contribute to the encoding of sensory inputs (Manita et al., 2017). Because of their slow kinetics NMDA-spikes/plateau potentials might participate in the generation of persistent firing states (Wang, 1999). Persistent activity likely underlies short-term retention of stimulus-related information (Constantinidis et al., 2018;Zylberberg et al., 2017), a putative mechanism of working memory (Riley et al., 2015). In the PFC, a brain region involved in working memory (Riley, et al., 2015), D2R-dependent LTD of NMDAR-mediated transmission reduces the temporal integration of incoming hippocampal inputs with possible repercussions on pyramidal cell firing and PFC function (Banks, et al., 2015). This effect could be counterbalanced by D1Rs that induce NMDAR LTP and enhance temporal integration of synaptic inputs (Seamans et al., 2001). 5HT1A and 5HT2A receptors also bidirectionally modulate NMDARs (Barre, et al., 2016;Yuen, et al., 2008;Yuen, et al., 2005;Zhong, et al., 2008). 5HT2A receptors in the PFC have recently been implicated in cognitive processes and associative learning (Barre, et al., 2016), but it is unclear whether this action is due to pre- or postsynaptic modulation of NMDAR. At the mf-CA3 synapse, mGluR5-dependent LTP and mGluR1-dependent MDAR LTD modulate the output firing of CA3 pyramidal cells induced by presynaptic bursts of activity (Hunt, et al., 2013). There is also evidence that NMDAR LTP at this synapse can trigger homo- and heterosynaptic metaplasticity (Hunt, et al., 2013;Rebola et al., 2011). Both, the increase of CA3 output and metaplasticity triggered by NMDAR LTP could have important implications in hippocampal computations and in the creation/dismantling of CA3 neuronal assemblies during memory storage (Hunt, et al., 2013), consistent with a role of CA3 NMDARs in memory retrieval (Nakazawa et al., 2002). Thus, by regulating NMDAR-transmission and plasticity, group I mGluRs, and presumably other GPCRs, could contribute significantly to memory processing in the hippocampus. While GPCR modulation of NMDARs can strongly affect important brain functions and computations, the diverse effects of GPCRs on neuronal function impose significant challenges for understanding the precise impact of GPCR modulation of NMDARs on behavior. Future work will be required to develop novel pharmacological and optical tools that modulate specific signaling pathways linking GPCRs to NMDARs with high spatiotemporal resolution.
Modulation of NMDARs by GPCRs in brain disease
Modulation of NMDAR by GPCRs has been proposed to contribute to neuropsychiatric diseases like autism and schizophrenia. NMDAR dysfunction has been strongly linked to autism spectrum disorder, highlighting NMDAR modulation as a potential therapeutic target for autistic patients (Lee et al., 2015). Modulation of NMDAR using allosteric modulators of mGluR5 seems to ameliorate hallmark symptoms of autism such as impaired social behavior and repetitive behavior (Silverman et al., 2010;Won et al., 2012). In Fmr1 KO mice, excessive mGluR5 surface mobility impairs NMDAR-mediated transmission and mGluR-dependent LTD and leads to cognitive dysfunction (Aloisi, et al., 2017). Both pharmacological blockade and genetic deletion of mGluR5 abolish NMDAR LTP (Harnett, et al., 2009;Hunt, et al., 2013;Jia, et al., 1998;Kotecha, et al., 2003a;Kwon, et al., 2008;Rebola, et al., 2008), suggesting that impaired modulation of NMDARs by mGluR5 could be involved in fragile X syndrome.
Modulation of NMDAR by DA receptors might play an important role in schizophrenia (Javitt, 2010). Both D2Rs and NMDARs have been strongly implicated in schizophrenia (Howes et al., 2015). Antipsychotic drugs that target D2Rs are still one of the most effective treatments for schizophrenia, and NMDAR antagonists such as ketamine and phencyclidine (PCP) can mimic both the positive and negative symptoms of schizophrenia (Javitt, 2010;Javitt et al., 1991). This evidence strongly supports the dopamine-glutamate crosstalk hypothesis of schizophrenia (Howes, et al., 2015). It also suggests that alterations of the DA receptor-mediated regulation of NMDARs could contribute to some symptoms of schizophrenia and that this interaction could be a therapeutic target. Targeting GPCRs with higher selectivity has been one of the main challenges in the development of new therapies for neuropsychiatric diseases. Allosteric modulators of GPCRs represent a novel approach that is gaining attention in the treatment of diseases such as schizophrenia and autism disorder (Conn et al., 2014;Foster et al., 2017). Allosteric modulators target GPCRs on a different binding site from the endogenous ligand (the orthosteric site) and can enhance (positive allosteric modulator, PAM) or inhibit (negative allosteric modulator, NAM) the effects of endogenous ligands on their target GPCRs. PAMs and NAMs can also have intrinsic activity independent of agonist binding to the orthosteric site (Conn, et al., 2014). In addition, GPCRs possess multiple conformational states that are linked to the activation of distinct signaling pathways (e.g. G-protein-dependent vs G-protein-independent). Allosteric modulators can stabilize GPCRs in a specific conformational state and thus bias the signaling pathway of a receptor in a specific manner. This approach might be employed in the future to treat NMDAR-linked disorders without directly targeting the NMDAR itself but by binding to GPCRs that modulate NMDAR properties.
Conclusions and future directions
By changing the number and functional properties of NMDARs, several GPCRs strongly modulate NMDAR-mediated processes, including synaptic transmission and plasticity, and by this means significantly modify brain function. Depending on the context –e.g. brain area, synapse type, developmental stage– and extent of GPCR activation, the same GPCR may engage different intracellular pathways and as a result have diverse effects on NMDARs. In addition, different GPCRs and their signaling cascades can converge in a synaptic compartment. Thus, coincident activation of multiple GPCRs may have synergistic or opposite effects on NMDARs. Because many GPCRs are coupled to the same G-protein type (i.e. Gs, Gi/o, Gq), the pathways involved in the modulation of NMDARs are highly redundant. However, GPCRs with common G-protein coupling can modulate different downstream targets (i.e. AMPAR vs NMDAR) and thus be more selective than previously thought. NMDARs and GPCRs have been implicated in neuropsychiatric diseases and might be key targets for the development of new therapeutic strategies. Biased allosteric modulators could be employed to target specific GPCR pathways that are selectively involved in the modulation of NMDARs, thus avoiding harmful off-target effects.
While the molecular mechanisms that link GPCRs to NMDAR have been extensively investigated, several questions remain unanswered. It is unclear whether release of neuromodulators with physiological patterns of activity in vivo would yield similar results to those observed with pharmacological application of exogenous agonists in vitro. Classic pharmacological tools do not allow manipulation of GPCRs with high spatiotemporal resolution. In addition, studying the effect of GPCR activation on NMDARs in subcellular compartments in vivo (i.e. dendrites, dendritic spines, presynaptic boutons) is challenging in part due to the poor resolution of most imaging techniques (e.g. two-photon calcium imaging). New tools could be combined in order to achieve the spatiotemporal resolution required to measure and manipulate GPCR activity. Optogenetic activation of specific neuromodulatory inputs can mimic the release of endogenous neuromodulators, and light-activated molecules such as caged compounds and photoswitchable ligands (Paoletti et al., 2019;Spangler et al., 2017) could be combined with the measurement of subthreshold and/or population activity with genetically encoded voltage-indicators (Abdelfattah et al., 2019;Kwon et al., 2017).
Several outstanding questions about the modulation of NMDAR by GPCRs remain unanswered, including the role of heterodimerization and/or crosstalk of GPCRs targeting NMDARs. Multiple GPCRs can be expressed in the same neuron and even in the same subcellular compartment (e.g. dendritic spines, presynaptic terminals) (Hunt, et al., 2013;Lur, et al., 2015;Tebano, et al., 2005). Although the functional relevance of GPCRs heterodimers is still matter of debate (Gurevich et al., 2018), heterodimerization and/or simultaneous activation of different GPCRs might lead to the activation of unexpected signaling cascades that normally would not be engaged by single protomers (Lohse, 2010;Prezeau, et al., 2010). It is currently unknown whether gliotransmitters released by astrocytes could influence NMDAR via GPCRs. Astrocytes can release both glutamate and ATP (Harada, et al., 2015), which can target mGluRs and adenosine receptors (upon ATP conversion to adenosine), respectively. However it is unclear whether modulation of NMDARs by astrocytes occurs through activation of GPCRs by gliotransmitters. Lastly, it would be important to know whether GPCRs could also modify the metabotropic function of NMDARs.
GPCRs are powerful modulators of NMDAR function. While several mechanisms involved in the GPCR-mediated modulation of NMDAR properties have been elucidated, we are still far from understanding the exact role of this modulation in brain function and disease. The development of new optical and pharmacological tools that allow controlling GPCRs with high spatiotemporal resolution will not only advance our understanding on the role of NMDARs in circuit function and behavior, but may also reveal novel targets to control NMDAR dysregulations implicated in brain disorders.
HIGHLIGHTS.
GPCRs are strong modulators of NMDAR function throughout the brain
Modulation of NMDARs by GPCRs affects neuronal integrative properties and can induce metaplasticity
Multiple GPCRs can coexist at the same synapse and shape NMDAR function in an complex manner
Novel pharmacological and optical tools that target GPCRs will help to achieve precise spatiotemporal control of NMDARs
Acknowledgments
This work was supported by the NIH (R01-MH081935, R01-DA17392, R01-NS113600 to P.E.C.). We thank Coralie Berthoux, Hannah Monday, Kaoutsar Nasrallah and Michelle Gulfo for critically reading and editing the manuscript. We apologize to all investigators whose work we could not cite due to space limitations.
Footnotes
Disclosure Statement: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdelfattah AS, Kawashima T, Singh A, Novak O, Liu H, Shuai Y, Huang YC, Campagnola L, et al. (2019), Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 365:699–704. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF (1996), Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19:126–130. [DOI] [PubMed] [Google Scholar]
- Aloisi E, Le Corf K, Dupuis J, Zhang P, Ginger M, Labrousse V, Spatuzza M, Georg Haberl M, et al. (2017), Altered surface mGluR5 dynamics provoke synaptic NMDAR dysfunction and cognitive defects in Fmr1 knockout mice. Nat Commun 8:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman TK, Maki BA, Ruffino TJ, Kasperek EM, Popescu GK (2014), Separate intramolecular targets for protein kinase A control N-methyl-D-aspartate receptor gating and Ca2+ permeability. J Biol Chem 289:18805–18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniksztejn L, Bregestovski P, Ben-Ari Y (1991), Selective activation of quisqualate metabotropic receptor potentiates NMDA but not AMPA responses. Eur J Pharmacol 205:327–328. [DOI] [PubMed] [Google Scholar]
- Anwyl R (1999), Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29:83–120. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Magro P, Roberts R, Wang RY (1999), A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci 11:2917–2934. [DOI] [PubMed] [Google Scholar]
- Banks PJ, Burroughs AC, Barker GR, Brown JT, Warburton EC, Bashir ZI (2015), Disruption of hippocampalprefrontal cortex activity by dopamine D2R-dependent LTD of NMDAR transmission. Proc Natl Acad Sci U S A 112:11096–11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre A, Berthoux C, De Bundel D, Valjent E, Bockaert J, Marin P, Becamel C (2016), Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A 113:E1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu-Laroche L, Harnett MT (2018), Dendritic Spines Prevent Synaptic Voltage Clamp. Neuron 97:7582 e73. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR (2011), The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. [DOI] [PubMed] [Google Scholar]
- Beazely MA, Tong A, Wei WL, Van Tol H, Sidhu B, MacDonald JF (2006), D2-class dopamine receptor inhibition of NMDA currents in prefrontal cortical neurons is platelet-derived growth factor receptordependent. J Neurochem 98:1657–1663. [DOI] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U (2002), Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci 22:9679–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger BD, Stein L (1969), An analysis of the learning deficits produced by scopolamine. Psychopharmacologia 14:271–283. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD (2003), The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42:33–84. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M (2004), Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84:835–867. [DOI] [PubMed] [Google Scholar]
- Bhouri M, Farrow PA, Motee A, Yan X, Battaglia G, Di Menna L, Riozzi B, Nicoletti F, et al. (2014), mGlu1 receptor-induced LTD of NMDA receptor transmission selectively at Schaffer collateral-CA1 synapses mediates metaplasticity. J Neurosci 34:12223–12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigford GE, Chaudhry NS, Keane RW, Holohean AM (2012), 5-Hydroxytryptamine 5HT2C receptors form a protein complex with N-methyl-D-aspartate GluN2A subunits and activate phosphorylation of Src protein to modulate motoneuronal depolarization. J Biol Chem 287:11049–11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Goddard GV, Riives M (1983), Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J Physiol 334:475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Pin JP (1999), Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J 18:1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ (2005), Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28:574–582. [DOI] [PubMed] [Google Scholar]
- Bouvier G, Larsen RS, Rodriguez-Moreno A, Paulsen O, Sjostrom PJ (2018), Towards resolving the presynaptic NMDA receptor debate. Curr Opin Neurobiol 51:1–7. [DOI] [PubMed] [Google Scholar]
- Branco T, Clark BA, Hausser M (2010a), Dendritic discrimination of temporal input sequences in cortical neurons. Science 329:1671–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Hausser M (2010b), The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol 20:494–502. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G (1998), Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur J Neurosci 10:2887–2895. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS (1998), Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol 79:82–94. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS (2006), Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE 2006:pe20. [DOI] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG (2010), GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron 66:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Greengard P, Yan Z (2004), Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci U S A 101:2596–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Lee CF, Chern Y (2014), Adenosine receptor neurobiology: overview. Int Rev Neurobiol 119:1–49. [DOI] [PubMed] [Google Scholar]
- Chen Y, Granger AJ, Tran T, Saulnier JL, Kirkwood A, Sabatini BL (2017), Endogenous Galphaq-Coupled Neuromodulator Receptors Activate Protein Kinase A. Neuron 96:1070–1083 e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Meiler J, Niswender CM (2014), Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat Rev Drug Discov 13:692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP (1997), Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Funahashi S, Lee D, Murray JD, Qi XL, Wang M, Arnsten AFT (2018), Persistent Spiking Activity Underlies Working Memory. J Neurosci 38:7020–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L (1999), Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409:187–209. [DOI] [PubMed] [Google Scholar]
- Dascal N (2001), Ion-channel regulation by G proteins. Trends Endocrinol Metab 12:391–398. [DOI] [PubMed] [Google Scholar]
- Dore K, Aow J, Malinow R (2015), Agonist binding to the NMDA receptor drives movement of its cytoplasmic domain without ion flow. Proc Natl Acad Sci U S A 112:14705–14710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore K, Aow J, Malinow R (2016), The Emergence of NMDA Receptor Metabotropic Function: Insights from Imaging. Front Synaptic Neurosci 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J (1974), Human memory and the cholinergic system. A relationship to aging? Arch Neurol 30:113–121. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir R, et al. (2003), Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem 87:922–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K (1998), Serotonin inhibits the induction of long-term potentiation in rat primary visual cortex. Prog Neuropsychopharmacol Biol Psychiatry 22:983–997. [DOI] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K (1999), Stimulation of the 5-HT1A receptor selectively suppresses NMDA receptor-mediated synaptic excitation in the rat visual cortex. Brain Res 827:225–228. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P (2005), SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 8:635–641. [DOI] [PubMed] [Google Scholar]
- Fernandez de Sevilla D, Buno W (2010), The muscarinic long-term enhancement of NMDA and AMPA receptor-mediated transmission at Schaffer collateral synapses develop through different intracellular mechanisms. J Neurosci 30:11032–11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, Casado V, Fuxe K, et al. (2002), Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A 99:11940–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS (2002), Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol 88:3010–3020. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Conn PJ (2017), Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 94:431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Canals M, Torvinen M, Terasmaa A, Marcellino D, Goldberg SR, Staines W, et al. (2005), Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci 26:209–220. [DOI] [PubMed] [Google Scholar]
- Garaycochea J, Slaughter MM (2016), GABAB receptors enhance excitatory responses in isolated rat retinal ganglion cells. J Physiol 594:5543–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gean PW, Huang CC, Lin JH, Tsai JJ (1992), Sustained enhancement of NMDA receptor-mediated synaptic potential by isoproterenol in rat amygdalar slices. Brain Res 594:331–334. [DOI] [PubMed] [Google Scholar]
- Giessel AJ, Sabatini BL (2010), M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron 68:936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Zito K, Hell JW (2016), Non-ionotropic signaling by the NMDA receptor: controversy and opportunity. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2018), GPCRs and Signal Transducers: Interaction Stoichiometry. Trends Pharmacol Sci 39:672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Kamiya T, Tsuboi T (2015), Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front Neurosci 9:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Bernier BE, Ahn KC, Morikawa H (2009), Burst-timing-dependent plasticity of NMDA receptormediated transmission in midbrain dopamine neurons. Neuron 62:826–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney SC, Rowan M, Anwyl R (2006), Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to longterm potentiation by low intracellular calcium buffering. J Neurosci 26:1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Balasubramaniam R, Collingridge GL (1993), Carbachol can potentiate N-methyl-D-aspartate responses in the rat hippocampus by a staurosporine and thapsigargin-insensitive mechanism. Neurosci Lett 162:165–168. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL (2001), Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol 431:331–346. [DOI] [PubMed] [Google Scholar]
- Heuss C, Gerber U (2000), G-protein-independent signaling by G-protein-coupled receptors. Trends Neurosci 23:469–475. [DOI] [PubMed] [Google Scholar]
- Heuss C, Scanziani M, Gahwiler BH, Gerber U (1999), G-protein-independent signaling mediated by metabotropic glutamate receptors. Nat Neurosci 2:1070–1077. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL (2010), Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu I, Hori N, Katsuda N, Ishihara T (1989), Effect of anticholinergic drug on long-term potentiation in rat hippocampal slices. Brain Res 482:194–197. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR (2002), Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev 54:527–559. [DOI] [PubMed] [Google Scholar]
- Howes O, McCutcheon R, Stone J (2015), Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 29:97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR (2002), Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Be 71:533–554. [DOI] [PubMed] [Google Scholar]
- Hunt DL, Castillo PE (2012), Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol 22:496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DL, Puente N, Grandes P, Castillo PE (2013), Bidirectional NMDA receptor plasticity controls CA3 output and heterosynaptic metaplasticity. Nat Neurosci 16:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci GJ, Popescu GK (2018), Kinetic models for activation and modulation of NMDA receptor subtypes. Curr Opin Physiol 2:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC (2010), Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci 47:4–16. [PubMed] [Google Scholar]
- Javitt DC, Zukin SR (1991), Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J (1998), Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem 5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Mao LM, Wang JQ (2013), Differential regulation of CaMKIIalpha interactions with mGluR5 and NMDA receptors by Ca(2+) in neurons. J Neurochem 127:620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Xue B, Mao LM, Wang JQ (2015), Metabotropic glutamate receptor 5 upregulates surface NMDA receptor expression in striatal neurons via CaMKII. Brain Res 1624:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Son GH, Winters BL, Kim MJ, Whitcomb DJ, Dickinson BA, Lee YB, Futai K, et al. (2010), Muscarinic receptors induce LTD of NMDAR EPSCs via a mechanism involving hippocalcin, AP2 and PSD-95. Nat Neurosci 13:1216–1224. [DOI] [PubMed] [Google Scholar]
- Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin JP (1995), Molecular, functional, and pharmacological characterization of the metabotropic glutamate receptor type 5 splice variants: comparison with mGluR1. J Neurosci 15:3970–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Worley PF (2007), Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci U S A 104:6055–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Jang HJ, Cho KH, Hahn SJ, Kim MJ, Yoon SH, Jo YH, Kim MS, et al. (2006), Serotonin inhibits the induction of NMDA receptor-dependent long-term potentiation in the rat primary visual cortex. Brain Res 1103:49–55. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, MacDonald JF (2003a), Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem 278:27742–27749. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, MacDonald JF (2003b), Signaling molecules and receptor transduction cascades that regulate NMDA receptor-mediated synaptic transmission. Int Rev Neurobiol 54:51–106. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF (2002), A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron 35:1111–1122. [DOI] [PubMed] [Google Scholar]
- Kristiansen K (2004), Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther 103:21–80. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE (2008), Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron 57:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T, Sakamoto M, Peterka DS, Yuste R (2017), Attenuation of Synaptic Potentials in Dendritic Spines. Cell Rep 20:1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Harley CW (1985), The action of norepinephrine in the dentate gyrus: beta-mediated facilitation of evoked potentials in vitro. Brain Res 358:210–220. [DOI] [PubMed] [Google Scholar]
- Ladepeche L, Dupuis JP, Bouchet D, Doudnikoff E, Yang L, Campagne Y, Bezard E, Hosy E, et al. (2013), Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proc Natl Acad Sci U S A 110:18005–18010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, et al. (2001a), Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci 4:382–390. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Zheng X, Bennett MV, Zukin RS (2001b), Activation of metabotropic glutamate receptor 1 accelerates NMDA receptor trafficking. J Neurosci 21:6058–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J (2009), Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325:756–760. [DOI] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS (2010), SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci 30:242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS (2007), NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Choi SY, Kim E (2015), NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol 20:8–13. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, et al. (2002), Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell 111:219–230. [DOI] [PubMed] [Google Scholar]
- Letellier M, Park YK, Chater TE, Chipman PH, Gautam SG, Oshima-Takago T, Goda Y (2016), Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc Natl Acad Sci U S A 113:E2685–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Liu G, Hu JL, Gao WJ, Huang YQ (2010), Dopamine D(1) receptor-mediated enhancement of NMDA receptor trafficking requires rapid PKC-dependent synaptic insertion in the prefrontal neurons. J Neurochem 114:62–73. [DOI] [PubMed] [Google Scholar]
- Li YH, Han L, Wu KLK, Chan YS (2017), Activation of 5-HT7 receptors reverses NMDA-R-dependent LTD by activating PKA in medial vestibular neurons. Neuropharmacology 123:242–248. [DOI] [PubMed] [Google Scholar]
- Liu W, Yuen EY, Allen PB, Feng J, Greengard P, Yan Z (2006a), Adrenergic modulation of NMDA receptors in prefrontal cortex is differentially regulated by RGS proteins and spinophilin. Proc Natl Acad Sci U S A 103:18338–18343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, et al. (2006b), Modulation of D2R-NR2B interactions in response to cocaine. Neuron 52:897–909. [DOI] [PubMed] [Google Scholar]
- Lohse MJ (2010), Dimerization in GPCR mobility and signaling. Curr Opin Pharmacol 10:53–58. [DOI] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P (1996), Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci 8:1488–1500. [DOI] [PubMed] [Google Scholar]
- Lur G, Higley MJ (2015), Glutamate Receptor Modulation Is Restricted to Synaptic Microdomains. Cell Rep 12:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC (2012), NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manita S, Miyakawa H, Kitamura K, Murayama M (2017), Dendritic Spikes in Sensory Perception. Front Cell Neurosci 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ (1998), eceptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A 95:11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Segal M (1990), Acetylcholine potentiates responses to N-methyl-D-aspartate in the rat hippocampus. Neurosci Lett 113:62–65. [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M (1992), The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol 447:513–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks CR, Shonesy BC, Wang X, Stephenson JR, Niswender CM, Colbran RJ (2018), Activated CaMKIIalpha Binds to the mGlu5 Metabotropic Glutamate Receptor and Modulates Calcium Mobilization. Mol Pharmacol 94:1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS (2005), G-protein signaling: back to the future. Cell Mol Life Sci 62:551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisett RA, Mott DD, Lewis DV, Swartzwelder HS, Wilson WA (1991), GABAB-receptor-mediated inhibition of the N-methyl-D-aspartate component of synaptic transmission in the rat hippocampus. J Neurosci 11:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Rouaud E, Sinet PM, Potier B, Jouvenceau A, Dutar P, Videau C, Epelbaum J, et al. (2006), A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell 5:267–274. [DOI] [PubMed] [Google Scholar]
- Murphy JA, Stein IS, Lau CG, Peixoto RT, Aman TK, Kaneko N, Aromolaran K, Saulnier JL, et al. (2014), Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J Neurosci 34:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, Malinow R (2013), Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci U S A 110:4027–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, et al. (2002), Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Cuartero MI, Palenzuela R, Draffin JE, Konomi A, Serra I, Colie S, Castano-Castano S, et al. (2019), Astrocytic p38alpha MAPK drives NMDA receptor-dependent long-term depression and modulates long-term memory. Nat Commun 10:2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard S, Engberg I, Flatman JA (1986), Serotonin facilitates NMDA responses of cat neocortical neurones. Acta Physiol Scand 128:323–325. [DOI] [PubMed] [Google Scholar]
- Neuman RS, Harley CW (1983), Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res 273:162–165. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP (2005), SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8:642–649. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP (2011), Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60:1017–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Liu F, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P (2003), Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci U S A 100:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ (2010), Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg W, Wirkner K, Assmann H, Richter M, Illes P (1998), Adenosine A2A receptors inhibit the conductance of NMDA receptor channels in rat neostriatal neurons. Amino Acids 14:33–39. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Wirkner K, Illes P (1997), Effect of adenosine and some of its structural analogues on the conductance of NMDA receptor channels in a subset of rat neostriatal neurones. Br J Pharmacol 122:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JJ, Rowan MJ, Anwyl R (1994), Long-lasting enhancement of NMDA receptor-mediated synaptic transmission by metabotropic glutamate receptor activation. Nature 367:557–559. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Miyata M, Hashimoto K, Tabata T, Kishimoto Y, Fukaya M, Kase D, Kassai H, et al. (2014), The synaptic targeting of mGluR1 by its carboxyl-terminal domain is crucial for cerebellar function. J Neurosci 34:2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE (2004), Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol 92:2027–2039. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH (2006), Glia-derived Dserine controls NMDA receptor activity and synaptic memory. Cell 125:775–784. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q (2013), NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14:383–400. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ellis-Davies GCR, Mourot A (2019), Optical control of neuronal ion channels and receptors. Nat Rev Neurosci 20:514–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlos NJ, Friedman PA (2017), GPCR Signaling and Trafficking: The Long and Short of It. Trends Endocrinol Metab 28:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddie CJ, Davies HA, Colyer FM, Stewart MG, Rodriguez JJ (2008), Dendritic colocalisation of serotonin1B receptors and the glutamate NMDA receptor subunit NR1 within the hippocampal dentate gyrus: an ultrastructural study. J Chem Neuroanat 36:17–26. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zhao J, Gu QH, Chen RQ, Xu Z, Yan JZ, Wang SH, Liu SY, et al. (2010), Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus 20:646–658. [DOI] [PubMed] [Google Scholar]
- Polsky A, Mel B, Schiller J (2009), Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. J Neurosci 29:11891–11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezeau L, Gomeza J, Ahern S, Mary S, Galvez T, Bockaert J, Pin JP (1996), Changes in the carboxyl-terminal domain of metabotropic glutamate receptor 1 by alternative splicing generate receptors with differing agonist-independent activity. Mol Pharmacol 49:422–429. [PubMed] [Google Scholar]
- Prezeau L, Rives ML, Comps-Agrar L, Maurel D, Kniazeff J, Pin JP (2010), Functional crosstalk between GPCRs: with or without oligomerization. Curr Opin Pharmacol 10:6–13. [DOI] [PubMed] [Google Scholar]
- Rahman S, Neuman RS (1993), Activation of 5-HT2 receptors facilitates depolarization of neocortical neurons by N-methyl-D-aspartate. Eur J Pharmacol 231:347–354. [DOI] [PubMed] [Google Scholar]
- Raman IM, Tong G, Jahr CE (1996), Beta-adrenergic regulation of synaptic NMDA receptors by cAMPdependent protein kinase. Neuron 16:415–421. [DOI] [PubMed] [Google Scholar]
- Rebola N, Carta M, Lanore F, Blanchet C, Mulle C (2011), NMDA receptor-dependent metaplasticity at hippocampal mossy fiber synapses. Nat Neurosci 14:691–693. [DOI] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C (2008), Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57:121–134. [DOI] [PubMed] [Google Scholar]
- Rebola N, Srikumar BN, Mulle C (2010), Activity-dependent synaptic plasticity of NMDA receptors. J Physiol 588:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Baskys A, Carlen PL (1988), The effects of serotonin on N-methyl-D-aspartate and synaptically evoked depolarizations in rat neocortical neurons. Brain Res 456:286–292. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, et al. (2000), Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol 417:181–194. [PubMed] [Google Scholar]
- Riley MR, Constantinidis C (2015), Role of Prefrontal Persistent Activity in Working Memory. Front Syst Neurosci 9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders H, Berends M, Major G, Goldman MS, Lisman JE (2013), NMDA and GABAB (KIR) conductances: the “perfect couple” for bistability. J Neurosci 33:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Nicoll RA, Roche KW (2013), Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist 19:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Schiller Y (2001), NMDA receptor-mediated dendritic spikes and coincident signal amplification. Curr Opin Neurobiol 11:343–348. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ (2001), Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A 98:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN (2010), Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 35:976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, et al. (2006), Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci 9:501–510. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS (1994), D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci U S A 91:5720–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P (1998), A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci 18:10297–10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler SM, Bruchas MR (2017), Optogenetic approaches for dissecting neuromodulation and GPCR signaling in neural circuits. Curr Opin Pharmacol 32:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM (1985), Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci 5:2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Otaky N (1994), Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res 643:10–16. [DOI] [PubMed] [Google Scholar]
- Stein IS, Gray JA, Zito K (2015), Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage. J Neurosci 35:12303–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Gro MC, Schwarzschild MA, Chen JF, et al. (2005), Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-Daspartate effects. J Neurochem 95:1188–1200. [DOI] [PubMed] [Google Scholar]
- Thiele A (2013), Muscarinic signaling in the brain. Annu Rev Neurosci 36:271–294. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O’Dell TJ (1996), Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 17:475–482. [DOI] [PubMed] [Google Scholar]
- Tigaret CM, Chamberlain SEL, Sadowski J, Hall J, Ashby MC, Mellor JR (2018), Convergent Metabotropic Signaling Pathways Inhibit SK Channels to Promote Synaptic Plasticity in the Hippocampus. J Neurosci 38:9252–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torvinen M, Kozell LB, Neve KA, Agnati LF, Fuxe K (2004), Biochemical identification of the dopamine D2 receptor domains interacting with the adenosine A2A receptor. J Mol Neurosci 24:173–180. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, et al. (2010), Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62:405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL (2012), Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, et al. (1999), Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23:583–592. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF (1998), Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21:717–726. [DOI] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY (2010), Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasefi MS, Kruk JS, Heikkila JJ, Beazely MA (2013a), 5-Hydroxytryptamine type 7 receptor neuroprotection against NMDA-induced excitotoxicity is PDGFbeta receptor dependent. J Neurochem 125:26–36. [DOI] [PubMed] [Google Scholar]
- Vasefi MS, Kruk JS, Liu H, Heikkila JJ, Beazely MA (2012), Activation of 5-HT7 receptors increases neuronal platelet-derived growth factor beta receptor expression. Neurosci Lett 511:65–69. [DOI] [PubMed] [Google Scholar]
- Vasefi MS, Yang K, Li J, Kruk JS, Heikkila JJ, Jackson MF, MacDonald JF, Beazely MA (2013b), Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Mol Brain 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, O’Donnell P (2001), D(1) dopamine receptors potentiate nmda-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex 11:452–462. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Gu Z, Yan Z (2003), Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J Neurosci 23:9852–9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ (1999), Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J Neurosci 19:9587–9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Mitchell SJ (2008), Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11:790–798. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Assmann H, Koles L, Gerevich Z, Franke H, Norenberg W, Boehm R, Illes P (2000), Inhibition by adenosine A(2A) receptors of NMDA but not AMPA currents in rat neostriatal neurons. Br J Pharmacol 130:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, et al. (2012), Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 486:261–265. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, et al. (1998), Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron 21:707–716. [DOI] [PubMed] [Google Scholar]
- Yang K, Jackson MF, MacDonald JF (2014), Recent progress in understanding subtype specific regulation of NMDA receptors by G Protein Coupled Receptors (GPCRs). Int J Mol Sci 15:3003–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi PL, Chang FC, Tsai JJ, Hung CR, Gean PW (1995), The involvement of metabotropic glutamate receptors in long-term depression of N-methyl-D-aspartate receptor-mediated synaptic potential in the rat hippocampus. Neurosci Lett 185:207–210. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z (2008), Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem 283:17194–17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z (2005), Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci 25:5488–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan L, Ren Y, Liang J, Lin R, Feng Q, Zhou J, Hu F, et al. (2016), Presynaptic Excitation via GABAB Receptors in Habenula Cholinergic Neurons Regulates Fear Memory Expression. Cell 166:716–728. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS (1999), Protein kinase C potentiation of N-methyl-Daspartate receptor activity is not mediated by phosphorylation of N-methyl-D-aspartate receptor subunits. Proc Natl Acad Sci U S A 96:15262–15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Yuen EY, Yan Z (2008), Modulation of neuronal excitability by serotonin-NMDA interactions in prefrontal cortex. Mol Cell Neurosci 38:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylberberg J, Strowbridge BW (2017), Mechanisms of Persistent Activity in Cortical Circuits: Possible Neural Substrates for Working Memory. Annu Rev Neurosci 40:603–627. [DOI] [PMC free article] [PubMed] [Google Scholar]